Abstract
Janus kinases (Jaks) play an essential role in cytokine signaling and have been reported to regulate plasma membrane expression of their cognate receptors. In this study, we examined whether Jak3 and the common γ chain (γc) reciprocally regulate their plasma membrane expression. In contrast to interleukin-2Rα, γc localized poorly to the plasma membrane and accumulated in endosomal-lysosomal compartments. However, γc was expressed at comparable levels on the surface of cells lacking Jak3, and plasma membrane turnover of γc was independent of Jak3. Nonetheless, overexpression of Jak3 enhanced accumulation of γc at the plasma membrane. Without γc, Jak3 localized in the cytosol, whereas in the presence of the receptor, it colocalized with γc in endosomes and at the plasma membrane. Although the Jak FERM domain is necessary and sufficient for receptor binding, the requirement for full-length Jak3 in γc membrane trafficking was remarkably stringent; using truncation and deletion mutants, we showed that the entire Jak3 molecule was required, although kinase activity was not. Thus, unlike other cytokine receptors, γc does not require Jak3 for receptor membrane expression. However, full-length Jak3 is required for normal trafficking of this cytokine receptor/Jak pair, a finding that has important structural and clinical implications.
The Janus family of protein tyrosine kinases (Jaks) is a small family consisting of Jak1, Jak2, Jak3, and Tyk2 (11, 15, 19, 23). These kinases are structurally unique in possessing a carboxy-terminal kinase domain, along with a pseudokinase domain, which gave the Jaks their name. The pseudokinase domain, as its name implies, lacks catalytic activity but has essential regulatory functions (4, 29). Jaks also have an SH2-like domain, but the ability of this region to bind phosphotyrosine has not been established. The amino terminus of Jaks comprises a band-four-point-one, ezrin, radixin, moesin (FERM) domain, which is critical for binding cytokine receptors (7, 8, 23).
Many lines of evidence ranging from mutant cell lines to knockout mice and patients with immunodeficiency indicate that Jaks are essential for signaling via type I and type II cytokine receptors (6, 14, 15, 23). In addition, it has long been appreciated that for some receptors, Jaks also appear to be required for membrane localization of the cognate receptor. That is, the earliest study showing that a Jak, in this case Tyk2, is essential for signaling via alpha/beta interferon (IFN-α/β) also demonstrated that IFN-α receptor subunit 1 (IFNAR1) was poorly expressed on the surface of cells lacking this kinase (35). More recently, it has been reported that Tyk2 impedes constitutive endocytosis and degradation of IFNAR1 (25). This internalization of IFNAR1 is dependent upon the intracellular membrane proximal region (amino acids 480 to 520), since deletion of this region allowed stable surface expression (25). The requirement for Tyk2 for membrane localization of this receptor is most evident in human fibrosarcoma cell lines lacking Tyk2 (7, 26). However, in mice made deficient in Tyk2 by gene targeting, it has been reported that receptor expression is normal (12), although the apparent differences between humans and mice may reflect technical problems related to anti-receptor antibodies. Tyk2 has also been reported to enhance the surface expression of interleukin-10R2 (IL-10R2) (25).
Plasma membrane localization of the erythropoietin receptor (EpoR) also requires expression of its cognate Jak, Jak2, although the mechanism appears to be different. EpoR/Jak2 association occurs in the endoplasmic reticulum (ER) and the Jak has chaperone function; in the absence of Jak2, the EpoR fails to traffic to the plasma membrane efficiently. A requirement for Jak has also been reported for the localization of the Oncostatin M receptor to the plasma membrane, although the underlying mechanism has not been elucidated (24). In summary, data from several systems strongly argue for the importance of Jaks in regulating cytokine receptors. However, there are cell- and species-specific differences in this requirement, and a common mechanism for controlling membrane expression has not yet been identified.
Unlike other cytokine receptors and Jaks, the common γ chain (γc) and Jak3 have limited distribution, both being predominantly expressed in cells of the hematopoietic lineage (3, 13). Also in contrast to other cytokine receptor-Jak pairs is the selective association of γc and Jak3. To the best of our knowledge, γc interacts exclusively with Jak3 and the converse is also the case. This contention is also supported by genetic data; the phenotype of severe combined immunodeficiency (SCID) associated with γc deficiency is identical to that of Jak3 deficiency (18, 28). These properties are very useful if one wishes to examine whether Jak3 regulates the membrane expression of γc or vice versa. Moreover, mouse and human cells lacking γc, Jak3, or both are available. We therefore set out to determine whether Jak3 and γc regulate each other's subcellular localization.
In the present study, by using fluorescent fusion proteins and live cell imaging, we show that, in contrast to the IL-2Rα subunit, γc localized inefficiently to the plasma membrane and accumulated predominantly in endosomal and lysosomal compartments. Measuring receptor surface expression with flow cytometry shows that Jak3 is not required for the expression of γc at the plasma membrane, and its presence or absence does not influence receptor internalization. Nonetheless, overexpression of Jak3 promotes accumulation of γc at the plasma membrane. Conversely, in the absence of γc, Jak3 localized to the cytosol, and importantly, the entire Jak3 molecule is required for the proper localization of this cytokine receptor-Jak pair. These findings demonstrate that the plasma membrane expression of cytokine receptors is not universally dependent upon the cognate Jak. These findings also have important implications for our understanding of Jak structure and indicate that the requirements for proper subcellular localization of Jaks are surprisingly stringent.
MATERIALS AND METHODS
Antibodies and reagents.
Brefeldin A (BFA; Sigma, St. Louis, Mo.) dissolved in ethanol and cycloheximide (CHX; Sigma) dissolved in distilled water were used at final concentrations of 2 and 20 μg/ml, respectively. Alexa-568 goat-anti-mouse immunoglobulin G (IgG) and Alexa-568 goat-anti-rat IgG were purchased from Molecular Probes (Eugene, Oreg.). TGN38, early endosomal antigen 1 (EEA1), lysosomal membrane protein 1 (Lamp1), Rab5, CD132 (IL-2Rγ), R-phycoerythrin (PE)-conjugated monoclonal rat anti-human CD132 and PE-conjugated rat IgG2b antibodies were purchased from BD Transduction Laboratories/BD Pharmingen (San Diego, Calif.). 7G7, anti-IL-2R (receptor) α chain (anti-Tac) monoclonal antibody (MAb) was obtained from D. Nelson (National Cancer Institute, Bethesda, Md.). The following antibodies were purchased: anti-IL-2Rγc (Santa Cruz Biotechnology, Santa Cruz, Calif.) and anti-actin MAb (Chemicon International, Temecula, Calif.). The rabbit antisera against the carboxy [anti-Jak3(C)] or amino [anti-Jak3(N)] termini of human or murine Jak3 were described previously (5).
Plasmid constructs and fusion proteins.
All Jak3 fusion protein constructs were generated by PCR amplification with human Jak3-pME18s as a template (5). PCR products were first subcloned into pCR4-Topo vector for TA cloning (Invitrogen, Carlsbad, Calif.). Inserts were then excised and subcloned into the EcoRI-SacII sites of pEGFP-N3 (JH7-6), the KpnI-EcoRI sites (JH7-5), or the XhoI-SacII sites (full-length Jak3) of pEGFP-N1 (Clontech Laboratories, Palo Alto, Calif.). PCR amplification of full-length Jak3 was done in two steps. An EcoRI restriction site was inserted in position 1806 by silent mutation of Phe602 (TTT→TTC); after restriction digestion, the two fragments of 1.8 and 1.6 kb were subcloned sequentially into the XhoI-EcoRI sites and EcoRI-SacII sites of the pEGFP-N1 vector. The common γ chain of the human IL-2 receptor was generated by PCR amplification with the IL-2Rγ-pME18s construct as a template (27) and subsequently cloned into XhoI-BamHI sites of pHcRed-N1, pEYFP-N1, or pEGFP-N1 (Clontech). The following Jak3 residues were mutated by using the QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, Calif.): Y100C, D949N, K556A, K855A, R402H, E639K, C759R, Y980F, Y981F, and YY980/981FF. The Jak3ΔJH1-green fluorescent protein (GFP) mutant carrying a deletion of the entire JH1 domain was generated by introducing a SacII restriction site at nucleotide 2440 in the Jak3-GFP construct, followed by SacII digestion (removing amino acids 821 to 1096) and religation. All constructs derived by PCR were verified by sequencing. The pEYFP-Golgi expression plasmid was purchased from Clontech.
Cells, transfection, and microscopy.
COS-7 and HeLa cells were cultured at 37°C in 5% CO2 in Dulbecco modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml (complete medium). Epstein-Barr virus (EBV)-transformed human B cells from healthy donors or Jak3-SCID patients were cultured in complete RPMI 1640 medium. Media, antibiotics, and FBS were obtained from Biofluids (Rockville, Md.). Cells from patient 1, homozygous for a missense mutation of Y100→C (18), express Jak3 at low levels but the mutant Jak3 does not bind γc (1, 36). Cells from patient 2 are homozygous for a 151-bp deletion within the pseudokinase domain resulting in a frameshift and premature termination, and these cells do not express Jak3 protein (18).
For immunohistochemistry and confocal microscopy, cells were seeded on coverslips or grown in 4.3-cm2 chambers (two-well Lab-Tek chambered coverglass system; Nalge Nunc, Naperville, Ill.) and transiently transfected with 1 to 2 μg of plasmid by using FuGENE6 transfection reagent (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. EBV-transformed human B cells were transfected by electroporation with the human B-cell Nucleofector kit (Amaxa Biosystems, Cologne, Germany) according to the commercial protocol. At 20 to 24 h after transfection, cells were fixed in 4% paraformaldehyde (Sigma) for 20 min at 4°C, washed, permeabilized in phosphate-buffered saline containing 0.04% saponin (Sigma) and 1% bovine serum albumin (BSA) for 1 h, and then stained with the appropriate primary antibody in the same solution for 2 h, followed by staining with the secondary antibody for 45 min. Coverslips were mounted on glass slides in Vectashield mounting medium (Vector Laboratories, Burlingame, Calif.). For imaging of live cells expressing fluorescent protein constructs, two-well chambers were used to obtain the parallel and nearly simultaneous imaging of two separately transfected cell populations under identical experimental conditions. Cells were imaged in phenol red-free Dulbecco modified Eagle medium supplemented with 20 mM HEPES (pH 7.4), 10% FBS, l-glutamine, and antibiotics and then imaged 20 to 24 h after transfection by using an Olympus Fluoview FV500 confocal microscope with a ×40 1.3 NA or a ×60 1.4 NA Uplan apochromatic objective lens (Olympus America, Inc.) or a Zeiss LSM 510 confocal microscope with a ×40 or a ×63 1.4 NA objective lens (Carl Zeiss, Jena, Germany). An argon laser was used for excitation of pEGFP and pEYFP at wavelengths of 488 and 514 nm, respectively, whereas a helium-neon laser (568 nm, Olympus; 543 nm, Zeiss) was used for excitation of HcRed or Alexa-568. Cells expressing γc-GFP and Golgi-pEYFP were imaged on the Zeiss 510, by using the argon laser at wavelengths of 488 and 514 nm for excitation and the Meta detector for recording of the emission. The overlapping spectra of GFP and YFP emission were separated in post-image analysis with the Zeiss software for linear unmixing. Images were acquired with settings, allowing signal detection in the linear range below saturation. For double staining, sequential acquisitions were performed to avoid cross talk between the two channels. The images presented are representative of at least three independent experiments.
Flow cytometry.
To measure surface expression of γc in HeLa or COS-7 cells (106/condition) transfected with either GFP alone or γc-GFP or γc-GFP with Jak3-pME18s (molar ratio of 1:3), cells were incubated with anti-human CD132-PE or the appropriate isotype control for 30 min at 4°C. GFP-positive cells, indicating cells transfected either with GFP or γc-GFP, were gated for analysis of R-PE fluorescence. To measure the half-life of γc at the plasma membrane, 106 EBV-transformed normal human B cells or B cells from Jak3 SCID patients were treated with BFA or CHX for the indicated times prior to staining with anti-human CD132-PE.
Western blotting and immunoprecipitation.
Splenocytes from C57BL/6 or Jak3−/− mice and EBV-transformed B cells from Jak3 SCID and X-SCID patients or healthy donors were lysed in buffer containing 50 mM Tris-HCl (pH 7.5), 300 mM NaCl, 2 mM EDTA, 0.5% Triton X-100 (Sigma), 200 μM Na3VO4, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml, and 2.5 μM p-nitrophenyl-p-guanidinobenzoate (NPGB). Lysates were electrophoresed, transferred to nitrocellulose membranes, and immunoblotted with antibodies to Jak3, γc, and actin. For coimmunoprecipitation experiments, COS-7 cells were transiently transfected with Tac-γc (previously described by Zhou et al. [36]) and the indicated Jak3-GFP fusion proteins by using FuGENE6 (Roche Diagnostics, Mannheim, Germany) and then lysed in buffer containing 0.875% Brij 97 and 0.125% NP-40 (Sigma) as detergent. Clarified lysates were immunoprecipitated with anti-Tac. Lysates and immunoprecipitates were boiled, subjected to sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis, and electrotransferred onto nitrocellulose membranes (Schleicher & Schuell), followed by immunoblotting with anti-Jak3.
RESULTS
Jak3 is not required for γc membrane localization.
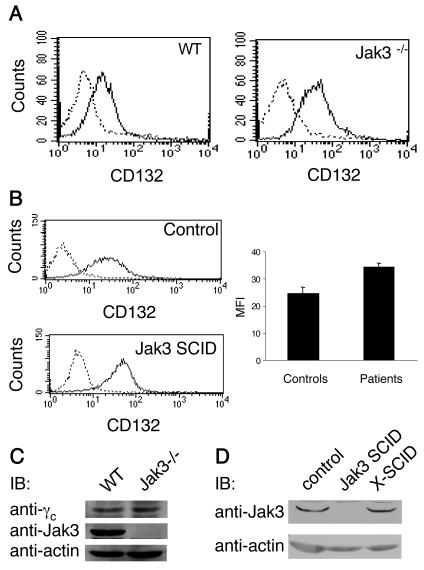
Findings from multiple cytokine receptor studies argue for the requirement of the relevant Jak to permit membrane expression of the respective cytokine receptor (10, 24, 25). We first set out to determine whether this requirement was also relevant for the localization of γc to the cell surface. To this end, we examined γc surface expression on cells obtained from Jak3-deficient mice. As determined by flow cytometry, the plasma membrane γc levels were not impaired by the absence of Jak3 (Fig. 1A); on the contrary, the level of expression was slightly increased, a finding consistent with a previous report (33). Because of potential species-specific differences in the reported Jak chaperone function, we next examined whether Jak3-independent membrane expression of γc was unique to mouse cells or present in both mice and humans. As depicted in Fig. 1B, human lymphocytes that lack Jak3 also had enhanced, not diminished, cell surface γc levels.
FIG. 1.
Jak3-independent surface expression of γc. (A and B) Comparison of γc cell surface levels on splenocytes from wild-type C57BL/6 versus Jak3−/− mice (A) and EBV-transformed B cells from normal individuals versus cells from Jak3 SCID patients (B). Cells were stained with R-PE anti-CD132 (IL-2Rγ), and γc surface expression was determined by flow cytometry. Dashed lines depict staining with isotype control; black solid lines indicate anti-CD132 staining. The bar graph in panel B shows a quantification of γc surface expression on cell lines from three different Jak3 SCID patients and three normal controls. Levels of γc and Jak3 protein are not influenced by each other's presence or absence. (C and D) Splenocytes from wild-type C57BL/6 versus Jak3−/− mice (C) and EBV-transformed B cells from normal individuals, Jak3 SCID, or X-SCID patients (D) were lysed, and proteins from the clarified lysates were separated on SDS-polyacrylamide gels, transferred to nitrocellulose membranes, and blotted with anti-IL-2Rγc, anti-Jak3 (C), or antiactin antibodies.
We considered the possibility that elevated γc surface levels were the result of increased total γc protein, and we therefore assessed total cellular γc levels by immunoblotting. However, Fig. 1C shows that the levels of γc protein were not altered by the presence or absence of Jak3. Conversely, total Jak3 levels were not affected by the presence or absence of γc (Fig. 1D). Similar to experiments with endogenous kinase and receptor, the lack of effect of γc and Jak3 on each others' protein expression was as evident in transient-transfection experiments with COS-7 cells (data not shown).
γc is poorly expressed on the cell surface compared to IL-2Rα.
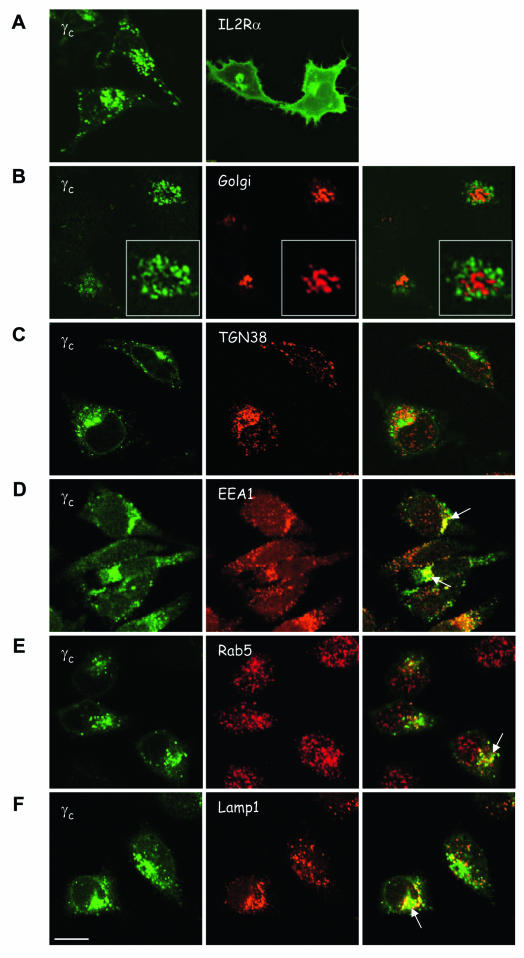
Given that γc was expressed in the absence of Jak3 and in view of the fact that this behavior is distinct from that of some other cytokine receptors, we thought it important to study the subcellular distribution of γc. We therefore generated fluorescent fusion proteins to examine intracellular localization by confocal microscopy. Upon transfection in HeLa (Fig. 2) or COS-7 cells (not shown), which lack endogenous γc and Jak3, γc accumulated in vesicular structures and localized inefficiently to the cell surface relative to the intracellular pool (Fig. 2A, left panel). Nonetheless, γc was also readily detectable on the cell surface by flow cytometry (see below). The IL-2 receptor complex consists of three receptor subunits: IL-2Rα, IL-2Rβ, and γc. Given the predominantly intracellular distribution of γc, we compared its subcellular localization to another component of the IL-2R complex, IL-2Rα. The latter accumulated abundantly on the cell surface of HeLa and COS-7 cells and in perinuclear compartments (Fig. 2A, right panel), which contrasts sharply with the poor membrane localization of γc and its accumulation in vesicular compartments. To investigate the subcellular distribution pattern of γc, we cotransfected γc with a Golgi marker or stained the γc-transfected cells with antibodies against various organelles. In contrast to a report by Huang et al. (10), which concluded that the EpoR accumulates mostly in the ER in the absence of Jak2, we did not observe colocalization of γc with markers for the Golgi complex, trans-Golgi network (TGN) (Fig. 2B-C) or ER (not shown). Rather, as shown in Fig. 2D to F, γc accumulated in early endosomes and lysosomes, as reflected by its partial colocalization with EEA1, Rab5, and Lamp1. In summary, our data therefore indicate that γc did not efficiently localize to the cell surface compared to another subunit of the IL-2R complex. The accumulation of γc in endosomal and lysosomal compartments is consistent with previous data (9, 20) but is different from the reported localization of the EpoR in the Golgi-ER.
FIG. 2.
Subcellular distribution of γc. HeLa cells were transfected with human γc-GFP (A to F, left panels) or IL-2Rα-GFP (A, right panel). Cells were cotransfected with pEYFP-Golgi marker (B, red) or cells were fixed, permeabilized, and stained with antibodies against various organelles, followed by Alexa-568-coupled secondary antibody (C to F, green). All cells were imaged in confocal mode. (C) TGN38, antibody to the TGN (red); (D) EEA1, antibody to EEA1 (red); (E) anti-Rab5, antibody to early endosomes (red); (F) anti-Lamp1, antibody to lysosomes (red). The white arrows indicate colocalization of γc-GFP with endosomes (D and E) or lysosomes (F). Scale bar, 20 μm.
Upon overexpression, Jak3 and γc enhance each other's membrane localization.
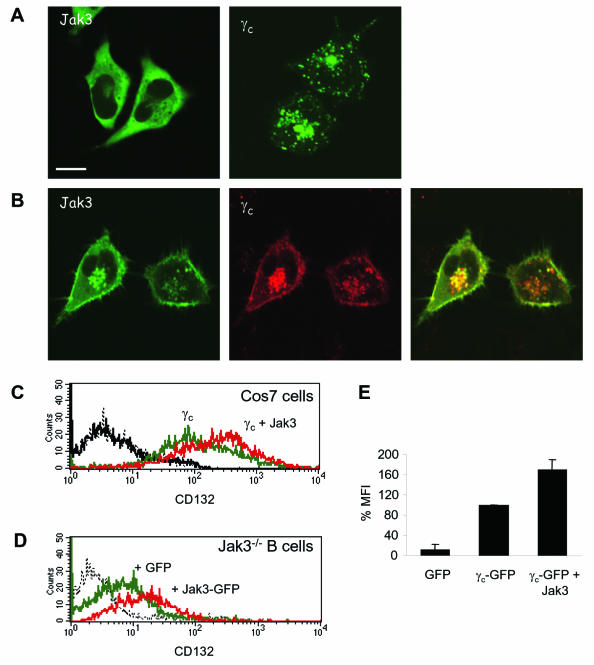
In contrast to other cytokine receptors, we found Jak3 not to be required for γc membrane expression (Fig. 1). However, we were nonetheless struck by the inefficient membrane localization of γc (Fig. 2). We therefore sought to determine whether reexpression of Jak3 in cells lacking this kinase would influence γc membrane localization and, conversely, whether γc influenced Jak3 subcellular distribution. As demonstrated in Fig. 3A, upon transfection in HeLa cells Jak3 was diffusely distributed in the cytosol without apparent intrinsic ability to localize to the plasma membrane. Interestingly, cotransfection of γc with Jak3 had effects on both proteins. First, Jak3 was now detected at the plasma membrane (Fig. 3B). In addition, γc also accumulated more at the cell surface (compare Fig. 3B and A). We next used flow cytometry to confirm and quantify the apparent enhanced membrane localization of γc by Jak3. As shown in Fig. 3C, γc was detectable at the cell surface of transfected COS-7 cells in the absence of Jak3 (mean fluorescence intensity [MFI] of 107.74 compared to cells transfected with GFP alone [MFI = 4.8]). However, cotransfection with Jak3 increased receptor surface levels (Fig. 3D, MFI = 208.91); the quantitation of this increase obtained from three independent experiments is shown in Fig. 3E. These results indicated that, although γc did not require Jak3 for its cell surface localization, artificial expression of this kinase clearly enhanced receptor trafficking to the membrane. To ascertain that this alteration in subcellular distribution was pertinent to lymphocytes, we performed analogous experiments in Jak3-deficient cell lines from a SCID patient. As indicated above, γc was expressed at higher levels on cells lacking Jak3, nonetheless, expression of Jak3 further augmented γc cell surface levels (Fig. 3D); the effect of Jak3 on γc was evident both in wild-type and Jak3-deficient cells. Importantly, we observed the same effects in transfected lymphocytes (Fig. 3D) as in COS-7 cells (Fig. 3C), indicating that Jak3 can positively influence γc membrane expression, although it is clearly not required.
FIG. 3.
Coexpression of Jak3 and γc increases membrane localization of both. HeLa cells were transfected with human Jak3 or γc individually (A) or together (B) and analyzed by confocal microscopy. In panel A, the left panel shows homogeneous cytoplasmic distribution of Jak3-GFP and the right panel depicts the endosomal-lysosomal and poor plasma membrane localization of γc-GFP. Panel B shows Jak3-GFP in green and γc-HcRed in red. The merged image of the two signals is shown on the right. Scale bar, 20 μm. (C and D) COS-7 cells, transfected with GFP-vector (black), γc-GFP alone (green), or γc-GFP plus Jak3-pME18s (molar ratio of 1:3; red) (C) and human Jak3-deficient EBV-transformed B cells, transfected with GFP-vector (green) or Jak3-GFP (red) (D) were stained after 20 h with R-PE-labeled anti-human CD132 antibody (anti-IL-2Rγ) and then analyzed by flow cytometry. Dashed lines depict staining with isotype control. GFP-positive cells were gated for analysis of PE fluorescence (γc cell surface expression). (E) Quantification of γc surface expression in COS-7 cells transfected with γc-GFP alone or γc-GFP and Jak3. The diagram shows the percentage of ΔMFI for the indicated constructs obtained from three independent experiments.
Membrane half-life of γc is independent of Jak3.
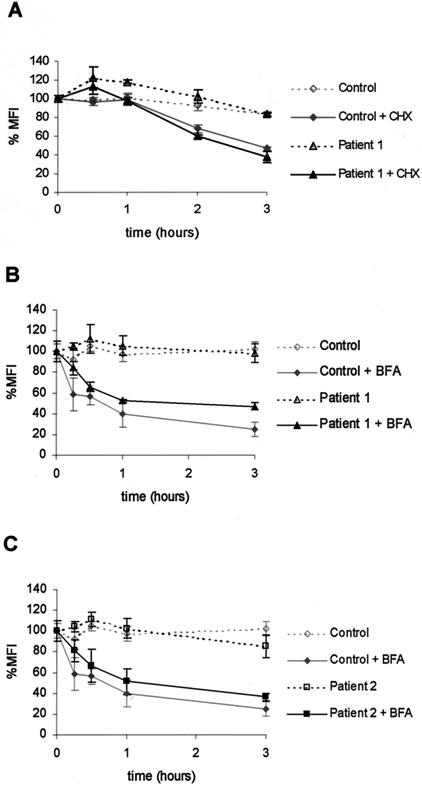
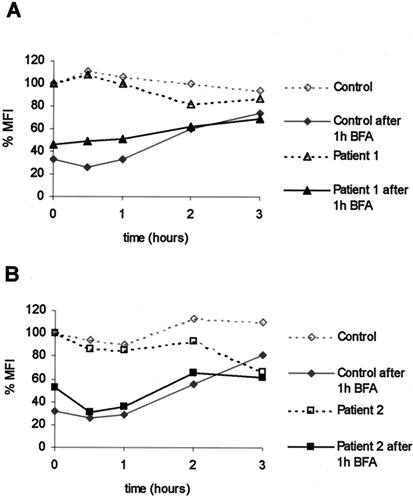
Although γc is detectable at the cell surface in the absence of Jak3, our data also indicated that γc localized poorly to the plasma membrane compared to the IL-2Rα. Therefore, we next considered the possibility that Jak3 might influence receptor half-life and that an effect of Jak3 would be more evident if we perturbed receptor expression. For instance, the degradation of IFNAR1 after CHX treatment was reduced in the presence of Tyk2 (25). We therefore treated normal and Jak3-deficient human B cells with CHX to prevent the synthesis of new receptors. Employing flow cytometry, we found a rapid decrease of γc surface levels, reaching 50% of the control level at approximately 2 h of incubation with CHX (Fig. 4A). However, no differences between normal human B cells and Jak3-deficient B cells were detected (Fig. 4A), indicating that the presence or absence of Jak3 did not influence the loss of γc surface expression after de novo protein synthesis was blocked. As an alternative approach we also used the fungal metabolite BFA, which reversibly blocks anterograde ER-to-Golgi traffic, resulting in accelerated redistribution of the Golgi into the ER (16). BFA treatment led to a decrease in γc surface levels similar to CHX, reaching 50% of the control level after approximately 45 min (Fig. 4B and C). Three independent experiments produced similar results. Treatment of cells with ethanol alone (vehicle control) did not influence γc surface expression (data not shown). Again, the absence of Jak3 (Fig. 4B and C) did not influence the loss of γc from the membrane; these results stand in contrast to the data pertaining to IFNAR1 (25).
FIG. 4.
Membrane turnover of γc is independent of Jak3. EBV-transformed human B-cell lines from normal individuals (control) and Jak3-SCID patients were treated with either CHX (20 μg/ml) (A) or BFA (2 μg/ml) (B and C) for the time indicated, fixed, and stained with anti-human CD132-PE antibody to determine γc surface levels by flow cytometry. The experiment was done in triplicate for each time point. The MFI indicates the channel number in a linear scale, which corresponds to the MFIs obtained for a particular antibody. The ΔMFI was calculated for each sample by subtracting the geometric mean fluorescence of the corresponding isotype control from those relative to the CD132-specific antibody. Cells from patient 1 (in panels A and B) are homozygous for a missense mutation Y100→C (18); this mutant version of Jak3 is expressed but does not bind to γc (1, 36). Cells from patient 2 (C) are homozygous for a 151-bp deletion in the pseudokinase domain, leading to a frameshift and premature termination (18); this Jak3 variant is not expressed.
Since the effect of BFA is reversible, we used BFA washout experiments to examine whether the presence of Jak3 affected the reappearance of γc at the cell surface. As shown in Fig. 5A, the membrane expression of γc was reduced after BFA treatment for 1 h. The cells were then reincubated in medium without BFA, and γc membrane expression returned to normal levels after 2 to 3 h (Fig. 5A). Three independent experiments produced similar results. As is evident in Fig. 5A and B, the absence of Jak3 in EBV-transformed B cells from Jak3 SCID patients did not affect the rate of reappearance of γc on the cell surface.
FIG. 5.
Recovery of γc expression on the cell surface is independent of Jak3. EBV-transformed human B-cell lines from normal individuals (control) and Jak3-SCID patients (see Fig. 4) were treated with BFA for 1 h, washed three times with ice-cold phosphate-buffered saline-2% FBS, and recultured in complete RPMI at 37°C for the indicated time points. The reappearance of γc at the cell membrane was measured by flow cytometry (as described in Fig. 4).
We conclude from these experiments that, there is no strict chaperone function of Jak3 for γc. In overexpression systems Jak3 can influence γc's subcellular distribution. However, in lymphocytes under basal and perturbed conditions, γc expression and its rate of appearance and disappearance on the cell surface are all independent of Jak3. Conversely, however, it is very clear that Jak3's membrane localization is entirely dependent upon γc. This then afforded us an opportunity to carefully examine the requirements for Jak3/γc interaction and trafficking to the plasma membrane.
The Jak3 FERM domain is necessary but not sufficient for proper membrane distribution of Jak3; full-length Jak3 is required.
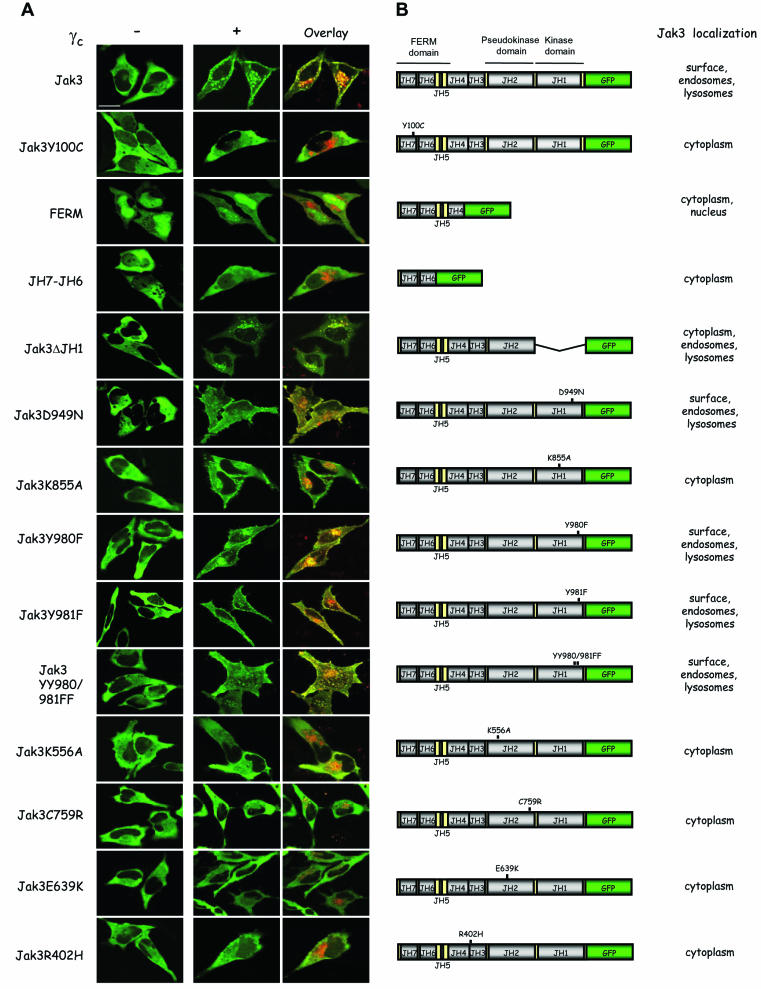
Work from multiple cytokine receptors, including γc, has argued for the importance of the Jak FERM domain in mediating receptor association (5, 7, 8, 36). A caveat with the coassociation studies is that many were done by using recombinant proteins or by coimmunoprecipitation of overexpressed proteins; these in vitro associations have not been carefully examined in live cells. To address this issue, we generated a series of Jak3-GFP fusion proteins to assess their ability to interact with γc and traffic to the plasma membrane. As shown in Fig. 6A (first row), full-length Jak3 colocalized with γc at the plasma membrane. In contrast, a variant of Jak3 bearing a SCID patient-derived mutation in the FERM domain (patient 1) failed to colocalize with γc (Fig. 6A, second row; Jak3Y100C). As a result, this mutant form of Jak3 was cytosolic, and γc accumulated in endosomal and lysosomal compartments, a finding consistent with the importance of the FERM domain in mediating receptor association. As shown in Fig. 6A, however, the isolated FERM domain (third row), as well as the JH7-JH6 domain (fourth row), did not support plasma membrane localization of Jak3. In fact, another mutant that included all but the kinase domain of Jak3 did not traffic properly either, although it colocalized with γc (Fig. 6A, fifth row; Jak3ΔJH1). Since the Jak3 mutant lacking the kinase domain did not traffic properly, we next considered the possibility that catalytic activity was required for this property. A Jak3 mutant in which the critical aspartate residue involved in the phosphotransferase reaction is mutated (Jak3D949N) promoted γc membrane expression (Fig. 6A, sixth row). In contrast, another mutation in the kinase domain, K855A, which affects the lysine residue that binds ATP-Mg, failed to traffic normally (Fig. 6A, seventh row; Jak3K855A). Thus, it appeared that seemingly minor Jak3 mutations had significant effects on the ability of Jak3 to localize with γc to the plasma membrane. Therefore, we next analyzed previously described tyrosine mutations within the putative activation loop of the Jak3 kinase domain (37). The activation loop residues Y980 and Y981 disparately regulate Jak3 kinase activity (37). However, none of the three mutants (Y980F, Y981F, and YY980/981FF) influenced normal trafficking and all promoted Jak3/γc membrane localization (Fig. 6A, eighth to tenth rows; Jak3Y980F, Jak3Y981F, and Jak3YY980/981FF).
FIG. 6.
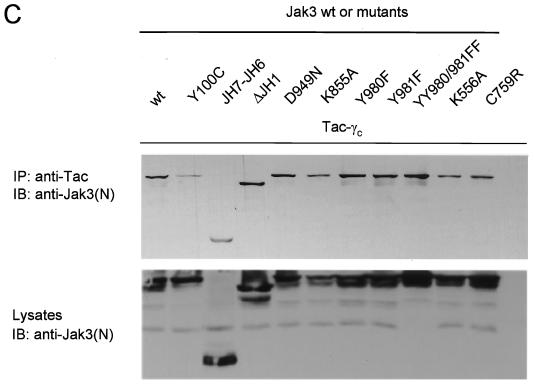
Strict structural requirements for proper Jak3 membrane localization but not coimmunoprecipitation with γc. (A) HeLa cells were transfected with the indicated Jak3 constructs with (+) or without (−) γc and then analyzed by confocal microscopy. The middle panels show the green channel; the right panels show a merge of the two signals after cotransfection of the indicated Jak3-GFP constructs with γc-HcRed. Scale bar, 20 μm. (B) Schematic presentation of the various constructs and a synopsis of the localization of the mutant Jaks. (C) COS-7 cells were transfected with Tac-γc and GFP fusion proteins of wild-type Jak3 or various mutants. Lysates were immunoprecipitated (IP) with anti-Tac and blotted with anti-Jak3(N), an anti-peptide Jak3 directed against the amino terminus (top panel). Expression of the different Jak3 constructs were analyzed by immunoblotting (IB) with anti-Jak3(N) (bottom panel).
Since some but not all Jak3 mutants influenced subcellular localization, we analyzed various other mutants. The pseudokinase domain has a site analogous to K855A, but whether it is functional or not has not been determined. As shown in Fig. 6A (eleventh row; Jak3K556A), this mutant failed to traffic properly. In addition, two other point mutations in the pseudokinase domain, a patient-derived mutation, C759R (2, 36) (Fig. 6A, twelfth row), and E639K, a mutation analogous to the gain-of-function Hopscotch mutation (17) (Fig. 6A, thirteenth row), failed to traffic normally. Jaks also have an SH2-like domain, but the ability of this domain to bind phosphotyrosine has not been established. A patient-derived mutation in this region, however, blocked the ability of Jak3 to promote γc membrane expression (Fig. 6A, bottom row; Jak3R402H). The localization of Jak3 and its mutants, cotransfected with γc, is summarized in Fig. 6B.
As a control, we also examined the coimmunoprecipitation of various Jak3 mutants with the cytoplasmic part of γc. As reported previously, the Y100C mutant associated poorly with γc compared to wild-type Jak3 (Fig. 6C, lane 2 versus lane 1) (1). Conversely, previous work has documented that the FERM domain is necessary and sufficient for receptor association as measured by coimmunoprecipitation. As is evident in Fig. 6C, the JH7-JH6 and ΔJH1 mutants (lanes 3 and 4) still bound γc, although they did not traffic properly within the cell (Fig. 6A). In addition, a number of Jak3 missense mutations all coprecipitated with γc as efficiently as the wild-type, and this in vitro association contrasts with their subcellular distribution.
DISCUSSION
Jaks are essential for cytokine receptor signaling; however, the extent to which they regulate receptor expression is less clear. In the present study we show that murine and human lymphocytes do not require Jak3 for expression of γc on the cell surface.
The EpoR, when overexpressed, is mostly retained in the ER, destined for degradation; only a small fraction exits the ER and is localized at the plasma membrane (21, 32). Coexpression of Jak2 enhances EpoR Golgi processing and surface expression. Similarly, the oncostatin M receptor, when overexpressed, also accumulates mainly in the ER (24). We did not find this to be the case for γc; it showed no coassociation with the Golgi complex or ER.
IFNAR1 and IL-10R2 also do not accumulate in the ER; rather, they accumulate in early endosomes and lysosomes (25). In this respect, γc behaves similarly. However, IFNAR1 is poorly expressed on the cell surface in the absence of Tyk2, which is thought to be important in regulating endocytosis of surface expressed receptor (25). γc, however, seems to be regulated differently. It localizes to the cell surface in the absence of Jak3, and its disappearance from or reappearance to the membrane is also not influenced by the kinase. Nonetheless, transfection of cells with Jak3 did upregulate γc membrane accumulation, a finding consistent with studies of the EpoR and IFNAR1. The basis for the similarities and differences in the behavior of cytokine receptors is unclear at the present time. Presumably, it will ultimately be possible to classify cytokine receptors based on their trafficking and Jak dependence. It will be interesting to understand the molecular mechanisms underlying these distinct behaviors. It is tempting to speculate that it will be possible to identify motifs within the cytosolic domains of the receptors that explain their trafficking.
Consistent with a previous study, the present study also shows that Jak3-deficient lymphocytes express higher levels of γc at the cell surface. One explanation of these results could be that the presence of Jak3 influences total γc protein levels rather than surface levels. However, as shown in Fig. 1C, the levels of γc protein were not altered by the presence or absence of Jak3. One might have concluded, therefore, that Jak3 negatively regulates its receptor. However, reconstitution experiments did not diminish receptor expression. On the contrary, Jak3 reconstitution in COS-7 cells or EBV-transformed B cells from Jak3 SCID patients enhanced γc surface levels even further, a finding consistent with studies of other cytokine receptors. A third possibility is that the cells have an activated phenotype. In fact, T cells from Jak3−/− mice do express activation markers (30, 31, 34), but B-cell lines from Jak3 SCID patients are heterogeneous and do not express activation markers; thus, this may not be the explanation. However, the enhanced receptor surface expression is likely a compensatory effect due to the lack of cytokine signaling, although it is notable that the total levels of γc are not increased. Thus, the exact mechanism underlying the enhanced membrane localization of γc remains obscure at this time.
Previous findings, as well as the present study, document that, like IL-2Rβ, γc is predominantly localized to endosomal and lysosomal compartments and has a short half-life on the plasma membrane. Given this distribution of γc, it is likely that the membrane expression of γc is highly regulated, both positively and negatively, by molecular chaperones. In view of the fact that the first 40 cytosolic amino acids of γc are reportedly sufficient for its internalization and degradation but not sufficient for Jak3 interaction, the major regulator would not be expected to be a Jak (20). The results provided by the present study, a finding consistent with the preceding data, firmly establish that Jak3 is not the major regulator of γc trafficking. Our data also show that overexpression of Jak3 can influence receptor expression, but whether there are physiologic or pathological settings in which this element of control is important remains to be determined; we could find no evidence of this at present.
The second major finding of the present study is that γc is critical for Jak3 plasma membrane localization, and the structural requirements of the association of kinase with γc are quite stringent. In the absence of γc, Jak3 was diffusely expressed in the cytosol with nuclear exclusion and apparently had no intrinsic ability to localize to the plasma membrane. Previous work has established that the Jak3 FERM domain is necessary and sufficient for receptor binding, as measured by the association of recombinant proteins (5, 36); this was confirmed in the experiment depicted in Fig. 6C. However, we found that the FERM domain was not sufficient to promote Jak3/γc localization at the plasma membrane in live cells. We therefore investigated in detail the structural and functional characteristics of the full-length Jak3 molecule that would allow for normal trafficking of Jak3/γc. Several Jak3 mutants, including the K855A and K556A mutants, failed to traffic normally. However, not all Jak3 mutations interfere with trafficking, i.e., a different kinase-deficient Jak3 mutant (D949N) was fully capable of promoting Jak3/γc membrane localization. Previously described tyrosine mutations in the putative activation loop of Jak3 (37) also did not impede the trafficking and were capable of facilitating the membrane localization of the receptor-kinase complex.
At present, there are more than 25 mutations described in Jak3-SCID patients, which may result in the reduction of Jak3 protein expression and loss of kinase activity (22). We tested some of these patient-derived mutations for intracellular trafficking and found that Jak3/γc interaction and trafficking were very sensitive to Jak3 mutations. All of the patient mutations abolished normal Jak3/γc membrane localization. It is apparent from these site-directed and naturally occurring Jak3 mutations that, although Jak3 is not an absolute requirement for γc localization to the plasma membrane, the converse is true: γc association is absolutely required for the ability of Jak3 to traffic to the plasma membrane. Jak structure clearly plays a critical role in the appropriate localization of the Jak3/γc complex, and aberrant trafficking is likely another contributor to the pathogenesis of SCID. Assessing the ability of Jak3 to properly localize with γc in living cells appears to be a very sensitive indicator of proper Jak structure.
In summary, the data in the present study indicate that, unlike other well-studied cytokine receptor-Jak pairs, Jak3 is not absolutely required for the plasma membrane expression of its cognate receptor γc, even though transfection of cells with Jak3 can promote γc membrane localization. Dissecting the molecular basis for the behaviors of the different cytokine receptors will clearly be an interesting area of future research. Conversely, γc is indispensable for Jak3 membrane localization. Moreover, the ability of Jak3 to properly traffic with γc to the cell surface requires the full-length Jak3 but does not depend on functional kinase activity. Nonetheless, multiple mutations in Jak3 do affect trafficking, indicating the complex interactions of Jak3 and γc. This is another area that clearly needs to be investigated more thoroughly, hopefully with more detailed structural studies. Understanding the molecular basis for the reciprocal regulation of γc and Jak3 will be important given the critical in vivo functions of both proteins and their clinical significance.
Acknowledgments
All imaging was done in the Light Imaging Section, National Institute of Arthritis and Musculoskeletal and Skin Diseases. We are grateful to Kristien J. M. Zaal and Evelyn Ralston for excellent technical assistance with confocal microscopy and helpful discussions. The cDNAs encoding the IL-2R subunits were kindly provided by Warren Leonard (NHLBI, NIH). We thank Juan Bonifacino (NICHD, NIH) for critical reading of the manuscript and helpful suggestions.
REFERENCES
- 1.Cacalano, N. A., T. S. Migone, F. Bazan, E. P. Hanson, M. Chen, F. Candotti, J. J. O'Shea, and J. A. Johnston. 1999. Autosomal SCID caused by a point mutation in the N terminus of Jak3: mapping of the Jak3-receptor interaction domain. EMBO J. 18:1549-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Candotti, F., S. A. Oakes, J. A. Johnston, S. Giliani, R. F. Schumacher, P. Mella, M. Fiorini, A. G. Ugazio, R. Badolato, L. D. Notarangelo, F. Bozzi, P. Macchi, D. Strina, P. Vezzoni, R. M. Blaese, J. J. O'Shea, and A. Villa. 1997. Structural and functional basis for JAK3-deficient severe combined immunodeficiency. Blood 90:3996-4003. [PubMed] [Google Scholar]
- 3.Cao, X., C. A. Kozak, Y. J. Liu, M. Noguchi, E. O'Connell, and W. J. Leonard. 1993. Characterization of cDNAs encoding the murine interleukin 2 receptor (IL-2R) gamma chain: chromosomal mapping and tissue specificity of IL-2R gamma chain expression. Proc. Natl. Acad. Sci. USA 90:8464-8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, M., A. Cheng, F. Candotti, Y. J. Zhou, A. Hymel, A. Fasth, L. D. Notarangelo, and J. J. O'Shea. 2000. Complex effects of naturally occurring mutations in the JAK3 pseudokinase domain: evidence for interactions between the kinase and pseudokinase domains. Mol. Cell. Biol. 20:947-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, M., A. Cheng, Y. Q. Chen, A. Hymel, E. P. Hanson, L. Kimmel, Y. Minami, T. Taniguchi, P. S. Changelian, and J. J. O'Shea. 1997. The amino terminus of JAK3 is necessary and sufficient for binding to the common gamma chain and confers the ability to transmit interleukin 2-mediated signals. Proc. Natl. Acad. Sci. USA 94:6910-6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gadina, M., D. Hilton, J. A. Johnston, A. Morinobu, A. Lighvani, Y. J. Zhou, R. Visconti, and J. J. O'Shea. 2001. Signaling by type I and II cytokine receptors: ten years after. Curr. Opin. Immunol. 13:363-373. [DOI] [PubMed] [Google Scholar]
- 7.Gauzzi, M. C., G. Barbieri, M. F. Richter, G. Uze, L. Ling, M. Fellous, and S. Pellegrini. 1997. The amino-terminal region of Tyk2 sustains the level of interferon alpha receptor 1, a component of the interferon alpha/beta receptor. Proc. Natl. Acad. Sci. USA 94:11839-11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haan, C., H. Is'harc, H. M. Hermanns, H. Schmitz-Van de Leur, I. M. Kerr, P. C. Heinrich, J. Grotzinger, and I. Behrmann. 2001. Mapping of a region within the N terminus of Jak1 involved in cytokine receptor interaction. J. Biol. Chem. 276:37451-37458. [DOI] [PubMed] [Google Scholar]
- 9.Hemar, A., A. Subtil, M. Lieb, E. Morelon, R. Hellio, and A. Dautry-Varsat. 1995. Endocytosis of interleukin 2 receptors in human T lymphocytes: distinct intracellular localization and fate of the receptor alpha, beta, and gamma chains. J. Cell Biol. 129:55-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang, L. J., S. N. Constantinescu, and H. F. Lodish. 2001. The N-terminal domain of Janus kinase 2 is required for Golgi processing and cell surface expression of erythropoietin receptor. Mol. Cell 8:1327-1338. [DOI] [PubMed] [Google Scholar]
- 11.Ihle, J. 2001. Pathways in cytokine regulation of hematopoiesis. Ann. N. Y. Acad. Sci. 938:129-130. [DOI] [PubMed] [Google Scholar]
- 12.Karaghiosoff, M., H. Neubauer, C. Lassnig, P. Kovarik, H. Schindler, H. Pircher, B. McCoy, C. Bogdan, T. Decker, G. Brem, K. Pfeffer, and M. Muller. 2000. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity 13:549-560. [DOI] [PubMed] [Google Scholar]
- 13.Kawamura, M., D. W. McVicar, J. A. Johnston, T. B. Blake, Y. Q. Chen, B. K. Lal, A. R. Lloyd, D. J. Kelvin, J. E. Staples, J. R. Ortaldo, et al. 1994. Molecular cloning of L-JAK, a Janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes. Proc. Natl. Acad. Sci. USA 91:6374-6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerr, I. M., A. P. Costa-Pereira, B. F. Lillemeier, and B. Strobl. 2003. Of JAKs, STATs, blind watchmakers, jeeps, and trains. FEBS Lett. 546:1-5. [DOI] [PubMed] [Google Scholar]
- 15.Leonard, W. J. 2001. Role of Jak kinases and STATs in cytokine signal transduction. Int. J. Hematol. 73:271-277. [DOI] [PubMed] [Google Scholar]
- 16.Lippincott-Schwartz, J., L. Yuan, C. Tipper, M. Amherdt, L. Orci, and R. D. Klausner. 1991. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell 67:601-616. [DOI] [PubMed] [Google Scholar]
- 17.Luo, H., P. Rose, D. Barber, W. P. Hanratty, S. Lee, T. M. Roberts, A. D. D'Andrea, and C. R. Dearolf. 1997. Mutation in the Jak kinase JH2 domain hyperactivates Drosophila and mammalian Jak-Stat pathways. Mol. Cell. Biol. 17:1562-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macchi, P., A. Villa, S. Giliani, M. G. Sacco, A. Frattini, F. Porta, A. G. Ugazio, J. A. Johnston, F. Candotti, J. J. O'Shea, et al. 1995. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature 377:65-68. [DOI] [PubMed] [Google Scholar]
- 19.Manning, G., D. B. Whyte, R. Martinez, T. Hunter, and S. Sudarsanam. 2002. The protein kinase complement of the human genome. Science 298:1912-1934. [DOI] [PubMed] [Google Scholar]
- 20.Morelon, E., and A. Dautry-Varsat. 1998. Endocytosis of the common cytokine receptor gamma chain. Identification of sequences involved in internalization and degradation. J. Biol. Chem. 273:22044-22051. [DOI] [PubMed] [Google Scholar]
- 21.Neumann, D., M. H. Yuk, H. F. Lodish, and G. Z. Lederkremer. 1996. Blocking intracellular degradation of the erythropoietin and asialoglycoprotein receptors by calpain inhibitors does not result in the same increase in the levels of their membrane and secreted forms. Biochem. J. 313(Pt. 2):391-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Notarangelo, L. D., P. Mella, A. Jones, G. de Saint Basile, G. Savoldi, T. Cranston, M. Vihinen, and R. F. Schumacher. 2001. Mutations in severe combined immune deficiency (SCID) due to JAK3 deficiency. Hum. Mutat. 18:255-263. [DOI] [PubMed] [Google Scholar]
- 23.O'Shea, J. J., M. Gadina, and R. D. Schreiber. 2002. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell 109(Suppl.):S121-S131. [DOI] [PubMed] [Google Scholar]
- 24.Radtke, S., H. M. Hermanns, C. Haan, H. Schmitz-Van De Leur, H. Gascan, P. C. Heinrich, and I. Behrmann. 2002. Novel role for Janus kinase 1 in the regulation of oncostatin M receptor surface expression. J. Biol. Chem. 277:11237-11305.. [DOI] [PubMed] [Google Scholar]
- 25.Ragimbeau, J., E. Dondi, A. Alcover, P. Eid, G. Uze, and S. Pellegrini. 2003. The tyrosine kinase Tyk2 controls IFNAR1 cell surface expression. EMBO J. 22:537-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richter, M. F., G. Dumenil, G. Uze, M. Fellous, and S. Pellegrini. 1998. Specific contribution of Tyk2 JH regions to the binding and the expression of the interferon alpha/beta receptor component IFNAR1. J. Biol. Chem. 273:24723-24729. [DOI] [PubMed] [Google Scholar]
- 27.Russell, S. M., J. A. Johnston, M. Noguchi, M. Kawamura, C. M. Bacon, M. Friedmann, M. Berg, D. W. McVicar, B. A. Witthuhn, O. Silvennoinen, et al. 1994. Interaction of IL-2Rβ and γc chains with Jak1 and Jak3: implications for XSCID and XCID. Science 266:1042-1045. [DOI] [PubMed] [Google Scholar]
- 28.Russell, S. M., N. Tayebi, H. Nakajima, M. C. Riedy, J. L. Roberts, M. J. Aman, T. S. Migone, M. Noguchi, M. L. Markert, R. H. Buckley, et al. 1995. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science 270:797-800. [DOI] [PubMed] [Google Scholar]
- 29.Saharinen, P., K. Takaluoma, and O. Silvennoinen. 2000. Regulation of the Jak2 tyrosine kinase by its pseudokinase domain. Mol. Cell. Biol. 20:3387-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saijo, K., S. Y. Park, Y. Ishida, H. Arase, and T. Saito. 1997. Crucial role of Jak3 in negative selection of self-reactive T cells. J. Exp. Med. 185:351-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sohn, S. J., K. A. Forbush, N. Nguyen, B. Witthuhn, T. Nosaka, J. N. Ihle, and R. M. Perlmutter. 1998. Requirement for Jak3 in mature T cells: its role in regulation of T-cell homeostasis. J. Immunol. 160:2130-2138. [PubMed] [Google Scholar]
- 32.Supino-Rosin, L., A. Yoshimura, H. Altaratz, and D. Neumann. 1999. A cytosolic domain of the erythropoietin receptor contributes to endoplasmic reticulum-associated degradation. Eur. J. Biochem. 263:410-419. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki, K., H. Nakajima, Y. Saito, T. Saito, W. J. Leonard, and I. Iwamoto. 2000. Janus kinase 3 (Jak3) is essential for common cytokine receptor gamma chain (γc)-dependent signaling: comparative analysis of γc, Jak3, and γc and Jak3 double-deficient mice. Int. Immunol. 12:123-132. [DOI] [PubMed] [Google Scholar]
- 34.Thomis, D. C., and L. J. Berg. 1997. Peripheral expression of Jak3 is required to maintain T lymphocyte function. J. Exp. Med. 185:197-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velazquez, L., K. E. Mogensen, G. Barbieri, M. Fellous, G. Uze, and S. Pellegrini. 1995. Distinct domains of the protein tyrosine kinase tyk2 required for binding of interferon-alpha/beta and for signal transduction. J. Biol. Chem. 270:3327-3334. [DOI] [PubMed] [Google Scholar]
- 36.Zhou, Y. J., M. Chen, N. A. Cusack, L. H. Kimmel, K. S. Magnuson, J. G. Boyd, W. Lin, J. L. Roberts, A. Lengi, R. H. Buckley, R. L. Geahlen, F. Candotti, M. Gadina, P. S. Changelian, and J. J. O'Shea. 2001. Unexpected effects of FERM domain mutations on catalytic activity of Jak3: structural implication for Janus kinases. Mol. Cell 8:959-969. [DOI] [PubMed] [Google Scholar]
- 37.Zhou, Y. J., E. P. Hanson, Y. Q. Chen, K. Magnuson, M. Chen, P. G. Swann, R. L. Wange, P. S. Changelian, and J. J. O'Shea. 1997. Distinct tyrosine phosphorylation sites in JAK3 kinase domain positively and negatively regulate its enzymatic activity. Proc. Natl. Acad. Sci. USA 94:13850-13855. [DOI] [PMC free article] [PubMed] [Google Scholar]