Abstract
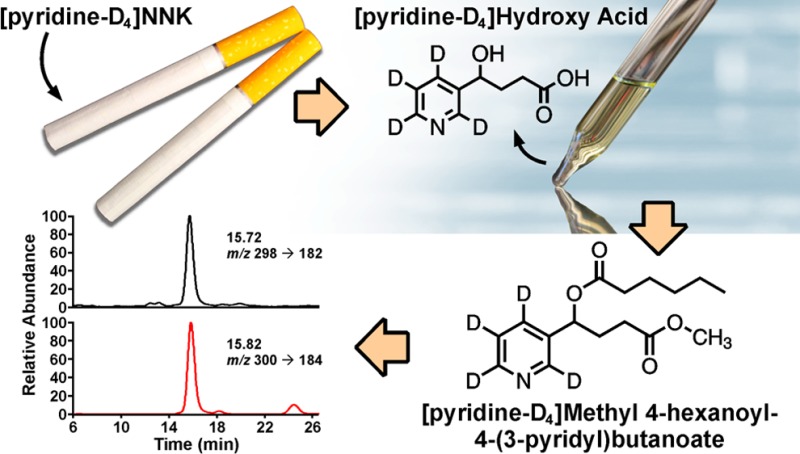
4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK, 1) is a potent tobacco-specific lung carcinogen believed to play a key role in the development of lung cancer in smokers. Metabolic activation of NNK to DNA damaging reactive intermediates proceeds via α-hydroxylation pathways. The end products of these pathways are excreted in the urine of smokers as 4-oxo-4-(3-pyridyl)butanoic acid (keto acid, 3) and 4-hydroxy-4-(3-pyridyl)butanoic acid (hydroxy acid, 4). The sum of these biomarkers (after NaBH4 treatment), referred to as total hydroxy acid, could potentially be used to measure the extent of NNK metabolic activation in smokers. However, the same metabolites are formed from nicotine; therefore, there is a need to distinguish the NNK- and nicotine-derived keto and hydroxy acid in smokers’ urine. We previously developed a unique methodology based on the use of [pyridine-D4]NNK ([D4]1), which metabolizes to the correspondingly labeled biomarkers. In this study, we developed a sensitive and reproducible assay for the detection and quantitation of total [pyridine-D4]hydroxy acid ([D4]4) in human urine. A two-step derivatization approach was used to convert [D4]4 to [pyridine-D4]methyl 4-hexanoyl-4-(3-pyridyl)butanoate ([D4]6), and an LC-ESI-MS/MS method was developed for the analysis of this derivative with excellent sensitivity, accuracy, and precision. The robustness and reproducibility of the assay was further confirmed by its application for the analysis of urine samples from 87 smokers who smoked [D4]1-containing cigarettes for 1 week. The measured level averaged 130 fmol/mL urine. The developed assay can be used in future studies that may require evaluation of the relative efficiency of NNK metabolic activation in humans.
Introduction
Tobacco use is among the most prevalent, albeit preventable, human carcinogen exposures. Cigarette smoking causes up to 90% of lung cancer, the most common cause of cancer death in the United States, resulting in a projected 159,480 deaths in 2013.1,2 Tobacco-specific nitrosamines (TSNA) are among the most significant tobacco carcinogens; multiple studies clearly document their strong carcinogenicity in laboratory animals as well as the occurrence of substantial amounts of these carcinogens in both unburned tobacco and tobacco smoke.3−8 One of the most prevalent of these compounds, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK, 1), is a remarkably effective lung carcinogen in laboratory animals, inducing lung tumors in rodents independent of the route of administration.3,9 Results of numerous investigations indicate that NNK is a causative agent for lung cancer in smokers.3,6,10−12 Exposure to NNK may also be causally related to nasal, oral, liver, pancreatic, and cervical cancers.3,6,9,13−16 NNK together with the related nitrosamine NNN have been classified as Group 1 carcinogens by the International Agency for Research on Cancer (IARC).9
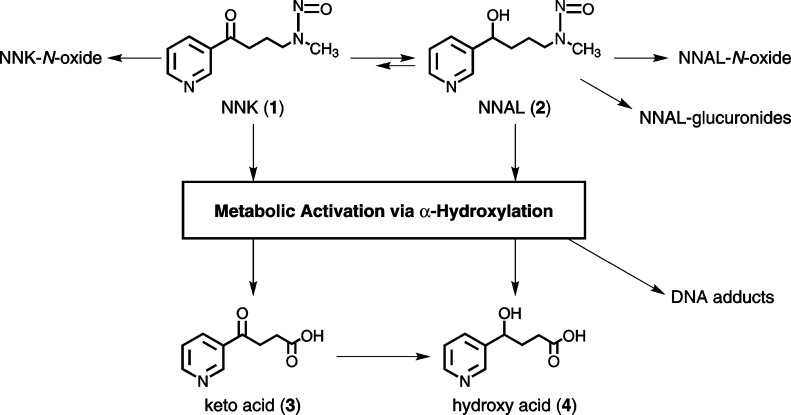
NNK requires metabolic activation to exert its carcinogenic effects.3 Therefore, the risk of lung cancer in smokers may be affected by the relative extent of NNK metabolic activation and detoxification, with those who activate NNK more extensively being at higher risk. Studies in laboratory animals show that NNK is metabolized by three major routes: carbonyl reduction, pyridine oxidation, and α-hydroxylation.3,17−20 Carbonyl reduction produces 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL, 2) (Scheme 1), which undergoes metabolic transformations similar to those of NNK, except for the formation of NNAL-glucuronides (NNAL-Glucs), an important pathway of NNK and NNAL detoxification.3,20−23 The sum of NNAL and its glucuronides can be measured in urine as “total NNAL,” which is an established biomarker of NNK exposure in humans.24−26 NNAL can also be partially converted back to NNK; however, the NNK-NNAL equilibrium favors NNAL in rodents, primates, and humans.19−21,27−29 The second process, pyridine-N-oxidation, is a minor metabolic pathway in humans29 and, therefore, is not expected to play an important role in the relative balance of metabolic activation and detoxification of NNK. The third major route, α-hydroxylation, leads to the formation of DNA methylating and pyridyloxobutylating intermediates, and thus represents metabolic activation of NNK and NNAL (Scheme 1). Formation of DNA adducts from the reactive pyridyloxobutyl metabolite has been well established; some of these adducts have miscoding properties and can activate oncogenes.30−33 The major end products of the NNK and NNAL α-hydroxylation pathway are 4-oxo-4-(3-pyridyl)butanoic acid (keto acid, 3) and 4-hydroxy-4-(3-pyridyl)butanoic acid (hydroxy acid, 4); urinary excretion of these metabolites has been shown in rodents, primates, and humans.20,21,34,35 Thus, the balance between urinary NNAL-Glucs, as a biomarker of NNK exposure and detoxification, and the sum of urinary 3 and 4, as a biomarker of NNK metabolic activation, could potentially provide a useful index for the relative efficiency of these major pathways in individual smokers.
Scheme 1. Overview of Three Major in Vivo Pathways of NNK Metabolism.
Adapted with permission from ref (38). Copyright 2008 American Association for Cancer Research. A detailed scheme of NNK metabolism is available elsewhere.3
The main obstacle to using 3 and 4 as biomarkers of NNK metabolic activation is nicotine in cigarette smoke, which also generates these metabolites and is present at more than 1,000-fold higher levels than NNK.36,37 Thus, most 3 and 4 in smokers’ urine results from nicotine metabolism.35 Therefore, in our previous study we developed a novel approach to specifically identify NNK-derived 3 and 4 by using deuterium-labeled NNK.38 In that study, we added [pyridine-D4]NNK ([D4]1) to a commercial cigarette, which originally contained significantly lower levels of NNK than regular brands, and then analyzed urine samples collected from individuals who smoked these cigarettes.38 After treating urine with NaBH4 to convert 3 to 4, we used liquid chromatography–electrospray ionization–tandem mass spectrometry (LC-ESI-MS/MS) to distinguish the [D4]1-derived urinary [pyridine-D4]hydroxy acid ([D4]4) from the corresponding unlabeled metabolite and demonstrated for the first time that NNK is metabolically activated in smokers.38
Future application of our unique methodology can potentially provide important insights into the role of interindividual differences in NNK metabolic activation in cancer risk. However, the analytical procedure for the analysis of ([D4]4) in our original study of 20 smokers was not optimized for robust application in larger trials. Therefore, our purpose in this study was to develop an improved LC-ESI-MS/MS method for the quantitation of total [D4]4 in human urine.
Experimental Procedures
Caution:
[Pyridine-D4]NNK ([D4]1) is carcinogenic and mutagenic and should be handled with extreme care, using personal protective equipment in a well-ventilated hood at all times.
Chemicals
Compounds 3 and 4 were purchased from Toronto Research Chemicals (Toronto, ON), and [pyridine-D4]ethyl nicotinate and [13C6]ethyl nicotinate were purchased from Cambridge Isotope Laboratories (Tewksbury, MA) and Moravek Biochemicals (Brea, CA), respectively. Other reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO). All aqueous solutions were prepared using H2O purified from a 0.22 μm Millipore system (Billerica, MA). [D4]1 was synthesized as previously described.38
[Pyridine-D4]Hydroxy Acid ([D4]4) and [13C6]Hydroxy Acid ([13C6]4)
These were synthesized by adaptation of a previously described procedure.39 Briefly, [pyridine-D4]ethyl nicotinate or [13C6]ethyl nicotinate (0.75 mmol) was added to a suspension of NaH (3.7 mmol, 60% dispersion in mineral oil) in 5 mL of refluxing benzene. After the addition of ethanol (0.5 mL), diethyl succinate (3.75 mmol) was added into the stirred refluxing mixture dropwise, and the reaction was allowed to take place for 45 min. The mixture was then cooled in an ice bath, and 0.5 mL of 5 M HCl was added slowly with constant stirring, which was continued for another 10 min after the addition was completed. The aqueous phase was extracted with benzene and, after discarding the organic layer, adjusted to pH 9 by the addition of NH4OH and extracted again with chloroform. The chloroform layer was then dried over Na2SO4 and concentrated under diminished pressure to obtain the crude diethyl α-nicotinyl succinate as a brown oil. This crude product was heated under reflux in 2 mL of 5 M H2SO4 for 36 h. After the solution was cooled to room temperature, NaOH was added to adjust the pH to 4–5, and the precipitated crude [D4]3 or [13C6]3 was collected for further purification by HPLC using a linear gradient from 100% 15 mM NH4OAc to 20% 15 mM NH4OAc and 80% CH3CN over 50 min, at 0.7 mL/min. A Luna C18 column (250 × 4.6 mm, 5 μm particle size, Phenomenex) was used, and the UV detector was set at 254 nm. The products were collected at 17 min, as determined by the retention time of unlabeled 3. The products were treated with NaBH4 to produce [D4]4 and [13C6]4, which were purified using the same HPLC system and collected at 15 min, based on the retention time of unlabeled 4. The solvent in the collected HPLC fractions was removed by rotary evaporation, and the products were dried overnight under vacuum yielding [D4]4 and [13C6]4 [0.6 mg of each (11%)]. Co-injection on HPLC of each product with 4 gave only one peak, and the purity of each was determined to be approximately 98%. MS analysis (20 eV) of each product in the positive ESI mode further confirmed their identity: [D4]4, m/z 186 [M + H]+ (relative intensity 52%) and m/z 168 (100%); and [13C6]4, m/z 188 [M + H]+ (63%) and m/z 170 (100%).
[Pyridine-D4]Methyl 4-Hexanoyl-4-(3-pyridyl)butanoate ([D4]6)
Briefly, 1.13 mg (6.10 μmol) of [D4]4, synthesized as described above, was incubated in 1 mL of 3% (v/v) concentrated H2SO4 in MeOH at room temperature overnight. The reaction mixture was neutralized with 2 mL of 0.6 M NaHCO3. Samples were then loaded onto 5 mL ChemElut cartridges (Agilent Tech.), and the [D4]4 methyl ester was eluted with 25 mL of CH2Cl2. After the removal of solvent, 100 μL of 50 mg/mL DMAP solution in toluene and 500 μL of hexanoic anhydride were added, and the second esterification reaction was allowed to take place at 70 °C for 20 min. After cooling to room temperature, 1.5 mL of hexane/EtOAc (90:10) was added, and the product was extracted thrice with 1 mL of 1 N HCl. The aqueous phase containing the product was neutralized (NaHCO3), and the ester was extracted with 3 × 5 mL hexane/EtOAc (90:10), concentrated, and purified by preparative HPLC, yielding [D4]6 (27%) 95% pure, MS (in the positive ESI mode) m/z 298 [M + H]+ (15%), m/z 182 (100%), and m/z 122 (27%); 1H NMR (CDCl3, no TMS added, 500 MHz): δ 0.88 (3H, t, J = 7.0 Hz, 6′-CH3), 1.23–1.34 (4H, m, 4′-CH2, 5′-CH2), 1.61 (2H, m, 3′-CH2), 2.12–2.29 (2H, m, 3-CH2), 2.31–2.40 (4H, m, 2-CH2, 2′-CH2), 3.67 (3H, s, COO–CH3), 5.82 (1H, dd, J = 8.2, 5.5 Hz, 4-CH).
[13C6]Methyl 4-Hexanoyl-4-(3-pyridyl)butanoate ([13C6]6)
The same method as that described for [D4]6 was used for the preparation of [13C6]6 (36%), 95% pure, m/z 300 [M + H]+ (5%), m/z 184 (100%), and m/z 124 (82%). [D4]6 and [13C6]6 were stored frozen at −20 °C until use.
Subjects and Urine Collection
Smokers’ urine samples analyzed here were collected from subjects recruited for a chemoprevention trial that will be described elsewhere. The study and the urine sample collection were approved by the University of Minnesota Institutional Review Board (Study # 0712M22651). As part of the trial design, subjects were asked to stop smoking their usual cigarettes and switch to the study cigarettes to which [D4]1 was added. The cigarettes were prepared as previously described, and their use was approved by the US Food and Drug Administration (IND 74037).38 Subjects were asked to collect a 24-h urine sample after a 7-day period of smoking cigarettes containing [D4]1 and bring the sample to the Tobacco Research Programs clinic at the University of Minnesota; these urine samples were analyzed here. Study subjects kept urine at room temperature during the 24-h collection and delivered it to the clinic the next day. An experiment in which urine of a study subject was incubated overnight at 37 °C showed no degradation of hydroxy acid. In the laboratory, urine samples were kept at −20 °C until analysis. Subjects further followed the trial protocol, which will be described elsewhere along with data on additional biomarkers including creatinine. Additional samples collected by the subjects in the study were not analyzed for this report.
Analysis of Total [Pyridine-D4]Hydroxy Acid ([D4]4) in Smokers’ Urine
Treatment with NaBH4 and Initial Purification
Five microliters of 0.1 ng/μL [13C6]4 internal standard solution was added to 3 mL of urine, followed by 0.6 mL of 1 M NaOH solution and 0.3 mL of basic NaBH4 solution (7.2 mg NaBH4 in 0.3 mL of 0.1 M NaOH). The sample was mixed by vortexing and allowed to stand for at least 2 h at room temperature to convert all [D4]3 to [D4]4. Excess NaBH4 was destroyed by adding 1 M HCl dropwise, and the pH of the sample was adjusted to 6.5–7.5. The volume was reduced to 1 mL (SpeedVac), and the sample was applied to a 200 mg/3 mL Strata-X cartridge (Phenomenex) activated with 2 mL of MeOH. The cartridge was washed with 1 mL of H2O (which was shown not to remove the analyte), and [D4]4 was eluted by applying another 5 mL of H2O to the cartridge. The sample was dried overnight, using a SpeedVac.
First Derivatization (Conversion to Methyl Ester)
On the next day, 1 mL of freshly prepared 3% (v/v) concentrated H2SO4 in MeOH was added to the dry residue, followed by sonication and vortexing to completely dissolve the sample. The mixture was allowed to stand at room temperature for at least 2 h to convert [D4]4 to its methyl ester. Then, 2 mL of 50 mg/mL NaHCO3 was added dropwise to adjust the pH to 7.0–8.0. The sample was loaded onto a 5 mL ChemElut cartridge (Agilent Tech.), and the [D4]4 methyl ester was eluted with 2 × 8 mL of CH2Cl2. The eluant was concentrated on a SpeedVac for 1.5 h, and 500 μL of CH2Cl2 was immediately added to the dry residue. This was applied to a CH2Cl2-activated BondElut cartridge (Agilent Tech.), which was washed with 1 mL of CH2Cl2 and 1 mL of CH2Cl2/EtOAc (50:50) sequentially. The [D4]4 methyl ester was finally eluted with 2 mL of 100% EtOAc and dried by SpeedVac.
Second Derivatization (Conversion to [D4]6) and Final Purification
Immediately after drying, 10 μL of DMAP (50 mg/mL in toluene) and 50 μL of hexanoic anhydride were added. After sonication and vortex mixing, the mixture was incubated at 70 °C for 20 min, producing [D4]6. To extract the product into the aqueous phase, 0.5 mL of hexane/EtOAc (90:10) and 0.5 mL of 1 N HCl were added sequentially with thorough vortex mixing (30 s) after each addition. The mixture was centrifuged at 500g for 2 min, and the aqueous phase containing the analyte was separated. This extraction was repeated once, and the aqueous phases from both extractions were combined and applied to an activated 60 mg Oasis MCX cartridge (Waters Corp.), which was washed with 3 mL of 1 N HCl, 3 mL of MeOH/1 N HCl (40:60), and 3 mL of H2O/MeOH/NH4OH (80:15:5), sequentially. The hexanoate [D4]6 was then eluted with 10 mL of H2O/MeOH/NH4OH (30:65:5) and dried overnight (SpeedVac). The residue was dissolved in 2 × 100 μL of CH3CN, filtered on a Spin-X centrifuge tube filter (0.45 μm, Corning Life Sciences), and transferred to a 300 μL fused LC autosampler vial with insert. The sample was finally dried on a SpeedVac and kept frozen at −20 °C until LC-ESI-MS/MS analysis.
LC-ESI-MS/MS Analysis
The dried sample was redissolved in 12 μL of H2O and analyzed on a TSQ Vantage system (Thermo Scientific) interfaced with an Agilent 1100 capillary HPLC system and an Agilent 1100 micro autosampler. A Zorbax Eclipse PAH column (150 × 0.5 mm, 3.5 μm particle size) was maintained at 40 °C throughout the separation. The chromatography was performed with a CH3CN and 15 mM NH4OAc solvent system at a flow rate of 10 μL/min. A linear gradient started with 60% aqueous phase and 40% CH3CN and changed to 44.3% aqueous phase and 55.7% CH3CN over 22 min. The gradient was then ramped to 35% aqueous phase and 65% CH3CN over 3 min, and returned to the initial condition in 2 min. The column was equilibrated for 10 min before the next injection. The total run time was 37 min per sample.
The mass spectrometer was operated in the positive ion electrospray mode with selective reaction monitoring (SRM). The transitions m/z 298 [M + H]+ → m/z 122 [M–(CH3(CH2)4COO + COOCH3 + H)]+ and m/z 298 [M + H]+ → m/z 182 [M–CH3(CH2)4COO]+ were used for [D4]6, and the corresponding transitions m/z 300 → m/z 124 and m/z 300 → m/z 184 were used for [13C6]6. The mass resolution was set at 0.7 amu for Q1 and Q3. The scan width was set at 0.4 amu. The collision gas Ar was at 1.1 mTorr. The collision energy (C.E.) was 30 eV for m/z 298 → m/z 122 and m/z 300 → m/z 124 transitions, and 20 eV for m/z 298→ m/z 182 and m/z 300 → m/z 184 transitions. The heated capillary temperature was 285 °C, and the spray voltage was 3000 mV.
Results
Method Development
The purpose of this study was to develop a sensitive and reproducible assay for the analysis of [D4]4 in the urine of smokers who smoked cigarettes containing [D4]1. Overall, our method includes the reduction of urinary [D4]3 to [D4]4, followed by conversion of the highly polar [D4]4 to a stable methyl ester hexanoate ([D4]6, Figure 1), which can be further purified and analyzed by LC-ESI-MS/MS with excellent separation efficiency and ionization selectivity. The method is summarized in Scheme 2.
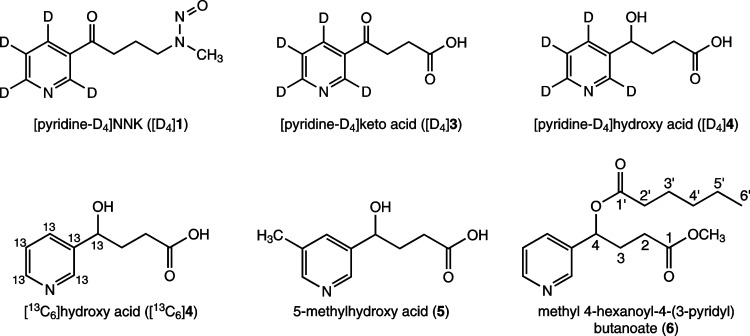
Figure 1.
Structures of some compounds discussed in this article. Carbons that covalently bond to hydrogens are labeled to assign NMR peaks for 6. See Experimental Procedures, Chemicals for details.
Scheme 2. Analytical Procedure for the Quantification of [D4]4 in Human Urine Samples.
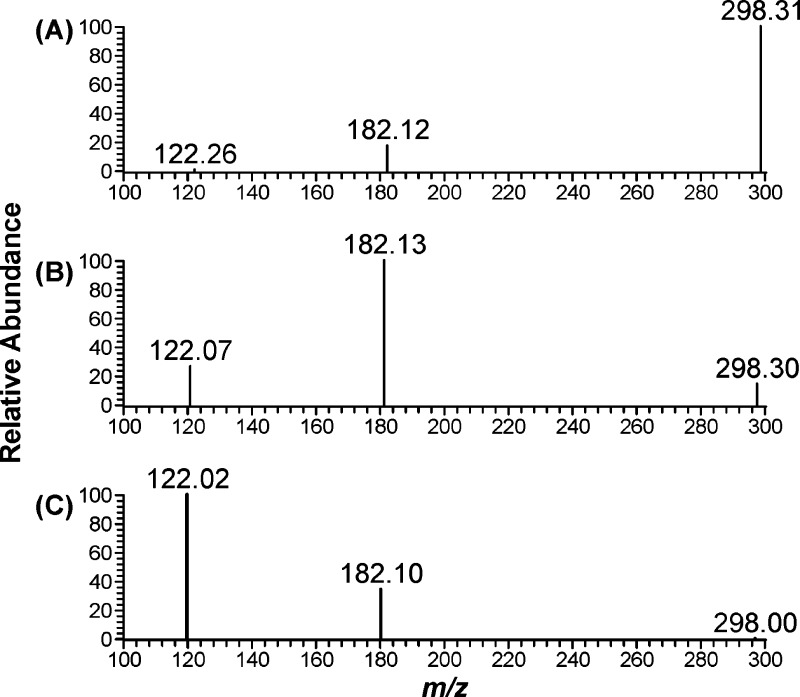
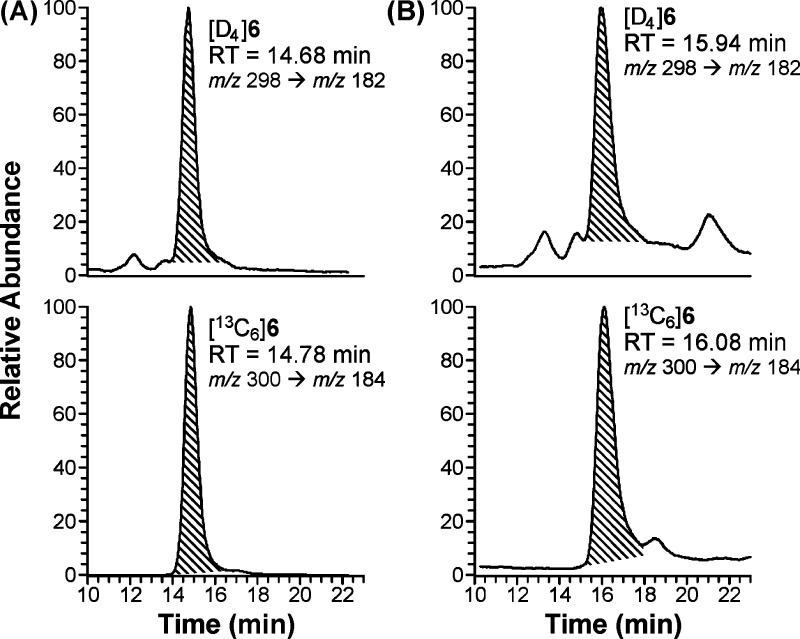
The LC-ESI-MS/MS conditions were developed using standard aqueous solutions containing [D4]6 and [13C6]6. The product ion spectra of [D4]6 were obtained by collision-induced dissociation of the ion at m/z 298 [M + H]+ as shown in Figure 2. We examined a range of collision energy values to investigate the fragmentation pattern. The protonated molecular ion (m/z 298) that was observed at 10 eV (Figure 2A) decreased at 20 eV (Figure 2B) and was barely observed at 30 eV (Figure 2C). A base peak with m/z 182 [M – CH3(CH2)4COO]+ was observed at 20 eV and a base peak with m/z 122 [M – CH3(CH2)4COO – COOCH3 – H]+ was observed at 30 eV. The transition m/z 298 → m/z 182 (20 eV) was chosen for quantitation because of the overall better peak shape and sensitivity both in nonsmokers’ urine samples to which [D4]4 was added and in smokers’ urine. The transition m/z 298 → m/z 122 (30 eV) was monitored to confirm the identity of the analyte in urine samples. The corresponding transitions for [13C6]6 are m/z 300 → m/z 184 and m/z 300 → m/z 124.
Figure 2.
Product scan of [D4]6 at different collision energy values used in the method development: A, 10 eV; B, 20 eV; and C, 30 eV.
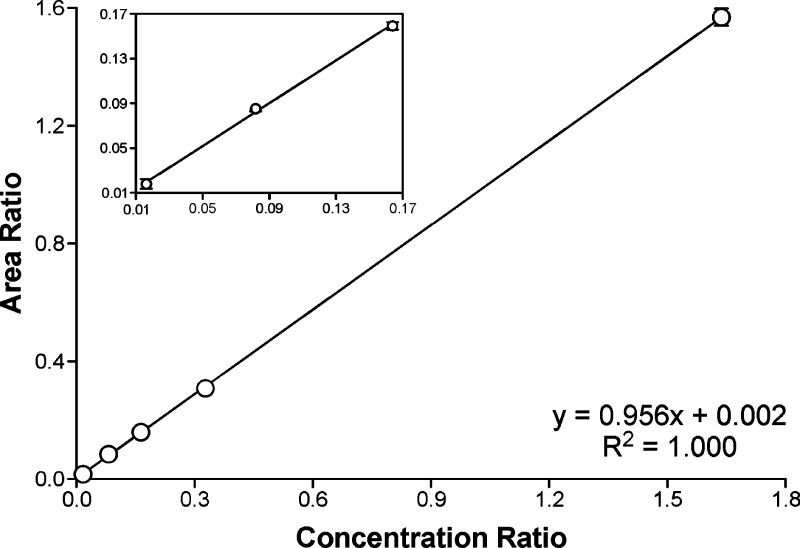
The calibration curve for the quantitation of [D4]6 was built by preparing a series of standard mixes containing varying ratios of [D4]6 to [13C6]6 (internal standard). As can be seen from Figure 3, which shows the calibration curve constructed from five standard mixes (six measurements performed for each on different days), excellent linearity was observed for the analyte in the range of 0.84 fmol ∼84 fmol [D4]6.
Figure 3.
Calibration curve for the quantitation of [D4]6 by LC-ESI-MS/MS. The amount of [13C6]6 (internal standard) was maintained at 51.1 pmol on column, while the amount of [D4]6 ranged from 0.84 fmol to 84 fmol on column. The X axis plots the concentration ratio of [D4]6 to [13C6]6. The Y axis plots the peak area ratio from the corresponding transitions, m/z 298 → m/z 182 and m/z 300 → m/z 184. Each data point represents the average of six measurements performed on different days with the error bars indicating standard deviation.
Method Characteristics
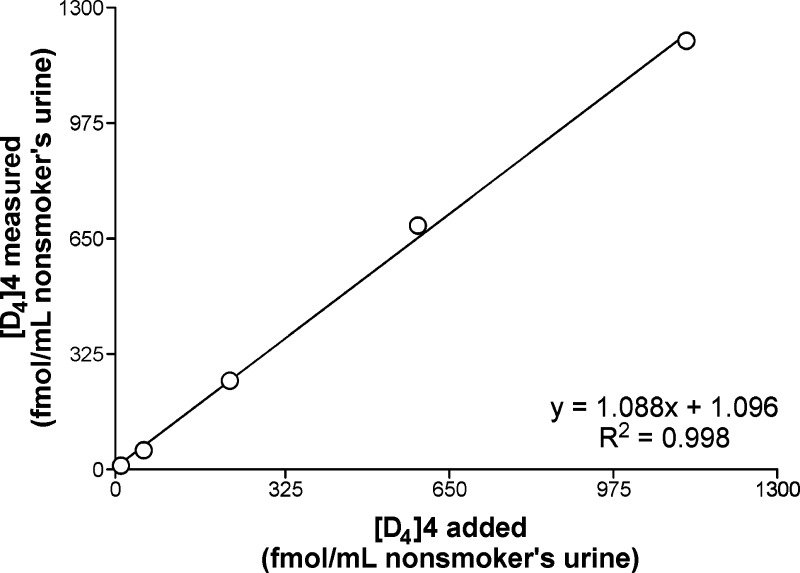
To determine precision, accuracy, recovery, and limit of detection of the assay, we used a nonsmoker’s urine to which a known amount of [D4]4 was added. A typical LC-ESI-MS/MS trace obtained in this analysis is shown in Figure 4A. The intraday precision of the assay was assessed by analyzing six aliquots of a nonsmoker’s urine mixed with [D4]4 at 360 fmol/mL urine. The measured mean level of [D4]4 was 342 ± 8 (SD) fmol/mL (coefficient of variation (CV) = 2.1%). The interday precision was determined by analyzing positive controls, aliquots of a nonsmoker’s urine mixed with [D4]4 at 225 fmol/mL urine, with six sets of assays performed during method characterization. The concentration of [D4]4 in positive controls averaged 226 ± 10 (SD) fmol/mL (CV = 4.6%). Assay accuracy was determined by analyzing a nonsmoker’s urine to which increasing amounts of [D4]4 were added, ranging from 11.3 fmol/mL to 1.12 pmol/mL. The accuracy of the measurements ranged from 99.0% to 115.8%. As demonstrated in Figure 5, the added and measured levels of [D4]4 were highly correlated (R2 = 0.9983). This experiment was performed at the method development step and was further included with each set of smokers’ urine samples analyzed later in the study. For the 20 sets of accuracy analyses performed in total, the average accuracy was 100.1% ± 14.7% (n = 100), and the average recovery of the [13C6]4 internal standard was 32.1% ± 27.6% (n = 100). Additional accuracy experiments with each sample set allowed us to determine the precision of the assay at [D4]4 levels that are lower than those used in the initial precision test and in the positive controls. At 11.3 fmol/mL [D4]4 addition, which is below the lowest level measured in smokers’ urine samples, the coefficient of variation was 14.2% . At the next [D4]4 addition level, 56.6 fmol/mL urine, the coefficient of variation was 5.8%, similar to that determined for the positive controls. At a 3:1 signal-to-noise ratio, the limit of detection (LOD) was 10 fmol/mL in nonsmokers’ urine to which [D4]4 was added. The limit of quantitation (LOQ) at a 5:1 signal-to-noise ratio was 25 fmol/mL and was established based on smokers’ urine analyzed by this method.
Figure 4.
Typical LC-ESI-MS/MS chromatograms obtained upon analysis of A, a nonsmoker’s urine (3 mL) to which 168 fmol of [D4]4 was added, and B, urine of a study subject who smoked cigarettes containing [D4]1.
Figure 5.
Relationship between added and measured levels of [D4]4 in a nonsmoker’s urine: method accuracy analysis.
Application of the Assay to the Analysis of Smokers’ Urine
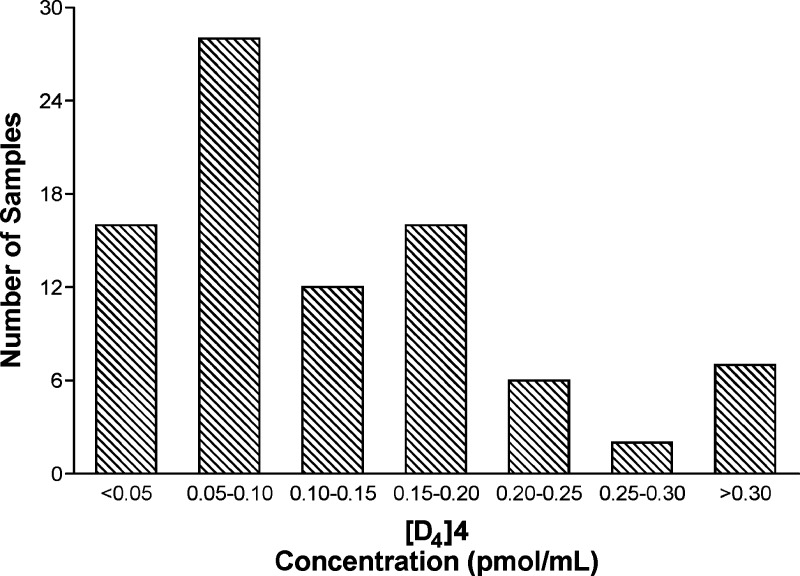
The method was applied to the analysis of [D4]4 in the urine of 87 smokers who smoked at least 10 [D4]1-containing cigarettes per day for at least 1 week. During sample preparation, a negative control, a positive control, and the accuracy test samples, all nonsmoker’s urine with or without [D4]4, were included with each set of smokers’ urine samples. Recovery of [13C6]4 was similar in nonsmokers’ and smokers’ urine, indicating that the high levels of unlabeled 4 that are present in smokers’ urine do not interfere with the analyses. Small peaks with retention time and m/z values matching [D4]4 were occasionally observed in the nonsmoker’s negative control samples and also in a pooled urine sample from smokers who did not smoke [D4]1; however, the level of this background interference in all cases was below LOD (∼1/3 LOD). In smokers who smoked [D4]1-containing cigarettes, urinary [D4]4 concentrations ranged from 25 fmol/mL to 390 fmol/mL urine, averaging 130 fmol/mL. Results for individual urine samples are summarized in Supporting Information, Table S1. A typical trace from an analysis of a smoker’s urine is shown in Figure 4B. The distribution of the measured [D4]4 concentrations in these urine samples is illustrated in Figure 6.
Figure 6.
Histogram demonstrating the distribution of [D4]4 levels in 87 urine samples from study subjects who smoked cigarettes containing [D4]1 for 1 week. For the levels in individual samples see Supporting Information, Table S1.
Discussion
Evaluation of the metabolic activation of the tobacco-specific lung carcinogen NNK in individual smokers may potentially provide important insights for understanding interindividual variation in the risk of developing lung cancer due to exposure to tobacco products. The addition of [D4]1 to special study cigarettes followed by the measurement of urinary total [D4]4 in people who smoke these cigarettes is a promising novel approach that can be used for this purpose. This approach requires a reliable and sensitive methodology for the measurement of the deuterium-labeled urinary biomarkers. In this study, we developed a sensitive and highly reproducible LC-ESI-MS/MS method for the analysis of [D4]4 in human urine. The method is characterized by excellent sensitivity, precision, accuracy, and recovery.
The original method for the analysis of [D4]4 that was used in our previous study included the conversion of the acid to its methyl ester to enable a purification step that cannot be applied without the esterification; this was followed by hydrolysis of the ester back to [D4]4, which was analyzed by LC-ESI-MS/MS.38 The resulting chromatographic traces were characterized by relatively high background noise and variable recovery of the analyte. In this study, we modified our protocol by including the second derivatization step after the conversion of [D4]4 to its methyl ester, thus producing [D4]6, a stable methyl ester hexanoate.40 This additional esterification decreases the polarity of the resulting product, allowing for more efficient removal of the multitude of polar interfering compounds present in the urine matrix, which ultimately results in better chromatography. Furthermore, the ionization efficiency in the ESI source is improved due to both decreased compound polarity and reduced matrix suppression. The second esterification also increases the molecular weight of the analyte by 98 amu, which increases the selectivity in the MS detection since most interfering compounds from the urine matrix have lower molecular weights. Another important modification is that in the new protocol, we applied the isotope dilution approach by using [13C6]4 as the internal standard, instead of 5-methylhydroxy acid (5, Figure 1) which was used in our previous assay.38 This approach ensures more accurate quantitation of the analyte as compared to the use of a surrogate internal standard. As shown in Figure 4, the analysis of both the nonsmoker’s urine to which [D4]4 was added and the urine of a smoker who switched to [D4]1-containing cigarettes, produced clean chromatograms with well-resolved peaks for [D4]6 and [13C6]6.
The concentration range of urinary total [D4]4 obtained in the current study for the 87 subjects who smoked [D4]1-containing cigarettes for 1 week is lower than that reported previously. Thus, in our previous study, the levels of urinary total [D4]4 ranged from 0.43 to 8.7 pmol/mL urine, averaging 2.8 pmol/mL in 20 smokers who smoked cigarettes to which [D4]1 was added at the same level as in the present study.38 This could be due to differences in the analytical procedures and internal standards used in the two studies, as well as the differences in the brand of study cigarettes used (Marlboro Virginia Blend in this study vs Quest cigarettes in the previous study), which may have affected the resulting smoking rates (21 vs 28 cigarettes per day in the current and the previous study, respectively), and other potential differences between the two studies and/or subject characteristics. We applied our new method to reanalyze a small set of urine samples that were available from the previous study and stored at −20 °C since its completion. We selected samples that, according to our previous analyses, contained high levels of [D4]4: 3.5, 4.2, and 8.8 pmol/mL urine. Reanalysis of these samples by the new method produced 1.4, 1.7, and 3.1 pmol/mL [D4]4, respectively. While there are discrepancies in urinary [D4]4 levels measured by the two methods, which could be due to a combination of the method differences and the prolonged storage of the samples from the previous study (at least 6 years), the levels determined by the new method in the urine collected in the previous study are still higher than those measured in the current study. Importantly, the method presented here has been thoroughly characterized and shows a broad dynamic range of accurate [D4]4 quantification, making it applicable in future studies.
NNK intake in smokers, nonsmokers exposed to secondhand smoke, and in smokeless tobacco users has been extensively analyzed by measuring urinary total NNAL, the end-product of the NNK carbonyl reduction pathway.41 It was also shown that pyridine-N-oxidation is only a minor metabolic pathway in humans.29 In contrast, the information on the extent of NNK α-hydroxylation in humans is extremely limited. It has been established that some of the adducts produced by pyridyloxobutylation of DNA or globin release 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB).42,43 While several studies detected and quantified HPB-releasing DNA and globin adducts in humans,44−46 it is not clear how much of the total measured level of these adducts is derived from NNK versus the related tobacco carcinogen N′-nitrosonornicotine, which shares common metabolic pathways with NNK and leads to the formation of the same adducts.47 Moreover, it is not clear how DNA or globin adduct levels can be compared to the urinary products of NNK detoxification, in order to estimate the balance between the two pathways in the same individual. Therefore, availability of a specific urinary biomarker of NNK metabolic activation offers practical advantages for investigation of the relative extent of NNK metabolic activation and detoxification in humans. Our previous study developed a unique approach that provided such a biomarker, and our current report describes a sensitive and reproducible assay for the measurement of this biomarker in future studies.
A limitation of this study is that we did not quantify [D4]3 and [D4]4 separately. However, the sum of the keto and hydroxy acids is an informative aggregate biomarker for the quantitation of the total efficiency of the NNK and NNAL α-hydroxylation pathways, and is expected to be used in future large-scale studies. The separate analysis of [D4]3 and [D4]4 can be easily achieved by measuring [D4]4 in the same urine sample without the NaBH4 reduction and then subtracting the measured amount from the total [D4]4 determined by the method described here.38 It is also important to note that, because the levels of natural NNK in regular cigarettes are higher than the levels of [D4]1 added to study cigarettes, the amount of the NNK-derived hydroxy acid in the urine of regular smokers may be higher than the levels of [D4]4 measured in people who smoke cigarettes containing [D4]1. However, the ultimate goal of the [D4]1 approach is not to provide absolute quantification of NNK metabolic activation but to compare the relative ratio of biomarkers reflecting metabolic activation and detoxification pathways within an individual.
In summary, we have developed a sensitive and robust assay for the quantitation of total [D4]4 in smokers’ urine. The assay incorporates a two-step derivatization procedure and LC-ESI-MS/MS analysis to achieve excellent precision, accuracy, recovery, and limit of detection. Analysis of smokers’ urine indicated that levels of [D4]4 as low as 26 fmol/mL can be quantified. The results of this work further confirm that [D4]4 could be used as the urinary biomarker to study NNK metabolic activation. The broad dynamic range of this assay will be very useful in large studies, especially those that may deal with potentially lower efficiency of NNK metabolic activation in smokers due to polymorphisms in NNK metabolizing genes or in chemoprevention trials.
Acknowledgments
We thank Bradley Hochalter for valuable discussions in developing the assay, Dr. Peter Villalta and Brock Matter for help with mass spectrometry, Katrina Yershova for technical support, Tobacco Research Programs staff for subject recruitment and sample collection, and Adam T. Zarth for help with acquiring NMR spectra. We also thank Bob Carlson for editorial assistance.
Glossary
Abbreviations
- Amu
atomic mass unit
- CE
collision energy
- DMAP
4-dimethylaminopyridine
- HPB
4-hydroxy-1-(3-pyridyl)-1-butanone
- IARC
International Agency for Research on Cancer
- LC-ESI-MS/MS
liquid chromatography–electrospray ionization–tandem mass spectrometry
- LOD
limit of detection
- LOQ
limit of quantification
- NNAL
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol
- NNAL-Gluc
glucuronide conjugates of NNAL
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- NNN
N′-nitrosonornicotine
- PAH
polycyclic aromatic hydrocarbons
- SRM
selective reaction monitoring
- TSNA
tobacco-specific N-nitrosamines
Supporting Information Available
Results for individual urine samples. This material is available free of charge via the Internet at http://pubs.acs.org.
This study was supported by NCI grants CA-122244 and CA-138338. LC-ESI-MS/MS experiments were carried out in the Analytical Biochemistry core facility of the Masonic Cancer Center, University of Minnesota, supported in part by NCI grant CA-77598.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- American Cancer Society (2013) Cancer Facts and Figures 2013, American Cancer Society, Atlanta, GA.
- Siegel R.; Naishadham D.; Jemal A. (2013) Cancer statistics, 2013. CA Cancer J. Clin. 63, 11–30. [DOI] [PubMed] [Google Scholar]
- Hecht S. S. (1998) Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol. 11, 559–603. [DOI] [PubMed] [Google Scholar]
- Hoffmann D.; Brunnemann K. D.; Prokopczyk B.; Djordjevic M. V. (1994) Tobacco-specific N-nitrosamines and Areca-derived N-nitrosamines: chemistry, biochemistry, carcinogenicity, and relevance to humans. J. Toxicol. Environ. Health 41, 1–52. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder B.; Bartsch H. (1996) Tobacco-specific nitrosamines. Eur. J. Cancer Prev. 5, 33–38. [PubMed] [Google Scholar]
- International Agency for Research on Cancer (2004) Tobacco Smoke and Involuntary Smoking, in IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 83, IARC, Lyon, France. [PMC free article] [PubMed] [Google Scholar]
- Harris J. E. (2001) Smoke yields of tobacco-specific nitrosamines in relation to FTC tar level and cigarette manufacturer: analysis of the Massachusetts Benchmark Study. Public Health Rep. 116, 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I.; Knezevich A.; Zhang L.; Watson C. H.; Hatsukami D. K.; Hecht S. S. (2012) Carcinogenic tobacco-specific N-nitrosamines in US cigarettes: three decades of remarkable neglect by the tobacco industry. Tob. Control 21, 44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (2007) Smokeless Tobacco and Some Tobacco-Specific N-Nitrosamines, in IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 89, IARC, Lyon, France. [PMC free article] [PubMed] [Google Scholar]
- Hecht S. S. (1999) Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst. 91, 1194–1210. [DOI] [PubMed] [Google Scholar]
- Church T. R.; Anderson K. E.; Caporaso N. E.; Geisser M. S.; Le C. T.; Zhang Y.; Benoit A. R.; Carmella S. G.; Hecht S. S. (2009) A prospectively measured serum biomarker for a tobacco-specific carcinogen and lung cancer in smokers. Cancer Epidemiol. Biomarkers Prev. 18, 260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J.-M.; Koh W.-P.; Murphy S. E.; Fan Y.; Wang R.; Carmella S. G.; Han S.; Wickham K.; Gao Y.-T.; Yu M. C.; Hecht S. S. (2009) Urinary levels of tobacco-specific nitrosamine metabolites in relation to lung cancer development in two prospective cohorts of cigarette smokers. Cancer Res. 69, 2990–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Sundqvist K.; Belinsky S. A.; Castonguay A.; Tjälve H.; Grafström R. C. (1993) Metabolism and macromolecular interaction of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in cultured explants and epithelial cells of human buccal mucosa. Carcinogenesis 14, 2383–2388. [DOI] [PubMed] [Google Scholar]
- Prokopczyk B.; Hoffmann D.; Bologna M.; Cunningham A. J.; Trushin N.; Akerkar S.; Boyiri T.; Amin S.; Desai D.; Colosimo S.; Pittman B.; Leder G.; Ramadani M.; Henne-Bruns D.; Beger H. G.; El-Bayoumy K. (2002) Identification of tobacco-derived compounds in human pancreatic juice. Chem. Res. Toxicol. 15, 677–685. [DOI] [PubMed] [Google Scholar]
- Prokopczyk B.; Cox J. E.; Hoffmann D.; Waggoner S. E. (1997) Identification of tobacco-specific carcinogen in the cervical mucus of smokers and nonsmokers. J. Natl. Cancer Inst. 89, 868–873. [DOI] [PubMed] [Google Scholar]
- Prokopczyk B.; Trushin N.; Leszczynska J.; Waggoner S. E.; El-Bayoumy K. (2001) Human cervical tissue metabolizes the tobacco-specific nitrosamine, 4- (methylnitrosamino)-1-(3-pyridyl)-1-butanone, via alpha-hydroxylation and carbonyl reduction pathways. Carcinogenesis 22, 107–114. [DOI] [PubMed] [Google Scholar]
- Tjälve H. (1991) The tissue distribution and the tissue specificity of bioactivation of some tobacco-specific and some other N-nitrosamines. Crit. Rev. Toxicol. 21, 265–294. [DOI] [PubMed] [Google Scholar]
- Tjälve H., Löfberg B., Castonguay A., Trushin N., and Hecht S. S. (1986) Perinatal Disposition and Metabolism in Mice and Hamsters of Some N-Nitrosamines Present in Tobacco and Tobacco Smoke, in Mechanisms in Tobacco Carcinogenesis (Hoffmann D., and Harris C. C., Eds.) pp 179–195, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York. [Google Scholar]
- Castonguay A.; Tjälve H.; Trushin N.; d’Argy R.; Sperber G. (1985) Metabolism and tissue distribution of tobacco-specific N-nitrosamines in the marmoset monkey (Callithrix jacchus). Carcinogenesis 6, 1543–1550. [DOI] [PubMed] [Google Scholar]
- Hecht S. S.; Trushin N.; Reid-Quinn C. A.; Burak E. S.; Jones A. B.; Southers J. L.; Gombar C. T.; Carmella S. G.; Anderson L. M.; Rice J. M. (1993) Metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in the patas monkey: pharmacokinetics and characterization of glucuronide metabolites. Carcinogenesis 14, 229–236. [DOI] [PubMed] [Google Scholar]
- Morse M. A.; Eklind K. I.; Toussaint M.; Amin S. G.; Chung F.-L. (1990) Characterization of a glucuronide metabolite of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and its dose-dependent excretion in the urine of mice and rats. Carcinogenesis 11, 1819–1823. [DOI] [PubMed] [Google Scholar]
- Upadhyaya P.; Carmella S. G.; Guengerich F. P.; Hecht S. S. (2000) Formation and metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol enantiomers in vitro in mouse, rat and human tissues. Carcinogenesis 21, 1233–1238. [PubMed] [Google Scholar]
- Upadhyaya P.; Kenney P. M. J.; Hochalter J. B.; Wang M.; Hecht S. S. (1999) Tumorigenicity and metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) enantiomers and metabolites in the A/J mouse. Carcinogenesis 20, 1577–1582. [DOI] [PubMed] [Google Scholar]
- Carmella S. G.; Akerkar S.; Richie J. P. Jr.; Hecht S. S. (1995) Intraindividual and interindividual differences in metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in smokers’ urine. Cancer Epidemiol. Biomarkers Prev. 4, 635–642. [PubMed] [Google Scholar]
- Carmella S. G.; Akerkar S.; Hecht S. S. (1993) Metabolites of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in smokers’ urine. Cancer Res. 53, 721–724. [PubMed] [Google Scholar]
- Carmella S. G.; Han S.; Fristad A.; Yang Y.; Hecht S. S. (2003) Analysis of total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Cancer Epidemiol. Biomarkers Prev. 12, 1257–1261. [PubMed] [Google Scholar]
- Adams J. D.; LaVoie E. J.; Hoffmann D. (1985) On the pharmacokinetics of tobacco-specific N-nitrosamines in Fischer rats. Carcinogenesis 6, 509–511. [DOI] [PubMed] [Google Scholar]
- Adams J. D.; LaVoie E. J.; O’Mara-Adams K. J.; Hoffmann D.; Carey K. D.; Marshall M. V. (1985) Pharmacokinetics of N′-nitrosonornicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in laboratory animals. Cancer Lett. 28, 195–201. [DOI] [PubMed] [Google Scholar]
- Carmella S. G.; Borukhova A.; Akerkar S. A.; Hecht S. S. (1997) Analysis of human urine for pyridine-N-oxide metabolites of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a tobacco-specific lung carcinogen. Cancer Epidemiol. Biomarkers Prev. 6, 113–120. [PubMed] [Google Scholar]
- Hecht S. S.; Villalta P. W.; Sturla S. J.; Cheng G.; Yu N.; Upadhyaya P.; Wang M. (2004) Identification of O2-substituted pyrimidine adducts formed in reactions of 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone and 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanol with DNA. Chem. Res. Toxicol. 17, 588–597. [DOI] [PubMed] [Google Scholar]
- Wang M.; Cheng G.; Sturla S. J.; Shi Y.; McIntee E. J.; Villalta P. W.; Upadhyaya P.; Hecht S. S. (2003) Identification of adducts formed by pyridyloxobutylation of deoxyguanosine and DNA by 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone, a chemically activated form of tobacco-specific carcinogens. Chem. Res. Toxicol. 16, 616–626. [DOI] [PubMed] [Google Scholar]
- Lao Y.; Yu N.; Kassie F.; Villalta P. W.; Hecht S. S. (2007) Formation and accumulation of pyridyloxobutyl DNA adducts in F344 rats chronically treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem. Res. Toxicol. 20, 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya P.; Kalscheuer S.; Hochalter J. B.; Villalta P. W.; Hecht S. S. (2008) Quantitation of pyridylhydroxybutyl-DNA adducts in liver and lung of F-344 rats treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem. Res. Toxicol. 21, 1468–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann D.; Castonguay A.; Rivenson A.; Hecht S. S. (1981) Comparative carcinogenicity and metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and N′-nitrosonornicotine in Syrian golden hamsters. Cancer Res. 41, 2386–2393. [PubMed] [Google Scholar]
- Hecht S. S.; Hatsukami D. K.; Bonilla L. E.; Hochalter J. B. (1999) Quantitation of 4-oxo-4-(3-pyridyl)butanoic acid and enantiomers of 4-hydroxy-4-(3-pyridyl)butanoic acid in human urine: a substantial pathway of nicotine metabolism. Chem. Res. Toxicol. 12, 172–179. [DOI] [PubMed] [Google Scholar]
- Adams J. D.; O’Mara K. J.; Hoffmann D. (1987) Toxic and carcinogenic agents in undiluted mainstream smoke and sidestream smoke of different types of cigarettes. Carcinogenesis 8, 729–731. [DOI] [PubMed] [Google Scholar]
- Counts M. E.; Hsu F. S.; Laffoon S. W.; Dwyer R. W.; Cox R. H. (2004) Mainstream smoke constituent yields and predicting relationships from a worldwide market sample of cigarette brands: ISO smoking conditions. Regul. Toxicol. Pharmacol. 39, 111–134. [DOI] [PubMed] [Google Scholar]
- Stepanov I.; Upadhyaya P.; Feuer R.; Jensen J.; Hatsukami D. K.; Hecht S. S. (2008) Extensive metabolic activation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in smokers. Cancer Epidemiol. Biomarkers Prev. 17, 1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKennis H.; Shwartz S. L.; Turnbull L. B.; Tamaki E.; Bowman E. R. (1964) The metabolic formation of gamma-(3-pyridyl)-gamma-hydroxybutyric acid and its possible intermediary role in the mammalian metabolism of nicotine. J. Biol. Chem. 239, 3981–3989. [PubMed] [Google Scholar]
- Jacob P. III; Havel C.; Lee D. H.; Yu L.; Eisner M. D.; Benowitz N. L. (2008) Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Anal. Chem. 80, 8115–8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S. S. (2002) Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis 23, 907–922. [DOI] [PubMed] [Google Scholar]
- Hecht S. S.; Spratt T. E.; Trushin N. (1988) Evidence for 4-(3-pyridyl)-4-oxobutylation of DNA in F344 rats treated with the tobacco specific nitrosamines 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and N′-nitrosonornicotine. Carcinogenesis 9, 161–165. [DOI] [PubMed] [Google Scholar]
- Peterson L. A.; Hecht S. S. (1991) O6-Methylguanine is a critical determinant of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone tumorigenesis in A/J mouse lung. Cancer Res. 51, 5557–5564. [PubMed] [Google Scholar]
- Foiles P. G.; Akerkar S. A.; Carmella S. G.; Kagan M.; Stoner G. D.; Resau J. H.; Hecht S. S. (1991) Mass spectrometric analysis of tobacco-specific nitrosamine-DNA adducts in smokers and nonsmokers. Chem. Res. Toxicol. 4, 364–368. [DOI] [PubMed] [Google Scholar]
- Hölzle D.; Schlöbe D.; Tricker A. R.; Richter E. (2007) Mass spectrometric analysis of 4-hydroxy-1-(3-pyridyl)-1-butanone-releasing DNA adducts in human lung. Toxicology 232, 277–285. [DOI] [PubMed] [Google Scholar]
- Stepanov I.; Muzic J.; Le C. T.; Sebero E.; Villalta P.; Ma B.; Jensen J.; Hatsukami D.; Hecht S. S. (2013) Analysis of 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB)-releasing DNA adducts in human exfoliated oral mucosa cells by liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem. Res. Toxicol. 26, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S. S. (1999) DNA adduct formation from tobacco-specific N-nitrosamines. Mutat. Res. 424, 127–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.