Abstract
The ability to walk independently with the velocity and endurance that permit home and community activities is a highly regarded goal for neurological rehabilitation after stroke. This pilot study explored a functional magnetic resonance imaging (fMRI) activation paradigm for its ability to reflect phases of motor learning over the course of locomotor rehabilitation-mediated functional gains. Ankle dorsiflexion is an important kinematic aspect of the swing and initial stance phase of the gait cycle. The motor control of dorsiflexion depends in part on descending input from primary motor cortex. Thus, an fMRI activation paradigm using voluntary ankle dorsiflexion has face validity for the serial study of walking-related interventions. Healthy control subjects consistently engaged contralateral primary sensorimotor cortex (S1M1), supplementary motor area (SMA), premotor (PM) and cingulate motor (CMA) cortices, and ipsilateral cerebellum. Four adults with chronic hemiparetic stroke evolved practice-induced representational plasticity associated with gains in speed, endurance, motor control, and kinematics for walking. For example, an initial increase in activation within the thoracolumbar muscle representation of S1M1 in these subjects was followed by more focused activity toward the foot representation with additional pulses of training. Contralateral CMA and the secondary sensory area also reflected change with practice and gains. We demonstrate that the supraspinal sensorimotor network for the neural control of walking can be assessed indirectly by ankle dorsiflexion. The ankle paradigm may serve as an ongoing physiological assay of the optimal type, duration, and intensity of rehabilitative gait training.
Keywords: Functional magnetic resonance imaging, Motor control, Gait, Rehabilitation, Stroke, Foot movements
Introduction
Functional neuroimaging techniques, including positron emission tomography (PET), transcranial magnetic stimulation (TMS), and functional magnetic resonance imaging (fMRI), have been employed to study the recovery of motor skills primarily of the upper extremity after stroke (Calautti and Baron, 2003). Few studies have related behavioral gains to changes within cerebral sensorimotor regions of interest (ROIs) over the course of a specific therapy or general approach to rehabilitation (Carey et al., 2002; Johansen-Berg et al., 2002; Liepert et al., 2000; Nelles et al., 2001; Ward et al., 2003b; Wittenberg et al., 2003). To do so convincingly, investigators could employ (1) a well-defined rehabilitation strategy that emphasizes the practice of functionally important movements, (2) an activation paradigm during neuroimaging that incorporates components of the movements being retrained, (3) changes in activated regions of interest over time in relation to the intensity or duration of the rehabilitation strategy, and (4) behavioral outcome measures that monitor, with adequate sensitivity, the gains over time that are relevant to what was practiced (Dobkin, 2003b). If such studies reveal that changing patterns of activation evolve with behavioral gains during a task-specific therapy, such as a strategy for the practice of walking, then the fMRI activation paradigm may also serve as a predictor of outcomes beyond what may be ascertained by clinical features alone.
The ability to walk at the speeds and distances needed for home and community ambulation is an especially important and readily measured outcome after hemiplegic stroke and spinal cord injury (SCI). Six months after stroke, patients with persistent hemiparesis walk approximately one third as fast and only 40% the distance of age-matched healthy persons (Pohl et al., 2004). Additional gait training can improve walking speed and endurance (Sullivan et al., 2002). Can functional neuroimaging reflect training-related reorganization and provide a physiological insight into the optimal dose of additional gait training? Walking is not feasible during fMRI but has been performed during near-infrared spectroscopy (NIRS) (Miyai et al., 2001, 2002), 18fluorodeoxyglucose PET (Dobkin, 1996), and single photon emission computerized tomography (Fukuyama et al., 1997). Bilateral cortical activity was found in primary and secondary motor cortices and the cerebellum. Standing, assessed by PET, also revealed expected cerebellar contributions for postural control (Ouchi et al., 1999). Cerebral activity has been assessed while subjects imagine walking, but the cerebral resources used by an individual to visualize ambulation are open to wide variations across subjects. Prior studies have used toe, foot, or knee movements during fMRI (Debaere et al., 2001; Kollias et al., 2001; Lafleur et al., 2002; Leherricy et al., 1998; Lotze et al., 2000, 2003b; Luft et al., 2002; Sabbah et al., 2002; Sahyoun et al., 2004) and PET (Johannsen et al., 2001; Roelcke et al., 1997) to investigate motor control and somatotopy in normal and neurologically impaired subjects. We have been evaluating ankle dorsiflexion as a potential marker for gains in motor control of the lower extremity during rehabilitation and as a substitute for multijoint walking movements of the affected leg in patients with stroke and SCI (Dobkin, 2000; Dobkin and Sullivan, 2001; Dobkin et al., 2000).
Ankle dorsiflexion has reasonable face validity as an fMRI activation paradigm in relation to behavioral changes in the ability to walk. Ankle dorsiflexion is a critical component of the gait cycle (Dobkin, 2003a). The ankle dorsiflexes at heel strike upon initiation of the stance phase and throughout the swing phase. Heel strike is as uniquely human as walking with an upright posture (Capaday, 2002). For fMRI studies of the leg, the contribution of cortical projections for ankle movement makes ankle dorsiflexion a potentially practical way to assess the integrated cortical, subcortical, and spinal sensorimotor network involved with gains in skilful walking (Dobkin, 2003a; Sahyoun et al., 2004).
We present control data for active ankle dorsiflexion, which revealed consistent voxel activations within the nodes of the sensorimotor network. To evaluate potential relationships between activations in patients and their ability to walk, we examined the cortical maps of adults who had chronic hemiparesis from stroke. We then tested the effects of repeated bouts of a specific strategy for locomotor therapy on activity-dependent reorganization and behavioral outcomes. We present this pilot data to support the rationale for future use of the fMRI paradigm and its behavioral correlates for studies of the efficacy of interventions for walking. In addition, we begin to demonstrate the potential to use elicited patterns of activation to anticipate the capacity of the locomotor nodes to remodel in response to a specific therapy, which offers new insights into the optimal duration and intensity of rehabilitation therapy.
Methods
Subjects
Subjects were identified within the Neurologic Rehabilitation and Research Program at the University of California Los Angeles. They signed consents approved by the institutional review board. The fMRI studies were performed at the Ahmanson-Lovelace Brain Mapping Center at UCLA.
Procedures
Hemiparetic subjects received 2-week blocks of training (six sessions) for 10 weeks and an fMRI study at the end of each block.
Head motion tends to be greater in older than younger subjects and in patients with hemiparesis compared to healthy control subjects (Seto et al., 2001). Bite plates to control head motion may not be safe for patients after stroke and tend not to limit rostrocaudal motion from the hips and paraspinal muscles. Pelvic restraints have not eliminated the 2- to 3-mm coupling of head and trunk motion with ankle dorsiflexion or plantarflexion (Seto et al., 2001). We limited superior–inferior translation motion to less than 1 mm as subjects dorsiflexed at the ankle while wearing a polypropylene ankle-foot orthosis (AFO) articulated at the ankle joint. The device lifts the foot and calf an inch above the MRI gantry to prevent local muscle contractions from creating rostrocaudal leg motion. The frictionless AFO ankle joint permits 5° of plantar flexion and 10° of dorsiflexion. Velcro straps placed across the dorsal surface of the AFO hold the heel and forefoot in place. During scanning, the head was immobilized by foam pads and tape.
Ankle dorsiflexion paradigm
For voluntary ankle movement, subjects practiced two sets of five isolated dorsiflexor movements of 10°. An assistant visually inspected and palpated the leg muscles inside the scanning environment to monitor for synergistic and mirror movements and provided cues to eliminate extraneous movements after the first set. We have also used electromyography of upper and lower leg muscles outside of the scanner to detect any spread of muscle activity. Active foot dorsiflexion may be accompanied by slight simultaneous large toe dorsiflexion. Subjects kept their eyes open, watching a visual signal. A blinking green square containing the word “MOVE” for voluntary movement and a blinking red square with the word “REST” paced each block and helped subjects maintain their attention. An assistant remained in the room at all times with one hand pressing down on the proximal portion of the brace to help prevent motion artifact. For the passive movement paradigm, the assistant gently dorsiflexed the foot in the brace with one hand, while holding the proximal brace against the scanner table, at the same rate and with the same visual cues viewed by subject and assistant.
All studies were performed using a block design with 30 s of rest alternating with 30 s of cued voluntary or passive ankle dorsiflexion for 10 repetitions (0.3–0.4 Hz). We present passive movement data for the control subjects only. This rate is similar to that of dorsiflexor movements during walking at casual speeds. The rate was also slow enough not to evoke synergistic muscle contractions. We found no differences in regions activated or in peak activation within contralateral primary sensorimotor cortex (S1M1) or supplementary motor area (SMA) for rates of dorsiflexion between 8 and 12 per 30 s (data not shown). Thus, the frequency, direction, and degrees of motion during ankle dorsiflexion were held constant for all voluntary and passive movements. The force of voluntary movement was relatively constant over time of improvement in that the only requirement of the subject was to overcome the minimal friction of resistance of the articulated ankle device and ankle soft tissues, equivalent to 10° of dorsiflexion against gravity. Each activation paradigm lasted 4.5 min with four epochs of movement.
Movement homunculus
To determine the location of movement representations that may become active over the course of behavioral gains, we built a homunculus based on four control subjects who were able to isolate the following movements. Using the same block design, subjects performed rest versus movement at 0.4 Hz for 10° of toe dorsiflexion and then ankle dorsiflexion against no resistance while in the articulated ankle device, then a just visible distal quadriceps contraction about the knee that did not extend to any hip movement, and then an isolated contraction of the low thoracolumbar paraspinal and lateral oblique abdominal muscles, all on the right hemibody. Subjects practiced outside the scanner. Most of our control subjects could not isolate the unilateral back muscles by visual and palpation inspection outside the scanner, so they were not asked to perform these movements.
Data acquisition
fMRI was obtained using a Siemens Sonata 1.5-T (Erlangen, Germany) scanner optimized for brain studies. A three-dimensional high-resolution T1-weighted data set [1 × 1 × 1 mm voxels, repetition time (TR) = 1970 ms, echo time (TE) = 4.38, inversion time (TI) = 1100, flip angle = 15°, matrix = 256 × 256] was acquired for each volunteer. The T2-weighted echoplanar images (EPIs) [matrix = 64 × 64, 3 × 3 × 4 mm voxels, TR = 2500 ms, TE = 60 ms, flip angle = 80° ] included 25 axial slices with a slice thickness of 4- and 1-mm gap oriented parallel to the anterior–posterior commissure line and covering the whole brain. A total of 108 volumes were acquired.
Functional sessions began with an initial sagittal localizer scan, followed by autoshimming to maximize field homogeneity. To normalize spatial relations and help pinpoint regions of interest for the analysis of data within subjects, a set of high-resolution (25 contiguous axial slices, 1.5 × 0.8 mm in-plane × 4 mm thick, 1-mm skip) inversion time T1-weighted echo-planar images [TE =15 ms; TR = 600 ms; flip angle = 90°, matrix = 128 × 256] were acquired. The coregistered functional series lasted 4.5 min per run.
Data analysis
Imaging data were analyzed using FSL (FMRIB Software Library, Release 3.1, University of Oxford, www.fmrib.ox.ac.uk/fsl) (Smith et al., 2001). All volumes were realigned spatially to the middle volume to correct for residual head movement. Each subject's functional scan was coregistered to their high-resolution scan using a seven-parameter rigid-body transformation, and data were spatially smoothed using a 5-mm Gaussian filter.
For each functional scan, a voxelwise t test contrasted active and rest states within each voxel over time. The results were expressed as a cluster-based Z-map. All activation maps were inspected for evidence of misregistration (e.g., edge effects, rims of activity along the cortical surface, absence of activity). We used a cutoff value of Z = 2.3, (corresponding to a P value of 0.01, corrected) to calculate the mean number of activated pixels above threshold in a priori defined regions associated with motor control. These regions include contralateral and ipsilateral primary motor (M1), primary sensory (S1), cingulate motor (CMA), supplementary motor area (SMA), premotor (PM), prefrontal, and secondary somatosensory cortices (SII). Anatomic landmarks were used to define these regions of interest (ROIs) for each subject. S1M1 was defined by the paracentral lobule, medial to the juncture of the superior frontal sulcus and the precentral sulcus (anterior) and the ascending branch of the cingulate sulcus (posterior). SMA was defined as the portion of the mesial premotor cortex lying behind the vertical plane passing through the anterior commissure. CMA was defined by the cingulate cortex from the ascending branch of cingulate sulcus (posterior) and behind the vertical plane passing through the anterior commissure (anterior). The numbers of voxels activated for each subject within each ROI were then averaged for each group. For control subjects, each Z-map was registered to a standard template based on the Montreal Neurological Institute (MNI) reference brain supplied by FSL. These coordinates were converted into stereotaxic space (Talairach and Tournoux, 1988) using a nonlinear transformation (www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html).
Locomotor training
Body weight-supported treadmill training (BWSTT) is a task-oriented approach for ambulation (Barbeau, 2003; Dobkin, 1999). The strategy allows investigators to manipulate the intensity and duration of task-oriented practice, as well as variables such as walking speed, number of steps practiced, and kinematics. The therapist employs different levels of weight support and treadmill speeds, and most importantly, assists the step pattern with physical and verbal cues to optimize the temporal, kinematic, and kinetic parameters of the step cycle (Sullivan et al., 2002). Treadmill speeds averaged 1.5–2.5 mph, which may be too fast for practice by disabled subjects undergoing conventional therapy over ground. Practice on the treadmill continued with training over ground for a total of 1 h of activity. Other training strategies that provide the practice parameters most associated with learning a new skill (Dobkin, 1999; Duncan et al., 2003; Winstein et al., 2004) can also benefit patients.
Behavioral outcomes
Walking speed for a 50-ft walk and walking distance in 6 min were performed at the fastest pace at which each subject felt safe before each fMRI study. These tasks are valid and reliable measures of walking skills (Steffen et al., 2002). The Fugl-Meyer Assessment was also carried out. It is a valid and reliable measure of selective movement control for patients with stroke (Gladstone et al., 2002). Temporal features of the gait cycle were measured using five integrated accelerometers (Min-iSun LLC, Fresno, CA). One was placed on the chest, one on each anterior thigh, and one under each foot. Behavioral and fMRI studies were performed at two baselines and after 2, 4, and 8 weeks of training.
Results
Healthy control subjects
Twelve subjects, ages 25 to 70 years old (mean age 55), were studied during active ankle dorsiflexion. Fig. 1 shows the distribution of movement representations. For voluntary dorsiflexion, activations were found in contralateral S1M1, SMA, and CMA, as well as bilateral SII and contralateral more than ipsilateral thalamus, basal ganglia and dorsolateral prefrontal cortices (dlpfc), and ipsilateral cerebellar hemisphere. For contrast, we also performed passive ankle dorsiflexion in the same device at the same rate. Overlapping activity was found in contralateral S1M1, SMA, and CMA, and bilateral SII and prefrontal cortices. Passive movement produced lower peak activations over a more confined area than voluntary dorsiflexion, except within bilateral SII. Tables 1A and 1B tabulate the Talairach coordinates of activity in the ROIs.
Fig. 1.
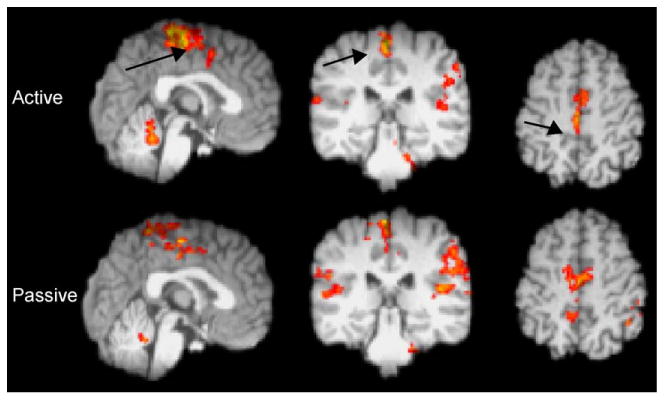
fMRI activations for 12 healthy controls during voluntary and passive ankle dorsiflexion. Left hemisphere on the left in axial view. Arrows at S1M1 on sagittal, coronal, and axial views.
Table 1A.
Talairach coordinates of local maxima of cortical clusters showing significant (P < 0.01, corrected for multiple comparisons) activity associated with voluntary movement of the right ankle
| Functional area | Area | Contralateral | Z | Ipsilateral | Z | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| x | y | z | x | y | z | ||||
| M1S1 | BA 4 | −1 | −32 | 56 | 5.42 | – | – | – | – |
| SMA | BA 6 | −3 | −21 | 48 | 5.23 | – | – | – | – |
| SII | BA 40 | −34 | −38 | 14 | 4.13 | 43 | −29 | 18 | 4.55 |
| Insula | BA 6, 13 | −41 | 4 | −2 | 3.92 | 34 | 10 | −2 | 4.24 |
| Cingulate | BA 24 | −10 | −7 | 42 | 4.86 | – | – | – | – |
| Inferior parietal | BA 40 | – | – | – | – | 52 | −27 | 25 | 4.03 |
| Thalamus | LPN | −17 | −21 | 11 | 4.64 | – | – | – | – |
| Cerebellum | Anterior | −1 | −46 | −24 | 4.28 | 6 | −42 | −22 | 4.46 |
| Pons | −10 | −40 | −31 | 3.92 | 15 | −38 | −30 | 4.13 | |
| Table 1B: Talairach coordinates of local maxima of cortical clusters showing significant (P < 0.01, corrected for multiple comparisons) activity associated with passive movement of the right ankle | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Functional area | Area | Contralateral | Z | Ipsilateral | Z | ||||
|
|
|
||||||||
| x | y | z | x | y | z | ||||
| M1 | BA 4 | −4 | −34 | 58 | 4.41 | – | – | – | – |
| SMA | BA 6 | −4 | −21 | 59 | 3.99 | – | – | – | |
| SI | BA 3, 1, 2 | −13 | −38 | 58 | 3.91 | – | – | – | |
| SII | BA 40 | −41 | −29 | 12 | 3.88 | 40 | −30 | 12 | 4.01 |
| Insula | BA 6, 13 | 48 | 6 | 4 | 3.75 | ||||
| Inferior parietal | BA 40 | – | – | – | – | 41 | −50 | 43 | 4.01 |
| Thalamus | LPN | 31 | 3 | −5 | 3.11 | ||||
| Cerebellum | Anterior | – | – | – | – | 3 | −42 | −24 | 3.71 |
| Pons | – | – | – | – | 10 | −38 | −27 | 3.09 | |
Healthy control homunculus
Fig. 2 shows the locations of peak activations on an axial view for a subject during rest versus movement at 0.4 Hz for toe dorsiflexion, ankle dorsiflexion, quadriceps contraction, and low thoracolumbar paraspinal muscle contraction, all on the right hemibody. The paraspinal muscles include segmental deep multifidus groups and nonsegmental long fibers of the iliocostalis dorsi and lumbrum and the longissimus dorsi. Voluntary movement in the subjects tested also activated the oblique thoracoabdominal muscles. The study revealed differences in the peak sites of activity, all of which are regions that may participate in a representational expansion for ankle movement. For example, the most lateral activation from the midline occurs for low back groups, just medial to the representation for the wrist in M1S1 in this axial plane.
Fig. 2.
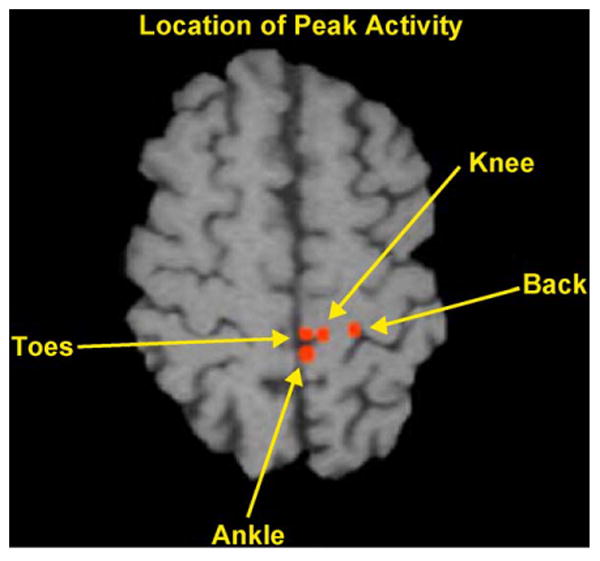
Homunculus of peak BOLD signal during isolated right-sided movement of the thoracolumbar paraspinal muscles, quadriceps, tibialis anterior, and extensor hallicus in a normal subject. Left hemisphere is on the right of the axial slice.
Reorganization with training
Four chronic hemiparetic patients described in Table 2 received 1 h of BWSTT and practice walking over ground 3 days a week in blocks of 2-week sessions for 10 weeks. Despite their longstanding disability, training-induced behavioral gains evolved, along with adaptations within the motor network for control of the ankle.
Table 2.
Data for hemiparetic adult subjects shown in Fig. 3
| Subject | Months since stroke | Location of lesion | Fugl-Meyer initial leg motor score (max = 34) | F-M at 24 sessions | Initial walking speed (cm/s) | Speed after 12 sessions (cm/s) | Speed after 30 sessions (cm/s) |
|---|---|---|---|---|---|---|---|
| 1 | 18 | Left cortical/subcortical | 15 | 23 | 65 | 90 | 112 |
| 2 | 12 | Right internal capsule | 17 | 25 | 55 | 70 | 92 |
| 3 | 45 | Right basis pontis | 18 | 27 | 70 | 85 | 102 |
| 4 | 53 | Right basal ganglia/capsule | 12 | 22 | 60 | 82 | 115 |
Fig. 3 compares the evoked activations for midline structures within these subjects during voluntary dorsiflexion of the affected lower extremity at baseline and after 6 (2 weeks) and 12 (4 weeks), 18 (6 weeks) and 30 (10 weeks) sessions of training. The hemiparetic subjects activated fewer voxels at baseline with voluntary dorsiflexion than control subjects in M1S1, consistent with having available fewer cortical motor neurons for the task. Fig. 4 shows the normalized voxel changes for three bilateral ROIs at baseline and after each bout of training. The three subjects with a subcortical stroke evolved increases in counts within contralateral S1M1 and cingulate motor area by 2–6 weeks of training, followed by declines as behavioral gains reached a plateau. Over the time of functional gains, activation increased, then decreased in the low back representation of S1M1 in subjects 2, 3, and 4. Ipsilateral S1M1 decreased its activity as training gains were made. For Subject 1, greater activity evolved posterior and lateral to the rim of the frontal infarct, peaking at the last study. Other regions of interest were transiently more and then less active, including secondary sensory cortex bilaterally and CMA contralateral to the paretic ankle, and in 3 of 4 subjects, in the contralateral prefrontal area before behavioral gains reached a plateau.
Fig. 3.
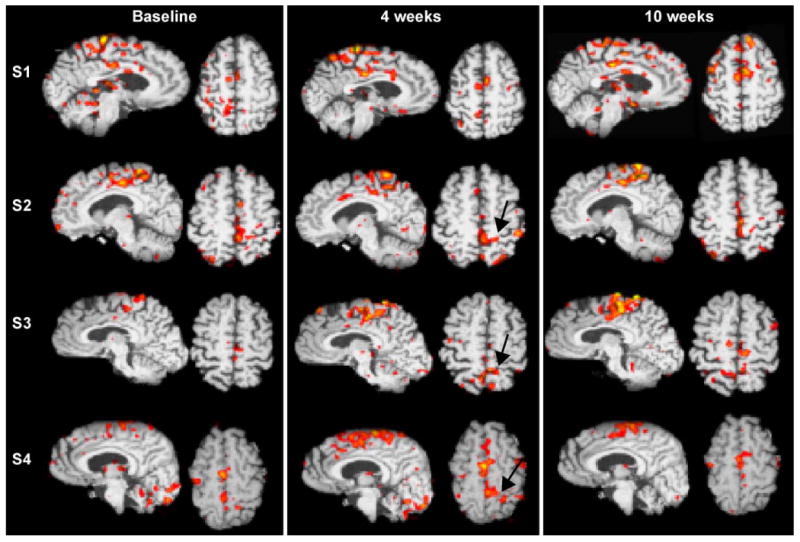
Serial single level sagittal and axial (left cerebrum on left) fMRI studies of Subjects (S) 1– 4 in Table 2 as they voluntarily dorsiflex the paretic ankle over the course of making behavioral gains with rehabilitation therapy for walking. Arrows show the representation for the thoracolumbar paraspinal muscles.
Fig. 4.
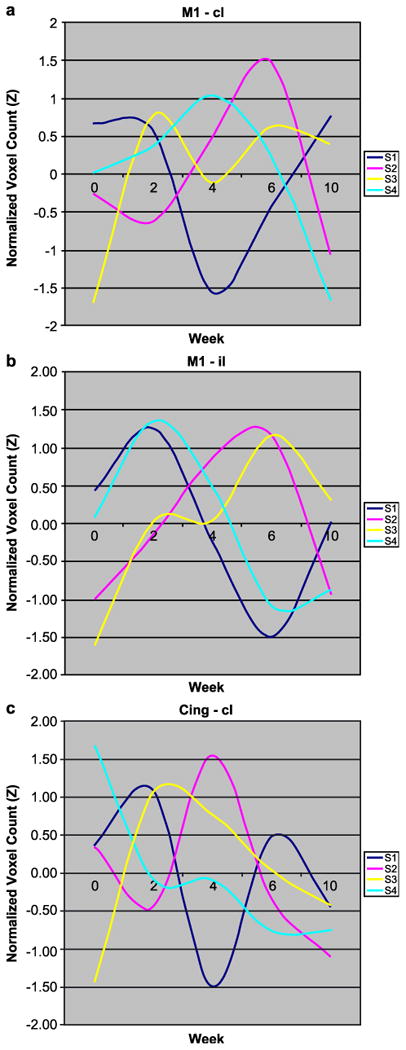
The number of voxels activated (Z > 2.3, P = 0.01) within the manually drawn regions of interest for each subject in Fig. 3 were tabulated and normalized at baseline for follow-up at 2, 4, 6, and 10 weeks of rehabilitation for walking. M1 (contralateral, cl) to the paretic foot (a) and ipsilateral (il) (b) consistently showed a rise, followed by a decline in activated voxels as behavioral gains progressed for the Subjects 2, 3, and 4 with subcortical strokes. A similar rise followed by a decline was evident in cingulate motor area cl (c). Subject 1, who had a frontal infarct, had a pattern of decreasing activation. The responses in supplementary motor areas changed little over time.
On fMRI, no significant differences were found in the locations and z scores of activity for dorsiflexion of the affected or unaffected ankle at repeated baseline studies performed 2 weeks apart before training. Stability in the location and magnitude of activations for the unaffected ankle was found throughout training, which adds to the weight of evidence that the BOLD responses for the affected ankle represent real changes in a stable MR environment.
Performance measures
Clinical and statistically significant gains in 50-ft walking speed and walking distance in 6 min were made by the group between baseline and sessions 12 and 30 (P = 0.001, fixed effects model) for mean walking speed and mean distance walked (1300 ± 202 ft). Scores on the Fugl-Meyer Assessment of selective motor control for the lower extremities also improved significantly across sessions (P = 0.001). The ratio of single limb to double limb stance time became more equal for each foot by 30 sessions as well, changing to a higher ratio as walking speed increased. A higher ratio is also present in healthy control subjects as walking speed increases. In addition, pulling acceleration of the affected thigh decreased toward normal forces and acceleration force of the foot increased toward normal levels over the course of bouts of training for each subject. The four subjects developed heel strike rather than foot flat onset of the stance phase by the end of the first pulse of therapy.
Discussion
We tested an fMRI activation paradigm for its ability to reflect the changing phases of improving motor control for walking. The active movement paradigm repeated over the time of training maintained the same frequency of dorsiflexion. It also aimed for a consistent minimal force within the constrained kinematics provided by a minimally resistant, dorsiflexion-only ankle device. The healthy control fMRI data for voluntary and passive ankle dorsiflexion are similar to prior reports in the location of ROIs (Sahyoun et al., 2004). Passive movement produced less activation within S1M1 and SMA than voluntary ankle dorsiflexion, but more activity in SII.
In this small pilot study of diverse subjects, some general relationships were found between the evolution of patterns of the BOLD response during an fMRI paradigm of kinematically reproducible ankle dorsiflexion and gains in motor control for walking over the course of pulses of locomotor training. The hemiparetic subjects improved their walking speed and endurance with the first pulse of 2–4 weeks of therapy. The induced pattern of fMRI activation, such as an increase in the magnitude or representational extent for the movement, raised the possibility that further behavioral gains were feasible. It is unlikely that additional physical therapy would have been offered these subjects under usual rehabilitation practices, given the initial solid behavioral gains. Subsequent improvement in walking speed and endurance evolved with a second pulse of task-specific therapy. The fMRI pattern in contralateral S1M1, for example, increased and then decreased over the time of training. Contralateral CMA and bilateral SII increased in magnitude of activity with successful training, then declined by the time of the final increment in gains. Dorsolateral prefrontal cortex was transiently active before three subjects reached a behavioral plateau.
Future studies may want to focus on these regions in relationship to stages of attainment of maximal motor skills. All of our subjects made significant gains in parameters of walking over time of treatment. It will be of value to identify patients who do not make functional gains, to determine if fMRI patterns of activation differ from those in subjects who do improve.
Ankle dorsiflexion then proved to be a valid fMRI activation paradigm in parallel with greater motor control for walking that in part depends upon better flexor movements of the affected leg. After a moderately severe hemiparetic stroke, gait velocity is especially slowed by paresis of the affected hip and knee extensors, but asymmetries between the affected and unaffected leg relate best to poor ankle dorsiflexion and hypertonicity of the ankle plantarflexors (Hsu et al., 2003). A cluster analysis of the temporal-spatial characteristics and kinematic parameters of gait patterns over the first 6 months after onset of hemiplegic stroke revealed the negative impact of weakness of the tibialis anterior on walking pattern and velocity (Mulroy et al., 2003). Over the time of improved walking, as peak ankle dorsiflexor activity during the swing phase increases, walking velocity increases. Of course, other affected muscles contribute to the four or more general types of hemiparetic gait deviations that are a focus of rehabilitation (Dobkin, 2003a).
Behavioral measures of walking
Walking-related disability is common after hemiplegic stroke. At least 40% of patients who return home after inpatient rehabilitation are too disabled to walk at home and especially in the community 4–6 months after onset of hemiparesis (Lord et al., 2004). This persistently disabled group was the focus of our pilot study. To relate fMRI activity to the success of rehabilitation training, sensitive and valid measures of function are necessary.
Functional scales for level of independence in walking after stroke have a ceiling effect. Instead, we used walking speed among our measures because it reflects the overall pattern of gait, safety from falling, and likelihood of walking in the community (Lord et al., 2004; Steffen et al., 2002). Unrestricted community ambulation after stroke requires walking speeds of 80 ± 15 cm/s (Perry et al., 1995). A prospective observational study of 185 patients admitted for inpatient rehabilitation after stroke found that gait velocity at discharge reached only 55 cm/s (45 cm/s = 1 mph) (Baer and Smith, 2001). For a timed 6-min walk, another one of our measures, hemiparetic persons walk about 40% the distance of community-dwelling elders (Pohl et al., 2004). Thus, stroke leaves most victims walking well below the facility needed for community mobility.
Our subjects were limited community walkers at onset of training, long after they had completed conventional rehabilitation. Walking speed and distance walked in 6 min were clinically meaningful and sensitive outcome measures. Remarkably, the Fugl-Meyer, which measures balance and lower extremity motor control, also significantly improved in these chronically paretic subjects. Thus, they had latent motor abilities brought out by task-specific training. In addition, greater single to double limb stance times, improved temporal symmetries during stance and swing between the paretic and uninvolved leg, and a decrease in the pulling acceleration of the thigh and increase for the foot were found. These behavioral measures appear to offer potentially important insights into training-induced changes in the motor control for walking. The combination of specific activity-induced fMRI findings, improved motor control on the Fugl-Meyer, and improved gait pattern argue for neural-mediated behavioral gains, in addition to a contribution from greater cardiovascular fitness (not measured in this pilot study) that enabled higher walking speeds and distances. In future trials, these outcome measures could be combined in a principle components analysis to measure change over time and relate improvements or lack of gains to patterns of fMRI activity (Ward et al., 2002a). Thus, the outcome measures appeared to be relevant to the conceptual basis for the fMRI activation paradigm, sensitive for change over the time of rehabilitation, and clinically meaningful.
Representations of the locomotor system
Much scientific emphasis has been placed upon the spinal cord's contributions to reciprocal stepping in mammals. Some reviews even suggest that pattern generation and spinal plasticity is the most important aspect of locomotor control in humans (Harkema, 2001). Many postural and automatic aspects of the neural control for quadrupedal walking are managed at the level of the thoracolumbar motor pools and lumbosacral locomotor circuitry, which probably includes central pattern generators (CPG) for stepping (Edgerton and Roy, 2002; Grillner, 2003). After a low thoracic spinal cord transection, the hindlimb motor pools of cats and other mammals can learn to conduct stepping on a moving treadmill belt at various speeds if provided truncal support and hindlimb loading by pulling down the tail. The animals cannot walk over ground (Rossignol et al., 2002). Thus, the CPG requires supraspinal input for even simple locomotion on flat surfaces. During normal quadrupedal walking, direct corticospinal projections to the lumbosacral alpha-motoneuron and interneuron pools, along with spinal inputs from brainstem locomotor regions, seg-mental lumbosacral afferents, and local spinal mechanisms aid in the initiation, maintenance, and coordination of rhythmic stepping (Pearson, 2000; Schallert and Woodlee, 2003). The specific role of spinal organization and reorganization for walking after stroke requires further study in animal models and physiological studies in patients.
Most likely, cortical influences are stronger in human bipeds than mammalian quadrupeds (Nielsen, 2003). Cortical involvement in gait is critical to the conceptual basis for the ankle dorsiflexor fMRI paradigm. Studies in humans have identified potential ROIs that are likely to be modulated by cognitive and physical cues during rehabilitation training for walking.
The fMRI signal preferentially provides information about intracortical processing and inputs to brain regions related to a specific task, rather than to the output of a region (Logothetis et al., 2001). Locomotor inputs that are not related to sound and vision initially arise from spinal segmental sensory information associated with the kinematics, kinetics, and temporal features of movements during skills practice. Sensory feedback from cutaneous and proprioceptive afferents alters corrective reactions, for example, at the level of the cord as well as within the cortex via a transcortical reflex pathway. Some sensory inputs in humans differ in their impact on spinal feedback compared to quadrupeds. For example, the drive from length-sensitive muscle afferents during ankle plantarflexion in the stance phase is a unique signal in man and critical for efficient bipedal walking. Such sensory drives for motor reorganization presumably played an important role in the brain-behavior changes we observed through our training and fMRI activation paradigms.
Bipedal walking required a number of evolutionary musculo-skeletal adaptations and accompanying neural computations to manage human locomotion (Nielsen, 2003). In a demonstration of the greater involvement of cortical motor neurons in humans, TMS was found to inhibit the corticospinal contribution to the electromyographic activity of large leg muscles during walking, presumably via monosynaptic projections to spinal motor neurons (Petersen et al., 2001). Other studies in humans are especially relevant to the neuroimaging of ankle dorsiflexor adaptations resulting from rehabilitation interventions for ambulation. TMS reveals greater activation of corticospinal input to the tibialis anterior muscle compared to the gastrocnemius in normal subjects who walk on a treadmill (Capaday, 2002; Capaday et al., 1991; Petersen et al., 2001). The spinal motoneurons for the ankle dorsiflexors, like the wrist extensors, have much stronger connections from M1 than found for the wrist flexors and ankle plantar flexors (Bawa et al., 2002). For voluntary tasks that require attention to the amount of motor activity of the ankle movers, M1 motoneurons appear equally linked to the segmental spinal motor pools of the flexors and extensors (Capaday et al., 1991). This finding suggests that activation of M1 is coupled to the timing of spinal locomotor activity in a task-dependent fashion. Specific M1 neurons also represent the contralateral paraspinal muscles and innervate the spinal motor pools for the bilateral abdominal muscles (Murayama et al., 2001). Residual overlapping representations between paraspinal and proximal and distal leg muscle/movement representations (Fig. 3) play a role in postural control during walking. These interconnected neurons may increase their synaptic efficacy by activity-dependent reorganization with gait retraining.
SI has a key role in both the storage and retrieval of representations of sensory information (Harris et al., 2001). SII connects to area 3b, posterior parietal, and prefrontal cortex, regions that became more active with training in most of our impaired subjects. Other cortical regions also participate in aspects of motor control. The SMA is directly connected to M1 and to the spinal cord, whereas pre-SMA connects prefrontal cortex. Anticipation of making an ankle movement compared to preparation for the movement revealed less activation in the medial wall within the SMA and CMA (Sahyoun et al., 2004). Visual feedback needed for walking increases the excitability of M1 neurons (Schubert et al., 1999). With an isometric contraction of the tibialis anterior or gastrocnemius muscles, the bilateral superior parietal (BA 7) and premotor BA 6 are active during PET scanning, probably as a result of an increase in cortical control of initiation and maintenance of the contraction (Johannsen et al., 2001). Parietal and dorsolateral frontal regions may be more active during stages of learning, depending upon the practice strategy. We found increases within these regions after the first pulse of training in Subjects 1 and 3 (data not shown).
The cerebellum also plays an important role in creating and selecting the internal models necessary for movements (Houk and Wise, 1995). Purkinje cells are rhythmically active throughout the step cycle (Orlovsky et al., 1999) for timing and performance aspects of motor control. Neurons of the fastigial and interpositus nuclei burst primarily during the flexor phase of stepping, which includes activation of the tibialis anterior. Inputs from alpha and gamma motor neurons and Ia interneurons, as well as from segmental dorsal root afferents, are copied not only to the cerebellum, but also to cortical motoneurons and to the locomotor regions of the dorsolateral midbrain and pons during walking and ankle dorsiflexion (Jueptner and Weiller, 1998; Muir and Steeves, 1997; Poppele and Bosco, 2003). Such activity, especially linked to the flexors of the leg, adds to the face validity of using an ankle dorsiflexor paradigm during fMRI for voluntary or passive movement. All of these areas are potential ROIs for future studies of strategies to improve walking after stroke.
More complex foot movements
Regional activations may differ when the lower extremity movement paradigm goes beyond simple repetitive dorsiflexion against no resistance. Electrical neuromuscular stimulation of the quadriceps muscle in healthy subjects, for example, leads to a rather linear increase in activated voxels in M1S1, cingulate gyrus, and cerebellum with increasing intensity of the stimulus (Smith et al., 2003), not unlike how an increase in frequency of finger tapping augments network activity. A similar correlation was found between the level of electromyographic activity in the tibialis anterior and regional cerebral blood flow in the cerebellum and M1 (Johannsen et al., 2001). This PET study also found differences in the location of peak activations between isometric dorsiflexor and plantar contraction compared to isotonic contraction.
In a study of interlimb coordination, overlap between the representations for wrist and ankle flexion–extension movements, within the spatial sensitivity of 15O-butanol PET, was reported in the contralateral ventral and dorsal premotor area, SMA, the parietal operculum, and the posterior parietal cortex (Ehrsson et al., 2000). These interactions could be used during training. For example, rhythmical arm swing may reinforce rhythmical stepping after SCI (Grasso et al., 2004). Learning a serial reaction time task that requires sequential ankle dorsiflexion–plantarflexion movements initially activates the bilateral dorsal premotor cortex and cerebellum and contralateral inferior parietal lobule, in addition to the motor network (Lafleur et al., 2002). When the sequential movements are imagined after practice, the rostral anterior cingulate and medial orbitofrontal cortex and striatum are active. A corticocerebellar network is involved in developing the cognitive strategies and motor routines necessary to execute a sequence of foot (and perhaps multijoint leg) movements, and presumably contributes to relearning motor control for walking (Lafleur et al., 2002). Practice leads to frontostriatal deposition of learned actions, allowing for explicit retrieval and long-term maintenance. Gait retraining is likely to require these systems. During the process of successful motor relearning, transient fMRI activations within these regions may be detectable.
Bipedal walking clearly requires a highly integrated sensorimotor network, which includes the human corticomotoneuronal pathway. This network of afferent feedback signals, spinal circuits, and descending pyramidal, extrapyramidal, and brainstem controllers permits great flexibility for the motor control of automatic and more complex lower extremity activities for bipedal walking. The cortical links may be especially important and adaptable for motor learning after stroke. All of these nodes become potential ROIs when using the ankle dorsiflexion fMRI paradigm over the time of locomotor training. Future studies can add more complex activation paradigms to further study relationships between regional activity and behavioral components of gains associated with a training strategy for walking.
Locomotor training-induced plasticity
One repeated-measures functional imaging study of gait has been reported. Investigators assessed the recovery of walking in eight subjects 3–4 months after a hemiparetic stroke using optical imaging with NIRS (Miyai et al., 2003). Regional activation by foot movements alone during NIRS is similar to that of walking on a treadmill when arm and truncal movements are subtracted out (Miyai et al., 2001). Patients were tested before and after inpatient rehabilitation while standing or stepping with partial body weight support on a treadmill. An increase in ipsilesional M1S1 and premotor cortex activity correlated with gains in walking cadence and the swing phase of gait. Premotor and prefrontal cortex may be important for purposeful modification of walking to improve performance. As several of our subjects trained, prefrontal activity increased at one fMRI session, suggesting that we captured a step in which greater working memory activity for motor learning contributed to regaining motor control.
Differences across subjects in regional activations after stroke have many interacting causes. These may include age, handedness, variations between individuals in the strength of residual nodes in a network, and the neural and kinematic strategies for movement that subjects adapt after the stroke. The operational task used during fMRI to test for activity-dependent plasticity may be affected by a changing motor strategy to accomplish the same movement. Given that the isolated ankle dorsiflexor movement was not practiced in the scanner or during therapy and that ankle dorsiflexion was only one of many components practiced during gait training, we do not believe that the evolution of training-induced fMRI signals was influenced by changes in the behavioral strategy for isolated dorsiflexion during scanning. More likely, locomotor training accounted for most of the differences in regional activity. After their stroke, our subjects had learned to walk, each with his or her strategy that included a variety of gait pattern deviations. With the pulses of additional therapy provided by this study, the hemiparetic subjects adapted different strategies that included ankle dorsiflexion at heel strike, which required greater motor control.
In our subjects, representational expansions and contractions within regions of interest such as S1M1 reflect a time- and experience-dependent change in the effective connectivity of interacting regions. Sensory feedback from the ensemble of axial and limb proprioceptive afferents would be expected to influence cortical synaptic efficacy and representational plasticity (Yakovenko et al., 2002). Cortical motor areas that encode distal leg movements during walking may redirect their motor commands to control more proximal segments of the body and leg and generate new patterns of muscle activity to produce rather typical kinematics for the trajectory of the ankle during walking (Grasso et al., 2004). Thus, a lateral expansion of the foot representation toward the hip and paraspinal area, just medial to the representation for the shoulder, is not unexpected, but this is the first description of such a change after stroke and confirms our previous findings after SCI (Dobkin, 2000; Dobkin et al., 2000). With further practice, peak activation moved more medial into its usual distribution. Serial imaging studies over the time of gains in upper extremity function tend to reveal an early increase in task-related cortical activation followed by a decrease that may approach a healthy control subject's distribution of BOLD contrast (Calautti and Baron, 2003; Ward et al., 2002a). Such evidence of recruitment or higher levels of motor neuron excitability followed by lesser activity occurs in other examples of motor learning (Boroojerdi et al., 2001; Karni et al., 1998). The key to capturing such variations is to obtain interim fMRI scans during training. We found for our walking strategy that scanning may be needed after every 6–12 training sessions.
We found a highly reproducible level and location of BOLD signal when we limited the degree of dorsiflexion to 10° at 10 repetitions per 30 s against no resistance. The signal was also consistent in location and magnitude in healthy control subjects for variations from 8–15 repetitions, which falls within the casual walking speed range for frequency of dorsiflexion in patients with stroke. For future investigations of subjects who are undergoing a walking intervention and can dorsiflex the ankle, serial fMRI studies could also employ a fixed percentage of maximum speed or force of dorsiflexion, but this may lead to greater motion artifact and be applicable to only a modest number of impaired subjects. Our pilot studies aimed specifically at finding an activation paradigm that was most likely to be applicable to the majority of hemiparetic patients. Isolated voluntary ankle dorsiflexion, however, may be difficult for patients with upper motor neuron lesions. Passive movement is independent of performance ability, yet may reflect the capacity for sensory adaptations to training. Future studies of passive ankle dorsiflexion will determine if this paradigm can substitute for voluntary movement in subjects who cannot dorsiflex the ankle. Passive movement has shown some potential in several other neuroimaging studies (Loubinoux et al., 2003; Reddy et al., 2002) with significant correlations between dynamic sensorimotor activations for hand function in bilateral inferior BA 40 and continued gains.
Functional relevance
The functional relevance of the evolution of the BOLD signal in our subjects is found in the behavioral gains associated with massed practice of walking that emphasized a reduction of deviations from a normal gait pattern. More consistent use of the ankle dorsiflexors during the stance and swing phases of gait evolved. Task-specific reduction in activation in contralateral sensorimotor regions, including S1M1, CMA, and SII, evolved in response to pulses of specific kinematic retraining, as demonstrated in normal subjects for S1M1 (Morgen et al., 2004). Walking speed, which serves as an overall reflection of kinematics, kinetics, and the temporal features of stance and swing times improved as well. Our subjects also improved in the symmetry of the stance and swing phases of gait. Thus, as in other functional imaging studies that relied on hand and finger movements in patients who showed behavioral gains after upper extremity therapy (Carey et al., 2002; Johansen-Berg et al., 2002; Wittenberg et al., 2003), the changes in the BOLD signal before and after locomotor training likely represent training-induced synaptic plasticity.
Our pilot study adds to the modest literature in humans and the large literature in animals (Schallert and Woodlee, 2003), suggesting that behavioral enrichment procedures that prominently encourage self-initiation of stepping will facilitate plasticity and motor function after brain or spinal cord injury. Specific mechanisms of action for such adaptations may include modulation of Hebbian plasticity and induction of long-term potentiation at a molecular and morphological level, such as neuronal cell precursor proliferation and migration, growth and retraction of dendritic spines, production of neurotrophins, and other biological responses to motor learning (Biernaskie et al., 2004; Cramer and Chopp, 2000; Frost et al., 2003; Gomez-Pinilla et al., 2002; Johansson and Belichenko, 2002; Kleim et al., 2002; Lotze et al., 2003a; Morgen et al., 2004; Rossini et al., 2003; Schaechter and Cramer, 2003; Uy et al., 2003; van Praag et al., 1999). The rapid changes in our subjects are most consistent with representational plasticity via engaged latent synapses, followed by greater learning-associated synaptic efficacy.
Conclusions
Our pilot study suggests that an ankle dorsiflexion fMRI paradigm can assay activity-induced plasticity associated with behavioral gains during training to walk after stroke. More patients must be evaluated to determine whether changes in patterns of ROI activity over the course of rehabilitation can be used to predict gains in motor control, reflected by improved walking speed, endurance, and temporal features of the gait pattern. This aim requires serial fMRI and behavioral studies across patients with a variety of lesion volumes and locations. Subjects must also be trained until their regional activations and behavioral measures become stable, before one can look for meaningful early patterns in retrospect. If one or more reliable brain-behavior relationships that we describe are confirmed, early patterns of evoked BOLD signals during ankle dorsiflexion may serve as a valuable physiological marker of the capacity for near-term and ongoing reorganization-associated rehabilitative gains. Used this way, fMRI may provide insight into optimal types of task-specific therapies for motor learning, as well as the duration of training necessary to maximize the utility of a rehabilitation strategy (Dobkin, 2003b; Matthews and Jezzard, 2004).
Acknowledgments
Studies were sponsored by the Larry L. Hillblom Foundation and the National Institutes of Health under RO1 HD39629 and T32 HD07416, and RR12169, RR13642 and RR08655.
References
- Baer G, Smith M. The recovery of walking ability and subclassification of stroke. Physiother Res Int. 2001;6:135–144. doi: 10.1002/pri.222. [DOI] [PubMed] [Google Scholar]
- Barbeau H. Locomotor training in neurorehabilitation: emerging rehabilitation concepts. Neurorehabilitation Neural Repair. 2003;17:3–11. doi: 10.1177/0888439002250442. [DOI] [PubMed] [Google Scholar]
- Bawa P, Chalmers G, Stewart H, Eisen A. Responses of ankle extensor and flexor motoneurons to transcranial magnetic stimulation. J Neurophysiol. 2002;88:124–132. doi: 10.1152/jn.2002.88.1.124. [DOI] [PubMed] [Google Scholar]
- Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. Neurobiol Dis. 2004;24:1245–1254. doi: 10.1523/JNEUROSCI.3834-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroojerdi B, Ziemann U, Chen R, Butefisch C, Cohen L. Mechanisms underlying human motor system plasticity. Muscle Nerve. 2001;24:602–613. doi: 10.1002/mus.1045. [DOI] [PubMed] [Google Scholar]
- Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke. 2003;34:1553–1566. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- Capaday C. The special nature of human walking and its neural control. Trends Neurosci. 2002;25:370–376. doi: 10.1016/s0166-2236(02)02173-2. [DOI] [PubMed] [Google Scholar]
- Capaday C, Lavoie B, Barbeau H, Schneider C, Bonnard M. Studies on the corticospinal control of human walking: I. Responses to focal transcranial magnetic stimulation of the motor cortex. J Neurophysiol. 1999;81:129–139. doi: 10.1152/jn.1999.81.1.129. [DOI] [PubMed] [Google Scholar]
- Carey J, Kimberley T, Lewis S, Auerbach E, Dorsey L, Rundquist P, et al. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- Cramer S, Chopp M. Recovery recapitulates ontogeny. Trends Neurosci. 2000;23:265–271. doi: 10.1016/s0166-2236(00)01562-9. [DOI] [PubMed] [Google Scholar]
- Debaere F, Swinnen S, Beatse E, Sunaert S, Van Hecke P, Duysens J. Brain areas involved in interlimb coordination: a distributed network. NeuroImage. 2001;14:947–958. doi: 10.1006/nimg.2001.0892. [DOI] [PubMed] [Google Scholar]
- Dobkin B. Recovery of locomotor control. Neurologist. 1996;2:239–249. [Google Scholar]
- Dobkin B. Overview of treadmill locomotor training with partial body weight support: a neurophysiologically sound approach whose time has come for randomized clinical trials. Neurorehabilitation Neural Repair. 1999;13:157–165. [Google Scholar]
- Dobkin B. Spinal and supraspinal plasticity after incomplete spinal cord injury: correlations between functional magnetic resonance imaging and engaged locomotor networks. In: Seil F, editor. Progress in Brain Research. Vol. 128. Elsevier, Amsterdam: 2000. pp. 99–111. [DOI] [PubMed] [Google Scholar]
- Dobkin B. The Clinical Science of Neurologic Rehabilitation. Oxford Univ. Press; New York: 2003a. [Google Scholar]
- Dobkin B. Functional MRI: a potential physiologic indicator for stroke rehabilitation interventions. Stroke. 2003b;34:e23–e24. doi: 10.1161/01.str.0000071140.00153.05. [DOI] [PubMed] [Google Scholar]
- Dobkin B, Sullivan K. Sensorimotor cortex plasticity and locomotor and motor control gains induced by body weight-supported treadmill training after stroke. Neurorehabilitation Neural Repair. 2001;15:258. [Google Scholar]
- Dobkin B, Davis B, Bookheimer S. Functional magnetic resonance imaging assesses plasticity in locomotor networks. Neurology. 2000;54(suppl. 3):A8. [Google Scholar]
- Duncan P, Studenski S, Richards L, Golub S, Lai S, Reker D, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34:2173–2180. doi: 10.1161/01.STR.0000083699.95351.F2. [DOI] [PubMed] [Google Scholar]
- Edgerton V, Roy R. Paralysis recovery in human and model systems. Curr Opin Neurobiol. 2002;12:658–667. doi: 10.1016/s0959-4388(02)00379-3. [DOI] [PubMed] [Google Scholar]
- Ehrsson H, Naito E, Geyer S, Amunts K, Zilles K, Forssberg H, et al. Simultaneous movements of upper and lower limbs are coordinated by motor representations that are shared by both limbs: a PET study. Eur J Neurosci. 2000;12:3385–3398. doi: 10.1046/j.1460-9568.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- Frost S, Barbay S, Friel K, Plautz E, Nudo R. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2003;89:3205–3214. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- Fukuyama H, Ouchi Y, Matsuzaki S, Nagahama Y, Yamauchi H, Ogawa M, et al. Brain functional activity during gait in normal subjects: a SPECT study. Neurosci Lett. 1997;228:183–186. doi: 10.1016/s0304-3940(97)00381-9. [DOI] [PubMed] [Google Scholar]
- Gladstone D, Danells C, Black S. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabilitation Neural Repair. 2002;16:232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy R, Molteni R, Edgerton V. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Grasso R, Ivanenko YP, Zago M, Molinari M, Scivoletto G, Castellano V, et al. Distributed plasticity of locomotor pattern generators in spinal cord injured patients. Brain. 2004;127:1019–1034. doi: 10.1093/brain/awh115. [DOI] [PubMed] [Google Scholar]
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev, Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- Harkema S. Neural plasticity after human spinal cord injury: application of locomotor training to the rehabilitation of walking. Neuroscientist. 2001;7:455–468. doi: 10.1177/107385840100700514. [DOI] [PubMed] [Google Scholar]
- Harris J, Petersen R, Diamond M. The cortical distribution of sensory memories. Neuron. 2001;30:315–318. doi: 10.1016/s0896-6273(01)00300-2. [DOI] [PubMed] [Google Scholar]
- Houk J, Wise S. Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: their role in planning and controlling action. Cereb Cortex. 1995;5:95–110. doi: 10.1093/cercor/5.2.95. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Tang PF, Jan MH. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch Phys Med Rehabil. 2003;84:1185–1193. doi: 10.1016/s0003-9993(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Johannsen P, Christensen L, Sinkjaer T, Nielsen J. Cerebral functional anatomy of voluntary contractions of ankle muscles in man. J Physiol. 2001;535(2):397–406. doi: 10.1111/j.1469-7793.2001.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Dawes H, Guy C, Smith S, Wade D, Matthews P. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125:2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- Johansson B, Belichenko P. Neuronal plasticity and dendritic spines: effect of environmental enrichment on intact and postischemic rat brain. J Cereb Blood Flow Metab. 2002;22:89–96. doi: 10.1097/00004647-200201000-00011. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Weiller C. A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain. 1998;121:1437–1449. doi: 10.1093/brain/121.8.1437. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Hipolito C, Jezzard P, Adams M. The acquisition of skilled motor performance: fast and slow experiencedriven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim J, Barbay S, Cooper N, Hogg T, Reidel C, Remple M, et al. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- Kollias S, Alkadhi H, Jaermann T, Crelier G, Hepp-Raymond M-C. Identification of multiple nonprimary motor cortical areas with simple movements. Brain Res Rev. 2001;36:185–195. doi: 10.1016/s0165-0173(01)00094-7. [DOI] [PubMed] [Google Scholar]
- Lafleur M, Jackson P, Malouin F, Richards C, Evans A, Doyon J. Motor learning produces parallel dynamic functional changes during the execution and imagination of sequential foot movements. NeuroImage. 2002;16:142–157. doi: 10.1006/nimg.2001.1048. [DOI] [PubMed] [Google Scholar]
- Lehericy S, van de Moortele PF, Lobel E, Paradis AL, Vidailhet M, Le Bihan D, et al. Somatotopical organization of striatal activation during finger and toe movement: a 3-T functional magnetic resonance imaging study. Ann Neurol. 1998;44:398–404. doi: 10.1002/ana.410440319. [DOI] [PubMed] [Google Scholar]
- Liepert J, Bauder H, Miltner W, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- Logothetis N, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Lord S, McPherson K, McNaughton H, Rochester L, Weatherall M. Community ambulation after stroke: how important and obtainable is it and what measures appear predictive? Arch Phys Med Rehabil. 2004;85:234–239. doi: 10.1016/j.apmr.2003.05.002. [DOI] [PubMed] [Google Scholar]
- Lotze M, Erb M, Flor H, Huelsmann E, Godde B, Grodd W. fMRI evaluation of somatotopic representation in human primary motor cortex. NeuroImage. 2000;11:473–481. doi: 10.1006/nimg.2000.0556. [DOI] [PubMed] [Google Scholar]
- Lotze M, Braun C, Birbaumer N, Anders S, Cohen L. Motor learning elicited by voluntary drive. Brain. 2003a;126:866–872. doi: 10.1093/brain/awg079. [DOI] [PubMed] [Google Scholar]
- Lotze M, Kaethner R, Erb M, Cohen L, Grodd W, Topka H. Comparison of representational maps using functional magnetic resonance imaging and transcranial magnetic stimulation. Clin Neurophysiol. 2003b;114:306–312. doi: 10.1016/s1388-2457(02)00380-2. [DOI] [PubMed] [Google Scholar]
- Loubinoux I, Carel C, Pariente J, Dechaumont S, Albucher JF, Marque P, Manelfe C, et al. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. NeuroImage. 2003;20:2166–2180. doi: 10.1016/j.neuroimage.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Luft A, Smith G, Forrester L, Whitall J, Macko R, Hanley D. Comparing brain activation associated with isolated upper and lower limb movement across corresponding joints. Hum Brain Mapp. 2002;17:131–140. doi: 10.1002/hbm.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P, Jezzard P. Functional magnetic resonance imaging. J Neurol, Neurosurg Psychiatry. 2004;75:6–12. [PMC free article] [PubMed] [Google Scholar]
- Miyai I, Tanabe H, Sase I, Eda H, Oda I, Konishi I, et al. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. NeuroImage. 2001;14:1186–1192. doi: 10.1006/nimg.2001.0905. [DOI] [PubMed] [Google Scholar]
- Miyai I, Yagura H, Oda I, Konishi I, Eda H, Suzuki T, et al. Premotor cortex is involved in restoration of gait in stroke. Ann Neurol. 2002;52:188–194. doi: 10.1002/ana.10274. [DOI] [PubMed] [Google Scholar]
- Miyai I, Yagura H, Hatakenaka M, Oda I, Konishi I, Kubota K. Longitudinal optical imaging study for locomotor recovery after stroke. Stroke. 2003;34:2866–2870. doi: 10.1161/01.STR.0000100166.81077.8A. [DOI] [PubMed] [Google Scholar]
- Morgen K, Kadom N, Sawaki L, Tessitore A, Ohayon J, Frank J, et al. Kinematic specificity of cortical reorganization associated with motor training. NeuroImage. 2004;21:1182–1187. doi: 10.1016/j.neuroimage.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Muir G, Steeves J. Sensorimotor stimulation to improve locomotor recovery after spinal cord injury. Trends Neurosci. 1997;20:72–77. doi: 10.1016/s0166-2236(96)10068-0. [DOI] [PubMed] [Google Scholar]
- Mulroy S, Gronley J, Weiss W, Newsam C, Perry J. Use of cluster analysis for gait pattern classification of patients in the early and late recovery phases following stroke. Gait Posture. 2003;18:114–125. doi: 10.1016/s0966-6362(02)00165-0. [DOI] [PubMed] [Google Scholar]
- Murayama N, Lin YY, Salenius S, Hari R. Oscillatory interaction between human motor cortex and trunk muscles during isometric contraction. NeuroImage. 2001;14:1206–1213. doi: 10.1006/nimg.2001.0907. [DOI] [PubMed] [Google Scholar]
- Nelles G, Jentzen W, Jueptner M, Muller S, Diener H. Arm training induced brain plasticity in stroke studies with serial positron emission tomography. NeuroImage. 2001;13:1146–1154. doi: 10.1006/nimg.2001.0757. [DOI] [PubMed] [Google Scholar]
- Nielsen J. How we walk: central control of muscle activity during human walking. Neuroscientist. 2003;9:195–204. doi: 10.1177/1073858403009003012. [DOI] [PubMed] [Google Scholar]
- Orlovsky G, Deliagina T, Grillner S. Neuronal Control of Locomotion: From Mollusc to Man. Oxford Univ. Press; Oxford, England: 1999. [Google Scholar]
- Ouchi Y, Okada Y, Yoshikawa E, Nobezawa S, Futatsubashi M. Brain activation during maintenance of standing postures in humans. Brain. 1999;122:329–338. doi: 10.1093/brain/122.2.329. [DOI] [PubMed] [Google Scholar]
- Pearson K. Neural adaptation in the generation of rhythmic behavior. Annu Rev Physiol. 2000;62:723–753. doi: 10.1146/annurev.physiol.62.1.723. [DOI] [PubMed] [Google Scholar]
- Perry J, Garrett M, Gromley J, Mulroy S. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–989. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- Petersen N, Butler J, Marchand-Pauvert V, Fisher R, Ledebt A, Pyndt H, et al. Suppression of EMG activity by transcranial magnetic stimulation in human subjects during walking. J Physiol. 2001;532(2):651–656. doi: 10.1111/j.1469-7793.2001.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl P, Perera S, Duncan P, Maletsky R, Whitman R, Studenski S. Gains in distance walking in a 3-month follow-up poststroke: what changes? Neurorehabilitation Neural Repair. 2004;18:30–36. doi: 10.1177/0888439003260494. [DOI] [PubMed] [Google Scholar]
- Poppele R, Bosco G. Sophisticated spinal contributions to motor control. Trends Neurosci. 2003;26:269–276. doi: 10.1016/S0166-2236(03)00073-0. [DOI] [PubMed] [Google Scholar]
- Reddy H, Narayanan S, Woolrich M, Mitsumori T, Lapierre Y, Arnold D, et al. Functional brain reorganization for hand movement in patients with multiple sclerosis: defining distinct effects of injury and disability. Brain. 2002;125:2646–2657. doi: 10.1093/brain/awf283. [DOI] [PubMed] [Google Scholar]
- Roelcke U, Curt A, Otte A, Missimer J, Maguire R, Dietz V, et al. Influence of spinal cord injury on cerebral sensorimotor systems: a PET study. J Neurol, Neurosurg Psychiatry. 1997:61–65. doi: 10.1136/jnnp.62.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Bouyer L, Barthelemy D, Langlet C, Leblond H. Recovery of locomotion in the cat following spinal cord lesions. Brain Res Rev. 2002;40:257–266. doi: 10.1016/s0165-0173(02)00208-4. [DOI] [PubMed] [Google Scholar]
- Rossini P, Calautti C, Pauri F, Baron JC. Post-stroke plastic reorganization in the adult brain. Lancet Neurol. 2003;2:493–502. doi: 10.1016/s1474-4422(03)00485-x. [DOI] [PubMed] [Google Scholar]
- Sabbah P, De Schonen S, Leveque C, Gay S, Pfefer F, Nioche C, et al. Sensorimotor cortical activity in patients with complete spinal cord injury: a functional magnetic resonance imaging study. J Neurotrauma. 2002;19:53–60. doi: 10.1089/089771502753460231. [DOI] [PubMed] [Google Scholar]
- Sahyoun C, Floyer-Lea A, Johansen-Bereg H, Matthews P. Towards an understanding of gait control: brain activation during the anticipation, preparation and execution of foot movements. NeuroImage. 2004;21:568–575. doi: 10.1016/j.neuroimage.2003.09.065. [DOI] [PubMed] [Google Scholar]
- Schaechter J, Cramer S. Effects of experience after stroke on brain and behavior. Neurol Rep. 2003;27:67–74. [Google Scholar]
- Schallert T, Woodlee M. Brain-dependent movements and cerebral-spinal connections: key targets of cellular and behavioral enrichment in CNS. J Rehabil Res Dev. 2003;40:9–18. doi: 10.1682/jrrd.2003.08.0009. [DOI] [PubMed] [Google Scholar]
- Schubert M, Curt A, Colombo G, Berger W, Dietz V. Volun tary control of human gait: conditioning of magnetically evoked motor responses in a precision stepping task. Exp Brain Res. 1999;126:583–588. doi: 10.1007/s002210050767. [DOI] [PubMed] [Google Scholar]
- Seto E, Sela G, McIlroy W, Black S, Staines W, Bronskill M, et al. Quantifying head motion associated with motor tasks used in fMRI. NeuroImage. 2001;14:284–297. doi: 10.1006/nimg.2001.0829. [DOI] [PubMed] [Google Scholar]
- Smith S, Bannister P, Beckmann C, Brady M, Clare S, Flitney D, et al. FSL: new tools for functional and structural brain image analysis. Seventh International Conference on Functional Mapping of the Human Brain 2001 [Google Scholar]
- Smith G, Alon G, Roys S, Gullapalli R. Functional MRI determination of a dose–response relationship to lower extremity neuromuscular electrical stimulation in healthy subjects. Exp Brain Res. 2003;150:33–39. doi: 10.1007/s00221-003-1405-9. [DOI] [PubMed] [Google Scholar]
- Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: six-minute walk test, berg balance scale, timed up and go test, and gait speeds. Phys Ther. 2002;82:128–137. doi: 10.1093/ptj/82.2.128. [DOI] [PubMed] [Google Scholar]
- Sullivan K, Knowlton B, Dobkin B. Step training with body weight support: effect of treadmill speed and practice paradigms on post-stroke locomotor recovery. Arch Phys Med Rehabil. 2002;83:683–691. doi: 10.1053/apmr.2002.32488. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: A 3-Dimensional Proportional System, An Approach to Cerebral Imaging. Thieme Medical; Stuttgart: 1988. [Google Scholar]
- Uy J, Ridding MC, Hillier S, Thompson P, Miles T. Does induction of plastic change in motor cortex improve leg function after stroke? Neurology. 2003;61:982–984. doi: 10.1212/01.wnl.0000078809.33581.1f. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage F. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Ward N, Brown M, Thompson A, Frackowiak R. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003a;126:1–21. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward N, Brown N, Thompson A, Frackowiak R. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003b;126:1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstein C, Rose D, Tan S, Lewthwaite R, Chui H, Azen S. A randomized controlled comparison of upper-extremity rehabilitation strategies in acute stroke: a pilot study of immediate and long-term outcomes. Arch Phys Med Rehabil. 2004;85:620–628. doi: 10.1016/j.apmr.2003.06.027. [DOI] [PubMed] [Google Scholar]
- Wittenberg GF, Chen R, Ishii K, Bushara KO, Taub E, Gerber LH, et al. Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabilitation Neural Repair. 2003;17:48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- Yakovenko S, Mushahwar V, VanderHorst V, Holstege G, Prochazka A. Spatiotemporal activation of lumbosacral motoneurons in the locomotor step cycle. J Neurophysiol. 2002;87:1542–2555. doi: 10.1152/jn.00479.2001. [DOI] [PubMed] [Google Scholar]


