Abstract
Purpose
To evaluate the safety, tolerability, and intraocular pressure (IOP)-lowering effect of Latrunculin-B (Lat-B), a marine macrolide that disrupts the actin cytoskeleton, in patients with ocular hypertension (OHT) or early primary open-angle glaucoma (POAG).
Methods
In this Phase I, multicenter, double-masked, randomized, placebo-controlled, ascending-dose study, subjects with bilateral OHT or early POAG (>22 mm Hg) received one of four concentrations of INS115644 (Lat-B ophthalmic solutions, 0.005%, 0.01%, 0.02%, or 0.05%) in one eye over 3 days (5 single-dose instillations, separated by 12 hours). One eye was randomly assigned to active drug, the other to placebo. IOP was measured prior to treatment initiation (day 0) and on days 1 and 3.
Results
Baseline IOPs were 22.9 ± 2.4 mm Hg and 23.5 + 3.1 mm Hg in the 0.02% and 0.05% dose groups, respectively. At 4 hours post instillation of the first dose, 0.02% INS115644 reduced IOP from baseline (mean ± SE) by 3.8 ± 0.7 mm Hg (P = 0.002) and 0.05% by 3.9 ± 1.0 mm Hg (P = 0.004). A maximum IOP decrease of 24% was noted at 4 hours after the fifth instillation of 0.02%. Adjusting for diurnal baseline and IOP in the contralateral, placebo-treated eye, the maximal 12-hour hypotensive effect was 4.0 ± 0.5 mm Hg (adjusted mean ± SE), a 17% decrease, following the fifth instillation of 0.02% (day 3). Adverse events were few and consisted mainly of mild redness, irritation, and a transient, clinically insignificant increase (≤2.5%) in central corneal thickness.
Conclusions
In OHT or POAG patients, twice daily Lat-B significantly lowered IOP compared with contralateral, placebo-treated eyes, with few and mild ocular adverse events.
Translational Relevance
Lat-B may be a potential therapeutic agent for glaucoma.
Keywords: intraocular pressure, trabecular meshwork, cytoskeleton
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide.1 Nearly 70 million people worldwide, 1% to 4% of the population older than 45 years of age, might be affected.2 Many have the disease without knowing it. As the population ages and life expectancy increases, estimates indicate an increase in the number of persons with POAG in the United States, from 2.71 million in 2011 to 7.32 million in 2050.3 The cost of treatment was estimated to exceed $5 billion annually in 2006, in the United States alone.4
Lowering intraocular pressure (IOP) reduces the risk of disease progression5–7 and controlling IOP with topical eye drops continues to be the most common first-line therapy. Most patients will need more than one medication, from more than one class of compound, to control their IOP. The most commonly prescribed drugs act on either the ciliary process epithelia to reduce aqueous humor production (beta-adrenergic receptor antagonists) or the ciliary muscle cells to increase uveoscleral outflow (prostaglandin analogs that bind to and activate prostaglandin F [FP] receptors).8,9 Drugs that target the trabecular meshwork (TM) and Schlemm's canal (SC) include cholinomimetics, which increase TM/SC outflow facility by contracting the ciliary muscle (CM), in turn distorting the TM so as to increase facility; and epinephrine, which acts directly on beta-2 adrenergic receptors in the TM/SC cells to increase TM facility (perhaps by disturbing the TM/SC cytoskeleton).10 Neither class is widely used clinically because of local (cholinomimetics) or both local and systemic (epinephrine) side effects, though they remain an alternative for patients refractory to conventional therapeutics, notably those with exfoliation syndrome/glaucoma and pigmentary glaucoma. Although cholinomimetics and epinephrine are approved and available, they are used infrequently, and there are currently no drugs commonly used clinically that directly target the TM.
In healthy eyes, the molecular pathways involved in uveoscleral and trabecular outflow and their physiologic effects work together to stimulate contraction and relaxation of the CM and TM/inner wall of Schlemm's canal (IWSC), providing finely-tuned control of outflow.11–13 Interference with TM/IWSC cellular contractility, and cell–cell or cell–extracellular matrix adhesion, whether by inhibiting the Rho kinase or myosin light-chain kinase cascades or by disrupting actin microfilaments (whether by direct disruption or inhibition of their assembly) can result in increased outflow facility in live nonhuman primates10,14,15 and in both human and primate organ-cultured anterior segments.16–20
Several Rho kinase inhibitor compounds are in development21–26 aiming to lower IOP by targeting trabecular outflow. Another novel agent targeting the TM is latrunculin B (Lat-B), which originates from Latrunculia (now Negombata) magnifica, a sponge from the Red Sea. Latrunculins inhibit the assembly of actin microfilaments by 1:1 molecular binding of free actin monomers in the cell cytoplasm.27,28 This results in cellular relaxation, and loosened cell–cell junctions and weakened focal contacts between cells and the surrounding matrix. Lat-B increases outflow facility in human19 and monkey (Gabelt, unpublished data 2011) organ-cultured anterior segments, and in live, nonhuman primates.14,15
The mechanism of the Lat-B facility increase is thought to be due to changes in the structural geometry of the TM29 as a consequence of reduction in cell–cell and cell–matrix adhesion, along with impairment of cellular contractility. Treating the normotensive monkey eye topically with Lat-B causes a substantial reduction in IOP.30,31 Administration of Lat-B into the anterior chamber of live monkey eyes causes a dose-dependent increase in outflow facility14 due to relaxation of the TM and SC, which expands the area available for fluid outflow and reduces resistance to fluid flow through/across the system.29 The facility increases after intracameral exchange infusion and topical eyedrop administration of several cytoskeletal agents (H-7 and Lat-A) are reversible, indicating that they are due to transient alterations in cellular contractility and cytoskeletal organization rather than irreversible toxicity.32,33 This is an important safety consideration for a potential antiglaucoma medication. Additionally, intravitreal administration of Lat-B in monkeys, at doses that increase outflow facility and lower IOP when given intracamerally, had no effect on retinal vascular permeability, retinal electrophysiology, or the angiographic appearance of the retina.34 This is also true for H-7, a Rho kinase/myosin light-chain kinase inhibitor.
The current study reports the first human trial of Lat-B for the treatment of ocular hypertension and POAG. The objectives of this study were to evaluate the safety, tolerability, and IOP-lowering efficacy of INS115644 Ophthalmic Solution in subjects presenting with bilateral ocular hypertension or early primary open-angle glaucoma (POAG).
Methods
This was a multicenter, double-masked, randomized, placebo-controlled, ascending dose Phase I/II study designed to evaluate the safety, tolerability and efficacy of four doses of Lat-B Ophthalmic Solution (Inspire Pharmaceuticals designation as INS115644) in subjects with bilateral ocular hypertension or early POAG.
Fourteen subjects were enrolled in each of four cohorts evaluating four concentrations of INS115644 (0.005%, 0.01%, 0.02%, and 0.05%). In each cohort, 10 patients were randomized to receive INS115644 in one eye and placebo in the contralateral eye, with the active-treated eye chosen at random. Additionally, four subjects in each cohort had placebo administered to both eyes. Once randomized, five single-dose instillations of INS115644/placebo or placebo alone were given over 3 days with each instillation separated by approximately 12 hours. Each dose was delivered as one drop in each eye by a designated member of the study staff. IOP was measured prior to initiation of treatment (day 0) and on days 1 and 3 at approximately 8 AM (predose days 1 and 3) and 10 AM, and 12, 4, and 8 PM. Following the completion of each cohort (0.005%, 0.01%, and 0.02%) an unmasked analysis of all safety and tolerability assessments performed through day 7 (±2 days) was conducted and reviewed by a data monitoring committee (DMC) to determine if it was appropriate to continue to the next dose/cohort.
Subjects enrolled in this study were 18 to 75 years of age, had a diagnosis of bilateral ocular hypertension or early POAG, and had open healthy-appearing anterior chamber angles (grade 3 or 4). For the purposes of this study, early POAG was defined as focal nonfull thickness rim thinning with no visual field (VF) changes or small isolated nasal step or paracentral scotoma or Seidel's scotoma. The entry requirements for IOP were unmedicated IOP of 22 to 30 mm Hg at 8 AM (<4 mm Hg difference between eyes). Other entry requirements included best-corrected visual acuity (BCVA) in in both eyes of at least +0.5 LogMAR or better as assessed by Early Treatment Diabetic Retinopathy Study charts; corneal endothelial cell counts of at least 2000 cells/mm2 (measured by noncontact specular microscopy) with normal morphology, and the ability and willingness to give signed informed consent and to follow study instructions.
Excluded from the study were individuals with closed angle, exfoliation syndrome, pigment dispersion, or secondary glaucoma; a history of glaucoma surgery (trabeculectomy, cryotherapy, etc.); cataract surgery within the past 3 months; progressive retinal or optic nerve disease apart from glaucoma, or history of chronic corticosteroid (topical or intraocular) use within the past month. Female subjects who were pregnant, lactating, or of childbearing potential and not using an acceptable method of birth control were excluded.
The screening examination included a physical exam, measurement of vital signs, complete blood count, chemistry panel and urinalysis assessment, visual acuity measurement (Early Treatment Diabetic Retinopathy Study charts), IOP, slit-lamp biomicroscopy, dilated ophthalmoscopy, corneal staining, automated visual fields (Humphrey program 24-2 SITA-standard strategy), ultrasonic pachymetry (contact), noncontact specular microscopy, anterior segment (nondilated) digital photos, the Roth 28 hue color vision test, and pupil measurement. Qualified individuals using topical ocular hypotensive underwent a washout as follows: prostaglandin analogues and β-adrenoceptor antagonists (6 weeks), α-adrenergic agonists and epinephrine-related medications (5 weeks), and pilocarpine or carbonic anhydrase inhibitors (7 days).
The key safety and efficacy endpoints were change in IOP, incidence of adverse events, change in endothelial cell count and morphology, change in corneal thickness, changes in slit-lamp, and fundus exam and change in visual acuity with and without manifest refraction.
Statistical methods: For all analyses, subject-level covariates were summarized within each cohort by subject classification (active-placebo versus placebo-placebo). Eye-level covariates were summarized by eye classification within subject classification. Categorical variables were generally summarized by the number and percentage of subjects in each category. Continuous variables were summarized using N, mean, SD, median, minimum, and maximum values. IOP for each day and time point, as well as diurnal values on days 1, 3, and 8, were summarized using Wilcoxon Signed-Rank test to assess treatment differences between eyes in subjects who received active treatment. Mean change values adjusted for baseline were generated using a mixed-effects model within cohort. The model included fixed effects for eyes (active eye, placebo eye from Active-Placebo subjects, and placebo eyes from Placebo-Placebo subjects) and baseline IOP with a random effect for subject. If the parameter between placebo eyes from Active-Placebo subjects and Placebo-Placebo subjects was nonsignificant, the adjusted means were presented for active-treated eyes versus placebo eyes.
All research followed the tenets of the Declaration of Helsinki and informed consent was obtained from the subjects after explanation of the nature and possible consequences of the study and where applicable, the research was approved by the institutional human experimentation committee or institutional review board (IRB).
Results
Patient Disposition and Demographics
A total of 56 individuals were enrolled as study patients. Of these, 40 were assigned to the intent-to-treat group, of which 38 patients completed the study (Table 1). One noncompleting patient was in the 2.5% protocol violation group and the other was in the 2.5% adverse event group.
Table 1.
Enrollment Statistics and Demographics Disposition by Treatment Group

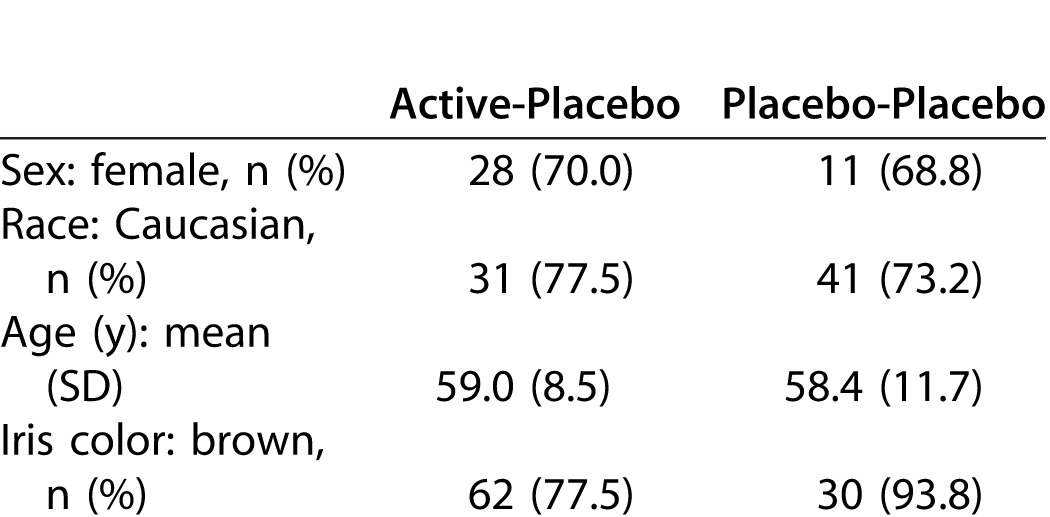
The prestudy subject-level demographics and background characteristics (intent-to-treat population) are shown in Table 2.
Table 2.
Subject-Level Demographics and Background Characteristics
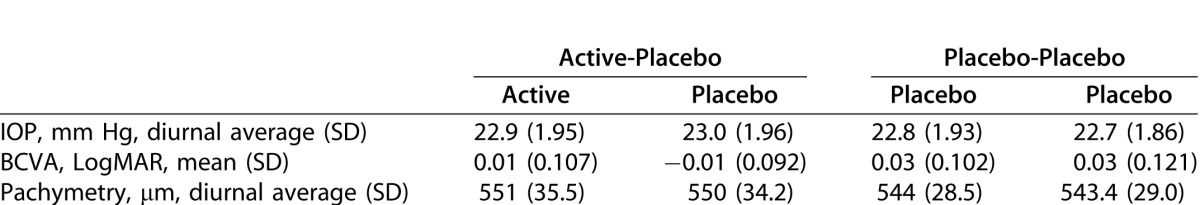
Eye-level demographics and background characteristics (intent-to-treat population) are summarized in Table 3.
Table 3.
Eye-Level Demographics and Background Characteristics
Safety
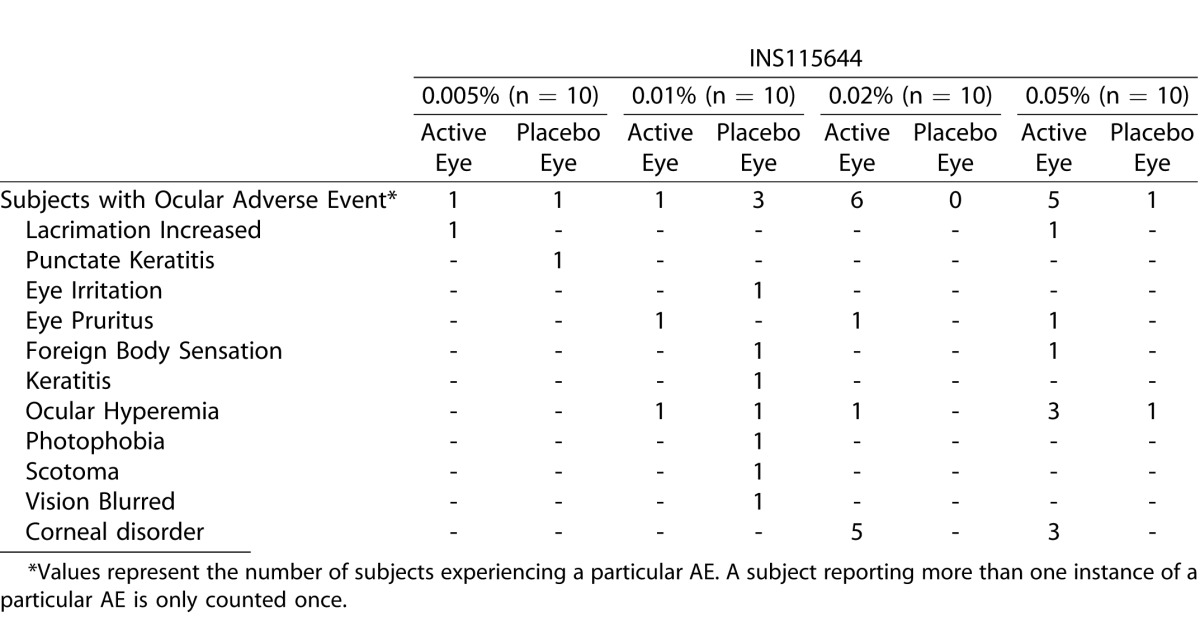
A few and mild ocular adverse events, consisting mainly of redness and irritation indicate that INS115644 was generally well tolerated. The incidence of ocular adverse events in the safety population is summarized in Table 4. The safety population included all subjects receiving at least one dose of study medication (active or placebo) in the study.
Table 4.
Incidence of Ocular Adverse Events
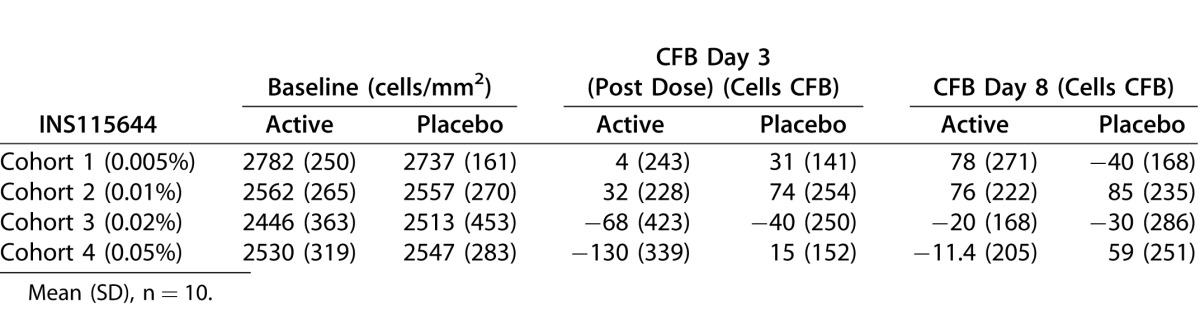
Transient changes in central corneal thickness were noted 4 hours post instillation. Increases were seen at the 0.005%, 0.02%, and 0.05% doses, while a decrease was seen at the 0.01% dose. The largest change was an increase of less than or equal to 2.5% at the 0.05% dose. Though there were statistically significant time points at each dose level, the changes in central corneal thickness were considered clinically insignificant. Table 5 summarizes the observed values and change from baseline measured by specular microscopy.
Table 5.
Specular Microscopy: Observed Values and Change From Baseline
IOP-Lowering Effect
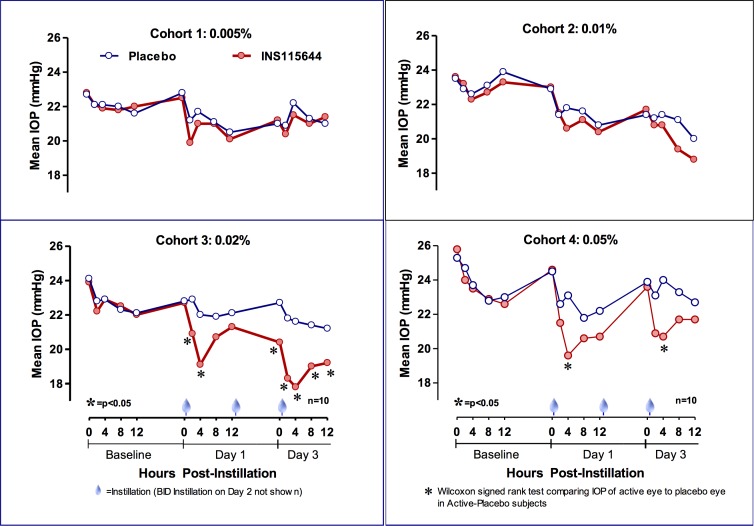
Baseline IOPs were 22.9 ± 2.4 mm Hg and 23.5 ± 3.1 mm Hg in the 0.02% and 0.05% dose groups, respectively. Following the first instillation, 0.02% INS115644 reduced IOP (mean ± SD) by 3.8 ± 2.25 mm Hg (P = 0.002), and 0.05% INS115644 by 3.9 ± 3.1 mm Hg (P = 0.004) from baseline (17% reduction in IOP) at 4 hours post instillation. After the fifth instillation (day 3) IOP decreased from baseline by 5.4 ± 2.4 mm Hg (P = 0.004), a 24% reduction in IOP, in the 0.02% cohort and 2.8 ± 2.7 mm Hg (P = 0.02), a 12% reduction, in the 0.05% cohort at 4 hours post instillation (Fig. 1). INS115644 did not significantly lower IOP in the treated eyes compared with contralateral, placebo-control eyes at the two lowest doses.
Figure 1.
Effects of INS115644 on IOP in subjects with OHT and POAG. Following the fifth instillation (day 3) IOP decreased from baseline by 5.4 ± 2.4 mm Hg (P = 0.004) and 2.8 ± 2.7 mm Hg (P = 0.02) in the 0.02% and 0.05% cohorts, respectively, at 4 hours post instillation. Wilcoxon Signed-Rank test comparing IOP of active eye to placebo eye in Active-Placebo subjects.
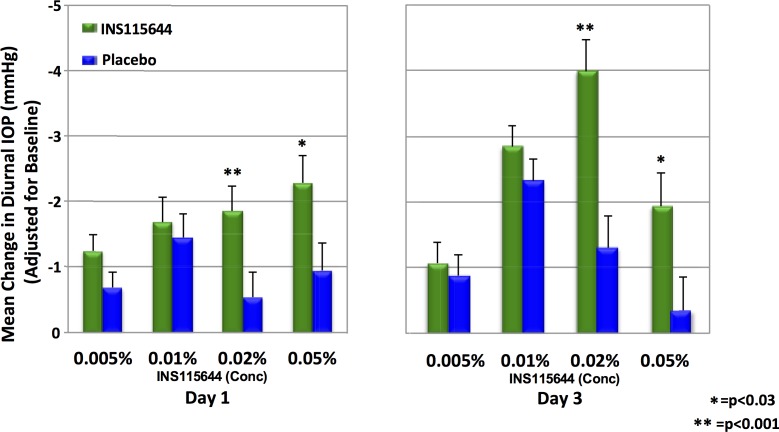
Adjusting for diurnal baseline and IOP in the contralateral, placebo-treated eye, a maximal 12-hour hypotensive effect of 4.0 ± 0.5 mm Hg following the fifth instillation of 0.02% INS115644 was noted on day 3 (Fig. 2). A diurnal curve was assembled at baseline (IOPs were collected at 8 and 10 AM, 12, 4, and 8 PM). The two time points with the greatest IOP decrease compared with baseline were 4 hours post dose on days 1 and 3. At the 0.05% dose level a 3.9 mm Hg, or 17% decrease, was seen on day 1 and a 2.8 mm Hg, or 12% decrease, was seen on day 5. At the 0.02% dose level a 3.7 mm Hg, or 16% decrease, was seen at day 1 and a 5.4 mm Hg, or 24% decrease, was seen at day 5. Compared with the same baseline time-of-day IOP, a maximum decrease of 24% was noted at 4 hours post fifth instillation of 0.02% INS115644.
Figure 2.
Effects of INS115644 on average diurnal IOP in subjects with OHT and POAG. Diurnal IOP was calculated from the mean measurements taken at 0 (8 AM) and 2, 4, 8 and 12 hours post instillation on day 1 or day 3.
Discussion
In this first-in-human 3-day, ascending dose Phase 1 study designed to evaluate the safety and tolerability of INS115644 (Lat-B) in patients with OHT or POAG, twice-daily INS115644 demonstrated ocular hypotensive efficacy in patients with elevated IOP. The two highest doses significantly lowered IOP at multiple time points compared with contralateral, placebo-control eyes. The greatest decreases, 17% to 24%, compared with the same baseline time-of-day, occurred 4 hours after instillation of the first and fifth doses at the two highest concentrations (0.05% and 0.02%, respectively). Significant IOP reductions were sustained for 12 hours post instillation of the fifth dose at the 0.02% concentration, with a maximal 12-hour hypotensive effect of 4.0 ± 0.5 mm Hg, a 17% decrease, after adjusting for diurnal baseline and IOP in the contralateral, placebo-treated eye. The 0.02% concentration provided more significantly reduced IOP at more time points than the 0.05% concentration suggesting 0.02% as approximately the top of the dose response range.
While the reported IOP-lowering effects are not unusually large, further studies are needed to determine the scope of Lat-B's potential clinical use, given the novel mechanism of action and potential to be additive to both secretory suppression and uveoscleral outflow enhancement drugs. The 12-hour maximal duration of action would indicate the need for twice daily dosing if used alone and perhaps even for adjunctive usage. Further studies to reduce the number of daily doses might include formulation improvements (to increase corneal penetration and bioavailability), sustained release options, modifying the molecule itself (Lat-B 2.0) or implantable delivery devices.
Few and mild ocular adverse events, consisting mainly of short-lived redness and irritation indicate that INS115644 was generally well tolerated. There were no systemic safety issues in the present study. The most common abnormality was conjunctival erythema/hyperemia, which occurred in both active- and placebo-treated eyes at most evaluations, including baseline. Only one patient was discontinued for an adverse event. This patient was treated with the 0.01% concentration and experienced several ocular events, namely, hyperemia, photophobia, keratitis, and eye irritation. The adverse event of ocular hyperemia has been reported previously in several clinical trials with Rho kinase inhibitors.21,22 It was suggested that the hyperemia could be the result of blood vessel relaxation, as ROCK inhibition induces smooth muscle relaxation.22 Hyperemia was not noted in the preclinical pharmacology monkey studies, perhaps because it is hard to see the vessels through the heavily-pigmented anterior conjunctiva and hard to see the more posterior but less pigmented conjunctiva. Monkey physiology data did indicate that Lat-B dose-dependently relaxes intraocular smooth muscles. Pupils dilated after phenylephrine administration dilated further relative to the contralateral controls 85 minutes after topical administration of 20 μL of 0.02% Lat-B. After pilocarpine was infused intramuscularly, the control pupils constricted but the pupils after 0.02% Lat-B did not. Although 0.02% Lat-B almost completely inhibited the miotic response to pilocarpine, it inhibited the accommodative response to pilocarpine by only up to 25%. This discrepancy might be a pharmacodynamic issue; drug levels reaching the CM might be less than those reaching the iris sphincter muscle.
A transient, clinically insignificant increase in central corneal thickness of less than or equal to 2.5% was noted 4 hours post instillation, while corneal endothelial cell density remained unchanged. In monkeys, a single dose of 20 μL of 500 μM (∼0.02%) Lat-B administered topically, produced a transient increase in corneal thickness of approximately 10% at 3.5 hours post dose and 6% at hour 6. Midperipheral corneal thickness also increased 2 to 5 hours after Lat-B administration in monkeys, with the greatest increase, 6%, at 3 hours.31 In separate studies, corneal thickness was not changed after 1 or 9 dose(s) of 0.01% Lat-B.30 Reversible abnormal corneal endothelial cell morphology, from specular microscopy observations, was noted in the higher dose groups. In monkey studies, by electron microscopy, the morphology of the corneal endothelium was unchanged (all junctional elements, including tight junctions, adherens junctions, and gap junctions were retained) after intracameral exchange and perfusion with 0.5 μM Lat-B, a concentration that affected the TM and increased outflow facility.29
Combination drug effects tested during in vivo monkey experiments in which H-7 was administered by AC exchange and cytochalasin B (CB) doses were given by bolus AC injection demonstrated that combining subthreshold doses of CB and a maximal dose of H-7 produced facility increases significantly higher than those produced by H-7 alone, and combining a near-maximal dose of CB and a maximal dose of H-7 produced a facility increase of greater magnitude than either alone.35 These findings could indicate that the pathways by which H-7 and CB increase outflow facility converge at some point, resulting in deterioration of actin microfilaments in the TM and alterations of cell–cell and/or cell–extracellular matrix adhesions. Alternatively, the findings could indicate that H-7 and CB may increase facility at least in part by different mechanisms with CB-induced cellular contractions disrupting cell–cell adhesion,36 complimenting H-7's predominant effects on cell–extracellular matrix adhesion.37 Additive IOP lowering effects of Lat-B combined with Rho-kinase inhibitors would need to be studied directly with these specific compounds, but it is possible that the mechanisms of action would be complementary and the combination would enhance IOP lowering effects.
Altering the actin microfilament system (by cytochalasins, latrunculins, etc.) or actomyosin contractility (by myosin light-chain kinase or Rho-kinase inhibitors; by over-expression of caldesmon, which in essence uncouples actin from myosin; or C3, which affects actin-myosin interactions by inhibiting Rho-GTP (guanosine triphosphate) at the beginning of the Rho activation cascade) reduces outflow resistance in nonhuman primates in vivo and/or in vitro. Morphological studies done with Lat-B and other cytoskeletal agents show that there are key common effects: relaxation of the TM and expansion of the JCT, and relaxation and flattening and spreading out of the SC inner wall endothelial cells, and consequent dilation of the canal.10,15
The clean slit-lamp exams in our patients, the reversibility of the TM effect, the absence of anterior segment adverse events and the healthy retina studies in monkeys, all bode well for Lat-B's safety clinical use. Our findings demonstrate that INS115644 may be a safe topical agent that is effective in reducing reduces IOP in OHT and POAG patients. The magnitude of the IOP-lowering effect of INS115644 Ophthalmic Solution observed in this study warrants further investigation in larger clinical trials. With its unique mechanism of action, this drug has the potential to positively add to current pharmacological interventions.
Acknowledgments
Supported by Inspire Pharmaceuticals and by grants from the National Institutes of Health/National Eye Institute (EY02698, University of Wisconsin-Madison Core Grant for Vision Research (P30 EY016665); Research to Prevent Blindness, Inc., New York, NY, unrestricted departmental award; Ocular Physiology Research and Education Foundation.
Disclosure: C.A. Rasmussen: None; R. Ritch: (F); P.L. Kaufman: (F), (C), (P); R. Haque: Inspire Pharma. (E); R.K. Brazzell: Inspire Pharma. (E); J.L. Vittitow: Inspire Pharma. (E), (P)
References
- 1.Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in 2002. Bull WHO. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006:90.262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vajaranant TS, Wu S, Torres M, Varma RA. 40-year forecast of the demographic shift in primary open-angle glaucoma in the United States. Invest Ophthalmol Vis Sci. 2012;53:2464–2466. doi: 10.1167/iovs.12-9483d. [DOI] [PubMed] [Google Scholar]
- 4.Lee PP, Walt JG, Doyle JJ, et al. A multicenter, retrospective pilot study of resource use and costs associated with severity of disease in glaucoma. Arch Ophthalmol. 2006;124:12–19. doi: 10.1001/archopht.124.1.12. [DOI] [PubMed] [Google Scholar]
- 5.Bengtsson B, Leske MC, Hyman L, Heijl A. Early Manifest Glaucoma Trial Group. Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:205–209. doi: 10.1016/j.ophtha.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 6.Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 7.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal tension glaucoma and patients with therapeutically reduced intraocular pressure. Am J Ophthalmol. 1998;126:487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 8.Ghate D, Edelhauser HF. Barriers to glaucoma drug delivery. J Glaucoma. 2008;17:147–156. doi: 10.1097/IJG.0b013e31814b990d. [DOI] [PubMed] [Google Scholar]
- 9.Medeiros FA, Weinreb RN. Medical backgrounders: glaucoma. Drugs Today. 2002;38:563. doi: 10.1358/dot.2002.38.8.704676. [DOI] [PubMed] [Google Scholar]
- 10.Tian B, Kaufman PL. Comparisons of actin filament disruptors and Rho kinase inhibitors as potential antiglaucoma medications. Expert Rev Ophthalmol. 2012;7:177–187. doi: 10.1586/eop.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stamer W, Lei Y, Boussommier-Calleja A, Overby D. Ethier C: eNOS, a pressure-dependent regulator of intraocular pressure. Invest Ophthalmol Vis Sci. 2011;52:9438–9444. doi: 10.1167/iovs.11-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufman PL, Rasmussen CA. Advances in glaucoma treatment and management: outflow drugs. Invest Ophthalmol Vis Sci. 2012;53:2495–500. doi: 10.1167/iovs.12-9483m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman PL. Enhancing trabecular outflow by disrupting the actin cytoskeleton, increasing uveoscleral outflow with prostaglandins, and understanding the pathophysiology of presbyopia interrogating Mother Nature: asking why, asking how, recognizing the signs, following the trail. Exp Eye Res. 2008;86:3–17. doi: 10.1016/j.exer.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson JA, Tian B, Geiger B, Kaufman PL. Effect of latrunculin-B on outflow facility in monkeys. Exp Eye Res. 2000;70:307–313. doi: 10.1006/exer.1999.0797. [DOI] [PubMed] [Google Scholar]
- 15.Tian B, Gabelt BT, Geiger B, Kaufman PL. The role of the actomyosin system in regulating trabecular fluid outflow. Exp Eye Res. 2009;88:713–717. doi: 10.1016/j.exer.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao PV, Deng P, Maddala R, Epstein DL, Li CY, Shimokawa H. Expression of dominant negative Rho-binding domain of Rho-kinase in organ cultured human eye anterior segments increases aqueous humor outflow. Mol Vis. 2005;11:288–297. [PubMed] [Google Scholar]
- 17.Gabelt BT, Hu Y, Vittitow JL, et al. Caldesmon transgene expression disrupts focal adhesions in HTM cells and increases outflow facility in organ-cultured human and monkey anterior segments. Exp Eye Res. 2006;82:935–944. doi: 10.1016/j.exer.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Hu Y, Gabelt BT, Kaufman PL. Monkey organ-cultured anterior segments: technique and response to H-7. Exp Eye Res. 2006;82:1100–1108. doi: 10.1016/j.exer.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Ethier CR, Read AT, Chan DW. Effects of latrunculin-B on outflow facility and trabecular meshwork structure in human eyes. Invest Ophthalmol Vis Sci. 2006;47:1991–1998. doi: 10.1167/iovs.05-0327. [DOI] [PubMed] [Google Scholar]
- 20.Lu Z, Overby DR, Scott PA, Freddo TF, Gong H. The mechanism of increasing outflow facility by rho-kinase inhibition with Y-27632 in bovine eyes. Exp Eye Res. 2008;86:271–281. doi: 10.1016/j.exer.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams RD, Novack GD, van Haarlem T, Kopczynski C. AR-12286 Phase 2A Study Group. Ocular hypotensive effect of the Rho kinase inhibitor AR-12286 in patients with glaucoma and ocular hypertension. Am J Ophthalmol. 2011;152:834–841.e1. doi: 10.1016/j.ajo.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Tanihara H, Inatani M, Honjo M, Tokushige H, Azuma J, Araie M. Intraocular pressure–lowering effects and safety of topical administration of a selective ROCK inhibitor, SNJ-1656, in healthy volunteers. Arch Ophthalmol. 2008;126:309–315. doi: 10.1001/archophthalmol.2007.76. [DOI] [PubMed] [Google Scholar]
- 23.Tanihara H, Inoue T, Yamamoto T, Kuwayama Y, Abe H, Araie M. K-115 Clinical Study Group. Phase 2 randomized clinical study of a Rho kinase inhibitor, K-115, in primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 2013;156:731–736. doi: 10.1016/j.ajo.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Novack GD. Rho kinase inhibitors for the treatment of glaucoma. Drugs Future. 2013;38:107–113. [Google Scholar]
- 25.Kopczynski CC, Epstein DL. Emerging trabecular outflow drugs. J Ocul Pharmacol Ther. 2014;30:85–87. doi: 10.1089/jop.2013.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue T, Tanihara H. Rho-associated kinase inhibitors: a novel glaucoma therapy. Prog Retin Eye Res. 2013;37:1–12. doi: 10.1016/j.preteyeres.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Spector I, Shochet NR, Blasberger D, Kashman Y. Latrunculins–novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell Motil Cytoskeleton. 1989;13:127–44. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
- 28.Spector I, Shochet NR, Kashman Y, Groweiss A. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science. 1983;219:493–495. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- 29.Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL. Latrunculin B effects on trabecular meshwork and corneal endothelial morphology in monkeys. Exp Eye Res. 2006;82:236–246. doi: 10.1016/j.exer.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Okka M, Tian B, Kaufman PL. Effects of latrunculin B on outflow facility, intraocular pressure, corneal thickness, and miotic and accommodative responses to pilocarpine in monkeys. Trans Am Ophthalmol Soc. 2004;102:251–257. [PMC free article] [PubMed] [Google Scholar]
- 31.B Peterson JA, Tian B, McLaren JW, Hubbard WC, Geiger B, Kaufman PL. Latrunculins' effects on intraocular pressure, aqueous humor flow, and corneal endothelium. Invest Ophthalmol Vis Sci. 2000;41:1749–1758. [PubMed] [Google Scholar]
- 32.Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL. Functional and structural reversibility of H-7 effects on the conventional aqueous outflow pathway in monkeys. Exp Eye Res. 2004;78:137–150. doi: 10.1016/j.exer.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Peterson JA, Tian B, Bershadsky AD, et al. Latrunculin-A increases outflow facility in the monkey. Invest Ophthalmol Vis Sci. 1999;40:931–941. [PubMed] [Google Scholar]
- 34.Kiland JA, Miller CL, Kim CB, et al. Effect of H-7 and Lat-B on retinal physiology. Curr Eye Res. 2006;31:441–455. doi: 10.1080/02713680600672185. [DOI] [PubMed] [Google Scholar]
- 35.Tian B, Gabelt BT, Geiger B, Kaufman PL. Combined effects of H-7 and cytochalasin B on outflow facility in monkeys. Exp Eye Res. 1999;68:649–655. doi: 10.1006/exer.1998.0647. [DOI] [PubMed] [Google Scholar]
- 36.Citi S, Volberg T, Bershadsky AD, Denisenko N, Geiger B. Cytoskeletal involvement in the modulation of cell±cell junctions by the protein kinase inhibitor H-7. J Cell Sci. 1994;107:683–692. [PubMed] [Google Scholar]
- 37.Tian B, Kaufman PL, Volberg T, Gabelt BT, Geiger B. H-7 disrupts the actin cytoskeleton and increases outflow facility. Arch Ophthalmol. 1998;116:633–643. doi: 10.1001/archopht.116.5.633. [DOI] [PubMed] [Google Scholar]