Abstract
The trioxacarcins are polyoxygenated, structurally complex natural products that potently inhibit the growth of cultured human cancer cells. Here we describe syntheses of trioxacarcin A, DC-45-A1, and structural analogs by latestage, stereoselective glycosylation reactions of fully functionalized, differentially protected aglycon substrates. Key issues addressed in this work include the identification of an appropriate means to activate and protect each of the two 2-deoxysugar components, trioxacarcinose A and trioxacarcinose B, as well as a viable sequencing of the glycosidic couplings. The convergent, component-based sequence we present allows for rapid construction of structurally diverse, synthetic analogs that would be inaccessible by any other means, in amounts required to support biological evaluation. Analogs arising from modification of four of five modular components are assembled in 11 steps or fewer. The majority of these are found to be active in antiproliferative assays using cultured human cancer cells.
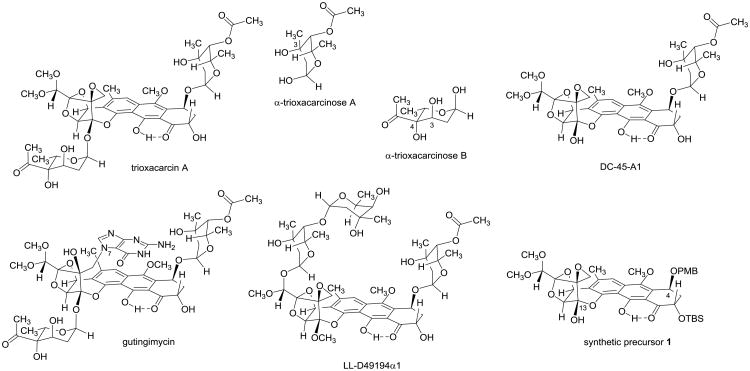
The trioxacarcins are bacterial metabolites of remarkable structural complexity that broadly inhibit the growth of cultured bacterial and eukaryotic cells.1–5 A number of unusual chemical features characterize the family, including a rigid, highly oxygenated polycyclic skeleton with a fused spiro epoxide function, as many as five ketal or hemiketal groups (three of them within a span of four contiguous carbon atoms) and one or more unusual glycosidic residues, eponymously identified as “trioxacarcinoses”. The most potent family member yet identified, trioxacarcin A (Figure 1), displays subnanomolar IC70 values in a number of different human cancer cell lines. Its extraordinary antiproliferative effects are believed to derive from the fact that trioxacarcin A efficiently and irreversibly alkylates G residues of duplex DNA, forming a covalent bond between the exocyclic carbon atom of the spiro epoxide function and N7 of the G residue that is alkylated. Both the DNA lesion and the product of depurination that is formed from it upon heating, a 1:1 adduct of guanine and trioxacarcin A (“gutingimycin”),6 have been crystallographically characterized in seminal work from researchers at the University of Göttingen.7
Figure 1. Trioxacarcin A and Structural Relatives.
Trioxacarcin A, a complex bacterial metabolite with potent growth-inhibitory properties, contains the unusual glycosidic residues trioxacarcinose A and trioxacarcinose B, whereas the related natural product DC-45-A1 contains only the trioxacarcinose A residue. Gutingimycin, also a bacterial isolate, is a covalent adduct of trioxacarcin A and guanine. Other close relatives of the trioxacarcins with distinct glycosylation patterns are known, as exemplified here by LL-D49194α1. In previous work in our laboratory, we prepared the differentially protected synthetic precursor 1, which we anticipated would enable synthetic access to the trioxacarcin class by selective, late-stage functionalization reactions.
Although so far as we are aware trioxacarcins have not been explored clinically in human cancer therapy, a phase I study of a closely related natural product with a distinct glycosylation pattern, LL-D49194α1 (Figure 1), was conducted in the early 1990s in 15 patients with diverse metastatic cancers that were refractory to existing therapies.8 While one patient in this study with colon cancer responded with improvement in performance that was sustained for 6 months, a fatality associated with cardiotoxicity led to suspension of the trial. A retrospective analysis suggested that the murine models that were used to determine dosing in the trial poorly predicted human pharmacokinetics; drug exposures ≥4-fold higher than anticipated were observed in patients. These insights would no doubt inform future clinical evaluations of trioxacarcins and their analogs, and suggest that one objective for structural refinement of the class, were this feasible, would be to identify analogs with diminished cardiotoxicity while retaining or enhancing antineoplastic effects. Structurally modified trioxacarcins might also facilitate the preparation of antibody-drug conjugates (ADCs), which are of considerable interest in light of a number of recent clinical successes of ADCs in cancer therapy.9 Although at least one natural trioxacarcin is available by fermentation (Maskey et al. reported the isolation of 257 mg of trioxacarcin A from 50 L of culture broth),5 we believed that a fully synthetic approach would greatly expand the number and diversity of trioxacarcins available for study beyond the relatively small pool of structures accessible through semi-synthesis.3
At the onset of our studies in 2005 the development of a scalable, practical and easily diversifiable synthetic route to molecules as complex as the trioxacarcins seemed an insurmountably difficult challenge. Inspired by this challenge, and the potential impacts a solution to the problem might have upon human medicine, we were led to embark upon a careful retrosynthetic analysis. From this, three criteria emerged as necessary and sufficient for a viable process: a practical solution must be modular, employing components of similar synthetic complexity, as well as highly convergent and scalable (the latter criterion being mitigated to some extent by the extraordinary potencies of the trioxacarcins). As an important first step toward this end in 2011 we reported a six-step route to a differentially protected trioxacarcin aglycon (“synthetic precursor 1” in Figure 1) by the assembly of three components of similar complexity.10 We had anticipated that this precursor would serve directly as a substrate for the introduction of carbohydrates such as trioxacarcinose B by glycosylation of the hemiketal hydroxyl group at position C13 or, after cleavage of the p-methoxybenzyl (PMB) ether protective group, would provide a substrate for selective introduction of carbohydrates such as trioxacarcinose A by glycosidation of the hydroxyl group liberated at position C4, which we expected to be more reactive. This expectation was borne out in practice.
In the present work we establish an appropriate means to protect and glycosidic subunits derived from trioxacarcinose A and trioxacarcinose B (Figure 1), to allow for their efficient and stereocontrolled coupling to a fully functionalized aglycon core, as well as a viable sequencing of the glycosylation reactions. Both carbohydrate subunits contain free hydroxyl groups that lie in a 1,3-diaxial relationship with an α-anomeric linkage to the core, and the issue of whether to protect these alcohols, and if so how, was of some concern, since it was easy to imagine many protection schemes that would disfavor α-glycosylation through steric interactions, but much less obvious how to promote the desired stereochemical outcome. We were particularly concerned about the feasibility of forming the “trehalose” glycosidic bond11 that links trioxacarcinose B to the sterically hindered and, we anticipated, poorly reactive hemiketal function of the polycyclic core. As outlined in Results below, we learned that in the case of coupling of trioxacarcinose A, protection of the 3-hydroxyl group was unnecessary, whereas for successful coupling of trioxacarcinose B protection of the 3-hydroxyl substituent was essential (protection of the tertiary alcohol function at position C4 was not required). We also learned that orthogonal modes of glycosidic activation were necessary for the sequential glycosidic couplings leading to trioxacarcin A, and that the ordering of the coupling reactions was critical. We also describe the modification of our completed route to trioxacarcin A to prepare fully synthetic analogs that would not be accessible by other means, and demonstrate that the majority of these analogs exhibit antiproliferative activity in assays using cultured human cancer cells.
Results
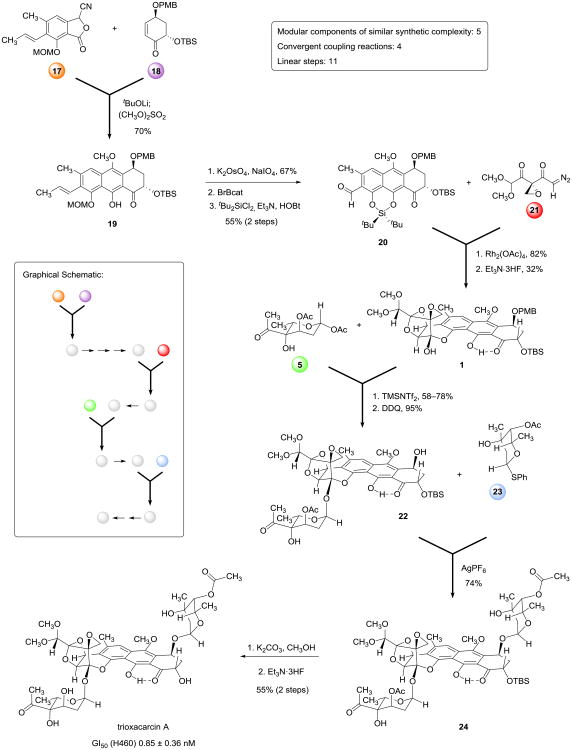
To pursue glycosylation studies we first refined our existing route to the key synthetic precursor 1,10 improving the efficiencies of both convergent coupling reactions (as detailed in the Supplementary Information), and prepared 690 mg of this substance in diastereomerically pure form in a single batch, a >10-fold scaling of our previously published route. The 6-step sequence with current yields is briefly summarized in the first part of Figure 4 below, which depicts the complete route to trioxacarcin A, and is reproduced in order to facilitate discussion of analog synthesis later, where the structures of the initial coupling components are varied.
Figure 4. Modular synthesis of trioxacarcin A.
An 11-step synthesis of trioxacarcin A was achieved by the convergent assembly of five modular components of similar structural complexity, each denoted with a colored circle. This strategy provides a general route to analogs with deep-seated structural variations.
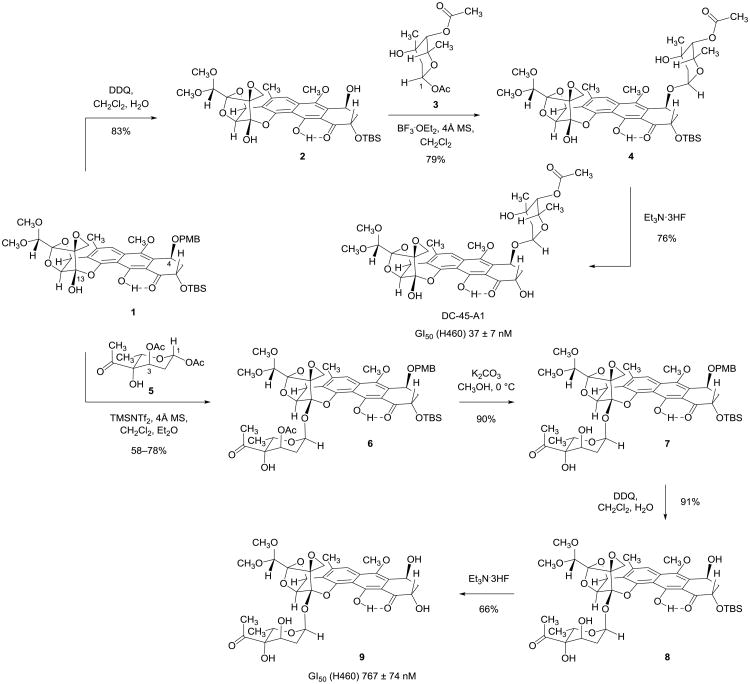
We began our glycosylation studies by investigating the selective introduction of trioxacarcinose A onto the C4-hydroxyl group of the diol substrate 2, formed in 83% yield upon exposure of the synthetic precursor 1 to 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) in dichloromethane–water (Figure 2). The trioxacarcinose A residue is common to many of the naturally occurring trioxacarcins, including the monoglycoside DC-45-A1, the bisglycoside trioxacarcin A, and the trisglycoside LL-D49194α1 (Figure 1).12 We elected to explore initial couplings with 1-O-acetyl trioxacarcinose A (3, Figure 2), which is available in multi-gram amounts by a short and practical sequence.13 We found that when a mixture of diol 2 (1 equiv), glycosyl donor 3 (2 equiv, a 1:12 mixture of α- and β-anomers, respectively), and powdered 4-Å molecular sieves in dichloromethane at −40 °C was treated with boron trifluoride etherate (1.0 equiv) as promoter the α-monoglycoside 4 was formed in 79% yield (23 mg). The β-configured glycosylation product was not observed. Cleavage of the tert-butyldimethylsilyl ether protecting group occurred upon exposure of the α-monoglycoside 4 to an excess of triethylamine trihydrofluoride (30 equiv) in acetonitrile at 23 °C (16 h), affording DC-45-A1 in 76% yield after purification by RP-HPLC (4.6 mg). 1H- and 13C-NMR, FT-IR, and MS data from the synthetic material were in agreement with values reported for the natural product.3 Definitive confirmation of identity was achieved by comparison of our synthetic product with an authentic sample of DC-45-A1; the samples were indistinguishable spectroscopically and chromatographically (TLC co-spot, RP-HPLC coinjection).
Figure 2. Syntheses of DC-45-A1 and the trioxacarcinose B-containing monoglycoside 9.
Stereoselective glycosylation reactions enabled preparation of monoglycosides containing either trioxacarcinose A or B residues in sequences of three and four steps, respectively. Importantly, both glycosidations preserve the spirocyclic epoxide function.
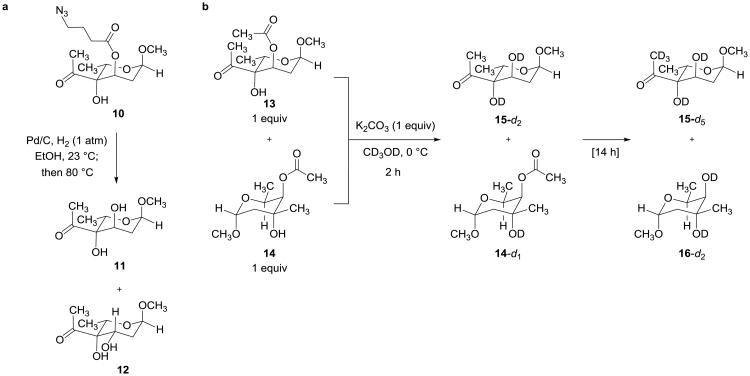
We next investigated the more challenging coupling chemistry that would enable formation of the α-trehalose-type linkage between the polycyclic core of substrate 1 and a suitably activated and protected trioxacarcinose B residue. Attempts to glycosidate substrate 1 with trioxacarcinose B donors bearing a free 3-hydroxyl group were not successful, and so we elected to protect the 3-hydroxyl function. In evaluating different protective groups, esters were given priority because they were considered to minimize a potentially destabilizing 1,3-diaxial steric interaction with the nucleophile in the required α-glycosidic bond formation. Because the trioxacarcinose A residue present in many natural trioxacarcins bears an acetate ester we initially sought to use an ester group other than acetate for protection of position C3 of the trioxacarcinose B donor, in order to avoid a problem of redundant functionality. 4-Azidobutyryl seemed a well-differentiated ester protective group; to evaluate it we first conducted a model study to explore conditions for its removal (Figure 3a). We observed that attempted deprotection of model substrate 1014 (hydrogenolysis in ethanol at 23 °C using Pd/C and 1 atm H2, followed by brief heating of the intermediate 4-aminobutyryl ester at 80 °C, to induce its cleavage) gave rise to a complex product mixture, from which both the expected C3-alcohol (11) and its epimer (12) were isolated (Figure 3a). The latter product was reasoned to have arisen from a retroaldol fragmentation-aldol cyclization sequence. This result demonstrated that 4-azidobutyryl was unsuitable as a protective group in the present context, and further suggested that the failure of our earlier efforts to introduce trioxacarcinose B using donors with free 3-hydroxyl groups might have been due to destruction of the sugar in paths initiated by (Lewis-acid promoted) retroaldol fragmentation. We were therefore led to examine in detail whether differentiation of the 3-hydroxyl group of trioxacarcinose B was actually required. A competition experiment suggested that it was not (Figure 3b). 1H NMR analysis (500 MHz, CD3OD) of a 1:1:1 mixture of methyl α-3-O-acetyl trioxacarcinoside B (13), methyl α-trioxacarcinoside A (14), and potassium carbonate showed that complete deprotection of the 3-O-acetyl protective group of the trioxacarcinose B residue occurred within 2 h at 0 °C to give methyl α-trioxacarcinoside B (15), while the acetate ester within the methyl α-trioxacarcinose A residue was unaffected (Figure 3b). Retrospectively, it is easy to rationalize the much greater stability of the α-trioxacarcinose A acetate ester toward methanolysis, for it is flanked on both sides by methyl groups, whereas the 3-O-acetyl protective group of the trioxacarcinose B residue has only one flanking methyl group.
Figure 3. Model studies leading to selection of O-acetyl for protection of the 3-hydroxyl group of trioxacarcinose B.
a, Hydrogenolysis of methyl α-3-O-(4′-azidobutyryl)trioxacarcinoside B (10)14 followed by brief heating produced a complex mixture containing both the desired product 11 and the epimerized product 12. The latter product likely arose from a retroaldol fragmentation-aldol cyclization sequence. b, Treatment of a mixture of methyl α-3-O-acetyl trioxacarcinoside B (13) and methyl β-trioxacarcinoside A (14) with potassium carbonate in methanol-d4 led to complete deprotection of the acetate ester of 13 within 2 h at 0 °C without affecting the more hindered acetate ester of methyl β-trioxacarcinoside A (14). Complete deprotection of both esters occurred upon prolonged stirring at 0 °C.
With evidence that 1-O-acetyl-3-O-acetyl trioxacarcinose B (5) might provide a viable donor en route to fully synthetic bisglycosides such as trioxacarcin A, we began to optimize the conditions for coupling this activated sugar with the differentially protected synthetic precursor 1 (Figure 2). We found that Lewis acids such as trimethylsilyl trifluoromethanesulfonate (TMSOTf) and N-trimethylsilyl-bis(trifluoromethanesulfonyl)imide (TMSNTf2)15 were preferred activating reagents. Over the course of several experiments we determined that the highest yields of coupled product were obtained when the ratio of glycosyl donor (5) to Lewis acid was ∼1.5:1. In an early successful coupling we treated a suspension of crushed, activated 4-Å molecular sieves, substrate 1 (1 equiv, 0.18 mM), and a large excess of donor 5 (30 equiv) in dichloromethane–ether (3:1) at −78 °C with excess TMSOTf (20 equiv)16,17 and obtained the α-glycoside 6 as the sole product in 78% yield after purification by RP-HPLC (30 mg, “example 1” in the Supplementary Information). By increasing the concentration of substrate 1 we could reduce the number of equivalents of donor 5 and Lewis acid that were necessary to achieve full conversion (8.5 equiv 5, “example 2,” 58% yield, 120 mg; 5.0 equiv 5, “example 3,” 63% yield, 65 mg; both employing TMSNTf2 as Lewis acid; see Supplementary Information). All attempts to couple substrate 1 with 3-O-acetyl trioxacarcinose B donors containing alternative anomeric substituents, groups such as 1-fluoro (α:β ∼ 1:3), 1-phenylthio (α:β ∼ 1:1.5), and 1-O-(4′-pentenyl) (α:β ∼ 1:1),14 were unsuccessful under a wide range of conditions explored.
Deprotection of the trioxacarcinose B-coupled product 6 was achieved in three steps (Figure 2). First, methanolysis of the acetate ester of the trioxacarcinose B residue using potassium carbonate as base afforded the alcohol 7 in 90% yield. Removal of the PMB ether protective group (DDQ, 91% yield) gave the benzylic alcohol 8; subsequent cleavage of the tert-butyldimethylsilyl ether group within this product (Et3N·3HF, 66% yield) then provided the novel trioxacarcinose B monoglycoside 9 as a yellow-green foam after careful purification by RP-HPLC.
With methods to couple (independently) both trioxacarcinoses A and B to polycyclic core substrates, we sought to coordinate the two couplings in order to prepare the bisglycoside trioxacarcin A. While in principle these transformations could be conducted in either sequence, in practice, it proved necessary to conduct the more challenging glycosidation with the trioxacarcinose B donor first, for when we attempted to glycosidate the trioxacarcinose A-containing monoglycoside 4 (Figure 2) with the trioxacarcinose B donor 5 using TMSOTf as promoter (conditions found to be effective for glycosidation of substrate 1) we observed only products arising from cleavage of the existing α-glycosidic bond. To investigate the alternative coupling sequence we first prepared the glycosyl acceptor 22 by removing the PMB ether protective group within monoglycoside 6 (DDQ, 1.1 equiv, 95% yield, Figure 4, middle). Attempted glycosylations of 22 with 1-O-acetyl trioxacarcinose A as donor (3) using Lewis-acidic promoters such as TMSOTf or boron trifluoride–etherate provided the desired α,α-bisglycoside 24 alongside a by-product in which the trioxacarcinose B residue had been cleaved (i.e., the monoglycoside 4 depicted in Figure 2). We therefore explored orthogonal activation conditions, and observed that addition of silver hexafluorophosphate (6.0 equiv) to a mixture of substrate 22 (65 mg, 1 equiv), 1-phenylthiotrioxacarcinoside A (23, 3.0 equiv, a 1:2.4 mixture of α- and β- anomers, respectively), 2,6-di-tert-butyl-4-methylpyridine (8.0 equiv) and powdered 4-Å molecular sieves in dichloromethane at 0 °C led to complete glycosylation within 1 h (Figure 4).18,19 The α,α-bisglycoside 24 was obtained as a greenish-yellow foam after purification by RP-HPLC (58 mg, 74%).
Selective cleavage of the trioxacarcinose B acetate ester protective group occurred upon exposure of the α,α-bisglycoside 24 to potassium carbonate for 1 h in ice-cold methanol (higher temperatures led to decomposition). 1H NMR analysis of the yellow-orange residue confirmed that methanolysis of the trioxacarcinose B acetate ester had occurred cleanly, and showed no evidence of any competing cleavage of the trioxacarcinose A acetate ester, as anticipated from model studies (Figure 3b above). The product was not purified, but was subjected to immediate deprotection with Et3N·3HF in acetonitrile at 23 °C (16 h). After isolation by RP-HPLC pure trioxacarcin A was obtained as an orange-red powder (23 mg, 55% yield over two steps). Spectroscopic data (1H NMR, 13C NMR, FT-IR, CD, and MS) were found to be identical with data we obtained from an authentic sample of natural trioxacarcin A.4,20 We observed that trioxacarcin A, like its precursor 24, is unstable toward base in alcoholic solvents; decomposition is evidenced by the formation of a deep burgundy-red solution (solutions of pure trioxacarcin A in ethanol and chloroform are typically orange).
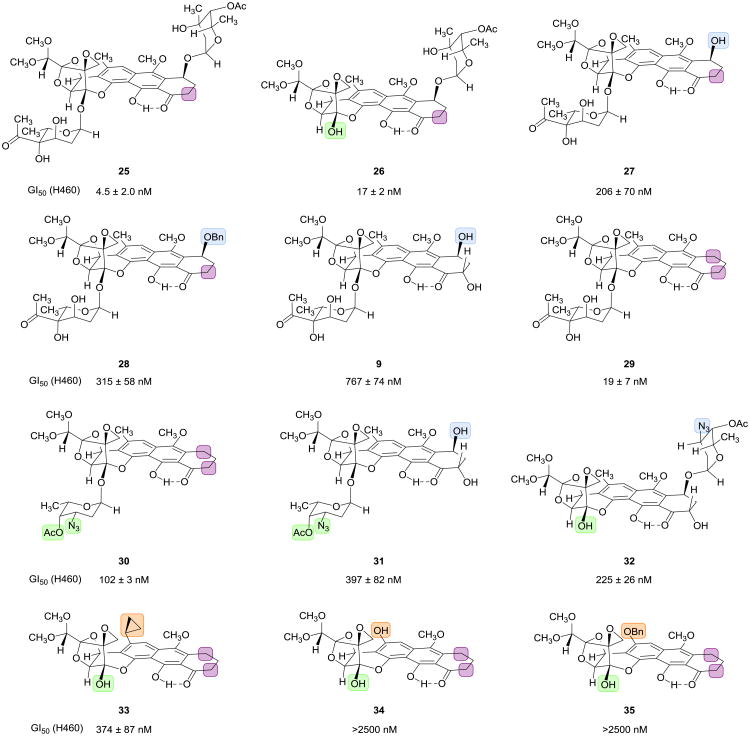
Having established a modular 11-step sequence for the assembly of trioxacarcin A from 5 components of similar synthetic complexity (identified with circles in Figure 4) we next sought to demonstrate the versatility of the sequence for the synthesis of analogs by component variation. Deep-seated structural modifications were introduced by variation of four of the five key building blocks (the cyanophthalide 17, the cyclohexenone 18, the trioxacarcinose B donor 5, and the trioxcarcinose A donor 23); the synthetic sequence used to prepare each analog was otherwise the same as the route to trioxacarcin A illustrated in Figure 4 (experimental details are provided in the Supplementary Information). Figure 5 depicts 12 compounds representative of more than 30 analogs that we have prepared to date by component variation. The analogs are depicted in a manner that highlights their structural differences relative to trioxacarcin A, using color to trace the origins of the variations to the components that were modified (identified in Figure 4). Below each structure in Figure 5 are listed average GI50 values21 we obtained in growth-inhibitory assays using cultured H460 cells, a human lung cancer cell line that is known to be especially sensitive to trioxacarcin A (we confirmed this, obtaining a GI50 value of 0.85 ± 0.36 nM for trioxacarcin A; DC-45-A1 was found to have a GI50 value of 37 ± 7nM—we are unaware of any previous measurements of growth-inhibitory activity for this natural product in any cell line).5 Of the 12 analogs depicted in Figure 5, 10 were found to be sub-micromolar inhibitors of cancer cell growth, a proportion that is representative of the larger pool of compounds synthesized. A detailed discussion of structure-activity relationships is beyond the scope of the present work, but it is evident that considerable structural variation around the polycyclic scaffold is tolerated.
Figure 5. Fully synthetic trioxacarcin analogs.
We prepared non-natural trioxacarcin analogs by varying 4 of the 5 modular components used in our synthesis of trioxacarcin A. Colored rectangles highlight structural differences relative to trioxacarcin A. Each of the analogs pictured was prepared in 11 or fewer chemical steps starting from modified modular components. The analogs prepared would be difficult or impossible to obtain by semi-synthesis.
Discussion
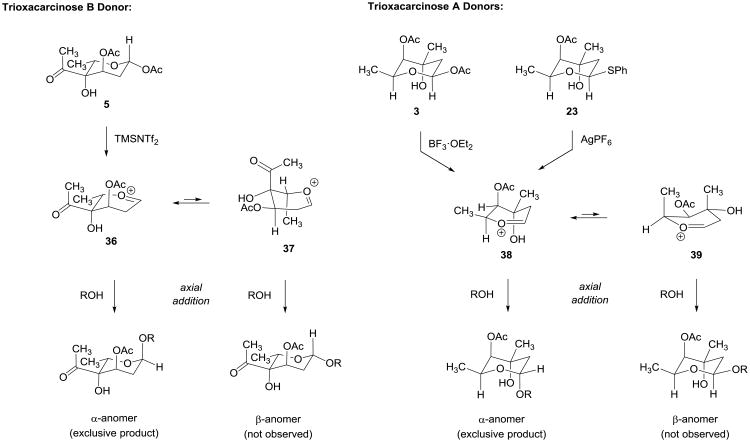
Conformational and stereoelectronic arguments can be advanced to explain the high α-selectivities observed in glycosylation reactions of trioxacarcinose A and B donors. We believe that axial addition of the glycosyl acceptor to the oxocarbenium ion 36 is relatively less impeded by 1,3-diaxial steric interactions than the alternative addition to the half-chair conformer 37 (Figure 6). Conformer 36 is also favored stereoelectronically by virtue of its axially disposed 3-acetoxy substituent.22–26 The stereochemical outcome of the glycosylation reaction is not that expected if the 3-acetoxy group had participated, as often occurs.27–28 A reviewer has suggested that this may be taken as evidence that the stereochemistry of the glycosylation reaction is under thermodynamic control, which we do not dispute. Similar considerations apply to explain the highly α-selective couplings of trioxacarcinose A donors 3 and 23. A remarkable and essential feature of glycosylation reactions of both trioxacarcinose A and B donors is that they are compatible with the spiro epoxide function of the many different core substrates containing it that we have examined. Evidence suggests that the epoxy group efficiently alkylates only nucleophiles that benefit from pi-stacking interactions with the aromatic core, such as a guanosine base of duplex DNA.29
Figure 6. Rationalizations of the stereochemical outcome of glycosylation reactions with trioxacarcinose A and B donors.
Axial addition to the stereoelectronically favored half-chair 36 is relatively less impeded by steric interactions than addition to the alternative conformer 37, offering one rationalization for the high α-selectivities observed in glycosylation reactions of trioxacarcinose B donor 5 (see text for another). Similar considerations apply to explain the highly α-selective couplings of trioxacarcinose A donors 3 and 23.
The synthetic sequence to trioxacarcin A we describe proceeds in 11 linear steps, four of these convergent coupling reactions, and assembles five components of comparable synthetic complexity: the cyanophthalide 17, the cyclohexenone 18, the epoxydiazodiketone 21, the trioxacarcinose A donor 23, and the trioxacarcinose B donor 5. Each of these components has been synthesized by a short scalable sequence in multi-gram amounts. A great virtue of the component-based assembly strategy that we have pursued both here and elsewhere30 is that deep-seated structural modifications can be introduced and explored relatively rapidly by independent modification of components, as illustrated by the subset of structures presented in Figure 5. Simultaneous structural variation of more than one component leads to multiplicative expansion of the pool of structures accessible for study.
Supplementary Material
Acknowledgments
T.M. acknowledges the Austrian Science Fund for a Schrödinger postdoctoral fellowship (FWF J3000-B11). D.J.S. acknowledges the Department of Defense for a National Defense Science and Engineering Graduate Fellowship. Financial support from the National Institutes of Health (Grant CA047148) is gratefully acknowledged. We thank Matthew Shair for helpful discussions. We thank Andreas Schumacher for assistance in the preparation of compounds 27 and 28, as detailed in the Supplementary Information. We gratefully acknowledge a gift of authentic samples of trioxacarcin A and DC-45-A1 from research scientists at Pfizer Research and Development in Groton, CT, as well as a gift of an authentic sample of trioxacarcin A from George Sheldrick at Universität Göttingen.
Footnotes
Author contributions: T.M., D.J.S., and A.G.M. conceived the synthetic route. T.M. and D.J.S. conducted all experimental work and analyzed the results. T.M., D.J.S., and A.G.M. wrote the manuscript.
Additional information: The authors declare no competing financial interests.
Supplementary information and chemical compound information accompany this paper at www.nature.com/naturechemistry.
References
- 1.Tomita F, Tamaoki T, Morimoto M, Fujimoto K. Trioxacarcins, novel antitumor antibiotics. I. Producing organism, fermentation and biological activities. J Antibiot. 1981;34:1519–1524. doi: 10.7164/antibiotics.34.1519. [DOI] [PubMed] [Google Scholar]
- 2.Tamaoki T, Shirahata K, Iida T, Tomita F. Trioxacarcins, novel antitumor antibiotics. II. Isolation, physico-chemical properties and mode of action. J Antibiot. 1981;34:1525–1530. doi: 10.7164/antibiotics.34.1525. [DOI] [PubMed] [Google Scholar]
- 3.Shirahata K, Iida T. U.S. Patent 4,459,291. Compounds having antibiotic activity, processes for their preparation, pharmaceutical compositions containing them and their use as medicaments. 1984
- 4.Tomita F, et al. U.S. Patent 4,511,560. Antibiotic substances DC-45, and their use as medicaments. 1985
- 5.Maskey RP, et al. Anti-cancer and antibacterial trioxacarcins with high anti-malaria activity from a marine Streptomycete and their absolute stereochemistry. J Antibiot. 2004;57:771–779. doi: 10.7164/antibiotics.57.771. [DOI] [PubMed] [Google Scholar]
- 6.Maskey RP, Sevvana M, Uson I, Helmke E, Laatsch H. Gutingimycin: A highly complex metabolite from a marine streptomycete. Angew Chem Int Ed. 2004;43:1281–1283. doi: 10.1002/anie.200352312. [DOI] [PubMed] [Google Scholar]
- 7.Pfoh R, Laatsch H, Sheldrick GM. Crystal structure of trioxacarcin A covalently bound to DNA. Nucleic Acids Res. 2008;36:3508–3514. doi: 10.1093/nar/gkn245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassidy J, et al. Phase I clinical study of LL-D49194α1 with retrospective pharmacokinetic investigations in mice and humans. Cancer Chemoth Pharm. 1993;31:395–400. doi: 10.1007/BF00686154. [DOI] [PubMed] [Google Scholar]
- 9.Gerber HP, Koehn FE, Abraham RT. The antibody-drug conjugate: an enabling modality for natural product-based cancer therapeutics. Nat Prod Rep. 2013;30:625–639. doi: 10.1039/c3np20113a. [DOI] [PubMed] [Google Scholar]
- 10.Švenda J, Hill N, Myers AG. A multiply convergent platform for the synthesis of trioxacarcins. Proc Natl Acad Sci USA. 2011;108:6709–6714. doi: 10.1073/pnas.1015257108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myers AG, Gin DY, Rogers DH. Synthetic studies of the tunicamycin antibiotics. Preparation of (+)-tunicaminyluracil, (+)-tunicamycin-V, and 5′-epi-tunicamycin-V. J Am Chem Soc. 1994;116:4697–4718. [Google Scholar]
- 12.Maiese WM, et al. LL-D49194 antibiotics, a novel family of antitumor agents: Taxonomy, fermentation and biological properties. J Antibiot. 1990;43:253–258. doi: 10.7164/antibiotics.43.253. [DOI] [PubMed] [Google Scholar]
- 13.Smaltz DJ, Švenda J, Myers AG. Diastereoselective additions of allylmetal reagents to free and protected syn-α,β-dihydroxyketones enable efficient synthetic routes to methyl trioxacarcinoside A. Org Lett. 2012;14:1812–1815. doi: 10.1021/ol300377a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magauer T, Myers AG. Short and efficient synthetic route to methyl α-trioxacarcinoside B and anomerically activated derivatives. Org Lett. 2011;13:5584–5587. doi: 10.1021/ol202315m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathieu B, Ghosez L. N-trimethylsilyl-bis(trifluoromethanesulfonyl)imide: A better carbonyl activator than trimethylsilyl triflate. Tetrahedron Lett. 1997;38:5497–5500. [Google Scholar]
- 16.Kimura Y, Suzuki M, Matsumoto T, Abe R, Terashima S. Trimethylsilyl trifluoromethanesulfonate (trimethylsilyl triflate) as an excellent glycosidation reagent for anthracycline synthesis: Simple and efficient synthesis of optically pure 4-demethoxydaunorubicin. Chem Lett. 1984;4:501–504. [Google Scholar]
- 17.Kimura Y, Suzuki M, Matsumoto T, Abe R, Terashima S. Novel glycosidation of 4-demethoxyanthracyclinones by the use of trimethylsilyl triflate. Syntheses of optically-active 4-demethoxydaunorubicin and 4-demethoxyadriamycin. B Chem Soc Jpn. 1986;59:423–431. [Google Scholar]
- 18.Lear MJ, Yoshimura F, Hirama M. A direct and efficient α-selective glycosylation protocol for the kedarcidin sugar, L-mycarose: AgPF6as a remarkable activator of 2-deoxythioglycosides. Angew Chem Int Ed. 2001;40:946–949. doi: 10.1002/1521-3773(20010302)40:5<946::AID-ANIE946>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 19.Ren F, Hogan PC, Anderson AJ, Myers AG. Kedarcidin chromophore: Synthesis of its proposed structure and evidence for a stereochemical revision. J Am Chem Soc. 2007;129:5381–5383. doi: 10.1021/ja071205b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirahata K, Iida T, Hirayama N. Symposium on the Chemistry of Natural Products. 1981;24:199–206. [Google Scholar]
- 21.Boyd MR, Pauli KD. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Develop Res. 1995;34:91–109. [Google Scholar]
- 22.Romero JAC, Tabacco SA, Woerpel KA. Stereochemical reversal of nucleophilic substitution reactions depending upon substituent: Reactions of heteroatom-substituted six-membered-ring oxocarbenium ions through pseudoaxial conformers. J Am Chem Soc. 2000;122:168–169. [Google Scholar]
- 23.Ayala L, Lucero CG, Romero JAC, Tabacco SA, Woerpel KA. Stereochemistry of nucleophilic substitution reactions depending upon substituent: Evidence for electrostatic stabilization of pseudoaxial conformers of oxocarbenium ions by heteroatom substituents. J Am Chem Soc. 2003;125:15521–15528. doi: 10.1021/ja037935a. [DOI] [PubMed] [Google Scholar]
- 24.Chamberland S, Ziller JW, Woerpel KA. Structural evidence that alkoxy substituents adopt electronically preferred pseudoaxial orientations in six-membered ring dioxocarbenium ions. J Am Chem Soc. 2005;127:5322–5323. doi: 10.1021/ja050830i. [DOI] [PubMed] [Google Scholar]
- 25.Yang MT, Woerpel KA. The effect of electrostatic interactions on conformational equilibria of multiply substituted tetrahydropyran oxocarbenium ions. J Org Chem. 2009;74:545–553. doi: 10.1021/jo8017846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedersen CM, Nordstrøm LU, Bols M. “Super armed” glycosyl donors: conformational arming of thioglycosides by silylation. J Am Chem Soc. 2007;129:9222–9235. doi: 10.1021/ja071955l. [DOI] [PubMed] [Google Scholar]
- 27.Crich D, Hu T, Cai F. Does neighboring group participation by non-vicinal esters play a role in glycosylation reactions? Effective probes for the detection of bridging intermediates. J Org Chem. 2008;73:8942–8953. doi: 10.1021/jo801630m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiesner K, Tsai TYR, Jin H. On cardioactive steroids. XVI. Stereoselective β-glycosylation of digitoxose: The synthesis of digitoxin. Helv Chim Acta. 1985;68:300–314. [Google Scholar]
- 29.Fitzner A, Frauendorf H, Laatsch H, Diederichsen U. Formation of gutingimycin: Analytical investigation of trioxacarcin A-mediated alkylation of dsDNA. Anal Bioanal Chem. 2008;390:1139–1147. doi: 10.1007/s00216-007-1737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun C, et al. A robust platform for the synthesis of new tetracycline antibiotics. J Am Chem Soc. 2008;130:17913–17927. doi: 10.1021/ja806629e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.