Abstract
The organ crosstalk can be defined as the complex biological communication and feedback between distant organs mediated via cellular, molecular, neural, endocrine and paracrine factors. In the normal state, this crosstalk helps to maintain homeostasis and optimal functioning of the human body. However, during disease states this very crosstalk can carry over the influence of the diseased organ to initiate and perpetuate structural and functional dysfunction in the other organs. Heart performance and kidney function are intimately interconnected, and the communication between these organs occurs through a variety of bidirectional pathways. The cardiorenal syndrome (CRS) is defined as a complex pathophysiological disorder of the heart and the kidneys whereby acute or chronic dysfunction in one organ may induce acute or chronic dysfunction in the other organ. In particular, CRS type 1 is characterized by a rapid worsening of the cardiac function leading to acute kidney injury. This clinical condition requires a more complex management given its more complicated hospital course and higher mortality. A lot of research has emerged in the last years trying to explain the pathophysiology of CRS type 1 which remains in part poorly understood. This review primarily focuses on the hemodynamic and nonhemodynamic mechanisms involved in this syndrome.
Key Words: Cardiorenal syndrome, Organ crosstalk, Heart and renal dysfunction, Acute kidney injury, Hemodynamic mechanisms
Introduction
The so-called organ crosstalk refers to the complex cellular communication, conveyed by afferent vagal neurons and circulating myeloid and lymphoid cells, between distant systems in the organism. This physiological crosstalk is necessary to preserve regular homeostasis and normal functioning of the different organs. However, in the diseased state, this strong connection can carry over the influence and signaling of the damaged organ and can induce structural and functional dysfunctions in other organs [1,2].
Heart performance and kidney function are strictly interconnected, and the communication between the two organs occurs through a variety of pathways, including perfusion, filling pressure and neurohormonal activity [3]. The dynamic connections between both acute and chronic cardiac dysfunction and acute and chronic kidney disease are well recognized and classified as the cardiorenal syndrome (CRS) by the consensus conference of the Acute Dialysis Quality Initiative (ADQI) [4,5].
The current definition has been expanded into 5 subtypes of which the etymology reflects the primary and secondary pathology, the time frame as well as the simultaneous cardiac and renal co-dysfunction secondary to systemic disease [5]. The classification is not intended to be stationary; it is acknowledged that many patients may transition between different CRS subtypes during the course of their disease [6].
The CRS type 1 is characterized by a rapid worsening of the cardiac function leading to acute kidney injury (AKI). Increases in serum creatinine (sCr) ranging from 0.1 to 0.5 mg/dl and of 25-50% from baseline have been used to define this type of CRS. Other definitions include changes in the estimated glomerular filtration rate (eGFR; e.g., a decrease in eGFR of 25%), changes in sCr and/or urine output, or changes in blood urea nitrogen. Different studies also considered variable time frames for the ascertainment of this end point. Most commonly, the period of observation is within the hospital admission [7].
Recognizing the limitations of having varied definitions, CRS type 1 occurs in approximately 25-33% of patients admitted with acute decompensate heart failure and follows ischemic or nonischemic cardiac events [8]. It is an important consequence of hospitalization with a myriad of implications in terms of diagnosis, prognosis and a more complex management [4,5]. Furthermore, patients who develop AKI after an acute cardiac event have a significantly high morbidity and mortality, an increased stroke risk, longer hospitalization and a greater incidence of readmission [9]. Currently, no pharmacological agents have been proven to prevent AKI, and the mortality rate of patients with severe AKI has not declined in the recent decades [2,7]. The pathophysiology of CRS type 1 is multifaceted and involves numerous factors [10]. In recent years, there has been growing interest in organ-organ interactions as a way of understanding the underlying pathophysiology of concomitant heart and renal dysfunction (fig. 1). The complexity of this syndrome presents a key challenge for singular diagnostic and treatment approaches. A lot of research has emerged in the last years trying to explain the pathophysiology of CRS type 1 which remains in part poorly understood. This review primarily focuses on the hemodynamic and nonhemodynamic mechanisms involved in this syndrome.
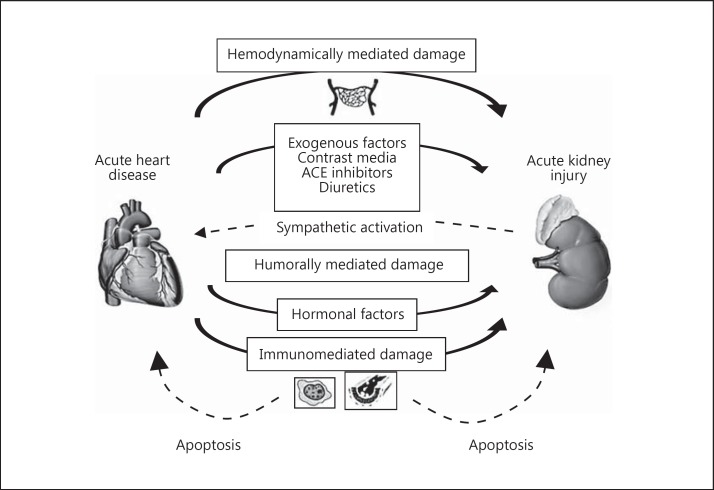
Fig. 1.
CRS type 1 is characterized by an acute worsening of heart function leading to AKI and/or dysfunction. Acute cardiac events that may contribute to AKI include acute decompensated heart failure, acute coronary syndrome, cardiogenic shock and cardiac surgery-associated low cardiac output syndrome.
Hemodynamic Mechanisms of Heart-Kidney Crosstalk in CRS Type 1
CRS type 1 most frequently appears in the setting of acute decompensated heart failure [11] and follows ischemic (cardiac surgery, myocardial infarction) or nonischemic (valve dysfunction, aortic dissection, pulmonary embolism) cardiac events [9]. Any of these clinical conditions can cause worsening renal function, which is related to increased hospital stay, morbidity and mortality [4,5,12,13,14,15,16,17,18,19,20,21]. Risk predictors for this complication include reduced baseline renal function, diabetes and previous episodes of heart failure [22].
At the onset of acute heart failure (AHF), particularly with the presence of systolic dysfunction and decreased cardiac output, kidney arterial underfilling and increased venous congestion are expected complications leading to a decreased GFR [23]. A lower kidney perfusion in the setting of AHF overactivates the renin-angiotensin-aldosterone system, promoting water and sodium retention, which will contribute to systemic and kidney hypertension and consequently endothelial and glomerular injury [7]. Additionally, angiotensin II and aldosterone have profibrotic and proinflammatory properties that further contribute to kidney damage, which is more pronounced in CRS type 2.
Patients with AHF often develop hyponatremia, which is an independent predictor of mortality and repeated hospitalizations for decompensation [24]. Heart failure-associated hyponatremia is predominantly hypervolemic and a marker of congestion, which is an important factor contributing to AKI. In these patients, hyponatremia can be effectively considered as an index of general neurohumoral activation including the nonosmotic release of vasopressin and higher levels of plasma renin, angiotensin II, aldosterone and catecholamines.
Some drugs commonly prescribed for the treatment of AHF can also contribute to the development of AKI by disturbing systemic and kidney hemodynamics. Diuretics are recommended to control dyspnea and edema, but their use may be complicated by excessive intravascular volume depletion and further compromise kidney perfusion [25,26]. Diuretic resistance may also complicate the clinical picture of CRS type 1 by acutely or chronically increasing sodium retention [27]. ACE inhibitors, angiotensin receptor blockers and aldosterone receptor antagonists are included in the treatment of heart failure. However, they affect kidney hemodynamics, and their use must be carefully monitored to avoid AKI in decompensated patients.
Another important iatrogenic nephrotoxin in AHF and acute coronary syndromes is radiocontrast medium for imaging procedures [7]. Iodinated contrast agents induce intense and prolonged vasoconstriction at the corticomedullary junction of the kidney and directly impair the autoregulatory capacity of the kidney through a reduction in nitric oxide (NO) synthesis [28,29]. These effects, coupled with the direct tubular toxicity of iodinated radiocontrast agents lead to overt acute tubular necrosis and contrast-induced AKI.
Nonhemodynamic Mechanisms of Heart-Kidney Crosstalk in CRS Type 1
In addition to hemodynamic pathways, other mechanisms have been described to be involved in the pathogenesis of CRS type 1, including the activation of the sympathetic nervous system, innate and adaptive immunity, inflammation and impaired balance between reactive oxygen species (ROS) and NO production [30].
Humoral signaling is one of the essential precipitating mechanisms in human disease crosstalk. Immune response orchestrates the eradication of pathogens, tissue healing and generation. Uncontrolled inflammatory processes involving one organ may cause distant organ damage. The activation of proinflammatory networks leads to combined inter- and intracellular changes, including alterations in gene expression and methylation, apoptosis induction, rapid and unmitigated cell decline, system dysfunction and, in the worst outcome, death [30].
Humoral cardiorenal mediators are cytokines, chemokines, and growth factors which are responsible for both inter- and intracellular modifications. Signaling pathways activated by damaged cardiac or renal cells promote innate immune mechanisms and strengthen adaptive immune processes.
Role of Immune Response in Heart-Kidney Crosstalk
The immune system plays a pivotal role both in the host defense against harmful agents and in the tolerance to other antigens. Dysregulation of the immune response may result in disorders of the innate and adaptive immune system as well as in inflammation [31]. Recent studies have highlighted the importance of both the innate and adaptive immune response in heart-kidney crosstalk.
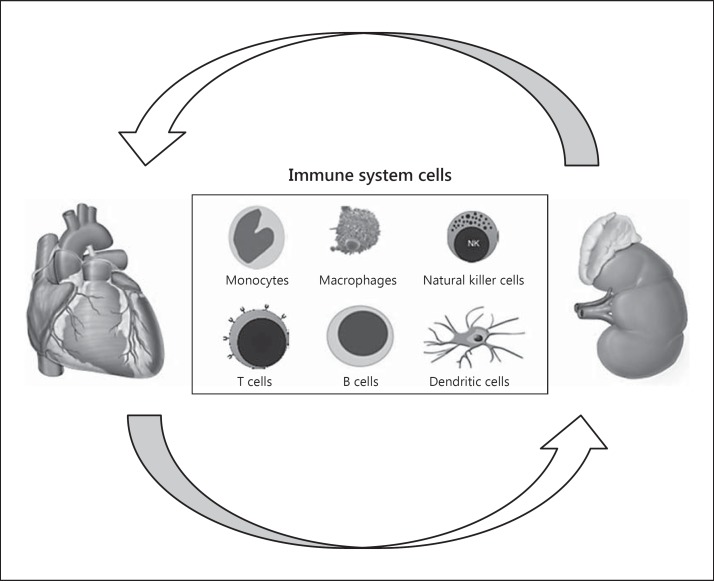
Neutrophils, monocytes/macrophages, dendritic cells (DCs), natural killer cells and natural killer T cells, which are part of the innate immune system, are able to immediately defend the host from infection states in a nonantigenic-specific manner (fig. 2). Innate immune cells serve as sentinel cells that are prestationed in tissues and continuously monitor their microenvironment for signs of distress. Conversely, the adaptive immune system is a second line of defense and becomes responsive to specific antigens at cellular and humoral stages. The adaptive immune response includes dendritic cell maturation and antigen presentation, CD4+ and CD8+ lymphocyte proliferation and stimulation as well as interactions between T and B lymphocytes [30].
Fig. 2.
Cellular types involved in CRS type 1.
The adaptive immune system is stimulated by the specificity of antigen receptors on B and T lymphocytes that react to several antigenic molecular structures. Once stimulated, B cells produce specific antibodies, perform opsonization to encourage phagocytosis and activate the complement system [32].
Proposed initiators of the innate immune response during AKI include the activation of Toll-like receptors and the release of ROS and reactive nitrogen species (RNS) as well as mitochondrial products [33]. Inflammatory mechanisms stimulate the expression of adhesion molecules in renal endothelial cells, which leads to the deposition of immune complexes and vascular stiffening [32,34]. Early inflammatory response is triggered by the direct contact between renal vascular endothelial cells and injurious agents [35], which are responsible for the disorganization of the endothelial integrity, producing a partial disappearance of cell-cell borders and disruption of cell-cell contacts. Thus, endothelial disintegration increases vascular permeability and facilitates leukocyte infiltration and inflammation in the renal parenchyma.
Monocytes play a pivotal role in the coordination of the inflammatory pathways in the pathogenesis of heart failure [36]. However, it remains unclear if they have a reparative role or if their effects are rather detrimental to the myocardium. Importantly, monocytes are not homogenous and comprise subsets with a unique phenotype and function, and this may be important in understanding their specific role in the pathogenesis of heart failure [37,38,39]. Indeed, activated monocytes and macrophages are the major source of cytokines responsible for heart inflammatory mechanisms [36,39,40].
Immune-mediated damage, immune dysregulations and alterations in the immune response have been postulated as potential specific mechanisms involved in the pathogenesis of CRS type 1. Recently, Virzì et al. [41] explored the premise of an immune-mediated process in the pathophysiology of this syndrome and showed marked apoptotis in monocytes incubated with CRS type 1 plasma. The specific immune responses during AKI facilitate and enhance distant heart-kidney crosstalk; in particular, inflammation of renal tissue stimulates the expression of adhesion molecules in endothelial cells, which leads to the deposition of immune complexes and vascular stiffening in kidney disease [30].
Cell Signaling, Cytokines, Chemokines and Inflammation
Inflammation is produced by eicosanoid and cytokine release from injured or infected cells. Common cytokines include interleukins responsible for the communication between white blood cells, chemokines, which promote chemotaxis, and interferons, which have antiviral effects. The unbalance of cytokine and chemokine networks is involved in several inflammatory diseases. Persisting exposure to proinflammatory factors damages tissues, impairs organ function and is lethal in the organ crosstalk [30].
Cytokines, chemokines and eicosanoids mediate cellular responses and interact with genome-encoded receptors expressed on monocytes, macrophages, mast cells, astrocytes and other cells of the innate immune system [42]. Upregulation of humoral factors by injured cells leads to the activation of the Toll/interleukin (IL)-1 superfamily. Members of this superfamily possess a conserved domain able to activate nuclear factor kB, which translocates to the nucleus causing an upregulation of the inflammatory cytokine expression.
Over the past 30 years, there has been growing evidence on the role of the inflammatory response in the pathogenesis of AHF. Cytokines have been found to be produced by cardiomyocytes following ischemic or mechanical stimuli, but also by innate immune response [43,44,45]. In the setting of AHF, an immune dysregulation may occur. In addition to myocyte damage, cytokines may trigger distant organ damage such as AKI. In the kidney, the renal tubular epithelium is a major site of cell injury and death during AKI. Furthermore, numerous studies have suggested that renal epithelial cells play a central role in inflammation during AKI and in the handling of inflammatory mediators and their resulting efflux into the systemic circulation. These cells contribute to the circulating levels of inflammatory mediators by different mechanisms including epigenetic processes [30,46,47,48].
Oxidative Stress
Oxidative stress is a common pathway involved in cellular dysfunction, tissue injury and organ failure. Injury caused by oxidative stress occurs in many clinical scenarios involving ischemia and reperfusion such as organ transplantation, hemorrhagic shock, myocardial infarction and cerebral vascular accidents, hypertension, diabetes, metabolic syndrome and nephrolithiasis [49,50,51].
Oxidative loss of redox homeostasis in ROS and RNS results in an immune system activation and in a proinflammatory and profibrotic milieu via distinct mechanisms that stimulate renal and cardiovascular structural and functional abnormalities [52,53]. The link between oxidative stress and inflammatory pathways is poorly understood [53]. Cardiorenal pathophysiological interactions render the heart and kidney vulnerable to loss of control over normal cellular oxidative reactions. Although physiological levels of ROS are required for normal cellular function, overproduction of ROS will result in deleterious effects. It is important to underline that in every cardiorenal crosstalk, oxidative stress and injury to the heart and kidneys may play a potentially reversible role. These mechanisms describe a final common pathway for tissue damage and organ failure. The development and progression of CRS lead to and amplify oxidative stress production [54]. Thus, a better understanding of the cellular and mitochondrial pathways involved in both renal and cardiac oxidative stress is essential in order to allow the development of novel and more effective therapeutic strategies able to improve survival and prognosis in CRS patients [4,54].
Apoptosis and Cellular Death
Apoptosis is a controlled and physiological mechanism of the regulation of cell populations in a programmed pattern in prenatal development and aging [55]. Apoptosis also occurs as a defense mechanism such as in immune reactions or when cells are damaged by a disease or toxic agents [56]. However, an alteration in the regulation of cell death by apoptosis may negatively affect the mechanism of host defense [55]. Furthermore, aberrant regulations of the apoptotic pathways is the central abnormality in many clinical settings such as cancer, neurodegenerative disorders, ischemic diseases, myocardial infarction, heart failure and some renal disease [57,58,59,60]. Although there is a wide range of stimuli that can trigger apoptosis in physiological and pathological conditions, not all cells will necessarily die in response to the same stimulus [61]. The best characterized endogenous promoters of apoptosis are death receptors containing a death domain, such as receptors for TNF-α, FasL and TNF-related apoptosis-inducing ligand (TRAIL) [62,63]. Recently, inhibitors of TNF-α and FasL have been successfully used in cell lines and animal models of AKI [64,65,66]. Multiple factors are involved in the development of AKI during heart failure. There is increasing evidence showing that sustained loss of cardiac cells by apoptosis can induce cardiac dysfunction and ultimately lead to heart failure. In addition, apoptotic pathways and caspases seem to have direct effects on cardiomyocyte contractility and remodeling, cleaving myocardiac contractile proteins and promoting systolic dysfunction [67,68]. Recent evidence has suggested that immune-mediated mechanisms are implicated in the pathogenesis of CRS type 1 [41,69]. It has been recently shown that plasma from CRS type 1 patients induced apoptosis in monocytes with an activation of caspase cascade; furthermore, IL-6 levels were significantly higher in these patients when compared with healthy controls and heart failure patients without kidney impairment [41,69]. In a different study, Virzì et al. [69] demonstrated that TNF-α levels, detected in monocyte cell supernatants, were significantly elevated in cells treated by plasma from heart failure and CRS type 1 patients compared with supernatants from cells treated by plasma from healthy subjects; in the cell supernatants obtained from monocytes of patients with CRS type 1, the levels of the proinflammatory cytokines IL-6 and IL-18 were significantly higher than in monocyte supernatants of heart failure patients and controls. This is the first piece of evidence that CRS type 1 is an inflammatory disorder, and the contribution of the inflammatory pathways in its pathogenesis is essential for distant organ damage and the heart-kidney crosstalk.
Conclusion
In conclusion, CRS type 1 is a complex and multidimensional entity which is common in clinical practice and significantly impacts patients' morbidity and mortality. Many studies confirm that immune systems, inflammation, oxidative stress and apoptosis may contribute to the final nonhemodynamic pathways of organ dysfunction in the heart-kidney crosstalk. Cytokines may be produced by different tissues and cleared by the kidney. The inability to metabolize or clear cytokines in AKI and in CRS type 1 may lead to increased inflammation and a general dysregulation.
In the last years, a significant number of genomics, transcriptomics and proteomics as well as metabolomics studies became available characterizing altered kidney or cardiovascular functions, but combined analyses of both failing organs have been rare. These studies are needed for a better understanding of the heart-kidney crosstalk and for delineating molecular features hypothetically involved in CRS crosstalk at molecular levels. Gaining a clear mechanistic understanding on how dysfunction of one organ can lead to distant organ dysfunction is critical in allowing the development of effective treatments.
The heart and kidney are players of the same game at different levels and by different and complex interactions. Moreover, the management of CRS type 1 is challenging because of the multitude and complexity of pathophysiological interactions between these two organs. The promising complete characterization of cellular and subcellular orchestration in the heart-kidney crosstalk in CRS type 1 could allow the identification of new targets of therapeutic strategies.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Acknowledgements
This work was supported by a research grant of the Veneto region (RSF No. 303/2009).
References
- 1.Ledoux P. Cardiorenal syndrome (in undetermined language) Avenir Med. 1951;48:149–153. [PubMed] [Google Scholar]
- 2.Molls RR, Rabb H. Limiting deleterious cross-talk between failing organs. Crit Care Med. 2004;32:2358–2359. doi: 10.1097/01.ccm.0000145957.97995.55. [DOI] [PubMed] [Google Scholar]
- 3.Viswanathan G, Gilbert S. The cardiorenal syndrome: making the connection. Int J Nephrol. 2011;2011:283137. doi: 10.4061/2011/283137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. 2012;60:1031–1042. doi: 10.1016/j.jacc.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 5.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 6.Bagshaw SM, Cruz DN, Aspromonte N, Daliento L, Ronco F, Sheinfeld G, Anker SD, Anand I, Bellomo R, Berl T, Bobek I, Davenport A, Haapio M, Hillege H, House A, Katz N, Maisel A, Mankad S, McCullough P, Mebazaa A, Palazzuoli A, Ponikowski P, Shaw A, Soni S, Vescovo G, Zamperetti N, Zanco P, Ronco C. Epidemiology of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant. 2010;25:1406–1416. doi: 10.1093/ndt/gfq066. [DOI] [PubMed] [Google Scholar]
- 7.Cruz DN. Cardiorenal syndrome in critical care: the acute cardiorenal and renocardiac syndromes. Adv Chronic Kidney Dis. 2013;20:56–66. doi: 10.1053/j.ackd.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Haase M, Muller C, Damman K, Murray PT, Kellum JA, Ronco C, McCullough PA. Pathogenesis of cardiorenal syndrome type 1 in acute decompensated heart failure: workgroup statements from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI) Contrib Nephrol. 2013;182:99–116. doi: 10.1159/000349969. [DOI] [PubMed] [Google Scholar]
- 9.Ismail Y, Kasmikha Z, Green HL, McCullough PA. Cardio-renal syndrome type 1: epidemiology, pathophysiology, and treatment. Semin Nephrol. 2012;32:18–25. doi: 10.1016/j.semnephrol.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Bozkurt B, Mann DL, Deswal A. Biomarkers of inflammation in heart failure. Heart Fail Rev. 2010;15:331–341. doi: 10.1007/s10741-009-9140-3. [DOI] [PubMed] [Google Scholar]
- 11.Eren Z, Ozveren O, Buvukoner E, Kaspar E, Degertekin M, Kantarci G. A single-centre study of acute cardiorenal syndrome: incidence, risk factors and consequences. Cardiorenal Med. 2012;2:168–176. doi: 10.1159/000337714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 13.Bhandari S, Jain P. Management of acute coronary syndrome in chronic kidney disease. J Assoc Physicians India. 2012;60:48–51. [PubMed] [Google Scholar]
- 14.Givertz MM, Teerlink JR, Albert NM, Westlake Canary CA, Collins SP, Colvin-Adams M, Ezekowitz JA, Fang JC, Hernandez AF, Katz SD, Krishnamani R, Stough WG, Walsh MN, Butler J, Carson PE, Dimarco JP, Hershberger RE, Rogers JG, Spertus JA, Stevenson WG, Sweitzer NK, Tang WH, Starling RC. Acute decompensated heart failure: update on new and emerging evidence and directions for future research. J Card Fail. 2013;19:371–389. doi: 10.1016/j.cardfail.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Lazaros G, Tsiachris D, Tousoulis D, Patialiakas A, Dimitriadis K, Roussos D, Vergopoulos E, Tsioufis C, Vlachopoulos C, Stefanadis C. In-hospital worsening renal function is an independent predictor of one-year mortality in patients with acute myocardial infarction. Int J Cardiol. 2012;155:97–101. doi: 10.1016/j.ijcard.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 16.Amin AP, Spertus JA, Reid KJ, Lan X, Buchanan DM, Decker C, Masoudi FA. The prognostic importance of worsening renal function during an acute myocardial infarction on long-term mortality. Am Heart J. 2010;160:1065–1071. doi: 10.1016/j.ahj.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Granfeldt A. Organ dysfunction following regional and global ischemia/reperfusion. Intervention with postconditioning and adenocaine. Dan Med J. 2012;59:B4496. [PubMed] [Google Scholar]
- 18.Hsieh MJ, Chen YC, Chen CC, Wang CL, Wu LS, Wang CC. Renal dysfunction on admission, worsening renal function, and severity of acute kidney injury predict 2-year mortality in patients with acute myocardial infarction. Circ J. 2013;77:217–223. doi: 10.1253/circj.cj-12-0539. [DOI] [PubMed] [Google Scholar]
- 19.Silverberg D, Wexler D, Blum M, Schwartz D, Iaina A. The association between congestive heart failure and chronic renal disease. Curr Opin Nephrol Hypertens. 2004;13:163–170. doi: 10.1097/00041552-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Cole RT, Masoumi A, Triposkiadis F, Giamouzis G, Georgiopoulou V, Kalogeropoulos A, Butler J. Renal dysfunction in heart failure. Med Clin North Am. 2012;96:955–974. doi: 10.1016/j.mcna.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Udani SM, Koyner JL. The effects of heart failure on renal function. Cardiol Clin. 2010;28:453–465. doi: 10.1016/j.ccl.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, Love TE, Aban IB, Shlipak MG. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. The severe cardiorenal syndrome: ‘Guyton revisited’. Eur Heart J. 2005;26:11–17. doi: 10.1093/eurheartj/ehi020. [DOI] [PubMed] [Google Scholar]
- 24.Packer M, Lee WH, Kessler PD. Preservation of glomerular filtration rate in human heart failure by activation of the renin-angiotensin system. Circulation. 1986;74:766–774. doi: 10.1161/01.cir.74.4.766. [DOI] [PubMed] [Google Scholar]
- 25.Aspromonte N, Cruz DN, Valle R, Bonello M, Tubaro M, Gambaro G, Marchese G, Santini M, Ronco C. Metabolic and toxicological considerations for diuretic therapy in patients with acute heart failure. Expert Opin Drug Metab Toxicol. 2011;7:1049–1063. doi: 10.1517/17425255.2011.586629. [DOI] [PubMed] [Google Scholar]
- 26.Aspromonte N, Cruz DN, Valle R, Ronco C. Management and monitoring of haemodynamic complications in acute heart failure. Heart Fail Rev. 2011;16:575–581. doi: 10.1007/s10741-011-9229-3. [DOI] [PubMed] [Google Scholar]
- 27.Ellison DH. Diuretic therapy and resistance in congestive heart failure. Cardiology. 2001;96:132–143. doi: 10.1159/000047397. [DOI] [PubMed] [Google Scholar]
- 28.McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51:1419–1428. doi: 10.1016/j.jacc.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 29.Tumlin J, Stacul F, Adam A, Becker CR, Davidson C, Lameire N, McCullough PA. Pathophysiology of contrast-induced nephropathy. Am J Cardiol. 2006;98:14K–20K. doi: 10.1016/j.amjcard.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Virzi GM, Day S, de Cal M, Vescovo G, Ronco C. Heart-kidney crosstalk and role of humoral signaling in critical illness. Crit Care. 2014;18:201. doi: 10.1186/cc13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 32.White LE, Hassoun HT. Inflammatory mechanisms of organ crosstalk during ischemic acute kidney injury. Int J Nephrol. 2012;2012:505197. doi: 10.4061/2012/505197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chakraborti T, Mandal A, Mandal M, Das S, Chakraborti S. Complement activation in heart diseases. Role of oxidants. Cell Signal. 2000;12:607–617. doi: 10.1016/s0898-6568(00)00111-x. [DOI] [PubMed] [Google Scholar]
- 34.McElrath MJ, Steinman RM, Cohn ZA. Latent HIV-1 infection in enriched populations of blood monocytes and T cells from seropositive patients. J Clin Invest. 1991;87:27–30. doi: 10.1172/JCI114981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molitoris BA, Sutton TA. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int. 2004;66:496–499. doi: 10.1111/j.1523-1755.2004.761_5.x. [DOI] [PubMed] [Google Scholar]
- 36.Vaduganathan M, Greene SJ, Butler J, Sabbah HN, Shantsila E, Lip GY, Gheorghiade M. The immunological axis in heart failure: importance of the leukocyte differential. Heart Fail Rev. 2013;18:835–845. doi: 10.1007/s10741-012-9352-9. [DOI] [PubMed] [Google Scholar]
- 37.Coggins M, Rosenzweig A. The fire within: cardiac inflammatory signaling in health and disease. Circ Res. 2012;110:116–125. doi: 10.1161/CIRCRESAHA.111.243196. [DOI] [PubMed] [Google Scholar]
- 38.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–173. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wrigley BJ, Lip GY, Shantsila E. The role of monocytes and inflammation in the pathophysiology of heart failure. Eur J Heart Fail. 2011;13:1161–1171. doi: 10.1093/eurjhf/hfr122. [DOI] [PubMed] [Google Scholar]
- 40.Satoh M, Minami Y, Takahashi Y, Nakamura M. Immune modulation: role of the inflammatory cytokine cascade in the failing human heart. Curr Heart Fail Rep. 2008;5:69–74. doi: 10.1007/s11897-008-0012-2. [DOI] [PubMed] [Google Scholar]
- 41.Virzì GM, Torregrossa R, Cruz DN, Chionh CY, de Cal M, Soni SS, Dominici M, Vescovo G, Rosner MH, Ronco C. Cardiorenal syndrome type 1 may be immunologically mediated: a pilot evaluation of monocyte apoptosis. Cardiorenal Med. 2012;2:33–42. doi: 10.1159/000335499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benoist C, Mathis D. Mast cells in autoimmune disease. Nature. 2002;420:875–878. doi: 10.1038/nature01324. [DOI] [PubMed] [Google Scholar]
- 43.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST) Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 44.Aukrust P, Ueland T, Lien E, Bendtzen K, Muller F, Andreassen AK, Nordoy I, Aass H, Espevik T, Simonsen S, Froland SS, Gullestad L. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;83:376–382. doi: 10.1016/s0002-9149(98)00872-8. [DOI] [PubMed] [Google Scholar]
- 45.Amione P, Ricci E, Topo F, Izzo L, Pirovano R, Rega V, Cocci C, Masina M. Comparison of Allevyn Adhesive and Biatain Adhesive in the management of pressure ulcers. J Wound Care. 2005;14:365–370. doi: 10.12968/jowc.2005.14.8.26819. [DOI] [PubMed] [Google Scholar]
- 46.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida M, Honma S. Regeneration of injured renal tubules. J Pharmacol Sci. 2014;124:117–122. doi: 10.1254/jphs.13r12cp. [DOI] [PubMed] [Google Scholar]
- 48.Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li PL, Zhang Y. Cross talk between ceramide and redox signaling: implications for endothelial dysfunction and renal disease. Handb Exp Pharmacol. 2013;216:171–197. doi: 10.1007/978-3-7091-1511-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan SR. Stress oxidative: nephrolithiasis and chronic kidney diseases. Minerva Med. 2013;104:23–30. [PubMed] [Google Scholar]
- 51.Mandavia CH, Aroor AR, Demarco VG, Sowers JR. Molecular and metabolic mechanisms of cardiac dysfunction in diabetes. Life Sci. 2013;92:601–608. doi: 10.1016/j.lfs.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic Biol Med. 2010;48:1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feltes CM, Van Eyk J, Rabb H. Distant-organ changes after acute kidney injury. Nephron Physiol. 2008;109:p80–p84. doi: 10.1159/000142940. [DOI] [PubMed] [Google Scholar]
- 54.Rubattu S, Mennuni S, Testa M, Mennuni M, Pierelli G, Pagliaro B, Gabriele E, Coluccia R, Autore C, Volpe M. Pathogenesis of chronic cardiorenal syndrome: is there a role for oxidative stress? Int J Mol Sci. 2013;14:23011–23032. doi: 10.3390/ijms141123011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen JJ, Duke RC, Fadok VA, Sellins KS. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- 56.Norbury CJ, Hickson ID. Cellular responses to DNA damage. Annu Rev Pharmacol Toxicol. 2001;41:367–401. doi: 10.1146/annurev.pharmtox.41.1.367. [DOI] [PubMed] [Google Scholar]
- 57.Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 58.Solary E, Dubrez L, Eymin B. The role of apoptosis in the pathogenesis and treatment of diseases. Eur Respir J. 1996;9:1293–1305. doi: 10.1183/09031936.96.09061293. [DOI] [PubMed] [Google Scholar]
- 59.Grams ME, Rabb H. The distant organ effects of acute kidney injury. Kidney Int. 2012;81:942–948. doi: 10.1038/ki.2011.241. [DOI] [PubMed] [Google Scholar]
- 60.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int. 2011;80:29–40. doi: 10.1038/ki.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 63.Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 64.Sanz AB, Justo P, Sanchez-Nino MD, Blanco-Colio LM, Winkles JA, Kreztler M, Jakubowski A, Blanco J, Egido J, Ruiz-Ortega M, Ortiz A. The cytokine TWEAK modulates renal tubulointerstitial inflammation. J Am Soc Nephrol. 2008;19:695–703. doi: 10.1681/ASN.2007050577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Misseri R, Meldrum DR, Dinarello CA, Dagher P, Hile KL, Rink RC, Meldrum KK. TNF-alpha mediates obstruction-induced renal tubular cell apoptosis and proapoptotic signaling. Am J Physiol Renal Physiol. 2005;288:F406–F411. doi: 10.1152/ajprenal.00099.2004. [DOI] [PubMed] [Google Scholar]
- 66.Hamar P, Song E, Kokeny G, Chen A, Ouyang N, Lieberman J. Small interfering RNA targeting Fas protects mice against renal ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2004;101:14883–14888. doi: 10.1073/pnas.0406421101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chandrashekhar Y, Sen S, Anway R, Shuros A, Anand I. Long-term caspase inhibition ameliorates apoptosis, reduces myocardial troponin-I cleavage, protects left ventricular function, and attenuates remodeling in rats with myocardial infarction. J Am Coll Cardiol. 2004;43:295–301. doi: 10.1016/j.jacc.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 68.Broberg CS, Bax BE, Okonko DO, Rampling MW, Bayne S, Harries C, Davidson SJ, Uebing A, Khan AA, Thein S, Gibbs JS, Burman J, Gatzoulis MA. Blood viscosity and its relationship to iron deficiency, symptoms, and exercise capacity in adults with cyanotic congenital heart disease. J Am Coll Cardiol. 2006;48:356–365. doi: 10.1016/j.jacc.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 69.Virzi GM, de Cal M, Cruz DN, Bolin C, Vescovo G, Ronco C. Type 1 cardiorenal syndrome and its possible pathophysiological mechanisms (in Italian) G Ital Nefrol. 2012;29:690–698. [PubMed] [Google Scholar]