Abstract
Background
Cardiovascular disease such as coronary artery disease, stroke and congestive heart failure, is a leading cause of death worldwide. A postulated risk factor is elevated circulating total homocysteine (tHcy) levels which is influenced mainly by blood levels of cyanocobalamin (vitamin B12), folic acid (vitamin B9) and pyridoxine (vitamin B6). There is uncertainty regarding the strength of association between tHcy and the risk of cardiovascular disease.
Objectives
To assess the clinical effectiveness of homocysteine-lowering interventions (HLI) in people with or without pre-existing cardiovascular disease.
Search methods
We searched The Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library (issue 3 2008), MEDLINE (1950 to August 2008), EMBASE (1988 to August 2008), and LILACS (1982 to September 2, 2008). We also searched in Allied and Complementary Medicine (AMED; 1985 to August 2008), ISI Web of Science (1993 to August 2008), and the Cochrane Stroke Group Specialised Register (April 2007). We hand searched pertinent journals and the reference lists of included papers. We also contacted researchers in the field. There was no language restriction in the search.
Selection criteria
We included randomised clinical trials (RCTs) assessing the effects of HLI for preventing cardiovascular events with a follow-up period of 1 year or longer. We considered myocardial infarction and stroke as the primary outcomes. We excluded studies in patients with end-stage renal disease.
Data collection and analysis
We independently performed study selection, risk of bias assessment and data extraction. We estimated relative risks (RR) for dichotomous outcomes. We measured statistical heterogeneity using I2. We used a random-effects model to synthesise the findings.
Main results
We included eight RCTs involving 24,210 participants with a low risk of bias in general terms. HLI did not reduce the risk of non-fatal or fatal myocardial infarction, stroke, or death by any cause (pooled RR 1.03, 95% CI 0.94 to 1.13, I2 = 0%; pooled RR 0.89, 95% CI 0.73 to 1.08, I2 = 15%); and pooled RR 1.00 (95% CI 0.92 to 1.09, I2: 0%), respectively.
Authors’ conclusions
Results from available published trials suggest that there is no evidence to support the use of HLI to prevent cardiovascular events.
Medical Subject Headings (MeSH): Angina Pectoris [prevention & control], Cardiovascular Diseases [*prevention & control], Hyperhomocysteinemia [*therapy], Myocardial Infarction [prevention & control], Randomized Controlled Trials as Topic, Stroke [prevention & control], Vitamin B Complex [*therapeutic use]
MeSH check words: Humans
BACKGROUND
Description of the condition
The burden of cardiovascular disease
Cardiovascular disease (CVD) is the number one cause of death worldwide (Jamison 2006; WHO 2002). It covers a wide array of disorders, including diseases of the cardiac muscle and of the vascular system supplying the heart, brain and other vital organs (Gaziano 2006). The most common causes of CVD morbidity and mortality are ischaemic heart disease, stroke, and congestive heart failure (Gaziano 2006).
The burden of CVD is significant and ischaemic heart disease is the single largest cause of death in developed countries. Moreover, it is one of the main contributors to death in developing countries (Table 1; Gaziano 2006). This knowledge is useful when developing strategies for reducing morbidity, mortality and costs (Kahn 2008; Math 2007).
Table 1.
Burden of deaths attributable to cardiovascular diseases (%) (From Gaziano 2006)
| Region | % |
|---|---|
| Sub-Saharan Africa, parts of all regions excluding high-income regions | 5 to 10 |
| South Asia, southern East Asia and the Pacific, parts of Latin America and the Caribbean | 15 to 35 |
| Europe and Central Asia, northern East Asia and the Pacific, Latin America and the Caribbean, Middle East and North Africa, and urban parts of most low-income regions (especially India) |
> 50 |
| High-income countries, parts of Latin America and the Caribbean | < 50 |
The major risk factors for CVD include tobacco use, high blood pressure, high blood glucose, lipid abnormalities, obesity, and physical inactivity (Epstein 1996; Gaziano 2006; Narayan 2006; Rodgers 2006; WHO 2002; Willet 2006). However, there are other risk factors for CVD called ‘emergent or new risk factors’ (NACB 2009).
Homocysteine as a risk factor for cardiovascular disease
One of the emergent risk factors is elevated circulating total homocysteine (tHcy) levels. Homocysteine is an amino acid (Hackam 2003; NACB 2009; Nygård 1997; Prasad 1999; Selhub 2006; Ueland 1992) and several observational studies have shown that tHcy is a risk factor for CVD (Casas 2005; Danesh 1998; Davey Smith 2003; Eikelboom 1999; Ford 2002; Guthikonda 2006; Jacobsen 2005; HSC 2002; Nygård 1997; Refsum 1998; Splaver 2004; Stampfer 1992; Wald 2002; Wang 2005). The public significance of raised circulating tHcy has been considered recently (Shelhub 2008).
In 1962, it was hypothesised that increased levels of tHcy might cause vascular disease: the homocysteine theory of arteriosclerosis (McCully 2005). The pathways through which tHcy may cause damage to endothelial cells and lead to atherosclerosis have been widely described (Ferretti 2006; Jacobsen 2006; Jakubowski 2000; Jakubowski 2004; Jakubowski 2008; Obeid 2009; Riksen 2005; Zhou 2009).
Circulating tHcy is composed by protein (albumin)-homocysteine mixed disulfide, sulfhydryl form and low molecular weight disulfides (Mudd 2000). The normal levels of tHcy are close to 10 μmol/L (Mudd 2000). Hyperhomocysteinemia (HHcy) is defined as the presence of an abnormally elevated concentration of plasma or serum tHcy (Mudd 2000). However, there is some controversy to about defining the degree of HHcy. Fasting tHcy concentrations between 12 and 30 μmol/L are commutable termed mild or moderate, while intermediate HHcy includes levels between 31 to 100 μmol/L, and severe HHcy reflects values > 100 μmol/L (Maron 2006). In the general population, the prevalence of HHcy is between 5% and 10% (Refsum 1998). However, rates may be as high as 30-40% in the elderly population (Selhub 1993). According to the results of population-based studies, up to 10% of events due to CAD may be attributable to elevated circulating tHcy levels (Boushey 1995).
Description of the intervention
B-complex vitamins: cyanocobalamin (B12), folic acid (B9) and pyridoxine (B6) as a supplement.
How the intervention might work
The B-complex vitamins are required for transformation, excretion or both steps in the tHcy metabolism pathway (Castro 2006; Fowler 2005; Per a-Kaján 2007; Ramakrishnan 2006). Supplementation with B-complex vitamins reduces the tHcy levels (Clarke 2007; HLTC 2005). There is some ambiguity regarding the function of pyridoxine (vitamin B6): vitamin B6 supplementation has been shown to lower tHcy levels after methionine load, which is an experimental situation and it is believed that it is a weak determinant of circulating tHcy levels. However, at least two studies showed the contrary (Gori 2007; Sofi 2008).
Why it is important to do this review
Elevated circulating tHcy is a powerful prognostic marker of mortality and CVD events in patients with pre-existing CVD risk factors, but evidence is not sufficient to conclude that moderately raised tHcy causes CVD (Refsum 2006). Recently, a meta-analysis concluded that “each increase of 5 μmol/L in tHcy levels increases the risk of CHD events by approximately 20%, independently of traditional CHD risk factors” (Humphrey 2008).
Many studies have shown that supplementation with B-complex vitamins reduces the circulating tHcy (Folsom 1998; HLTC 2005; Jacques 1999; Robinson 1998; Verhaar 2002). However, there is uncertainty regarding the strength of association between tHcy and the risk of CVD. There is some evidence from controlled trials that the postulated association may be weaker than previously believed (B-VTTC 2006). According to National Institutes of Health the annuals sales of supplements in the United States are at about $23 billion, “a substantial share of which is spent on vitamins and minerals” (NIH 2006). This economic burden could be reduced if the true role of tHcy in CVD is determined. Unfortunately, to our knowledge, we lack the data of the savings which might explain how often these supplements are taken for the specific purpose of lowering tHcy levels.
The first systematic review on this issue included twelve randomised controlled trials (RCT) (16,958 participants) that compared folic acid supplementation with either placebo or usual care for a minimum duration of six months found no benefit of the intervention (Bazzano 2006). Limitations of this systematic review are: it only evaluated a folic acid supplementation; there is a risk of publication bias (only included search in MEDLINE); it evaluated studies with a follow-up period of less than 12 months. A second systematic review assessed the impact of folic acid (vitamin B9) for preventing stroke (Wang 2007). They concluded that the intervention is beneficial in reducing the risk of stroke (RR 0.82, 95% CI 0.68 to 1.00). However, this systematic review did not describe statistical heterogeneity (I2), it included papers with short follow up (less than six months), the forest plot is absent, sensitivity analysis data were not shown, and the intervention group also included regimens incorporating cyanocobalamin (B12) and pyridoxine (B6) vitamins in combination with folic acid (Wang 2007). Spence 2007 suggested also that folic acid could reduce stroke incidence. His argument was based on HOPE-2 2006 and a sub-study from VISP 2004.
Five RCT comparing B6, B9 and B12 vitamins to placebo demonstrated that homocysteine-lowering interventions (HLI) are not effective for preventing CVD (NORVIT 2006, HOPE-2 2006; VISP 2004; WAFACS 2008; WENBIT 2008). Thus, controversy remains on whether tHcy is or not a marker or causal agent of the CVD (Guilland 2003; Moat 2008; McNulty 2008; Potter 2008b; Ueland 2000).
This review aims to address the question: what is the clinical effectiveness and safety of HLI compared to placebo or low-dose vitamins B6, B9 and B12 for preventing cardiovascular events?
OBJECTIVES
To determine whether HLI in those with and without pre-existing cardiovascular disease:
(a) are effective for preventing cardiovascular events and death (all cause mortality);
(b) are safe for preventing cardiovascular events;
(c) differ in efficacy or safety.
METHODS
Criteria for considering studies for this review
Types of studies
Randomised controlled trials with a follow-up period of 1 year or longer.
Types of participants
Adults (> 18 years) at risk of or with established cardiovascular disease. Studies with patients with end-stage renal disease were excluded.
Types of interventions
The interventions considered were vitamins B6, B9 or B12 given alone or in combination, at any dosage and via any administration route. Comparisons were made with placebo, or with differing regimes of vitamins B6, B9 or B12. When the included population was at risk, combinations of HLI with standard treatment (such as antihypertensives and statins) were considered as long as the same standard treatment was given to the control.
Types of outcome measures
Primary outcomes
(1) Non-fatal or fatal myocardial infarction.
(2) Non-fatal or fatal stroke (ischemic or hemorrhagic).
Secondary outcomes
(1) First unstable angina pectoris episode requiring hospitalisation.
(2) Hospitalisation for heart failure.
(3) Mortality by any cause.
(4) Serious or non-serious adverse events.
We defined serious adverse events according to the International Conference on Harmonisation (ICH) Guidelines (ICH-GCP 1997) as any event that leads to death, is life-threatening, requires hospitalisation or prolongation of existing hospitalisation and/or results in persistent or significant disability. All other adverse events will be considered non-serious.
Search methods for identification of studies
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library (issue 3 2008), MEDLINE (1950 to August 2008), EMBASE (1988 to the August 2008), LILACS (1982 to September 2, 2008), Allied and Complementary Medicine (AMED; 1985 to August 2008) and ISI Web of Science (1993 to August 2008). The search strategies are in Appendix 1. We searched the Cochrane Heart Group Controlled Trials Register and asked the Cochrane Stroke group and the Cochrane Complementary Medicine group to check their Specialised Registers. We also checked the reference lists of all trials identified by the above methods. No language restrictions were applied.
The following web sites also were searched: http://www.clinicaltrials.gov (September 2, 2008).
http://controlled-trials.com/isrctn/search.asp (September 2, 2008).
We contacted authors and researchers to obtain further details for unpublished studies.
Data collection and analysis
Two authors, Arturo Martí-Carvajal (AMC) and Ivan Solà (IS) assessed each reference identified by the searches to see if they meet the inclusion criteria. AMC, IS and GS independently assessed the risk of bias of the trials according to the Cochrane Handbook (Higgins 2008). We examined the adequacy of the methods used to generate the allocation sequence; the concealment of allocation; and the level of blinding (clinician, participants). We also evaluated the risk associated with dropouts, as estimated by the percentage of participants lost.
We used the following definitions:
Generation of the allocation sequence
Adequate, if the allocation sequence was generated by a computer or random number table. Drawing of lots, tossing of a coin, shuffling of cards, or throwing dice will be considered as adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure.
Unclear, if the trial was described as randomised, but the method used for the allocation sequence generation was not described.
Inadequate, if a system involving dates, names, or admittance numbers was used for the allocation of patients. These studies are known as quasi-randomised and will be excluded from the present review when assessing beneficial effects.
Allocation concealment
Adequate, if the allocation of patients involved a central independent unit, on-site locked computer, identically appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed envelopes.
Unclear, if the trial was described as randomised, but the method used to conceal the allocation was not described.
Inadequate, if the allocation sequence was known to the investigators who assigned participants or if the study was quasi-randomised.
Blinding (or masking)
Blinding has been assessed for all main outcomes together and has been characterised as:
Adequate, if the participants of the trial were blinded to the intervention.
Unclear, if there is no information on blinding.
Not performed, if the participants were not blinded to the intervention.
We did not use blinding of outcome assessors as possible source of bias. According to a recent study (Wood 2008), this might be irrelevant when the outcomes are subjective as it is the case in this review.
Follow-up
Adequate, if the numbers and reasons for drop-outs and withdrawals in all intervention groups were described and where comparable between groups.
Unclear, if the report gave the impression that there had been no drop-outs or withdrawals, but it is unclear whether the analysis included missing data in an adequate manner
Inadequate, if the number or reasons for drop-outs and withdrawals was either unbalanced between groups, differ in reason or was high enough to alter the effect of the intervention. To judge the later, we compared to proportion of dropouts to the event rate.
We grouped the studies into two categories; low risk of bias when the allocation concealment was adequate and participants and the providers of care where blinded to the allocation and high risk of bias otherwise.
AMC, IS and Dimitris Lathyris (DL) extracted data and Georgia Salanti checked for discrepancies.
The first author of the paper was contacted if data were missing. We extracted data on the number of participants by allocated treatment group, irrespectively of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded from treatment or follow up. If we were not able to do so, we recorded for each study whether the results pertained to an intention-to-treat analysis or to available-cases analysis.
We quantified statistical heterogeneity using I2, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). We summarized the findings using a random-effects model, as differences were anticipated in terms of interventions and patients. In case of significant heterogeneity we have planned to identify possible causes of heterogeneity by exploring the effect of participant, intervention and study design characteristics.
Subgroup analysis was performed according to the type of intervention.
We conducted a sensitivity analysis comparing the results using all studies and using only those of low risk of bias. We assessed whether the review was possibly subject to publication bias by using a funnel plot.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
We identified 4,318 references from our search strategy. From these we excluded 4,299 references after examining the title and abstract because they were not relevant. Full reprints of the remaining 19 references were obtained for more detailed examination. This led to the identification of four further publications from their references lists. We finally included 8 RCT (24,210 participants) in 11 articles published between 2002 to 2008. These are described in Characteristics of included studies. The length of follow-up ranged from 1 to 7.3 years. They varied in size, characteristics of participant populations, duration, drug dosage, and experimental design.
Three studies were classified as ongoing studies (SEARCH 2000, SU.FOL.OM3 2003, VITATOPS 2002). These are described in the Characteristics of ongoing studies.
Included studies
Seven trials were conducted in patients with prior cardiovascular heart disease (CVD) such as coronary artery disease, myocardial infarction, stable angina, unstable angina, stroke, intermittent claudication (CHAOS 2002; FOLARDA 2004; GOES 2003; HOPE-2 2006; NORVIT 2006; WAFACS 2008; WENBIT 2008); and a further study included explicitly patients with history of nondisabling cerebral infarction (VISP 2004).
Seven trials included patients with at least one the following known cardiovascular risk factors: diabetes mellitus, hypertension elevated total cholesterol, current smoking and low HDL cholesterol (FOLARDA 2004; GOES 2003; HOPE-2 2006; NORVIT 2006; VISP 2004; WAFACS 2008; WENBIT 2008). This aspect was unclear for CHAOS 2002. WAFACS 2008 included patients with three or more coronary risk factors. One trial explicitly excluded patients with previously-known HHcy (total plasma homocysteine > 18 μmol/L) (FOLARDA 2004).
HOPE-2 2006 included patients without history of CHD. WAFACS 2008 was conducted only with female patients.
Six trials were conducted with more than 1000 patients (CHAOS 2002; HOPE-2 2006; NORVIT 2006; VISP 2004; WAFACS 2008; WENBIT 2008).
Seven studies were controlled with placebo (CHAOS 2002; HOPE-2 2006; NORVIT 2006; WAFACS 2008; WENBIT 2008) or standard care (FOLARDA 2004; GOES 2003) while one study was a randomised controlled trial (VISP 2004) that compared high versus low dose of HLI.
Three trials only included vitamin B9 as intervention (CHAOS 2002; FOLARDA 2004; GOES 2003). Five trials included B6, B9 and B12 vitamins, in any combination, as intervention (HOPE-2 2006; NORVIT 2006; VISP 2004; WAFACS 2008; WENBIT 2008). Further details can be found in Table 2.
Table 2.
Composition of intervention and control groups
| Study | Year | Folic acid (mg/d) | Vitamin B12 (mg/d) | Vitamin B6 (mg/d) | Control |
|---|---|---|---|---|---|
| CHAOS-2 | 2002 | 5 | None | None | Placebo |
| FOLARDA | 2004 | 5 | None | None | Standard care |
| GOES | 2003 | 0.5 | None | None | Standard care |
| HOPE-2 | 2006 | 2.5 | 1 | 50 | Placebo |
| NORVIT | 2006 | Group A: 0.8; Group B: 0.8; Gruop C: none | Group A: 0.4; Group B: 0.4; Group C: none | Group A: none; Group B: none; Group C: 40 | Placebo |
| VISP | 2004 | 2.5 | 0.4 | 25 | Folic acid: 0.02; Vit.B12: 0.06; Vita B6: 0.2 |
| WAFACS | 2008 | 2.5 | 1 | 50 | Placebo |
| WENBIT | 2008 | Group A: 0.8; Group B: 0.8; Gruop C: none | Group A: 0.4; Group B: 0.4; Group C: none | Group A: none; Group B: none; Group C: 40 | Placebo |
FOLARDA 2004; GOES 2003; HOPE-2 2006; NORVIT 2006; WAFACS 2008, and WENBIT 2008 described lipid-lowering drugs used as concomitant medication.
Two trials (VISP 2004; WAFACS 2008) were conducted in fortified population described as “…nutritional intervention programme with a specifically defined target, and fortified food products are expected to become a main source of the specific added nutrient” (Wirakartakusumah 1998). One further trial was performed with mixed population (HOPE-2 2006), and the rest were carried out in not-fortified population (CHAOS 2002; FOLARDA 2004; GOES 2003; NORVIT 2006; WENBIT 2008).
Seven studies had composite outcomes in their analyses (CHAOS 2002; FOLARDA 2004; GOES 2003; HOPE-2 2006; NORVIT 2006; WAFACS 2008; WENBIT 2008). Four studies included revascularization or other vascular procedure (CHAOS 2002; GOES 2003; WAFACS 2008; WENBIT 2008). Seven included stroke as end point (FOLARDA 2004; GOES 2003; HOPE-2 2006; NORVIT 2006; VISP 2004; WAFACS 2008; WENBIT 2008). All assessed the impact of the intervention in the myocar-dial infarction rates. None of the studies included pectoris angina as component of composite outcomes. (see also in Table 3).
Table 3.
Components of the composite outcomes
| Study | Year | Components |
|---|---|---|
| CHAOS-2 | 2002 | Non-fatal myocardial infarction; cardiovascular death; unplanned revascularization |
| FOLARDA | 2004 | Cardiovascular death (sudden death; fatal recurrent myocardial infarction; fatal stroke; death from cardiovascular) |
| GOES | 2003 | Death from cardiovascular cause; death from non-cardiovascular cause; recurrent myocardial infarction; invasive coronary procedures; stroke; other vascular surgery |
| HOPE-2 | 2006 | Death from cardiovascular; myocardial infarction; stroke. |
| NORVIT | 2006 | Myocardial infarction (fatal and non-fatal); stroke (fatal and non-fatal); sudden death attributed to coronary heart disease |
| WAFACS | 2008 | Myocardial infarction; stroke; revascularization; death from cardiovascular cause |
| WENBIT | 2008 | All-cause death; nonfatal acute myocardial infarction; acute hospitalisation for unstable angina pectoris; non-fatal thromboembolic stroke |
Seven studies reported sample size calculation (FOLARDA 2004; GOES 2003; HOPE-2 2006; NORVIT 2006; VISP 2004; WAFACS 2008; WENBIT 2008). They used 80% or 90% power to detect between 20% and 50% reduction in end points.
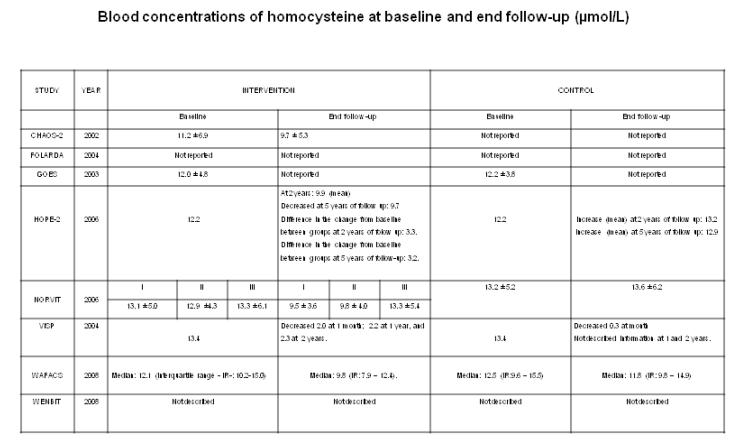
Blood concentrations of tHcy at baseline were reported in seven RCTs (CHAOS 2002; GOES 2003; HOPE-2 2006; NORVIT 2006; VISP 2004; WAFACS 2008; WENBIT 2008). Five RCTs reported the tHcy levels at the end follow-up (CHAOS 2002; HOPE-2 2006; NORVIT 2006; VISP 2004; WAFACS 2008). WENBIT 2008 described tHcy levels during intervention: 10.8 (SD, 4.5) μmol/L at baseline versus to 7.6 (SD, 2.2) μmol/L after one year of the intervention (B9 and B12 groups). There were not changes in the B6 and placebo groups. CHAOS 2002 did not report tHcy levels at baseline and end follow-up in the control arm. GOES 2003 reported tHcy levels at baseline and end follow-up only for the intervention arm and not for the control. FOLARDA 2004 did not measure the circulating tHcy levels in any of the groups. Figure 1 shows further details.
Figure 1.
Blood homocysteine levels.
Definitions used for defining myocardial infarction, stroke, unstable angina, and death (any) are described in Table 4.
Table 4.
Definitions of myocardial infarction (MI), stroke, unstable angina, and death.
| Study | Year | MI | Stroke | Unstable angina | Death | Notes | |
|---|---|---|---|---|---|---|---|
| CHAOS-2 | 2002 | Not available. | Not evaluated in this study. | Not evaluated in this study. | Not available. | ||
| FOLARDA | 2004 | Creatinine kinase elevation at least two times the upper limit of normal and in addition one of the following: On EKG, new pathological Q-waves in at least two contiguous leads or new left bundle branch block or chest pain longer than 30 min | Unequivocal signs of focal or global neurological deficit with sudden onset was present with a duration greater than 24 h and which were judged to be vascular in origin | Recurrence of typical or atypical anginal pain at rest or at effort with the appearance of a ST segment change >0.1mV and/or T wave inversion in at least 2 of 12 leads | Not available. | ||
| GOES | 2003 | Two out of three criteria should be positive: chest pain lasting _30 min, creatine kinase elevation _2 times the upper limit of normal or new pathological Q waves of 0.04-s duration or 25% of the corresponding R-wave amplitude, both in at least two contiguous leads | Not available. | Not available. | Not available. | ||
| HOPE-2 | 2006 | Two of the following three criteria were met: typical symptoms, increased cardiac-enzyme levels, and diagnostic electrocardiographic changes$ | Focal neurologic deficit lasting more than 24 hours. Computed tomography or magnetic resonance imaging was recommended to identify the type of stroke (ischaemic or hemorrhagic). When these tools were not available, the stroke was classified as of uncertain type | Not available. | Cardiovascular causes were unexpected deaths presumed to be due to ischaemic cardiovascular disease and occurring within 24 hours after the onset of symptoms without clinical or postmortem evidence of another cause, deaths from myocardial infarction or stroke within 7 days after the event, deaths associated with cardiovascular interventions within 30 days after cardiovascular surgery or within 7 days after percutaneous interventions, and deaths from congestive heart failure, arrhythmia, pulmonary embolism, or ruptured aortic aneurysm. Deaths from uncertain causes were presumed to be due to cardiovascular causes | $ Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined - a consensus document of the joint European Society of Cardiology/ American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000;36: 959-69. [Erratum, J Am Coll Cardiol 2001;37:973.]: source not available. | |
| NORVIT | 2006 | See supplementary appendix: www.nejm.org | See supplementary appendix: www.nejm.org | See supplementary appendix: www.nejm.org | See supplementary appendix: www.nejm.org | Definitions are very longer to resume here. | |
| VISP | 2004 | New ECG changes including Q waves or marked ST-T changes plus abnormal cardiac enzymes, cardiac symptoms plus abnormal enzymes, or symptoms plus hyperacute ECG changes resolving with thrombolysis | Evidence of sudden onset of focal neurologic deficit lasting at least 24 hours accompanied by an increased NIHSS Score in an area that was previously normal. When the sudden onset of symptoms lasting at least 24 hours was not accompanied by an increased NIHSS Score in an area that was previously normal, then recurrent stroke was diagnosed using cranial CT or MRI evidence of new infarction consistent with the clinical presentation | Not available. | Not available. | ||
| WAFACS | 2008 | According to World Health Organizations’s criteria. | A new neurologic deficit of sudden onset that persisted for more than 24 jours or until death within 24 hours | Not available. | Due to cardiovascular disease was confirmed by examinations of autopsy reports, death certificates, medical records, and information obtained from the next kin or other family members. Death from any cause was confirmed by the end point committee on the basis of a death certificate |
||
| WENBIT | 2008 | According to The Joint European Society of Cardiology/ American College of Cardiology Committee. Eur Heart J. 2000;21:1502-13 | According to Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR et al. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee). J Am Coll Cardiol. 2001;38: 2114-30 | According to Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR et al. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee). J Am Coll Cardiol. 2001; 38: 2114-30 | If death occurred within 28 days after the onset of an event, the event was classified as fatal. |
We identified 3 duplicate publications associated to three included studies (for a detailed description see Table 5). GOES 2003 has an associated publication with the results corresponding to 42 months of follow. Spence et al. published one reanalysis for VISP 2004. Finally, WAFACS 2008 trialists published a preliminary report.
Table 5.
Secondary publication
| Included Study | Reference | Objective | Data added addendum |
|---|---|---|---|
| GOES 2003 | Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: results of the Goes extension study. Heart 2005;91:1213-4 | To report the results of the study with a mean (SD) follow up of 42 (10) months. | Hcy has to be interpreted as a modifiable risk marker without apparent clinically salutary effects on hard clinical outcomes in patients with stable coronary artery disease |
| VISP 2004 | Spence JD, Bang H, Chambless LE, Stampfer MJ. Vitamin Intervention For Stroke Prevention trial: an efficacy analysis. Stroke 2005;36:2404-09 | This RCT was an efficacy analysis limited to patients most likely to benefit from the treatment, based on hypotheses arising from evidence developed since VISP was initiated | In the era of folate fortification, B12 plays a key role in vitamin therapy for total homocysteine. Higher doses of B12, and other treatments to lower total homocysteine may be needed for some patients |
| WAFACS 2008 | Redbeg RF, Block PC. A randomised trial of folic acid and B-vitamins in the secondary prevention of cardiovascular events in women: Results from the Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS). ACC Cardiosource Review Journal. 2006;16(7):52-58 | This RCT is a preliminary report of WAFAC study. |
Excluded studies
Nine studies did not fulfil inclusion criteria and were excluded (Bazzano 2006; FINEST 2006; Lange 2004; Lonn 2007; PACIFIC 2002; Swiss 2002; Vesin 2007; Wang 2007; Wierzbicki 2007). The reasons for exclusion are described in the Characteristics of excluded studies.
Risk of bias in included studies
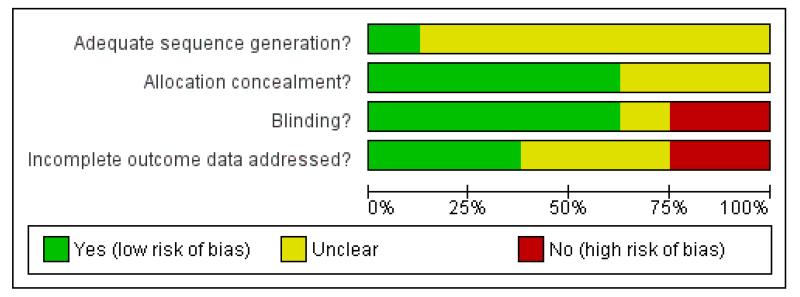
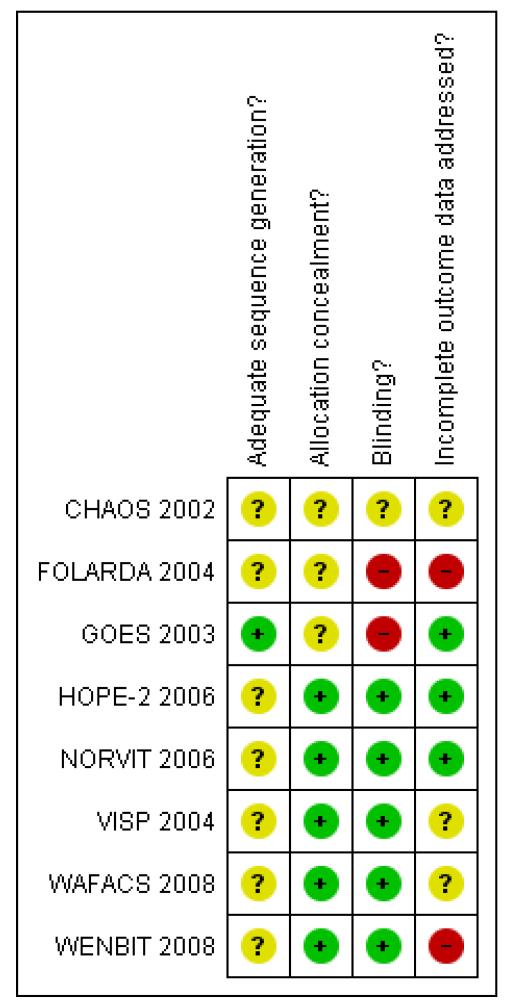
The risk of bias in the included trials is summarised in Figure 2 and Figure 3, and detailed in the Characteristics of included studies tables.
Figure 2.
Methodological quality graph: review authors’ judgements about each methodological quality item presented as percentages across all included studies.
Figure 3.
Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Of the included trials, five had an adequate allocation concealment (HOPE-2 2006; NORVIT 2006; VISP 2004; WAFACS 2008; WENBIT 2008). The rest did not provide further information on this domain (CHAOS 2002; FOLARDA 2004; GOES 2003).
Five trials were conducted using blinding for participants and providers of care (HOPE-2 2006; NORVIT 2006; VISP 2004; WAFACS 2008; WENBIT 2008). Regarding CHAOS 2002, we received information from one of the authors that the trial was double blinded.
Effects of interventions
See: Summary of findings for the main comparison
Results were based on 24,210 participants, and summarised in the Summary of findings for the main comparison .
Primary outcomes
Myocardial infarction (non-fatal or fatal)
-
HLI versus placebo group or conventional care (7 RCTs; 20,561 participants; 1,834 events):
There was no significant difference in non-fatal or fatal myocardial infarction between intervention and placebo or conventional care groups (RR 1.03, 95% CI 0.94 to 1.13; P =0.50, I2 = 0%). (CHAOS 2002; FOLARDA 2004; GOES 2003; HOPE-2 2006; NORVIT 2006; WAFACS 2008; WENBIT 2008). (see ‘Analysis 1.1’).
-
HLI (high dose) versus HLI (low dose) (1 study; 3,649 participants; 153 events):
There was no significant difference in non-fatal or fatal myocardial infarction between intervention and control groups (RR 0.90, 95% CI 0.66 to 1.23, P =0.50). (VISP 2004).(see ‘Analysis 1.1’).
Stroke (non-fatal or fatal)
LI versus placebo group (5 RCTs; 18,086 participants; 572 events)
There was no significant difference in non-fatal or fatal stroke between intervention and placebo groups (RR 0.89, 95%CI 0.73 to 1.08, P = 0.45, I2 = 15%). (FOLARDA 2004; HOPE-2 2006; NORVIT 2006; WAFACS 2008; WENBIT 2008) (see ‘Analysis 1.2’).
HLI (high dose) versus HLI (low dose): (1 study; 3,649 participants; 300 events).
There was no significant difference in non-fatal or fatal myocardial infarction between intervention and control groups (RR 1.04, 95% CI 0.84 to 1.29, P =0.73) (VISP 2004), see Analysis 1.2.
Secondary outcomes
First unstable angina pectoris episode requiring hospitalisation
There was no significant difference in first unstable angina pectoris episode requiring hospitalisation between intervention and placebo groups (RR 0.98, 95% CI 0.80 to 1.21, P = 0.87, I2 = 66%), see Analysis 1.3. This outcome was reported by FOLARDA 2004; HOPE-2 2006; NORVIT 2006; WENBIT 2008 (4 RCTs; 12,644 participants; 1,378 events).
Hospitalisation for heart failure
There was no significant difference in hospitalisation for heart failure between intervention and placebo groups (RR 1.16, 95% CI 0.96 to 1.41, P = 0.13) (5,522 participants; 374 events) (HOPE-2 2006).
Mortality by any cause
HLI versus placebo group: (6 RCTs, 18,679 participants; 1986 events).
There was no significant difference in mortality by any cause between intervention and placebo groups (RR 1.00, 95% CI 0.92 to 1.09, P = 0.96, I2: 0%) (FOLARDA 2004; GOES 2003; HOPE-2 2006; NORVIT 2006; WAFACS 2008; WENBIT 2008), see Analysis 1.4.
HLI (high dose) versus HLI (low dose): (1 RCT, 3,649 participants; 216 events).
There was no significant difference in mortality for any cause between intervention and control groups (RR 0.86, 95% CI 0.66 to 1.11, P = 0.24) (VISP 2004), see Analysis 1.4.
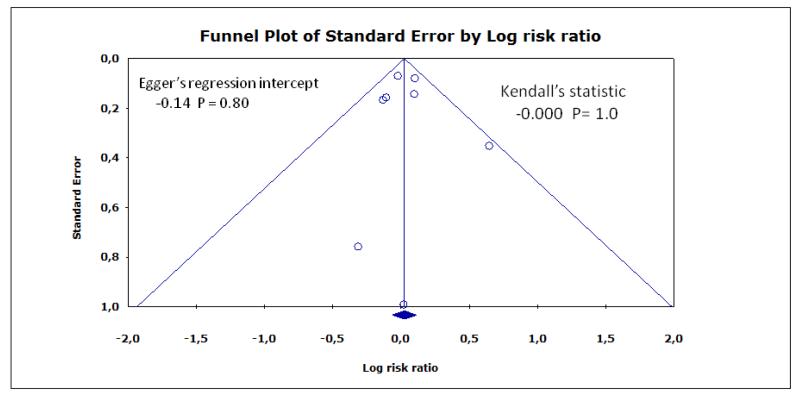
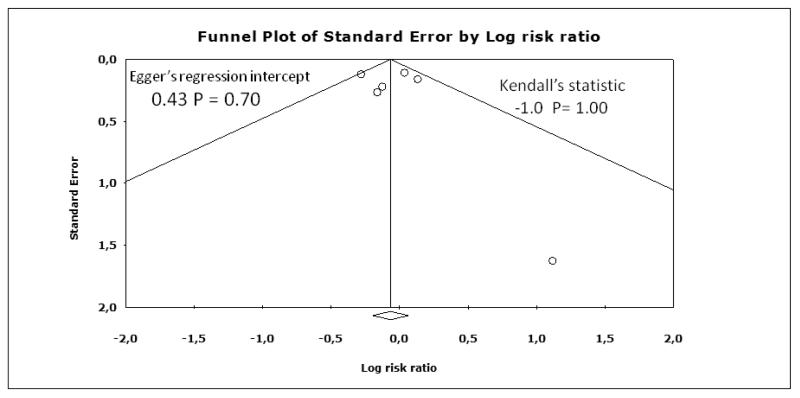
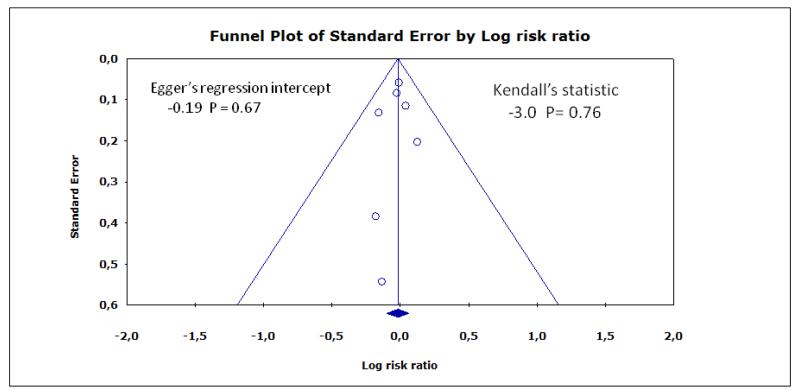
Funnel plots for myocardial infarction, stroke, and death are shown in Figure 4, Figure 5, Figure 6, respectively. Although the apparent asymmetry in the funnel plots is not necessarily associated with publication bias, there is a mild indication that small negative studies are missing. However, this should not be interpreted as a clear evidence of publication bias, mainly due to small number of included studies.
Figure 4.
Funnel plot for myocardial infarction.
Figure 5.
Funnel plot for stroke
Figure 6.
Funnel plot for death
Serious or non-serious adverse events
Three studies reported data on safety (HOPE-2 2006; NORVIT 2006; WENBIT 2008); they included 12,361 participants, and 996 events. The pooled estimate (RR) for cancer (HOPE-2 2006; NORVIT 2006; WENBIT 2008) was 1.06 (95% CI 0.90 to 1.25, P = 0.49, I2: 21%), see Analysis 1.5.
Subgroup and sensitivity analysis
The heterogeneity for all main outcomes was low as conveyed by the I2 values. Moreover, very few studies have been included to allow subgroup analysis. Therefore we did not pursue extensive investigation as previously planned. Due to lack of important heterogeneity fixed and random effects models give the same results for the main outcomes. Sensitivity analysis including only trials of low risk of bias (HOPE-2 2006; NORVIT 2006; WAFACS 2008; WENBIT 2008) gave the following results:
Primary outcomes
Myocardial infarction (non-fatal or fatal)
HLI versus placebo group (4 RCTs; 17,803 participants; 1,774 events)
There was no significant difference in non-fatal or fatal myocardial infarction between intervention and placebo groups (RR 1.02, 95% CI 0.93 to 1.13, P = 0.60, I2 =0%), see Analysis 2.1.
Stroke (non-fatal or fatal)
HLI versus placebo group (4 RCTs; 17,803 participants; 571 events)
There was no significant difference in non-fatal or fatal stroke between intervention and placebo groups (RR 0.89, 95% CI 0.72 to 1.09, P = 0.27, I2 =28%), see Analysis 2.2.
Secondary outcomes
First unstable angina pectoris episode requiring hospitalisation
HLI versus placebo group: (3 RCTs; 14,713 participants; 1364 events).
There was no significant difference in first unstable angina pectoris episode requiring hospitalisation between intervention and placebo groups (RR 1.06, 95% CI 0.79 to 1.42, P = 0.95, I2: 76%), see Analysis 2.3.
Mortality by any cause
HLI versus placebo group: (4 RCTs, 17,803 participants; 1,947 events).
There was no significant difference in mortality for any cause between intervention and placebo groups (RR 1.00, 95% CI 0.92 to 1.09, P = 0.99, I2: 0%), see Analysis 2.4.
DISCUSSION
Summary of main results
This review of HLI (B vitamins) for preventing cardiovascular events found eight randomised controlled trials, and their critical appraisal does not support the use of those interventions. We did not find significant differences on incidence of myocardial infarction (fatal or non-fatal), stroke (fatal or non fatal) or mortality by any cause. The data of the included studies (24,210 participants) are very consistent. Participants differed somewhat in cardiovascular risk levels (with established CVD or at high risk of CVD), baseline tHcy levels, access to foods fortified with folic acid or not, different dosages of vitamin and control groups, and treatment periods varying from 2 to 7 years.
Overall completeness and applicability of evidence
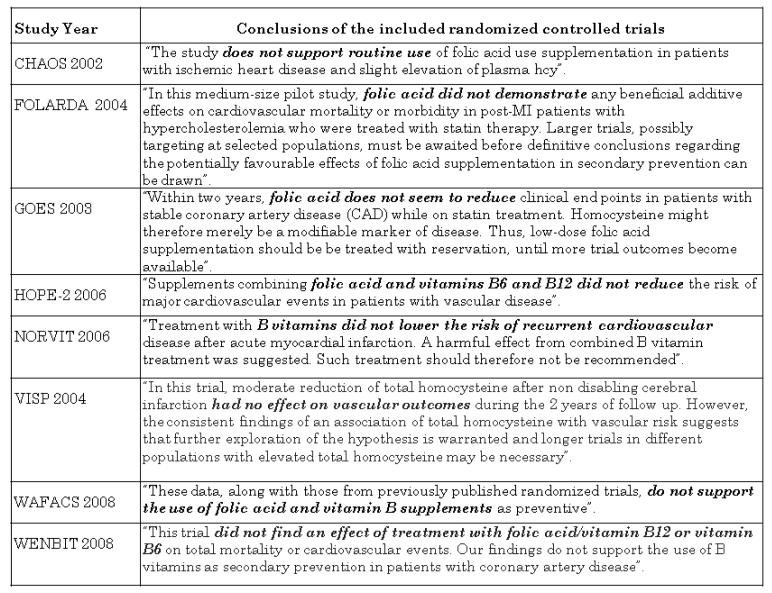
From the 1960’s, there is a ‘homocysteine theory of arteriosclerosis’ (McCully 2005). Evidence from several epidemiological observational studies suggested that elevated circulating tHcy levels are related to a higher risk of coronary heart disease, stroke and peripheral vascular disease. However, observational studies could be influenced by known biases. The literature on plausible biological mechanisms for supporting those probable causal relationship between raised tHcy levels and CVD is abundant and up-to-date (Abahji 2007; Ferretti 2006; Jacobsen 2005; Jakubowski 2000; Jakubowski 2004; Jakubowski 2008; Moens 2007; Riksen 2005; Splaver 2004; Tanriverdi 2007; Wang 2005; Zhou 2009). Further, it has been shown that supplementation with folic acid reduces circulating tHcy levels (Mirarefin 2007; PACIFIC 2002; Sato 2002). As the case might be similar to this previously observed when studying the effect of estrogens (Hulley 1998) and antioxidant vitamins (Vivekananthan 2003) used for preventing cardiovascular disease, randomised evidence is important before establishing the postulated association. All included RCT in this review, each one separately or summarised found not evidence of benefit (Figure 7) (CHAOS 2002; FOLARDA 2004; GOES 2003; HOPE-2 2006; NORVIT 2006; VISP 2004; WAFACS 2008; WENBIT 2008). As emphasised by Dusitanond 2005 referring to a sub-study of an ongoing trial (VITATOPS 2002): “elevated tHcy is a non causal marker of increased vascular risk”.
Figure 7.
Conclusions from the included RCT in this Cochrane review
Marcus et al studied the apparent lack of efficacy in “Homocysteine lowering and cardiovascular disease risk: lost in translation” (Marcus 2007). The reasons might be found at the molecular level. Firstly, it might be the autoantibodies against N-homocysteinylated proteins demonstrated in patients with premature coronary artery disease and stroke (Beł towski 2005; Jacobsen 2005; Jakubowski 2005; Per a-Kaján 2007). Undas 2006 showed that tHcy-related autoimmune response is resistant to folic acid administration in patients with coronary heart disease; but in healthy subjects there was a reduction of the circulating tHcy levels with a step down of those antibodies. Secondly, the tHcy-related autoimmune response is caused by a metabolite called homocysteine-thiolactone which modifies proteins such as fibrinogen, low-density lipoprotein, high-density lipoprotein, albumin, haemoglobin, and ferritin and generates that immune response causing autoantibodies against N-homocysteinylated proteins. It enhances thrombosis (Jakubowski 2008; Jakubowski 2009; ). Thirdly, high levels of tHcy generate an inflammatory response in endothelial cells, this reaction is mediated by cytokines, molecules that are necessary for inter-cellular communications, and they are involved in the atherothrombotic process (Libby 1995; Poddar 2001; Su 2005).
A research was recently conducted to assess the effect of homocysteine reduction by B-vitamin supplementation on inflammatory markers (Peeters 2007). The study demonstrated a reduction of the tHcy concentration without effect on cytokine levels (Peeters 2007). Therefore, the hypothesis suggesting that high circulating tHcy increases arteriosclerotic or thrombotic risk through vascular inflammation is questionable (Peeters 2007). Similiar findings were found in a sub-study of WENBIT 2008. The authors stated that “homocysteine-lowering therapy with B-vitamins does not affect levels of inflammatory markers associated with atherogenesis” (Bleie 2007). These findings and the results from RCTs had stimulated studies with non-vitamin therapy to reassess the homocysteine theory of atherosclerosis (Urquhart 2007). Lastly, HLI improving effects on endothelial function have been found to be significant in longer trials only (Potter 2008a).
Folic acid fortification is mandatory only in the USA from 1998.The objective was to lower serum homocysteine concentration and vascular disease risk (Koehler 1997). However only minor effect on tHcy-related mortality was observed (Anderson 2004). Also, high folic acid supplementation could mask the diagnosis of the vitamin B12 deficiency (Rampersaud 2003), a major determinant of elevated tHcy levels and increased carotid plaque area (Robertson 2005). Voluntary fortification policies in healthy adults assessed in Europe showed increased circulating levels of folate related B-complex vitamins and reduced tHcy levels, but possible effects on cardiovascular outcomes were not evaluated (Hoey 2007; Winkels 2008). Only three of the analysed studies in our meta-analysis refer to folate fortification (HOPE-2 2006, VISP 2004, WAFACS 2008). They were all performed in the United States of America. Although, folate fortification reduced the prevalence of low folate levels in the control groups and the levels of homocysteine were either unchanged or reduced, the influence on primary outcomes results (death from cardiovascular causes, myocardial infraction, stroke) was not important. We believe that further evaluation of the effect of folic acid fortification on cardiovascular diseases is necessary.
It is interesting to mention that HOPE-2 2006 study (which showed a significant reduction of stroke) was the only study to use an adequate dose of vitamin B12. The results of this RCT regarding stroke should be interpreted with caution. First, in HOPE-2 2006 combination pills of B6, B9 and B12 vitamins were used, therefore the reduction of stroke events could not be attributed to the adequate dosing of vitamin B12 since it was not delivered alone. Second, authors of HOPE-2 2006 stated that the stroke results should be interpreted with caution as the number of strokes in their study were much lower than the number of coronary events, the confidence intervals around estimated risks were wide, and the results were not adjusted for the multiplicity of outcomes compared.
The VISP subgroup analysis is a post hoc subgroup analysis of a randomised trial and should be interpreted with caution (Spence 2005). It is possible that a particular group of patients may benefit from intake of adequate doses of B6, B9 and B12 but further studies should be performed in order to arrive to conclusive results. Metabolic vitamin B12 deficiency exists in 20% of people over age 65 (Andrès 2004), and is defined either by elevation of methylmalonic acid, or in folate-replete subjects, by elevation of tHcy levels above 14 μmol/L. The VISP subgroup analysis (Spence 2005) , which showed that when patients with vitamin B12 deficiency and those with renal failure were excluded from the analysis showed a 35% reduction of stroke, death and coronary events with high-dose vs low dose therapy, comparing those with adequate vitamin B12 absorption (serum vitamin B12 above the median) with those with vitamin B12 levels below the median. If future studies are to be conducted, they should take into account the key issue of vitamin B12 deficiency which becomes increasingly more common with age, as does stroke.
This review found a non-significantly increased incidence of cancer-associated HLI (HOPE-2 2006; NORVIT 2006; WENBIT 2008). By advice of its safety committee, WENBIT 2008 was stopped because numerous participants were alerted by the increased risk of cancer reported in NORVIT 2006. Biochemical mechanisms for explaining this relationship between vitamin B9 and colorectal cancer had been described (Loscalzo 2006; Riksen 2005, Cole 2007). The role for folate in carcinogenesis required further research (Ulrich 2006).
Ueland and Clarke suggest to “…defer judgment on the clinical and public health relevance of the “homocysteine hypothesis” until the results of the additional ongoing trials of B-vitamins are available” (Clarke 2007; Ueland 2007). Durga 2005 pointed out that “ it is prudent for public health policy makers to await trials…before deciding on vitamin B9 fortification for the general population”. It is unclear how many participants will be needed in order to accept the evidence that supplementation of vitamin B9 alone or in combination with vitamin B12 and/or vitamin B6 does not prevent cardiovascular events. Similar to the use of streptokinase to prevent death following myocardial infarction (Yusuf 1985), it is possible that the results of the ongoing trials (SEARCH 2000; SU.FOL.OM3 2003; VITATOPS 2002) would reduce the confidence intervals of the pooled estimate for the outcomes of interest; however it is not very likely that they will change the overall conclusion.
Quality of the evidence
Despite the differences in the methodology, participant and doses the results of studies appear to be consistent regarding the effect of the intervention (B-complex vitamins versus placebo group or conventional care) on myocardial infarction, stroke and death. All large studies were at low risk of bias for allocation concealment and blinding and the large total sample size adds confidence to the conclusions of this review.
However, only a few trials clearly described what HHcy is and determined the circulating tHcy levels during the experiment. This review excluded end-stage renal patients as it is addressed in another Cochrane review ( Nigwekar 2009). The impact of losses to follow-up was unclear in many trials and the variability of the interventions across trials might limit generalisability.
Potential biases in the review process
Publication bias represents a major threat to the validity of systematic reviews, particularly reviews of small trials. However, this review has a low risk of publication bias, due to the meticulous trial search. Inconclusive studies have been identified and if more had been identified, the conclusions would be even more sceptical regarding the effect of the intervention (Figure 4; Figure 5; Figure 6).
Agreements and disagreements with other studies or reviews
Despite the methodology characteristics differences, this review found the same findings that Bazzano 2006. Also, two studies performed in individuals with advanced chronic kidney disease who had high tHcy levels demonstrated that HLI did not improve survival or reduce the incidence of vascular disease (Jamison 2007; Vianna 2007). This means that in people with variable tHcy levels (low or high), the B-complex vitamin intervention does not supply benefit to reduce the risk of CVD or death. We could conclude that the tHcy hypothesis for atherothrombotic cardiovascular disease has not been validated (Kaul 2006).
The conclusion of this review contradicted by the findings in Wang 2007. This disagreement is due to several methodology differences between both systematic reviews. However, it is remarkable that Wang 2007 only studied stroke as outcome. All of the studies included in Wang 2007 found no benefit for reducing the risk of stroke. This review also included one RCT that choose “multiple vitamin/mineral supplement” as the intervention and this may be a confounder (Mark 1996). In addition Wang 2007 did not show the individual data from each of the included RCTs. This meant that statistical heterogeneity could not be assessed and information needed to examine methodological quality, to determine its risk of bias, was absent.
AUTHORS’ CONCLUSIONS
Implications for practice
This Cochrane review provides evidence that HLI do not prevent cardiovascular events. The results are based on eight studies (24,210 participants) assessing vitamins B6, B9 or B12 (B-complex vitamins), given alone, or in combination, at any dosage compared with placebo or standard care, or with different regimes of vitamins B6, B9 or B12. The included trials did not show a benefit in preventing cardiovascular events in patients at risk of or with prior cardiovascular events. Therefore prescription of these interventions is not justified, unless new evidence from large high quality trials alters this conclusion.
Implications for research
The results from three ongoing trials will help consolidate or challenge the current conclusions regarding the effects of HLI. The association between the apparent lack of effectiveness of the intervention and adequate dosage of vitamin B12 might require further investigation.
PLAIN LANGUAGE SUMMARY.
B-complex vitamin therapy for preventing cardiovascular events
Cardiovascular disease is the number one cause of death worldwide. The most common causes of its morbidity and mortality are ischaemic heart disease, stroke and congestive heart failure. Many people with cardiovascular diseases may be asymptomatic, and might have high risk for developing a myocardial infarction, angina pectoris, stroke (ischaemic, haemorrhagic or both). ‘Emergent or new risk factors’ for cardiovascular disease have been recently added to the established risk factors (diabetes mellitus, high blood pressure, active smoker, adverse blood lipid profile). One of these risk factors is an elevated circulating total homocysteine (tHcy) levels. Homocysteine is an amino acid, and its levels in blood are influenced by blood levels of B-complex vitamins: cyanocobalamin (B12), folic acid (B9) and pyridoxine (B6). High tHcy levels are associated with an increased risk for atherosclerotic diseases. Hence, it has been suggested that B vitamins supplementation might reduce the risk of myocardial infarction, stroke, angina pectoris. Preventive strategies might include healthy people with low or high risk for developing cardiovascular disease (primary prevention) and people with an established cardiovascular disease (secondary prevention). In this review we included eight randomised controlled trials equivalent to 24,210 participants. We found no evidence that homocysteine-lowering interventions, in the form of supplements of vitamins B6, B9 or B12 given alone or in combination, at any dosage compared with placebo or standard care, prevents myocardial infarction, stroke, or reduces total mortality in participants at risk or with established cardiovascular disease.
ACKNOWLEDGEMENTS
We thank Dr. Joey Kwong and Ms Margaret Burke from the Cochrane Heart Group for their assistance. We sincerely thank the peer reviewers for their great suggestions that improved the quality of this review.
Thanks for Ms Carmen Verônica Abdala from BIREME/OPS/OMS for her help to develop the strategy of the search in LILACS.
We thank Dr. Agustín Ciapponi and Dr. Ricardo Hidalgo for their help during the preparation of the protocol of this Cochrane review.
SOURCES OF SUPPORT
Internal sources
No sources of support supplied
External sources
-
Iberoamerican Cochrane Centre, Spain.
Academic
-
Cochrane Heart Group, UK.
Academic
SUMMARY OF FINDINGS FOR THE MAIN COMPARISON
Homocysteine-lowering treatments for preventing cardiovascular events compared to placebo or standard care
Patient or population: patients with preventing cardiovascular events
Settings: Patients with known risk factors
Intervention: Homocysteine-lowering treatments
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Control | Homocysteine-lowering treatments | |||||
| Myocardial infarction | Low risk population |
RR 1.03 (0.94 to 1.13) |
20561 (7 studies) |
⊕⊕⊕○ moderate1 |
||
| 50 per 1000 |
51 per 1000 (47 to 56) |
|||||
| High risk population | ||||||
| 100 per 1000 |
103 per 1000 (94 to 113) |
|||||
| Stroke | Low risk population |
RR 0.89 (0.73 to 1.08) |
18086 (5 studies) |
⊕⊕⊕○ moderate |
||
| 50 per 1000 |
44 per 1000 (37 to 54) |
|||||
| High risk population | ||||||
| 100 per 1000 |
89 per 1000 (73 to 108) |
|||||
| Death by any cause | Low risk population |
RR 1 (0.92 to 1.09) |
18679 (6 studies) |
⊕⊕⊕○ moderate |
||
| 50 per 1000 |
50 per 1000 (46 to 55) |
|||||
| High risk population | ||||||
| 100 per 1000 |
100 per 1000 (92 to 109) |
|||||
| Serious adverse events (Cancer) | Low risk population |
RR 1.06 (0.9 to 1.25) |
12361 (3 studies) |
⊕⊕⊕○ moderate |
||
| 10 per 1000 |
11 per 1000 (9 to 12) |
|||||
| High risk population | ||||||
| 50 per 1000 |
53 per 1000 (45 to 62) |
|||||
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk ratio;
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
The interventions have different characteristis across studies
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Design: randomised, double blind, placebo-controlled trial. Multicentre study. Follow up period: mean of 1.7 years. |
|
| Participants | N: 1882 patients randomised (folic acid: 942 versus placebo: 940 patients). Sex: not reported. Age: not reported. Homocysteine levels at baseline (treatment group) (μmol/L): 11.2 ± 6.9 μmol/L Inclusion criteria (one of the following): Positive coronary angiogram; Admission with myocardial infarction or unstable angina. Exclusion criteria: Not reported. |
|
| Interventions | Intervention: folic acid 5 mg per day. Control: placebo in addition to usual drugs. Treatment duration: 2 years. |
|
| Outcomes | Composite outcome: myocardial infarction, revascularization, death from cardiovascular cause | |
| Notes | Sponsors: Not available. Other: Data not yet fully published. Results on the table corresponds to conference proceedings. Homocysteine levels were only collected in two participating centres |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | No information reported about this domain. Described as randomised. |
| Allocation concealment? | Unclear | No information reported about this domain. |
| Blinding? All outcomes |
Unclear | Described as double blinded. However, the information was obtained from the final report (abstract) |
| Incomplete outcome data addressed? All outcomes |
Unclear | Flow of participants during trial was not reported. |
| Methods | Design: randomised, open label, standard care-controlled trial. Multicentre study. Follow-up period: 1 year. |
|
| Participants | N: 283 randomised patients (folic acid: 140 versus standard care: 143). Sex (% male): folic acid: 69% versus standard care: 70%. Age (mean): folic acid: 59 years versus standard care: 59 Homocysteine levels at baseline: not reported Inclusion criteria (one of the following): Myocardial infarction. Total cholesterol value at admission or within 24 hours after onset of symptoms: > 6.5 μmol/L (251 mg/dL), Elevation of CK-MB at least 2 times upper the limit of normal function, Markedly increased chest pain lasting more than 30 minutes or classical ECG changes Exclusion criteria: Age under 18 years, Use of lipid lowering agents within the previous 3 months, High triglyceride levels > 4.5 μmol/L, Known familial dyslipidaemia, Low vitamin B12 levels, Hyperhomocysteinaemia (total plasma homocysteine > 18 μmol/L) or a known disturbed methionine loading test (total plasma homocysteine > 47 μmol/L), Severe renal failure (serum creatinine >180 μmol/L), Hepatic disease, Severe heart failure (New York Heart Association class IV), Scheduled percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) operation |
|
| Interventions | Intervention: Folic acid: 5 mg per day. Treatment was initiated at least 1 day prior for hospital discharge, and no later of 14 days after the myocardial infarction. The treatment continued for 1 year. Patients in this group also received statin therapy (fluvastatin, 40 mg per day). The clinician had at its discretion the prescription of additional prophylactic medication (aspirin, beta-blocking agents, and/or ACE inhibitors) Control: Standard care: Statin therapy (fluvastatin, 40 mg per day). The clinician had at its discretion the prescription of additional prophylactic medication (aspirin, beta-blocking agents, and/or ACE inhibitors) Treatment duration: 1 year. |
|
| Outcomes | Cardiovascular death (sudden death, fatal recurrent MI, fatal stroke, and other cardiovascular deaths). Non-cardiovascular death. Recurrent MI. Recurrent ischaemia requiring hospitalisation or revascularization (PCI, CABG) |
|
| Notes | Study phase: III Sample calculation a priori: Sample size calculation to detect (80% power and 5% significance level) a 50% reduction in clinical events in that kind of patients, assuming a one-year event rate of 30%. These numbers resulted in an estimation of 120 patients per group. Analyses conducted in ITT basis. Sponsors: AstraZeneca, The Netherlands, Working group on Cardiovascular research (WCN), The Netherlands. One author is an Established Investigator of the Netherlands Heart Foundation Other: Author did not perform homocysteine-level measures during the study |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | No complete information reported on this domain. Quote: ‘patients were randomised…’ |
| Allocation concealment? | Unclear | No information reported about this domain. |
| Blinding? All outcomes |
No | ‘… treatment with open label folic acid […] or not’ |
| Incomplete outcome data addressed? All outcomes |
No | 23 patients discontinued treatment and no information is given |
| Methods | Design: randomised, open-label, controlled trial. Single centre study. Follow-up period: 1 year. |
|
| Participants | N: 593 randomised patients (folic acid: 300 versus standard care: 293). Sex (% males): folic acid: 76% versus standard care: 80%. Age (mean±SD): folic acid: 64.9± 9.9 versus standard care: 65.5± 9.7 Homocysteine levels at baseline: not reported Inclusion criteria: Myocardial infarction, Coronary artery lesions (>60%) on coronary angiography, Percutaneous coronary intervention (PCI), Coronary artery bypass graft surgery (CABG), Patients had to be stable, with no invasive vascular procedures scheduled, Statin therapy for at least three months, Taking any form of vitamin B-containing medication, regularly or sporadic Exclusion criteria: Age < 18 years, History of low vitamin B12 levels, Therapy for hyperhomocysteinemia, Severe renal failure, or any other treatment for renal disease, Hepatic disease, Severe heart failure (New York Heart Association functional class IV), Serious illness that would exclude follow-up time of at least three years |
|
| Interventions | Intervention:folic acid: 0.5 mg per day. Control group: Standard care Intensive follow-up and treatment of risk factors, with counselling provided by a qualified nurse. Statin dosage was increased when necessary. Dietary counselling was provided, and smoking discouraged Treatment duration: not reported. |
|
| Outcomes | Primary outcome (composite): Composite: vascular death (sudden death, fatal recurrent MI, fatal stroke, and other cardiovascular deaths), Non cardiovascular death, Recurrent MI; Invasive coronary procedures (PCI, CABG); Cerebrovascular accident or transient ischemic attack; Any other vascular surgery (carotid endarterectomy, abdominal aneurysmectomy, or peripheral vascular surgery including limb amputation for vascular reasons) Secondary: Hospitalization for unstable angina |
|
| Notes | Study phase: III Sample size calculation a priori: Sample size calculation (80% power and 5% significance level) to detect a 50% reduction in clinical events in that kind of patients, assuming a two-years event rate of 15%. These numbers resulted in an estimation of 300 patients per group. Analyses conducted in ITT basis Sponsors: Trial with public funding (Stichting Paracard). Other: The trial allowed the entry of patients taking vitamin B supplementation. These patients showed higher levels of serum folate and lower levels of homocysteine |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: ‘A computer program randomly allocated patients […] to treatment’ |
| Allocation concealment? | Unclear | No information reported about this domain. |
| Blinding? All outcomes |
No | ‘… treatment with open label folic acid […] or standard care’ |
| Incomplete outcome data addressed? All outcomes |
Yes | After randomization, 12 patients per group withdrew the study but were followed and included in the final analysis |
| Methods | Design: randomised, double-blind, placebo-controlled trial. Multicentre international study (13 countries; 145 centres). Follow-up period: five years. |
|
| Participants | N: 5522 patients randomised (vitamin: 2758 versus group: 2764 patients). Sex (% males): vitamin: 71.1% versus placebo: 72.4%. Age (mean±SD): vitamin: 68.8 ± 7.1 versus placebo: 68.9± 6.8 Homocysteine level at baseline: 12.2 μmol/L (1.6 mg/L) Inclusion criteria: Men and women aged >55 years, Hstory of vascular disease (coronary, cerebrovascular, or peripheral vascular) or diabetes and additional risk factors for atherosclerosis, Irrespective of their homocysteine levels, from countries with mandatory folate fortification of food (Canada and the United States) and countries without mandatory folate fortification (Brazil, western Europe, and Slovakia) Exclusion criteria: Patients taking vitamin supplements containing more than 0.2 mg of folic acid per day |
|
| Interventions | Intervention: Multivitamin therapy with 2.5 mg of folic acid, 50 mg of vitamin B6, and 1 mg of vitamin B12 per day Control: Matching placebo daily Treatment duration: 5 years. |
|
| Outcomes | Primary outcome (composite): Death from cardiovascular causes, myocardial infarction, stroke Secondary outcomes: Total Ischaemic events (composite of death from cardiovascular causes, myocardial infarction, stroke, hospitalisation for unstable angina, and revascularization), Death from any cause, Hospitalization for unstable angina or congestive heart failure, Revascularization, Incidence and death for cancer. Other outcomes: transient ischaemic attacks, venous thromboembolic events, fractures |
|
| Notes | Study phase: III, registered (ClinicalTrials.gov number NCT00106886) Sample calculation a priori: Sample size calculation to detect between a 17 and a 20% (80% and 90% power, respectively) reduction in the risk rate of primary endpoint over 5 years of follow up (assuming an annual event rate of 4% in the placebo group). These numbers resulted in an estimation of 5000 patients. Analyses conducted in ITT basis Sponsors: Public funding (Canadian Institutes of Health Research). The study medication was provide by Jamieson Laboratories. They were not involved in the design, execution, analysis, or reporting of the trial results |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | No information reported about this domain. |
| Allocation concealment? | Yes | Centralised telephone randomisation (accessible 24h a day). |
| Blinding? All outcomes |
Yes | Vitamins manufactured to be indistinguishable in colour, weight or ability to be dissolved in water |
| Incomplete outcome data addressed? All outcomes |
Yes | 21 patients in the treatment group and 16 in the placebo not completed the study. Vital status know for the 99.3% of the sample. |
| Methods | Design: Randomised, double-blind, placebo-controlled trial, with factorial design. Multicentre study. Follow-up period: 3.5 years. |
|
| Participants | N: 3749 patients randomised (folic acid, vitamins B6 and B12: 937 versus folic acid, vitamin B12: 935 versus vitamin B6: 934 versus placebo: 943) Sex (% male) Folic acid, vitamins B6 and B12: 73% Folic acid, vitamin B12: 74% Vitamin for B6: 73% Placebo: 75% Age (mean±SD, years) Folic acid, vitamins B6 and B12: 63.6±11.9 Folic acid, vitamin B12: 63.2±11.6 Vitamin B6: 62.5±11.7 Placebo: 62.6±11.4 years Inclusion criteria: Men and women aged 30 to 85 years, History of acute myocardial infarction within seven days before randomisation Exclusion criteria: Coexisting disease associated with a life expectancy < 4 years, Prescribed treatment with B vitamins or untreated vitamin B deficiency, Inability to follow the protocol, as judged by the investigator |
|
| Interventions | Intervention: Folic acid (group 1): 0.8 mg; vitamin B12: 0.4 mg; vitamin B6: 40 mg per day Folic acid (group 2): 0.8 mg; vitamin B12: 0.4 mg per day Vitamin B6 (group 3): 40 mg per day Control: Placebo. Medication was delivered in single capsules taken once per day. For the first two weeks after study entry patients in groups 1 and 2 received an additional folic acid dose (5 mg) per day, whereas the other two groups received placebo Treatment duration: Not clearly described. |
|
| Outcomes | Primary outcome (composite): Recurrent myocardial infarction, stroke, and sudden death attributed to coronary artery disease Secondary outcomes: Myocardial infarction, Unstable angina pectoris requiring hospitalisation, Coronary revascularization with percutaneous coronary intervention or coronary-artery bypass grafting, Stroke, Death from any cause. Incident cases of cancer. |
|
| Notes | Study phase: III, registered (ClinicalTrials.gov number NCT00266487) Sample calculation a priori: Sample size calculation to detect a 20% relative reduction in the rate of primary endpoint (assuming a 25% of endpoints in the placebo group). These numbers resulted in an estimation of 3500 patients assuming in 750 primary events The calculation of the sample size was based on data from previous Scandinavian trials, assuming the three-year rate of the primary end point would be 25 percent in the placebo group. The planned enrolment of 3500 patients, with an average follow-up of 3.0 years, was expected to result in 750 primary events and give the study a statistical power of more than 90 percent to detect a 20 percent relative reduction in the rate of the primary end point, given a two-sided alpha value of 0.05 Sponsors: Public and governmental funding. Supported by the Norwegian Research Council, the Council on Health and Rehabilitation, the University of Tromso, the Norwegian Council on Cardiovascular Disease, the Northern Norway Regional Health Authority, the Norwegian Red Cross, the Foundation to Promote Research into Functional Vitamin B12 Deficiency, and an unrestricted private donation The study medication was provide by Alpharma. The sponsors had no role in the design, conduct, or reporting of the study |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | No information reported about this domain. |
| Allocation concealment? | Yes | The manufacturer provided study sites centrally blocks of medication assigned in numerical order |
| Blinding? All outcomes |
Yes | Vitamins manufactured to be indistinguishable in colour, weight or ability to be dissolved in water |
| Incomplete outcome data addressed? All outcomes |
Yes | 11% of patients stopped the medication. 94% attended the final visit, but data on mortality was available for the entire sample. Incomplete outcome data for 20 patients. Patients that had not completed the planned follow up were followed by phone or consulted for vital status |
| Methods | Design: Randomised, double-blind, placebo-controlled trial. Multicentre international study. Follow-up period: 2 years. |
|
| Participants | N: 3680 patients randomised (high dose: 1827 versus low dose: 1853 patients) Sex (% male): high dose: 62.3% versus low dose: 62.8%. Age (mean±SD): high dose: 66.4 (10.8) versus low dose: 66.2 (10.8) Inclusion criteria: Nondisabling ischemic stroke(Modified Rankin Stroke Scale 3): Onset 120 days before randomization. Focal neurological deficit of likely atherothrombotic origin, classified as ischemic stroke by questionnaire/algorithm or confirmed as new cerebral infarction consistent with symptoms by cranial computed tomography or brainmagnetic resonance imaging, Total homocysteine level 25th percentile for North American stroke population, Age: ≥ 35 years, Accessibility for follow-up, Agreement to take study medication and not take other multivitamins or pills containing folic acid or vitamin B6, Written informed consent. Exclusion criteria: Potential sources of emboli (atrial fibrillation within 30 days of stroke, prosthetic cardiac valve, intracardiac thrombus or neoplasm, or valvular vegetation), Other major neurological illness that would obscure evaluation of recurrent stroke, Life expectancy 2 years, Renal failure requiring dialysis, Untreated anemia or untreated vitamin B12 deficiency, Systolic blood pressure 185mmHg or diastolic blood pressure 105 mm Hg on 2 readings 5 minutes apart at time of eligibility determination, Refractory depression, severe cognitive impairment, or alcoholism or other substance abuse, Use within the last 30 days of medications that affect total homocysteine level (methotrexate, tamoxifen, levodopa, niacin, or phenytoin) or bile acid sequestrants that can decrease folate levels, Childbearing potential, Participation in another trial with active intervention, General anesthesia or hospital stay of 3 days, any type of invasive cardiac instrumentation, or endarterectomy, stent placement, thrombectomy, or any other endovascular treatment of carotid artery within 30 days prior to randomization or scheduled to be performed within 30 days after randomization |
|
| Interventions | High dose multivitamin therapy 2.5 mg folic acid; 0.4 mg vitamin B12; 25 mg vitamin B6 per day Low dose multivitamin therapy 20 micrograms folic acid; 6 micrograms vitamin B12; 200 micrograms vitamin B6 per day Co-interventions: Risk factor control education Aspirin (325 mg/d). Duration Treatment: Not described |
|
| Outcomes | Primary outcome: Recurrent cerebral infarction. Secondary outcomes: Coronary heart disease, including: myocardial infarction requiring hospitalisation; coronary revascularization; and fatal coronary heart disease Death. |
|
| Notes | Study phase: III Sample size calculation a priori: Sample size calculation (80% power a .05 significance level for a 2-sided test) to detect a 30% reduction in the rate of primary endpoint over 2 years of follow up (assuming a 8% of events in the first year and a 4% at the second year, with a 20% of losses of follow up). These numbers resulted in an estimation of 1800 patients per group. Trialists planned up to 6 interim analyses Sponsors: Supported by the National Institute of Neurological Disorders and Stroke (grant RO1 NS34447). The study medication was provided by Roche Inc. They had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript Other: Elegible patients were tested for treatment compliance giving a 1-month low dose vitamins. Only persons taking at least 75% of treatment were randomised |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | The allocation of participants was programmed by the statistical coordinating center, encrypted, and entered into a data entry program installed on a studycomputer at each site |
| Allocation concealment? | Yes | Allocation programmed by the statistical coordinating centre. All the information on assignment were encrypted an entered in computers in study sites. After verification of eligibility participants were assigned in 1 of 20 medication codes |
| Blinding? All outcomes |
Yes | The drug distributor centre bottled and distributed the vitamins, that were manufacture to be indistinguishable in colour, weight or ability to be dissolved in water The primary endpoint was reviewed by a local neurologist and two external independent review neurologists |
| Incomplete outcome data addressed? All outcomes |
Unclear | 132 patients in the low dose group, and 133 in the high dose were lost to follow up. Of these 18 and 13 patients respectively had no contact after randomisation, and were not included in the analysis. 186 patients in the low dose group, and 179 in the high dose discontinued the assigned treatment Patients that had not completed the planned follow up were invited to an exit visit |
| Methods | Design: Randomised, double-blind, placebo-controlled trial, with factorial design Multicentre study Follow-up period: 7.3 years. |
|
| Participants | N: 5442 randomised patients (vitamin group: 2721 patients; placebo group: 2721 patients) Sex: Women health professionals Age (mean [Standar deviation])] years Active group: 62.8 (8.8). Control group: 62.8 (8.8). Inclusion criteria 1.- Women 2. Age: 40 years or older 3.- Postmenopausal or had no intention of becoming pregnant 4.- History of CVD or had at least 3 cardiac risk factors Exclusion criteria: 1.- Cancer (excluding nonmelanoma skin cancer) within the past 10 years. 2.- Serious non-cardiovascular disease. 3.- Warfarin or other anticoagulants use. |
|
| Interventions | Intervention: Folic acid: 2.5 mg; vitamin B12: 1 mg; vitamin B6: 50 mg per day Control: Matching placebo per day. Co-interventions: vitamin C, vitamin E, ß-carotene. Treatment duration: not clearly reported. |
|
| Outcomes | Primary outcome (composite): Incident myocardial infarction, stroke, coronary revascularization procedures (coronary artery bypass grafting or percutaneous coronary intervention) and cardiovascular mortality Secondary outcomes: Myocardial Infarction rate, Stroke rate, Total coronary heart disease events (MI, coronary revascularization, and death from coronary heart disease) |
|
| Notes | Study phase: III, registered (ClinicalTrials.gov number NCT00000541) The information in this table was kindly supplied by Dr. Nancy Cook who was the statistician for the WACS and WAFACS studies (June 23, 2008) The WACS study was a 2×2×2 factorial trial of three anti-oxidants, vitamins C, E and beta-carotene. Randomization of the 8171 participants into the 8 treatment groups took place from June 1995 to October 1996, and was conducted using blocks of size 16 within 5-year age groups. The folate/B6/B12 arm was added in April 1998, and the 5442 participants who were willing and eligible were randomised (at one time) using blocks of size 8 within strata defined by age and the other treatment arms. Participants were sent yearly supplies of calendar packs containing the study medications or matching placebo pills that were identical in appearance. All medical records were reviewed by an Endpoints Committee that was blinded to treatment assignment Sample calculation a priori: Sample size with a 91.5% power to detect a 20% reduction in in the primary end point (major vascular events). For the end points of total CHD (defined as nonfatal MI, CHD death, or revascularization), MI, and stroke, the minimum detectable risk reduction with 80% power ranges from 19% to 32%. It was used a two sided significance level of 0.05 Sponsor: Public funding and from several industry sources. Grant HL47959 from the National Heart, Lung, and Blood Institute of the National Institutes of Health. Vitamin E and its placebo were supplied by Cognis Corporation (La-Grange, Illinois) All other agents and their placebos were supplied by BASF Corporation (Mount Olive, New Jersey). Pill packaging was provided by Cognis and BASF. They did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript Other: The analyses of the end points were done only for those confirmed outcomes. However, there was an additional 43 records deaths for total mortality |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Blocks randomisation with a size of 8 generated by computer, stratified by age |
| Allocation concealment? | Yes | Central randomisation. Patients were sent yearly supplies of calendar packs containing their medication or matching placebos identical in appearance |
| Blinding? All outcomes |
Yes | All study investigators, personnel, and participants were unaware of the participants’ treatment assignments’. Patients were sent packs containing medication or matching placebos identical in appearance An independent committee monitored the ‘safety and overall quality and scientific integrity’ of the trial, that was blinded to treatment assignment. All the information was supplied by Nancy Cook (WACS statistician, June 23th, 2008) |
| Incomplete outcome data addressed? All outcomes |
Unclear | Unknown vital status for 194 patients in the folic acid group and for 207 patients in the placebo group. All the patients were included in the primary analysis, but not described how |
| Methods | Design: Randomised, double-blind, placebo-controlled trial, with factorial design Multicentre study. Follow-up period: 4 years. |
|
| Participants | N: 3096 patients randomised (folic acid, vitamins B6 and B12: 772 versus folic acid, vitamin B12: 772 versus vitamin B6: 772 versus placebo: 780) Sex (% male) Folic acid, vitamins B6 and B12: 81.2% Folic acid, vitamin B12: 80.4% Vitamin B6: 80.2% Placebo: 76.5% Age (mean±SD, years): Folic acid, vitamins B6 and B12: 61.7±10.3 Folic acid, vitamin B12: 61.3±10.0 Vitamin B6: 61.4±9.7 Placebo: 62.0±9.9 Inclusion criteria: Age: 18 years or older, Undergoing coronary angiography for suspected CAD and/or aortic valve stenosis at the 2 university hospitals in western Norway Exclusion criteria: Unavailability for follow-up, Participation in other trials, Hisytory of alcohol abuse, serious mental illness, or cancer |
|
| Interventions | Intervention: Folic acid (group 1): 0.8 mg; vitamin B12: 0.4 mg; vitamin B6: 40 mg per day Folic acid (group 2): 0.8 mg; vitamin B12: 0.4 mg per day Vitamin B6 (group 3): 40 mg per day Control: Placebo. Co-interventions: statins, insulin, aspirin, clopidogrel, Beta-Blockers, ACE inhibitors/ARBs, calcium channel blockers, loop diuretics, oral antidiabetics, medication for Chronic Obstructive Pulmonar Disease Duration Treatment: Not described. |
|
| Outcomes | Primary outcome (composite): All-cause death, nonfatal acute myocardial infarction, acute hospitalisation for unstable angina pectoris, and nonfatal thromboembolic stroke Secondary outcomes: Acute Myocardial Infarction, Acute hospitalisation for angina pectoris, Stable angina pectoris with angiographically verified progression, Myocardial revascularization procedures, Stroke, Incident cases of cancer. |
|
| Notes | Study phase: III, registered (ClinicalTrials.gov number NCT00354081) Sample calculation a priori: Sample of 3088 participants to detect a 20% reduction in the primary end point during 4 years of follow-up with a statistical power of 80% at a 2-sided significance level of .05 Sponsors: the Advanced Research Program and Research Council of Norway, the Norwegian Foundation for Health and Rehabilitation, the Norwegian Heart and Lung Patient Organisation, the Norwegian Ministry of Health and Care Services, the Western Norway Regional Health Authority, the Department of Heart Disease at Haukeland University Hospital, Locus for Homocysteine and Related Vitamins at the University of Bergen, Locus for Cardiac Research at the University of Bergen, the Foundation to Promote Research Into Functional Vitamin B12 Deficiency, Bergen, Norway, and Alpharma Inc, Copenhagen, Denmark The study medication was provide by Alpharma which had no access to study data, and did not participate in data analysis or interpretation or in the preparation, review, or approval of the manuscript Other: The first 90 participants were randomised before undergoing angiography in order to ensure no effects on blood indexes from the invasive procedure. Subsequent participants were randomised after baseline angiography This trial was stopped by no beneficial effects and suggesting increased risk of cancer by B vitamin treatment |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | 2×2 factorial design with block randomisation with a size of 20 |
| Allocation concealment? | Yes | Centralised independently by the manufacturer (Alpharma). Study nurses received coded boxes provided to participants in numerical order. The codes were kept by the manufacturer until eligibility data was complete |
| Blinding? All outcomes |
Yes | Vitamins manufacture to be indistinguishable in colour, weight or ability to be dissolved in water. Endpoints adjudicated by an independent committee unaware of patient’s assignment |
| Incomplete outcome data addressed? All outcomes |
No | Data on 6 patients (0.2% from the sample) withdrew consent to participate in the trial and were excluded from the analysis. Due to the media impact of the NORVIT interim results 692 patients were asked to stop the medication. Outcome data available for the 86% of patients at the final visit |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bazzano 2006 | Systematic review. |
| FINEST 2006 | RCT with a follow up less than one year. |
| Lange 2004 | RCT with a follow up less than one year. |
| Lonn 2007 | Review. |
| PACIFIC 2002 | RCT with a follow up lesser than one year. |
| Swiss 2002 | RCT with a follow less than one year. |
| Vesin 2007 | Review. |
| Wang 2007 | Systematic review. |
| Wierzbicki 2007 | Review. |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | SEARCH: Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine |
| Methods | |
| Participants | Ages: 18 to 80 years. Genders: both Inclusion criteria: Prior myocardial infarction; statin therapy indicated; no clear indication for folic acid. Exclusion criteria: No clear contraindication to study treatments; Screening plasma total cholesterol <3.5 μmol/L in patient already on statin therapy, or <4.5 μmol/L in patient not on statin therapy; Chronic liver disease; Severe renal disease or evidence of renal impairment ; Inflammatory muscle disease; Concurrent treatment with fibrates or high-dose niacin; Concurrent treatment with cyclosporin (or condition likely to result in organ transplantation and the need for cyclosporin), nefazodone, methotrexate, systemic azole antifungal or systemic macrolide antibiotics; child bearing potential; No other predominant medical problem (other than coronary heart disease [CHD]) which might limit compliance with 5 years of study treatment |
| Interventions | Simvastatin (20mg versus 80mg daily). Folic acid 2mg plus vitamin B12 1mg daily. |
| Outcomes | Primary: Major vascular events (MVE): non-fatal myocardial infarction, coronary death, stroke or arterial revascularization Secondary: MVEs separately in year 1 and in later years; MVEs in patients subdivided into 3 groups by baseline low-density lipoprotein (LDL); MVEs in presence and absence of the other factorial treatment ; major coronary events; total strokes |
| Starting date | July 1998 |
| Contact information | Rory Collins, MB BS FRCP, Study Director, University of Oxford |
| Notes | Region: United Kingdom (Oxford). Study Design: prevention, randomised, double-Blind, dose comparison, factorial assignment, safety/efficacy study This study is no longer recruiting patients. Total enrolment: 12064. Phase III. ClinicalTrials.gov Identifier: NCT00124072. Sponsors and collaborators: University of Oxford; Merck. |
| Trial name or title | Double-blind randomised placebo-controlled secondary prevention trial to test the impact of supplementation with folate, vitamin B6 and B12 and/or omega-3 fatty acids on the prevention of recurrent ischemic events in subjects with atherosclerosis in the coronary or cerebral arteries |
| Methods | |
| Participants | Patients with myocardial infarction, unstable angina pectoris or ischemic stroke. Population without mandatory folate fortification of food. Age: 45 and 80 years |
| Interventions | Folate plus vitamin B6 and B12 and/or omega-3 fatty acids and/or placebo |
| Outcomes | Recurrent ischemic diseases. |
| Starting date | 2003 |
| Contact information | Galan P (s_galan@vcnam.cnam.fr) Unité de Surveillance et d’Epidémiologie Nutritionnelle, Institut Scientifique et Technique de la Nutrition et de l’Alimentation, Paris |
| Notes | * Study design: Double-blind randomised placebo-controlled. Region: France. Total enrolment: 3,000 patients. Follow-up: 5 years. Sources: Faeh D et al. SWISS MED WKLY 2006;136:745-756. Galan P et al. J Nutr Health Aging. 2003;7(6):428-35. |
| Trial name or title | A Study of VITAmins TO Prevent Stroke |
| Methods | |
| Participants | Patients with stroke or TIA in the previous 7 months. Inclusion Criteria: Patients presenting within seven months of stroke (Ischaemic or hemorrhagic) or TIA. Agree to take study medication Be geographically accessible for follow-up. Provide written informed consent. Exclusion Criteria: Taking folic acid or B6 on medical advice. Use of vitamin supplements containing folate, B6 or B12 (unless patient agrees to take study medication instead of the vitamin supplements which they usually take). Taking Methotrexate for any reason. Pregnancy or women of child-bearing potential who are at risk of pregnancy. Limited life expectancy. |
| Interventions | Folic acid: 2mg Vitamin B6: 25mg Vitamin B12: 500 micrograms |
| Outcomes | Primary: Non-fatal stroke Non-fatal myocardial infarction Death due to vascular causes. Secondary: TIA Revascularization procedures Dementia Depression. |
| Starting date | November 1998 |
| Contact information | Julia Pizzi +61 8 9224 7004 VITATOPS@health.wa.gov.au Michelle Tang +61 8 9224 3336 VITATOPS@health.wa.gov.au |
| Notes | Region: Australia: Study Design: prevention, randomised, double-blind, placebo control, parallel assignment, safety/efficacy study Sample size calculation: To reliably identify a 15% reduction in relative risk of the primary outcome event from 8% to 6.8% per year with an alpha of 0.05 and power of 80%, 8,000 patients need to be randomised and followed-up for an average of two years Current progress: As of November, 2004, more than 4,400 patients have been randomised in 73 centres in 19 countries in five continents: Australia, Austria, Belgium, Brazil, Hong Kong, Italy, Malaysia, Moldova, Netherlands, New Zealand, Pakistan, Philippines, Portugal, Republic of Georgia, Serbia & Monte Negro, Singapore, Sri Lanka, United Kingdom, and United States |
Authors asked to the author for getting complete data; however, they did not send the information.
NA: not available.
DATA AND ANALYSES
Comparison 1.
Homocysteine-lowering treatment versus other (any comparisons)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Myocardial infarction | 8 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Homocysteine-lowering versus placebo | 7 | 20561 | Risk Ratio (M-H, Random, 95% CI) | 1.03 [0.94, 1.13] |
| 1.2 Homocysteine-lowering treatment at high dose versus low dose | 1 | 3649 | Risk Ratio (M-H, Random, 95% CI) | 0.90 [0.66, 1.23] |
| 2 Stroke | 6 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Homocysteine-lowering treatment versus placebo | 5 | 18086 | Risk Ratio (M-H, Random, 95% CI) | 0.89 [0.73, 1.08] |
| 2.2 Homocysteine-lowering treatment at high dose versus low dose | 1 | 3649 | Risk Ratio (M-H, Random, 95% CI) | 1.04 [0.84, 1.29] |
| 3 First unstable angina pectoris episode requiring hospitalization | 4 | 12644 | Risk Ratio (M-H, Random, 95% CI) | 0.98 [0.80, 1.21] |
| 4 Death by any cause | 7 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Homocysteine-lowering treatment versus placebo | 6 | 18679 | Risk Ratio (M-H, Random, 95% CI) | 1.00 [0.92, 1.09] |
| 4.2 Homocysteine-lowering treatments at high dose versus low dose | 1 | 3649 | Risk Ratio (M-H, Random, 95% CI) | 0.86 [0.66, 1.11] |
| 5 Serious adverse events (Cancer) | 3 | 12361 | Risk Ratio (M-H, Random, 95% CI) | 1.06 [0.90, 1.25] |
Comparison 2.
Homocysteine-lowering treatment versus other (Sensitivity analysis)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Myocardial infarction | 4 | 17803 | Risk Ratio (M-H, Random, 95% CI) | 1.02 [0.93, 1.13] |
| 1.1 Trials with low risk of bias (mixed populations) | 3 | 12361 | Risk Ratio (M-H, Random, 95% CI) | 1.04 [0.94, 1.15] |
| 1.2 Trials with low risk of bias (only women included) | 1 | 5442 | Risk Ratio (M-H, Random, 95% CI) | 0.88 [0.63, 1.22] |
| 2 Stroke | 4 | 17803 | Risk Ratio (M-H, Random, 95% CI) | 0.89 [0.72, 1.09] |
| 2.1 Trials with low risk of bias (mixed populations) | 3 | 12361 | Risk Ratio (M-H, Random, 95% CI) | 0.79 [0.65, 0.97] |
| 2.2 Trials with low risk of bias (only women included) | 1 | 5442 | Risk Ratio (M-H, Random, 95% CI) | 1.14 [0.83, 1.57] |
| 3 First unstable angina pectoris episode requiring hospitalization | 3 | 12361 | Risk Ratio (M-H, Random, 95% CI) | 0.99 [0.79, 1.24] |
| 4 Death by any cause | 4 | 17803 | Risk Ratio (M-H, Random, 95% CI) | 1.00 [0.92, 1.09] |
| 4.1 Trials with low risk of bias (mixed populations) | 3 | 12361 | Risk Ratio (M-H, Random, 95% CI) | 1.01 [0.91, 1.12] |
| 4.2 Trials with low risk of bias (only women included) | 1 | 5442 | Risk Ratio (M-H, Random, 95% CI) | 0.98 [0.83, 1.15] |
Analysis 1.1. Comparison 1 Homocysteine-lowering treatment versus other (any comparisons), Outcome 1 Myocardial infarction
Review: Homocysteine lowering interventions for preventing cardiovascular events
Comparison: 1 Homocysteine-lowering treatment versus other (any comparisons)
Outcome: 1 Myocardial infarction
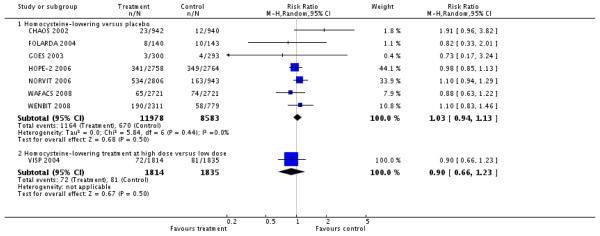 |
Analysis 1.2. Comparison 1 Homocysteine-lowering treatment versus other (any comparisons), Outcome 2 Stroke
Review: Homocysteine lowering interventions for preventing cardiovascular events
Comparison: 1 Homocysteine-lowering treament versus other (any comparisons)
Outcome: 2 Stroke
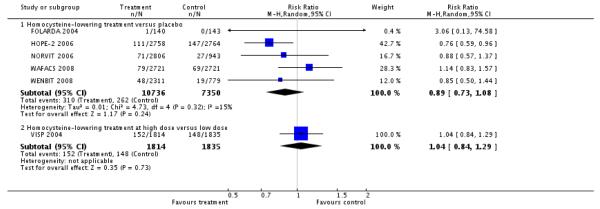 |
Analysis 1.3. Comparison 1 Homocysteine-lowering treatment versus other (any comparisons), Outcome 3 First unstable angina pectoris episode requiring hospitalization
Revies: Homocysteine lowering interventions for preventing cardiovascular events
Comparison: 1 Homocysteine-lowering treatment versus other (any comparisons)
Outcome: 3 First unstable angina pectoris episode requiring hospitalization
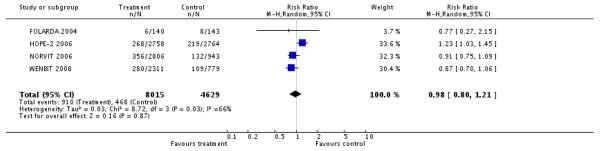 |
Analysis 1.4. Comparison 1 Homocysteine-lowering treatment versus other (any comparisons), Outcome 4 Death by any cause
Review: Homocysteine lowering interventions for preventing cardiovascular events
Comparison: 1 Homocysteine-lowering treatment versus other (any comparisons)
Outcome: 4 Death by any cause
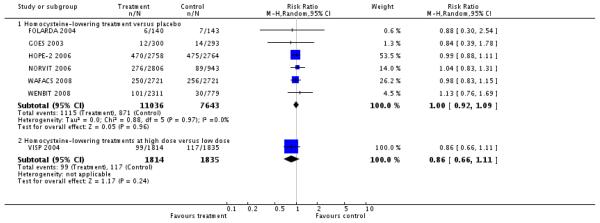 |
Analysis 1.5. Comparison 1 Homocysteine-lowering treatment versus other (any comparisons), Outcome 5 Serious adverse events (Cancer)
Review: Homocysteine lowering interventions for preventing cardiovascular events
Comparison: 1 Homocysteine-lowering treatment versus other (any comparisons)
Outcome: 5 Serious adverse events (Cancer)
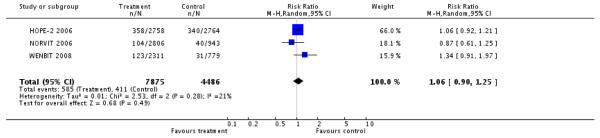 |
Analysis 2.1. Comparison 2 Homocysteine-lowering treatment versus other (Sensitivity Analysis), Outcome 1 Myocardial infarction
Review: Homocysteine lowering interventions for preventing cardiovascular events
Comparison: 2 Homocysteine-lowering treatment versus other (Sensitivity analysis)
Outcome: 1 Myocardial infarction
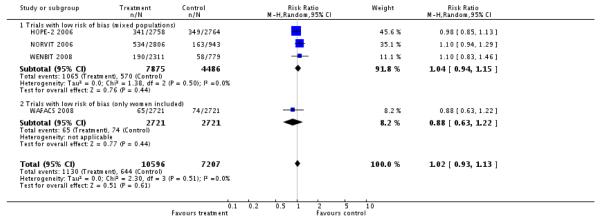 |
Analysis 2.2. Comparison 2 Homocysteine-lowering treatment versus other (Sensitivity Analysis), Outcome 2 Stroke
Review: Homocysteine lowering interventions for preventing cardiovascular events
Comparison: 2 Homocysteine-lowering treatment versus other (Sensitivity analysis)
Outcome: 2 Stroke
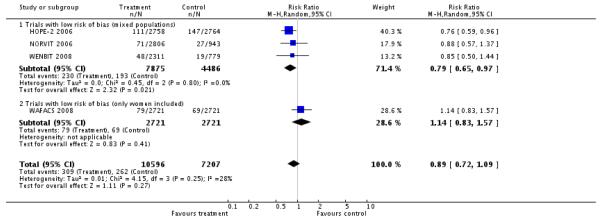 |
Analysis 2.3. Comparison 2 Homocysteine-lowering treatment versus other (Sensitivity Analysis), Outcome 3 First unstable angina pectoris episode requiring hospitalization
Review: Homocysteine lowering interventions for preventing cardiovascular events
Comparison: 2 Homocysteine-lowering treatment versus other (Sensitivity analysis)
Outcome: 3 First unstable angina pectoris episode requiring hospitalization
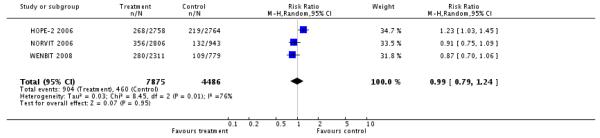 |
Analysis 2.4. Comparison 2 Homocysteine-lowering treatment versus other (Sensitivity analysis), Outcome 4 Death by any cause
Review: Homocysteine lowering interventions for preventing cardiovascular events
Comparison: 2 Homocysteine-lowering treatment versus other (Sensitivity analysis)
Outcome: 4 Death by any cause
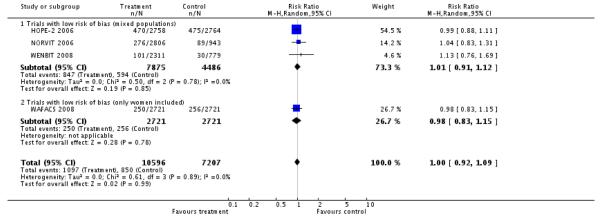 |
HISTORY
Protocol first published: Issue 3, 2007
Review first published: Issue 4, 2009
Appendix 1. Search strategies
CENTRAL (The Cochrane Library 2008, Issue 3)
#1 MeSH descriptor Vitamin B Complex explode all trees
#2 “vitamin b*”
#3 folic next acid in Title, Abstract or Keywords
#4 folate* in Title, Abstract or Keywords
#5 (homocyst* near/6 lower* )
#6 (homocyst* near/6 reduc* )
#7 pyridoxin*
#8 cobalamin*
#9 cyanocobalamin*
#10 pyridoxol*
#11 MeSH descriptor Vitamins this term only
#12 (vitamin* and homocyst* )
#13 multivitamin*
#14 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13)
#15 MeSH descriptor Cardiovascular Diseases this term only
#16 MeSH descriptor Myocardial Ischemia explode all trees
#17 MeSH descriptor Brain Ischemia explode all trees
#18 MeSH descriptor Cerebrovascular Disorders this term only
#19 (coronary near/6 disease )
#20 angina
#21 myocardial next infarct*
#22 heart next infarct*
#23 (stroke or strokes )
#24 (cerebr* near/6 accident* )
#25 (cerebr* near/6 infarct* )
#26 (brain near/6 infarct* )
#27 apoplexy
#28 cardiovascular next disease*
#29 (cardiovascular near/6 event* )
#30 MeSH descriptor Hyperhomocysteinemia explode all trees
#31 hyperhomocyst*
#32 cva
#33 (#15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25)
#34 (#26 or #27 or #28 or #29 or #30 or #31 or #32)
#35 (#33 or #34)
#36 (#14 and #35)
Lilacs (accessed through Biblioteca Virtual em Saúde)
((Pt ENSAYO CONTROLADO ALEATORIO OR Pt ENSAYO CLINICO CONTROLADO OR Mh ENSAYOS CONTROLADOS ALEATORIOS OR Mh DISTRIBUCIÓN ALEATORIA OR Mh METODO DOBLE CIEGO OR Mh METODO SIMPLECIEGO OR Pt ESTUDIO MULTICÉNTRICO) or ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT ((Ct ANIMALES OR Mh ANIMALES OR Ct CONEJOS OR Ct RATÓN OR MH Ratas OR MH Primates OR MH Perros OR MH Conejos OR MH Porcinos) AND NOT (Ct HUMANO AND Ct ANIMALES)) [Palavras] and MH Vitamina B 12 OR Cobamidas OR Hidroxocobalamina OR Complejo Vitamínico B OR Ácido Fólico OR Ácidos Pteroilpoliglutámicos OR Tetrahidrofolatos OR Formiltetrahidrofolatos OR Vitamina B 6 OR Piridoxal OR Fosfato de Piridoxal OR Piridoxamina OR Piridoxina OR Homocisteína OR Vitaminas or TW vitamin$ or tw cobalamin$ or tw cianocobalamin$ or tw cyanocobalam$ or tw cobamid$ or tw hidroxocobalam$ or tw Hydroxocobalam$ or ((tw complejo or tw complex$) and tw vitamin$ and tw b) or (tw acid$ and (tw folic$ or tw ptero$)) or tw Tetrahidrofolatos or tw Formiltetrahidrofolatos or (tw vitamin$ or (tw b or tw b6 or tw b12)) or tw Piridoxal or tw Pyridoxal or ((tw Fosfat$ or tw phosphate$) and (tw Piridoxal or tw pyridoxal)) or tw Piridox$ or tw Pyridox$ or tw Homocisteína or tw Homocysteine) AND (MH Enfermedades Cardiovasculares or Isquemia Miocárdica or Ex C14.280.647$ or Isquemia Encefálica or Ex C10.228.140.300.150$ or Trastornos Cerebrovasculares or hiperhomocisteinemia or Accidente Cerebrovascular or ((tw apoplexia or tw derrame or tw trastorno$ or tw accident$ or tw acidente or tw stroke$ or tw disease$ or tw enfermedad$ or tw doenca$ or tw event$ or tw infart$ or tw isquemia or tw disorder$) and (tw miocardio or tw myocard$ or tw cerebr$ or tw cardiovascul$ or tw heart or tw cardiovascul$ or tw encefal$)) or tw hyperhomocyst$ or tw hiperhomocisteinemia) [Palavras]
MEDLINE (accessed through Ovid)
exp Vitamin B Complex/ (104482)
vitamin b.tw. (4924)
folic acid.tw. (9923)
folate$.tw. (13932)
((homocystein$ or homocystin$) adj3 (low$ or reduc$)).tw. (1436)
pyridoxin$.tw. (3952)
cobalamin$.tw. (2802)
cyanocobalamin$.tw. (1001)
pyridoxol$.tw. (85)
Vitamins/ (14164)
or/1-10 (126718)
Cardiovascular Diseases/ (64258)
exp Myocardial Ischemia/ (291587)
exp Brain Ischemia/ (62826)
Cerebrovascular Disorders/ (41502)
(coronary adj3 disease$).tw. (79742)
angina.tw. (36699)
myocardial infarct$.tw. (107782)
heart infarct$.tw. (708)
heart attack$.tw. (2655)
(stroke or strokes).tw. (89789)
(cerebr$ adj3 (accident$ or infarct$)).tw. (15976)
(brain adj3 infarct$).tw. (3151)
apoplexy.tw. (1922)
(cardiovascular adj2 (disease$ or event$)).tw. (60351)
Hyperhomocysteinemia/ (2497)
hyperhomocyst?in?emi$.tw. (3501)
or/12-27 (553136)
11 and 28 (6360)
randomized controlled trial.pt. (264114)
controlled clinical trial.pt. (79970)
Randomized controlled trials/ (56782)
random allocation/ (62626)
double blind method/ (100106)
single-blind method/ (12467)
or/30-35 (445752)
exp animal/ not humans/ (3351598)
36 not 37 (417326)
clinical trial.pt. (457582)
exp Clinical Trials as Topic/ (211137)
(clin$ adj25 trial$).ti,ab. (151447)
((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab. (96859)
placebos/ (28068)
placebo$.ti,ab. (113353)
random$.ti,ab. (424142)
research design/ (54217)
or/39-46 (942009)
47 not 37 (874873)
38 or 48 (899775)
49 and 29 (1537)
EMBASE (accessed through Ovid)
exp Vitamin B Group/ (75410)
vitamin b.tw. (1818)
folic acid.tw. (6853)
folate$.tw. (11356)
((homocystein$ or homocystin$) adj3 (low$ or reduc$)).tw. (1389)
pyridoxin$.tw. (2405)
cobalamin$.tw. (2261)
cyanocobalamin$.tw. (597)
pyridoxol$.tw. (41)
Vitamins/ (9853)
or/1-10 (88156)
Cardiovascular Diseases/ (69944)
exp ischemic heart disease/ (223039)
exp Coronary Artery Disease/ (81147)
exp Brain Ischemia/ (42318)
cerebrovascular disease/ (15529)
stroke/ (63052)
cerebrovascular accident/ (21560)
(coronary adj3 disease$).tw. (68830)
angina.tw. (29346)
myocardial infarct$.tw. (82436)
heart infarct$.tw. (773)
heart attack$.tw. (1913)
(stroke or strokes).tw. (81585)
(cerebr$ adj3 (accident$ or infarct$)).tw. (13910)
(brain adj3 infarct$).tw. (2838)
apoplexy.tw. (970)
(cardiovascular adj2 (disease$ or event$)).tw. (54102)
Hyperhomocysteinemia/ (4343)
hyperhomocyst?in?emi$.tw. (3426)
or/12-30 (467011)
11 and 31 (7063)
controlled clinical trial/ (50501)
random$.tw. (378547)
randomized controlled trial/ (161864)
follow-up.tw. (340013)
double blind procedure/ (70310)
placebo$.tw. (107162)
placebo/ (117448)
factorial$.ti,ab. (7812)
(crossover$ or cross-over$).ti,ab. (38508)
(double$ adj blind$).ti,ab. (83051)
(singl$ adj blind$).ti,ab. (7269)
assign$.ti,ab. (104756)
allocat$.ti,ab. (33066)
volunteer$.ti,ab. (96369)
Crossover Procedure/ (20586)
Single Blind Procedure/ (7760)
or/33-48 (983879)
32 and 49 (1637)
Allied and Complementary Medicine - AMED (accessed through Ovid)
exp Vitamin B Complex/ (216)
vitamin b.tw. (181)
folic acid.tw. (102)
folate$.tw. (62)
((homocystein$ or homocystin$) adj3 (low$ or reduc$)).tw. (18)
pyridoxin$.tw. (39)
cobalamin$.tw. (12)
cyanocobalamin$.tw. (3)
pyridoxol$.tw. (0)
Vitamins/ (771)
or/1-10 (1061)
(coronary adj3 disease$).tw. (863)
angina.tw. (226)
myocardial infarct$.tw. (559)
heart infarct$.tw. (0)
heart attack$.tw. (47)
(stroke or strokes).tw. (4972)
(cerebr$ adj3 (accident$ or infarct$)).tw. (1762)
(brain adj3 infarct$).tw. (39)
apoplexy.tw. (59)
(cardiovascular adj2 (disease$ or event$)).tw. (1148)
hyperhomocyst?in?emi$.tw. (13)
exp cardiovascular disease/ (10808)
or/12-23 (12172)
11 and 24 (95)
randomized controlled trial.pt. (1200)
randomised controlled trial.pt. (67)
random$.tw. (9697)
factorial$.tw. (223)
(crossover$ or cross over$).tw. (617)
placebo$.tw. (2128)
(double$ adj blind).tw. (1270)
(singl$ adj blind$).tw. (361)
assign$.tw. (2815)
allocat$.tw. (1211)
volunteer$.tw. (2403)
double blind method/ (386)
random allocation/ (288)
exp clinical trials/ (2938)
or/26-39 (15394)
25 and 40 (10)
ISI Web of Science
# 11 TS=(#10 and (random* or blind* or placebo* or comparative or comparison or prospective or controlled or trial or evaluation or rct))
# 10 #7 or #8 or #9
# 9 TS=(#6 and (“cerebrovascular accident*” or hyperhomocyst*))
# 8 TS=(#6 and (angina or stroke or strokes or cva or infarction*))
# 7 TS=(#6 and (cardiovascular or myocardial or coronary or cardiac or “heart disease*”))
# 6 #1 or #2 or #3 or #4 or #5
# 5 TS=(homocyst* same (lower* or reduc*))
# 4 TS=(vitamin* and homocyst*)
# 3 TS=folate*
# 2 TS=“vitamin B”
# 1 TS=(pyridoxin* or cobalamin* or cyanocobalamin* or pyridoxol* or “folic acid”)
Footnotes
DECLARATIONS OF INTEREST Ivan Solà, Dimitrios Lathyris and Georgia Salanti: None known.
In 2004 Arturo Martí-Carvajal was employed by Eli Lilly to run a four hours workshop on ‘how to critically appraise clinical trials on osteoporosis and how to teach this’. This activity was not related to his work with The Cochrane Collaboration or any Cochrane review.
In 2007 Arturo Martí-Carvajal was employed by Merck to run a four hours workshop ‘how to critically appraise clinical trials and how to teach this’. This activity was not related to his work with The Cochrane Collaboration or any Cochrane review.
References to studies included in this review
- CHAOS 2002 {published data only} .Baker F, Picton D, Blackwood S, Hunt J, Erskine M, Dyas M, et al. Blinded comparison of folic acid and placebo in patients with ischaemic heart disease: an outcome trial. Circulation. 2002;106(Suppl II):741. [Google Scholar]
- FOLARDA 2004 {published data only} .Liem AH, van Boven AJ, Veeger NJ, Withagen AJ, Robles de Medina RM, Tijssen JG, et al. Efficacy of folic acid when added to statin therapy in patients with hypercholesterolemia following acute myocardial infarction: a randomised pilot trial. International Journal of Cardiology. 2004;93:175–9. doi: 10.1016/j.ijcard.2003.02.001. [MEDLINE: 14975544] [DOI] [PubMed] [Google Scholar]
- GOES 2003 {published data only} .*; Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: effects on clinical outcomes. Journal of the American College of Cardiology. 2003;41:2105–13. doi: 10.1016/s0735-1097(03)00485-6. [MEDLINE: 12821232] [DOI] [PubMed] [Google Scholar]; Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: results of the Goes extension study. Heart. 2005;91:1213–4. doi: 10.1136/hrt.2004.035030. [MEDLINE: 16103563] [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPE-2 2006 {published data only} .Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. New England Journal of Medicine. 2006;354:1567–77. doi: 10.1056/NEJMoa060900. [MEDLINE: 16531613] [DOI] [PubMed] [Google Scholar]
- NORVIT 2006 {published data only} .Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. New England Journal of Medicine. 2006;354:1578–88. doi: 10.1056/NEJMoa055227. [MEDLINE: 16531614] [DOI] [PubMed] [Google Scholar]
- VISP 2004 {published data only} .Spence JD, Bang H, Chambless LE, Stampfer MJ. Vitamin Intervention For Stroke Prevention trial: an efficacy analysis. Stroke. 2005;36:2404–09. doi: 10.1161/01.STR.0000185929.38534.f3. [DOI] [PubMed] [Google Scholar]; *; Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–75. doi: 10.1001/jama.291.5.565. [MEDLINE: 14762035] [DOI] [PubMed] [Google Scholar]
- WAFACS 2008 {published data only} .*; Albert CM, Cook NR, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027–36. doi: 10.1001/jama.299.17.2027. [MEDLINE: 18460663] [DOI] [PMC free article] [PubMed] [Google Scholar]; Redbeg RF, Block PC. A randomized trial of folic acid and B-vitamins in the secondary prevention of cardiovascular events in women: Results from the Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS) ACC Cardiosource Review Journal. 2006;16(7):52–58. [Google Scholar]
- WENBIT 2008 {published data only} .Ebbing M, Bleie Ø , Ueland PM, Nordrehaug JE, Nilsen DW, Vollset SE, et al. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA. 2008;300:795–804. doi: 10.1001/jama.300.7.795. [PUBMED: 18714059] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Bazzano 2006 {published data only} .Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA. 2006;296:2720–6. doi: 10.1001/jama.296.22.2720. [DOI] [PubMed] [Google Scholar]
- FINEST 2006 {published data only (unpublished sought but not used)} .Nevado J. [accessed Oct 12th 2007];Folate Intervention in Non-ST elevation myocardial infarction and unstable angina: a randomised placebo-controlled trial. 2006 http://controlled-trials.com/ISRCTN30249553/homocysteine.
- Lange 2004 {published data only} .Lange H, Suryapranata H, De Luca G, Börner C, Dille J, Kallmayer K, et al. Folate therapy and in-stent restenosis after coronary stenting. New England Journal of Medicine. 2004;350:2673–81. doi: 10.1056/NEJMoa032845. [MEDLINE: 15215483] [DOI] [PubMed] [Google Scholar]
- Lonn 2007 {published data only} .Lonn E. Homocysteine in the prevention of ischemic heart disease, stroke and venous thromboembolism: therapeutic target or just another distraction? Current Opinion in Hematology. 2007;14:481–7. doi: 10.1097/MOH.0b013e3282c48bd8. [MEDLINE: 17934354] [DOI] [PubMed] [Google Scholar]
- PACIFIC 2002 {published data only} .Neal B, MacMahon S, Ohkubo T, Tonkin A, Wilcken D, PACIFIC Study Group Dose-dependent effects of folic acid on plasma homocysteine in a randomized trial conducted among 723 individuals with coronary heart disease. European Heart Journal. 2002;23:1509–15. doi: 10.1053/euhj.2002.3161. [MEDLINE: 12395803] [DOI] [PubMed] [Google Scholar]
- Swiss 2002 {published data only} .Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: the Swiss Heart study: a randomized controlled trial. JAMA. 2002;288:973–9. doi: 10.1001/jama.288.8.973. [MEDLINE: 12190367] [DOI] [PubMed] [Google Scholar]
- Vesin 2007 {published data only} .Vesin C, Mairesse S, Iaria P, Blacher J. Efficacy of folic acid in prevention of cerebrovascular disease [Efficacite de l’acide folique dans la prevention cerebrovasculaire] Medecine des Maladies Metaboliques. 2007;1:50–2. [EMBASE: 2007490543] [Google Scholar]
- Wang 2007 {published data only} .Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, et al. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. 2007;369:876–82. doi: 10.1016/S0140-6736(07)60854-X. [MEDLINE: 17544768] [DOI] [PubMed] [Google Scholar]
- Wierzbicki 2007 {published data only} .Wierzbicki AS. Homocysteine and cardiovascular disease: a review of the evidence. Diabetes & Vascular Disease Research. 2007;4:143–50. doi: 10.3132/dvdr.2007.033. [MEDLINE: 17654449] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- SEARCH 2000 {published data only} .MacMahon M, Kirkpatrick C, Cummings CE, Clayton A, Robinson PJ, Tomiak RH, et al. A pilot study with simvastatin and folic acid/vitamin B12 in preparation for the Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Nutrition, Metabolism, and Cardiovascular Diseases. 2000;10:195–203. [PubMed] [Google Scholar]
- SU.FOL.OM3 2003 {published data only} .Galan P, de Bree A, Mennen L, Potier de Courcy G, Preziozi P, Bertrais S, et al. Background and rationale of the SU.FOL.OM3 study: double-blind randomized placebo-controlled secondary prevention trial to test the impact of supplementation with folate, vitamin B6 and B12 and/or omega-3 fatty acids on the prevention of recurrent ischemic events in subjects with atherosclerosis in the coronary or cerebral arteries. The Journal of Nutrition, Health & Aging. 2003;7:428–35. [PubMed] [Google Scholar]
- VITATOPS 2002 {unpublished data only} .The VITATOPS (Vitamins to Prevent Stroke) Trial The VITATOPS (Vitamins to Prevent Stroke) Trial: rationale and design of an international, large, simple, randomised trial of homocysteine-lowering multivitamin therapy in patients with recent transient ischaemic attack or stroke. Cerebrovascular Diseases. 2002;13:120–6. doi: 10.1159/000047761. [DOI] [PubMed] [Google Scholar]
Additional references
- Abahji 2007 .Abahji TN, Nill L, Ide N, Keller C, Hoffmann U, Weiss N. Acute hyperhomocysteinemia induces microvascular and macrovascular endothelial dysfunction. Archives of Medical Research. 2007;38:411–6. doi: 10.1016/j.arcmed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Anderson 2004 .Anderson JL, Jensen KR, Carlquist JF, Bair TL, Horne BD, Muhlestein JB. Effect of folic acid fortification of food on homocysteine-related mortality. American Journal of Medicine. 2004;116:158–64. doi: 10.1016/j.amjmed.2003.10.024. [MEDLINE: 14749159] [DOI] [PubMed] [Google Scholar]
- Andrès 2004 .Andrès E, Loukili NH, Noel E, Kaltenbach G, Abdelgheni MB, Perrin AE, et al. Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ : Canadian Medical Association journal = journal de l’Association Medicale Canadienne. 2004;171(3):251–9. doi: 10.1503/cmaj.1031155. [PUBMED: 15289425] [DOI] [PMC free article] [PubMed] [Google Scholar]
- B-VTTC 2006 .B-Vitamin Treatment Trialists’ Collaboration Homocysteine-lowering trials for prevention of cardiovascular events: a review of the design and power of the large randomized trials. American Heart Journal. 2006;151:282–7. doi: 10.1016/j.ahj.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Beł towski 2005 .Beł towski J. Protein homocysteinylation: a new mechanism of atherogenesis? Postȩ py higieny i medycyny doś wiadczalnej. 2005;59:392–404. [MEDLINE: 16106241] [PubMed] [Google Scholar]
- Bleie 2007 .Bleie Ø , Semb AG, Grundt H, Nordrehaug JE, Vollset SE, Ueland PM, et al. Homocysteine-lowering therapy does not affect inflammatory markers of atherosclerosis in patients with stable coronary artery disease. Journal of Internal Medicine. 2007;262:244–53. doi: 10.1111/j.1365-2796.2007.01810.x. [MEDLINE: 17645592] [DOI] [PubMed] [Google Scholar]
- Boushey 1995 .Boushey CJ, Beresford SAA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–57. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- Casas 2005 .Casas JP, Bautista LE, Smeeth L, Sharma P, Hingorani AD. Homocysteine and stroke: evidence on a causal link from mendelian randomisation. Lancet. 2005;365:224–32. doi: 10.1016/S0140-6736(05)17742-3. [DOI] [PubMed] [Google Scholar]
- Castro 2006 .Castro R, Rivera I, Blom HJ, Jakobs C, Tavares de Almeida I. Homocysteine metabolism, hyperhomocysteinaemia and vascular disease: an overview. Journal of Inherited Metabolic Disease. 2006;29:3–20. doi: 10.1007/s10545-006-0106-5. [DOI] [PubMed] [Google Scholar]
- Clarke 2007 .Clarke R, Lewington S, Sherliker P, Armitage J. Effects of B-vitamins on plasma homocysteine concentrations and on risk of cardiovascular disease and dementia. Current Opinion in Clinical Nutrition and Metabolic Care. 2007;10:32–9. doi: 10.1097/MCO.0b013e328011aa71. [MEDLINE: 17143052] [DOI] [PubMed] [Google Scholar]
- Cole 2007 .Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–9. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- Danesh 1998 .Danesh J, Lewington S. Plasma homocysteine and coronary heart disease: systematic review of published epidemiological studies. Journal of Cardiovascular Risk. 1998;5:229–32. [MEDLINE: 9919470] [PubMed] [Google Scholar]
- Davey Smith 2003 .Davey Smith G, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? International Journal of Epidemiology. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- Durga 2005 .Durga J, Verhoef P. Let them eat cake. Current Nutrition & Food Science. 2005;1:35–40. [Google Scholar]
- Dusitanond 2005 .Dusitanond P, Eikelboom JW, Hankey GJ, Thom J, Gilmore G, Loh K, et al. Homocysteine-lowering treatment with folic acid, cobalamin, and pyridoxine does not reduce blood markers of inflammation, endothelial dysfunction, or hypercoagulability in patients with previous transient ischemic attack or stroke: a randomized substudy of the VITATOPS trial. Stroke. 2005;36:144–6. doi: 10.1161/01.STR.0000150494.91762.70. [MEDLINE: 15569860] [DOI] [PubMed] [Google Scholar]
- Eikelboom 1999 .Eikelboom JW, Lonn E, Genest J, Jr, Hankey G, Yusuf S. Homocyst(e)ine and cardiovascular disease: a critical review of the epidemiologic evidence. Annals of Internal Medicine. 1999;131:363–75. doi: 10.7326/0003-4819-131-5-199909070-00008. [DOI] [PubMed] [Google Scholar]
- Epstein 1996 .Epstein FH. Cardiovascular disease epidemiology : a journey from the past into the future. Circulation. 1996;93:1755–64. doi: 10.1161/01.cir.93.9.1755. [DOI] [PubMed] [Google Scholar]
- Ferretti 2006 .Ferretti G, Bacchetti T, Negre-Salvayre A, Salvayre R, Dousset N, Curatola G. Structural modifications of HDL and functional consequences. Atherosclerosis. 2006;184:1–7. doi: 10.1016/j.atherosclerosis.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Folsom 1998 .Folsom AR, Nieto FJ, McGovern PG, Tsai MY, Malinow MR, Eckfeldt JH, et al. Prospective study of coronary heart disease incidence in relation to fasting total homocysteine, related genetic polymorphisms, and B vitamins: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 1998;98:204–10. doi: 10.1161/01.cir.98.3.204. [DOI] [PubMed] [Google Scholar]
- Ford 2002 .Ford ES, Smith SJ, Stroup DF, Steinberg KK, Mueller PW, Thacker SB. Homocyst(e)ine and cardiovascular disease: a systematic review of the evidence with special emphasis on case-control studies and nested case-control studies. International Journal of Epidemiology. 2002;31:59–70. doi: 10.1093/ije/31.1.59. [MEDLINE: 11914295] [DOI] [PubMed] [Google Scholar]
- Fowler 2005 .Fowler B. Homocysteine: overview of biochemistry, molecular biology, and role in disease processes. Seminars in Vascular Medicine. 2005;5:77–86. doi: 10.1055/s-2005-872394. [MEDLINE: 16047261] [DOI] [PubMed] [Google Scholar]
- Gaziano 2006 .Gaziano T, Reddy KS, Paccaud F, Horton S, Chaturvedi V. Cardiovascular Disease. In: Jamison DT, et al., editors. Disease control priorities in developing countries. 2nd Edition The World Bank and Oxford University Press; Washington DC; New York: 2006. [Google Scholar]
- Gori 2007 .Gori AM, Sofi F, Marcucci R, Giusti B, Franco Gensini G, Abbate R. Association between homocysteine, vitamin B (6) concentrations and inflammation. Clinical Chemistry and Laboratory Medicine. 2007;45:1728–36. doi: 10.1515/CCLM.2007.347. [MEDLINE: 17990948] [DOI] [PubMed] [Google Scholar]
- Guilland 2003 .Guilland JC, Favier A, Potier de Courcy G, Galan P, Hercberg S. Hyperhomocysteinemia: an independent risk factor or a simple marker of vascular disease? 2. Epidemiological data. Pathologie-Biologie. 2003;51:111–21. doi: 10.1016/s0369-8114(03)00105-6. [MEDLINE: 12801809] [DOI] [PubMed] [Google Scholar]
- Guthikonda 2006 .Guthikonda S, Haynes WG. Homocysteine: role and implications in atherosclerosis. Current Atherosclerosis Reports. 2006;8:100–6. doi: 10.1007/s11883-006-0046-4. [DOI] [PubMed] [Google Scholar]
- Hackam 2003 .Hackam DG, Anand SS. Emerging risk factors for atherosclerotic vascular disease: a critical review of the evidence. JAMA. 2003;290:932–40. doi: 10.1001/jama.290.7.932. [DOI] [PubMed] [Google Scholar]
- Higgins 2003 .Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2008 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.0. The Cochrane Collaboration; 2008. Available from www.cochrane-handbook.org. [Google Scholar]
- HLTC 2005 .Homocysteine Lowering Trialists’ Collaboration Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. American Journal of Clinical Nutrition. 2005;82:806–12. doi: 10.1093/ajcn/82.4.806. [DOI] [PubMed] [Google Scholar]
- Hoey 2007 .Hoey L, McNulty H, Askin N, Dunne A, Ward M, Pentieva K, et al. Effect of a voluntary food fortification policy on folate, related B vitamin status, and homocysteine in healthy adults. American Journal of Clinical Nutrition. 2007;86:1405–13. doi: 10.1093/ajcn/86.5.1405. [MEDLINE: 17991653] [DOI] [PubMed] [Google Scholar]
- HSC 2002 .Homocysteine Studies Collaboration Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–22. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- Hulley 1998 .Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Humphrey 2008 .Humphrey LL, Fu R, Rogers K, Freeman M, Helfand M. Homocysteine level and coronary heart disease incidence: a systematic review and meta-analysis. Mayo Clinic Proceedings. 2008;83:1203–12. doi: 10.4065/83.11.1203. [DOI] [PubMed] [Google Scholar]
- ICH-GCP 1997 .International Committee on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. Code of Federal Regulations & International Conference on Harmonisation Guidelines; Philadelphia, US. Barnett International/PAREXEL; 1997. [Google Scholar]
- Jacobsen 2005 .Jacobsen DW, Catanescu O, Dibello PM, Barbato JC. Molecular targeting by homocysteine: a mechanism for vascular pathogenesis. Clinical Chemistry & Laboratory Medicine. 2005;43:1076–83. doi: 10.1515/CCLM.2005.188. [DOI] [PubMed] [Google Scholar]
- Jacobsen 2006 .Jacobsen DW. Homocysteine targeting of plasma proteins in hemodialysis patients. Kidney International. 2006;69:787–89. doi: 10.1038/sj.ki.5000235. [DOI] [PubMed] [Google Scholar]
- Jacques 1999 .Jacques PF, Selhub J, Bostom AG, Wilson PWF, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. New England Journal of Medicine. 1999;340:1449–54. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]
- Jakubowski 2000 .Jakubowski H. Homocysteine thiolactone: metabolic origin and protein homocysteinylation in humans. Journal of Nutrition. 2000;130(2S Suppl):377S–81S. doi: 10.1093/jn/130.2.377S. [DOI] [PubMed] [Google Scholar]
- Jakubowski 2004 .Jakubowski H. Molecular basis of homocysteine toxicity in humans. Cellular and Molecular Life Sciences. 2004;61:470–87. doi: 10.1007/s00018-003-3204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski 2005 .Jakubowski H. Anti-N-homocysteinylated protein autoantibodies and cardiovascular disease. Clinical Chemistry & Laboratory Medicine. 2005;43:1011–4. doi: 10.1515/CCLM.2005.177. [DOI] [PubMed] [Google Scholar]
- Jakubowski 2008 .Jakubowski H. The pathophysiological hypothesis of homocysteine thiolactone-mediated vascular disease. Journal of Physiology and Pharmacology. 2008;59(Suppl 9):155–67. [MEDLINE: 19261978] [PubMed] [Google Scholar]
- Jakubowski 2009 .Jakubowski H, Perla-Kaján J, Finnell RH, Cabrera RM, Wang H, Gupta S, et al. Genetic or nutritional disorders in homocysteine or folate metabolism increase protein N-homocysteinylation in mice. The Faseb Journal. 2009;23:1721–7. doi: 10.1096/fj.08-127548. [MEDLINE: 19204075] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison 2006 .Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al. Priorities in health. The World Bank; Washington DC: 2006. [PubMed] [Google Scholar]
- Jamison 2007 .Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, et al. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA. 2007;298:1163–70. doi: 10.1001/jama.298.10.1163. [DOI] [PubMed] [Google Scholar]
- Kahn 2008 .Kahn R, Robertson RM, Smith R, Eddy D. The impact of prevention on reducing the burden of cardiovascular disease. Circulation. 2008;118:576–85. doi: 10.1161/CIRCULATIONAHA.108.190186. [DOI] [PubMed] [Google Scholar]
- Kaul 2006 .Kaul S, Zadeh AA, Shah PK. Homocysteine hypothesis for atherothrombotic cardiovascular disease: Not validated. Journal of the American College of Cardiology. 2006;48:914–23. doi: 10.1016/j.jacc.2006.04.086. [DOI] [PubMed] [Google Scholar]
- Koehler 1997 .Koehler KM, Pareo-Tubbeh SL, Romero LJ, Baumgartner RN, Garry PJ. Folate nutrition and older adults: challenges and opportunities. Journal of the American Dietetic Association. 1997;97:167–73. doi: 10.1016/S0002-8223(97)00044-8. [MEDLINE: 9020245] [DOI] [PubMed] [Google Scholar]
- Libby 1995 .Libby P, Sukhova G, Lee RT, Galis ZS. Cytokines regulate vascular functions related to stability of the atherosclerotic plaque. Journal of Cardiovascular Pharmacology. 1995;25(Suppl 2):9–12. doi: 10.1097/00005344-199500252-00003. [MEDLINE: 8699871] [DOI] [PubMed] [Google Scholar]
- Loscalzo 2006 .Loscalzo J. Homocysteine Trials - Clear Outcomes for Complex Reasons. New England Journal of Medicine. 2006;354:1629–32. doi: 10.1056/NEJMe068060. [DOI] [PubMed] [Google Scholar]
- Marcus 2007 .Marcus J, Sarnak MJ, Menon V. Homocysteine lowering and cardiovascular disease risk: lost in translation. Canadian Journal of Cardiology. 2007;23(9):707–10. doi: 10.1016/s0828-282x(07)70814-0. [MEDLINE: 17622392] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark 1996 .Mark SD, Wang W, Fraumeni JF, Jr, Li JY, Taylor PR, Wang GQ, et al. Lowered risks of hypertension and cerebrovascular disease after vitamin/mineral supplementation: the Linxian Nutrition Intervention Trial. American Journal of Epidemiology. 1996;143:658–64. doi: 10.1093/oxfordjournals.aje.a008798. [MEDLINE: 8651227] [DOI] [PubMed] [Google Scholar]
- Maron 2006 .Maron BA, Loscalzo J. Homocysteine. Clinics in Laboratory Medicine. 2006;26:591–609. doi: 10.1016/j.cll.2006.06.008. [MEDLINE: 16938586] [DOI] [PubMed] [Google Scholar]
- Math 2007 .Math MV, Balasubramaniam P. Steps to control the burden of cardiovascular disease in the Indian Subcontinent. The Indian Journal of Medical Research. 2007;125:590–1. [PubMed] [Google Scholar]
- McCully 2005 .McCully KS. Hyperhomocysteinemia and arteriosclerosis: historical perspectives. Clinical Chemistry & Laboratory Medicine. 2005;43:980–6. doi: 10.1515/CCLM.2005.172. [DOI] [PubMed] [Google Scholar]
- McNulty 2008 .McNulty H, Pentieva K, Hoey L, Ward M. Homocysteine, B-vitamins and CVD. The Proceeding of the Nutrition Society. 2008;67:232–7. doi: 10.1017/S0029665108007076. [MEDLINE: 18412997] [DOI] [PubMed] [Google Scholar]
- Mirarefin 2007 .Mirarefin M, Aminpour A, Fakhrzadeh H, Tahbaz F, Abadi A. The effect of folate fortified bread on homocysteine, folate and vitamin B12 status among hyperhomocysteinemic subjects: Tehran homocysteine study. Iranian Journal of Diabetes and Lipid Disorders. 2007;7:229–37. [Google Scholar]
- Moat 2008 .Moat SJ. Plasma total homocysteine: instigator or indicator of cardiovascular disease? Annals of Clinical Biochemistry. 2008;45(Pt 4):345–8. doi: 10.1258/acb.2008.008053. [MEDLINE: 18583617] [DOI] [PubMed] [Google Scholar]
- Moens 2007 .Moens AL, Claeys MJ, Wuyts FL, Goovaerts I, Van Hertbruggen E, Wendelen LC, et al. Effect of folic acid on endothelial function following acute myocardial infarction. American Journal of Cardiology. 2007;99:476–81. doi: 10.1016/j.amjcard.2006.08.057. [DOI] [PubMed] [Google Scholar]
- Mudd 2000 .Mudd SH, Finkelstein JD, Refsum H, Ueland PM, Malinow MR, Lentz SR, et al. Homocysteine and its disulfide derivatives: a suggested consensus terminology. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:704–6. doi: 10.1161/01.atv.20.7.1704. [MEDLINE: 10894806] [DOI] [PubMed] [Google Scholar]
- NACB 2009 .Myers GL, Christenson RH, Cushman M, Ballantyne CM, Cooper GR, Pfeiffer CM, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice guidelines: emerging biomarkers for primary prevention of cardiovascular disease. Clinical Chemistry. 2009;55:378–84. doi: 10.1373/clinchem.2008.115899. [MEDLINE: 19106185] [DOI] [PubMed] [Google Scholar]
- Narayan 2006 .Narayan KMV, Zhang P, Kanaya AM, Williams DE, Engelgau MM, Imperatore G, et al. Diabetes: the pandemic and potential solutions. In: Jameson DT, editor. Disease control priorities in developing countries. 2nd Edition The World Bank and Oxford University Press; Washington, Oxford: 2006. [Google Scholar]
- Nigwekar 2009 .Nigwekar SU, Cass A, Gallagher MP, Jardine MJ, Kang A, Kulshrestha S, et al. Interventions for lowering plasma homocysteine levels in dialysis patients. Cochrane Database of Systematic Reviews. 2009;(Issue 2) doi: 10.1002/14651858.CD004683.pub4. Art. No.: CD004683. DOI: 10.1002/14651858.CD004683.pub3. [DOI: 10.1002/14651858] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH 2006 .National Health Institutes NIH State-of-the-Science Conference Statement on Multivitamin/Mineral Supplements and Chronic Disease Prevention. Annals of Internal Medicine. 2006;145:364–71. doi: 10.7326/0003-4819-145-5-200609050-00136. [DOI] [PubMed] [Google Scholar]
- Nygård 1997 .Nygård O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. New England Journal of Medicine. 1997;337:230–6. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- Obeid 2009 .Obeid R, Herrmann W. Homocysteine and lipids: S-adenosyl methionine as a key intermediate. FEBS Letters. 2009;583:1215–25. doi: 10.1016/j.febslet.2009.03.038. [MEDLINE: 19324042] [DOI] [PubMed] [Google Scholar]
- Peeters 2007 .Peeters AC, van Aken BE, Blom HJ, Reitsma PH, den Heijer M. The effect of homocysteine reduction by B-vitamin supplementation on inflammatory markers. Clinical Chemistry and Laboratory Medicine. 2007;45:54–8. doi: 10.1515/CCLM.2007.021. [DOI] [PubMed] [Google Scholar]
- Per a-Kaján 2007 .Per a-Kaján J, Twardowski T, Jakubowski H. Mechanisms of homocysteine toxicity in humans. Amino Acids. 2007;32:561–72. doi: 10.1007/s00726-006-0432-9. [MEDLINE: 17285228] [DOI] [PubMed] [Google Scholar]
- Poddar 2001 .Poddar R, Sivasubramanian N, DiBello PM, Robinson K, Jacobsen DW. Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: implications for vascular disease. Circulation. 2001;103:2717–23. doi: 10.1161/01.cir.103.22.2717. [MEDLINE: 11390343] [DOI] [PubMed] [Google Scholar]
- Potter 2008a .Potter K, Hankey GJ, Green DJ, Eikelboom J, Jamrozik K, Arnolda LF. The effect of long-term homocysteine-lowering on carotid intima-media thickness and flow-mediated vasodilation in stroke patients: a randomized controlled trial and meta-analysis. BMC Cardiovascular Disorders. 2008;8:24. doi: 10.1186/1471-2261-8-24. [MEDLINE: 18803866] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter 2008b .Potter K. Homocysteine and cardiovascular disease: should we treat? The Clinical Biochemist. Reviews. 2008;29:27–30. [MEDLINE: 18566666] [PMC free article] [PubMed] [Google Scholar]
- Prasad 1999 .Prasad K. Homocysteine, a risk factor for cardiovascular disease. International Journal of Angiology. 1999;8:76–86. doi: 10.1007/BF01616850. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan 2006 .Ramakrishnan S, Sulochana KN, Lakshmi S, Selvi R, Angayarkanni N. Biochemistry of homocysteine in health and diseases. Indian Journal of Biochemistry & Biophysics. 2006;43:275–83. [MEDLINE: 17133733] [PubMed] [Google Scholar]
- Rampersaud 2003 .Rampersaud GC, Kauwell GP, Bailey LB. Folate: a key to optimizing health and reducing disease risk in the elderly. Journal of the American College of Nutrition. 2003;22:1–8. doi: 10.1080/07315724.2003.10719270. [MEDLINE: 12569109] [DOI] [PubMed] [Google Scholar]
- Refsum 1998 .Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annual Review of Medicine. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- Refsum 2006 .Refsum H, Nurk E, Smith AD, Ueland PM, Gjesdal CG, Bjelland I, et al. The Hordaland Homocysteine Study: a community-based study of homocysteine, its determinants, and associations with disease. Journal of Nutrition. 2006;136(6 Suppl):1731S–40S. doi: 10.1093/jn/136.6.1731S. [DOI] [PubMed] [Google Scholar]
- Riksen 2005 .Riksen NP, Rongen GA, Boers GH, Blom HJ, van den Broek PH, Smits P. Enhanced cellular adenosine uptake limits adenosine receptor stimulation in patients with hyperhomocysteinemia. Arteriosclerosis, Thrombosis & Vascular Biology. 2005;25:109–14. doi: 10.1161/01.ATV.0000150651.85907.69. [DOI] [PubMed] [Google Scholar]
- Robertson 2005 .Robertson J, Iemolo F, Stabler SP, Allen RH, Spence JD. Vitamin B12, homocysteine and carotid plaque in the era of folic acid fortification of enriched cereal grain product products. Canadian Medical Association Journal. 2005;172:1569–73. doi: 10.1503/cmaj.045055. [MEDLINE: 15939916] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson 1998 .Robinson K, Arheart K, Refsum H, Brattstrom L, Boers G, Ueland P, et al. Low circulating folate and vitamin B6 concentrations: risk factors for stroke, peripheral vascular disease, and coronary artery disease. European COMAC Group. Circulation. 1998;97:437–43. doi: 10.1161/01.cir.97.5.437. [DOI] [PubMed] [Google Scholar]
- Rodgers 2006 .Rodgers A, Lawes CMM, Gaziano TA, Vos T. The growing burden of risk from high blood pressure, cholesterol, and bodyweight. In: Jameson DT, editor. Disease control priorities in developing countries. 2nd Edition The World Bank and Oxford University Press; Washington, New York: 2006. [Google Scholar]
- Sato 2002 .Sato Y, Kaji M, Kondo I, Yoshida H, Satoh K, Metoki N. Hyperhomocysteinemia in Japanese patients with convalescent stage ischemic stroke : effect of combined therapy with folic acid and mecobalamine. Journal of the Neurological Sciences. 2002;202:65–8. doi: 10.1016/s0022-510x(02)00210-1. [DOI] [PubMed] [Google Scholar]
- Selhub 1993 .Selhub J, Jacques PF, Wilson PWF, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA. 1993;270:2693–8. doi: 10.1001/jama.1993.03510220049033. [DOI] [PubMed] [Google Scholar]
- Selhub 2006 .Selhub J. The many facets of hyperhomocysteinemia: studies from the Framingham cohorts. Journal of Nutrition. 2006;136(6 Suppl):1726S–30S. doi: 10.1093/jn/136.6.1726S. [DOI] [PubMed] [Google Scholar]
- Shelhub 2008 .Shelhub J. Public health significance of elevated homocysteine. Food and Nutrition Bulletin. 2008;29(Suppl 2):116–25. doi: 10.1177/15648265080292S116. [MEDLINE: 18709886] [DOI] [PubMed] [Google Scholar]
- Sofi 2008 .Sofi F, Marcucci R, Bolli P, Giambene B, Sodi A, Fedi S, et al. Low vitamin B6 and folic acid levels are associated with retinal vein occlusion independently of homocysteine levels. Atherosclerosis. 2008;198:223–7. doi: 10.1016/j.atherosclerosis.2007.09.009. [MEDLINE: 17945240] [DOI] [PubMed] [Google Scholar]
- Spence 2005 .Spence JD, Bang H, Chambless LE, Stampfer MJ. Vitamin Intervention For Stroke Prevention trial: an efficacy analysis. Stroke. 2005;36:2404–09. doi: 10.1161/01.STR.0000185929.38534.f3. [DOI] [PubMed] [Google Scholar]
- Spence 2007 .Spence JD. Homocysteine-lowering therapy: a role in stroke prevention? The Lancet Neurology. 2007;6:830–8. doi: 10.1016/S1474-4422(07)70219-3. [MEDLINE: 17706567] [DOI] [PubMed] [Google Scholar]
- Splaver 2004 .Splaver A, Lamas GA, Hennekens CH. Homocysteine and cardiovascular disease: biological mechanism, observational epidemiology, and the need for randomised trials. American Heart Journal. 2004;148:34–40. doi: 10.1016/j.ahj.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Stampfer 1992 .Stampfer MJ, Malinow MR, Willett WC, Newcomer LM, Upson B, Ullmann D, et al. prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA. 1992;268:877–81. [PubMed] [Google Scholar]
- Su 2005 .Su SJ, Huang LW, Pai LS, Liu HW, Chang KL. Homocysteine at pathophysiologic concentrations activates human monocyte and induces cytokine expression and inhibits macrophage migration inhibitory factor expression. Nutrition. 2005;21:994–1002. doi: 10.1016/j.nut.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Tanriverdi 2007 .Tanriverdi H, Evrengul H, Enli Y, Kuru O, Seleci D, Tanriverdi S, et al. Effect of homocysteine-induced oxidative stress on endothelial function in coronary slow-flow. Cardiology. 2007;107:313–20. doi: 10.1159/000099068. [DOI] [PubMed] [Google Scholar]
- Ueland 1992 .Ueland PM, Refsum H, Brattstrom L. Plasma homocysteine and cardiovascular disease. In: Francis RB, editor. Atherosclerotic cardiovascular disease hemostasis and endothelial function. Marcel Dekker; New York: 1992. [Google Scholar]
- Ueland 2000 .Ueland PM, Refsum H, Beresford SA, Vollset SE. The controversy over homocysteine and cardiovascular risk. American Journal of Clinical Nutrition. 2000;72:324–32. doi: 10.1093/ajcn/72.2.324. [MEDLINE: 10919921] [DOI] [PubMed] [Google Scholar]
- Ueland 2007 .Ueland PM, Clarke R. Homocysteine and cardiovascular risk: considering the evidence in the context of study design, folate fortification, and statistical power. Clinical Chemistry. 2007;53:807–9. doi: 10.1373/clinchem.2007.085480. [DOI] [PubMed] [Google Scholar]
- Ulrich 2006 .Ulrich CM, Potter JD. Folate supplementation: too much of a good thing? Cancer Epidemiology, Biomarkers & Prevention. 2006;15:189–93. doi: 10.1158/1055-9965.EPI-152CO. [DOI] [PubMed] [Google Scholar]
- Undas 2006 .Undas A, Stepien E, Glowacki R, Tisonczyk J, Tracz W, Jakubowski H. Folic acid administration and antibodies against homocysteinylated proteins in subjects with hyperhomocysteinemia. Thrombosis and Haemostasis. 2006;96:342–7. doi: 10.1160/TH06-04-0228. [DOI] [PubMed] [Google Scholar]
- Urquhart 2007 .Urquhart BL, Freeman DJ, Spence JD, House AA. Mesna as a nonvitamin intervention to lower plasma total homocysteine concentration: implications for assessment of the homocysteine theory of atherosclerosis. Journal of Clinical Pharmacology. 2007;47:991–7. doi: 10.1177/0091270007303767. [DOI] [PubMed] [Google Scholar]
- Verhaar 2002 .Verhaar MC, Stroes E, Rabelink TJ. Folates and cardiovascular disease. Arteriosclerosis, Thrombosis & Vascular Biology. 2002;22:6–13. doi: 10.1161/hq0102.102190. [DOI] [PubMed] [Google Scholar]
- Vianna 2007 .Vianna AC, Mocelin AJ, Matsuo T, Morais-Filho D, Largura A, Delfino VA, et al. Uremic hyperhomocysteinemia: a randomized trial of folate treatment for the prevention of cardiovascular events. Hemodialysis International. 2007;11:210–6. doi: 10.1111/j.1542-4758.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- Vivekananthan 2003 .Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361:2017–23. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- Wald 2002 .Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325:1202–6. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang 2005 .Wang H, Tan H, Yang F. Mechanisms in homocysteine-induced vascular disease. Drug Discovery Today: Disease Mechanisms. 2005;2:25–31. [Google Scholar]
- Wang 2007 .Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, et al. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. The Lancet. 2007;369:1876–82. doi: 10.1016/S0140-6736(07)60854-X. [DOI] [PubMed] [Google Scholar]
- WHO 2002 .World Health Organization . The world health report: reducing risks, promoting healthy life. World Health Organization; Geneva: [accessed 01 01 2007]. 2002. Available at http://www.who.int/whr/2002/en/whr02.en.pdf. [Google Scholar]
- Willet 2006 .Willett WC, Koplan JP, Nugent R, Dusenbury C, Puska P, Gaziano TA. Prevention of chronic disease by means of diet and lifestyle changes. In: Jameson DT, editor. Disease control priorities in developing countries. 2nd Edition The World Bank and Oxford University Press; New York, Oxford: 2006. [Google Scholar]
- Winkels 2008 .Winkels RM, Brouwer IA, Clarke R, Katan MB, Verhoef P. Bread cofortified with folic acid and vitamin B-12 improves the folate and vitamin B-12 status of healthy older people: a randomized controlled trial. American Journal of Clinical Nutrition. 2008;88:348–55. doi: 10.1093/ajcn/88.2.348. [MEDLINE: 18689370] [DOI] [PubMed] [Google Scholar]
- Wirakartakusumah 1998 .Wirakartakusumah MA, Hariyadi P. Food and Nutrition Bulletin. Vol. 19. United Nations University Press; Tokyo, Japan: 1998. Technical aspects of food fortification. [Google Scholar]
- Wood 2008 .Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336(7644):601–5. doi: 10.1136/bmj.39465.451748.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf 1985 .Yusuf S, Collins R, Peto R, Furberg C, Stampfer MJ, Goldhaber SZ, et al. Intravenous and intracoronary fibrinolytic therapy in acute myocardial infarction: overview of results on mortality, reinfarction and side-effects from 33 randomized controlled trials. European Heart Journal. 1985;6:556–85. doi: 10.1093/oxfordjournals.eurheartj.a061905. [DOI] [PubMed] [Google Scholar]
- Zhou 2009 .Zhou J, Austin RC. Contributions of hyperhomocysteinemia to atherosclerosis: Causal relationship and potential mechanisms. Biofactors. 2009;35:120–9. doi: 10.1002/biof.17. [MEDLINE: 19449439] [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study