Abstract
Objective
The aim of this study was to confirm the effects of chronic kidney disease (CKD) and anemia on physical function and to clarify whether the interaction between CKD and anemia has an additive effect.
Design
Eligible subjects were chronic heart failure (HF) patients who were discharged between March 2007 and August 2009. A total of 102 chronic HF patients (33% females; mean age: 68 ± 14 years) were enrolled in the present study. CKD was defined as an estimated glomerular filtration rate of <60 ml/min/1.73 m2, and anemia was defined as a hemoglobin level of <12 g/dl in males and of <11 g/dl in females. The Short Physical Performance Battery (SPPB) was used to assess physical function.
Results
The adjusted mean SPPB score was lower in patients with both CKD and anemia than in those with neither of the diseases or with either disease alone (p < 0.05).
Conclusion
This study found that CKD and anemia are independently associated with reduced physical function.
Key Words: Chronic kidney disease, Anemia, Physical function, Heart failure
Introduction
Chronic kidney disease (CKD) and anemia are common comorbidities in patients with heart failure (HF) [1]. Several studies have shown that patients with CKD and/or anemia are more likely to have physical dysfunction and exercise intolerance [2,3,4,5,6]. Odden et al. [2] suggested that the impact of CKD and anemia on physical function is complicated because of their symptoms and overlap with aging and other common comorbidities such as malnutrition and HF. Other studies have found an association between anemia and physical function in patients with chronic HF or severe CKD [7,8].
Most of the previous studies evaluated exercise capacity using the symptom-limited cardiopulmonary exercise test as a physical function test [7,8]. However, the number of patients who cannot undergo this test is increasing because of comorbidities and/or age. Recently, there has been wide use of physical performance tests in community-dwelling elderly patients with chronic diseases such as renal disease, cancer and HF. In particular, the Short Physical Performance Battery (SPPB) was used for elderly patients with HF [2,3,4]. The SPPB is an easy test for evaluating physical function even in advanced-age or frail patients with comorbidities because there are no special modalities and special skills necessary to assess the SPPB. Moreover, the SPPB score has been widely used as a predictor of disability and readmission for patients with HF [9,10]. The aim of this study was to confirm the effects of CKD and anemia on physical function, and to clarify whether the interaction between CKD and anemia has an additive effect.
Subjects and Methods
Patients with HF who underwent the SPPB at discharge between March 2007 and August 2009 were eligible for the study. A total of 102 HF patients (33% females; mean age: 68 ± 14 years) were enrolled in the present study. Patients on hemodialysis were excluded. All participants provided their written informed consent, and the protocol was approved by the institutional review boards.
Definitions of CKD and Anemia
CKD was defined as an estimated glomerular filtration rate (eGFR) of <60 ml/min/1.73 m2, which is defined as CKD stage 3 as suggested by the National Kidney Foundation classification [11]. The eGFR was calculated using the following formula: 194 × serum creatinine-1.094 × age-0.287 (× 0.739 for females), which was recommended by the Japanese Society of Nephrology [12]. Hemoglobin (Hb) levels were measured in the hematology laboratory, and anemia was defined as an Hb level of <12 g/dl for males or <11 g/dl for females [13].
SPPB Score
Physical function was assessed by the SPPB at discharge. SPPB scores ranged from 0 to 12, with a higher score representing better physical function. The SPPB consists of the standing balance, gait speed and chair stand tests, as previously described in detail [14].
In the standing balance test, the patients were asked to attempt to maintain their feet in side-by-side, semitandem (heel of one foot beside the big toe of the other foot) and tandem (heel of one foot directly in front of the other foot) standing positions for 10 s each. Scoring was as follows: ‘0’ if a patient was unable to do or hold the side-by-side standing position for 10 s; ‘1’ if a patient could hold the side-by-side standing position for 10 s but was unable to hold the semitandem position for 10 s; ‘2’ if a patient could hold the semitandem position for 10 s but was unable to hold the full tandem position for >3 s; ‘3’ if a patient could stand in the full tandem position for 3-9.99 s, and ‘4’ if a patient could stand in the full tandem position for 10 s.
In the gait speed test, a 4-meter walk at a comfortable speed was timed and scored according to quartiles for the length of time required. The faster time of 2 walks was used for scoring as follows: 0 = unable to do the test; 1 = 8.70 s; 2 = 6.20-8.70 s; 3 = 4.82-6.20 s, and 4 = ≤4.82 s.
In the chair stand test, the patients were asked to fold their arms across their chests and stand up from a sitting position once. If successfully rising from the chair, the patients were asked to stand up and sit down 5 times, as quickly as possible. Quartiles for the length of time required for this test were used for scoring as follows: 0 = unable to do the test or required >60 s; 1 = 16.7 s; 2 = 13.70-16.69 s; 3 = 11.20-13.69 s, and 4 = ≤11.19 s.
The scores for all three tests were summed up to give the total SPPB score.
Statistical Analyses
This study evaluated the differences in baseline clinical characteristics between patients with and those without CKD. Similarly, this study also examined the differences in baseline clinical characteristics between patients with and those without anemia. Two groups were compared using the unpaired t test for continuous variables and the χ2 test for dichotomous variables.
To investigate the level-dependent effect of eGFR and Hb on physical function, this study stratified patients by eGFR (stage 4: <30; stage 3: 30 to <60; stage 2: 60 to <90; stage 1: ≥90 ml/min/1.73 m2) and by Hb level (<10; 10 to <11; 11 to <12; 12 to <13, and ≥13 g/dl).
Analysis of variance with Bonferroni post hoc testing was employed to evaluate physical function across the Hb and eGFR subgroups. Linear regression analysis was used to determine the independent association of the primary predictors with each outcome after adjustment for the clinical characteristics. Stepwise regression was used with a criterion of p < 0.05 for inclusion. Models that evaluated CKD as the primary predictor were adjusted for anemia, and models evaluating anemia as the primary predictor were adjusted for CKD. The adjusted mean physical function was calculated on the basis of the parameter estimates from the multivariate linear regression models. This study evaluated the differences in adjusted mean physical function between patients with and those without CKD or anemia, as well as between patients with neither condition, those with either condition and those with both conditions. This study also aimed to examine the effects of left ventricular systolic dysfunction on physical function in HF patients. Therefore, we analyzed the difference in SPPB scores between HF patients with preserved ejection fraction (HFpEF; left ventricular ejection fraction, LVEF, >40%) and HF patients with reduced ejection fraction (HFrEF; LVEF ≤40%). All statistical analyses were performed using the statistical software SPSS version 19.0 (Chicago, Ill., USA).
Results
Clinical Characteristics
Of the 102 eligible patients, 70 (69%) had CKD and 42 (41%) had anemia. The age, rate of hypertension and rate of anemia were significantly higher in patients with CKD than in those without (table 1). The age, LVEF and rate of CKD were significantly higher in patients with anemia than in those without. Moreover, the left ventricular diastolic and systolic diameters were significantly lower in patients with anemia than in those without (table 2).
Table 1.
Comparison of baseline clinical characteristics of chronic HF patients with and those without CKD
| CKD (n = 70) | No CKD (n = 32) | p value | |
|---|---|---|---|
| Age, years | 71 ± 13 | 61 ± 14 | <0.01 |
| Male/female, n | 43/27 | 25/7 | 0.07 |
| BMI | 22.4 ± 3.7 | 21.5 ± 3 | 0.24 |
| Etiology, n | |||
| Ischemic | 24 | 10 | 0.11 |
| Valvular | 12 | 7 | |
| Myopathy | 14 | 13 | |
| Hypertensive | 5 | 0 | |
| Arrhythmia | 5 | 1 | |
| Others | 10 | 1 | |
| Comorbidity, % | |||
| Diabetes mellitus | 40 | 34 | 0.37 |
| Hypertension | 61 | 28 | <0.01 |
| Dyslipidemia | 39 | 34 | 0.42 |
| LVEF, % | 38 ± 14.0 | 36 ± 14 | 0.41 |
| Left ventricular diastolic dimension, mm | 54 ± 10.0 | 56 ± 11 | 0.55 |
| Left ventricular systolic dimension, mm | 48 ± 12.0 | 46 ± 12 | 0.74 |
| Left atrial dimension, mm | 44 ± 9.0 | 43 ± 10 | 0.53 |
| E/E’ | 24.3 ± 13.0 | 21.7 ± 10.7 | 0.32 |
| Hb, g/dl | 12.3 ± 2.2 | 13.1 ± 1.9 | 0.07 |
| Anemia, % | 49 | 25 | 0.03 |
| Serum creatinine, mg/dl | 1.2 ± 0.4 | 0.8 ± 0.1 | <0.01 |
| eGFR, ml/min/1.73 m2 | 44.5 ± 12.8 | 77.8 ± 13.1 | <0.01 |
| Albumin, mg/dl | 3.4 ± 0.4 | 3.6 ± 0.5 | 0.62 |
| Brain natriuretic peptide, pg/dl | 898 ± 778.0 | 650 ± 765 | 0.18 |
Values denote means ± SD unless specified otherwise. E/E, = Ratio of mitral early diastolic peak flow velocity (E) to tissue Doppler early mitral annular diastolic velocity (E').
Table 2.
Comparison of baseline clinical characteristics of chronic HF patients with and those without anemia
| Anemia (n = 42) | No Anemia (n = 60) | p value | |
|---|---|---|---|
| Age, years | 72 ± 15 | 65 ± 12 | <0.01 |
| Male/female, n | 26/16 | 42/18 | 0.40 |
| BMI | 21.5 ± 5.3 | 22.6 ± 3.6 | 0.13 |
| Etiology, n | |||
| Ischemic | 15 | 19 | 0.08 |
| Valvular | 12 | 7 | |
| Myopathy | 6 | 21 | |
| Hypertensive | 1 | 4 | |
| Arrhythmia | 3 | 6 | |
| Others | 6 | 2 | |
| Comorbidity, % | |||
| Diabetes mellitus | 43 | 35 | 0.53 |
| Hypertension | 50 | 52 | 1.00 |
| Dyslipidemia | 38 | 37 | 1.00 |
| LVEF, % | 42 ± 14 | 34 ± 14 | <0.01 |
| Left ventricular diastolic dimension, mm | 52 ± 10 | 56 ± 10 | 0.05 |
| Left ventricular systolic dimension, mm | 41 ± 11 | 47 ± 12 | <0.01 |
| Left atrial dimension, mm | 45 ± 11 | 43 ± 8 | 0.56 |
| E/E’ | 25.5 ± 13.0 | 22.1 ± 12.0 | 0.18 |
| Hb, g/dl | 10.5 ± 1.0 | 14.0 ± 1.4 | 0.01 |
| Serum creatinine, mg/dl | 1.2 ± 0.5 | 1.0 ± 0.4 | 0.03 |
| eGFR, ml/min/1.73 m2 | 49.5 ± 20.3 | 58.3 ± 19.3 | 0.03 |
| CKD, % | 79 | 62 | 0.05 |
| Albumin, mg/dl | 3.4 ± 0.4 | 3.6 ± 0.5 | 0.56 |
| Brain natriuretic peptide, pg/dl | 846 ± 734.0 | 650 ± 812 | 0.79 |
Values denote means ± SD unless specified otherwise. E/E’ = Ratio of mitral early diastolic peak flow velocity (E) to tissue Doppler early mitral annular diastolic velocity (E').
Physical Function
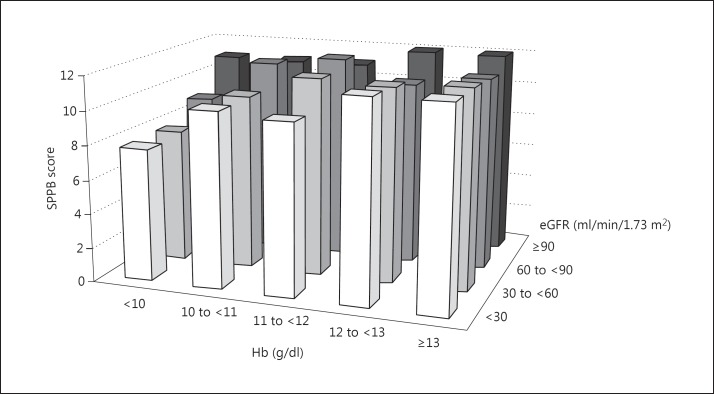
In the univariate analyses, both CKD (SPPB score: 11.6 ± 0.7 without vs. 10.1 ± 2.3 with CKD; p < 0.01) and anemia (SPPB score: 11.3 ± 1.3 without vs. 9.3 ± 2.6 with anemia; p < 0.01) were significantly associated with SPPB score. Figure 1 indicates the effects of Hb and eGFR on SPPB score, with the category of Hb on the x-axis, the category of eGFR on the y-axis and the mean SPPB scores on the z-axis. There were no significant differences between the subcategories.
Fig. 1.
Unadjusted association of eGFR and Hb level with SPPB score.
Multivariate adjustment clearly showed additive effects of the interaction between CKD and anemia on the SPPB score (table 3). The adjusted mean SPPB score was significantly lower in patients with both CKD and anemia than in those with neither CKD nor anemia or in those with either CKD or anemia.
Table 3.
Comparison of adjusted mean SPPB scores
| Patients, n | Adjusted mean SPPB score | 95% CI | p value | |
|---|---|---|---|---|
| Without CKD | 32 | 11.6 | 10.94 – 12.28 | <0.01 |
| With CKD | 70 | 9.8 | 9.31 – 10.22 | |
| Without anemia | 42 | 11.2 | 10.72 – 11.67 | <0.01 |
| With anemia | 60 | 9.2 | 8.61 – 9.73 | |
| Neither CKD nor anemia | 30 | 11.5 | 10.82 – 12.14 | |
| Either CKD or anemia | 40 | 11.0a | 10.48 – 11.52 | |
| Both CKD and anemia | 32 | 8.59a, b | 8.01 – 9.18 | |
p < 0.05 vs. neither CKD nor anemia
p < 0.05 vs. either CKD or anemia.
There was no significant difference in SPPB scores between the HFpEF and HFrEF groups (10.4 ± 2.1 vs. 10.7 ± 2.0; p = 0.493). On the other hand, both the HFpEF and HFrEF groups showed that the SPPB scores in patients with both CKD and anemia were significantly lower than those in the group with neither CKD nor anemia and in the group with either CKD or anemia (p < 0.05; tables 4, 5).
Table 4.
Comparison of adjusted mean SPPB scores for HFrEF patients
| HFrEF patients (LVEF ≤40%; n = 58) |
||||
|---|---|---|---|---|
| n | adjusted mean | 95% CI | p value | |
| Without CKD | 21 | 11.3 | 0.341 – 2.734 | 0.123 |
| With CKD | 37 | 10.1 | ||
| Without anemia | 21 | 11.2 | 0.501 – 2.971 | <0.01 |
| With anemia | 37 | 9.5 | ||
| Neither CKD nor anemia | 23 | 11.1 | 9.139 – 12.725 | |
| Either CKD or anemia | 22 | 11.0 | 10.222 – 11.975 | |
| Both CKD and anemia | 13 | 9.6a,b | 8.192 – 10.932 | |
p < 0.05 vs. neither CKD nor anemia
p < 0.05 vs. either CKD or anemia.
Table 5.
Comparison of adjusted mean SPPB scores for HFpEF patients
| HFpEF patients (LVEF ≤40%; n = 44) |
||||
|---|---|---|---|---|
| n | adjusted mean | 95% CI | p value | |
| Without CKD | 11 | 11.2 | 0.382 – 1.906 | 0.186 |
| With CKD | 33 | 10.4 | ||
| Without anemia | 21 | 11.1 | 0.237 – 2.582 | 0.101 |
| With anemia | 23 | 9.9 | ||
| Neither CKD nor anemia | 7 | 11.8 | 10.409 – 13.187 | |
| Either CKD or anemia | 18 | 10.9 | 10.035 – 11.939 | |
| Both CKD and anemia | 19 | 9.1a, b | 8.061 – 10.154 | |
p < 0.05 vs. neither CKD nor anemia;
p < 0.05 vs. either CKD or anemia.
Discussion
The present study showed that both CKD and anemia were independently related to physical function, and the combined presence of CKD and anemia in chronic HF patients was associated with additive deterioration in physical function compared with the presence of CKD or anemia alone.
Effects of CKD and Anemia on Physical Function
The causes of low physical function in patients with CKD have not been clarified. Previous studies have found an association between malnutrition and low physical function in patients with CKD [7,8,15]; however, we found no association between CKD and serum albumin. Our study subjects received any cardiac rehabilitation during their hospital stays and underwent the physical performance test at discharge; therefore, our study population did not include patients who could not undergo any cardiac rehabilitation due to hemodynamic instability or patients who transferred to another institute such as a rehabilitation hospital or nursing home to receive hospital care or rehabilitation. Therefore, we thought the reason for the different results for nutritional status in patients with and those without CKD was a selection bias.
Activated inflammatory responses have been demonstrated in patients with CKD [16]; our study did not collect inflammatory biomarkers. These inflammatory responses were proposed to explain an association of CKD with skeletal muscle abnormalities and lower physical function [17,18]. Those reports suggested that inflammation caused a loss of protein stores and muscle wasting because the serum levels of proinflammatory cytokines might be caused to increase by developed CKD or the presence of comorbidity.
Recently, there have been many studies examining the relationship between anemia and low physical function in HF patients [19]. Additionally, efforts to improve Hb levels using erythropoiesis-stimulating agents (ESA) and oral or intravenous (IV) iron, and even IV iron without ESA, have shown positive effects on hospitalization, New York Heart Association functional class, cardiac and renal function, quality of life and exercise capacity [20]. Therefore, we thought that HF patients limited in their activities of daily living due to severe anemia were better treated with EPO, ESA and oral or IV iron, or even IV iron without ESA, but most studies are poorly powered and therefore of limited validity, and the aim of Hb correction is also not well defined. Anemia has been suggested to be a marker of loss of lean body mass, presence of comorbidity and elevated inhibitory cytokine levels [21,22]. Anemia and elevated inhibitory cytokine levels are determinants of skeletal muscle abnormality which might lead to physical dysfunction [22].
The present study showed that the relationship between anemia and low physical function was independent of CKD. In addition, it demonstrated an additive effect of CKD and anemia on physical function in HF patients in general as well as in the HFpEF and HFrEF subgroups. After adjusting for other covariates, HF patients – as well as patients from either the HFpEF or HFrEF subgroup – with both CKD and anemia had lower physical function than those with neither CKD nor anemia or those with either CKD or anemia. Low physical function in patients with both CKD and anemia was explained by abnormal energy metabolism, increased catabolism and malnutrition [23].
Cardiorenal Anemia Syndrome
Silverberg et al. [24,25,26,27,28] first proposed the ‘cardiorenal anemia syndrome’, a vicious circle that appears to be present in HF patients, in which HF itself causes both anemia and CKD. CKD increases the severity of anemia, and anemia and CKD can further worsen HF, which again aggravates anemia and CKD, and so on.
In the present study, HF patients with CKD and anemia had lower physical function, confirming previous findings. However, we did not evaluate exercise capacity and daily physical activity, which are counted as factors of physical function. HF patients with both CKD and anemia are at higher risk of physical functional deterioration. Therefore, a more detailed clinical research protocol is needed to explore effective interventions for preventing physical functional deterioration in HF patients.
Limitations of the Study
In our study, we did not find significant differences in SPPB scores (fig. 1), although the adjusted mean SPPB scores showed significant differences between the groups with neither CKD nor anemia, with either CKD or anemia and with both CKD and anemia. We suspect that this divergence was caused by our small sample size. As only 102 patients were included, the subgroups consisted of only a few patients each. Therefore, we assume that the small sample size may have caused type II errors (false negatives). We have to ascertain the effects of the subcategories of CKD and anemia on SPPB scores in the future.
Conclusion
In summary, this study found that CKD and anemia were independently associated with reduced physical function in HF patients. Further studies are needed to determine the cause of this lowered physical function and to explore whether there are effective interventions for HF patients with CKD and anemia.
References
- 1.Silverberg DS, Wexler D, Iaina A, et al. The interaction between heart failure and other heart diseases, renal failure, and anemia. Semin Nephrol. 2006;26:296–306. doi: 10.1016/j.semnephrol.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Odden MC, Whooley MA, Shlipak MG. Association of chronic kidney disease and anemia with physical capacity: the Heart and Soul Study. J Am Soc Nephrol. 2004;15:2908–2915. doi: 10.1097/01.ASN.0000143743.78092.E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heiwe S, Tollbäck A, Clyne N. Twelve weeks of exercise training increases muscle function and walking capacity in elderly predialysis patients and healthy subjects. Nephron. 2001;88:48–56. doi: 10.1159/000045959. [DOI] [PubMed] [Google Scholar]
- 4.Clyne N, Jogestrand T, Lins LE, et al. Progressive decline in renal function induces a gradual decrease in total hemoglobin and exercise capacity. Nephron. 1994;67:322–326. doi: 10.1159/000187987. [DOI] [PubMed] [Google Scholar]
- 5.Rocco MV, Gassman JJ, Wang SR, et al. Cross-sectional study of quality of life and symptoms in chronic renal disease patients: the Modification of Diet in Renal Disease Study. Am J Kidney Dis. 1997;29:888–896. doi: 10.1016/s0272-6386(97)90463-7. [DOI] [PubMed] [Google Scholar]
- 6.Shidler NR, Peterson RA, Kimmel PL. Quality of life and psychosocial relationships in patients with chronic renal insufficiency. Am J Kidney Dis. 1998;32:557–566. doi: 10.1016/s0272-6386(98)70017-4. [DOI] [PubMed] [Google Scholar]
- 7.Macdougall IC, Lewis NP, Saunders MJ, et al. Long-term cardiorespiratory effects of amelioration of renal anaemia by erythropoietin. Lancet. 1990;335:489–493. doi: 10.1016/0140-6736(90)90733-l. [DOI] [PubMed] [Google Scholar]
- 8.McMahon LP, McKenna MJ, Sangkabutra T, et al. Physical performance and associated electrolyte changes after haemoglobin normalization: a comparative study in haemodialysis patients. Nephrol Dial Transplant. 1999;14:1182–1187. doi: 10.1093/ndt/14.5.1182. [DOI] [PubMed] [Google Scholar]
- 9.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puthoff ML. Outcome measures in cardiopulmonary physical therapy: short physical performance battery. Cardiopulm Phys Ther J. 2008;19:17–22. [PMC free article] [PubMed] [Google Scholar]
- 11.National Kidney Foundation. NKF-K/DOQI clinical practice guidelines for anemia of chronic kidney disease: update 2000. Am J Kidney Dis. 2001;37:S182–S238. doi: 10.1016/s0272-6386(01)70008-x. [DOI] [PubMed] [Google Scholar]
- 12.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 13.Maggioni AP, Opasich C, Anand I, et al. Anemia in patients with heart failure: prevalence and prognostic role in a controlled trial and in clinical practice. J Card Fail. 2005;11:91–98. doi: 10.1016/j.cardfail.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 15.Harris LE, Luft FC, Rudy DW, et al. Clinical correlates of functional status in patients with chronic renal insufficiency. Am J Kidney Dis. 1993;21:161–166. doi: 10.1016/s0272-6386(12)81087-0. [DOI] [PubMed] [Google Scholar]
- 16.Shlipak MG, Fried LF, Crump C, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92. doi: 10.1161/01.cir.0000042700.48769.59. [DOI] [PubMed] [Google Scholar]
- 17.Wang XH, Du J, Klein JD, et al. Exercise ameliorates chronic kidney disease-induced defects in muscle protein metabolism and progenitor cell function. Kidney Int. 2009;76:751–759. doi: 10.1038/ki.2009.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fried LF, Lee JS, Shlipak M, et al. Chronic kidney disease and functional limitation in older people: health, aging and body composition study. J Am Geriatr Soc. 2006;54:750–756. doi: 10.1111/j.1532-5415.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 19.Anand I, McMurray JJ, Whitmore J, et al. Anemia and its relationship to clinical outcome in heart failure. Circulation. 2004;110:149–154. doi: 10.1161/01.CIR.0000134279.79571.73. [DOI] [PubMed] [Google Scholar]
- 20.Silverberg DS. The role of erythropoiesis stimulating agents and intravenous (IV) iron in the cardio renal anemia syndrome. Heart Fail Rev. 2010;16:609–614. doi: 10.1007/s10741-010-9194-2. [DOI] [PubMed] [Google Scholar]
- 21.Horwich TB, Fonarow GC, Hamilton MA, et al. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol. 2002;39:1780–1786. doi: 10.1016/s0735-1097(02)01854-5. [DOI] [PubMed] [Google Scholar]
- 22.Mancini DM, Katz SD, Lang CC, et al. Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation. 2003;107:294–299. doi: 10.1161/01.cir.0000044914.42696.6a. [DOI] [PubMed] [Google Scholar]
- 23.Silverberg DS, Wexler D, Blum M, et al. Erythropoietin should be part of congestive heart failure management. Kidney Int. 2003;87(suppl):S40–S47. doi: 10.1046/j.1523-1755.64.s87.7.x. [DOI] [PubMed] [Google Scholar]
- 24.Silverberg DS, Wexler D, Iaina A. The role of anemia in the progression of congestive heart failure: is there a place for erythropoietin and intravenous iron? J Nephrol. 2004;17:749–761. [PubMed] [Google Scholar]
- 25.Silverberg DS, Wexler D, Iaina A, et al. Anaemia management in cardio renal disease. J Ren Care. 2010;36(suppl 1):86–96. doi: 10.1111/j.1755-6686.2010.00164.x. [DOI] [PubMed] [Google Scholar]
- 26.Silverberg DS, Wexler D, Blum M, et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000;35:1737–1744. doi: 10.1016/s0735-1097(00)00613-6. [DOI] [PubMed] [Google Scholar]
- 27.Silverberg DS, Wexler D, Blum M, et al. The correction of anemia in severe resistant heart failure with erythropoietin and intravenous iron prevents the progression of both the heart and the renal failure and markedly reduces hospitalization. Clin Nephrol. 2002;58(suppl 1):S37–S45. [PubMed] [Google Scholar]
- 28.Silverberg DS, Wexler D, Sheps D, et al. The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J Am Coll Cardiol. 2001;37:1775–1780. doi: 10.1016/s0735-1097(01)01248-7. [DOI] [PubMed] [Google Scholar]