Abstract
Lysophosphatidic acid (LPA) activates a family of cognate G protein-coupled receptors and is involved in various pathophysiological processes. However, it is not clearly understood how these LPA receptors are specifically coupled to their downstream signaling molecules. This study found that LPA2, but not the other LPA receptor isoforms, specifically interacts with Na+/H+ exchanger regulatory factor2 (NHERF2). In addition, the interaction between them requires the C-terminal PDZ domain-binding motif of LPA2 and the second PDZ domain of NHERF2. Moreover, the stable expression of NHERF2 potentiated LPA-induced phospholipase C-β (PLC-β) activation, which was markedly attenuated by either a mutation in the PDZ-binding motif of LPA2 or by the gene silencing of NHERF2. Using its second PDZ domain, NHERF2 was found to indirectly link LPA2 to PLC-β3 to form a complex, and the other PLC-β isozymes were not included in the protein complex. Consistently, LPA2-mediated PLC-β activation was specifically inhibited by the gene silencing of PLC-β3. In addition, NHERF2 increases LPA-induced ERK activation, which is followed by cyclooxygenase-2 induction via a PLC-dependent pathway. Overall, the results suggest that a ternary complex composed of LPA2, NHERF2, and PLC-β3 may play a key role in the LPA2-mediated PLC-β signaling pathway.
Lysophosphatidic acid (LPA) is released by activated platelets and by a wide variety of mammalian cells, including adipocytes, fibroblasts, and endothelial cells, as well as by several types of cancer cells (11, 36). Generated LPA acts as both an autocrine and paracrine of cellular signaling in cells and elicits numerous physiological responses such as cell proliferation, survival, chemotaxis, platelet aggregation, smooth muscle contractions, and tumor cell invasion (34).
LPA binds to members of the family of G-protein-coupled receptor (GPCR), which are localized on the plasma membrane. To date, three subtypes of the LPA receptor (EDG2, EDG4, and EDG7) have been cloned (1, 4, 18, 22) and have been renamed LPA1, LPA2, and LPA3 according to the IUPHAR nomenclature system. Upon LPA stimulation, the LPA receptors interact with the heterotrimeric G-proteins (Gq/11, and Gi/o) and trigger the GDP/GTP exchange of their α subunits. Subsequently, the dissociated Gα and βγ subunits activate multiple effector systems, including phospholipase C-β (PLC-β)/protein kinase C (PKC)/Ca2+, RAS-Raf-1-mitogen-activated protein (MAPK), and phosphatidylinositol 3-kinase, but inhibit the adenylyl cyclase-cyclic AMP pathway (3, 4, 8, 22, 23). The LPA receptors also activate the small GTPase, RhoA through G12/13, which leads to stress fiber formation and a focal adhesion assembly (8, 23). These results suggest that heterotrimeric G proteins play a key role in LPA-induced cell signaling. In addition to the heterotrimeric G proteins, recent reports have suggested that the PDZ (PSD-95/Disk-large/ZO-1) domain-containing proteins play a role in regulating GPCR-mediated signaling (15).
The Na+/H+ exchanger regulatory factor (NHERF) family proteins, i.e., NHERF1 and NHERF2, regulate intracellular signal transduction by a PDZ domain-mediated interaction with multiple target proteins and/or by the ERM-binding region-mediated interactions with the actin-binding proteins, i.e., ezrin, radixin, or moesin (6, 43, 45). The NHERF family proteins contain two tandem PDZ domains, which are protein-protein interaction domains that are associated with the specific C-terminal motifs on the target proteins (43, 45). It has been reported that the first PDZ domain of NHERF1 preferentially binds to the motif, D-S/T-X-L, at the end of target proteins, such as β2-adrenergic receptor (D-S-L-L), P2Y1 purinergic receptor (D-T-S-L), and cystic fibrosis transmembrane regulator (D-T-R-L) (16). In addition, by screening a random peptide library, it was demonstrated that the first PDZ domain of NHERF1 avidly binds to a consensus motif (S/T-R/Y-L) and the second PDZ domain of NHERF1 interacts with a different amino acid sequence (S-S/T-W-L) (44). This suggests that the two PDZ domains of the NHERF family proteins may have a distinct peptide-binding specificity. Although the binding specificity of NHERF2 has not been determined, several reports have suggested that NHERF2 may have a distinct binding specificity and physiological function that are not duplicated by NHERF1 (10, 21, 24, 47). In our previous study, it was observed that NHERF2 but not NHERF1 specifically interacts with PLC-β3 and plays a key role in PLC-β3 activation by the PDZ domain-mediated interaction (21). By means of the PDZ domain-mediated interaction, NHERF1 and/or NHERF2 interact directly with the C-terminal PDZ domain-binding motifs of several GPCRs, including the β2-adrenergic receptor (17), the parathyroid hormone 1 receptor (31), and the P2Y1 purinergic receptor (16). In addition to these GPCRs, two isoforms of LPA receptors, LPA1 and LPA2, also harbor a PDZ-binding motif (H-S-V-V and D-S-T-L, respectively) in their carboxyl termini. However, it is not known whether both LPA receptors and PLC-β3 are included in a protein complex by the PDZ domain-mediated interaction and whether LPA-induced PLC-β activation is specifically regulated by the NHERF family proteins.
The present study provides the first evidence that NHERF2 specifically interacts with LPA2 but not with the other LPA receptor isotypes. In addition, it is shown that NHERF2 physically links PLC-β3 to LPA2 and that the resultant ternary complex is crucial for determining the amplitude and the specificity of LPA2-mediated PLC-β activation.
MATERIALS AND METHODS
Materials.
Lysophosphatidic acid (1-oleoyl-2-hydroxy-sn-glycerol-3-phosphate) was purchased from Biomol (Plymouth Meeting, Pa.). Fluo-3/AM, Lipofectamine, FuGene 6, and DSP (dithiobis[succinimidylpropionate]) were from Molecular Probes, Invitrogen (Carlsbad, Calif.), Boehringer Mannheim (Mannheim, Germany), and Pierce (Rockford, Ill.), respectively. The cell culture dishes and plates were obtained from BD Bioscience (Falcon). The serum and medium were obtained from HyClone (Logan, Utah) and Gibco-BRL, and the other chemicals were purchased from Sigma (St. Louis, Mo.) in extrapure grade.
Antibodies for immunoblotting.
Rabbit polyclonal anti-NHERF1 and anti-NHERF2 antibody were generated as previously described (29). The anti-glutathione S-transferase (GST) and anti-MBP antibodies were purchased from New England Biolabs, and anti-Flag antibody (M2 clone) was purchased from Sigma. In addition, the specific antibodies to PLC-β1, PLC-β3, and phosphorylated extracellular signal-regulated kinase (ERK) were obtained from the Santa Cruz Co., and the anti-COX-2 antibody was acquired from Caymen Chemicals (Ann Arbor, Mich.).
Cell culture and transfection.
Monolayer cultures of the COS-7 cells (from Gibco-BRL), HeLa cells, and Rat-1 cells (from the American Type Culture Collection) were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum. The cells were grown at 37°C in a humidified atmosphere containing 5% CO2. For the transient expression, either COS-7 cells or HeLa cells were plated at a density of 1.5 × 104 cells/cm2. The next day, the cells were transfected with ca. 1 to 5 μg of the total plasmid DNA by using a Lipofectamine reagent according to the manufacturer's instructions (Invitrogen). For the stable expression of the various NHERF2 constructs, Rat-1 cells were transfected by using the FuGene 6 reagent and the positive clones were then selected by using the culture medium supplemented with the G418 reagents. The stable expression level of the NHERF2 constructs was examined by Western blot analysis.
Plasmid constructions and site-directed mutagenesis.
Initially, the human LPA2/EDG4 cDNA was cloned as a mutant form (GenBank accession number AF011466) (1). Thereafter, Bandoh et al. and Contos et al. identified a G deletion, in the 3′ region of the previous cloned cDNA, which results in a frame-shift mutation in the LPA2/EDG4 C-terminal region (3, 9). The corrected version of the LPA2/EDG4 cDNA was deposited by them to the GenBank (GenBank accession number AF233092). The present study used the Flag-tagged versions of the wild-type human LPA1, LPA2, and LPA3, which were kindly provided by J. Aoki (Tokyo University, Tokyo, Japan). It was also confirmed by automatic sequencing that the nucleotide sequence of the wild-type LPA2 matched the sequence deposited in the GenBank (accession number AF233092), perfectly. In order to express the cytoplasmic tail (CT) of LPA1, LPA2, or LPA3 in Escherichia coli, these cDNAs were also used as a template for PCR. The pairs of primers used for PCR were 5′-CCG GCC TTC CAG CGC AGT GAGT AAC CCC ACC GG-3′ and 5′-ACGC GTCGAC CTA AAC CAC AGA GTG ATC ATT GCT G-3′ for LPA1, 5′-CCG GAA TTC CTC CGC CAG TCC ACC CGC GAG TC-3′ and 5′-ACGC GTC GAC CTA AAG GGT GGA GTC CAT CAG TGG-3′for LPA2, and 5′-CCG GAA TTC TTC TCT CAG GAG AAC CCA GAG AGG C-3′ and 5′-ACGC GTC GAC TTA GGA AGT GCT TTT ATT GCA GAC TG-3′ for LPA3. The PCR products were digested with EcoRI and SalI enzyme and inserted into either pGEX4T-1 (Amersham Pharmacia Biotech) or into pMAL-C2x (New England Biolabs). Various NHERF2 and NHERF1 constructs were generated as described previously (21). The site-directed mutagenesis was performed by the PCR method (19). In order to substitute the Leu351 with Ala (the DSTA mutant), the PCRs were performed by using a wild-type LPA2 template DNA and the reverse primer 5′-ATG CGC GGC CGC CTA AGC GGT GGA GTC CAT CAG-3′. All of the mutations were verified by sequence analysis by using an ABI automated sequencer (Applied Biosystems). It was also confirmed that no mutation on the other site(s) had occurred.
Pull-down assay.
Pull-down assays were performed by the recombinant proteins fused to either GST or the His6 epitope. The lysates were sonicated and centrifuged at 10,000 × g for 20 min. Equal amounts of the tissue or cell extracts were incubated with either the Sepharose-bound GST-fusion proteins or the Ni2+-nitrilotriacetic acid resin-bound His6-tagged proteins. The beads and lysates were rocked for 90 min at 4°C. After incubation, the beads were washed three times in a lysis buffer (50 mM HEPES [pH 7.4] and 150 mM NaCl plus 1% Triton X-100). The bound proteins were eluted with a 1× sodium dodecyl sulfate (SDS) sample buffer supplemented with 50 mM dithiothreitol and 30 mM EDTA, separated on SDS-10% polyacrylamide gels, transferred to nitrocellulose membrane, and analyzed by standard Western blotting procedures.
Immunoprecipitation.
The cells were lysed in a lysis buffer, allowed to immunoreact with the appropriate antibodies, and immobilized onto either the protein A-Sepharose or α-Flag affinity resin. The immunocomplexes were collected by the centrifugation at 1,000 × g and then washed four times with 1 ml of a lysis buffer. The resulting precipitates were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and were analyzed by using Western blot analysis.
Ca2+ imaging analysis by confocal laser microscopy.
Rat-1 cells were plated for Ca2+ imaging analysis in the adherent cells. One day later, the cells were incubated with Fluo-3/AM (1 μM; Molecular Probes) for 30 min and washed twice with 1 ml of a prewarmed Ca2+-free Locke's solution (158.4 mM NaCl, 5.6 mM KCl, 1.2 mM MgCl2, 5 mM HEPES [pH 7.3], 10 mM glucose, 0.2 mM EGTA). The intracellular calcium-dependent fluorescence was imaged under a Zeiss LSM510 confocal laser microscope. The fluorescence intensity traces from the individual cells were obtained by monitoring the average overall intensity of the cell each by using Zeiss laser-scanning microscopy software. Fluo-3 was excited by the 488-nm line from a krypton-argon laser, and the emission fluorescence was monitored at 515 nm. For the Fluo-3 measurements, the contrast and brightness levels were maintained at constant settings.
Knock-down by using siRNA transfections.
Small interfering RNA (siRNA) duplexes directed against NHERF1 (nucleotides 451 to 469), NHERF2 (nucleotides 862 to 880), PLC-β1 (nucleotides 374 to 392), and PLC-β3 (nucleotides 483 to 501) were synthesized by Dhamarcon, Inc. (Lafayette, Colo.). The presynthesized control siRNA duplexes (Luciferase GL3 Duplex) were also purchased and used as the control oligonucleotides. HeLa cells were transfected with 20 nM oligonucleotide by using Oligofectamine in serum-free conditions according to the manufacturer's instructions. At 4 h after transfection, the cells were washed and supplemented with fresh medium containing 10% fetal bovine serum. The cells were incubated for 72 h prior to use.
Adenoviral infections.
The recombinant adenovirus, expressing the Flag-LPA2, was generated and amplified in HEK-293T cells, which was purified over CsCl gradients. HeLa cells that were grown to confluence in six-well plates were infected at a multiplicity of infection (MOI) of 10 for 4 h in the serum-containing medium. In order to control for any nonspecific effects of viral infection on HeLa cell signaling, monolayers were infected in parallel with the adenovirus containing the empty shuttle vector. The infected cells were then used to measure the level of PLC activation, as described below.
Measurement of total IPs.
HeLa or Rat-1 cells were seeded at a density of 1.5 × 104 cells/cm2. The HeLa cells were either transfected with 1 μg of the LPA2 receptor plasmids with Lipofectamine or infected with the recombinant adenovirus expressing the LPA2 receptor at an MOI of 10. After 24 h, either Rat-1 cells or HeLa cells were incubated with 1 μCi of [3H]inositol (NEN Life Science Products)/well in 1 ml of inositol-free medium for 24 h at 37°C. After incubation with 10 mM LiCl for 20 min, the cells were treated with the agonists for 20 min and then fixed by adding ice-cold 5% perchloric acid. The accumulated [3H]inositol phosphates (IPs) were determined as described previously (21). All of the assays were duplicated, and the radioactivity of the eluants was normalized to the radioactivity of the total extract.
RESULTS
LPA2 interacts with NHERF2 in isoform-specific manner.
NHERF2 is classified as a type I PDZ domain-containing protein, which recognizes the S/T-X-I/V/L* motifs (“X” stands for any amino acid, and the asterisk indicates the C-terminal residue) (20). It was reported that several target proteins of NHERF2 harbor a Leu residue in their C terminus (7, 10, 21, 24, 47). Interestingly, LPA2 contains the D-S-T-L motif at its C terminus, whereas LPA1 and LPA3 have H-S-V-V and K-S-T-S motifs, respectively. This information raised the possibility that the molecular interaction may happen between NHERF2 and the specific isoform of LPA receptor. In order to examine this possibility, pull-down assays from rat kidney extracts were performed by using GST-fused CT of the LPA receptors. NHERF2 was coprecipitated with GST-LPA2-CT but with neither GST-LPA1-CT nor GST-LPA3-CT (Fig. 1A). In order to determine whether NHERF2 associates with the full-length LPA2 receptor in vivo, the interaction between LPA2 and NHERF2 was then examined by the coimmunoprecipitation method. NHERF2 was cotransfected with the Flag-tagged forms of either LPA1 or LPA2 in the COS-7 cells, the cell lysates were allowed to immunoreact with the anti-Flag antibody. Consistently, NHERF2 was coimmunoprecipitated by Flag-LPA2 but not by Flag-LPA1 (Fig. 1B), suggesting a specific association of LPA2 with NHERF2.
FIG. 1.
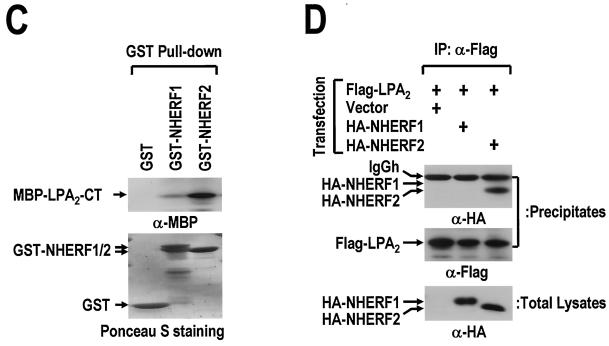
LPA2 interacts with NHERF2 in an isoform-specific manner. (A) NHERF2 binds to the CT of LPA2. The GST-fused CTs of three LPA receptor isoforms (GST-LPA1-CT, GST-LPA2-CT, and GST-LPA3-CT) and GST (4 μg) were immobilized onto glutathione beads, and incubated with lysates (3 mg) from rat kidney. The resulting precipitates were subjected to SDS-PAGE and analyzed by Western blot analysis with anti-NHERF2 antibody (upper panel) or by Ponceau S staining (lower panel). (B) Flag-LPA2, but not Flag-LPA1, coimmunoprecipitates NHERF2. Flag-LPA1 or Flag-LPA2 was cotransfected with NHERF2 into COS-7 cells, as indicated. The cell lysates were immunoprecipitated with anti-Flag antibody. The resulting precipitates were analyzed by Western blot analysis with anti-NHERF2 antibody (upper panel). The same blot was reprobed with anti-Flag antibody to show the amount of precipitated LPA receptors (middle panel). The NHERF2 expression levels in the total cell lysates are also shown (bottom panel). (C) Preferential interaction of GST-fused NHERF2 to LPA2-CT. MBP-fused LPA2-CT (MBP-LPA2-CT) was incubated with GST or GST-fused NHERF isoforms (GST-NHERF1 and GST-NHERF2), which were immobilized on GSH beads. The amounts of MBP-LPA2-CT bound to the GST-fused proteins were measured by SDS-PAGE and after Western blot analysis with anti-MBP antibody (upper) or Ponceau S staining (lower). (D) HA-NHERF2, but not HA-NHERF1, is coimmunoprecipitated with LPA2. Either HA-NHERF1 or HA-NHERF2 was transiently coexpressed with Flag-LPA2 in COS-7 cells. The cell lysates were immunoprecipitated with anti-Flag antibody. After being washed, the resulting precipitates and total lysates were subjected to SDS-PAGE after immunoblot analysis with anti-HA antibody or anti-Flag antibody, as indicated. These results are representative of at least two independent experiments.
The NHERF family contains two subtypes, NHERF1 and NHERF2 (43). NHERF1 and NHERF2 were further examined to determine whether they commonly bind to LPA2. The immobilized GST-fused proteins were incubated with the MBP-fused LPA2-CT (Fig. 1C). It was found that MBP-LPA2 CT has a strong preference for NHERF2 over NHERF1. In addition, this result was confirmed by immunoprecipitation with Flag-LPA2 from the lysates of COS-7 cells cotransfected with the HA-NHERF constructs. HA-NHERF2 was found to interact significantly with Flag-LPA2, whereas HA-NHERF1 was not detected, which was attributed to its low affinity for LPA2 (Fig. 1D). These results suggest that LPA2 binding is specific for NHERF2 but not for NHERF1.
The interaction between LPA2 and NHERF2 requires both the second PDZ domain of NHERF2 and the C-terminal PDZ-binding motif of LPA2.
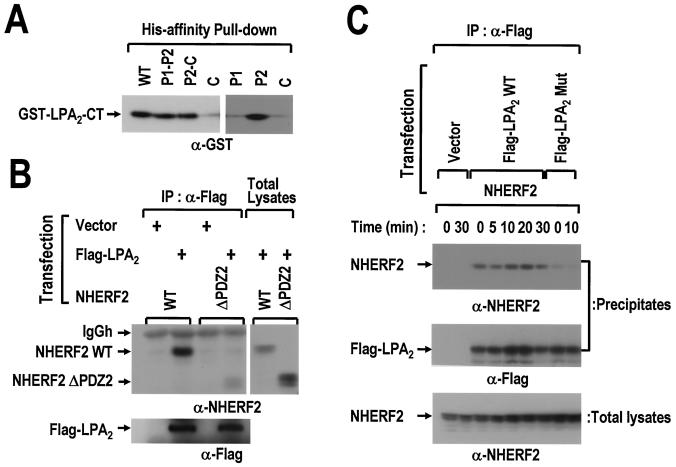
First, we attempted to determine the binding region of NHERF2 that are essential for the LPA2 association. Two different deletion mutants (P1-P2 and P2-C), which were truncated at the C or N terminus, and three fragments (P1, P2, and C), which contain the first PDZ, second PDZ, or ERM-binding domains individually, were expressed in His6-tagged forms. Several fragments of NHERF2 were immobilized onto a Ni2+-nitrilotriacetic acid resin and incubated with the GST-LPA2-CT (Fig. 2A). The P1-P2 mutant, which contains both PDZ domains, was found to interact equivalently with the wild-type NHERF2. Interestingly, the deletion of the first PDZ domain from the N terminus did not affect the association with LPA2. Further successive analysis, using the fragmented versions of NHERF2 such as P1, P2, and C, revealed that LPA2-CT binds to P2 fragment specifically. These results suggest that the second PDZ domain of NHERF2 is required for the interaction with LPA2. The binding region of NHERF2 was further confirmed in vivo by comparing the wild-type NHERF2 with the second PDZ domain-deleted mutant. As shown in Fig. 2B, Flag-LPA2 coimmunoprecipitated wild-type NHERF2 but not the NHERF2 mutant with the second PDZ domain deleted, suggesting that LPA2 forms a molecular complex with NHERF2 via its second PDZ domain.
FIG. 2.
The interaction between LPA2 and NHERF2 requires both the second PDZ domain of NHERF2 and the C-terminal PDZ-binding motif of LPA2. (A) Six His-NHERF2 (WT) and its fragments (P1, the first PDZ domain; P2, the second PDZ domain; and C, the C-terminal fragment of NHERF2) were immobilized on Ni2+-affinity resin and subsequently incubated with the GST-LPA2-CT. The amounts of GST-LPA2-CT bound to the immobilized proteins were measured by SDS-PAGE, followed by Western blot analysis with anti-GST antibody. (B) Flag-LPA2 was cotransfected with either wild-type NHERF2 (NHERF2 WT) or the second PDZ-deleted form (NHERF2 ΔPDZ2), as indicated. The cell lysates were immunoprecipitated with anti-Flag antibody. The resulting precipitates were analyzed by Western blot analysis with the anti-NHERF2 antibody (left, upper) to detect the precipitated NHERF2 WT or NHERF2 ΔPDZ2. The same blot was reprobed with anti-Flag antibody (left, lower) to show the amount of precipitated LPA2. The expression levels of NHERF2 WT and of NHERF2 ΔPDZ2 within the total cell lysates are also shown (right). (C) NHERF2 was cotransfected with wild-type Flag-LPA2 (Flag-LPA2 WT) or its mutant form (Flag-LPA2 Mut) into COS-7 cells, as indicated. The transfected cells were serum deprived for 24 h prior to treatment with 1 μM LPA in 0.1% BSA for the indicated times. After being washed with ice-cold phosphate-buffered saline (PBS), the cells were lysed and immunoprecipitated with anti-Flag antibody. The resulting precipitates (top, middle) and total lysates (bottom) were analyzed by Western blot analysis with either anti-NHERF2 antibody or anti-Flag antibody, as indicated. These results are representative of three independent experiments.
Second, we examined whether or not the interaction between LPA2 and NHERF2 essentially depends on the C-terminal PDZ-binding motif of LPA2. The wild-type Flag-LPA2 was compared to its mutant, in which the C-terminal Leu residue was substituted with Ala. As expected, a point mutation (L→A) in the PDZ-binding motif of LPA2 abolished the interaction with NHERF2 (Fig. 2C). Furthermore, the effect of agonist stimulation on the interaction between two molecules was examined. LPA stimulation did not induce any considerable change in the LPA2-NHERF2 interaction through all of the time points examined. Therefore, these results indicate that LPA2 exists in a molecular complex with NHERF2 in resting cells regardless of the LPA stimulation.
The stable expression of NHERF2 increases LPA-induced PLC-β activation in Rat-1 cells.
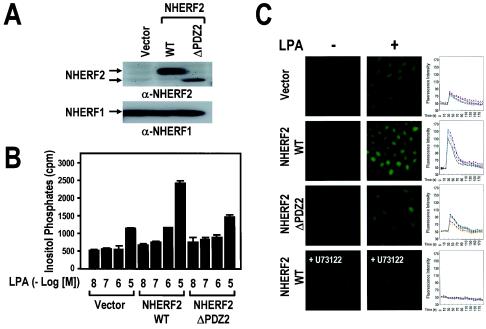
In order to reveal the functional role of NHERF2 in LPA signaling, Rat-1 cell lines overexpressing either wild-type NHERF2 (NHERF2 wild type [NHERF2 WT]) or the NHERF2 mutant with the second PDZ domain deleted (NHERF2 ΔPDZ2) were established (Fig. 3A). In these cell lines, the NHERF1 expression levels were not altered by NHERF2 expression. Previously, it was demonstrated that LPA induces the LPA receptor-mediated PLC-β activation, which is followed by intracellular Ca2+ mobilization in a variety of cells (4, 8, 22, 23). Interestingly, we found that LPA-induced IP generation was markedly potentiated by the expression of the wild-type NHERF2. In contrast to the wild-type NHERF2, the second PDZ domain-deleted mutant of NHERF2 did not make any change on LPA-induced IP generation, suggesting the importance of the second PDZ domain in the function of NHERF2 (Fig. 3B). Consistently, LPA-induced Ca2+ mobilization was markedly augmented by the wild-type NHERF2 expression but not by either the vector or the NHERF2 mutant expression (Fig. 3C). Furthermore, intracellular Ca2+ mobilization was completely abolished by the presence of 5 μM U73122, which is a specific PLC inhibitor. These results indicate that NHERF2 expression potentiates the LPA-induced PLC-β activation, followed by intracellular Ca2+ mobilization in Rat-1 cells.
FIG. 3.
NHERF2 increases LPA-induced IP generation and subsequent Ca2+ mobilization in Rat-1 cells. (A) Rat-1 cells were stably transfected with vector alone (Vector), NHERF2 WT, and the second PDZ domain-deleted mutant (NHERF2 ΔPDZ2), individually. The NHERF2 (upper) and NHERF1 (lower) expression levels were determined by Western blot analysis with the specific antibodies against each isoform of NHERF, as indicated. (B) Rat-1 cell lines were split at a density of 3 × 105 cells/well, which were then loaded with 1 μCi of [3H]inositol/ml for 24 h at 37°C and stimulated with LPA at the indicated concentrations. The generation of [3H]IPs was determined as described in Materials and Methods. The data are presented as means ± the standard errors (SE) of three separate experiments performed in duplicate. (C) LPA-induced Ca2+ mobilization in Rat-1 cell lines. Rat-1 cells were loaded with 1 μM Fluo-3/AM for 30 min at 37°C, washed twice with prewarmed Ca2+-free Locke's solution, and then stabilized for 5 min under a microscope. Fluorescence confocal microscopic images of the cytosolic Ca2+ mobilization of Rat-1 cell lines were captured sequentially before (−) and after (+) the addition of 10 μM LPA (magnification, ×200) and in the presence or absence of 5 μM U73122, as indicated. Ca2+-sensitive fluorescence images of each of the Rat-1 cells were subjected to densitometry over time (right). The results shown are those of a single experiment that is representative of three experiments performed with independent preparations.
NHERF2 is required for the efficient LPA2-mediated PLC-β signaling in HeLa cells.
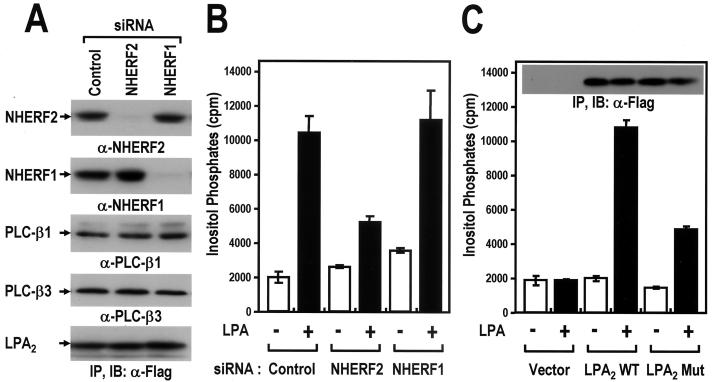
The functional role of NHERF2 was further confirmed in HeLa cells, in which NHERF2 was expressed endogenously. In addition, HeLa cells are ideal for assessing the LPA2-mediated PLC activation because these cells show low levels of a background LPA response and thus allow LPA2 signaling to be studied after transfection. In order to modulate the NHERF2 expression level in the HeLa cells, the RNAi technique, which specifically silences the expression of the endogenous genes in mammalian cells, was used. When transfected with the siRNA duplexes directed against the respective NHERF isoforms, the NHERF1 and NHERF2 expression levels were significantly decreased (Fig. 4A). This effect of the siRNA transfection was specific for the respective isoforms since it had no effect on the expressions of the other NHERF isoform. In addition, the expressions of PLC-β isoforms or Flag-LPA2 were not affected by siRNA transfection. Importantly, the knock-down of NHERF2 resulted in a marked decrease in LPA2-mediated IP generation, suggesting the functional significance of NHERF2 in LPA2-mediated PLC-β activation in HeLa cells (Fig. 4B). Furthermore, in good agreement with the selective interaction of NHERF2 with LPA2 (Fig. 1D), LPA2-mediated IP generation was affected specifically by the knock-down of NHERF2 but not by that of NHERF1. This suggests that NHERF2 plays an important role in LPA2-mediated PLC-β activation.
FIG. 4.
NHERF2 is required for the efficient LPA2-mediated PLC-β activation in HeLa cells. (A) Gene silencing of either the NHERF1 or NHERF2 with siRNAs. HeLa cells were transfected with siRNAs directed against NHERF1 or NHERF2 or with a control siRNA (Luciferase GL3), as described in Materials and Methods. One day after the siRNA transfection, the cells were split at a density of 2 × 105 cells/well. After 24 h, the cells were infected with the recombinant adenovirus expressing Flag-LPA2. After 24 h postinfection, the HeLa cells were lysed and analyzed by Western blot with the specific antibodies as indicated. These results were representative of three independent experiments. (B) Effects of NHERF2 gene silencing on LPA2-mediated PLC-β activation in HeLa cells. After 12 h postinfection, the infected cells were labeled with 1 μCi of [3H]inositol/ml for 12 h at 37°C, treated with either 0.1% BSA only (−) or 1 μM LPA-BSA conjugates (+), and then analyzed for [3H]IPs as described in Materials and Methods. The data are presented as means ± the SE of three separate experiments performed in duplicate. (C) The effects of a mutation at the C-terminal PDZ-binding motif of LPA2 on LPA-induced IPs generation in HeLa cells. HeLa cells were transfected with the indicated constructs (Vector, control vector; LPA2 WT, wild-type LPA2; LPA2 Mut, mutant LPA2). At 24 h posttransfection, the cells were labeled with [3H]inositol for 12 h and treated with either 0.1% BSA only (−) or 1 μM LPA-BSA conjugates (+). The generation of IPs was analyzed as described previously. The results are presented as means ± SE obtained from three separate experiments performed in duplicate. In addition, the expressions of Flag-LPA2 WT and Flag-LPA2 Mut were analyzed by immunoprecipitation and subsequent Western blot analysis with anti-Flag antibody (inside the graph).
The importance of the direct interaction between LPA2 and NHERF2 was next examined by comparing the wild-type LPA2 with its mutant, which was defective in terms of the interaction with NHERF2. The wild-type LPA2 or its mutant (-DSTA) was transfected into HeLa cells. Upon treatment with 1 μM LPA, IP accumulation increased markedly in the wild-type LPA2-transfected cells, whereas its mutant (-DSTA) showed an ∼70% reduction in IP accumulation (Fig. 5C). Despite the significant difference in the LPA-induced PLC activation, the expression levels of the wild-type LPA2 and of its mutant were similar (Fig. 5C), and both endogenous PLC-β and NHERF2 expression levels were unaffected by the transfection of LPA2 (data not shown). Therefore, the observed difference in the activation of PLC-β may have been caused by a point mutation in the PDZ-binding motif of LPA2. These results indicate that LPA2-mediated PLC-β activation is modulated by the PDZ-binding motif-dependent interaction of LPA2 with NHERF2.
FIG. 5.
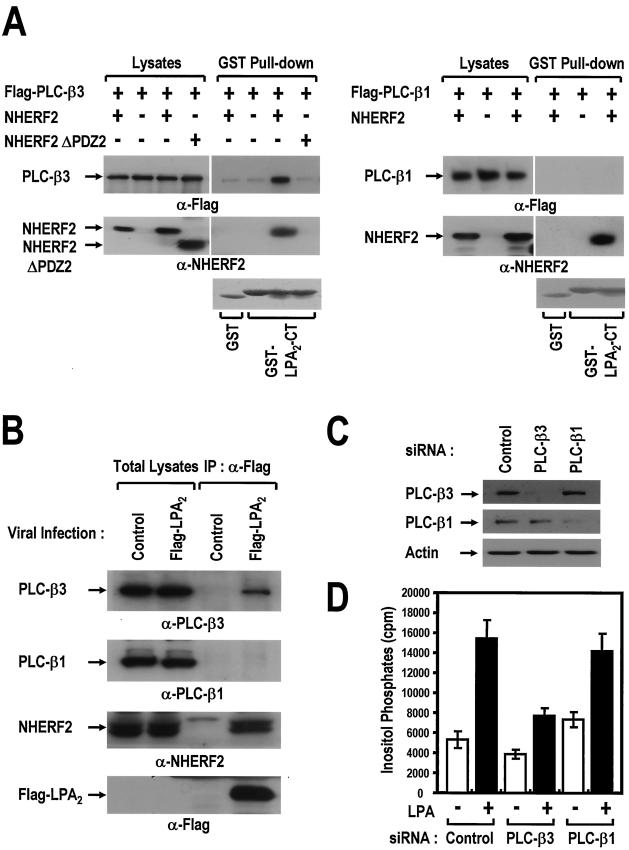
NHERF2 couples LPA2 to PLC-β3 specifically. (A) LPA2 forms a molecular complex with PLC-β3 in a NHERF2-dependent manner. The NHERF2 constructs (NHERF2, NHERF2 WT; NHERF2 ΔPDZ2, the second PDZ domain-deleted form) were cotransfected in combination with either Flag-PLC-β3 (left) or Flag-PLC-β1 (right) as indicated. After 2 days, COS-7 cells were washed and incubated with PBS containing 0.5 mM DSP (Pierce), which is a cell-permeable cross-linker, for 30 min. After the residual DSP was blocked with PBS containing 50 mM Tris buffer, the cleared lysates were subjected to a pull-down assay using GST-LPA2-CT immobilized onto GSH beads. After a washing step, the resulting precipitates were subjected to SDS-PAGE and then analyzed by Western blot analysis with anti-Flag antibody (top) and anti-NHERF2 antibody (middle) or by Ponceau S staining (bottom) as indicated. The results are representative of three independent experiments. (B) Specific coimmunoprecipitation of PLC-β3 and not of PLC-β1 with Flag LPA2. HeLa cells (at a density of 6 × 106 cells/150-mm dish) were infected with either the recombinant Flag-LPA2 adenovirus or control empty virus at an MOI of 10. At 24 h postinfection, the infected cells were incubated with PBS containing 0.5 mM DSP for 30 min. After the cell lysates were blocked with 50 mM Tris buffer, they were immunoprecipitated with anti-Flag antibody. After a washing step, the resulting precipitates were analyzed with the specific antibodies as indicated. (C) Gene silencing of PLC-β isoforms with specific siRNAs. HeLa cells were transfected with siRNAs directed against PLC-β1 or PLC-β3, along with control siRNA (Luciferase), as described in Materials and Methods. After 3 days, the HeLa cells were lysed and then analyzed by Western blot analysis with specific antibodies, as indicated. (D) PLC-β3 is functionally coupled to LPA2. At 24 h after siRNA transfection, the cells were split at a density of 2 × 105 cells/35-mm well and infected with recombinant adenovirus expressing Flag-LPA2 for 4 h. At 12 h after viral infection, the infected cells were labeled with 1 μCi of [3H]inositol/ml for 12 h and treated with either 0.1% BSA only (−) or 1 μM LPA-BSA conjugates (+). The generation of [3H]IPs was analyzed as described in Materials and Methods. The results are presented as means ± the SE of three experiments performed in duplicate.
NHERF2 couples LPA2 to PLC-β3 specifically.
In a previous study, it was demonstrated that NHERF2 is associated with PLC-β3 (21). Moreover, several reports have suggested that NHERF forms a multimeric complex within the cells due to the dimerization of the PDZ domains (30, 33, 47). Therefore, NHERF2 was examined to determine whether it enables LPA2 to be linked physically to PLC-β3. NHERF2 was cotransfected in combination with Flag-tagged PLC-β isoforms into COS-7 cells and treated with 0.5 mM DSP, which is a cell permeable cross-linker, as described by Lau and Hall (30). Thereafter, the lysates were subjected to a pull-down assay with GST-LPA2-CT and GST as the control. As shown in Fig. 5A, PLC-β3 was coprecipitated with GST-LPA2 CT in the presence of NHERF2 but not in its absence. On the other hand, PLC-β1 was not detected despite the presence of NHERF2. In a good agreement with the above results, it was found that Flag-LPA2 coimmunoprecipitated endogenous PLC-β3, along with NHERF2 in HeLa cells. In contrast, PLC-β1 was not detected in the precipitates (Fig. 5B). This suggests that NHERF2 mediates the physical and specific interaction of LPA2 with PLC-β3 but not with PLC-β1.
LPA2 was then examined to determine whether it is functionally coupled to PLC-β3 isoform. When analyzed for the endogenous PLC-β isoforms, HeLa cells were found to express both PLC-β1 and PLC-β3 isoforms (Fig. 5B), whereas the other PLC-β isoforms were not detected by Western blot analysis (data not shown). The present study developed siRNAs, which were directed against respective PLC-β isoforms (Fig. 5C). After transfection with the siRNA duplexes, the expressions of either PLC-β1 or PLC-β3 were specifically reduced to ca. 80 to 90% of the control cell level. Upon LPA stimulation, accumulation of the IPs was severely affected by PLC-β3 knock-down but not by PLC-β1 knock-down (Fig. 5D). Therefore, these results indicate the functional and specific coupling between LPA2 and PLC-β3 isoform.
NHERF2 potentiates LPA-induced ERK activation and COX-2 induction.
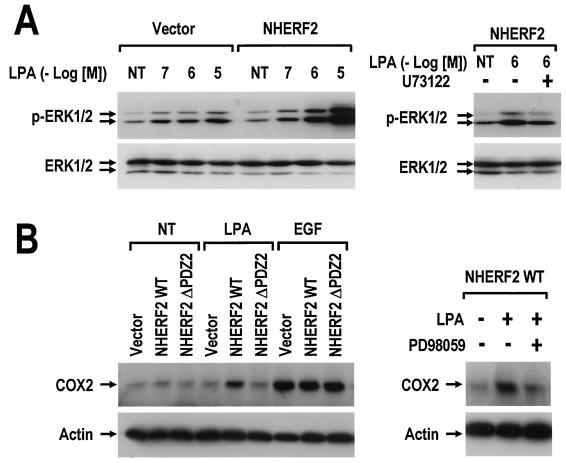
The potential influence of NHERF2 expression on MAPK pathway was next examined. As shown in Fig. 6A, LPA-induced ERK activation was strongly enhanced by NHERF2 expression in Rat-1 cells. Moreover, the potentiation of ERK activity was reversed by pretreating the cells with 5 μM U73122, which is a PLC inhibitor. This suggests that an increase in PLC-β signaling is responsible for the NHERF2-dependent amplification of ERK activation. In addition, the present study examined whether or not the enhanced ERK activation has an effect on the expression of the downstream target genes. Cyclooxygenase-2 (COX-2) is an inducible form of cyclooxygenase and is known to be upregulated in a MAPK-dependent manner (39). In response to 5 μM LPA in 0.1% bovine serum albumin (BSA), COX-2 expression was strongly induced by NHERF2 expression in Rat-1 cells but not by either the vector or the second PDZ-deleted form (Fig. 6B). In contrast, epidermal growth factor (EGF)-induced COX-2 expression was similar in the three cell lines, suggesting a specific role of NHERF2 in LPA signaling. Moreover, this induction of the COX-2 gene was blocked by PD98059, which is a specific inhibitor of the MEK/ERK signaling pathway, indicating the involvement of the ERK pathways in this process. These results suggest that NHERF2-dependent potentiation of PLC-β signaling augments the LPA-induced ERK activation and the subsequent COX-2 induction in Rat-1 cells.
FIG. 6.
NHERF2 potentiates LPA-induced ERK activation and COX-2 induction. (A) Rat-1/vector and Rat-1/NHERF2 WT cells were serum deprived for 24 h and then treated with the LPA-BSA conjugates at the indicated concentrations for 10 min. Independently, Rat-1/NHERF2 WT cells were pretreated with dimethyl sulfoxide (−) or 5 μM U73122 (+) for 20 min and then treated with 1 μM LPA in 0.1% BSA for 10 min. The cell lysates (20 μg) were subjected to SDS-PAGE and analyzed with either anti-pERK antibody or anti-ERK antibody. The results shown are representative of three independent experiments. (B) Serum-deprived Rat-1 cell lines (vector, NHERF2 WT, and NHERF2 ΔPDZ2) were treated with 5 μM LPA or with 50 ng of EGF/ml for 10 h at 37°C. In an independent experiment, Rat-1/NHERF2 WT cells were pretreated with dimethyl sulfoxide (−) or 30 μM PD98059 (+) for 30 min and then treated with 5 μM LPA for 10 h at 37°C. The cell lysates (20 μg) were subjected to SDS-PAGE and analyzed by Western blot with anti-COX-2 antibody or anti-actin antibody as indicated. The results shown are those of a single experiment, which is representative of three experiments performed with independent preparations.
DISCUSSION
NHERF2-dependent regulation of LPA2-mediated PLC-β activation.
In the present study, we demonstrated that NHERF2 specifically interacts with LPA2 in an isoform-specific manner. Although it is known that NHERF1 and NHERF2 share the majority of their target proteins (16, 17, 29, 33), there is a line of evidence suggesting a specific association of NHERF2 with several target molecules, such as plasma membrane Ca2+ ATPase isoform 2b (PMCA2b), PLC-β3, TAZ, α-actinin-4, and serum/glucocorticoid-regulated protein kinase (7, 10, 21, 24, 27, 47). It is therefore interesting that NHERF2 specifically regulates the LPA2 signaling through the selective interaction. Therefore, these results provide the evidence that NHERF2 has a distinctive role in signal transduction that is not duplicated by NHERF1. LPA has been reported to stimulate PLC-β activation via its cognate receptors: LPA1, LPA2, and LPA3 (3, 4, 22, 23). In addition, when examined in mouse embryo fibroblast cells, which were derived from knockout mice, both LPA1 and LPA2 were shown to mediate PLC-β activation in response to LPA stimulation (8). These reports suggest that the LPA receptors commonly have an intrinsic ability to induce PLC-β activation in various types of cells. However, they appear to vary in the relative strength of agonist-induced PLC-β signaling events, involving Ca2+ mobilization and PKC activation, according to the cell types examined (3, 4, 8, 12, 22, 23). In this regard, it is interesting that NHERF2 interacts specifically with LPA2 and may provide a mechanism by which LPA2 intensifies PLC-β activation. Therefore, these results suggest that LPA receptor-mediated PLC-β activation might be differentially regulated by the specific coupling with not only the G proteins but also additional regulatory factors, such as NHERF2.
Although it is not known how NHERF2 increases the LPA-induced PLC-β activity, we suggest that NHERF2 acts as a scaffolding protein. It was shown here that PLC-β3 is included in the same protein complex with LPA2 and NHERF2 (Fig. 5A and B). We previously reported that PLC-β3 contains a C-terminal PDZ domain-binding motif and directly associates with the second PDZ domain of NHERF2 (21). In the absence of NHERF2, both LPA2 and PLC-β3 may be nonassociated, which limits the signal transduction between them. In contrast, in the presence of NHERF2, LPA2 and PLC-β3 may be physically coupled in the protein complex, thus enabling LPA2 to trigger PLC-β3 signaling efficiently. However, these findings raise a question as to how both PLC-β3 and LPA2 bind to the same region (the second PDZ domain) of NHERF2. One possible explanation is that LPA2 and PLC-β3 bind to different NHERF2 molecules in the same protein complex. Recently, several reports have demonstrated that NHERF1 or NHERF2 dimerizes via the PDZ domain-mediated interactions (30, 33, 47). Therefore, these results suggest that the self-association of NHERF2 may be essential for the formation of a protein complex containing LPA2 and PLC-β3.
NHERF2-dependent specific coupling of LPA2 to PLC-β3.
Mammals possess four PLC-β isoforms, namely, β1 to β4 (37), which are known to have quite distinct physiological roles (26). We found here that LPA2 is coupled to PLC-β3 in an isoform-specific manner. A large body of evidence suggests that the PLC-β isoforms are selectively activated by extracellular GPCR agonists (2, 14, 41, 42). It has been generally assumed that this selectivity might be due to either a cell type-specific distribution of PLC-β isoforms or the G-protein subclass involved (37). However, both PLC-β1 and PLC-β3 are commonly expressed in several cell lines, including Rat-1 and HeLa cells, which were used in the present study. With respect to the G-protein sensitivity, it is known that both PLC-β1 and PLC-β3 are stimulated by GTPγS-bound Gαq to similar extents in vitro (40). Furthermore, LPA2-mediated PLC activation is either insensitive or partially sensitive to pertussis toxin according to the cells examined, which suggests that LPA2 couples to Gαq primarily to activate PLC-β (4, 23). In this regard, neither the differential distribution of PLC-β isoforms nor the distinct activation of the G-protein subclass is likely to be a satisfactory explanation for the PLC-β selectivity of LPA2. Therefore, isoform-selective coupling appears to involve a mechanism different from those described previously. In light of our pull-down assay results (Fig. 5A and B), which show that LPA2 can form a ternary complex with PLC-β3 but not with PLC-β1, the signaling specificity is probably conferred by the selective scaffolding of the sequential signal transducers. This selectivity of the PLC-β isoform is consistent with the higher degree of preference of NHERF2 for PLC-β3 over PLC-β1, as was reported previously (21). Accordingly, such interactions with NHERF2 may also influence the specificity of LPA2-mediated PLC-β activation by holding LPA2 and PLC-β3 in close proximity.
Potential physiological consequence of NHERF2-dependent regulation of LPA2 signaling.
PLC-β is involved in MAPK activation by IP3-dependent Ca2+ mobilization and DAG-dependent PKC activation (5, 35). Moreover, GPCR agonists, including LPA and carbachol, have been shown to activate ERK in a PLC-dependent manner (25, 32). The present study found that NHERF2 potentiates the LPA-induced ERK activation, followed by COX-2 induction in Rat-1 cells via a PLC-dependent pathway. It is known that COX-2 is upregulated in a MAPK-dependent manner in response to cytokines, growth factors, and other stimuli and plays a key role as a mediator of proliferation and inflammation (28, 39). Furthermore, whereas NHERF2 upregulates LPA-driven COX-2 induction, EGF-driven induction is unaffected. This indicates that NHERF2 specifically increases LPA-stimulated COX-2 induction by interacting directly with LPA2, since EGF receptor does not contain a PDZ domain-binding motif at its C terminus. Therefore, these results suggest that NHERF2 may play an important role in regulating LPA2-mediated downstream signaling such as the PLC-β/ERK/COX-2 pathway. LPA and its receptors are known to be multifunctional in a variety of normal cell physiologies (34, 36). In addition, several reports have suggested a role for LPA/LPA2 signaling in the pathogenesis, such as in tumor formation and cancer development (11, 13, 38, 46). In this regard, the current observation that NHERF2 potentiates the LPA2 signaling events such as PLC-Ca2+/PKC, ERK, and COX-2 induction implies a broad connection of NHERF2 in the regulation of LPA/LPA2-mediated pathological, physiological responses, including cell proliferation, inflammation, and tumor progression.
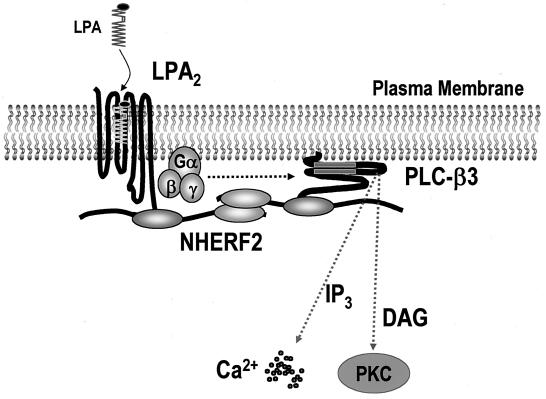
In summary, it was found that LPA2 interacts with NHERF2 in an isoform-specific manner and provided evidence that NHERF2 functions as a molecular scaffold, which defines the specificity and efficiency of LPA2-mediated PLC-β3 activation. A schematic view of LPA2 signaling emerging from the present study is depicted in Fig. 7. The results suggest that a ternary complex of LPA2, NHERF2, and PLC-β3 plays a pivotal role in the organization and modulation of the LPA2-mediated PLC signaling pathway.
FIG. 7.
Schematic view of the NHERF2-dependent regulation of LPA2-mediated PLC-β3 signaling. Prior to agonist stimulation, both LPA2 and PLC-β3 directly interact with NHERF2, which is localized to the plasma membrane. NHERF2, which is multimerized by a PDZ-PDZ interaction, clusters LPA2 and PLC-β3 in close proximity, thereby creating spatially compact signaling complexes beneath the plasma membrane. Consequently, the LPA2-NHERF2-PLC-β3 complex enables LPA2 to transduce its signal to PLC-β3 with efficiency and specificity.
Acknowledgments
We thank Junken Aoki (Tokyo University) for kindly providing three LPA receptors constructs. We also are grateful to Yasuyuki Igarashi (Hokkaido University), and Jerold Chun (The Scripps Research Institute) for helpful discussions and invaluable comments.
This study was supported by grants from the Korea Health 21 R&D Project (00-PJ1-PG1-CH13-0005), Ministry of Health and Welfare, Seoul, Republic of Korea, and the 21C Frontier R&D Program (M101KB010001-02K0201-01710), Center for Functional Analysis of Human Genome, Seoul, Republic of Korea.
REFERENCES
- 1.An, S., T. Bleu, O. G. Hallmark, and E. J. Goetzl. 1998. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J. Biol. Chem. 273:7906-7910. [DOI] [PubMed] [Google Scholar]
- 2.Arthur, J. F., S. J. Matkovich, C. J. Mitchell, T. J. Biden, and E. A. Woodcock. 2001. Evidence for selective coupling of α1-adrenergic receptors to phospholipase C-β1 in rat neonatal cardiomyocytes. J. Biol. Chem. 276:37341-37346. [DOI] [PubMed] [Google Scholar]
- 3.Bandoh, K., J. Aoki, A. Taira, M. Tsujimoto, H. Arai, and K. Inoue. 2000. Lysophosphatidic acid (LPA) receptors of the EDG family are differentially activated by LPA species: structure-activity relationship of cloned LPA receptors. FEBS Lett. 478:159-165. [DOI] [PubMed] [Google Scholar]
- 4.Bandoh, K., J. Aoki, H. Hosono, S. Kobayashi, T. Kobayashi, K. Murakami-Murofushi, M. Tsujimoto, H. Arai, and K. Inoue. 1999. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J. Biol. Chem. 274:27776-27785. [DOI] [PubMed] [Google Scholar]
- 5.Beekman, A., B. Helfrich, P. A. Bunn, Jr., and L. E. Heasley. 1998. Expression of catalytically inactive phospholipase C-β disrupts phospholipase C-β and mitogen-activated protein kinase signaling and inhibits small cell lung cancer growth. Cancer Res. 58:910-913. [PubMed] [Google Scholar]
- 6.Bretscher, A., D. Chambers, R. Nguyen, and D. Reczek. 2000. ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu. Rev. Cell Dev. Biol. 16:113-143. [DOI] [PubMed] [Google Scholar]
- 7.Chun, J., T. Kwon, E. Lee, P. G. Suh, E. J. Choi, and S. S. Kang. 2002. The Na+/H+ exchanger regulatory factor 2 mediates phosphorylation of serum- and glucocorticoid-induced protein kinase 1 by 3-phosphoinositide-dependent protein kinase 1. Biochem. Biophys. Res. Commun. 298:207-215. [DOI] [PubMed] [Google Scholar]
- 8.Contos, J. J., I. Ishii, N. Fukushima, M. A. Kingsbury, X. Ye, S. Kawamura, J. H. Brown, and J. Chun. 2002. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2). Mol. Cell. Biol. 22:6921-6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contos, J. J., and J. Chun. 2000. Genomic characterization of the lysophosphatidic acid receptor gene, lp(A2)/Edg4, and identification of a frameshift mutation in a previously characterized cDNA. Genomics 64:155-169. [DOI] [PubMed] [Google Scholar]
- 10.DeMarco, S. J., M. C. Chicka, and E. E. Strehler. 2002. Plasma membrane Ca2+ ATPase isoform 2b interacts preferentially with Na+/H+ exchanger regulatory factor 2 in apical plasma membranes. J. Biol. Chem. 277:10506-10511. [DOI] [PubMed] [Google Scholar]
- 11.Erickson, J. R., Y. Hasegawa, X. Fang, A. Eder, M. Mao, T. Furui, J. Aoki, A. Morris, and G. B. Mills. 2001. Lysophosphatidic acid and ovarian cancer: a paradigm for tumorigenesis and patient management. Prostaglandins 64:63-81. [DOI] [PubMed] [Google Scholar]
- 12.Fang, X., S. Yu, J. L. Tanyi, Y. Lu, J. R. Woodgett, and G. B. Mills. 2002. Convergence of multiple signaling cascades at glycogen synthase kinase-3: Edg receptor-mediated phosphorylation and inactivation by lysophosphatidic acid through a protein kinase C-dependent intracellular pathway. Mol. Cell. Biol. 22:2099-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goetzl, E. J., H. Dolezalova, Y. Kong, Y. L. Hu, R. B. Jaffe, K. R. Kalli, and C. A. Conover. 1999. Distinctive expression and functions of the type 4 endothelial differentiation gene-encoded G protein-coupled receptor for lysophosphatidic acid in ovarian cancer. Cancer Res. 59:5370-5375. [PubMed] [Google Scholar]
- 14.Gonzalez-Yanes, C., J. Santos-Alvarez, and V. Sanchez-Margalet. 2001. Pancreastatin, a chromogranin A-derived peptide, activates Gα16 and phospholipase C-β2 by interacting with specific receptors in rat heart membranes. Cell. Signal 13:43-49. [DOI] [PubMed] [Google Scholar]
- 15.Hall, R. A., and R. J. Lefkowitz. 2002. Regulation of G protein-coupled receptor signaling by scaffold proteins. Circ. Res. 91:672-680. [DOI] [PubMed] [Google Scholar]
- 16.Hall, R. A., L. S. Ostedgaard, R. T. Premont, J. T. Blitzer, N. Rahman, M. J. Welsh, and R. J. Lefkowitz. 1998. A C-terminal motif found in the β2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. Proc. Natl. Acad. Sci. USA 95:8496-8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall, R. A., R. T. Premont, C. W. Chow, J. T. Blitzer, J. A. Pitcher, A. Claing, R. H. Stoffel, L. S. Barak, S. Shenolikar, E. J. Weinman, S. Grinstein, and R. J. Lefkowitz. 1998. The β2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature 392:626-630. [DOI] [PubMed] [Google Scholar]
- 18.Hecht, J. H., J. A. Weiner, S. R. Post, and J. Chun. 1996. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J. Cell Biol. 135:1071-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 20.Hung, A. Y., and M. Sheng. 2002. PDZ domains: structural modules for protein complex assembly. J. Biol. Chem. 277:5699-5702. [DOI] [PubMed] [Google Scholar]
- 21.Hwang, J. I., K. Heo, K. J. Shin, E. Kim, C. H. Yun, S. H. Ryu, H. S. Shin, and P. G. Suh. 2000. Regulation of phospholipase C-β3 activity by Na+/H+ exchanger regulatory factor 2. J. Biol. Chem. 275:16632-16637. [DOI] [PubMed] [Google Scholar]
- 22.Im, D. S., C. E. Heise, M. A. Harding, S. R. George, B. F. O'Dowd, D. Theodorescu, and K. R. Lynch. 2000. Molecular cloning and characterization of a lysophosphatidic acid receptor, Edg-7, expressed in prostate. Mol. Pharmacol. 57:753-759. [PubMed] [Google Scholar]
- 23.Ishii, I., J. J. Contos, N. Fukushima, and J. Chun. 2000. Functional comparisons of the lysophosphatidic acid receptors, LP(A1)/VZG-1/EDG-2, LP(A2)/EDG-4, and LP(A3)/EDG-7 in neuronal cell lines using a retrovirus expression system. Mol. Pharmacol. 58:895-902. [DOI] [PubMed] [Google Scholar]
- 24.Kanai, F., P. A. Marignani, D. Sarbassova, R. Yagi, R. A. Hall, M. Donowitz, A. Hisaminato, T. Fujiwara, Y. Ito, L. C. Cantley, and M. B. Yaffe. 2000. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 19:6778-6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keely, S. J., S. O. Calandrella, and K. E. Barrett. 2000. Carbachol-stimulated transactivation of epidermal growth factor receptor and mitogen-activated protein kinase in T(84) cells is mediated by intracellular Ca2+, PYK-2, and p60src. J. Biol. Chem. 275:12619-12625. [DOI] [PubMed] [Google Scholar]
- 26.Kim, D., K. S. Jun, S. B. Lee, N. G. Kang, D. S. Min, Y. H. Kim, S. H. Ryu, P. G. Suh, and H. S. Shin. 1997. Phospholipase C isozymes selectively couple to specific neurotransmitter receptors. Nature 389:290-293. [DOI] [PubMed] [Google Scholar]
- 27.Kim, J. H., W. Lee-Kwon, J. B. Park, S. H. Ryu, C. H. Yun, and M. Donowitz. 2002. Ca2+-dependent inhibition of Na+/H+ exchanger 3 (NHE3) requires an NHE3-E3KARP-α-actinin-4 complex for oligomerization and endocytosis. J. Biol. Chem. 277:23714-23724. [DOI] [PubMed] [Google Scholar]
- 28.Koki, A., N. K. Khan, B. M. Woerner, A. J. Dannenberg, L. Olson, K. Seibert, D. Edwards, M. Hardy, P. Isakson, and J. L. Masferrer. 2002. Cyclooxygenase-2 in human pathological disease. Adv. Exp. Med. Biol. 507:177-184. [DOI] [PubMed] [Google Scholar]
- 29.Lamprecht, G., E. J. Weinman, and C. H. Yun. 1998. The role of NHERF and E3KARP in the cAMP-mediated inhibition of NHE3. J. Biol. Chem. 273:29972-29978. [DOI] [PubMed] [Google Scholar]
- 30.Lau, A. G., and R. A. Hall. 2001. Oligomerization of NHERF-1 and NHERF-2 PDZ domains: differential regulation by association with receptor carboxyl termini and by phosphorylation. Biochemistry 40:8572-8580. [DOI] [PubMed] [Google Scholar]
- 31.Mahon, M. J., M. Donowitz, C. C. Yun, and G. V. Segre. 2002. Na+/H+ exchanger regulatory factor 2 directs parathyroid hormone 1 receptor signalling. Nature 417:858-861. [DOI] [PubMed] [Google Scholar]
- 32.Mattingly, R. R., V. Saini, and I. G. Macara. 1999. Activation of the Ras-GRF/CDC25Mm exchange factor by lysophosphatidic acid. Cell. Signal 11:603-610. [DOI] [PubMed] [Google Scholar]
- 33.Maudsley, S., A. M. Zamah, N. Rahman, J. T. Blitzer, L. M. Luttrell, R. J. Lefkowitz, and R. A. Hall. 2000. Platelet-derived growth factor receptor association with Na+/H+ exchanger regulatory factor potentiates receptor activity. Mol. Cell. Biol. 20:8352-8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moolenaar, W. H., O. Kranenburg, F. R. Postma, and G. C. Zondag. 1997. Lysophosphatidic acid: G-protein signalling and cellular responses. Curr. Opin. Cell Biol. 9:168-173. [DOI] [PubMed] [Google Scholar]
- 35.Nebigil, C. G. 1997. Suppression of phospholipase C-β, -γ, and -δ families alters cell growth and phosphatidylinositol 4,5-bisphosphate levels. Biochemistry 36:15949-15958. [DOI] [PubMed] [Google Scholar]
- 36.Pages, C., M. F. Simon, P. Valet, and J. S. Saulnier-Blache. 2001. Lysophosphatidic acid synthesis and release. Prostaglandins Other Lipid Mediat. 64:1-10. [DOI] [PubMed] [Google Scholar]
- 37.Rhee, S. G. 2001. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 70:281-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulte, K. M., A. Beyer, K. Kohrer, S. Oberhauser, and H. D. Roher. 2001. Lysophosphatidic acid, a novel lipid growth factor for human thyroid cells: overexpression of the high-affinity receptor edg4 in differentiated thyroid cancer. Int. J. Cancer 92:249-256. [DOI] [PubMed] [Google Scholar]
- 39.Sheng, H., C. S. Williams, J. Shao, P. Liang, R. N. DuBois, and R. D. Beauchamp. 1998. Induction of cyclooxygenase-2 by activated Ha-ras oncogene in Rat-1 fibroblasts and the role of mitogen-activated protein kinase pathway. J. Biol. Chem. 273:22120-22127. [DOI] [PubMed] [Google Scholar]
- 40.Smrcka, A. V., and P. C. Sternweis. 1993. Regulation of purified subtypes of phosphatidylinositol-specific phospholipase C-β by G protein α and βγ subunits J. Biol. Chem. 268:9667-9674. [PubMed] [Google Scholar]
- 41.Strassheim, D., and C. L. Williams. 2000. P2Y2 purinergic and M3 muscarinic acetylcholine receptors activate different phospholipase C-β isoforms that are uniquely susceptible to protein kinase C-dependent phosphorylation and inactivation. J. Biol. Chem. 275:39767-39772. [DOI] [PubMed] [Google Scholar]
- 42.Strassheim, D., P. Y. Law, and H. H. Loh. 1998. Contribution of phospholipase C-β3 phosphorylation to the rapid attenuation of opioid-activated phosphoinositide response. Mol. Pharmacol. 53:1047-1053. [PubMed] [Google Scholar]
- 43.Voltz, J. W., E. J. Weinman, and S. Shenolikar. 2001. Expanding the role of NHERF, a PDZ-domain containing protein adapter, to growth regulation. Oncogene 20:6309-6314. [DOI] [PubMed] [Google Scholar]
- 44.Wang, S., R. W. Raab, P. J. Schatz, W. B. Guggino, and M. Li. 1998. Peptide binding consensus of the NHE-RF-PDZ1 domain matches the C-terminal sequence of cystic fibrosis transmembrane conductance regulator (CFTR). FEBS Lett. 427:103-108. [DOI] [PubMed] [Google Scholar]
- 45.Weinman, E. J., C. Minkoff, and S. Shenolikar. 2000. Signal complex regulation of renal transport proteins: NHERF and regulation of NHE3 by PKA. Am. J. Physiol. Renal Physiol. 279:F393-F399. [DOI] [PubMed] [Google Scholar]
- 46.Xu, Y., Z. Shen, D. W. Wiper, M. Wu, R. E. Morton, P. Elson, A. W. Kennedy, J. Belinson, M. Markman, and G. Casey. 1998. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA 280:719-723. [DOI] [PubMed] [Google Scholar]
- 47.Yun, C. C., Y. Chen, and F. Lang. 2002. Glucocorticoid activation of Na+/H+ exchanger isoform 3 revisited: the roles of SGK1 and NHERF2. J. Biol. Chem. 277:7676-7683. [DOI] [PubMed] [Google Scholar]