Abstract
Data indicate that interleukin (IL)-1β and tumor necrosis factor-α (TNFα) are involved in the regulation of non-rapid eye movement sleep (NREMS). Previous studies demonstrate that mice lacking the IL-1β type 1 receptor spend less time in NREMS during the light period, whereas mice lacking the p55 (type 1) receptor for TNFα spend less time in NREMS during the dark period. To further investigate roles for IL-1β and TNFα in sleep regulation we phenotyped sleep and responses to sleep deprivation of mice lacking both the IL-1β receptor 1 and TNFα receptor 1 (IL-1R1/TNFR1 KO). Male adult mice (IL-1R1/TNFR1 KO, n = 14; B6129SF2/J, n = 14) were surgically instrumented with EEG electrodes and with a thermistor to measure brain temperature. After recovery and adaptation to the recording apparatus, 48 h of undisturbed baseline recordings were obtained. Mice were then subjected to 6 h sleep deprivation at light onset by gentle handling. IL-1R1/TNFR1 KO mice spent less time in NREMS during the last 6 h of the dark period and less time in rapid eye movement sleep (REMS) during the light period. There were no differences between strains in the diurnal timing of delta power during NREMS. However, there were strain differences in the relative power spectra of the NREMS EEG during both the light period and the dark period. In addition, during the light period relative power in the theta frequency band of the REMS EEG differed between strains. After sleep deprivation, control mice exhibited prolonged increases in NREMS and REMS, whereas the duration of the NREMS increase was shorter and there was no increase in REMS of IL-1R1/TNFR1 KO mice. Delta power during NREMS increased in both strains after sleep deprivation, but the increase in delta power during NREMS of IL-1R1/TNFR1 KO mice was of greater magnitude and of longer duration than that observed in control mice. These results provide additional evidence that the IL-1β and TNFα cytokine systems play a role in sleep regulation and in the alterations in sleep that follow prolonged wakefulness.
Keywords: REM sleep, NREM sleep, Sleep deprivation, Delta power, Theta power, IL-1β, TNFα
1. Introduction
Cytokines are a family of proteins known best as signaling molecules involved in responses to, and regulation of, local and systemic inflammatory responses. Although most cytokines have been discovered in the peripheral immune system, the presence of several cytokines and their receptors within the central nervous system (CNS) has been widely demonstrated (Breder et al., 1988; Benveniste, 1992; Schöbitz et al., 1994; Eriksson et al., 2000). We now know cytokines are involved in multiple central nervous system (CNS) processes (Dantzer, 1994; Krueger et al., 2001; Dantzer, 2006).With respect to the regulation of sleep, two cytokines, interleukin-1β (IL-1β) and tumor necrosis factor α (TNFα), have been studied extensively. Data demonstrate these cytokines are involved in the regulation of normal, physiological non-rapid eye movement sleep (NREMS) in the absence of immune challenge (Krueger et al., 2001; Opp, 2005).
Neurons immunoreactive for IL-1β and TNFα and their receptors are located in brain regions implicated in the regulation of sleep-wake behavior (Breder et al., 1988, 1993). In several species, central or peripheral administration of these cytokines increases the amount of time animals spend in NREMS (Krueger et al., 2001; Toth and Opp, 2002) and enhances EEG slow oscillations (<5 Hz) (Opp and Imeri, 2001), often referred to as delta power. Delta power during NREMS is widely regarded as a measure of the intensity or depth of sleep (Borbély, 1982). In addition, prolonged wake-fulness (sleep deprivation) is associated with enhanced production of IL-1β and TNFα (Mackiewicz et al., 1996; Taishi et al., 1998). Interfering with the normal actions of IL-1β or TNFα by inhibiting or inactivating these cytokines with antibodies, receptor antagonists or soluble receptors reduces spontaneous NREMS (Opp et al., 1992; Opp and Krueger, 1994a; Takahashi et al., 1996b,c).
The molecular steps by which IL-1β and TNFα contribute to the regulation of NREMS are still not fully understood. Two types of membrane receptors have been characterized for each of these cytokines. IL-1β signals through the type 1 receptor (IL-1R1), whereas the IL-1 type 2 receptor lacks an intracellular domain, does not signal, and acts as a decoy receptor (Colotta et al., 1993; Sims et al., 1993). The extracellular domains of the two TNFα receptors share significant sequence identity, whereas their intracellular domains exhibit striking structural differences reflecting different signaling pathways and functions (Hohmann et al., 1990; Holtmann and Neurath, 2004).
Studies of receptor knockout (KO) mice indicate that NREMS of mice lacking the IL-1R1 is not altered by administration of IL-1β. However, these same animals do spend more time in NREMS when given TNFα (Fang et al., 1998). IL-1R1 KO mice also spend less time in spontaneous NREMS during the dark period than do strain-control mice (Fang et al., 1998). Similarly, mice lacking the p55 (type 1) receptor for TNFα (TNFR1) do not exhibit excess NREMS if given exogenous TNFα. These mice do, however, have robust NREMS responses to IL-1β (Fang et al., 1997). Spontaneous NREMS and REMS of TNFR1 KO mice is reduced during the light period, although sleep during the dark period appears normal (Fang et al., 1997). These data suggest that IL-1β and TNFα might differentially contribute to sleep regulation since each of these KO strains has reduced NREMS during different times of the day. To further investigate roles for, and mutual influences of, the IL-1β and TNFα systems in sleep regulation, we characterized in this study sleep and responses to sleep deprivation in a strain of mice lacking both IL-1R1 and TNFR1 (IL-1R1/TNFR1 KO).
2. Methods
2.1. Animals
Breeding pairs of 129S-Tnfrsf1atmiImxIl1r1tmiImx/J(IL-1R1/TNFR1 KO) were purchased from the Jackson Laboratory (Bar Harbor, ME). A breeding colony was established at the University of Michigan under the supervision of the Unit for Laboratory Animal Medicine. IL-1R1/ TNFR1 KO mice are homozygous for both Tnfrsf1atmiIm (formerly Tnfr1tmiIm, p55 deficient) and Il1r1tmiImx targeted mutations and they do not express IL1-R1 or TNFR1. The KO strain was derived from a mixed C57BL/6 × 129 background. Control mice were F2 hybrids derived from C57BL/6J × 129S1/SvImJ crossing. Although these animals are an approximate genetic match for the IL-1R1/TNFR1 KO mice, they are considered by the mouse geneticists at the Jackson Laboratory to be suitable controls and have been used as control animals in studies using the IL-1R1/TNFR1 KO mice (Chen et al., 1999; Brito et al., 1999; Moreland et al., 2001; Lucey et al., 2002).
Adult male (25–30 g at time of surgery; n = 14) IL-1R1/TNFR1 KO mice were brought from the breeding colony to the recording room one week prior to surgery. The B6129SF2/J control mice (n = 14) were purchased from the Jackson Laboratory. These mice were also housed in the recording room for a minimum of one week prior to surgery. All mice were individually housed in standard cages and kept in temperature controlled chambers at 29 ± 1 °C, a temperature approximately in the middle of the thermoneutral zone of mice (Gordon and White, 1985; Rudaya et al., 2005). Lights were maintained on a 12:12 h light–dark cycle and food and water were provided ad libitum. All the procedures involving the use of animals were approved by the University of Michigan Committee on Care and Use of Animals in accordance with the US Department of Agriculture Animal Welfare Act, and the Public Health Service policy on Humane Care and Use of Laboratory Animals.
2.2. Surgical procedure
Under deep general anesthesia (isoflurane: 4% induction, 2% maintenance) mice were implanted epidurally with three stainless steel screws placed over frontal and parietal brain cortices (relative to bregma, Frontal: AP, 1.0; DL, 1.0; Parietal: AP, −2.5; DL, 1.5). These screws served as electrodes for electroencephalographic (EEG) recording. A calibrated 10-kΩ thermistor (AB6E3-GC16KA103L, Thermometrics, CA) was also implanted between the dura mater and the skull over the parietal cortex to measure cortical brain temperature (Tbr). The leads from the electrodes and the thermistor were soldered to the pins of a plastic connector that was embedded in dental acrylic. The surgical wound was treated with a topical analgesic ointment (L.M.X.4, Lidocaine 4%, Ferndale IP, Inc., MI) and a triple antibiotic ointment (Neomycin and Polymixin B sulfates and Bacitracin Zinc, E. Fougera & Co., NY). The animals were allowed at least 12 days of recovery after the surgical procedures. On the fifth post-surgical day, the mice were connected to the recording apparatus via a flexible tether for habituation.
2.3. Recording apparatus
Signals from the EEG electrodes were amplified (factor of 5000) and filtered (line filter: Notch type, 60 Hz; low frequencies filter: −6 db, 0.3 Hz; high frequencies filter: −6 db, 30 Hz) by means of commercially available amplifiers (Grass Telefactor, West Warwick, RI; model P511AC). The signal from the thermistor was amplified by means of a DC powered custom made bridge (Intec, UK). The output from all thermistors was calibrated prior to implantation using a water bath and a precision digital thermometer. Motor activity was detected using an infrared sensor housed in an observation unit that also contained a camera (BioBserve, GmbH, Bonn, Germany). As such mice could be observed during both the light and dark periods by means of a closed-circuit video system. Movements detected by the infrared sensor were converted to a voltage output, the magnitude of which was directly related to the magnitude of movements detected. All the signals were subjected to analog-to-digital conversion (A/D board: PCI:3033E,National Instruments, TX) with 16-bit precision at a sampling rate of 128 Hz for EEG, 1 Hz for Tbr and 8 Hz for motor activity. The digitized EEG waveform, Tbr samples, and integrated values for motor activity were stored as binary files until further processing.
Determination of arousal state was made by visual scoring of EEG, body movements and brain temperature in 10-s epochs using custom software (ICELUS, M. Opp, University of Michigan) written in LabView for Windows (National Instruments, TX). The EEG signal was subjected to fast Fourier transformation (FFT), yielding power spectra between 0.5 and 30 Hz in 0.5-Hz frequency bins. EEG spectral frequency bands of interest were defined as delta (0.5–4.5 Hz) and theta (6.0–9.0 Hz). Arousal state was classified as previously described as NREMS, rapid eye movement sleep (REMS), or WAKE on the basis of state-dependent changes in multiple parameters, including the EEG, body movements and brain temperature (Opp and Krueger, 1994a; Opp, 1998). Briefly, wakefulness was defined on the basis of a low-amplitude, mixed-frequency (delta, theta) EEG accompanied by body movements. Increases in brain temperature occur as a function of activity. NREM sleep was identified by an increased absolute EEG amplitude, integrated values for the delta frequency band greater than those for theta, and lack of body movements. Upon entry into NREMS, there is a regulated decrease in brain temperature until it reaches an asymptote. REM sleep was characterized by a low-amplitude EEG, with integrated values for the delta frequency band less than those for the theta frequency band. There is a rapid increase in brain temperature at the transition from NREMS to REMS. During the assignment of arousal state, any epoch containing movement artifact or electrical noise was tagged and excluded from subsequent spectral analyses. Sleep architecture was also determined on the basis of previously defined criteria that were adapted to the 10-s epochs used in this study (Opp, 1998). The criteria used to determine sleep architecture included a minimum number of consecutive epochs spent in a given behavioral state. Thus, brief (<20-s) episodes of NREMS, REMS, or WAKE were not counted as bouts.
Spectral characteristics of the EEG were further analyzed offline. We used FFT to obtain absolute EEG power spectra for each animal and each behavioral state. Power spectra were computed for the 5 consecutive 2 s segments of the artifact-free EEG that comprised the 10-s scoring epoch. These 5 spectra were averaged to provide 1 spectrum for the entire 10-s epoch. Spectra from artifact-free epochs were then averaged for each hour within the states of NREMS, REMS, or WAKE. Because of the inherent differences between animals in EEG signals, hourly average state-specific spectra were subjected to a normalization procedure. To obtain a reference value, total state-specific power was computed from the averaged spectra by summing across all frequency bins from 0.5 to 30 Hz. Separate reference values were computed from average spectra obtained during the 12 h light and 12 h dark period. Relative EEG power density in each frequency bin was then expressed as a percentage of the reference EEG power across all the frequency bins for that behavioral state during that portion of the light:dark cycle. This is a modification of a method that has been previously used for analysis of strain differences in EEG power (Franken et al., 1998).
Delta power during NREMS was also determined within each hour. In this case, the power density values from the frequency bins encompassing 0.5–4.5 Hz range for every artifact-free epoch scored as NREMS were averaged.
2.4. Experimental protocol
After recovery from surgery and adaptation to the recording apparatus, two 24 h undisturbed baseline recordings were obtained. After this 48 h recording period mice were sleep deprived for 6 h beginning at light onset. Sleep deprivation was achieved by gentle handling. Laboratory personnel constantly observed the mice and whenever behavioral signs of sleep were observed novel objects were introduced to the animals or acoustic and tactile stimuli were given. A subsequent undisturbed 18 h period was recorded in order to assess the dynamics of sleep recovery in these mouse strains.
2.5. Statistical analysis
Results are presented as means ± SEM. Tests for statistical significance were performed using SPSS for Windows. Between-strain comparisons for time spent in each behavioral state, motor activity, Tbr and delta power during NREMS were carried out by means of a mixed model analysis for repeated measures, using a first-order regression covariance structure where time (hours) was used as repeated measure. Between-strain comparisons of power spectra for each behavioral state were performed by means of a univariate general linear model analysis with strain and frequency bin as fixed effects and power in each frequency bin as the dependent variable. Within-strain comparisons for time spent in each behavioral state, motor activity, Tbr and delta power during NREMS were performed by means of a general linear model for repeated measures. For time spent in each behavioral state, motor activity, Tbr and delta power during NREMS, both between- and within-strain comparisons were performed on 6 h time blocks.
3. Results
3.1. Spontaneous sleep-wake behavior
Baseline recordings were analyzed to determine spontaneous sleep-wake behavior of IL-1R1/TNFR1 KO and B6129SF2/J mice. Due to technical problems with the miniature thermistors we were able to acquire Tbr signals only from half of the animal pool resulting in sample sizes for this parameter of n = 7 for B6129SF2/J and n = 7 for the IL-1R1/TNFR1 KO. There were no significant differences among any parameters obtained during the two baseline recording days within experimental groups, and as such the data for the two 24 h periods were averaged to provide control values.
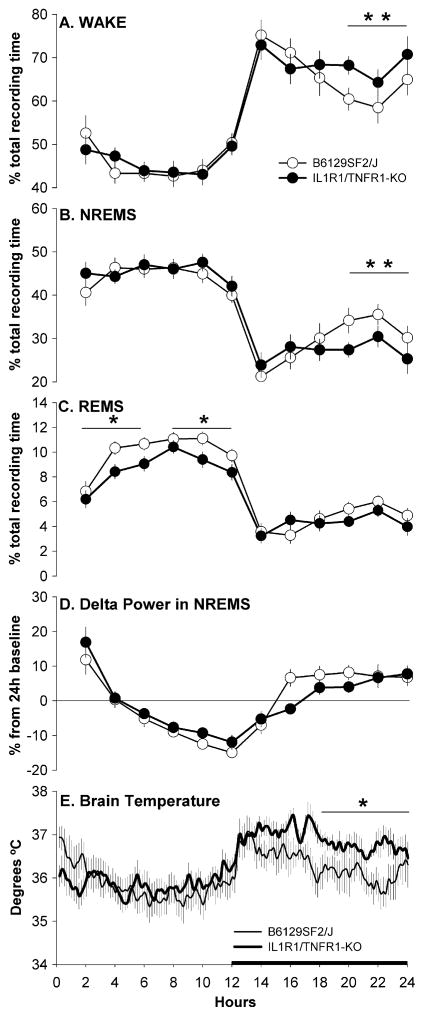
During undisturbed baseline periods, both strains of mice showed clear diurnal variation in sleep-wake behavior spending less time in wakefulness and more time in both NREMS and REMS during the light phase than during the dark phase of the 24 h cycle (Table 1 and Fig. 1A–C). Despite the fact that the amount of time spent in wake-fulness across the 24 h was similar between strains (Table 1), IL-1R1/TNFR1 KO mice were awake more than control mice during the last 6 h of the dark period (Fig. 1A). Significant differences between the two strains were not detected in the 24 h amount of NREMS or in the frequency or duration of NREMS bouts (Table 1). However IL-1R1/ TNFR1 KO mice spent significantly less time in NREMS during the last 6 h of the dark period (Fig. 1B). Across the 24 h, IL-1R1/TNFR1 KO mice spent less time in REMS than B6129SF2/J control mice (Table 1). The reduction in REMS expression was limited to the 12 h light period (Fig. 1C) and the analysis of sleep architecture revealed that this difference was due to fewer REMS bouts rather than a reduced REMS bout duration (Table 1). No differences were detected between strains in the number of transitions from one vigilance state to another (Table 1), indicating that lack of these cytokine receptors did not alter normal consolidation of sleep-wake behavior in these animals.
Table 1.
Sleep parameters determined from B6129SF2/J (n = 14) and IL-1R1/TNFR1 KO mice (n = 14) during undisturbed baseline conditions
| Sleep parameter and mouse strain | Light period | Dark period | Total 24-h period |
|---|---|---|---|
| Wake duration (% recording time) | |||
| B6129SF2/J | 46.0 ± 1.5 | 66.0 ± 2.2 | 56.0 ± 1.5 |
| IL-1R1/TNFR1 KO | 46.0 ± 1.4 | 68.7 ± 2.0 | 57.3 ± 1.5 |
| Wake bouts (number/h) | |||
| B6129SF2/J | 8.8 ± 0.5 | 5.2 ± 0.4 | 7.0 ± 0.3 |
| IL-1R1/TNFR1 KO | 8.3 ± 0.6 | 4.8 ± 0.3 | 6.5 ± 0.4 |
| Wake bout duration (min) | |||
| B6129SF2/J | 3.9 ± 0.3 | 13.7 ± 1.5 | 8.8 ± 0.7 |
| IL-1R1/TNFR1 KO | 4.5 ± 0.6 | 16.0 ± 1.9 | 10.3 ± 1.0 |
| NREMS duration (% recording time) | |||
| B6129SF2/J | 44.0 ± 1.6 | 29.4 ± 2.0 | 36.7 ± 1.7 |
| IL-1R1/TNFR1 KO | 45.3 ± 1.3 | 27.0 ± 1.6 | 36.2 ± 1.4 |
| NREMS bouts (number/h) | |||
| B6129SF2/J | 12.0 ± 0.8 | 7.2 ± 0.7 | 9.6 ± 0.7 |
| IL-1R1/TNFR1 KO | 12.9 ± 0.6 | 7.4 ± 0.5 | 10.2 ± 0.4 |
| NREMS bout duration (min) | |||
| B6129SF2/J | 2.1 ± 0.2 | 2.1 ± 0.2 | 2.1 ± 0.2 |
| IL-1R1/TNFR1 KO | 2.2 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.1 |
| REMS duration (% recording time) | |||
| B6129SF2/J | 9.9 ± 0.3 | 4.6 ± 0.3 | 7.3 ± 0.2 |
| IL-1R1/TNFR1 KO | 8.6 ± 0.4* | 4.2 ± 0.4 | 6.5 ± 0.3* |
| REMS bouts (number/h) | |||
| B6129SF2/J | 5.4 ± 0.4 | 2.8 ± 0.5 | 4.1 ± 0.4 |
| IL-1R1/TNFR1 KO | 4.4 ± 0.2* | 2.4 ± 0.2 | 3.4 ± 0.2 |
| REMS bout duration (min) | |||
| B6129SF2/J | 1.3 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.1 |
| IL-1R1/TNFR1 KO | 1.2 ± 0.0 | 0.9 ± 0.1 | 1.0 ± 0.0 |
| Transitions (number/h) | |||
| B6129SF2/J | 40.1 ± 0.6 | 24.1 ± 2.3 | 32.1 ± 1.6 |
| IL-1R1/TNFR1 KO | 38.8 ± 2.8 | 22.7 ± 1.6 | 30.8 ± 1.9 |
Values are means ± SEM. Single asterisk (*) indicates a statistical difference of p < 0.05.
Fig. 1.
Percentage of recording time spent in Wake (A) NREMS (B) and REMS (C); EEG delta power (0.5–4.0 Hz) during artifact-free epochs of NREMS (D); brain temperature (E). Values were obtained from B6129SF/J control mice (sleep n = 14, open symbols; brain temperature n = 7, thin line) and IL-1R1/TNFR1 KO mice (sleep n = 14, filled symbols; brain temperature n = 7, thick line) during undisturbed baseline recordings. For visual clarity, data (means ± SEM) for sleep parameters are presented in 2 h intervals whereas brain temperature data are presented in 10 min intervals. Values for EEG delta power during NREMS (D) are presented as percentage from the 24 h mean (depicted by the zero line). Statistics were performed on 6 h time blocks. Single asterisk (*) indicates a statistical difference of p < 0.05 whereas double asterisk (**) indicates a statistical difference of p < 0.01. The black bar on the X-axis indicates the dark portion of the light–dark cycle.
As observed for arousal state, Tbr exhibited a normal diurnal variation in both strains, with lower temperatures during the light phase and higher temperatures during the dark phase of the light–dark cycle (Fig. 1E). Tbr of IL-1R1/TNFR1 KO mice during the dark period were higher than those of B6129SF2/J control mice, reaching a statistically significant difference during the last 6 h of the dark period (Fig. 1E).
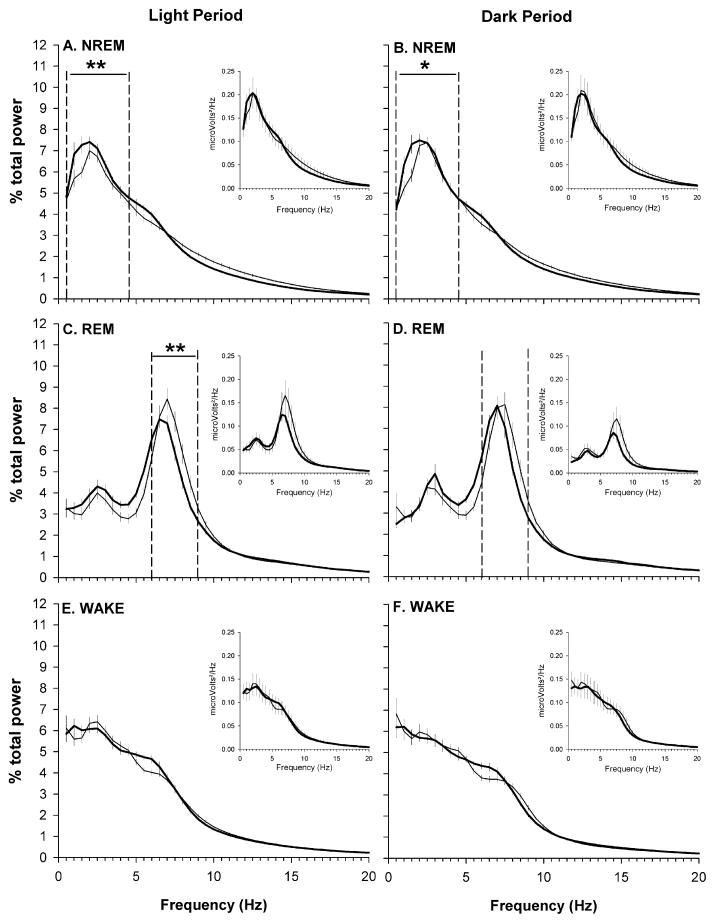
The EEG was analyzed for both strains. EEG delta power (0.5–4.5 Hz) during NREMS exhibited a diurnal rhythm in both IL-1R1/TNFR1 KO and B6129SF2/J mice (Fig. 1D). There were no differences in the diurnal timing of delta power during NREMS. Absolute spectra (0.5–30 Hz) within each behavioral state did not differ between strains (Fig. 2, inset panels). However, because there is inherent variability in the EEG signal between animals which may mask biologic differences, we also analyzed relative power spectra after a normalization procedure (see Section 2). Analysis of normalized spectra revealed that NREM-specific spectra differ in frequency distribution between IL-1R1/TNFR1 KO mice and B6129SF2/J mice. These differences in relative power spectra during NREMS were apparent during both the light period and the dark period (Fig. 2A and B). Relative to control mice, the contribution of the slower frequencies to the total power of NREM power spectra was greater in IL-1R1/TNFR1 KO mice, specifically in the frequency range defining delta power (0.5–4.5 Hz) (Fig. 2A and B). REMS-specific spectra during the light period also differed between strains. The frequencies in the range defining theta power (6–9 Hz) contribute less to the total power in IL-1R1/TNFR1 KO mice as compared to B6129SF2/J mice (Fig. 2C). No other relative power spectra differed between strains (Fig. 2D–F).
Fig. 2.
State-specific EEG power spectra obtained from undisturbed B6129SF2/J control mice (n = 14, thin line) and IL-1R1/TNFR1 KO mice (n = 14, thick line) during both the light (A, C and E) and the dark (B, D and F) periods of the 24 h light–dark cycle. Spectral analyses were conducted on frequencies from 0.5 to 30 Hz, but graphic presentation is limited to frequencies from 0.5 to 20 Hz. Plots depict absolute spectra (insets) or normalized spectra. Spectra were normalized as a percentage of total power across all frequencies for specific behavioral states within the 12 h light or dark period (see Section 2), and are plotted as mean ± SEM for each frequency bin. Statistical analyses were performed on bins in the delta (0.5–4.5 Hz) and theta (6.0–9.0 Hz) frequency bands for NREMS and REMS, respectively. A single asterisk (*) indicates a statistical difference of p < 0.05, whereas double asterisks (**) denote statistical differences of p < 0.01.
3.2. Responses to sleep deprivation
Sleep deprivation was effective. During the 6 h deprivation period, B6129SF2/J mice spent on average 2.9 ± 1.4 min in NREMS and IL-1R1/TNFR1 KO mice 2.9 ± 0.8 min in NREMS. No mouse of either strain entered REMS during the 6 h deprivation period.
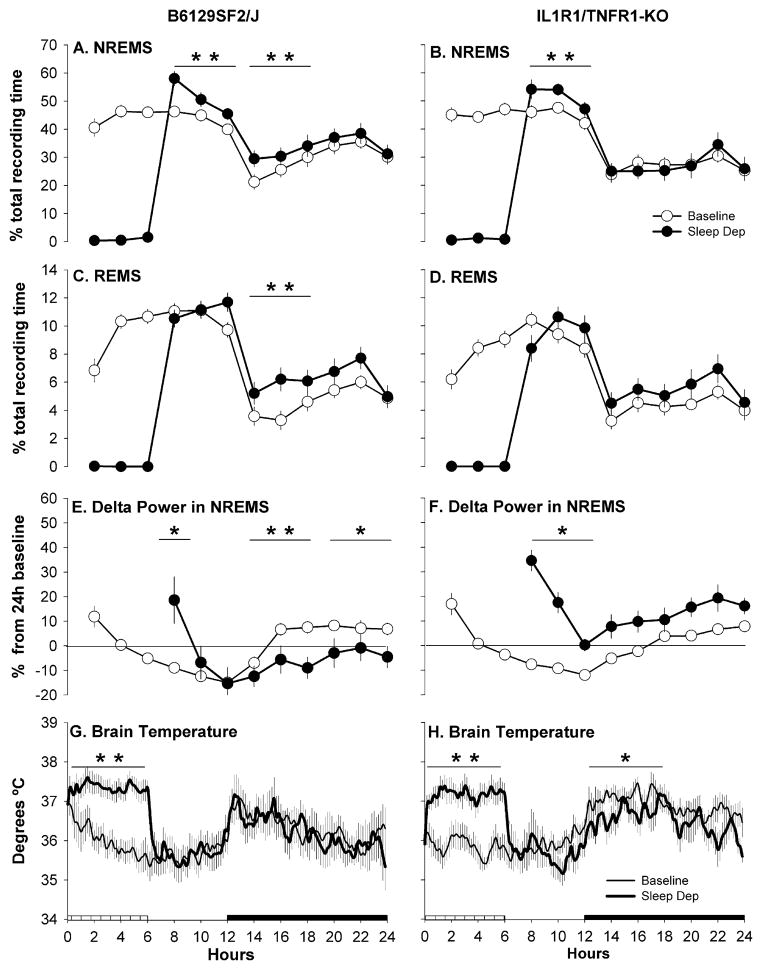
B6129SF2/J mice responded to sleep deprivation with a rebound in NREMS, REMS and EEG delta power during NREMS (Figs. 3 and 4). The increase in NREMS was apparent during the 6 h remainder of the light period and during the first 6 h of the dark period (Fig. 3A), whereas REMS was enhanced only during the subsequent dark period. REMS was increased during the first 6 h of the dark period after sleep deprivation, and there was a tendency for increased REMS during the entire 12 h dark period (Fig. 3C). The rebound in delta power during NREMS in B6129SF2/J mice was observed immediately after sleep deprivation. The dynamic of this response was particularly quick with the sleep deprivation-induced increase in EEG delta power during NREMS dissipating within the first 2 h of the recovery period (Fig. 3E). During the subsequent dark period a significant undershoot (negative rebound) in delta power during NREMS occurred. Tbr of B6129SF2/J mice recorded during the 18 h recovery period following sleep deprivation did not differ from that recorded during corresponding baseline conditions (Fig. 3G).
Fig. 3.
Percentage of recording time spent in NREMS (A and B) and REMS (C and D); EEG delta power (0.5–4.0 Hz) during artifact-free epochs of NREMS (E and F); brain temperature (G and H). Values were obtained from B6129SF/J control mice (sleep n = 14; brain temperature n = 7: right column) and IL-1R1/TNFR1 KO mice (sleep n = 14; brain temperature n = 7: left column) during undisturbed baseline recordings (open symbols) and during and after sleep deprivation (filled symbols). For visual clarity, data (means ± SEM) for sleep parameters are presented as in 2 h intervals whereas brain temperature data are presented in 10 min intervals. Values for EEG delta power during NREMS (E and F) are presented as percentage from the 24 h average baseline value (depicted by the zero line). Statistics were performed on 6 h time blocks. Single asterisk (*) indicates a statistical difference of p < 0.05 whereas double asterisk (**) indicates a statistical difference of p < 0.01. The crosshatched bar on the X-axis indicates the sleep deprivation period whereas the black bar indicates the dark portion of the light–dark cycle.
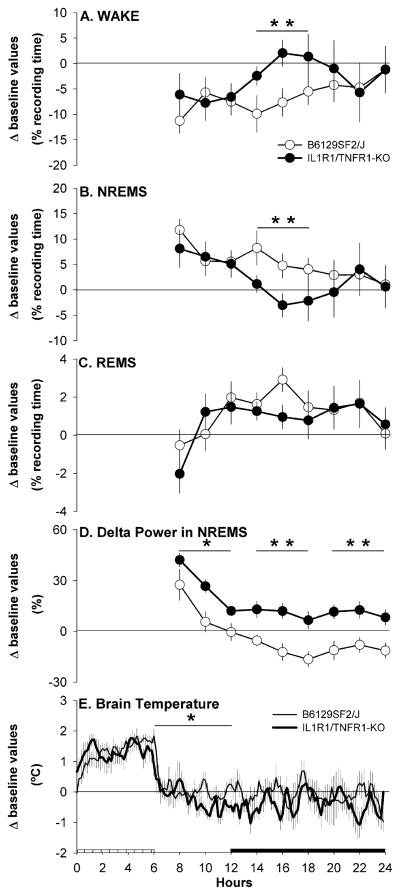
Fig. 4.
Mouse strain differences from baseline values (depicted by the zero lines) after sleep deprivation in: percentage time spent in Wake (A) NREMS (B) and REMS (C); EEG delta power (0.5–4.0 Hz) during artifact-free epochs of NREMS (D); brain temperature (E). Values were obtained from B6129SF/J control mice (sleep n = 14, open symbols; brain temperature n = 7, thin line) and IL-1R1/TNFR1 KO mice (sleep n = 14, filled symbols; brain temperature n = 7, thick line). For visual clarity, data (means ± SEM) for sleep parameters are presented in 2 h intervals as whereas brain temperature data are presented in 10 min intervals. Statistics were performed on 6 h time blocks. Single asterisk (*) indicates a statistical difference of p < 0.05 whereas double asterisk (**) indicates a statistical difference of p < 0.01. The crosshatched bar on the X-axis indicates the sleep deprivation period whereas the black bar indicates the dark portion of the light–dark cycle.
IL-1R1/TNFR1 KO responded to sleep deprivation with increases in NREMS and in delta power during NREMS (Figs. 3 and 4). However, in contrast to B6129SF2/J mice, IL-1R1/TNFR1 KO mice did not exhibit a REMS rebound after sleep deprivation (Fig. 3D). The increase in NREMS expression was limited to the first 6 h after sleep deprivation; there were no significant differences from baseline values in the percentage of recording time that IL-1R1/TNFR1KO mice spent in NREMS during the remainder of the post-deprivation recording period (Fig. 3B). The sleep deprivation-induced increase in delta power during NREMS dissipated during the first 6 h period following the sleep deprivation. During the subsequent dark period, delta power values tended to be greater than during comparable control periods, but this increase in delta power did not achieve statistical significance (Fig. 3F). Although there was a tendency for reduced Tbr in IL-1R1/TNFR1KO mice for the entire 18 h post-deprivation period, Tbr differed significantly from corresponding baseline values only during the first 6 h of the dark period (Fig. 3H).
To directly compare the impact of sleep deprivation on subsequent sleep-wake behavior of B6129SF2/J and IL-1R1/TNFR1KO mice, differences were calculated from each strain’s baseline values for Wake, NREMS, REMS, delta power during NREMS and Tbr (Fig. 4). Differences in the amount of time spent awake and in NREMS between the two strains were apparent during the first 6 h of the dark period, with IL-1R1/TNFR1KO mice spending more time awake and less time in NREMS (Fig. 4A and B). No differences between the two strains were detected in REMS during the period after sleep deprivation (Fig. 4C). Analysis of delta power during NREMS indicated a clear difference in the dynamic of the rebound between the two strains. The rate of dissipation of delta power was similar for both the control and IL-1R1/ TNFR1 KO mice showing a progressive decrease across the 18 h post-deprivation. However delta power during NREMS was significantly higher in IL-1R1/TNFR1KO mice compared to B6129SF2/J mice for the duration of the recovery period (Fig. 4D). Tbr values were significantly lower in IL-1R1/TNFR1KO mice with respect to the control strain during the 6 h period immediately following sleep deprivation (Fig. 4E).
4. Discussion
Results of this study demonstrate a unique sleep phenotype in mice lacking both IL-1β type 1 receptor and the TNFα type 1 receptor. Relative to control mice, these IL-1R1/TNFR1KO mice spend less time in NREMS during the dark period and less time in REMS during the light period. Furthermore, the increase in NREMS duration of IL-1R1/TNFR1 KO mice after sleep deprivation is attenuated, providing additional evidence in support of the role of endogenous IL-1β and TNFα in the regulation of sleep-wake behavior (Krueger et al., 2001; Opp, 2005). Nevertheless, the lack of both IL-1R1 and TNFR1 results in a sleep phenotype that differs from expected on the basis of sleep of mice lacking one or the other of these cytokine receptors. Because IL-1R1 KO mice spend less time in NREMS during the dark period (Fang et al., 1998), whereas TNFR1 KO mice spend less time in both NREMS and REMS during the light period (Fang et al., 1997), we expected the IL-1R1/TNFR1 KO mice to exhibit less NREMS and REMS during both light and dark periods. However, the IL-1R1/TNFR1 KO mice spend less time in REMS only during the 12 h of the light period and less time in NREMS only during the last 6 h of the dark period.
These differences from the hypothesized sleep phenotype of the IL-1R1/TNFR1 KO mice also were unexpected because IL-1β and TNFα induce each other’s production, and the simultaneous inhibition of both of these cytokines results in slightly greater sleep loss than inhibition of either one alone (Takahashi et al., 1999). We cannot exclude the possibility that the limited difference in NREMS between the two strains might be due to the alterations of other systems during development, i.e. developmental compensation. B6129SF2/J mice were chosen as the control strain since IL-1R1/TNFR1 KO mice were derived from a mixed C57BL/6 × 129 background. However, the genetic composition of animals in which genes of interest have been ablated differs from the genetic composition of a strain produced by crossing two lines (Gerlai, 1996; Gingrich and Hen, 2000). Furthermore, the consequences of lack of the IL-1R1 and TNFR1 genes during development may differ from responses elicited by the acute blockade of IL-1β and/or TNFα receptors, since animals might have developed compensatory mechanism for the loss of the two receptors. The possible developmental compensation for the lack of the IL-1R1 and TNFR1 genes seems likely because sleep is a fundamental behavior that is regulated by redundant and interacting biochemical and neuroanatomical pathways. However, these caveats aside, the use of animals in which genes have been genetically ablated has proven to be a powerful tool in determining the genetic basis of complex CNS processes and behavior (see Gerlai, 1996 for review) including sleep (e.g. Tobler et al., 1996; Chemelli et al., 1999).
The decrease in REMS of our IL-1R1/TNFR1 KO mice is consistent with that observed in TNFR1 KO mice (Fang et al., 1997) and in mice lacking TNFR2 or the TNF ligand (Deboer et al., 2002). Although little is known about mechanisms underlying the interaction between cytokines and the regulation of REMS, multiple studies demonstrate that the administration of cytokines such as IL-1, IL-2, IL-15, IL-18 or TNFα inhibits REMS (see Opp, 2005 for review). Conversely, antagonizing endogenous cytokines in healthy animals with receptor antagonist, soluble receptors or antibodies either has no effect on REMS (Opp and Krueger, 1994a; Takahashi et al., 1995a, 1997) or only slightly reduces REMS (Takahashi et al., 1995b). It has been previously hypothesized that the reduction in REMS of TNFR1 KO mice (Fang et al., 1997) could result indirectly from the concomitant reduction in NREMS; reductions in NREMS limit the potential to enter REMS because normally REMS is entered from NREMS. Although results of this study do not provide information with respect to mechanisms by which cytokines alter REMS, our data demonstrate that mice lacking signaling receptors for IL-1β and TNFα spend less time in REMS, and that these changes in REMS are not temporally related to alterations in NREMS. The fact that reductions in REMS of the IL-1R1/TNFR1 KO mice are independent from reductions in NREMS is consistent with previous observations by Deboer et al. (2002) of mice lacking TNFR2 or the TNF ligand. Collectively, these data suggest that IL-1β and TNFα are differentially involved in the regulation of REMS and NREMS.
The responses to sleep deprivation in B6129SF2/J mice are similar to those previously reported for C57BL/6J or 129 mouse strains (Franken et al., 1999; Huber et al., 2000; Deboer et al., 2002; Morrow and Opp, 2005; Jhaveri et al., 2007). Relative to the control strain, IL-1R1/TNFR1 KO mice exhibited a reduced recuperative response to sleep loss with a shorter increase in NREMS and no increase in REMS. These results implicate IL-1β and TNFα systems in alterations in sleep amounts that follow prolonged wake-fulness, and are in agreement with previous observations that increases in sleep observed after sleep deprivation are attenuated if animals are pretreated with inhibitors of these two cytokines (Opp and Krueger, 1994a,b; Takahashi et al., 1996a, 1997). The fact that IL-1R1/TNFR1 KO mice had a short rebound in NREMS was not unexpected because of the redundancy of sleep control mechanisms. Substances implicated in sleep following prior wakefulness, such as adenosine or nitric oxide (NO), are released during wakefulness as the product of neuronal activity. Although IL-1β and TNFα increase the synthesis of these transmitters, the release of adenosine and NO is not strictly dependent upon the activation of these two cytokine receptors (Zhu et al., 2006). For example, TNFR2 signaling activates NF-κB (Hohmann et al., 1990; Holtmann and Neurath, 2004), which also promotes production of other substances that can lead to synthesis of NO, such as for example cyclo-oxygenase-2 (COX-2) (Tsai et al., 2002). COX-2 is involved in the production of prostaglandin D2 (PGD2), and nitric oxide synthase-2 (Xie et al., 1994), which have been implicated as mediators of IL-1-induced alterations in NREMS. Mice lacking TNFR2 exhibit a rebound in delta power during NREMS after sleep deprivation (Deboer et al., 2002). As such, it is not possible at this time to categorically exclude TNFR2 as a mediator of effects of TNFα on sleep.
No differences between the two strains of mice were detected in the diurnal timing of delta power during NREMS during undisturbed spontaneous sleep. However, analysis of relative power spectra revealed that in IL-1R1/ TNFR1 KO mice frequencies in the range defining delta power contribute to NREMS total power to a greater extent than observed in control mice. Delta power during NREMS is now widely accepted as an indicator of the depth or quality of sleep (Borbély, 1982). The relative increase in delta power frequencies during NREMS of IL-1R1/TNFR1 KO mice suggests that the reduction in NREMS duration during the dark period could be compensated by NREMS of greater intensity. It is also possible that NREMS of the IL-1R1/TNFR1 KO mice during the light period is of greater intensity than that of control mice, which could also contribute to compensation for reduced NREMS duration during the dark period. In contrast, analysis of the REMS power spectrum revealed that the theta power frequency range contributes less to total power of this spectrum in IL-1R1/TNFR1 KO mice than in control mice. This reduction in relative theta power during REMS is apparent only during the light period, which is the time of the day during which IL-1R1/TNFR1 KO mice spend less time in REMS compared to B6129SF2/J. Collectively, data from this study suggest that the lack of IL-1R1 and TNFR1affects both the quantity and quality of both NREMS and REMS.
After sleep deprivation, delta power during NREMS in both mouse strains increased. The alterations in delta power during NREMS of B6129SF2/J mice after sleep deprivation at light onset in this study are similar to those previously reported for other mouse strains (Franken et al., 1999; Huber et al., 2000; Deboer et al., 2002; Morrow and Opp, 2005; Jhaveri et al., 2007); there is an initial increase in delta power during NREMS immediately after sleep deprivation, which is followed by a significant reduction in delta power during NREMS during the subsequent dark period. In IL-1R1/TNFR1 KO mice, the sleep deprivation-induced increase in delta power during NREMS is greater and more prolonged compared to control mice. A similar response has been previously described in rat when the IL-1 system is targeted by intracerebroventricular (ICV) administration of anti-IL-1 antibodies; EEG power density values are twice those recorded after sleep deprivation and ICV injection of vehicle (Opp and Krueger, 1994a). Because delta power during NREMS is a measure of the depth of sleep, the prolonged increase in this sleep parameter observed after sleep deprivation suggests that IL-1R1/ TNFR1 KO mice might recover NREMS loss by sleeping more intensely rather than by spending more time in NREMS. This dissociation between NREMS amount and delta power during NREMS after sleep deprivation is consistent with previous observations (Kapás et al., 1996; Yasuda et al., 2005) and suggests that the regulation of NREMS is independent, in part, from the regulation of EEG slow wave activity characterizing delta power.
The effects of IL-1β and TNFα on delta power are not well understood. IL-1β increases the amplitude of EEG slow waves during NREMS in rabbit and rat, but these changes are dependent on the dose and on the time of administration (Shoham et al., 1987; Opp et al., 1991). Whereas central administration of TNFα enhances slow wave oscillations during NREMS (Takahashi et al., 1996c, 1997), intraperitoneal injection of TNFα increases NREMS duration without a concomitant increase of EEG delta power (Fang et al., 1997, 1998; Kubota et al., 2002). Moreover, both IL-1β and TNFα enhance EEG delta power in a dose-dependent manner when locally applied to the surface of the cerebral cortex, but do not alter NREMS duration under these conditions (Yoshida et al., 2004, 2005). Taken together, these observations suggest that the effects of these two cytokines on delta power are complex and may be dissociated from the duration of time spent in NREMS. Although additional studies are necessary to determine the exact nature of cytokine regulation/modulation of delta power, data derived from mice lacking signaling receptors for IL-1β and TNFα suggest the different dynamics of delta power during NREMS after sleep deprivation in B6129SF2/J and IL-1R1/TNFR1 KO mice may be due to the actions of these cytokines.
Data derived from in vitro and in vivo studies indicate IL-1β and TNFα alter GABAergic and glutamatergic neu-rotransmission both through Ca2+ mobilization (Casamenti et al., 1999; De et al., 2002, 2003; Zhu et al., 2006) and regulation of cell-surface expression of GABAA and AMPA receptors (Beattie et al., 2000; Stellwagen et al., 2005; Serantes et al., 2006; Lai et al., 2006). The previously published data from Krueger and colleagues using direct application of these cytokines to the cortical surface indicate the actions of IL-1β and TNFα on delta power during NREMS are dose-dependent and biphasic. The modulating action of IL-1β and TNFα on neurotransmission of GABA and glutamate, which is probably lacking in IL-1R1/ TNFR1KO mice, might somehow be responsible for the negative rebound in delta power during NREMS observed during the dark period following sleep deprivation. The demonstration that the GABAergic system and AMPA receptors are involved in the regulation of cortical synchronization of neuronal activity (Steriade et al., 1991; Kim et al., 1997; Bazhenov et al., 1999; Steriade, 2003) provides additional supporting evidence for this hypothesis. Additional studies are necessary to determine if this hypothesis is correct.
Mechanisms regulating the diurnal timing of changes in Tbr do not appear to be altered by genetic ablation of IL-1R1 and TNFR1. During undisturbed baseline recording conditions, increasing Tbr at the transition from the light period to the dark period occur at the same time in both mouse strains. However, the light:dark variation in amplitude of changes in Tbr is greater in IL-1R1/TNFR1 KO mice than in control animals. The increase in amplitude of diurnal changes in Tbr of IL-1R1/TNFR1 KO mice is due to elevated brain temperatures during the dark period. The increases in Tbr of IL-1R1/TNFR1 KO mice during the dark period generally correspond to those periods during which these mice spend more time awake and less time in NREMS. Tbr does not differ between these mouse strains during the light period, during which time the IL-1R1/TNFR1 KO mice spend less time in REMS. As such, it is likely that elevated Tbr of IL-1R1/TNFR1 KO mice during the dark period is activity dependent rather than reflective of a change in mechanisms by which the amplitude of diurnal changes in Tbr is regulated.
Tbr of control mice is not altered during the 18 h post-sleep deprivation period. This lack of impact of sleep deprivation on Tbr is of interest because sleep is increased and wakefulness reduced for 12 h after sleep deprivation. The combination of increased sleep and reduced wakefulness in mice is expected to result in a lower body temperature due to a reduction in activity. In somewhat of a contrast, Tbr of IL-1R1/TNFR1 KO mice is modestly reduced relative to corresponding baseline values during the dark period following sleep deprivation, even though the amount of time spent in NREMS and wakefulness is normal. The potential ramifications of these differences in Tbr of these mice during undisturbed baseline conditions and periods of reduced activity following sleep deprivation are not yet apparent.
In conclusion, lack of signaling receptors for both IL-1R1 and TNFR1 results in a sleep phenotype that differs from expected on the basis of sleep of mice lacking only one of these cytokine receptors. Data presented in this study contribute to the large literature demonstrating a role for IL-1β and TNFα in the regulation of physiological sleep-wake behavior. Furthermore, this study also contributes to the literature indicating IL-1β and TNFα are involved in responses to prolonged wakefulness, in terms of amount of time spent in arousal states, in the cortical EEG as indexed by delta power, and in Tbr.
Acknowledgments
The assistance of Jill Priestley, Ashley Talsma, Sara Waugh and Melissa Olivadoti in sleep depriving mice is gratefully acknowledged. This work was supported, in part, by the National Institutes of Health, HL080972 and GM067189, and by the Department of Anesthesiology.
References
- Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Self-sustained rhythmic activity in the thalamic reticular nucleus mediated by depolarizing GABAA receptor potentials. Nat Neurosci. 1999;2:168–174. doi: 10.1038/5729. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Benveniste EN. Inflammatory cytokines within the central nervous system: sources, function, and mechanisms of action. Am J Physiol. 1992;263:C1–C16. doi: 10.1152/ajpcell.1992.263.1.C1. [DOI] [PubMed] [Google Scholar]
- Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Breder CD, Dinarello CA, Saper CB. Interleukin-1 immuno-reactive innervation of the human hypothalamus. Science. 1988;240:321–324. doi: 10.1126/science.3258444. [DOI] [PubMed] [Google Scholar]
- Breder CD, Tsujimoto M, Terano Y, Scott DW, Saper CB. Distribution and characterization of tumor necrosis factor-α-like immuno-reactivity in the murine central nervous system. J Comp Neurol. 1993;337:543–567. doi: 10.1002/cne.903370403. [DOI] [PubMed] [Google Scholar]
- Brito BE, O’Rourke LM, Pan Y, Anglin J, Planck SR, Rosenbaum JT. IL-1 and TNF receptor-deficient mice show decreased inflammation in an immune complex model of uveitis. Invest Ophthalmol Vis Sci. 1999;40:2583–2589. [PubMed] [Google Scholar]
- Casamenti F, Prosperi C, Scali C, Giovannelli L, Colivicchi MA, Faussone-Pellegrini MS, Pepeu G. Interleukin-1[beta] activates forebrain glial cells and increases nitric oxide production and cortical glutamate and GABA release in vivo: implications for Alzheimer’s disease. Neuroscience. 1999;91:831–842. doi: 10.1016/s0306-4522(98)00680-0. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chen CP, Hertzberg M, Jiang Y, Graves DT. Interleukin-1 and tumor necrosis factor receptor signaling is not required for bacteria-induced osteoclastogenesis and bone loss but is essential for protecting the host from a mixed anaerobic infection. Am J Pathol. 1999;155:2145–2152. doi: 10.1016/S0002-9440(10)65532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F, Re F, Muzio M, Bertini R, Polentarutti N, Sironi M, Giri JG, Dower SK, Sims JE, Mantovani A. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokines and behavior. The Physiologist. 1994;37:A4–A5. [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Neurol Clin. 2006;24:441–460. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De A, Churchill L, Obal F, Jr, Simasko SM, Krueger JM. GHRH and IL1beta increase cytoplasmic Ca(2+) levels in cultured hypothalamic GABAergic neurons. Brain Res. 2002;949:209–212. doi: 10.1016/s0006-8993(02)03157-8. [DOI] [PubMed] [Google Scholar]
- De A, Krueger JM, Simasko SM. Tumor necrosis factor alpha increases cytosolic calcium responses to AMPA and KCl in primary cultures of rat hippocampal neurons. Brain Res. 2003;981:133–142. doi: 10.1016/s0006-8993(03)02997-4. [DOI] [PubMed] [Google Scholar]
- Deboer T, Fontana A, Tobler I. Tumor necrosis factor (TNF) ligand and TNF receptor deficiency affects sleep and the sleep EEG. J Neurophysiol. 2002;88:839–846. doi: 10.1152/jn.2002.88.2.839. [DOI] [PubMed] [Google Scholar]
- Eriksson C, Nobel S, Winblad B, Schultzberg M. Expression of interleukin 1α and β, and interleukin 1 receptor antagonist mRNA in the rat central nervous system after peripheral administration of lipopolysaccharides. Cytokine. 2000;12:423–431. doi: 10.1006/cyto.1999.0582. [DOI] [PubMed] [Google Scholar]
- Fang J, Wang Y, Krueger JM. Mice lacking the TNF 55 kDa receptor fail to sleep more after TNFα treatment. J Neurosci. 1997;17:5949–5955. doi: 10.1523/JNEUROSCI.17-15-05949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Wang Y, Krueger JM. Effects of interleukin-1 beta on sleep are mediated by the type I receptor. Am J Physiol. 1998;274:R655–R660. doi: 10.1152/ajpregu.1998.274.3.R655. [DOI] [PubMed] [Google Scholar]
- Franken P, Malafosse A, Tafti M. Genetic variation in EEG activity during sleep in inbred mice. Am J Physiol. 1998;275:R1127–R1137. doi: 10.1152/ajpregu.1998.275.4.R1127. [DOI] [PubMed] [Google Scholar]
- Franken P, Malafosse A, Tafti M. Genetic determinants of sleep regulation in inbred mice. Sleep. 1999;22:155–169. [PubMed] [Google Scholar]
- Gerlai R. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 1996;19:177–189. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- Gingrich JA, Hen R. The broken mouse: the role of development, plasticity and environment in the interpretation of phenotypic changes in knockout mice. Curr Opin Neurobiol. 2000;10:146–152. doi: 10.1016/s0959-4388(99)00061-6. [DOI] [PubMed] [Google Scholar]
- Gordon CJ, White EC. Temporal response of neurons to ambient heating in the preoptic and septal area of the unanesthetized rabbit. Comp Biochem Physiol A. 1985;82:879–884. doi: 10.1016/0300-9629(85)90500-6. [DOI] [PubMed] [Google Scholar]
- Hohmann HP, Brockhaus M, Bauerle PA, Remy R, Kolbeck R, van Loon APGM. Expression of the type A and B tumor necrosis factor (TNF) receptor is independently regulated, and both receptors mediate activation of the transcription factor NF-κB. J Biol Chem. 1990;265:22409–22417. [PubMed] [Google Scholar]
- Holtmann MH, Neurath MF. Differential TNF-signaling in chronic inflammatory disorders. Curr Mol Med. 2004;4:439–444. doi: 10.2174/1566524043360636. [DOI] [PubMed] [Google Scholar]
- Huber R, Deboer T, Tobler I. Effects of sleep deprivation on sleep and sleep EEG in three mouse strains: empirical data and simulations. Brain Res. 2000;857:8–19. doi: 10.1016/s0006-8993(99)02248-9. [DOI] [PubMed] [Google Scholar]
- Jhaveri KA, Trammell RA, Toth LA. Effect of environmental temperature on sleep, locomotor activity, core body temperature and immune responses of C57BL/6J mice. Brain Behav Immun. 2007 doi: 10.1016/j.bbi.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapás L, Book AA, Schweitzer JB, Wiley RG, Krueger JM. The effects of immunolesions of nerve growth factor-receptive neurons by 192 IgG-saporin on sleep. Brain Res. 1996;712:53–59. doi: 10.1016/0006-8993(95)01431-4. [DOI] [PubMed] [Google Scholar]
- Kim U, Sanchez-Vives MV, McCormick DA. Functional dynamics of GABAergic inhibition in the thalamus. Science. 1997;278:130–134. doi: 10.1126/science.278.5335.130. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Obal FJ, Fang J, Kubota T, Taishi P. The role of cytokines in physiological sleep regulation. Ann NY Acad Sci. 2001;933:211–221. doi: 10.1111/j.1749-6632.2001.tb05826.x. [DOI] [PubMed] [Google Scholar]
- Kubota T, Li N, Guan Z, Brown RA, Krueger JM. Intrapreoptic microinjection of TNF-alpha enhances non-REM sleep in rats. Brain Res. 2002;932:37–44. doi: 10.1016/s0006-8993(02)02262-x. [DOI] [PubMed] [Google Scholar]
- Lai AY, Swayze RD, El Husseini A, Song C. Interleukin-1 beta modulates AMPA receptor expression and phosphorylation in hippocampal neurons. J Neuroimmunol. 2006;175:97–106. doi: 10.1016/j.jneuroim.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Lucey EC, Keane J, Kuang PP, Snider GL, Goldstein RH. Severity of elastase-induced emphysema is decreased in tumor necrosis factor-alpha and interleukin-1beta receptor-deficient mice. Lab Invest. 2002;82:79–85. doi: 10.1038/labinvest.3780397. [DOI] [PubMed] [Google Scholar]
- Mackiewicz M, Sollars PJ, Ogilvie MD, Pack AI. Modulation of IL-1β gene expression in the rat CNS during sleep deprivation. Neuroreport. 1996;7:529–533. doi: 10.1097/00001756-199601310-00037. [DOI] [PubMed] [Google Scholar]
- Moreland JG, Fuhrman RM, Wohlford-Lenane CL, Quinn TJ, Benda E, Pruessner JA, Schwartz DA. TNF-alpha and IL-1 beta are not essential to the inflammatory response in LPS-induced airway disease. Am J Physiol Lung Cell Mol Physiol. 2001;280:L173–L180. doi: 10.1152/ajplung.2001.280.1.L173. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Opp MR. Sleep-wake behavior and responses of interleukin-6-deficient mice to sleep deprivation. Brain Behav Immun. 2005;19:28–39. doi: 10.1016/j.bbi.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Opp MR. Rat strain differences suggest a role for corticotropin-releasing hormone in modulating sleep. Physiol Behav. 1998;63:67–74. doi: 10.1016/s0031-9384(97)00390-9. [DOI] [PubMed] [Google Scholar]
- Opp MR. Cytokines and sleep. Sleep Med Rev. 2005;9:355–364. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Opp MR, Imeri L. Rat strains that differ in corticotropin-releasing hormone production exhibit different sleep-wake responses to interleukin 1. Neuroendocrinology. 2001;73:272–284. doi: 10.1159/000054644. [DOI] [PubMed] [Google Scholar]
- Opp MR, Krueger JM. Anti-interleukin-1β reduces sleep and sleep rebound after sleep deprivation in rats. Am J Physiol. 1994a;266:R688–R695. doi: 10.1152/ajpregu.1994.266.3.R688. [DOI] [PubMed] [Google Scholar]
- Opp MR, Krueger JM. Interleukin-1 is involved in responses to sleep deprivation in the rabbit. Brain Res. 1994b;639:57–65. doi: 10.1016/0006-8993(94)91764-7. [DOI] [PubMed] [Google Scholar]
- Opp MR, Obál F, Krueger JM. Interleukin-1 alters rat sleep: temporal and dose-related effects. Am J Physiol. 1991;260:R52–R58. doi: 10.1152/ajpregu.1991.260.1.R52. [DOI] [PubMed] [Google Scholar]
- Opp MR, Postlethwaite AE, Seyer JM, Krueger JM. Interleukin 1 receptor antagonist blocks somnogenic and pyrogenic responses to an interleukin 1 fragment. Proc Natl Acad Sci USA. 1992;89:3726–3730. doi: 10.1073/pnas.89.9.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Roma-novsky AA. Thermoregulatory responses to lipopolysac-charide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1244–R1252. doi: 10.1152/ajpregu.00370.2005. [DOI] [PubMed] [Google Scholar]
- Schöbitz B, De Kloet ER, Holsboer F. Gene expression and function of interleukin 1, interleukin 6 and tumor necrosis factor in the brain. Prog Neurobiol. 1994;44:397–432. doi: 10.1016/0301-0082(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Serantes R, Arnalich F, Figueroa M, Salinas M, Andres-Mateos E, Codoceo R, Renart J, Matute C, Cavada C, Cuadrado A, Montiel C. Interleukin-1beta enhances GABAA receptor cell-surface expression by a phosphatidylinositol 3-kinase/Akt pathway: relevance to sepsis-associated encephalopathy. J Biol Chem. 2006;281:14632–14643. doi: 10.1074/jbc.M512489200. [DOI] [PubMed] [Google Scholar]
- Shoham S, Davenne D, Cady AB, Dinarello CA, Krueger JM. Recombinant tumor necrosis factor and interleukin 1 enhance slow-wave sleep. Am J Physiol. 1987;253:R142–R149. doi: 10.1152/ajpregu.1987.253.1.R142. [DOI] [PubMed] [Google Scholar]
- Sims JE, Gayle MA, Slack JL, Alderson MR, Bird TA, Giri JG, Colotta F, Re F, Mantovani A, Shanebeck K, Grabstein KH, Dower SK. Interleukin 1 signaling occurs exclusively via the type I receptor. Proc Natl Acad Sci USA. 1993;90:6155–6159. doi: 10.1073/pnas.90.13.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. The corticothalamic system in sleep. Front Biosci. 2003;8:d878–d899. doi: 10.2741/1043. [DOI] [PubMed] [Google Scholar]
- Steriade M, Dossi RC, Nunez A. Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: cortically induced synchronization and brainstem cholin-ergic suppression. J Neurosci. 1991;11:3200–3217. doi: 10.1523/JNEUROSCI.11-10-03200.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taishi P, Chen Z, Hansen MK, Zhang J, Fang J, Krueger JM. Sleep-associated changes in interleukin-1β mRNA in the brain. J Interferon Cytokine Res. 1998;18:793–798. doi: 10.1089/jir.1998.18.793. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Fang J, Kapás L, Wang Y, Krueger JM. Inhibition of brain interleukin-1 attenuates sleep rebound after sleep deprivation in rabbits. Am J Physiol. 1997;273:R677–R682. doi: 10.1152/ajpregu.1997.273.2.R677. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kapás L, Fang J, Krueger JM. An anti-tumor necrosis factor antibody suppresses sleep in rats and rabbits. Brain Res. 1995a;690:241–244. doi: 10.1016/0006-8993(95)00609-t. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kapás L, Fang J, Krueger JM. Somnogenic relationships between tumor necrosis factor and interleukin-1. Am J Physiol. 1999;276:R1132–R1140. doi: 10.1152/ajpregu.1999.276.4.R1132. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kapás L, Fang J, Seyer JM, Wang Y, Krueger JM. An interleukin-1 receptor fragment inhibits spontaneous sleep and muramyl dipeptide-induced sleep in rabbits. Am J Physiol. 1996a;271:R101–R108. doi: 10.1152/ajpregu.1996.271.1.R101. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kapas L, Krueger JM. A tumor necrosis factor (TNF) receptor fragment attenuates TNF-alpha- and muramyl dipeptide-induced sleep and fever in rabbits. J Sleep Res. 1996b;5:106–114. doi: 10.1046/j.1365-2869.1996.d01-63.x. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kapás L, Seyer JM, Wang Y, Krueger JM. Inhibition of tumor necrosis factor attenuates physiological sleep in rabbits. Neuroreport. 1996c;7:642–646. doi: 10.1097/00001756-199601310-00063. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Tooley DD, Kapás L, Fang J, Seyer JM, Krueger JM. Inhibition of tumor necrosis factor in the brain suppresses rabbit sleep. Pflügers Archiv Eur J Physiol. 1995b;431:155–160. doi: 10.1007/BF00410186. [DOI] [PubMed] [Google Scholar]
- Tobler I, Gaus SE, Deboer T, Achermann P, Fischer M, Rülicke T, Moser M, Oesch B, McBrides PA, Manson JC. Altered circadian activity rhythms and sleep in mice devoid of prion protein. Nature. 1996;380:639–642. doi: 10.1038/380639a0. [DOI] [PubMed] [Google Scholar]
- Toth LA, Opp MR. Infection and sleep. In: Lee-Chiong T, Carskadon MA, Sateia M, editors. Sleep Medicine. Hanley & Belfus, Inc; Philadelphia: 2002. pp. 77–84. [Google Scholar]
- Tsai SH, Liang YC, Chen L, Ho FM, Hsieh MS, Lin JK. Arsenite stimulates cyclooxygenase-2 expression through activating IkappaB kinase and nuclear factor kappaB in primary and ECV304 endothelial cells. J Cell Biochem. 2002;84:750–758. doi: 10.1002/jcb.10096. [DOI] [PubMed] [Google Scholar]
- Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- Yasuda T, Yoshida H, Garcia-Garcia F, Kay D, Krueger JM. Interleukin-1beta has a role in cerebral cortical state-dependent electroencephalographic slow-wave activity. Sleep. 2005;28:177–184. doi: 10.1093/sleep/28.2.177. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Peterfi Z, Garcia-Garcia F, Kirkpatrick R, Yasuda T, Krueger JM. State-specific asymmetries in EEG slow wave activity induced by local application of TNF[alpha] Brain Res. 2004;1009:129–136. doi: 10.1016/j.brainres.2004.02.055. [DOI] [PubMed] [Google Scholar]
- Zhu G, Okada M, Yoshida S, Mori F, Ueno S, Wakabayashi K, Kaneko S. Effects of interleukin-1beta on hippocampal glutamate and GABA releases associated with Ca2+-induced Ca2+ releasing systems. Epilepsy Res. 2006;71:107–116. doi: 10.1016/j.eplepsyres.2006.05.017. [DOI] [PubMed] [Google Scholar]