Abstract
Molecular mechanisms for the dorso-ventral patterning and interventricular septum formation in the embryonic heart are unknown. To investigate a role of Hand1/eHAND in cardiac chamber formation, we generated Hand1/eHAND knock-in mice where Hand1/eHAND cDNA was placed under the control of the MLC2V promoter. In Hand1/eHAND knock-in mice, the outer curvature of the right and left ventricles expanded more markedly. Moreover, there was no interventricular groove or septum formation, although molecularly, Hand1/eHAND knock-in hearts had two ventricles. However, the morphology of the inner curvature of the ventricles, the atrioventricular canal, and the outflow tract was not affected by Hand1/eHAND expression. Furthermore, expression of Hand1/eHAND in the whole ventricles altered the expression patterns of Chisel, ANF, and Hand2/dHAND but did not affect Tbx5 expression. In contrast, the interventricular septum formed normally in transgenic embryos overexpressing Hand1/eHAND in the right ventricle but not in the boundary region. These results suggested that Hand1/eHAND is involved in expansion of the ventricular walls and that absence of Hand1/eHAND expression in the boundary region between the right and left ventricles may be critical in the proper formation of the interventricular groove and septum. Furthermore, Hand1/eHAND is not a master regulatory gene that specifies the left ventricle myocyte lineage but may control the dorso-ventral patterning in concert with additional genes.
In vertebrate cardiac development, dorso-ventral (DV) patterning, as well as antero-posterior (AP) patterning, plays an essential role in the transformation of the linear heart tube into the four-chambered heart (7, 9). The linear heart tube is polarized along the AP axis, composed of five primordial segments: inflow tract (IFT), common atrium, atrioventricular canal (AVC), primitive ventricle, and outflow tract (OFT). Each segment is controlled by different developmental programs, characterized by specific gene expression profiles (2, 9, 20). In addition to the AP polarity, DV patterning has recently received attention. During cardiac looping, the ventricular chambers expand from the ventral surface of the heart tube (8). Trabeculae form only at the outer curvature of the heart tube, whereas the inner curvature remains smooth walled (7). Christoffels et al. and Lamers and Moorman proposed a two-step model for chamber formation in the embryonic heart (7, 13). First, the primary myocardium is induced, which makes up the linear heart tube. Second, the chamber myocardium is specified on the ventral side of the heart tube, acquiring an additional and presumably more advanced transcriptional program. In the looping heart, the specified chamber myocardium located at the outer curvature expands rapidly while the myocardium of the inner curvature, as well as the IFT, AVC, and OFT, retains the functional and molecular properties of the primary myocardium that has a limited proliferative capacity (7, 13). As a result, the atrial and ventricular chambers balloon out along the outer curvature (9). This ballooning model provides a view that DV, as well as AP, patterning information is critical for chamber specification. However, molecular mechanisms for the expansion of the chamber walls and the DV patterning of the embryonic heart are unknown.
Hand1/eHAND is a potential candidate gene. Hand1/eHAND is expressed on the ventral surface in the caudal half of the linear heart tube and predominantly at the outer curvature of the left ventricle (LV) in the looping heart while the gene is absent at the inner curvature (1, 21, 24). Therefore, its expression is highly restricted along the DV as well as the AP axis. Tetraploid-rescued Hand1/eHAND null embryos displayed a single ventricle, suggesting that the gene may play a critical role in specification or proliferation of LV myocytes during ballooning (17).
Moreover, the ballooning of chamber walls may be closely related to the formation of the interventricular septum (IVS). In the ballooning model, the structures flanking the atrial and ventricular chambers do not expand and retain the tubular shape, contributing to the proper AV septation and alignment of the IFT and OFT (7, 13). However, in this model, it is not clear what determines the boundary between the right ventricle (RV) and LV. The myocardium at the interventricular groove (IVG) is not the primary but working myocardium according to this model, but this region does not expand. It is totally unknown what molecular mechanisms determine the location of the IVS and IVG.
In this study, we examined a role of Hand1/eHAND in the DV patterning of the embryonic heart and the IVS formation. For this purpose, we knocked in the Hand1/eHAND gene to the mouse myosin light chain 2V (MLC2V) locus. We demonstrated that Hand1/eHAND enhanced expansion of chamber walls and that absence of Hand1/eHAND expression in the IVG may be critical in the proper formation of the IVS.
MATERIALS AND METHODS
Gene targeting.
From a 129SvJ bacterial artificial chromosome library, 3-kb upstream and 6-kb downstream fragments of the coding region of the MLC2V gene were isolated. The upstream 3-kb fragment, FLAG-tagged mouse Hand1/eHAND cDNA, the human growth hormone poly(A) signal, and the 6-kb downstream fragment were ligated into pPNTloxPneo (15). The targeting vector was linearized with NotI for transfection.
RW4 embryonic stem (ES) cells (Incyte Genomics, St. Louis, Mo.) isolated from the 129SvJ strain were cultured on mouse embryonic fibroblast feeder layers in high-glucose Dulbecco's modified Eagle medium containing 20% fetal calf serum and 103 U of leukemia inhibitory factor/ml. ES cells (1.0 × 107) were electroporated with 30 μg of the linearized targeting vector. Electroporated ES cells were cultured on neomycin-resistant feeder cells with 300 μg of G418/ml and 2 μM ganciclovir for 8 days. Two hundred eight drug-resistant colonies were isolated, and Southern hybridization demonstrated that four clones contained the correctly targeted allele at the MLC2V locus.
These clones were electroporated with 5 μg of the Cre-expressing vector pCre-Pac (KURABO, Osaka, Japan). After electroporation, cells were cultured on feeder cells with 1.7 μg of puromycin/ml for 2 days. Single colonies were picked up in duplicate, and neomycin-sensitive colonies were amplified and genotyped by Southern blotting. Two correctly targeted clones were injected into blastocysts from C57BL/6J mice. Male chimeras were bred with female C57BL/6J mice to test for germ line transmission. All animal procedures were approved by the Animal Research Committee, Graduate School of Medicine, Kyoto University.
Genotyping of progeny.
DNA was isolated from tail biopsy specimens of weaned mice, yolk sacs, or placentas. PCR and Southern hybridization were performed to genotype embryos and mice. The primers used for detection of the targeted allele were 5′-TCCGCCTCACCTACAACTGC-3′ and 5′-ACAGAAGGGGGTCACCGTGG-3′.
Generation of transgenic mice.
The 250-bp rat MLC2V promoter (10) was synthesized by PCR and was ligated to FLAG-tagged mouse Hand1/eHAND cDNA with the human growth hormone poly(A) signal. The identity of the synthesized promoter was confirmed by DNA sequencing. The creation of transgenic mice was done in a standard manner. F0 embryos were dissected at embryonic day 11.5 (E11.5), and genotyping was performed by PCR on DNA isolated from the yolk sacs. PCR primer pairs used for detection of the transgenes were 5′-TGCTGTCAGCCCAATTAG-3′ and 5′-GGCTGCAGTCCTCCTCTTCCTCCCCCTC-3′.
In situ hybridization.
In situ hybridization was performed as described previously (23). Briefly, embryos were fixed in 4% paraformaldehyde at 4οC overnight, dehydrated through graded ethanol and xylene, and embedded in paraffin wax. Sections of 6-μm thickness were hybridized with [35S]CTP-labeled riboprobe at 55°C overnight. After hybridization, they were treated with RNase A, washed, and dehydrated through graded ethanol, and emulsion autoradiography was performed. Probes for α-cardiac actin, atrial natriuretic factor (ANF), Hand1/eHAND, Hand2/dHAND, MEF2C, TEF-1, Nkx2.5, and N-myc were described previously (23). An EagI-EcoRI fragment of the 3′ untranslated region (3′ UTR) of Hand1/eHAND was used to detect endogenous Hand1/eHAND expression. A probe for Tbx5 (3) was kindly provided by Benoit G. Bruneau (University of Toronto, Toronto, Canada). A probe for Chisel (16) was synthesized by reverse transcription-PCR. The identity of the probe was confirmed by DNA sequencing.
Immunohistochemistry.
After rehydration, paraffin sections of embryos were autoclaved in 10 mM EDTA (pH 8.0) at 121οC for 10 min, blocked with an avidin-biotin blocking kit (Vector, Burlingame, Calif.), and incubated with biotinylated mouse anti-FLAG M2 monoclonal antibody (Sigma, St. Louis, Mo.) (1:200) overnight at 4°C. After incubation, sections were washed and incubated with streptavidin-horseradish peroxidase (Nichirei, Tokyo, Japan), and peroxidase activity was detected with 3,3′-diaminobenzidine.
RESULTS
Generation of Hand1/eHAND KI mice.
To investigate a role of Hand1/eHAND in the DV patterning of the embryonic heart, we generated mice expressing Hand1/eHAND in the whole ventricles. For this purpose, we employed a knock-in (KI) strategy to place Hand1/eHAND cDNA into the genomic locus of MLC2V, since this gene is expressed in ventricular myocytes throughout development, and heterozygous knock-out mice for MLC2V were reported to display no obvious phenotype (5). After the first round of homologous recombination, the FLAG-tagged Hand1/eHAND cDNA and the pgk-neo cassette flanked by two loxP sites were inserted into the MLC2V locus (Fig. 1A). Four correctly targeted clones were identified (Fig. 1B). We then removed the pgk-neo cassette by transiently expressing the Cre recombinase (Fig. 1C). After the second round of recombination, two ES clones were injected into C57BL/6 blastocysts. We crossed male chimeras with female C57BL/6 to check for germ line contribution of ES cells by screening for the presence of agouti offspring. Two germ line chimeras were obtained, but none of their offspring carried the KI allele (0 of 20 agouti offspring), indicating that Hand1/eHAND KI mice were embryonically lethal.
FIG. 1.
(A) Targeting strategy. The structure of the MLC2V locus and the targeting vector are shown first and second, respectively. The mutated locus after homologous recombination is shown third, and the modified locus by Cre recombination is shown at the bottom. ATG is the transcriptional start site. The closed arrowheads represent the loxP sites. B, BamHI; H, HindIII; X, XbaI. (B) Genotyping of ES cell clones after homologous recombination. Genomic DNA was digested with XbaI and analyzed by Southern blotting. The 5′ probe (a BamHI-HindIII fragment) was used. Hybridization with the 5′ probe revealed the expected 5.5- and 6.5-kb fragments from the wild-type and targeted alleles, respectively. (C) Genotyping of ES clones after Cre recombination. Genomic DNA was digested with BamHI and analyzed by Southern blotting. Hand1/eHAND cDNA was used as a probe. The expected 4-kb fragment from the original targeted allele and the 2-kb fragment from the Cre mutated allele were revealed. Fragments from the wild-type allele for Hand1/eHAND were also detected (not shown on this figure). (D, E, and F) Immunohistochemistry with an anti-FLAG antibody. FLAG-tagged Hand1/eHAND protein was expressed in the nuclei of ventricular cells, whereas the expression was not detected in atrial cells in Hand1/eHAND KI embryos (E). FLAG-tagged Hand1/eHAND protein was expressed in the whole ventricle (F). A, atrium; V, ventricle. Bars, 100 μm.
Morphological and histological analysis of Hand1/eHAND KI embryos.
To investigate the timing of lethality, we examined litters from a germ line chimera, all of whose offspring had agouti coat color. At E9.5 and E10.5, Hand1/eHAND KI embryos were indistinguishable from wild-type littermates. However, Hand1/eHAND KI embryos showed slight growth retardation at E11.5 and were severely retarded at E12.5, and PCR analysis of the placenta of absorbed embryos at E14.5 revealed that all absorbed embryos carried the KI allele. Viable embryos at E14.5 were all wild type. These results indicated that Hand1/eHAND KI embryos died between E12.5 and E14.5.
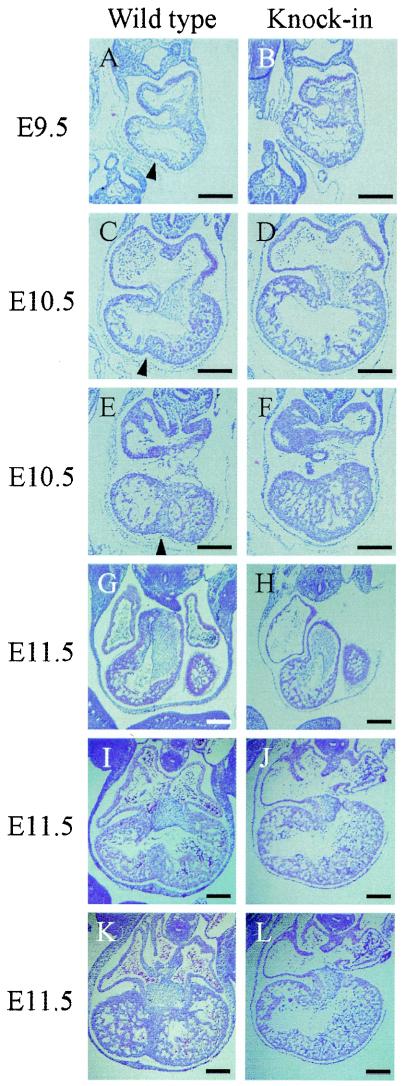
Histological examination at E9.5 revealed that trabeculation and endocardial cushion formation occurred normally in the hearts of Hand1/eHAND KI embryos. Hand1/eHAND KI and wild-type embryos were indistinguishable except that there was no IVG in Hand1/eHAND KI hearts (Fig. 2A and B). At E10.5, ventricular chambers, particularly the RVs, balloon out more markedly in Hand1/eHAND KI embryos, although their ventricles were single chambers, lacking the IVG and IVS (Fig. 2D and F). In contrast, IVS formation was clearly observed in wild-type littermates (Fig. 2C and E). The morphology of the inner curvature, AVC, and OFT was comparable between Hand1/eHAND KI and wild-type embryos (Fig. C, D, G, and H). At E11.5, no IVG or IVS formation was observed in Hand1/eHAND KI embryos (Fig. 2J and L), whereas the IVS was well developed in wild-type hearts (Fig. 2I and K). The compact zone myocardium was thinner in Hand1/eHAND KI embryos, suggesting that the embryonic lethality may be due to heart failure caused by poor development of the compact zone myocardium.
FIG. 2.
Histological analysis of wild-type and Hand1/eHAND KI embryos from E9.5 to E11.5. Hematoxylin- and eosin-stained sections of wild-type (A, C, E, G, I, and K) and Hand1/eHAND KI (B, D, F, H, J, and L) embryos are shown. At E9.5, trabeculation, endocardial cushion formation, and looping normally occurred in Hand1/eHAND KI embryos (B). Note the absence of the IVG in Hand1/eHAND KI embryos. The IVG can be observed in wild-type embryos (arrowhead in panel A). At E10.5, the outer curvature expanded more markedly in Hand1/eHAND KI embryos (C and D). The difference was more evident in the RV. There was no IVG or IVS formation in Hand1/eHAND KI embryos (D and F). At E11.5, Hand1/eHAND KI embryos exhibited a single ventricle with complete absence of the IVS and IVG (J and L). Endocardial cushion formation in the OFT was comparable between wild-type (G) and Hand1/eHAND KI (H) embryos. In KI embryos, the development of the AVC was disturbed (J and L). The arrowheads in panels A, C, and E indicate the IVG. Bars, 200 μm.
Gene expression in Hand1/eHAND KI heart.
We first examined expression of Hand1/eHAND in Hand1/eHAND KI and wild-type embryos. In wild types, Hand1/eHAND was expressed in the outer curvature of the LV and the OFT at E9.5 and E10.5 (Fig. 3A and C). Weak expression of Hand1/eHAND was also observed in the outer curvature of the RV. At E11.5, Hand1/eHAND expression was down-regulated (Fig. 3E). Notably, Hand1/eHAND expression was absent at the IVG and IVS throughout development in wild-type embryos (Fig. 3A, C, and E). In contrast, Hand1/eHAND was expressed in the whole ventricle as well as in the AVC and OFT in Hand1/eHAND KI embryos (Fig. 3B, D, F, and H). The expression level was still high at E11.5 (Fig. 3F). To confirm the expression of the FLAG-tagged Hand1/eHAND protein, we also performed immunohistochemistry with an anti-FLAG antibody. The FLAG-tagged Hand1/eHAND protein was detected in cardiac myocytes of the whole ventricle in Hand1/eHAND KI embryos (Fig. 1D, E, and F) but not in wild types (data not shown).
FIG. 3.
Expression of Hand1/eHAND in wild-type and Hand1/eHAND KI embryos. In wild-type embryos, Hand1/eHAND was expressed in the outer curvature of the LV at E9.5 (arrows in panel A). Weak expression was observed in the outer curvature of the RV at E10.5 and E11.5 (C, E, and G). Hand1/eHAND expression was also detected in the distal part of the OFT (G). Note the absence of Hand1/eHAND expression in the IVG (arrowheads in panels A, C, and E). In Hand1/eHAND KI embryos, Hand1/eHAND was expressed in the whole ventricle, including the inner curvature, as well as in the AVC and the proximal part of the OFT (B, D, F, and H). In spite of the ectopic Hand1/eHAND expression, the inner curvature, AVC, or OFT did not expand outwards in Hand1/eHAND KI embryos (D, F, and H). Bars, 200 μm.
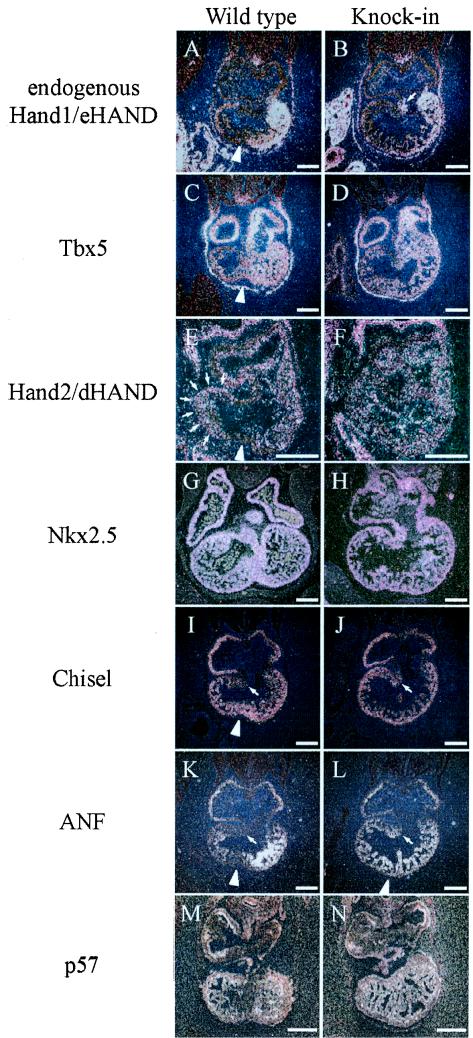
We next examined endogenous Hand1/eHAND expression using the 3′ UTR of Hand1/eHAND as a probe, since the 3′ UTR is not included in the FLAG-tagged Hand1/eHAND cDNA. Endogenous Hand1/eHAND expression was confined to the left side of the single ventricle in Hand1/eHAND KI embryos, and the expression level was comparable to that in wild-type embryos (Fig. 4A and B), indicating that there was a clear distinction between the left and right sides of the ventricle at the molecular level, although there was no IVG or IVS formation. Moreover, the left-side expression of Tbx5 was not disturbed in Hand1/eHAND KI embryos (Fig. 4C and D), further supporting the observation that the right and left sides of the ventricle were molecularly distinctive in Hand1/eHAND KI embryos. Furthermore, endogenous Hand1/eHAND expression was detected in the AVC in Hand1/eHAND KI embryos (Fig. 4B), suggesting that a positive feedback regulation of Hand1/eHAND may exist in the AVC.
FIG. 4.
Expression of cardiac transcription factors and molecular markers for the chamber myocardium. Expression of endogenous Hand1/eHAND (A and B), Tbx5 (C and D), Hand2/dHAND (E and F), Nkx2.5 (G and H), Chisel (I and J), ANF (K and L), and p57 (M and N) are shown. Endogenous Hand1/eHAND expression was only detected in the left half of the ventricle in Hand1/eHAND KI embryos (B). The Tbx5 expression gradient with higher expression in the LV was not disturbed in Hand1/eHAND KI embryos (D). Expression of Hand2/dHAND in the RV (E) was almost abolished in Hand1/eHAND KI embryos (F). Note the absence of Hand2/dHAND expression in the IVG in wild-type embryos (arrowhead in panel E). Nkx2.5 expression was comparable between wild-type (G) and Hand1/eHAND KI (H) embryos. Chisel expression was also detected in the inner curvature and AVC in Hand1/eHAND KI embryos (J). Note the absence of ANF expression in the IVG (arrowhead in panel K) and inner curvature (arrow in panel K) in wild-type embryos (K). ANF expression was up-regulated in the RV and inner curvature in Hand1/eHAND KI embryos (L). ANF was also expressed at the region where the IVS was expected to form (arrowhead in panel L). Expression of p57 was detected only in the trabecular layer both in wild-type and Hand1/eHAND KI embryos (M and N). Bars, 200 μm.
Chisel and ANF are regarded as molecular markers for the working myocardium (7). Chisel was expressed in the atrium and the outer curvature of the ventricle but was absent in the inner curvature and the AVC in wild-type embryos (Fig. 4I) (7, 16). Interestingly, in Hand1/eHAND KI embryos, Chisel was also expressed in the inner curvature and AVC, suggesting that Chisel expression was dependent on Hand1/eHAND (Fig. 4J). In wild-type embryos, ANF was strongly expressed in the trabecular layer of the LV and weaker expression was observed in the atrium and the trabecular layer of the RV. ANF expression was not observed in the inner curvature, the AVC, or the IVG in wild-type embryos (Fig. 4K). In Hand1/eHAND KI embryos, ANF expression in the RV was up-regulated and the expression was also detected in the inner curvature but not in the AVC, indicating that ANF expression in the RV and the inner curvature was regulated by Hand1/eHAND (Fig. 4L).
We further examined expression of transcription factors known to play critical roles in cardiac development. While expression of Nkx2.5 (Fig. 4G and H) and MEF2C (data not shown) was comparable, Hand2/dHAND expression in the RV was down-regulated in Hand1/eHAND KI embryos (Fig. 4E and F), suggesting that Hand1/eHAND may suppress Hand2/dHAND expression. What is the molecular mechanism for thin myocardium in Hand1/eHAND KI embryos? Inactivation of N-myc or TEF-1 in mice resulted in thin myocardium (4, 6, 14), but these genes were normally expressed in Hand1/eHAND KI embryos (data not shown). Homozygous Splotch mutant mice that lack the transcription factor Pax3 also showed thin myocardium. p57, a cyclin-dependent kinase inhibitor normally expressed in the trabecular layer, was also expressed in the compact zone layer in the mutant embryos, suggesting precocious cardiomyocyte differentiation in Splotch mutants (11). We thus investigated expression of p57 in Hand1/eHAND KI embryos, but p57 expression was detected only in the trabecular layer both in Hand1/eHAND KI and wild-type embryos (Fig. 4M and N).
Normal IVS formation in transgenic embryos overexpressing Hand1/eHAND in the RV.
The defect in the IVS formation in Hand1/eHAND KI embryos may be due to nonspecific effects of Hand1/eHAND overexpression. To further examine the significance of the absence of Hand1/eHAND expression in the boundary region, we generated and analyzed transgenic embryos overexpressing Hand1/eHAND in the RV by using the MLC2V promoter (Fig. 5A and B) (10, 18). As expected, the IVS formed normally in these transgenic embryos (Fig. 5D). Immunohistochemistry revealed FLAG-tagged Hand1/eHAND expression in the RV but not in the boundary region (Fig. 5C). These results indicated that the absence of Hand1/eHAND expression in the boundary region was critical for the proper formation of the IVS.
FIG. 5.
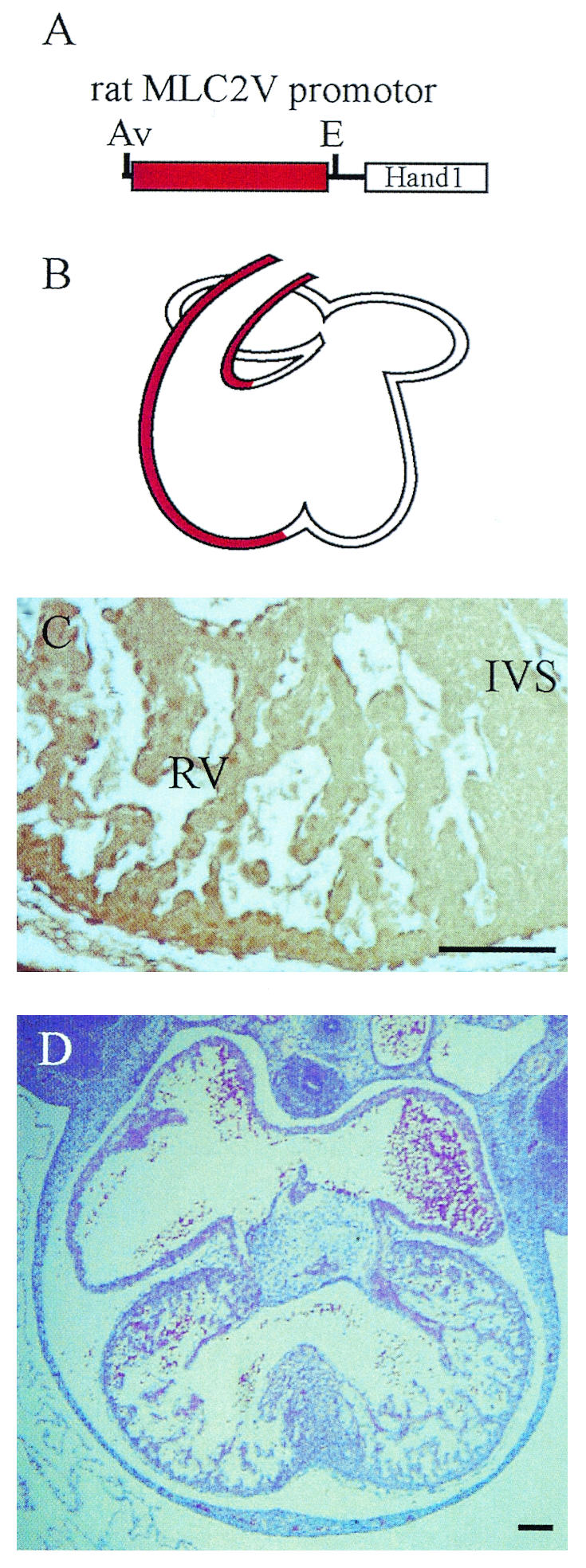
MLC2V-Hand1/eHAND transgenic mice. (A) Schematic representation of the transgene. (B) The MLC2V promoter drives transgene expression in the RV and OFT but not in the boundary region. (C) Immunohistochemistry with an anti-FLAG antibody revealed FLAG-tagged Hand1/eHAND protein expression in the RV but not in the IVS. (D) In MLC2V-Hand1/eHAND transgenic embryos, the IVS formed normally. Av, AvaII; E, EcoRI. Bars, 100 μm.
DISCUSSION
Septum formation is one of the critical steps in the transformation of a linear heart tube into a four-chambered heart. Morphologically, it has been pointed out that the boundary region between the LV and RV does not expand during the formation of the muscular IVS (Fig. 6A) (19). When the outer curvatures on each side of the narrow boundary region keep expanding, the two walls will eventually fuse, forming a septum (19). However, the molecular mechanism for expansion of the ventricular walls has never been elucidated. Hand1/eHAND KI embryos had a morphologically single ventricle, but there were distinctive LV and RV at the molecular level. Therefore, forced expression of Hand1/eHAND in the whole ventricle resulted in expansion of the entire ventricular wall including the boundary region (Fig. 6B). Although it is possible that overexpression of Hand1/eHAND may have affected the phenotype, the absence of Hand1/eHAND expression in the boundary region was critical in the development of the IVG and IVS (Fig. 6A) because transgenic embryos expressing Hand1/eHAND in the RV and LV, but not in the boundary region, exhibited normal formation of the IVS (Fig. 6C).
FIG. 6.
Schematic presentation of ventricular expansion and IVS formation. (A) IVS formation in normal hearts. The outer curvatures of the LV and RV expand outwards. For the proper formation of the IVS, the boundary region should not expand. Hand1/eHAND and Hand2/dHAND may regulate expansion of the LV and RV, respectively. Note the absence of Hand1/eHAND and Hand2/dHAND expression in the boundary region. (B) In Hand1/eHAND KI hearts, the boundary region also expanded outward. As a result, the IVS did not form properly. (C) In MLC2V-Hand1/eHAND transgenic embryos, while the Hand1/eHAND transgene was expressed in the RV and endogenous Hand1/eHAND was expressed in the LV, Hand1/eHAND expression was absent in the boundary region. In these transgenic embryos, the boundary region did not expand outwards and the IVS formed normally.
Ectopic expression of Hand1/eHAND in the entire RV resulted in more marked expansion of the outer curvature of the RV. Together with the result that Hand1/eHAND expression caused expansion of the boundary region, it is likely that Hand1/eHAND is involved in expansion of the ventricular walls. Then, which gene(s) regulate ballooning of the RV during normal cardiac development? Specific hypoplasia of the RV soon after cardiac looping in Hand2/dHAND knock-out embryos suggested a role of Hand2/dHAND in the expansion of the RV (21). Notably, Hand2/dHAND expression was also absent in the boundary region (Fig. 4E), thus suggesting a possibility that absence of Hand1/eHAND and Hand2/dHAND expression in the boundary region may be essential for the IVG and IVS formation (Fig. 6A).
Does Hand1/eHAND control the DV patterning of the embryonic heart? Interestingly, Chisel and ANF, molecular markers for the working myocardium (7), were ectopically expressed in the inner curvature and/or the AVC in Hand1/eHAND KI embryos. However, the inner curvature or the AVC did not expand morphologically. These results indicated that Hand1/eHAND regulated expression of molecular markers for the working myocardium but that additional gene(s) normally expressed in the outer curvature may be required for expansion of the chamber walls together with Hand1/eHAND.
The results of this study also gave insight into the hierarchical and combinatorial molecular cascade that controls cardiac development. Forced expression of Hand1/eHAND in the RV down-regulated Hand2/dHAND expression. It is possible that expression of Hand2/dHAND in the LV may be suppressed by high expression of Hand1/eHAND in the normal embryonic heart. However, Hand1/eHAND expression in the whole ventricles did not disturb the Tbx5 expression gradient or endogenous Hand1/eHAND expression. Therefore, it is unlikely that Hand1/eHAND is the most upstream gene that specifies the LV myocyte lineage.
Between E10.5 and E11.5, cardiac myocytes undergo rapid cell division, resulting in doubling of cardiac mass (11). By E10.5, Hand1/eHAND KI embryos were indistinguishable from wild-type embryos except that they lacked the IVG and IVS. At E11.5, the compact zone layer of the Hand1/eHAND KI hearts were thin, suggesting that heart failure due to poor development of the compact zone layer may have caused the embryonic lethality. What is the mechanism for thin myocardium in Hand1/eHAND KI embryos? N-myc, TEF-1, and p57 were normally expressed in Hand1/eHAND KI embryos, suggesting that there may be other mechanism(s). At E11.5, Hand1/eHAND expression was obviously down-regulated in wild-type embryos, while strong expression of Hand1/eHAND persisted in Hand1/eHAND KI embryos. Thus, down-regulation of Hand1/eHAND at the mid-stage of cardiac development may be important for the proper formation of the compact zone myocardium. Although it may seem inconsistent that Hand1/eHAND enhanced expansion of the ventricular chambers at E10.5 but that overexpression of Hand1/eHAND at E11.5 disturbed proliferation of the compact zone myocardium, fine-tuning of Hand1/eHAND expression at each stage may be required for the proper development of the embryonic heart. It is also possible that different mechanisms may exist to regulate expansion of the ventricular chambers and thickening of ventricular walls.
Recently, Takeuchi et al. reported that Tbx5 may determine the position of the IVS in chicken and mouse embryonic hearts (22). When Tbx5 was overexpressed in the whole ventricles, the Hand1/eHAND expression domain was expanded to the RV, resulting in a lack of IVS formation. Their study suggested that Tbx5 may control Hand1/eHAND expression and that in the chicken heart, the boundary of the Tbx5 and Tbx20 expression domains may determine the position of the IVS (22). Together with the results of our study, it was likely that the function of Tbx5 in the expansion of the ventricular walls and the IVS formation in murine hearts was mediated through eHAND. Moreover, since Tbx20 is uniformly expressed in the LV and RV (12) and Tbx5 is not expressed in the boundary region between the LV and RV in the normal murine hearts, the absence of Tbx5 and Hand1/eHAND expression in the boundary region may be critical in the proper formation of the IVS in murine cardiac development.
In summary, expression of Hand1/eHAND enhanced expansion of chamber walls, and absence of Hand1/eHAND expression in the boundary region may be essential for the proper formation of the IVG and IVS. Moreover, additional factors normally expressed in the outer curvature may determine the DV patterning of the embryonic heart in concert with Hand1/eHAND.
Acknowledgments
We gratefully acknowledge Keiko Kobayashi and Ayumi Hosotani for technical assistance. We also thank Benoit G. Bruneau for providing a cDNA probe.
This work was supported by research grants from the Ministry of Education, Science, Sports, and Culture of Japan (grants 13045019, 13832003, and 15590738 [to M.T.] and 12CE2006 and 13307034 [to T.K.]), research grants from the Ministry of Health, Labor, and Welfare of Japan (Comprehensive Research on Aging and Health grant no. H14-choju-012 [to T. Kita]), and the grant provided by the Ichiro Kanehara Foundation (to M.T.).
REFERENCES
- 1.Biben, C., and R. P. Harvey. 1997. Homeodomain factor Nkx2-5 controls left/right asymmetric expression of bHLH gene eHand during murine heart development. Genes Dev. 11:1357-1369. [DOI] [PubMed] [Google Scholar]
- 2.Bruneau, B. G. 2002. Transcriptional regulation of vertebrate cardiac morphogenesis. Circ. Res. 90:509-519. [DOI] [PubMed] [Google Scholar]
- 3.Bruneau, B. G., G. Nemer, J. P. Schmitt, F. Charron, L. Robitaille, S. Caron, D. A. Conner, M. Gessler, M. Nemer, C. E. Seidman, and J. G. Seidman. 2001. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell 106:709-721. [DOI] [PubMed] [Google Scholar]
- 4.Charron, J., B. A. Malynn, P. Fisher, V. Stewart, L. Jeannotte, S. P. Goff, E. J. Robertson, and F. W. Alt. 1992. Embryonic lethality in mice homozygous for a targeted disruption of the N-myc gene. Genes Dev. 6:2248-2257. [DOI] [PubMed] [Google Scholar]
- 5.Chen, J., S. W. Kubalak, S. Minamisawa, R. L. Price, K. D. Becker, R. Hickey, J. Ross, Jr., and K. R. Chien. 1998. Selective requirement of myosin light chain 2v in embryonic heart function. J. Biol. Chem. 273:1252-1256. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Z., G. A. Friedrich, and P. Soriano. 1994. Transcriptional enhancer factor 1 disruption by a retroviral gene trap leads to heart defects and embryonic lethality in mice. Genes Dev. 8:2293-2301. [DOI] [PubMed] [Google Scholar]
- 7.Christoffels, V. M., P. E. Habets, D. Franco, M. Campione, F. de Jong, W. H. Lamers, Z. Z. Bao, S. Palmer, C. Biben, R. P. Harvey, and A. F. Moorman. 2000. Chamber formation and morphogenesis in the developing mammalian heart. Dev. Biol. 223:266-278. [DOI] [PubMed] [Google Scholar]
- 8.de Jong, F., S. Viragh, and A. F. Moorman. 1997. Cardiac development: a morphologically integrated molecular approach. Cardiol. Young 7:131-146. [Google Scholar]
- 9.Harvey, R. P. 1999. Seeking a regulatory roadmap for heart morphogenesis. Semin. Cell Dev. Biol. 10:99-107. [DOI] [PubMed] [Google Scholar]
- 10.Henderson, S. A., M. Spencer, A. Sen, C. Kumar, M. A. Siddiqui, and K. R. Chien. 1989. Structure, organization, and expression of the rat cardiac myosin light chain-2 gene. Identification of a 250-base pair fragment which confers cardiac-specific expression. J. Biol. Chem. 264:18142-18148. [PubMed] [Google Scholar]
- 11.Kochilas, L. K., J. Li, F. Jin, C. A. Buck, and J. A. Epstein. 1999. p57Kip2 expression is enhanced during mid-cardiac murine development and is restricted to trabecular myocardium. Pediatr. Res. 45:635-642. [DOI] [PubMed] [Google Scholar]
- 12.Kraus, F., B. Haenig, and A. Kispert. 2001. Cloning and expression analysis of the mouse T-box gene tbx20. Mech. Dev. 100:87-91. [DOI] [PubMed] [Google Scholar]
- 13.Lamers, W. H., and A. F. Moorman. 2002. Cardiac septation: a late contribution of the embryonic primary myocardium to heart morphogenesis. Circ. Res. 91:93-103. [DOI] [PubMed] [Google Scholar]
- 14.Moens, C. B., B. R. Stanton, L. F. Parada, and J. Rossant. 1993. Defects in heart and lung development in compound heterozygotes for two different targeted mutations at the N-myc locus. Development 119:485-499. [DOI] [PubMed] [Google Scholar]
- 15.Nagy, A., C. Moens, E. Ivanyi, J. Pawling, M. Gertsenstein, A. K. Hadjantonakis, M. Pirity, and J. Rossant. 1998. Dissecting the role of N-myc in development using a single targeting vector to generate a series of alleles. Curr. Biol. 8:661-664. [DOI] [PubMed] [Google Scholar]
- 16.Palmer, S., N. Groves, A. Schindeler, T. Yeoh, C. Biben, C. C. Wang, D. B. Sparrow, L. Barnett, N. A. Jenkins, N. G. Copeland, F. Koentgen, T. Mohun, and R. P. Harvey. 2001. The small muscle-specific protein Csl modifies cell shape and promotes myocyte fusion in an insulin-like growth factor 1-dependent manner. J. Cell Biol. 153:985-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riley, P., L. Anson-Cartwright, and J. C. Cross. 1998. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat. Genet. 18:271-275. [DOI] [PubMed] [Google Scholar]
- 18.Ross, R. S., S. Navankasattusas, R. P. Harvey, and K. R. Chien. 1996. An HF-1a/HF-1b/MEF-2 combinatorial element confers cardiac ventricular specificity and established an anterior-posterior gradient of expression. Development 122:1799-1809. [DOI] [PubMed] [Google Scholar]
- 19.Sadler, T. W. 2004. Langman's medical embryology, 9th ed. Lippincott William & Wilkins, Baltimore, Md.
- 20.Srivastava, D., and E. N. Olson. 2000. A genetic blueprint for cardiac development. Nature 407:221-226. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava, D., T. Thomas, Q. Lin, M. L. Kirby, D. Brown, and E. N. Olson. 1997. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat. Genet. 16:154-160. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi, J. K., M. Ohgi, K. Koshiba-Takeuchi, H. Shiratori, I. Sakaki, K. Ogura, Y. Saijoh, and T. Ogura. 2003. Tbx5 specifies the left/right ventricles and ventricular septum position during cardiogenesis. Development 130:5953-5964. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka, M., Z. Chen, S. Bartunkova, N. Yamasaki, and S. Izumo. 1999. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development 126:1269-1280. [DOI] [PubMed] [Google Scholar]
- 24.Thomas, T., H. Yamagishi, P. A. Overbeek, E. N. Olson, and D. Srivastava. 1998. The bHLH factors, dHAND and eHAND, specify pulmonary and systemic cardiac ventricles independent of left-right sidedness. Dev. Biol. 196:228-236. [DOI] [PubMed] [Google Scholar]