Abstract
Rehabilitation after hemiplegic stroke has typically relied on the training of patients in compensatory strategies. The translation of neuroscientific research into care has led to new approaches and renewed promise for better outcomes. Improved motor control can progress with task-specific training incorporating increased use of proximal and distal movements during intensive practice of real-world activities. Functional gains are incorrectly said to plateau by 3–6 months. Many patients retain latent sensorimotor function that can be realised any time after stroke with a pulse of goal-directed therapy. The amount of practice probably best determines gains for a given level of residual movement ability. Clinicians should encourage patients to build greater strength, speed, endurance, and precision of multijoint movements on tasks that increase independence and enrich daily activity. Imaging tools may help clinicians determine the capacity of residual networks to respond to a therapeutic approach and help establish optimal dose-response curves for training. Promising adjunct approaches include practice with robotic devices or in a virtual environment, electrical stimulation to increase cortical excitability during training, and drugs to optimise molecular mechanisms for learning. Biological strategies for neural repair may augment rehabilitation in the next decade.
Rehabilitation of patients with hemiplegia after stroke has been limited for decades by a lack of theory-driven strategies leading to successful clinical trials with improvement in motor skills for daily activities. Recent therapeutic approaches have begun to build on methods to manipulate the remarkable adaptability or plasticity of the brain in response to task-specific practice, drugs, robotic trainers, and other ways to augment motor learning.1 In this review, I offer conceptual bases for a clinical science of neurorehabilitation and emphasise mechanisms and procedures to improve walking and arm movement to lessen disability and limitations in daily life. One goal is to diminish the pessimism of physicians regarding the effect of additional rehabilitation and to provide them with sensible advice to offer patients who seek to improve motor skills at any time after stroke.
The art of rehabilitation
Stroke is among the most common causes of adult-onset disability. 70–85% of first strokes are accompanied by hemiplegia.1 6 months after stroke, only 60% of people with hemiparesis who need inpatient rehabilitation have achieved functional independence in simple activities of daily living (ADL) such as toileting and walking short distances.2,3 Patients with sensorimotor and visual-field loss are much more dependent on carers than those with pure motor impairments, but even the latter may walk too slowly to participate in out-of-home activities or may be unable to integrate the use of an affected arm into personal care.
Rehabilitation for hemiplegic stroke includes organised multidisciplinary, supportive services that begin 48 h after onset in stable patients. Inpatient and outpatient rehabilitation works to the advantage of patients and families in a general sense, but the effectiveness of each component of care falls short of evidence-based practice standards. The training of patients to compensate with the unaffected arm or leg has been a mainstay of rehabilitation. Physical, occupational, and speech therapists primarily build skills and change the environment to maintain patients at home with as little carer support as possible. Problem-solving, supportive social and psychological services, removal of architectural barriers to mobility, braces and other orthotics, and devices such as wheelchairs and walkers continue to play an important part in helping patients adapt to disability.
Well-designed clinical trials that deal with critical conceptual and therapeutic issues have developed substantially only in the past 5–10 years.4 As of 2002, about 125 clinical trials of therapies for stroke rehabilitation had been designed as randomised trials with blinded procedures for the measurement of outcomes.1,5,6 Most trials examined the organisation, location, or intensity of general rehabilitation services, the prevention and management of medical complications,1,7,8 and support for community reintegration; for example, organised care in a stroke unit improves outcomes,9 and home services may help prevent deterioration.10 Small pilot trials have also examined specific drug, cognitive, or physical approaches to therapy, but their design prevents generalisation of results. Very few trials have looked into the optimum intensity and duration of a specific intervention. Without studies of dose-response interactions, interventions may be stopped before rehabilitations gains peak.
Neuroscientific bases for rehabilitation
Within the past 12 years, empirical bases for rehabilitative practices have developed in parallel to the growth of neuroscientific knowledge about basic mechanisms for motor control, cognition, learning, and memory. This neurobiology of rehabilitation (panel 1) may lead to improved methods for early and late therapeutic interventions to lessen physical and cognitive impairments and functional disabilities.11 A brief introduction to this rapidly growing subject will help clinicians appreciate some of the elements that may increase or limit gains.
Panel 1. Neurobiological rehabilitation strategies (approaches that manipulate brain processes and behaviours).
Restitution of activity in neurons and networks associated with internal biological events—eg, gene expression and resolution of detrimental conditions such as oedema, heme toxicity, calcium and sodium ionic fluxes, diminished neurotransmitter release, and transynaptic failure in regions remote but connected to damaged neurons (diaschisis)
Extrinsic activity-induced adaptations in partly spared networks within a distributed system for motor and cognitive function, elicited by practice that is progressive and motivating and drives synaptic activity for learning
Extrinsic pharmacological replacement of neurotransmitters from interrupted cortical projections by use of neuromodulators and molecules that improve synaptic efficacy and long-term potentiation or activate gene expression for growth of dendritic sprouts and spines
Endogenous and exogenous biological manipulations of gene expression, axon regeneration, axon guidance, dendritic sprouting, synaptogenesis, and cell replacement
The bases for the acquisition, retention, and retrieval of information in the healthy brain are no different from those in patients with stroke, who have a lesser complement of intact neural pathways. For example, long-term potentiation is one of the most likely molecular mechanisms by which synapses and groups of neurons encode new information to represent a movement skill (procedural memory) or retain information about facts and events (declarative memory). Long-term potentiantion develops from repeated associated inputs, called Hebbian synaptic learning, onto neurons in the motor cortex as they participate, for example, in the coding of a new sequence of finger movements for playing the piano. Those neurons, and others linked to them in related sensorimotor regions of the brain and spinal cord, come to represent the acquired skilled movements. The same neurons participate in other movements as well. Various neurotransmitters may excite or inhibit this learning. For example, lorazepam may impair the acquisition of a new motor skill by activating the GABA receptor, without interfering with the ability to do a previously learned task.12
The neurobiology of rehabilitation-induced neural adaptations has developed from experiments in animal models13–16 and from neurophysiological and neuroimaging studies in patients after focal brain lesions.17 Early gains after stroke occur with the resolution of oedema, ionic fluxes, inflammatory and oxidative processes, and impaired neurotransmitter actions. In addition, residual tissue and genes like those expressed during brain development serve as a potential substrate for reorganisation that may be modulated by rehabilitation therapies.18–21
Experience and training induce physiological and morphological plasticity after stroke (panel 2).1 Stronger synaptic connections arise between groups of neurons that represent more skilled movement.22 Cortical neurons discharge at different rates depending on the direction, acceleration, and force of movement into space for reaching or stepping.23 With new demands and training, these neurons can represent different movements. Sensory feedback, such as proprioception, has a crucial effect on motor ability at cortical and spinal levels as it reshapes sensorimotor integration. Central controllers for movements interact with the peripheral motor system and environment and use input from these interactions to plan and modify a movement.24,25
Panel 2. Steps for motor learning after stroke.
Progressively train components of goal-oriented, skilled movement tasks
Reinforce behaviour by a specified learning framework
Optimise kinematic, force, acceleration, directional, and temporal sensorimotor components of movement for feedback into the sensorimotor network
Repetitive practice under varying conditions for procedural or episodic learning
Evolution of neuronal representational maps for skilled movement by unmasking of latent synapses and recruitment of areas needed when task difficulty increases
Greater neuronal membrane excitability and synaptic efficacy of connections within related primary and secondary sensorimotor cortices and spinal motor pools
Morphological changes in dendritic branches and spines associated with long-term potentiation and long-term depression
Adaptation of the remaining cortical, subcortical, and spinal network for skilled movement that takes into account the mechanical properties of the limb and estimates motor commands from signals associated with intended limb motion, spatial targets, and goals
The capacity of spared networks and assemblies of neurons to contribute to partial restoration of skilled behaviours is critical to improving the use of the affected arm or leg. Functional neuroimaging studies show that the best gains after stroke generally coincide with greater engagement of spared primary regions that ordinarily contribute to the control of a behaviour.26 Reorganisation also occurs within bilaterally distributed networks that are normally only needed for complex activities. Homologous sensorimotor structures of the uninjured hemisphere, for example, commonly develop a larger role. The brain's reward systems within the basal ganglia,27 tweaked by motivation and feedback, and its working memory regions in the frontal lobe, become important contributors to the use of informational cues provided by therapists to improve motor skills. Gains may also arise from within subcomponents of hierarchical systems, such as the cortical and spinal organisations for certain semiautomatic movements within the usual workspace of each limb—such as stepping, reaching for a nearby object, or bringing the hand to the mouth for feeding.28–30
Clinicians, therefore, can take advantage of the injured brain's residual distributed system. This resource for neurorestoration primarily deploys changes in synaptic plasticity in response to motor learning. Well-defined training methods are essential for optimum manipulation of rehabilitation-induced neural adaptations that produce behavioural gains.
Arm training
Forms of practice characterise all rehabilitation efforts to regain motor control. To some degree, most approaches encourage the practice of feasible submovements and larger sequences of action by varied means to target better multijoint movement patterns. Constraint-induced movement therapy (CIMT) progressively shapes more functionally useful movements with an operant approach, coupled to simultaneous restraint of the unaffected arm.31 A standard set of tasks for reaching, grasping, and pinching are used. The conceptual basis for CIMT is the idea that learned nonuse of the affected hand is common after the completion of formal rehabilitation because of pain, slow and high-effort attempts to use the arm, and ease of use of the unaffected arm. Forced use of the arm throughout the day and formal training for 6 h a day for 2 weeks during CIMT are recommended, but less intensity may work as well. Many patients make up to half their improvement in the first several days of practice because they have substantial latent motor control. Treatment with CIMT so far only seems beneficial if patients have a minimum of 10° of voluntary wrist and finger extension; perhaps fewer than 10% of outpatients can benefit from this massed-practice approach.
Forced use tends to negate other approaches for training, such as bimanual arm practice.32 Also, published information on motor learning suggests that blocks of massed practice may result in less need for memory retrieval over the course of training, which could lead to less retention.33 Spaced practice with some interval between sessions may lead to better retention of a skill. A well-designed, multicentre trial of CIMT in patients with moderate and more severe wrist and finger impairment 3–9 months after stroke will be reported this year.34
Some concern about massed practice in the first few days after stroke has been raised after the experimental finding in rats that more neurons may be damaged or the size of the infarct increased by early overuse of a paretic limb.35,36 However, the level of exercise of a rat running on a rotating wheel is much greater than that a patient could possibly experience. In general, exercise by rodents starting several days after an induced stroke and in healthy rodents has positive effects on mechanisms of plasticity, such as production of brain-derived neurotrophic factor.37 However, these results in caged inbred rats do not prove that this exercise phenomenon occurs in human beings.
Task-oriented functional training customises therapy for repetitive practice of tasks by use of whatever proximal and distal function is present. This approach uses motor learning principles of spaced practice and intermittent feedback to facilitate real-world activities. Tasks that are relevant to a patient's daily life, and hence are motivating, are done in random order to optimise learning.38 A pilot trial that compared conventional therapy for the arm with functional task practice and strengthening for an additional 20 h during 4–6 weeks of inpatient rehabilitation found both short and long-term gains in motor control for those who received the more focused interventions.39
Approaches for walking
One of the more common complaints from patients who are living at home 6 months after stroke is that they cannot yet walk safely and efficiently in the community.40 Walking-related disability is common. The likelihood of recovering the ability to walk 150 foot (45 m) without physical assistance depends, in part, on the type of stroke-related impairment (table).2 The Copenhagen study41 showed that 80% of survivors of acute stroke who were initially unable to walk reached their best function within 6 weeks and 95% within 11 weeks. Independent walking for 150 feet was achieved by 34% of survivors who were dependent on admission and 60% of those who initially required assistance. Thus, improvement on the basis of this common functional measure of walking, which is included in the Barthel index and Functional Independence Measure, is maximum by 12 weeks after stroke. By 12 weeks physical therapy has typically been stopped and 40% of patients who return home are too disabled to walk.40 Many people with ambulatory hemiparesis do not walk safely. They have four times the risk of falls and ten times the risk of hip fractures of healthy people. Just as importantly, these patients tend to walk at less than a half normal casual walking speed and for only modest distances.
Table. Recovery (%) of independent walking for 150 feet by impairment group2.
| Initial impairment | Able to walk at onset | 1 month | 3 months | 6 months |
|---|---|---|---|---|
| Motor | 18 | 50 | 75 | 85 |
| Sensorimotor | 10 | 48 | 72 | 72 |
| Motor, hemianopia | 7 | 28 | 68 | 75 |
| Sensorimotor, hemianopia | 3 | 16 | 33 | 38 |
A study of 147 people 6 months after stroke revealed that those with hemiparesis who walk at 25 cm/s (SD 10) achieve only household ambulation. Restricted community ambulation generally required walking speeds of 40–79 cm/s. Unrestricted community ambulation was most likely for those who walked at 80 cm/s (SD 15).40,42 Only 18% of people achieved unlimited community ambulation. Across prospective observational studies and interventional experiments, walking speeds at the end of inpatient rehabilitation range from 25–60 cm/s. Normal casual walking speed for healthy adults is 2·5–3·0 miles per hour (about 130 cm/s).43 This functional limitation highlights the need for better solutions.
Task-oriented practice of walking
In research studies over the past 10 years, exercise and rehabilitation strategies to improve mobility have become more task specific, intensive, and progressive in their demands.1,44 When well-defined interventions to improve walking have been tested in selected groups of patients in randomised clinical trials, gains are common for people who received progressive interventions within 3 months of a stroke.44,45 Conventional physiotherapy—with or without treadmill activities and of modest intensity—have also led to improved walking skills, including level of independence and walking speed, when started 6–18 months after stroke.46–53 Although patients who make large gains tend to be among those with more modest impairments, these studies confirm the potential to improve function by trying a pulse of goal-directed physical therapy at any time after stroke.
One approach for massed practice is treadmill training with partial support of body weight. This strategy is developed from studies of cats with transected spinal cords,54 but this theoretical basis seems less translatable to humans than most reports suggest. Many postural and automatic features of the neural control for quadrupedal walking are managed at the level of the lumbar motor pools, which probably includes the conservation of a subcomponent of the locomotor system called central pattern generators. This system facilitates automatic reciprocal flexor and extensor stepping movements of the hindlimbs.55,56 Remarkably, cats have been trained to walk on a moving treadmill belt with trunk support after a low thoracic cord transection with their dorsal and ventral roots intact, but they cannot walk over ground.57 Human beings, however, probably depend less than other mammals on pattern generation. Bipedal walking required a number of evolutionary musculoskeletal adaptations and accompanying neural computations to manage human locomotion.58 For example, human beings must land on the heel and roll forward to the ball of the foot to push off for greater energy efficiency; quadrupeds do not. Supraspinal motor regions are quite active in human beings during over ground or treadmill walking as revealed by functional imaging techniques.59,60
Despite its theoretical basis, body-weight-supported treadmill training (BWSTT) has had a disappointing effect on walking outcomes when compared with conventional training of the same duration, at least in randomised clinical trials begun within 2 months of onset of hemiplegia. In these trials during inpatient rehabilitation, patients were trained at slow treadmill speeds (from 0·2–1·0 mph) and body-weight support was used only until patients could step at slow speeds without assistance, rather than using support to enable practice at faster speeds.45,61–64 Training at fast treadmill speeds leads to fast walking over ground.51,65 Patients with hemiparesis can safely exercise at a level of effort that provides a conditioning response.47 The energy cost of walking may decrease by 50% as walking speeds increase from 40 cm/s to 150 cm/s.66
BWSTT may have a greater effect in patients with more chronic hemiparesis who walk with a poor gait pattern and slowly (less than 80 cm/s) beyond the time usually allotted for inpatient and outpatient rehabilitation.51,61,67 Important features of training include practice at treadmill speeds consistently faster than the subject can walk over ground and physical and verbal cues from therapists who help to optimise the symmetry of stepping, joint angles, and timing of loading and unloading each leg by manipulating the legs and pelvis. No randomised clinical trial has been done. A recent Cochrane review of 11 trials with a total of 458 participants concluded that a well-designed large-scale study to assess BWSTT after stroke is urgently needed.68
An approach such as BWSTT is not a stand-alone therapy. It must be complemented by training on various surfaces that further help patients recapture motor control for a better gait pattern, as well as by working on strengthening, conditioning, and balance. Physicians can encourage patients to work on these features of walking. One simple technique to rapidly improve the symmetry and speed of walking is to have the patient more consciously try to push the leg that is in mid-stance into the floor and, at that moment, pull the other leg from the hip to initiate its swing forward. This load and swing cue improves attention to the timing of loading the stance leg (particularly the unaffected one) and swinging the other (especially the affected leg). The strategy is supported by data from studies of sensory triggers for rhythmic stepping in human beings and other mammals.69,70
Spasticity
Clinicians may spend needless effort in trying to treat increases in muscle tone, despite the fact that spasticity is not causing any fuctional disability. Clinically significant spasticity is rather uncommon after stroke.71 The loss of independent movements, such as hip flexion and knee extension for walking or elbow extension for reaching with simultaneous wrist extension (movement out of synergy), is commonly thought to be treatable by interventions that decrease muscle tone. Impairment in making a reaching movement, such as raising the hemiparetic arm to reach while extending the elbow, seems to reflect a problem in motor control for isolation of individualised movements, not due to spasticity.72 Muscle tone is poorly related with functional disability. Indeed, bad motor control—as assessed by paresis, impaired dexterity, and fatigability, along with tissue changes in muscle73—is usually more limiting than increased tone.1
Hypertonicity that leads to dystonic flexor postures of the hand and inversion of the foot is perhaps most commonly caused by pain, overuse, and impaired postural control that drives flexor or extensor activity. Immediate medical and rehabilitation management will prevent dystonic postures. A brief course of antispasticity drugs may also help, but continued drug use should be reassessed at least quarterly. Selective serotonin reuptake inhibitors and dopamine blockers can sometimes increase hypertonicity, so they should be stopped. Antispasticity drugs and locally injected botulinum toxin rarely improve function of the arms. Studies that report efficacy for the hand and arm rely on scales that do not address functional limitations.74 Also, trials do not combine the intervention with range of motion, weight bearing, and other rehabilitation interventions that may eliminate the need to denervate a muscle every 3–4 months. Botulinum toxin injected into the tibialis posterior and toe plantar flexors may improve the gait of patients with plantar flexion and inversion that limits heel strike and stance, but must be followed by stretching and gait training.
How much training?
Many small trials reveal motor and behavioural improvements in patients with chronic disability after a pulse of therapy aimed at a specific impairment or functional goal at any time after a stroke (panel 3).51,61,65,75–82 An ethical challenge for the providers of therapies is to find common agreement with patients about realistic functional and quality-of-life goals. Clinicians should test adjunct strategies on patients who have at least modest motor control before deciding that no further gains can be made.
Panel 3. Queries before starting a trial of therapy in chronic stroke.
Has the patient clearly reached a plateau in goal-directed therapy for a given level of motor and cognitive function?
What is a realistic outcome for an additional intervention?
What new strategies can be drawn from the rehabilitation and scientific literature?
What form of assessment will serve as a practical, but sensitive measure of progress toward the functional goal?
What will be the balance between therapist-directed treatment and home-based practice?
How will the necessary duration and intensity of treatment be determined?
What criteria will be used by the patient and therapist to decide that the new strategy offers no further benefit?
The intensity and duration of therapy varies widely in studies. The decision about frequency and duration of therapy is, in many cases, made entirely on the basis of what is practical for patients and investigators in a research study or on the number of treatment sessions covered by payments from a third party. Some general guidelines can be drawn. Most studies that led to a very significant 10 point gain on the Fugl–Meyer motor assessment of selective movements or greater strength provided specific treatment by a therapist for 12–60 h, running from 1–6 h a day, 3–5 days a week for 2–12 weeks. Most likely, the amount of goal-directed therapy, rather than the daily intensity, is the most important factor.83 Many outpatient clinical trials offer the experimental intervention for 1 h/day, 3 days a week for 2–3 months.44 We have found that task-specific functional therapy for the arm leads to significant gains in motor control and strength if patients are allowed to continue treatment until no further gains are made for up to ten additional sessions.84 Within the context of research studies, our laboratory routinely improves the walking speed of patients with chronic hemiparesis by 30–50% with 3 h a week for 4–6 weeks of BWSTT or other intensive practice, plus home practice. Continued training in 2–4 week increments improves the gait pattern in many patients until a plateau is reached at 24–36 sessions (figure).85 More studies with open-ended length until a planned endpoint are needed.
Figure.
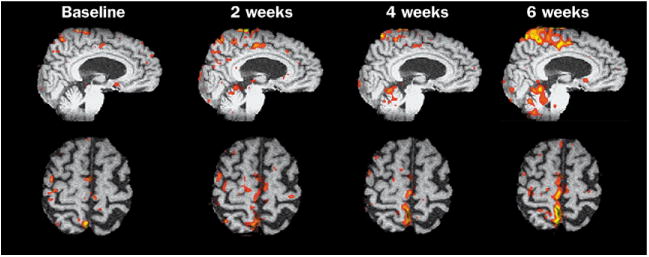
Functional MRI series with the blood-oxygen-level dependent signal and analysis of regions of interest during voluntary ankle dorsiflexion. The patient had chronic hemiparesis after a subcortical stroke 14 months earlier and still walked at less than 65 cm/s. The behavioural changes from baseline to the end of the first 12 training sessions were significant, so further therapy would probably not have been offered. The increase in fMRI activity, however, suggested ongoing recruitment within primary sensorimotor cortex (S1M1). Therapy was extended another six sessions in 2 weeks to see if gains in walking and recruitment had reached a plateau. There was a 20% increase in walking speed and improved motor control. The fMRI study at 6 weeks revealed an expansion of the foot representation medially into the representation for the back and hip muscles. Also, greater cerebellar and cingulate motor cortex activity developed. Two additional bouts of therapy led to greater motor control of the ankle during walking and focusing, rather than expanding fMRI activity within M1, consistent with greater synaptic efficacy (not shown).
Patients are more likely to improve if they practice tasks at home in convenient blocks of 20 min a few times a day. It is most motivating for them to select tasks they wish to add to their daily activities, such as turning a key in a lock, reaching for cups and silverware until they empty the shelf, pulling shirts on hangers from a rack, and typing with whichever fingers function at increasing speed and accuracy. They can also repeat standing up from a chair and sitting down three to five times each time they do have to get up to build thigh and gluteal strength. Indeed, proximal weakness of the affected and unaffected hip and knee muscles is common because patients are less active due to the hemiparesis, use a wheelchair for outdoor mobility, or have side-effects of drugs—such as a statin-induced myopathy. Strengthening exercises are possible. Clinical trials reveal efficacy without causing complications, such as pain or spasticity.86–88 Weights, theraband elastic strips, and treadmill walking or stationary bicycling allow training at home. The success of strengthening with resistance exercises with weights for two sets of 12 repetitions against about 50% of the maximum single repetition load requires three sessions a week for 8–12 weeks. Isometric exercise against moderate resistance is a safe approach for patients who are frail and cognitively impaired. Patients may improve their walking speed by timing themselves walking 50 m several times a week, pushing to gradually accomplish this in 45 s while a helper assists for safety if necessary. Physicians and family can also motivate patients to gradually build their endurance for walking the distances necessary for community activities, for example, by monitoring the distance walked in 10 min.
Functional neuroimaging in rehabilitation
The strength of the relations between therapeutic practice by patients with brain lesions, mechanisms of activity-dependent plasticity, and changes in the brain maps of neuronal activity acquired by techniques of functional imaging are not fully understood. More work has gone into proving plasticity after stroke without a specific rehabilitation intervention than into trying to extract the elements of training that induce plasticity in relation to behavioural gains. In addition, very few studies have related important functional gains to specific patterns of functional MRI activity.26
Functional imaging, however, may offer clinicians insight into how to better engage a network in the hope of improving rehabilitation training techniques. For example, the magnitude of the blood-oxygen-level-dependent signal in functional MRI studies is greater within M1 and the supplementary motor cortex when the eyes gaze in the direction of the hand doing a sequential motor task than when looking at the inactive hand. Activity within the dorsal premotor cortex for the affected hand after stroke reveals its contribution as a substrate of functional gains for use of the hand, bolstered by connections to visuomotor regions.89 Thus, focused visual input may be an important feature of training. Transcranial magnetic stimulation (TMS) and functional MRI studies over the time of a treatment strategy could take snapshots of a network, to ensure it was engaged by the intervention. Imaging could also be done during a verbal or visuospatial working memory task to help estimate a patient's residual neural resources for learning and potential to employ mental imagery and observation of arm actions as forms of practice.90,91
PET, functional MRI, and TMS92 has detected functional reorganisation as a patient's sensorimotor and cognitive experience was manipulated, primarily in studies of arm recovery.26,93 For walking, near-infrared spectroscopy94 during stepping on a treadmill and functional MRI during ankle dorsiflexion (figure)85,95,96 serve as markers of training-induced plasticity. Improved motor control of the leg and faster walking speed were accompanied by recruitment of sensorimotor system neurons and representational changes. Longitudinal functional MRI mapping studies may offer insight into the optimal intensity or duration of a task-specific therapy.85 Mapping of brain-activity patterns may also help to predict the capacity of a movement representation to reorganise in response to a particular therapy soon after the start of the intervention. For example, TMS of the hand representation in M1 of the affected hemisphere reveals less excitability after stroke than stimulation of the unaffected side. Increased excitability of affected M1 with greater symmetry between the two sides in the first several sessions of task-oriented training of the hemiparetic arm was associated with long-term gains in the Fugl–Meyer score for motor control.84
Neuroaugmentation
Even with optimised approaches and intensities of physical, occupational, language, and cognitive rehabilitation, other manipulations may be needed to increase gains. These interventions require further study but build on growing research rationales.
Neuropharmacological adjuncts
Cascades of molecular interactions underlie activity-dependent plasticity and skills learning. Many of these processes involve the major neurotransmitters.97 After stroke, dopamine, acetylcholine, serotonin, and norepinephrine may be interrupted or downregulated in their projections to cortex from sites of origin in the brainstem. This may contribute to diaschisis, the failure of transynaptic neurotransmission. Clinicians may soon identify individuals with genotypes that also reduce central aminergic or cholinergic tone. These “low-aminergic” patients may benefit, for example, from amphetamine after a brain injury.98 Pertinent to clinical practice is the possibility that commonly used drugs may change neurotransmitter concentrations or block their receptors, leading to iatrogenic loss of tone. Stroke trials suggest at least a transient decline in function from antiepileptic drugs (which affect cognitive processing), dopamine blockers such as antipsychotics, and α2 adrenergic drugs.99
Augmentation of one of these chemical transmitters, especially activation of NMDA receptors,100 may drive long-term potentiation and optimise activity-dependent relearning of skills after stroke. Available acetylcholinesterase inhibitors may aid both declarative and procedural learning.16,101 Among their functions, acetylcholine projections from the nucleus basalis transmit behaviourally relevant sensory information. Dopaminergic drugs such as levodopa,102 as well as drugs that increase the availability of norepinephrine, such as methylphenidate103 and amphetamine,104,105 have shown some efficacy in stroke-rehabilitation trials when combined with physical or language therapy. Dopaminergic projections from the ventral tegmental tract relate a reward to the cognitive effort of the task, which reinforces associative learning. Norepinephrine projections from the locus coeruleus also modulate the saliency of sensory inputs, attention, and features of memory.106 Drugs that act on these neurotransmitter receptors improve task-specific signalling.107 Selective serotonin reuptake inhibitors have modestly improved motor learning in healthy people and patients with stroke for arm tasks, as the drugs increased regional brain activity.108,109 Studies with TMS suggest that selective serotonin reuptake inhibitors increase the excitability of corticospinal neurons.110
Learning and synaptic plasticity are also modulated by other molecules, such as cAMP response element-binding protein (CREB), which can be activated by drugs.111 Pharmaceutical research is very active in the development of “memory” drugs for Alzheimer's disease that could also enable task-specific learning. The propensity for responsiveness to a drug may be predicted by fMRI and PET activation studies112 and by TMS.113,114 These tools, then, may help clinicians choose a drug for an individualised approach to augment rehabilitation.115
Electrical neurostimulation
Motor gains and limited functional gains have been elicited by electrical neuromuscular stimulation triggered by feedback. One approach stimulates a weak muscle, such as the wrist extensors, on the basis of the amplitude of the electromyographic signal during attempted voluntary movement of a joint.32,116 Other biofeedback techniques may help patients activate a muscle or joint movement, although there are few data. Direct neuromuscular electrical stimulation over the surface of key muscles for a grasp and pinch movement may improve motor skills when combined with task-oriented practice.117 Functional electrical stimulation is also used to activate paretic muscles timed to a movement, such as contraction of the tibialis anterior to clear the foot during the swing phase of walking. Injectable BION electrodes triggered by an external transmitter have shown potential as a muscle-stimulating neuroprosthesis.118
Cortical plasticity has been modulated for up to several hours in animals and human beings by peripheral-nerve and direct cortical stimulation, independently and in combination.119 These methods include cortical motor stimulation by repetitive TMS,120 direct electrode array stimulation over the dura of M1,121 repetitive peripheral nerve stimulation,122 and paired associative stimulation with a TMS pulse immediately after a peripheral nerve stimulus.123,124 Mechanisms of action include an increase in NMDA excitation, a decrease in GABA inhibition, an increase in neuronal excitability, and Hebbian associative synaptic learning. In a similar vein, local anaesthesia of a portion of the affected hand rapidly changed representational plasticity and improved function.125 The inducing of anaesthesia in the unaffected hand seemed to reverse inhibition of the unaffected hemisphere on the affected side,126 leading to transient gains in hand strength.127 Practice combined with carefully chosen stimulation may come into clinical use to augment short-term learning and function.
Robotic trainers and virtual-reality practice
These adjuncts may maximise the intensity and convenience of task-oriented practice. Computerised virtual reality-based activities have a reasonable conceptual basis for rehabilitation.128–130 A virtual-reality system may include a computer or television screen that shows the virtual environment, a device on the patient's hand that records movement direction and acceleration as he or she reaches into cyberspace, and motivational games that encourage reaching, grasping, and manipulation. People can watch and attempt to mimic a movement that may activate a class of mirror neurons to drive visuomotor neuroplasticity.131 The investigator can give quantitative feedback about the accuracy and speed of movement. A few small trials suggest possible effectiveness for these forms of practice.132
For reaching or walking, a range of clever automated assistive devices are in development.133 They may offer more intensive practice opportunities without increasing time spent on supervision by the treating therapist. Robotic devices can also measure motor skills, especially during movements of the whole limb. For the arm, devices have been limited to shoulder and elbow movements. Results from small trials point more to improved strength79 than to improved function.134,135 For walking, a stepper device produced equal results to conventional gait training in terms of walking speed.136 Future trials of the devices should seek better definition of the optimal intensity and duration of treatment.
Neural Repair
Animal models of stroke and spinal-cord injury reveal the potential for clinicians to modulate similar acute and chronic responses for repair.137 Biological interventions may promote regeneration of cells, axons, and neural circuitry. Endogenous neurons and glia from the subventricular zone near an infarct may proliferate and migrate toward the stroke in response to local signals. Exogenous stem cells, neural progenitors, bone-marrow stromal cells,138 and other types139 offer hope for restoration of cells and their proteins for repair.140 These cells may bridge an injury site to enable greater connectivity. Stem cells and engineered fibroblasts are readily engineered to make brain-derived neurotrophic factor and acetylcholine, for example, which could allow placement of the growth factor or neurotransmitter where needed. Pharmacological and immunological approaches may target growth-cone receptors to provide signals for regeneration or to block inhibitory factors in the environment.141 However, incorporation of new neurons and axons that augment functional recovery will also depend on rehabilitation therapies, especially those that drive activity-dependent plasticity. Without such drives, these new elements and cortical and subcortical projections may not become incorporated into learning networks.36,142,143
Conclusions
Rehabilitation after stroke must continue to address serious functional limitations, such as walking speed and distance that permit community activities and better use of a hemiparetic arm and hand. A growing understanding of the molecules and physiology of neuroplasticity during motor-skills learning has made an exciting contribution to new strategies for stroke rehabilitation. Clinicians and scientists can now design and test therapies that manipulate cerebral adaptations to lessen impairments, disabilities, and functional limitations. Functional MRI, TMS, and other physiological windows on brain function may offer guidance about whether relevant networks are engaged and manipulated over the time that a training, pharmacological, or biological intervention proceeds. Insights may be gained about the optimal intensity and duration of therapies and about how different therapies interact. This information may one day help individualise rehabilitation approaches and lessen the need for large clinical trials.
The recent emphasis of neurological rehabilitation on basic and clinical scientific research, theory-based interventions, better clinical-trial designs and outcome measures, systematic testing of interventions, and task-oriented therapies at any time after stroke will better realise the potential of millions of disabled people.
Search strategy and selection criteria.
Articles were identified by searches of MEDLINE with the words “rehabilitation”, “stroke”, “motor control”, and “recovery of function”, from 1990–2004, from the author's files and textbooks. Only references published in English were used.
Acknowledgments
Role of the funding source: Research in my laboratory contributing to this review is supported by the National Institutes of Health at the National Institute of Child Health and Human Development (HD39629) and the National Institute of Neurological Disorders and Stroke (HD0741), the Larry L Hillblom Foundation, and the Nathan Shapell Foundation. None of these funding sources had a role in the preparation of this review or in the decision to submit it for publication.
Footnotes
Conflict of interest: I have no conflicts of interest.
References
- 1.Dobkin B. The clinical science of neurologic rehabilitation. New York: Oxford University Press; 2003. [Google Scholar]
- 2.Patel A, Duncan P, Lai S, Studenski S. The relation between impairments and functional outcomes poststroke. Arch Phys Med Rehabil. 2000;81:1357–63. doi: 10.1053/apmr.2000.9397. [DOI] [PubMed] [Google Scholar]
- 3.Jorgensen H, Nakayama H, Raaschou H, et al. Outcome and time course of recovery in stroke, part I: time course. Arch Phys Med Rehabil. 1995;76:406–12. doi: 10.1016/s0003-9993(95)80568-0. [DOI] [PubMed] [Google Scholar]
- 4.Dobkin B. Focused stroke rehabilitation programs do not improve outcome. Arch Neurol. 1989;46:701–03. doi: 10.1001/archneur.1989.00520420123035. [DOI] [PubMed] [Google Scholar]
- 5.Gresham C, Duncan P, Stason W, et al. Post-stroke rehabilitation: assessment, referral, and patient management: clinical practice guideline No 16, report no 95-0663. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; 1995. [PubMed] [Google Scholar]
- 6.Teasell R, Foley N, Bhogal S, S M. An evidence-based review of stroke rehabilitation. Top Stroke Rehabil. 2003;10:29–58. doi: 10.1310/8YNA-1YHK-YMHB-XTE1. [DOI] [PubMed] [Google Scholar]
- 7.Roth E, Lovell L, Harvey R, Heinemann A, Semik P, Diaz S. Incidence of and risk factors for medical complications during stroke rehabilitation. Stroke. 2001;32:523–9. doi: 10.1161/01.str.32.2.523. [DOI] [PubMed] [Google Scholar]
- 8.Stineman M, Ross R, Maislin G, Fiedler R, Granger C. Risks of acute hospital transfer and mortality during stroke rehabilitation. Arch Phys Med Rehabil. 2003;84:712–18. doi: 10.1016/s0003-9993(02)04850-5. [DOI] [PubMed] [Google Scholar]
- 9.Stroke Unit Trialists' Collaboration. The Cochrane Library. 2. Chichester, UK: John Wiley & Sons, Ltd; 2004. Organised inpatient (stroke unit) care for stroke (Cochrane Review) [Google Scholar]
- 10.Legg L. Outpatient Service Trialists: Rehabilitation therapy services for stroke patients living at home: systematic review of randomised trials. Lancet. 2004;363:352–56. doi: 10.1016/S0140-6736(04)15434-2. [DOI] [PubMed] [Google Scholar]
- 11.Dobkin B. In: The neurobiology of rehabilitation. Kaler SNN, editor. Understanding and optimizing human development from cells to patients to populations New York; New York Acad Sci: in press. [Google Scholar]
- 12.Donchin O, Sawaki L, Madupu G, Cohen LG, Shadmehr R. Mechanisms influencing acquisition and recall of motor memories. J Neurophysiol. 2002;88:2114–23. doi: 10.1152/jn.2002.88.4.2114. [DOI] [PubMed] [Google Scholar]
- 13.Frost S, Barbay S, Friel K, Plautz E, Nudo R. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2003;89:3205–14. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- 14.Bury S, Jones T. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the “unaffected” forelimb and training-induced dendritic structural plasticity in the motor cortex. J Neurosci. 2002;22:8597–606. doi: 10.1523/JNEUROSCI.22-19-08597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmichael S. Plasticity of cortical projections after stroke. Neuroscientist. 2003;9:64–73. doi: 10.1177/1073858402239592. [DOI] [PubMed] [Google Scholar]
- 16.Conner J, Culberson A, Packowski C, Chiba A, Tuszynski M. Lesions of the basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron. 2003;38:819–29. doi: 10.1016/s0896-6273(03)00288-5. [DOI] [PubMed] [Google Scholar]
- 17.Rossini P, Calautti C, Pauri F, Baron JC. Post-stroke plastic reorganization in the adult brain. Lancet Neurol. 2003;2:493–502. doi: 10.1016/s1474-4422(03)00485-x. [DOI] [PubMed] [Google Scholar]
- 18.Dobkin B. Activity-dependent learning contributes to motor recovery. Ann Neurol. 1998;44:158–60. doi: 10.1002/ana.410440204. [DOI] [PubMed] [Google Scholar]
- 19.Wei L, Erinjeri J, Rovainen C, Woolsey T. Collateral growth and angiogenesis around cortical stroke. Stroke. 2001;32:2179–84. doi: 10.1161/hs0901.094282. [DOI] [PubMed] [Google Scholar]
- 20.Katsman D, Zheng J, Spinelli K, Carmichael S. Tissue microenvironments within functional cortical subdivisions adjacent to focal stroke. J Cereb Blood Flow Metab. 2003;23:997–1009. doi: 10.1097/01.WCB.0000084252.20114.BE. [DOI] [PubMed] [Google Scholar]
- 21.Croquelois A, Wintermark M, Reichhart M, Meuli R, Bogousslavsky J. Aphasia in hyperacute stroke: language follows brain penumbra dynamics. Ann Neurol. 2003;54:321–29. doi: 10.1002/ana.10657. [DOI] [PubMed] [Google Scholar]
- 22.Nudo R, Wise B, SiFuentes F, Milliken G. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–94. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 23.Sergio L, Kalaska J. Systematic changes in motor cortex cell activity with arm posture during directional isometric force generation. J Neurophysiol. 2003;89:212–28. doi: 10.1152/jn.00016.2002. [DOI] [PubMed] [Google Scholar]
- 24.Todorov E, Jordan M. Optimal feedback control as a theory of motor coordination. Nat Neurosci. 2002;5:1226–35. doi: 10.1038/nn963. [DOI] [PubMed] [Google Scholar]
- 25.Scott S, Norman K. Computational approaches to motor control and their potential for interpreting motor dysfunction. Curr Opin Neurol. 2003;16:693–98. doi: 10.1097/01.wco.0000102631.16692.71. [DOI] [PubMed] [Google Scholar]
- 26.Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke. 2003;34:1553–66. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- 27.Graybiel A, Aosaki T, Flaherty A, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–31. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- 28.Dietz V. Spinal cord pattern generators for locomotion. Clin Neurophysiol. 2003;114:1379–89. doi: 10.1016/s1388-2457(03)00120-2. [DOI] [PubMed] [Google Scholar]
- 29.Bizzi E, Tresch M, Saltiel P, d'Avella A. New perspectives on spinal motor systems. Nat Rev Neurosci. 2000;1:101–08. doi: 10.1038/35039000. [DOI] [PubMed] [Google Scholar]
- 30.Graziano M, Taylor C, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34:841–51. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- 31.Taub E, Uswatte G, Elbert T. New treatments in neurorehabilitation founded on basic research. Nat Rev Neurosci. 2002;3:228–36. doi: 10.1038/nrn754. [DOI] [PubMed] [Google Scholar]
- 32.Cauraugh JH, Kim SB. Stroke motor recovery: active neuromuscular stimulation and repetitive practice schedules. J Neurol Neurosurg Psychiatry. 2003;74:1562–66. doi: 10.1136/jnnp.74.11.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt R, Wrisbert C. Motor learning and performance: a problem-based learning approach. Champaign, IL: Human Kinetics; 2000. [Google Scholar]
- 34.Winstein C, Miller J, Blanton S, et al. Methods for a multi-site randomized trial to investigate the effect of constraint-induced movement therapy in improving upper extremity function among adults recovering from a cerebrovascular stroke. Neurorehabil Neural Repair. 2003;17:137–52. doi: 10.1177/0888439003255511. [DOI] [PubMed] [Google Scholar]
- 35.Bland S, Pillai R, Aronowski J, Grotta J, Schallert T. Early overuse and disuse of the affected forelimb after moderately severe intraluminal suture occlusion of the middle cerebral artery in rats. Behav Brain Res. 2001;126:33–41. doi: 10.1016/s0166-4328(01)00243-1. [DOI] [PubMed] [Google Scholar]
- 36.Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabiliitative experience declines with time after focal ischemic brain injury. Neurobiol Dis. 2004;24:1245–54. doi: 10.1523/JNEUROSCI.3834-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Praag H, Kempermann G, Gage F. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–98. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 38.Hanlon R. Motor learning following unilateral stroke. Arch Phys Med Rehabil. 1996;77:811–15. doi: 10.1016/s0003-9993(96)90262-2. [DOI] [PubMed] [Google Scholar]
- 39.Winstein C, Rose D, Tan S, Lewthwaite R, Chui H, Azen S. A randomized controlled comparison of upper-extremity rehabilitation strategies in acute stroke: a pilot study of immediate and long-term outcomes. Arch Phys Med Rehabil. 2004;85:620–28. doi: 10.1016/j.apmr.2003.06.027. [DOI] [PubMed] [Google Scholar]
- 40.Lord S, McPherson K, McNaughton H, Rochester L, Weatherall M. Community ambulation after stroke: how important and obtainable is it and what measures appear predictive? Arch Phys Med Rehabil. 2004;85:234–39. doi: 10.1016/j.apmr.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Jorgensen H, Nakayama H, Raaschou H, Olsen T. Recovery of walking function in stroke patients: The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:27–32. doi: 10.1016/s0003-9993(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 42.Perry J, Garrett M, Gromley J, Mulroy S. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–89. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 43.Bohannon R. Comfortable and maximum walking speed of adults aged 20 to 79 years: reference values and determinants. Age Ageing. 1997;26:15–19. doi: 10.1093/ageing/26.1.15. [DOI] [PubMed] [Google Scholar]
- 44.Duncan P, Studenski S, Richards L, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34:2173–80. doi: 10.1161/01.STR.0000083699.95351.F2. [DOI] [PubMed] [Google Scholar]
- 45.Visintin M, Barbeau H, Korner-Bitensky N, Mayo N. A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke. 1998;29:1122–28. doi: 10.1161/01.str.29.6.1122. [DOI] [PubMed] [Google Scholar]
- 46.Green J, Forster A, Bogle S, Young J. Physiotherapy for patients with mobility problems more than 1 year after a stroke: a randomised controlled trial. Lancet. 2002;359:199–203. doi: 10.1016/S0140-6736(02)07443-3. [DOI] [PubMed] [Google Scholar]
- 47.Macko R, Smith G, Dobrovolny C, Sorkin J, Goldberg A, Silver K. Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehabil. 2001;82:879–84. doi: 10.1053/apmr.2001.23853. [DOI] [PubMed] [Google Scholar]
- 48.Dean C, Richards C, Malouin F. Task-related circuit training improves performance of locomotor tasks in chronic stroke: a randomized, controlled pilot trial. Arch Phys Med Rehabil. 2000;81:409–17. doi: 10.1053/mr.2000.3839. [DOI] [PubMed] [Google Scholar]
- 49.Dam M, Tonin P, Casson S, et al. The effects of long-term rehabilitation therapy on poststroke hemiplegic patients. Stroke. 1993;24:1886–91. doi: 10.1161/01.str.24.8.1186. [DOI] [PubMed] [Google Scholar]
- 50.Werner C, Bardeleben A, Mauritz KH, Kirker S, Hesse S. Treadmill training with partial body weight support and physiotherapy in stroke patients: a preliminary comparison. Eur J Neurol. 2002;9:639–44. doi: 10.1046/j.1468-1331.2002.00492.x. [DOI] [PubMed] [Google Scholar]
- 51.Sullivan K, Knowlton B, Dobkin B. Step training with body weight support: Effect of treadmill speed and practice paradigms on post-stroke locomotor recovery. Arch Phys Med Rehabil. 2002;83:683–91. doi: 10.1053/apmr.2002.32488. [DOI] [PubMed] [Google Scholar]
- 52.Ada L, Dean CM, Hall JM, Bampton J, Crompton S. A treadmill and overground walking program improves walking in persons residing in the community after stroke: a placebo-controlled, randomized trial. Arch Phys Med Rehabil. 2003;84:1486–91. doi: 10.1016/s0003-9993(03)00349-6. [DOI] [PubMed] [Google Scholar]
- 53.Yagura H, Miyai I, Seike Y, Suzuki T, Yanagihara T. Benefit of inpatient multidisciplinary rehabilitation up to 1 year after stroke. Arch Phys Med Rehabil. 2003;84:1687–91. doi: 10.1053/s0003-9993(03)00286-7. [DOI] [PubMed] [Google Scholar]
- 54.Barbeau H. Locomotor training in neurorehabilitation: emerging rehabilitation concepts. Neurorehabil Neural Repair. 2003;17:3–11. doi: 10.1177/0888439002250442. [DOI] [PubMed] [Google Scholar]
- 55.Edgerton V, Roy R. Paralysis recovery in human and model systems. Curr Opin Neurobiol. 2002;12:658–67. doi: 10.1016/s0959-4388(02)00379-3. [DOI] [PubMed] [Google Scholar]
- 56.Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci. 2003;4:573–86. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- 57.Rossignol S, Bouyer L, Barthelemy D, Langlet C, Leblond H. Recovery of locomotion in the cat following spinal cord lesions. Brain Res Rev. 2002;40:257–66. doi: 10.1016/s0165-0173(02)00208-4. [DOI] [PubMed] [Google Scholar]
- 58.Nielsen J. How we walk: Central control of muscle activity during human walking. Neuroscientist. 2003;9:195–204. doi: 10.1177/1073858403009003012. [DOI] [PubMed] [Google Scholar]
- 59.Dobkin B. Recovery of locomotor control. Neurologist. 1996;2:239–49. [Google Scholar]
- 60.Miyai I, Tanabe H, Sase I, et al. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. NeuroImage. 2001;14:1186–92. doi: 10.1006/nimg.2001.0905. [DOI] [PubMed] [Google Scholar]
- 61.Hesse S, Bertelt C, Jahnke M, Baake P, Mauritz K. Treadmill training with partial body weight support compared with physiotherapy in nonambulatory hemiparetic patients. Stroke. 1995;26:976–81. doi: 10.1161/01.str.26.6.976. [DOI] [PubMed] [Google Scholar]
- 62.Nilsson L, Carlsson J, Danielsson A, et al. Walking training of patients with hemiparesis at an early stage after stroke: a comparison of walking training on a treadmill with body weight support and walking training on the ground. Clin Rehabil. 2001;15:515–27. doi: 10.1191/026921501680425234. [DOI] [PubMed] [Google Scholar]
- 63.Kosak M, Reding M. Comparison of partial body weight-supported treadmill gait training versus aggressive bracing assisted walking post stroke. Neurorehabil Neural Repair. 2000;14:13–19. doi: 10.1177/154596830001400102. [DOI] [PubMed] [Google Scholar]
- 64.Lennihan L, Wootten M, Wainwright M, Tenteromano L, McMahon D, Cotier J. Treadmill with partial body-weight support versus conventional gait training after stroke. Arch Phys Med Rehabil. 2003;84:E5. [Google Scholar]
- 65.Pohl M, Mehrholz J, Ritschel C, Ruckriem S. Speed-dependent treadmill training in ambulatory hemiparetic stroke patients. Stroke. 2002;33:553–58. doi: 10.1161/hs0202.102365. [DOI] [PubMed] [Google Scholar]
- 66.Hesse S, Weerner C, Paul T, Bardeleben A, Chaler J. Influence of walking speed on lower limb muscle activity and energy consumption during treadmill walking of hemiparetic patients. Arch Phys Med Rehabil. 2001;82:1547–50. doi: 10.1053/apmr.2001.26607. [DOI] [PubMed] [Google Scholar]
- 67.Hesse S, Bertelt C, Schaffrin A, Malezic M, Mauritz K. Restoration of gait in nonambulatory hemiparetic patients by treadmill training with partial body-weight support. Arch Phys Med Rehabil. 1994;75:1087–93. doi: 10.1016/0003-9993(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 68.Moseley AM, Stark A, Cameron ID, Pollock A. The Cochrane Library. 2. Chichester, UK: John Wiley & Sons, Ltd; 2004. Treadmill training and body weight support for walking after stroke (Cochrane Review) [Google Scholar]
- 69.Dietz V, Duysens J. Significance of load receptor input during locomotion: a review. Gait Posture. 2000;11:102–10. doi: 10.1016/s0966-6362(99)00052-1. [DOI] [PubMed] [Google Scholar]
- 70.Harkema S, Hurley S, Patel U, Dobkin B, Edgerton V. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol. 1997;77:797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- 71.Sommerfeld D, Eek E, Svensson AK, Holmqvist L, von Arbin M. Spasticity after stroke. Stroke. 2004;35:134–39. doi: 10.1161/01.STR.0000105386.05173.5E. [DOI] [PubMed] [Google Scholar]
- 72.Zackowski K, Dromerick A, Sahrmann S, Thach W, Bastian A. How do strength, sensation, spasticity and joint individuation relate to the reaching deficits of people with chronic hemiparesis? Brain. 2004;127:1035–46. doi: 10.1093/brain/awh116. [DOI] [PubMed] [Google Scholar]
- 73.Lieber R, Einarsson F, Friden J. Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extracellular matrix material. Muscle Nerve. 2003;28:464–71. doi: 10.1002/mus.10446. [DOI] [PubMed] [Google Scholar]
- 74.Brashear A, Gordon M, Elovic E, et al. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke. N Engl J Med. 2002;347:395–400. doi: 10.1056/NEJMoa011892. [DOI] [PubMed] [Google Scholar]
- 75.Bonan I, Yelnik A, Colle F, et al. Reliance on visual information after stroke, part II: effectiveness of a balance rehabilitation program with visual cue deprivation after stroke, a randomized controlled trial. Arch Phys Med Rehabil. 2004;85:274–78. doi: 10.1016/j.apmr.2003.06.016. [DOI] [PubMed] [Google Scholar]
- 76.Pulvermuller F, Neininger B, Elbert T, et al. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001;32:1621–26. doi: 10.1161/01.str.32.7.1621. [DOI] [PubMed] [Google Scholar]
- 77.Carlomagno S, Pandolfi M, Labruna L, Colombo A, Razzano C. Recovery from moderate aphasia in the first year poststroke: effect of type of therapy. Arch Phys Med Rehabil. 2001;82:1073–80. doi: 10.1053/apmr.2001.25155. [DOI] [PubMed] [Google Scholar]
- 78.Musso M, Weiller C, Kiebel S. Training-induced brain plasticity in aphasia. Brain. 1999;122:1781–90. doi: 10.1093/brain/122.9.1781. [DOI] [PubMed] [Google Scholar]
- 79.Fasoli S, Krebs I, Stein J, et al. Effects of robotic therapy on motor impairment and recovery in chronic stroke. Arch Phys Med Rehabil. 2003;84:477–82. doi: 10.1053/apmr.2003.50110. [DOI] [PubMed] [Google Scholar]
- 80.Wolf S, Blanton S, Baer H, Breshears J, Butler A. Repetitive task practice: a critical review of constraint-induced movement therapy in stroke. Neurologist. 2002;8:325–38. doi: 10.1097/01.nrl.0000031014.85777.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pizzamiglio L, Perani D, Cappa S, Vallar G, Paolucci S, Fazio F. Recovery of neglect after right hemisphere damage. Arch Neurol. 1998;55:561–68. doi: 10.1001/archneur.55.4.561. [DOI] [PubMed] [Google Scholar]
- 82.Frassinetti F, Angeli V, Meneghello F, Avanzi S, Ladavas E. Long-lasting amelioration of visuospatial neglect by prism adaptation. Brain. 2002;125:608–23. doi: 10.1093/brain/awf056. [DOI] [PubMed] [Google Scholar]
- 83.Page SJ, Sisto S, Levine P, McGrath RE. Efficacy of modified constraint-induced movement therapy in chronic stroke: a single-blinded randomized controlled trial. Arch Phys Med Rehabil. 2004;85:14–18. doi: 10.1016/s0003-9993(03)00481-7. [DOI] [PubMed] [Google Scholar]
- 84.Koski L, Mernar T, Dobkin B. Immediate and long-term changes in corticomotor output response to rehabilitation: correlation with functional improvements in chronic stroke. Neurorehabil Neural Repair. doi: 10.1177/1545968304269210. in press. [DOI] [PubMed] [Google Scholar]
- 85.Dobkin B, Firestine A, West M, Saremi K, Woods R. Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. NeuroImage. doi: 10.1016/j.neuroimage.2004.06.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith G, Silver K, Goldberg A, Macko R. “Task-oriented” exercise improves hamstring strength and spastic reflexes in chronic stroke patients. Stroke. 1999;30:2112–18. doi: 10.1161/01.str.30.10.2112. [DOI] [PubMed] [Google Scholar]
- 87.Teixeira-Salmela L, Olney S, Nadeau S. Muscle strengthening and physical conditioning to reduce impairment and disability in chronic stroke survivors. Arch Phys Med Rehabil. 1999;80:1211–18. doi: 10.1016/s0003-9993(99)90018-7. [DOI] [PubMed] [Google Scholar]
- 88.Weiss A, Suzuki T, Bean J, Fielding RA. High intensity strength training improves strength and functional performance after stroke. Am J Phys Med Rehabil. 2000;79:369–76. doi: 10.1097/00002060-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 89.Fridman E, Hanakawa T, Chung M, Hummel F, Leiguarda R, Cohen L. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–58. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- 90.Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- 91.Malouin F, Belleville S, Richards C, Desrosiers J, Doyon J. Working memory and mental practice outcomes after stroke. Arch Phys Med Rehabil. 2004;85:177–83. doi: 10.1016/s0003-9993(03)00771-8. [DOI] [PubMed] [Google Scholar]
- 92.Liepert J, Bauder H, Miltner W, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–16. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 93.Ward N, Brown M, Thompson A, Frackowiak R. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:1–21. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miyai I, Yagura H, Hatakenaka M, Oda I, Konishi I, Kubota K. Longitudinal optical imaging study for locomotor recovery after stroke. Stroke. 2003;34:2866–70. doi: 10.1161/01.STR.0000100166.81077.8A. [DOI] [PubMed] [Google Scholar]
- 95.Dobkin B, Davis B, Bookheimer S. Functionalmagnetic resonance imaging assesses plasticity n locomotor networks. Neurology. 2000;4(suppl 3):A8. [Google Scholar]
- 96.Sahyoun C, Floyer-Lea A, Johansen-Bereg H, Matthews P. Towards an understanding of gait control: brain activation during the anticipation, preparation and execution of foot movements. NeuroImage. 2004;21:568–75. doi: 10.1016/j.neuroimage.2003.09.065. [DOI] [PubMed] [Google Scholar]
- 97.Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111:815–35. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- 98.Mattay V, Goldberg T, Fera F, et al. Catechol 0-methyltransferace val 158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–91. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Goldstein L, Dromerick A, Good D, et al. Possible time window for the detrimental effects of drugs on poststroke recovery. Neurology. 2002;58(suppl 3):A5–A6. [Google Scholar]
- 100.Dinse H, Ragert P, Pleger B, Schwenkreis P, Tegenthoff M. Pharmacological modulation of perceptual learning and associated cortical reorganization. Science. 2003;301:91–94. doi: 10.1126/science.1085423. [DOI] [PubMed] [Google Scholar]
- 101.Sawaki L, Boroojerdi B, Kaelin-Lang A, et al. Cholinergic influences on use-dependent plasticity. J Neurophysiol. 2002;87:166–71. doi: 10.1152/jn.00279.2001. [DOI] [PubMed] [Google Scholar]
- 102.Scheidtmann K, Fries W, Muller F, Koenig E. Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: a prospective, randomised, double-blinded study. Lancet. 2001;358:787–90. doi: 10.1016/S0140-6736(01)05966-9. [DOI] [PubMed] [Google Scholar]
- 103.Grade C, Redford B, Chrostowski J, Toussaint L, Blackwell B. Methylphenidate in early poststroke reovery: a double-blind, placebo-controlled study. Arch Phys Med Rehabil. 1998;79:1047–50. doi: 10.1016/s0003-9993(98)90169-1. [DOI] [PubMed] [Google Scholar]
- 104.Walker-Batson D, Smith P, Curtis S, Unwin H, Greenlee R. Amphetamine paired with physical therapy accelerates motor recovery after stroke. Stroke. 1995;26:2254–59. doi: 10.1161/01.str.26.12.2254. [DOI] [PubMed] [Google Scholar]
- 105.Sawaki L, Cohen L, Classen J, Davis B, Butefisch C. Enhancement of use-dependent plasticity by d-amphetamine. Neurology. 2002;59:1262–64. doi: 10.1212/wnl.59.8.1262. [DOI] [PubMed] [Google Scholar]
- 106.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 107.Bao S, Chan V, Merzenich M. Cortical remodelling induced by activity in ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- 108.Loubinoux I, Pariente J, Boulanouar K, et al. A single dose of the serotonin neurotransmission agonist paroxetine enhances motor output: double-blind, placebo-controlled, fMRI study in healthy subjects. NeuroImage. 2002;15:26–36. doi: 10.1006/nimg.2001.0957. [DOI] [PubMed] [Google Scholar]
- 109.Pariente J, Loubinoux I, Carel C, et al. Fluoxetine modulates motor performance and cerebral activation of patients recovering from stroke. Ann Neurol. 2001;50:718–29. doi: 10.1002/ana.1257. [DOI] [PubMed] [Google Scholar]
- 110.Ilic T, Korchounov A, Ziemann U. Complex modulation of human motor cortex excitability by the specific serotonin re-uptake inhibitor sertraline. Neurosci Lett. 2002;319:116–20. doi: 10.1016/s0304-3940(01)02563-0. [DOI] [PubMed] [Google Scholar]
- 111.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–23. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 112.Mattay V, Tessitore A, Callicott J, et al. Dopaminergic modulation of cortical function in patients with Parkinson's disease. Ann Neurol. 2002;51:156–64. doi: 10.1002/ana.10078. [DOI] [PubMed] [Google Scholar]
- 113.Boroojerdi B, Ziemann U, Chen R, Butefisch C, Cohen L. Mechanisms underlying human motor system plasticity. Muscle Nerve. 2001;24:602–13. doi: 10.1002/mus.1045. [DOI] [PubMed] [Google Scholar]
- 114.Butefisch C, Davis B, Sawaki L, et al. Modulation of use-dependent plasticity by d-amphetamine. Ann Neurol. 2002;51:59–68. doi: 10.1002/ana.10056. [DOI] [PubMed] [Google Scholar]
- 115.Thiel C. Cholinergic modulation of learning and memory in the human brain as detected with functional neuroimaging. Neurobiol Learn Mem. 2003;80:234–44. doi: 10.1016/s1074-7427(03)00076-5. [DOI] [PubMed] [Google Scholar]
- 116.Cauraugh J, Light K, Kim S, Thigpen M, Behrman A. Chronic motor dysfunction after stroke: recovering wrist and finger extension by electromyography-triggered neuromuscular stimulation. Stroke. 2000;31:1360–64. doi: 10.1161/01.str.31.6.1360. [DOI] [PubMed] [Google Scholar]
- 117.Alon G, McBride S, Ring H. Improving selected hand functions using a noninvasive neuroprosthesis in persons with chronic stroke. J Stroke Cerebrovasc Dis. 2002;11:99–106. doi: 10.1053/jscd.2002.127107. [DOI] [PubMed] [Google Scholar]
- 118.Salter AC, Bagg S, Creasy J, et al. First clinical experience with BION implants for therapeutic electrical stimulation. Neuromodulation. 2004;7:38–47. doi: 10.1111/j.1525-1403.2004.04005.x. [DOI] [PubMed] [Google Scholar]
- 119.Dobkin B. Do electrically stimulated sensory inputs and movements lead to long-term plasticity and rehabilitation gains? Curr Opin Neurol. 2003;16:685–92. doi: 10.1097/01.wco.0000102622.38669.ac. [DOI] [PubMed] [Google Scholar]
- 120.Fraser C, Power M, Hamdy S, et al. Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron. 2002;34:831–40. doi: 10.1016/s0896-6273(02)00705-5. [DOI] [PubMed] [Google Scholar]
- 121.Plautz E, Barbay S, Frost S, et al. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003;25:801–10. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- 122.Conforto A, Kaelin-Lang A, Cohen L. Increase in hand muscle strength of stroke patients after somatosensory stimulation. Ann Neurol. 2002;51:122–25. doi: 10.1002/ana.10070. [DOI] [PubMed] [Google Scholar]
- 123.Stefan K, Kunesch E, Cohen L, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–84. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- 124.Uy J, Ridding MC, Hillier S, Thompson P, Miles T. Does induction of plastic change in motor cortex improve leg function after stroke? Neurology. 2003;61:982–84. doi: 10.1212/01.wnl.0000078809.33581.1f. [DOI] [PubMed] [Google Scholar]
- 125.Muellbacher W, Richards C, Ziemann U, et al. Improving hand function in chronic stroke. Arch Neurol. 2002;59:1278–82. doi: 10.1001/archneur.59.8.1278. [DOI] [PubMed] [Google Scholar]
- 126.Murase N, Duque J, Mazzocchio R, Cohen L. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–09. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 127.Werhahn KJ, Mortensen J, Van Boven RW, Zeuner KE, Cohen LG. Enhanced tactile spatial acuity and cortical processing during acute hand deafferentation. Nat Neurosci. 2002;5:936–38. doi: 10.1038/nn917. [DOI] [PubMed] [Google Scholar]
- 128.Nair DG, Purcott KL, Fuchs A, Steinberg F, Kelso JA. Cortical and cerebellar activity of the human brain during imagined and executed unimanual and bimanual action sequences: a functional MRI study. Cogn Brain Res. 2003;15:250–60. doi: 10.1016/s0926-6410(02)00197-0. [DOI] [PubMed] [Google Scholar]
- 129.Decety J. Can motor imagery be used as a form of therapy? J NIH Res. 1995;7:47–48. [Google Scholar]
- 130.Jack D, Boian R, Merians A, et al. Virtual reality-enhanced stroke rehabilitation. IEEE Trans Neural Syst Rehabil Eng. 2001;9:308–18. doi: 10.1109/7333.948460. [DOI] [PubMed] [Google Scholar]
- 131.Iacoboni M, Woods R, Brass M, Bekkering H, Mazziottal J, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–28. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- 132.Holden M, Dyar T. Virtual environment training: a new tool for neurorehabilitation. Neurol Report. 2002;26:62–74. [Google Scholar]
- 133.Hesse S, Schmidt H, Werner C, Bardeleben A. Upper and lower extremity robotic devices for rehabilitation and for studying motor control. Curr Opin Neurol. 2003;16:705–10. doi: 10.1097/01.wco.0000102630.16692.38. [DOI] [PubMed] [Google Scholar]
- 134.Lum P, Burgar C, Majimundar M, Van der Loos M. Robot-assisted movement training compared to conventional therapy techniques for the rehabilitation of upper limb motor function following stroke. Arch Phys Med Rehabil. 2002;83:952–59. doi: 10.1053/apmr.2001.33101. [DOI] [PubMed] [Google Scholar]
- 135.Hesse S, Schulte-Tigges G, Konrad M, Bardelen A, Werner C. Robot-assisted arm trainer for the passive and active practice of bilateral forearm and wrist movements in hemiparetic subjects. Arch Phys Med Rehabil. 2003;84:915–20. doi: 10.1016/s0003-9993(02)04954-7. [DOI] [PubMed] [Google Scholar]
- 136.Werner C, von Frankenberg S, Treig T, Konrad M, Hesse S. Treadmill training with partial body weight support and electromechanical gait trainer for restoration of gait in subacute stroke patients. Stroke. 2002;33:2895–901. doi: 10.1161/01.str.0000035734.61539.f6. [DOI] [PubMed] [Google Scholar]
- 137.Dobkin B, Havton L. Basic advances and new avenues in therapy of spinal cord injury. Annu Rev Med. 2004;55:255–82. doi: 10.1146/annurev.med.55.091902.104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–11. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 139.Kondziolka D, Wechsler L, Achim C. Neural transplantation for stroke. J Clin Neurosci. 2002;9:225–30. doi: 10.1054/jocn.2001.1043. [DOI] [PubMed] [Google Scholar]
- 140.Rothstein J, Snyder E. Reality and immortality—neural stem cells for therapies. Nat Biotech. 2004;22:283–85. doi: 10.1038/nbt0304-283. [DOI] [PubMed] [Google Scholar]
- 141.Emerick A, Neafsey E, Schwab M, Kartje G. Functional reorganization of the motor cortex in adult rats after cortical lesion and treatment with monoclonal antibody IN-1. J Neurosci. 2003;23:4826–30. doi: 10.1523/JNEUROSCI.23-12-04826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schabitz WR, Berger C, Kollmaar R, et al. Effect of brain-derived neurotrophic factor treatment and forced use on functional recovery after small cortical ischemia. Stroke. 2004;35:992–97. doi: 10.1161/01.STR.0000119754.85848.0D. [DOI] [PubMed] [Google Scholar]
- 143.Johansson B, Belichenko P. Neuronal plasticity and dendritic spines: effect of environmental enrichment on intact and postischemic rat brain. J Cereb Blood Flow Metab. 2002;22:89–96. doi: 10.1097/00004647-200201000-00011. [DOI] [PubMed] [Google Scholar]


