Abstract
Despite the availability of blood pressure (BP)-lowering medications and dietary education, hypertension is still poorly controlled in the chronic kidney disease (CKD) population. As glomerular filtration rate declines, the number of medications required to achieve BP targets increases, which may lead to reduced patient adherence and therapeutic inertia by the clinician. Home BP monitoring (HBPM) has emerged as a means of improving diagnostic accuracy, risk stratification, patient adherence, and therapeutic intervention. The definition of hypertension by HBPM is an average BP >135/85 mm Hg. Twelve readings over the course of 3-5 days are sufficient for clinical decision making. Diagnostic accuracy is especially important in the CKD population as approximately half of these patients have either white coat hypertension or masked hypertension. Preliminary data suggest that HBPM outperforms office BP monitoring in predicting progression to end-stage renal disease or death. When combined with additional support such as telemonitoring, medication titration, or behavioral therapy, HBPM results in a sustained improvement in BP control. HBPM must be adapted to provide information on the phenomena of nondipping (absence of nocturnal fall in BP) and reverse dipping (paradoxical increase in BP at night). These diurnal patterns are more prevalent in the CKD population and are important cardiovascular risk factors. Ambulatory BP monitoring provides nocturnal BP readings and unlike HBPM may be reimbursed by Medicare when certain criteria are met. Further studies are needed to determine whether HBPM is cost-effective in the current US healthcare system.
Key Words: Treatment of hypertension, Chronic kidney disease, Hemodialysis, Peritoneal dialysis, Kidney transplantation
Home Blood Pressure Monitoring Target in Chronic Kidney Disease
For the past 10 years, the Joint National Committee on hypertension (JNC) recommended an office blood pressure monitoring (OBPM) target of 130/80 mm Hg or less in chronic kidney disease (CKD) [1]. Recently, JNC 8 loosened this target to 140/90 mm Hg, the same as for the general population <60 years [2]. This new recommendation reflects the lack of robust data supporting the lower blood pressure (BP) target. Accordingly, recent clinical practice guidelines for BP management in CKD have suggested a target of ≤130/80 mm Hg only in the setting of severe albuminuria [3]. Multiple studies have addressed the cutoff value for hypertension by home BP monitoring (HBPM). Methods include setting the value as greater than the 95th percentile of HBPM among individuals who were normotensive by conventional OBPM. Others have used regression analysis to determine the HBPM that corresponds to an OBPM of 140/90 mm Hg [4]. Tsuji et al. [5] based their recommendation of 137/84 mm Hg on 5-year mortality data from their trial of HBPM in residents of Ohasama, Japan. The International Database of Home Blood Pressure in relation to Cardiovascular Outcome (IDHOCO) has compiled data of over 6,000 participants from population studies of HBPM in Japan, Finland, Uruguay, and Greece. Over a follow-up period of 8.3 years, they calculated HBPM levels that had a similar 10-year risk as OBPM thresholds. By their analysis, the thresholds for JNC 7 stages 1 and 2 hypertension are 130/85 and 145/90 mm Hg, respectively [6]. The first value is slightly lower than the hypertension definition of the JNC 7 and European Society of Hypertension Practice Guidelines of HBPM >135/85 mm Hg [1,7]. The HBPM definition was not addressed in the JNC 8 guidelines [2].
Risk Stratification
Hypertension is the most important risk factor for cardiovascular (CV) disease, ischemic stroke, and intracerebral hemorrhage [8]. In addition, hypertension is the second leading cause of CKD, and elevated BP accelerates the decline in glomerular filtration rate (GFR) over time in those with CKD from other etiologies [9]. The goal of detection and treatment of hypertension in the population is to reduce adverse outcomes. HBPM is more accurate than OBPM, using ambulatory BP monitoring (ABPM) as the standard [4,10]. Recent literature has also shown that HBPM is superior to OBPM in predicting left ventricular hypertrophy, fatal or nonfatal CV events, and all-cause mortality in the general population [11,12,13]. These differences are magnified as risk increases and are higher in men and with increasing age and systolic BP (SBP) [4]. The previously described IDHOCO has recently reported on the association of white coat hypertension, masked hypertension, and sustained hypertension with a composite CV outcome, including CV mortality, nonfatal myocardial infarction, surgical and percutaneous coronary revascularization, heart failure, pacemaker implantation, and stroke [14]. Over a mean follow-up of 8.3 years, masked hypertension was shown to be a significant risk factor for the composite CV outcome. The results are consistent with previous reports using ABPM. Interestingly, this study also found that the risk of the composite outcome was higher in untreated patients with white coat hypertension. Unfortunately, OBPM was assessed during a single visit, and different HBPM schedules were used in the individual population studies, limiting the generalizability of the data [14]. Future studies from this database will address (1) concordance between morning and evening BP, (2) variability of HBPM and heart rate, and (3) pulse pressure [15]. The studies included in the database represent the general population in their respective vicinities, but it is possible that the large sample size may allow a subgroup analysis of patients with CKD. Rather than focusing the attention on identifying novel biomarkers of cardiac risk, accurate stratification of patients based on established risk factors is a pragmatic contemporary clinical approach.
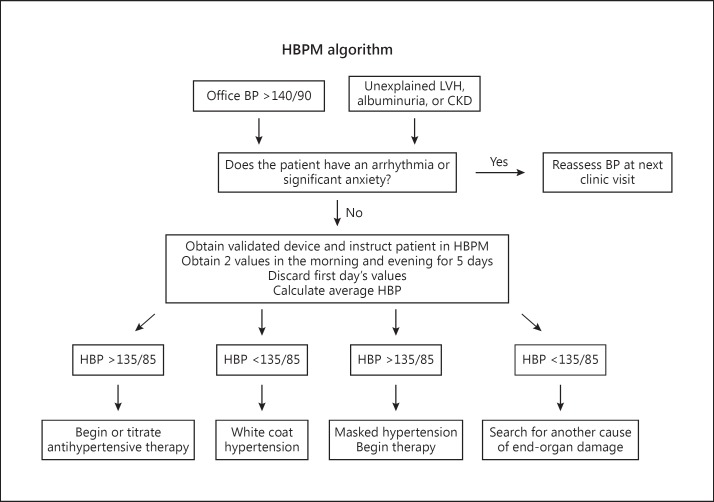
The prevalence of hypertension increases with declining GFR and was estimated at 83% in the CKD population in the Modification of Diet in Renal Disease (MDRD) Study [9]. Of these individuals, only 56% had controlled hypertension as defined by BP <140/90 mm Hg [16]. Unfortunately, OBPM is an inadequate diagnostic tool in this population as evidenced by masked hypertension rates of approximately 25% and white coat hypertension rates of 29% [17]. OBPM performs especially poorly in African-Americans with hypertensive nephrosclerosis. In the AASK trial, 70% of those with controlled OBPM were found to have hypertension by ABPM [18]. Masked hypertension has been shown to have a significantly higher CV risk than normotension [19]. HBPM may also correlate better with renal outcomes than OBPM. A small study showed that the albumin excretion rate correlated with HBPM but not OBPM [20]. In a study of 217 US veterans with CKD, HBPM performed better than OBPM in predicting progression to end-stage renal disease or death over a 3.5-year period [21]. HBPM should be considered for any patient with new-onset hypertension by OBPM or left ventricular hypertrophy, albuminuria, or unexplained CKD (fig. 1).
Fig. 1.
Flow diagram for the use of HBPM. LVH = Left ventricular hypertrophy.
BP Variability and Nocturnal Readings
Data are emerging that multiple BP readings through the day may provide information on BP trends that predict prognosis more precisely. These metadata capture BP variability and patterns of nondipping (absence of nocturnal fall in BP) and reverse dipping (paradoxical increase in BP at night). SBP variability has been linked with a lower GFR [22]. In one study of diabetic patients with albuminuria, SBP variability significantly correlated with a higher incidence of coronary artery disease and retinopathy [23]. However, it is currently unclear whether SBP variability correlates with progression of CKD [24].
The prevalence of nondipping and reverse dipping is also higher in the CKD population and increases with declining GFR. Nondippers have a significantly higher rate of stroke and CV mortality independent of the mean 24-hour ABPM [25]. Furthermore, obstructive sleep apnea (OSA) may cause nocturnal rises in BP through hypoxemia, sympathetic activation, and disruption of normal sleep [26]. Continuous positive airway pressure therapy decreases CV mortality in patients with OSA [27]. Thus, detection of the nondipping status is not only of prognostic importance, but may identify patients who should be screened for OSA. The Omron 747-1C-N has a memory-equipped clock that can be utilized as a timer to obtain BP readings at night and has been used in at least one clinical trial [28]. Since dipping status may vary from night to night, HBPM offers the advantage of measurements over multiple nights. See table 1 for a comparison of OBPM, HBPM, and ABPM.
Table 1.
Methods of BP measurement
| Method | Advantages | Disadvantages |
|---|---|---|
| OBPM | Most commonly used in RCTs and long-term outcome trials | Highly variable |
| Observer bias | ||
| Reimbursed with office visit | May be inaccurate in white coat hypertension and masked hypertension | |
| HBPM | Stronger predictor of hypertensive end-organ damage than OBPM | Requires training and device calibration |
| Improves adherence and BP control, especially when combined with additional supportive measures | Out of pocket patient expense | |
| Unreliable in atrial fibrillation | ||
| Detects white coat and masked hypertension | May exacerbate anxiety disorder and obsessive behavior | |
| Wide availability and low cost | ||
| ABPM | Stronger predictor of hypertensive end-organ damage than OBPM | Expensive |
| Cumbersome for the user | ||
| Most reliable way to assess nondipping and reverse dipping | Strict criteria for reimbursement | |
| Limited availability | ||
| Detects white coat and masked hypertension | ||
Adherence and Management
HBPM is attractive as a means of reducing pill burden in those with white coat hypertension and improving adherence in patients with sustained hypertension. Hypertension is an insidious disease that may be completely asymptomatic until it causes end-organ damage. Patients often cite the lack of symptoms as a reason for nonadherence to a BP regimen, and the estimated adherence varies between 50-70% [29]. HPBM gives the patient a way of monitoring medication efficacy and may act as positive reinforcement [30]. In a study of 250 patients in Spain, adherence to an antihypertensive regimen was 92% in the HPBM group as compared to 74% in the control group [31]. It is also useful in avoiding unnecessary therapy in white coat hypertension. In the Treatment of Hypertension Based on Home or Office Blood Pressure (THOP) trial, 400 European patients with a diastolic BP (DBP) >95 mm Hg were randomized to HBPM or OBPM antihypertensive titration per protocol. At 1 year, more patients in the home BP group (25.6 vs. 11.3%) were able to discontinue medications, but the average BP was also higher by 4.9/2.9 mm Hg [32]. The target DBP was <90 mm Hg, although guidelines suggest a home DBP target <85 mm Hg, which may have accounted for the higher home BP. Identification of white coat hypertension also led to more medication discontinuation in the Home Versus Office Measurement, Reduction of Unnecessary Treatment Study (HOMERUS), which included 430 patients, used oscillometric devices for both OBPM and HBPM, and assessed both SBP and DBP control. Unlike THOP, HOMERUS found that uncontrolled hypertension was more frequent in the OBPM group and a statistically higher percentage of patients in the HBPM group met their BP target (74 vs. 50%) [33].
HBPM generates more BP readings that may help overcome therapeutic inertia on the part of the clinician and allow more rapid titration of medications. In a large European study by McManus et al. [34], titration of medications occurred every 2 months in the group randomized to HBPM and telemonitoring. The usual care group saw a physician on average 3.5 times/year and had a significantly higher office BP at 1 year. These results have been reproduced in a large US Veterans Administration study that included 48% African-Americans. Patients had better BP control at 1 month with HBPM and medication titration every 6 weeks as necessary. The improvement was sustained at 18 months in those who were hypertensive at baseline [35]. Pharmacist management of antihypertensive medications may be equally efficacious [36]. Further, a meta-analysis of 52 prospective comparative trials of HBPM with or without intervention compared to OBPM found HBPM combined with additional support results in an average lowering of SBP by 3.4-8.9 mm Hg and DBP by 1.9-4.4 mm Hg. There was no significant difference between usual care and HBPM at 1 year in the absence of additional support such as telemonitoring, medication titration, or behavioral therapy [37]. These trials support the use of HBPM with interventions to achieve BP targets. Most trials excluded patients with a creatinine >2.0 mg/dl (177 mmol/l), except the study by McManus et al. [34], which included 10% of the patients with CKD. Further studies are needed in the CKD population.
Cost Benefit
Due to the heterogeneity of study outcomes and healthcare systems, there is insufficient data to determine the cost-effectiveness of HBPM for both the diagnosis and management of hypertension. In the HOMERUS trial, identification of white coat hypertension and reduced medication costs amounted to a savings of USD 135/person/year [33]. The benefits of HBPM in hypertension management are sustained when combined with additional support such as nursing or physician feedback. These costs will vary based on the method of intervention and type of clinicians utilized. The validated device used in the majority of studies (Omron HBPM 7 series) was noted to cost USD 59/person/year. The price of approved devices ranges between USD <50-100 and can be found at bloodpressureuk.org and bhsoc.org [38,39]. In contrast, the median price of an ABPM device is USD 1,600 [40]. An analysis of the Ohasama study, a large Japanese trial of HBPM as a diagnostic and prognostic instrument showed that this approach is cost-effective, but it did not assess HBPM as a management tool, and therefore did not include costs of additional nurse or physician support [41]. In a simulation model using the Framingham risk score, risk factor inputs from the Health Survey of England 2006, and estimates of risk reduction with antihypertensive therapy from a meta-analysis of 147 trials, both HBPM and ABPM were cost-effective with ABPM being the most cost-effective [40]. The majority of savings result from a reduction of therapy in patients with white coat hypertension as medications comprise the majority of costs for the long-term care of hypertension [40,42]. Though HBPM is a better predictor of CV risk and is an effective means of achieving BP goals when combined with behavioral or medication interventions, long-term studies are needed to determine whether this translates into improved CV outcomes and increased quality-adjusted life years. Improved outcomes conceptually provide the basis for the cost-effectiveness of HBPM as a management tool without relying on the savings from medication discontinuation.
Reimbursement
Medicare does not currently reimburse HBPM. Thus, clinicians can use HBPM only for patients who are able to purchase the monitor out of pocket. Since the purchase does not require a prescription, the patient runs the risk of buying a device that has never been validated. Physicians also do not have the possibility to bill specifically for the time spent instructing the patient on the proper technique or review patient data in the absence of an office visit. Medicare reimburses for ABPM only if strict criteria are met: (1) OBPM >140/90 mm Hg, (2) at least two BP measurements taken outside the office <140/90 mm Hg, and (3) no evidence of end-organ damage [43]. Charges allowed by Medicare to confirm the diagnosis of white coat hypertension vary from USD 70-105, which includes interpretation by the physician [42].
Implementation
The American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association released guidelines on the use of HBPM in 2008. The European Society of Hypertension released updated guidelines in 2010. Both recommend the use of an oscillometric approved upper arm device, since finger and wrist devices are less accurate and in general more dependent on the arm position [7]. An updated list of validated devices are available via web link [38,39,44]. Prior to use, the patient should bring the monitor to the clinic to check patient technique and monitor accuracy. The inflatable bladder of the cuff should be at least 80% of the upper arm circumference. The patient should sit comfortably for 5 min with his/her arm resting on a table prior to taking a measurement. The cuff should be placed approximately 2-3 cm above the bend of the elbow at the level of the heart. Results should be written down immediately if the device is not memory equipped [7]. Patients should perform two measurements in the morning and evening on 3-5 consecutive days with the first day's readings excluded due to disproportionately high values on day 1 [45]. Twelve values are sufficient to make clinical decisions [42].
Patients with arrhythmias such as atrial fibrillation should not use HBPM as results may not be accurate. In addition, HBPM should be discouraged in patients with severe anxiety that may worsen with the detection of high values. Though arterial stiffness is increased in patients with diabetes and CKD, the difference between oscillometric and mercury-based measurements is small. The Omron M3 Intellisense monitor has been validated in CKD stages 3-5 (average GFR 31 ml/min/1.73 m2), and the Microlife 3AC1-1PC has been validated in hemodialysis (HD) populations [46]. The IDHOCO includes data from five large population studies of HBPM versus OBPM, and using these results will further elucidate the number and timing of HBPM readings required for the prediction of CV risk [15].
Dialysis and Transplant
HD patients have substantial swings in BP. BP measurements in the predialysis, postdialysis, and intradialytic period may not correlate with standardized BP measurements used in large randomized controlled trials (RCTs) from which BP goals are derived [47]. The timing of the target BP measurement is a challenging clinical concern in this population [48]. The mixed data regarding outcomes in achieving clinical practice guideline-recommended BP targets may stem from the use of these suboptimal measurements to guide ultrafiltration and antihypertensive therapy [49]. Data suggest that HBPM results may be a stronger predictor of CV outcomes in the HD population [50]. As in the general population, HBPM correlates better with ABPM. In a study of 140 hemodialysis patients, Agarwal et al. [51] found that HBPM (13-15 readings over 1 week) outperformed predialysis and postdialysis BP in predicting 44-hour interdialytic ABPM. HBPM measurements were more reproducible and had a decreased lag time in BP response to probing dry weight based on clinical signs and symptoms [51]. In addition, an RCT of 17 HD patients found that HBPM with nursing intervention resulted in significantly lower average weekly BP compared to usual care [52]. HBPM has not been extensively studied in the peritoneal dialysis population. A study of 32 peritoneal dialysis patients demonstrated that HBPM correlated with ABPM but was not superior to OBPM [53].
In the kidney transplant population, approximately 60-80% of the patients are hypertensive, and many immunosuppressive agents elevate BP [54]. Poor control not only has detrimental CV effects for the recipient but also is an independent risk factor for graft failure [55]. Agena et al. [56] found that HBPM correlated better with ABPM than with OBPM in kidney recipients between 1 and 10 years after transplantation. More study is needed to determine the prognostic value of HBPM in graft failure, CV outcomes, and its utility in the management of hypertension in this population.
Clinical Trial Design
The superiority of HBPM over OBPM in predicting CV outcomes allows for further study of controversial topics in hypertension management including the optimal level of dietary sodium restriction, chronotherapy, and stenting for atherosclerotic renal artery stenosis. Asayama et al. [57] completed a 5-year study of CV outcomes of tight versus usual BP control guided by HBPM. Patients were included if they fell within a prespecified BP range by HBPM during a 2-week run-in period off therapy. A memory-equipped device was employed, and patients took measurements once per day within 1 h of awakening. Drug therapy was based on the average of morning readings over the 5 days immediately preceding the doctor's visit. The study dropout rate was 35% over the 5 years, and the authors did not provide reasons for discontinuation. There was also no information on the percentage of participants with incomplete data. Nevertheless, this study supports the feasibility of long-term trials using HBPM once daily [57]. HBPM was also practical in a study of elderly persons >70 years of age with CKD who were asked to record two daily values for 7 days every 6 months. At a mean follow-up time of 3 years, 79 of 104 participants had HBPM information available. Participants who started dialysis, died within 1 year or had inadequate HBPM data, comprised the 25 participants who were excluded from the analysis. The authors did not provide a breakdown of the number of patients who met exclusionary criteria [58]. The ideal number of BP readings to assess a clinically significant BP response in trials remains to be defined. In the Ohasama study, the accuracy of HBPM improved with the increasing number of measurements [59]. A memory-equipped device was employed in both studies and is essential to ensure data integrity.
Therapeutic Inertia
One of the benefits of HBPM is that it may help overcome therapeutic inertia, since the practitioner has more information available to make clinical decisions. However, this relies on the active engagement of the healthcare team that may include nurses, pharmacists, and physicians. Though adherence may improve with HPBM alone, additional support such as telemonitoring results in sustained benefit.
Conclusion
HBPM is a simple, inexpensive tool that correlates better with ABPM, the gold standard, than OBPM. It is useful in diagnosing white coat hypertension and masked hypertension and adds prognostic value in predicting the progression of CKD to end-stage renal disease. It is a promising therapeutic tool in the titration of antihypertensives and has demonstrated superiority to OBPM in controlling BP when combined with supportive measures. Validated, memory-equipped devices are widely available and can be programmed to provide nocturnal readings. Long-term US studies are needed to determine the cost-effectiveness within our unique healthcare system.
References
- 1.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 2.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 3.Taler SJ, Agarwal R, Bakris GL, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for management of blood pressure in CKD. Am J Kidney Dis. 2013;62:201–213. doi: 10.1053/j.ajkd.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verberk W, Kroon A, Kessels A, de Leeuw P. Home blood pressure measurement: a systematic review. J Am Coll Cardiol. 2005;46:743–751. doi: 10.1016/j.jacc.2005.05.058. [DOI] [PubMed] [Google Scholar]
- 5.Tsuji I, Imai Y, Nagai K, et al. Proposal of reference values for home blood pressure measurement: prognostic criteria based on a prospective observation of the general population in Ohasama, Japan. Am J Hypertens. 1997;10:409–418. [PubMed] [Google Scholar]
- 6.Niiranen TJ, Asayama K, Thijs L, et al. Outcome-driven thresholds for home blood pressure measurement: international database of home blood pressure in relation to cardiovascular outcome. Hypertension. 2013;61:27–34. doi: 10.1161/HYPERTENSIONAHA.111.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parati G, Stergiou G, Asmar R, et al. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens. 2008;26:1505–1526. doi: 10.1097/HJH.0b013e328308da66. [DOI] [PubMed] [Google Scholar]
- 8.Wilson P. Established risk factors and coronary artery disease: the Framingham Study. Am J Hypertens. 1994;7:7S–12S. doi: 10.1093/ajh/7.7.7s. [DOI] [PubMed] [Google Scholar]
- 9.Buckalew VJ, Berg R, Wang S, et al. Prevalence of hypertension in 1,795 subjects with chronic renal disease: the modification of diet in renal disease study baseline cohort. Modification of Diet in Renal Disease Study Group. Am J Kidney Dis. 1996;28:811–821. doi: 10.1016/s0272-6386(96)90380-7. [DOI] [PubMed] [Google Scholar]
- 10.Stergiou GS, Karpettas N, Kapoyiannis A, Stefanidis CJ, Vazeou A. Home blood pressure monitoring in children and adolescents: a systematic review. J Hypertens. 2009;27:1941–1947. doi: 10.1097/HJH.0b013e32832ea93e. [DOI] [PubMed] [Google Scholar]
- 11.Ohkubo T, Imai Y, Tsuji I, et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens. 1998;16:971–975. doi: 10.1097/00004872-199816070-00010. [DOI] [PubMed] [Google Scholar]
- 12.Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–1783. doi: 10.1161/01.CIR.0000160923.04524.5B. [DOI] [PubMed] [Google Scholar]
- 13.Drawz P, Abdalla M, Rahman M. Blood pressure measurement: clinic, home, ambulatory, and beyond. Am J Kidney Dis. 2012;60:449–462. doi: 10.1053/j.ajkd.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stergiou GS, Asayama K, Thijs L, et al. Prognosis of white-coat and masked hypertension: international database of home blood pressure in relation to cardiovascular outcome. Hypertension. 2014;63:675–682. doi: 10.1161/HYPERTENSIONAHA.113.02741. [DOI] [PubMed] [Google Scholar]
- 15.Niiranen T, Thijs L, Asayama K, et al. The International Database of Home blood pressure in relation to Cardiovascular Outcome (IDHOCO): moving from baseline characteristics to research. Hypertens Res. 2012;35:1072–1079. doi: 10.1038/hr.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peralta C, Hicks L, Chertow G, et al. Control of hypertension in adults with chronic kidney disease in the United States. Hypertension. 2005;45:1119–1124. doi: 10.1161/01.HYP.0000164577.81087.70. [DOI] [PubMed] [Google Scholar]
- 17.Andersen M, Khawandi W, Agarwal R. Home blood pressure monitoring in CKD. Am J Kidney Dis. 2005;45:994–1001. doi: 10.1053/j.ajkd.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Gabbai F, Rahman M, Hu B, et al. Relationship between ambulatory BP and clinical outcomes in patients with hypertensive CKD. Clin J Am Soc Nephrol. 2012;7:1770–1776. doi: 10.2215/CJN.11301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hänninen M, Niiranen T, Puukka P, Johansson J, Jula A. Prognostic significance of masked and white-coat hypertension in the general population: the Finn-Home Study. J Hypertens. 2012;30:705–712. doi: 10.1097/HJH.0b013e328350a69b. [DOI] [PubMed] [Google Scholar]
- 20.Mule G, Caimi G, Cottone S. Value of home blood pressures as predictor of target organ damage in mild arterial hypertension. J Cardiovasc Risk. 2002;9:123–129. doi: 10.1177/174182670200900208. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal R, Andersen M. Prognostic importance of clinic and home blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69:406–411. doi: 10.1038/sj.ki.5000081. [DOI] [PubMed] [Google Scholar]
- 22.Romero MJ. Home blood pressure variability: a new target to monitor in chronic kidney disease patients with low eGFR? Hypertens Res. 2013;36:673–675. doi: 10.1038/hr.2013.53. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura M, Kato Y, Tanaka T, et al. Significance of estimating the glomerular filtration rate for the management of hypertension in type 2 diabetes with microalbuminuria. Hypertens Res. 2013;36:705–710. doi: 10.1038/hr.2013.22. [DOI] [PubMed] [Google Scholar]
- 24.Okada T, Matsumoto H, Nagaoka Y, Nakao T. Association of home blood pressure variability with progression of chronic kidney disease. Blood Press Monit. 2012;17:1–7. doi: 10.1097/MBP.0b013e32834f7125. [DOI] [PubMed] [Google Scholar]
- 25.Cohen D, Younghong H, Townsend R. Ambulatory blood pressure in chronic kidney disease. Curr Hypertens Rep. 2013;15:160–166. doi: 10.1007/s11906-013-0339-2. [DOI] [PubMed] [Google Scholar]
- 26.Wolf J, Hering D, Narkiewicz K. Non-dipping pattern of hypertension and obstructive sleep apnea syndrome. Hypertens Res. 2010;33:867–871. doi: 10.1038/hr.2010.153. [DOI] [PubMed] [Google Scholar]
- 27.Doherty L, Kiely J, Swan V, McNicholas W. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127:2076–2084. doi: 10.1378/chest.127.6.2076. [DOI] [PubMed] [Google Scholar]
- 28.Chonan K, Kikuya M, Araki T, et al. Device for the self-measurement of blood pressure that can monitor blood pressure during sleep. Blood Press Monit. 2001;6:203–205. doi: 10.1097/00126097-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Sabate E. Adherence to Long-Term Therapies: Evidence for Action. Geneva, World Health Organization, 2003.
- 30.Johnson A, Taylor D, Sackett D, et al. Self-recording of blood pressure in the management of hypertension. Can Med Assoc J. 1978;119:1034–1039. [PMC free article] [PubMed] [Google Scholar]
- 31.Márquez-Contreras E, Martell-Claros N, Gil-Guillén V, et al. Compliance Group of the Spanish Society of Hypertension (SEE) Efficacy of a home blood pressure monitoring programme on therapeutic compliance in hypertension: the EAPACUM-HTA study. J Hypertens. 2006;24:169–175. doi: 10.1097/01.hjh.0000198023.53859.a2. [DOI] [PubMed] [Google Scholar]
- 32.Den Hond E, Staessen JA, Celis H, et al. Treatment of Hypertension Based on Home or Office Blood Pressure (THOP) Trial Investigators. Antihypertensive treatment based on home or office blood pressure – the THOP trial. Blood Press Monit. 2004;9:311–314. doi: 10.1097/00126097-200412000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Verberk W, Kroon A, Kessels A, et al. Home versus Office blood pressure Measurements: Reduction of Unnecessary treatment Study: rationale and study design of the HOMERUS trial. Blood Press. 2003;12:326–333. doi: 10.1080/08037050310022405. [DOI] [PubMed] [Google Scholar]
- 34.McManus R, Mant J, Bray E, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet. 2010;376:163–172. doi: 10.1016/S0140-6736(10)60964-6. [DOI] [PubMed] [Google Scholar]
- 35.Bosworth H, Powers B, Olsen M, et al. Home blood pressure management and improved blood pressure control: results from a randomized controlled trial. Arch Intern Med. 2011;171:1173–1180. doi: 10.1001/archinternmed.2011.276. [DOI] [PubMed] [Google Scholar]
- 36.Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310:46–56. doi: 10.1001/jama.2013.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uhlig K, Patel K, Ip S, Kitsios G, Balk E. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Ann Intern Med. 2013;159:185–194. doi: 10.7326/0003-4819-159-3-201308060-00008. [DOI] [PubMed] [Google Scholar]
- 38.Blood pressure monitors validated for home use. 2012. www.bhsoc.org (accessed January 14, 2014).
- 39.Clinically validated home blood pressure monitors. 2008. www.bloodpressureuk.org (accessed January 14, 2014).
- 40.Lovibond K, Jowett S, Barton P, et al. Cost-effectiveness of options for the diagnosis of high blood pressure in primary care: a modelling study. Lancet. 2011;378:1219–1230. doi: 10.1016/S0140-6736(11)61184-7. [DOI] [PubMed] [Google Scholar]
- 41.Fukunaga H, Ohkubo T, Kobayashi M, et al. Cost-effectiveness of the introduction of home blood pressure measurement in patients with office hypertension. J Hypertens. 2008;26:685–690. doi: 10.1097/HJH.0b013e3282f42285. [DOI] [PubMed] [Google Scholar]
- 42.Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:10–29. doi: 10.1161/HYPERTENSIONAHA.107.189010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tunis S, Kendall P, Londner M, Whyte J. Medicare Coverage Policy – Decisions: Ambulatory Blood Pressure Monitoring (#CAG-00067N): Decision Memorandum. Washington DC, 2001.
- 44.Sphygmomanometers for self-measurement of blood pressure. 2013. www.dableeducational.org (accessed January 14, 2014).
- 45.Stergiou G, Skeva I, Zourbaki A, Mountokalakis T. Self-monitoring of blood pressure at home: how many measurements are needed? J Hypertens. 1998;16:725–731. doi: 10.1097/00004872-199816060-00002. [DOI] [PubMed] [Google Scholar]
- 46.Thompson A, Eguchi K, Reznik M, Shah S, Pickering T. Validation of an oscillometric home blood pressure monitor in an end-stage renal disease population and the effect of arterial stiffness on its accuracy. Blood Press Monit. 2007;12:227–232. doi: 10.1097/MBP.0b013e328108f544. [DOI] [PubMed] [Google Scholar]
- 47.Rahman M, Griffin V, Kumar A, Manzoor F, Wright JJ, Smith M. A comparison of standardized versus ‘usual’ blood pressure measurements in hemodialysis patients. Am J Kidney Dis. 2002;39:1226–1230. doi: 10.1053/ajkd.2002.33395. [DOI] [PubMed] [Google Scholar]
- 48.Levin N, Kotanko P, Eckardt K, et al. Blood pressure in chronic kidney disease stage 5D – report from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney Int. 2010;77:273–284. doi: 10.1038/ki.2009.469. [DOI] [PubMed] [Google Scholar]
- 49.Tentori F, Hunt W, Rohrscheib M, et al. Which targets in clinical practice guidelines are associated with improved survival in a large dialysis organization? J Am Soc Nephrol. 2007;18:2377. doi: 10.1681/ASN.2006111250. [DOI] [PubMed] [Google Scholar]
- 50.Alborzi P, Patel N, Agarwal R. Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol. 2007;2:1228–1234. doi: 10.2215/CJN.02250507. [DOI] [PubMed] [Google Scholar]
- 51.Agarwal R, Satyan S, Alborzi P. Home blood pressure measurements for managing hypertension in hemodialysis patients. Am J Nephrol. 2009;30:126–134. doi: 10.1159/000206698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kauric-Klein Z, Artinian N. Improving blood pressure control in hypertensive hemodialysis patients. CANNT J. 2007;17:24–28. 31–36. [PubMed] [Google Scholar]
- 53.Wang M, Tseng C, Tsai W, Huang J. Blood pressure and left ventricular hypertrophy in patients on different peritoneal dialysis regimens. Perit Dial Int. 2001;21:36–42. [PubMed] [Google Scholar]
- 54.Mangray M, Vella J. Hypertension after kidney transplant. Am J Kidney Dis. 2011;57:331. doi: 10.1053/j.ajkd.2010.10.048. [DOI] [PubMed] [Google Scholar]
- 55.Kasiske B, Anjum S, Shah R, et al. Hypertension after kidney transplantation. Am J Kidney Dis. 2004;43:1071–1081. doi: 10.1053/j.ajkd.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 56.Agena F, Prado Edos S, Souza P, et al. Home blood pressure (BP) monitoring in kidney transplant recipients is more adequate to monitor BP than office BP. Nephrol Dial Transplant. 2011;26:3745–3749. doi: 10.1093/ndt/gfr143. [DOI] [PubMed] [Google Scholar]
- 57.Asayama K, Ohkubo T, Metoki H, et al. Cardiovascular outcomes in the first trial of antihypertensive therapy guided by self-measured home blood pressure. Hypertens Res. 2012;35:1102–1110. doi: 10.1038/hr.2012.125. [DOI] [PubMed] [Google Scholar]
- 58.Okada T, Nakao T, Matsumoto H, et al. Prognostic significance of home blood pressure control on renal and cardiovascular outcomes in elderly patients with chronic kidney disease. Hypertens Res. 2009;32:1123–1129. doi: 10.1038/hr.2009.165. [DOI] [PubMed] [Google Scholar]
- 59.Ohkubo T, Asayama K, Kikuya M, et al. How many times should blood pressure be measured at home for better prediction of stroke risk? Ten-year follow-up results from the Ohasama study. J Hypertens. 2004;22:1099–1104. doi: 10.1097/00004872-200406000-00009. [DOI] [PubMed] [Google Scholar]