Abstract
Since previous numbers-needed-to-treat (NNT) and relative risk reduction (RRR) report, a few studies were published to evaluate prophylactic effectiveness of neuromuscular training for anterior cruciate ligament (ACL) injury in female athletes. The purpose of the current analyses was to determine the effectiveness of neuromuscular training interventions in reducing both non-contact and overall ACL injury risk in female athletes through RRR and NNT. The keywords ‘knee’, ‘anterior cruciate ligament’, ‘ACL’, ‘prospective’, ‘neuromuscular’, ‘training’, ‘female’ and ‘prevention’ were searched to find studies published from 1995 to 2011 in PubMed and EBSCO (CINAHL, Health source, MEDLINE and SPORT Discus). Inclusion criteria required that relevant studies: recruited physically active young girls as subjects, documented the number of ACL injuries, employed a neuromuscular training intervention, and used a prospective controlled study design. The numbers of non-contact and overall ACL injuries, subjects and observation time period were used to calculate RRR and NNT for each study. A total of 12 studies met the inclusion criteria. There was a 73.4% (95% CI 62.5% to 81.1%) and 43.8% (95% CI 28.9% to 55.5%) of RRR for non-contact and overall ACL injuries. From the NNT analysis, it was determined that, respectively, 108 (95% CI 86 to 150) and 120 (95% CI 74 to 316) individuals would need to be trained to prevent one non-contact or one overall ACL injury over the course of one competitive season. Although the RRR analysis indicated prophylactic benefits of neuromuscular training, the relatively large NNT indicated that many athletes are needed to prevent one ACL injury. A future direction to reduce NNT and improve the efficiency of ACL injury-prevention strategies is to develop a screening system for identifying at-risk athletes.
INTRODUCTION
Each year, it is estimated that 250 000 anterior cruciate ligament (ACL) reconstruction surgeries are performed in the USA.1 The average cost associated with ACL injuries, including diagnostic tests, surgery and rehabilitation, is conservatively estimated to be $17 000 per each reconstructive case from 1999 data.2 In sum, the financial burdens associated with ACL reconstruction surgery is estimated to be more than $2 billion annually.1 Time lost from ACL injury can be 6 months3,4 or longer. In addition to these substantial financial and time costs associated with ACL injury, various negative consequences have been documented such as mood disturbance5 as well as increased risks of a second ACL injury.6,7 Specifically, female athletes who suffer ACL injuries are more likely to experience premature osteoarthritis8 and a reduced quality of life because of limited knee function.9
Approximately 70% of ACL injuries occur with a non-contact mechanism,10–12 and the rate of ACL injury occurrence in female athletes is higher in cutting, jumping and pivoting sports compared with males.13–15 Risk factors14–17 associated with neuromuscular control are potentially modifiable and may reduce the risk of non-contact ACL injury.18 Since the 1990s, several prospective cohort studies have been performed to determine the effect of neuromuscular training interventions targeted to reduce ACL, knee and other lower-extremity injuries.19–32 Studies often utilised single or limited training modes in their neuromuscular training interventions such as plyometric exercises, balance exercises or a combination of both.19,22,23 More comprehensive approaches have been initiated recently, which consist of a combination of different types of exercises such as plyometrics, strengthening, stretching and balancing training. The ‘Dynamic Neuromuscular Analysis (DNA) training’,18 ‘Prevent Injury and Enhance Performance (PEP)’,24,29 ‘11’28 and ‘11+’31 programmes are examples of comprehensive neuromuscular training protocols. In addition, some components of the newly developed neuromuscular training protocols include sports-specific exercises.30 However, prophylactic effectiveness of those neuromuscular training programmes have shown mixed results.
To assess the effectiveness of various neuromuscular training programmes, Grindstaff et al33 applied relative risk reduction (RRR) and number-needed-to-treat (NNT) analyses on the available neuromuscular training cohort studies that aimed to reduce ACL injury in female athletes. The analyses demonstrated a 70% total RRR in subjects in the intervention groups compared with those in control groups. Furthermore, the NNT analysis concluded that 89 was the minimum number of athletes needed to prevent one non-contact ACL injury per competitive season.33 However, since this previous assessment of the effectiveness of neuromuscular training,33 the number of large-scale cohort studies has nearly doubled, which warranting reassessment of the effectiveness of ACL injury reduction achieved through neuromuscular training interventions. Therefore, the purpose of the current analysis was to provide an up-to-date analysis aimed at determining the effectiveness of neuromuscular training interventions designed to reduce both non-contact and overall ACL injury risk in female athletes through RRR and NNT.
METHODS
Literature search
A literature search was performed using PubMed and EBSCO (CINAHL, MEDLINE and SPORT Discus) database from 1995 to 2011 in January 2012. The keywords searched were performed by applying a combination of following words: ‘knee’, ‘anterior cruciate ligament’, ‘ACL’, ‘prospective’, ‘neuromuscular’, ‘training’, ‘female’ and ‘prevention’ (table 1). Studies were limited to English language, human subject investigations. The following inclusionary criteria were applied: (1) the number of ACL injury incidents were reported, (2) a neuromuscular training intervention that aimed to reduce ACL incidence was applied, (3) a control group was used, (4) a prospective controlled trial study design was employed and (5) females were included as subjects. Abstracts, posters and unpublished data were excluded. Literature found by the keyword search was screened in a step-by-step procedure based on the above inclusionary criteria. During this process, a potential inclusion of studies that hold very similar characteristics of the above five inclusionary criteria was considered. Egger’s regression was used to examine a potential risk of publication bias.
Table 1.
Stepped PubMed/EBSCO host search strategy with the number of studies
| Step | Strategy | PubMed | EBSCO |
|---|---|---|---|
| #17 | Search (#11) AND (#16) | 390 | 59 |
| #16 | Search (#12) OR (#13) OR (#14) OR (#15) | 23899 | 18522 |
| #15 | Search ‘preventing’ [TIAB] | 4916 | 996 |
| #14 | Search ‘preventive’ [TIAB] | 2373 | 2143 |
| #13 | Search ‘prevent’ [TIAB] | 8219 | 1660 |
| #12 | Search ‘prevention’ [TIAB] | 12956 | 1367 |
| #11 | Search(#5) AND (#10) | 4151 | 2185 |
| #10 | Search (#6) OR (#7) OR (#8) OR (#9) | 73052 | 62290 |
| #9 | Search ‘female’ [TIAB] | 14981 | 15682 |
| #8 | Search ‘training’ [TIAB] | 9806 | 24398 |
| #7 | Search ‘neuromuscular’ [TIAB] | 1412 | 2278 |
| #6 | Search ‘prospective’ [TIAB] | 50692 | 20738 |
| #5 | Search (#1) OR (#2) OR (#3) OR (#4) | 12480 | 53232 |
| #4 | Search ‘ACL’ [TIAB] | 411 | 1001 |
| #3 | Search ‘anterior cruciate ligament’ [TIAB] | 554 | 4001 |
| #2 | Search ‘knee’ [TIAB] | 4827 | 17026 |
| #1 | Search ‘injury’ [TIAB] | 7824 | 33140 |
Date were limited from 1 January 1995 to 31 December 2011. Language was limited in English. Species were limited in humans. Sex was limited in female. CINAHL, MEDLINE and SPORT Discus were included in the EBSCO search. TIAB, title and abstract.
Quality of methodology evaluation method
The Physiotherapy Evidence Database (PEDro) scale is a widely used measurement tool and was employed to analyse methodological quality of the included studies.20–30,32 Two reviewers independently examined the methodological quality of each study using the PEDro scale. Discrepancies between reviewers were settled by arbitration and consensus.
Level of evidence and strength of recommendation assessment method
To evaluate the quality of the current analysis, the Centre of Evidence Based Medicine (CEBM)-Level of Evidence was implemented. The CEBM-Level of Evidence is used to assess the research design quality of the included studies and facilitates the generation of a grade of strength of recommendation for the current analyses.
Data extraction
The number of ACL occurrences in each group (control and intervention), the number of athletes in each group (control and intervention) and athletic exposures were extracted from each study. Whether or not the ACL injuries were contact or non-contact in nature was also extracted from each study. When the mechanism of injury was not documented as either contract or non-contact, an email was sent to the corresponding author in the original paper asking for the mechanism of the ACL injuries. From studies that had both male and female subjects,21,25 only data regarding female subjects were utilised. To calculate athletic exposure data, the number of hours and days of participation were extracted from each study. Each day of participation was estimated as 2 h and then converted to hours of participation as used in previous studies.33
Operational calculations
The number of ACL injuries, number of athletes and athletic exposures in both the intervention and control groups were extracted from each study and used to calculate the NNT and RRR. Initially, the control event rate (CER) and intervention event rate (IER) were calculated
CER=# ACL-injured subjects in control group/total number of subjects in the control group
IER=# ACL-injured subjects in intervention group/total number of subjects in the intervention group
Absolute risk reduction (ARR), the absolute difference in event rate between control and intervention groups, was then calculated:
The inverse of the ARR is used to calculate NNT and was based on the number of athletes across one competitive season. It is mathematically expressed as
A positive NNT value represents a beneficial preventive effect because of the intervention and is referred to as NNT to benefit (NNTB). Conversely, a negative NNT value is indicative of a harmful effect and is referred to as NNT to harm (NNTH). If the ARR is zero, the NNT values would approach infinity (∞), indicating no beneficial or harmful effects and meaning that an infinite number of athletes might have been needed to demonstrate the benefit or harm from the given intervention.
RRR was then calculated using the following formula:
The RRR value indicates the percentage by which the intervention reduces risk compared to the controls. Positive RRR values suggest reduced risk by the given intervention. In contrast, negative RRR values indicate increased risk compared with the controls. In addition, 95% CI were calculated for all NNT and RRR values.34 A set of matrix laboratory (MATLAB) codes were made and used for the NNT and RRR calculation with 95% CI.
RESULTS
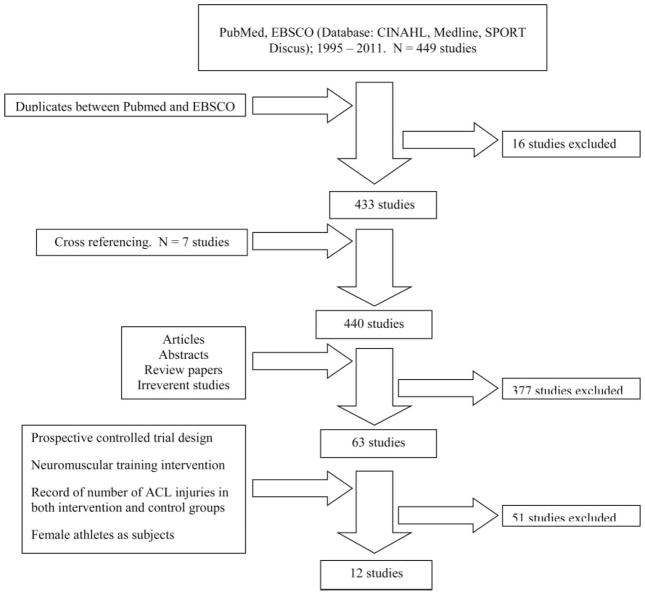
A total of 440 unique publications were collected including cross-referenced studies, and 11 studies met the inclusionary criteria. One study that did not completely fulfil the inclusion criteria because of an absence of control group due to the study design was actually included since the study met the purpose of current project. Thus, a total of 12 studies were included in the current analyses (figure 1). The neuromuscular training of each study is summarised in table 2 and the relevant methodological quality as evaluated by the PEDro scores is expressed in table 3. The non-contact and overall ACL injury incidence rates in each group, NNT, RRR and 95% CI were reported in tables 4 and 5. The mean PEDro score was 4.3/10 for the 12 reviewed studies. Two studies25,28 were rated as high as 7/10 while two studies26,27 were classified 2/10 in PEDro score.
Figure 1.
Flow chart of literature search.
Table 2.
Summary of reviewed studies including study design, the Physiotherapy Evidence Database score, sports, number of teams, ages, type, length, frequency and duration
| Reference (year) | Study design | Level of evidence | Sports | Number of teams | Ages | Type | Length | Frequency | Duration |
|---|---|---|---|---|---|---|---|---|---|
| Hewett et al (1999)21 | Prospective non-randomised cohort | 2b | Soccer, volleyball and basketball | 15 teams (control) and 15 teams (intervention) | 14–18 year (range) | Stretching, plyometrics, weight training | 60–90 min | 3 days per week in preseason | 6 weeks |
| Soderman et al (2000)22 | Prospective randomised control | 2b | Soccer | 6 teams (control) and 7 teams (intervention) | C:20.4 ±5.4 year and I: 20.4 ±4.6 year (mean) | Balance with dynadiscs, balance boards | 10–15 min | Each day for 30 days and 3 days per week rest of the season | 6 months |
| Heidt et al (2000)20 | Prospective randomised control | 1b | Soccer | 258 individuals (control) and 42 individuals (intervention) | 14–18 year (range) | Cardiovascular, plyometrics, strength, flexibility, agility, sports specific drills | 75 min | 3 days per week in preseason | 7 weeks |
| Myklebust et al (2003)23 | Prospective non-randomised cross over | 2b | Handball | 60 teams (first year), 58 teams (second year) and 52 teams (third year) | 21–22 year (mean) | Balance with mats, wobble boards | 15 min | 3 days per week for 5–7 weeks. Once a week for rest of the season | One competitive handball season (5 months) |
| Mandelbaum et al (2005)24 | Prospective non-randomised cohort | 2b | Soccer | 207 teams (control) and 97 teams (intervention) | 14–18 year (range) | Basic warm-up, stretching, strengthening, plyometrics, agility | 20 min | 2–3 times per week in in-season | 3 competitive soccer seasons (12 weeks per season) |
| Olsen et al (2005)25 | Prospective cluster randomised controlled | 1b | Handball | 59 teams(control) and 61 teams (intervention) | 16–17 year (mean) | Warm-up, technique, balance, strength, power | 15–20 min | 15 consecutive sessions. Once a week for rest of the season | One competitive handball season (5 months) |
| Petersen et al (2005)26 | Prospective matched cohort | 2b | Handball | 10 teams(control) and 10 teams (intervention) | C:19.8 and I: 19.4 year (mean) | Education, balance-board exercise, jump training | 10 min | 3 times per week in preseason. Once per week for rest of the season | 8 weeks |
| Pfeiffer et al (2006)27 | Prospective non-randomised cohort | 2b | Soccer, volleyball and basketball | 69 teams(control) and 43 teams (intervention) | 14–18 year (range) | Plyometrics | 20 min | 2 times per week in in-season | One competitive season (4–5 months) |
| Steffen et al (2008)28 | Prospective block randomised controlled | 1b | Soccer | 51 teams (control) and 58 teams (intervention) | 15.4 year (mean) | Core stability, balance, plyometrics | 15 min | 15 consecutive sessions. Once a week for rest of the season | 7.5 months |
| Gilchrist et al (2008)29 | Prospective cluster randomised controlled | 1b | Soccer | 35 teams (control) and 26 teams (intervention) | C:19.9 and I: 19.9 year (mean) | Basic warm-up, stretching, strengthening, plyometrics, agility | 20 min | 3 times per week in in-season | One competitive soccer season (4–5 months) |
| Kiani et al (2010)30 | Prospective cluster non-randomised cohort | 2b | Soccer | 49 teams (control) and 48 teams (intervention) | C: 15.0 and I: 14.7 year (mean) | Core stability, strengthening, balance | 20–25 min | 2 days per week for 2 months. Once a week for rest of the season | 9 months |
| LaBella et al (2011)32 | Prospective cluster randomised controlled | 1b | Soccer and basketball | 53 teams (control) and 53 teams (intervention) | C: 16.2 and I: 16.2 year (mean) | Strengthening, plyometrics, balance, agility | 20 min | 3 times per week in pre- and in-season | One competitive season (8–17 weeks) |
Although the study was a randomised controlled design, the follow-up rate was low (51.2%). Therefore, the level of evidence was rated as 2b.
Table 3.
Physiotherapy Evidence Database scores of the reviewed studies
| Reviewed studies | Total scores | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hewett et al21* | 3 | – | X | X | X | |||||||
| Soderman et al22 | 4 | – | X | X | X | X | ||||||
| Heidt et al20 | 5 | – | X | X | X | X | X | |||||
| Myklebust et al23* | 5 | – | X | X | X | X | X | |||||
| Mandelbaum et al24* | 3 | – | X | X | X | |||||||
| Olsen et al25 | 7 | – | X | X | X | X | X | X | X | |||
| Petersen et al26* | 2 | – | X | X | ||||||||
| Pfeiffer et al27* | 2 | – | X | X | ||||||||
| Steffen et al28 | 7 | – | X | X | X | X | X | X | X | |||
| Gilchrist et al29 | 4 | – | X | X | X | X | ||||||
| Kiani et al30* | 4 | – | X | X | X | X | ||||||
| LaBella et al32 | 6 | – | X | X | X | X | X | X |
X, ‘yes’ score and blank, ‘no’ score. PEDro scale is optimised for evaluation of randomised control trails, thus the PEDro assessment score for the non-randomised control should be interrupted with caution. Studies with *Are not randomised trial. 1, Eligibility criteria specified; 2, random allocation of subjects; 3, allocation concealed; 4, similar groups at baseline; 5, blinding of subjects; 6, blinding of intervention providers; 7, blinding of outcome assessors; 8, outcomes obtained from 85% of subjects; 9, use of intent-to-treat analysis if protocol violated; 10, between-group statistical comparison; 11, point measures and measures of variability.
Table 4.
Relative risk reduction and numbers-needed-to-treat analyses for non-contact anterior cruciate ligament injury
| Reference | Non-contact ACL injury in group | Female athletes per group | Non-contact ACL incidence rates* | RRR (95% CI) | NNT to benefit (NNTB)†, NNT to harm (NNTH)‡ (95% CI) |
|---|---|---|---|---|---|
| Hewett et al | |||||
| Control | 5 | 463 | 0.11 | 100 | 93 NNTB |
| Intervention | 0 | 366 | 0 | (NA) | (50 NNTB to 723) |
| Myklebust et al | |||||
| Control | 18 | 942 | 0.09 | 38.8 | 135 NNTB |
| Intervention (first year only) | 10 | 855 | 0.03 | (−0.9, 62.9) | (53 NNTB to ∞ to 255 NNTH) |
| Mandelbaum et al | |||||
| Control | 67 | 3818 | 0.24 | 81.9 | 70 NNTB |
| Intervention (first and second year total) | 6 | 1885 | 0.04 | (60.9, 91.6) | (52 NNTB to 106) |
| Olsen et al | |||||
| Control | 5 | 778 | 0.06 | 80.7 | 193 NNTB |
| Intervention | 1 | 808 | 0.01 | (−15.3, 96.8) | (89 NNTB to ∞ to 1079 NNTH) |
| Petersen et al | |||||
| Control | 5 | 142 | 0.21 | 100 | 28 NNTB |
| Intervention | 0 | 134 | 0 | (NA) | (16 NNTB to 205) |
| Pfeiffer et al | |||||
| Control | 3 | 862 | 0.04 | −49.4 | 582 NNTH |
| Intervention | 3 | 577 | 0.08 | (−233.4, 33.1) | (188 NNTB to ∞ to 114 NNTH) |
| Gilchrist et al | |||||
| Control | 10 | 583 | 0.18 | 86.3 | 68 NNTB |
| Intervention | 2 | 852 | 0.06 | (51.5, 96.1) | (39 NNTB to 265) |
| Steffen et al | |||||
| Control | 2 | 947 | 0.03 | −32.4 | 1462 NNTH |
| Intervention | 3 | 1073 | 0.05 | (−171.1, 35.4) | (277 NNTB to ∞ to 201 NNTH) |
| Kiani et al | |||||
| Control | 5 | 729 | 0.08 | 100 | 146 NNTB |
| Intervention | 0 | 777 | 0 | (NA) | (78 NNTB to 1153) |
| LaBella et al | |||||
| Control | 6 | 755 | 0.48 | 65.9 | 191 NNTB |
| Intervention | 2 | 737 | 0.10 | (−13.5, 89.7) | (80 NNTB to ∞ to 470 NNTH) |
| Total | |||||
| Control | 126 | 10019 | 0.14 | 73.4 | 108 NNTB |
| Intervention | 27 | 8064 | 0.04 | (62.5, 81.1) | (86 NNTB to 150) |
Relative incidence rate (per 1000 A*h).
NNT to benefit (NNTB) represents a beneficial preventive effect of the intervention.
NNT to harm (NNTH) indicates harmful effect of the intervention. Infinity (∞) means no beneficial or harmful effects.
Table 5.
Relative risk reduction and numbers-needed-to-treat analyses for overall anterior cruciate ligament injury
| Reference | Overall ACL injury in group | Female athletes per group | Overall ACL incidence rates* | RRR (95% CI) | NNT to benefit (NNTB)†, NNT to harm (NNTH)‡ (95% CI) |
|---|---|---|---|---|---|
| Hewett et al | |||||
| Control | 5 | 463 | 0.11 | 49.4 | 187 NNTB |
| Intervention | 2 | 366 | 0.06 | (−63.7, 84.4) | (58 NNTB to ∞ to 149 NNTH) |
| Soderman et al | |||||
| Control | 1 | 78 | 0.12 | −403.2 | 19 NNTH |
| Intervention | 4 | 62 | 0.68 | (−712.8, −211.6) | (70 NNTB to ∞ to 9 NNTH) |
| Heidt et al | |||||
| Control | 8 | 258 | NA § | 23.2 | 139 NNTB |
| Intervention | 1 | 42 | (−498.3, 90.1) | (17 NNTB to ∞ to 22 NNTH) | |
| Myklebust et al | |||||
| Control | 29 | 942 | 0.14 | 12.6 | 257 NNTB |
| Intervention (First only) | 23 | 855 | 0.06 | (−19.0, 35.9) | (52 NNTB to ∞ to 86 NNTH) |
| Olsen et al | |||||
| Control | 9 | 778 | 0.10 | 67.9 | 127 NNTB |
| Intervention | 3 | 808 | 0.03 | (14.4, 88.0) | (61 NNTB to ∞ to 1334 NNTH) |
| Petersen et al | |||||
| Control | 5 | 142 | 0.21 | 78.8 | 36 NNTB |
| Intervention | 1 | 134 | 0.04 | (−27.4, 96.5) | (17 NNTB to ∞ to 170 NNTH) |
| Gilchrist et al | |||||
| Control | 18 | 583 | 0.34 | 73.4 | 44 NNTB |
| Intervention | 7 | 852 | 0.20 | (50.0, 85.8) | (27 NNTB to 136) |
| Steffen et al | |||||
| Control | 5 | 947 | 0.08 | 29.4 | 644 NNTB |
| Intervention | 4 | 1073 | 0.06 | (−46.8, 66.0) | (135 NNTB to ∞ to 231 NNTH) |
| Total | |||||
| Control | 80 | 4191 | 0.14 | 43.8 | 120 NNTB |
| Intervention | 45 | 4192 | 0.06 | (28.9, 55.5) | (74 NNTB to 316) |
Relative incidence rate (per 1000 A*h).
NNT to benefit (NNTB) represents a beneficial preventive effect of the intervention.
NNT to harm (NNTH) indicates harmful effect of the intervention. Infinity (∞) means no beneficial or harmful effects.
Exposure information was not available in the original report.
Summaries of included studies
Hewett et al in 199921 (PEDro score 3, level of evidence 2b)
This research team used a prospective cluster study design and provided 6 weeks of neuromuscular training, consisting of weight training, plyometrics and flexibility, to a total of 43 teams (volleyball, soccer and basketball) from area high schools. Each neuromuscular training session lasted 60–90 min and took place three times per week for 6 weeks. Certified athletic trainers and physical therapists gave technique instructions and the training sessions progressed through three phases: (I) technique phase, (II) fundamental phase and (III) performance phase. The 15 girls’ teams that received the intervention (6 weeks of neuromuscular training) consisted of 366 athletes: 185 volleyball (50.5%), 97 soccer (26.5%) and 84 basketball (23%) players. An ACL incidence rate of the intervention group was 0.06 per 1000 h of Athletic-Exposure (1000 h AE) in the intervention group and 0.11 per 1000 h AE in the control group.
Soderman et al in 200022 (PEDro score 4, level of evidence 2b)
This prospective randomised controlled trial provided 10–15 min of balance training utilising dynadiscs and balance boards to a total of 221 soccer players for 6 months. After randomisation, 121 athletes (seven teams) were assigned to the intervention group and 100 athletes (six teams) were assigned to the control group. The athletes in the intervention group were asked to perform the balance training with balance boards everyday for the first month. After the first month, training was decreased to 3 days per week. This study reported an ACL incidence rate of 0.68 per 1000 h AE in the intervention group and 0.12 per 1000 h AE in the control group.
Heidt et al in 200020 (PEDro score 5, level of evidence 1b)
This research group employed a 75-min long custom-made speed and agility programme to 42 randomly selected high-school-age soccer players for a total of 21 sessions (first session is an orientation) over 7 weeks. The randomly selected subjects in the intervention group commuted to a local fitness gym to perform the intervention programme in preseason. Over the course of 4 months, an ACL injury rate in the intervention group was 2.38% and 3.10% in the control group. This study did not record or report exposure data.
Myklebust et al in 200323 (PEDro score 5, level of evidence 2b)
A 3-year prospective cross-over study (the first year was an observational year, whereas the two subsequent years were intervention periods) recruited a total of 1705 female handball athletes playing for the top three Norwegian handball leagues. A 15-min session of balance exercises with mats and wobble boards was implemented 3 days per week in the initial 5–7 weeks, which was subsequently reduced to once a week for the remainder of the handball season (~5 months). During the study period, an ACL incidence rate were: control (Year 1) 0.14 per 1000 h AE, intervention (Year 2) 0.13 per 1000 h AE and intervention (Year 3) 0.09 per 1000 h AE were recorded.
Mandelbaum et al in 200524 (PEDro score 3, level of evidence 2b)
Using a prospective cluster cohort study design, the research team applied a neuromuscular and proprioceptive programme to a total of 1885 female soccer players (1041 subjects in first year and 844 in second year) and compared the number of ACL injuries with age- and skill-matched controls. The programme was 20 min in duration, which consisted of education, basic warm-up, stretching for trunk and lower extremity, strengthening for trunk and lower extremity and plyometrics, and was performed two to three sessions per week. This investigation reported an ACL incidence rate of 0.04 per 1000 h AE in the intervention group and 0.24 per 1000 h AE in the control group over two competitive soccer seasons.
Olsen et al in 200525 (PEDro score 7, level of evidence 1b)
With cluster randomised controlled trial design, a 15–20 min long structured warm-up programme was implemented to improve awareness of neuromuscular control, balance and strength of knees and ankles in running, cutting and landing techniques in Norwegian handball players (808 subjects in the intervention group). The structured warm-up programme had four different exercises (warm-up, technique, balance and strength and power), and each exercise was progressed with increasing the level of difficulty. The structured warm-up programme was performed in 15 consecutive sessions and then once a week during one competitive Norwegian handball season. During the study period, an ACL incidence rate in the intervention group was 0.03 per 1000 h AE, whereas the control group was of 0.10 per 1000 h AE.
Petersen et al in 200526 (PEDro score 2, level of evidence 2b)
A prospective cohort study incorporated 10 min of injury-prevention training into a team warm-up. The injury-prevention training programme consisted of improving awareness of injury mechanisms and prevention strategies, balance-board exercises and jump training and was executed three times per week in the preseason and once a week in competition period. Lower-extremity injuries were tracked in 134 female handball players who performed the training programme and compared with age-matched and skill-matched controls. The study reported an ACL incidence rate 0.04 per 1000 h AE in the intervention group and 0.21 per 1000 AE in the control group.
Pfeiffer et al in 200627 (PEDro score 2, level of evidence 2b)
This research team implemented a 20 min plyometric-based exercise programme twice a week in high-school female soccer, volleyball and basketball athletes for 2 years. The plyometric-based exercise programme, ‘Knee Ligament Injury Prevention’ (KLIP), was developed by various healthcare practitioners and experts. A total of 577 athletes (43 teams) were classified in the intervention group and 862 athletes (69 teams) were categorised into the control group. During the investigation, an ACL incidence rate of 0.08 per 1000 h AE in the intervention group and 0.04 per 1000 AE in the control group were documented.
Steffen et al in 200828 (PEDro score 7, level of evidence 1b)
Using a cluster-randomised controlled trial (113 teams, 2100 players) this research team prescribed 15 min of a structured warm-up programme called ‘11’. The 11, which consisted of core stability, balance, plyometrics and hamstrings strengthening exercises, was applied to 1073 young female soccer players (51 teams) for the first 15 consecutive sessions and once a week for the remaining 7½ months. The study documented an ACL incidence rate of 0.06 per 1000 h AE in the intervention and 0.08 per 1000 h AE in the control group.
Gilchrist et al in 200829 (PEDro score 4, level of evidence 1b)
In the randomised cluster-controlled study, investigators applied the 20 min long programme, previously reported by Mandelbaum et al,24 to high-level college female soccer teams. The intervention and control groups were paired and formed a cluster. The clustered pairs were purposefully allocated different geographic regions throughout the USA. Then, one cluster of each region was randomly selected for the study. Soccer players (583 players, 26 teams) classified in the intervention group performed the programme three times per week for the entire fall soccer season (12 weeks). An ACL incidence rate in the intervention group was 0.20 per 1000 h AE, whereas the ACL incidence rate in the control group was 0.34 per 1000 h AE.
Kiani et al in 201030 (PEDro score 4, level of evidence 2b)
This prospective cluster-control trial (97 teams, 1506 players) included a 20–25 neuromuscular regimen consisting of a running warm-up, isometric contraction of lower extremity muscle groups, balance exercises with jump components, strengthening of lower extremities and core stability to 777 young soccer players (48 teams) 2 days per week for the 2 month preseason and once a week during 6 months of in-season sessions. This study reported an ACL incidence report of 0 per 1000 h AE in the intervention group and 0.08 per 1000 h AE ACL incident rate in the control group.
LaBella et al in 201132 (PEDro score 6, level of evidence 1b)
Using a randomised cluster-controlled design, investigators applied a programme called ‘Knee Injury Prevention Program’ (KIPP). A total of 737 athletes: 321 soccer (43.6%) and 416 basketball (56.4%) players practised the KIPP, which comprised 20 min of progressive strengthening, plyometric, balance and agility exercises three times per week for one competitive season. Over the course of the study, an ACL incidence rate of 0.10 per 1000 h AE was documented in the intervention group and 0.48 per 1000 h AE ACL incidence rate was documented in the control group.
Data synthesis
For synthesis of the12-included studies, the RRR for non-contact ACL injury from the reviewed studies was 73.4% (95% CI 62.5% to 81.1%) in subjects who performed the assigned neuromuscular training programmes compared with subjects who were allocated in the control group (table 4). The RRR for overall ACL injuries was 43.8% (95% CI 28.9% to 55.5%) for subjects in the intervention group compared with subjects who were in the control group (table 5). The NNT analysis indicated that it was necessary to intervene upon 108 athletes (95% CI=NNTB 86–150) in order to prevent one non-contact ACL injury (table 4). For overall ACL injuries, the NNT analysis indicated that 120 athletes (95% CI=NNTB 74–316) are needed to participate in neuromuscular training programmes to show prophylactic effects of preventing one injury (table 5).
Bias assessment
The bias assessment for the 12-included studies was performed using Egger’s regression. Egger’s regression intercept was −0.29 (95% CI −2.20, 1.61, p=0.37, one tailed), indicating that publication bias was not detected in the current analysis.
Evidence synthesis
The CEBM-Level of Evidence of each study is listed in table 1. The CEBM level of evidence can further generate a grade of strength of recommendation based on the level of consistent evidence, which consists of A–D. In the current analysis, five of the included studies were rated as level 1b, while seven studies were rated as level 2b. On the basis of consistency of the results from included studies, the strength of recommendation grade for the current evidence is B (consistent level of 2 or 3 studies or extrapolations from level 1 studies).
DISCUSSION
The aim of this systematic review of the literature was to identify effectiveness of neuromuscular training programmes in preventing non-contact and overall ACL injury incidence in large-scale studies published from 1995 to 2011 using RRR and NNT. The RRR for non-contact ACL injury was 73.4% and 43.8% for overall ACL injuries, with CI that do not encompass zero. It can be interpreted that female athletes who performed a given neuromuscular training programme have 73.4% less risk to suffer a non-contact ACL injury compared with those who did not perform NMT. Similarly, 43.8% of overall ACL injury risk reduction can be obtained in female athletes who performed neuromuscular training compared with those who served in a control group. To our knowledge, this may be the first study to report prophylactic effects of NMT on the overall ACL injury risk.
Recent ACL injury classification recommendations suggest four different types of ACL injury: direct contact, indirect contact, classic non-contact and other non-contact.35 The non-contact ACL injuries resulted from an individual’s own movements without contact by another person or object, which typically disturbed by some types of perturbation.35 The classical non-contact ACL injury mechanism involves cognitive perturbation, which is defined as a disruption to the planned motor task that requires a rapid update to the intended motor control plan.35 Unexpected sudden movement or position give perturbation to one’s cognition, and the ACL is torn, which is often observed in athletic setting. Conversely, other non-contact ACL injuries occur in simple activities in daily living, sometimes without any specific mechanism and seemingly no cognitive perturbation was applied.35 The other two types of ACL injury mechanisms, direct and indirect contact, involves physical perturbation either direct to the knee joint or other body parts at the time of or immediately before the injury.35 Since both of the classic and other non-contact ACL injury mechanisms do not entail the physical perturbation, it was assumed that the prophylactic effects of neuromuscular training are only applicable to non-contact ACL injury. However, the current analysis identified prophylactic effects to overall ACL injuries, which include direct and indirect contact mechanisms, in addition to the classic and other non-contact ACL injuries.
Two previous studies,16,36 that analysed large-scale neuromuscular training interventions targeted to reduce ACL incidence, demonstrated lower RRR than the current analysis. A meta-analysis of a total of seven randomised controlled and prospective cohort studies that aimed to reduce ACL injuries among female athletes by neuromuscular training interventions showed 60% of RRR (95% CI 40% to 73%) between athletes in the intervention and control groups.36 This analysis did not separate ACL injuries based on the mechanism (either non-contact or contact): thus, it can be inferred that 60% of RRR is a reflection of neuromuscular training for a combination of non-contact and overall ACL injuries. Another RRR study based on five prospective neuromuscular training intervention trials aimed to reduce ACL injuries in female athletes documented 70% of RRR (95% CI 54% to 80%).16 The study included only non-contact ACL cases for the analysis; therefore, it is interpreted that the neuromuscular training can effectively reduce 70% of non-contact ACL risks in female athletes compared with subjects in the control groups. The current analysis included several recently published studies, and 73.4% (95% CI 62.5% to 81.1%) of RRR for non-contact ACL injury were comparable with the previous reports. Based on the RRR numbers, it can be interpreted that it is possible to prevent approximately three-quarters of non-contact ACL injuries by applying a neuromuscular training intervention (table 4). Furthermore, the current analysis found that neuromuscular training can effectively reduce overall ACL injury risks by 43.8% (95% CI 28.9% to 55.5%) (table 5).
Through examining neuromuscular training programmes that demonstrated >73.4% and 43.8% of RRR in non-contact and overall ACL injuries,21,24–26,29,30 a few common characteristics were observed. It appears that those programmes combined multiple types of exercises instead of one neuromuscular training type.21,24–26,29,30 Strengthening, plyometric and balance exercises were primarily employed in those programmes.21,24–26,29,30 Unlike those programmes, several studies that included in the current analysis did not show high RRR rates applied a single type of neuromuscular training.22,23,27 Two studies22,23 implemented a set of balance exercises and one study27 tested the effectiveness of plyometric exercises. Synthesising the information altogether, providing one type of neuromuscular exercise is not adequate to generate a prophylactic effect; however, combining multiple types of exercises seems to enhance the effectiveness of neuromuscular training in female athletes.
In contrast, the NNT values obtained from the current analysis were quite different from the previously reported values. The current NNT analysis demonstrated 108 (95% CI=NNT 86 to 150) and 120 (95% CI=NNT 74 to 316) for non-contact and overall ACL incidences. It is interpreted that 108 athletes are needed to prevent one non-contact ACL injury as well as 120 athletes for overall ACL injury. Previous research in NNT to reduce the risk of non-contact ACL injury was reported at NNT=89 (95% CI=NNT 66 to 136), which is notably fewer than the current analysis.33 The higher NNT value for overall ACL injury prevention in the current study may be a result of the inclusion of studies that did not demonstrate favourable prophylactic effects to the subjects in intervention groups. For instance, the previous NNT analysis33 did not include a prospective randomised control study22 published in 2000. The study actually observed more ACL incidence in the intervention group instead of the control group (table 5). Additionally, a recently published study with a cluster randomised controlled trial design28 was not included in the previous report, but was included in the current analysis. The study did not demonstrate strong prophylactic neuromuscular training effects in the intervention group (tables 4 and 5). From a study-quality standpoint, the previously published NNT analysis33 had one randomised controlled trial,25 whereas the current analysis comprises five randomised controlled trials.20,25,28,29,32 Another analysis is imperative to find a link between the quality of the study and number of ACL injuries. However, these study results were likely to influence the higher NNT values in the current analysis compared with previous reports.
Coaches and clinicians may be hesitant to implement an intervention programme with an NNT of 108 for non-contact ACL injuries and 120 for overall ACL injuries. The time commitment for preventing one ACL injury may appear too substantial. For instance, to generate neuromuscular training prophylactic effects, a female soccer team that consists of 20 players needs to keep performing a neuromuscular training programmes for over five competitive seasons (20 players×5 seasons=NNT 100) to prevent one non-contact ACL injury. Estimating that there are approximately 15 players on one handball team, eight competitive seasons (15 athletes×8 seasons=NNT 120) are required to reach the NNT 120, which is the estimated number needed to prevent one overall ACL injury. The lengthy time commitment for preventing one ACL injury may not be a primary interest of coaches and health-care providers. Additionally, most neuromuscular training programmes take approximately 15–20 min to complete,23–25,27,29,32 which coaches may feel is ‘too much’, especially during in-season. In fact, most studies performed in Europe reduced the frequency of the neuromuscular training session during in-season compared with preseason.22,23,25,26,28,30 Those factors may lead to a difficulty of neuromuscular training programme inception and potentially low compliance. In fact, several reviewed studies pointed that low compliance of assigned neuromuscular training programmes as a limitation of the studies.23,28
A potential approach to improve compliance to injury-prevention intervention is to explain additional benefits associated with neuromuscular training. One of the reviewed studies demonstrated not only the positive effects on ACL injury, but also significantly lower rates of injury to other knee ligaments, and reduced moderate and major acute knee or ankle injuries rate in those who performed neuromuscular training compared with those who did not.25 Similarly, fewer overall knee injuries were reported in subjects in the intervention group compared with the control group in one reviewed study.30 Another reviewed study also showed lower ankle sprain injury rates in the intervention group.32 Performance enhancement is an added benefit to neuromuscular training as demonstrated in one investigation which used paediatric aged girls and boys (mean age=10±1-years-old) revealed improvements in balance and vertical jump height after 9 weeks of injury-prevention programme implementation.37 This study stated that those performance changes may help gaining support from coaches and potentially increase compliance to the preventive neuromuscular training.
The current analysis generated effectiveness of neuromuscular training as an intervention to prevent non-contact and overall ACL injuries. However, a significant number of athletes are needed to demonstrate prophylactic effects of the neuromuscular training; thus, the next logical step is to establish a method to screen athletes for injury risk. To detect potential at-risk athletes for future ACL injury, the current gold standard is usage of a three-dimensional laboratory-based motion analysis system. This system is specialised to capture the three (sagittal, frontal and transverse) plane kinematic motions with high-frequency cameras. However, it requires expensive equipment, extensive time and skillful biomechanists to analyse the data. To make the screening more efficient and applicable to larger populations, development of a valid and reliable tool with low cost and high efficiency is ideal. Several screening methods, aimed to identify at-risk athletes for future ACL injury without the three-dimensional laboratory motion analysis, were developed and introduced in recent publications.38–43
In place of the expensive three-dimensional motion analysis cameras, several screening tools were recently introduced using two-dimensional cameras. A landing error scoring system (LESS) was a clinical screening tool generated by Padua and his research team.39 Two standard video cameras are placed to capture the athlete’s landing kinematics from sagittal and frontal plane views. The landing patterns captured by the video cameras were examined and provided a total error score. Another tool developed by Myer and colleagues used a nomogram scale, which consists of total points from a combination of static (body mass and tibia length) and dynamic (knee valgus motion, knee flexion range of motion, quadriceps/hamstring strength ratio) measures captured with two standard video cameras.42 Also, a study conducted by Stensrud et al43 utilised a standard video camera to capture an image of knee joint alignment in the frontal view during dynamic movements. However, validation of those two-dimensional video screening tools for a clinical use is warranted in future studies.
Limitations
Several limitations to this study should be stated. Although each study was carefully reviewed, only half21,24,27,29,30 of the reviewed studies (5/12) documented the nature of the ACL injury mechanism. The lead author contacted the corresponding authors of each study. However, only one28 of the six studies who did not document the nature of the injury mechanism responded with full information. When the corresponding authors did not respond to the question, the status of ACL injury mechanism was cited from the previously published study.33 The lead author contacted a primary author of the study and assured accuracy of the ACL mechanism information presented.33
One study23 did not meet one of the inclusion criteria, which was a presence of control group; however, the study was included in the current analysis. The study had a large sample size with a good methodological quality (PEDro score 5/10) so that it was too difficult to exclude. The study implemented a cross-over study design instead of prospective cohort design and the intervention periods were actually 2 years followed by 1 year of control period. Therefore, the current analysis extracted only 1 year (first year) of intervention and control period.
Wide varieties of neuromuscular training programmes were noted across the reviewed studies (table 2). Frequency, duration and intensity varied across studies; therefore, even though the RRR 73.7% and 43.8% for non-contact and contact ACL injuries were found, it was difficult to point out what frequency, duration and intensity would maximise the neuromuscular training prophylactic effect to reduce future ACL injury risk among female athletes. Furthermore, different types of neuromuscular training were applied to different sports, ages and study designs. All of those variables made this analysis challenging to identify imperative aspects of neuromuscular training.
CONCLUSION
A review of 12 large-scale neuromuscular training studies aimed to lower ACL injuries in female athletes demonstrated an RRR of 73.4% and 43.8% for non-contact and overall contact ACL injury, respectively. Neuromuscular training may reduce non-contact ACL injury risk and overall ACL injury risk, which includes contact ACL mechanisms (direct and indirect). NNT analysis estimated that 120 athletes need to perform a neuromuscular training programme to prevent one overall ACL injury. Similarly, the NNT estimated that 108 athletes need to execute a neuromuscular training programme to prevent one non-contact ACL injury. Although the current analysis demonstrated prophylactic effectiveness of neuromuscular training, the NNT values yielded high NNT values, which may cause a difficulty in gaining support from a coaching staff. However, several studies documented benefits of neuromuscular training beyond ACL injury prevention, which include reduction in other knee and ankle injuries and performance improvement. Another possible direction to reduce the NNT is to identify at-risk athletes using two-dimensional camera systems, which have begun showing potential, but more studies are needed for these tools to be implemented in clinical use. Future training prophylactic effects to specific populations (gender, age and sports) as well as pursue more efficient methods to identify at-risk athletes. The resulting findings could lead to a more desirable outcome for ACL injury prevention and could promote safe and long-lasting athletic participation in a physically active population.
What is already known on this topic
Previous study reported a 70% (95% CI 54% to 80%) relative risk reduction (RRR) of non-contact anterior cruciate ligament (ACL) injury by neuromuscular training (NMT) in female athletes based on six individual studies.
Previously reported numbers needed to treat for non-contact ACL injury was 89 (95% CI 66 to 136) in female athletes based on six individual studies.
What this paper adds
A review of 12 individual studies demonstrated prophylactic effects of neuromuscular training (NMT) by a relative risk reduction (RRR) of 73.4% (95% CI 63% to 81%) for non-contact anterior cruciate ligament (ACL) injury in female athletes.
From the needed-to-treat (NNT) analysis, it determined that 108 (95% CI 86 to 150) individuals are necessary to prevent one non-contact ACL injury in female population by NMT intervention.
The current study showed NMT reduces overall ACL injury, as demonstrated by an RRR of 43.8% (29% to 56%) in female athletes.
The NNT analysis suggested 120 (95% CI 74 to 316) individuals to prevent one overall ACL injury in female athletes by NMT intervention.
Acknowledgments
The authors would like to thank Ms Catherine P Starnes for her statistical expertise and guidance for this project. All authors are independent of any commercial funder, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. We would like to thank you and the reviewers for the excellent comments that have influenced a much better presentation for the current manuscript.
Funding The authors would like to acknowledge funding support from National Institutes of Health Grant R01-AR049735, R01-AR055563 and R01-AR056259.
Footnotes
Provenance and peer review Not commissioned; externally peer reviewed.
Contributors DS was involved in conception and design, acquisition of data, drafting the article, critical revision for important intellectual content, and collection and assembly of data and final approval of the study. GDM was involved in conception and design, critical revision for important intellectual content, provision of study materials and patients, admin, technical and logistic support and final approval of the study. JMM was involved in acquisition of data, analysis and interpretation of data, drafting the article, statistical expertise and final approval of the study. THE was involved in concept and design, critical revision for important intellectual content, provision of study materials and patients, obtaining funding, admin, technical and logistic support and final approval of the study.
References
- 1.Silvers HJ, Mandelbaum BR. Prevention of anterior cruciate ligament injury in the femlae athlete. Br J Sports Med. 2007;41(Suppl 1):i52–9. doi: 10.1136/bjsm.2007.037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottlob CA, Baker CL, Jr, Pellissier JM, et al. Cost effectiveness of anterior cruciate ligament reconstruction in young adults. Clin Orthop Relat Res. 1999:272–82. [PubMed] [Google Scholar]
- 3.Cimino F, Volk BS, Setter D. Anterior cruciate ligament injury: diagnosis, management, and prevention. Am Fam Physician. 2010;82:917–22. [PubMed] [Google Scholar]
- 4.Dugan SA. Sports-related knee injuries in female athletes: what gives? Am J Phys Med Rehabil. 2005;84:122–30. doi: 10.1097/01.phm.0000154183.40640.93. [DOI] [PubMed] [Google Scholar]
- 5.Morrey MA, Stuart MJ, Smith AM, et al. A longitudinal examination of athletes’ emotional and cognitive responses to anterior cruciate ligament injury. Clin J Sport Med. 1999;9:63–9. doi: 10.1097/00042752-199904000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Wright RW, Dunn WR, Amendola A, et al. Risk of tearing the intact anterior cruciate ligament in the contralateral knee and rupturing the anterior cruciate ligament graft during the first 2 years after anterior cruciate ligament reconstruction: a prospective Moon cohort study. Am J Sports Med. 2007;35:1131–3. doi: 10.1177/0363546507301318. [DOI] [PubMed] [Google Scholar]
- 7.Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38:1968–78. doi: 10.1177/0363546510376053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louboutin H, Debarge R, Richou J, et al. Osteoarthritis in patients with anterior cruciate ligament rupture: a review of risk factors. Knee. 2009;16:239–44. doi: 10.1016/j.knee.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Lohmander LS, Ostenberg A, Englund M, et al. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–52. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 10.Agel J, Olson DE, Dick R, et al. Descriptive epidemiology of collegiate women’s basketball injuries: National Collegiate Athletic Association Injury Surveillance System, 1988–1989 through 2003–2004. J Athl Train. 2007;42:202–10. [PMC free article] [PubMed] [Google Scholar]
- 11.Boden BP, Dean GS, Feagin JA, Jr, et al. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23:573–8. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- 12.Boden BP, Sheehan FT, Torg JS, et al. Noncontact anterior cruciate ligament injuries: mechanisms and risk factors. J Am Acad Orthop Surg. 2010;18:520–7. doi: 10.5435/00124635-201009000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arendt EA, Agel J, Dick R. Anterior cruciate ligament injury patterns among collegiate men and women. J Athl Train. 1999;34:86–92. [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin LY, Albohm MJ, Arendt EA, et al. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006;34:1512–32. doi: 10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- 15.Renstrom P, Ljungqvist A, Arendt E, et al. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med. 2008;42:394–412. doi: 10.1136/bjsm.2008.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hewett TE, Ford KR, Myer GD. Anterior cruciate ligament injuries in female athletes: part 2, a meta-analysis of neuromuscular interventions aimed at injury prevention. Am J Sports Med. 2006;34:490–8. doi: 10.1177/0363546505282619. [DOI] [PubMed] [Google Scholar]
- 17.Hewett TE, Myer GD, Ford KR. Anterior cruciate ligament injuries in female athletes: part 1, mechanisms and risk factors. Am J Sports Med. 2006;34:299–311. doi: 10.1177/0363546505284183. [DOI] [PubMed] [Google Scholar]
- 18.Hewett TE, Myer GD, Ford KR, et al. Dynamic neuromuscular analysis training for preventing anterior cruciate ligament injury in female athletes. Instr Course Lect. 2007;56:397–406. [PubMed] [Google Scholar]
- 19.Caraffa A, Cerulli G, Projetti M, et al. Prevention of anterior cruciate ligament injuries in soccer. A prospective controlled study of proprioceptive training. Knee Surg Sports Traumatol Arthrosc. 1996;4:19–21. doi: 10.1007/BF01565992. [DOI] [PubMed] [Google Scholar]
- 20.Heidt RS, Jr, Sweeterman LM, Carlonas RL, et al. Avoidance of soccer injuries with preseason conditioning. Am J Sports Med. 2000;28:659–62. doi: 10.1177/03635465000280050601. [DOI] [PubMed] [Google Scholar]
- 21.Hewett TE, Lindenfeld TN, Riccobene JV, et al. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27:699–706. doi: 10.1177/03635465990270060301. [DOI] [PubMed] [Google Scholar]
- 22.Soderman K, Werner S, Pietila T, et al. Balance board training: prevention of traumatic injuries of the lower extremities in female soccer players? A prospective randomized intervention study. Knee Surg Sports Traumatol Arthrosc. 2000;8:356–63. doi: 10.1007/s001670000147. [DOI] [PubMed] [Google Scholar]
- 23.Myklebust G, Engebretsen L, Braekken IH, et al. Prevention of anterior cruciate ligament injuries in female team handball players: a prospective intervention study over three seasons. Clin J Sport Med. 2003;13:71–8. doi: 10.1097/00042752-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Mandelbaum BR, Silvers HJ, Watanabe DS, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33:1003–10. doi: 10.1177/0363546504272261. [DOI] [PubMed] [Google Scholar]
- 25.Olsen OE, Myklebust G, Engebretsen L, et al. Exercises to prevent lower limb injuries in youth sports: cluster randomised controlled trial. BMJ. 2005;330:449. doi: 10.1136/bmj.38330.632801.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen W, Braun C, Bock W, et al. A controlled prospective case control study of a prevention training program in female team handball players: the German experience. Arch Orthop Trauma Surg. 2005;125:614–21. doi: 10.1007/s00402-005-0793-7. [DOI] [PubMed] [Google Scholar]
- 27.Pfeiffer RP, Shea KG, Roberts D, et al. Lack of effect of a knee ligament injury prevention program on the incidence of noncontact anterior cruciate ligament injury. J Bone Joint Surg Am. 2006;88:1769–74. doi: 10.2106/JBJS.E.00616. [DOI] [PubMed] [Google Scholar]
- 28.Steffen K, Myklebust G, Olsen OE, et al. Preventing injuries in female youth football —a cluster-randomized controlled trial. Scand J Med Sci Sports. 2008;18:605–14. doi: 10.1111/j.1600-0838.2007.00703.x. [DOI] [PubMed] [Google Scholar]
- 29.Gilchrist J, Mandelbaum BR, Melancon H, et al. A randomized controlled trial to prevent noncontact anterior cruciate ligament injury in female collegiate soccer players. Am J Sports Med. 2008;36:1476–83. doi: 10.1177/0363546508318188. [DOI] [PubMed] [Google Scholar]
- 30.Kiani A, Hellquist E, Ahlqvist K, et al. Prevention of soccer-related knee injuries in teenaged girls. Arch Intern Med. 2010;170:43–9. doi: 10.1001/archinternmed.2009.289. [DOI] [PubMed] [Google Scholar]
- 31.Soligard T, Myklebust G, Steffen K, et al. Comprehensive warm-up programme to prevent injuries in young female footballers: cluster randomised controlled trial. BMJ. 2008;337:a2469. doi: 10.1136/bmj.a2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labella CR, Huxford MR, Grissom J, et al. Effect of neuromuscular warm-up on injuries in female soccer and basketball athletes in urban public high schools. Arch Pediatr Adolesc Med. 2011;165:1033–40. doi: 10.1001/archpediatrics.2011.168. [DOI] [PubMed] [Google Scholar]
- 33.Grindstaff TL, Hammill RR, Tuzson AE, et al. Neuromuscular control training programs and noncontact anterior cruciate ligament injury rates in female athletes: a numbers-needed-to-treat analysis. J Athl Train. 2006;41:450–6. [PMC free article] [PubMed] [Google Scholar]
- 34.Bender R. Calculating cofidence intervals for the number needed to treat. Controlled Clin Trial. 2001;22:102–10. doi: 10.1016/s0197-2456(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 35.Marshall SW. Recommendation for defining and classifying anterior cruciate ligament injuries in epidemiologic studies. J Athl Train. 2010;45:516–18. doi: 10.4085/1062-6050-45.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoo JH, Lim BO, Ha M, et al. A meta-analysis of the effect of neuromuscular training on the prevention of the anterior cruciate ligament injury in female athletes. Knee Surg Sports Traumatol Arthrosc. 2010;18:824–30. doi: 10.1007/s00167-009-0901-2. [DOI] [PubMed] [Google Scholar]
- 37.DiStefano LJ, Padua DA, Blackburn JT, et al. Integrated injury prevention program improves balance and vertical jump height in children. J Strength Cond Res. 2010;24:332–42. doi: 10.1519/JSC.0b013e3181cc2225. [DOI] [PubMed] [Google Scholar]
- 38.McLean SG, Walker K, Ford KR, et al. Evaluation of a two dimensional analysis method as a screening and evaluation tool for anterior cruciate ligament injury. Br J Sports Med. 2005;39:355–62. doi: 10.1136/bjsm.2005.018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Padua DA, Marshall SW, Boling MC, et al. The Landing Error Scoring System (LESS) is a valid and reliable clinical assessment tool of jump-landing biomechanics: the JUMP-ACL study. Am J Sports Med. 2009;37:1996–2002. doi: 10.1177/0363546509343200. [DOI] [PubMed] [Google Scholar]
- 40.Kagaya Y, Kawasaki W, Fujii Y, et al. Valdiation of a two-dimensional motion analysis technique for quantifying dynamic knee valgus during a drop landing by comparisons to data from three-dimensional analysis. Jpn J Phys Fitness Sports Med. 2010;59:407–14. [Google Scholar]
- 41.Hayashi Y, Ishibashi Y, Tsuda E, et al. Two-dimensional and three-dimensional motion analysis of lower limb alignment and joint kinematics during a jump landing task. Jpn Clin Sports Med J. 2008;16:24–9. [Google Scholar]
- 42.Myer G, Ford KR, Khoury J, et al. Three-dimensioal motion analysis validation of a clinic-based nomorgram designed to identify high ACL injury risk in female athletes. Phys Sportsmed. 2011;39:78–84. doi: 10.3810/psm.2011.02.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stensrud S, Myklebust G, Kristianslund E, et al. Correlation between two-dimensional video analysis and subjective assessment in evaluating knee control among elite female team handball players. Br J Sports Med. 2011;45:589–95. doi: 10.1136/bjsm.2010.078287. [DOI] [PubMed] [Google Scholar]