Abstract
Patients with squamous cell carcinoma of the head and neck (HNSCC) are usually treated by a multimodal approach with surgery and/or radiochemotherapy as the mainstay of local–regional treatment in cases with advanced disease. Both chemotherapy and radiation therapy have the disadvantage of causing severe side effects, while the clinical outcome of patients diagnosed with HNSCC has remained essentially unchanged over the last decade. The potential of immunotherapy is still largely unexplored. Here the authors review the current status of the art and discuss the future challenges in HNSCC treatment and prevention.
Keywords: cancer vaccines, CRT, EGFR, HNSCC, HPV
Squamous Cell Carcinoma of the Head and Neck
Head and neck squamous cell carcinoma (HNSCC) is a serious disease with a broad impact. It is the sixth most common cancer in the world, affecting over 500,000 people. In the United States it accounts for more deaths annually than cervical cancer, malignant melanoma, or Hodgkin lymphoma [1, 2]. HNSCC refers to a collection of squamous cell carcinomas that arise from the upper aerodigestive tract epithelium, mainly the lip, oral cavity, oropharynx, nasopharynx, hypopharynx, and larynx. In fact, 90% of cancers found in the aerodigestive tract are squamous cell carcinomas [3]. Epidemiologically, HNSCC incidence displays an uneven geographical distribution. This was thought to be related to geographic differences in exposure to associated risk factors, such as alcohol or tobacco consumption and the prevalence of HPV [4]. HPV infections are hypothesized to be contracted during sexual activity [5, 6]. Heavy tobacco use exerts the greatest influence on risk of developing HNSCC. Users of tobacco have a 5- to 25-fold increased risk of developing HNSCC [3]. The male-to-female incidence rates are as high as 2:1, but this ratio has been on a steady decline due to the increasing number of female alcohol and tobacco users over the past two decades [3].
HNSCC remains localized to the head and neck for months to years. As the tumor progresses and becomes invasive, local tissue invasion is mostly followed by metastasis to regional lymph nodes. Distant lymphatic metastases tend to occur late, and hematogenous metastases are rarely associated with large persistent tumors or immunodeficiency.
We outline here the immunological treatment options available and envisioned for clinical use in the treatment of HNSCC, and we propose a new possible algorithm using immunological therapy to debulk the tumor-mass, kill micro-metastases, and allow a lower dose of chemotherapy to achieve better cytoreduction.
Biomarkers for HNSCC
Biomarkers are very useful oncologic tools: firstly, they allow for early cancer detection in diagnostic screenings, secondly they can be used as prognostic factors to guide clinical decisions, and, lastly, they can be valuable monitoring tools during patients’ follow-up. Currently, a number of potential biomarkers for HNSCC have been identified. However, as of yet, no biomarker and assay techniques have been developed that satisfy requirements for appropriate clinical usage.
One recent study by Lallement et al. analyzed 23 publications on biomarkers in HNSCC, identifying 9 genes that displayed frequent alterations with clinical significance [7]. This study highlighted 3 transcriptional markers that were of special interest: IL1RN, MAL, and MMP1 when tested individually were shown in tissue samples to have sensitivity of 93% and a specificity of 91%. No additional benefit was found when markers where combined [7]. However, no clinically relevant correlations were identified between gene expression and clinical prognostic factors. The authors suggested this could be due to the fact that the detected biomarker deregulations occurred early in the carcinogenesis process, making them detectable in all stages of HNSCC. The authors also analyzed the same biomarkers in saliva washes, achieving reducing sensitivity. Here, even though MMP1 only had a sensitivity of 20%, the specificity rose to 100%, as it was never detected in a healthy patient [7]. The authors reported that advances in saliva collection and technical changes of the procedure may be able to increases the sensitivity of this technique in the future. This would be a large step forward clinically, as it would possibly allow for the detection of oral HNSCC through a mouth rinse [7]. Table 1 summarizes the principal findings concerning HNSCC biomarkers.
TABLE 1.
Potential biomarkers for HNSCC
| Gene | qRT-PCR | Function | Reference |
|---|---|---|---|
| CDH11 | X | Integral membrane protein: cadherin-11 (cell–cell adhesion) | [82] |
| SPARC | X | Extracellular matrix-associated protein osteonectin (influences extracellular matrix synthesis, changes cell shape) |
[7, 82] |
| POSTN | X | Periostin (ligand for various intergrins) | [82] |
| TNC | X | Extracellular matrix protein tenascin (regulates cell adhesion) | [82] |
| TGM3 | X | Transglutaminase 3 (crosslinks intracellular structural proteins, important in cell envelope formation) |
[7, 82] |
| FABP5 | X | Fatty acid-binding protein (expressed in epidermis and endothelial cells of microvasculature of different organs) |
[83] |
| MIF | X | Macrophage inhibitory factor (works on CD74) | [83] |
| Il1RN | X | Il-1 receptor antagonist | [7] |
| MAL | X | Myelin and lymphocyte protein (linked to lipid raft in cell membranes influencing membrane fluidity, fusion, adhesion and cell signaling; associated with lack of cisplatin sensitivity in ovarian cancer) |
[7, 84] |
| MMP1 | X | Matrix metallopeptidase 1 (interstitial collagenase) | [7] |
Traditional Treatments and Advances
Until recently, three treatment modalities existed for HNSCC: surgery, chemotherapy, and radiation. These three treatment options are still widely used, but a great utility has come from changing the timing of their use and combinatorial approaches, specifically induction chemotherapy, concurrent chemoradiation therapy (CTR), and postoperative chemotherapy. Induction chemotherapy is given before surgery or radiation, and CRT is given during radiotherapy. Postoperative chemotherapy is given after initial surgical or radiotherapy and is also called adjuvant therapy. Additionally, due to the serious side effects and limited success of traditional modalities, targeted therapies are urgently needed.
Treatment recommendation for early HNSCC, correlating to stage I or II cancer, has remained constant over the last several years. Stage I/II cancers have a good prognosis and have been treated successfully with single modalities. Therefore, the treatment of choice is surgery or radiation [1, 8].
Locally advanced and regional disease, characterized by stage III and IV HNSCC, require multiple modalities. Locally advanced and regional disease should be treated with surgery, radiation, and chemotherapy. In particular, chemotherapy should be given as chemoradiotherapy (CRT). Recent studies have clearly shown the benefits of CRT compared with radiation alone [9], and it has been proven to increase regional control and survival, but it has not been linked to decreased metastatization [3, 8, 9]. Another treatment option that should be strongly considered in locally advanced and regional HNSCC is induction chemotherapy. Recently, it has been shown to increase survival by 5% [8, 10], reduce metastases, and increase local control of the primary tumor [8, 10]. The ability of induction chemotherapy to shrink primary tumors becomes especially important in large advanced local disease. Here, the tumor size can be reduced enough to turn an otherwise unresectable lesion into a tumor for which surgery may be possible [8, 10].
Although induction chemotherapy appears to be a good treatment option in stage III/IV disease, other factors must be considered before its administration. Treatment with induction chemotherapy is not without risk and has been associated with neutropenia, febrile neutropenia, and diarrhea. Currently, benefits have been shown to be present only in patients with good performance status who could withstand chemotherapy. This is concurrent with findings that induction chemotherapy is especially useful in younger patients and shows decreased benefits as the patient’s age increases [3, 8]. Places where the use of induction chemotherapy should be considered by clinicians as a treatment option are in young patients and those with good performance status, large primary or regional involvement, and symptomatic disease [8].
Postoperative chemotherapy has gained recent acceptance in specific situations. It should be offered to patients who have been found to have high risk factors after surgery. Factors qualifying for postoperative chemotherapy are positive margins, extracapsular invasion and the involvement of many lymph nodes. In cases where these risk factors have been found, the use of postoperative chemotherapy has been demonstrated to increase progression-free survival and decrease locoregional reoccurrence. It has shown little effect on metastasis [8].
Radiation therapy (RT) remains a key modality in treating all stages of HNSCC. However, new techniques involving altering the dose and frequency of irradiation have been developed with good results, yielding better local and regional control then traditional RT, but have not been shown to reduce metastases [3]. Hyperfractionated RT consists of giving patients treatments more often but at lower doses. A typical fractionated radiation schedule would include 2-3 doses of 1.2 Gy of radiation for 7 weeks; a total of 81.6 Gy is delivered by this technique [3]. It is thought that decreased levels of irradiation at increased intervals decreases late toxicity. It has also been proven to increase survival [8].
Accelerated RT involves giving patients more irradiation in a shorter period of time. Patients are given 1.6 Gy of irradiation twice a day for 6 weeks. This results in a total delivery of 67.2 Gy, but it is delivered in short time frame [3]. Accelerated RT is thought to work by decreasing the time that cancer cells have to repopulate between radiation treatments [8]. Accelerated and hyperfractionated RT has been shown to increase survival compared to traditional RT. However, benefits from alternative RT have been found to be greater in hyperfractionated RT than accelerated RT [8].
Recurrent or metastatic HNSCC has a dismal prognosis. This can be treated with the intent to cure by reirradiating the site or by excision using a cyber knife [3]. Standard treatment for high-volume reoccurrence that is inoperable or has been previously irradiated, plus metastatic disease, consists mainly of platin-based chemotherapy [3, 9]. Combinations of platin drugs with other traditional chemotherapeutics such as5-fluorouracil and taxols have not been shown to improve results of treatment compared to single agent platin based therapy[3]. Those with platin-resistant tumor have few options. New hope for treatment of recurrent and metastatic HNSCC comes from targeted therapy. Targeted therapy has many advantages over traditional approaches, including relative tolerability and increased activity on metastasis [3, 8]. Cetuximab and immunotherapy are both targeted therapies and will be addressed in this paper. Cetuximab has already been shown to improve survival in recurrent disease when given with cisplatin and immunotherapy holds great potential for further scientific development and application [3, 8].
Obstacles to Traditional Approaches to Treatment of HNSCC
Although great efforts have been made to improve traditional HNSCC treatments modalities, such as surgery, radiation, and chemotherapy, many issues persist. Limited increases in survival times have occurred over the last decade and current treatments have serious side effects [9, 11]. Surgery and radiation may leave patients disfigured and without the use of complex head and neck functions. Chemotherapy can be just as devastating to the patient, resulting in severe internal organ damage and dysfunction. Platins, in particular, have been liked to neuropathy, nephrotoxicity, hearing loss, nausea, and vomiting [12]. This toxicity is unacceptable for some patients and limits its use. Secondly, though the advance of chemoradiotherapy has led to large increases in local and regional control, there has been little improvement in metastatic control [13, 14]. The solution to both of these problems may come from the new and expanding field of immunotherapy.
Immunotherapy is more specific and less toxic than traditional therapies and it also induces memory responses that could yield long-term tumor immunosurveillance. By continually scanning for and destroying cancerous cells, immunotherapy may decrease the incidence of relapses and increase long-term disease-free survival. All forms of immunotherapy rely on the antigenic properties of the tumor.
Targeted Therapy
EGFR Treatment
The epidermal growth factor receptor (EGFR) is a cell surface receptor with tyrosine kinase activity. This receptor has been linked to HNSCC to proliferation, angiogenesis, and progression through the cell cycle [1, 9]. It is also a marker of poor prognosis [1, 9]. Due to the oncologic nature of the protein, it can be inferred that a blockade of this receptor should inhibit these cancerous properties. Cetuximab and other monoclonal antibodies of this class work at the external binding site while tyrosine kinase inhibitors block EGFR kinase activity.
Cetuximab is a chimeric human-murine monoclonal antibody [3]. Its main mechanism of action is a competitive blockade of the EGFR, hampering the binding of the ligands EGF and TGF-α. Interestingly, both have been shown to be elevated almost universally in HNSCC [3]. A different EGFR-targeted therapy is based on drugs of the class of tyrosine kinase inhibitors. These include gefitinib and erlotinib. They work directly on tyrosine kinases, preventing downstream signaling, but they have been shown to be less effective than anti-EGFR antibodies. Cetuximab was able to improve overall survival and progression-free survival in locally advanced and regional disease when given with other modalities [1, 3, 9]. Cetuximab has found an important niche in platin-resistant recurrent or metastatic disease. Here, it is the last remaining option for many patients. It is also given in combination with cisplatin in non-platin-resistant recurrences with some increased survival benefits [3].
The EGFRvIII is the most common genomic variant of EGFR and it is frequently detected in human malignancies such as colorectal cancer, glioma, glioblastoma, and HNSCC [15-20]. To date, EGFRvIII is known to be expressed in a minority of HNSCC patients, compared with EGFR, yet it is an independent prognostic marker [21]. EGFRvIII displays a unique extracellular domain with a mutant glycine residue, which dramatically hampers the binding affinity of monoclonal antibodies recognizing EGFR. Unlike EGFR, EGFRvIII is able to initiate intracellular signaling in the absence of TGF-α since its protein kinase domain is constitutively phosphorylated. EGFRvIII-mediated anti-apoptotic signals are critical for HNSCC survival. Since EGFRvIII is selectively expressed by tumor cells, it is an ideal immunotherapy target. Conjugating toxins to monoclonal antibodies binding the EGFRvIII extracellular domain (such as the MR1 conjugate) has been shown to afford protection against glioblastoma in animal models [22]. An ongoing phase I clinical trial is investigating the use of MR-1 immunotoxin in patients with glioblastoma. The use of anti-EGFRvIII immunotoxins in HNSCC is likely to be proven useful, since EGFRvIII signaling is the main mechanism of resistance to anti-EGFR therapy in HNSCC [23].
Bispecific T-cell-engager (BiTE) Antibodies and Antibody–Drug Conjugates (ADCs) in HNSCC
Tumor-specific humanized or chimeric antibodies act by triggering antibody-dependent cellular cytotoxicity (ADCC) through Fcγ receptors on immune cells. While NK-cells, neutrophils, or macrophages naturally bind to antibodies, T cells lack Fcγ receptors and are therefore unresponsive to these treatments [24]. Since cytotoxic T cells are able to mount efficacious responses against tumors, a new class of antibodies has been developed [25], termed bispecific T-cell engagers (BiTE), which are able to activate of T cells by inducing CD3 clustering [26]. Interestingly, most BiTEs target the same antigens that had previously been validated with standard monoclonal antibodies, such as anti-EGFR for HNSCC [27].
ADCs are new-generation biomolecules able to specifically deliver drugs to cancer cells. They are currently under evaluation for the treatment of HNSCC. Besides the anti-EGFRvIII immunotoxin (discussed above), promising results were obtained using anti-CD44 monoclonal antibodies [28, 29]. Further improvements of the anti-CD44 antibody were obtained by the use of mAbs specific for CD44v6, a variant overexpressed in HNSCC. An anti-CD44v6 antibody, named U3 [30], conjugated to the radioisotope rhenium-186 (186Re), was shown to stabilize disease in HNSCC patients [31]. To overcome the issue of antibody immunogenicity, a humanized form of U3, named bivatuzumab (BIWA-4), was developed. Technetium-99m (99mTC) BIWA-4 was shown to result in stabilized disease in 50% of patients at the maximum tolerated dose [32]. BIWA-4 conjugated to the cytotoxic drug mertansine in HNSCC patients produced dose-limiting toxicities (skin disorders, epidermal necrolysis) [28], due to the presence of CD44v6 in the skin [33]. However, in a study on breast cancer patients, stable disease was achieved in 50% of cases [34]. Despite BIWA-4 clinical trials were stopped because of the occurrence of skin-related toxicities, the antibody-drug conjugate showed disease stabilization and/or tumor regression in both HNSCC and metastatic breast cancer [35].
Immunotherapy and HNSCC
As described above, most patients with advanced disease are currently treated with a combination of surgery, radiation, and/or chemotherapy with serious side effects. The possibility of destroying more malignant cells by increasing the chemotherapeutic dose or prolonging radiation exposure is limited by the nonspecific organ toxicity.
Immunological therapy is not only more specific and less toxic, but it may also induce memory responses that could yield long-term tumor immunosurveillance and reduce the incidence of relapses, thus increasing long-term disease-free survival. All immunotherapy approaches rely on the antigenic properties of the tumor.
Tumor-associated Antigens
Immunotherapy is based on the theory that antigenically activated lymphocytes in the human body patrol tissue and recognize and eliminate malignant cells. This has been confirmed many times over the last several decades [36-38]. For lymphocytes to be able to recognize tumors, certain antigens must either be overexpressed in tumor cells or exist only in tumor cells and relatively few other areas. Cancer testis antigens (CTA) are a unique group of antigens that are expressed specifically on tumor cells and are otherwise restricted to expression in male germ cells and relatively few other locations [37, 39]. CTA are a particular subgroup of tumor-associated antigens (TAA) [40]. Because TAA are both the initiator of the immune response against the tumor and the target for cytotoxic T lymphocytes (CTLs), their discovery and validation is at the backbone of immunotherapy [40]. Table 2 references the TAAs that have been found in HNSCC and highlights the percentage of tumor locations and their expression rate above that found in normal tissue.
TABLE 2.
CTAs found in SCHHN
| Gene | Function | Prevalence of CTA in HNSCC (%) |
Average tumor CTA expression level |
Gene location |
Reference |
|---|---|---|---|---|---|
| MAGEA1 (CT1.1) | Unknown | 63 | 100–1000× | Xq28 | [40, 85, 86] |
| MAGEA2 (CT1.2) | Unknown | 69 | 100–1000× | XXq28 | [40, 85, 86] |
| MAGEA3 (CT1.3) | Unknown | 69 | >1000× | Xq28 | [40] |
| MAGEA4 (CT1.4) | Possible pro-apoptotic activity |
56 | 100–1000× | Xq28 | [40, 85, 86] |
| MAGEA6 (CT1.6) | Unknown | 63 | >1000× | Xq28 | [40, 85, 86] |
| MAGEA9 (CT1.9) | Unknown | 63 | >1000× | Xq28 | [40, 85, 86] |
| MAGEA10 (CT1.10) | Unknown | 63 | 100–1000× | Xq28 | [40, 85, 86] |
| MAGEA12 (CT1.12) | Unknown | 50 | 100-1000× | Xq28 | [40, 86] |
| MAGEB (CT3) | Unknown | 81 | 100–1000× | Xp21.3 | [40, 86] |
| MAGEB1 (CT3.1) | Unknown | 50 | 10–100× | Xp21.3 | [40, 86] |
| MAGEB2 (CT3.2) | Unknown | 50 | >1000× | Xp21.3 | [40, 86] |
| MAGEB3 (CT3.5) | Unknown | 19 | 10–100× | Xp21.3 | [40, 86] |
| MAGEB4 (CT3.6) | Unknown | — | — | Xp21.3 | [40, 86] |
| MAGEB6 (CT3.4) | Unknown | 44 | 100–1000× | Xp21.3 | [40, 86] |
| MAGEB18 | Unknown | 19 | 10–100× | Xp21.3 | [40, 86] |
| MAGEC1 (CT7.1) | Unknown but reported to interact with NY-ESO-1 |
31 | 100–1000× | Xq26Xq27 | [40, 86] |
| MAGEC2 (CT10) | Unknown | 38 | 100–1000× | Xq27 | [40, 86] |
| MAGEC3 (CT7.2) | Unknown | 6 | 10–100× | Xq26Xq27 | [40, 86] |
| GAGE (CT4) | Unknown | 56 | 100–1000× | Xp11.23 | [40, 86] |
| BAGE (CT2) | Unknown | 6 | 10–100× | 21p11.1 | [40, 86] |
| SAGE (CT14) | Unknown | 25 | 100–1000× | Xq26 | [40, 86] |
| CAGE (CT26) | Unknown | 19 | 100–1000× | [40, 86] | |
| XAGE1 (CT12) | Unknown | 13 | 100,1000× | Xp11.22 | [40, 86] |
| Xp11.21 | |||||
| LAGE2 (CT6.1) | Unknown | 13 | 100–1000× | [40, 86] | |
| SSX (CT5) | Transcriptional regulator |
19 | 100–1000× | Xp11.23 | [40, 86] |
| CSAG (CT24.2) | Unknown | 88 | 100–1000 | Xq28 | [40, 86] |
| SCP1 (CT8) | Unknown | 25 | 10–100 | [40, 86] | |
| SPANXC (CT11.3) | Unknown | 31 | 100–1000 | Xq27.1 | [40, 86] |
| TPTE (CT44) | Important in spermatogen- esis and endocrine function of testis |
6 | 100–1000 | [40] | |
| BORIS (CT27) | Unknown | 31 | <1000 | [40, 86] | |
| BRDT (CT9) | Role in sper- matogenesis |
31 | 100–1000 | 1p22.1 | [40, 86] |
| ADAM2 (CT15) | Participates in sperm-egg membrane binding |
— | — | 8p11.2 | [40, 86] |
| SP17 (#1)(CT22) | Unknown | — | — | [40, 86] | |
| SP17 (#2)(CT22) | Unknown | — | — | [40, 86] | |
| MMA (CT25) | Unknown | 13 | 100–1000 | [40, 86] | |
| HOM-TES85 (CT28) | Unknown | 13 | 10–100 | [40] | |
| TPBG (5T4) | Unknown | 6 | 10–100 | [40, 86] | |
| HCA661 (CT32) | Unknown | 69 | 100–1000 | [40, 86] | |
| MORC (CT33) | Unknown but required for spermatogen- esis |
19 | 10–100 | 3q13 | [40, 86] |
| NXF2 (CT39) | Exhibits RNA export activity in male germ cell and neurons |
31 | 100–1000 | Xq22.1 | [40, 86] |
| LIP1 (CT17) | Unknown | — | — | 21q11.2 | [40, 86] |
| CTAGE (CT21) | Unknown | 6 | 10–100 | 18p11.2 | [40, 86] |
| NY-SAR-35 (CT37) | Unknown | — | — | [40, 86] | |
| FTHL17 (CT38) | Unknown | 6 | 100–1000 | Xp21 | [40, 86] |
| Potential Non-CTA TAA Immunotherapy Targets in HNSCC | |||||
| p53 | [40] | ||||
| Her2/Neu | [40] | ||||
| BCL2α | [40] | ||||
| Livin | [40] | ||||
| Surviving | [40] | ||||
| PHAMM | [40] | ||||
| Htert | [40] | ||||
| IL-13Rα2 | [40,83] | ||||
| BXL-XL | [40] | ||||
| MCL1 | [40] | ||||
| MELK | [40] | ||||
| DPPA2 | [40] | ||||
| KM-HN-1 | [40] | ||||
| CD24 | [83] | ||||
| CD44 | [83] | ||||
| CD74 | [83] | ||||
| HSP27 | [83] | ||||
Categories of Immunotherapy
All cancer immunotherapy shares the same end goal of increasing the host’s immune response to tumor cells. It places special emphases on increasing the activity of CTLs capable of killing cells expressing TAAs. However, different types of immunotherapy accomplish this goal through different processes, and they potentially afford the maximal benefits before, during, or after traditional treatments, while vaccinations against tumor antigens also have a prophylactic activity. Currently, immunotherapy can be divided into three broad categories as follows.
Increasing the Nonspecific Immune System
This type of immunotherapy relies on the theory that increases in the general activity of the immune system will lead to increased levels of naturally occurring T cells. A number of these T cells should be able to react to TAAs on cancerous cells causing a cell-mediated destruction of the cancerous tissue. Previous studies showed that increased levels of T cells in tumor tissues led to increased control of the neoplasm [36,38,41]. Therapies included in this category are often based on cytokines such as IL-2, IL-12, and IFN-α. Some therapies, such as IRX-2, use a combination of these cytokines. IRX-2 is a biological product that contains multiple cytokines produced from phytohemagglutinin stimulated mononuclear cells. In particular, it contains IL-1, IL-2, IL-6, IL-8, TNF-α, INF-γ, G-CSF, and GM-CSF. This study consists of giving an initial dose of cyclophosphamide, a 2-week course of IRX-2, followed by a 3-week course of indomethacin and zinc supplementation. The study includes patients with advanced disease and those who requested this treatment for adjuvant therapy. Initial doses of cyclophosphamide were used to inhibit T suppressor cells, indomethacin was used to block immunosuppression due to prostaglandins synthesized by tumor cells, and zinc was used to reverse cellular immunodeficiency [42, 43]. A trial of IRX-2 administered prior to surgery to 27 previously untreated patients with stage II-IV HNSCC showed great promise. IRX-2 showed minimal acute toxicity. Tumor responses (graded as 12% decrease on blinded CT review) were seen in 16% of patients and 74% of patient’s tumors either had reductions or remained stable in size. Decreases in lymph node infiltration were also observed. Two-year survival was estimated at 72% and disease-free survival estimates were 67%. Both were increased compared to the 81 treatment-matched controls in this study [42, 43].
Active Immunization
The second broad category of immunotherapy is active immunization of the tumor-bearing host. This strategy is designed to increase and activate preexisting anti-tumor T cells. The approach, in general, has not been as successful as adoptive transfer of activated immune T cells in patients with preexisting tumors [36]. However, barriers to this approach are undergoing more intensive research, which may increase efficacy. The two most common forms of tumor vaccines that are used to develop active immunity are based on peptides or dendritic cells. Both work by taking advantage of TAAs to create a cell-mediated response.
Peptide-based vaccines are made of antigens which are expressed in HNSCC. When antigens are injected into the tumor site, they are processed by APCs, such as macrophages and dendritic cells, and are then presented to T cells. This process increases the naturally existing subpopulation of active T cells and results in an increased cell-mediated destruction of tumor cells. An ongoing phase I clinical trial is assessing the safety and dosing of a peptide vaccine made of MAGE-A and HPV-16 antigens for the use in treatment of HNSCC [38,42].
Dendritic cell vaccines are a new generation of tumor vaccines. Currently, dendritic cell vaccines are of more interest than peptide vaccines in the treatment of HNSCC. This is because dendritic cell vaccines are the most powerful method of inducing active immunity [37]. By either pulsing dendritic cells with antigens or using gene therapy to transduce genes expressing antigens, dendritic cells can be reprogrammed to present TAAs to host lymphocytes [44,45] after injection into the site of the tumor with a compliment of activating adjuvants.
In April 2010, the APC–containing vaccine sipuleucel-T (Provenge) was the first cancer vaccine approved by the FDA [46]. Currently, sipuleucel-T is approved for use in the treatment of metastatic castration-resistant prostate cancer. This approval was based on three phase III clinical studies, the most important of which was the IMPCAT trial. Sipuleucel-T was shown to have a median survival improvement of 4.1 months when compared to placebo [46-48]. Side effects appeared low, with 83% of enrolled patients able to continue their lives without restrictions. The most common side effect was flu-like symptoms which were found in 3.5% of those enlisted [46-48]. The approval of sipuleucel-T is a breakthrough in the field of tumor immunotherapy. It proved that immunotherapy holds potential in cancer treatment. Phase I clinical trials are currently exploring dendritic cell vaccines in HNSCC (Table 3).
TABLE 3.
Immunotherapy, vaccines, and cytokines currently in development for HNSCC
| Agents | Phase | Status | Primary objective/brief description |
|---|---|---|---|
| Cyclophosphamide fludarabine T-cell infusion Interleukin-2 |
Phase INCT00937300 | Recruiting N = 6 | The aim of this study is to investigate the toxicity and immune response of therapy with tumor infiltrating lymphocytes as adjuvant treatment for head and neck cancer after primary operation and radiotherapy. To accomplish these goals, patients will receive a single treatment consisting of conditioning chemotherapy for 7 days (cyclophosphamide for 2 days and fludarabine for 5 days), intravenous infusion of a high number of in vitro expanded tumor infiltrating lymphocytes followed by 2 weeks with daily low-dose interleukine-2. Patients will be evaluated for toxicity and immune response. |
| P53 | Phase 1 NCT00404339 | Recruiting N = 50 | To determine the toxicity of intranodally injected autologous dendritic cells loaded with wild-type p53 peptides with or without T-helper peptide epitopes in patients with HNSCC. |
| Dendritic cell | Phase 1 NCT00492947 | Recruiting N = 10 | To determine safety and efficacy of dendritic cell vaccines in HNSCC. |
| PV701 | Phase INCT00081211 | Complete N = 30 | To determine the effectiveness of intratumoral PV701 in treating patients who have advanced or recurrent unresectable HNSCC. |
| MAGE-A3 HPV-16 | Phase INCT00704041 | Recruiting N =48 | To test the safety of experimental cancer vaccines made ofMAGE-A3 and HPV-16 antigens. To test which doses best stimulate the immune system |
| Recombinant fowlpox-TRICOM |
Phase INCT00021424 | Complete N = 20 | To access safety and dosing of intralesional immunotherapy with a recombinant avipox virus engineered to express a triad of co-stimulatory molecules. |
| Allovectin-7 | Phase III NCT00050388 | Complete | To determine if Allovectin-7, an experimental gene-based immunotherapy, can shrink head and neck tumors. |
| HPVE6E7 Peptide | Phase INCT00019110 | Complete N = 40 | To determine safety of and presence of endogenous cellular immune response to HPV viral antigens E6 and E7. |
| Ras Peptides IL-2 GM-CSF |
Phase IINCT00019331 | Complete N = 60 | To study the effectiveness of Ras vaccine therapy plus interleukin-2 and/or sargramostim in treating adults who have metastatic solid tumors. |
| IL-2 | Phase III NCT00002702 | Recruiting N = 260 | To study surgery and radiation therapy alone to see how well it works compared to surgery, radiation therapy, and interleukin-2 in treating patients with squamous cell carcinoma of the mouth or oropharynx. |
| IRX-2 Cyclophosphamide Indomethacin Zinc |
Phase IINCT00210470 | Ongoing | To determine efficacy of IRX-2. IRX-2 is a biological product that contains multiple cytokines produced from phytohemagglutinin stimulated mononuclear cells. The IRX-2 regimen to be studied is the combination of a 2-week course of IRX-2 itself, and an initial dose of cyclophosphamide and a 3-week course of indomethacin and zinc supplementation. Some patients in study were treated with neoadjuvant therapy and some were treated with advanced disease. |
Notes. All data from Clinicaltrials.gov [42]
Adoptive T-cell Transfer
The last and most efficacious form of immunotherapy is adoptive T-cell transfer. This involves the transfer of activated immune T cells, which are capable of recognizing cancer cells and destroying them. This technique is so efficacious that it has been shown to induce cancer regression in 50–70% of patients with metastatic melanoma [36,49,50].
Most of the efficacy of adoptive T-cell transfer stems from to the possibility of selecting highly reactive T cells. In a normal host, most of the T cells capable of identifying and destroying cancer cells have a low affinity for TAAs. This occurs because the natural expression of TAAs in noncancerous host tissue causes TAAs to be presented to T cells in the thymus during negative selection [36]. The process of negative selection removes T cells that have high reactivity toward the antigens as part of the body’s natural defense against autoimmunity. The majority of T cells that remain in the body after negative selection have a low affinity for TAAs. Most of the highly reactive T cells undergo apoptosis [36]. Because cancer vaccines can activate only naturally occurring T cells, a great majority of the effector T cells activated through their use will have a low affinity for the cancer cells [36]. Adoptive immunity can overcome this by selecting highly reactive T cells ex vivo. These cells can then be proliferated before reinjection.
In June 2009, a phase I clinical trial assessing the safety and efficacy of adoptive T-cell therapy in HNSCC began at the Herlev Hospital in Denmark [42]. This study is assessing the immune response to tumor-infiltrating lymphocytes (TILs) as adjuvant treatments for head and neck cancer after primary operation and radiotherapy. To accomplish these goals, patients will receive a single treatment consisting of conditioning chemotherapy for 7 days (cyclophosphamide for 2 days and fludarabine for 5 days), intravenous infusion of a high number of in vitro expanded tumor infiltrating lymphocytes, followed by 2 weeks with daily low-dose IL-2. Historically, TILs have been exploited successfully in the therapy of melanoma [51-54]. Recently, the feasibility of ex vivo expanding relevant numbers of tumor-specific TILs from patients with HNSCC was studied. TIL bulk cultures were established from HNSCC lesions by high-dose IL-2, then stimulated with anti-CD3 antibody and feeder tumor cells. The study showed that TILs could be expanded from 80% of patients in 17 days [55]. Since infection with high-risk human papilloma virus (HPV) is associated with HNSCC and viral proteins E6 and E7 are specifically expressed by tumor cells, they are ideal targets for T-cell adoptive transfer therapies. It has recently been shown that E6/E7 specific CD4+ lymphocytes could be generated in vitro by introduction of HPV16E6/E7-specific TCRs into circulating CD4+ cells [56]. These findings indicate that TCR transfer is a promising technique to generate ex vivo HPV16 specific CD4+ lymphocytes that could be adoptively transferred to patients suffering from HPV16-induced tumors, including cervical cancer and HNSCC. Finally, a currently ongoing clinical trial will investigate the toxicity and immunologic response of TILs adoptive transfer as adjuvant therapy for HNSCC after surgical debulking and radiotherapy (identifying number NCT00937300, www.clinicaltrials.gov). Patients receive a single conditioning chemotherapy for 1 week, followed by intravenous infusion of in vitro expanded TILs and daily low-dose interleukine-2 for additional 2 weeks. Results from this study are expected by the end of 2012.
Solutions to Past Problems and the Current Standings of Immunotherapy in Cancer Treatment
Although many barriers still exist to successful implementation of immunotherapy, we believe that with the increasing knowledge of pathology and recent technological advances, the barriers can be overcome. In particular, we feel that the invention of recent techniques, such as profound lymphodepletion, and the concurrent use of specific cytokines and chemotherapy with immunotherapy will allow for immunotherapy to overcome local immunologic suppression and permit the effective destruction of cancer cells.
Lymphodepletion by total-body irradiation or chemotherapy is emerging as a key pretreatment for immunotherapy. It allows for substantial increases in the efficacy of adoptive cell transfer for two reasons [36,57,58]. First, lymphodepletion removes suppressor cells, such as T regs and CD8+ suppressor cells. Without the presence of these cells creating anergy at the tumor site, transferred cells are able to appropriately attack tumor cells [36,57-59]. Secondly, lymphodepletion removes endogenous low-affinity CD8+ T cells and NK cells. These cells can act as sinks by stealing important homeostatic cytokines, such as IL-7 and IL-15, from newly transferred high-affinity T cells. Without the presence of native cells, which possess low-affinity T-cell receptors, the high-affinity transferred T cells can use cytokines to their full potential, increasing levels of cellular-mediated tumor destruction [36,58].
A recently murine model showed that increases in efficacy obtained through lymphodepletion in adoptive T-cell therapy are in direct proportion to the degree that the subject was lympho-depleted. In this study, as the conditioning levels of lymphodepletion increased, so did the efficacy of the adoptive T-cell transfer. Further studies must be completed to titrate conditioning levels in order to create an appropriate tradeoff balance between increased efficacy of treatment and total body harm by irradiation [58].
Current clinical research, which takes into account the aforementioned barriers, is starting to see impressive results in several clinical trials. In a phase I/II trial investigating the efficacy and safety of immunotherapy in the treatment of malignant melanoma, a regression was seen in 50% of the patients treated [58]. In this trial, lympho-depletion was given before adoptive T-cell therapy with concurrent IL-2 [58].
HPV Vaccines in HNSCC Immunotherapy
A subgroup of oropharyngeal squamous-cell carcinoma is caused by HPV-16 infection [60]. These malignancies are characterized by the expression of the viral oncoproteins E6 that blocks p53, and E7, and inactivates pRb [61]. It has been shown that HPV-positive HNSCC patients have better prognosis than HPV-negative patients [62], but the reason for this difference is unclear [63]. However, a recent meta-analysis study [64] focusing on HPV infection and HNSCC risk and survival demonstrated that the prevalence of HPV-positive HNSCC is 22% (with 86.7% of HPV-16 genotype) and that an inverse correlation exists between HPV infection and p53 inactivating mutations. The study also highlighted that HPV-positive HNSCC represents a separate and peculiar biologic entity that likely will require the establishment of different therapies. In June 2009, the results of the first large phase III international clinical trial investigating the prognostic significance of HPV and p16 status in oropharyngeal cancers were published [62]: the study confirmed that HPV-positive patients with p16-positive tumors have better prognosis than patients with HPV-negative and p16-negative cancers. Further, it has been reported that HPV infection reduces the correlation between EGFR hyperexpression and poor prognosis [65]; therefore, an HPV infection test should be included in the clinical evaluation of EGFR expression levels as a prognostic marker.
Based on these very recent findings, HPV evaluation and stratification will be needed in future clinical trials. Accordingly, the 2008 National Cancer Institute’s State of the Science Meeting has clearly established that HPV status implies serious concerns in HNSCC future clinical trial designs and statistical evaluations [66]. Finally, a highly potential treatment opportunity comes from the observation of HPV-associated HNSCC: anti-HPV vaccines such as Cervarix and Gardasil, currently FDA-approved for the prevention of HPV-associated cervical cancer [67-71], are worthy of careful evaluation to prevent HPV infections, also in HNSCC and as therapeutic tools for the management of both HNSCC locoregional recurrence and metastatic disease. Clinical trials evaluating the association between HPV-vaccine and HNSCC incidence and prognosis will be required to provide the rationale for a HPV-based HNSCC preventive vaccination strategy.
Experimental Algorithm for HNSCC
The key to progress in developing immunotherapeutic treatment of HNSCC is the development of an algorithm for its use. Because removing or not offering standard treatment to patients is not possible based on the limited knowledge of the potential success that immunotherapy may possess, we suggest its use in concurrence with current standards of care. An algorithm based on multiple therapies is also beneficial because immunotherapy lends itself to synergy at two different levels. Firstly, it synergizes with traditional therapies by successfully filling the deficiencies that they possesses. Here, it provides a relatively nontoxic treatment option with the capability to control metastasis and provide long-term immunosurveillance. By working together, almost all forms of HNSCC can be treated efficiently. Secondly, recent studies have shown that chemotherapy and radiation can increase CTL-mediated tumor cell destruction, increasing the efficacy of immunotherapy [72, 73].
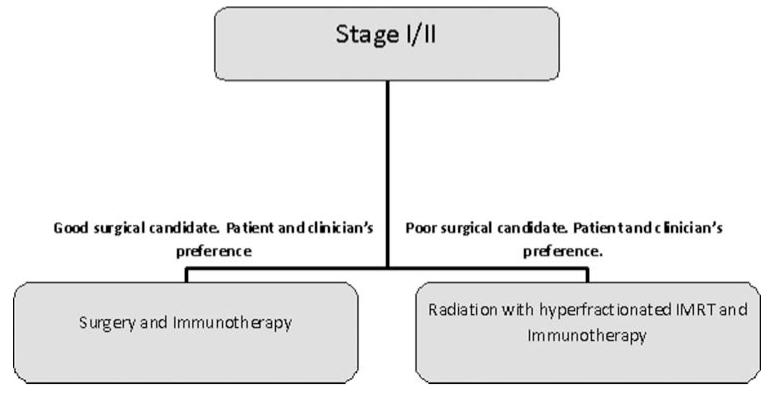
Stage I/II
Currently stage I/II HNSCC is treated with monotherapy of surgery or radiation with a 90% cure rate. Though these treatment modalities offer a good prognosis, we think that the addition of immunotherapy to either will have benefits (Figure 1). Considering the small size of early-stage lesions, we suggest that the isolation and expansion of TILs could be challenging, while the use of IRX-2 and/or active immunization with MAGE-A and HPV16 E6/E7 antigens are likely to be the best options. Firstly, we hope that immunotherapy can further increase survival rates for this stage. A 90% survival rate is good unless you are the 10% not included in the statistic. Secondly, immunotherapy’s ability to debulk tumors would allow less invasive surgery and more focused radiation. Ultimately, this leads to the goal of decreasing morbidity during the treatment of this delicate area.
FIGURE 1.
Experimental algorithm for stage I/II HNSCC.
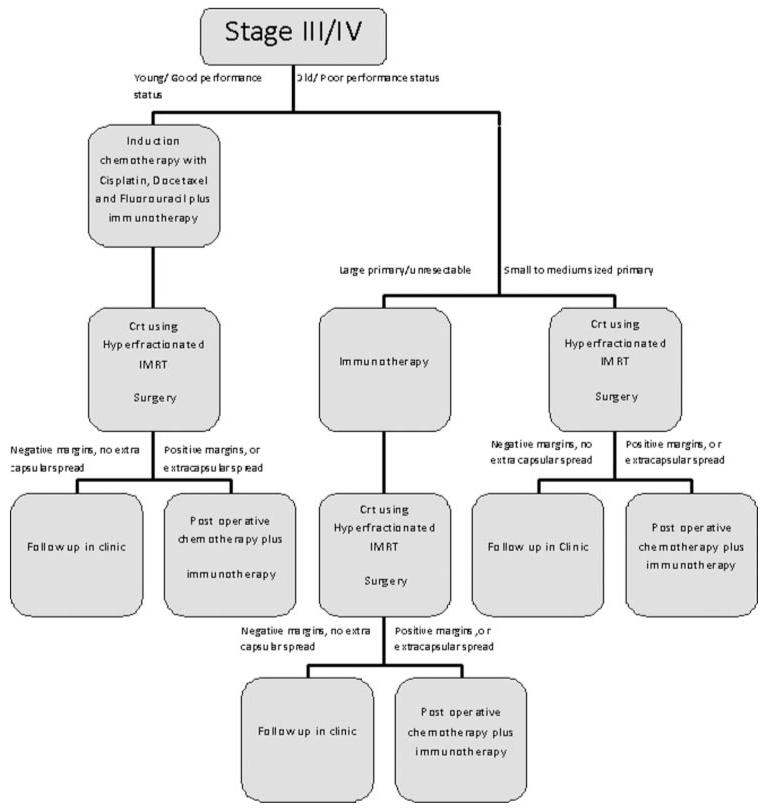
Stage III/IV
In our proposed algorithm for treatment of stage III/IV cancers, patients would first be broken into two groups. The first arm would include young patients with good performance status. The second arm would contain older patients with poor performance status.
The first arm, consisting of young patients, would be given induction chemotherapy consisting of docetaxel, fluorouracil, and cisplatin. They would also receive immunotherapy at this time. For these patients we suggest the use of dendritic cell-based tumor vaccines closely resembling sipuleucel-T. Indeed, this approach is expected to afford a powerful and long-lasting immune response, but it could be hampered by the immune senescence process [74,75]; therefore it will be best exploited in young subjects with good general performance status [76-78]. Induction chemotherapy has already been shown to increase survival when given in this subset of patients. We feel that immunotherapy would compliment chemotherapy by decreasing the bulk of the primary tumor and helping to destroy micrometastasis and distant spread. By decreasing the size of the primary tumor, surgery should result in less morbidity. Also, this cytoreduction should allow for less chemotherapy to be used with equal results. By attacking micrometastasis and distant spread with immunotherapy, our algorithm (Figure 2) aims to increase survival. Metastasis is one area that is currently not attacked well by other treatment options.
FIGURE 2.
Experimental algorithm for stage III/IV HNSCC.
After induction chemotherapy and immunotherapy, patients would undergo CRT and surgery. This radiation treatment will help control the primary tumor and locoregional disease. Clinicians should use hyperfractionated IMRT when giving radiation due to the increased survival and decreased morbidity associated with both treatment options compared to older techniques.
Lastly, patients would be postoperatively staged by new TNM staging guidelines. If extracapsular spread is found, or pathology reports positive margins, the patient would be given postoperative chemotherapy and immunotherapy. This will control disease that was not destroyed during previous treatments. Immunotherapy will again break host anergy against tumors and allow for body wide attack of tumor cells.
The tumors of second arm of our proposed treatment guideline, consisting of older patients with poor performance status, would be assessed for size. Patients with large tumors, or tumors in which surgery would be difficult, would first be given immunotherapy. This process fulfills the role of induction chemotherapy. Induction chemotherapy cannot be given to these patients because the added toxicities of chemotherapy outweigh the benefits. Immunotherapy will reduce the size of the tumor, allowing for less morbidity during surgery. It will also hopefully increase survival by its control of metastasis and locally advanced disease. Without immunotherapy, these areas could not be adequately targeted in this patient subpopulation. To this goal, best outcomes are likely to be obtained by a combination of cytokines and active immunization. After immunotherapy, patients with large tumors should be treated with CRT and surgery in the same fashion as that seen in the guidelines for first arm of the treatment. They should also go under identical postoperative staging and further treatment based on staging results.
Older patients with small to medium-sized tumors should directly undergo CRT and surgery. If patients are found to have extracapsular spread or positive margins, immunotherapy and postoperative chemotherapy should be given. If a patient’s comorbidities prevent chemotherapy, only immunotherapy should be given (Figure 2). We suggest the use of recombinant TCR-transduced tumor-specific T cells previously expanded from TILs, which will be collected after surgery with high yield.
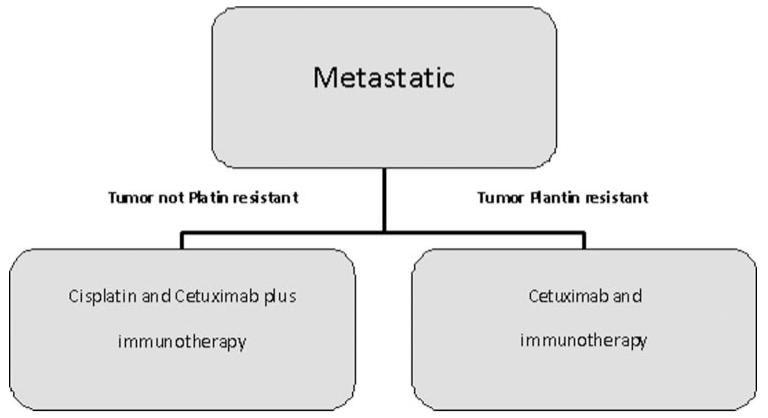
Metastatic
Metastatic HNSCC is currently treated for palliation with platin and cetuximab. We hope that the addition of immunotherapy to this regime will extend survival and possibly treat to cure (Figure 3). We hold that active immunization followed by adoptive T-cell transfer and (whenever possible according to the patient performance status) supported by cytokine therapy will be the required regimen [79] to generate a sustained and prolonged immune response against multiple metastatic sites [80,81].
FIGURE 3.
Experimental algorithm for metastatic HNSCC
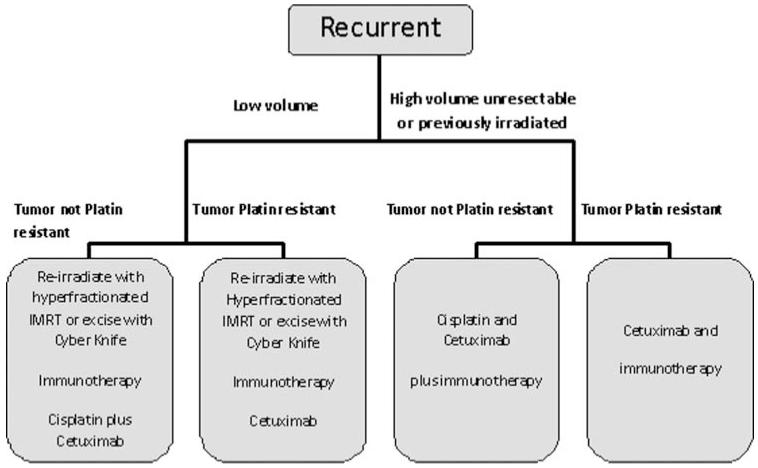
Recurrent
Recurrent HNSCC currently has a dismal prognosis and is treated the same as metastatic HNSCC unless it is low volume. If recurrence is small, our guideline recommends reirradiation or cyber knife excision with intent to cure. This is followed by immunotherapy, cisplatin, and cetuximab (Figure 4). If tumor is platin resistant, platins would not be given. Since the ultimate goal for recurrent HNSCC therapy is improving patients’ quality of life, we suggest that the use of potentially aggressive immunotherapies would not be indicated. Rather, we believe that a cytokine cocktail-based support to increase naturally occurring responses will be the best option.
FIGURE 4.
Experimental algorithm for recurrent HNSCC.
Conclusions
Due to limited increases in survival time over the last decade and the severe morbidity that is seen during the treatment of HNSCC, we urgently need to explore further therapeutic options. This study introduces several innovations and proposes a possible future algorithmic guideline for treatment of HNSCC through immunotherapy.
Immunotherapy has the potential to achieve great results in treating HNSCC and was previously shown to be successful in the treatment of liver carcinomas, malignant melanoma, and prostate carcinoma. By harnessing the host’s immune system, immunotherapy can attack primary and secondary cancer tissues.
We believe that immunological therapy could become a new addition to the standard treatment of HNSCC for the following reasons. (1) It can debulk the tumor mass while destroying the tumor by means of a cellular response. This allows for decreased morbidity during secondary treatment. It also allows for direct local and regional control of the tumor. (2) It should be able to control the satellite lesions and micro-metastases more efficiently because of its more cell-specific nature. This destruction of cells should increase survival and allow for decreased levels of chemotherapy to achieve an equal response. (3) The systemic immune response created by immunotherapy may help target distant metastasis. This stage is currently untreatable and has a poor prognosis. (4) The possible activation of memory responses could lead to much-needed long-term tumor immunosurveillance, and thus reduce the incidence of relapses.
Acknowledgments
We thank Teri Fields for assistance in revising this manuscript. This review is partially based on research that is supported by NIH grants RC2 CA148298-01 and RO1 CA74397-11 (to WMK). This review has been partially supported by the Associate Dean of the Oncology Programs at TTUHSC. The Billy and Ruby Power Endowment for Cancer Research (MCI) and Laura W. Bush Institute for Women’s Health(MCI) and Mrs J. Avery “Janie” Rush Endowed Chair for Excellence in WH and Oncology (MCI).
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- [1].Cognetti DM, Weber RS, Lai SY. Head and neck cancer: an evolving treatment paradigm. Cancer. 2008;113:1911–1932. doi: 10.1002/cncr.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- [3].Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83:489–501. doi: 10.4065/83.4.489. [DOI] [PubMed] [Google Scholar]
- [4].Nguyen NP, Chi A, Nguyen LM, et al. Human papillomavirus-associated oropharyngeal cancer: a new clinical entity. QJM. 2010;103:229–236. doi: 10.1093/qjmed/hcp176. [DOI] [PubMed] [Google Scholar]
- [5].Heck JE, Berthiller J, Vaccarella S, et al. Sexual behaviours and the risk of head and neck cancers: a pooled analysis in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. Int J Epidemiol. 2010;39:166–181. doi: 10.1093/ije/dyp350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Goon PK, Stanley MA, Ebmeyer J, et al. HPV & head and neck cancer: a descriptive update. Head Neck Oncol. 2009;1:36. doi: 10.1186/1758-3284-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lallemant B, Evrard A, Combescure C, et al. Clinical relevance of nine transcriptional molecular markers for the diagnosis of head and neck squamous cell carcinoma in tissue and saliva rinse. BMC Cancer. 2009;9:370. doi: 10.1186/1471-2407-9-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359:1143–1154. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- [9].Langer CJ. Targeted therapy in head and neck cancer: state of the art 2007 and review of clinical applications. Cancer. 2008;112:2635–2645. doi: 10.1002/cncr.23521. [DOI] [PubMed] [Google Scholar]
- [10].Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- [11].Psyrri A, Fountzilas G. Advances in the treatment of locally advanced non-nasopharyngeal squamous cell carcinoma of the head and neck region. Med Oncol. 2006;23:1–15. doi: 10.1385/MO:23:1:1. [DOI] [PubMed] [Google Scholar]
- [12].Tsao AS, Garden AS, Kies MS, et al. Phase I/II study of docetaxel, cisplatin, and concomitant boost radiation for locally advanced squamous cell cancer of the head and neck. J Clin Oncol. 2006;24:4163–4169. doi: 10.1200/JCO.2006.05.7851. [DOI] [PubMed] [Google Scholar]
- [13].Fung C, Grandis JR. Emerging drugs to treat squamous cell carcinomas of the head and neck. Expert Opin Emerg Drugs. 2010;17:17. doi: 10.1517/14728214.2010.497754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shinoto M, Shioyama Y, Sasaki T, et al. Clinical results of definitive chemoradiotherapy for patients with synchronous head and neck squamous cell carcinoma and esophageal cancer. Am J Clin Oncol. 2010;3:3. doi: 10.1097/COC.0b013e3181e84b4b. [DOI] [PubMed] [Google Scholar]
- [15].Choi BD, Archer GE, Mitchell DA, et al. EGFRvIII-targeted vaccination therapy of malignant glioma. Brain Pathol. 2009;19:713–723. doi: 10.1111/j.1750-3639.2009.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li G, Mitra S, Wong AJ. The epidermal growth factor variant III peptide vaccine for treatment of malignant gliomas. Neurosurg Clin N Am. 2010;21:87–93. doi: 10.1016/j.nec.2009.08.004. [DOI] [PubMed] [Google Scholar]
- [17].Li G, Wong AJ. EGF receptor variant III as a target antigen for tumor immunotherapy. Expert Rev Vaccines. 2008;7:977–985. doi: 10.1586/14760584.7.7.977. [DOI] [PubMed] [Google Scholar]
- [18].Uribe P, Gonzalez S. Epidermal growth factor receptor (EGFR) and squamous cell carcinoma of the skin: molecular bases for EGFR-targeted therapy. Pathol Res Pract. 2011;207:337–342. doi: 10.1016/j.prp.2011.03.002. [DOI] [PubMed] [Google Scholar]
- [19].Valentini AM, Pirrelli M, Caruso ML. EGFR-targeted therapy in colorectal cancer: does immunohistochemistry deserve a role in predicting the response to cetuximab? Curr Opin Mol Ther. 2008;10:124–131. [PubMed] [Google Scholar]
- [20].Wikstr CJ, Hale LP, Batra SK, et al. Monoclonal antibodies against EGFRvIII are tumor specific and react with breast and lung carcinomas and malignant gliomas. Cancer Res. 1995;55:3140–3148. [PubMed] [Google Scholar]
- [21].Tinhofer I, Klinghammer K, Weichert W, et al. Expression of amphiregulin and EGFRvIII affect outcome of patients with squamous cell carcinoma of the head and neck receiving cetuximab-docetaxel treatment. Clin Cancer Res. 2011;17:5197–5204. doi: 10.1158/1078-0432.CCR-10-3338. [DOI] [PubMed] [Google Scholar]
- [22].Ochiai H, Archer GE, Herndon JE, 2nd, et al. EGFRvIII-targeted immunotoxin induces antitumor immunity that is inhibited in the absence of CD4 +and CD8+ T cells. Cancer Immunol Immunother. 2008;57:115–121. doi: 10.1007/s00262-007-0363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dreier A, Barth S, Goswami A, et al. Cetuximab induces mitochondrial translocalization of EGFRvIII, but not EGFR: involvement of mitochondria in tumor drug resistance? Tumour Biol. 2011;11:11. doi: 10.1007/s13277-011-0248-4. [DOI] [PubMed] [Google Scholar]
- [24].Weiner GJ. Monoclonal antibody mechanisms of action in cancer. Immunol Res. 2007;39:271–278. doi: 10.1007/s12026-007-0073-4. [DOI] [PubMed] [Google Scholar]
- [25].Baeuerle PA, Kufer P, Bargou R. BiTE: Teaching antibodies to engage T-cells for cancer therapy. Curr Opin Mol Ther. 2009;11:22–30. [PubMed] [Google Scholar]
- [26].Wolf E, Hofmeister R, Kufer P, et al. BiTEs: bispecific antibody constructs with unique anti-tumor activity. Drug Discov Today. 2005;10:1237–1244. doi: 10.1016/S1359-6446(05)03554-3. [DOI] [PubMed] [Google Scholar]
- [27].Lutterbuese R, Schaller E, Burghart E, Sriskandarajah M, Raum T, Rau D, Mangold S, Cierpka R, Guller B, Lutterbuese P, Baeuerle PA, Kufer P. Conversion of cetuximab, panitumumab, trastuzumab and omalizumab into T-cell-engaging BiTE antibodies creates novel drug candidates of high potency. Proc Am Assoc Cancer Res. 2008;99 Abs 2402. [Google Scholar]
- [28].Tijink BM, Buter J, R de Bree, et al. A phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus. Clin Cancer Res. 2006;12:6064–6072. doi: 10.1158/1078-0432.CCR-06-0910. [DOI] [PubMed] [Google Scholar]
- [29].Sauter A, Kloft C, Gronau S, et al. Pharmacokinetics, immunogenicity and safety of bivatuzumab mertansine, a novel CD44v6-targeting immunoconjugate, in patients with squamous cell carcinoma of the head and neck. Int J Oncol. 2007;30:927–935. [PubMed] [Google Scholar]
- [30].Van Hal NL, Van Dongen GA, Ten Brink CB, et al. Sequence variation in the monoclonal-antibody-U36-defined CD44v6 epitope. Cancer Immunol Immunother. 1997;45:88–92. doi: 10.1007/s002620050406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Colnot DR, Ossenkoppele GJ, Roos JC, et al. Reinfusion of unprocessed, granulocyte colony-stimulating factor-stimulated whole blood allows dose escalation of 186Relabeled chimeric monoclonal antibody U36 radioimmunotherapy in a phase I dose escalation study. Clin Cancer Res. 2002;8:3401–3406. [PubMed] [Google Scholar]
- [32].Borjesson PK, Postema EJ, Roos JC, et al. Phase I therapy study with (186)Re-labeled humanized monoclonal antibody BIWA 4 (bivatuzumab) in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2003;9:3961S–3972S. [PubMed] [Google Scholar]
- [33].Mackay CR, Terpe HJ, Stauder R, et al. Expression and modulation of CD44 variant isoforms in humans. J Cell Biol. 1994;124:71–82. doi: 10.1083/jcb.124.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rupp U, Schoendorf-Holl E, Eichbaum M, et al. Safety and pharmacokinetics of bivatuzumab mertansine in patients with CD44v6-positive metastatic breast cancer: final results of a phase I study. Anticancer Drugs. 2007;18:477–485. doi: 10.1097/CAD.0b013e32801403f4. [DOI] [PubMed] [Google Scholar]
- [35].Platt VM, Szoka FC., Jr. Anticancer therapeutics: targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor. Mol Pharm. 2008;5:474–486. doi: 10.1021/mp800024g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rosenberg SA. Overcoming obstacles to the effective immunotherapy of human cancer. Proc Natl Acad Sci U S A. 2008;105:12643–12644. doi: 10.1073/pnas.0806877105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chiriva-Internati M, Cobos E, Kast WM. Advances in immunotherapy of multiple myeloma: from the discovery of tumor-associated antigens to clinical trials. Int Rev Immunol. 2007;26:197–222. doi: 10.1080/08830180701365966. [DOI] [PubMed] [Google Scholar]
- [38].Rapidis AD, Wolf GT. Immunotherapy of head and neck cancer: current and future considerations. J Oncol. 2009;2009:346345. doi: 10.1155/2009/346345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chiriva-Internati M, Yu Y, Mirandola L, et al. Cancer testis antigen vaccination affords long-term protection in a murine model of ovarian cancer. PLoS One. 2010;5:e10471. doi: 10.1371/journal.pone.0010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Weinert BT, Krishnadath KK, Milano F, et al. Real-time PCR analysis of genes encoding tumor antigens in esophageal tumors and a cancer vaccine. Cancer Immun. 2009;9:9. [PMC free article] [PubMed] [Google Scholar]
- [41].Boni A, Muranski P, Cassard L, et al. Adoptive transfer of allogeneic tumor-specific T cells mediates effective regression of large tumors across major histocompatibility barriers. Blood. 2008;112:4746–4754. doi: 10.1182/blood-2008-07-169797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].U. S. N. I. o. Health Clinical Trials. 2010:2010. [Google Scholar]
- [43].Wolf FWGT, Dolan R. IRX-2: promising new immunotherapy for head and neck cancer; Proceeding of 7th internation Conference on Head and Neck Cancer of American Head and Neck Society; San Francisco, Calif, USA. 2008. [Google Scholar]
- [44].Chiriva-Internati M, Liu Y, Salati E, et al. Efficient generation of cytotoxic T lymphocytes against cervical cancer cells by adeno-associated virus/human papillomavirus type 16 E7 antigen gene transduction into dendritic cells. Eur J Immunol. 2002;32:30–38. doi: 10.1002/1521-4141(200201)32:1<30::AID-IMMU30>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- [45].Steinman RM, Mellman I. Immunotherapy: bewitched, bothered, and bewildered no more. Science. 2004;305:197–200. doi: 10.1126/science.1099688. [DOI] [PubMed] [Google Scholar]
- [46].Brower V. Approval of provenge seen as first step for cancer treatment vaccines. J Natl Cancer Inst. 2010;102:1108–1110. doi: 10.1093/jnci/djq295. [DOI] [PubMed] [Google Scholar]
- [47].Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10:580–593. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Higano CS, Small EJ, Schellhammer P, et al. Sipuleucel-T. Nat Rev Drug Discov. 2010;9:513–514. doi: 10.1038/nrd3220. [DOI] [PubMed] [Google Scholar]
- [49].Disis ML, Bernhard H, Jaffee EM. Use of tumour-responsive T cells as cancer treatment. Lancet. 2009;373:673–683. doi: 10.1016/S0140-6736(09)60404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gervois N, Heuze F, Diez E, et al. Selective expansion of a specific anti-tumor CD8 +cytotoxic T lymphocyte clone in the bulk culture of tumor-infiltrating lymphocytes from a melanoma patient: cytotoxic activity and T cell receptor gene rearrangements. Eur J Immunol. 1990;20:825–831. doi: 10.1002/eji.1830200417. [DOI] [PubMed] [Google Scholar]
- [52].Melief CJ, Kast WM. T-cell immunotherapy of cancer. Res Immunol. 1991;142:425–429. doi: 10.1016/0923-2494(91)90042-h. [DOI] [PubMed] [Google Scholar]
- [53].Wong RA, Alexander RB, Puri RK, et al. In vivo proliferation of adoptively transferred tumor-infiltrating lymphocytes in mice. J Immunother. 1991;10:120–130. doi: 10.1097/00002371-199104000-00006. [DOI] [PubMed] [Google Scholar]
- [54].Yang JC, Rosenberg SA. Current approaches to the adoptive immunotherapy of cancer. Adv Exp Med Biol. 1988;233:459–467. doi: 10.1007/978-1-4899-5037-6_50. [DOI] [PubMed] [Google Scholar]
- [55].Junker N, Andersen MH, Wenandy L, et al. Bimodal ex vivo expansion of T cells from patients with head and neck squamous cell carcinoma: a prerequisite for adoptive cell transfer. Cytotherapy. 2011;13:822–834. doi: 10.3109/14653249.2011.563291. [DOI] [PubMed] [Google Scholar]
- [56].Scholten KB, Turksma AW, Ruizendaal JJ, et al. Generating HPV specific T helper cells for the treatment of HPV induced malignancies using TCR gene transfer. J Transl Med. 2011;9:147. doi: 10.1186/1479-5876-9-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Heemskerk B, Liu K, Dudley ME, et al. Adoptive cell therapy for patients with melanoma, using tumor-infiltrating lymphocytes genetically engineered to secrete interleukin-2. Hum Gene Ther. 2008;19:496–510. doi: 10.1089/hum.2007.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wrzesinski C, Paulos CM, Kaiser A, et al. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J Immunother. 2010;33:1–7. doi: 10.1097/CJI.0b013e3181b88ffc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- [61].Rampias T, Sasaki C, Weinberger P, et al. E6 and e7 gene silencing and transformed phenotype of human papillomavirus 16-positive oropharyngeal cancer cells. J Natl Cancer Inst. 2009;101:412–423. doi: 10.1093/jnci/djp017. [DOI] [PubMed] [Google Scholar]
- [62].Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- [64].Dayyani F, Etzel CJ, Liu M, et al. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC) Head Neck Oncol. 2010;2:15. doi: 10.1186/1758-3284-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Adelstein DJ, Ridge JA, Gillison ML, et al. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting, November 9-10, 2008, Washington, D.C. Head Neck. 2009;31:1393–1422. doi: 10.1002/hed.21269. [DOI] [PubMed] [Google Scholar]
- [67].Munoz N, Manalastas R, Jr, Pitisuttithum P, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24-45 years: a randomised, double-blind trial. Lancet. 2009;373:1949–1957. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- [68].Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- [69].Schwarz TF, Spaczynski M, Schneider A, et al. Immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15-55 years. Vaccine. 2009;27:581–587. doi: 10.1016/j.vaccine.2008.10.088. [DOI] [PubMed] [Google Scholar]
- [70].Munoz N, Kjaer SK, Sigurdsson K, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102:325–339. doi: 10.1093/jnci/djp534. [DOI] [PubMed] [Google Scholar]
- [71].Palefsky JM. Human Papillomavirus-related disease in men: not just a women’s issue. J Adolescent Health. 2010;46:S12–S19. doi: 10.1016/j.jadohealth.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kaneno R, Shurin GV, Tourkova IL, et al. Chemomodulation of human dendritic cell function by antineoplastic agents in low noncytotoxic concentrations. J Transl Med. 2009;7:58. doi: 10.1186/1479-5876-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Gelbard A, Garnett CT, Abrams SI, et al. Combination chemotherapy and radiation of human squamous cell carcinoma of the head and neck augments CTL-mediated lysis. Clin Cancer Res. 2006;12:1897–1905. doi: 10.1158/1078-0432.CCR-05-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Grubeck-Loebenstein B, Della Bella S, Iorio AM, et al. Immunosenescence and vaccine failure in the elderly. Aging Clin Exp Res. 2009;21:201–209. doi: 10.1007/BF03324904. [DOI] [PubMed] [Google Scholar]
- [75].Shurin MR, Shurin GV, Chatta GS. Aging and the dendritic cell system: implications for cancer. Crit Rev Oncol Hematol. 2007;64:90–105. doi: 10.1016/j.critrevonc.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ressing ME, van Driel WJ, Brandt RM, et al. Detection of T helper responses, but not of human papillomavirus-specific cytotoxic T lymphocyte responses, after peptide vaccination of patients with cervical carcinoma. J Immunother. 2000;23:255–266. doi: 10.1097/00002371-200003000-00010. [DOI] [PubMed] [Google Scholar]
- [77].van Driel WJ, Ressing ME, Kenter GG, et al. Vaccination with HPV16 peptides of patients with advanced cervical carcinoma: clinical evaluation of a phase I-II trial. Eur J Cancer. 1999;35:946–952. doi: 10.1016/s0959-8049(99)00048-9. [DOI] [PubMed] [Google Scholar]
- [78].Warrino DE, Olson WC, Knapp WT, et al. Disease-stage variance in functional CD4(+) T-cell responses against novel pan-human leukocyte antigen-D region presented human papillomavirus-16 E7 epitopes. Clin Cancer Res. 2004;10:3301–3308. doi: 10.1158/1078-0432.CCR-03-0498. [DOI] [PubMed] [Google Scholar]
- [79].Dallal RM, Lotze MT. Immunotherapy of metastasis. Surg Oncol Clin N Am. 2001;10:433–447. [PubMed] [Google Scholar]
- [80].Nothelfer EM, Zitzmann-Kolbe S, Garcia-Boy R, et al. Identification and characterization of a peptide with affinity to head and neck cancer. J Nucl Med. 2009;50:426–434. doi: 10.2967/jnumed.108.058123. [DOI] [PubMed] [Google Scholar]
- [81].Walsh JE, Lathers DM, Chi AC, et al. Mechanisms of tumor growth and metastasis in head and neck squamous cell carcinoma. Curr Treat Options Oncol. 2007;8:227–238. doi: 10.1007/s11864-007-0032-2. [DOI] [PubMed] [Google Scholar]
- [82].Choi P, Jordan CD, Mendez E, et al. Examination of oral cancer biomarkers by tissue microarray analysis. Arch Otolaryngol Head Neck Surg. 2008;134:539–546. doi: 10.1001/archotol.134.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Han J, Kioi M, Chu WS, et al. Identification of potential therapeutic targets in human head & neck squamous cell carcinoma. Head Neck Oncol. 2009;1:27. doi: 10.1186/1758-3284-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lee PS, Teaberry VS, Bland AE, et al. Elevated MAL expression is accompanied by promoter hypomethylation and platinum resistance in epithelial ovarian cancer. Int J Cancer. 2010;126:1378–1389. doi: 10.1002/ijc.24797. [DOI] [PubMed] [Google Scholar]
- [85].Muller-Richter UD, Dowejko A, Reuther T, et al. Analysis of expression profiles of MAGE-A antigens in oral squamous cell carcinoma cell lines. Head Face Med. 2009;5:10. doi: 10.1186/1746-160X-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Online CTDatabase . Vol. 2010: Ludwig Institute for Cancer Research. 2010. [Google Scholar]