Abstract
STUDY QUESTION
Do teenage girls with a history of menstrual irregularity and/or elevated androgen levels in adolescence exhibit an increased risk of polycystic ovary syndrome (PCOS) and/or infertility later on in adulthood?
SUMMARY ANSWER
Our results suggest that menstrual irregularity and/or elevated androgen levels at 16 years are still associated with symptoms of PCOS at 26 years as well as infertility problems at 26 years but not with decreased pregnancy or delivery rates at 26 years.
WHAT IS KNOWN ALREADY
Hyperandrogenaemia is associated with menstrual irregularity, hirsutism, acne and potentially higher risk for PCOS, but there are few follow-up studies investigating whether adolescent hyperandrogenaemia and/or menstrual irregularity are an early sign of PCOS.
STUDY DESIGN, SIZE, DURATION
A prospective population-based cohort study was conducted using two postal questionnaires targeting girls in the Northern Finland Birth Cohort 1986 (NFBC1986, n = 4567). The NFBC1986 comprises all expected births from the year 1986 in the two northernmost provinces of Finland. Collection of the database was performed at the age of 16 and 26. The 16-year and 26-year questionnaires included one question about the regularity and length of the menstrual cycle. The 26-year questionnaire also included questions about symptoms of PCOS, reproduction and infertility problems.
PARTICIPANTS, SETTING, METHODS
The response rates for the questionnaires were 80% (n = 3669) at 16 years and 50% (n = 2270) at 26 years. At 15–16 years, of 2448 girls, 709 (29%) girls reported menstrual irregularity (symptomatic girls) and 1739 (71%) had regular periods (non-symptomatic girls). After combining data from the two questionnaires a total of 2033 girls were included in the analyses. The χ2 and Student's t-test was used to compare reproductive outcome and prevalence of clinical hyperandrogenaemia, PCOS and infertility at 26 years between the study groups. Univariate and multivariate logistic regression models were employed to estimate the association of menstrual irregularity at 16 years with clinical hyperandrogenaemia, PCOS and infertility at 26 years.
MAIN RESULTS AND THE ROLE OF CHANCE
At follow-up, the proportion of symptomatic girls who had conceived at least once (68.0 versus 67.9%) and had delivered at least one child (25.7 versus 28.1%) was similar to the non-symptomatic women and the groups had similar miscarriage rates (11.6 versus 12.1%). Logistic regression analyses indicated that menstrual irregularity at 16 years was associated with an increased risk of menstrual irregularity [adjusted odds ratio (OR) 1.37, 95% confidence interval (CI) 1.00–1.88, P = 0.050], PCOS (adjusted OR 2.91, 95% CI 1.74–4.84, P < 0.001) and infertility problems (adjusted OR 2.07, 95% CI 1.16–3.76, P = 0.013) at 26 years. At 26 years, women with PCOS (P = 0.013), hirsutism (P = 0.001) and acne (P < 0.001) exhibited significantly higher values of free androgen index (FAI) at 16 years than control women. There was a significant linear trend in the higher FAI quartiles at 16 years towards higher prevalence of PCOS (P = 0.005), hirsutism (P < 0.001) and acne (P < 0.001) at 26 years. Only 10.5% of the girls with menstrual irregularity at 16 years had PCOS at 26 years.
LIMITATIONS, REASONS FOR CAUTION
The diagnosis of menstrual irregularity was based on a self-reported questionnaire, thus introducing a risk of information bias in reporting the symptoms. Moreover, ovarian ultrasonography was not available to aid the diagnosis of PCOS and there was no clinical evaluation of hyperandrogenism. The relatively low rate of participation to the questionnaire at 26 years may also have biased the results.
WIDER IMPLICATIONS OF THE FINDINGS
Our findings confirm that menstrual irregularity and/or elevated androgen levels are already present in adolescence in women with PCOS and infertility in later life, which strengthens the importance of early identification of menstrual irregularity.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by grants from the Finnish Medical Society Duodecim, the North Ostrobothnia Regional Fund, the Academy of Finland, the Sigrid Juselius Foundation, University Hospital Oulu and University of Oulu, the European Commission and the Medical Research Council, UK, Welcome Trust (089549/Z/09/Z). None of the authors have any conflict of interest.
Keywords: polycystic ovary syndrome, hyperandrogenaemia in adolescence, hirsutism, menstrual irregularity, oligo-amenorrhea
Introduction
Hyperandrogenaemia is associated with menstrual irregularity, hirsutism and acne and it has been suggested to increase risk for metabolic disorders in women (Franks, 1995). Hyperandrogenaemia is also an important marker of polycystic ovary syndrome (PCOS), a common heterogeneous endocrine disorder affecting 5–10% of women of reproductive age, depending on the population and the diagnostic criteria applied (Franks, 1995). Menstrual irregularity in adolescence has been shown to be a good marker of hyperandrogenaemia and it has been proposed to lead to the development of PCOS in adulthood (Lewy et al., 2001; Pinola et al., 2012). In addition, adolescent girls with irregular menstrual cycles have higher androgen levels than girls with regular menstrual cycle (Venturoli et al., 1986, 1995; van Hooff et al., 2004; Pinola et al., 2012).
PCOS is commonly associated with anovulation, and previous studies have shown that adult women with PCOS have a smaller family size and more infertility problems than healthy women (Koivunen et al., 2008; Roos et al., 2011). An association between biochemical hyperandrogenaemia and unfavourable reproductive potential has been reported (Apter and Vihko, 1990). There are, however, few longitudinal studies on the association of early menstrual irregularity and/or elevated androgen levels in adolescence with PCOS, fertility and reproductive health later in life (Southam and Richart, 1966; Apter and Vihko, 1990; Venturoli et al., 1995; van Hooff et al., 2004; Chung et al., 2011; West et al., 2013).
The aim of this study was to explore the association of reproductive health (diagnosis of PCOS, infertility problems, rates of pregnancies, miscarriages and deliveries) in early adulthood (at 26 years) with a history of menstrual irregularity and/or elevated androgen levels at 16 years.
Materials and Methods
Study population
The population derives from the prospective Northern Finland Birth Cohort 1986 (NFBC 1986) members followed up since fetal life. In 1986, 9362 mothers, who had an expected date of birth between 1 July 1985 and 30 June 1986, gave birth to 9432 live born children (47 stillbirths) in two northernmost provinces of Finland.
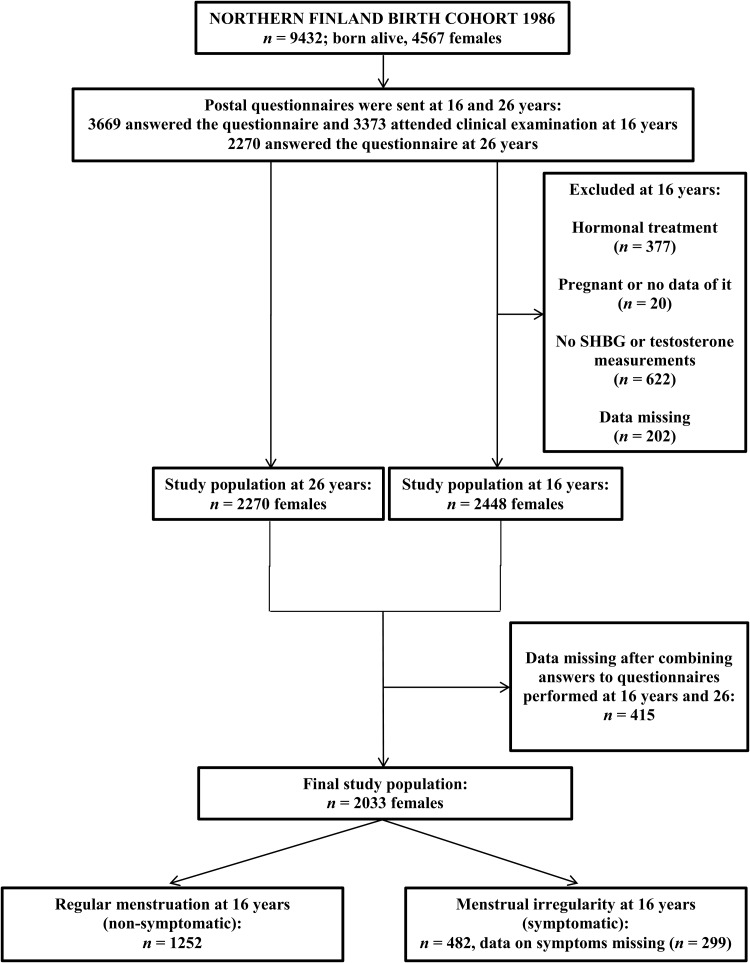
The girls who were alive and traceable (n = 4567) were traced for the 16-year follow-up including a postal questionnaire and an invitation to a clinical examination. Of these, 3669 (80%) answered the questionnaire and 3373 (74%) attended this clinical examination and gave fasting blood samples. Twins and triplets, pregnant girls, hormonal contraceptive users, and subjects with incomplete data were excluded from the study. At this stage, the study population consisted of 2448 singleton females (53.6% of those eligible). At 26 years, young women (n = 4503) were sent another questionnaire with questions on socio-demographic and other health background factors mainly about reproduction, menstruation and infertility. Overall 2270 (50%) women responded to the questionnaire. After combining data from the 16- and 26-year follow-up questionnaires, the final study sample included 2033 singleton females. The flow chart of the study is presented in Fig. 1.
Figure 1.
Flow chart of the study population.
The questionnaires included questions on the regularity and length of the menstrual cycle, pregnancies, deliveries, miscarriages, infertility and acne (Fig. 2). At 16 years, the girls who answered ‘yes’ to the question 1 on oligo-amenorrhea were considered as ‘symptomatic’. The women who answered ‘no’ were considered as ‘non-symptomatic’. At 26 years, the women who marked ≥1 to at least one of the four options (question 2) were considered to have been pregnant at some point of their lives.
Figure 2.
Questions from the 16- and 26-year questionnaire.
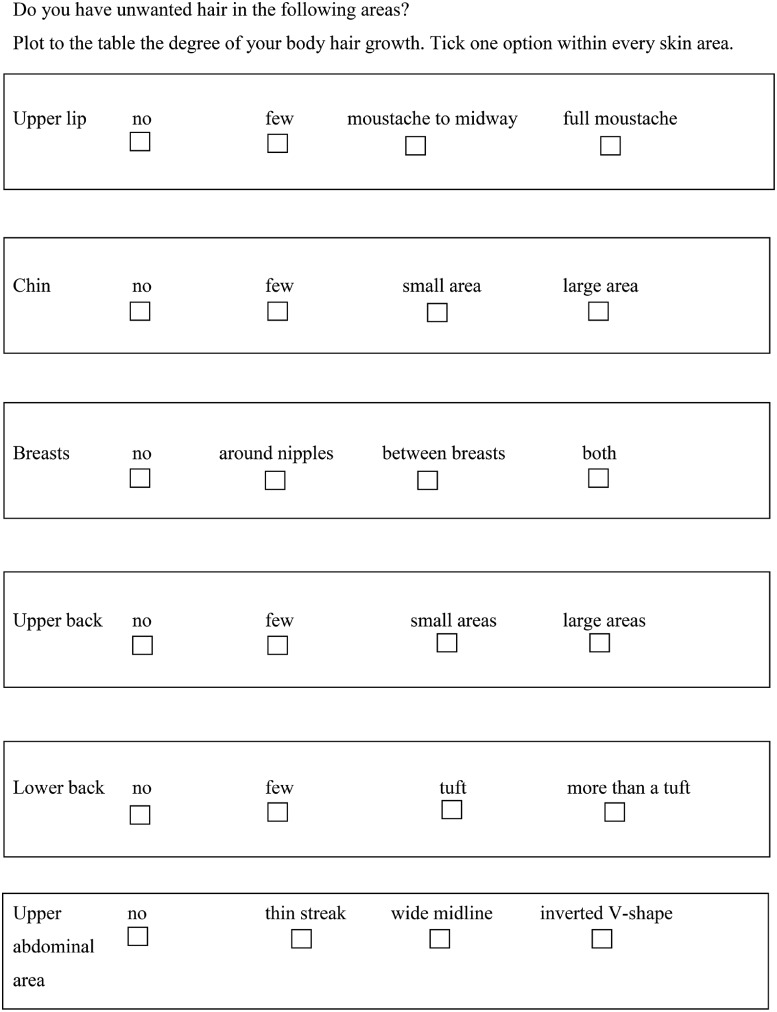
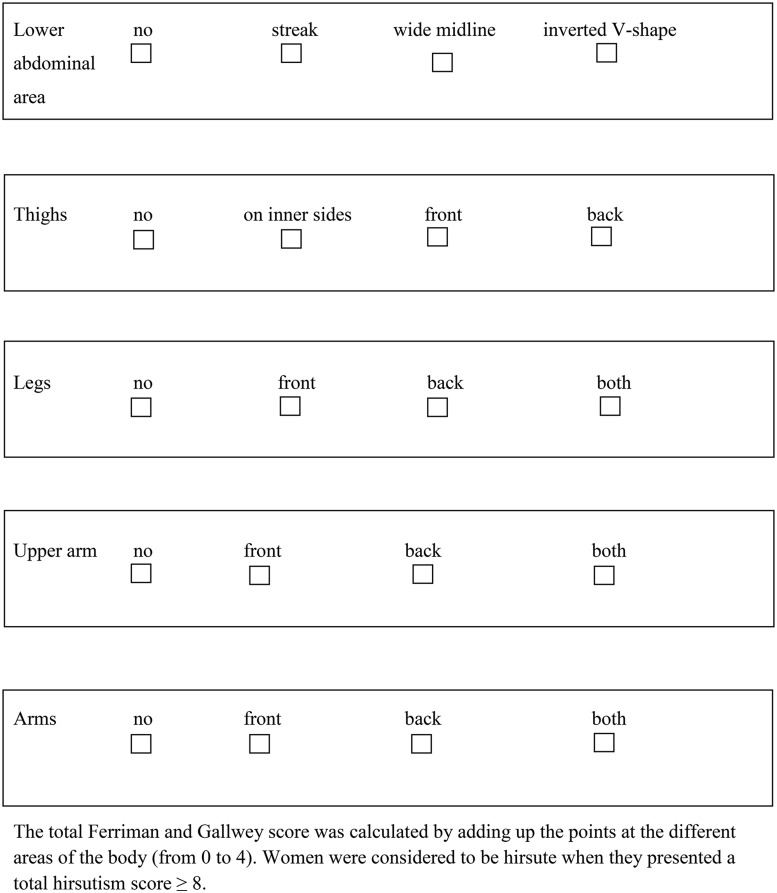
Hirsutism score was self-estimated with a modified Ferriman–Gallwey score assessment that was sent together with the questionnaire (Fig. 3). Women were considered to be hirsute when they presented a total hirsutism score ≥8 according to the Ferriman and Gallwey chart (Ferriman and Gallwey, 1961).
Figure 3.
Modified Ferriman and Gallwey score.
In the 26-year questionnaire (Fig. 2, question 5), women who reported a menstrual cycle longer than 35 days (option 3), extremely irregular (option 4) or had amenorrhoea (option 5) were considered to have ‘menstrual irregularity’. Women on hormonal contraception were informed in the questionnaire to report menstrual patterns during the year before using any hormonal contraception. Women who had not been pregnant by the age of 26 were excluded from the miscarriage analysis. Also women who had achieved pregnancy with infertility treatments were included in the pregnancy and delivery analyses. PCOS at 26 years was diagnosed either by a physician (women who reported ‘yes’ to the question 6) and/or by the presence of both menstrual irregularity and hirsutism in the questionnaire, which is consistent with both the National Institutes of Health and the Rotterdam criteria for diagnosis of PCOS (Zawadski and Dunaif, 1992; Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group 2004). At 26 years, women who had been investigated for infertility were categorized as having ‘infertility problems’ (question 3).
At 16 and 26 years, BMI was calculated as the ratio of weight (kg) and height squared (m2). The waist-to-hip ratio (WHR) was assessed as the ratio between the circumferences of the waist (at the level midway between lowest rib margin and the iliac crest) and the hip (at the widest trochanters). Annual household income was enquired about in the 16-year questionnaire, and to enable comparison of households of different sizes and structures, household consumption units (Organisation for Economic Co-operation and Development scale) were calculated by assigning the first adult in the household a value of 1.0 unit, with additional adults (>17 years) receiving 0.7 and each child (≤17 years) 0.5 units (United Nations, Statistical Office 1977).
The Ethics Committee of the Northern Ostrobothnia Hospital District approved this study and written informed consent was obtained from all subjects.
Laboratory methods
At 16 years, serum samples for assay of testosterone were analysed by using Agilent triple quadrupole 6410 LC/MS equipment with an electrospray ionization source operating in positive-ion mode (Agilent Technologies, Wilmington, DE, USA). Multiple reaction monitoring was used to quantify testosterone by using trideuterated testosterone (d3-testosterone), with the following transitions: m/z 289.2 to 97 and 289.2 to 109 for testosterone and 292.2 to 97 and 292.2 to 109 for d3-testosterone. The intra-assay coefficients of variation (CVs) of the method were 5.3, 1.6 and 1.2% for testosterone at 0.6, 6.6 and 27.7 nmol/l, respectively. The inter-assay CVs were 5.3, 4.2 and 1.0% for the respective concentrations. Serum sex hormone-binding globulin (SHBG) was analysed by time-resolved fluoroimmunoassay (AutoDelfia, PerkinElmer, Turku, Finland). The free androgen index (FAI) was calculated by using the equation 100 × testosterone (nmol/l)/SHBG (nmol/l).
Statistical methods
The Chi squared (χ2) test was used to compare reproductive outcome (pregnancies, deliveries and miscarriages) and prevalence of clinical hyperandrogenaemia, PCOS and infertility problems at 26 years between the study groups. Comparisons of continuous variables with normal distribution (numbers of pregnancies, deliveries and miscarriages) were made by using Student's t-test. Univariate and multivariate logistic regression models were employed to estimate the association of menstrual irregularity at 16 years with clinical hyperandrogenaemia, PCOS and infertility problems at 26 years. The results are reported as odds ratios (ORs) with 95% confidence intervals (95% CIs). The data were adjusted for the following variables at 16 years: consumption of alcohol as never/occasionally (no) or ≥1 days per month (yes); smoking as never/≤1 days per week (no) or ≥2 days per week (yes); annual income per consumption unit, and age at menarche (supplemented with results from the 26-year questionnaire) and BMI as continuous variable. The data were also adjusted for the potential confounding factors at 26 years (consumption of alcohol, smoking and BMI). The distributions of laboratory and anthropometric measurements were skewed both among the cases and controls; therefore, the difference in distributions was tested by using nonparametric Mann–Whitney U-test. The hormone levels in the cases and controls are presented as medians and lower (25th) and upper quartiles (75th) (interquartile range, IQR). Results from the whole study population were also stratified into serum testosterone, SHBG and FAI quartiles and the χ2 test (linear-by-linear association) was used to compare the categorical variables across these quartiles. A P-value <0.05 was considered significant. Statistical analyses were performed by using IBM SPSS Statistics 20.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Reproductive outcome at 26 years
Women reporting menstrual irregularity suggestive of anovulatory cycles at 16 years became pregnant at least once, and had at least one delivery or one miscarriage as often as women with regular menstruation at 16 years, and had similar mean pregnancy, delivery and spontaneous abortion rates at 26 years (Table I). Excluding women using hormonal contraceptives did not alter the results (data not shown).
Table I.
Reproductive outcome in girls with or without menstrual irregularity at 16 years of age.
| Menstrual irregularitya at 16 years | Nb | Reproductive outcome at 26 years |
P-value | |
|---|---|---|---|---|
| No pregnancies (N/%) | At least one pregnancy (N/%) | |||
| No | 1252 | 402/32.1 | 850/67.9 | |
| Yes | 482 | 154/32.0 | 328/68.0 | 0.950 |
| No deliveries (N/%) | At least one delivery (N/%) | |||
| No | 1252 | 900/71.9 | 352/28.1 | |
| Yes | 482 | 358/74.3 | 124/25.7 | 0.318 |
| No miscarriagesc (N/%) | At least one miscarriagec (N/%) | |||
| No | 850 | 747/87.9 | 103/12.1 | |
| Yes | 328 | 290/88.4 | 38/11.6 | 0.801 |
| Number of pregnancies [mean (SD)] | ||||
| No | 1252 | 0.71 (1.18) | ||
| Yes | 482 | 0.64 (1.10) | 0.249 | |
| Number of deliveries [mean (SD)] | ||||
| No | 1252 | 0.47 (0.88) | ||
| Yes | 482 | 0.44 (0.86) | 0.410 | |
| Number of miscarriagesc [mean (SD)] | ||||
| No | 850 | 0.16 (0.48) | ||
| Yes | 328 | 0.15 (0.46) | 0.712 | |
The significance tests used are the Chi-squared (χ2) test and Student's t-test.
aIn the 26-year questionnaire, women who reported menstruation cycle longer than 35 days (option 3), extremely irregular (option 4) or had amenorrhoea (option 5) were considered to have ‘menstrual irregularity’.
bThe numbers of non-symptomatic and symptomatic women in separate analyses varies due to non-response to some items.
cWomen who had not been pregnant by the age of 26 were excluded from the miscarriage analysis.
Prevalence of clinical hyperandrogenaemia, PCOS and infertility problems at 26 years
Women with menstrual irregularity at 16 years had a significantly higher prevalence of menstrual irregularity suggestive of anovulatory cycles, PCOS (self-reported menstrual irregularity and hirsutism at 26 years and/or PCOS diagnosis made by the physician) and infertility problems at 26 years than women with regular menstrual cycle (Table II). The results remained significant after excluding women on hormonal contraception at 26 years (menstrual irregularity: 27.7 versus 20.3%, respectively, P = 0.012) (PCOS: 14.6 versus 4.4%, P < 0.001) (infertility problems: 10.5 versus 6.4%, P = 0.032). The symptomatic and non-symptomatic girls at 16 years had a similar prevalence of hirsutism and acne at 26 years (Table II), but after excluding women on hormonal contraception at 26 years, the symptomatic 16-year-old girls exhibited a higher prevalence of hirsutism at 26 years (25.4 versus 19.5%, P = 0.049).
Table II.
Prevalence of clinical symptoms of hyperandrogenaemia, polycystic ovary syndrome (PCOS) and infertility problems at 26 years of age in girls with or without menstrual irregularity at 16 years.
| Menstrual irregularity at 16 years | Na | Clinical symptoms of hyperandrogenaemia, PCOS and infertility problems at 26 years |
P-value | |
|---|---|---|---|---|
| No menstrual irregularity (N/%) | Menstrual irregularity (N/%) | |||
| No | 1236 | 972/78.6 | 264/21.4 | |
| Yes | 475 | 334/70.3 | 141/29.7 | <0.001 |
| No PCOS (N/%) | PCOSb (N/%) | |||
| No | 1214 | 1163/95.8 | 51/4.2 | |
| Yes | 475 | 425/89.5 | 50/10.5 | <0.001 |
| No PCOS diagnosis (N/%) | PCOS diagnosis (N/%) | |||
| No | 1214 | 1196/98.5 | 18/1.5 | |
| Yes | 475 | 447/94.1 | 28/5.9 | <0.001 |
| Non-symptomatic (N/%) | Menstrual irregularity and hirsutism (N/%) | |||
| No | 1214 | 1180/97.2 | 34/2.8 | |
| Yes | 475 | 446/93.9 | 29/6.1 | 0.001 |
| No hirsutism (N/%) | Hirsutism (N/%) | |||
| No | 1142 | 994/82.7 | 198/17.3 | |
| Yes | 445 | 352/79.1 | 93/20.9 | 0.100 |
| No acne (N/%) | Acne (N/%) | |||
| No | 1247 | 782/62.7 | 465/37.3 | |
| Yes | 482 | 292/60.6 | 190/39.4 | 0.413 |
| No infertility problems (N/%) | Infertility problems (N/%) | |||
| No | 1174 | 1125/95.8 | 49/4.2 | |
| Yes | 459 | 427/93.0 | 32/7.0 | 0.019 |
The significance test used is the Chi-squared (χ2) test.
aThe number of non-symptomatic and symptomatic women in separate analyses varies due to non-response to some items.
bWomen with either self-reported PCOS diagnosis or both menstrual irregularity and hirsutism.
Menstrual irregularity at 16 years had a sensitivity of 50.5%, a specificity of 73.2%, a likelihood ratio for a positive result of 1.9 and a likelihood ratio for a negative result of 0.7 for predicting PCOS at 26 years.
Logistic regression analyses
Menstrual irregularity at 16 years was associated with an increased risk of menstrual irregularity at 26 years. After adjusting for potential confounders (consumption of alcohol, smoking, annual income per consumption unit, age at menarche and BMI) the association remained significant. In both univariate and multivariate analyses, women with menstrual irregularity problems at 16 years were at a higher risk of having been diagnosed with PCOS and of suffering from infertility at 26 years than women with a regular cycle (Table III). The data were also adjusted for the potential confounders (consumption of alcohol, smoking and BMI) at 26 years and the results remained unchanged.
Table III.
Risk of clinical symptoms of hyperandrogenaemia, PCOS and infertility problems at 26 years of age in girls with or without menstrual irregularity at 16 years.
| Menstrual irregularity at 16 years | Clinical symptoms of hyperandrogenaemia, PCOS and infertility problems at 26 years |
|||
|---|---|---|---|---|
| Crude OR (95% CI) | P-value | Adjusteda OR (95% CI) | P-value | |
| Menstrual irregularity (no/yes) | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.55 (1.22–1.97) | <0.001 | 1.37 (1.00–1.88) | 0.050 |
| PCOSb (no/yes) | ||||
| No | 1.00 | 1.00 | ||
| Yes | 2.68 (1.79–4.03) | <0.001 | 2.91 (1.74–4.84) | <0.001 |
| Self-reported PCOS diagnosis (no/yes) | ||||
| No | 1.00 | 1.00 | ||
| Yes | 4.16 (2.28–7.60) | <0.001 | 5.53 (2.61–11.69) | <0.001 |
| Menstrual irregularity and hirsutism (no/yes) | ||||
| No | 1.00 | 1.00 | ||
| Yes | 2.26 (1.36–3.75) | 0.002 | 2.06 (1.06–4.02) | 0.034 |
| Hirsutism (no/yes) | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.26 (0.96–1.66) | 0.100 | 1.29 (0.92–1.81) | 0.141 |
| Acne (no/yes) | ||||
| No | 1.00 | |||
| Yes | 1.09 (0.88–1.36) | 0.413 | 1.16 (0.89–1.52) | 0.272 |
| Infertility problems (no/yes) | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.72 (1.092.72) | 0.021 | 2.07 (1.16–3.67) | 0.013 |
The analyses were performed with univariate and multivariate logistic regression.
OR, odds ratio; CI, confidence interval.
aAdjusted for consumption of alcohol (no/yes), smoking (no/yes), BMI (as continuous variable), annual income per consumption unit (as continuous variable) and age at menarche (as continuous variable).
bWomen with either self-reported PCOS diagnosis or both menstrual irregularity and hirsutism.
Adolescent serum testosterone levels and symptoms of PCOS at 26 years
Women suffering from PCOS at 26 years exhibited significantly higher serum concentrations of testosterone (P = 0.011) and FAI values (P = 0.013), and women with hirsutism had significantly higher FAI values (P = 0.001) and lower concentration of SHBG (P = 0.003) at 16 years than non-symptomatic women. Women with acne at 26 years had higher serum concentration of testosterone (P = 0.041), higher FAI values (P < 0.001) and lower levels of SHBG (P = 0.003) at 16 years than women with no acne (Table IV).
Table IV.
Hormonal parameters at 16 years of age in women with or without clinical symptoms of hyperandrogenaemia, PCOS and infertility problems at 26 years.
| Testosterone (nmol/l) | P-value | SHBG (nmol/l) | P-value | FAI | P-value | |
|---|---|---|---|---|---|---|
| Menstrual irregularity | ||||||
| No (n = 1174–1175) | 1.64 (1.30, 2.05) | 52.0 (37.1, 73.6) | 3.13 (2.03, 4.68) | |||
| Yes (n = 350–351) | 1.67 (1.28, 2.00) | 0.725 | 54.9 (38.3, 76.6) | 0.222 | 3.11 (2.03, 4.42) | 0.378 |
| PCOSa | ||||||
| No (n = 1415–1417) | 1.63 (1.29, 2.02) | 52.8 (37.6, 74.4) | 3.09 (2.01, 4.54) | |||
| Yes (n = 92) | 1.82 (1.43, 2.25) | 0.011 | 49.8 (34.1, 71.0) | 0.297 | 3.65 (2.31, 5.55) | 0.013 |
| Hirsutism | ||||||
| No (n = 1157–1159) | 1.64 (1.30, 2.03) | 53.7 (38.0, 77.7) | 2.97 (1.95, 4.53) | |||
| Yes (n = 266) | 1.69 (1.33, 2.08) | 0.218 | 48.8 (34.6, 66.8) | 0.003 | 3.47 (2.26, 5.05) | 0.001 |
| Acne | ||||||
| No (n = 961–962) | 1.62 (1.28, 2.00) | 54.6 (38.5, 78.1) | 2.93 (1.93, 4.42) | |||
| Yes (n = 577–578) | 1.71 (1.34, 2.07) | 0.041 | 49.5 (35.7, 68.5) | 0.003 | 3.42 (2.22, 4.93) | <0.001 |
| Infertility problems | ||||||
| No (n = 1384–1386) | 1.64 (1.30, 2.03) | 52.4 (37.3, 73.6) | 3.15 (2.03, 4.61) | |||
| Yes (n = 74) | 1.71 (1.39, 2.23) | 0.337 | 58.2 (37.8, 86.8) | 0.332 | 3.03 (2.10, 5.17) | 0.870 |
The values are presented as medians and interquartile ranges (IQR) (from 25th to 75th percentile). The significance test used is the Mann–Whitney U-test. The number of individuals in separate analyses varies due to non-response to some items or blood sample failures.
SHBG, sex hormone-binding globulin; FAI, free androgen index.
aWomen with either self-reported PCOS diagnosis or both menstrual irregularity and hirsutism.
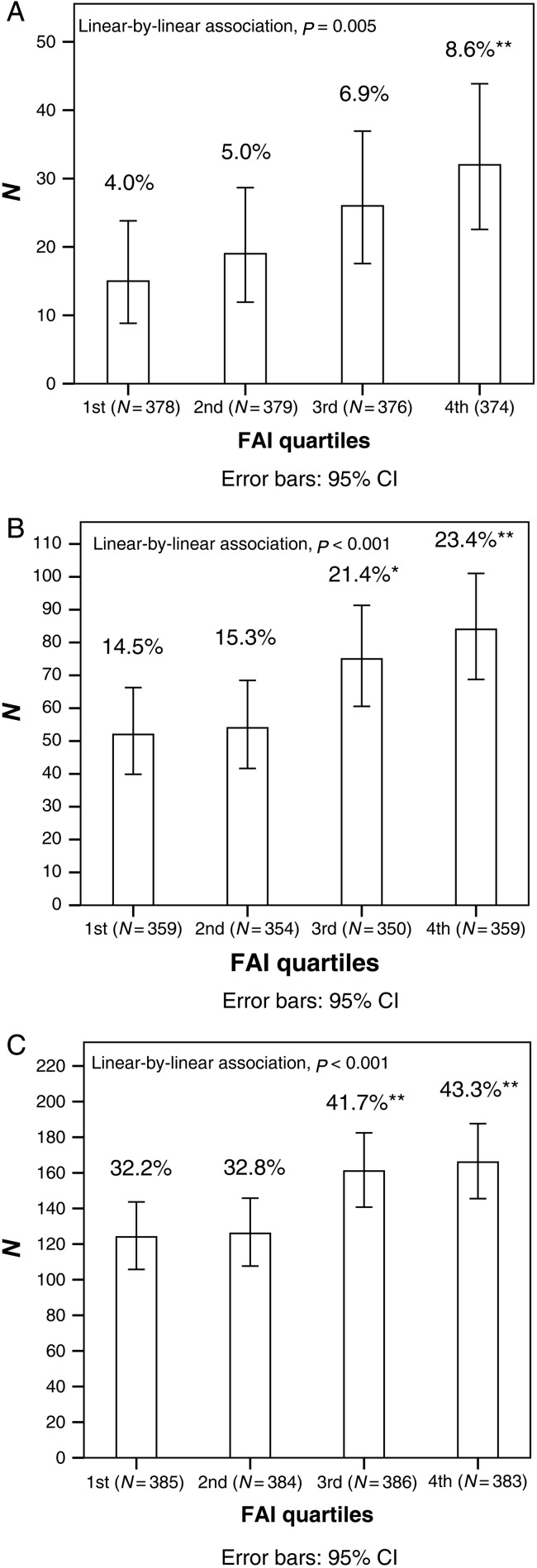
After stratifying the whole study population into testosterone, SHBG and FAI quartiles at 16 years, there was a significant linear trend in the higher testosterone quartiles towards higher prevalence of PCOS (from the first quartile 4.2% to fourth quartile 8.6%, P = 0.008) and acne (from 34.0 to 40.5%, P = 0.036) at 26 years. Occurrence of hirsutism (from 14.2 to 22.7%, P = 0.005) and acne (from 31.4 to 41.4%, P = 0.001) at 26 years increased significantly from the highest SHBG quartile to the lowest SHBG quartile (data not shown). There was a significant linear trend in the higher FAI quartiles towards higher prevalence of PCOS (from 4.0 to 8.6%, P = 0.005), hirsutism (from 14.5 to 23.4%, P < 0.001) and acne (from 32.2 to 43.3%, P < 0.001) at 26 years (Fig. 4).
Figure 4.
Prevalence (%) of PCOS (A), hirsutism (B) and acne (C) at 26 years in the different FAI quartiles at 16 years. The P-values represent the linear relationship in prevalence of PCOS, hirsutism and acne across the FAI quartiles. The total number of individuals in the quartiles is presented in parentheses. *χ2 test compared with the first quartile, P < 0.05. **χ2 test compared with the first quartile, P < 0.01.
Hormonal and anthropometric parameters in the group of girls with menstrual irregularity at 16 years
The women with menstrual irregularity at 16 years and PCOS at 26 years already had higher serum concentrations of testosterone (P = 0.001) and FAI values (P = 0.046) at 16 years than women with menstrual irregularity at 16 years but without PCOS at 26 years. There was, however, no difference between these two groups as regards BMI, WHR or change in BMI during the follow-up (Table V). Women with menstrual irregularity both at 16 years and 26 had significantly lower BMI [median (IQR)] at 16 years [19.4 (18.1–20.9) versus 20.8 (19.2–22.5), P < 0.001] and 26 [20.9 (19.6, 22.0) versus 23.6 (21.6, 26.5), P < 0.001], WHR [0.83 (0.77, 0.88) versus 0.85 (0.79, 0.90), P = 0.009] at 26 years and increase in BMI [1.65 (0.73, 2.81) versus 2.70 (0.88, 4.65), P < 0.001] during the follow-up than symptomatic women with regular menstrual cycles at 26 years. The two groups did not differ as regards serum concentrations of testosterone or SHBG, or FAI value (all data not shown).
Table V.
Hormonal and anthropometric parameters of girls categorized by menstrual irregularity at the start of the study and with or without PCOS 10 years later.
| Menstrual irregularityA No PCOSB (n = 362–410) |
Menstrual irregularityA PCOSB,b (n = 43–50) |
P-value | |||
|---|---|---|---|---|---|
| Median (IQR) | Na | Median (IQR) | Na | ||
| Testosterone (nmol/l) | 1.63 (1.31, 2.06) | 367 | 1.95 (1.63, 2.37) | 43 | 0.001 |
| SHBG (nmol/l) | 48.7 (36.1, 64.9) | 368 | 46.7 (31.4, 64.7) | 43 | 0.699 |
| FAI | 3.33 (2.29, 4.95) | 367 | 4.04 (2.73, 5.89) | 43 | 0.046 |
| BMI at 16 years | 20.3 (18.8, 22.0) | 399 | 20.3 (19.0, 22.8) | 45 | 0.620 |
| WHR at 16 years | 0.76 (0.74, 0.79) | 397 | 0.77 (0.73, 0.80) | 44 | 0.916 |
| Change in BMI between age 16 and 26 | 2.30 (0.79, 4.02) | 384 | 2.27 (1.13, 6.00) | 45 | 0.348 |
| BMI at 26 years | 22.5 (20.5, 25.1) | 410 | 22.0 (20.1, 29.4) | 50 | 0.784 |
| WHR at 26 years | 0.84 (0.79, 0.90) | 362 | 0.85 (0.79, 0.89) | 47 | 0.867 |
The values are presented as medians and IQR (from 25th to 75th percentile). The significance test used is the Mann–Whitney U-test.
WHR, waist-to-hip ratio.
AMenstrual irregularity: at start of study (i.e. age 16 years).
BPCOS: data at 10 years after start of study (i.e. age 26 years).
aThe number of individuals in separate analyses varies due to non-response to some items.
bWomen with either self-reported PCOS diagnosis or both menstrual irregularity and hirsutism.
Discussion
Menstrual irregularity is a common gynaecological condition affecting especially women in early reproductive life. The present study suggests that at 26 years the frequency of persistent menstrual irregularity and PCOS was significantly higher in women with previous menstrual irregularity at 16 years, compared with girls with a regular menstrual cycle. The girls who exhibited the greatest degree of androgen excess at 16 years were also at higher risk for retaining the diagnosis of PCOS and clinical signs of hyperandrogenaemia, such as hirsutism and acne, later in life. Although symptomatic girls were investigated more often for infertility than non-symptomatic girls, adolescent menstrual irregularity was not linked to an increased risk of childlessness in early adulthood.
Comparison with other studies
Several studies have reported the prevalence of menstrual irregularity in adolescence, but there are few follow-up studies, most of them of short duration (2–3 years) (Southam and Richart, 1966; Apter and Vihko, 1990; Venturoli et al., 1995; van Hooff et al., 2004; Chung et al., 2011), demonstrating the effects of adolescent menstrual irregularity in early adulthood. In the present study the follow-up was 10 years, which allowed us to investigate longer-term consequences of menstrual irregularity on reproductive health in a large cohort of 2033 females.
In agreement with previous cross-sectional and follow-up studies (Dahlgren et al., 1992; Hudecova et al., 2009), the symptomatic girls at 16 years gave birth successfully to at least one child as often, conceived at least once as often and had similar pregnancy, delivery rates and prevalence of spontaneous abortions, as the non-symptomatic girls until the age of 26. Importantly, these findings are also in line with our previous results from another Finnish follow-up cohort (the Northern Finnish Birth Cohort 1966), showing that the impact of oligo-amenorrhea and hirsutism on fertility and family size was limited at 31 years. At a more advanced age, however, the women with both oligo-amenorrhea and hirsutism did not quite match the parity of healthy non-symptomatic women despite more frequent infertility treatments (Koivunen et al., 2008; West et al., 2013). Another follow-up study has also demonstrated that androgen concentrations before pregnancy were higher in females who subsequently had no pregnancies than in those who had been pregnant (Apter and Vihko, 1990). The failure to demonstrate any difference in pregnancy and birth rates in the present study may be due to the fact that the first questionnaire picked only the girls with menstrual irregularity and not hirsutism, and that the second questionnaire was performed in very early adulthood when the impact of menstrual irregularity on fertility rates is not yet fully manifested. In the present study, the symptomatic girls were also investigated for infertility significantly more frequently than the non-symptomatic girls suggesting that infertility treatment may restore normal reproductive capacity in these young women.
In contrast to previous studies performed in small selected groups of women with an established PCOS diagnosis and attending infertility clinics (Sagle et al., 1988; Watson et al., 1993; Glueck et al., 1999; Wang et al., 2002), we found no increased risk of spontaneous abortions among symptomatic girls. Interestingly, however, our results were also similar to those obtained in our previous cohort studies (Koivunen et al., 2008; West et al., 2013).
Variability in adolescent menstrual cycles is wide: according to a World Health Organization study of 3073 girls, 10% of them had >60 days between their first and second menses, and 7% had a first-cycle length of 20 days (Anonymous 1986). The prevalence of menstrual irregularities in adolescents depends also on the definition used for irregular menstrual cycle and age at the time of the questionnaire [from 12% (Nwankwo et al., 2010) to almost 30% (Anonymous 1986)]. The present 10-year follow-up revealed that the presence of menstrual irregularity was significantly higher at 26 years among the girls with menstrual irregularity at 16 years. In line with these results, a recent 4-year follow-up study has shown an almost 3-fold risk for persistent menstrual irregularity among girls with long cycle lengths at first consultation (Chung et al., 2011). Similarly, a 3-year follow-up study, which documented changes in menstrual pattern between ages 15 and 18 years in the general population, reported a 31-fold risk for oligo-amenorrhea among adolescents with oligo-amenorrhea at first consultation (van Hooff et al., 2004). In another study, about half of the adolescents with oligo-amenorrhoea at first control remained oligo-amenorrhoeic during an 8-year follow-up (Southam and Richart, 1966).
In the present results, however, after adjustment for age at menarche, the risk for persistent menstrual irregularity in the symptomatic women remained barely significant. One possible explanation could be that the symptomatic girls at 16 years entered menarche 5 months later and probably reached full maturation of the hypothalamic–pituitary–ovarian axis later than the non-symptomatic girls, thus leading to an underestimation of the association between menstrual irregularity at 16 and 26 years. The reproductive system takes ∼2 years after menarche to mature before adolescents have regular ovulatory cycles (Anonymous, 1986; Apter et al., 1978). Moreover, a retrospective study of menstrual disturbances in Chinese adolescents demonstrated that the proportion of menstrual irregularities decreased significantly from the first to seventh year after menarche and that the main drop occurred at the second year (Chan et al., 2009). In line with this hypothesis, in the present study, 70% of the girls with menstrual irregularity at 16 years recovered regular cycles at 26 years. The girls who retained menstrual irregularity at 26 years were significantly leaner at 16 and 26 years and had a smaller gain in weight than symptomatic girls without menstrual irregularity, suggesting a hypothalamic aetiology for at least some the menstrual disorders.
In the whole study population, the women who had PCOS, hirsutism or acne at 26 years already exhibited higher FAI values at 16 years than the women without such conditions, and the girls in the highest FAI quartiles at 16 years had the highest prevalence of PCOS, hirsutism and acne at 26 years. It is generally accepted that puberty is characterized by an increase in insulin resistance, which might induce symptoms of PCOS more often at this age, and that the symptoms disappear after the pubertal period with subsequent normalization of insulin resistance (Amiel et al., 1986). In the present study, only 10.5% of the girls with menstrual irregularity at 16 years had PCOS at 26 years. We expected that these girls would have been heavier and experienced a greater weight gain during the follow-up than the girls without PCOS, but this was not the case. Comparison of anthropometric and hormonal parameters revealed that the girls with menstrual irregularity at 16 years who retained the diagnosis of PCOS at 26 years did not differ as regards BMI and WHR at 16 or 26 years, nor did they exhibit the greatest increase in BMI during the follow-up. All these findings suggest that the two pivotal criteria for the diagnosis of PCOS, namely hyperandrogenism and irregular cycles, are already present in adolescence and persist in early adulthood, independent of weight and weight gain in the women with the full phenotype of PCOS. In line with this, a previous study showed that adolescents with irregular menstrual cycles had significantly higher free testosterone concentrations during the first and the last longitudinal examination compared with control adolescents (Venturoli et al., 1995). Earlier reports have also stated that the hormonal characteristics and menstrual pattern that are formed during adolescence persist at least until the age of 30 (Southam and Richart, 1966; Apter and Vihko, 1990). However, according to our results, the predictive value of these two symptoms seems to be too low to justify an early intervention to avoid adult PCOS.
Strengths and limitations
The strength of our study is that it has been conducted on a large population-based, unselected birth cohort. We were able to use a self-reported symptoms based approach with information on menstrual cycle to investigate the association of adolescent menstrual irregularity with a risk for PCOS and PCOS-related symptoms in young adulthood. The longitudinal nature of the study allows us to follow the reproductive characteristics of this population in later life. The study population was also remarkably homogenous as regards ethnicity (all were Caucasians) and residence during the study period. As previously published, at 16 years both symptomatic and non-symptomatic girls were statistically comparable as regards to alcohol consumption, smoking habits, socio-economic status, BMI, fasting serum insulin and glucose levels, high sensitivity C-reactive protein (hs-CRP) and lipids [serum levels of total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL) and triglycerides] and insulin sensitivity, measured by HOMA-S (Pinola et al., 2012).
A limitation of our study is that the documenting of symptoms of PCOS was based on questionnaires as regards acne and hirsutism, which might be over-reported by self-estimation and therefore possibly narrowed the differences between the study groups. Hirsutism, however, was also checked with a detailed modified questionnaire. Ovarian ultrasonography was not available to aid the diagnosis of PCOS, but we have previously demonstrated (using the same questionnaire) in a similar cohort at age 31 (the Northern Finland Birth Cohort 1966) that self-reported menstrual irregularity and hirsutism can identify most women with the typical endocrine and metabolic profile of PCOS (Taponen et al., 2003, 2004). We were also able to take into account potential confounding factors at 16 and 26 years. The relatively low rate of response to the questionnaire at 26 years may also have biased the results. The lower response rate to the questionnaire at 26 years of age was possibly linked to the fact that, in contrast to the questionnaire at 16 years, the subjects had most likely moved away from their parents' household and received no encouragement to reply to the questionnaire. Furthermore, the large size of the study population may easily generate statistically significant but not necessarily clinically relevant differences between the study groups. In the present study menstrual irregularity at 16 years was a moderately weak predictor of PCOS, although the ORs in the logistic regression models indicated statistical significance.
Conclusions and policy implications
The present findings show that an irregular menstrual cycle and hyperandrogenism in adolescence seem to be early manifestations of PCOS and its symptoms in later life. However, the predictive value of these symptoms seems to be too low to justify an early clinical intervention, such as changes in lifestyle, in order to prevent adult PCOS. Importantly, girls presenting with menstrual irregularity at 16 years were more likely to suffer from infertility problems at 26 years than the non-symptomatic girls, but did not display any increased risk of spontaneous abortions and had at least one birth as often as non-symptomatic girls at 26 years. These results suggest that fertility can be restored by infertility treatment in early adulthood, which is an important finding as regards the prognosis of infertility in this particular population. Additional longitudinal reports are needed to clarify these relations and to determine the extent to which adolescent menstrual irregularity is associated with an increased risk of later morbidity to reproductive or metabolic disorders.
Authors' roles
L.M.-P., J.S.T. and S.W. conceived and designed the study. A.B. and S.W. analysed the data and A.B., M.-R.J., H.L., L.M.-P., J.S.T. and S.W. contributed to its interpretation. K.P. and A.R. analysed the samples and participated to the interpretation of the results. S.W. drafted the manuscript and all authors participated in the revision process and have approved this submission for publication. A.B., S.W. and L.M.-P. had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This work was supported by grants from the Finnish Medical Society Duodecim, the North Ostrobothnia Regional Fund, the Academy of Finland (project grants 104781, 120315, 129269, 1114194, SALVE), the Sigrid Juselius Foundation, University Hospital Oulu, Biocenter, University of Oulu, Finland (75617), the European Commission (EURO-BLCS, Framework 5 award QLG1-CT-2000–01643), Welcome Trust (089549/Z/09/Z to HL, M.-R.J. and S.F.) and the Medical Research Council, UK (PrevMetSyn/SALVE and G0802782 to S.F.).
Conflict of interest
None declared.
Acknowledgements
Sample quality controls, biobank up-keeping and aliquotting were performed in the National Public Health Institute, Biomedicum Helsinki, Finland and this study was financially supported by the Academy of Finland and Biocentrum Helsinki. We thank Ms. Outi Tornwall and Ms. Minttu Jussila.
References
- World Health Organization multicenter study on menstrual and ovulatory patterns in adolescent girls. II. Longitudinal study of menstrual patterns in the early postmenarcheal period, duration of bleeding episodes and menstrual cycles. World Health Organization Task Force on Adolescent Reproductive Health. J Adolesc Health Care. 1986;7:236–244. [PubMed] [Google Scholar]
- Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med. 1986;315:215–219. doi: 10.1056/NEJM198607243150402. [DOI] [PubMed] [Google Scholar]
- Apter D, Vihko R. Endocrine determinants of fertility: serum androgen concentrations during follow-up of adolescents into the third decade of life. J Clin Endocrinol Metab. 1990;71:970–974. doi: 10.1210/jcem-71-4-970. [DOI] [PubMed] [Google Scholar]
- Apter D, Viinikka L, Vihko R. Hormonal pattern of adolescent menstrual cycles. J Clin Endocrinol Metab. 1978;47:944–954. doi: 10.1210/jcem-47-5-944. [DOI] [PubMed] [Google Scholar]
- Chan SS, Yiu KW, Yuen PM, Sahota DS, Chung TK. Menstrual problems and health-seeking behaviour in Hong Kong Chinese girls. Hong Kong Med J. 2009;15:18–23. [PubMed] [Google Scholar]
- Chung PW, Chan SS, Yiu KW, Lao TT, Chung TK. Menstrual disorders in a Paediatric and Adolescent Gynaecology Clinic: patient presentations and longitudinal outcomes. Hong Kong Med J. 2011;17:391–397. [PubMed] [Google Scholar]
- Dahlgren E, Johansson S, Lindstedt G, Knutsson F, Oden A, Janson PO, Mattson LA, Crona N, Lundberg PA. Women with polycystic ovary syndrome wedge resected in 1956 to 1965: a long-term follow-up focusing on natural history and circulating hormones. Fertil Steril. 1992;57:505–513. doi: 10.1016/s0015-0282(16)54892-4. [DOI] [PubMed] [Google Scholar]
- Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–1447. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- Glueck CJ, Wang P, Fontaine RN, Sieve-Smith L, Tracy T, Moore SK. Plasminogen activator inhibitor activity: an independent risk factor for the high miscarriage rate during pregnancy in women with polycystic ovary syndrome. Metab Clin Exp. 1999;48:1589–1595. doi: 10.1016/s0026-0495(99)90250-0. [DOI] [PubMed] [Google Scholar]
- Hudecova M, Holte J, Olovsson M, Sundstrom Poromaa I. Long-term follow-up of patients with polycystic ovary syndrome: reproductive outcome and ovarian reserve. Hum Reprod. 2009;24:1176–1183. doi: 10.1093/humrep/den482. [DOI] [PubMed] [Google Scholar]
- Koivunen R, Pouta A, Franks S, Martikainen H, Sovio U, Hartikainen AL, McCarthy MI, Ruokonen A, Bloigu A, Jarvelin MR, et al. Fecundability and spontaneous abortions in women with self-reported oligo-amenorrhea and/or hirsutism: Northern Finland Birth Cohort 1966 Study. Hum Reprod. 2008;23:2134–2139. doi: 10.1093/humrep/den136. [DOI] [PubMed] [Google Scholar]
- Lewy VD, Danadian K, Witchel SF, Arslanian S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2001;138:38–44. doi: 10.1067/mpd.2001.109603. [DOI] [PubMed] [Google Scholar]
- Nwankwo TO, Aniebue UU, Aniebue PN. Menstrual disorders in adolescent school girls in Enugu, Nigeria. J Pediatr Adolesc Gynecol. 2010;23:358–363. doi: 10.1016/j.jpag.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Pinola P, Lashen H, Bloigu A, Puukka K, Ulmanen M, Ruokonen A, Martikainen H, Pouta A, Franks S, Hartikainen AL, et al. Menstrual disorders in adolescence: a marker for hyperandrogenaemia and increased metabolic risks in later life? Finnish general population-based birth cohort study. Hum Reprod. 2012;27:3279–3286. doi: 10.1093/humrep/des309. [DOI] [PubMed] [Google Scholar]
- Roos N, Kieler H, Sahlin L, Ekman-Ordeberg G, Falconer H, Stephansson O. Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: population based cohort study. BMJ. 2011;343:6309. doi: 10.1136/bmj.d6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Sagle M, Bishop K, Ridley N, Alexander FM, Michel M, Bonney RC, Beard RW, Franks S. Recurrent early miscarriage and polycystic ovaries. BMJ. 1988;297:1027–1028. doi: 10.1136/bmj.297.6655.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southam AL, Richart RM. The prognosis for adolescents with menstrual abnormalities. Am J Obstet Gynecol. 1966;94:637–645. doi: 10.1016/0002-9378(66)90398-x. [DOI] [PubMed] [Google Scholar]
- Taponen S, Martikainen H, Jarvelin MR, Laitinen J, Pouta A, Hartikainen AL, Sovio U, McCarthy MI, Franks S, Ruokonen A. Hormonal profile of women with self-reported symptoms of oligomenorrhea and/or hirsutism: Northern Finland birth cohort 1966 study. J Clin Endocrinol Metab. 2003;88:141–147. doi: 10.1210/jc.2002-020982. [DOI] [PubMed] [Google Scholar]
- Taponen S, Martikainen H, Jarvelin MR, Sovio U, Laitinen J, Pouta A, Hartikainen AL, McCarthy MI, Franks S, Paldanius M, et al. Metabolic cardiovascular disease risk factors in women with self-reported symptoms of oligomenorrhea and/or hirsutism: Northern Finland Birth Cohort 1966 Study. J Clin Endocrinol Metab. 2004;89:2114–2118. doi: 10.1210/jc.2003-031720. [DOI] [PubMed] [Google Scholar]
- van Hooff MH, Voorhorst FJ, Kaptein MB, Hirasing RA, Koppenaal C, Schoemaker J. Predictive value of menstrual cycle pattern, body mass index, hormone levels and polycystic ovaries at age 15 years for oligo-amenorrhoea at age 18 years. Hum Reprod. 2004;19:383–392. doi: 10.1093/humrep/deh079. [DOI] [PubMed] [Google Scholar]
- Venturoli S, Porcu E, Fabbri R, Paradisi R, Gammi L, Passarini M, Orsini LF, Flamigni C. Ovarian multifollicularity, high LH and androgen plasma levels, and anovulation are frequent and strongly linked in adolescent irregular cycles. Acta Endocrinol. 1986;111:368–372. doi: 10.1530/acta.0.1110368. [DOI] [PubMed] [Google Scholar]
- Venturoli S, Porcu E, Fabbri R, Pluchinotta V, Ruggeri S, Macrelli S, Paradisi R, Flamigni C. Longitudinal change of sonographic ovarian aspects and endocrine parameters in irregular cycles of adolescence. Pediatr Res. 1995;38:974–980. doi: 10.1203/00006450-199512000-00024. [DOI] [PubMed] [Google Scholar]
- Wang JX, Davies MJ, Norman RJ. Obesity increases the risk of spontaneous abortion during infertility treatment. Obes Res. 2002;10:551–554. doi: 10.1038/oby.2002.74. [DOI] [PubMed] [Google Scholar]
- Watson H, Kiddy DS, Hamilton-Fairley D, Scanlon MJ, Barnard C, Collins WP, Bonney RC, Franks S. Hypersecretion of luteinizing hormone and ovarian steroids in women with recurrent early miscarriage. Hum Reprod. 1993;8:829–833. doi: 10.1093/oxfordjournals.humrep.a138149. [DOI] [PubMed] [Google Scholar]
- West S, Vähäsarja M, Bloigu A, Pouta A, Franks S, Hartikainen A-L, Järvelin M-R, Corbett S, Vääräsmäki M, Morin-Papunen L. The impact of self-reported oligo-amenorrhea and hirsutism on fertility and lifetime reproductive success: results from the Northern Finland Birth Cohort 1966. Hum Reprod. 2013;29:628–633. doi: 10.1093/humrep/det437. [DOI] [PubMed] [Google Scholar]
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif AGJ, Haseltine F, editors. Polycystic Ovary Syndrome. Boston: Blackwell Scientific; 1992. pp. 377–384. [Google Scholar]