Abstract
Clinical data registries are commonly used worldwide and are implemented for a variety of purposes ranging from physician or facility clinic logs for tracking patients, collecting outcomes data, to measuring quality improvement or safety of medical devices. In the United States, the Food and Drug Administration has used data collected through registries to facilitate the drug and device regulatory process, ongoing surveillance during the product life-cycle, and for disease appraisals. Furthermore, the Centers for Medicare and Medicaid Services, in certain instances, base registry participation and submitting data to registries as factors for reimbursement decisions. The purpose of this article is to discuss the use of clinical data registries, the role that medical specialty societies, in particular the American Society of Plastic Surgeons and The Plastic Surgery Foundation, can have in the development and management of registries, and the opportunities for registry use in Plastic Surgery. As outcomes data are becoming essential measures of quality healthcare delivery, participating in registry development and centralized data collection has become a critical effort for Plastic Surgery to engage in to proactively participate in the national quality and performance measurement agenda.
Keywords: data registry, medical specialty society, national databases
Introduction
The Agency for Healthcare Research and Quality (AHRQ) defines a patient registry as “an organized system that uses observational study methods to collect uniform data (clinical and other) to evaluate specified outcomes for a population defined by a particular disease, condition, or exposure, and that serves one or more predetermined scientific, clinical, or policy purposes (1).” Registry use occurs on a variety of levels ranging from clinic logs to international disease databases. Data collected often include demographic information such as gender and age, medical history, diagnostic information, procedure and device specifics, and clinical outcomes. The uniform collection of these data creates a better understanding of practice characteristics, treatments, diseases and outcomes (1). In their most practical use, registries can help identify which patients have a certain condition or disease, or follow patients receiving certain devices or treatments (1).
However, registries are not limited to simply collecting a log of patients. They are increasingly being developed to collect clinical, procedure and outcome information associated with the care of particular patient populations. Registry data are used to describe practice patterns, measure procedure outcomes, facilitate medical device surveillance, and also serve as a repository of clinical data for future studies or generate hypotheses for future research. In the surgical specialties this has been seen with an increasing frequency of scientific publications using data from Society of Thoracic Surgeons’ (STS) Adult Cardiac Surgery Database and the American College of Surgeons’ (ACS) National Surgical Quality Improvement Program (NSQIP) (2–5). In Plastic Surgery, data from the American Society of Plastic Surgeon’s (ASPS) Tracking Operations and Outcomes for Plastic Surgeons (TOPS) registry was recently used to study national complication rates associated with abdominoplasty and breast augmentation (6).
As registry development and use continues to gain popularity, public/private partnerships are being established to leverage existing infrastructures and expertise. In late 2011, the American College of Cardiology (ACC) and the STS established the Transcatheter Valve Therapies (TVT) registry in partnership with the Food and Drug Administration (FDA) (7). Also in 2011, the ASPS, The Plastic Surgery Foundation (PSF) and the FDA began developing a registry to characterize women who have received breast implants and have been diagnosed with Anaplastic Large Cell Lymphoma (ALCL) (8–9). These partnerships, and others like them, aim to streamline the collection and analysis of information related to new devices and procedures and assist medical device manufacturers and the FDA in surveillance of safety and efficacy.
The utility of registries will likely grow as advancements in health information technologies make data submission, aggregation and analysis more efficient and less burdensome. Other factors influencing the growth of registry development include policy issues such as the 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act, including the adoption of electronic medical record (EMR) systems, as well as anticipated changes in healthcare reimbursement, quality reporting requirements such as the Physician Quality Reporting System, and performance-based certification and licensure. These changes are creating an environment where developing and using information technologies will help healthcare providers demonstrate that their outcomes are meeting a satisfactory level of patient care quality. The anticipated use of EMRs and their potential to integrate with registries will reduce the burden of data entry on individual sites, allowing for a more seamless and expedient transfer of patient data.
Medical Specialty Society Sponsored Registries
Medical specialty societies are organizations that represent networks of physicians. These organizations often exist to provide services to their members in the areas of advocacy, education, and practice management. Based on their close interaction with their members and their national and international reach, medical specialty societies are uniquely positioned to collect and manage data related to the type of care their members provide. Medical specialty societies are able to connect with the members directly at meetings and symposia, as well as using direct-to member correspondences.
Medical specialty societies have sponsored and developed registries for years. Table 1 provides an example of several active medical society-sponsored registries (2–5, 10–31). Many of the registries listed were developed for multiple purposes but commonly collect data on outcomes, safety and the effectiveness of devices and procedures. These registries range from tracking patients, devices and procedure outcomes, to tracking patient reported outcomes and their attitudes towards their procedures. In addition to using the registries to meet their members’ data reporting and benchmarking needs, societies often use registry data to identify gaps in care and from there, work towards developing quality improvement activities.
Table 1.
Examples of Medical Specialty Society Affiliated Registries
| Medical Specialty Society | Registry | Year Established | Number of Sites Contributed | Records Collected |
|---|---|---|---|---|
|
| ||||
| American Academy of Orthopedic Surgeons | American Joint Replacement Registry | 2010 | >51 | >30,000 arthroplasty procedures |
|
| ||||
| American College of Cardiology | National Cardiovascular Data Registry | 1997 | ||
| CathPCI: 1,580 | CathPCI: 15,000,000 | |||
| ICD: 1,710 | ICD: 900,000 | |||
| CARE: 189 | CARE: 20,000 | |||
| ACTION-GWTG: 771 | ACTION-GWTG: 425,000 | |||
| PINNACLE: 1,000 | PINNACLE: 3,000,000 | |||
| IMPACT: 58 | IMPACT: 9,000 | |||
|
| ||||
| American College of Cardiology/ Society of Thoracic Surgeons | TVT Registry7 | 2011 | 145 | 2,000 Patient records |
|
| ||||
| American College of Surgeons | NSQIP8 | 1994 | >400 US sites 26 international sites |
Number not available |
|
| ||||
| American Society of Plastic Surgeons | TOPS9 | 2002 | 1,292 | 1,030,000 Plastic Surgery procedures |
|
| ||||
| Society of Thoracic Surgeons | Society of Thoracic Surgeons Adult Cardiac Surgery Database | 1989 | 1,100 participant sites 3,000 surgeons |
>4,700,000 records |
Registry of diagnostic cardiac catheterizations and percutaneous coronary interventions
Registry of implantable cardioverter defibrillators and leads
Registry of carotid artery revascularization and endarterectomy procedures
Registry of acute coronary syndrome patients
Registry of coronary artery disease, hypertension, heart failure and atrial fibrillation in the outpatient setting
Registry of improving pediatric and adult congenital treatment
Transcatheter Valve Therapies Registry
National Surgical Quality Improvement Program
Tracking Operations and Outcomes in Plastic Surgery
Registries require the use of standardized data elements and definitions for meaningful comparisons and analysis. The ACC has formed an extensive registry infrastructure through its National Cardiovascular Data Registry (NCDR). Established in 1997, NCDR is comprised of six sub-registries that together contain clinical information on millions of records (11). The sub-registries within the NCDR provide cardiovascular data for physician quality improvement. These data have been used by government agencies, educational institutions and companies. For example, the ACTION Registry®-GWTG, an outcomes-based, quality improvement program allows hospitals to apply clinical guideline recommendations in their facilities, and provides them with tools to meet their quality improvement goals (12, 23–27). The registry provides reports to their physicians to identify areas of success and opportunities for improvement, as well as for documenting the results of quality improvement efforts (12). Similarly, the STS established the STS National Database for cardiothoracic surgery. The STS database was established in 1989 and is comprised of sub-registries (2–5). The STS Adult Cardiac Surgery Database has over 1,100 participating sites and has data on over 4.7 million procedures. The ACS NSQIP was created in 1994, and since then has had more than 400 sites participating within the United States, and more than 25 sites participating internationally (13–14). The ACC, ACS and STS registries provide their respective membership networks with valuable tools for the purpose of performance measurement and quality improvement (2–5, 13–31). In some instances participation in these registries is associated with procedure reimbursement.
In addition to their registries being used for member reporting and quality improvement activities, medical specialty societies together with the FDA, have initiated collaborations with device manufacturers for the purpose of facilitating post-approval data collection on approved medical devices. The FDA has been involved in the development of several device-specific registries in cardiology, orthopedics and ophthalmology (32). In 2012, the ASPS, The PSF and FDA started work to establish a national registry for breast implants (33). Resulting from safety and efficacy concerns with synthetic mesh used for pelvic organ prolapse, the American College of Obstetricians and Gynecologists (ACOG) and the American Urogynecologic Society (AUS) have proposed a national registry to track outcomes for all patients receiving vaginal mesh implants (34). The ACOG and AUS plan to work with the FDA on this project. The FDA plans to continue to facilitate the creation of these types of registries for the purpose of assessing the real-world performance of medical products and procedures (32).
Creating individual registries to meet the post-market surveillance needs of a specific manufacturer or a specific product type may not be efficient or economical. For targeted areas, developing a nationwide medical device registry may be more efficient and cost effective. Using medical specialty societies to develop clinical data networks, it is likely that specialty specific data can be collected faster and from a broad constituency, thus providing comprehensive real world information on safety, efficacy, and treatment options.
National Plastic Surgery Registries
Breast Implant Registries
In the late 1990s and early 2000s, numerous national and international registries were established to collect information on breast implant devices. In 1998, a breast implant registry was established in Australia to track breast implant patients and identify potential problems (35). In 1999, the Danish Registry for Plastic Surgery of the Breast was established to develop a national system for collecting preoperative, perioperative, and postoperative data on women undergoing breast implantation, breast reduction, or mastopexy (36–37). In 2000, The PSF (formerly the Plastic Surgery Educational Foundation) established the North American Breast Implant Registry (NaBIR) (38). NaBIR collected data on the patterns of use and reasons for re-operations associated with breast implants (38). Data on over 51,000 surgeries were included in NaBIR when it was replaced in 2007 by the TOPS registry’s breast implant module. Since then, nearly 65,000 additional procedures have been entered into that module. In 2002, an international registry was established with participation from Brazil, Australia, Mexico, Canada, Israel and Germany.
In 2010, reports surfaced of a possible association between ALCL and breast implants (8–9, 40–41). More recently there were concerns about an increased rupture rate associated with Poly Implants Prostheses (PIP) implants, a French breast implant not approved in the United States (35, 39, 42). These two events have contributed to new efforts worldwide to establish registries for the ongoing surveillance of breast implants. In the United States, the ASPS, The PSF and FDA established a Cooperative Research and Development Agreement for the purpose of establishing a National Breast Implant Registry, as well as a registry to increase the scientific data on ALCL in women with breast implants (8). Outside the United States, the Australian Society of Plastic Surgery and the Danish Society of Plastic Surgery are developing new breast registries (43). It is anticipated that additional international plastic surgery societies will establish similar registries. The formation of a new international collaboration of breast registry activities will help establish an international network of national plastic surgery societies that are developing breast implant registries for the purpose of learning best practices and possibly aggregating data to answer questions of safety and efficacy outcomes for patients with breast implants (44).
TOPS - Procedure and Outcome Registry
In the United States, ASPS sponsors and manages the TOPS registry. TOPS is a national registry of plastic surgery procedures and outcomes used to track procedures and assess 30-day post-operative outcomes. Since its inception in 2002, over 1,600 ASPS members have contributed over 600,000 physician cases and over 1 million plastic surgery procedures to the registry. TOPS is an integral part of ASPS and The PSF efforts and is utilized in many of the society’s key initiatives, including monitoring clinical outcomes and emerging trends, supporting research and educational programs, developing evidence-based practice parameters, and compilation of the National Clearinghouse of Annual Plastic Surgery Statistics (45).
TOPS was developed when it became apparent to the plastic surgery community that there was limited information available regarding surgical outcomes that could assess the value of care for plastic surgery procedures. Clinical outcomes information for accountability in provision of care were beginning to be demanded. The scientific literature that was available could not be translated to the plastic surgery population at large due to significant variations and limitations in how the data were being collected. It was also widely recognized that not having a national repository for plastic surgery procedural and outcomes information meant that as a specialty, Plastic Surgery did not have a way to provide data to respond to the needs of the members and the external environment. Plastic surgeons also expressed concerns and frustrations of being overwhelmed with requests for data from the specialty organizations they were members of as well as from hospital, state and regulatory organizations. TOPS provides ASPS member plastic surgeons with a mechanism to track demographic, procedural, and outcomes information to help physicians benchmark and evaluate their practice.
TOPS uses an electronic data capture interface to collect common demographic, risk factor, procedural and 30-day outcome data elements. These data allow registry users to evaluate outcomes based on patient comorbidities and risk factors including body mass index, tobacco use, and diabetes, in addition to reporting and tracking the rate of surgical incidences occurring post-operatively such as types of infection (wound disruption, incisional surgery site), hematoma, deep vein thromboembolism/ pulmonary embolism, or an unplanned return to the hospital/emergency department. TOPS is designed to incorporate modules for the collection of additional data based on specific procedures of special interest to the ASPS and its members. Modules currently exist for breast implant, lipoplasty and bariatric surgery procedures.
TOPS electronically collects patient reported outcomes from breast augmentation and reconstruction patients. The BREAST-Q©, a validated patient-reported outcome instrument for breast surgery patients can be seamlessly incorporated into a surgeon’s practice and provides plastic surgeons with an important metric for documenting clinical performance appraisal and improvement by measuring the effectiveness of breast reconstruction, reduction and augmentation based on the patients’ responses (46-48). As patients complete the BREAST-Q through TOPS, reports and dynamic customized graphs and charts can be generated in order to assess patient satisfaction with breast, satisfaction with outcome, psychosocial well-being, physical well-being, sexual well-being, and satisfaction with process of care in his or her own practice compared to aggregate TOPS data (46–48). Currently, over 200 ASPS member surgeons use the BREAST-Q through TOPS. BREAST-Q integration within the TOPS system has established a framework for future patient reported outcomes instruments to be incorporated within the registry.
The ASPS continually evaluates member and organizational needs related to clinical data collection and makes upgrades to TOPS to improve the program’s value to member surgeons, including enhanced data entry, querying and benchmark reporting functions. TOPS has several real-time self-assessment reports such as the Demographics Benchmark Report, Risk Factor Benchmark Report, and Outcome Benchmark Report that allow users to benchmark and measure their performance against all TOPS users and by practice type (i.e. solo practice). Upgrades have also been made that allow for data transfer from EMR and practice management programs.
Limitations of Registries and Solutions
The value of a registry depends greatly on the quality of its data. One of the biggest concerns associated with registry data is data completeness. Because registry data collection and follow-up are often less structured, when compared to randomized clinical trials (RCT), the impact that missing data have on registry data analysis must always be considered. To mitigate these concerns, electronic edit checks during data entry are commonly used. Some registry sponsors conduct random data audits to validate the quality of the data. Furthermore, physician and institution participation are essential components to ensuring that useful data are collected in a clinical registry. Successful registries implemented by ACC, STS and others have likely benefited from having participation associated with reimbursement or quality reporting requirements. Given that many procedures performed in Plastic Surgery do not qualify for government or insurance reimbursement, additional participation strategies must be developed to encourage widespread use. Educating and engaging plastic surgery residents in routine collection of procedural and outcomes data using tools like TOPS would aid in building this culture in Plastic Surgery. Additionally, as EMR use increases, data entry should become less redundant and burdensome. ASPS’ TOPS registry has recently developed integrations for receiving data directly from certain EMR systems. It is envisioned that this type of integration will become commonplace in the years ahead.
Similarly, the increase in connectivity with patients through social media outlets like Twitter and Facebook enhance the ability to have higher rates of patient follow-up than relying on phone calls or mailing surveys to registry participants. Patient reported outcomes are a crucial piece of the registry puzzle.
Needs, Implications and Vision for the Future
As evidence-based medicine continues to set the standard for clinical decision-making, it will be crucial for plastic surgeons to have an efficient mechanism for collecting clinical information from patients over a longitudinal period of time and benchmark their patient outcomes and complications data against other groups of participants. Solo and private practices have limited options available to them to produce meaningful reports or compare and judge their performance relative to their colleagues. Registries can provide a framework for all practice types to assess individual performance compared to the aggregate (49).
With over 18 million cosmetic and reconstructive procedures performed in 2011, plastic surgeons continue to generate valuable procedure data (45). One of the greatest challenges in developing plastic surgery registries is the diverse set of procedures performed and patients seen by plastic surgeons. For many medical specialties, procedures take place in hospitals and centers that have dedicated staff to provide patient and case information to registries. Conversely, many plastic surgery procedures are routinely performed in outpatient facilities, in private practice settings. Additionally, many plastic surgery procedures are elective, and therefore, not covered by insurance and not collected by administrative databases. These factors contribute heavily into the difficulty faced when collecting plastic surgery procedural data.
We agree with others that developing appropriately designed registries may help address and be the best means of collecting data for research and quality improvement in specialties such as Plastic Surgery where RCTs are not always feasible (49). Examples from other medical specialty societies including Cardiology and Surgery provide insight into how to successfully develop a national registry infrastructure for Plastic Surgery. The good news is that Plastic Surgery is not starting from scratch. The efforts started by ASPS over a decade ago by establishing TOPS has positioned Plastic Surgery well for meeting the data collection demands of plastic surgeons, national organizations and government agencies. The current infrastructure maintained by ASPS and The PSF allows for efficient development and management of multiple registries and data repository that could address the strategic issues facing the specialty, including the need for procedure, device, or outcome-specific data. Developing a coordinated clinical data infrastructure in conjunction with the ability to collect patient reported outcomes will provide plastic surgeons with valid clinical, surgical, and patient reported information that can be used for multiple purposes.
The ASPS and The PSF are currently developing a portfolio of registries that address strategic areas impacting Plastic Surgery practice as well as a vision for a centralized infrastructure that supports the comprehensive data collection needs for plastic surgeons, plastic surgery organizations and specialty as a whole (Figure 1). Establishing a national infrastructure will enable the specialty to efficiently capture clinical data for multiple purposes from multiple sources. In turn these data can be useful for identifying gaps in care, developing guidelines and performance measures, and meeting the reporting requirements of certifying organizations, other plastic surgery organizations, and state medical boards or hospitals. By developing a national framework to collect, integrate and store information for quick access, the easier it will be for plastic surgeons to answer the questions and address critical issues that the specialty faces in pursuit of improving the quality of patient care.
FIGURE 1.
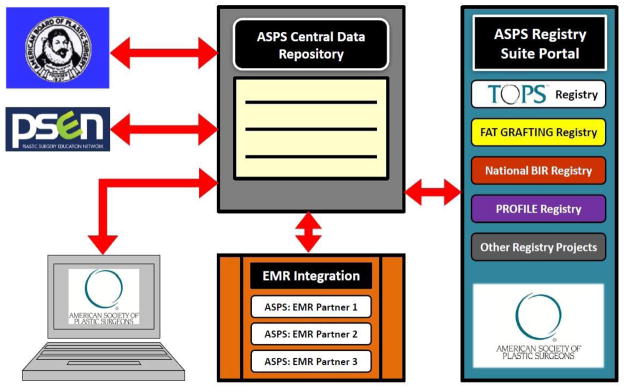
National Plastic Surgery Centralized Data Repository and Data Integration Vision
Conclusion
It is becoming increasingly evident that registries are the way of the future in device, disease, and procedural outcomes evaluation. Sientra’s breast implant device approval in March 2012 included, as a condition of approval, the participation in a national breast implant registry (50). Moving forward, it appears imminent that registry participation will be mandated by the government and other agencies for multiple purposes. It will be imperative that Plastic Surgery is an active participant in the development of these registries, before the registry content is decided for the specialty. Plastic Surgery needs to develop a culture of outcomes assessment and submit data for the benefit of the specialty. In return, the ASPS and The PSF are taking leadership roles to make this as easy as possible, but all stakeholders will have to participate to make Plastic Surgery a shining example of its commitment to deliver quality care.
Acknowledgments
Financial Disclosure and Products:
Research supported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K24 AR053120 (to K.C.C.).
Keith M. Hume, MA – reports no commercial associations or financial disclosures
Catherine A. Crotty, MPH – reports no commercial associations or financial disclosures
Christopher J. Simmons, BS – reports no commercial associations or financial disclosures
Michael W. Neumeister, MD – reports no commerical associations or financial disclosures
Kevin C. Chung, MD, MS – reports no commerical associations or financial disclosures
References
- 1.Gliklich RE, Dreyer NA, editors. AHRQ Publication No.10- EHC049. 2. Rockville, MD: Agency for Healthcare Research and Quality; Sep, 2010. Registries for Evaluating Patient Outcomes: A User’s Guide. (Prepared by Outcome DEcIDE Center [Outcome Sciences, Inc. d/b/a Outcome] under Contract No. HHSA29020050035I TO3.) [PubMed] [Google Scholar]
- 2.Brennan JM, Edwards FH, Zhao Y, et al. Early anticoagulation of bioprosthetic aortic valves in older patients: results from the Society of Thoracic Surgeons Adult Cardiac Surgery National Database. Am Coll Cardiol. 2012 Sep 11;60(11):971–7. doi: 10.1016/j.jacc.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 3.Brennan JM, Edwards FH, Zhao Y, et al. Long-term survival after aortic valve replacement among high-risk elderly patients in the United States: insights from the Society of Thoracic Surgeons Adult Cardiac Surgery Database, 1991 to 2007. Circulation. 2012 Sep 25;126(13):1621–9. doi: 10.1161/CIRCULATIONAHA.112.091371. [DOI] [PubMed] [Google Scholar]
- 4.Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg. 2012 Sep;256(3):487–93. doi: 10.1097/SLA.0b013e318265819c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dibardino DJ, Pasquali SK, Hirsch JC, et al. Effect of sex and race on outcome in patients undergoing congenital heart surgery: An analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Annals of Thoracic Surgery. 2012 Aug 9; doi: 10.1016/j.athoracsur.2012.05.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alderman AK, Collins ED, Streu R, et al. Benchmarking outcomes in plastic surgery: National complication rates for abdominoplasty and breast augmentation. Plastic and Reconstructive Surgery. 2009 Dec;124:2127–33. doi: 10.1097/PRS.0b013e3181bf8378. [DOI] [PubMed] [Google Scholar]
- 7.Home. [Accessed December 6, 2012]; Available at: https://www.ncdr.com/TVT/Home/Default.aspx.
- 8. [Accessed December 6, 2012];ASPS collaborates with FDA to establish breast implant registry. Available at: http://www.plasticsurgery.org/news-and-resources/press-release-archives/2011-pressrelease-archives/asps-collaborates-with-fda-to-establish-breast-implant-registry.html.
- 9. [Accessed January 14, 2013];Anaplastic Large Cell Lymphoma (ALCL) In Women with Breast Implants: Preliminary FDA Findings and Analyses. Available at: http://www.fda.gov/downloads/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/UCM240003.pdf.
- 10.Smith MA, Smith WT. The American Joint Replacement Registry. Orthopedic Nursing. 2012 Sep-Oct;31(5):296–299. doi: 10.1097/NOR.0b013e31826649b6. [DOI] [PubMed] [Google Scholar]
- 11.NCDR. [Accessed December 2, 2012]; Available at: http://www.ncdr.com/webncdr/common/
- 12.Home. [Accessed December 4, 2012]; Available at: https://www.ncdr.com/webncdr/action/
- 13.Participants || ACS NSQIP. [Accessed November 16, 2012]; Available at: http://site.acsnsqip.org/participants/
- 14.ACS NSQIP || Surgical Quality Improvement. [Accessed December 4, 2012]; Available at: http://site.acsnsqip.org/
- 15.Federspiel JJ, Mudrick DW, Shah BR, et al. Patterns and predictors of stress testing modality after percutaneous coronary stenting: Data from the NCDR (®) JACC Cardiovascular Imaging. 2012 Oct;5(10):969–80. doi: 10.1016/j.jcmg.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawkins BM, Kennedy KF, Giri J, et al. Pre-procedural risk quantification for carotid stenting using the CAS score: A report from the NCDR CARE registry. Journal of the American College of Cardiology. 2012 Oct 23;60(17):1617–22. doi: 10.1016/j.jacc.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 17.Messenger JC, Ho KK, Young CH, et al. NCDR Science and Quality Oversight Committee Data Quality Workgroup. The National Cardiovascular Data Registry (NCDR) data quality brief: The NCDR Data Quality Program in 2012. Electrophysiology studies in patients undergoing ICD implantation: findings from the NCDR®. Journal of the American College of Cardiology. 2012 Oct 16;60(16):1484–8. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Parikh NI, Honeycutt EF, Roe MT, et al. Left and co-dominant coronary artery circulations are associated with higher in-hospital mortality among patients undergoing percutaneous coronary intervention for acute coronary syndromes: Report from the National Cardiovascular Database Cath Percutaneous Coronary Intervention (CathPCI) Registry. Circ Cardiovascular Qualitative Outcomes. 2012 Oct 30; doi: 10.1161/CIRCOUTCOMES.111.964593. [DOI] [PubMed] [Google Scholar]
- 19.Dehmer GJ, Weaver D, Roe MT, et al. A contemporary view of diagnostic cardiac catheterization and percutaneous coronary intervention in the United States: A report from the CathPCI Registry of the National Cardiovascular Data Registry, 2010 through June 2011. Journal of the American College of Cardiology. 2012 Nov 13;60(20):2017– 31. doi: 10.1016/j.jacc.2012.08.966. [DOI] [PubMed] [Google Scholar]
- 20.Subherwal S, Peterson ED, Dai D, et al. Temporal trends in and factors associated with bleeding complications among patients undergoing percutaneous coronary intervention: A report from the National Cardiovascular Data CathPCI Registry. Journal of the American College of Cardiology. 2012 May 22;59(21):1861–9. doi: 10.1016/j.jacc.2011.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng A, Wang Y, Berger RD, et al. Electrophysiology studies in patients undergoing ICD implantation: findings from the NCDR®. Pacing Clinical Electrophysiology. 2012 Aug;35(8):912–8. doi: 10.1111/j.1540-8159.2012.03441.x. [DOI] [PubMed] [Google Scholar]
- 22.Dewland TA, Pellegrini CN, Wang Y, et al. Dual-chamber implantable cardioverter-defibrillator selection is associated with increased complication rates and mortality among patients enrolled in the NCDR implantable cardioverter-defibrillator registry. Journal of the American College of Cardiology. 2011 Aug 30;58(10):1007–13. doi: 10.1016/j.jacc.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 23.Shah RV, Holmes D, Anderson M, et al. Risk of heart failure complication during hospitalization for acute myocardial infarction in a contemporary population: Insights from the National Cardiovascular Data ACTION Registry. Circ Heart Fail. 2012 Oct 9; doi: 10.1161/CIRCHEARTFAILURE.112.968180. [DOI] [PubMed] [Google Scholar]
- 24.Chin CT, Wang TY, Li S, et al. Comparison of the prognostic value of peak creatine kinase-MB and troponin levels among patients with acute myocardial infarction: a report from the Acute Coronary Treatment and Intervention Outcomes Network Registry-get with the guidelines. Clinical Cardiology. 2012;35(7):424–9. doi: 10.1002/clc.21980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guzman LA, Li S, Wang TY, et al. Differences in treatment patterns and outcomes between Hispanics and non-Hispanic Whites treated for ST-segment elevation myocardial infarction: results from the NCDR ACTION Registry-GWTG. Journal of the American College of Cardiology. 2012 Feb 7;59(6):630–1. doi: 10.1016/j.jacc.2011.10.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson ED, Roe MT, Chen AY, et al. The NCDR ACTION Registry-GWTG: transforming contemporary acute myocardial infarction clinical care. Heart. 2010 Nov;96(22):1798–802. doi: 10.1136/hrt.2010.200261. [DOI] [PubMed] [Google Scholar]
- 27.Diercks DB, Kontos MC, Chen AY, et al. Utilization and impact of pre-hospital electrocardiograms for patients with acute ST-segment elevation myocardial infarction: data from the NCDR (National Cardiovascular Data Registry) ACTION (Acute Coronary Treatment and Intervention Outcomes Network) Registry. Journal of the American College of Cardiology. 2009 Jan 13;53(2):161–6. doi: 10.1016/j.jacc.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 28.May DC, Fiocchi F, Kehoe K, Wright JS, Mullen JB, Oetgen WJ. Improving cardio care in the outpatient setting: implementing the PINNACLE Registry in a single-specialty practice. Physician Exec. 2011 Nov-Dec;37(6):38–40. 42. [PubMed] [Google Scholar]
- 29.Chan PS, Maddox TM, Tang F, Spinler S, Spertus JA. Practice-level variation in warfarin use among outpatients with atrial fibrillation (from the NCDR PINNACLE program) American Journal of Cardiology. 2011 Oct 15;108(8):1136–40. doi: 10.1016/j.amjcard.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oetgen WJ, Mullen JB, Mirro MJ. Electronic health records, the PINNACLE registry, and quality care. Archives of Internal Medicine. 2011 May 23;171(10):953–4. doi: 10.1001/archinternmed.2011.189. author reply 954. [DOI] [PubMed] [Google Scholar]
- 31.STS National Database. [Accessed December 6, 2012]; Available at: http://www.sts.org/national-database.
- 32. [Accessed December 19, 2012];CDRH Reports > National Medical Device Postmarket Surveillance Plan. Available at: http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/CDRHReports/ucm301912.htm.
- 33. [Accessed January 14, 2013];January/February 2013 Reader. Available at: http://asps.imirus.com/Mpowered/book/vpsn13/i1/p8.
- 34. [Accessed October 5, 2012];ACOG - Joint Recommendations Issued on Use of Vaginal Mesh for POP. Available at: http://www.acog.org/About_ACOG/News_Room/News_Releases/2011/Joint_Recommendations_Issued_on_Use_of_Vaginal_Mesh_for_POP.
- 35. [Accessed January 14, 2013];Doctors call breast implant register overhaul | The Australian. Available at: http://www.theaustralian.com.au/news/health-science/doctors-call-for-breast-implant-register-overhaul/story-e6frg8y6-1226288783275.
- 36.Henriksen T, Holmich L, Friis S, McLaughlin J, Fryzek J, Hoyer A, et al. The Danish registry for plastic surgery of the breast: Establishment of a nationwide registry for prospective follow-up, quality assessment, and investigation of breast surgery. Plastic and Reconstructive Surgery. 111:2182–2189. doi: 10.1097/01.PRS.0000060111.19272.8D. [DOI] [PubMed] [Google Scholar]
- 37.Hvilson G, Holmich L, Henriksen T, Lipworth L, McLaughlin J, Friis S. Local Complications after Cosmetic Breast Augmentation: Results from the Danish Registry for Plastic Surgery of the Breast. Plastic and Reconstructive Surgery. :919–925. doi: 10.1097/PRS.0b013e3181b0389e. [DOI] [PubMed] [Google Scholar]
- 38. [Accessed December 6, 2012];Findings of the North American Breast Implant Registry. Available at: https://asps.confex.com/asps/2003am/techprogram/paper_3335.htm.
- 39. [Accessed December 6, 2012];Breast implant registry scandal shows regulators in dark on risk. Available at: http://www.reuters.com/article/2011/12/30/us-breastimplants-registryidUSTRE7BS18P20111230.
- 40.Kim B, Roth C, Chung KC, et al. Anaplastic Large Cell Lymphoma and Breast Implants: A Systematic Review. Plastic Reconstr Surg. 2011;127(6):2141–2150. doi: 10.1097/PRS.0b013e3182172418. [DOI] [PubMed] [Google Scholar]
- 41.Kim B, Roth C, Chung KC, et al. Anaplastic Large Cell Lymphoma and Breast Implants: Results from a Structured Expert Consultation Process. Plastic Reconstr Surg. 2011;128(3):629–39. doi: 10.1097/PRS.0b013e31821f9f23. [DOI] [PubMed] [Google Scholar]
- 42. [Accessed December 6, 2012];PIP Breast Implants. Available at: http://www.dailymail.co.uk/health/article-2081089/PIP-breast-implants-Government-adviser-says-50k-women-SHOULD-removed.html.
- 43. [Accessed December 8, 2012];Patients and Consumers—Breast Implant Registry. Available at http://www.plasticsurgery.org.au/patients-and-consumers/breast-implant-registry/
- 44.Cooter R. Breast device registry update. Paper presented at: 2012 Annual Meeting of the American Society of Plastic Surgeons; October 2012; New Orleans, LA. [Google Scholar]
- 45. [Accessed January 14, 2013];2011 Plastic Surgery Procedural Statistics American Society of Plastic Surgeons. Available at: http://www.plasticsurgery.org/News-and-Resources/2011-Statistics-.html.
- 46.Cano SJ, Klassen AF, Scott AM, Cordeiro PG, Pusic AL. The BREAST Q©: Further validation in independent clinical samples. Plast Reconstr Surg. 2012;129:293–302. doi: 10.1097/PRS.0b013e31823aec6b. [DOI] [PubMed] [Google Scholar]
- 47.Klassen AF, Pusic AL, Scott A, Klok J, Cano SJ. Satisfaction and quality of life in women who undergo breast surgery: a qualitative study. BMC Womens Health. 2009 May 1;9:11. doi: 10.1186/1472-6874-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124:345–53. doi: 10.1097/PRS.0b013e3181aee807. [DOI] [PubMed] [Google Scholar]
- 49.Drolet BC, Lorenzi NM. Registries and evidence-based medicine in craniofacial and plastic surgery. J Craniofac Surg. 2012 Jan;23(1):301–3. doi: 10.1097/SCS.0b013e318241dbee. [DOI] [PubMed] [Google Scholar]
- 50. [Accessed December 6, 2012];Press Announcement. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm295437.htm.


