Abstract
HDL-replacement therapy is a promising new treatment strategy involving the acute administration of HDL to rapidly stabilize patients at imminent risk for developing a myocardial infarction, such as those with acute coronary syndrome. This review will first focus on the anti-atherogenic mechanisms for HDL, such as the stimulation of the reverse cholesterol transport pathway, and then discuss the other potential beneficial biological effects of HDL on atherosclerosis. The various types of HDL-replacement therapies that are being investigated and developed will be reviewed and ongoing clinical trials and other possible clinical indications for HDL-replacement therapy besides the prevention of myocardial infarction will also be described. Finally, HDL-replacement therapy will be put into perspective by summarizing the current gaps in our knowledge of HDL metabolism and identifying challenges for future research in this area.
Keywords: apolipoprotein A–I, atherosclerosis, cholesterol, coronary heart disease, HDL, lipoprotein, therapy
It has been over 50 years since the respective pro- and anti-atherogenic effects of LDL and HDL were first described [1], but the main focus has been on developing agents for decreasing LDL-cholesterol (LDL-C). There is now great interest in identifying new agents for raising HDL or for improving its function, with the expectation that a combined approach of simultaneously lowering LDL and raising HDL will be more effective in reducing cardiovascular events than only lowering LDL, which in most trials reduces clinical events by only approximately 30% [2–5]. Currently, the most effective drug for increasing HDL is niacin but its use has been limited because of side effects, although newer slower release formulations of niacin coupled with selective prostaglandin D2 receptor antagonists may ameloriate this problem [6]. Cholesteryl ester transfer protein inhibitors are also an effective way to elevate HDL but the recent setbacks of the late-stage clinical trials of torcetrapib, perhaps due to unanticipated off-target effects, has delayed the development of this class of drug [7–9].
Besides creating small-molecule-type drugs, a new treatment strategy has been described for acutely raising HDL that involves the infusion of synthetic or reconstituted HDL or HDL mimetics [10–12]. This treatment approach can be viewed as a replacement-type therapy because, as opposed to the chronic use of a drug to increase endogenous levels of HDL, HDL or HDL mimetics from an exogenous source are administered to a patient. The rationale behind this therapy is that it has been observed that HDL infusion can have a surprisingly rapid effect in reducing plaque volume in both animal models and humans, and thus it could quickly stabilize patients with acute coronary syndrome, who are at great risk of myocardial infarction [13,14]. In this review, we will first discuss the anti-atherogenic mechanisms of HDL. Next, we will review the different types of HDL-replacement agents that are currently being investigated, as well as their possible clinical indications besides the prevention of myocardial infarction.
HDL protective mechanisms
Reverse cholesterol transport
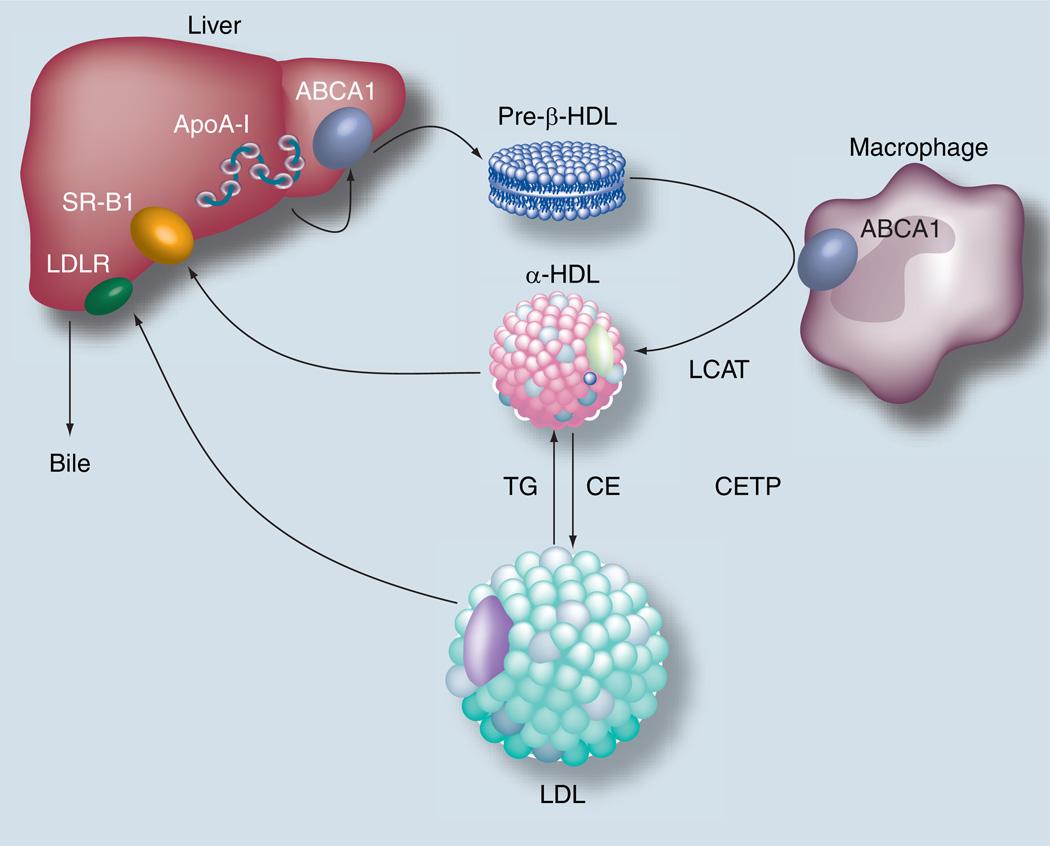
It has long been considered that the main atheroprotective mechanism of HDL is related to its ability to facilitate the reverse cholesterol transport pathway (Figure 1), the pathway by which excess cholesterol from peripheral cells, such as macrophages, in the vessel wall is transported to the liver for excretion [15]. The first step in this pathway begins with the biogenesis of HDL, which occurs mostly in the liver [16] and also in the intestine [17]. Phospholipids and most likely cholesterol are added to ApoA-I, the main protein constituent of HDL, by the ATP-binding cassette (ABCA1) transporter [18]. ApoA-I is secreted from the liver and intestine in a relatively lipid-poor state and acquires additional lipid in the extracellular space by the ABCA1 transporter, forming nascent discoidal type structures, which have a pre-β-type migration on agarose gels [19]. These nascent HDL particles can then acquire additional cholesterol and phospholipid after interaction with the ABCA1 transporter on peripheral cells. Macrophages in particular are heavily dependent upon this pathway because in the absence of a functional ABCA1 transporter, as occurs in Tangier disease, there is a marked accumulation of cholesteryl esters in macrophages. Other transporters, in particular ABCG1 [20] and scavenger receptor class B type I (SR-BI) [21], may also participate in the efflux of excess cholesterol from cells. In addition, there is a spontaneous bidirectional exchange of cholesterol between cell membranes and extracellular lipoproteins [15]. These alternative pathways differ from ABCA1 in that they largely promote cholesterol efflux to mature lipid rich forms of HDL, which are spherical in shape and migrate in the α-position during electrophoresis. There appears to be synergy and coordinate regulation between these different cholesterol efflux pathways, at least for ABCA1 and ABCG1 [22,23]. Knocking out both genes in mice results in a much more profound increase in macrophage cholesteryl ester accumulation than knocking out just one of the genes.
Figure 1. Reverse cholesterol transport pathway.
ApoA-I acquires lipid in the liver and intestine to form nascent discoidal shaped HDL. This form of HDL can then efflux cholesterol from peripheral cells and return it back to the liver for excretion into the bile. This can occur either by hepatic uptake by SR-BI or by uptake of LDL after cholesteryl esters are transferred to LDL from HDL by CETP.
ABCA1: ATP-binding cassette transporter A–I; ApoA-I: Apolipoprotein A–I; CE: Cholesteryl esters; CETP: Cholesteryl ester transfer protein; LCAT: Lecithin:cholesterol acyltransferase; LDLR: LDL receptor; SR-BI: Scavenger receptor B–I; TG: Trigylcerides.
Lecithin:cholesterol acyltransferase (LCAT) is a serum enzyme produced mostly by the liver (Figure 1). It is believed to play a critical role in the reverse cholesterol transport pathway by converting cholesterol to cholesteryl esters [24], which partition into the core of HDL and convert discoidal shaped HDL to the more mature spherical shaped HDL, the predominant form found in serum [25]. The esterification of cholesterol, which increases its hydrophobicity, also prevents the spontaneous back exchange of cholesterol from HDL to cells, thus facilitating its net removal from cells.
The next step of this pathway involves the delivery of cholesterol to the liver (Figure 1). This has been proposed to occur by a direct route via the SR-BI receptor, which promotes the selective uptake of cholesteryl esters from HDL [26]. In addition, at least in rabbits, approximately half of cholesterol on HDL is returned indirectly to the liver by the hepatic uptake of LDL, after cholesteryl esters from HDL are transferred to LDL and other apoB-containing lipoproteins in exchange for triglyceride by the cholesteryl ester transfer protein [27]. Hepatic cholesterol can then be directly excreted into the bile or excreted after first being converted to a bile salt, which is largely regulated by 7-α hydroxylase [28].
Pleiotropic anti-atherogenic effects of HDL
In addition to promoting reverse cholesterol transport, there have been many reports of other salutary effects of HDL in reducing atherosclerosis, which are listed in Box 1. Almost every step in the pathogenesis of atherosclerosis appears to be favorably affected by HDL, which may account for its potent effect in preventing atherosclerosis. In the plasma compartment, HDL has been shown to prevent endothelial dysfunction. For example, it inhibits the expression of adhesion proteins by endothelial cells [29], which mediate the initial attachment and infiltration of monocytes into early plaques. HDL also has favorable effects on the vasomotor tone of vessels, by promoting the NO production of endothelial cells [30], which increases vasodilation and suppresses smooth muscle cell proliferation in plaques. HDL reduces platelet activation [31] and promotes fibrinolysis [32], and thus may inhibit the formation of a thrombus over ruptured plaques. It may also reduce intraplaque hemorrhage and plaque progression. Inside the vascular wall, HDL appears to have anti-inflammatory effects by reducing proinflammatory cytokine and chemokine production [33–36]. It also transports several anti-oxidants and enzymes that can break down and neutralize lipid hydroperoxides [37]. Furthermore, it can sequester oxidized lipids and thus may reduce the formation and cell damage from oxidized LDL.
Box 1. Pleiotropic effects of HDL.
Recent findings have also established a connection between HDL and other disorders associated with cardiovascular disease, such as metabolic syndrome and diabetes. It was discovered that apoA-I activates AMP-activated protein kinase (AMPK), and thus stimulates glucose uptake in myocytes [38]. Consistent with this finding, apoA-I knockout (K/O) mice were found to be insulin resistant and have increased fat content. It was recently demonstrated that mice with selective impairment of ABCA1 in β-cells have impaired glucose tolerance and defective insulin secretion [39]. ABCA1 is a binding partner of apoA-I and the mechanism connecting ABCA1 deficiency and β-cell impairment was found to be accumulation of cholesterol in β-cells due to its defective removal. Furthermore, HDL in diabetic patients has been shown to be altered in some way so that it is dysfunctional. For example, HDL is enriched in triglyceride in diabetic patients [40], leading to HDL that is less effective in counteracting the inhibition of endothelium-dependent vasorelaxation induced by oxidized LDL [41]. HDL from diabetic patients also has decreased ability to metabolize oxidized palmitoyl-arachido-nylphosphatidy l-choline, a major product of LDL oxidation and a paraoxonase-1 substrate, and is impaired in ABCA-I-mediated cholesterol cellular efflux [42]. Infusion of reconstituted HDL in diabetic patients was shown to restore endothelial function by increasing NO production [43].
It is perhaps surprising that there is such a multitude of mechanisms for the atheroprotective effect of HDL, particularly for those processes that are seemingly unrelated to cholesterol metabolism. As has been previously proposed, this may be due to a dual role of lipoproteins in innate immunity [44–46]. It is also important to note that HDL is a complex and heterogeneous collection of lipoproteins, with multiple subfractions [47,48]. It also contains over 40 different proteins, each of which could potentially alter cell function [49]. HDL also transports many different types of lipids, some of which are delivered to cells and are known to be potent bioactive molecules, such as sphinosine-1-phosphate [50]. Furthermore, the ability of HDL to remove excess cholesterol from cells can lead to many changes in cell function and gene expression. This is not unexpected given the abundance of cholesterol in cells and its importance in the structure and function of membrane proteins. For example, removal of excess cholesterol from cells facilitates signaling by the TGF-β receptor, which reduces cell proliferation in plaques [51,52]. Other recent studies have shown that reducing the cholesterol content of macrophages prevents their apoptosis and results in numerous gene expression changes, some of which may lead to the migration of macrophages out of the vessel wall [53]. The reduction of cholesterol in plaques from HDL may, therefore, not only be from cholesterol efflux from cells in the plaque but also from the egress of cholesterol-loaded cells from the plaque and perhaps may also be due to a reduction in the infiltration and retention of lipoproteins in the vessel wall. In addition to cholesterol, HDL can also promote the efflux of oxysterols from cells that are proinflammatory and proapoptotic [54]. Thus, some of the pleiotropic effects of HDL may still be related to its ability to efflux lipid from cells. However, the relative in vivo importance of all these potential beneficial effects of HDL in reducing atherosclerosis is not known and remains a critical unresolved question in HDL research.
HDL-replacement agents
A list of the possible HDL-replacement agents, which are at various stages of development, is shown in Table 1. Depending on the source material they can be classified as either synthetic or as autologous/homologous HDL isolated from patients. Based on the components of HDL, the synthetic forms of HDL-replacement agents can be further subclassified into apolipoproteins, phospholipids and enzymes.
Table 1.
HDL-replacement agents.
| Type | Agent | Ref. |
|---|---|---|
| Synthetic/purified | ||
| ApoA-I | ApoA-I Milano (ETC-216) | [64] |
| Wild-type ApoA-I (CSL-111) | [78] | |
| Trimeric ApoA-I | [79] | |
| Apo A–I mimetic peptides | D-4F | [85–88] |
| 5A | [92,94] | |
| Enzymes | LCAT | [97] |
| Phospholipids | PC-LUVs | [99,104] |
| Oral PC | [105] | |
| Autologouslhomologous | ||
| Cholesterol extraction treatment | [106] | |
| Phospholipid enrichment treatment | [107] | |
LCAT: Lecithin:cholesterol acyltransferase; LUV: Large unilamellar vesicles; PC: Phosphatidylcholine.
ApoA-I
Apolipoprotein A–I is by far the most common protein component of HDL and thus is a natural choice in developing a therapeutic HDL-replacement agent. As discussed previously, apoA-I is known to play a central role in the ability of HDL to extract excess cholesterol from peripheral cells and return it to the liver for excretion. The first studies involving the repeated infusion of HDL were conducted in cholesterol-fed rabbits [55]. In these pioneering studies, it was found that the intravenous administration of homologous rabbit HDL into cholesterol-fed rabbits blocked the progression of atherosclerotic plaques and could also promote plaque regression. Many subsequent studies of purified apoA-I reconstituted with phospholipids have confirmed these findings in various animal models of atherosclerosis, and they also revealed that even a single infusion of HDL can have a significant favorable impact on plaque composition and volume [56]. As discussed previously, the rapid effect of HDL on plaques is probably not only a consequence of stimulating cholesterol efflux, but also the ability of HDL to decrease inflammation and to mediate the other pleiotropic effects of HDL, which are preserved with reconstituted HDL. In human studies, infusion of reconstituted HDL has been shown to raise HDL-cholesterol (HDL-C) [57], to increase fecal sterol excretion [58] and to counteract endothelial dysfunction [59]. By contrast, infusion of lipid-free apoA-I increased the phospholipid content on HDL but did not substantially increase HDL-C [60]. These early studies led to the idea of using apoA-I reconstituted with phospholipid as a therapeutic agent.
ApoA-IMilano, which was the first apoA-I mutation described [61], contains an Arg to Cys mutation at residue 173 and is associated with low HDL. This change in the protein enables it to form homodimers and heterodimers with other proteins, such as apoA-II [62]. This also changes the physical and biological properties of the protein and may alter its cholesterol efflux properties [63,64]. The free sulfhydryl of ApoA-IMilano may also increase its antioxidant effect [65,66]. These potential beneficial features of ApoA-IMilano were examined because it was found that, despite the fact that heterozygous carriers for this mutation have low HDL, they appeared to have increased longevity and less carotid intimal thickness when compared with a cohort with similarly low levels of HDL [67,68]. All of these findings generated interest in the use of ApoA-IMilano as a therapeutic agent (ETC-216), which is a recombinant form of this protein produced in Escherichia coli and complexed with phosphatidylcholine [69]. ETC-216 was first produced and investigated by Esperion Therapeutics and later further developed by Pfizer.
In the first, and so far only, clinical study of the effect of ETC-216 in humans, atherosclerosis was assessed by intravascular ultrasound on patients with acute coronary syndrome. In this trial, 57 patients were given weekly infusions of ETC-216 at 15 and 45 mg/kg or placebo for 5 weeks and were assessed by intravascular ultrasound at baseline and after the 5-week treatment period. The average decrease in plaque volume for the ETC-216 treatment group was 4.2% compared with baseline, whereas there was a slight increase in plaque volume of 0.14% in the placebo group, which was statistically significantly different from the treatment group. Other secondary measures, such as absolute change in plaque volume and maximum atheroma thickness, also showed a favorable statistically significant improvement. Based on the analysis of the position of the external elastic membrane, atheroma volume in the most diseased segments was reduced by 10.9% on average after treatment with ETC-216 [70]. However, the reduction in plaque volume was less than that observed in some animal studies, which may be a consequence of differences in plaque composition between animals and humans, such as the relative greater abundance of extracellular lipid in human plaques. Nevertheless, the observed change in plaque volume after only five treatments with ETC-216 is more than the change seen in plaque volume with the use of statins for several years [71,72]. Although not determined in this study, treatment with ETC-216 may have also changed plaque composition, as has been observed in animal studies [73], which may translate into a greater effect in reducing clinical events than what was observed for the reduction in plaque volume. A recent intravascular ultrasound study of rabbit coronary vessels has revealed that perhaps higher doses of ETC-216 may be even more effective [74]. At a low dose of 5 or 10 mg/kg, ETC-216 inhibited plaque progression, but it was not until at least the 20 mg/kg dose that plaque regression was observed and even greater regression was observed at the highest dose of 150 mg/kg. Since ETC-216 was well tolerated and showed promising efficacy in decreasing plaque size even in the first small clinical study, future clinical trials of ETC-216 at higher doses are warranted.
Commonwealth Serum Laboratories (CSL), an Australian company that specializes in the preparation and purification of blood products, is developing wild-type apoA-I as a therapeutic product. ApoA-I is purified from human plasma and is also reconstituted with soybean phosphatidylcholine at a 1:150 molar ratio, using a cholate dialysis method and is referred to as CSL-111. Although ApoA-IMilano may have some increased atheroprotective properties compared with wild-type apoA-I [75], this has not been firmly established, and it is clear from numerous epidemiologic and animal studies that wild-type apoA-I is also atheroprotective. In addition, given the relatively large amount of recombinant ApoA-IMilano that was used in the initial clinical trials of ETC-216 (15 and 45 mg/kg), purification of wild-type apoA-I from plasma may be a more economical source of material. Several preclinical studies examining the effect of CSL-111 on cholesterol efflux and on endothelial function have shown that it behaves similarly to reconstituted HDL prepared with ApoA-IMilano [76,77]. Similar to the clinical study conducted with ETC-216, the effect of treatment with CSL-111 on patients with acute coronary syndrome by intravascular ultrasound was studied in a trial called ERASE [78]. However, the study was significantly larger (n = 183) and involved only four treatments at either 40 or 80 mg/kg. The overall reduction in plaque volume, which was the primary end point in this study, was similar (3.4%) to observations in the ETC-216 trial (4.2%), but it did not reach statistical significance when compared with the placebo group. However, the reduction in plaque volume did show a statistically significant decrease when compared with the individual baseline for each patient and other secondary end points, such as plaque characterization index and coronary score, also showed a statistically significant improvement after treatment with CSL-111. Treatment with CSL-111, however, was associated with transient elevation in serum transaminases, particularly at higher doses, which was not observed with ETC-216. This has been proposed to be due to a large surge of cholesterol acutely delivered to the liver by the administered HDL, but it could have also been due to the residual cholate that was used in the phospholipid reconstitution step for preparing CSL-111 but not for ETC-216. Owing to differences in the treatment regimen and baseline patient characteristics of the ERASE trial and the ETC-216 study, it is not clear whether there is a difference in the efficacy of the two forms of apoA-I. Future larger studies based on clinical end points, such as myocardial infarction, are necessary to establish the utility of either agent.
Another HDL-replacement product based on apoA-I, which is being developed Roche, is trimeric apoA-I [79]. As its name implies, trimeric apoA-I has three copies of apoA-I per complex and is produced in E. coli. This is achieved by attaching the trimerization domain of the human tetranectin sequence to the amino terminal end of apoA-I, which allows the modified apoA-I to trimerize. The rationale behind the design of this protein is that this will increase the size of the complex and reduce its clearance by renal filtration [80,81]. ApoA-I is only 28 kDa in size and is below the limit for glomerular filtration. When bound to HDL, however, apoA-I is not filtered but when HDL undergoes remodeling by the various lipoprotein modifying enzmes, apoA-I can dissociate from HDL and be removed by the kidney. In cell-culture studies, trimeric apoA-I has been demonstrated to promote lipid efflux by the ABCA1 transporter, and in fact, it may be superior to wild-type apoA-I. The administration of trimeric apoA-I, reconstituted with phospholipid, to cholesterol-fed LDLR K/O mice did not change total lesion area but did reduce the severity of the lesions, as determined by histologic analysis. Treatment with trimeric apoA-I also did not have a significant effect on plasma lipids. No clinical trials of trimeric apoA-I have been reported to date.
ApoA-I mimetic peptides
A potential major limitation of HDL-replacement therapy with apoA-I is the relatively large amount of protein that has to be given to achieve a clinical effect. Most recombinant or purified therapeutic proteins are potent biological molecules, such as hormones, enzymes or cytokines, which are active when administered in nanogram to milligram quantities. In the clinical trials of reconstituted ApoA-IMilano or wild-type apoA-I, as much as 5 g of the protein was used per dose and multiple treatments were given. Creating such large quantities of recombinant or purified protein suitable for injection into humans is a challenge. In addition, the reconstitution of apoA-I with phospholipids creates another level of complexity, particularly because multiple forms of apoA-I-phospholipid complexes are typically generated during this process and the biological properties and therapeutic benefit of these different forms of reconstituted HDL are not understood. Another problem is the intravenous route of administration needed for reconstituted apoA-I, which makes it impractical for it to be used as a long-term treatment. These problems have stimulated interest in the use of synthetic amphipathic peptides that are relatively short and inexpensive to produce and can potentially be delivered orally. In addition, by the use of structure-function studies it should be possible to optimize the biological features of such peptides to mimic and maybe even improve upon the biological properties of apoA-I.
One of the first apoA-I mimetic peptides designed was the 18A peptide [82]. It was initially produced not as a therapeutic agent but to understand the structure of amphipathic helices, which is the main structural motif on apolipoproteins [83]. Amphipathic helices are simply α-helical protein segments with one face of the helix containing hydrophobic amino acids, whereas the other side contains more polar or charged amino acids. This enables apolipoproteins to bind to lipids and to stabilize the structure of lipoproteins. Short peptides, containing amphipathic helices, have also been shown in a nonstereoselective manner to promote the efflux of cholesterol from cells by the ABCA1 transporter [84], which as already discussed, is believed to be one of the main anti-atherogenic mechanisms of HDL. Most of the work in this area has been carried out with the D-4F peptide, so-called because it is composed of d-amino acids, enabling it to be orally available, since the d-amino acids make the peptide resistant to proteolysis in the gut. It is otherwise similar to the 18A helix, except for the fact that four residues on the hydrophobic face of this helix were changed to phenylalanine (F), which is more hydrophobic than the original residues. In an early study of this peptide, it was shown that the inclusion of D-4F in the water of apoE-K/O mice protected them against atherosclerosis [85]. This occurred despite the fact that the oral bioavailability of the peptide is relatively low and the concentration of the peptide in the plasma compartment was considerably lower than apoA-I. It does, however, promote the remodeling of endogenous HDL and the formation of pre-β HDL [86]. In addition, D-4F appears to have potent anti-inflammatory properties by the sequestration of oxidized lipids, which may be the main mechanism by which it confers protection against atherosclerosis [87].
A Phase I clinical trial of D-4F was reported recently [88]. In this study, 50 subjects at high risk of, or presenting with, coronary heart disease received a single oral dose of D-4F, ranging from 30 to 500 mg/day. The oral bioavailability of the peptide was low: less than 1% was absorbed and peak plasma levels only reached between 1 and 16 ng/ml. D-4F was well tolerated, with no major adverse events attributed to the peptide, and there were no signs of toxicity based on clinical laboratory testing. Consistent with observations from animal studies [89], oral administration of the peptide had no appreciable effect on the level of the various lipoproteins, but at the highest doses it did improve the anti-inflammatory property of HDL. This was measured by determining the ability of fast protein liquid chromatography-purified HDL from patients to inhibit LDL-induced monocyte chemotactic activity in cultures of human aortic endothelial cells. Despite these promising preclinical studies and the apparent safety of D-4F, the l-stereoisomer of this peptide, referred to as L-4F, is now being developed as an intravenous agent by Novartis.
ApoA-I mimetic peptides, which have been reviewed recently [90], are currently an active area of investigation by both research labs and several large pharmaceutical companies and it is likely that other peptides will shortly undergo evaluation in clinical trials. Given the multitude of possible beneficial effects of HDL on atherosclerosis, an important unresolved issue in this area is the best rationale for the design of such peptides. The majority of the new peptides currently being investigated appear to be based on optimizing their ability to efflux cholesterol from cells [91–93].
The presence of an amphipathic helix appears to be a key motif that is necessary for these peptides to promote cholesterol efflux, but peptides with too high a lipid affinity may act nonspecifically as detergents and can be cytotoxic. The undesirable features of these peptides are limited in the 5A peptide, which is a bihelical amphipathic peptide that pairs one high lipid-affinity helix with a low lipid-affinity helix, and as apoA-I only, promotes cholesterol efflux by the ABCA1 transporter [92]. The 5A peptide has been shown to increase reverse cholesterol transport in rats and to reduce atherosclerosis in apoE K/O mice [94]. Other apoA-I mimetic peptides that have been designed specifically to either activate LCAT [201], to act as antioxidants [95] or to increase the hepatic uptake of lipoproteins have also been described [96].
Lecithin cholesterol acyltransferase
In addition to apolipoproteins, HDL has several associated enzymes that play a key role in lipoprotein metabolism. One such enzyme is LCAT, which is also associated with other lipoproteins but, as has already been discussed, plays a major role in HDL metabolism. By the esterification of cholesterol, LCAT promotes the unidirectional flux of cholesterol from cells to HDL and the cholesteryl esters formed by LCAT are ultimately delivered to the liver for excretion. In addition, in the absence of LCAT, HDL can not fully mature and there is a build up of pre-β HDL, which is presumably hypercatabolized, leading to an overall decrease in HDL. In rabbits [97] and in cholesteryl ester transfer protein-transgenic mice [98], the overexpression of LCAT has been shown to be atheroprotective. No clinical trials have been reported but AlphaCore Pharma is investigating recombinant LCAT as a possible therapeutic agent for enhancing reverse cholesterol transport. As there appears to be synergy between LCAT and apoA-I in terms of HDL levels, the coadministration of LCAT along with apoA-I or an apoA-I mimetic peptide may be a very effective approach for reducing atherosclerosis.
Phospholipids
The other main component of HDL besides proteins is, of course, lipid and lipid-based HDL-replacement therapies have also been described. In fact, the use of lipids, in particular phospholipids, as therapeutic agents for reducing atherosclerosis precedes the use of apoA-I as a therapeutic agent [99]. The rationale behind this strategy is that in vitro phospholipid vesicles, in the absence of any apolipoprotein, can promote the efflux of cholesterol from cells [100]. This probably occurs by a passive aqueous diffusion process, whereby any cholesterol that spontaneously desorbs from the cell surface can then bind to any extracellular acceptor capable of binding cholesterol, such as albumin, cyclodextrin or phospholipid vesicles [15]. Unlike apoA-I or apoA-I mimetic peptides, phospholipid vesicles do not specifically promote cholesterol efflux by the ABCA1 transporter, which is upregulated in cholesterol-loaded macrophages. Recently, the ABCG1 transporter (which can efflux cholesterol to phospholipid vehicles) was also found to be upregulated in cholesterol-loaded macrophages. However, it may be unnecessary to specifically promote cholesterol efflux from cells in plaques and then to deliver it to the liver for excretion in order to reduce atherosclerosis. Recent cholesterol balance studies in various animal models of genes involved in HDL metabolism have questioned the proposed tight linkage between HDL levels and the net cholesterol excretion in stool [101]. It may be that the initial efflux step of cholesterol from peripheral cells is more relevant to the atheroprotective ability of HDL than its ability for promoting hepatic cholesterol excretion into the stool. Since the cholesterol content of plaques is relatively small compared with total body tissues pools, any cholesterol removed from plaques by phospholipid vesicles would be expected to re-equilibrate with the much larger tissue pools, thus reducing total cholesterol levels in plaques. Another mechanism by which phospholipid vesicles may be atheroprotective is that they may alter endogenous lipoproteins and/or promote the shuttling of cholesterol between HDL and liposomes, which depending on their size, may be too large to enter the vessel wall. In fact, it has been shown previously that the introduction of dimyristyl phosphatidylchline vesicles into plasma can significantly alter HDL to create pre-β-like HDL particles, which then can promote lipid efflux by the ABCA1 transporter [102].
Intravenous infusion of a large quantity of phospholipid (300 mg/kg) every other day for a 20-day period has been shown to reduce atherosclerotic lesion area in rabbits and to reduce the cholesterol content of the vessel wall by nearly 50% [103]. In these studies, large unilamellar vesicles of phosphatidylcholine were used, which may help to stabilize them from clearance and/or degradation in the plasma compartment in the absence of any associated protein. One concern with this approach is that although infused phospholipid vesicles are cleared by the liver, they are primarily removed by Kupfer cells [104] and uptake by other macrophages in the vessel wall could potentially accelerate atherosclerosis. Phospholipid large unilamellar vesicles (LUVs) were being investigated by Esperion Therapeutics and then later by Pfizer after it was acquired, but presently it does not appear to be actively being developed as a therapeutic agent.
Large amounts of oral soy phospholipids have also been shown to significantly increase HDL in clinical trials performed by Liponex Inc. The mechanism is not completely known but most likely linoleic acid released from the ingested phospholipids stimulates the transcription factor PPAR-γ [105]; thus, the mechanism for oral phospholipids is unrelated to what has been proposed for phospholipid vesicle infusion [99].
Autologous/homologous HDL
In addition to using largely synthetic components of HDL as therapeutic agents, a strategy has been described by Lipid Sciences Inc. based on the collection of either autologous HDL from a patient or homologous HDL from a donor, followed by a treatment to improve its anti-atherogenic properties and then its reinfusion into the patient [106]. This involves the treatment of plasma collected from a patient with organic solvents to selectively extract cholesterol from HDL. It has been established previously that the phospholipid–cholesterol ratio is a critical factor in the overall capacity of HDL to remove and bind cholesterol [15]. The removal of cholesterol from HDL and reinfusion back into patients should alter the equilibrium between tissue cholesterol and circulating cholesterol in plasma lipoproteins to favor increased reverse cholesterol transport. In addition, the delipidation of cholesterol from HDL has been shown to transform the mature lipid-rich forms of HDL to pre-β-like HDL. This type of HDL-replacement therapy is now undergoing Phase I testing. One possible limitation of this approach may be the amount of plasma that can be delipidated per treatment. It may be insufficient to significantly alter overall cholesterol flux in the body. Repeat treatments and/or automating the lipid extraction step so that it can perhaps be coupled to a plasmapheresis-like device to treat large plasma volumes may overcome this limitation.
A different approach, involving the phospholipid enrichment of HDL, has also been described but has only been tested in vitro [107]. During this treatment, HDL is incubated in the presence of phospholipid vesicles along with cholate, which are later removed by dialysis. This results in a significant enrichment of phospholipid on HDL and increases its phospholipid–cholesterol ratio. HDL treated in this way has been shown to have improved ability for promoting cholesterol efflux from cells. Treatment of plasma by this method and reinfusion could be beneficial in reducing plaque size and or composition but has not yet been tested in vivo in either animals or humans.
Potential clinical indications for HDL-replacement therapy
To date, although the main focus for HDL-replacement therapy has been the prevention of myocardial infarction in patients with acute coronary syndrome, here we will review other possible clinical indications (Box 2). Presently, most of these other potential applications have been investigated only in small pilot studies. The ability of HDL to alter many different common disease processes, such as inflammation and oxidation (Box 1), does suggest, however, that HDL-replacement therapy may be valuable for multiple clinical disorders.
Box 2. Potential clinical indications for HDL-replacement therapy.
Peripheral vascular disease
Besides reducing atherosclerotic plaque in the coronary artery vasculature, HDL-replacement therapy is also being considered for reducing plaque in other vascular sites that result in disease, such as peripheral vascular disease. Currently, there is an ongoing clinical trial examining the effect of reconstituted HDL from CSL on peripheral vascular disease. Patients scheduled for percutaneous superficial femoral artery revascularization for leg claudication are treated with HDL (CSL-111; 80 mg/kg) or a vehicle control prior to surgery. The effect of the treatment is determined 5–7 days later by a histological and chemical analysis of the surgically excised plaques. A preliminary report of the study indicated that a single HDL treatment decreased femoral plaque endothelial VCAM1 expression, oxygen free-radical generation and lipid accumulation [108].
Thrombotic stroke
Low levels of HDL have also been implicated in the development of thrombotic stroke due to increased plaque formation in carotid arteries [109], hence this is another potential indication for HDL-replacement therapy. Patients who are not good candidates for carotid endarterctomy surgery could potentially benefit from such treatment or it could be used as an adjunctive therapy prior to surgical treatment. A small pilot study examining the effect of a single infusion of HDL (CSL-111; 80 mg/kg) on thrombomodularity gene expression changes on plaque later removed by carotid endar-terectomy has also revealed a beneficial effect of HDL treatment in terms of a reduced level of tissue factor gene expression [110]. A large, double-blind placebo-controlled trial is now planned to examine the benefit of HDL for this indication.
Myocardial infarction
HDL-replacement therapy may also have a role after the development of myocardial infarction [111]. There has been a great effort to find agents that would decrease the ischemia/reperfusion injury that occurs after a myocardial infarction, but so far no clinically useful agents have been found. Interestingly, it has been observed that patients who have a myocardial infarction appear to have a better recovery from the event if they have high HDL [112,113], which suggests a possible role of HDL in limiting ischemia/reperfusion injury. In isolated rat hearts [114] and in an intact rabbit heart injury model [115], it has been shown that HDL infusion immediately before or right after cardiac ischemia is protective and limits the size of the infarct. The 37pA apoA-I mimetic peptide reconstituted with phospholipid was also shown to be protective [116]. The mechanism of action of HDL in this disorder is unlikely to be related to any enhancement of reverse cholesterol transport given the rapidity of the damage that follows ischemia and is probably related to one of the pleiotropic effects of HDL (Box 1). It was found in these studies that HDL treatment increases prostacyclin production, thus increasing blood flow. It also decreases TNF-α cytokine production and reduces the expression of VCAM1 by endothelial cells. HDL has also been shown to be protective against ischemia/reperfusion injury in multiple organs following hemorrhagic shock [117] and from damage due to organ-specific ischemia, such as the kidney [118], intestine [119] and brain [120].
Alzheimer’s disease
There are many links between cholesterol, HDL metabolism and the pathogenesis of Alzheimer’s disease (AD) [121]. The brain is the most cholesterol-rich organ in the human body and HDL is the only lipoprotein found in the CNS [122,123], where it is known to play a key role in synaptogenesis and in the maintenance of synapse plasticity of the hippocampus [124]. The level of HDL-C in cerebrospinal fluid of Alzheimer’s disease patients is lower than that of the control subjects [125], suggesting that a low HDL in the cerebrospinal fluid may be a risk factor for AD. In addition, the E4 polymorphism of apoE, the main HDL protein in cerebrospinal fluid, is a known AD risk factor [126]. Epidemiological studies have also suggested that an elevated serum total cholesterol level is a risk factor for AD and mild cognitive impairment [127]. Finally, several studies have also shown that a reduced cellular cholesterol level reduces amyloid β-protein generation [128,129].
Based on all these findings statins were tested in clinical trials for AD patients and showed some benefit in improving cognitive function [130–132]. Amphipathic peptides based on apoE have also been shown, in mouse models of AD and brain injury, to reduce β-amyloid protein production and inflammation from microglial activation [133]. The D-4F peptide, when added to the water of a rat model for AD, has also been shown to reduce CNS inflammation and improve cognitive function [134]. No human studies on HDL-replacement therapy for AD have been described.
Chronic renal disease
Recently, it has been appreciated that hyperlipidemia can contribute to the development of chronic renal disease [135]. Although the underlying pathological process of atherosclerosis and chronic renal disease are different, there are some similarities, such as the deposition and accumulation of excess lipid in the glomerulus. LDLR K/O mice on a high-fat diet have been shown to develop renal dysfunction and treatment with the apoA-I mimetic peptide, D-4F, produced a considerable effect in this model [136]. It improved renal function, as assessed by decreased lipid deposition and inflammation in the glomerulus. Since patients with chronic renal failure have a marked increase risk in atherosclerosis and their main cause of mortality is in fact cardiovascular disease [137], this population of patients in particular may benefit from HDL-replacement therapy. It could potentially help both their renal disease and possibly prevent the development of atherosclerosis.
Septic shock
Septic shock, which has an extremely high mortality and for which there are currently limited treatment options, is largely mediated by endotoxins [138,139]. HDL has been shown in both in vitro and in vivo studies to avidly bind and neutralize endotoxins, such as lipopolysaccharides [140,141], and the first efforts related to the purification and production of therapeutic HDL were for its possible treatment in septic shock. Furthermore, HDL is known to be reduced in septic shock [142], in a magnitude positively correlated with the severity of the illness.
Administration of HDL in various animal models has been shown to reduce mortality [143,144]. Synthetic amphipathic helical peptides have also been shown in mice to reduce the proinflammatory HDL changes that occur during sepsis [145,146]. In healthy subjects, infusion of HDL has been shown to reduce the procoagulant state caused by endotoxin exposure and to reduce monocyte activation and cytokine production [147,148]. To date, no human clinical trials of HDL infusion for septic shock have been described but perhaps the availability of the new HDL-replacement agents described in Table 1 may reignite interest in this area.
Expert commentary & five-year view
Great progress has been made in understanding HDL metabolism, which has made possible the development of various HDL-replacement agents that are currently being explored. However, there are still many unresolved issues concerning how to use HDL as a therapeutic agent. First, despite a renewed interest in HDL research we still do not completely understand how HDL protects against atherosclerosis. The prevailing hypothesis is that it potentiates the reverse cholesterol transport pathway but the other beneficial effects of HDL may also contribute to its atheroprotective function. A better understanding of how HDL prevents atherosclerosis is critical in the design and optimization of HDL-replacement agents and also in monitoring the clinical efficacy of such drugs or treatments. This is a particularly important issue for short apoA-I mimetic peptides, which may not share all of the features or biological properties of the full length apoA-I molecule. The majority of HDL-replacement agents have employed a combination of an apolipoprotein or peptide mimic with a phospholipid, which makes the production and preparation of these agents more difficult and expensive. It is still not clear from most studies to what degree the added phospholipid contributes to the observed protection against atherosclerosis. The effect of different types of phospholipids and/or the optimum ratio of lipid–protein has also not been systematically investigated. In both animal and human studies, the route of administration of these agents has been primarily intravenous, which would limit the use of this type of treatment to the acute stabilization of patients. Other approaches, such as oral administration or subcutaneous injection, may be possible, particularly for lipid-free apoA-1 mimetic peptides (e.g., D-4F).
The most important issue is that only early-stage clinical trials have been completed for the various HDL-replacement agents. Although data from preclinical animal studies are encouraging and the various treatments examined to date appear to be safe, the efficacy of this approach has not been fully investigated and no studies based on clinical end points have been reported. Therefore, much work remains to be carried out before HDL-replacement therapy can be added to our standard treatment approach for cardiovascular disease, but it shows great promise and research in this area has already greatly added to our basic understanding of HDL metabolism.
Key issues.
New treatment approaches besides lowering LDL levels are needed to more effectively treat coronary heart disease.
HDL reduces atherosclerosis by increasing reverse cholesterol transport but probably also via other mechanisms.
Besides small-molecule drugs for raising HDL, infusion of HDL or HDL mimetics may be useful for the rapid stabilization of patients.
ApoA-l, the main protein component of HDL, reconstituted with phospholipids, can quickly reduce plaque size after infusion.
Small peptide mimics of apoA-l appear to share the same biological properties of full-length apoA-l and thus may be useful as therapeutic agents, similar to ApoA-1.
Other possible HDL-replacement therapies include phospholipids, lecithin:cholesterol acyltransferase and manipulation of autologous or homologous HDL.
Besides preventing myocardial infarction, HDL-replacement therapy may have other indications, such as peripheral vascular disease, thrombotic stroke, ischemia/reperfusion injury and septic shock.
Large-scale clinical trials with clinical end points must be conducted to establish the efficacy of HDL-replacement therapy.
Acknowledgments
Research by authors AT Remaley and M Amar is supported by intramural research funds from National Heart, Lung, and Blood Institute, National Institutes for Health, Bethesda, MD, USA. They are also patent holders of the described 5A apoA-I mimetic peptide.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Alan T Remaley, National Institutes of Health, Lipoprotein Metabolism Section, Pulmonary and Vascular Medicine Branch, National Heart, Lung, and Blood Institute, Building 10, Room 7N-115, 10 Center Drive, Bethesda, MD 20892-21508, USA.
Marcelo Amar, National Institutes of Health, Lipoprotein Metabolism Section, Pulmonary and Vascular Medicine Branch, National Heart, Lung, and Blood Institute, Bethesda, MD, USA.
Dmitri Sviridov, Baker Heart Research Institute, Melbourne, Australia.
References
- 1.Barr DP, Russ EM, Eder HA. Protein–lipid relationships in human plasma. II. In atherosclerosis and related conditions. Am. J. Med. 1951;11:480–493. doi: 10.1016/0002-9343(51)90183-0. [DOI] [PubMed] [Google Scholar]
- 2.Chhabria MT, Suhagia BN, Brahmkshatriya PS. HDL elevation and lipid lowering therapy: current scenario and future perspectives. Recent Patents Cardiovasc. Drug Discov. 2007;2:214–227. doi: 10.2174/157489007782418973. [DOI] [PubMed] [Google Scholar]
- 3.Wong NC. Novel therapies to increase apolipoprotein AI and HDL for the treatment of atherosclerosis. Curr. Opin. Investig. Drugs. 2007;8:718–728. [PubMed] [Google Scholar]
- 4.Sacks FM. The relative role of low-density lipoprotein cholesterol and high-density lipoprotein cholesterol in coronary artery disease: evidence from large-scale statin and fibrate trials. Am. J. Cardiol. 2001;88:N14–N18. doi: 10.1016/s0002-9149(01)02147-6. [DOI] [PubMed] [Google Scholar]
- 5.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 6.Guyton JR. Niacin in cardiovascular prevention: mechanisms, efficacy, and safety. Curr. Opin. Lipidol. 2007;18:415–420. doi: 10.1097/MOL.0b013e3282364add. [DOI] [PubMed] [Google Scholar]
- 7.Joy T, Hegele RA. Is raising HDL a futile strategy for atheroprotection? Nat. Rev. Drug Discov. 2008;7:143–155. doi: 10.1038/nrd2489. [DOI] [PubMed] [Google Scholar]
- 8.Kastelein JJ, van Leuven SI, Burgess L, et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N. Engl. J. Med. 2007;356:1620–1630. doi: 10.1056/NEJMoa071359. [DOI] [PubMed] [Google Scholar]
- 9.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 10.Brewer HB, Jr, Remaley AT, Neufeld EB, Basso F, Joyce C. Regulation of plasma high-density lipoprotein levels by the ABCA1 transporter and the emerging role of high-density lipoprotein in the treatment of cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2004;24:1755–1760. doi: 10.1161/01.ATV.0000142804.27420.5b. [DOI] [PubMed] [Google Scholar]
- 11.Newton RS, Krause BR. HDL therapy for the acute treatment of atherosclerosis. Atheroscler. Suppl. 2002;3:31–38. doi: 10.1016/s1567-5688(02)00044-2. [DOI] [PubMed] [Google Scholar]
- 12.Conca P, Franceschini G. Synthetic HDL as a new treatment for atherosclerosis regression: has the time come? Nutr. Metab. Cardiovasc. Dis. 2008;18(4):329–335. doi: 10.1016/j.numecd.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Calabresi L, Sirtori CR, Paoletti R, Franceschini G. Recombinant apolipoprotein A–I Milano for the treatment of cardiovascular diseases. Curr. Atheroscler. Rep. 2006;8:163–167. doi: 10.1007/s11883-006-0054-4. [DOI] [PubMed] [Google Scholar]
- 14.Marchesi M, Sirtori CR. Therapeutic use of the high-density lipoprotein protein and peptides. Expert Opin. Investig. Drugs. 2006;15:227–241. doi: 10.1517/13543784.15.3.227. [DOI] [PubMed] [Google Scholar]
- 15.Yancey PG, Bortnick AE, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 2003;23:712–719. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- 16.Basso F, Freeman L, Knapper CL, et al. Role of the hepatic ABCA1 transporter in modulating intrahepatic cholesterol and plasma HDL cholesterol concentrations. J. Lipid Res. 2003;44:296–302. doi: 10.1194/jlr.M200414-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Brunham LR, Kruit JK, Iqbal J, et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo . J. Clin. Invest. 2006;116:1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nofer JR, Remaley AT. Tangier disease: still more questions than answers. Cell. Mol. Life Sci. 2005;62:2150–2160. doi: 10.1007/s00018-005-5125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oram JF, Vaughan AM. ATP-binding cassette cholesterol transporters and cardiovascular disease. Circ. Res. 2006;99:1031–1043. doi: 10.1161/01.RES.0000250171.54048.5c. [DOI] [PubMed] [Google Scholar]
- 20.Cavelier C, Lorenzi I, Rohrer L, von Eckardstein A. Lipid efflux by the ATP-binding cassette transporters ABCA1 and ABCG1. Biochim. Biophys. Acta. 2006;1761:655–666. doi: 10.1016/j.bbalip.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Williams DL, Connelly MA, Temel RE, et al. Scavenger receptor BI and cholesterol trafficking. Curr. Opin. Lipidol. 1999;10:329–339. doi: 10.1097/00041433-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Out R, Hoekstra M, Habets K, et al. Combined deletion of macrophage ABCA1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler. Thromb. Vasc. Biol. 2008;28:258–264. doi: 10.1161/ATVBAHA.107.156935. [DOI] [PubMed] [Google Scholar]
- 23.Yvan-Charvet L, Ranalletta M, Wang N, et al. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santamarina-Fojo S, Lambert G, Hoeg JM, Brewer HB., Jr Lecithin-cholesterol acyltransferase: role in lipoprotein metabolism, reverse cholesterol transport and atherosclerosis. Curr. Opin. Lipidol. 2000;11:267–275. doi: 10.1097/00041433-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Asztalos BF, Schaefer EJ, Horvath KV, et al. Role of LCAT in HDL remodeling: investigation of LCAT deficiency states. J. Lipid Res. 2007;48:592–599. doi: 10.1194/jlr.M600403-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Connelly MA, Williams DL. SR-BI and HDL cholesteryl ester metabolism. Endocr. Res. 2004;30:697–703. doi: 10.1081/erc-200043979. [DOI] [PubMed] [Google Scholar]
- 27.de Grooth GJ, Klerkx AH, Stroes ES, Stalenhoef AF, Kastelein JJ, Kuivenhoven JA. A review of CETP and its relation to atherosclerosis. J. Lipid Res. 2004;45:1967–1974. doi: 10.1194/jlr.R400007-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Bjorkhem I, Lund E, Rudling M. Coordinate regulation of cholesterol 7 α-hydroxylase and HMG-CoA reductase in the liver. Subcell. Biochem. 1997;28:23–55. doi: 10.1007/978-1-4615-5901-6_2. [DOI] [PubMed] [Google Scholar]
- 29.Barter PJ, Rye KA. Relationship between the concentration and and-atherogenic activity of high-density lipoproteins. Curr. Opin. Lipidol. 2006;17:399–403. doi: 10.1097/01.mol.0000236365.40969.af. [DOI] [PubMed] [Google Scholar]
- 30.Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circ. Res. 2006;98:1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- 31.Lerch PG, Spycher MO, Doran JE. Reconstituted high density lipoprotein (rHDL) modulates platelet activity in vitro and ex vivo . Thromb. Haemost. 1998;80:316–320. [PubMed] [Google Scholar]
- 32.Norata GD, Banfi C, Pirillo A, et al. Oxidised-HDL3 induces the expression of PAI-1 in human endothelial cells. Role of p38MAPK activation and mRNA stabilization. Br. J. Haematol. 2004;127:97–104. doi: 10.1111/j.1365-2141.2004.05163.x. [DOI] [PubMed] [Google Scholar]
- 33.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ. Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 34.Navab M, Anantharamaiah GM, Fogelman AM. The role of high-density lipoprotein in inflammation. Trends Cardiovasc. Med. 2005;15:158–161. doi: 10.1016/j.tcm.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Ansell BJ, Watson KE, Fogelman AM, Navab M, Fonarow GC. High-density lipoprotein function recent advances. J. Am. Coll. Cardiol. 2005;46:1792–1798. doi: 10.1016/j.jacc.2005.06.080. [DOI] [PubMed] [Google Scholar]
- 36.Navab M, Ananthramaiah GM, Reddy ST, et al. The double jeopardy of HDL. Ann. Med. 2005;37:173–178. doi: 10.1080/07853890510007322. [DOI] [PubMed] [Google Scholar]
- 37.Navab M, Ananthramaiah GM, Reddy ST, et al. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J. Lipid Res. 2004;45:993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Han R, Lai R, Ding Q, et al. Apolipoprotein A–I stimulates AMP-activated protein kinase and improves glucose metabolism. Diabetologia. 2007;50:1960–1968. doi: 10.1007/s00125-007-0752-7. [DOI] [PubMed] [Google Scholar]
- 39.Brunham LR, Kruit JK, Pape TD, et al. β-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat. Med. 2007;13:340–347. doi: 10.1038/nm1546. [DOI] [PubMed] [Google Scholar]
- 40.Mastorikou M, Mackness M, Mackness B. Defective metabolism of oxidized phospholipid by HDL from people with type 2 diabetes. Diabetes. 2006;55:3099–3103. doi: 10.2337/db06-0723. [DOI] [PubMed] [Google Scholar]
- 41.Persegol L, Verges B, Foissac M, Gambert P, Duvillard L. Inability of HDL from type 2 diabetic patients to counteract the inhibitory effect of oxidised LDL on endothelium-dependent vasorelaxation. Diabetologia. 2006;49:1380–1386. doi: 10.1007/s00125-006-0244-1. [DOI] [PubMed] [Google Scholar]
- 42.Passarelli M, Tang C, McDonald TO, et al. Advanced glycation end product precursors impair ABCA1-dependent cholesterol removal from cells. Diabetes. 2005;54:2198–2205. doi: 10.2337/diabetes.54.7.2198. [DOI] [PubMed] [Google Scholar]
- 43.Nieuwdorp M, Vergeer M, Bisoendial RJ, et al. Reconstituted HDL infusion restores endothelial function in patients with type 2 diabetes mellkus. Diabetologia. 2008;51:1081–1084. doi: 10.1007/s00125-008-0975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi K, Lopez LR, Shoenfeld Y, Matsuura E. The role of innate and adaptive immunity to oxidized low-density lipoprotein in the development of atherosclerosis. Ann. NY Acad. Sci. 2005;1051:442–454. doi: 10.1196/annals.1361.086. [DOI] [PubMed] [Google Scholar]
- 45.Villarroel F, Bastias A, Casado A, Amthauer R, Concha MI. Apolipoprotein A–I, an antimicrobial protein in Oncorhynchus mykiss: evaluation of its expression in primary defence barriers and plasma levels in sick and healthy fish. Fish Shellfish Immunol. 2007;23:197–209. doi: 10.1016/j.fsi.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 46.James RW. A long and winding road: defining the biological role and clinical importance of paraoxonases. Clin. Chem. Lab. Med. 2006;44:1052–1059. doi: 10.1515/CCLM.2006.207. [DOI] [PubMed] [Google Scholar]
- 47.Movva R, Rader DJ. Laboratory assessment of HDL heterogeneity and function. Clin. Chem. 2008;54:788–800. doi: 10.1373/clinchem.2007.101923. [DOI] [PubMed] [Google Scholar]
- 48.Remaley AT, Warnick GR. High density lipoprotein: what is the best way to measure its anti-atherogenic potential? Expert Opin. Med. Diagn. 2008;2(7):773–788. doi: 10.1517/17530059.2.7.773. [DOI] [PubMed] [Google Scholar]
- 49.Vaisar T, Pennathur S, Green PS, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J. Clin. Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nofer JR, van der GM, Tolle M, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J. Clin. Invest. 2004;113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen CL, Huang SS, Huang JS. Cholesterol modulates cellular TGF-β responsiveness by altering TGF-β binding to TGF-β receptors. J. Cell. Physiol. 2008;215:223–233. doi: 10.1002/jcp.21303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen CL, Liu IH, Fliesler SJ, Han X, Huang SS, Huang JS. Cholesterol suppresses cellular TGF-β responsiveness: implications in atherogenesis. J. Cell. Sci. 2007;120:3509–3521. doi: 10.1242/jcs.006916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trogan E, Feig JE, Dogan S, et al. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc. Natl Acad. Sci. USA. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terasaka N, Wang N, Yvan-Charvet L, Tall AR. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc. Natl Acad. Sci. USA. 2007;104:15093–15098. doi: 10.1073/pnas.0704602104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J. Clin. Invest. 1990;85:1234–1241. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaul S, Rukshin V, Santos R, et al. Intramural delivery of recombinant apolipoprotein A–I Milano/phospholipid complex (ETC-216) inhibits in-stent stenosis in porcine coronary arteries. Circulation. 2003;107:2551–2554. doi: 10.1161/01.CIR.0000074042.19447.B1. [DOI] [PubMed] [Google Scholar]
- 57.Carlson LA. Effect of a single infusion of recombinant human proapolipoprotein A–I liposomes (synthetic HDL) on plasma lipoproteins in patients with low high density lipoprotein cholesterol. Nutr. Metab. Cardiovasc. Dis. 1995;5:85–91. [Google Scholar]
- 58.Eriksson M, Carlson LA, Miettinen TA, Angelin B. Stimulation of fecal steroid excretion after infusion of recombinant proapolipoprotein A–I. Potential reverse cholesterol transport in humans. Circulation. 1999;100:594–598. doi: 10.1161/01.cir.100.6.594. [DOI] [PubMed] [Google Scholar]
- 59.Bisoendial RJ, Hovingh GK, Levels JH, et al. Restoration of endothelial function by increasing high-density lipoprotein in subjects with isolated low high-density lipoprotein. Circulation. 2003;107:2944–2948. doi: 10.1161/01.CIR.0000070934.69310.1A. [DOI] [PubMed] [Google Scholar]
- 60.Nanjee MN, Crouse JR, King JM, et al. Effects of intravenous infusion of lipid-free apo A–I in humans. Arterioscler. Thromb. Vasc. Biol. 1996;16:1203–1214. doi: 10.1161/01.atv.16.9.1203. [DOI] [PubMed] [Google Scholar]
- 61.Franceschini G, Sirtori CR, Capurso A, Weisgraber KH, Mahley RW. A–I Milano apoprotein. Decreased high density lipoprotein cholesterol levels with significant lipoprotein modifications and without clinical atherosclerosis in an Italian family. J. Clin. Invest. 1980;66:892–900. doi: 10.1172/JCI109956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weisgraber KH, Rall SC, Jr, Bersot TP, Mahley RW, Franceschini G, Sirtori CR. Apolipoprotein A–I Milano. Detection of normal A–I in affected subjects and evidence for a cysteine for arginine substitution in the variant A–I. J. Biol. Chem. 1983;258:2508–2513. [PubMed] [Google Scholar]
- 63.Franceschini G, Calabresi L, Chiesa G, et al. Increased cholesterol efflux potential of sera from ApoA-I Milano carriers and transgenic mice. Arterioscler. Thromb. Vasc. Biol. 1999;19:1257–1262. doi: 10.1161/01.atv.19.5.1257. [DOI] [PubMed] [Google Scholar]
- 64.Calabresi L, Vecchio G, Longhi R, et al. Molecular characterization of native and recombinant apolipoprotein A–I Milano dimer. The introduction of an interchain disulfide bridge remarkably alters the physicochemical properties of apolipoprotein A–I. J. Biol. Chem. 1994;269:32168–32174. [PubMed] [Google Scholar]
- 65.Bielicki JK, Oda MN. Apolipoprotein A–I (Milano) and apolipoprotein A–I (Paris) exhibit an antioxidant activity distinct from that of wild-type apolipoprotein A–I. Biochemistry. 2002;41:2089–2096. doi: 10.1021/bi011716p. [DOI] [PubMed] [Google Scholar]
- 66.Jia Z, Natarajan P, Forte TM, Bielicki JK. Thiol-bearing synthetic peptides retain the antioxidant activity of apolipoprotein A–I (Milano) Biochem. Biophys. Res. Commun. 2002;297:206–213. doi: 10.1016/s0006-291x(02)02143-5. [DOI] [PubMed] [Google Scholar]
- 67.Franceschini G, Sirtori CR, Bosisio E, et al. Relationship of the phenotypic expression of the A–I Milano apoprotein with plasma lipid and lipoprotein patterns. Atherosclerosis. 1985;58:159–174. doi: 10.1016/0021-9150(85)90063-2. [DOI] [PubMed] [Google Scholar]
- 68.Sirtori CR, Calabresi L, Franceschini G, et al. Cardiovascular status of carriers of the apolipoprotein A–I (Milano) mutant: the Limone sul Garda study. Circulation. 2001;103:1949–1954. doi: 10.1161/01.cir.103.15.1949. [DOI] [PubMed] [Google Scholar]
- 69.Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 70.Nicholls SJ, Tuzcu EM, Sipahi I, et al. Relationship between atheroma regression and change in lumen size after infusion of apolipoprotein A–I Milano. J. Am. Coll. Cardiol. 2006;47:992–997. doi: 10.1016/j.jacc.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 71.Nissen SE. Effect of intensive lipid lowering on progression of coronary atherosclerosis: evidence for an early benefit from the Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) trial. Am. J. Cardiol. 2005;96:F61–F68. doi: 10.1016/j.amjcard.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 72.Nissen SE. Effect of intensive lipid lowering on progression of coronary atherosclerosis: evidence for an early benefit from the Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) trial. Am. J. Cardiol. 2005;96(5A):61F–68F. doi: 10.1016/j.amjcard.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 73.Ibanez B, Vilahur G, Cimmino G, et al. Rapid change in plaque size, composition, and molecular footprint after recombinant apolipoprotein A–I Milano (ETC-216) administration: magnetic resonance imaging study in an experimental model of atherosclerosis. J. Am. Coll. Cardiol. 2008;51:1104–1109. doi: 10.1016/j.jacc.2007.09.071. [DOI] [PubMed] [Google Scholar]
- 74.Parolini C, Marchesi M, Lorenzon P, et al. Dose-related effects of repeated ETC-216 (recombinant apolipoprotein A–I Milano/1-palmitoyl-2-oleoyl phosphatidylcholine complexes) administrations on rabbit lipid-rich soft plaques: in vivo assessment by intravascular ultrasound and magnetic resonance imaging. J. Am. Coll. Cardiol. 2008;51:1098–1103. doi: 10.1016/j.jacc.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 75.Kaul S, Shah PK. ApoA-I Milano/phospholipid complexes emerging pharmacological strategies and medications for the prevention of atherosclerotic plaque progression. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005;5:471–479. doi: 10.2174/156800605774962004. [DOI] [PubMed] [Google Scholar]
- 76.Spieker LE, Sudano I, Hurlimann D, et al. High-density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation. 2002;105:1399–1402. doi: 10.1161/01.cir.0000013424.28206.8f. [DOI] [PubMed] [Google Scholar]
- 77.Nanjee MN, Cooke CJ, Garvin R, et al. Intravenous apoA-I/lecithin discs increase pre-β-HDL concentration in tissue fluid and stimulate reverse cholesterol transport in humans. J. Lipid Res. 2001;42:1586–1593. [PubMed] [Google Scholar]
- 78.Tardif JC, Gregoire J, L’Allier PL, et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 79.Graversen JH, Laurberg JM, Andersen MH, et al. Trimerization of apolipoprotein A–I retards plasma clearance and preserves and atherosclerotic properties. J. Cardiovasc. Pharmacol. 2008;51:170–177. doi: 10.1097/FJC.0b013e31815ed0b9. [DOI] [PubMed] [Google Scholar]
- 80.Brinton EA, Eisenberg S, Breslow JL. Human HDL cholesterol levels are determined by apoA-I fractional catabolic rate, which correlates inversely with estimates of HDL particle size. Effects of gender, hepatic and lipoprotein lipases, triglyceride and insulin levels, and body fat distribution. Arterioscler. Thromb. 1994;14:707–720. doi: 10.1161/01.atv.14.5.707. [DOI] [PubMed] [Google Scholar]
- 81.Lee JY, Timmins JM, Mulya A, et al. HDLs in apoA-I transgenic Abcal knockout mice are remodeled normally in plasma but are hypercatabolized by the kidney. J. Lipid Res. 2005;46:2233–2245. doi: 10.1194/jlr.M500179-JLR200. [DOI] [PubMed] [Google Scholar]
- 82.Anantharamaiah GM, Jones JL, Brouillette CG, et al. Studies of synthetic peptide analogs of the amphipathic helix. Structure of complexes with dimyristoyl phosphatidylcholine. J. Biol. Chem. 1985;260:10248–10255. [PubMed] [Google Scholar]
- 83.Segrest JP, Garber DW, Brouillette CG, Harvey SC, Anantharamaiah GM. The amphipathic α helix: a multifunctional structural motif in plasma apolipoproteins. Adv. Protein Chem. 1994;45:303–369. doi: 10.1016/s0065-3233(08)60643-9. [DOI] [PubMed] [Google Scholar]
- 84.Remaley AT, Thomas F, Stonik JA, et al. Synthetic amphipathic helical peptides promote lipid efflux from cells by an ABCA1-dependent and an ABCA1-independent pathway. J. Lipid Res. 2003;44:828–836. doi: 10.1194/jlr.M200475-JLR200. [DOI] [PubMed] [Google Scholar]
- 85.Anantharamaiah G, Navab M, Reddy ST, et al. Synthetic peptides: managing lipid disorders. Curr. Opin. Lipidol. 2006;17:233–237. doi: 10.1097/01.mol.0000226114.89812.75. [DOI] [PubMed] [Google Scholar]
- 86.Navab M, Anantharamaiah GM, Reddy ST, et al. Oral D-4F causes formation of pre-β high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation. 2004;109:3215–3220. doi: 10.1161/01.CIR.0000134275.90823.87. [DOI] [PubMed] [Google Scholar]
- 87.Anantharamaiah GM, Mishra VK, Garber DW, et al. Structural requirements for antioxidative and anti-inflammatory properties of apolipoprotein A–I mimetic peptides. J. Lipid Res. 2007;48:1915–1923. doi: 10.1194/jlr.R700010-JLR200. [DOI] [PubMed] [Google Scholar]
- 88.Bloedon LT, Dunbar R, Duffy D, et al. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J. Lipid Res. 2008;49:1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Navab M, Anantharamaiah GM, Reddy ST, Fogelman AM. Apolipoprotein A–I mimetic peptides and their role in atherosclerosis prevention. Nat. Clin. Pract. Cardiovasc. Med. 2006;3:540–547. doi: 10.1038/ncpcardio0661. [DOI] [PubMed] [Google Scholar]
- 90.Sethi AA, Amar M, Shamburek RD, Remaley AT. Apolipoprotein AI mimetic peptides: possible new agents for the treatment of atherosclerosis. Curr. Opin. Investig. Drugs. 2007;8:201–212. [PubMed] [Google Scholar]
- 91.Vedhachalam C, Narayanaswami V, Neto N, et al. The C-terminal lipid-binding domain of apolipoprotein E is a highly efficient mediator of ABCAl-dependent cholesterol efflux that promotes the assembly of high-density lipoproteins. Biochemistry. 2007;46:2583–2593. doi: 10.1021/bi602407r. [DOI] [PubMed] [Google Scholar]
- 92.Remaley A, Stonik J, Fairwell T, Demosky SJ, Neufeld EB, Brewer HB. Asymmetry in lipid affinity of multihelical amphipathic peptides: an important structural determinant for specificity of ABCAl-dependent cholesterol efflux by peptides [abstract] Circulation. 2004;110:243. [Google Scholar]
- 93.Natarajan P, Forte TM, Chu B, Phillips MC, Oram JF, Bielicki JK. Identification of an apolipoprotein A–I structural element that mediates cellular cholesterol efflux and stabilizes ATP binding cassette transporter Al. J. Biol. Chem. 2004;279:24044–24052. doi: 10.1074/jbc.M400561200. [DOI] [PubMed] [Google Scholar]
- 94.Voogt J, Turner S, Chang B, et al. Effect of the 5A bi-helical ApoA-I mimetic peptide on cholesterol efflux and global reverse cholesterol transport in vivo [abstract]; Atlanta, GA, USA. Presented at: ATVB Annual Meeting; 2008. Apr, pp. 16–18. [Google Scholar]
- 95.Nguyen SD, Jeong TS, Sok DE. Apolipoprotein A–I-mimetic peptides with antioxidant actions. Arch. Biochem. Biophys. 2006;451:34–42. doi: 10.1016/j.abb.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 96.Gupta H, White CR, Handattu S, et al. Apolipoprotein E mimetic peptide dramatically lowers plasma cholesterol and restores endothelial function in watanabe heritable hyperlipidemic rabbits. Circulation. 2005;111:3112–3118. doi: 10.1161/CIRCULATIONAHA.104.497107. [DOI] [PubMed] [Google Scholar]
- 97.Brousseau ME, Kauffman RD, Herderick EE, et al. LCAT modulates atherogenic plasma lipoproteins and the extent of atherosclerosis only in the presence of normal LDL receptors in transgenic rabbits. Arterioscler. Thromb. Vasc. Biol. 2000;20:450–458. doi: 10.1161/01.atv.20.2.450. [DOI] [PubMed] [Google Scholar]
- 98.Foger B, Chase M, Amar MJ, et al. Cholesteryl ester transfer protein corrects dysfunctional high density lipoproteins and reduces aortic atherosclerosis in lecithin cholesterol acyltransferase transgenic mice. J. Biol. Chem. 1999;274:36912–36920. doi: 10.1074/jbc.274.52.36912. [DOI] [PubMed] [Google Scholar]
- 99.Williams KJ, Werth VP, Wolff JA. Intravenously administered lecithin liposomes: a synthetic anti-atherogenic lipid particle. Perspect. Biol. Med. 1984;27:417–431. doi: 10.1353/pbm.1984.0031. [DOI] [PubMed] [Google Scholar]
- 100.Rodrigueza WV, Pritchard PH, Hope MJ. The influence of size and composition on the cholesterol mobilizing properties of liposomes in vivo . Biochim. Biophys. Acta. 1993;1153:9–19. doi: 10.1016/0005-2736(93)90270-a. [DOI] [PubMed] [Google Scholar]
- 101.Dietschy JM, Turley SD. Control of cholesterol turnover in the mouse. J.Biol. Chem. 2002;277:3801–3804. doi: 10.1074/jbc.R100057200. [DOI] [PubMed] [Google Scholar]
- 102.Hajj HH, Blain S, Boucher B, Denis M, Krimbou L, Genest J. Structural modification of plasma HDL by phospholipids promotes efficient ABCAl-mediated cholesterol release. J. Lipid Res. 2005;46:1457–1465. doi: 10.1194/jlr.M400477-JLR200. [DOI] [PubMed] [Google Scholar]
- 103.Rodrigueza WV, Klimuk SK, Pritchard PH, Hope MJ. Cholesterol mobilization and regression of atheroma in cholesterolfed rabbits induced by large unilamellar vesicles. Biochim. Biophys. Acta. 1998;1368:306–320. doi: 10.1016/s0005-2736(97)00198-3. [DOI] [PubMed] [Google Scholar]
- 104.Roerdink F, Dijkstra J, Hartman G, Bolscher B, Scherphof G. The involvement of parenchymal, Kupffer and endothelial liver cells in the hepatic uptake of intravenously injected liposomes. Effects of lanthanum and gadolinium salts. Biochim. Biophys. Acta. 1981;677:79–89. doi: 10.1016/0304-4165(81)90148-3. [DOI] [PubMed] [Google Scholar]
- 105.Pandey NR, Sparks DL. Phospholipids as cardiovascular therapeutics. Curr. Opin. Investig. Drugs. 2008;9:281–285. [PubMed] [Google Scholar]
- 106.Brewer HB, Alaupovic P, Kostner G, et al. Selective plasma HDL delipidation and reinfusion: a new approach for acute HDL therapy in the treatment of cardiovascular disease. Circulation. 2004;110:51–52. [Google Scholar]
- 107.Pownall HJ. Detergent-mediated phospholipidation of plasma lipoproteins increases HDL cholesterophilicity and cholesterol efflux via SR-BI. Biochemistry. 2006;45:11514–11522. doi: 10.1021/bi0608717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shaw J, Blombery P, Lyon S, Hosp A. Administration of intravenous high density lipoprotein leads to acute changes in human atherosclerotic plaque in vivo [abstract] Circulation. 2007;116:678. [Google Scholar]
- 109.Bloomfield Rubins H, Davenport J, Babikian V, et al. Reduction in stroke with gemfibrozil in men with coronary heart disease and low HDL cholesterol: the Veterans Affairs HDL Intervention Trial (VA-HIT) Cirulation. 2001;103:2828–2833. doi: 10.1161/01.cir.103.23.2828. [DOI] [PubMed] [Google Scholar]
- 110.Nasr HH, Loftus IM, Sayed S, et al. A single dose of reconstituted high density lipoprotein reduces expression of tissue-factor in human carotid atherosclerotic plaques. Circulation. 2007;116 II_33 (Abstract) [Google Scholar]
- 111.Calabresi L, Gomaraschi M, Rossoni G, Franceschini G. Synthetic high density lipoproteins for the treatment of myocardial ischemia/reperfusion injury. Pharmacol. Ther. 2006;111:836–854. doi: 10.1016/j.pharmthera.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 112.Goldbourt U, Cohen L, Neufeld HN. High density lipoprotein cholesterol: prognosis after myocardial infarction. The Israeli Ischemic Heart Disease Study. Int. J. Epidemiol. 1986;15:51–55. doi: 10.1093/ije/15.1.51. [DOI] [PubMed] [Google Scholar]
- 113.Berge KG, Canner PL, Hainline A., Jr High-density lipoprotein cholesterol and prognosis after myocardial infarction. Circulation. 1982;66:1176–1178. doi: 10.1161/01.cir.66.6.1176. [DOI] [PubMed] [Google Scholar]
- 114.Rossoni G, Gomaraschi M, Berti F, Sirtori CR, Franceschini G, Calabresi L. Synthetic high-density lipoproteins exert cardioprotective effects in myocardial ischemia/reperfusion injury. J.Pharmacol. Exp. Ther. 2004;308:79–84. doi: 10.1124/jpet.103.057141. [DOI] [PubMed] [Google Scholar]
- 115.Marchesi M, Booth EA, Davis T, Bisgaier CL, Lucchesi BR. Apolipoprotein A–I Milano and 1-palmitoyl-2-oleoyl phosphatidylcholine complex (ETC-216) protects the in vivo rabbit heart from regional ischemia-reperfusion injury. J. Pharmacol. Exp. Ther. 2004;311:1023–1031. doi: 10.1124/jpet.104.070789. [DOI] [PubMed] [Google Scholar]
- 116.Gomaraschi M, Calabresi L, Rossoni G, et al. Anti-inflammatory and cardioprotective activities of synthetic high-density lipoprotein containing apolipoprotein A–I mimetic peptides. J. Pharmacol. Exp. Ther. 2008;324:776–783. doi: 10.1124/jpet.107.129411. [DOI] [PubMed] [Google Scholar]
- 117.Cockerill GW, McDonald MC, Mota-Filipe H, Cuzzocrea S, Miller NE, Thiemermann C. High density lipoproteins reduce organ injury and organ dysfunction in a rat model of hemorrhagic shock. FASEB J. 2001;15:1941–1952. doi: 10.1096/fj.01-0075com. [DOI] [PubMed] [Google Scholar]
- 118.Thiemermann C, Patel NS, Kvale EO, et al. High density lipoprotein (HDL) reduces renal ischemia/reperfusion injury. J. Am. Soc. Nephrol. 2003;14:1833–1843. doi: 10.1097/01.asn.0000075552.97794.8c. [DOI] [PubMed] [Google Scholar]
- 119.Cuzzocrea S, Dugo L, Patel NS, et al. High-density lipoproteins reduce the intestinal damage associated with ischemia/reperfusion and colitis. Shock. 2004;21:342–351. doi: 10.1097/00024382-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 120.Paterno R, Ruocco A, Postiglione A, Hubsch A, Andresen I, Lang MG. Reconstituted high-density lipoprotein exhibits neuroprotection in two rat models of stroke. Cerebrovasc. Dis. 2004;17:204–211. doi: 10.1159/000075792. [DOI] [PubMed] [Google Scholar]
- 121.Michikawa M. Cholesterol paradox: is high total or low HDL cholesterol level a risk for Alzheimer’s disease? J. Neurosci. Res. 2003;72:141–146. doi: 10.1002/jnr.10585. [DOI] [PubMed] [Google Scholar]
- 122.Fagan AM, Younkin LH, Morris JC, et al. Differences in the Ap40/Ap42 ratio associated with cerebrospinal fluid lipoproteins as a function of apolipoprotein E genotype. Ann. Neurol. 2000;48:201–210. [PubMed] [Google Scholar]
- 123.Pitas RE, Boyles JK, Lee SH, Hui D, Weisgraber KH. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J. Biol. Chem. 1987;262:14352–14360. [PubMed] [Google Scholar]
- 124.Mauch DH, Nagler K, Schumacher S, et al. CNS synaptogenesis promoted by gliaderived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 125.Merched A, Xia Y, Visvikis S, Serot JM, Siest G. Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer’s disease. Neurobiol. Aging. 2000;21:27–30. doi: 10.1016/s0197-4580(99)00103-7. [DOI] [PubMed] [Google Scholar]
- 126.Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S. Apolipoprotein-E polymorphism and Alzheimers-disease. Lancet. 1993;342:697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- 127.Kivipelto M, Helkala EL, Hanninen T, et al. Midlife vascular risk factors and late-life mild cognitive impairment - a population-based study. Neurology. 2001;56:1683–1689. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- 128.Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of β-amyloid in hippocampal neurons. Proc. Natl Acad. Sci. USA. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fassbender K, Simons M, Bergmann C, et al. Simvastatin strongly reduces levels of Alzheimer’s disease β-amyloid peptides A β 42 and A β 40 in vitro and in vivo . Proc. Natl Acad. Sci. USA. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bonn V, Cheung RC, Chen B, Taghibiglou C, Van Iderstine SC, Adeli K. Simvastatin, an HMG-CoA reductase inhibitor induces the synthesis and secretion of apolipoprotein AI in HepG2 cells and primary hamster hepatocytes. Atherosclerosis. 2002;163:59–68. doi: 10.1016/s0021-9150(01)00754-7. [DOI] [PubMed] [Google Scholar]
- 131.Chong PH, Kezele R, Franklin C. High-density lipoprotein cholesterol and the role of statins. Circ. J. 2002;66:1037–1044. doi: 10.1253/circj.66.1037. [DOI] [PubMed] [Google Scholar]
- 132.Vega GL, Weiner MF, Lipton AM, et al. Reduction in levels of 24S-hydroxycholesterol by statin treatment in patients with Alzheimer disease. Arch. Neurol. 2003;60:510–515. doi: 10.1001/archneur.60.4.510. [DOI] [PubMed] [Google Scholar]
- 133.Wang H, Durham L, Dawson H, et al. An apolipoprotein E-based therapeutic improves outcome and reduces Alzheimer’s disease pathology following closed head injury: evidence of pharmacogenomic interaction. Neuroscience. 2007;144:1324–1333. doi: 10.1016/j.neuroscience.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 134.Buga GM, Frank JS, Mottino GA, et al. D-4F decreases brain arteriole inflammation and improves cognitive performance in LDL receptor-null mice on a Western diet. J. Lipid Res. 2006;47:2148–2160. doi: 10.1194/jlr.M600214-JLR200. [DOI] [PubMed] [Google Scholar]
- 135.Trevisan R, Dodesini AR, Lepore G. Lipids and renal disease. J. Am. Soc. Nephrol. 2006;17:S145–S147. doi: 10.1681/ASN.2005121320. [DOI] [PubMed] [Google Scholar]
- 136.Buga GM, Frank JS, Mottino GA, et al. D-4F reduces E06 immunoreactivity, SREBP-1c mRNA levels, and renal inflammation in LDL receptor-null mice fed a Western diet. J. Lipid Res. 2008;49:192–205. doi: 10.1194/jlr.M700433-JLR200. [DOI] [PubMed] [Google Scholar]
- 137.Baber U, Toto RD, de Lemos JA. Statins and cardiovascular risk reduction in patients with chronic kidney disease and end-stage renal failure. Am. Heart J. 2007;153:471–477. doi: 10.1016/j.ahj.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 138.Opal SM, Scannon PJ, Vincent JL, et al. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis. 1999;180:1584–1589. doi: 10.1086/315093. [DOI] [PubMed] [Google Scholar]
- 139.Wray GM, Foster SJ, Hinds CJ, Thiemermann C. A cell wall component from pathogenic and non-pathogenic gram-positive bacteria (peptidoglycan) synergises with endotoxin to cause the release of tumour necrosis factor-α, nitric oxide production, shock, and multiple organ injury/dysfunction in the rat. Shock. 2001;15:135–142. doi: 10.1097/00024382-200115020-00010. [DOI] [PubMed] [Google Scholar]
- 140.Emancipator K, Csako G, Elin RJ. In vitro inactivation of bacterial endotoxin by human lipoproteins and apolipoproteins. Infect. Immun. 1992;60:596–601. doi: 10.1128/iai.60.2.596-601.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Eggesbo JB, Hjermann I, Hostmark AT, Kierulf P. LPS induced release of IL-1 β, IL-6, IL-8 and TNF-α in EDTA or heparin anticoagulated whole blood from persons with high or low levels of serum HDL. Cytokine. 1996;8:152–160. doi: 10.1006/cyto.1996.0022. [DOI] [PubMed] [Google Scholar]
- 142.Gordon BR, Parker TS, Levine DM, et al. Low lipid concentrations in critical illness: implications for preventing and treating endotoxemia. Crit. Care Med. 1996;24:584–589. doi: 10.1097/00003246-199604000-00006. [DOI] [PubMed] [Google Scholar]
- 143.Hubsch AP, Casas AT, Doran JE. Protective effects of reconstituted high-density lipoprotein in rabbit gram-negative bacteremia models. J. Lab. Clin. Med. 1995;126:548–558. [PubMed] [Google Scholar]
- 144.Levine DM, Parker TS, Donnelly TM, Walsh A, Rubin AL. In vivo protection against endotoxin by plasma high density lipoprotein. Proc. Natl. Acad. Sci. USA. 1993;90:12040–12044. doi: 10.1073/pnas.90.24.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Garber DW, Datta G, Chaddha M, et al. A new synthetic class A amphipathic peptide analogue protects mice from diet-induced atherosclerosis. J. Lipid Res. 2001;42:545–552. [PubMed] [Google Scholar]
- 146.Datta G, White CR, Chaddha M, Palgunachari MN, Anantharamaiah GM, Smythis L. The ApoA-I mimetic peptide, 4F, inhibits LPS-induced inflammatory reactions through dysregulation of the NFkB activation pathway. Circulation. 2007 (Abstract) [Google Scholar]
- 147.Pajkrt D, Doran JE, Koster F, et al. Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J. Exp. Med. 1996;184:1601–1608. doi: 10.1084/jem.184.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pajkrt D, Lerch PG, van der Poll T, et al. Differential effects of reconstituted high-density lipoprotein on coagulation, fibrinolysis and platelet activation during human endotoxemia. Thromb. Haemost. 1997;77:303–307. [PubMed] [Google Scholar]
Patent
- 201.Dassuec J-L, Sekul R, Bitner K, Cornut I, Metz G, Dufourcq J. 1999 WO09916459. [Google Scholar]