Abstract
Wnt glycoproteins play essential roles in the development of metazoan organisms. Many Wnt proteins, such as Wnt1, activate the well-conserved canonical Wnt signaling pathway, which results in accumulation of β-catenin in the cytosol and nucleus. Other Wnts, such as Wnt5a, activate signaling mechanisms which do not involve β-catenin and are less well characterized. Dishevelled (Dvl) is a key component of Wnt/β-catenin signaling and becomes phosphorylated upon activation of this pathway. In addition to Wnt1, we show that several Wnt proteins, including Wnt5a, trigger phosphorylation of mammalian Dvl proteins and that this occurs within 20 to 30 min. Unlike the effects of Wnt1, phosphorylation of Dvl in response to Wnt5a is not concomitant with β-catenin stabilization, indicating that Dvl phosphorylation is not sufficient to activate canonical Wnt/β-catenin signaling. Moreover, neither Dickkopf1, which inhibits Wnt/β-catenin signaling by binding the Wnt coreceptors LRP5 and -6, nor dominant-negative LRP5/6 constructs could block Wnt-mediated Dvl phosphorylation. We conclude that Wnt-induced phosphorylation of Dvl is independent of LRP5/6 receptors and that canonical Wnts can elicit both LRP-dependent (to β-catenin) and LRP-independent (to Dvl) signals. Our data also present Dvl phosphorylation as a general biochemical assay for Wnt protein function, including those Wnts that do not activate the Wnt/β-catenin pathway.
The Wnt family of secreted signaling factors is a major group of developmental regulators in metazoan species from hydra to humans (16, 52, 58). Their functions include crucial roles in the morphogenesis and patterning of a wide variety of tissues during mammalian development and in tissue homeostasis in the adult. At the cellular level, Wnt proteins have been shown to regulate cell proliferation, apoptosis, differentiation, self-renewal of stem cells, and cell fate determination (16, 52, 95). In addition, deregulated Wnt signaling can contribute to tumorigenesis: aberrant activation of intracellular Wnt signaling is one of the most common signaling abnormalities observed in human cancer (12, 26, 62, 75).
The founder members of the Wnt gene family, Wnt1 and Wingless, were first identified as an oncogene in mouse mammary tumors and as a segment polarity gene in Drosophila melanogaster, respectively (57, 64). Subsequent studies have revealed as many as 19 different Wnt genes in vertebrates. All of these encode secreted glycoproteins containing a conserved domain of approximately 300 amino acids that is punctuated by 21 to 23 cysteine residues with a characteristic spacing pattern (13, 58). Many of these proteins have been shown to act via the well-characterized canonical Wnt signaling pathway (also known as the Wnt/β-catenin pathway) (20, 52). Activation of this pathway is initiated by binding of Wnt proteins to cell surface receptors composed of a member of the Frizzled (Fzd) protein family and one of the LDL receptor-related proteins LRP-5 or LRP-6 (8, 32, 50, 83). Signaling from Wnt receptors proceeds through the proteins Dishevelled (Dsh/Dvl) and Axin, leading to inactivation of a cytoplasmic protein complex that normally catalyzes the phosphorylation and subsequent destruction of β-catenin (9, 52, 62). Canonical Wnt signaling thus induces stabilization of cytosolic β-catenin. A fraction of β-catenin then enters the nucleus, binds to transcription factors such as those of the LEF-1/TCF family, and modulates the transcription of specific target genes (23).
A variety of experimental systems have been used to characterize the canonical Wnt signaling pathway and have provided powerful assays for studying individual components. These assays include the induction of secondary axes in Xenopus laevis embryos, morphological transformation of mouse mammary cells, stabilization of cytosolic β-catenin, and activation of TCF-dependent transcriptional reporters in transfected cells (14, 37, 73, 77). Although many Wnts display consistent activity in these assays, some members of the family fail to activate the Wnt/β-catenin pathway, and there is considerable evidence that certain Wnts signal via alternative, noncanonical mechanisms. The prototypical example of such a noncanonical Wnt is Wnt5a. Unlike many members of the Wnt family, mammalian Wnt5a is widely expressed in embryonic tissues (98). When injected into Xenopus embryos, Wnt5a RNA does not normally induce axis duplication but instead interferes with morphogenetic movements of the convergent extension (CE) process that occurs during gastrulation (22, 54). Targeted gene disruption in the mouse has shown that Wnt5a is required for the elongation of axial structures and may influence the proliferation of precursor cells (98), while analysis of human WNT5A expression has revealed frequent overexpression of the gene in cancers of the lung, breast, and prostate and in melanomas (19, 33, 43). Moreover, Wnt5a has been reported to promote cell motility and invasion of metastatic melanoma cells (92), suggesting that Wnt5a signaling could be a potential target for anticancer therapies.
The mechanisms of signaling by Wnt5a, and of noncanonical Wnt signaling in general, are much less clearly understood than those which result in β-catenin stabilization. At least two distinct pathways of noncanonical Wnt signaling have been proposed: the planar cell polarity (PCP) pathway and the Wnt/Ca2+ pathway (40, 53). The latter pathway is implicated in the specification of ventral cell fates in Xenopus embryos and in regulating the adhesive properties of gastrulating cells. It is thought to result in release of intracellular Ca2+, activation of protein kinase C and calcium-dependent kinases and nuclear import of the transcription factor NF-AT (39, 68, 72). A specific subset of Fzd proteins has been associated with Wnt/Ca2+ signaling, but the role of individual Wnt proteins is unclear. The PCP pathway, originally defined by genetic analysis in Drosophila, controls the lateral polarity of epithelial cells in a plane orthogonal to their apical-basal axis (1). Such planar polarity is required, for example, for the alignment of bristles in the adult Drosophila epidermis. In vertebrates, a similar mechanism appears to govern the CE movements that elongate columns of mesodermal and neuroectodermal cells during gastrulation (9, 29, 35, 53). The PCP/CE pathway shares at least two key components with the canonical Wnt/β-catenin pathway, namely Fzd and Dsh, but its downstream components are distinct (3, 9, 85, 88, 89). These components may include the small G-protein RhoA, the RhoA-associated kinase ROCK/Drok (10, 80, 97), and possibly the c-Jun N-terminal kinase (JNK) (10, 46, 79, 99). Although Fzd is required for correct planar polarity, no specific Wnt protein has been implicated in PCP signaling in Drosophila. In zebrafish, however, mutation of Wnt11/silberblick specifically disrupts CE movements in embryogenesis, implying that the PCP/CE pathway can indeed be dependent on Wnt ligands (29, 35, 63).
It is notable that the cytoplasmic protein Dsh/Dvl is an essential component of both the Wnt/β-catenin pathway and the Wnt/PCP pathway. Dsh also interacts with the signaling protein Notch (4) and may be a protein that serves to integrate different signals and/or channel them to different pathways. In mammals there are three homologs of Drosophila Dsh, named Dvl1, -2, and -3 (70, 82, 102). Each Dsh/Dvl protein is composed of three conserved modular domains: an amino-terminal DIX domain, a central PDZ domain, and a carboxy-terminal DEP domain (9). These domains are differentially required for the different signaling functions of Dsh/Dvl (5, 9, 10).
Previous studies have shown that Dsh/Dvl undergoes phosphorylation in response to Wnt signals that stabilize β-catenin (65, 101). Although the functional significance of this phosphorylation is unclear, a number of candidate Dsh/Dvl kinases have been described, and in some cases Dvl phosphorylation has been implicated as an activating step that allows Dvl to signal downstream to β-catenin (61, 81, 86, 94). Here we report that Dvl phosphorylation is a direct signaling consequence of both functional classes of Wnt protein, those that stabilize β-catenin and those that do not. This phosphorylation is thus not sufficient to mediate canonical Wnt/β-catenin signaling. We show that the phosphorylation of Dvl by Wnt proteins is not dependent on the Wnt coreceptors LRP5/6 and, hence, reflects a signal transduction event distinct from that which leads to β-catenin stabilization. Our data also indicate that Dvl phosphorylation provides a reliable biochemical assay for Wnt5a and other Wnts that do not signal via β-catenin. This should facilitate further analysis of signaling by this important category of Wnt proteins.
MATERIALS AND METHODS
Plasmids.
cDNAs encoding mouse LRP5 and LRP6 were kindly provided by Fred Hess (15, 30). LRP5 Ex and LRP6 Ex are secreted forms of the LRP5 and LRP6 extracellular domains, comprising amino acids 1 to 1387 and 1 to 1372, respectively. LRP6Δ173 is a transmembrane protein comprising amino acids 1 to 1440 and has the majority of the LRP6 intracellular domain deleted (11). cDNAs encoding each of the above were subcloned into the expression vector pcDNA3.1/Myc-His (Invitrogen), thus introducing Myc epitopes and poly-histidine tags at the C terminus.
Murine Fzd8 and rat Fzd1 cDNAs were kindly provided by Jeremy Nathans and Robert Nissenson, respectively (18, 91). Fzd1 Ex and Fzd8 Ex are secreted forms of the entire extracellular domains (amino acids 1 to 309 and 1 to 270, respectively), each with a C-terminal Myc tag, and were expressed by subcloning into pcDNA3 (Invitrogen). Dkk1 cDNA, a gift from Stuart Aaronson (6), was subcloned into the expression vector pcDNA3.1/V5-His (Invitrogen), such that a V5 epitope was introduced at the C terminus of the protein.
Plasmid vectors containing cDNAs for ΔN-Arrow (amino acids 1445 to 1678), Dfz2, and Wingless for expression in Drosophila S2 cells were as previously described (11).
Cell culture and conditioned media.
Human embryonic kidney cell lines 293T and BOSC 23 (60), Rat2 fibroblasts, and the murine cell lines 10T1/2, L, and L/Wnt3a were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (Life Technologies, Inc.). L cells and L/Wnt3a were kindly provided by Karl Willert and Roel Nusse (96). Drosophila Schneider2 cells were maintained in Schneider's medium (Gibco-BRL) containing 15% heat-inactivated fetal bovine serum at 22°C and atmospheric CO2.
Cell lines stably expressing Wnt proteins, LRP6, or Dvl2 derivatives were generated by infection with recombinant retroviruses based on the vector pLNCX. BOSC 23 packaging cells were transiently transfected with the relevant constructs, and helper-free virus stocks were harvested 48 h later (60). Approximately 50 to 100 individual G418-resistant colonies were pooled to generate the infected cell lines used. Coculture of Wnt-expressing Rat2 cells with Rat2 cells transduced with exogenous Dvl2 derivatives (see Fig. 3B, below) was initiated at a 1:1 ratio on the day of seeding, and cell lysates were harvested 2 days later. S2 cells stably expressing Dfz2 were generated by transfecting with full-length Dfz2 cDNA in the heat shock promoter vector pPHygroHS and selecting the cells in medium containing 200 μg of hygromycin B (Roche)/ml.
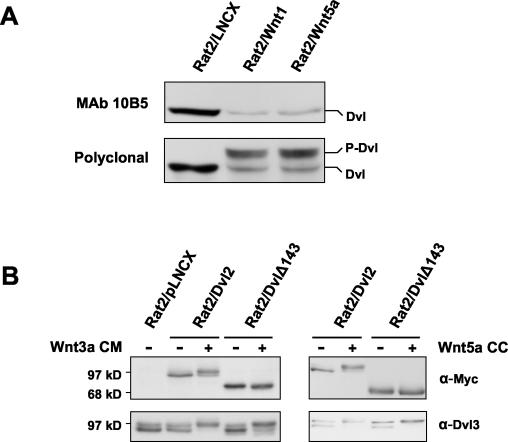
FIG. 3.
Canonical and noncanonical Wnts induce phosphorylation of a common region of Dvl2. (A) Phosphorylation of Dvl2 induced by either Wnt1 or Wnt5a masks the epitope for recognition by monoclonal antibody 10B5. Whole-cell extracts from Rat2 cells stably expressing the control vector LNCX, Wnt1, or Wnt5a were analyzed for Dvl2 phosphorylation by Western blotting using either monoclonal antibody 10B5 (upper panel) or a polyclonal Dvl2 antibody (lower panel). 10B5 recognizes an epitope within amino acids 594 to 736 of human Dvl2. (B) The Dvl2 derivative DvlΔ143, lacking amino acids 594 to 736, is not phosphorylated in response to signaling by Wnt3a or Wnt5a, but full-length Dvl2 shows a mobility shift. Rat2 cells stably expressing either Dvl2 or DvlΔ143 were either treated for 2 h with control or Wnt3a CM or were cocultured (CC) with control (−) or Wnt5a-producing Rat2 cells. Myc-tagged exogenous Dvl phosphorylation was analyzed by Western blotting using an anti-Myc antibody. Phosphorylation of endogenous Dvl3 was monitored as a positive control.
To obtain conditioned medium (CM) containing Wnt1 or Wnt5a, Rat2 cells infected with LNC-Wnt1, LNC-Wnt5a, or the empty vector LNCX were plated at a density of 5 × 105 per 10-cm dish and grown to 80 to 90% confluence. The medium was then replaced with Dulbecco's modified Eagle's medium containing 1% fetal bovine serum. Once the cells reached confluence, CM was harvested, centrifuged for 10 min at 2,000 × g, and concentrated 10-fold using Centriplus YM-10 columns (Millipore). CM containing Wnt3a, harvested from L/Wnt3a cells (96), was collected in standard growth medium and used unconcentrated. Dkk1 CM was obtained from transiently transfected 293T cells. At 20 h after transfection the medium was replaced, and CM was harvested 24 h later.
Transfections and TOPflash assays.
293T and S2 cells were transfected by using calcium phosphate precipitation methods as described previously (11) and harvested 48 h later for analysis of β-catenin or Armadillo levels. TOPflash assays with pRL-TK as an internal standard were carried out in transfected 293T cells as described previously (31). Firefly and Renilla luciferase activities were separately measured using the Dual Luciferase reagent kit (Promega) and a Monolight 2010 luminometer (Analytical Luminescence Laboratory). Coculture of transiently transfected 293T cells with Wnt-expressing cells was achieved by adding an approximately equal number of Rat2/Wnt1 or control Rat2/LNCX cells to the cultures 24 h after transfection.
Assays of β-catenin levels by Western blotting.
Immunoblot analysis of cytosolic β-catenin was carried out essentially as previously described (25). Briefly, cells were lysed in a Dounce homogenizer and cytosolic fractions were prepared by centrifugation (25). Protein concentrations were determined using the Bio-Rad protein assay reagent (Bio-Rad Laboratories). Aliquots containing 5 μg of protein were fractionated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Millipore). β-Catenin was detected with a mouse monoclonal antibody (C19220; Transduction Laboratories). Equal loading of the samples was confirmed by probing the blots with either mouse anti-GSK3β antibody (Transduction Laboratories) or with anti-β-tubulin antibodies. Expression of Myc-tagged Fzd1, Fzd8, LRP5, LRP6, and Dvl2 derivatives was monitored by analysis with mouse anti-Myc antibody 9E10 (Santa Cruz Biotechnology). The tagged Dkk1 construct was detected with mouse anti-V5 antibody (Invitrogen).
Armadillo protein levels were determined after lysis of S2 cells in boiling SDS buffer (10 mM Tris-HCl [pH 7.4], 2% SDS) and homogenization by passage through a 23-gauge needle. Protein levels were determined using the bicinchoninic acid reagent (Pierce). A 50-μg aliquot of protein was analyzed by Western blotting using mouse monoclonal anti-Armadillo antibody N2 7A1 (Developmental Studies Hybridoma Bank) and a rabbit anti-Dishevelled antibody generously provided by R. Nusse, Stanford University (101).
Determination of Dvl phosphorylation status.
Cells were washed with Tris-buffered saline and lysed in radioimmunoprecipitation assay buffer (10 mM Na2HPO4 [pH 7.2], 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 2 mM EDTA, 50 mM NaF, 0.2 mM sodium orthovanadate, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml, and 1 mM phenylmethylsulfonyl fluoride). Protein concentrations were determined using Bio-Rad DC protein assay reagents (Bio-Rad Laboratories). Forty-microgram samples of cell lysate were analyzed by SDS-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. Dvl was detected with a rabbit polyclonal antibody generated against a recombinant glutathione S-transferase fusion protein encoding amino acids 90 to 246 of Dvl1. This antibody can recognize all three Dvl isoforms on Western blotting, as determined by transfection of cells with individual Dvl cDNAs. Antibodies used for specific detection of individual Dvl isoforms were mouse anti-Dvl1 (3F12; Santa Cruz), rabbit polyclonal anti-Dvl2 (H-75; Santa Cruz), mouse monoclonal anti-Dvl2 (10B5; Santa Cruz), and mouse anti-Dvl3 (4D3; Santa Cruz).
RESULTS
Wnt5a expression causes Dvl phosphorylation but not β-catenin stabilization.
In order to investigate the effects of canonical and noncanonical Wnt signals on Dvl phosphorylation, we first used retroviral vectors to generate Rat2 fibroblasts stably expressing either Wnt1 (Rat2/Wnt1) or Wnt5a (Rat2/Wnt5a). The status of Dvl in these cell lines was analyzed by Western blotting using an antibody that recognizes all three mammalian Dvls. Stable expression of either Wnt1 or Wnt5a induced a shift in mobility of the Dvl band relative to that observed in cells infected with the control vector (Fig. 1A). This mobility shift was similar to that described by others as a result of phosphorylation of Dvl in response to canonical Wnt signals that stabilize β-catenin (42, 65), but this has not been reported in response to Wnt proteins that elicit noncanonical signals. To confirm that the upper band corresponded to a more highly phosphorylated form of Dvl, extracts of cells expressing Wnt5a were treated with lambda phosphatase in the presence or absence of phosphatase inhibitors (Fig. 1B). In parallel with the analysis of Dvl mobility in Fig. 1A, β-catenin levels in the same cell lines were analyzed in cytosolic extracts. As expected, Wnt1 expression led to accumulation of β-catenin but Wnt5a did not (Fig. 1A). This demonstrates that the phosphorylation of Dvl mediated by Wnt5a is not sufficient to activate the Wnt/β-catenin pathway. Similar results that dissociate Dvl phosphorylation from β-catenin stabilization were also obtained when Wnt1 and Wnt5a were stably expressed in other cell lines, such as mouse 10T1/2 cells (data not shown).
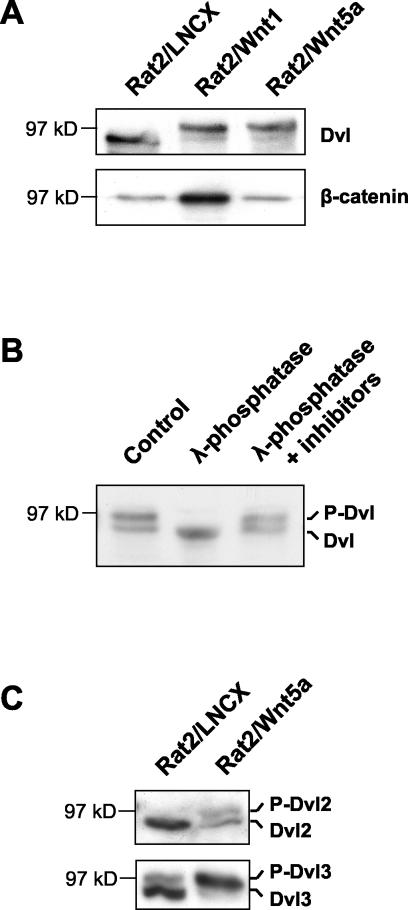
FIG. 1.
Wnt5a causes Dvl phosphorylation but not β-catenin stabilization. (A) Stable expression of either Wnt1 or Wnt5a leads to Dvl phosphorylation, but only Wnt1 causes β-catenin stabilization. Whole-cell extracts (upper panel) and cytosolic extracts (lower panel) from Rat2 cells stably expressing the control vector LNCX, Wnt1, or Wnt5a were analyzed by Western blotting with antibodies against Dvl proteins or β-catenin, as indicated. (B) Western analysis of Dvl in Rat2/Wnt5a cell extracts either untreated (control), treated with λ-phosphatase, or treated with phosphatase in the presence of phosphatase inhibitors. The mobility shift upon phosphatase treatment confirms that the upper Dvl band in Rat2/Wnt5a cells is hyperphosphorylated. For simplicity, the two forms of Dvl protein are here referred to as unphosphorylated (Dvl) and phosphorylated Dishevelled (P-Dvl). (C) Wnt5a induces phosphorylation of both Dvl2 and Dvl3. Extracts from control Rat2/LNCX and from Rat2/Wnt5a cells were analyzed by Western blotting using Dvl2-specific and Dvl3-specific antibodies. Dvl2 and Dvl3 appear to comigrate on SDS-polyacrylamide gels.
To determine which individual member(s) of the Dvl family is phosphorylated in response to Wnt5a, we also analyzed cell extracts from control and Rat2/Wnt5a cells with antibodies that distinguish individual Dvl family members. We were unable to detect endogenous Dvl1 expression in the cells using the available antibodies. However, both Dvl2 and Dvl3 were detected, and both were found to undergo Wnt-induced phosphorylation (Fig. 1C).
Dvl phosphorylation is a signaling response common to several Wnt proteins.
We next wished to determine whether the observed Dvl phosphorylation was a rapid signaling response to soluble Wnt proteins. Accordingly, we harvested Wnt CM from either Wnt1- or Wnt5a-expressing cells and used this to stimulate parental Rat2 cells. Exposure to either Wnt1 or Wnt5a CM for 2 h CM was sufficient to induce hyperphosphorylation of Dvl (Fig. 2A). As in the experiment shown in Fig. 1, however, only Wnt1 CM was able to induce β-catenin stabilization. A similar, but more complete, induction of Dvl phosphorylation was observed in 10T1/2 cells after treatment with Wnt5a CM (Fig. 2B). We also tested the ability of other Wnt proteins to induce Dvl phosphorylation in CM transfer assays or by expression of the relevant Wnt cDNA. All of those tested, specifically Wnt3a, Wnt4, and Wnt11, were able to elicit the response (data not shown) (see Fig. 4). While Wnt3a is known to elicit canonical Wnt/β-catenin signals, Wnt11 is associated with noncanonical Wnt signaling (29, 59, 73). This suggests that induction of Dvl phosphorylation may be a universal signaling response to Wnt proteins, irrespective of their ability to stabilize β-catenin.
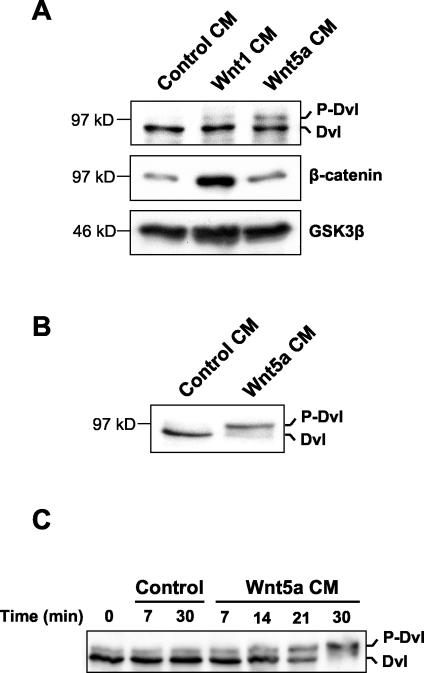
FIG. 2.
CM from Wnt5a-expressing cells induces Dvl phosphorylation. (A) Rat2 fibroblasts were treated for 2 h with CM from Rat2 cells expressing control vector, Wnt1, or Wnt5a. Extracts were then analyzed as described for Fig. 1A for phosphorylation of Dvl and cytosolic β-catenin. Analysis of cytosolic GSK3β protein levels provided a loading control. (B) Treatment of 10T1/2 fibroblasts with Wnt5a CM also induces Dvl phosphorylation. (C) Kinetics of Dvl phosphorylation induced by Wnt5a. 10T1/2 fibroblasts were treated with either control or Wnt5a CM for the indicated times. Cells were then lysed, and extracts were analyzed by Western blotting with Dvl antibodies as described for panels A and B.
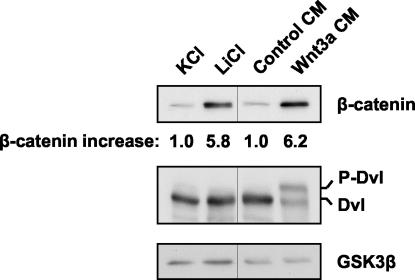
FIG. 4.
Lithium treatment stabilizes β-catenin but has no effect on Dvl phosphorylation. Rat2 cells were treated for 2 h with 40 mM KCl, 40 mM LiCl, control CM, or Wnt3a CM. Extracts were then analyzed as described for Fig. 1A for cytosolic β-catenin levels, Dvl phosphorylation, and cytosolic GSK3β as a loading control. The relative increases in β-catenin levels determined by densitometry are indicated.
We next studied the kinetics of Dvl phosphorylation in more detail, using 10T1/2 cells as targets in view of their robust response. An increase in the hyperphosphorylated form of Dvl was detected within approximately 20 min of treatment with Wnt5a CM and reached a maximum by 30 min (Fig. 2C). The kinetics of Dvl phosphorylation in response to Wnt3a were similar to those observed with Wnt5a (data not shown). These results are consistent with the phosphorylation of Dvl being a direct effect of Wnt signal transduction in mammalian cells and suggest that the signaling mechanism involved may be common to Wnts that stabilize β-catenin and those that do not.
The electrophoretic mobility shift in Dvl observed above was similar in response to Wnt1 and Wnt5a (Fig. 1A and 2A), and expression of both Wnts simultaneously did not result in a discernible supershift (data not shown). This is consistent with the possibility that both categories of Wnt protein induce phosphorylation of Dvl on the same sites. To investigate this further, we made use of the chance observation that a particular monoclonal antibody directed against Dvl2 is unable to recognize the Wnt-induced phosphorylated form of Dvl2. Immunoblot analysis of Rat2/Wnt1 and Rat2/Wnt5a cells with this antibody showed a disappearance of unphosphorylated Dvl2 but no concomitant appearance of the phosphorylated form detected by the polyclonal antibody (Fig. 3A). This implies that the epitope for this antibody is masked by phosphorylation. Since the epitope masking was observed in response to both Wnt1 and Wnt5a, the relevant site of phosphorylation is apparently common to both signals.
The monoclonal antibody described above recognizes an epitope within the C-terminal domain of Dvl2. To investigate the importance of this domain, we generated cells expressing a Myc-tagged Dvl2 derivative with the C-terminal 143 amino acids deleted (DvlΔ143). Upon stimulation of these cells with either Wnt3a, which induces β-catenin stabilization, or with Wnt5a, which does not, we observed no mobility shift in DvlΔ143 (Fig. 3B). In contrast, Myc-tagged full-length Dvl2 displayed Wnt-induced phosphorylation. Thus, the C-terminal domain of Dvl2 is critically required for Wnt-induced mobility shifting and may contain the major sites of Wnt-mediated phosphorylation of Dvl2 in our system. In these and other experiments, we failed to observe differences in the behavior of Dvl proteins phosphorylated in response to Wnt1, Wnt3a, or Wnt5a. This suggests that both categories of Wnt signal activate similar phosphorylation events on Dvl proteins.
β-Catenin can be stabilized without inducing Dvl phosphorylation.
The results above demonstrate that the Dvl phosphorylation we observed in response to Wnt5a is not sufficient to cause β-catenin stabilization (Fig. 1 and 2). In previous studies, however, Dsh/Dvl phosphorylation has been associated with canonical Wnt signaling (42, 61, 101). We therefore wished to test the possibility that Dvl phosphorylation might be a consequence of canonical Wnt/β-catenin signaling downstream of β-catenin. To do this we employed the GSK3β inhibitor lithium chloride, which can stabilize β-catenin and thus activate downstream signaling in the absence of a Wnt protein signal (28). As shown in Fig. 4, treatment of cells with LiCl caused stabilization of β-catenin but had no apparent effect on Dvl. In contrast, parallel treatment of the cells with Wnt3a CM induced both β-catenin stabilization and Dvl phosphorylation. These data argue that Dvl phosphorylation is not downstream of β-catenin in the Wnt/β-catenin pathway and suggest that it may result from an independent signaling mechanism.
DN Fzd, but not DN LRP proteins, can inhibit Dvl phosphorylation.
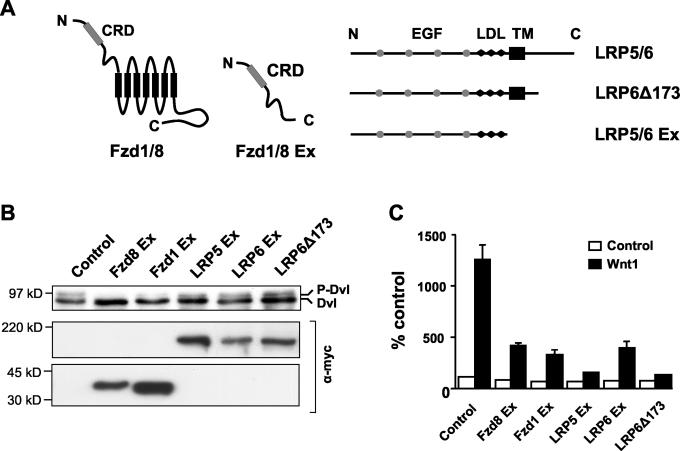
Having shown that Dvl phosphorylation is a common response to Wnt signaling, we were able to use this as an assay to investigate receptors involved in transducing Wnt signals to Dvl. To determine whether both Fzd and LRP5 and -6 receptor components were required for Dvl phosphorylation, we analyzed the capacity of different dominant-negative (DN) derivatives of these receptors to inhibit Dvl hyperphosphorylation. These DN receptors lack the intracellular sequences of the protein but retain the Wnt-binding domain (Fig. 5A) and are presumed to work by preventing interaction of the cognate receptors with ligand (11, 32, 44, 83, 90). Certain mammalian cell lines, including 293T cells, have high basal levels of phosphorylated Dvl, most likely due to endogenous expression of Wnt proteins. Thus, we transiently transfected 293T cells with either DN Fzd or DN LRP constructs and analyzed their effects on basal Dvl phosphorylation. Our results showed that Fzd8 Ex and to a lesser extent Fzd1 Ex, but not LRP5 or -6 DN proteins, were able to abolish basal Dvl hyperphosphorylation, although all constructs were expressed at similar levels (Fig. 5B). In contrast, DN LRP5 and DN LRP6 constructs were as effective as DN Fzds in inhibiting Wnt1-induced transcription from the β-catenin/TCF-dependent reporter pTOPflash (Fig. 5C) and Wnt1-induced accumulation of cytosolic β-catenin (data not shown) (11). Therefore, these data suggest that while LRP proteins are essential upstream components of the Wnt/β-catenin pathway, they may not be required for Dvl phosphorylation.
FIG. 5.
DN Frizzled, but not DN LRP5 or -6, can inhibit Dvl phosphorylation. (A) Diagram of Fzd and LRP5 and -6 constructs used in this study. The extracellular cysteine-rich domain (CRD) and the seven transmembrane domains (black rectangles) are indicated for Fzd 1 and 8, as are the epidermal growth factor (EGF) repeat domains (grey circles), LDL receptor repeats (LDL; black lozenges), and transmembrane domain (TM; black rectangle) for LRP5/6. (B) 293T cells were transfected with vectors encoding the indicated Fzd or LRP5/6 DN constructs. Dvl phosphorylation was analyzed by Western blotting as described in the legend for Fig. 1A. Expression of the epitope-tagged Frizzled and LRP derivatives was confirmed with an anti-Myc antibody. Only Fzd8 Ex, and to a lesser extent Fzd1 Ex, inhibited Dvl phosphorylation. (C) Both DN Frizzled and LRP5 and -6 proteins can inhibit TCF/β-catenin-dependent transcription. 293T cells were transfected with vectors encoding the indicated Fzd or LRP5/6 DN constructs, in the absence (white bars) or presence (black bars) of a Wnt1-expressing construct, together with the TCF/β-catenin-responsive reporter pTOPflash. The cells were also transfected with a Renilla luciferase plasmid, pRL-TK, as an internal control. Luciferase activities were measured, and TOPflash values were normalized to Renilla values. Results shown are means + standard deviations of six replicates.
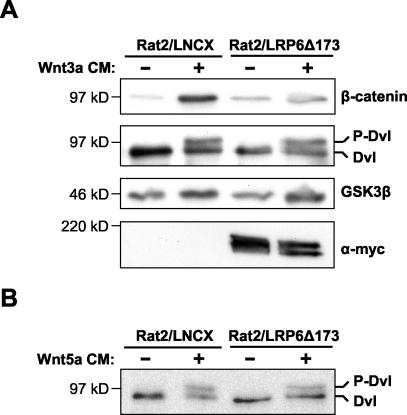
To investigate this further, we generated Rat2 cells stably expressing the DN LRP6 protein LRP6Δ173 (Rat2/LRP6Δ173) by retroviral infection. Treatment of Rat2/LRP6Δ173 cells with Wnt3a CM induced an increase in Dvl phosphorylation similar to that induced by Wnt3a in control cells infected with empty vector (Rat2/LNCX) (Fig. 6A). However, in the same experiment, DN LRP6 prevented the increase in cytosolic β-catenin induced by treatment with Wnt3a CM (Fig. 6A). In addition, LRP6Δ173 had no effect on Dvl phosphorylation triggered by Wnt5a (Fig. 6B). Taken together, these results strongly suggest that LRP receptors are not required for Wnt-mediated phosphorylation of Dvl.
FIG. 6.
DN LRP does not inhibit Wnt3a- or Wnt5a-induced Dvl phosphorylation. (A) Rat2 cells stably expressing either LRP6Δ173 or the control vector LNCX were treated for 2 h with either control or Wnt3a CM. Cytosolic β-catenin levels and Dvl phosphorylation were analyzed by Western blotting as described in the legend for Fig. 1A. LRP6Δ173 blocked the Wnt-induced stabilization of β-catenin but did not block the phosphorylation of Dvl. GSK3β was used as a loading control, and expression of the Myc epitope-tagged LRP6Δ173 protein was confirmed using an anti-Myc antibody. (B) Rat2 cells stably expressing LRP6Δ173 or empty vector were treated for 2 h with either control or Wnt5a CM. Whole-cell lysates were analyzed for Dvl phosphorylation as described for panel A.
Dickkopf1 inhibits the Wnt/β-catenin pathway but does not block Dvl phosphorylation.
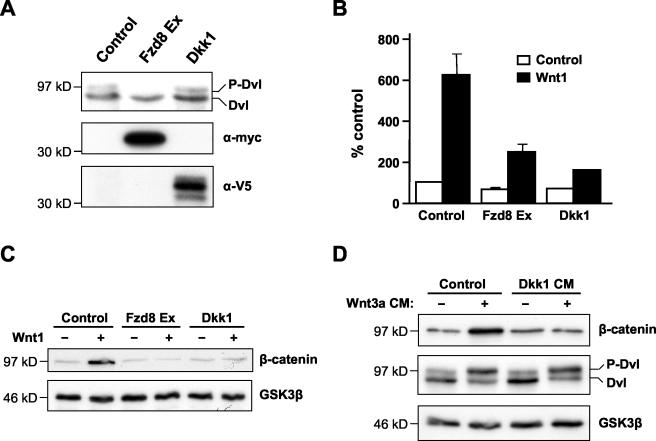
Dickkopf (Dkk) proteins constitute a family of extracellular modulators of Wnt signaling which display high-affinity interactions with LRP5 and -6. It has been shown by several groups that Dkk1 binding to LRP5/6 specifically inhibits Wnt/β-catenin signaling (6, 49, 71). Our results suggested that Dvl hyperphosphorylation is independent of LRP5 or -6 receptors. If so, we reasoned that it should not be blocked by Dkk1. To test this hypothesis, we transiently transfected 293T cells with plasmids encoding either Dkk1 or Fzd8 Ex as a positive control. While Fzd8 Ex blocked basal Dvl phosphorylation, Dkk1 did not (Fig. 7A). However, in a parallel experiment both constructs were able to inhibit Wnt1-induced β-catenin stabilization and β-catenin/TCF-dependent transcription (Fig. 7B and C). We also found that the ability of Wnt3a CM to induce Dvl phosphorylation in 10T1/2 cells was unaffected by treatment of the cells with Dkk1 CM, while the same Dkk1 treatment completely blocked the stabilization of cytosolic β-catenin triggered by Wnt3a CM (Fig. 7D). Collectively, these data indicate that Dvl hyperphosphorylation is independent of LRP5/6 protein signaling and imply that other Wnt receptor components, such as Fzds, are responsible for transducing Wnt signals that result in Dvl phosphorylation.
FIG. 7.
Dkk1 inhibits the Wnt/β-catenin pathway but does not block Dvl phosphorylation. (A) Dkk1 does not inhibit endogenous Dvl phosphorylation. 293T cells were transfected with vectors encoding either Dkk1 or Fzd8 Ex, and Dvl phosphorylation was analyzed by Western blotting. Expression of epitope-tagged Dkk1 and Fzd8 Ex was confirmed using anti-V5 and anti-Myc antibodies, respectively. (B) Dkk1 inhibits TCF/β-catenin-dependent transcription. 293T cells were transfected with vectors encoding Dkk1 or Fzd8 Ex, together with pTOPflash and pRL-TK. These cells were then cocultured with Rat2 cells stably expressing either Wnt1 (black bars) or empty vector (white bars). Luciferase assays were performed as described for Fig. 5C. Results shown are the mean + standard deviation of six replicates. (C) Dkk1 inhibits Wnt1-dependent β-catenin stabilization. 293T cells were transfected either with empty vector or vector encoding Wnt1, in the absence or presence of vectors encoding Dkk1 or Fzd8 Ex. Cytosolic β-catenin was analyzed by Western blotting as described before, and GSK3β was used as a loading control. (D) Dkk1 inhibits Wnt3a-mediated β-catenin stabilization but not Dvl phosphorylation. 10T1/2 cells were pretreated for 2 h with either control or Dkk1 CM, and this was then supplemented with control or Wnt3a CM for a further 2 h. Cell extracts were analyzed for β-catenin levels and Dvl phosphorylation as described above.
Activated LRP6/Arrow can stabilize β-catenin/Armadillo without a detectable change in Dvl/Dsh phosphorylation.
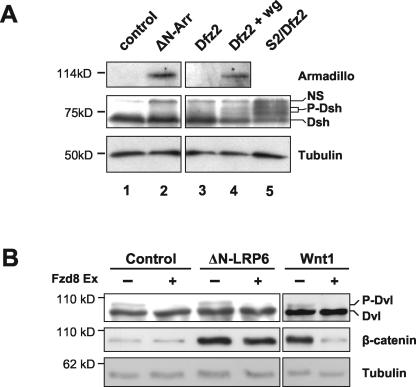
Several groups have shown that activated forms of LRP5, LRP6, or their Drosophila homolog Arrow, can be generated by deletion of their extracellular domain and that such activated mutants can initiate canonical Wnt/β-catenin signaling in transfected cells (11, 48-50, 69). The above results indicating that Wnt-induced Dvl phosphorylation is independent of LRP5/6 led to a prediction that activated forms of LRP5/6/Arrow may be able to stabilize β-catenin without affecting Dvl phosphorylation. To test this, we first turned to Drosophila S2 cells, a cell line in which N-terminally truncated Arrow (ΔN-Arr) can stabilize the β-catenin homolog Armadillo in a ligand-independent and Frizzled-independent manner (11). While coexpression of Dfrizzled2 and Wingless led to detection of hyperphosphorylated forms of Dishevelled in S2 cells, we did not observe equivalent phosphorylated forms in cells expressing ΔN-Arr (Fig. 8A).
FIG. 8.
Activated LRP6/Arrow can stabilize β-catenin/Armadillo without an apparent change in Dvl/Dsh phosphorylation. (A) A truncated form of Drosophila Arrow (ΔN-Arr) activates signaling to Armadillo (Arm) in S2 cells with no evidence of Dishevelled (Dsh) phosphorylation. Drosophila S2 cells were transiently transfected with control vector (lane 1), ΔN-Arr (lane 2), Dfrizzled2 (Dfz2; lane 3), or Dfz2 plus Wingless (Wg; lane 4). Cellular levels of Arm and phosphorylation of Dsh were analyzed by Western blotting, and β-tubulin provided a loading control. Dishevelled is constitutively phosphorylated in S2 cells stably transfected with Dfz2 (lane 5), allowing identification of two bands corresponding to phosphorylated Dsh. These lie below a nonspecific background band (labeled NS) in each lane. While Wg induces Arm stabilization and Dsh phosphorylation (lane 4), ΔN-Arr induces Arm stabilization only (lane 2). (B) ΔN-LRP6 causes stabilization of β-catenin but does not induce Dvl phosphorylation. 293T cells were transiently transfected with ΔN-LRP6, Wnt1, or control vector, in the presence or absence of Fzd8 Ex. Whole-cell and cytosolic extracts were analyzed for Dvl phosphorylation and β-catenin levels, respectively. Tubulin levels provided a loading control. Fzd8 Ex abolishes basal Dvl phosphorylation in all cases but does not prevent β-catenin stabilization in ΔN-LRP6-transfected cells.
To confirm this finding in a mammalian cell system, we performed similar experiments using human 293T cells. Since these cells display a detectable level of endogenous phospho-Dishevelled, we coexpressed the soluble Wnt-inhibitory protein Fzd8 Ex to reduce this background, as in Fig. 5B. This prevented Wnt1 from signaling to β-catenin but, as expected, equivalent signaling by ΔN-LRP6 was unaffected (Fig. 8B). Importantly, expression of ΔN-LRP6 resulted in stabilization of β-catenin without detectable phosphorylation of Dvl (Fig. 8B). Together these results support the notion that constitutively active forms of LRP5/6/Arrow can initiate signaling that stabilizes β-catenin without an apparent change in the phosphorylation state of Dishevelled.
DISCUSSION
In this paper we have shown that Wnt proteins induce hyperphosphorylation of Dishevelled proteins in mammalian cells, leading to a mobility shift that is detectable within 30 min. Certain Wnts, such as Wnt1 and Wnt3a, concurrently activate the canonical Wnt signaling pathway, leading to stabilization of β-catenin. In contrast, Wnt5a induces phosphorylation of Dvl but has no effect on β-catenin. Thus, Dvl phosphorylation appears to be a common signaling consequence of several Wnt proteins, regardless of their capacity to stabilize β-catenin. Unlike Wnt signaling to β-catenin, we show that the ability to phosphorylate Dvl is independent of the Wnt coreceptor LRP5/6. These data indicate that certain Wnt proteins are capable of stimulating two distinct signaling pathways simultaneously.
Dishevelled in canonical Wnt signaling.
The segment polarity gene product Dishevelled (Dsh) is required for patterning of the Drosophila embryonic epidermis by the Wnt protein Wingless (36). This process is a classic example of canonical Wnt signaling regulating development (16). The mammalian homologs of Dishevelled, Dvl1, -2, and -3, are similarly implicated in canonical Wnt/β-catenin signaling in vertebrates (42, 78). While genetic data and other studies place Dvl/Dsh as a key cytoplasmic intermediate between Wnt receptors and the Axin-APC complex that regulates β-catenin stability (55, 76, 78), it is not clear whether biochemical modification of Dvl protein is a requirement for signal transmission. Wnt-induced phosphorylation of Dsh was first reported by Yanagawa et al., who showed that Wingless caused increased phosphorylation of Dsh in Drosophila cells and that this correlated with the accumulation of Armadillo (101). Subsequently, three distinct kinases have been described which are able to phosphorylate Dsh/Dvl and whose activity has been implicated to various extents in Wnt signal transduction (93). Both casein kinase I (CKI) and CK2 have been shown to associate with and phosphorylate Dsh in vitro and in vivo (61, 94). A third candidate Dsh/Dvl kinase is PAR-1, also known as EMK-1 and MARK2 (7, 21, 27). PAR-1 can phosphorylate Dsh/Dvl in vitro and is physically associated with Dsh, and its kinase activity is modulated by treatment of Drosophila cells with Wg protein (81).
Overexpression of these putative Dvl kinases can elevate β-catenin levels and induce secondary axis formation in Xenopus embryos (61, 67) and potentiate β-catenin-LEF1-dependent transcription in mammalian cells (81). Together with the phosphorylation of Dvl by Wnt ligand-initiated signaling, these observations link the induction of Dvl phosphorylation with stabilization of β-catenin and raise the possibility that phosphorylation is the means by which Dvl is activated in canonical Wnt signal transduction downstream of the Wnt receptor complex. This notion is supported by evidence that kinase-defective mutants of the putative Dvl kinases, or inhibitory RNA-derived mutant phenocopies, can inhibit canonical Wnt signaling in vitro and in vivo (61, 67, 81). The data we present here confirm that Wnt proteins such as Wnt1 and Wnt3a, which mediate canonical β-catenin signaling, are able to induce Dvl phosphorylation. In addition, however, such phosphorylation was induced by Wnt5a and Wnt11, proteins that typically do not result in signaling to β-catenin. Thus, our data indicate that Wnt-induced Dvl phosphorylation is not sufficient to mediate β-catenin stabilization.
Despite the ability of activated forms of LRP6 to stabilize β-catenin without a detectable increase in Dvl phosphorylation, it remains possible that phosphorylation of Dvl might be an obligatory step in canonical signaling mediated by Wnt ligands. The kinetics of this phosphorylation, as judged by mobility shift assays, were slower than might be expected for a proximal event required upstream of β-catenin stabilization, which is thought to begin within minutes of Wnt stimulation (50, 87). Although not definitive, these data do not favor a receptor-proximal role for Dvl phosphorylation in a simple linear pathway of signaling to β-catenin. Using LiCl to inhibit GSK3β activity, we also tested the possibility that Dvl phosphorylation might occur downstream of β-catenin stabilization, but no evidence of this was observed.
Current models of Wnt signal transduction involve formation of a ternary complex between a Wnt ligand, a member of the Frizzled family of transmembrane proteins, and either the LDL receptor-related protein LRP6 or the related protein LRP5 (11, 50, 83, 103). While Wnt-Frizzled interactions may also be involved in noncanonical Wnt signaling events, the LRP5/6 moiety appears to be specifically required for the canonical pathway and may be an intracellular signal-initiating component of the receptor complex. The secreted protein Dkk1 binds to LRP5 and LRP6 and acts as a specific inhibitor of Wnt-LRP signaling (6, 49, 71). We show here that while Wnt-induced phosphorylation of Dvl is blocked by a dominant-negative Frizzled, it is unaffected by either Dkk1 or DN LRP6, each of which abolish Wnt-mediated signaling to β-catenin. In addition, activated forms of LRP6/Arrow stabilized β-catenin without inducing a detectable shift in Dvl mobility. Collectively, these results indicate that Wnt-induced Dvl phosphorylation is LRP independent and thus is achieved through a signal transduction event distinct from that leading to stabilization of β-catenin.
Data from other systems are consistent with the above notion that Dvl phosphorylation is uncoupled from β-catenin stabilization. For example, overexpression of Fz or Dfz2 in Drosophila S2 cells leads to hyperphosphorylation of Dsh, but this is not sufficient to stabilize the β-catenin homolog Armadillo (8, 56, 86, 94). In addition, a recent study of signaling by rat Fzd9 overexpressed in mammalian cells showed that certain Fzd9 mutants induce Dvl1 phosphorylation but not stabilization of β-catenin (34).
Dishevelled in noncanonical Wnt signaling.
There is extensive evidence that Wnt5a and other family members are capable of signaling via β-catenin-independent, noncanonical pathways (16, 29, 40, 52, 54). Components of one such pathway appear to be required both for PCP in Drosophila tissues and for CE movements during vertebrate embryogenesis (1, 35, 53). Genetic evidence in the fly demonstrates that Dsh/Dvl, as well as Frizzled, is required for these patterning events (1, 38). It is therefore possible that Dvl phosphorylation is an intrinsic component of such a pathway. Indeed, PCP signaling in Drosophila results in phosphorylation of Dsh (3, 5), and it has been reported that Dvl phosphorylation in Xenopus correlates with CE but not with axis duplication, a β-catenin-mediated process (65). The latter observation may appear surprising in view of the reports discussed above that associate Dvl phosphorylation with canonical Wnt signaling in Xenopus (61, 67, 81).
There are several ways to reconcile the apparent conflict between data that associate Dvl phosphorylation with canonical (Wnt/β-catenin) signaling and those that associate it with noncanonical (Wnt/PCP) signaling. First, a potential source of confusion is that some of the Dvl kinases may be capable of pleiotropic effects on other components of the Wnt/β-catenin pathway. For example, CKI has been shown to phosphorylate Axin, APC, β-catenin, and TCF3, as well as Dvl, leading in most cases to destabilization of β-catenin and inhibition of canonical signaling (2, 24, 41, 47, 51, 66, 100). Thus, experiments in which kinase expression leads to Dvl phosphorylation and altered canonical signaling may be confounded by the promiscuity of the kinases. A second possibility is that distinct kinases and/or distinct phosphorylation sites on Dvl may be involved in phosphorylation of Dvl in the different Wnt pathways. Although this is difficult to exclude completely, the forms of Dvl phosphorylated in response to canonical and noncanonical Wnts showed the same electrophoretic mobility in our experiments, and stimulation by both classes of Wnt simultaneously did not result in any further shift in mobility (unpublished data). Moreover, our experiments with a monoclonal antibody whose epitope on Dvl2 is masked by phosphorylation indicate that at least one of the sites of Wnt-induced Dvl modification is common to both classes of Wnt signal.
In light of these observations, we favor a model in which phosphorylation of Dsh/Dvl is a common feature of more than one Wnt-induced signaling pathway and may be necessary but not sufficient for downstream signals in each case. Indeed, an empirical conclusion from our study is that Dvl phosphorylation is a common cellular response to Wnt proteins, regardless of whether they elicit canonical or noncanonical signals. The ability of Dkk1 to inhibit both β-catenin stabilization and TCF-dependent transactivation but not Dvl phosphorylation demonstrates that Wnts which stabilize β-catenin, such as Wnt1 and Wnt3a, signal both via an LRP-dependent mechanism (to stabilize β-catenin) and an LRP-independent one (to phosphorylate Dvl). This important finding supports the possibility that most Wnt proteins have phenotypic consequences beyond those mediated by β-catenin.
General model of Wnt signaling.
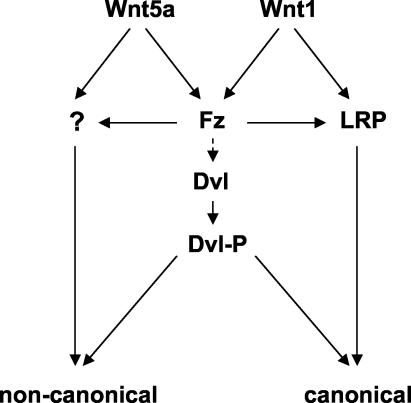
As discussed above, the transmembrane proteins LRP5 or LRP6 are essential components of the Wnt/β-catenin signaling pathway, in addition to the Frizzled component of Wnt receptors. It has been reported that Wnts induce the binding of LRP5/6 to Axin, leading to Axin degradation and β-catenin stabilization (50, 84). Moreover, truncated forms of LRP5 or -6 lacking the extracellular domain can act as constitutively active signaling molecules, activating the β-catenin pathway in a ligand-independent and Frizzled-independent manner (11, 49, 50, 84). Recent data suggest that Dvl is not required for signaling from LRP6, although it is necessary for Wnt ligand-mediated canonical signaling (45). This implies that in Wnt-mediated canonical signaling, Dvl must either function upstream of LRP5/6 or else in a parallel pathway. This model is consistent with our observations that Dvl phosphorylation occurs through an LRP-independent mechanism, that truncated LRP6/Arrow can signal to β-catenin without phosphorylating Dvl, and that Wnt-induced Dvl phosphorylation is not sufficient for β-catenin stabilization. If phosphorylation of Dvl is required for Wnt-mediated signaling, we speculate that it constitutes part of a parallel pathway that modulates signaling to either the canonical or noncanonical pathways but is not sufficient for either. A diagram illustrating this model is shown in Fig. 9.
FIG. 9.
Model illustrating Dvl phosphorylation as a common response to Wnt proteins that activate distinct signaling pathways. Wnt signaling via the canonical β-catenin pathway requires both Fzd and LRP5/6, while noncanonical Wnt signaling requires Fzd and possibly additional receptors as yet uncharacterized. For simplicity, we have indicated that signals leading to phosphorylation of Dvl are derived from Fzd alone. We propose that Dvl phosphorylation is a common response to both the Wnt1 class and Wnt5a class of Wnt proteins and that it potentiates Wnt signaling via either the canonical or noncanonical pathways. Which of these pathways dominates in a given circumstance may depend on the involvement of Wnt coreceptor components in addition to Fzd and possibly on the subcellular distribution of Dvl. The reported association between different subcellular locations of Dvl protein and different signaling pathways suggests that this might be a key determinant (17).
Dvl phosphorylation as an assay for noncanonical Wnt signaling.
Irrespective of its precise role in canonical and/or noncanonical Wnt signaling, the phosphorylation of Dvl by Wnt signals provides a direct and consistent biochemical assay for Wnt proteins that do not stabilize β-catenin in cell culture. Although several potential assays for noncanonical Wnt signaling are suggested by studies of such signaling in whole embryos and other systems (39, 72, 74), few such assays have been shown to be consistently applicable in mammalian cell culture. It has been reported that Wnt5a can elicit modest JNK activation in NIH 3T3 fibroblasts (99), but we have been unsuccessful in detecting Wnt5a-mediated activation of this kinase in Rat2 or 10T1/2 cells, in which Wnt5a clearly induced Dvl phosphorylation (unpublished data). Since phosphorylation of Dvl is a response to extracellular Wnt proteins, this assay should facilitate identification of specific receptor components that mediate the relevant signal transduction. It will also be important to determine which kinase(s) is responsible for Wnt5a-induced Dvl phosphorylation and how such kinases are activated by Wnt5a receptor components. The recent implication of Wnt5a signaling in the invasive properties of melanoma underscores just one example of the importance of understanding the molecular basis of signal transduction by this poorly understood class of Wnt proteins (92).
Acknowledgments
This work was supported by NIH grant CA47207 (to A.M.C.B.), a fellowship from the Ministerio de Educacion, Cultura, y Deportes of Spain (to J.M.G.-S.), U.S. Army Medical Research and Materiel Command fellowships DAMD17-99-1-9388 (to K.B.) and DAMD17-02-1-0359 (to L.A.C.-S.), NIH MSTP grant GM67739, and funding from GlaxoSmithKline Pharmaceuticals, Inc.
We acknowledge Mikhail Semenov for contributions to an early phase of these studies and a role in producing one of the antibodies used. We are grateful to Tamara Weissman and Ana Chavarri for technical assistance, to Alberto Muñoz for reagents and advice, and to Louise Howe, Anne Muesch, Maya Elbert, and Paul Wilson, for helpful discussions.
REFERENCES
- 1.Adler, P. N. 2002. Planar signaling and morphogenesis in Drosophila. Dev. Cell 2:525-535. [DOI] [PubMed] [Google Scholar]
- 2.Amit, S., A. Hatzubai, Y. Birman, J. S. Andersen, E. Ben-Shushan, M. Mann, Y. Ben-Neriah, and I. Alkalay. 2002. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 16:1066-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Axelrod, J. D. 2001. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 15:1182-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axelrod, J. D., K. Matsuno, S. Artavanis-Tsakonas, and N. Perrimon. 1996. Interaction between Wingless and Notch signaling pathways mediated by Dishevelled. Science 271:1826-1832. [DOI] [PubMed] [Google Scholar]
- 5.Axelrod, J. D., J. R. Miller, J. M. Shulman, R. T. Moon, and N. Perrimon. 1998. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 12:2610-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bafico, A., G. Liu, A. Yaniv, A. Gazit, and S. A. Aaronson. 2001. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat. Cell Biol. 3:683-686. [DOI] [PubMed] [Google Scholar]
- 7.Bessone, S., F. Vidal, Y. Le Bouc, J. Epelbaum, M. T. Bluet-Pajot, and M. Darmon. 1999. EMK protein kinase-null mice: dwarfism and hypofertility associated with alterations in the somatotrope and prolactin pathways. Dev Biol. 214:87-101. [DOI] [PubMed] [Google Scholar]
- 8.Bhanot, P., M. Brink, C. H. Samos, J. C. Hsieh, Y. Wang, J. P. Macke, D. Andrew, J. Nathans, and R. Nusse. 1996. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 382:225-230. [DOI] [PubMed] [Google Scholar]
- 9.Boutros, M., and M. Mlodzik. 1999. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech. Dev. 83:27-37. [DOI] [PubMed] [Google Scholar]
- 10.Boutros, M., N. Paricio, D. I. Strutt, and M. Mlodzik. 1998. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94:109-118. [DOI] [PubMed] [Google Scholar]
- 11.Brennan, K., J. M. Gonzalez-Sancho, L. A. Castelo-Soccio, L. R. Howe, and A. M. C. Brown. Truncated mutants of the putative Wnt receptor LRP6/Arrow can stabilize beta-catenin independently of Frizzled proteins. Oncogene, in press. [DOI] [PMC free article] [PubMed]
- 12.Brown, A. M. C. 2001. Wnt signaling in breast cancer: have we come full circle? Breast Cancer Res. 3:351-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown, A. M. C., J. Papkoff, Y. K. T. Fung, G. M. Shackleford, and H. E. Varmus. 1987. Identification of protein products encoded by the proto-oncogene int-1. Mol. Cell. Biol. 7:3971-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown, A. M. C., R. A. Wildin, T. J. Prendergast, and H. E. Varmus. 1986. A retrovirus vector expressing the putative mammary oncogene int-1 causes partial transformation of a mammary epithelial cell line. Cell 46:1001-1009. [DOI] [PubMed] [Google Scholar]
- 15.Brown, S. D., R. C. Twells, P. J. Hey, R. D. Cox, E. R. Levy, A. R. Soderman, M. L. Metzker, C. T. Caskey, J. A. Todd, and J. F. Hess. 1998. Isolation and characterization of LRP6, a novel member of the low density lipoprotein receptor gene family. Biochem. Biophys. Res. Commun. 248:879-888. [DOI] [PubMed] [Google Scholar]
- 16.Cadigan, K. M., and R. Nusse. 1997. Wnt signaling: a common theme in animal development. Genes Dev. 11:3286-3305. [DOI] [PubMed] [Google Scholar]
- 17.Capelluto, D. G., T. G. Kutateladze, R. Habas, C. V. Finkielstein, X. He, and M. Overduin. 2002. The DIX domain targets dishevelled to actin stress fibres and vesicular membranes. Nature 419:726-729. [DOI] [PubMed] [Google Scholar]
- 18.Chan, S. D., D. B. Karpf, M. E. Fowlkes, M. Hooks, M. S. Bradley, V. Vuong, T. Bambino, M. Y. Liu, C. D. Arnaud, G. J. Strewler, et al. 1992. Two homologs of the Drosophila polarity gene frizzled (fz) are widely expressed in mammalian tissues. J. Biol. Chem. 267:25202-25207. [PubMed] [Google Scholar]
- 19.Crnogorac-Jurcevic, T., E. Efthimiou, P. Capelli, E. Blaveri, A. Baron, B. Terris, M. Jones, K. Tyson, C. Bassi, A. Scarpa, and N. R. Lemoine. 2001. Gene expression profiles of pancreatic cancer and stromal desmoplasia. Oncogene 20:7437-7446. [DOI] [PubMed] [Google Scholar]
- 20.Dale, T. C. 1998. Signal transduction by the Wnt family of ligands. Biochem. J. 329:209-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drewes, G., A. Ebneth, U. Preuss, E. M. Mandelkow, and E. Mandelkow. 1997. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell 89:297-308. [DOI] [PubMed] [Google Scholar]
- 22.Du, S. J., S. M. Purcell, J. L. Christian, and R. T. Moon. 1995. Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Mol. Cell. Biol. 15:2625-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eastman, Q., and R. Grosschedl. 1999. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr. Opin. Cell Biol. 11:233-240. [DOI] [PubMed] [Google Scholar]
- 24.Gao, Z. H., J. M. Seeling, V. Hill, A. Yochum, and D. M. Virshup. 2002. Casein kinase I phosphorylates and destabilizes the beta-catenin degradation complex. Proc. Natl. Acad. Sci. USA 99:1182-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giarre, M., M. V. Semenov, and A. M. C. Brown. 1998. Wnt signaling stabilizes the dual function protein beta-catenin in diverse cell types. Ann. N. Y. Acad. Sci. 857:43-55. [DOI] [PubMed] [Google Scholar]
- 26.Giles, R. H., J. H. van Es, and H. Clevers. 2003. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta 1653:1-24. [DOI] [PubMed] [Google Scholar]
- 27.Guo, S., and K. J. Kemphues. 1995. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell 81:611-620. [DOI] [PubMed] [Google Scholar]
- 28.Hedgepeth, C. M., L. J. Conrad, J. Zhang, H. C. Huang, V. M. Lee, and P. S. Klein. 1997. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev. Biol. 185:82-91. [DOI] [PubMed] [Google Scholar]
- 29.Heisenberg, C. P., M. Tada, G. J. Rauch, L. Saude, M. L. Concha, R. Geisler, D. L. Stemple, J. C. Smith, and S. W. Wilson. 2000. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405:76-81. [DOI] [PubMed] [Google Scholar]
- 30.Hey, P. J., R. C. Twells, M. S. Phillips, N. Yusuke, S. D. Brown, Y. Kawaguchi, R. Cox, X. Guochun, V. Dugan, H. Hammond, M. L. Metzker, J. A. Todd, and J. F. Hess. 1998. Cloning of a novel member of the low-density lipoprotein receptor family. Gene 216:103-111. [DOI] [PubMed] [Google Scholar]
- 31.Howe, L. R., H. C. Crawford, K. Subbaramaiah, J. A. Hassell, A. J. Dannenberg, and A. M. C. Brown. 2001. PEA3 is up-regulated in response to Wnt1 and activates the expression of cyclooxygenase-2. J. Biol. Chem. 276:20108-20115. [DOI] [PubMed] [Google Scholar]
- 32.Hsieh, J. C., A. Rattner, P. M. Smallwood, and J. Nathans. 1999. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc. Natl. Acad. Sci. USA 96:3546-3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iozzo, R. V., I. Eichstetter, and K. G. Danielson. 1995. Aberrant expression of the growth factor Wnt-5a in human malignancy. Cell Regul. 55:3495-3499. [PubMed] [Google Scholar]
- 34.Karasawa, T., H. Yokokura, J. Kitajewski, and P. J. Lombroso. 2002. Frizzled-9 is activated by Wnt-2 and functions in Wnt/beta-catenin signaling. J. Biol. Chem. 277:37479-37486. [DOI] [PubMed] [Google Scholar]
- 35.Kilian, B., H. Mansukoski, F. C. Barbosa, F. Ulrich, M. Tada, and C. P. Heisenberg. 2003. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech. Dev. 120:467-476. [DOI] [PubMed] [Google Scholar]
- 36.Klingensmith, J., R. Nusse, and N. Perrimon. 1994. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev. 8:118-130. [DOI] [PubMed] [Google Scholar]
- 37.Korinek, V., N. Barker, P. J. Morin, D. van Wichen, R. de Weger, K. W. Kinzler, B. Vogelstein, and H. Clevers. 1997. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 275:1784-1787. [DOI] [PubMed] [Google Scholar]
- 38.Krasnow, R. E., L. L. Wong, and P. N. Adler. 1995. Dishevelled is a component of the frizzled signaling pathway in Drosophila. Development 121:4095-4102. [DOI] [PubMed] [Google Scholar]
- 39.Kuhl, M., L. C. Sheldahl, C. C. Malbon, and R. T. Moon. 2000. Ca2+/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J. Biol. Chem. 275:12701-12711. [DOI] [PubMed] [Google Scholar]
- 40.Kuhl, M., L. C. Sheldahl, M. Park, J. R. Miller, and R. T. Moon. 2000. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 16:279-283. [DOI] [PubMed] [Google Scholar]
- 41.Lee, E., A. Salic, and M. W. Kirschner. 2001. Physiological regulation of beta-catenin stability by Tcf3 and CK1epsilon. J. Cell Biol. 154:983-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee, J. S., A. Ishimoto, and S. Yanagawa. 1999. Characterization of mouse dishevelled (Dvl) proteins in Wnt/Wingless signaling pathway. J. Biol. Chem. 274:21464-21470. [DOI] [PubMed] [Google Scholar]
- 43.Lejeune, S., E. L. Huguet, A. Hamby, R. Poulsom, and A. L. Harris. 1995. Wnt5a cloning, expression, and up-regulation in human primary breast cancers. Clin. Cancer Res. 1:215-222. [PubMed] [Google Scholar]
- 44.Leyns, L., T. Bouwmeester, S.-H. Kim, S. Piccolo, and E. M. DeRobertis. 1997. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell 88:747-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li, L., J. Mao, L. Sun, W. Liu, and D. Wu. 2002. Second cysteine-rich domain of Dickkopf-2 activates canonical Wnt signaling pathway via LRP-6 independently of dishevelled. J. Biol. Chem. 277:5977-5981. [DOI] [PubMed] [Google Scholar]
- 46.Li, L., H. Yuan, W. Xie, J. Mao, A. M. Caruso, A. McMahon, D. J. Sussman, and D. Wu. 1999. Dishevelled proteins lead to two signaling pathways. Regulation of LEF-1 and c-Jun N-terminal kinase in mammalian cells. J. Biol. Chem. 274:129-134. [DOI] [PubMed] [Google Scholar]
- 47.Liu, C., Y. Li, M. Semenov, C. Han, G. H. Baeg, Y. Tan, Z. Zhang, X. Lin, and X. He. 2002. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108:837-847. [DOI] [PubMed] [Google Scholar]
- 48.Liu, G., A. Bafico, V. K. Harris, and S. A. Aaronson. 2003. A novel mechanism for Wnt activation of canonical signaling through the LRP6 receptor. Mol. Cell. Biol. 23:5825-5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mao, B., W. Wu, Y. Li, D. Hoppe, P. Stannek, A. Glinka, and C. Niehrs. 2001. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 411:321-325. [DOI] [PubMed] [Google Scholar]
- 50.Mao, J., J. Wang, B. Liu, W. Pan, G. H. Farr III, C. Flynn, H. Yuan, S. Takada, D. Kimelman, L. Li, and D. Wu. 2001. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell 7:801-809. [DOI] [PubMed] [Google Scholar]
- 51.McKay, R. M., J. M. Peters, and J. M. Graff. 2001. The casein kinase I family in Wnt signaling. Dev. Biol. 235:388-396. [DOI] [PubMed] [Google Scholar]
- 52.Miller, J. R. 2002. The Wnts. Genome Biol. 3:reviews 3001.1-3001.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mlodzik, M. 2002. Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 18:564-571. [DOI] [PubMed] [Google Scholar]
- 54.Moon, R. T., R. M. Campbell, J. L. Christian, L. L. McGrew, J. Shih, and S. Fraser. 1993. Xwnt-5A: a maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development 119:97-111. [DOI] [PubMed] [Google Scholar]
- 55.Noordermeer, J., J. Klingensmith, N. Perrimon, and R. Nusse. 1994. Dishevelled and Armadillo act in the Wingless signalling pathway in Drosophila. Nature 367:80-83. [DOI] [PubMed] [Google Scholar]
- 56.Nusse, R., C. H. Samos, M. Brink, K. Willert, K. M. Cadigan, A. Wodarz, M. Fish, and E. Rulifson. 1997. Cell culture and whole animal approaches to understanding signaling by Wnt proteins in Drosophila. Cold Spring Harbor Symp. Quant. Biol. 62:185-190. [PubMed] [Google Scholar]
- 57.Nusse, R., and H. E. Varmus. 1982. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31:99-109. [DOI] [PubMed] [Google Scholar]
- 58.Nusse, R., and H. E. Varmus. 1992. Wnt genes. Cell 69:1073-1087. [DOI] [PubMed] [Google Scholar]
- 59.Pandur, P., M. Lasche, L. M. Eisenberg, and M. Kuhl. 2002. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature 418:636-641. [DOI] [PubMed] [Google Scholar]
- 60.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peters, J. M., R. M. McKay, J. P. McKay, and J. M. Graff. 1999. Casein kinase I transduces Wnt signals. Nature 401:345-350. [DOI] [PubMed] [Google Scholar]
- 62.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 14:1837-1851. [PubMed] [Google Scholar]
- 63.Rauch, G. J., M. Hammerschmidt, P. Blader, H. E. Schauerte, U. Strahle, P. W. Ingham, A. P. McMahon, and P. Haffter. 1997. Wnt5 is required for tail formation in the zebrafish embryo. Cold Spring Harbor Symp. Quant. Biol. 62:227-234. [PubMed] [Google Scholar]
- 64.Rijsewijk, F., M. Schuerman, E. Wagenaar, P. Parren, D. Weigel, and R. Nusse. 1987. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell 50:649-657. [DOI] [PubMed] [Google Scholar]
- 65.Rothbacher, U., M. N. Laurent, M. A. Deardorff, P. S. Klein, K. W. Cho, and S. E. Fraser. 2000. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J. 19:1010-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rubinfeld, B., D. A. Tice, and P. Polakis. 2001. Axin-dependent phosphorylation of the adenomatous polyposis coli protein mediated by casein kinase 1epsilon. J. Biol. Chem. 276:39037-39045. [DOI] [PubMed] [Google Scholar]
- 67.Sakanaka, C., P. Leong, L. Xu, S. D. Harrison, and L. T. Williams. 1999. Casein kinase 1 epsilon in the wnt pathway: regulation of beta-catenin function. Proc. Natl. Acad. Sci. USA 96:12548-12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saneyoshi, T., S. Kume, Y. Amasaki, and K. Mikoshiba. 2002. The Wnt/calcium pathway activates NF-AT and promotes ventral cell fate in Xenopus embryos. Nature 417:295-299. [DOI] [PubMed] [Google Scholar]
- 69.Schweizer, L., and H. Varmus. 2003. Wnt/Wingless signaling through beta-catenin requires the function of both LRP/Arrow and frizzled classes of receptors. BMC Cell Biol. 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Semenov, M. V., and M. Snyder. 1997. Human dishevelled genes constitute a DHR-containing multigene family. Genomics 42:302-310. [DOI] [PubMed] [Google Scholar]
- 71.Semenov, M. V., K. Tamai, B. K. Brott, M. Kuhl, S. Sokol, and X. He. 2001. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. 11:951-961. [DOI] [PubMed] [Google Scholar]
- 72.Sheldahl, L. C., M. Park, C. C. Malbon, and R. T. Moon. 1999. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr. Biol. 9:695-698. [DOI] [PubMed] [Google Scholar]
- 73.Shimizu, H., M. A. Julius, M. Giarre, Z. Zheng, A. M. Brown, and J. Kitajewski. 1997. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ. 8:1349-1358. [PubMed] [Google Scholar]
- 74.Slusarski, D. C., V. G. Corces, and R. T. Moon. 1997. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 390:410-413. [DOI] [PubMed] [Google Scholar]
- 75.Smalley, M. J., and T. C. Dale. 1999. Wnt signalling in mammalian development and cancer. Cancer Metastasis Rev. 18:215-230. [DOI] [PubMed] [Google Scholar]
- 76.Smalley, M. J., E. Sara, H. Paterson, S. Naylor, D. Cook, H. Jayatilake, L. G. Fryer, L. Hutchinson, M. J. Fry, and T. C. Dale. 1999. Interaction of axin and Dvl-2 proteins regulates Dvl-2-stimulated TCF-dependent transcription. EMBO J. 18:2823-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sokol, S., J. L. Christian, R. T. Moon, and D. A. Melton. 1991. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell 67:741-752. [DOI] [PubMed] [Google Scholar]
- 78.Sokol, S. Y. 1996. Analysis of Dishevelled signalling pathways during Xenopus development. Curr. Biol. 6:1456-1467. [DOI] [PubMed] [Google Scholar]
- 79.Strutt, D., R. Johnson, K. Cooper, and S. Bray. 2002. Asymmetric localization of frizzled and the determination of notch-dependent cell fate in the Drosophila eye. Curr. Biol. 12:813-824. [DOI] [PubMed] [Google Scholar]
- 80.Strutt, D. I., U. Weber, and M. Mlokzik. 1997. The role of RhoA in tissue polarity and frizzled signaling. Nature 387:292-295. [DOI] [PubMed] [Google Scholar]
- 81.Sun, T. Q., B. Lu, J. J. Feng, C. Reinhard, Y. N. Jan, W. J. Fantl, and L. T. Williams. 2001. PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nat. Cell Biol. 3:628-636. [DOI] [PubMed] [Google Scholar]
- 82.Sussman, D. J., J. Klingensmith, P. Salinas, P. S. Adams, R. Nusse, and N. Perrimon. 1994. Isolation and characterization of a mouse homolog of the Drosophila segment polarity gene dishevelled. Dev. Biol. 166:73-86. [DOI] [PubMed] [Google Scholar]
- 83.Tamai, K., M. Semenov, Y. Kato, R. Spokony, C. Liu, Y. Katsuyama, F. Hess, J. P. Saint-Jeannet, and X. He. 2000. LDL-receptor-related proteins in Wnt signal transduction. Nature 407:530-535. [DOI] [PubMed] [Google Scholar]
- 84.Tamai, K., X. Zeng, C. Liu, X. Zhang, Y. Harada, Z. Chang, and X. He. 2004. A mechanism for Wnt coreceptor activation. Mol. Cell 13:149-156. [DOI] [PubMed] [Google Scholar]
- 85.Theisen, H., J. Purcell, M. Bennett, D. Kansagara, A. Syed, and J. L. Marsh. 1994. dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development 120:347-360. [DOI] [PubMed] [Google Scholar]
- 86.Tomlinson, A., W. R. Strapps, and J. Heemskerk. 1997. Linking Frizzled and Wnt signaling in Drosophila development. Development 124:4515-4521. [DOI] [PubMed] [Google Scholar]
- 87.Van Leeuwen, F., C. H. Samos, and R. Nusse. 1994. Biological activity of soluble wingless protein in cultured Drosophila imaginal disc cells. Nature 368:342-344. [DOI] [PubMed] [Google Scholar]
- 88.Vinson, C. R., S. Conover, and P. N. Adler. 1989. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature 338:263-264. [DOI] [PubMed] [Google Scholar]
- 89.Wallingford, J. B., B. A. Rowning, K. M. Vogeli, U. Rothbacher, S. E. Fraser, and R. M. Harland. 2000. Dishevelled controls cell polarity during Xenopus gastrulation. Nature 405:81-85. [DOI] [PubMed] [Google Scholar]
- 90.Wang, S., M. Krinks, K. Lin, F. P. Luyten, and M. Moos. 1997. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell 88:757-766. [DOI] [PubMed] [Google Scholar]
- 91.Wang, Y., J. P. Macke, B. S. Abella, K. Andreasson, P. Worley, D. J. Gilbert, N. G. Copeland, N. A. Jenkins, and J. Nathans. 1996. A large family of putative transmembrane receptors homologous to the product of the Drosophila tissue polarity gene frizzled. J. Biol. Chem. 271:4468-4476. [DOI] [PubMed] [Google Scholar]
- 92.Weeraratna, A. T., Y. Jiang, G. Hostetter, K. Rosenblatt, P. Duray, M. Bittner, and J. M. Trent. 2002. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 1:279-288. [DOI] [PubMed] [Google Scholar]
- 93.Wharton, K. A., Jr. 2003. Runnin' with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev. Biol. 253:1-17. [DOI] [PubMed] [Google Scholar]
- 94.Willert, K., M. Brink, A. Wodarz, H. Varmus, and R. Nusse. 1997. Casein kinase 2 associates with and phosphorylates dishevelled. EMBO J. 16:3089-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Willert, K., J. D. Brown, E. Danenberg, A. W. Duncan, I. L. Weissman, T. Reya, J. R. Yates III, and R. Nusse. 2003. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423:448-452. [DOI] [PubMed] [Google Scholar]
- 96.Willert, K., S. Shibamoto, and R. Nusse. 1999. Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev. 13:1768-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Winter, C. G., B. Wang, A. Ballew, A. Royou, R. Karess, J. D. Axelrod, and L. Luo. 2001. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105:81-91. [DOI] [PubMed] [Google Scholar]
- 98.Yamaguchi, T. P., A. Bradley, A. P. McMahon, and S. Jones. 1999. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126:1211-1223. [DOI] [PubMed] [Google Scholar]
- 99.Yamanaka, H., T. Moriguchi, N. Masuyama, M. Kusakabe, H. Hanafusa, R. Takada, S. Takada, and E. Nishida. 2002. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 3:69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yanagawa, S., Y. Matsuda, J. S. Lee, H. Matsubayashi, S. Sese, T. Kadowaki, and A. Ishimoto. 2002. Casein kinase I phosphorylates the Armadillo protein and induces its degradation in Drosophila. EMBO J. 21:1733-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yanagawa, S., F. Van Leeuwen, A. Wodarz, J. Klingensmith, and R. Nusse. 1995. The Dishevelled protein is modified by Wingless signaling in Drosophila. Genes Dev. 9:1087-1097. [DOI] [PubMed] [Google Scholar]
- 102.Yang, Y., N. Lijam, D. J. Sussman, and M. Tsang. 1996. Genomic organization of mouse Dishevelled genes. Gene 180:121-123. [DOI] [PubMed] [Google Scholar]
- 103.Zorn, A. M. 2001. Wnt signalling: antagonistic Dickkopfs. Curr. Biol. 11:R592-R595. [DOI] [PubMed] [Google Scholar]