Abstract
Objectives
To investigate mechanisms of reduced susceptibility to commonly used antibiotics in Prevotella cultured from patients with cystic fibrosis (CF), patients with invasive infection and healthy control subjects and to determine whether genotype can be used to predict phenotypic resistance.
Methods
The susceptibility of 157 Prevotella isolates to seven antibiotics was compared, with detection of resistance genes (cfxA-type gene, ermF and tetQ), mutations within the CfxA-type β-lactamase and expression of efflux pumps.
Results
Prevotella isolates positive for a cfxA-type gene had higher MICs of amoxicillin and ceftazidime compared with isolates negative for this gene (P < 0.001). A mutation within the CfxA-type β-lactamase (Y239D) was associated with ceftazidime resistance (P = 0.011). The UK CF isolates were 5.3-fold, 2.7-fold and 5.7-fold more likely to harbour ermF compared with the US CF, UK invasive and UK healthy control isolates, respectively. Higher concentrations of azithromycin (P < 0.001) and clindamycin (P < 0.001) were also required to inhibit the growth of the ermF-positive isolates compared with ermF-negative isolates. Furthermore, tetQ-positive Prevotella isolates had higher MICs of tetracycline (P = 0.001) and doxycycline (P < 0.001) compared with tetQ-negative isolates. Prevotella spp. were also shown, for the first time, to express resistance nodulation division (RND)-type efflux pumps.
Conclusions
This study has demonstrated that Prevotella isolated from various sources harbour a common pool of resistance genes and possess RND-type efflux pumps, which may contribute to tetracycline resistance. The findings indicate that antibiotic resistance is common in Prevotella spp., but the genotypic traits investigated do not reflect phenotypic antibiotic resistance in every instance.
Keywords: resistance genes, β-lactamases, efflux pumps
Introduction
Prevotella spp. are opportunistic Gram-negative obligate anaerobic pathogens that are associated with polymicrobial infections in a multitude of sites throughout the body, including the respiratory tract.1 Although the role of these bacteria in the pathogenesis of chronic pulmonary infection is unclear, this genus has been repeatedly detected as a member of the respiratory microbiota in patients with cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD) or bronchiectasis using both culture and molecular methods.2–9 Antibiotics routinely used in the management of these chronic lung infections such as β-lactam, macrolide/lincosamide/streptogramin B (MLS) and tetracycline antibiotics have activity against these anaerobic bacteria. However, a recent study by our group detected resistance to antibiotics in each of these classes among Prevotella spp. from a range of sources.10
Antimicrobial susceptibility testing of Prevotella spp. takes several days to complete and the delay in reporting the susceptibility results may have negative clinical implications for the patient. Therefore, it is essential to better understand the mechanisms underlying resistance in Prevotella isolates and to determine whether the genotype can be used to predict phenotypic resistance in this genus. These data may enable clinicians to more rapidly select the most appropriate antibiotic if Prevotella spp. are to be treated as part of a polymicrobial infection such as that present in chronic pulmonary disease.
Three putative antibiotic resistance genes have been previously detected in Prevotella spp. cultured from non-CF patients: (i) a cfxA-type gene encoding a β-lactamase, which hydrolyses the β-lactam bond in some penicillin and cephalosporin antibiotics; (ii) ermF, encoding a methylase, which alters the binding site of MLS antibiotics; and (iii) tetQ, encoding a ribosomal protection protein, which prevents the action of tetracyclines.11,12 It is also recognized that mutations within β-lactamase-encoding genes may extend the enzyme's spectrum of activity;13 however, it has not yet been determined whether similar mutations exist within CfxA-type β-lactamases in Prevotella spp. Furthermore, bacterial efflux may contribute to resistance to multiple structurally diverse antimicrobial agents.14 Efflux pumps associated with clinically significant resistance in Gram-negative bacteria, including Pseudomonas aeruginosa and Bacteroides fragilis, belong to the resistance nodulation division (RND) family.14,15 The type of efflux pump expressed by Prevotella spp. is currently unknown.
In this study we investigated mechanisms of resistance to β-lactam, MLS and tetracycline antibiotics in a large number of clinical Prevotella isolates, including for the first time CF-associated Prevotella. We also determined whether the detection of specific genes, mutations within a β-lactamase-encoding gene and the expression of efflux pumps could predict phenotypic resistance.
Materials and methods
Clinical Prevotella isolates
The Prevotella isolates (n = 157) analysed in this study have been previously described in detail.10 Briefly, the isolates were split into four groups depending on their source (UK CF, n = 57; US CF, n = 23; UK invasive, n = 50; and UK healthy controls, n = 27). The CF isolates were cultured from adult (≥18 years) CF patients attending the adult CF clinic, Belfast, UK (sputum, n = 53; and plaque, n = 4) or paediatric (<18 years) CF patients attending the CF clinic, University of North Carolina at Chapel Hill, USA (sputum, n = 9; and bronchoalveolar lavage, n = 14). The UK invasive isolates were cultured from non-CF patients (of unknown age) with various infections including brain abscesses, kidney ulcers and infections identified on blood culture (n = 50) and were kindly provided Dr Valerie Hall, Anaerobe Reference Laboratory, Cardiff, UK. The remaining isolates were from UK adult (≥18 years) healthy control subjects (induced sputum, n = 27). Isolates were identified by 16S rRNA sequencing (Table S1, available as Supplementary data at JAC Online). Where available, information was obtained on azithromycin prescription (currently prescribed or not currently prescribed) at the time of sample collection.
PCRs
Genomic DNA extraction was performed using a ZR Fecal DNA KitTM (Zymo Research, Cambridge Biosciences, UK) in accordance with the manufacturer's instructions. Primer pairs for PCR assays are described elsewhere11,16,17 or were designed in Primer3 software (Table 1) and were purchased from Eurofins MWG Operon, UK. Each PCR was performed using the JumpStartTM Taq DNA Polymerase Kit with 10× reaction buffer without MgCl2 (Sigma-Aldrich, UK). The final reaction mixture (50 μL) contained 1× PCR buffer, 200 μM of each dNTP, 0.5 μM of each forward and reverse primer and 1.25 U of Taq polymerase. MgCl2 was added at the concentration specified in Table S2 (available as Supplementary data at JAC Online). DNA template (5 μL) was added and sterile DEPC-treated water (Ambion®, UK) was used to bring the reaction volume to 50 μL. Sterile DEPC-treated water (Ambion®, UK) replacing bacterial DNA was used in all PCR assays as a negative control. PCR was performed using a Veriti® Thermal Cycler (Applied Biosystems, UK). Table S2 provides details of the cycling parameters used. Amplicons were analysed by agarose gel electrophoresis and the resulting bands were visualized by UV luminescence (Gel DocTM, Bio-Rad, UK) using Quantity One software (Bio-Rad).
Table 1.
Primers used for PCR, qRT-PCR and sequencing experiments
| Target gene | Primer sequence (5′–3′) | Amplicon size (bp) | Reference |
|---|---|---|---|
| Antibiotic resistance genes | |||
| cfxA-type gene | AGCTGCTATCCTATCTACACC, CCACACTCATTCCTCGTTC | 214 | this study |
| ermF | CGGGTCAGCACTTTACTATTG, GGACCTACCTCATAGACAAG | 466 | 16 |
| tetQ | CATGGATCAGCAATGTTCAATATCGG, CCTGGATCCACAATGTATTCAGAGCGG | 460 | 17 |
| Amplification and sequencing of the full-length cfxA-type gene | |||
| cfxA-type gene | GAAAAAAACAGAAAAAAACAAATC, TTAAGATTTTACTGAAGTTTG | 966 | 11 |
| 5′ end of gene and 3′ end of gene | ACGCAGCAAATCTCTCACTG, TACGGAAGAGGAAATGTCGG | used for sequencing only | this study |
| Species-specific efflux pumps (PCR and/or qRT-PCR) | |||
| P. melaninogenica pump 1 (PCR and qRT-PCR) | GGTGGTAGCGGTCAGATTGT, TCGCCCATTTTAAGACCACT | 200 | this study |
| P. melaninogenica pump 2 (PCR and qRT-PCR) | CATCCAGAACGCTTTTCACA, TGTTGGCATTGCTAAAGACG | 151 | this study |
| P. salivae pump 1 (PCR) | CTCCTTGGCTGACGCTAAAG, GACTTATCAGGCAGCCGTTC | 211 | this study |
| P. salivae pump 2 (PCR) | CTTTTCCGCATTTCGATTGT, CATTGAGCTTCGCTTTAGCC | 157 | this study |
| P. salivae pump 1 (qRT-PCR) | TCCCATCATTCTGTGGGTTT, ATCCCGAACGCATATTGAAG | 186 | this study |
| P. salivae pump 2 (qRT-PCR) | ACAGGCCACGTATGAAGAGG, TCATGCACTTTCTGCACTCC | 174 | this study |
| P. histicola pump 1 (PCR and qRT-PCR) | GGCTCAAGCAAAGGCTAATG, CTTGTCGAACACCTGCTTCA | 248 | this study |
| P. histicola pump 2 (PCR and qRT-PCR) | TAAGGGAAAGACGCTTGTGC, TTGTTGGTGCTGACGAAGAG | 192 | this study |
| P. nigrescens pump 1 (PCR and qRT-PCR) | GCCCTAAACTCTGCACAAGC, CCTGAATGGGCAGTTTGAAT | 179 | this study |
| P. denticola pump 1 (PCR and qRT-PCR) | GATTACCGTAGACCCGCAGA, TTTCTCCGATTTCCTGATGG | 166 | this study |
| 16S rRNA reference gene (qRT-PCR) | TAAGCATCCCACCTGGGGAG, GCTGACGACAACCATGCAGC | 197 | this study |
Detection of antibiotic resistance genes
PCR screening assays were used for detection of the cfxA-type gene (penicillin and cephalosporin resistance), ermF (MLS resistance) and tetQ (tetracycline resistance). Prevotella pallens NCTC 13042 (cfxA2 and tetQ) and Prevotella bivia R24779 (ermF) were used as positive controls. Where available, MIC and susceptibility category (provided that anaerobic breakpoints were approved by the CLSI) of a range of antibiotics (amoxicillin, ceftazidime, co-amoxiclav, azithromycin, clindamycin, doxycycline and tetracycline), determined previously,10 were compared in the presence and absence of the relevant antibiotic resistance gene in the clinical isolates.
Analysis of the CfxA-type β-lactamase in clinical Prevotella isolates
Amplification of the full-length β-lactamase-encoding gene (966 bp) was carried out with 20 clinical isolates of Prevotella and P. pallens NCTC 13042, which were positive for the 214 bp fragment of the cfxA-type gene. PCR products were purified using a QIAquick® PCR Purification Kit (Qiagen, UK) in accordance with the manufacturer's instructions. Both strands of DNA were sequenced by Eurofins MWG Operon using appropriate primers (Table 1). The CfxA-type protein sequence was determined from a consensus DNA sequence. The deduced protein sequences of the CfxA-type β-lactamase from the clinical Prevotella isolates and P. pallens NCTC 13042 were compared between isolates deposited in GenBank and correlated with amino acid substitutions. The MICs of amoxicillin, ceftazidime and co-amoxiclav determined previously10 were also compared between isolates with amino acid substitutions within the β-lactamase protein sequence.
Detection of species-specific RND-type efflux pumps
Protein sequences of RND efflux pumps in P. aeruginosa PAO1 (MexAB-OprM, MexCD-OprJ, MexEF-OprN and MexXY-OprM) and B. fragilis ATCC 25285 (BmeABC1–8) were searched for homologies within five Prevotella genomes (Prevotella denticola F0289, Prevotella nigrescens ATCC 33563, Prevotella melaninogenica ATCC 25845, Prevotella histicola F0411 and Prevotella salivae DSM 15606) using protein BLAST. Species-specific primer pairs were designed to amplify the gene encoding the linker protein, a periplasmic membrane fusion protein,14 of the efflux pump operon found in the Prevotella genomes (Table 1). Clinical Prevotella isolates (n = 87) that belonged to these five species (P. denticola, n = 13; P. nigrescens, n = 12; P. melaninogenica, n = 34; P. histicola, n = 20; and P. salivae, n = 8) and two type strains (P. denticola NCTC 13067 and P. melaninogenica ATCC 25845) were selected and screened for the presence of species-specific RND-type efflux pumps.
Transcription levels of species-specific RND-type efflux pumps
A detailed description of the method used to prepare total RNA from stationary growth (optical density at 550 nm of 0.8–1.3) cultures (n = 26) is provided elsewhere.18 Clinical isolates were selected randomly. RNA integrity was verified on a 1% agarose gel. The quantitative real-time PCR (qRT-PCR) procedure was carried out as previously described,18 with several modifications for use with Prevotella spp. as follows: (i) 500 ng of total RNA was used for cDNA generation; (ii) the cycling conditions used were 10 min at 95°C followed by 40 cycles of 15 s at 95°C and 60 s at 60°C; and (iii) real-time PCR was performed in a final reaction volume of 20 μL containing 2.5 μL of cDNA (1 : 10 dilution), 10 μL of FastStart® Universal SYBR green master mix with ROX (Roche, UK), 0.2 μL of each forward and reverse primer (10 μM) and 7.1 μL of DEPC-treated water (Ambion®). The species-specific efflux pump primers that were used for qRT-PCR are listed in Table 1 and were designed from the gene encoding the linker protein of the efflux pump operon found in the Prevotella genomes. The comparative threshold cycle (CT) method (ΔΔCT) was used to determine the relative transcription levels using the 16S rRNA gene as the endogenous control. Data were analysed using Applied Biosystems 7500 software.
Inhibition of efflux pumps
Under strict anaerobic conditions clinical Prevotella isolates (n = 25) were inoculated onto supplemented brucella blood agar with or without an efflux pump inhibitor (EPI), Phe-Arg β-naphthylamide dihydrochloride (Sigma-Aldrich), added uniformly to the agar at a final concentration of 80 mg/L.19 The MICs of amoxicillin, azithromycin, ceftazidime, clindamycin, co-amoxiclav, doxycycline and tetracycline were then determined in the presence or absence of the EPI by Etest® (bioMérieux, France) according to the manufacturer's instructions.
Statistical analysis
MICs were compared in isolates that were positive versus negative for: (i) the cfxA-type gene (amoxicillin, ceftazidime and co-amoxiclav); (ii) ermF (azithromycin and clindamycin); and (iii) tetQ (tetracycline and doxycycline) using the Mann–Whitney test. Isolates were separated into groups depending on a Y239D substitution in the CfxA-type β-lactamase and amoxicillin, ceftazidime and co-amoxiclav MICs compared using the Mann–Whitney test. A relative risk (RR) analysis was performed to determine the risk of harbouring ermF in UK CF isolates relative to US CF, UK invasive and UK healthy control isolates. Isolates were grouped according to patient (UK CF, US CF and healthy control) and prescription of azithromycin (currently prescribed versus not currently prescribed) and a χ2 test with Yates correction was used to determine whether there was evidence of an association between ermF and current azithromycin prescription. The Wilcoxon signed rank test was used to compare the MICs before and after inhibition of the efflux pumps. All statistical analyses were carried out using an SPSS software package (SPSS Version 19; Chicago, IL, USA). A two-tailed P value <0.05 was considered statistically significant.
Results
The cfxA-type gene was common among Prevotella isolates from a range of sources and was associated with high MICs of β-lactam antibiotics
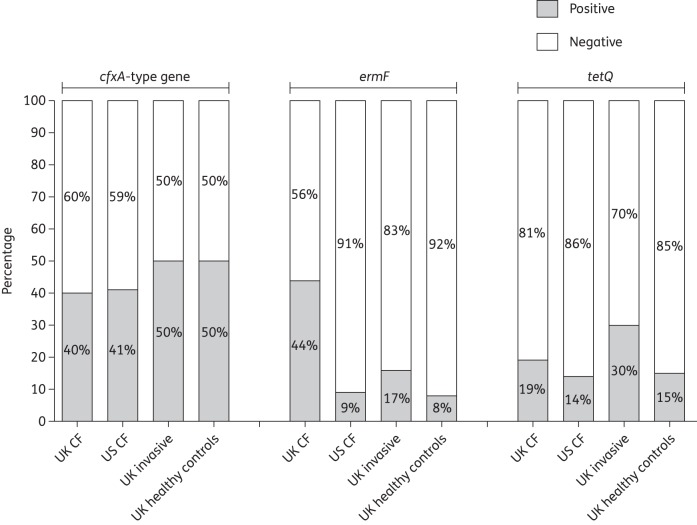
The cfxA-type gene codes for a class 2e β-lactamase13 and was detected in 70/155 (45%) isolates with similar proportions in each Prevotella group positive for the gene (Figure 1). The isolates positive for the cfxA-type gene had significantly higher MICs of amoxicillin and ceftazidime compared with isolates negative for the cfxA-type gene (P < 0.001, Mann–Whitney test) (Figure 2). Although this β-lactamase is typically inhibited by clavulanic acid,13 isolates positive for the cfxA-type gene also had significantly higher MICs of co-amoxiclav compared with isolates negative for the cfxA-type gene (P < 0.001; Figure 2). However, only 16/69 (23%) isolates positive for the cfxA-type gene were also categorized as resistant or intermediately resistant to co-amoxiclav.
Figure 1.
Prevalence of resistance genes (cfxA-type gene, ermF and tetQ) in each of the Prevotella groups.
Figure 2.
Comparison of susceptibility (MICs) in isolates positive and negative for associated resistance genes. In the box and whisker plots, the top and bottom boundaries of each box indicate the 75th and 25th quartile values, respectively, with the line inside the box representing the median (50th quartile). The ends of the whiskers indicate the range. Any isolates recorded as having an MIC greater than the maximum concentration (>256 mg/L) or lower than the minimum concentration (<0.016 mg/L) on the Etest® strip are shown as double the maximum concentration or half the lower concentration, respectively. *P < 0.05 and ≥0.001; **P < 0.001.
Prevotella isolates with a Y239D substitution in the CfxA-type β-lactamase protein sequence were more resistant to ceftazidime
The deduced amino acid sequences of the CfxA-type β-lactamase in 20 clinical isolates and one type strain (P. pallens NCTC 13042) were >98% similar. Several amino acid substitutions were observed when compared with CfxA from Bacteroides vulgatus (GenBank protein accession number AAB17891) (Table 2). Each of the deduced amino acid sequences possessed the K272E substitution common to CfxA2, and an additional substitution at residue 239 (Y239D/C/S) was observed more than once (Table 2). Given that the Y239D substitution was most common (Table 2; CfxA3), the MICs of amoxicillin, ceftazidime and co-amoxiclav were compared between isolates with this substitution (Table 2). Those clinical isolates with an aspartic acid (D) at this residue (n = 6) had significantly higher MICs of ceftazidime compared with those with tyrosine (Y) at this position (n = 11) (P = 0.011, Mann–Whitney test). No difference in the MICs of amoxicillin and co-amoxiclav was detected between the two groups.
Table 2.
Amino acid sequence analysis of 20 clinical Prevotella isolates and a type strain, P. pallens NCTC 13042, compared with CfxA from B. vulgatus AAB1789123
| Source | MIC (mg/L) |
Amino acid substitutions in CfxA | CfxA-type β-lactamase | Protein accession number of identical sequence | ||
|---|---|---|---|---|---|---|
| amoxicillin | ceftazidime | co-amoxiclav | ||||
| UK CF | 6 | 2 | 1.5 | K272E, L189F | NA | NA |
| UK CF | >256 | >256 | 64 | K272E, Y239S | NA | NA |
| UK CF | >256 | 256 | >256 | K272E, Y239S | NA | NA |
| UK CF | >256 | 128 | 3 | K272E, Y239D | CfxA3 | AAL79549 |
| UK CF | 32 | 2 | 12 | K272E | CfxA2 | ADD23513 |
| UK CF | 64 | 4 | 6 | K272E | CfxA2 | ADD23513 |
| UK CF | 64 | 3 | 12 | K272E | CfxA2 | ADD23513 |
| UK CF | 48 | >256 | 64 | K272E, Y239D | CfxA3 | AAL79549 |
| UK CF | 96 | >256 | 0.064 | K272E, Y239C | CfxA5 | AAV37206 |
| UK invasive | >256 | >256 | 3 | K272E, Y239D | CfxA3 | AAL79549 |
| UK invasive | >256 | 4 | 3 | K272E | CfxA2 | ADD23513 |
| UK invasive | >256 | >256 | 3 | K272E, Y239D | CfxA3 | AAL79549 |
| UK invasive | 64 | 1.5 | 0.125 | K272E | CfxA2 | ADD23513 |
| UK invasive | 48 | 3 | 0.064 | K272E, Y239D | CfxA3 | AAL79549 |
| UK invasive | >256 | >256 | 0.25 | K272E | CfxA2 | ADD23513 |
| UK healthy control | 192 | 32 | 0.25 | K272E | CfxA2 | ADD23513 |
| UK healthy control | 3 | 1.5 | 0.25 | K272E | CfxA2 | ADD23513 |
| UK healthy control | 4 | 0.25 | 0.032 | K272E | CfxA2 | ADD23513 |
| UK healthy control | 2 | 16 | 0.023 | K272E | CfxA2 | ADD23513 |
| UK healthy control | >256 | >256 | 3 | K272E, Y239D | CfxA3 | AAL79549 |
| Type strain | 1.5 | 3 | 0.064 | K272E | CfxA2 | ADD23513 |
The UK CF isolates were more likely to harbour ermF compared with the other groups
The ermF gene was detected most frequently in the UK CF Prevotella isolates (44%; Figure 1). There was a significant difference in the proportion of ermF-positive isolates between the UK CF group and the US CF (RR 5.26, 95% CI 1.35–20.49, P = 0.005), UK invasive (RR 2.74, 95% CI 1.36–5.52, P = 0.004) and UK healthy control (RR 5.70, CI 1.46–22.29, P = 0.003) groups. The risk of UK CF isolates harbouring ermF was increased 5.3-fold, 2.7-fold and 5.7-fold relative to the US CF, UK invasive and UK healthy control isolates, respectively. Significantly higher concentrations of both azithromycin (P < 0.001, Mann–Whitney test) and clindamycin (P < 0.001) were required to inhibit the ermF-positive isolates compared with the ermF-negative isolates (Figure 2). Furthermore, 28/37 (76%) ermF-positive isolates were categorized as resistant to clindamycin. A significant association between a current patient prescription of azithromycin and the presence of ermF among clinical Prevotella isolates was also detected (χ2 = 31.534, P < 0.001); 11/27 (41%) and 9/46 (20%) isolates from patients currently and not currently prescribed azithromycin, respectively, were positive for the gene.
The tetQ gene was detected in similar proportions in each group and was associated with high MICs of tetracycline antibiotics
Thirty-two of 151 (21%) Prevotella isolates were positive for tetQ. This gene was detected in similar proportions in each group (Figure 1). Significantly higher concentrations of tetracycline (P = 0.001, Mann-Whitney test) and doxycycline (P < 0.001) were required to inhibit the growth of tetQ-positive compared with tetQ-negative isolates (Figure 2). Among the tetQ-positive isolates, 19/23 (83%) were categorized as resistant or intermediately resistant to tetracycline.
A high percentage of Prevotella isolates tested harboured one or more antibiotic resistance genes
Of the 151 Prevotella isolates tested for the three antibiotic resistance genes, 93 (62%) were positive for one or more of the antibiotic resistance genes by PCR (Figure S1, available as Supplementary data at JAC Online). Twenty-six isolates carried two resistance genes [cfxA-type gene and ermF, 13/26 (50%); cfxA-type gene and tetQ, 11/26 (42%); and ermF and tetQ, 2/26 (8%)] and 6/151 (4%) isolates of Prevotella were found to harbour all three resistance genes (Figure S1).
Prevotella spp. express RND-type efflux pumps, which may contribute to tetracycline resistance
Genes encoding a linker protein, inner membrane protein and outer membrane protein were detected within each of the five Prevotella genomes searched in silico and were organized as an operon similar to RND-type efflux pumps in other bacteria.14 At the time of our analysis a single efflux pump was identified in P. denticola F0289 and P. nigrescens ATCC 33563 and two efflux pumps were detected in P. melaninogenica ATCC 25845, P. histicola F0411 and P. salivae DSM 15606 (Table S3, available as Supplementary data at JAC Online).
The species-specific linker genes were present in the majority of Prevotella spp. isolates tested (83/87, 95%) by PCR. The remaining isolates (P. melaninogenica) were either negative by PCR for one (n = 2) or both (n = 2) of the predicted linker genes. The transcription levels of the species-specific efflux pumps were analysed in 25 clinical isolates and P. melaninogenica ATCC 25845 by qRT-PCR. Twenty-one of 25 (84%) isolates and the type strain expressed the expected linker genes (Figure S2, available as Supplementary data at JAC Online). For the remaining four isolates either no expression was detected (P. melaninogenica, n = 1; and P. histicola, n = 2) or one of two expected pumps was expressed (n = 1; Figure S2). The MICs of seven antibiotics in the presence or absence of an EPI was also investigated. A statistically significant decrease in the MICs of tetracycline (P = 0.001, Wilcoxon signed rank test) was detected in the presence of the EPI, with no statistically significant differences apparent for the remaining antibiotics (Figure 3). A further analysis of the tetracycline MIC data revealed that 17/25 (68%) Prevotella isolates were negative by PCR for tetQ. A significant decrease in MICs was detected among the tetQ-negative isolates in the presence of the EPI (P = 0.025, Wilcoxon signed rank test). In contrast, no statistical difference in the MIC of tetracycline was detected in the presence or absence of an EPI for the tetQ-positive isolates (n = 8).
Figure 3.
Antibiotic susceptibility (MICs) of Prevotella isolates (n = 25) in the absence or presence of the EPI Phe-Arg β-naphthylamide dihydrochloride. In the box and whisker plots, the top and bottom boundaries of each box indicate the 75th and 25th quartile values, respectively, with the line inside the box representing the median (50th quartile). The ends of the whiskers indicate the range. Any isolates recorded as having an MIC greater than the maximum concentration (>256 mg/L) or lower than the minimum concentration (<0.016 mg/L) on the Etest® strip are shown as double the maximum concentration or half the lower concentration, respectively.
Discussion
Prevotella spp. have been detected in the airway microbiota in diseases characterized by chronic infection, including CF, COPD and bronchiectasis.4,6,8 Although their role in infection and inflammation in these conditions has not been clearly defined, this obligate anaerobe may be a future target for antimicrobial treatment. In vitro antimicrobial susceptibility testing may be used clinically to guide the treatment of individual anaerobic isolates. Unfortunately, in vitro susceptibility testing of Prevotella spp. can be difficult to perform given the fastidious nature of this genus. A genotypic prediction of resistance may be an attractive alternative to susceptibility testing with some antibiotics and has proved useful to detect single antibiotic resistance gene targets associated with resistance, e.g. mecA associated with methicillin resistance in Staphylococcus aureus.20 Before genotypic prediction can be used, it is important to understand the mechanisms of resistance that are commonly associated with reduced susceptibility in the target bacterium. Therefore, in this study we investigated, in clinical Prevotella isolates, putative mechanisms of resistance to antibiotics used in the treatment of chronic lung infections. We also explored the relationship between the presence of specific antibiotic resistance genes, amino acid mutations within the CfxA-type β-lactamase and the expression of efflux pumps and in vitro antimicrobial susceptibility and determined whether the genotype could predict phenotypic resistance.
β-Lactamase production by bacteria is a key mechanism of resistance to β-lactam antibiotics, and the presence of a cfxA-type gene in the Prevotella isolates in this study was associated with high MICs of amoxicillin, as has previously been reported for Prevotella isolates from dental infection.21 In the current study, isolates harbouring the cfxA-type gene also had higher MICs of ceftazidime and co-amoxiclav compared with isolates negative for the gene. This is in contrast to the findings of a previous study, which found that all Prevotella isolates positive for the β-lactamase-encoding gene cfxA2 (n = 62) were amoxicillin resistant, but susceptible to ceftazidime and co-amoxiclav.11 Giraud-Morin et al.11 proposed that substrate specificity may be extended by mutations within the cfxA-type gene as it is recognized that mutations in other β-lactamase-encoding genes (e.g. TEM-type β-lactamases) widen substrate specificity.13 In the present study, when the CfxA-type amino acid sequence was analysed, the K272E substitution, common to CfxA2, was detected in all 20 isolates tested. However, additional substitutions at amino acid residue 239 were also found, which are characteristic of other CfxA2-like sequences (CfxA3 and CfxA5) suggesting that Prevotella isolates from a range of sources produce variants of the CfxA2 β-lactamase. Furthermore, the data indicate that a Y239D substitution within the CfxA2-type protein sequence is associated with significantly higher MICs of ceftazidime. Therefore, the presence of this substitution may reduce susceptibility to ceftazidime.
Resistance to MLS antibiotics in Prevotella spp. may be due to an ermF-encoded methylase, which alters the binding site of these antibiotics. Isolates positive for ermF were associated with higher MICs of azithromycin and clindamycin. In agreement with this, Xie et al.22 detected 13/19 (68%) clindamycin- and/or roxithromycin-resistant Prevotella isolates from dental abscesses to be positive for ermF. Furthermore, the risk of UK CF isolates harbouring ermF was much higher compared with those collected from other patient groups. This may reflect the fact that the UK CF isolates were cultured from adult CF patients, where long-term azithromycin is prescribed in a high percentage of patients compared with the paediatric US CF isolates or UK invasive and healthy control isolates, for whom the use of azithromycin is less likely. The hypothesis of higher resistance in Prevotella being linked to azithromycin use appears to be confirmed by the association between a current patient prescription of azithromycin and the presence of ermF among the Prevotella isolates. Ideally, we would like to investigate the prevalence of ermF in Prevotella isolates from young patients with CF from the UK in future studies. However, as bronchoscopy is not performed in young children in the UK as part of routine clinical practice, obtaining clinically relevant respiratory samples is challenging. The expression of ermF in these isolates could also be evaluated between CF patients treated with or without chronic azithromycin. However, this would also be difficult to do as most CF patients are prescribed long-term azithromycin therapy.
Between 14% and 30% of Prevotella isolates in each group were also positive for tetQ, which has been associated with resistance to tetracyclines, with tetQ-positive isolates having statistically higher MICs of both doxycycline and tetracycline. A previous study also reported that ∼25% of Prevotella isolates cultured from clinical specimens and the resident microbiota of healthy subjects were positive for tetQ.12 Similarly, Arzese et al.12 investigated tetQ and susceptibility to tetracycline, and reported that all tetQ-positive isolates (n = 14) were resistant to tetracycline.
The results of this study indicate that if one of the target resistance genes is detected in clinical Prevotella isolates, it is more likely that these isolates will have higher MICs of the associated antibiotics. However, susceptible isolates with low MICs were also positive for the target resistance gene; this suggests that the choice of antibiotic treatment cannot be based solely on the presence of the gene. This was particularly evident for co-amoxiclav, where only 23% of isolates positive for the cfxA-type gene were resistant or intermediately resistant to co-amoxiclav. There were no amino acid mutations identified within the CfxA-type β-lactamase that were associated with reduced susceptibility to this antibiotic. These data suggest that the cfxA-gene itself may not be a major mechanism of resistance to co-amoxiclav. However, the determination of co-amoxiclav susceptibility post-complementation of a susceptible Prevotella strain with mutated cfxA genes would be required to support this hypothesis.
We also investigated whether the expression of efflux pumps could contribute to antibiotic resistance in Prevotella spp. The constitutive expression or overexpression of efflux pumps by bacteria is an important putative resistance mechanism as it may contribute to the reduced susceptibility to structurally dissimilar antibiotics. This study is the first to report the type of efflux pump in the Prevotella genome and whether these efflux pumps were expressed and potentially contributing to antibiotic resistance in five different species. Although detected in higher numbers in other bacterial genomes,14,15 a maximum of two RND-type efflux pumps were detected within five Prevotella genomes, which were homologous to efflux pumps found in P. aeruginosa PAO1 and B. fragilis ATCC 25285. In P. aeruginosa, it is recognized that MexAB-OprM and MexXY-OprM efflux systems are constitutively active.14 In this study, 22/25 (88%) clinical Prevotella isolates tested demonstrated pump expression. It is also known that efflux pumps expressed by P. aeruginosa contribute to resistance to a range of antibiotics including β-lactams and tetracycline.14 In our study, the presence of an EPI resulted in a statistically significant decrease in the MICs of tetracycline, suggesting that this antibiotic is a potential substrate of Prevotella efflux pumps, especially in isolates negative for tetQ. Further work includes comparing the expression levels of efflux pumps in a large number of isolates susceptible, intermediately resistant and resistant to tetracycline across all the different species.
A number of Prevotella isolates in this study also had high MICs of an antibiotic, but lacked a cfxA-type gene, ermF or tetQ. Although it is feasible that these isolates harbour a novel resistance mechanism, it is also possible that the primers used in this study did not recognize the target antibiotic resistance genes, which could be due to mutations within the target sequence. Furthermore, we cannot rule out that Prevotella isolates may harbour more than one mechanism of resistance for the same antibiotics.
There are a number of limitations to this study. Single bacterial colonies from each clinical sample were isolated and those identified as Prevotella spp. were subsequently used in this study. We cannot discount that other genotypes of Prevotella spp., which may have a different susceptibility profile from that detected, were present in the same sample. Furthermore, the microenvironment in chronic lung infections may induce different metabolic conditions and affect in vivo susceptibility compared with our in vitro results; however, the current approach to testing susceptibility and detecting resistance genes mirrors techniques used frequently in clinical practice.
In summary, this study has shown that Prevotella isolated from chronic pulmonary infection, invasive infections and the healthy respiratory tract harbour a common pool of resistance genes and possess RND-type efflux pumps, which potentially contribute to a reduced susceptibility to tetracycline. The presence of a cfxA-type gene, ermF or tetQ was associated with significantly higher MICs of β-lactam, MLS and tetracycline antibiotics, respectively, and an amino acid substitution within the CfxA-type β-lactamase was associated with higher MICs of ceftazidime. The data suggest that the presence of these genes increases the likelihood of resistance, but absence does not imply susceptibility. There was also an association between current patient prescriptions of azithromycin and the presence of ermF among the CF Prevotella isolates, suggesting that antibiotic pressure may have contributed to the spread of ermF among these isolates.
Funding
This work was supported by grants from the Health and Social Care Research and Development, Public Health Agency, Northern Ireland, the Medical Research Council and the United States National Institutes of Health (grant numbers HL092964, HL084934 and 5R01 HL092964-04) through a US-Ireland Partnership Grant. M. M. T. was supported by a Health and Social Care Research and Development, Public Health Agency, Northern Ireland, funded UK National Institute for Health Research Career Scientist Award. L. J. S. and K. A. G. were supported by Department of Employment and Learning, Northern Ireland, Studentships.
Transparency declarations
None to declare.
Author contributions
L. J. S., B. S., M. M. T., D. F. G., T. S. and J. S. E. conceived and designed research. L. J. S., B. S., K. A. G., S. J. M., L. M. and J. H. performed research. L. J. S., B. S. and M. M. T. analysed data. L. J. S., B. S., M. M. T., M. S. M. and J. S. E. wrote the paper. M. C. W., D. F. G. and T. S. reviewed and revised the paper.
Supplementary data
Acknowledgements
We thank Mr Gerry McGrillen (School of Pharmacy, Queen's University Belfast, Belfast, UK) for technical assistance and Dr Valerie Hall (Anaerobe Reference Laboratory, Cardiff, UK) for kindly providing the UK invasive isolates.
References
- 1.Bahrani-Mougeot FK, Paster BJ, Coleman S, et al. Molecular analysis of oral and respiratory bacterial species associated with ventilator-associated pneumonia. J Clin Microbiol. 2007;45:1588–93. doi: 10.1128/JCM.01963-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tunney MM, Field TR, Moriarty TF, et al. Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. Am J Respir Crit Care Med. 2008;177:995–1001. doi: 10.1164/rccm.200708-1151OC. [DOI] [PubMed] [Google Scholar]
- 3.Tunney MM, Klem ER, Fodor AA, et al. Use of culture and molecular analysis to determine the effect of antibiotic treatment on microbial community diversity and abundance during exacerbation in patients with cystic fibrosis. Thorax. 2011;66:579–84. doi: 10.1136/thx.2010.137281. [DOI] [PubMed] [Google Scholar]
- 4.Tunney MM, Einarsson GG, Wei L, et al. The lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am J Respir Crit Care Med. 2013;187:1118–26. doi: 10.1164/rccm.201210-1937OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris JK, De Groote MA, Sagel SD, et al. Molecular identification of bacteria in bronchoalveolar lavage fluid from children with cystic fibrosis. Proc Natl Acad Sci USA. 2007;104:20529–33. doi: 10.1073/pnas.0709804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fodor AA, Klem ER, Gilpin DF, et al. The adult cystic fibrosis airway microbiota is stable over time and infection type, and highly resilient to antibiotic treatment of exacerbations. PLoS One. 2012;7:e45001. doi: 10.1371/journal.pone.0045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bittar F, Richet H, Dubus JC, et al. Molecular detection of multiple emerging pathogens in sputa from cystic fibrosis patients. PLoS One. 2008;3:e2908. doi: 10.1371/journal.pone.0002908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang YJ, Kim E, Cox MJ, et al. A persistent and diverse airway microbiota present during chronic obstructive pulmonary disease exacerbations. OMICS. 2010;14:9–59. doi: 10.1089/omi.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao J, Schloss PD, Kalikin LM, et al. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci USA. 2012;109:5809–14. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherrard LJ, Graham KA, McGrath SJ, et al. Antibiotic resistance in Prevotella species isolated from patients with cystic fibrosis. J Antimicrob Chemother. 2013;68:2369–74. doi: 10.1093/jac/dkt191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giraud-Morin C, Madinier I, Fosse T. Sequence analysis of cfxA2-like β-lactamases in Prevotella species. J Antimicrob Chemother. 2003;51:1293–6. doi: 10.1093/jac/dkg221. [DOI] [PubMed] [Google Scholar]
- 12.Arzese AR, Tomasetig L, Botta GA. Detection of tetQ and ermF antibiotic resistance genes in Prevotella and Porphyromonas isolates from clinical specimens and resident microbiota of humans. J Antimicrob Chemother. 2000;45:577–82. doi: 10.1093/jac/45.5.577. [DOI] [PubMed] [Google Scholar]
- 13.Bush K, Jacoby GA. Updated functional classification of β-lactamases. Antimicrob Agents Chemother. 2010;54:969–76. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piddock LJ. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev. 2006;19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueda O, Wexler HM, Hirai K, et al. Sixteen homologs of the Mex-type multidrug resistance efflux pump in Bacteroides fragilis. Antimicrob Agents Chemother. 2005;49:2807–15. doi: 10.1128/AAC.49.7.2807-2815.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung WO, Werckenthin C, Schwarz S, et al. Host range of the ermF rRNA methylase gene in bacteria of human and animal origin. J Antimicrob Chemother. 1999;43:5–14. doi: 10.1093/jac/43.1.5. [DOI] [PubMed] [Google Scholar]
- 17.Nikolich MP, Hong G, Shoemaker NB, et al. Evidence for natural horizontal transfer of tetQ between bacteria that normally colonize humans and bacteria that normally colonize livestock. Appl Environ Microbiol. 1994;60:3255–60. doi: 10.1128/aem.60.9.3255-3260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCaughey G, Gilpin DF, Schneiders T, et al. Fosfomycin and tobramycin in combination downregulate nitrate reductase genes narG and narH, resulting in increased activity against Pseudomonas aeruginosa under anaerobic conditions. Antimicrob Agents Chemother. 2013;57:5406–14. doi: 10.1128/AAC.00750-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baucheron S, Imberechts H, Chaslus-Dancla E, et al. The AcrB multidrug transporter plays a major role in high-level fluoroquinolone resistance in Salmonella enterica serovar Typhimurium phage type DT204. Microb Drug Resist. 2002;8:281–9. doi: 10.1089/10766290260469543. [DOI] [PubMed] [Google Scholar]
- 20.Bode LG, van Wunnik P, Vaessen N, et al. Rapid detection of methicillin-resistant Staphylococcus aureus in screening samples by relative quantification between the mecA gene and the SA442 gene. J Microbiol Methods. 2012;89:129–32. doi: 10.1016/j.mimet.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Iwahara K, Kuriyama T, Shimura S, et al. Detection of cfxA and cfxA2, the β-lactamase genes of Prevotella spp., in clinical samples from dentoalveolar infection by real-time PCR. J Clin Microbiol. 2006;44:172–6. doi: 10.1128/JCM.44.1.172-176.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie Y, Chen J, He J, et al. Antimicrobial resistance and prevalence of resistance genes of obligate anaerobes isolated from periodontal abscesses. J Periodontol. 2014;85:327–34. doi: 10.1902/jop.2013.130081. [DOI] [PubMed] [Google Scholar]
- 23.Parker AC, Smith CJ. Genetic and biochemical analysis of a novel Ambler class A β-lactamase responsible for cefoxitin resistance in Bacteroides species. Antimicrob Agents Chemother. 1993;37:1028–36. doi: 10.1128/aac.37.5.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.