Abstract
STUDY QUESTION
Is the ongoing pregnancy rate with a new aqueous formulation of subcutaneous progesterone (Prolutex®) non-inferior to vaginal progesterone (Endometrin®) when used for luteal phase support of in vitro fertilization?
SUMMARY ANSWER
In the per-protocol (PP) population, the ongoing pregnancy rates per oocyte retrieval at 12 weeks of gestation were comparable between Prolutex and Endometrin (41.6 versus 44.4%), with a difference between groups of −2.8% (95% confidence interval (CI) −9.7, 4.2), consistent with the non-inferiority of subcutaneous progesterone for luteal phase support.
WHAT IS KNOWN ALREADY
Luteal phase support has been clearly demonstrated to improve pregnancy rates in women undergoing in vitro fertilization (IVF). Because of the increased risk of ovarian hyperstimulation syndrome associated with the use of hCG, progesterone has become the treatment of choice for luteal phase support.
STUDY DESIGN, SIZE, DURATION
This prospective, open-label, randomized, controlled, parallel-group, multicentre, two-arm, non-inferiority study was performed at eight fertility clinics. A total of 800 women, aged 18–42 years, with a BMI of ≤30 kg/m2, with <3 prior completed assisted reproductive technology (ART) cycles, exhibiting baseline (Days 2–3) FSH of ≤15 IU/L and undergoing IVF at 8 centres (seven private, one academic) in the USA, were enrolled from January 2009 through June 2011.
PARTICIPANTS/MATERIALS, SETTING, METHODS
In total, 800 women undergoing IVF were randomized after retrieval of at least three oocytes to an aqueous preparation of progesterone administered subcutaneously (25 mg daily) or vaginal progesterone (100 mg bid daily). Randomization was performed to enrol 100 patients at each site using a randomization list that was generated with Statistical Analysis Software (SAS®). If a viable pregnancy occurred, progesterone treatment was continued up to 12 weeks of gestation.
MAIN RESULTS AND THE ROLE OF CHANCE
Using a PP analysis, which included all patients who received an embryo transfer (Prolutex = 392; Endometrin = 390), the ongoing pregnancy rate per retrieval for subcutaneous versus vaginal progesterone was 41.6 versus 44.4%, with a difference between groups of −2.8% (95% CI −9.7, 4.2), consistent with the non-inferiority of subcutaneous progesterone for luteal phase support. In addition, rates of initial positive β-hCG (56.4% subcutaneous versus 59.0% vaginal; 95% CI −9.5, 4.3), clinical intrauterine pregnancy with fetal cardiac activity (42.6 versus 46.4%; 95% CI −10.8, 3.2), implantation defined as number of gestational sacs divided by number of embryos transferred (33.2 versus 35.1%; 95% CI −7.6, 4.0), live birth (41.1 versus 43.1%; 95% CI −8.9, 4.9) and take-home baby (41.1 versus 42.6%; 95% CI −8.4, 5.4) were comparable. Both formulations were well-tolerated, with no difference in serious adverse events. Analysis with the intention-to-treat population also demonstrated no difference for any outcomes between the treatment groups.
LIMITATIONS, REASONS FOR CAUTION
The conclusions are limited to the progesterone dosing regimen studied and duration of treatment for the patient population examined in this study.
WIDER IMPLICATIONS OF THE FINDINGS
Subcutaneous progesterone represents a novel option for luteal phase support in women undergoing IVF who for personal reasons prefer not to use a vaginal preparation or who wish to avoid the side effects of vaginal or i.m. routes of administration.
STUDY FUNDING/COMPETING INTERESTS
The study was funded by Institut Biochimique SA (IBSA). CAJ, BC, ST and CJ are employees of IBSA. FH currently consults for IBSA.
TRIAL REGISTRATION NUMBER
Keywords: progesterone, luteal phase support, in vitro fertilization, RCT
Introduction
Luteal phase support has been clearly demonstrated to improve pregnancy rates in women undergoing IVF (van der Linden et al., 2011). Because of the increased risk of ovarian hyperstimulation syndrome (OHSS) associated with the use of hCG, progesterone has become the treatment of choice for luteal phase support.
During the course of IVF treatment, progesterone is typically administered by i.m. injection or vaginal insertion. With the exception of the synthetic progestin dydrogesterone (Ganesh et al., 2011), the oral route of administration has been demonstrated to be less effective compared with the i.m. or vaginal routes (van der Linden et al., 2011). Intramuscular injections of various oil-based progesterone formulations are painful, can lead to significant skin inflammation and, on occasion, the formation of sterile abscesses. In addition, none of the progesterone in oil formulations has been approved by the United States Food and Drug Administration (FDA) for use in IVF. In contrast, two vaginal preparations (a gel and a vaginal insert) are FDA-approved for luteal phase support in IVF. Although vaginal formulations are generally well-tolerated, this route of administration may be associated with vaginal irritation and discharge and may not be desirable to some women for this reason, or due to personal or cultural considerations. Consequently, an efficacious and well-tolerated alternative to the vaginal and i.m. routes of progesterone administration would potentially be welcomed by both reproductive endocrinologists and women undergoing IVF.
To provide such an option, a water-soluble formulation of progesterone was developed that can be administered by subcutaneous injection. This formulation was made possible by the formation of a molecular complex of progesterone and the starch hydroxypropyl-β-cyclodextrin, which permits solubility in water of the otherwise only lipid-soluble progesterone (Zoppetti et al., 2007). A pharmacokinetic study has demonstrated that this aqueous preparation is promptly absorbed, achieving higher peak serum progesterone concentrations compared with i.m. administration of the same dose of an oil-based formulation (Sator et al., 2013). Aqueous progesterone administered subcutaneously has been demonstrated to produce adequate endometrial decidualisation (secretory transformation) at a daily dose of 25 and 50 mg in women who were down-regulated with GnRH agonist and then treated with estradiol to develop the endometrium (de Ziegler et al., 2013).
The objective of the present study was to compare the safety, efficacy and tolerability of subcutaneous aqueous progesterone (Prolutex 25 mg; IBSA Institut Biochimique SA, Switzerland) with vaginal progesterone inserts (Endometrin 100 mg twice daily; Ferring Pharmaceuticals, Parsippany, NJ, USA) for luteal support in patients undergoing IVF. The primary end-point for assessing efficacy in this study was ongoing pregnancy rate at 12 weeks of gestation.
Materials and Methods
Study design
This prospective, open-label, randomized, controlled, parallel-group, multicentre, two-arm, non-inferiority study was performed at eight fertility clinics in the USA. A total of 800 patients undergoing IVF were randomized to 2 groups: subcutaneous aqueous progesterone (Prolutex) 25 mg SC daily (n = 400) or vaginal progesterone (Endometrin) 100 mg twice daily (n = 400). Each site was asked to enrol 100 patients with equal numbers assigned to Prolutex and Endometrin. The primary outcome was defined as ongoing pregnancy rate at 12 weeks of gestation.
Patients with infertility, planning to undergo IVF with or without ICSI, were selected for possible study inclusion between January 2009 and June 2011. The eligibility criteria were female, age 18–42 years, BMI ≤30 kg/m2, less than three prior completed assisted reproductive technology (ART) cycles, baseline (Days 2–3) FSH ≤15 IU/l and estradiol <80 pg/ml and a normal uterine cavity as demonstrated by recent hysteroscopy, sonohysterogram or hysterosalpingogram. Eligibility for randomization required that at least three oocytes were retrieved and that the patient planned to proceed with a fresh embryo transfer.
Significant exclusion criteria included cavity-distorting intramural fibroids or polyps of >1 cm that were not removed prior to cycle initiation, stage III or IV endometriosis, untreated hydrosalpinx, history of previous poor response to gonadotrophin stimulation resulting in cancellation of the ART cycle, recurrent miscarriage defined as three or more spontaneous pregnancy losses after the development of a gestational sac on transvaginal ultrasound, donated oocytes, thawed embryos, gestational carrier, preimplantation genetic diagnosis or screening, supplemental luteal phase estrogen and untreated thyroid disease, adrenal disease or thromboembolic disorder. All patients who were screened but excluded met one of these exclusion criteria.
IVF and embryo transfer were performed according to site-specific protocols. The majority of patients were down-regulated with oral contraceptive pills in the pretreatment cycle (95% in both groups). Ovarian stimulation protocols included GnRH agonist (long and flare protocols) and GnRH antagonist. Gonadotrophin treatment included recombinant or urinary FSH plus human menopausal gonadotrophin (HMG) at individualized doses as recommended by the treating physicians. Final oocyte maturation was achieved with 5000–10 000 IU of hCG, and oocyte retrieval was performed 34–36 h later in accordance with the protocol followed at each clinic. Transfer could occur at either the cleaved or blastocyst stage. The number of embryos transferred was at the discretion of the investigator and the patient. Endometrial thickness was assessed on the day of embryo transfer. Variations in the choice of stimulation protocol, hCG dose and formulation for oocyte maturation, stage of transfer and number of embryos transferred were allowed, so that the study would reliably compare the two forms of progesterone luteal phase support in real-world practice. We also wanted to allow the treating physicians to choose the protocol and stage of transfer best suited for each patient based on data from their own practices as well as from the medical literature.
The study was designed, conducted, recorded and reported in compliance with the principles of Good Clinical Practice guidelines. The study was approved by an Institutional Review Board with jurisdiction at each site, and informed consent was obtained from each participant. The trial was registered with clinicaltrials.gov with the identifier NCT00828191.
Progesterone treatment and follow-up
Women who expressed an interest in participating provided informed consent and were screened during IVF treatment if an initial review of records suggested there was a high likelihood that they would fulfil all the inclusion/exclusion criteria (Fig. 1). On the day of oocyte retrieval, after confirmation that at least three oocytes were retrieved, women were randomized to Prolutex or Endometrin. Randomization was performed at each site using a randomization list that was generated by the study statistician using Statistical Analysis Software (SAS®), with the list for each site including 100 subjects, randomized in blocks of 4 to either Prolutex or Endometrin (i.e. 25 blocks of 4 subjects in each block, with 2 women assigned to each of the 2 treatments in each block). Randomization and enrolment were implemented by the research coordinator at each site who assigned randomization numbers in sequential/numerical order from the site's randomization list generated by the study statistician. Prolutex was provided to the patient as multiple vials each containing 25 mg of lyophilized progesterone and a 1-ml syringe of sterile water for solubilization (now available as a ready-to-use solution in some European countries), whereas Endometrin was supplied as vaginal inserts with vaginal applicators in individually sealed foil pouches. The first dose of Prolutex was administered under supervision shortly before the woman was to be discharged and the first vaginal Endometrin insert was self-administered on the day of retrieval at home following discharge.
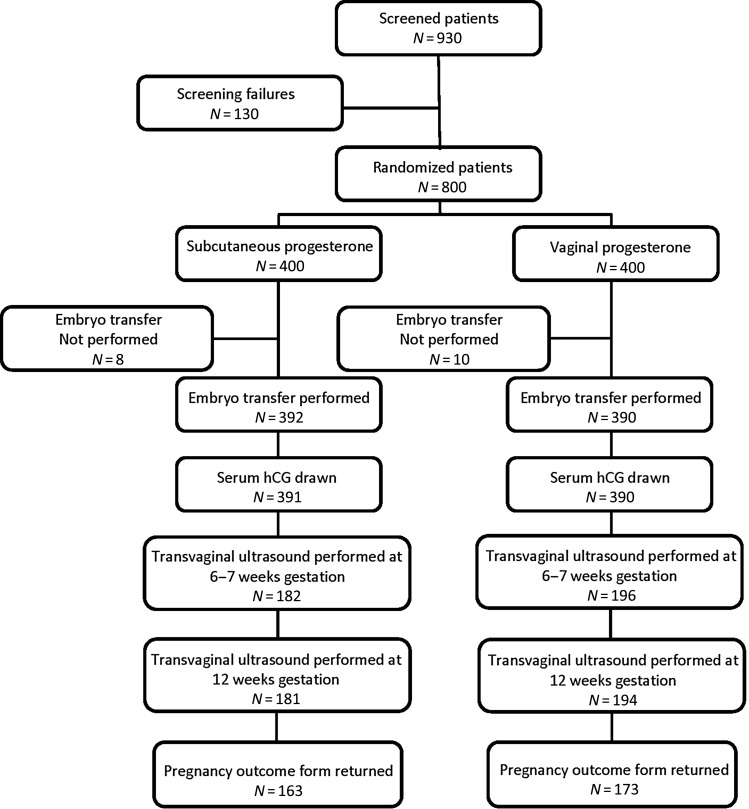
Figure 1.
Disposition of research subjects from screening to live birth.
A serum pregnancy test was performed at 15 ± 2 days after oocyte retrieval and, if positive, was repeated 2–3 days later. For patients with a rising β-hCG level, an ultrasound was performed at 6–7 weeks of gestation. If a viable intrauterine pregnancy was confirmed, the ultrasound was repeated at 12 weeks of gestation. Daily progesterone for luteal support was either continued to 12 weeks of gestation (a total of 10 weeks of progesterone administration) or discontinued if and when the pregnancy was found to be non-viable.
Throughout the study, women recorded all adverse events and concomitant medications in a diary. At each visit, specific questions were asked to obtain additional information on each particular route of administration. Women randomized to Prolutex were asked to describe the degree of itching and pain from injections, and the investigator assessed the last injection site using a rating scale for redness, tenderness, irritation and inflammation. Similarly, women randomized to Endometrin were asked about the presence of vaginal irritation, inflammation and itching, vaginal leakage and vaginal bleeding.
Patients with an ongoing pregnancy at the final visit were provided with a pregnancy outcome form for completion by their obstetrician following delivery. The patient was subsequently contacted by telephone 15 days after the expected delivery date if the completed form had not been received by that time.
Statistical analysis
The primary population for efficacy analyses was the per-protocol (PP) cohort, which included all women who underwent an embryo transfer, so chosen because women who did not receive an embryo could not get pregnant on protocol and therefore would not require luteal support. Moreover, treatment assignment could not have influenced outcome for those patients with regard to embryo availability for embryo transfer because Prolutex and Endometrin were administered only after oocyte retrieval, and any patient discontinuing progesterone due to issues related to product safety or tolerability after treatment initiation was included in the PP cohort as treatment failures. Analyses were also performed for the intention-to-treat (ITT) population (i.e. all women who received at least one dose of the assigned treatment) primarily for safety parameters, and for the population who became pregnant.
The sample size calculation assumed an ongoing pregnancy rate of 43% in both groups, a clinically significant difference of 10%, a two-sided alpha level of 0.05 and a beta level of 0.20 (80% power). Based on these assumptions, a total of 770 patients (385 per group) would be required to demonstrate non-inferiority.
The primary efficacy variable was the proportion of patients who had an ongoing viable pregnancy of 10 weeks after the start of progesterone treatment (12 weeks of gestation). The estimate of the difference in pregnancy rate between Prolutex and Endometrin with a corresponding two-sided 95% confidence interval (CI) was calculated using the standard formula for the large sample approximation of the standard error of the difference between two proportions. For the purpose of non-inferiority assessment, if the lower bound of the 95% CI of the difference was greater than −10%, then Prolutex was considered to be non-inferior to Endometrin.
For secondary continuous variables, statistical analyses were performed using analysis of variance models with a factor for treatment group (Prolutex versus Endometrin). For categorical variables, the effect of treatment group was analysed using the Fisher exact test or the Cochran–Mantel–Haenszel test, as appropriate. For comparisons involving multiple samples per patient (e.g. analyses where the sample units were transferred embryos or gestational sacs), Generalized Estimating Equation models with an independent covariance structure were used. The incidence of adverse events was compared using the two-sided Fisher exact test.
Simple logistic regression and multiple logistic regression models were used to assess the effect of baseline variables on pregnancy rates. In order to assess the effect of centres, a logistic regression model with factors for treatment (Prolutex versus Endometrin), centre and the treatment by centre interaction was employed.
Results
All 800 patients in this study were assigned to one of the two luteal support agents, and all received at least one dose of study medication or comparator (ITT cohort) (Fig. 1). Embryo transfer was not performed for 8 patients randomized to Prolutex (5 with failed fertilization or no progressing embryos, 2 for risk of OHSS and 1 with a lack of available sperm), and for 10 patients randomized to Endometrin (5 with failed fertilization or no progressing embryos, 4 for risk of OHSS, 1 for allergic reaction to tetracycline). If a patient did not receive an embryo, she was discontinued from the study and therefore did not complete the therapeutic plan (i.e. ∼15 days of luteal support and if pregnant at ∼15 days, treatment continuing for a total of 10 weeks). All patients who underwent embryo transfer were included in the PP cohort. There were four patients who discontinued treatment before the pregnancy test on Day 15 of treatment (including two in the Prolutex group who discontinued due to injection site reaction, and two in the Endometrin group who discontinued due to vaginal bleeding). These four patients who discontinued study medication before Day 15 of treatment did not violate the protocol and therefore were retained in the PP cohort as treatment failures to ensure an accurate estimate of product efficacy. There were five other patients in the PP Endometrin cohort who did not complete the therapeutic plan (three who switched to i.m. progesterone in oil, one who changed study medication and one who took a medication that was not allowed). These five patients were included in the PP analysis as treatment failures.
The Prolutex and Endometrin groups were well-matched with respect to baseline characteristics (Table I), and IVF treatment parameters (Table II). Women in both groups had a mean age of ∼34 years and a mean duration of infertility of ∼3 years. There was some ethnic diversity, with >20% Asian and 8.5% Hispanic women included. There were no significant differences between the treatment groups for age, race/ethnicity, BMI, infertility history, infertility diagnosis, basal FSH and estradiol levels, smoking, ovarian stimulation protocol, number of oocytes retrieved, number of embryos created and transferred, or stage of transfer. As noted in Table II, the majority of embryos were transferred at either the cleavage or blastocyst stage, with few transferred on Day 4. There were nine patients transferred on Day 2 (seven in the Prolutex group and two in the Endometrin group), four patients transferred on Day 6 (three in the Prolutex group and one in the Endometrin group) and one patient transferred on Day 7 (in the Prolutex group).
Table I.
Demographic characteristics of the intent-to-treat (ITT) study population.
| Prolutex (n = 400) | Endometrin (n = 400) | |
|---|---|---|
| Mean age in years (SD) | 34.3 (4.4) | 34.3 (4.5) |
| Race/ethnicity | ||
| African American | 2.5 | 3.5 |
| Asian | 21.5 | 20.3 |
| Caucasian | 67.3 | 66.3 |
| Hispanic | 8.5 | 8.5 |
| Other | 0.3 | 1.5 |
| Infertility diagnosis | ||
| Male factor | 49.0 | 50.8 |
| Tubal disease | 22.5 | 22.5 |
| Diminished ovarian reserve | 20.0 | 19.8 |
| Unexplained | 14.3 | 16.5 |
| Endometriosis | 11.0 | 7.8 |
| Polycystic ovarian syndrome | 10.5 | 8.3 |
| Anovulatory/ovarian dysfunction | 12.0 | 11.3 |
| Other | 3.8 | 4.5 |
| Duration of infertility in months (SD) | 35.8 (31.1) | 36.2 (27.1) |
| Nulliparity | 47.5 | 50.3 |
| Number prior miscarriages | ||
| 0 | 78.0 | 74.3 |
| 1 | 17.5 | 20.5 |
| 2 | 4.5 | 5.0 |
| Number prior completed IVF cycles | ||
| 0 | 80.0 | 82.3 |
| 1 | 16.3 | 13.8 |
| 2 | 3.8 | 3.5 |
| Body mass index (kg/m2) | ||
| Mean (SD) | 23.5 (3.1) | 23.7 (3.0) |
| Min–Max | 16.6–30.2 | 18.2–30.6 |
| Smoker | 2.0 | 2.3 |
| Baseline FSH in IU/l, mean (SD) | 7.1 (2.2) | 7.0 (2.2) |
| Min–Max | 0.2–14.5 | 1.3–14.9 |
Values are expressed as percentages unless otherwise noted.
Table II.
Fertility treatment parameters for the ITT study population.
| Prolutex (n = 400) | Endometrin (n = 400) | |
|---|---|---|
| Oral contraception pretreatment | 95.3 | 95.5 |
| Protocol (%) | ||
| GnRH agonist | 77.7 | 76.5 |
| Antagonist | 20.8 | 21.8 |
| Both (antagonist coast) | 1.5 | 1.8 |
| Trigger of oocyte maturation | ||
| Human (urinary) hCG | 86.0 | 87.0 |
| Recombinant hCG | 13.8 | 12.8 |
| Other | 0.3 | 0.3 |
| Total gonadotrophin dose in IUa | ||
| rFSH mean (SD) (# patients) | 2067 (1050) (381) | 2203 (1141) (378) |
| HMG mean (SD) (# patients) | 1081 (595) (375) | 1037 (551) (361) |
| hFSH mean (SD) (# patients) | 2449 (1302) (23) | 1852 (944) (23) |
| Number of oocytes retrieved, mean (SD) | 16.4 (8.9) | 15.7 (8.3) |
| ICSI (all or some of the mature oocytes) | 71.8 | 72.8 |
| Number of embryos created, mean (SD) | 9.2 (5.8) | 8.7 (5.6) |
| Endometrial thickness day of transfer in mm | ||
| mean (SD) | 10.9 (2.3) | 10.9 (2.6) |
| Day of embryo transfer, number (% in the timeframe) | ||
| Days 2–3 (cleaved) | 168 (42.9) | 171 (43.8) |
| Day 4 | 9 (2.3) | 8 (2.1) |
| Days 5–7 (blastocyst) | 215 (54.8) | 211 (54.1) |
| Number of embryos transferred (% with each number transferred except as noted for the mean) | ||
| Mean (SD, range) | 2.2 (0.8, 0–7) | 2.2 (0.8, 0–6) |
| 0 | 2.0 | 2.5 |
| 1 | 13.0 | 12.5 |
| 2 | 59.8 | 59.5 |
| 3 | 18.0 | 20.5 |
| 4 | 6.0 | 4.5 |
| ≥5 | 1.3 | 0.5 |
| Number embryos frozen, mean (SD) | 3.3 (4.1) | 2.8 (3.9) |
Values are expressed as percentages unless otherwise noted.
aOf the 800 patients, 56 received only rFSH, 3 received only HMG and 1 received only hFSH. Of the remaining patients, 690 received rFSH + HMG, 35 received hFSH + HMG and 15 received some other combination.
In the PP population, the ongoing pregnancy rates per oocyte retrieval at 12 weeks of gestation were comparable between the Prolutex and Endometrin groups (41.6 versus 44.4%), with a difference between groups of −2.8% (95% CI −9.7, 4.2). This finding is consistent with non-inferiority of Prolutex to Endometrin because the lower bound of the 95% CI of the difference between groups was greater than the pre-specified non-inferiority margin of −10%. Similar results were noted for Prolutex versus Endometrin in the ITT analysis (40.8 versus 43.3%), with a difference between groups of −2.5% (95% CI −9.4, 4.4).
Logistic regression analysis demonstrated that a number of variables were significantly correlated with outcome, including patient age, total stimulation dose, use of recombinant FSH, number of mature oocytes, total number of embryos created, percentage of available embryos transferred and day of embryo transfer. However, when these same variables were included in a multiple regression model, the only significant predictors were total stimulation dose (P < 0.001), use of recombinant FSH (P = 0.008) and day of transfer (P < 0.001), with patients given higher stimulation doses or recombinant FSH having lower pregnancy rates and those receiving Days 5–6 of transfer having higher pregnancy rates. The mean rFSH stimulatory dose (as a percentage of total dose) for non-pregnant versus pregnant women was 66.6 versus 61.8%, whereas the mean HMG dose (as a percentage of total dose) for non-pregnant versus pregnant women was 30.6 versus 34.1%, indicating that women in this study were more likely to become pregnant if a higher percentage of HMG and a lower percentage of rFSH were utilized in the stimulatory phase of the IVF cycle. Perhaps the reason patient age did not remain a predictor of outcome in the multiple regression model was that it is highly (positively) correlated with total gonadotrophin dosage, but the latter may be a more sensitive indicator of ongoing pregnancy as it will include younger patients who also required a high gonadotrophin dosage and whose ovarian reserve was therefore comparable with older patients. In another multiple regression model with treatment, centre and a treatment by centre interaction term included, there was an effect of centre as there frequently is in multicentre clinical trials (P = 0.003), but there was no effect of treatment (P = 0.428) or a treatment by interaction effect (0.944), the latter indicating there was no difference between centres in the relative efficacy of Prolutex and Endometrin.
No statistically significant differences between Prolutex and Endometrin were seen for any of the secondary efficacy variables, including rates of initial positive serum β-hCG, clinical pregnancy at 6–7 weeks of gestation, live birth, take-home baby, implantation, biochemical pregnancy loss or early pregnancy loss, irrespective of the study cohort evaluated (Table III). The numbers of women who achieved clinical pregnancy were 163 in the Prolutex group and 173 women in the Endometrin group. The take-home baby rate per embryo transfer (the most important outcome measure from the patient's perspective) for Prolutex versus Endometrin was 41.1 versus 42.6% (P = 0.72) in the PP population. The rates of biochemical pregnancy loss (positive β-hCG only without development of a gestational sac) and early clinical pregnancy loss (pregnancy loss up to 12 weeks of gestation after confirmation of a clinical pregnancy with fetal cardiac activity at 6–7 weeks of gestation) also did not differ between groups (Table III).
Table III.
Pregnancy and implantation rates by treatment group (in per cent), calculated for both the per-protocol (PP) and intent-to-treat (ITT) cohorts (PP/ITT, n = 392/400 for Prolutex, n = 390/400 for Endometrin).
| Prolutex | Endometrin | Difference versus vaginal (95% CI) (non-inferiority margin = 10%) | |
|---|---|---|---|
| Initial serum β-hCG positive | |||
| PP | 56.4 (221/392) | 59.0 (230/390) | −2.6 (−9.5, 4.3) |
| ITT | 55.3 (221/400) | 57.5 (230/400) | −2.2 (−9.1, 4.6) |
| Clinical pregnancy (6–7 weeks of gestation) | |||
| PP | 42.6 (167/392) | 46.4 (181/390) | −3.8 (−10.8, 3.2) |
| ITT | 41.8 (167/400) | 45.3 (181/400) | −3.5 (−10.4, 3.4) |
| Ongoing pregnancy (12 weeks of gestation—primary efficacy variable) | |||
| PP | 41.6 (163/392) | 44.4 (173/390) | −2.8 (−9.7, 4.2) |
| ITT | 40.8 (163/400) | 43.3 (173/400) | −2.5 (−9.4, 4.4) |
| Live birth | |||
| PP | 41.1 (161/392) | 43.1 (168/390) | −2.0 (−8.9, 4.9) |
| ITT | 40.3 (161/400) | 42.0 (168/400) | −1.7 (−8.6, 5.1) |
| Take-home baby | |||
| PP | 41.1 (161/392) | 42.6 (166/390) | −1.5 (−8.4, 5.4) |
| ITT | 40.3 (161/400) | 41.5 (166/400) | −1.3 (−8.1, 5.6) |
| Implantation (mean, SD) | |||
| PP | 33.2 (42.0) (N = 392) | 35.1 (40.9) (N = 390) | −1.8 (−7.6, 4.0) |
| Biochemical pregnancy lossa | |||
| 24.4 (54/221) | 21.3 (49/230) | 3.1 (−4.6, 10.9) | |
| Early pregnancy lossb | |||
| 2.4 (4/167) | 4.4 (8/181) | −2.0 (−5.9, 1.8) | |
Clinical pregnancy was defined as the presence of a gestational sac with a fetal heart beat at gestational age of 6–7 weeks. Implantation rate was defined as the number of gestational sacs divided by the number of embryos transferred for each individual patient then averaged. Early pregnancy loss was defined as loss up to 12 weeks of gestation after confirmation of fetal cardiac activity.
There was no statistically significant difference for any comparison between groups.
SD = standard deviation.
aCohort is comprised of patients who had a biochemical pregnancy.
bCohort is comprised of patients who were clinically pregnant at 6–7 weeks of gestation.
There was no evidence of a difference between Prolutex and Endometrin in women aged <35 years and women aged ≥35 years. In the PP population, the ongoing pregnancy rate for Prolutex versus Endometrin for women aged <35 years was 45.7 versus 50.5% (difference −4.7, 95% CI of the difference −14.1, 4.7). For women aged ≥35 years, the ongoing pregnancy rate for Prolutex versus Endometrin was 36.1 versus 36.9% (difference −0.8, 95% CI of the difference −11.0, 9.4). However, it should be noted, that similar to a previous study of luteal phase support (Doody et al., 2009), this study was not powered to examine differences in efficacy between treatment groups in specific age cohorts.
The number of adverse events and the proportion of study subjects experiencing at least one adverse event were similar between the two treatment groups. The most frequently reported adverse events, irrespective of treatment group, were abdominal pain and discomfort, constipation, diarrhoea, vaginal bleeding, OHSS, breast pain and tenderness, and headache. Adverse events, which could be associated with route of administration, occurred with similar frequency in each group. In the Prolutex group, 22.0% of women reported adverse events associated with injection, including injection site bruising, inflammation, oedema and injection site pain. In the Endometrin group, 14.5% reported side effects associated with vaginal administration including genital pruritus, vaginal discharge and vaginal pain/discomfort. In both groups, the majority of adverse reactions were reported as mild. Two patients in the Prolutex group experienced an injection site reaction before Day 15 of treatment and were discontinued from the study, and two in the Endometrin group were discontinued before the Day 15 pregnancy test due to vaginal bleeding.
Serious adverse events leading to cessation of progesterone treatment were mainly related to complications of pregnancy. Ectopic pregnancy was reported for five women (1.5%) in Prolutex group, and for four women (1.3%) in the Endometrin group. Non-viable intrauterine pregnancy was reported for 12 patients randomized to Prolutex (7 with spontaneous abortion, 4 with missed abortion and 1 with incomplete abortion) and for 12 patients randomized to Endometrin (9 with spontaneous abortion, 3 with missed abortion). Other serious adverse events that resulted in discontinuation were not related to progesterone treatment, including one ovarian torsion in the Prolutex group and one pelvic haematoma following oocyte retrieval in the Endometrin group.
Five patients in the Endometrin group were major protocol violators (three who switched to i.m. progesterone in oil, one who changed study medication and one who took a medication that was not allowed). In addition, two patients in the Endometrin group were later found to have completed >2 prior IVF cycles. In the Prolutex group, one patient was unintentionally randomized but did not receive study medication because only one oocyte was retrieved, and one patient had slightly lower creatinine clearance at study entry than required by the protocol (59.4 versus ≥60 ml/min) and was not excluded for this minor protocol violation.
Information was collected for 223 newborns (born to 163 mothers) in the Prolutex group and 228 newborns (born to 173 mothers) in the Endometrin group. There was one stillbirth in the Prolutex group and five stillbirths (from three pregnancies) in the Endometrin group. Neonatal demise after live birth was reported for one baby in the Prolutex group and for four babies (from two twin pregnancies) in the Endometrin group. Abnormalities were noted for eight babies in the Prolutex group (including club foot, Epstein abnormality, heart murmur, hydronephrosis, intraventricular haemorrhage and patent ductus arteriosis) and for two babies in the Endometrin group (Down's syndrome, neonatal tooth). There were no statistically significant differences for any of these outcomes, although it is acknowledged that the study was not specifically powered to detect differences in rates of stillbirth, neonatal death or birth defects.
Discussion
The findings of this study are consistent with the hypothesis that subcutaneous aqueous progesterone (Prolutex) of 25 mg daily is non-inferior to vaginal progesterone inserts (Endometrin) 100 mg twice daily for luteal phase support in women undergoing fresh embryo transfer IVF using autologous oocytes. This conclusion is based on the fact that the lower bound of the 95% CI for the treatment difference in the primary efficacy variable, ongoing pregnancy rate, was above the pre-specified non-inferiority limit of −10% for both the PP and ITT populations, a non-inferiority margin that was also employed for the original Endometrin study (Doody et al., 2009). There was also no difference between treatments for any of the secondary end-points, including rates of implantation, clinical pregnancy, live birth and take-home baby.
The findings in this study are consistent with a study comparing Prolutex with vaginal progesterone gel in patients undergoing in vitro fertilization in Europe (Lockwood et al., 2014). Of note, all of the cycles in the European study utilized the long agonist protocol and ICSI and few used pre-cycle treatment with oral contraceptives. The ongoing pregnancy and live birth rates reported herein were ∼10% higher than those reported by Lockwood et al., but both studies demonstrated non-inferiority of subcutaneous to vaginal progesterone. This consistency is reassuring given that there are differences in the patient population, approach to ovarian stimulation and likely other factors between the USA and Europe.
There is no agreement in the literature on the optimal route of progesterone administration during IVF (van der linden et al., 2011). Indeed, several observational studies have suggested that there is no difference in efficacy between i.m. and vaginal progesterone (Khan et al., 2009; Mitwally et al., 2010; Silverberg et al., 2012). A recent survey did, however, reveal geographic differences in the utilization of various routes of progesterone administration (Vaisbuch et al., 2012); worldwide, nearly two-third of IVF cycles utilize vaginal progesterone, whereas in North America, IM progesterone is utilized alone or with vaginal progesterone in 57% of cycles. Given that a single progesterone product is not preferentially administered during IVF, patient preference can and should be considered. Women prefer vaginal over IM progesterone because of the sequelae associated with the use of IM progesterone (Yanushpolsy et al., 2010). However, some women may prefer not to use the vaginal route for personal or cultural reasons. Subcutaneous administration avoids the concerns regarding vaginal administration and, unlike IM injections that are known to cause significant adverse reactions, the subcutaneous injections in this study were well-tolerated. Further, women undergoing IVF have practical experience with subcutaneous administration after using gonadotrophins earlier in the procedure. A reasonable approach is to consider providing women with choice, particularly when non-different efficacy has been demonstrated.
Analogous to the situation regarding route of administration, there is also no agreement in the literature on the optimal dose of progesterone for luteal phase support (van der Linden et al., 2011). There were several reasons for choosing the dose of Prolutex and Endometrin used in this study. One such reason was to use the minimum effective dose, and since both 25 and 50 mg daily doses of Prolutex led to decidualisation (secretory transformation) in reproductive-aged women who were down-regulated with GnRH agonist and treated with estradiol to develop the endometrium (de Ziegler et al., 2013), 25 mg was considered a reasonable choice. Regarding Endometrin, 100 mg twice daily was FDA-approved and was demonstrated to be effective in a large, randomized trial (Doody et al., 2009) and was chosen to also be consistent with the principle of choosing the lowest effective dose. Another reason is safety, and in a pharmacokinetic study, fewer skin reactions were seen with the 25 mg dose of Prolutex than either the 50 or 100 mg doses (Sator et al., 2013). This dose also relates to the daily production of progesterone, which has been estimated to be 25 mg (Strauss and Barbieri, 2004).
Analogous to the opinions regarding route and dose, there is no agreement in the literature on the optimal time to initiate progesterone treatment for luteal phase support (van der Linden et al., 2011). Part of this uncertainty is owing to the fact that the timing and duration of implantation are not precisely known (Paulson, 2011). In other studies, progesterone has been initiated on the day of retrieval in some or all patients (Baruffi et al., 2003; Mochtar et al., 2006), the day after retrieval (Doody et al., 2009; Yanushpolsky et al., 2010; Stadtmauer et al., 2013) or 2 days after retrieval (Silverberg et al., 2012), without an obvious impact on treatment effect. In our study, the first dose of subcutaneous progesterone was administered on the day of (but immediately after) oocyte retrieval, so that the administration could be performed under the supervision of a healthcare provider and the injection site could be observed to ensure that the subject did not experience a significant allergic reaction. Vaginal progesterone was also initiated on the day of oocyte retrieval but was not performed in the clinic to allow the vagina to recover following the retrieval procedure.
The strengths of this study include a large sample size, randomized study subjects, general applicability to the real-life practice of IVF due to broad inclusion criteria with no restrictions on the ovarian stimulation protocol, and a low drop-out rate. In addition, the subcutaneous administration of progesterone is novel. There has been only one other publication (Lockwood et al., 2014) providing clinical outcome data about this route of administration.
There are several limitations of this study. First, the findings are limited to one daily dose of subcutaneous progesterone (25 mg) and a twice-daily dose of vaginal progesterone (100 mg/dose). All patients were treated until 12 weeks of gestation, which is consistent with recently published studies (Doody et al., 2009; Stadtmauer et al., 2013) and international standard of care (Vaisbuch et al, 2012). Thus, it is not possible to know whether a shorter duration of treatment would have been equally effective, as has been suggested in other studies (Aboulghar et al, 2008; Kohls et al., 2012; Liu et al., 2012). Second, because women with a BMI of >30 were excluded, the findings from this study cannot be extrapolated to obese women, even though there were no significant differences between the progesterone treatment groups within the BMI range included in this study. Third, the study was open-label (not blinded). A placebo injection for the Endometrin group and a placebo vaginal insert for the Prolutex group (double-dummy design) could have been utilized, but this was not done for several reasons. The primary outcome measure for this study, ongoing clinical pregnancy, was non-subjective, and it would be highly unlikely that either physician or patient bias could affect study outcome (i.e. neither will be biased against the patient becoming pregnant). As detailed in Table II, there were no differences in treatment parameters between the Prolutex and Endometrin group, substantiating the assertion that the physicians treating the patients were not treating the patients differently based on study group assignment. In addition, double-dummy studies are difficult to execute, particularly with regard to dosing compliance. Although subjects had the opportunity to report all adverse events experience during the study, specific questions about vaginal symptoms were only asked of women taking Endometrin and specific questions about skin reactions were only asked of women taking Prolutex. Notwithstanding this possibility, the primary efficacy variable (ongoing pregnancy rate) is an objective measure that would not be influenced by investigator or subject bias.
Conclusion
This study demonstrated that a novel formulation of progesterone suitable for subcutaneous administration (Prolutex) was well-tolerated and non-inferior in efficacy to a vaginal insert (Endometrin) for luteal phase support in IVF. A subcutaneous formulation may be appealing to women who prefer to avoid the i.m. and vaginal routes of administration.
Authors' roles
V.L.B. contributed to the study design and study execution and wrote the manuscript. C.J. and C.A.J. contributed to study design, study execution, database review, and manuscript review and approval. K.D., R.F., B.Y., G.D.A., G.D., G.H. and M.S. contributed to study design, execution and manuscript review and approval. B.C. and S.T. contributed to study design, database review and manuscript review and approval. F.H. provided the statistical analysis plan and sample size calculations prior to study initiation, performed statistical analysis of the data and contributed to manuscript review and approval.
Funding
The study was funded by Institut Biochimique SA (IBSA). C.A.J., B.C., S.T. and C.J. are employees of IBSA. Each site participating in the study received funding from IBSA necessary to conduct the study. Funding to pay the Open Access Publication charges for this article was provided by Institut Biochimque (IBSA).
Conflict of interest
C.J., C.J., S.T. and B.C. are employees of IBSA. F.H. served as a statistical consultant for IBSA. Valerie Baker received travel support to attend a meeting. The remaining authors have no relevant conflict of interest.
Acknowledgements
Karen Purcell, MD contributed to study design and patient recruitment during the early phase of the study.
References
- Aboulghar MA, Amin YM, Al-Inany HG, Aboulghar MM, Mourad LM, Serour GI, Mansour RT. Prospective randomized study comparing luteal phase support for ICSI patients up to the first ultrasound compared with an additional three weeks. Hum Reprod. 2008;23:857–862. doi: 10.1093/humrep/den012. [DOI] [PubMed] [Google Scholar]
- Baruffi R, Mauri AL, Petersen CG, Felipe V, Franco JG., Jr Effects of vaginal progesterone administration starting on the day of oocyte retrieval on pregnancy rates. J Assisted Reprod Genet. 2003;20:517–520. doi: 10.1023/B:JARG.0000013653.54830.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ziegler D, Sator M, Binelli D, Leuratti C, Cometti B, Bourgain C, Fu YS, Garhofer G. A randomized trial comparing the endometrial effects of daily subcutaneous administration of 25 mg and 50 mg progesterone in aqueous preparation. Fertil Steril. 2013;100:860–866. doi: 10.1016/j.fertnstert.2013.05.029. [DOI] [PubMed] [Google Scholar]
- Doody KJ, Schnell VL, Foulk RA, Miller CE, Kolb BA, Blake EJ, Yankov VI. Endometrin for luteal phase support in a randomized, controlled, open-label, prospective in vitro fertilization trial using a combination of Menopur and Bravelle for controlled ovarian hyperstimulation. Fertil Steril. 2009;91:1012–1017. doi: 10.1016/j.fertnstert.2008.01.069. [DOI] [PubMed] [Google Scholar]
- Ganesh A, Chakravorty N, Mukherjee R, Goswami S, Chaudhury K, Chakravarty B. Comparison of oral dydrogestrone with progesterone gel and micronized progesterone for luteal support in 1,373 women undergoing in vitro fertilization: a randomized clinical study. Fertil Steril. 2011;95:1961–1965. doi: 10.1016/j.fertnstert.2011.01.148. [DOI] [PubMed] [Google Scholar]
- Khan N, Richter KS, Newsome TL, Blake EJ, Yankov VI. Matched-samples comparison of intramuscular versus vaginal progesterone for luteal phase support after in vitro fertilization and embryo transfer. Fertil Steril. 2009;91:2445–2450. doi: 10.1016/j.fertnstert.2008.03.072. [DOI] [PubMed] [Google Scholar]
- Kohls G, Ruiz F, Martinez M, Hauzman E, de la Fuente G, Pellicer A, Garcia-Velasco JA. Early progesterone cessation after in vitro fertilization/intracytoplasmic sperm injection: a randomized, controlled trial. Fertil Steril. 2012;98:858–862. doi: 10.1016/j.fertnstert.2012.05.046. [DOI] [PubMed] [Google Scholar]
- Liu XR, Mu HQ, Shi Q, Xiao XQ, Qi HB. The optimal duration of progesterone supplementation in pregnant women after IVF/ICSI: a meta-analysis. Reprod Biol Endocrinol. 2012;10:107. doi: 10.1186/1477-7827-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood G, Griesinger G, Cometti B 13 European centers. Subcutaneous progesterone versus vaginal progesterone gel for luteal phase support in in vitro fertilization: a noninferiority randomized controlled study. Fertil Steril. 2014;101:112–119. doi: 10.1016/j.fertnstert.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Mitwally MF, Diamond MP, Abuzeid M. Vaginal micronized progesterone versus intramuscular progesterone for luteal support in women undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2010;93:554–569. doi: 10.1016/j.fertnstert.2009.02.047. [DOI] [PubMed] [Google Scholar]
- Mochtar MH, Van Wely M, Van der Veen F. Timing luteal phase support in GnRH agonist down-regulated IVF/embryo transfer cycles. Hum Reprod. 2006;21:905–908. doi: 10.1093/humrep/dei437. [DOI] [PubMed] [Google Scholar]
- Paulson RJ. Hormonal induction of endometrial receptivity. Fertil Steril. 2011;96:530–535. doi: 10.1016/j.fertnstert.2011.07.1097. [DOI] [PubMed] [Google Scholar]
- Sator M, Radicioni M, Cometti B, Loprete L, Leuratti C, Schmidl D, Garhofer G. Pharmacokinetics and safety profile of a novel progesterone aqueous formulation administered by the s.c. route. Gynecol Endocrinol. 2013;29:205–208. doi: 10.3109/09513590.2012.736560. [DOI] [PubMed] [Google Scholar]
- Silverberg KM, Vaughn TC, Hansard LJ, Burger NZ, Minter T. Vaginal (Crinone 8%) gel vs. intramuscular progesterone in oil for luteal phase support in in vitro fertilization: a large prospective trial. Fertil Steril. 2012;97:344–348. doi: 10.1016/j.fertnstert.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Stadtmauer L, Silverberg KM, Ginsburg ES, Weiss H, Howard B. Progesterone vaginal ring versus vaginal gel for luteal support with in vitro fertilization: a randomized comparative study. Fertil Steril. 2013;99:1543–1549. doi: 10.1016/j.fertnstert.2012.12.052. [DOI] [PubMed] [Google Scholar]
- Strauss JFI, Barbieri R. Yen and Jaffe's Reproductive Endocrinology. 5th edn. Philadelphia, USA: Elsevier Saunders; 2004. The synthesis and metabolism of steroids hormones. [Google Scholar]
- Vaisbuch E, Leong M, Shoham Z. Progesterone support in IVF: is evidence-based medicine translated to clinical practice? A worldwide web-based survey. Reprod Biomed Online. 2012;25:139–145. doi: 10.1016/j.rbmo.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database of Systematic Reviews (Online) 2011 doi: 10.1002/14651858.CD009154.pub2. CD009154. [DOI] [PubMed] [Google Scholar]
- Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein M. Crinone vaginal gel is equally effective and better tolerated than intramuscular progesterone for luteal phase support in in vitro fertilization-embryo transfer cycles: a prospective randomized study. Fertil Steril. 2010;94:2596–2599. doi: 10.1016/j.fertnstert.2010.02.033. [DOI] [PubMed] [Google Scholar]
- Zoppetti GPN, Pizzutti M, Fini A, Giovani T, Comini S. Water soluble progesterone–hydroxypropyl-b-cyclodextrin complex for injectable formulations. J Incl Phenom Macrocycl Chem. 2007;57:283–288. [Google Scholar]