Abstract
Diminished vascular endothelial cell nitric oxide (NO) production is a major factor in the complex pathogenesis of diabetes mellitus. In this report, we demonstrate that insulin not only maintains endothelial NO production through regulation of endothelial nitric oxide synthase (eNOS), but also via the regulation of argininosuccinate synthase (AS), which is the rate-limiting step of the citrulline-NO cycle. Using serum starved, cultured vascular endothelial cells, we show that insulin up-regulates AS and eNOS transcription to support NO production. Moreover, we show that insulin enhances NO production in response to physiological cues such as bradykinin. To translate these results to an in vivo model, we show that AS transcription is diminished in coronary endothelial cells isolated from rats with streptozotocin (STZ) -induced diabetes. Importantly, we demonstrate restoration of AS and eNOS transcription by insulin treatment in STZ-diabetic rats, and show that this restoration was accompanied by improved endothelial function as measured by endothelium-dependent vasorelaxation. Overall, this report demonstrates, both in cell culture and whole animal studies, that insulin maintains vascular function, in part, through the maintenance of AS transcription, thus ensuring an adequate supply of arginine to maintain vascular endothelial response to physiological cues.
Keywords: AS, insulin, streptozotocin, eNOS, vasorelaxation
Introduction
The hallmark of endothelial dysfunction is the inability of endothelial cells to release nitric oxide (NO) in response to physiological cues that promote vasodilation [1]. NO production in endothelial cells is supported by the citrulline-NO cycle which is comprised of three enzymes: the arginine recycling enzymes argininosuccinate synthase (AS) and argininosuccinate lyase (AL), and endothelial NO synthase (eNOS) [2]. Various studies, both in vitro and in vivohave demonstrated that arginine recycling is required for the physiologically regulated production of endothelial NO [3; 4; 5; 6].
Endothelial NO is a readily diffusible free radical that stimulates soluble guanylyl cyclase in smooth muscle cells to regulate smooth muscle relaxation and blood vessel dilation. Consequently, impaired production of NO by endothelial cells contributes to hypertension [1]. Since endothelial NO plays a critical physiological role in controlling vasodilation, the regulation of eNOS has been extensively investigated to better understand the mechanisms which regulate NO production. Several physiological factors, such as acetylcholine, bradykinin, and insulin are known to regulate eNOS to increase NO production by vascular tissues. Acetylcholine and bradykinin are acute regulators recognized to promote NO production by increasing eNOS activity through phosphorylation at serine-1177 (bovine S1179) [7; 8]. Insulin is likewise known to stimulate eNOS activity to promote NO production through phosphorylation; however, insulin has also been acknowledged to support NO production via maintenance of eNOS transcription [9; 10].
Importantly, it is also known that the loss of insulin sensitivity, which underlies the cause of type 2 diabetes, results in serious vascular complications, including hypertension [11]. Because of this, the vasodilatory effects of insulin have been extensively studied and shown to be mediated essentially through NO signaling. For example, in type 2 diabetes where insulin signaling is impaired [12; 13; 14], treatment with sodium nitroprusside (SNP), an NO donor, improved vasodilation [15]. The association of endothelial dysfunction with type 2 diabetes [16; 17] is further supported by reports showing that therapeutic interventions used to alleviate the insulin resistance also ameliorated endothelial dysfunction [18; 19].
Unlike eNOS, however, there is a limited understanding of the regulation of AS, which catalyzes the rate-limiting step in the citrulline-NO cycle in endothelial cells. The current literature suggests that AS mRNA levels appear to be coordinately regulated by physiological factors that regulate eNOS to affect endothelial NO production [20; 21]. For example, the pro-inflammatory cytokine TNFα has been shown to coordinately decrease eNOS and AS transcription in endothelial cells [5], while the PPARγ agonist troglitazone, as well as sheer-stress, have been shown to coordinately increase eNOS and AS transcription [22]. In the work presented here, we investigated how insulin mediates its effects on the citrulline-NO cycle, not only by affecting eNOS expression, but also AS expression in order to ensure that the levels of available arginine are sufficient to support eNOS-catalyzed NO production. To examine this question, we investigated whether insulin supports the expression of AS mRNA in vascular endothelial cells; and if so, if changes in AS mRNA levels mediated by insulin coordinate with eNOS mRNA levels. Two systems were used to investigate this question; cultured bovine aortic vascular endothelial cells and coronary endothelial cells isolated from streptozotocin-induced type 1 diabetic rats.
Materials and methods
Cell Culture
Bovine aortic endothelial cells (BAEC) were cultured in complete DMEM (1 g/L glucose, Mediatech) containing 10% fetal bovine serum (Hyclone Laboratories), 100 units/ml penicillin and 100 µg/ml streptomycin (Mediatech) at 37°C and 5% CO2.
Rat STZ-Diabetic In Vivo Model
Insulin-dependent diabetes was induced in male Sprague-Dawley rats (approximately 300 grams) by injecting 65 mg/kg streptozotocin (STZ) (Sigma, in 0.1 M citrate buffer, pH 4.5) into the peritoneal cavity. Control rats received vehicle injection only. Three experimental groups of animals were followed for 21 days: (i) STZ-diabetic rats (STZ, n = 8); (ii) age-matched control non-diabetic rats treated with vehicle only (Controls, n = 16), and (iii) STZ-diabetic rats receiving subcutaneously delivered insulin (STZ + INS, n = 4, for each dose). Insulin was delivered via a slow release pellet (LinPlant) implanted under the dorsal skin of the neck according to the manufacturer’s (LinShin Canada) instructions. The size of the pellet was chosen to deliver consistent daily doses of insulin from 1-5 IU/day, allowing maintainance of normoglycemia or mild to moderate hyperglycemia, if desired, in the animals. The pellets provided insulin delivery for up to 56 days.
Non-fasting blood glucose concentration and body weight of all animals were measured weekly and when animals were sacrificed. Blood glucose was measured using a One-Touch Ultra glucose meter (LifeScan). At 21 days, animals were euthanized and the hearts removed for isolation of coronary endothelial cells (CEC) as described by Zuidema et al [23]. Briefly, ventricular tissue in 20mM HEPES was digested with Liberase Blendzyme 3 (final 0.02-0.07 mg/ml; Roche) and CEC were isolated using biotinylated PECAM-1antibody (Sertec) and streptavidin-coated M280 magnetic beads (Invitrogen). Endothelial cells bound to beads were isolated using a magnetic stand to collect CEC. CEC for total RNA isolation were frozen in RNAlater (Applied Biosystems).
NO Determinations
Confluent BAECs were serum starved for 16 hr in DMEM without phenol red, and in the presence of 0.1% BSA (Fraction V, Sigma)then treated with 10 nM insulin, 10 µM bradykinin, both or neither for 4 hours. The total nitrite from cell culture media was measured using the 2,3-diaminonaphthalene (DAN) assay and BMG Fluostar Galaxy spectrofluorometer using an excitation wavelength of 360 nm and emission wavelength of 405 nm. [24].
Western Blot Analysis
BAECs were serum starved as described above followed by treatment with 10 nM insulin for 2 hr and then harvested and lysed by scraping in RIPA buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1X protease inhibitors (Calbiochem) in PBS). Protein concentrations were determined by BCA assay (Pierce) and equal amounts of protein were resolved on 4-15% Tris-HCl SDS-polyacrylamide gels (Bio-Rad) and blotted onto Immobilon PVDF (Millipore) for immunoblotting. Primary antibodies used include anti-AS and anti-eNOS (BD Transduction Labs) and anti-GAPDH (Novus Biologicals). Secondary antibodies used were peroxidase-conjugated goat anti-mouse or anti-rabbit IgG (Jackson ImmunoResearch Labs). Blots were visualized by chemiluminescence using ECL reagent (Pierce) and exposed to film.
RNA Isolation and Quantitative RT-PCR
To measure steady state RNA expression of AS and eNOS, total RNA from cultured BAEC and isolated rat CEC was isolated using Tri Reagent according to the manufacturer’s recommendations (MRC) and as described previously [25]. RNA was treated with DNase (Ambion) and quantitated prior to reverse transcription with the High Capacity cDNA kit (Applied Biosystems) according to the manufacturer’s instructions. Quantitative RT-PCR (qRT-PCR) was performed using the following probe/primer sets: bovine AS sense (5′- TCAGCAAGGAGTTTGTGGAGGAGT-3′) AS antisense (5′- ACACATACTTGGCTCCTTCTCGCT-3′) AS probe (5′-FAM ATCCAGTCCAGCGCACTGTACCAGGABHQ-3′); bovine eNOS sense (5′- TACATGAGCACGGAGATTGG-3′) and eNOS antisense (5′- AGCACAGCCAGGTTGATCTC-3′) detected with sybr green; and GAPDH sense (5′- CATGTTTGTGATGGGCGTGAACCA-3′), GAPDH antisense (5′- TGATGGCGTGGACAGTGGTCATAA-3′), GAPDH probe (5′-ROXN ATTGTCAGCAATGCCTCCTGCACCACCAABHQ-3′). Data for bovine gene expression levels was normalized to GAPDH. Rat mRNA levels were detected with the following Taqman Gene Expression Assays (Applied Biosystems) AS: Ass1 argininosuccinate synthetase 1, Rn00565808_g1; eNOS: Nos3 NO synthase 3, Rn02132634_s1; Rplp2: ribosomal protein, large P2, Rn01479927_g1. Data for rat gene expression levels was normalized to Rplp2.
Aortic Ring Preparation and Vascular Reactivity Measurement
Endothelium-dependent vascular reactivity was assessed as previously described [26]. Briefly, abdominal aortic rings were fixed on two stainless steel wires, one attached to a force transducer and the other attached to a micrometer. The rings were then lowered into a bath containing Krebs bicarbonate buffer, equilibrated for 1.5 hours, followed by pre-constriction with norepinephrine (10−6 mol/L), and the concentration-response relationships to acetylcholine (Ach, 10−10~10−6 mol/L) were determined by cumulative addition of Ach in half-log increments directly to the bath. In all rings, NO-mediated relaxation was verified at the end of the experiment using 100 mmol/L sodium nitroprusside (SNP), a spontaneous NO donor used to assess endotheliumindependent relaxation of aortic rings. The contraction tension was recorded and vasorelaxation in response to Ach or SNP was calculated as percent reduction from norepinephrine-induced tension.
Statistical analysis
All in vitro data were from at least three independent experiments. Results were expressed as the mean +/− standard error. The student’s t-test was used to note statistical significance at p < 0.05 between means of treated and control groups.
Results
Insulin treatment promotes AS and eNOS transcription to support NO production
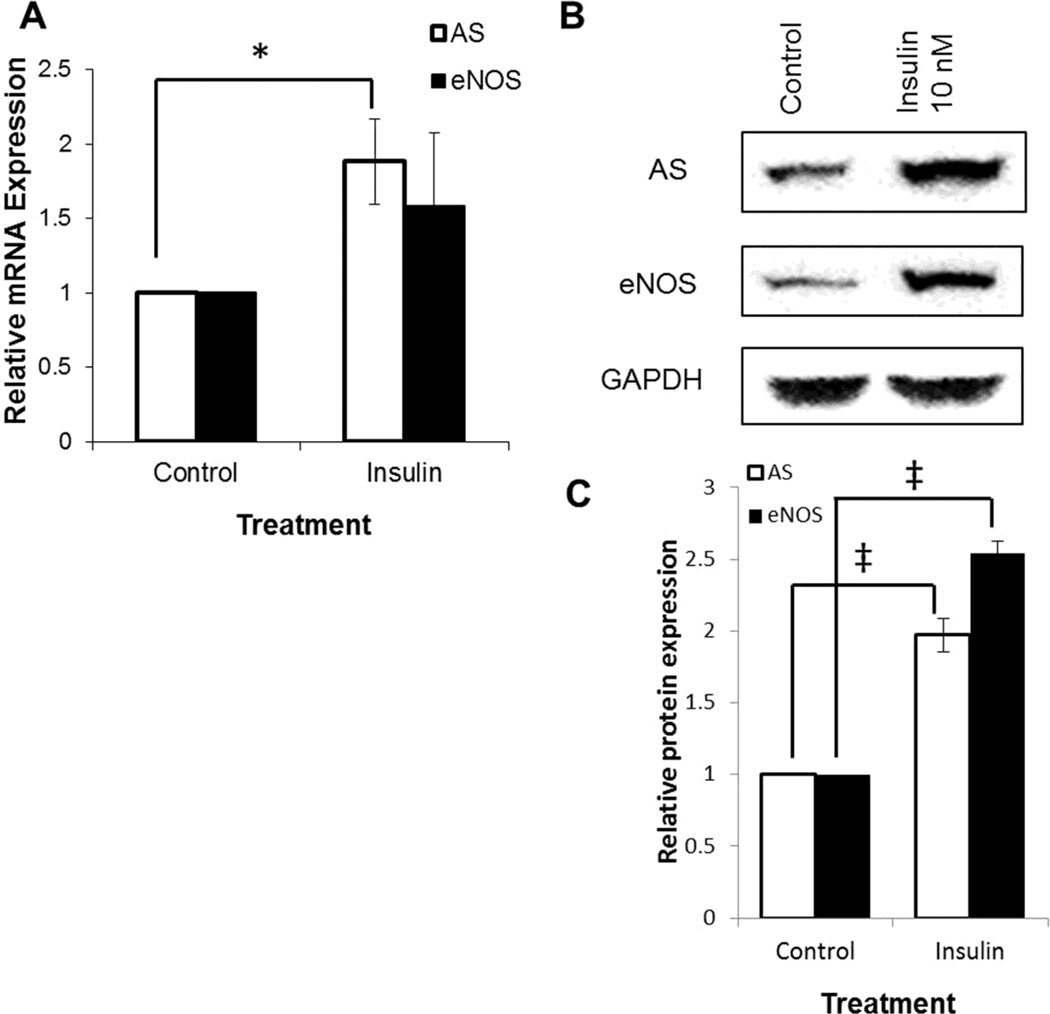
Since the expression of AS is necessary to support endothelial NO production [6; 27; 28; 29], we investigated whether the effects of insulin on vascular endothelial NO production may be mediated through AS, or whether the increase in NO production was simply due to established effects on eNOS activation [30; 31; 32]. To examine changes in AS transcription that may result from insulin treatment, BAECs were grown to confluence and then serum starved for 16 hours before treatment with insulin for 2 hours. RNA was prepared and quantitated by qRT-PCR, and as shown in Fig. 1A, there was almost a 2-fold increase in AS mRNA observed after treatment with 10 nM insulin. Similarly, eNOS mRNA levels increased 1.5-fold (near significance) in response to insulin treatment, and these results with eNOS were comparable to reported values [33].
Fig. 1.
Quantitation of eNOS and AS expression in cultured cells by qRT-PCR and western blotting. Confluent BAEC were serum starved for 16 hr and treated with 10 nM insulin for 2 hours. Control cells were untreated. (A) Total RNA, isolated from treated and control BAEC, was reverse transcribed and AS and eNOS mRNA levels were measured relative to GAPDH by qRT-PCR. (B) BAEC were lysed with RIPA buffer, equal amounts of protein were separated by SDS PAGE, and standard western blotting was performed using anti-AS, anti-eNOS, and anti- GAPDH antibodies. (C) Quantitation of the western blots relative to GAPDH expression respectively. (* p < 0.05, ‡p < 0.01)
With cultured endothelial cells, there was sufficient sample to verify whether mRNA levels corresponded to protein levels. Protein levels of AS and eNOS were determined by western blotting and, as shown in Fig. 1B & 1C, insulin treatment resulted in an increase in AS and eNOS protein that closely correlated with the corresponding mRNA changes. AS and eNOS expression increased 2.2-fold and 2.5-fold, respectively, as shown in Fig. 2B. These results demonstrated that insulin did indeed promote an increase in AS as well as eNOS expression.
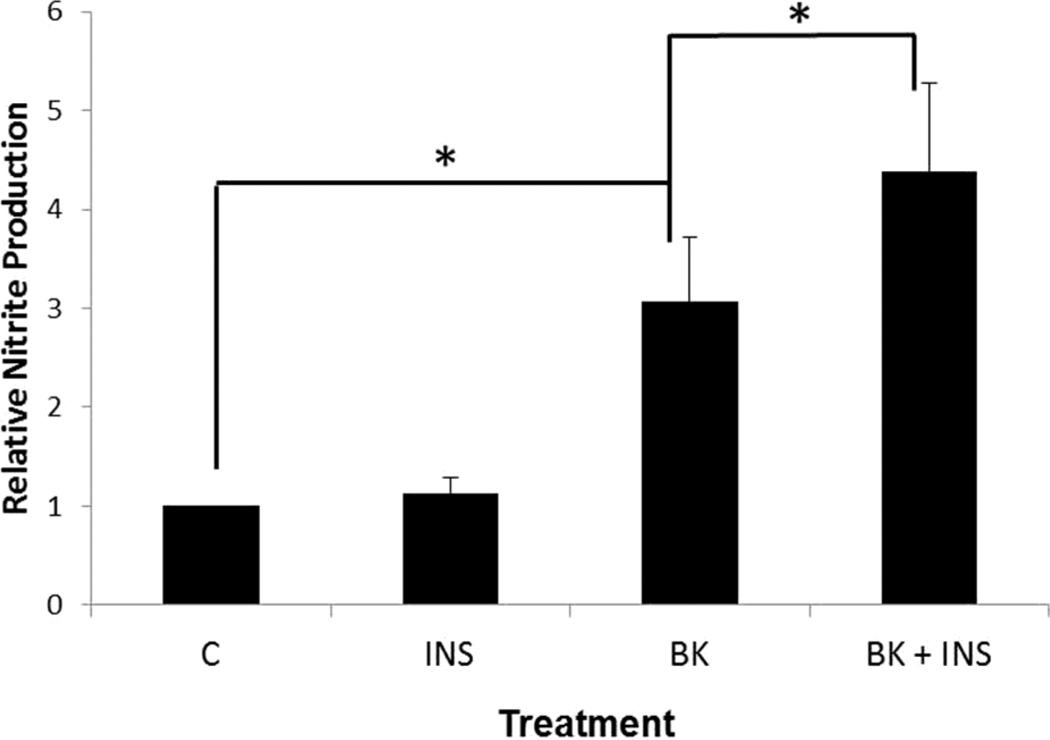
Fig. 2.
Measurement of NO production by stimulated BAEC. Confluent BAEC were serum starved 16 hr and NO production was stimulated by 10 µM bradykinin in the absence (BK) or presence (BK + INS) of 10 nM insulin for 4 hr. NO produced was compared to insulin alone (INS) and untreated BAEC (control). Total nitrite from cell culture media was measured using the 2,3-diaminonaphthalene (DAN) assay. Nitrite levels were normalized to total protein and reported as relative change from non-treated (control). (*p < 0.05)
To show that up-regulation of AS and eNOS expression by insulin corresponded to enhanced production of NO, cultured endothelial cells were treated with insulin under basal and stimulatory conditions. BAEC were serum starved for 16 hours and treated with or without insulin (10 nM) for 4 hours to measure the effect of insulin on basal NO levels. For stimulatory conditions, BAEC were again serum starved for 16 hours, and then treated with 10 µM bradykinin, with or without insulin (10 nM), for 4 hours. From both determinations, culture media was collected and nitrite, a stable metabolite of NO, was measured using the DAN assay. As shown in Fig. 2, no significant change in NO production was observed with treatment of insulin alone. However, under the stimulatory conditions of 10 µM bradykinin, insulin enhanced the levels of NO produced approximately 30% compared to cells treated with bradykinin alone.
Insulin restores AS expression in the streptozotocin-induced diabetic rat
To ensure that the insulin effects observed in cultured endothelial cells translate to vascular endothelial cells from whole animals, we investigated the effects of insulin on AS expression in the coronary endothelial cells isolated from STZ -treated diabetic rats. The STZ-diabetic rat is a well-characterized animal model of type 1 diabetes mellitus for metabolic studies [34; 35] and for evaluation of vascular function [20; 36], allowing us to address 1) whether treatment with insulin in STZ-induced diabetic rats restores AS expression, and 2) whether insulin restoration of AS expression restores endothelial function.
Because STZ selectively destroys insulin-producing beta cells of the pancreas [37], the effects of STZ treatment are to generate an animal which is insulin-dependent. This type 1 diabetes was confirmed in each animal by measuring serum glucose levels after treatment. Nonfasting hyperglycemia in the STZ-treated rats averaged 586.3 mg/dL blood glucose compared to 140.4 mg/dL blood glucose for the untreated, normal animals. Correspondingly, the hyperglycemia in the STZ-treated rats decreased with increasing doses of insulin that followed STZ treatment. For the ½ implant (25% normal insulin), the average level in blood glucose was found to average 462.8 mg/dL, a 21% reduction in blood glucose relative to STZ alone. For the 1 implant (50% normal insulin) blood glucose was reduced, on average, to 315.6 mg/dL for approximately 46% reduction in blood glucose; and for the 2 implants (100% normal insulin) the average blood glucose was found to be slightly below the values seen in the normal rats, at 124.5 mg/dL (78% reduction in blood glucose).
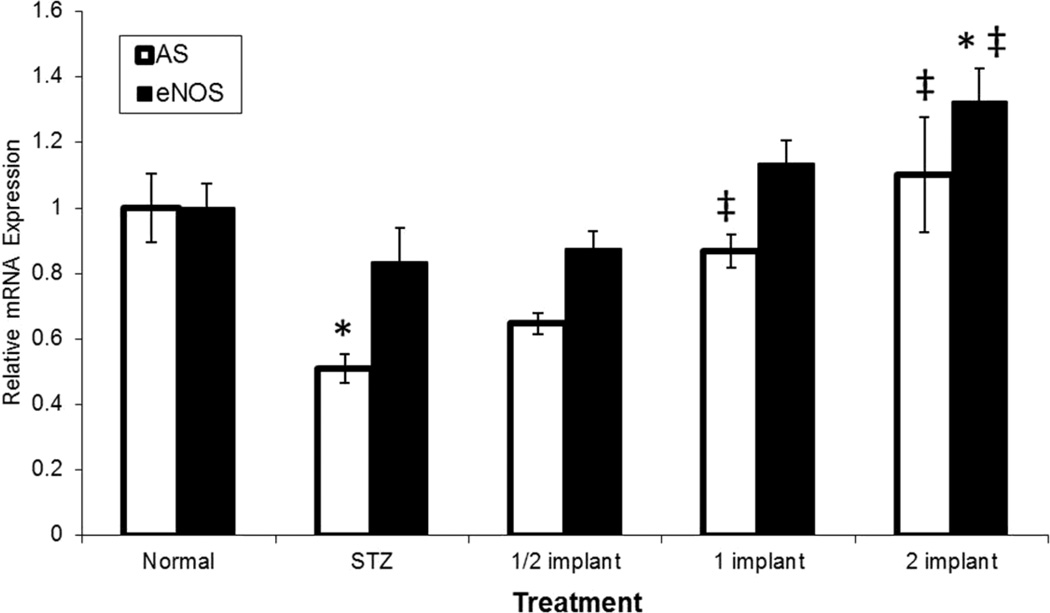
As shown in Fig. 3, qRT-PCR on the RNA from isolated coronary endothelial cells demonstrated a 50% decrease in AS mRNA levels in the STZ induced diabetic rat relative to control animals. A similar decrease, by approximately 20% in eNOS mRNA levels, was observed to result from STZ-treatment which was comparable with reported literature values [20]. Importantly, treatment with insulin was shown to restore both AS and eNOS transcription in a dose-dependent fashion, consistent with results from cultured endothelial cells where serum starved levels of AS and eNOS mRNAs were restored by treatment with insulin (Fig. 1).
Fig. 3.
Quantitation of eNOS and AS mRNA expression in STZ-induced diabetic rats by qRT-PCR. STZ-induced diabetic rats were compared to normal rats relative to insulin induced expression of AS and eNOS. Diabetic rats were randomly divided into four groups (n=4 per group). Three groups of diabetic rats had slow-release insulin pellets implanted under the dorsal skin of the neck at a dosage of 25% normal insulin (1/2 implant), 50% normal insulin (1 implant), and 100% normal insulin (2 implant) for 21 days. One group of diabetic rats received no implant (STZ). Non-diabetic (Normal) animals also received no implant. Total CEC RNA was reverse transcribed and AS and eNOS mRNA levels were quantitated relative to ribosomal protein, large P2 (RPLP2). (*p < 0.05 compared to control, ‡p < 0.05 compared to STZ alone)
Insulin restores acetycholine induced, NO mediated relaxation in vascular rings from streptozotocin induced diabetic rats
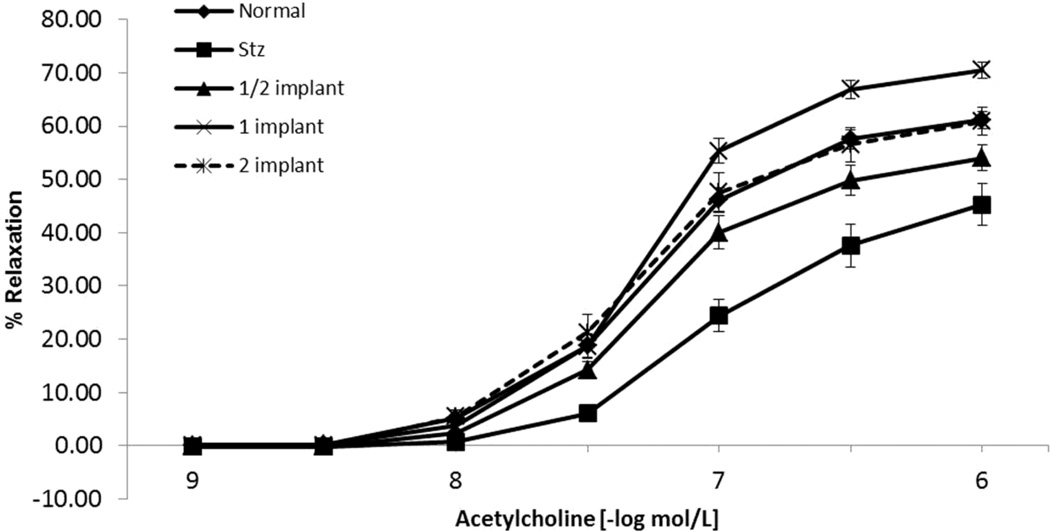
To demonstrate that reestablishment of AS and eNOS transcription relates to the physiological recovery of endothelial function, vasodilatory studies were carried out on isolated aortic rings from the STZ-treated rats. Not surprisingly, NO-dependent, acetylcholine-stimulated vasorelaxation was shown to be reduced in aortic rings taken from STZ-induced diabetic rats compared to those isolated from untreated animals (Fig. 4). Relaxation values (% of maximal non-endothelium-mediated relaxation by SNP) decreased from 61.21 ± 1.62% in the normal animals to 45.31 ± 3.97% in the STZ-diabetic animals. However, aortic vessel reactivity was shown to recover in rats treated with insulin implants as demonstrated by relaxation values of 54.03 ± 2.41% for ½ insulin implant, 70.53 ± 1.58% for 1 insulin implant, and 60.86 ± 2.65% for 2 insulin implants. The 1 insulin implant level actually improved vessel function to a greater extent than that observed in normal animals, consistent with its reduction in blood glucose.
Fig. 4.
Endothelium-dependent relaxation assessment of vascular rings isolated from treated STZ-induced diabetic rats. Rats were treated as described in Fig. 3. At 21 days, animals were euthanized and segments of abdominal aorta were dissected for vessel reactivity studies. Aortic rings were pre-constricted with norepinephrine and aortic vessel reactivity was measured in the presence of increasing concentrations of acetylcholine (10−10-10−6 M).
Discussion
Impairment of endothelial NO production plays a pivotal role in the pathogenesis of diabetes, compromising endothelial cell regulation of vascular function and homeostasis [38]. Therefore, deciphering the molecular mechanisms involved in the constitutive and stimulated production of NO by vascular endothelial cells is critical to understanding how physiological cues mediate their cardiovascular protective effects, thereby maintaining endothelial function, and how risk factors mediate their deleterious effects resulting in endothelial dysfunction.
In this study, the enhancement of AS expression by insulin in BAECs was shown to correspond to a similarly enhanced expression of eNOS, as well as to an augmented response to bradykinin, evidenced by increased NO production relative to bradykinin treatment alone. The fact that the response to NO stimulators, such a bradykinin, was enhanced by insulin suggests that one action of insulin may be to poise the endothelium to more effectively respond to physiological cues by promoting both AS and eNOS expression. This is important since the coordinate expression of AS is necessary to maintain an adequate source of arginine to support endothelial NO production [6]. If arginine availability is limited under conditions of no or limited insulin sensitivity, this could lead to enzymatic uncoupling of eNOS with subsequent production of reactive oxygen species [39].
Furthermore, using the STZ-treated diabetic rat, we showed that the absence of insulin resulted in diminished expression of AS mRNA, as well as eNOS mRNA; the latter having been well-established as important to insulin cardioprotective effects [30; 31; 32]. The loss of AS expression in coronary endothelial cells from STZ-treated diabetic rats suggested that insulin signaling is required to maintain a functional citrulline-NO cycle. Also noted was that aggressive insulin therapy with regards to the STZ-treated diabetic rats resulted in an increased expression of both eNOS and AS mRNA over that of the control with a matching improvement in vascular function.
In summary, this examination of insulin regulation of endothelial AS transcription provides an additional level of support for the physiological significance of AS in vascular endothelial NO production. These results suggest that insulin preserves optimal endothelial function by maintaining the appropriate expression of the major enzymatic constituents of the citrulline-NO cycle.
Highlights.
Comparison of insulin effects on argininosuccinate synthase and eNOS expression.
Insulin treatment restores argininosuccinate synthase mRNA expression.
Insulin promotes optimal expression of the citrulline-NO cycle components.
Acknowledgements
This work was supported by James and Esther King Biomedical Research Program Grant, DOH Florida; NIH R01 HL083153-01A2.
Abbreviations
- AS
argininosuccinate synthase
- eNOS
endothelial nitric oxide synthase
- NO
nitric oxide
- qRT-PCR
quantitative real time polymerase chain reaction
- CEC
coronary endothelial cells
- BAEC
bovine aortic endothelial cells
- STZ
streptozotocin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Potenza MA, Gagliardi S, Nacci C, Carratu MR, Montagnani M. Endothelial dysfunction in diabetes: from mechanisms to therapeutic targets. Curr Med Chem. 2009;16:94–112. doi: 10.2174/092986709787002853. [DOI] [PubMed] [Google Scholar]
- 2.Flam BR, Eichler DC, Solomonson LP. Endothelial nitric oxide production is tightly coupled to the citrulline-NO cycle. Nitric Oxide. 2007;17:115–121. doi: 10.1016/j.niox.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Erez A, Nagamani SC, Shchelochkov OA, Premkumar MH, Campeau PM, Chen Y, Garg HK, Li L, Mian A, Bertin TK, Black JO, Zeng H, Tang Y, Reddy AK, Summar M, O'Brien WE, Harrison DG, Mitch WE, Marini JC, Aschner JL, Bryan NS, Lee B. Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat Med. 17:1619–1626. doi: 10.1038/nm.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mun GI, Kim IS, Lee BH, Boo YC. Endothelial argininosuccinate synthetase 1 regulates nitric oxide production and monocyte adhesion under static and laminar shear stress conditions. J Biol Chem. doi: 10.1074/jbc.M110.180489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodwin B, Pendleton L, Levy M, Solomonson L, Eichler D. Tumor Necrosis Factor-{alpha} Reduces Substrate Availability for Nitric Oxide Production via Down-Regulation of Argininosuccinate Synthase. Am J Physiol Heart Circ Physiol. 2007;293:H1115–H1121. doi: 10.1152/ajpheart.01100.2006. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin BL, Solomonson LP, Eichler DC. Argininosuccinate synthase expression is required to maintain nitric oxide production and cell viability in aortic endothelial cells. J Biol Chem. 2004;279:18353–18360. doi: 10.1074/jbc.M308160200. [DOI] [PubMed] [Google Scholar]
- 7.Harris MB, Ju H, Venema VJ, Liang H, Zou R, Michell BJ, Chen ZP, Kemp BE, Venema RC. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem. 2001;276:16587–16591. doi: 10.1074/jbc.M100229200. [DOI] [PubMed] [Google Scholar]
- 8.Zecchin HG, Priviero FB, Souza CT, Zecchin KG, Prada PO, Carvalheira JB, Velloso LA, Antunes E, Saad MJ. Defective insulin and acetylcholine induction of endothelial cell-nitric oxide synthase through insulin receptor substrate/Akt signaling pathway in aorta of obese rats. Diabetes. 2007;56:1014–1024. doi: 10.2337/db05-1147. [DOI] [PubMed] [Google Scholar]
- 9.Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ, King GL. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo : a specific vascular action of insulin. Circulation. 2000;101:676–681. doi: 10.1161/01.cir.101.6.676. [DOI] [PubMed] [Google Scholar]
- 10.Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser (1179) J Biol Chem. 2001;276:30392–30398. doi: 10.1074/jbc.M103702200. [DOI] [PubMed] [Google Scholar]
- 11.Petrie JR, Ueda S, Webb DJ, Elliott HL, Connell JM. Endothelial nitric oxide production and insulin sensitivity. A physiological link with implications for pathogenesis of cardiovascular disease. Circulation. 1996;93:1331–1333. doi: 10.1161/01.cir.93.7.1331. [DOI] [PubMed] [Google Scholar]
- 12.Baron AD, Brechtel G, Johnson A, Fineberg N, Henry DP, Steinberg HO. Interactions between insulin and norepinephrine on blood pressure and insulin sensitivity. Studies in lean and obese men. J Clin Invest. 1994;93:2453–2462. doi: 10.1172/JCI117254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vollenweider P, Randin D, Tappy L, Jequier E, Nicod P, Scherrer U. Impaired insulin-induced sympathetic neural activation and vasodilation in skeletal muscle in obese humans. J Clin Invest. 1994;93:2365–2371. doi: 10.1172/JCI117242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, Andrews JW, Hayes JR. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:771–776. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Nieves B, Dunbar JC. Vascular dilatatory responses to sodium nitroprusside (SNP) and alpha-adrenergic antagonism in female and male normal and diabetic rats. Proc Soc Exp Biol Med. 1999;222:90–98. doi: 10.1111/j.1525-1373.1999.10000.x. [DOI] [PubMed] [Google Scholar]
- 16.Napoli C, Ignarro LJ. Nitric oxide and atherosclerosis. Nitric Oxide. 2001;5:88–97. doi: 10.1006/niox.2001.0337. [DOI] [PubMed] [Google Scholar]
- 17.Honing ML, Morrison PJ, Banga JD, Stroes ES, Rabelink TJ. Nitric oxide availability in diabetes mellitus. Diabetes Metab Rev. 1998;14:241–249. doi: 10.1002/(sici)1099-0895(1998090)14:3<241::aid-dmr216>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 18.Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci U S A. 2003;100:7996–8001. doi: 10.1073/pnas.1332551100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sechi LA, Melis A, Tedde R. Insulin hypersecretion: a distinctive feature between essential and secondary hypertension. Metabolism. 1992;41:1261–1266. doi: 10.1016/0026-0495(92)90019-7. [DOI] [PubMed] [Google Scholar]
- 20.Oyadomari S, Gotoh T, Aoyagi K, Araki E, Shichiri M, Mori M. Coinduction of endothelial nitric oxide synthase and arginine recycling enzymes in aorta of diabetic rats. Nitric Oxide. 2001;5:252–260. doi: 10.1006/niox.2001.0344. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin BL, Pendleton LC, Levy MM, Solomonson LP, E DC. Selective PPARγ Agonists Promote Vascular Endothelial NO Production Through the Up-regulation of Argininosuccinate Synthase. Molecular Endocrinology submitted. 2007 [Google Scholar]
- 22.Goodwin BL, Corbin KD, Pendleton LC, Levy MM, Solomonson LP, Eichler DC. Troglitazone up-regulates vascular endothelial argininosuccinate synthase. Biochem Biophys Res Commun. 2008;370:254–258. doi: 10.1016/j.bbrc.2008.03.089. [DOI] [PubMed] [Google Scholar]
- 23.Zuidema MY, Yang Y, Wang M, Kalogeris T, Liu Y, Meininger CJ, Hill MA, Davis MJ, Korthuis RJ. Antecedent hydrogen sulfide elicits an anti-inflammatory phenotype in postischemic murine small intestine: role of BK channels. Am J Physiol Heart Circ Physiol. 299:H1554–H1567. doi: 10.1152/ajpheart.01229.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleinhenz DJ, Fan X, Rubin J, Hart CM. Detection of endothelial nitric oxide release with the 2,3-diaminonapthalene assay. Free Radic Biol Med. 2003;34:856–861. doi: 10.1016/s0891-5849(02)01438-7. [DOI] [PubMed] [Google Scholar]
- 25.Pendleton LC, Goodwin BL, Flam BR, Solomonson LP, Eichler DC. Endothelial argininosuccinate synthase mRNA 5′-untranslated region diversity. Infrastructure for tissue-specific expression. J Biol Chem. 2002;277:25363–25369. doi: 10.1074/jbc.M111677200. [DOI] [PubMed] [Google Scholar]
- 26.Griffin KL, Laughlin MH, Parker JL. Exercise training improves endothelium-mediated vasorelaxation after chronic coronary occlusion. J Appl Physiol. 1999;87:1948–1956. doi: 10.1152/jappl.1999.87.5.1948. [DOI] [PubMed] [Google Scholar]
- 27.Xie L, Hattori Y, Tume N, Gross SS. The preferred source of arginine for high-output nitric oxide synthesis in blood vessels. Semin Perinatol. 2000;24:42–45. doi: 10.1016/s0146-0005(00)80054-3. [DOI] [PubMed] [Google Scholar]
- 28.Xie L, Gross SS. Argininosuccinate synthetase overexpression in vascular smooth muscle cells potentiates immunostimulant-induced NO production. J Biol Chem. 1997;272:16624–16630. doi: 10.1074/jbc.272.26.16624. [DOI] [PubMed] [Google Scholar]
- 29.Flam BR, Hartmann PJ, Harrell-Booth M, Solomonson LP, Eichler DC. Caveolar localization of arginine regeneration enzymes, argininosuccinate synthase, and lyase, with endothelial nitric oxide synthase. Nitric Oxide. 2001;5:187–197. doi: 10.1006/niox.2001.0340. [DOI] [PubMed] [Google Scholar]
- 30.Ritchie SA, Kohlhaas CF, Boyd AR, Yalla KC, Walsh K, Connell JM, Salt IP. Insulin-stimulated phosphorylation of endothelial nitric oxide synthase at serine-615 contributes to nitric oxide synthesis. Biochem J. 426:85–90. doi: 10.1042/BJ20091580. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Wang AX, Liu Z, Chai W, Barrett EJ. The trafficking/interaction of eNOS and caveolin-1 induced by insulin modulates endothelial nitric oxide production. Mol Endocrinol. 2009;23:1613–1623. doi: 10.1210/me.2009-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potenza MA, Addabbo F, Montagnani M. Vascular actions of insulin with implications for endothelial dysfunction. Am J Physiol Endocrinol Metab. 2009;297:E568–E577. doi: 10.1152/ajpendo.00297.2009. [DOI] [PubMed] [Google Scholar]
- 33.Fisslthaler B, Benzing T, Busse R, Fleming I. Insulin enhances the expression of the endothelial nitric oxide synthase in native endothelial cells: a dual role for Akt and AP-1. Nitric Oxide. 2003;8:253–261. doi: 10.1016/s1089-8603(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 34.Brosnan JT, Man KC, Hall DE, Colbourne SA, Brosnan ME. Interorgan metabolism of amino acids in streptozotocin-diabetic ketoacidotic rat. Am J Physiol. 1983;244:E151–E158. doi: 10.1152/ajpendo.1983.244.2.E151. [DOI] [PubMed] [Google Scholar]
- 35.Wu G, Meininger CJ. Impaired arginine metabolism and NO synthesis in coronary endothelial cells of the spontaneously diabetic BB rat. Am J Physiol. 1995;269:H1312–H1318. doi: 10.1152/ajpheart.1995.269.4.H1312. [DOI] [PubMed] [Google Scholar]
- 36.Pieper GM. Review of alterations in endothelial nitric oxide production in diabetes: protective role of arginine on endothelial dysfunction. Hypertension. 1998;31:1047–1060. doi: 10.1161/01.hyp.31.5.1047. [DOI] [PubMed] [Google Scholar]
- 37.Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976;193:415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- 38.Meininger CJ, Cai S, Parker JL, Channon KM, Kelly KA, Becker EJ, Wood MK, Wade LA, Wu G. GTP cyclohydrolase I gene transfer reverses tetrahydrobiopterin deficiency and increases nitric oxide synthesis in endothelial cells and isolated vessels from diabetic rats. Faseb J. 2004;18:1900–1902. doi: 10.1096/fj.04-1702fje. [DOI] [PubMed] [Google Scholar]
- 39.Huk I, Nanobashvili J, Neumayer C, Punz A, Mueller M, Afkhampour K, Mittlboeck M, Losert U, Polterauer P, Roth E, Patton S, Malinski T. L-arginine treatment alters the kinetics of nitric oxide and superoxide release and reduces ischemia/reperfusion injury in skeletal muscle. Circulation. 1997;96:667–675. doi: 10.1161/01.cir.96.2.667. [DOI] [PubMed] [Google Scholar]