Abstract
Iron-sulfur (Fe/S) proteins are located in mitochondria, cytosol, and nucleus. Mitochondrial Fe/S proteins are matured by the iron-sulfur cluster (ISC) assembly machinery. Little is known about the formation of Fe/S proteins in the cytosol and nucleus. A function of mitochondria in cytosolic Fe/S protein maturation has been noted, but small amounts of some ISC components have been detected outside mitochondria. Here, we studied the highly conserved yeast proteins Isu1p and Isu2p, which provide a scaffold for Fe/S cluster synthesis. We asked whether the Isu proteins are needed for biosynthesis of cytosolic Fe/S clusters and in which subcellular compartment the Isu proteins are required. The Isu proteins were found to be essential for de novo biosynthesis of both mitochondrial and cytosolic Fe/S proteins. Several lines of evidence indicate that Isu1p and Isu2p have to be located inside mitochondria in order to perform their function in cytosolic Fe/S protein maturation. We were unable to mislocalize Isu1p to the cytosol due to the presence of multiple, independent mitochondrial targeting signals in this protein. Further, the bacterial homologue IscU and the human Isu proteins (partially) complemented the defects of yeast Isu protein-depleted cells in growth rate, Fe/S protein biogenesis, and iron homeostasis, yet only after targeting to mitochondria. Together, our data suggest that the Isu proteins need to be localized in mitochondria to fulfill their functional requirement in Fe/S protein maturation in the cytosol.
Iron-sulfur (Fe/S) proteins perform central functions in catalysis, electron transport, and regulation of biological processes (1, 2). They possess an inorganic cofactor that, in its simplest version, consists of two or four iron and sulfur atoms, the so-called [2Fe-2S] or [4Fe-4S] clusters, respectively (3). During the past few years, complex machineries have been discovered that assist in the assembly of the Fe/S clusters and in their insertion into apoproteins. The nitrogen fixation (NIF) machinery is dedicated to the assembly of Fe/S clusters in the complex metalloprotein nitrogenase of nitrogen-fixing bacteria (13, 43). The sulfur mobilization (SUF) machinery is present in Bacteria, Archaea, and the apicoplast of parasites and particularly is required under oxidative stress and iron-limiting conditions (32, 40, 54). The ISC (iron-sulfur cluster) assembly machinery found in Bacteria and mitochondria of Eukarya comprises some 10 components (8, 14, 16, 31). In many bacteria, the encoding genes are frequently organized in clusters termed isc operons (67).
Functional studies of Fe/S protein biogenesis in eukaryotes have been performed with the model organism Saccharomyces cerevisiae, in which Fe/S cluster assembly takes place in mitochondria (see, e.g., references 15, 23, 26, 29, 33, 47, and 53). The presence of ISC homologues in virtually all eukaryotic species suggests that the process is highly conserved in Eukarya. Central players of the pathway are the cysteine desulfurase Nfs1p, which releases sulfur from cysteine, and the highly homologous proteins Isu1p and Isu2p, which serve as scaffolds for the assembly of the clusters (34). The chemistry of Fe/S cluster formation is still under investigation, mainly using ISC components isolated from bacteria (21, 39, 51, 66). One model proposes that ferrous iron associates with conserved cysteine residues of the Isu (or bacterial IscU) proteins. Complex formation with Nfs1p (or the bacterial homolog IscS or NifS) (48, 68) leads to the transfer of sulfur from a cysteine persulfide moiety on Nfs1p to a cysteine of Isu/IscU and the subsequent formation of the Fe/S cluster. Further components required for Fe/S cluster synthesis on the Isu proteins are an electron transfer chain consisting of the ferredoxin reductase Arh1p and the ferredoxin Yah1p, which likely transfer electrons to sulfur (S0) released from cysteine in order to form the sulfide (S2−) present in the cluster (26, 29). The loading of ferrous iron onto the Isu scaffold proteins is facilitated by their interaction with frataxin (yeast Yfh1p), a protein depleted in the neurodegenerative disease Friedreich's ataxia (7, 17, 65). Crucial proteins that are required later in biosynthesis are the heat shock proteins Ssq1p and Jac1p, which interact with Isu1p and Isu2p, and the mitochondrial glutaredoxin Grx5p (12, 22, 33, 44, 59, 60).
Fe/S proteins are found not only in mitochondria but also in the cytosol and the nucleus (16). Previous studies suggested that Fe/S protein maturation in the cytosol requires the function of mitochondria (see, e.g., references 5, 23, 26, and 29). For instance, it was shown that yeast Nfs1p was required inside the organelles for its functional involvement in cytosolic Fe/S protein biogenesis. Other components of the yeast mitochondrial ISC assembly machinery were also found to be essential for maturation of Fe/S proteins in the cytosol, such as the electron transfer chain Arh1p/Yah1p and Yfh1p. It was proposed that export of a yet unknown compound from mitochondria to the cytosol is facilitated by the ABC transporter Atm1p of the inner membrane, thereby supporting cytosolic Fe/S protein maturation (23, 31). Atm1p is needed specifically for cytosolic but not for mitochondrial Fe/S protein maturation and may be assisted by the sulfhydryl oxidase Erv1p of the intermembrane space and by the tripeptide glutathione (27, 50). Nevertheless, some ISC assembly components have been identified not only in mitochondria but also in the cytosol and nucleus (25, 37, 55, 56). Even though the extramitochondrial ISC proteins are present at low concentrations, these components may be responsible for Fe/S protein assembly and/or repair outside mitochondria. However, experimental evidence for the functionality of the cytosolic ISC components has not been provided yet. Interestingly, a function of extramitochondrial Nfs1p in the synthesis of thionucleotides of tRNA has been demonstrated recently (38; G. Kispal and U. Mühlenhoff, unpublished data).
In this report, we used yeast as a model system and asked questions about whether the scaffold proteins Isu1p and Isu2p participate in cytosolic Fe/S protein maturation and in which subcellular compartment they perform this possible function. By employing a yeast mutant that allowed the regulated expression of the ISU genes, we demonstrate that the Isu proteins support the de novo synthesis of Fe/S proteins not only in mitochondria but also in the cytosol. For the latter function, mitochondrial localization of the Isu proteins was essential. The fact that both the sulfur donor Nfs1p (23) and the Fe/S cluster assembly proteins Isu1p and Isu2p (this work) are required inside mitochondria to assist in cytosolic Fe/S protein maturation reinforces the concept of a primary function of mitochondria in Fe/S protein maturation in the entire cell.
MATERIALS AND METHODS
Yeast strains and cell growth.
The following strains of S. cerevisiae were used. W303-1A (MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100) served as the wild type. The ISU1 and ISU2 genes were deleted in wild-type cells by a PCR-based method employing a histidine-selective marker (62) (strains Δisu1 and Δisu2). Exchange of the endogenous promoter of the ISU1 gene for a galactose-inducible promoter in Δisu2 cells (strain Gal-ISU1/Δisu2) was performed as described by Lafontaine and Tollervey (http://www.mips.biochem.mpg.de/proj/yeast/info/tools/tollervey/pcr.html). The galactose-inducible promoter (GAL1-10) from plasmid pTL26 was cloned into pRS315 as a BamHI/HindIII fragment to yield plasmid pRS315-GAL1-10. Its LEU2/GAL1-10 cassette was used as target DNA for ISU1 promoter exchange by PCR using primers matching nucleotides −477 to −427 and 37 to −15 of the ISU1 gene. Growth of yeast cells was as detailed previously using rich (YP) or synthetic complete medium (49) and lactate medium (10) (with 0.1% glucose) containing the required carbon sources. For experiments involving cell labeling with radioactive 55Fe, no iron salt was added to the synthetic minimal medium (iron-poor medium).
Plasmids.
The different ISU1 constructs were expressed under the control of the MET25 promoter from a high-copy-number vector (p426Met25; constructed according to reference 36). ISU1 was cloned without the putative mitochondrial targeting sequence (yielding Isu1pΔ30; codons 31 to 165) or fused in frame with the Neurospora crassa F0-ATPase subunit 9 presequence (pSu9-Isu1pΔ30; pSu9, codons 1 to 69; ISU1, codons 31 to 165). To synthesize Isu1p with a negatively charged prepiece (DAE-Isu1pΔ30), the ISU1 gene (codons 31 to 165) was amplified by PCR with a 5′ primer additionally coding for the tripeptide DAE.
The human cDNAs encoding cytosolic hIsu1 (IMAGE clone 3617035; RZPD, Berlin, Germany) and mitochondrial hIsu2 (IMAGE clone 3900148) were used for complementation experiments. The coding sequences of hIsu1 and of the mature form of hIsu2 (codons 34 to 167) were cloned into the yeast vector p424GPD (36) or were fused to the mitochondrial presequence of subunit β of N. crassa F1-ATPase (pF1β; codons 1 to 40) before cloning into the same vector. These constructs were used to attach a C-terminal hemagglutinin (HA) epitope tag to the human Isu proteins. The coding sequences were inserted into vector p424GPD. The iscU gene from Escherichia coli was amplified by PCR using chromosomal DNA and cloned into the vector p416Met25 (36) either without modification or fused in frame to the coding sequence of N. crassa pSu9 presequence (encoding the fusion protein pSu9-IscU). All constructs were verified by DNA sequencing.
Miscellaneous methods.
The following published methods were used: manipulation of DNA and PCR (46), transformation of yeast cells (18), and isolation of yeast mitochondria and postmitochondrial supernatant (11). The mitochondrial iron content was measured with the iron-chelator bathophenanthroline-disulfonic acid (28). The labeling of yeast cells with radioactive iron (55Fe; 2 h for mitochondrial and 4 h for cytosolic Fe/S proteins) and the measurement of the 55Fe incorporation into various Fe/S proteins by immunoprecipitation and liquid scintillation counting were performed as described earlier (23, 35). Experiments were performed at least three times.
RESULTS
Generation of a mutant strain for regulated expression of ISU1.
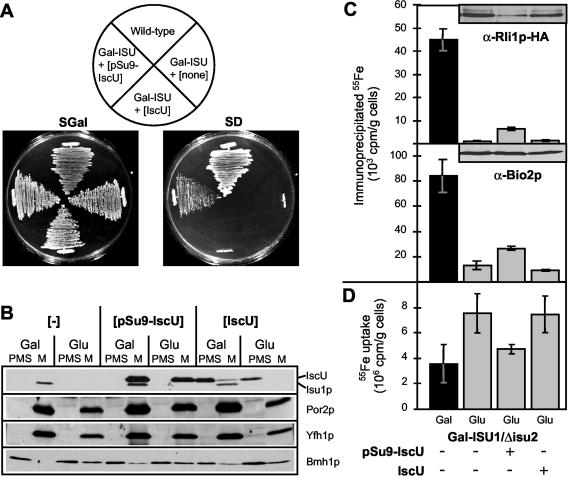
Yeast mutants were constructed in which either the ISU1 or ISU2 genes were deleted (Δisu1 and Δisu2 strains). In our strain background (W303), these mutants showed wild-type growth and did not display significant defects in mitochondrial and cytosolic Fe/S proteins (not shown). This indicated that Isu1p and Isu2p could replace each other and hence perform overlapping functions. To study the consequences of an Isu protein deficiency, we created a yeast strain that allowed the regulated expression of ISU1 in the absence of functional Isu2p. The Δisu2 strain was used to generate the mutant Gal-ISU1/Δisu2 in which the ISU1 gene is under the control of the GAL1-10 promoter, and thus Isu1p levels can be down-regulated by growth of cells in the absence of galactose. Gal-ISU1/Δisu2 cells grew at wild-type rates in the presence of galactose yet did not give rise to colonies on glucose-containing agar plates, indicating that depletion of Isu1p in the absence of Isu2p is lethal (Fig. 1A) (15, 47). When Gal-ISU1/Δisu2 cells were grown in liquid medium, they could undergo three to five duplications after transfer to medium containing glucose as the sole carbon source before growth arrest (not shown). Notably, the growth characteristics of Gal-ISU1/Δisu2 cells were similar to those of Gal-NFS1 cells (Fig. 1A) (23).
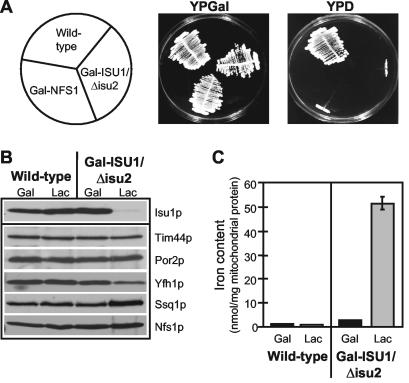
FIG. 1.
Deficiency of the Isu proteins impairs growth and results in mitochondrial iron accumulation in Gal-ISU1/Δisu2 cells. (A) Wild-type, Gal-NFS1 (23), and Gal-ISU1/Δisu2 cells were grown on agar plates with rich medium containing galactose (YPGal) or glucose (YPD) for 3 days at 30°C. (B) Mitochondria were isolated from wild-type and Gal-ISU1/Δisu2 cells grown in YPGal or lactate medium containing 0.1% glucose (Lac) (11). Equal amounts of protein (50 μg) were analyzed by immunostaining with antisera raised against Isu1p and the indicated mitochondrial proteins. (C) The non-heme, non-Fe/S iron content of isolated mitochondria was measured by the bathophenanthroline method. Standard deviations were derived from three independent experiments.
Mitochondria were isolated from Gal-ISU1/Δisu2 cells grown for 16 h in glucose-containing medium and analyzed by immunostaining. Almost quantitative depletion of Isu1p was seen relative to wild-type mitochondria or mitochondria isolated from Gal-ISU1/Δisu2 cells that were grown in the presence of galactose (Fig. 1B). The amounts of other mitochondrial proteins needed for preprotein or metabolite transport were not altered upon depletion of Isu1p. Proteins of the ISC assembly machinery were either slightly increased, such as Nfs1p or Ssq1p, or somewhat decreased, such as Yfh1p (yeast frataxin). We noted, however, that in the absence of Isu1p and Isu2p, the amounts and activities of several mitochondrial Fe/S proteins were decreased (not shown). This is consistent with earlier findings that showed a crucial role of the Isu proteins in mitochondrial Fe/S protein biogenesis (15, 34, 47). Further, Isu protein-depleted mitochondria accumulated 20-fold-larger amounts of iron than wild-type organelles (Fig. 1C). This phenotype is characteristic for yeast cells exhibiting defects in the assembly of cellular Fe/S proteins. Thus, the strain Gal-ISU1/Δisu2 is suitable for functional studies of the Isu proteins in Fe/S protein maturation.
The Isu proteins are required for de novo biogenesis of mitochondrial and cytosolic Fe/S proteins.
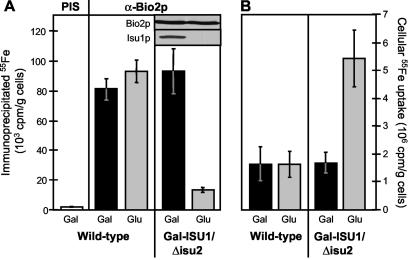
We first tested whether the Isu proteins are required for the de novo formation of mitochondrial Fe/S proteins and chose biotin synthase (Bio2p) as a model Fe/S protein (41). Wild-type and Gal-ISU1/Δisu2 cells were grown overnight in iron-poor minimal medium containing either galactose or glucose. The incorporation of 55Fe into overproduced Bio2p was analyzed by radiolabeling cells for 2 h with 55Fe in the presence of ascorbate, preparation of a cell extract, and immunoprecipitation of the Fe/S protein by using specific anti-Bio2p antibodies. The radioactivity associated with the immunobeads reflects the de novo assembly of an Fe/S cluster with the respective apoprotein and can be estimated by scintillation counting (23). Comparable amounts of radioactivity were associated with Bio2p in wild-type cells and in Gal-ISU1/Δisu2 cells grown in the presence of galactose (Fig. 2A). Only a small amount of radioactivity was coimmunoprecipitated with preimmune serum. Upon depletion of Isu1p by growth in glucose-containing medium, hardly any radioactivity was associated with Bio2p despite unchanged levels of the Bio2p polypeptide chain (inset). These data indicate that the role of the Isu proteins in maintaining the activity of mitochondrial Fe/S proteins (15, 47; not shown) is explained by their necessity for de novo Fe/S cluster synthesis. 55Fe uptake into Isu1p-depleted cells was increased about threefold (Fig. 2B), a finding compatible with the iron accumulation in mitochondria (see above).
FIG. 2.
Depletion of Isu1p in Gal-ISU1/Δisu2 cells causes defects in the de novo biogenesis of mitochondrial Fe/S proteins and increases cellular iron uptake. Wild-type and Gal-ISU1/Δisu2 cells were transformed with the plasmid p426GPD carrying the BIO2 gene coding for mitochondrial biotin synthase Bio2p. Cells were grown in iron-poor minimal medium containing galactose (Gal) or glucose (Glu). They were radiolabeled with [55Fe]iron chloride in the presence of 1 mM ascorbate, and a cell extract was prepared. The amount of Fe/S cluster incorporation into Bio2p was estimated by immunoprecipitation with Bio2p-specific antibodies (α-Bio2p) and quantitation of coimmunoprecipitated 55Fe by liquid scintillation counting. A control immunoprecipitation was performed with preimmune serum (PIS) to detect the background levels of 55Fe precipitation. The inset shows the amounts of Bio2p and Isu1p in Gal-ISU1/Δisu2 cells detected by immunostaining of cell extracts (50 μg). (B) The amounts of 55Fe uptake by the cells were quantified by liquid scintillation counting. Standard deviations were calculated from three independent experiments.
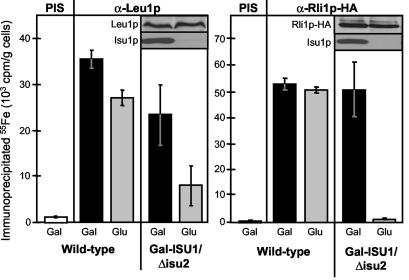
Next, de novo formation of cytosolic Fe/S proteins was studied by using isopropylmalate isomerase Leu1p and the essential Rli1p as model Fe/S proteins. The latter protein was overproduced as a hemagglutinin-tagged version (termed Rli1p-HA) (27). Upon depletion of Isu1p in Gal-ISU1/Δisu2 cells, a strong decrease of 55Fe/S cluster assembly on both Leu1p and Rli1p-HA was observed compared with that in wild-type cells or Gal-ISU1/Δisu2 cells containing Isu1p (Fig. 3). The levels of both proteins were not significantly altered upon depletion of Isu1p, as shown by immunostaining (insets in Fig. 3). These results demonstrate that the Isu proteins are also required for the biosynthesis of Fe/S proteins in the cytosol.
FIG. 3.
The Isu proteins are required for the maturation of cytosolic Fe/S proteins. Wild-type and Gal-ISU1/Δisu2 cells were transformed with the plasmid p426GPD carrying the gene encoding Rli1p-HA. Cell growth, radiolabeling with 55Fe, and immunoprecipitation using antibodies against Leu1p (α-Leu1p) or the HA tag (α-Rli1p-HA) were performed as described in the legend to Fig. 2A. Control immunoprecipitations were performed with preimmune serum (PIS). The insets show the amounts of Leu1p, Rli1p-HA, and Isu1p in Gal-ISU1/Δisu2 cells detected by immunostaining. Standard deviations were estimated from five independent experiments.
Failure to mislocalize Isu1p to the yeast cytosol.
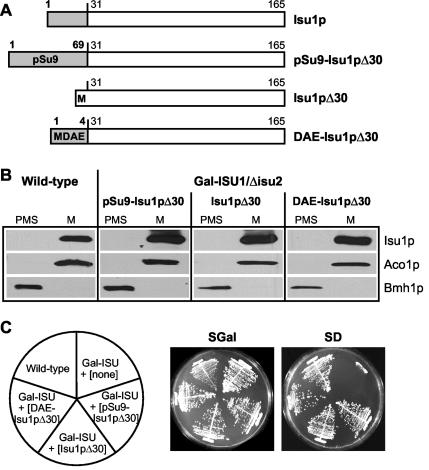
Both Isu1p and Isu2p were found to be located exclusively in the mitochondrial matrix in yeast (15, 47). Nevertheless, based on findings with yeast Nfs1p (37), it cannot be excluded from such cell fractionation data that minimal amounts of the Isu proteins may be located in other subcellular compartments. This fraction may be responsible for maturation of extramitochondrial Fe/S proteins. To study the potential activity of Isu1p outside mitochondria, we attempted to mislocalize the protein by removing its N-terminal mitochondrial targeting sequence. Upon synthesis of the truncated protein termed Isu1pΔ30 (Fig. 4A) in Isu1p-depleted Gal-ISU1/Δisu2 cells, Isu1pΔ30 was detectable only in mitochondria (Fig. 4B). As expected from this result, Isu1pΔ30 fully complemented the growth defect of Gal-ISU1/Δisu2 cells after depletion of endogenous Isu1p in glucose-containing medium (Fig. 4C). Apparently, Isu1p contains targeting information in parts other than the N terminus of its precursor form.
FIG. 4.
Isu1p lacking its N-terminal presequence is targeted efficiently to mitochondria. (A) Schematic description of various yeast Isu1p mutant proteins either carrying another N-terminal mitochondrial targeting sequence from the F0-ATPase subunit 9 of N. crassa (pSu9-Isu1pΔ30), lacking its N-terminal presequence (Isu1pΔ30), or containing a short negatively charged tripeptide (DAE-Isu1pΔ30) instead of the presequence. Numbers indicate the amino acid positions in the proteins. The white boxes represent wild-type mature Isu1p, and the light gray boxes represent the various N-terminal extensions. (B) Plasmids (p426Met25) carrying the indicated ISU1 mutants (described for panel A) were transformed into Gal-ISU1/Δisu2 cells, and cells were grown in glucose-containing minimal medium to deplete endogenous Isu1p. Mitochondria (M) and postmitochondrial supernatants (PMS) were isolated from wild-type cells and from Gal-ISU1/Δisu2 cells expressing the different ISU1 mutants (11). Equal amounts of protein (50 μg) were analyzed by immunostaining with specific antisera against Isu1p, the mitochondrial marker Aco1p, and the cytosolic marker Bmh1p. (C) Wild-type cells and Gal-ISU1/Δisu2 (Gal-ISU) cells transformed with plasmids encoding various Isu1p mutant proteins (indicated in parentheses and described for panel A) were incubated on synthetic complete minimal medium with either galactose (SGal) or glucose (SD) for 3 days at 30°C.
Inspection of the N terminus of mature Isu1p (predicted cleavage site after residue 27) by several mitochondrial presequence prediction programs (Mitoprot, TargetP, and Predotar) revealed a high (41 to 99%) probability of mitochondrial targeting of mature Isu1p. Thus, targeting information present in the N terminus of mature Isu1p may readily explain the efficient localization of this protein to mitochondria. Since the N terminus of mature Isu1p is highly conserved, it was not possible to introduce mutations into this region, since they might interfere with both targeting to mitochondria and the function of Isu1p. We therefore decided to add a short negatively charged N-terminal extension (DAE) to Isu1pΔ30. Negatively charged amino acids are incompatible with mitochondrial targeting (61), and consequently the mutant protein termed DAE-Isu1pΔ30 (Fig. 4A) was predicted to be targeted to mitochondria with rather low efficiency (3 to 15%) by presequence prediction programs. Surprisingly, expression of the gene encoding DAE-Isu1pΔ30 in Gal-ISU1/Δisu2 cells yielded mitochondrial localization of the protein and did not lead to any detectable amounts of DAE-Isu1pΔ30 in the cytosol (Fig. 4B). Synthesis of DAE-Isu1pΔ30 fully complemented the growth defect of Isu1p-depleted Gal-ISU1/Δisu2 cells (Fig. 4C). We did not further analyze the reason why Isu1p still was targeted exclusively to mitochondria, but apparently there exists targeting information in parts other than the N terminus of this protein.
The inability to mislocalize Isu1p to the cytosol precluded the detection of the functional location of Isu1p in cytosolic Fe/S protein maturation. Nevertheless, the data show that the protein contains multiple pieces of targeting information, including the N-terminal presequence, the N terminus of the mature part, and additional, internal information. It seems highly likely that the combination of these multiple targeting sequences ensures overwhelming localization of Isu1p to the mitochondrial matrix.
Bacterial IscU supports cellular Fe/S protein biogenesis after targeting to mitochondria.
To circumvent the inability to mislocate yeast Isu1p to the cytosol, we asked whether bacterial IscU proteins can replace the function of the yeast Isu protein homologues and allow localization of IscU to either mitochondria or the cytosol for use in functional studies. The E. coli gene iscU was cloned into a yeast expression vector with or without the coding sequence for the strong mitochondrial presequence of N. crassa F0-ATPase subunit 9 (pSu9). Production of mitochondrion-targeted pSu9-IscU in yeast resulted in partial complementation of the growth defect of Isu1p-depleted Gal-ISU1/Δisu2 cells (Fig. 5A). In contrast, no colony formation was found for IscU synthesized without a mitochondrial presequence, despite the fact that the IscU protein was detectable in the postmitochondrial supernatant, as analyzed by immunostaining (Fig. 5B). This finding provided further support for the essential function of the Isu/IscU proteins inside mitochondria.
FIG. 5.
Targeting of E. coli IscU to mitochondria partially restores the defects of Isu protein deficiency in Gal-ISU1/Δisu2 cells. (A) The gene encoding IscU from E. coli was inserted into the yeast vector p416Met25 either without (IscU) or with an N-terminal mitochondrial targeting sequence of N. crassa F0-ATPase subunit 9 (pSu9-IscU). Plasmids were transformed into Gal-ISU1/Δisu2 (Gal-ISU) cells, and cell growth was compared to that of wild-type cells or Gal-ISU1/Δisu2 cells with a plasmid lacking an insert (none) by incubation on agar plates containing synthetic complete medium with either galactose (SGal) or glucose (SD) for 3 days at 30°C. (B) Mitochondria (M) and postmitochondrial supernatants (PMS) were isolated from Gal-ISU1/Δisu2 cells described for panel A after growth in minimal medium containing galactose (Gal) or glucose (Glu). Subcellular localization of IscU was analyzed by immunostaining (50 μg of protein per lane) with antisera raised against yeast Isu1p, which exhibits cross-reactivity against E. coli IscU. The mitochondrial proteins Por2p and Yfh1p and cytosolic Bmh1p served as controls. (C) The cells described for panel A were transformed with the plasmid p424GPD carrying the BIO2 or the HA-tagged RLI1 genes. Cells were examined for de novo Fe/S protein maturation as described for Fig. 2A. The levels of Rli1p-HA and Bio2p were visualized by immunostaining (insets). (D) The cellular 55Fe uptake of the various cell types was quantified by liquid scintillation counting. Standard deviations were calculated from five independent experiments.
The function of bacterial IscU in the two cellular locations was examined by measuring the 55Fe incorporation into mitochondrial Bio2p and cytosolic Rli1p-HA as model Fe/S proteins with Isu1p-depleted Gal-ISU1/Δisu2 cells. Only mitochondrion-targeted but not cytosolic IscU supported Fe/S cluster association with both Bio2p and Rli1p (Fig. 5C). The efficiency of Fe/S protein formation by mitochondrial IscU was rather low but two- to fourfold higher than the levels of Fe/S cluster formation in Isu1p-depleted Gal-ISU1/Δisu2 cells. Further, the higher iron uptake observed in Isu1p-depleted Gal-ISU1/Δisu2 cells was almost fully reversed by mitochondrial IscU (Fig. 5D). Despite the incomplete functional replacement of the yeast Isu proteins by bacterial IscU, these proteins can be regarded as functional orthologues. Clearly, IscU requires mitochondrial localization for participation in both mitochondrial and cytosolic Fe/S protein biogenesis. These data provide evidence for the notion that the mitochondrial matrix is the functional location of the Isu proteins for maturation of Fe/S proteins, including those located in the cytosol.
Targeting of human Isu proteins to mitochondria restores the defects of Isu protein-depleted yeast cells.
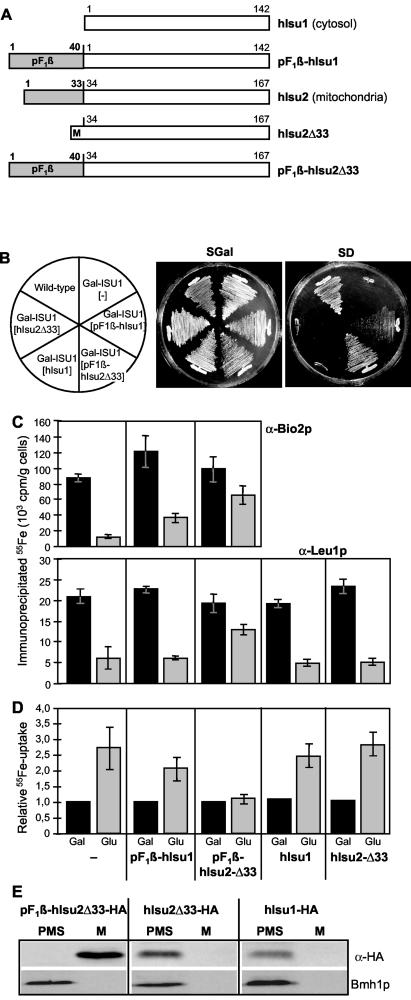
Since the complementation of the Isu protein deficiency by bacterial IscU was only partial, we asked whether a eukaryotic Isu protein would complement these defects more efficiently. We took advantage of the two human Isu protein homologues to study their functionality in Gal-ISU1/Δisu2 cells and to further explore the functional site of Isu proteins in eukaryotes. In humans, two cDNAs have been discovered that are transcribed from a single ISU gene (56). The predominant form (ca. 90 DNA sequence database entries) encodes a mitochondrial version of Isu protein (termed hIsu2). A minor form (seven expressed sequence tag [EST] entries; all derived from tumor cell lines) lacks the mitochondrial presequence due to a splice variation and gives rise to small amounts of an Isu protein isoform in the cytosol (termed hIsu1) (56). We first tested the functional complementation of Isu1p-depleted Gal-ISU1/Δisu2 cells by mitochondrion-targeted human Isu proteins. To achieve efficient targeting of the human proteins to yeast mitochondria, the endogenous N-terminal mitochondrial presequence of hIsu2 was replaced by the strong pF1β presequence (to yield pF1β-hIsu2Δ33 [Fig. 6A]), and hIsu1 was synthesized with an N-terminal pF1β presequence (pF1β-hIsu1). Synthesis of both proteins in Gal-ISU1/Δisu2 cells complemented the deficiency in the endogenous Isu proteins in a different manner. Almost-wild-type growth was observed for pF1β-hIsu2Δ33, whereas hardly any growth was seen for mitochondrion-localized pF1β-hIsu1 (Fig. 6B). A likely reason for the low activity of hIsu1 in mitochondria may be the lack of a conserved tyrosine residue at its N terminus. This residue is found in virtually all eukaryotic Isu and prokaryotic IscU protein family members, yet is lacking in cytosolic hIsu1 as a result of differential splicing of its mRNA.
FIG. 6.
Upon targeting to mitochondria, human Isu proteins restore the defects of Isu protein-depleted Gal-ISU1/Δisu2 cells. (A) Schematic description of human cytosolic hIsu1 and mitochondrial hIsu2 with and without mitochondrial targeting sequences. hIsu1 was used in native form or with the N-terminal mitochondrial targeting sequence of F1β-ATPase (pF1β-hIsu1). The presequence of hIsu2 was either deleted (hIsu2Δ33) or replaced with the presequence of F1β-ATPase (pF1β-hIsu2Δ33). (B) Gal-ISU1/Δisu2 (Gal-ISU) cells weretransformed with plasmids carrying either no gene or the genes for human hIsu1 and hIsu2 proteins indicated in panel A. These cells and wild-type cells were grown on agar plates containing synthetic complete medium with either galactose (SGal) or glucose (SD) for 3 days at 30°C. (C) Cells from panel B were transformed with the plasmid p426GPD carrying the BIO2 gene. After growth in the presence of galactose (Gal) or glucose (Glu), cell extracts were examined for de novo 55Fe/S cluster incorporation into mitochondrial Bio2p and cytosolic Leu1p as described for Fig. 2A. (D) The cellular 55Fe uptake of the various cell types was quantified by liquid scintillation counting. Data were normalized to the amounts of iron uptake into Isu1p-containing Gal-ISU1/Δisu2 cells (grown with galactose). Standard deviations were calculated from four independent experiments. (E) Plasmid p424GPD carrying the genes for HA-tagged versions of pF1β-hIsu2Δ33, hIsu2Δ33, or hIsu1 was transformed into Gal-ISU1/Δisu2 cells. After growth in minimal medium containing glucose, mitochondria (M) and postmitochondrial supernatants (PMS) were prepared. Equal amounts of protein (50 μg) were analyzed by immunostaining for the HA-tagged human Isu proteins and Bmh1p.
To analyze the function of the mitochondrion-targeted human Isu proteins in mitochondrial and cytosolic Fe/S protein biogenesis, we used the 55Fe incorporation into Bio2p and Leu1p, respectively, in Isu1p-depleted Gal-ISU1/Δisu2 cells. Mitochondrial pF1β-hIsu2Δ33 supported Fe/S cluster formation in both Fe/S apoproteins at almost wild-type efficiencies (75% of Isu1p-containing Gal-ISU1/Δisu2 cells [Fig. 6C]). Thus, yeast Isu proteins and human mitochondrial hIsu2 can be regarded as functional orthologues. Only a slight improvement of Fe/S cluster insertion into Bio2p and virtually no effect on Leu1p were observed for mitochondrion-targeted pF1β-hIsu1. Apparently, hIsu1 exhibits only a weak activity in Fe/S cluster biogenesis, despite its almost identical amino acid sequence compared to hIsu2Δ33. This finding is consistent with the slow growth of the corresponding cells (Fig. 6B). We also analyzed the cellular iron uptake, which is characteristically higher in Isu protein-depleted cells (Fig. 2B). Iron uptake was fully reversed to levels observed for Isu1p-containing Gal-ISU1/Δisu2 cells by synthesis of pF1β-hIsu2Δ33, but no significant effect was seen by pF1β-hIsu1, corroborating the results presented above (Fig. 6D).
We further tested whether the human Isu proteins might be functional in cytosolic Fe/S protein assembly, when localized to the yeast cytosol. To this end, cytosolic human hIsu1 was synthesized without modification in Gal-ISU1/Δisu2 cells, and the truncated version of hIsu2 lacking its N-terminal presequence (hIsu2Δ33) was used (Fig. 6A). No colony formation was observed when these two isoforms were produced without a mitochondrial presequence (Fig. 6B). These data show that mitochondrial localization was essential for functional replacement of the yeast Isu proteins by the human homologues. No positive influence of both cytosol-localized human Isu proteins was detectable for Fe/S cluster assembly on cytosolic Leu1p in Isu1p-deficient Gal-ISU1/Δisu2 cells, demonstrating that also for functional participation in cytosolic Fe/S protein biogenesis, the Isu proteins need to be localized in mitochondria (Fig. 6C). Moreover, the increased cellular 55Fe uptake was not altered upon synthesis of cytosol-targeted human Isu proteins (Fig. 6D).
Finally, the localization of the human Isu proteins in yeast was analyzed by transforming cells with plasmids carrying genes encoding HA-tagged human Isu proteins. Mitochondria and postmitochondrial supernatants were prepared and analyzed by immunostaining. The presequence-containing pF1β-hIsu2Δ33-HA was localized in mitochondria, whereas both hIsu2Δ33-HA and hIsu1-HA were found in the postmitochondrial supernatant fraction (Fig. 6E). These findings support the conclusion that only mitochondrion-targeted but not cytosol-localized human Isu proteins were functional in replacing the yeast Isu proteins. In summary, our data demonstrate that the Isu proteins perform their function in Fe/S protein biogenesis in mitochondria, for both mitochondrial and cytosolic Fe/S target proteins.
DISCUSSION
In vivo and in vitro evidence suggests that the members of the Isu/IscU protein family provide a scaffold for the de novo synthesis of an Fe/S cluster in mitochondria and bacteria (34, 39, 66). The central importance of these proteins becomes most evident from their functional interaction with several other proteins of the ISC assembly machinery that are needed either for Fe/S cluster synthesis (Nfs1p and frataxin) (17) or for later steps of Fe/S protein biosynthesis (Ssq1q and Jac1p) (12). Our present study adds another feature that establishes an important function of the Isu proteins for the entire cell. We demonstrate that the Isu proteins not only play a central role in mitochondrial Fe/S cluster metabolism but also are involved in the biosynthesis of the cytosolic Fe/S proteins Leu1p and Rli1p. Most probably, the Isu proteins participate in the biosynthesis of all (or at least most) cellular Fe/S proteins. Similar findings have been made for other members of the ISC assembly machinery, including Nfs1p, Yah1p, Arh1p, and frataxin (23, 26, 29, 35), underscoring the central task of mitochondria in the generation of cellular Fe/S proteins.
Several lines of evidence indicate that location of the Isu proteins inside the mitochondrial matrix is crucial for their function in maturation of both mitochondrial and cytosolic Fe/S proteins. First, Isu1p could not be mistargeted to the cytosol, a property that likely is due to the presence of multiple targeting sequences in this protein. As a consequence, Isu1p appears to be located only inside mitochondria. The same may be true for Isu2p, since the functions of both Isu proteins are fully overlapping, and no significant phenotypes of the single-gene-deletion strains Δisu1 and Δisu2 were evident in our strain background. Second, both bacterial and human Isu homologues can (at least partially) replace the yeast Isu proteins, yet only after targeting to mitochondria. Synthesis of both human and bacterial Isu proteins in the yeast cytosol did not support growth of yeast cells and most strikingly had no detectable influence on the biosynthesis of cytosolic Fe/S proteins. Thus, mitochondrial location of the Isu proteins is necessary for the maturation of cytosolic Fe/S proteins in yeast cells. Whether a cytosolic version of the Isu proteins may be required in addition to mitochondrion-located copies cannot be excluded with certainty, but it seems rather unlikely from the virtually quantitative location of the Isu proteins within yeast mitochondria.
The participation of the yeast Isu proteins and the sulfur donor Nfs1p in cellular Fe/S protein biogenesis is strikingly similar (23). Like the Isu proteins, the cysteine desulfurase performs its function in both mitochondrial and cytosolic Fe/S protein maturation inside mitochondria. Cytosolic location of Nfs1p does not give rise to Fe/S protein formation in the yeast cytosol. In addition to the essential mitochondrial function of Nfs1p, genetic studies have identified an indispensable task in the nucleus (37). Recent biochemical studies indicate that the nuclear form of Nfs1p is involved in thiouridine modification of tRNA and not in Fe/S metabolism (38; G. Kispal and U. Mühlenhoff, unpublished observation). Notably, IscS, the bacterial homologue of Nfs1p, provides sulfur for Fe/S clusters, tRNA, and thiamine, thus impressively illustrating the functional similarity of the eukaryotic and prokaryotic enzymes (19).
Mitochondrial function of the Isu proteins (this work) and Nfs1p (23) in cytosolic Fe/S protein maturation implies that mitochondria export a component required for Fe/S protein maturation in the cytosol. Presently, little is known about this putative export process and the chemical nature of the transported moiety. Potential exported components include an Fe/S cluster synthesized by the mitochondrial ISC machinery or a product of a mitochondrial Fe/S protein (31). Central players of the export pathway are the ABC transporter Atm1p of the mitochondrial inner membrane, the sulfhydryl oxidase Erv1p of the intermembrane space, and the tripeptide glutathione (23, 27, 50). Depletion of these components results in defects of cytosolic but not mitochondrial Fe/S proteins. This specific phenotype is distinct from the consequences of functional inactivation of components of the ISC assembly machinery (such as the Isu proteins or Nfs1p), resulting in defects in both mitochondrial and extramitochondrial Fe/S proteins (15, 23, 28, 47, 53; this study). Recently, yet another factor needed for cytosolic Fe/S protein maturation was identified (45). The cytosolic P-type ATPase Cfd1p was found to be involved in cytosolic but not mitochondrial Fe/S protein biosynthesis: i.e., inactivation of Cfd1p elicits a phenotype comparable to the defects in components of the mitochondrial ISC export machinery. It is tempting to speculate that Cfd1p cooperates with the mitochondrial ISC export machinery in maturation of cytosolic Fe/S proteins.
Several components of the yeast mitochondrial ISC assembly and export machineries including the two Isu proteins are encoded by essential genes (30). In fact, Fe/S protein biogenesis is the only known biochemical process (apart from biogenesis of the organelle by preprotein import, processing and folding) that renders mitochondria essential. Surprisingly, with the exception of Yah1p (26) no essential mitochondrial Fe/S protein is known to date that could explain this property. However, the yeast cytosol contains several essential Fe/S proteins, including Rli1p. The fact that mitochondrial location of the Isu proteins, Nfs1p, and other essential ISC proteins is required for Rli1p maturation provides a logical reason why these organelles may be indispensable for the eukaryotic cell. This requirement readily explains the essential character of the Isu proteins, of other central members of the mitochondrial ISC machineries, and of cytosolic Cfd1p, which also is encoded by an essential gene (31, 45). This view is further supported by recent data showing that even in organisms such as Giardia lamblia lacking classical mitochondria, Fe/S protein biogenesis appears to be compartmentalized (57). Giardia was shown to contain so-called mitosomes, double-membrane-bounded organelles derived from mitochondria by reductive evolution. Mitosomes lack classical functions of mitochondria (20, 63), but maintained the ISC assembly machinery as their prominent function, underlining the importance of mitochondria/mitosomes for Fe/S cluster biogenesis in the entire cell.
Fe/S protein biogenesis appears to be conserved in eukaryotes. Mammalian genomes contain homologues of virtually all components of the yeast ISC assembly and export machineries, and these proteins share extensive sequence similarity. Some of these components have been demonstrated to functionally replace each other, such as human and yeast Isu proteins (this study), human frataxin and Yfh1p (64), human ALR and Erv1p (27), or human ABC7 and its functional orthologue, Atm1p (9). Moreover, the largely increased mitochondrial iron uptake in mammalian cells harboring defective frataxin or ABC7 suggests close functional similarities between the yeast and mammalian systems (4, 24, 42). However, only a few functional studies on the biosynthesis of Fe/S proteins in higher eukaryotes have been reported so far. For instance, maturation of the cytosolic aconitase IRP1 depends on the function of energized mitochondria (i.e., organelles exhibiting a membrane potential [5]). This finding is strikingly similar to our observations made for yeast cells described above (23). Despite these conspicuous similarities, Fe/S protein biogenesis in human cells may be more complex than it is in yeast. Some components of the human ISC assembly machinery have been detected in the cytosol and the nucleus, even though at rather low concentrations (25, 55, 56). This raises several intriguing questions, including what contribution the human cytosolic ISC components might provide for cytosolic Fe/S protein maturation and what role human mitochondria might play in this process. We show here that mitochondrial hIsu2 can replace the endogenous yeast proteins after targeting to mitochondria, whereas the cytosolic human hIsu1 is hardly functional in yeast mitochondria, despite its sequence identity with mitochondrial hIsu2 (56). The reason for this striking functional difference is the lack of a conserved tyrosine at the N terminus of cytosolic hIsu1. Notably, hIsu1 is the only member of the large Isu/IscU protein family lacking this residue. The inherent functional defect combined with the low cytosolic concentration of hIsu1 questions the functionality of hIsu1 in cytosolic Fe/S cluster synthesis.
Counterparts of the splice variants of human Isu mRNA have not been identified in other higher eukaryotes so far. A recent systematic study has shown that splice variations are remarkably conserved in higher eukaryotes (6). On the basis of this study, it is expected that the same Isu splice variants exist in other organisms. Indeed, the general split gene structure of the Isu protein is conserved in, e.g., mice and rats, but we did not find any ESTs matching the N-terminal region of hIsu1. Instead, we identified a single mouse EST that deviates from the normal mitochondrial forms of murine Isu protein (represented by 30 ESTs encoded by two almost identical genes on chromosomes 5 and 19). However, the open reading frame lacks a start methionine at its N terminus, and hence, its mRNA may not give rise to a functional protein. Thus, at present, it is unclear whether mouse and other organisms contain a cytosolic form of the Isu proteins. If Fe/S cluster synthesis in human cells should use the ISC machinery in the cytosol, human cytosolic Isu1 and Nfs1 may likely need the support of other crucial ISC components. For instance, mammalian adrenodoxin and adrenodoxin reductase, homologues of yeast Yah1p and Arh1p, may be needed for biogenesis. However, these two proteins do not appear to be functional outside mitochondria, as they participate in partial reactions of steroid hormone biosynthesis only after steroid precursor import into mitochondria from the smooth endoplasmic reticulum (52).
Clearly, all this information is indirect and speculative and therefore does not clarify the interesting problem of mitochondrial versus cytosolic Fe/S cluster assembly in mammalian cells. The studies with yeast, including those presented here, strongly indicate that there is no duplication of the mitochondrial ISC assembly machinery in the cytosol. However, with respect to the apparent fundamental differences in the mechanisms of iron sensing, uptake, and storage in fungi and humans (58), it is not excluded that cytosolic Fe/S assembly may take a different path in mammalian systems. Solution of this problem will have to come from in vivo investigations of Fe/S cluster assembly in higher eukaryotes.
Acknowledgments
Our work was supported by grants from the Deutsche Forschungsgemeinschaft (Gottfried-Wilhelm Leibniz program), Sonderforschungsbereich 593, the European Commission (QLG1-CT-2001-00966), the Deutsche Humangenomprojekt, and Fonds der Chemischen Industrie.
REFERENCES
- 1.Beinert, H., and P. J. Kiley. 1999. Fe-S proteins in sensing and regulatory functions. Curr. Opin. Chem. Biol. 3:152-157. [DOI] [PubMed] [Google Scholar]
- 2.Beinert, H. 2000. Iron-sulfur proteins: ancient structures, still full of surprises. J. Bioinorg. Chem. 5:2-15. [DOI] [PubMed] [Google Scholar]
- 3.Beinert, H., R. H. Holm, and E. Münck. 1997. Iron-sulfur clusters: nature's modular, multipurpose structures. Science 277:653-659. [DOI] [PubMed] [Google Scholar]
- 4.Bekri, S., G. Kispal, H. Lange, E. Fitzsimons, J. Tolmie, R. Lill, and D. F. Bishop. 2000. Human ABC7 transporter: gene structure and mutation causing X-linked sideroblastic anemia with ataxia (XLSA/A) with disruption of cytosolic iron-sulfur protein maturation. Blood 96:3256-3264. [PubMed] [Google Scholar]
- 5.Bouton, C., M. J. Chauveau, S. Lazereg, and J. C. Drapier. 2002. Recycling of RNA binding iron regulatory protein 1 into an aconitase after nitric oxide removal depends on mitochondrial ATP. J. Biol. Chem. 277:31220-31227. [DOI] [PubMed] [Google Scholar]
- 6.Brett, D., H. Pospisil, J. Valcarcel, J. Reich, and P. Bork. 2002. Alternative splicing and genome complexity. Nat. Genet. 30:29-30. [DOI] [PubMed] [Google Scholar]
- 7.Chen, O. S., S. Hemenway, and J. Kaplan. 2002. Inhibition of Fe-S cluster biosynthesis decreases mitochondrial iron export: evidence that Yfh1p affects Fe-S cluster synthesis. Proc. Natl. Acad. Sci. USA 99:12321-12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig, E. A., and J. Marszalek. 2002. A specialized mitochondrial molecular chaperone system: a role in formation of Fe/S centers. Cell. Mol. Life Sci. 59:1658-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csere, P., R. Lill, and G. Kispal. 1998. Identification of a human mitochondrial ABC transporter, the functional orthologue of yeast Atm1p. FEBS Lett. 441:266-270. [DOI] [PubMed] [Google Scholar]
- 10.Daum, G., P. C. Böhni, and G. Schatz. 1982. Import of proteins into mitochondria: cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J. Biol. Chem. 257:13028-13033. [PubMed] [Google Scholar]
- 11.Diekert, K., A. I. P. M. deKroon, G. Kispal, and R. Lill. 2001. Isolation and sub-fractionation of mitochondria from the yeast Saccharomyces cerevisiae. Methods Cell Biol. 65:37-51. [DOI] [PubMed] [Google Scholar]
- 12.Dutkiewicz, R., B. Schilke, H. Kneiszner, W. Walter, E. A. Craig, and J. Marszalek. 2003. Ssq1, a mitochondrial Hsp70 involved in iron-sulfur (Fe/S) center biogenesis: similarities to and differences from its bacterial counterpart. J. Biol. Chem. 278:29719-29727. [DOI] [PubMed] [Google Scholar]
- 13.Frazzon, J., and D. R. Dean. 2002. Biosynthesis of the nitrogenase iron-molybdenum-cofactor from Azotobacter vinelandii. Metal Ions Biol. Syst. 39:163-186. [PubMed] [Google Scholar]
- 14.Frazzon, J., and D. R. Dean. 2003. Formation of iron-sulfur clusters in bacteria—an emerging field in bioinorganic chemistry. Curr. Opin. Chem. Biol. 7:166-173. [DOI] [PubMed] [Google Scholar]
- 15.Garland, S. A., K. Hoff, L. E. Vickery, and V. C. Culotta. 1999. Saccharomyces cerevisiae ISU1 and ISU2: members of a well-conserved gene family for iron-sulfur cluster assembly. J. Mol. Biol. 294:897-907. [DOI] [PubMed] [Google Scholar]
- 16.Gerber, J., and R. Lill. 2002. Biogenesis of iron-sulfur proteins in eukaryotes: components, mechanism and pathology. Mitochondrion 2:71-86. [DOI] [PubMed] [Google Scholar]
- 17.Gerber, J., U. Mühlenhoff, and R. Lill. 2003. An interaction between frataxin and Isu1/Nfs1 that is crucial for Fe/S cluster synthesis on Isu1. EMBO Rep. 4:906-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gietz, D., A. St. Jean, R. A. Woods, and R. H. Schiestl. 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20:1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kambampati, T., and C. T. Lauhon. 1999. IscS is a sulfurtransferase for the in vitro biosynthesis of 4-thiouridine in Escherichia coli tRNA. Biochemistry 38:16561-16568. [DOI] [PubMed] [Google Scholar]
- 20.Katinka, M. D., S. Duprat, E. Cornillot, G. Metenier, F. Thomarat, G. Prensier, V. Barbe, E. Peyretaillade, P. Brottier, P. Wincker, F. Delbac, H. El Alaoui, P. Peyret, W. Saurin, M. Gouy, J. Weissenbach, and C. P. Vivares. 2001. Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature 414:450-453. [DOI] [PubMed] [Google Scholar]
- 21.Kato, S., H. Mihara, T. Kurihara, Y. Takahashi, U. Tokumoto, T. Yoshimura, and N. Esaki. 2002. Cys-328 of IscS and Cys-63 of IscU are the sites of disulfide bridge formation in a covalently bound IscS/IscU complex: implications for the mechanism of iron-sulfur cluster assembly. Proc. Natl. Acad. Sci. USA 99:5948-5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, R., S. Saxena, D. M. Gordon, D. Pain, and A. Dancis. 2001. J-domain protein, Jac1p, of yeast mitochondria required for iron homeostasis and activity of Fe-S cluster proteins. J. Biol. Chem. 276:17524-17532. [DOI] [PubMed] [Google Scholar]
- 23.Kispal, G., P. Csere, C. Prohl, and R. Lill. 1999. The mitochondrial proteins Atm1p and Nfs1p are required for biogenesis of cytosolic Fe/S proteins. EMBO J. 18:3981-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamarche, J. B., M. Cote, and B. Lemieux. 1980. The cardiomyopathy of Friedreich's ataxia: morphological observations in 3 cases. Can. J. Neurol. Sci. 7:389-396. [DOI] [PubMed] [Google Scholar]
- 25.Land, T., and T. A. Rouault. 1998. Targeting of a human iron-sulfur cluster assembly enzyme, nifs, to different subcellular compartments is regulated through alternative AUG utilization. Mol. Cell 2:807-815. [DOI] [PubMed] [Google Scholar]
- 26.Lange, H., G. Kispal, A. Kaut, and R. Lill. 2000. A mitochondrial ferredoxin is essential for biogenesis of intra- and extra-mitochondrial Fe/S proteins. Proc. Natl. Acad. Sci. USA 97:1050-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lange, H., T. Lisowsky, J. Gerber, U. Mühlenhoff, G. Kispal, and R. Lill. 2001. An essential function of the mitochondrial sulfhydryl oxidase Erv1p/ALR in the maturation of cytosolic Fe/S proteins. EMBO Rep. 2:715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, J., M. Kogan, S. A. Knight, D. Pain, and A. Dancis. 1999. Yeast mitochondrial protein Nfs1p coordinately regulates iron-sulfur cluster proteins, cellular iron uptake, and iron distribution. J. Biol. Chem. 274:33025-33034. [DOI] [PubMed] [Google Scholar]
- 29.Li, J., S. Saxena, D. Pain, and A. Dancis. 2001. Adrenodoxin reductase homolog (Arh1p) of yeast mitochondria required for iron homeostasis. J. Biol. Chem. 276:1503-1509. [DOI] [PubMed] [Google Scholar]
- 30.Lill, R., K. Diekert, A. Kaut, H. Lange, W. Pelzer, C. Prohl, and G. Kispal. 1999. The essential role of mitochondria in the biogenesis of cellular iron-sulfur proteins. Biol. Chem. 380:1157-1166. [DOI] [PubMed] [Google Scholar]
- 31.Lill, R., and G. Kispal. 2000. Maturation of cellular Fe/S proteins: the essential function of mitochondria. Trends Biochem. Sci. 25:352-356. [DOI] [PubMed] [Google Scholar]
- 32.Loiseau, L., S. Ollagnier-de-Choudens, L. Nachin, M. Fontecave, and F. Barras. 2003. Biogenesis of Fe-S cluster by the bacterial Suf system: SufS and SufE form a new type of cysteine desulfurase. J. Biol. Chem. 278:38352-38359. [DOI] [PubMed] [Google Scholar]
- 33.Lutz, T., B. Westermann, W. Neupert, and J. M. Herrmann. 2001. The mitochondrial proteins Ssq1 and Jac1 are required for the assembly of iron sulfur clusters in mitochondria. J. Mol. Biol. 307:815-825. [DOI] [PubMed] [Google Scholar]
- 34.Mühlenhoff, U., J. Gerber, N. Richhardt, and R. Lill. 2003. Components involved in assembly and dislocation of iron-sulfur clusters on the scaffold protein Isu1p. EMBO J. 22:4815-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mühlenhoff, U., N. Richhardt, M. Ristow, G. Kispal, and R. Lill. 2002. The yeast frataxin homologue Yfh1p plays a specific role in the maturation of cellular Fe/S proteins. Hum. Mol. Genet. 11:2025-2036. [DOI] [PubMed] [Google Scholar]
- 36.Mumberg, D., R. Müller, and M. Funk. 1995. Yeast vectors for controlled expression of heterologous proteins in different genetic backgrounds. Gene 156:119-122. [DOI] [PubMed] [Google Scholar]
- 37.Nakai, Y., M. Nakai, H. Hayashi, and H. Kagamiyama. 2001. Nuclear localization of yeast Nfs1p is required for cell survival. J. Biol. Chem. 276:8314-8320. [DOI] [PubMed] [Google Scholar]
- 38.Nakai, Y., N. Umeda, T. Suzuki, M. Nakai, H. Hayashi, K. Watanabe, and H. Kagamiyama. 2004. Yeast Nfs1p is involved in thio-modification of both mitochondrial and cytoplasmic tRNAs. J. Biol. Chem. 279:12363-12368. [DOI] [PubMed] [Google Scholar]
- 39.Nuth, M., T. Yoon, and J. A. Cowan. 2002. Iron-sulfur cluster biosynthesis: characterization of iron nucleation sites for assembly of the [2Fe-2S]2+ cluster core in IscU proteins. J. Am. Chem. Soc. 124:8774-8775. [DOI] [PubMed] [Google Scholar]
- 40.Outten, F. W., M. J. Wood, F. M. Munoz, and G. Storz. 2003. The SufE protein and the SufBCD complex enhance SufS cysteine desulfurase activity as part of a sulfur transfer pathway for Fe-S cluster assembly in E. coli. J. Biol. Chem. 278:45713-45719. [DOI] [PubMed] [Google Scholar]
- 41.Pelzer, W., U. Mühlenhoff, K. Diekert, K. Siegmund, G. Kispal, and R. Lill. 2000. Mitochondrial Isa2p plays a crucial role in the maturation of cellular iron-sulfur proteins. FEBS Lett. 476:134-139. [DOI] [PubMed] [Google Scholar]
- 42.Puccio, H., D. Simon, M. Cossee, P. Criqui-Filipe, F. Tiziano, J. Melki, C. Hindelang, R. Matyas, P. Rustin, and M. Koenig. 2001. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat. Genet. 27:181-186. [DOI] [PubMed] [Google Scholar]
- 43.Rees, D. C., and J. B. Howard. 2000. Nitrogenase: standing at the crossroads. Curr. Opin. Chem. Biol. 4:559-566. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Manzaneque, M. T., J. Tamarit, G. Belli, J. Ros, and E. Herrero. 2002. Grx5 is a mitochondrial glutaredoxin required for the activity of iron/sulfur enzymes. Mol. Biol. Cell 13:1109-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roy, A., N. Solodovnikova, T. Nicholson, W. Antholine, and W. E. Walden. 2003. A novel eukaryotic factor for cytosolic Fe-S cluster assembly. EMBO J. 22:4826-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 47.Schilke, B., C. Voisine, H. Beinert, and E. Craig. 1999. Evidence for a conserved system for iron metabolism in the mitochondria of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 96:10206-10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwartz, C. J., O. Djaman, J. A. Imlay, and P. J. Kiley. 2000. The cysteine desulfurase, IscS, has a major role in in vivo Fe-S cluster formation in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:9009-9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194:3-21. [DOI] [PubMed] [Google Scholar]
- 50.Sipos, K., H. Lange, Z. Fekete, P. Ullmann, R. Lill, and G. Kispal. 2002. Maturation of cytosolic iron-sulfur proteins requires glutathione. J. Biol. Chem. 277:26944-26949. [DOI] [PubMed] [Google Scholar]
- 51.Smith, A. D., J. N. Agar, K. A. Johnson, J. Frazzon, I. J. Amster, D. R. Dean, and M. K. Johnson. 2001. Sulfur transfer from IscS to IscU: the first step in iron-sulfur cluster biosynthesis. J. Am. Chem. Soc. 123:11103-11104. [DOI] [PubMed] [Google Scholar]
- 52.Stocco, D. M. 2000. Intramitochondrial cholesterol transfer. Biochim. Biophys. Acta 1486:184-197. [DOI] [PubMed] [Google Scholar]
- 53.Strain, J., C. R. Lorenz, J. Bode, S. Garland, G. A. Smolen, D. T. Ta, L. E. Vickery, and V. C. Culotta. 1998. Suppressors of superoxide dismutase (SOD1) deficiency in Saccharomyces cerevisiae. Identification of proteins predicted to mediate iron-sulfur cluster assembly. J. Biol. Chem. 273:31138-31144. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi, Y., and U. Tokumoto. 2002. A third bacterial system for the assembly of iron-sulfur clusters with homologs in archaea and plastids. J. Biol. Chem. 277:28380-28383. [DOI] [PubMed] [Google Scholar]
- 55.Tong, W. H., G. N. Jameson, B. H. Huynh, and T. A. Rouault. 2003. Subcellular compartmentalization of human Nfu, an iron-sulfur cluster scaffold protein, and its ability to assemble a [4Fe-4S] cluster. Proc. Natl. Acad. Sci. USA 100:9762-9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tong, W. H., and T. Rouault. 2000. Distinct iron-sulfur cluster assembly complexes exist in the cytosol and mitochondria of human cells. EMBO J. 19:5692-5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tovar, J., G. Leon-Avila, L. B. Sanchez, R. Sutak, J. Tachezy, M. Van Der Giezen, M. Hernandez, M. Muller, and J. M. Lucocq. 2003. Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation. Nature 426:172-176. [DOI] [PubMed] [Google Scholar]
- 58.Van Ho, A., D. M. Ward, and J. Kaplan. 2002. Transition metal transport in yeast. Annu. Rev. Microbiol. 56:237-261. [DOI] [PubMed] [Google Scholar]
- 59.Voisine, C., Y. C. Cheng, M. Ohlson, B. Schilke, K. Hoff, H. Beinert, J. Marszalek, and E. A. Craig. 2001. Jac1, a mitochondrial J-type chaperone, is involved in the biogenesis of Fe/S clusters in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 98:1483-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voisine, C., B. Schilke, M. Ohlson, H. Beinert, J. Marszalek, and E. A. Craig. 2000. Role of the mitochondrial Hsp70s, Ssc1 and Ssq1, in the maturation of Yfh1. Mol. Cell. Biol. 20:3677-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Heijne, G. 1986. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 5:1335-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wach, A., A. Brachat, R. Poehlmann, and P. Phillipsen. 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10:1793-1808. [DOI] [PubMed] [Google Scholar]
- 63.Williams, B. A., R. P. Hirt, J. M. Lucocq, and T. M. Embley. 2002. A mitochondrial remnant in the microsporidian Trachipleistophora hominis. Nature 418:865-869. [DOI] [PubMed] [Google Scholar]
- 64.Wilson, R. B., and D. M. Roof. 1997. Respiratory deficiency due to loss of mitochondrial DNA in yeast lacking the frataxin homologue. Nat. Genet. 16:352-357. [DOI] [PubMed] [Google Scholar]
- 65.Yoon, T., and J. A. Cowan. 2003. Iron-sulfur cluster biosynthesis. Characterization of frataxin as an iron donor for assembly of [2Fe-2S] clusters in ISU-type proteins. J. Am. Chem. Soc. 125:6078-6084. [DOI] [PubMed] [Google Scholar]
- 66.Yuvaniyama, P., J. N. Agar, V. L. Cash, M. K. Johnson, and D. R. Dean. 2000. NifS-directed assembly of a transient [2Fe-2S] cluster within the NifU protein. Proc. Natl. Acad. Sci. USA 97:599-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng, L., V. L. Cash, D. H. Flint, and D. R. Dean. 1998. Assembly of iron-sulfur clusters. Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J. Biol. Chem. 273:13264-13272. [DOI] [PubMed] [Google Scholar]
- 68.Zheng, L., R. H. White, V. L. Cash, R. F. Jack, and D. R. Dean. 1993. Cysteine desulfurase acitivity indicates a role for NifS in metallocluster biosynthesis. Proc. Natl. Acad. Sci. USA 90:2754-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]