Abstract
A study now links platelet generation and cholesterol metabolism, providing new understanding of the mechanisms involved in thrombocytosis and atherogenesis. The authors show that the cholesterol transporter ABCG4 is highly expressed in bone marrow megakaryocyte progenitors, and in its absence, these cells have defective cholesterol efflux and increased proliferation, leading to increased megakaryocyte production, thrombocytosis and accelerated atherogenesis in atherosclerosis-prone mice (pages 586–594).
Platelets are small (2–3 µm in diameter) anucleated, highly specialized cells that are best known for their role in hemostasis and thrombosis. They participate in a diverse array of pathophysiological processes, including angiogenesis, tumor growth and metastasis, innate and adaptive immune responses, and chronic inflammation–associated pathologies such as atherosclerosis1. Platelets play an important part in all stages of atherosclerotic lesion development: initiation, progression and stability2. Notably, these cells have a crucial role in atherothrombosis and subsequent acute cardiovascular events triggered by plaque rupture or erosion. Control of platelet reactivity is regarded as a key target in the prevention of acute cardiovascular events and is currently achieved via inhibition of platelet activation, aggregation or both2,3.
Platelets are produced through a unique and highly unusual process in which the very large (up to 50–60 µm in diameter) polyploid precursor cell, called the megakaryocyte, sheds (or, according to an alternative hypothesis, disintegrates into) platelets. This process occurs mostly in bone marrow, where megakaryocytes extend long cytoplasmic processes into vascular sinusoids4. Normally, megakaryocytes account for a very low percentage of bone marrow cells. The increased demand for platelets stimulates megakaryocyte production in bone marrow, leading to increased platelet generation. Mutations leading to the dysregulation of megakaryocyte production can lead to either overproduction of platelets, as in essential thrombocytosis, or severe thrombocytopenia in conditions such as congenital amegakaryocytic thrombocytopenia5. Both conditions can be life threatening: thrombocytopenia can lead to death due to an increased risk of bleeding, and thrombocythemia can cause death through an increased risk of myocardial infarction and occlusive stroke. Owing to its translational potential, the regulation of megakaryocyte production is a very active area of research. Megakaryocyte production is regulated by multiple growth factors, of which thrombopoietin signaling through its receptor c-MPL is the best known and probably the most important.
Whereas thrombocythemia is a less well-known risk factor for thrombosis, disturbed lipoprotein and cholesterol metabolism is a major risk factor for atherosclerosis-associated thrombosis. Cholesterol is a key component of the cell membrane, and its concentration there has a profound effect on the membrane assembly of signaling protein complexes and, consequently, on cell function. Accordingly, mammalian cells have evolved complex feedback mechanisms to ensure a sufficient supply of cholesterol but prevent its excessive accumulation. In dyslipidemia and/or chronic inflammation, these homeostatic mechanisms fail in cells such as macrophages in atherosclerotic lesions. This can lead to an accumulation of macrophages in the artery wall and to atherosclerotic lesion progression, subsequent eventual rupture or erosion—thus triggering atherothrombosis. Interestingly, it has also been shown that dyslipidemia raises the risk of fatal thrombotic events by increasing platelet reactivity via several mechanisms2.
In this issue of Nature Medicine, Murphy et al.6 now connect two seemingly separate research areas, platelet production and cholesterol metabolism, and reveal a previously unknown mechanism by which megakaryopoiesis is regulated. They show that disturbed cholesterol metabolism in megakaryocyte precursor cells can lead to their enhanced proliferation, the expansion of the megakaryocyte pool and thrombocythemia in conditions when the concentration of low-density lipoprotein (LDL, a carrier of ‘bad’ cholesterol) in circulation is substantially increased. This results in an increased risk of thrombosis and accelerated development of atherosclerosis in a mouse model of the disease.
The new findings of Murphy et al.6 are based on studies from the past two decades that have brought about a clearer understanding of the mechanisms of reverse cholesterol transport and cellular cholesterol efflux, and thus of cholesterol overload in cells, especially in macrophages7. In particular, these studies implicated multiple members of a family of highly conserved cellular transmembrane proteins, ATP-binding cassette (ABC) transporters, in cellular lipid trafficking processes8,9. For example, two proteins of this family, ABCA1 and ABCG1, are responsible for the major part of macrophage cholesterol efflux to either protein acceptors such as apoA-I and apoE or high-density lipoprotein (HDL, a carrier of ‘good’ cholesterol). The crucial role of ABC transporters in cell function and physiology is evident from the pathologies associated with mutations of genes encoding members of this family.
Another member of the family, ABCG4, can promote the efflux of cellular cholesterol to HDL, but until recently no one had convincingly demonstrated the biological role for ABCG4. Murphy et al.6 now provide new insights into the function of ABCG4, and these findings stem from another recent interesting discovery. For half a century, it was known that there is a strong clinical connection between high blood leukocyte counts and a negative outcome of cardiovascular events10. Although this connection could theoretically be explained by leukocytosis being simply a marker of inflammation, studies in animals suggest that leukocytosis can be induced by dyslipidemia and then directly contribute to atherosclerosis and thrombosis. Several years ago, Alan Tall and his colleagues began to unravel the connection between hypercholesterolemia, cellular cholesterol efflux and leukocytosis. In a series of elegant studies, they demonstrated that deficient cholesterol efflux caused by the absence of expression of ABCA1 and ABCG1 leads to pronounced leukocytosis due to a dramatic expansion of the stem and progenitor cell population in the bone marrow11,12. These studies led to the conclusion that the traditional roles of HDL and ABC transporters in cholesterol efflux are mechanistically linked to known anti-inflammatory and immunosuppressive functions of HDL.
Murphy et al.6 now show that a defect in cholesterol efflux caused by ABCG4 deficiency in the bone marrow leads to thrombocytosis, a prothrombotic phenotype and accelerated atherosclerosis in atherosclerosis-prone hyperlipidemic LDL receptor–deficient mice. Surprisingly, ABCG4 was absent in platelets and in atherosclerotic lesions. Instead, the authors found that ABCG4 was highly expressed in bone marrow megakaryocyte progenitors. In the absence of ABCG4, these cells showed defective cholesterol efflux to HDL, increased cell surface expression of the thrombopoietin receptor (c-MPL), enhanced proliferation and megakarypoiesis (Fig. 1). Mechanistically, the authors showed that these effects could be explained by the reduced activity of the cholesterol- sensitive Src family kinase Lyn and interruption of a negative-feedback loop suppressing expression of c-MPL in the absence of ABCG4. Of clinical interest, Murphy et al.6 showed that HDL infusions reduced platelet counts in LDL receptor–deficient mice and in a mouse model of myeloproliferative neoplasm in an ABCG4-dependent fashion, strongly suggesting that HDL infusions may offer a new approach to controlling thrombocytosis and preventing thrombotic events associated with increased platelet production.
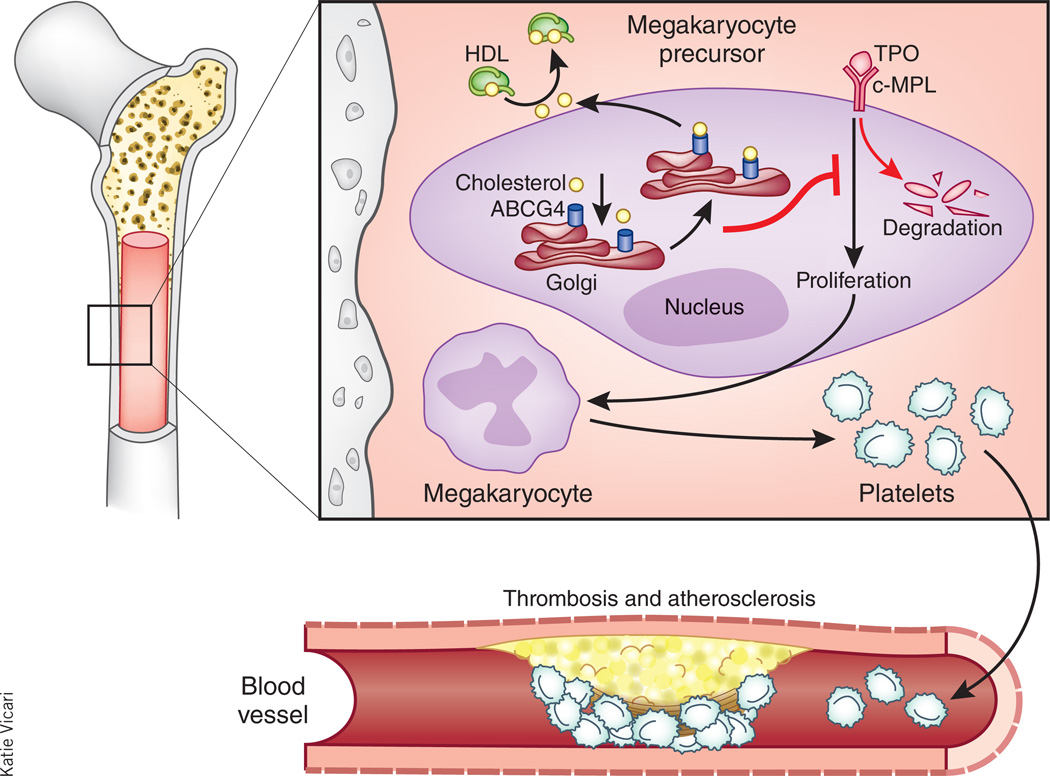
Figure 1.
ABCG4 in megakaryocyte precursor cells controls cell proliferation by mediating cholesterol efflux. Murphy et al.6 now show that ABCG4 promotes cholesterol efflux to HDL in megakaryocyte precursor cells (MPCs), reduces the concentration of cholesterol in their membranes and prevents thrombopoietin (TPO)–c-MPL signaling by promoting c-MPL degradation. In the absence of ABCG4, thrombopoietin expression and MPC proliferation are increased, leading to overproduction of megakaryocytes and platelets. This, in turn, promotes atherosclerosis and accelerated thrombosis.
This study may also suggest a new approach to treat platelet-related cardiovascular events. Current strategies are mostly based on the direct inhibition of platelet function and include platelet cyclooxygenase-1 inhibitor, platelet ADP receptor antagonists and antagonists of platelet fibrinogen receptor integrin αIIbβ3. However, there is still a need for alternative approaches that are based more on the induction of changes in platelet production and physiology, which are important events leading to coronary artery occlusion2,3. The disadvantage of the approach suggested by the work of Murphy et al.6 is that it requires intravenous infusion of recombinant HDL or lipid-free apoA-I, which is rapidly converted into HDL in circulation. However, new experimental oral medications, either those that stimulate apoA-I production or apoA-I mimetics, are already being tested in human trials and can overcome this limitation. Another interesting possibility is to assess the effect of tolimidone, an orally bioavailable allosteric Lyn kinase activator, on thrombocytosis in patients. As ABCG4 has a restricted pattern of tissue expression, it may also be of interest to test whether the reverse approach, namely the inhibition of ABCG4 function by specific blocking antibodies, can promote megakaryopoiesis and increase platelet counts in patients with thrombocytopenia in whom treatment with recombinant human thrombopoietin showed no benefit. It remains to be seen whether this ‘good cholesterol’ treatment can be applied beyond its potential role in atherosclerosis regression.
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Semple JW, et al. Nat. Rev. Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 2.Jackson SP. Nat. Med. 2011;17:1423–1436. doi: 10.1038/nm.2515. [DOI] [PubMed] [Google Scholar]
- 3.Coller BS. J. Thromb. Haemost. 2011;9:374–395. doi: 10.1111/j.1538-7836.2011.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Italiano JE., Jr Semin. Thromb. Hemost. 2013;39:15–24. doi: 10.1055/s-0032-1331157. [DOI] [PubMed] [Google Scholar]
- 5.Pang L, et al. J. Clin. Invest. 2005;115:3332–3338. doi: 10.1172/JCI26720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy AJ, et al. Nat. Med. 2013;19:586–594. doi: 10.1038/nm.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, et al. Curr. Opin. Cardiol. 2007;22:368–372. doi: 10.1097/HCO.0b013e3281ec5113. [DOI] [PubMed] [Google Scholar]
- 8.Oram JF, et al. Circ. Res. 2006;99:1031–1043. doi: 10.1161/01.RES.0000250171.54048.5c. [DOI] [PubMed] [Google Scholar]
- 9.Kusuhara H, et al. Pflugers Arch. 2007;453:735–744. doi: 10.1007/s00424-006-0134-x. [DOI] [PubMed] [Google Scholar]
- 10.Coller BS. Arterioscler. Thromb. Vasc. Biol. 2005;25:658–670. doi: 10.1161/01.ATV.0000156877.94472.a5. [DOI] [PubMed] [Google Scholar]
- 11.Yvan-Charvet L, et al. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy AJ, et al. J. Clin. Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]