Abstract
The first objective of this study was to determine the effects of physiological cyclic loading followed by unloaded recovery on the mechanical response of human intervertebral discs. The second objective was to examine how nucleotomy alters the disc’s mechanical response to cyclic loading. To complete these objectives, 15 human L5-S1 discs were tested while intact and subsequent to nucleotomy. The testing consisted of 10,000 cycles of physiological compressive loads followed by unloaded hydrated recovery. Cyclic loading increased compression modulus (3%) and strain (33%), decreased neutral zone modulus (52%), and increased neutral zone strain (31%). Degeneration was not correlated with the effect of cyclic loading in intact discs, but was correlated with cyclic loading effects after nucleotomy, with more degenerate samples experiencing greater increases in both compressive and neutral zone strain following cyclic loading. Partial removal of the nucleus pulposus decreased the compression and neutral zone modulus while increasing strain. These changes correspond to hypermobility, which will alter overall spinal mechanics and may impact low back pain via altered motion throughout the spinal column. Nucleotomy also reduced the effects of cyclic loading on mechanical properties, likely due to altered fluid flow, which may impact cellular mechanotransduction and transport of disc nutrients and waste. Degeneration was not correlated with the acute changes of nucleotomy. Results of this study provide an ideal protocol and control data for evaluating the effectiveness of a mechanically-based disc degeneration treatment, such as a nucleus replacement.
Keywords: Intervertebral Disc, Spine, Cyclic Loading, Nucleus Pulposus, Nucleotomy
Introduction
The intervertebral disc performs the mechanical roles of supporting loads, permitting motion, and dissipating energy. Disc degeneration is a strongly implicated cause of low back pain and within the U.S. results in annual costs over $100 billion (Katz, 2006). Degeneration is a multifaceted process that manifests early in the nucleus pulposus (NP) and subsequently extends to other disc components (Vernon Roberts et al., 2007). Despite disc degeneration prevalence, many current treatments are palliative only and ultimately fail (Andersson, 1999; Luo et al., 2004; Rajaee et al., 2012). Minimally invasive treatments of early disc degeneration, such as injectable NP replacements, are under development (Lewis, 2012; Malhotra et al., 2012; Reitmaier et al., 2012). For such mechanically based treatments to be successful they must replicate natural NP mechanical function. In healthy discs, proteoglycans create an osmotic pressure which draws fluid into the NP (Urban and McMullin, 1988). This fluid both directly supports compressive loads and places the collagen fibers of the annulus fibrosus in tension, permitting the disc to support loads while undergoing a wide range of motion.
Physiological cyclic compressive loading and unloaded recovery is an ideal testing modality for examining the loading contribution of the nucleus pulposus because it simulates the disc’s time-dependent mechanical response. The interplay between osmotic pressure and external loads causes 20–25% of the disc’s water content to be expressed and re imbibed daily (Sivan et al., 2006). Reduced hydration decreases disc height, increases stiffness, and increases range of motion, with properties returning to baseline after rehydration (Adams et al., 1990; Wilke et al., 1999). These diurnal changes have been replicated in several large animal models by compressive cyclic loading and unloaded recovery (Johannessen et al., 2004; Korecki et al., 2008; Thoreson et al., 2010). While animal and human discs are similar, these findings may not be representative of cyclic loading in human discs due to lower water and proteoglycan content in humans, varied applied loads and loading durations between studies, and differences in cartilage endplate (Beckstein et al., 2008; Demers et al., 2004; Showalter et al., 2012; Wilke et al., 1997; Zhang et al., 2014). Further, the majority of previous cyclic loading studies were designed to test damage mechanisms by employing high loads and number of cycles and by not including unloaded recovery (Adams and Hutton, 1985; Hasegawa et al., 1995). Disc hydration effects have also been studied using creep tests (Adams et al., 1987; Koeller et al., 1984). However, creep induces lower fluid flow rates than cyclic loading and thus doesn’t simulate the dynamic nature of disc loading (White and Malone, 1990). This study examines the contribution of the NP to disc loading by testing human discs undergoing a physiologically relevant testing modality, which is compressive cyclic loading with hydrated unloaded recovery.
The mechanical role of the nucleus pulposus can be evaluated by comparing the effect of cyclic loading on intact and partially nucleotomized samples. Nucleotomy, also known as discectomy, is a clinical treatment for disc herniation (Friedman, 1983; Kambin and Brager, 1987). The procedure alters disc mechanics by decreasing disc height, NP pressure, and stiffness while increasing range of motion and creep (Brinckmann and Grootenboer, 1991; Cannella et al., 2008; Frei et al., 2001; Goel et al., 1986; Ishihara et al., 1993; Meakin and Hukins, 2000; O’Connell et al., 2011). While the acute effects of nucleotomy have been determined, its impact on the disc’s fluid-flow related mechanical response has not.
The first study objective was to determine the effects of physiological cyclic loading followed by unloaded recovery on the mechanical response of human intervertebral discs. The second objective was to examine how nucleotomy alters the disc’s mechanical response to cyclic loading.
Methods
Sample Preparation
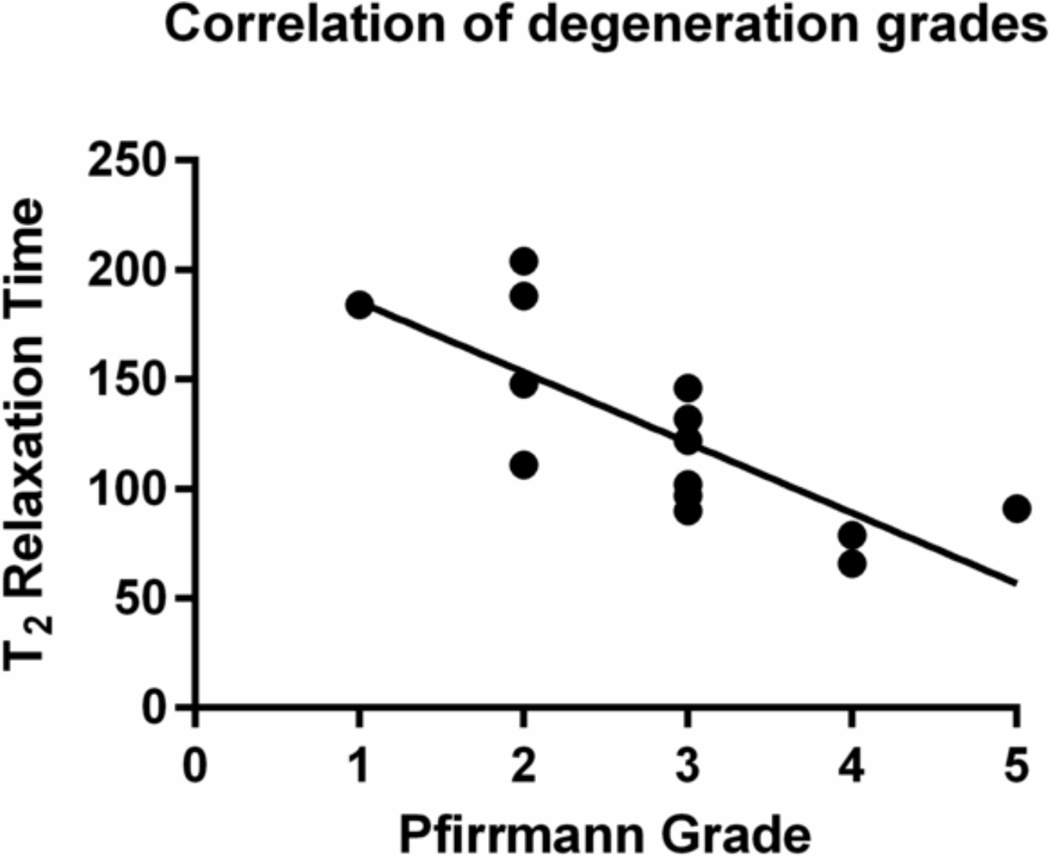
Fifteen human lumbar spine segments with a mean donor age of 50.1 years (22–75 years) were obtained from institutionally approved sources. These spines underwent MR imaging to determine degeneration grade and T2 relaxation times (Kerttula et al., 2001; Marinelli et al., 2010; Pfirrmann et al., 2001). The L5-S1 disc was selected for testing because herniations and discectomies are most common at this level (Weinstein et al., 2006). Samples had degeneration grades ranging from 1 to 5 and T2 relaxation times ranging from 66 to 204, with higher relaxation times indicating healthier discs. T2 relaxation time was not acquired for one sample. For the remaining 14 samples, T2 relaxation times correlated with Pfirrmann grades (r = −0.76) and provide a quantitative, continuous measurement of degeneration (Figure 1). Disc cross sectional area was measured from MR images. After imaging, L5-S1 bone disc bone segments were prepared by first removing the posterior elements and extraneous soft tissue. Although in vivo loads are shared between the disc and facets, posterior elements were removed in order to isolate the contributions of the disc in compression (Beckstein et al., 2008; Meakin and Hukins, 2000). Following dissection, 1.25 mm Kirschner wires were placed in the L5 vertebrae and sacrum. The L5-S1 discs were wedge-shaped, with a 13±4° angle between the L5 and S1 endplates, which likely results in some applied shear loads even during simple compression experiments. For consistency in mechanical testing, loads were applied perpendicular to the L5 vertebrae. This was ensured by potting the motion segments in PMMA with the L5 endplate parallel to the potting fixtures using fluoroscopic guidance. Samples were stored in a freezer at −20°C when not in use.
Figure 1. Pfirrmann vs T2.
T2 relaxation times are strongly correlated to Pfirrmann scores (r = −0.76, p = 0.001) for the human L5-S1 discs used in this study.
Mechanical testing
The mechanical test consisted of six steps (Figure 2). 1. Overnight hydration, also called initial hydration. 2. Measure mechanical parameters (Initial). 3. 10,000 cycles of compressive loading. 4. Measure mechanical parameters (Cyclic). 5. Overnight hydration, also called unloaded recovery. 6. Measure mechanical parameters (Recovery). During the first step, overnight hydration, samples were submerged in a 0.15 M PBS bath containing protease inhibitors (Phenylmethylsulfonyl Fluroide, N-Ethylmalemide Bioxtra, and Benzamidine Hydrochloride from Sigma Aldrich, St. Louis, MO) at 4°C for 16–24 hours. Second, mechanical parameters were measured by applying 60 cycles at 0.2 Hz between 0.48 MPa (734 ± 122N) in compression and 375 N in tension cycles, which is similar to previous studies (Beckstein et al., 2008). The stress of 0.48 MPa corresponds to one body weight or to low intensity activities such as sitting upright without support or relaxed standing (Elliott and Sarver, 2004; Nachemson, 1981; Wilke et al., 1999). The tensile load of 375 N was applied to all samples instead of a uniform stress to prevent sample pull-out from the PMMA for discs with large cross sectional areas while ensuring sufficient load to describe the mechanical parameters of interest. The third step was to apply 10,000 cycles of compressive loads at 2 Hz between 0.12 and 0.96 MPa (1467±244N) which are loads similar to moderate physical activities such as jogging or lifting a 10 kg weight (Nachemson, 1981; Wilke et al., 1999). The number of cycles and cycle frequency were selected for comparison with earlier studies and because at these loads are representative of moderate physical labor (Brinckmann et al., 1987; Johannessen et al., 2004). Fourth, mechanics were measured immediately after cyclic loading in the same manner as the second step. For the fifth and six steps, discs were rehydrated overnight followed by measurement of mechanical parameters.
Figure 2. Test Sequence.
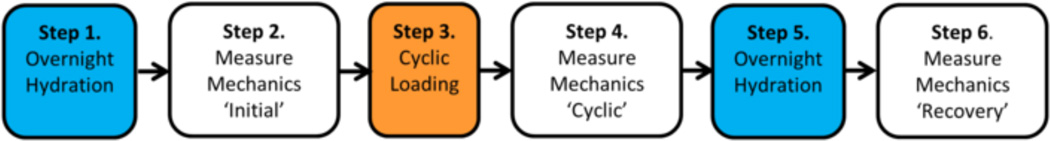
The mechanical testing procedure altered disc hydration. Discs are fully hydrated in a PBS bath containing protease inhibitors (Steps 1 and 5), and have reduced hydration after cyclic loading (Step 3). Mechanical parameters are measured after each change in hydration level (Steps 2, 4, and 6).
Samples were tested on an ElectroPuls E3000 test system (Instron, Norwood, MA). All tests were run using trimodal control, in which the system reaches the target peak load while running sine waves in position control. If cycle peak loads do not match the target loads, the peak positions of the sine wave are adjusted on the next cycle. During testing, target loads were reached between the 30th and 40th cycles. While this is more preconditioning than applied in many studies, previous work showed 20 cycles were needed to sufficiently precondition the disc for measuring mechanical parameters and preliminary studies for this work showed no difference in mechanical properties measured between the 20th and 50th cycles (Boxberger et al., 2006).
Nucleotomy
To evaluate the effect of nucleotomy, samples were tested using the 6-step method described above while intact and again following nucleotomy. Nucleotomy was performed by first hydrating the discs in PBS for eight hours. Next, a cruciate incision was created in the posterolateral annulus using a #11 scalpel blade. Afterwards, either 2 or 4 mm pituitary rongeurs were used to remove 1.71±0.38 g of nuclear material, which is approximately 50% of NP volume (Castro et al., 1992; Dullerud et al., 1993). Amount of NP removed was not correlated with sample degeneration (r = 0.19, p = 0.5). Following nucleotomy, samples were frozen until further testing.
Data Analysis
Disc area was measured from MR images by outlining the disc in a midaxial slice using the DICOM viewer OsiriX (Pixmeo, Switzerland). Disc height was calculated from a lateral fluoroscopic image of the disc by dividing disc area by the anteroposterior width using Matlab (MathWorks, Natick, MA) (O'Connell et al., 2007).
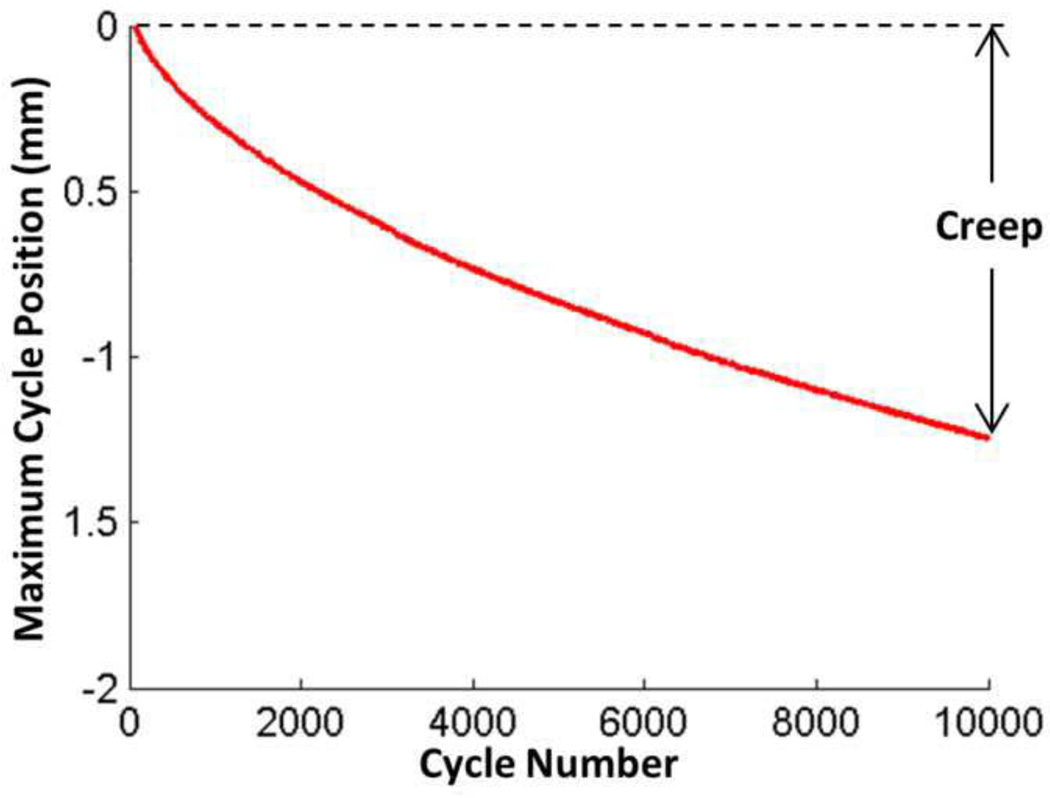
The maximum position of a single loading cycle decreases over the 10,000 cycles of compressive loading (Figure 3). Creep was defined as the decrease in maximum cycle position from cycle 50 to 10,000 of the cyclic loading and creep strain was calculated by dividing creep by initial disc height.
Figure 3. Creep Definition.
The maximum position of single cycle decreases throughout the 10,000 cycles of compressive loading. Creep is defined as the drop in maximum cycle displacement over 10,000 cycles, after which creep strain is calculated by dividing creep by initial disc height.
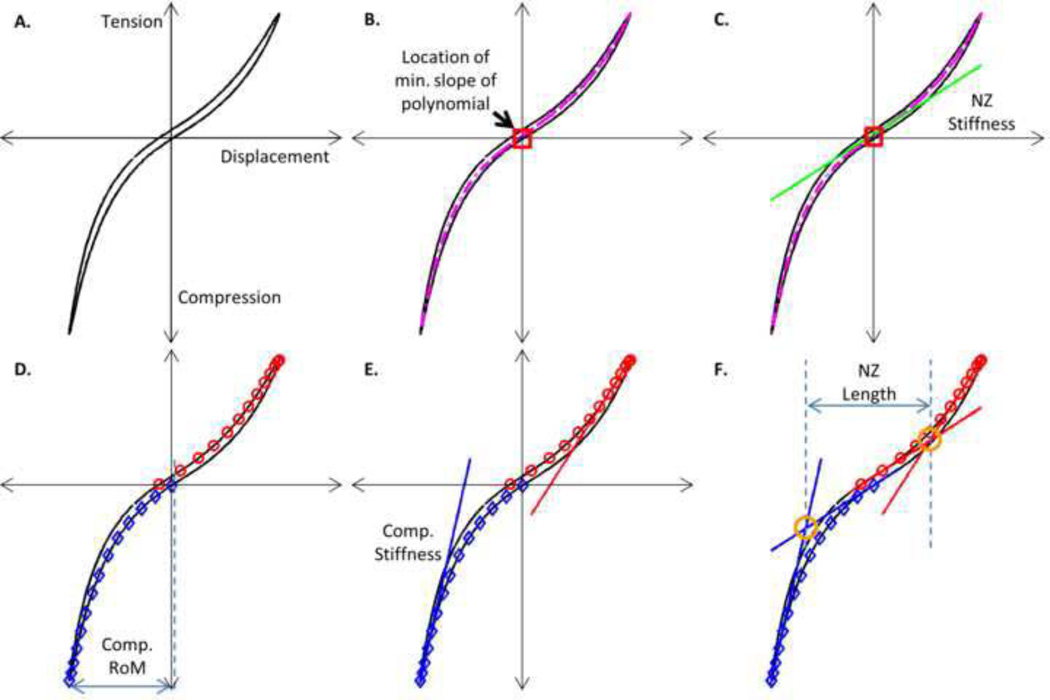
Mechanical parameters were measured on the 50th cycle of the mechanical measurement steps of the testing procedure (steps 2, 4, and 6) to ensure sufficient preconditioning and eliminate super hydration (Adams et al., 2000; Johannessen et al., 2006). Parameters were measured using a previously described custom Matlab program that performs a trilinear fit to the data (Figure 4A) (Beckstein et al., 2008). This program first determines the minimum slope location of a 5th order polynomial fit to the data (Figure 4B). Neutral zone stiffness was defined as the slope of the loading data at this location (Figure 4C). Next, the data was separated into tension and compression loading curves and compressive range of motion was defined as the displacement between 0 MPa and 0.48 MPa of applied stress (Figure 4D). Compressive stiffness was defined as the slope of the line fit through the data points above 80% of the maximum compressive load (Figure 4E). Neutral zone length was defined as the displacement between the intersection of the neutral zone line fit with the compression and tension curves (Figure 4F). Compression and neutral zone apparent modulus were calculated from stiffness by multiplying by disc height and then dividing by disc area. Similarly, compression and neutral zone strain were calculated by dividing the compression range of motion or neutral zone length by disc height. Intact disc height and area were used for this normalization.
Figure 4. Trilinear Fit.
Mechanical parameters are found using a trilinear fit of force-displacement curve from 50th cycle of the mechanical measurement steps (steps 2, 4, and 6 of the testing procedure). A. Original data B. The minimum slope of a 5th order polynomial fit is located. C. Neutral zone is fit through data at the point of minimum slope. Neutral zone stiffness is the slope of this line. D. Tension (red circles) and compression (blue diamonds) loading curves are separated from the data. Compression range of motion is the displacement between 0 and 0.48 MPa on the compression loading curve. E. Tension and compression line fits through the maximum 80% of the respective loading curves. Compression stiffness is the slope of the compression line. F. Lines are fit in the neutral zone regions of the tension and compression loading curves. Neutral zone length is the displacement between the intersection of these lines with either the tension and compression lines. All mechanical parameters are then normalized by initial disc area and height.
Statistics
The effects of loading history (Initial, Cyclic, and Recovery) and treatment (Intact and Nucleotomy) on the mechanical parameters were analyzed using a 2-Way Repeated Measures ANOVA. Significant loading history and treatment were examined with a Tukey’s or Sidak’s Multiple Comparison Test, respectively. Degeneration effects on cyclic loading were examined using linear correlations between T2 relaxation time and the percent change of parameters between loading history states. Similarly, degeneration effects on the changes caused by nucleotomy were analyzed by performing linear correlations between T2 relaxation time and the percent change between intact and nucleotomy parameter values at respective loading history state. To include the sample with no measured T2 relaxation time in the degeneration analysis, its T2 relaxation time was estimated based on its Pfirrmann grade and the regression line calculated from the other 14 samples. The effects of nucleotomy on disc height and creep strain were analyzed using paired Student’s t-test. Significance was set at p < 0.05.
Results
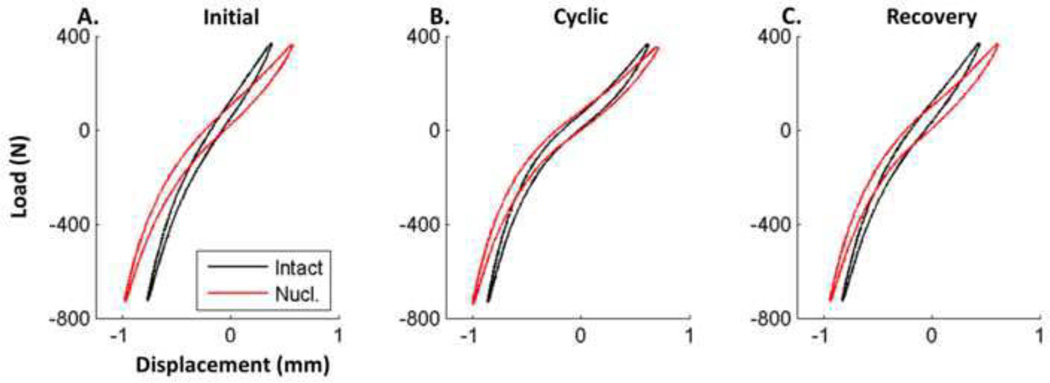
No visible tissue degradation was observed throughout the test. Intact disc area was 1528±254 mm2 and disc height was 8.6±2.3 mm. Qualitatively, both nucleotomy and cyclic loading affected the load displacement response of the disc (Figure 5). All mechanical data, with the exception of disc height, is normalized by disc geometry.
Figure 5. Force Displacement Curves.
Force displacement curves for a single sample over the course of the experiment. Cyclic loading increased range of motion and decreased neutral zone modulus, which recovered following hydration. Nucleotomy increased range of motion while decreasing stiffness and the amount of change in mechanical parameters caused by cyclic loading.
Effects of Cyclic Loading Followed by Hydrated Recovery
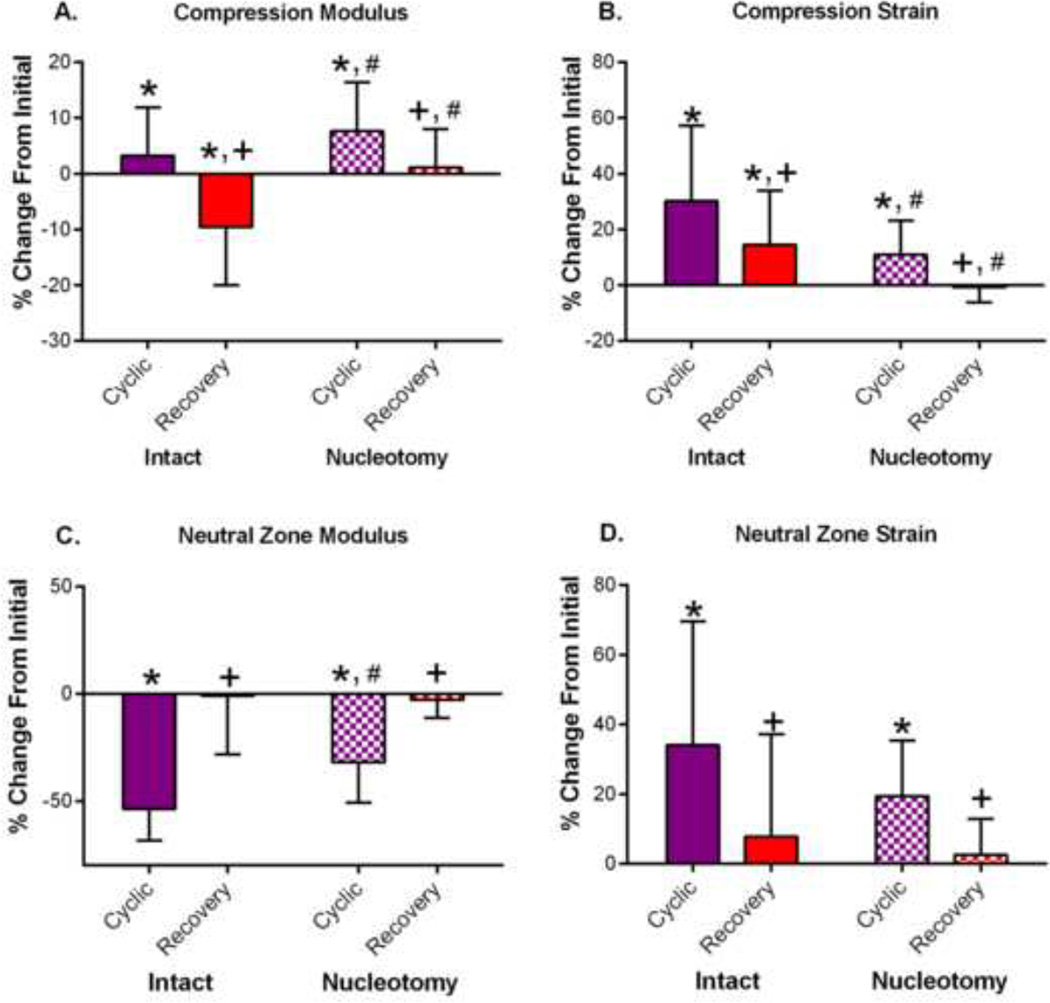
For intact samples, 10,000 cycles of compressive loading increased compressive modulus by 3% and increased compressive strain by 33% (Figure 6 A&B). Neutral zone properties were also affected by compressive cyclic loading, which decreased neutral zone modulus by 52% and increased neutral zone strain by 31% (Figure 6 C&D). Unloaded hydrated recovery caused neutral zone modulus and neutral zone strain returned to initial values (Figure 6 C&D). However, unexpectedly, compressive modulus decreased 9% from initial and compressive strain remained elevated by 16% after unloaded recovery compared to initial values (Figure 6 A&B).
Figure 6. Effect of Cyclic Loading.
Disc mechanical properties are altered by cyclic loading (cyclic) and generally recover after unloaded hydration (recovery). These changes mimic naturally occurring diurnal changes. Nucleotomy reduced the hydration effects on mechanical properties. In particular, nucleotomy reduced the percent change between the Initial and Cyclic hydration states for compressive strain (B), neutral zone modulus (C), and neutral zone strain (D). *p < 0.05 vs. initial, +p<0.05 vs. cyclic, # p<0.05 vs. intact.
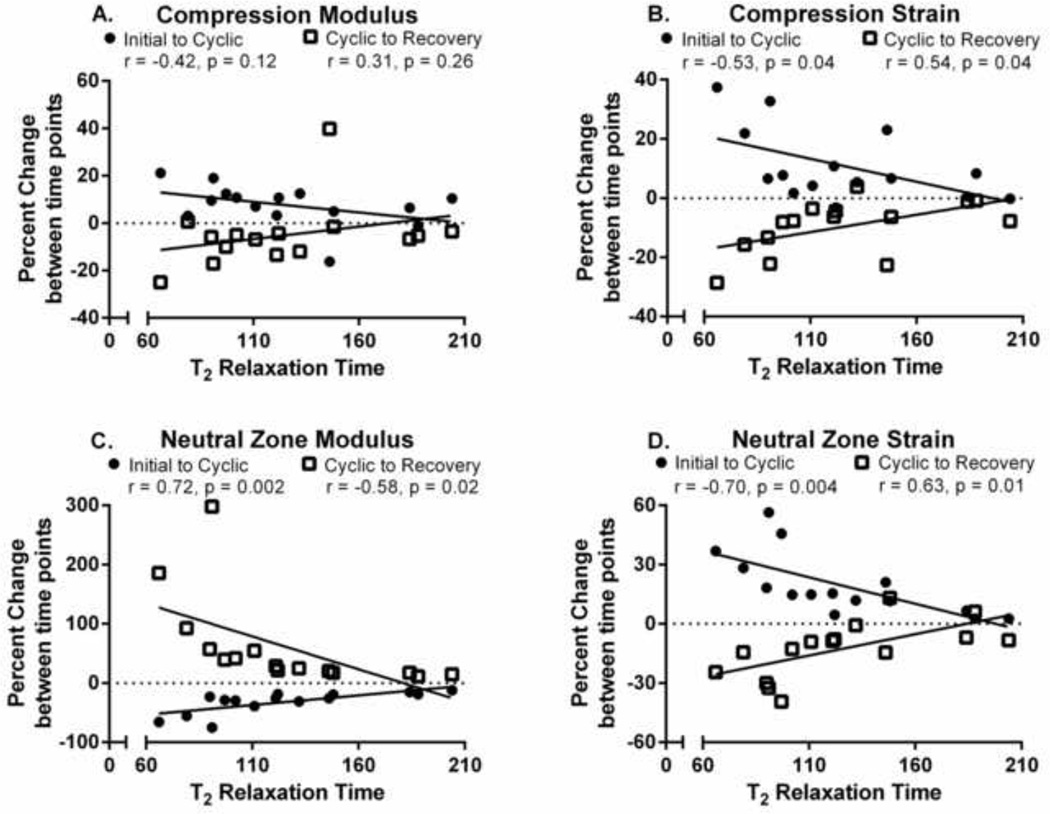
Degeneration did not significantly affect cyclic loading in intact samples (p > 0.05). However, following nucleotomy, degeneration influenced cyclic loading effects in compressive strain, neutral zone modulus, and neutral zone stiffness (Figure 7). Specifically, samples with lower T2 relaxation times (more degenerate samples) had greater increases in compressive strain between the initial and cyclic time points (r = −0.53, p = 0.04) and similarly had larger decreases in compressive strain between the cyclic and recovery time points (r = 0.54, p = 0.04) (Figure 7B). More degenerate samples also experienced greater changes in neutral zone mechanics. Low T2 relaxation times corresponded to greater decreases in percent change of neutral zone modulus (r = 0.72, p = 0.002) and increases in percent change of neutral zone strain (r = −0.70, p = 0.004) between the initial and cyclic time points (Figure 7 C&D). These greater changes between the initial and cyclic time points were matched by similar greater magnitudes of restoration towards initial values between the cyclic and recovery time points.
Figure 7. Effect of Degeneration on Cyclic Loading.
Impact of degeneration on the percent change of mechanical parameters caused by cyclic loading in nucleotomy samples. More degenerate samples (Lower T2 Relaxation Time) experienced non-significant increases in compression modulus (A) and significant increases in both compression and neutral zone strain (B&D) as a result of 10,000 cycles of compressive loading, and proportional decreases following unloaded recovery. More degenerate samples also experienced greater decreases in neutral zone modulus (C) following cyclic compressive loading and corresponding greater recoveries following unloaded recovery.
Effects of Nucleotomy
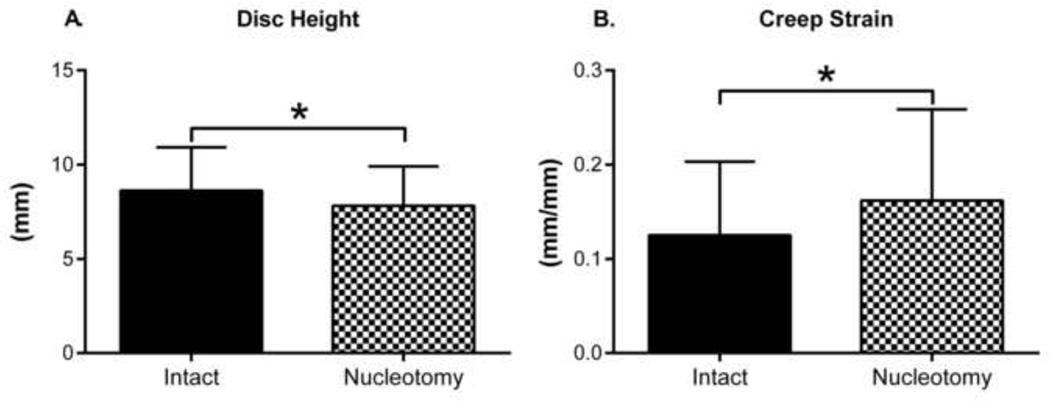
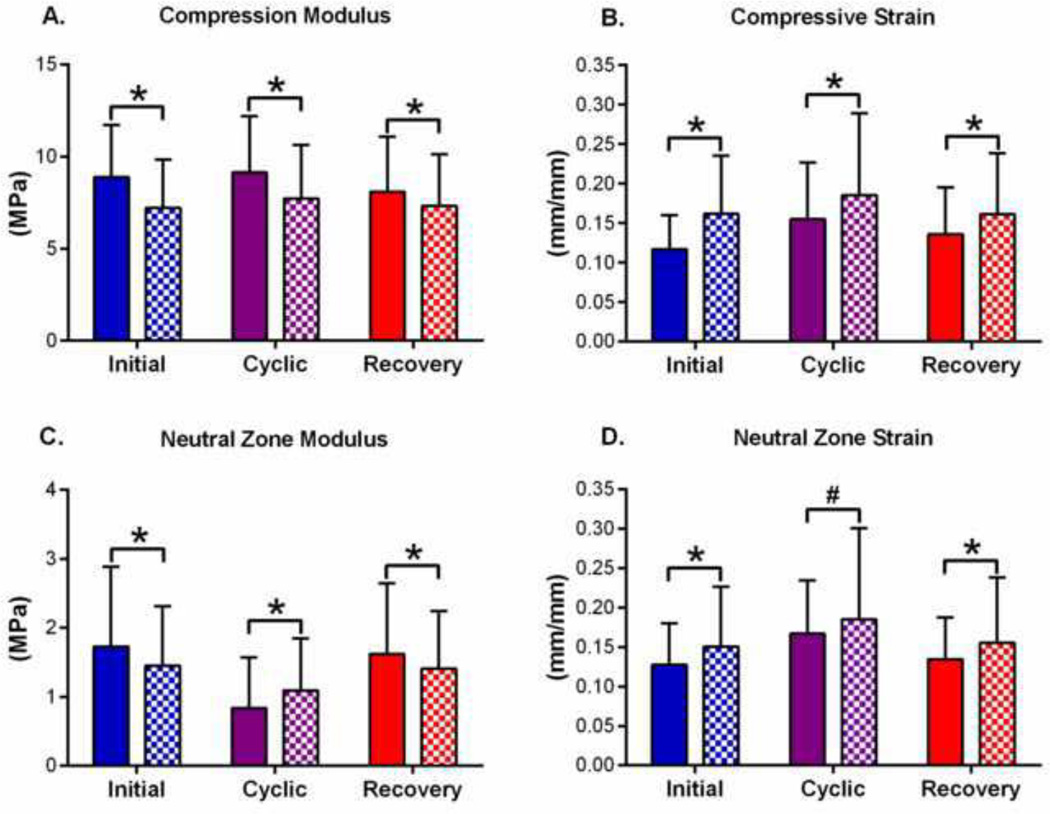
Nucleotomy reduced disc height by 9% and caused a 29% increase in creep strain (Figure 8). Compression apparent modulus decreased 19, 15, and 9% at the Initial, Cyclic, and Recovery time points, respectively (Figure 9A) and was accompanied by corresponding increases in compressive strain of 38, 19, and 19% (Figure 9B). Nucleotomy decreased neutral zone modulus by 16 and 13% (Figure 9C) at the initial and recovery time points, but increased it by 31% at the cyclic loading condition. The procedure also caused increases in neutral zone strain of 18, 11, and 15% at the three loading conditions (Figure 9D). These findings indicate that nucleotomy induces hypermobility regardless of the disc’s hydration state. Sample degeneration did not affect the changes caused by nucleotomy (p > 0.28).
Figure 8. Creep and Disc Height.
Nucleotomy decreased disc height and increased creep strain.
Figure 9. Effect of Nucleotomy.
Nucleotomy decreased compression modulus (A), increased compressive strain (B), decreased neutral zone modulus at the initial and recovery hydration states and increased neutral zone modulus at the cyclic hydration state (C), and increased neutral zone strain (D). These findings indicate that nucleotomy induces hypermobility regardless of the disc’s hydration state. Solid = Intact, Checkered = Nucleotomy. *p < 0.05, #p < 0.1
Following nucleotomy, cyclic loading affected the compressive and neutral zone mechanical properties in the same direction as the intact samples (e.g. compressive modulus increased for both intact and nucleotomy samples), but change in magnitudes were different. Specifically, after nucleotomy, cyclic loading increased compressive modulus by 7% and compressive strain by 15%. Neutral zone modulus decreased by 25% and neutral zone strain increased by 23%. For nucleotomy samples, all mechanical parameters returned to initial values following a period of unloaded hydrated recovery (Figure 6 A–D).
Interaction of Nucleotomy and Cyclic Loading
There was a significant interaction between nucleotomy and loading history for compression modulus, compression strain, and neutral zone modulus. Nucleotomy increased the magnitude of the percent increase in compression modulus caused by cyclic loading (Figure 2, step 3) from 3% for intact samples to 7% following nucleotomy (Figure 6A). However, the procedure also reduced the percent change caused by cyclic loading in compression strain from 33% for intact samples to 15% (Figure 6B) and percent change in neutral zone modulus decreased from 52% for intact samples to 25% following nucleotomy (Figure 6C). Nucleotomy also affected the trends caused by unloaded recovery (Figure 2, step 5). Compressive modulus decreased by 12% following unloaded recovery for intact samples, but was only 5% lower for nucleotomy samples. For intact samples, compressive strain decreased by 12% following unloaded recovery, which increased to 13% for nucleotomy samples. Unloaded recovery increased neutral zone modulus by 94% for intact samples, but only 29% for nucleotomy samples.
Discussion
The first study objective was to determine the effects of physiological cyclic loading followed by unloaded recovery on the mechanical response of human intervertebral discs. We determined that human discs were less affected by cyclic loading than animal models in previous studies and that cyclic loading affected neutral zone properties more than compressive properties. The second objective was to examine how nucleotomy alters the disc’s mechanical response to cyclic loading. We found that partial removal of the nucleus pulposus decreases intervertebral disc modulus while increasing strain (range of motion), which corresponds to hypermobility. Nucleotomy also mitigated cyclic loading effects on mechanical properties, indicating altered fluid flow. This altered fluid flow may in turn affect cellular mechanotransduction and transport of disc nutrients and waste.
In human discs, cyclic loading and hydrated recovery affected both compressive and neutral zone properties. For example, cyclic loading increased compressive modulus by 3% while recovery decreased it by 12% (Figure 6A). This is lower than the 15–33% changes seen in bovine and ovine tests (Johannessen et al., 2004; Korecki et al., 2008). However, the lower magnitudes were expected because the mechanical changes are due to redistribution of fluid within the disc and human discs have a lower fluid content than the animal discs (Adams et al., 1990; Johannessen et al., 2004).
Cyclic loading caused greater changes in neutral zone properties than compressive properties (Figure 6). Cyclic loading effects on neutral zone properties have not previously been reported; however, the role of the nucleus is more pronounced in the neutral zone than at higher loads (Cannella et al., 2008). Given that cyclic loading causes fluid to be either redistributed within or extruded from the disc and that the NP has higher water content than the annulus (Antoniou et al., 1996; Johannessen et al., 2004), higher fluid flow from the nucleus may explain why neutral zone properties were more affected by cyclic loading than compressive properties.
An intriguing result of the study is the limited role degeneration plays in affecting the disc’s mechanical response to cyclic loading and hydrated recovery. Degeneration was correlated with cyclic loading effects after nucleotomy (Figure 7), with more degenerate samples experiencing greater increases in both compressive and neutral zone strain following cyclic loading. However, degeneration was not correlated with the acute changes of nucleotomy or effect of cyclic loading in intact discs. The more disorganized annular structure of the degenerate samples likely permits greater redistribution of fluid than is possible for intact samples, which was magnified by the nucleotomy incision (Haefeli et al., 2006; Michalek and Iatridis, 2011). This finding has clinical relevance, indicating that nucleotomy causes more hypermobility in extended physiological loading for degenerate discs than non-degenerate discs.
The acute effects of nucleotomy were a loss of disc height, decrease in both compressive and neutral zone modulus, and increase in compressive and neutral zone range of motion (Figures 8&9). Decreased disc height and increased range of motion caused by nucleotomy indicate that in order to compensate for the disc’s altered mechanical loading following nucleus depressurization (via age-related proteoglycan loss, herniation, or discectomy) the remaining annulus and surrounding spinal elements must resist more motion. This could induce back pain by placing additional stresses on these innervated structures and advancing the degenerative cascade. In addition, spinal motion of the treated and adjacent levels may be altered if facet joints induce flexion moments within the disc. Also, the mechanical changes of nucleotomy are similar to the mechanical changes of natural degeneration. Thus, nucleotomy is a potential platform for evaluating mechanically based spinal treatments, such nucleus pulposus replacement (Omlor et al., 2012; Ruan et al., 2010; Woiciechowsky et al., 2012).
Our measured acute effects of nucleotomy are comparable to literature values. Specifically, our measured disc height loss of 0.8 mm for 1.71 g of NP removed is comparable to a reported height loss of 0.5 mm for 1.75 g removed (Wilke et al., 2001). Our observation that nucleotomy initially causes a 0.35 mm increase in compressive range of motion is lower than literature values of 0.6–0.8 mm (Frei et al., 2001; O’Connell et al., 2011). However, these studies also applied higher compressive loads, which may account for some of this difference. In addition, the unique geometry of the L5-S1 disc relative to the other lumbar levels used in the existing literature may further explain these differences. Our observed 20% decrease in compressive stiffness fits within the literature range of 3–50% decrease for trans-annular nucleotomy studies. (Cannella et al., 2008; Markolf and Morris, 1974). However, trans-endplate nucleotomy did not affect compressive stiffness (Johannessen et al., 2006), which suggests decreased compressive stiffness caused by nucleotomy may be more the result of the annular injury than the removal of the nucleus. This is corroborated by existing literature that shows the annulus plays a significant role in supporting compressive loads (Iatridis et al., 1998; Vresilovic et al., 2006).
It’s interesting that the NZ modulus at the cyclic time point is higher for the nucleotomy sample than for the intact samples (Figure 9C). The higher modulus after nucleotomy may be attributed to fluid flow through the annular nucleotomy incision. In intact samples, the annulus serves as a barrier for fluid flow to and from the nucleus. The incision may increase permeability or create a fluid flow channel in the injury region (Michalek and Iatridis, 2011). As a result, following nucleotomy, PBS from the bath may more freely enter and exit the disc. Thus, the neutral zone modulus of nucleotomy samples at the cyclic time point may be higher than intact samples because there is more PBS within the nuclear region.
In addition to the acute effects of nucleotomy, we also found that nucleotomy reduced cyclic loading effects. This is clearly seen in the reduction of percent change between cyclic and recovery values for compressive modulus (12 to 5%) and neutral zone modulus (94 to 29%) (Figure 6 A&C). Reduced effects of cyclic loading indicate altered fluid flow, which has two significant ramifications. First, it alters the stresses and strains experienced by the disc’s cells, potentially altering cellular mechanotransduction and subsequent protein synthesis (Setton and Chen, 2004). This in turn, may alter the discs biochemical composition and mechanical function (Baer et al., 2003). The second potential effect of altered fluid flow is modified nutrient delivery and waste removal. The intervertebral disc is a largely avascular tissue and, as a result, its cells are dependent on nutrient diffusion (Moore, 2000; Urban and Roberts, 2003). Thus, any alterations to the delivery mechanism have the potential to create wide-reaching effects.
The failure of compressive modulus and compressive strain to return to initial values for the intact samples (Figure 6A&B) indicates a study limitation in that cyclic loading may have caused some non recoverable damage to the annulus fibrosus. However, following annular nucleotomy, the compressive properties at the initial and recovery hydration states are identical. This suggests the damage caused to the intact samples is due to resisting fluid flow through the annulus, because following nucleotomy fluid had a less obstructed pathway. This potential annular damage was unexpected because it was not seen in previous ovine studies with similar loads or in the preliminary studies for this work (Johannessen et al., 2004; Malhotra et al., 2012). However, most of the human tissue used was moderately degenerated, in contrast with the non-degenerated samples used in animal studies. This initial degeneration may have caused the samples to be more susceptible to injury. In contrast to compressive properties, cyclic loading effects in neutral zone properties were completely recoverable following hydration.
This study has demonstrated that cyclic loading increases compressive modulus, compressive strain, and neutral zone strain while decreasing neutral zone modulus. These changes are the result of altered hydration levels caused by the cyclic loading protocol and are similar to naturally occurring diurnal changes. Nucleotomy reduced disc height and modulus while increasing range of motion. In addition to these acute changes, nucleotomy also reduced the difference between mechanical properties before and after cyclic loading. The observed changes in mechanical behavior induced by nucleotomy are similar to naturally occurring degenerative changes. Results of this study provide an ideal protocol and control data for evaluating the effectiveness of a mechanically-based disc degeneration treatment, such as a nucleus replacement.
Acknowledgements
We would like to thank Dhara B. Amin and Ian S. MacLean for performing some of the mechanical testing. Spines were acquired from National Disease Research Interchange, Philadelphia, PA and Platinum Training, Henderson, NV. This study was funded by NIH R01AR050052, NSF Graduate Research Fellowship #DGE-0822, and a Neurosurgery Research and Education Foundation grant. None of the funding agencies played a direct role in study design, data collection, or data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None of the authors have conflicts of interest to disclose.
References
- Adams MA, Dolan P, Hutton WC. Diurnal variations in the stresses on the lumbar spine. Spine. 1987;12:130–137. doi: 10.1097/00007632-198703000-00008. [DOI] [PubMed] [Google Scholar]
- Adams MA, Dolan P, Hutton WC, Porter RW. Diurnal changes in spinal mechanics and their clinical significance. Journal of Bone and Joint Surgery. 1990;72B:266–270. doi: 10.1302/0301-620X.72B2.2138156. [DOI] [PubMed] [Google Scholar]
- Adams MA, Freeman BJC, Morrison HP, Nelson IW, Dolan P. Mechanical initiation of intervertebral disc degeneration. Spine. 2000;25:1625–1636. doi: 10.1097/00007632-200007010-00005. [DOI] [PubMed] [Google Scholar]
- Adams MA, Hutton WC. Gradual disc prolapse. Spine. 1985;10:524–531. doi: 10.1097/00007632-198507000-00006. [DOI] [PubMed] [Google Scholar]
- Andersson GBJ. Epidemiological features of chronic low-back pain. The Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertbral disc: Evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. Journal of Clinical Investigation. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer AE, Laursen TA, Guilak F, Setton LA. The micromechanical environment of intervertebral disc cells determined by a finite deformation, anisotropic, and biphasic finite element model. Journal of Biomechanical Engineering. 2003;125:1–11. doi: 10.1115/1.1532790. [DOI] [PubMed] [Google Scholar]
- Beckstein JC, Sen S, Schaer TP, Vresilovic EJ, Elliott DM. Comparison of animal discs used in disc research to human lumbar disc: Axial compression mechanics and glycosaminoglycan content. Spine. 2008;33:E166–E173. doi: 10.1097/BRS.0b013e318166e001. [DOI] [PubMed] [Google Scholar]
- Boxberger JI, Sen S, Yerramalli CS, Elliott DM. Nucleus pulposus glycosaminoglycan content is correlated with axial mechanics in rat lumbar motion segments. Journal of Orthopaedic Research. 2006;24:1906–1915. doi: 10.1002/jor.20221. [DOI] [PubMed] [Google Scholar]
- Brinckmann P, Grootenboer H. Change of disc height, radial disc bulge, and intradiscal pressure from discectomy. An in vitro investigation on human lumbar discs. Spine. 1991;16:641–646. doi: 10.1097/00007632-199106000-00008. [DOI] [PubMed] [Google Scholar]
- Brinckmann P, Johannleweling N, Hilweg D, Biggemann M. Fatigue fracture of human lumbar vertebrae. Clin. Biomech. 1987;2:94–96. doi: 10.1016/0268-0033(87)90134-3. [DOI] [PubMed] [Google Scholar]
- Cannella M, Arthur A, Allen S, Keane M, Joshi A, Vresilovic E, Marcolongo M. The role of the nucleus pulposus in neutral zone human lumbar intervertebral disc mechanics. Journal of Biomechanics. 2008;41:2104–2111. doi: 10.1016/j.jbiomech.2008.04.037. [DOI] [PubMed] [Google Scholar]
- Castro WHM, Jerosch J, Halm H, Rondhuis J. How much nuclear material is removed with percutaneous nucleotomy. Zeitschrift Fur Orthopadie Und Ihre Grenzgebiete. 1992;130:467–471. doi: 10.1055/s-2008-1039654. [DOI] [PubMed] [Google Scholar]
- Demers CN, Antoniou J, Mwale F. Value and limitations of using the bovine tail as a model for the human lumbar spine. Spine. 2004;29:2793–2799. doi: 10.1097/01.brs.0000147744.74215.b0. [DOI] [PubMed] [Google Scholar]
- Dullerud R, Amundsen T, Johansen JG, Magnaes B. Lumbar percutaneous automated nucleotomy - technique, patient selection, and preliminary results. Acta Radiologica. 1993;34:536–542. [PubMed] [Google Scholar]
- Elliott DM, Sarver JJ. Young investigator award winner: Validation of the mouse and rat disc as mechanical models of the human lumbar disc. Spine. 2004;29:713–722. doi: 10.1097/01.brs.0000116982.19331.ea. [DOI] [PubMed] [Google Scholar]
- Frei H, Oxland TR, Rathonyi GC, Nolte LP. The effect of nucleotomy on lumbar spine mechanics in compression and shear loading. Spine. 2001;26:2080–2089. doi: 10.1097/00007632-200110010-00007. [DOI] [PubMed] [Google Scholar]
- Friedman WA. Percutaneous discectomy - an alternative to chemonucleolysis. Neurosurgery. 1983;13:542–547. doi: 10.1227/00006123-198311000-00010. [DOI] [PubMed] [Google Scholar]
- Goel VK, Nishiyama K, Weinstein JN, Liu YK. Mechanical properties of lumbar spinal motion segments as affected by partial disc removal. Spine. 1986;11:1008–1012. doi: 10.1097/00007632-198612000-00007. [DOI] [PubMed] [Google Scholar]
- Haefeli M, Kalberer F, Saegesser D, Nerlich AG, Boos N, Paesold G. The course of macroscopic degeneration in the human lumbar intervertebral disc. Spine. 2006;31:1522–1531. doi: 10.1097/01.brs.0000222032.52336.8e. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Turner C, Chen J, Burr DB. Effect of disc lesion on microdamage accumulation in lumbar vertebrae under cyclic compression loading. Clinical Orthopaedics & Related Research. 1995:190–198. [PubMed] [Google Scholar]
- Iatridis JC, Setton LA, Foster RJ, Rawlins BA, Weidenbaum M, Mow VC. Degeneration affects the anisotropic and nonlinear behaviors of human anulus fibrosus in compression. Journal of Biomechanics. 1998;31:535–544. doi: 10.1016/s0021-9290(98)00046-3. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Tsuji H, Hirano N, Ohshima H, Terahata N. Biorheological responses of the intact and nucleotomized intervertebral disks to compressive, tensile, and vibratory stresses. Clinical Biomechanics. 1993;8:250–254. doi: 10.1016/0268-0033(93)90034-F. [DOI] [PubMed] [Google Scholar]
- Johannessen W, Cloyd J, O'Connell G, Vresilovic E, Elliott D. Trans-endplate nucleotomy increases deformation and creep response in axial loading. Annals of Biomedical Engineering. 2006;34:687–696. doi: 10.1007/s10439-005-9070-8. [DOI] [PubMed] [Google Scholar]
- Johannessen W, Vresilovic EJ, Wright AC, Elliott DM. Intervertebral disc mechanics are restored following cyclic loading and unloaded recovery. Annals of Biomedical Engineering. 2004;32:70–76. doi: 10.1023/b:abme.0000007792.19071.8c. [DOI] [PubMed] [Google Scholar]
- Kambin P, Brager MD. Percutaneous posterolateral discectomy - anatomy and mechanism. Clinical Orthopaedics and Related Research. 1987:145–154. [PubMed] [Google Scholar]
- Katz JN. Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. Journal of Bone and Joint Surgery. 2006;88A:21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- Kerttula L, Kurunlahti M, Jauhiainen J, Koivula A, Oikarinen J, Tervonen O. Apparent diffusion coefficients and t2 relaxation time measurements to evaluate disc degeneration. A quantitative mr study of young patients with previous vertebral fracture. Acta Radiol. 2001;42:585–591. doi: 10.1080/028418501127347241. [DOI] [PubMed] [Google Scholar]
- Koeller W, Funke F, Hartmann F. Biomechanical behavior of human intervertebral discs subjected to long lasting axial loading. Biorheology. 1984;21:675–686. doi: 10.3233/bir-1984-21502. [DOI] [PubMed] [Google Scholar]
- Korecki CL, MacLean JJ, Iatridis JC. Dynamic compression effects on intervertebral disc mechanics and biology. Spine. 2008;33:1403–1409. doi: 10.1097/BRS.0b013e318175cae7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G. Nucleus pulposus replacement and regeneration/repair technologies: Present status and future prospects. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2012;100B:1702–1720. doi: 10.1002/jbm.b.32712. [DOI] [PubMed] [Google Scholar]
- Luo X, Pietrobon R, X Sun S, Liu GG, Hey L. Estimates and patterns of direct health care expenditures among individuals with back pain in the united states. Spine. 2004;29:79–86. doi: 10.1097/01.BRS.0000105527.13866.0F. [DOI] [PubMed] [Google Scholar]
- Malhotra NR, Han WM, Beckstein J, Cloyd J, Chen W, Elliott DM. An injectable nucleus pulposus implant restores compressive range of motion in the ovine disc. Spine. 2012;37:E1099–E1105. doi: 10.1097/BRS.0b013e31825cdfb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli NL, Haughton VM, Anderson PA. T2 relaxation times correlated with stage of lumbar intervertebral disk degeneration and patient age. American Journal of Neuroradiology. 2010;31:1278–1282. doi: 10.3174/ajnr.A2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markolf KL, Morris JM. The structural components of the intervertebral disc: A study of their contributions to the ability of the disc to withstand compressive forces. Journal of Bone and Joint Surgery. 1974;56A:675–687. [PubMed] [Google Scholar]
- Meakin JR, Hukins DWL. Effect of removing the nucleus pulposus on the deformation of the annulus fibrosus during compression of the intervertebral disc. Journal of Biomechanics. 2000;33:575–580. doi: 10.1016/s0021-9290(99)00215-8. [DOI] [PubMed] [Google Scholar]
- Michalek AJ, Iatridis JC. Penetrating annulus fibrosus injuries affect dynamic compressive behaviors of the intervertebral disc via altered fluid flow: An analytical interpretation. Journal of Biomechanical Engineering. 2011;133:084502. doi: 10.1115/1.4004915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RJ. The vertebral end-plate: What do we know? European Spine Journal. 2000;9:92–96. doi: 10.1007/s005860050217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachemson AL. Disc pressure measurements. Spine. 1981;6:93–97. doi: 10.1097/00007632-198101000-00020. [DOI] [PubMed] [Google Scholar]
- O'Connell GD, Vresilovic EJ, Elliott DM. Comparison of animals used in disc research to human lumbar disc geometry. Spine. 2007;32:328–333. doi: 10.1097/01.brs.0000253961.40910.c1. [DOI] [PubMed] [Google Scholar]
- O'Connell G, Malhotra NR, Vresilovic EJ, Elliott DM. The effect of nucleotomy and the dependence on degeneration of human intervertebral disc strain in axial compression. Spine. 2011;36:1765–1771. doi: 10.1097/BRS.0b013e318216752f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omlor G, Nerlich A, Lorenz H, Bruckner T, Richter W, Pfeiffer M, Gühring T. Injection of a polymerized hyaluronic acid/collagen hydrogel matrix in an in vivo porcine disc degeneration model. European Spine Journal. 2012;21:1700–1708. doi: 10.1007/s00586-012-2291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfirrmann CWA, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- Rajaee SS, Bae HW, Kanim LEA, Delamarter RB. Spinal fusion in the united states. Spine. 2012;37:67–76. doi: 10.1097/BRS.0b013e31820cccfb. [DOI] [PubMed] [Google Scholar]
- Reitmaier S, Wolfram U, Ignatius A, Wilke H-J, Gloria A, Martín-Martínez JM, Silva-Correia J, Miguel Oliveira J, Luís Reis R, Schmidt H. Hydrogels for nucleus replacement—facing the biomechanical challenge. Journal of the Mechanical Behavior of Biomedical Materials. 2012;14:67–77. doi: 10.1016/j.jmbbm.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Ruan DK, Xin H, Zhang C, Wang C, Xu C, Li C, He Q. Experimental intervertebral disc regeneration with tissue-engineered composite in a canine model. Tissue Engineering: Part A. 2010;16:2381–2389. doi: 10.1089/ten.TEA.2009.0770. [DOI] [PubMed] [Google Scholar]
- Setton LAP, Chen JP. Cell mechanics and mechanobiology in the intervertebral disc. Spine. 2004;29:2710–2723. doi: 10.1097/01.brs.0000146050.57722.2a. [DOI] [PubMed] [Google Scholar]
- Showalter BL, Beckstein JC, Martin JT, Beattie EE, Espinoza Orías AA, Schaer TP, Vresilovic EJ, Elliott DM. Comparison of animal discs used in disc research to human lumbar disc: Torsion mechanics and collagen content. Spine. 2012;37:E900–E907. doi: 10.1097/BRS.0b013e31824d911c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan S, Merkher Y, Wachtel E, Ehrlich S, Maroudas A. Correlation of swelling pressure and intrafibrillar water in young and aged human intervertebral discs. Journal of Orthopaedic Research. 2006;24:1292–1298. doi: 10.1002/jor.20144. [DOI] [PubMed] [Google Scholar]
- Thoreson O, Baranto A, Ekström L, Holm S, Hellström M, Swärd L. The immediate effect of repeated loading on the compressive strength of young porcine lumbar spine. Knee Surgery, Sports Traumatology, Arthroscopy. 2010;18:694–701. doi: 10.1007/s00167-009-1001-z. [DOI] [PubMed] [Google Scholar]
- Urban JPG, McMullin JF. Swelling pressure of the lumbar intervertebral disks - influence of age, spinal level, composition, and degeneration. Spine. 1988;13:179–187. doi: 10.1097/00007632-198802000-00009. [DOI] [PubMed] [Google Scholar]
- Urban JPG, Roberts S. Degeneration of the intervertebral disc. Arthritis Research & Therapy. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon-Roberts B, Moore RJ, Fraser RD. The natural history of age-related disc degeneration: The pathology and sequelae of tears. Spine. 2007;32:2797–2804. doi: 10.1097/BRS.0b013e31815b64d2. [DOI] [PubMed] [Google Scholar]
- Vresilovic EJ, Johannessen W, Elliott DM. Disc mechanics with trans-endplate partial nucleotomy are not fully restored following cyclic compressive loading and unloaded recovery. Journal of Biomechanical Engineering. 2006;128:823–829. doi: 10.1115/1.2354210. [DOI] [PubMed] [Google Scholar]
- Weinstein JN, Lurie JD, Tosteson TD, Skinner JS, Hanscom B, Tosteson ANA, Herkowitz H, Fischgrund J, Cammisa FP, Albert T, Deyo RA. Surgical vs nonoperative treatment for lumbar disk herniation. JAMA: The Journal of the American Medical Association. 2006;296:2451–2459. doi: 10.1001/jama.296.20.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TL, Malone TR. Effects of running on intervertebral disc height. Journal of Orthopaedic and Sports Physical Therapy. 1990;12:139–146. doi: 10.2519/jospt.1990.12.4.139. [DOI] [PubMed] [Google Scholar]
- Wilke HJ, Kavanagh S, Neller S, Haid C, Claes LE. Effect of a prosthetic disc nucleus on the mobility and disc height of the l4-5 intervertebral disc postnucleotomy. Journal of Neurosurgery. 2001;95:208–214. doi: 10.3171/spi.2001.95.2.0208. [DOI] [PubMed] [Google Scholar]
- Wilke HJ, Kettler A, Claes LE. Are sheep spines a valid biomechanical model for human spines? Spine. 1997;22:2365–2374. doi: 10.1097/00007632-199710150-00009. [DOI] [PubMed] [Google Scholar]
- Wilke HJ, Neef P, Caimi M, Hoogland T, Claes LE. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine. 1999;24:755–762. doi: 10.1097/00007632-199904150-00005. [DOI] [PubMed] [Google Scholar]
- Woiciechowsky C, Abbushi A, Zenclussen ML, Casalis P, Kruger JP, Freymann U, Endres M, Kaps C. Regeneration of nucleus pulposus tissue in an ovine intervertebral disc degeneration model by cell-free resorbable polymer scaffolds. Journal of tissue engineering and regenerative medicine. 2012 doi: 10.1002/term.1582. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lenart BA, Lee JK, Chen D, Shi P, Ren J, Muehleman C, Chen D, An HS. Histological features of endplates of the mammalian spine: From mice to men. Spine (Phila Pa 1976) 2014;39:E312–E317. doi: 10.1097/BRS.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]