Critical roles for estrogens in growth and development and in pathological conditions of bone, breast, and uterus are well established. Estrogens and estrogen receptor modulators bind to estrogen receptor α (ERα) and/or ERβ to form discrete molecular complexes that exert pleiotropic tissue-specific effects by modulating the expression of target genes. Ligand-bound ER functions as a key transcription factor in various molecular pathways, and modulation of ER expression levels is important in determining cellular growth potential. Recent advances have begun to illuminate the mechanisms by which cells control ER expression. Kos et al. reviewed the genomic organization of the human ERα gene promoter region (31). The human ERα gene, located on chromosome 6, was cloned in 1986 and spans approximately 300 kb; its eight coding regions are transcribed from at least seven promoters (31). Considering the complexity of the ER promoter, it is not surprising that numerous factors affect ERα expression. The aim of this paper is to review the molecular mechanisms by which various cellular factors modulate ERα expression. We have focused on highlighting the major players, including effectors of chromatin structure, hormones, and other relevant agents, and limited our discussion to findings in human systems. There are vast qualitative and quantitative data regarding whether or not, and to what extent, ERα is expressed in normal and neoplastic tissues; this topic is beyond the scope of this review and will not be covered. Moreover, little is currently known about the role of specific transcription factors in ER expression, and these factors will be mentioned only briefly. Henceforth, the terms ERα and ER will be used interchangeably; expression of ERβ will not be discussed.
EFFECTORS OF CHROMATIN STRUCTURE
Epigenetic regulation is an important mechanism by which cells modulate the expression of a variety of genes. Down-regulation of ERα gene expression has been attributed to methylation in a variety of tissues including colon, blood, lung, heart, prostate, and ovary (27-29, 35, 42, 44, 45, 52). This mechanism has been most extensively studied in breast cancer cell lines and tissue to explain the loss of ER expression and acquired hormone resistance associated with this disease. Studies have consistently demonstrated that, whereas normal breast tissues and ER+ breast cancer cell lines lack methylation of the ER gene, ER− breast cancer cell lines and tumors display extensive methylation (35, 44). DNA methyltransferase 1 (DNMT1) levels and activity are increased 2- to 10-fold in ER− breast cell lines, in agreement with the levels of methylation observed in these cells (44). The methylation inhibitor 5-aza-2′-cytidine (5-aza-dC) sequesters DNMT1 to 5-aza-dC-substituted DNA and inhibits DNMT1 action (17, 30). The use of 5-aza-dC resulted in demethylation of the ER CpG island and reexpression of ER mRNA and protein (18). Thus, methylation was established as playing a direct role in the mechanism of ER transcriptional regulation. Lapidus et al. utilized a methylation-specific PCR assay to map the ER CpG island (35). The degree of methylation of the ER gene in breast tumors exhibited an inverse association with ER status, with the lowest levels of methylation seen in ER+/progesterone receptor-positive (PR+) tumors, intermediate levels seen in ER+/PR− tumors, and the highest levels seen in ER−/PR− tumors. Interestingly, 35% of the ER+/PR+ tumors showed substantial methylation. Due to the heterogeneous nature of ER expression in breast cancers, it has been speculated that ER methylation in ER+ tumors might serve as a predictor of which tumors may recur as ER− tumors or those that will develop resistance to hormonal therapy (35).
In contrast to breast carcinomas, the role of methylation in ER down-regulation in endometrial tumors is less well defined. Two separate groups failed to determine a significant correlation between a lack of ER expression and hypermethylation of the ER gene (24, 41). However, two additional studies concluded that methylation was associated with ER status in endometrial carcinomas (51, 55). Importantly, Sasaki et al. analyzed the methylation status of three isoforms generated from promoters A, B, and C (51) (as defined in reference 22). Expression of isoforms ER-A and ER-B was observed in endometrial cancer cell lines, unlike isoform ER-C, which was not expressed in any cell lines tested. Whereas ER-A and ER-B were not methylated in cancerous or normal tissues, ER-C was methylated in 94% of tumor tissues. Thus, the ER-C transcript was shown to be inactivated in endometrial carcinomas, where its lack of expression was associated with methylation.
The incidence of ERα gene methylation has also been linked to the development of heart disease. In the cardiovascular system, estrogen operates through the ER to increase the synthesis of nitric oxide, which promotes blood vessel dilation and prevents inflammation caused by platelets (19). Post et al. discovered an increased frequency of ER methylation in coronary atherosclerotic plaques when compared to normal tissue (45). Notably, ER methylation increased with age in the atrium and was observed principally in smooth muscle cells.
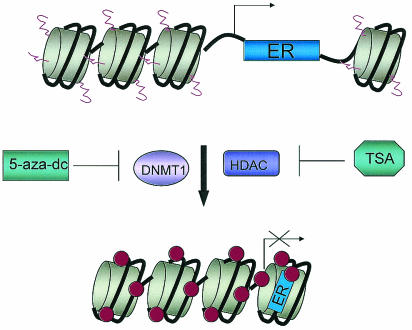
Numerous studies have postulated that methylation of the ER gene is not the sole mechanism for the inactivation of its expression (Fig. 1). In fact, there is increasing evidence that gene silencing by methylation also involves the assistance of histone deacetylases (HDAC) which remove acetyl groups from lysine residues on histones H3 and H4, resulting in the formation of a compact structure that diminishes gene activity (reviewed in reference 40). Yang et al. treated a panel of ER− breast cancer cell lines with trichostatin A (TSA), an HDAC inhibitor; ER mRNA was reexpressed in these cells in a time- and dose-dependent manner, with up to a fivefold increase in ER expression observed (69). However, the ER CpG island remained methylated with ER mRNA levels induced to only 1 to 10% of those seen in MCF7 and T47D ER+ cell lines (69). Additional studies have demonstrated that treatment of ER− cells with TSA and 5-aza-dC results in a synergistic effect on ER reexpression (5). One group observed a 300- to 400-fold induction of ER mRNA associated with increased acetylation of histones H3 and H4, decreased levels of DNMT1, and partial demethylation of the ER CpG island (70). If ER− cells were treated with either 5-aza-dC or TSA alone, however, 5-aza-dC only weakly activated ER expression, whereas TSA did not activate ER. Chen et al. established a clonal cell line derived from T47D cells (T47D:C4:2) that exhibited a loss of ER expression and responsiveness to hormones due to its maintenance in estrogen-free medium (7). Importantly, the ER CpG island in C4:2 cells was not methylated, indicating that methylation was not responsible for ER silencing in these cells. It was thus hypothesized that other steps may precede methylation, including a loss of transcriptional activators or the presence of factors that block the activity of methyltransferases (7).
FIG. 1.
Transcriptional regulation of the ER gene by epigenetic mechanisms. The ER gene is transcriptionally regulated by epigenetic mechanisms including methylation and acetylation. The methyltransferase DNMT1 transfers methyl groups (circles) to cytosine bases located within CpG islands in exon 1 and upstream regulatory regions of ER, thereby inhibiting transcription. Transcriptionally active ER is characterized by the presence of acetyl groups (wavy lines) on histones H3 and H4, which promotes an open, accessible chromatin conformation. Upon removal of the acetyl groups by HDAC, the chromatin becomes condensed where ER is now in a transcriptionally inactive state. The DNMT inhibitor 5-aza-dC and the HDAC inhibitor TSA work synergistically to allow the reexpression of ER in cells with methylated DNA and acetylated histones.
Two transcription factors, ER factor 1 (ERF-1) and ER-B factor 1 (ERBF-1), which regulate ER expression, potentially in conjunction with or as an alternative to methylation in breast cancer cell lines and tissues, have been described previously (13, 54, 71). ERF-1 has been shown to transactivate promoter A, whereas ERBF-1 is necessary for transcription from promoter B (defined in reference 71). Overexpression of ERF-1 in human mammary epithelial cells resulted in the generation of a DNase I-hypersensitive site in the untranslated region of exon 1 (54). Such sites are associated with regions of open chromatin conformations, thereby indicating that ERF-1 can not only transcriptionally regulate the ER gene but can also alter its chromatin structure (54). Yoshida et al. showed a negative correlation between the methylation status of both promoters A and B and ER expression (71). Upon demethylating treatment, no transcription from either promoter B or promoter A was seen in ER− MDA-MB-231 cells, which do not express ERBF-1 or ERF-1. However, promoter A transcripts, but not promoter B transcripts, were induced in ER− BT-20 cells. ERF-1 is expressed in this cell line, in contrast to the lack of expression of ERBF-1. Thus, it has been suggested that a loss of these factors may be important in preventing the reexpression of ER (71).
Another potential modulator of ER expression is mitogen-activated protein kinase (MAPK) kinase (MAPKK MEK), a downstream effector of growth factor signaling. Oh et al. recently demonstrated that hyperactivation of MAPKK in MCF7 cells leads to reduced levels of ER, whereby the inhibition of MAPKK activity led to its reversible up-regulation (43). Additionally, treatment of ER− breast cancer cell lines with 5-aza-cytidine resulted in both increased ER expression and a 9.4-fold decrease in MAPKK activity. If the MEK inhibitor U0126 was also added to the treated cells, MAPKK activity was further reduced 4.4-fold and ER levels increased by an additional 1.5-fold. Thus, these data provide evidence that the inactivation of MAPKK, together with the demethylation of the ER gene, promotes the reexpression of ER.
HORMONAL AND GROWTH FACTOR PATHWAYS
It is important to consider hormonal and growth factor effects on ER expression since one mechanism by which hormones and growth factors regulate the growth of various tissues is by modulating levels of transcription factors, such as the estrogen receptor, that then affect transcription of other genes. Signals transduced by the estrogen receptor affect the growth and metabolism of estrogen-responsive tissues; therefore, the level of ER transcription is an important determinant of cellular growth potential. Much of the research that has elucidated the mechanism of hormonal control of ER expression focuses on understanding how each hormonal system relates to the pathogenesis and pathophysiology of neoplasia, particularly of estrogen-dependent tumors. Table 1 summarizes the effects of the hormones and hormone receptor modulators, whose molecular mechanisms of action are discussed below, on ERα expression, while Fig. 2 graphically depicts the hormonal pathways involved.
TABLE 1.
Effects of hormones, hormone receptor modulators, and growth factors on ER expression
| Agent | Class/pathway | Tissue/cell linea | ER mRNA | ER protein | Reference(s) |
|---|---|---|---|---|---|
| Estradiol | Estrogen | MCF-7 | Increase | Decrease | 37, 49 |
| Endothelium | Increase | Decrease | 26, 64 | ||
| Hypothalamus | Increase | Increase | 58 | ||
| Osteobalst | Increase | 15, 16 | |||
| ICI 182 780 | Estrogen antagonist | MCF-7 | No effect | Decrease | 4 |
| Tamoxifen | MCF-7 | No effect | 67 | ||
| Uterus | Decrease | 9 | |||
| Raloxifene | SERM | MCF-7 | Increase | 67 | |
| ORG 2058 | Progesterone agonist | T47D | Decrease | 53 | |
| R5020 | Progesterone agonist | T47D and MCF-7 | Decrease | 1, 53 | |
| RU486 | Progesterone antagonist | T47D and uterus | Increase | 39, 53 | |
| Danazol | Progestogen/estrogen antagonist | PBMC | Decrease | Decrease | 20, 63 |
| Flutamide | Androgen antagonist | Prostate | Increase | 34 | |
| 1,25(OH)2D3 | Vitamin D | MCF-7 | Decrease | Decrease | 58, 62 |
| EB-1089 | Vitamin D agonist | MCF-7 | Decrease | Decrease | 62 |
| KH-1069 | Vitamin D agonist | MCF-7 | Decrease | Decrease | 62 |
| Ro 27-0574 | Vitamin D agonist | MCF-7 | Decrease | Decrease | 62 |
| Ro 23-7553 | Vitamin D agonist | MCF-7 | Decrease | Decrease | 62 |
| hCG | Gonadotropin/PKA | Ovary | Decrease | Decrease | 8 |
| EGF | Growth factor/PKB | MCF-7 | Decrease | Decrease | 11, 59 |
| D-Ala-GnRH | GnRH agonist/PKC | Ovary | Decrease | Decrease | 8 |
| Insulin | Insulin/IGF-1 | MCF-7 | Increase | 2 | |
| IGF-I | Insulin/IGF-I | MCF-7 | Decrease | Decrease | 60 |
PBMC, peripheral blood mononuclear cells.
FIG. 2.
Hormonal pathways involved in regulation of ER transcription. (A) Nuclear hormone effects on ER transcription. ERAG, estrogen receptor agonist; ERANT, estrogen receptor antagonist; PRAG, progesterone receptor agonist; T, testosterone; AR, androgen receptor; VDRAG, vitamin D receptor agonist; RXR, retinoid X receptor. (B) Protein hormone effects on ER transcription. HCG, human chorionic gonadotropin; INS, insulin; ↓ and ⊥, stimulation and inhibition within the nucleus, respectively.
NUCLEAR HORMONAL PATHWAYS
The human breast tumor cell line MCF7 has been used as a model of ERα action. Early reports of estradiol-induced “ER processing” in MCF7 cells suggested that agonist-induced receptor regulation also occurred in human breast ductal epithelium (25). Most recently, estradiol-induced ERα turnover mediated by the ubiquitin ligase 26S proteasome systems has been proposed as an integral part of the estrogen signaling pathway. In this model, rapid estradiol-induced ERα degradation is linked to ERα-mediated transcriptional responses (37, 49). This model predicts that ERα levels in mammary epithelium are maintained by new receptor synthesis.
Human vascular endothelial cells express ERα and contribute to an important vascular response to estradiol, the local generation of nitric oxide. Rapid proteasome-dependent down-regulation of ERα receptors was observed in human vascular endothelial cells in response to estradiol and was followed by the transcriptional activation of the ERα gene 4 h later and the repletion of ERα protein levels by 6 h (26, 64). These results indicate that acute degradation of ERα followed by an estradiol-dependent transcriptional activation of ERα mRNA is a general estradiol response.
Long-term exposure to estradiol also regulates steady-state ERα levels, and the clearest evidence for this type of control stems from investigations of nonreproductive estrogen target tissues including liver, brain, and peripheral nervous tissue. Most studies showed that estradiol maintains these tissues in an estrogen-responsive condition by up-regulating steady-state ERα expression levels. This view is supported by the presence of estrogen response elements in many of the ERα gene promoter regions (16). The ERα protein expressed in liver is transcribed primarily from the estrogen-inducible promoter E located 150 kb 5′ of the ERα translation initiation site. The splicing of DNA sequences from region E onto the ERα generates an ERα mRNA possessing a unique 5′ untranslated region with the potential for liver-specific regulation. Alternative promoter utilization as a means of transcriptional regulation of ERα gene expression promises to be a key factor in the tissue-specific regulation of ERα mRNA and was recently reviewed (48).
Though ERα is widely distributed in the brain, the hypothalamus is a brain region particularly enriched in ERα, and data obtained from this region illustrate the complexity of ERα regulation within the central nervous system. Within the human hypothalamus, the distribution of ERα is concentrated in the band of Broca, the medial mamillary nucleus, the medial preoptic area, the paraventricular nucleus, the lateral hypothalamus, the suprachiasmatic nucleus, and the ventromedial nucleus. Sex differences in ERα levels were observed in these nuclei, implying that estrogens and/or androgens regulate human hypothalamic ERα levels in a nucleus-specific manner (32). Specific evidence for estradiol-mediated up-regulation of ERα levels in the human suprachiasmatic nucleus (controlling circadian functions) has been shown previously (33). These few examples reveal that in the central nervous system, independent ERα gene regulation takes place in different juxtaposed cell types.
ERα is essential to normal bone metabolism and mediates the ability of estradiol to sustain and stimulate bone mineral density in both males and females (57). Studies of primary human osteoblast cell cultures revealed that the regulation of ERα gene expression in bone is tissue specific. As discussed previously, seven distinct ERα promoter regions are dispersed between +163 and −151 kb of the ERα transcription initiation sites (31). In osteoblasts, ERα transcription is initiated at a far 5′ upstream promoter F, ∼117 kb distant from the initiation sites A and C utilized in the human breast cancer cell line MCF7 and primary human aorta smooth muscle cells (14). Although promoter F lacks an estrogen response element consensus sequence common to the other ERα promoter regions, its expression is up-regulated by estradiol-ERα, suggesting that transcriptional regulation of the ERα gene in bone is unique (15, 16). Human osteoblastic cells express high levels of the ERα splice variant ERα46. The ERα46 variant lacks the A/B regions of the N-terminal domain (AF-1) of the full-length ERα66 gene because a splice acceptor in exon 2 rather than exon 1 is utilized (14). The signals controlling the alternative RNA processing of ERα are as yet unclear. However, ERα46 is biologically active and modulates tissue responses to estradiol through the formation of homodimers, heterodimers with ERα66, and heterodimers with ERβ.
The regulation of ERα by pharmacologic agents, phytoestrogens, and xenoestrogens has therapeutic and pathological implications (3). Although changes in ER levels in response to these various ERα ligands are undergoing study and characterization, there is still relatively little information regarding their ability to regulate ERα transcription. Down-regulation of ERα protein levels in response to the pure estrogen antagonist ICI 182,780 (Fulvesant) and other selective estrogen response modifiers (4-hydroxytamoxifen and GW7604, a carboxylated tamoxifen derivative) was studied in human breast tumor cell lines. Increased degradation of ligand-bound ERα occurred via ubiquitination and 26S proteasomal degradation pathways in the rank order ICI 182,780 ≫ estradiol ≫ GW7604 > 4-hydroxytamoxifen. A critical observation that distinguishes the pure estrogen antagonist ICI 182,780 from the potent ERα agonist estradiol is that ICI 182,780 fails to elicit a compensatory stimulation of ERα mRNA (4). Thus, ICI 182,780 caused a sustained depletion of ERα in mammary tumor cells, a therapeutically advantageous condition that precluded further estradiol-stimulated proliferation. Danazol, an antiestrogenic compound in long-term clinical use, has been shown to decrease ER expression in a variety of tissues (20, 63). Most notably, decreased ER mRNA and protein levels were found in the peripheral blood mononuclear cells of patients who were treated with danazol for endometriosis, and a run-on assay revealed that danazol decreased ER transcription (20).
Tamoxifen and raloxifene are clinically important selective estrogen receptor modulators (SERMs); neither drug down-regulated ERα levels in MCF7 cells growing in tissue culture, and in fact, tamoxifen stabilized the nuclear form of ERα. Tamoxifen treatment for 48 h caused no change in ERα mRNA levels in MCF7 cells, but raloxifene stimulated ERα mRNA two- to threefold (67). The basis for the increase in ERα mRNA in response to raloxifene is not known, and other SERMs showed a similar but more modest effect. Although in vivo administration of tamoxifen generally resulted in lower ER levels, there is no evidence that transcriptional regulation contributed to these effects. The in vivo actions of tamoxifen reflect a composite of its mixed agonist-antagonist activity and metabolism to 4-hydroxytamoxifen. When given to women for breast cancer treatment, tamoxifen can elicit a transient tumor “flare” response reflective of its estrogen agonist activity; however, chronic tamoxifen administration to women antagonizes breast tissue growth. In a study of human breast cancer patients previously treated with tamoxifen, endometrial hyperplasia and benign endometrial polyps were sampled immunochemically for ER levels, and ER levels were reduced compared with healthy, age-matched controls (9). The basis of an emerging model to predict the action of SERMs on ER turnover is the idea that different ligand-induced ERα conformations modulate the level of receptor ubiquitination and thus proteasomal degradation rates (46, 65, 68). This concept may drive the development of SERMs that selectively stabilize ERα or target it for destruction.
In contrast to the variable effects of estrogens, most studies consistently demonstrate that progesterone and progesterone agonists down-regulate ER expression. The PR agonist ORG 2058 decreased ER mRNA expression by 40% in breast cancer-derived T47D cells, while R5020, another PR agonist, decreased ER protein levels by 50% in T47D cells and to a lesser extent in MCF7 cells (1, 53). In support of these findings, the PR antagonist mifepristone (RU486) increased ER protein expression by two- to fourfold in T47D cells (53). Moreover, a single 200-mg dose of RU486 administered to nine regularly menstruating women two days after the luteinizing hormone peak resulted in intense nuclear staining for ER and PR in both endometrial gland and stromal cells, while faint or absent staining was noted in control cycles (no RU486) in the same women (39).
Androgens also modulate ER expression; prostatic stromal cells from patients with prostate cancer who were treated with androgen deprivation therapy had increased ER detected immunohistochemically compared to untreated patients, suggesting that androgens suppress ER expression in human prostatic stromal cells (34). These findings raise the intriguing possibility that androgens mitigate the breast cancer-enhancing effects of estrogens.
Finally, 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3], the biologically active form of vitamin D, is a potent inhibitor of breast cancer cell growth (10, 38, 56, 66). Swami et al. found that 1,25(OH)2D3 reduced MCF7 cell proliferation in a dose-dependent manner; furthermore, this antiproliferative effect correlated significantly with 1,25(OH)2D3-mediated ER suppression as measured by an E2 binding assay (62). In the same study, Northern and Western blot analyses revealed a transient decrease in ER mRNA and protein levels with nadirs at 24 and 48 h, respectively. Moreover, a transcription run-on assay confirmed a transient 1,25(OH)2D3-dependent depression of ER gene transcription, while cycloheximide did not prevent the decrease in mRNA, implying that ongoing protein synthesis is not required for 1,25(OH)2D3-mediated ER down-regulation. Finally, 1,25(OH)2D3 completely prevented the E2-mediated increase in functional PR and BRCA1 protein expression, suggesting that these are biologically relevant findings (62). Stoica et al. identified a vitamin D response element within the ER promoter; a series of experiments by that group, in which MCF7 cells were treated with 10 nM 1,25(OH)2D3, revealed a 60% decrease in ER gene transcription, a 50% decrease in steady-state ER mRNA levels, and a 50% decrease in ER protein levels (58). Stoica et al. also demonstrated a 1,25(OH)2D3-induced decrease in the number of ER binding sites, while the binding affinity of estradiol for the ER remained unchanged (58). Importantly, a mutation of the vitamin D response element inhibits the 1,25(OH)2D3 effects. Further evidence of a 1,25(OH)2D3-mediated decrease in ER expression levels includes correlative studies of EB-1089, KH-1060, Ro 27-0574, and Ro 23-7553, vitamin D analogues that suppress ER levels even more potently than 1,25(OH)2D3 (62).
PEPTIDE HORMONES AND GROWTH FACTORS
Human chorionic gonadotropin (hCG) decreases ER expression. Chiang et al. extensively studied hormonal regulation of ERα expression in human granulosa-luteal cells (hGLCs) in vitro (8). First, using a semiquantitative reverse transcription-PCR protocol, they were unable to detect changes in ER expression levels in hGLCs left to spontaneously luteinize in culture for up to 10 days. They then demonstrated a time- and dose-dependent decrease in ER mRNA levels in hGLCs treated with hCG for 24 h; however, the effect was not noted until the cells had been in culture for 7 days but persisted until day 10. Importantly, treatment of hGLCs with 8-bromo-cyclic AMP or forskolin also resulted in decreased ER mRNA expression, suggesting that hCG may activate the protein kinase A (PKA) signaling pathway. In addition, cotreatment of hGLCs with hCG plus a specific PKA or adenylate cyclase inhibitor prevented the decrease, providing further support for this theory. Finally, Western blot analysis confirmed that hCG, 8-bromo-cyclic AMP, and forskolin treatment results in lower ERα protein levels in hGLCs.
Overexpression of erbB2 (HER2) in advanced breast cancer is associated with hormone unresponsiveness, implicating the epidermal growth factor (EGF) pathway in ER regulation. Careful study of MCF7 cells treated with EGF revealed a decrease in ER binding sites without a change in ER binding affinity (11, 59). These results were extended to show that EGF decreased ER protein and mRNA, and this was due to a decrease in ER gene transcription (59). Tyrphostins and wormanin blocked the EGF effects, suggesting that phosphatidylinositol 3-kinase and PKB (Akt), the downstream targets of phosphatidylinositol 3-kinase, may mediate the EGF response (59).
The modulation of ER expression by gonadotropin-releasing hormone (GnRH) may depend on the tissue studied and the mode of delivery. A time- and dose-dependent decrease in ER mRNA levels was noted when hGLC cells were treated continuously for 24 h with the GnRH agonist d-Ala6-GnRH (GnRHa) (8). Interestingly, the decrease was noted on day 7 of culture, but no decrease was detected on day 1 or on day 10, indicating specific temporal regulation. Cotreatment of hGLCs with GnRHa plus the specific PKC inhibitor GF109203X inhibited the decrease in ER mRNA, suggesting that GnRH signals via the PKC pathway in the ovary. Western blot analysis in hGLCs revealed that GnRHa treatment also leads to lower ER protein levels.
Insulin and insulin-like growth factor I (IGF-I) signal via a complex cascade of proteins, including insulin substrate 1 (IRS-1), and have pleiotropic effects on ER biology. IGF-I and IRS-1 promote estrogen-independent growth of breast cancer cells, and increased levels of IGF-I and IRS-1 correlate with tumor progression and increasing tumor size. Insulin and E2 stimulate growth of MCF7 cells, and their effect is synergistic (2). However, in MCF7 cells that express antisense IRS-1 RNA, insulin failed to stimulate growth, whereas the growth effects of E2 alone or in combination with insulin were blunted (2). In addition, insulin and E2 each up-regulated ER protein expression and binding capacity in MCF7 cells. Interestingly, most cells that expressed antisense IRS-1 RNA demonstrated higher basal ER protein levels and a higher ER binding capacity; however, in those cells, insulin failed to up-regulate ER expression and E2 decreased ER protein levels (2). Finally, the effects of IGF-I were also examined in MCF7 cells; after treatment with IGF-I for just 3 h, a decrease in ER protein and mRNA expression was noted and was attributed to decreased ER gene transcription, with no change in ER binding affinity (60).
OTHER AGENTS
Several groups have examined the effects of the tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA) on ERα expression. Initial studies demonstrated that upon treatment of MCF7 cells with TPA, the number of estrogen receptors diminished in spite of an increase in total cellular protein (23). The reduction in ER levels corresponded to the inhibition of cell growth. However, TPA-resistant RPh4 cells showed no change in ER number. Saceda et al. verified the TPA-induced reduction in ER protein and also observed a decrease in steady-state mRNA levels to 20% of control levels (50). In the presence of cycloheximide, TPA retained the ability to down-regulate ER mRNA, indicating that mRNA levels were not affected by protein synthesis. Importantly, 100 nM TPA had no effect on gene transcription but decreased the half-life of ER mRNA, suggesting that TPA causes posttranscriptional destabilization (50). The mechanism of ER regulation by TPA was further defined when the ability of the PKC inhibitor H-7 to block TPA-mediated effects on ER was shown (50). Mezerezein, a tumor promoter and activator of PKC, reduced ER mRNA by 80% (36). Ree et al. extended these findings by showing that the RNA synthesis inhibitor actinomycin D increased the ER mRNA half-life from 3 to 12 h and eliminated the TPA-induced decrease in ER mRNA levels (47). However, studies by Lee et al. contradicted earlier observations (36, 50). By using 10 nM TPA in contrast to 100 nM TPA, Lee et al. discovered that TPA caused a decrease in the ER gene transcription rate prior to the observed reduction in ER mRNA (36). Furthermore, they did not see an effect on ER mRNA stability. Thus, these studies conflict on whether TPA mediates ER mRNA transcriptionally or posttranscriptionally. The effects of TPA on ER have also been examined in leukemia cells (12). In THP-1 cells derived from a patient with acute monocytic leukemia, 50 ng of TPA/ml stimulated ER expression, whereas untreated cells were ER negative. Approximately 60 to 75% of TPA-treated cells stained positively for ER, with the majority of ER seen within the nucleus (12).
Various other agents have been evaluated for their effects on ER regulation. For example, in MCF7 cells, the heavy metal cadmium decreases the levels of both ER protein and mRNA while stimulating cell growth (21). In vitro nuclear run-on experiments demonstrated that cadmium induced a 60% inhibition of ER gene transcription. Arsenic compounds also modulate the expression of ER (6, 61). In MCF7 cells, arsenite caused a concentration-dependent decrease in both ER protein and mRNA by up to 90%. Arsenic trioxide, used in the treatment of patients with acute promyelocytic leukemia, significantly reduced the levels of ER expression in both MCF7 and T47D cells when given at low doses. At higher doses, this agent caused cell death. If cells were switched to medium free of arsenic trioxide, ER levels were completely restored.
CONCLUSIONS
Effectors of chromatin structure, hormones, growth factors, and a variety of other agents implicate multiple cellular systems in the control of ERα expression. The dramatic increase in information about these agents provides investigators with an enhanced conceptual framework for the role of estrogen biology in health and disease. The factors reviewed above serve as tools that can be utilized to further accelerate the pace of discovery in this area. Moreover, the diversity of ER regulatory agents reflects the biologic importance of precisely coordinating the timing and extent of ER expression and suggests that investigators working in a number of fields can contribute relevant molecular knowledge that may result in the clinical use of novel molecular determinants of ER expression.
Acknowledgments
We are grateful to Richard Pestell for his critical review of the manuscript.
In part, this review is supported by NIH grants 1 K08 CA101875-01 (J.J.P.) and CA91149 (P.E.B.).
REFERENCES
- 1.Alexander, I. E., J. Shine, and R. L. Sutherland. 1990. Progestin regulation of estrogen receptor messenger RNA in human breast cancer cells. Mol. Endocrinol. 6:821-828. [DOI] [PubMed] [Google Scholar]
- 2.Ando, S., M.-L. Panno, M. Salerno, D. Sisci, L. Mauro, M. Lanzino, and E. Surmacz. 1998. Role of IRS-1 signaling in insulin-induced modulation of estrogen receptors in breast cancer cells. Biochem. Biophys. Res. Commun. 253:315-319. [DOI] [PubMed] [Google Scholar]
- 3.Belcher, S. M., and A. Zsarnovsky. 2001. Estrogenic actions in the brain: estrogen, phytoestrogens, and rapid intracellular signaling mechanisms. J. Pharmacol. Exp. Ther. 299:408-414. [PubMed] [Google Scholar]
- 4.Bentrem, D., R. Dardes, H. Liu, J. MacGregor-Schafer, J. Zapf, and V. Jordan. 2001. Molecular mechanism of action at estrogen receptor alpha of new clinically relevant antiestrogen (GW7604) related to tamoxifen. Endocrinology 142:838-846. [DOI] [PubMed] [Google Scholar]
- 5.Bovenzi, V., and R. L. Momparler. 2001. Antineoplastic action of 5-aza-2′-deoxycytidine and histone deacetylase inhibitor and their effect on the expression of retinoic acid receptor β and estrogen α receptor genes in breast carcinoma cells. Cancer Chemother. Pharmacol. 48:71-76. [DOI] [PubMed] [Google Scholar]
- 6.Chen, G.-C., L.-S. Guan, W.-L. Hu, and Z.-Y. Wang. 2002. Functional repression of estrogen receptor α by arsenic trioxide in human breast cancer cells. Anticancer Res. 22:633-638. [PubMed] [Google Scholar]
- 7.Chen, Z., A. Ko, J. Yang, and V. C. Jordan. 1998. Methylation of CpG island is not a ubiquitous mechanism for the loss of oestrogen receptor in breast cancer cells. Br. J. Cancer 77:181-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang, C.-H., K. W. Cheng, S. Igarashi, P. S. Nathwani, and P. C. K. Leung. 2000. Hormonal regulation of estrogen receptor α and β gene expression in human granulosa-luteal cells in vitro. J. Clin. Endocrinol. Metab. 85:3828-3839. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, I., Y. Beyth, M. M. Altaras, J. Shapira, R. Tepper, M. Cardoba, D. Yigael, A. Figer, A. Fishman, and J. Berenhein. 1997. Estrogen and progesterone receptor expression in postmenopausal tamoxifen-exposed endometrial pathologies. Gynecol. Oncol. 67:8-15. [DOI] [PubMed] [Google Scholar]
- 10.Colston, K. W., S. K. Chander, A. G. Mackey, and R. C. Coombes. 1992. Effects of synthetic vitamin D analogs on breast cancer cell proliferation in vivo and in vitro. Biochem. Pharmacol. 44:693-702. [DOI] [PubMed] [Google Scholar]
- 11.Cornier, E. M., M. F. Wolf, and V. C. Jordan. 1989. Decrease in estradiol-stimulated progesterone receptor production in MCF7 cells by EGF and possible clinical implications for paracrine-regulated breast cancer growth. Cancer Res. 49:576-580. [PubMed] [Google Scholar]
- 12.Cutolo, M., G. Carruba, B. Villaggio, D. A. Coviello, J.-M. Dayer, I. Campisi, M. Miele, R. Stefano, and L. A. M. Castagnetta. 2001. Phorbol diester 12-O-tetradecanoylphorbol 13-acetate (TPA) upregulates the expression of estrogen receptors in human THP-1 leukemia cells. J. Cell Biochem. 83:390-400. [DOI] [PubMed] [Google Scholar]
- 13.deConinck, E. C., L. A. McPherson, and R. J. Weigel. 1995. Transcriptional regulation of estrogen receptor in breast carcinomas. Mol. Cell. Biol. 15:2191-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denger, S., G. Reid, M. Kos, G. Flouriot, D. Parsch, H. Brand, K. S. Korach, V. Sonntag-Buck, and F. Gannon. 2001. ER-alpha gene expression in human primary osteoblasts: evidence for the expression of two receptor proteins. Mol. Endocrinol. 15:2064-2077. [DOI] [PubMed] [Google Scholar]
- 15.Denger, S., G. Reid, H. Brand, M. Kos, and F. Gannon. 2001. Tissue-specific expression of human ER-alpha and ER-beta in the male. Mol. Cell. Endocrinol. 178:155-160. [DOI] [PubMed] [Google Scholar]
- 16.Donaghue, C., B. R. Westley, and F. E. B. May. 1999. Selective promoter usage of the human estrogen receptor alpha gene and its regulation by estrogen. Mol. Endocrinol. 13:1934-1950. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson, A. T., P. M. Vertin, J. R. Spitzner, S. B. Baylin, M. T. Muller, and N. E. Davidson. 1997. Role of estrogen receptor gene demethylation and DNA methyltransferase-DNA adduct formation in 5-aza-2′-deoxycytidine-induced cytotoxicity in human breast cancer cells. J. Biol. Chem. 272:32260-32266. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson, A. T., R. G. Lapidus, S. B. Baylin, and N. E. Davidson. 1995. Demethylation of the estrogen receptor gene in estrogen receptor-negative breast cancer cells can reactivate estrogen receptor gene expression. Cancer Res. 55:2279-2283. [PubMed] [Google Scholar]
- 19.Fricher, J. 1999. Heart disease linked to estrogen-receptor gene methylation. Mol. Med. Today 5:505-506. [DOI] [PubMed] [Google Scholar]
- 20.Fujimoto, J., M. Hori, T. Itoh, S. Ichigo, M. Nishigake, and T. Tamaya. 1995. Danazol decreases transcription of estrogen receptor gene in human monocytes. Gen. Pharmacol. 26:507-516. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Morales, P., M. Saceda, N. Kenney, N. Kim, D. S. Salomon, M. M. Gottardis, H. B. Solomon, P. F. Sholler, V. C. Jordan, and M. B. Martin. 1994. Effect of cadmium on estrogen receptor levels and estrogen-induced responses in human breast cancer cells. J. Biol. Chem. 269:16896-16901. [PubMed] [Google Scholar]
- 22.Grandien, K. 1996. Determination of transcription start sites in the human estrogen receptor gene and identification of a novel, tissue-specific, estrogen receptor-mRNA isoform. Mol. Cell. Endocrinol. 116:207-212. [DOI] [PubMed] [Google Scholar]
- 23.Guilbaud, N., M. F. Pichon, J. C. Faye, F. Bayard, and A. Valette. 1988. Modulation of estrogen receptors by phorbol diesters in human breast MCF7 cell line. Mol. Cell. Endocrinol. 56:157-163. [DOI] [PubMed] [Google Scholar]
- 24.Hori, M., M. Iwasaki, J. Shimazaki, S. Inagawa, and M. Itabashi. 2000. Assessment of hypermethylated DNA in two promoter regions of the estrogen receptor alpha gene in human endometrial diseases. Gynecol. Oncol. 76:89-96. [DOI] [PubMed] [Google Scholar]
- 25.Horwitz, K. B., and W. L. McGuire. 1978. Nuclear mechanisms of estrogen action. Effects of estradiol and anti-estrogens on estrogen receptors and nuclear receptor processing. J. Biol. Chem. 253:8185-8191. [PubMed] [Google Scholar]
- 26.Ihionkhan, C. E., K. L. Chambliss, L. L. Gibson, L. D. Hahner, M. E. Medelsohn, and P. W. Shaul. 2002. Estrogen causes dynamic alterations in endothelial estrogen receptor expression. Circ. Res. 91:814-820. [DOI] [PubMed] [Google Scholar]
- 27.Issa, J. P., Y. L. Ottaviano, P. Celano, S. R. Hamilton, N. E. Davidson, and S. B. Baylin. 1994. Methylation of the oestrogen receptor CpG island links ageing and neoplasia in human colon. Nat. Genet. 7:536-540. [DOI] [PubMed] [Google Scholar]
- 28.Issa, J. P., B. A. Zehnbauer, C. I. Civin, M. I. Collector, S. J. Sharkis, N. E. Davidson, S. H. Kaufmann, and S. B. Baylin. 1996. The estrogen receptor CpG island is methylated in most hematopoietic neoplasms. Cancer Res. 56:973-977. [PubMed] [Google Scholar]
- 29.Issa, J. P., S. B. Baylin, and S. A. Belinsky. 1996. Methylation of the estrogen receptor CpG island in lung tumors is related to the specific type of carcinogen exposure. Cancer Res. 56:3655-3658. [PubMed] [Google Scholar]
- 30.Juttermann, R., E. Li, and R. Jaenisch. 1994. Toxicity of 5-aza-2′-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc. Natl. Acad. Sci. USA 91:11797-11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kos, M., G. Reid, S. Denger, and F. Gannon. 2001. Genomic organization of the human ER gene promoter region. Mol. Endocrinol. 15:2057-2063. [DOI] [PubMed] [Google Scholar]
- 32.Kruijver, F. P., R. Balesar, A. M. Espila, U. P. Unmehopa, and D. F. Swaab. 2002. ER-alpha distribution in the human hypothalamus in relation to sex and endocrine status. J. Comp. Neurol. 454:115-139. [DOI] [PubMed] [Google Scholar]
- 33.Kruijver, F. P., and D. F. Swaab. 2002. Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinology 75:296-305. [DOI] [PubMed] [Google Scholar]
- 34.Kruithof-Dekker, I. G., B. Tetu, P. J. A. Janssen, and T. H.Van Der Kwast. 1996. Elevated estrogen receptor expression in human prostatic stromal cells by androgen ablation therapy. J. Urol. 156:1194-1197. [PubMed] [Google Scholar]
- 35.Lapidus, R. G., S. J. Nass, K. A. Butash, F. F. Parl, S. A. Weitzman, J. G. Graff, J. G. Herman, and N. E. Davidson. 1998. Mapping of ER gene CpG island methylation by methylation-specific polymerase chain reaction. Cancer Res. 58:2515-2519. [PubMed] [Google Scholar]
- 36.Lee, C. S. L., A. deFazio, C. J. Ormandy, and R. L. Sutherland. 1996. Inverse regulation of oestrogen receptor and epidermal growth factor receptor gene expression in MCF7 breast cancer cells treated with phorbol ester. J. Steroid Biochem. Mol. Biol. 58:267-275. [DOI] [PubMed] [Google Scholar]
- 37.Lonard, D. M., Z. Nawaz, C. L. Smith, and B. W. O'Malley. 2000. The 26S proteasome is required for estrogen receptor alpha and coactivator turnover and for efficient estrogen receptor alpha transactivation. Mol. Cell 5:939-948. [DOI] [PubMed] [Google Scholar]
- 38.Love-Schimenti, C. D., D. F. C. Gibson, A. V. Ratnam, and D. D. Bikle. 1996. Antiestrogen potentiation of antiproliferative effects of vitamin D3 analogs in breast cancer cells. Cancer Res. 56:2789-2794. [PubMed] [Google Scholar]
- 39.Maentausta, O., P. Svalander, K. G. Danielsson, M. Bygdeman, and R. Vihko. 1993. The effects of an antiprogestin, mifepristone, and an antiestrogen, tamoxifen, on endometrial 17β-hydroxysteroid dehydrogenase and progestin and estrogen receptors during the luteal phase of the menstrual cycle: an immunohistochemical study. J. Clin. Endocrinol. Metab. 77:913-918. [DOI] [PubMed] [Google Scholar]
- 40.Nakao, M. 2001. Epigenetics: interaction of DNA methylation and chromatin. Gene 278:25-31. [DOI] [PubMed] [Google Scholar]
- 41.Navari, J. R., P. Y. Roland, P. Keh, H. B. Salvesen, L. A. Akslen, O. E. Iversen, S. Das, R. Kothari, S. Howey, and B. Phillips. 2000. Loss of estrogen receptor (ER) expression in endometrial tumors is not associated with de novo methylation of the 5′ end of the ER gene. Clin. Cancer Res. 6:4026-4032. [PubMed] [Google Scholar]
- 42.O'Doherty, A. M., S. W. Church, S. H. E. Russell, J. Nelson, and I. Hickey. 2002. Methylation status of oestrogen receptor-α gene promoter sequences in human ovarian epithelial cell lines. Br. J. Cancer 86:282-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh, A. S., L. A. Lorant, J. N. Holloway, D. L. Miller, F. G. Kern, and D. El-Ashry. 2001. Hyperactivation of MAPK induces loss of ERα expression in breast cancer cells. Mol. Endocrinol. 15:1344-1359. [DOI] [PubMed] [Google Scholar]
- 44.Ottaviano, Y. L., J. P. Issa, F. F. Parl, H. S. Smith, S. B. Baylin, and N. E. Davidson. 1994. Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res. 54:2552-2555. [PubMed] [Google Scholar]
- 45.Post, W. S., P. J. Goldschmidt-Clermont, C. C. Wilhide, A. W. Heldman, M. S. Sussman, P. Ouyang, E. E. Milliken, and J. P. Issa. 1999. Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc. Res. 43:985-991. [DOI] [PubMed] [Google Scholar]
- 46.Preisler-Mashek, M. T., N. Solodin, B. L. Stark, M. K. Tyriver, and E. T. Alarid. 2002. Ligand-specific regulation of proteasome-mediated proteolysis of estrogen receptor alpha. Am. J. Physiol. Endocrinol. Metab. 282:E891-E898. [DOI] [PubMed] [Google Scholar]
- 47.Ree, A. H., H. K. Knutsen, B. F. Landmark, W. Eskild, and V. Hansson. 1992. Downregulation of messenger ribonucleic acid (mRNA) for the estrogen receptor (ER) by phorbol ester requires ongoing RNA synthesis but not protein synthesis. Is hormonal control of ER mRNA degradation mediated by an RNA molecule? Endocrinology 131:1810-1814. [DOI] [PubMed] [Google Scholar]
- 48.Reid, G., S. Denger, M. Kos, and F. Gannon. 2002. Human estrogen receptor alpha: regulation by synthesis, modification and degradation. Cell. Mol. Life Sci. 59:821-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reid, G., M. R. Hubner, R. Metivier, H. Brand, S. Denger, D. Manu, J. Beaudouin, J. Ellenberg, and F. Gannon. 2003. Cyclic, proteasome-mediated turnover of unliganded and liganded ER alpha on responsive promoters is an integral feature of estrogen signaling. Mol. Cell 11:695-707. [DOI] [PubMed] [Google Scholar]
- 50.Saceda, M., C. Knabbe, R. B. Dickson, M. E. Lippman, D. Bronzert, R. K. Lindsey, M. M. Gottardis, and M. B. Martin. 1991. Post-transcriptional destabilization of estrogen receptor mRNA in MCF7 cells by 12-O-tetradecanoylphorbol-13-acetate. J. Biol. Chem. 266:17809-17814. [PubMed] [Google Scholar]
- 51.Sasaki, M., L. Kotcherguina, A. Dharia, S. Fujimoto, and R. Dahiya. 2001. Cytosine-phosphoguanine methylation of estrogen receptors in endometrial cancer. Cancer Res. 61:3262-3266. [PubMed] [Google Scholar]
- 52.Sasaki, M., Y. Tanaka, G. Perinchery, A. Dharia, I. Kotcherguina, S. Fujimoto, and R. Dahiya. 2002. Methylation and inactivation of estrogen, progesterone, and androgen receptors in prostate cancer. J. Natl. Cancer Inst. 94:384-390. [DOI] [PubMed] [Google Scholar]
- 53.Savoldi, G., F. Ferrari, G. Ruggeri, L. Sobek, A. Albertini, and D. Di Lorenzo. 1995. Progesterone agonists and antagonists induce down- and upregulation of estrogen receptors and estrogen inducible genes in human breast cancer cell lines. Int. J. Biol. Markers 10:47-54. [DOI] [PubMed] [Google Scholar]
- 54.Schuur, E. R., L. A. McPherson, G. P. Yang, and R. J. Weigel. 2001. Genomic structure of the promoters of the human estrogen receptor-α gene demonstrate changes in chromatin structure induced by AP2γ. J. Biol. Chem. 276:15519-15526. [DOI] [PubMed] [Google Scholar]
- 55.Shiozawa, T., K. Itoh, A. Horiuchi, I. Konishi, S. Fujii, and T. Nikaido. 2002. Downregulation of estrogen receptor by the methylation of the estrogen receptor gene in endometrial carcinoma. Anticancer Res. 22:139-143. [PubMed] [Google Scholar]
- 56.Simboli-Campbell, M., C. J. Narvaez, K. vanWeelden, M. Tenniswood, and J. E. Welsh. 1997. Comparative effects of 1,25(OH)2D3 and EB1089 on cell cycle kinetics and apoptosis in MCF7 breast cancer cells. Breast Cancer Res. Treat. 42:31-41. [DOI] [PubMed] [Google Scholar]
- 57.Smith, E. P., J. Boyd, G. R. Frank, H. Takahashi, R. M. Cohen, B. Specker, T. C. Williams, D. B. Lubahn, and K. S. Korach. 1994. Estrogen resistance caused by a mutation in the estrogen receptor gene. N. Engl. J. Med. 331:1056-1061. [DOI] [PubMed] [Google Scholar]
- 58.Stoica, A., M. Saceda, A. Fakhro, H. B. Solomon, B. D. Fenster, and M. B. Martin. 1999. Regulation of estrogen receptor-α gene expression by 1,25-dihydroxyvitamin D in MCF7 cells. J. Cell. Biochem. 75:640-651. [PubMed] [Google Scholar]
- 59.Stoica, A., M. Saceda, V. L. Doraiswamy, C. Coleman, and M. B. Martin. 2000. Regulation of estrogen receptor-α gene expression by epidermal growth factor. J. Endocrinol. 165:371-378. [DOI] [PubMed] [Google Scholar]
- 60.Stoica, A., M. Saceda, A. Fakhro, M. Joyner, and M. B. Martin. 2000. Role of insulin-like growth factor-I in regulating estrogen receptor-α gene expression. J. Cell. Biochem. 76:605-614. [DOI] [PubMed] [Google Scholar]
- 61.Stoica, A., E. Pentecost, and M. B. Marin. 2000. Effects of arsenite on estrogen receptor-α expression and activity in MCF7 breast cancer cells. Endocrinology 141:3595-3602. [DOI] [PubMed] [Google Scholar]
- 62.Swami, S., A. V. Krishnan, and D. Feldman. 2000. 1α,25-dihydroxyvitamin D3 downregulates estrogen receptor abundance and suppresses estrogen actions in MCF7 human breast cancer cells. Clin. Cancer Res. 6:3371-3379. [PubMed] [Google Scholar]
- 63.Tamaya T., K. Wada, H. Mori, and A. Imai. 1991. Different effects on oestrogen binding sites and anti-oestrogenic action of danazol and progesterone. Ann. Clin. Biochem. 28:250-252. [DOI] [PubMed] [Google Scholar]
- 64.Tschugguel, W., W. Dietrich, Z. Zhegu, F. Stonek, A. Kolbus, and J. C. Huber. 2003. Differential regulation of proteasome-dependent estrogen receptor alpha and beta turnover in cultured human uterine artery endothelial cells. J. Clin. Endocrinol. Metab. 88:2281-2287. [DOI] [PubMed] [Google Scholar]
- 65.Van Den Bemd, G. J., G. G. Kuiper, H. A. Pols, and J. P. VanLeeuwen. 1999. Distinct effects on the conformation of estrogen receptor alpha and beta by both the antiestrogens ICI 164,384 and ICI 182,780 leading to opposite effects on receptor stability. Biochem. Biophys. Res. Commun. 261:1-5. [DOI] [PubMed] [Google Scholar]
- 66.Vink-van Wijngaarden, T., H. Pols, C. Buurman, G. van den Bemd, L. Dorssers, J. Birkenhager, and J. van Leeuwen. 1994. Inhibition of breast cancer cell growth by combined treatment with vitamin D3 analogues and tamoxifen. Cancer Res. 54:5711-5717. [PubMed] [Google Scholar]
- 67.Wijayaratne, A. L., S. C. Nagel, L. A. Paige, D. J. Christensen, J. D. Norris, D. M. Fowlkes, and D. P. McDonnell. 1999. Comparative analyses of mechanistic differences among antiestrogens. Endocrinology 140:5828-5840. [DOI] [PubMed] [Google Scholar]
- 68.Wijayaratne, A. L., and D. P. McDonnell. 2001. The human estrogen receptor-alpha is a ubiquitinated protein whose stability is affected differentially by agonists, antagonists, and selective estrogen receptor modulators. J. Biol. Chem. 276:35684-35692. [DOI] [PubMed] [Google Scholar]
- 69.Yang, X., A. T. Ferguson, S. J. Nass, D. L. Phillips, K. A. Butash, S. M. Wang, J. G. Herman, and N. E. Davidson. 2000. Transcriptional activation of estrogen receptor α in human breast cancer cells by histone deacetylase inhibition. Cancer Res. 60:6890-6894. [PubMed] [Google Scholar]
- 70.Yang, X., D. L. Phillips, A. T. Ferguson, W. G. Nelson, J. G. Herman, and N. E. Davidson. 2001. Synergistic activation of functional estrogen receptor (ER)-α by DNA methyltransferase and histone deacetylase inhibition in human ER-α-negative breast cancer cells. Cancer Res. 61:7025-7029. [PubMed] [Google Scholar]
- 71.Yoshida, T., H. Eguchi, K. Nakachi, K. Tanimoto, Y. Higashi, K. Suemasu, Y. Iino, Y. Morishita, and S. Hayashi. 2000. Distinct mechanisms of loss of estrogen receptor α gene expression in human breast cancer: methylation of the gene and alteration of trans-acting factors. Carcinogenesis 12:2193-2201. [DOI] [PubMed] [Google Scholar]