SUMMARY
Apoptosis is a highly regulated form of cell death that controls normal homeostasis as well as the anti-tumor activity of many chemotherapeutic agents. Commitment to death via the mitochondrial apoptotic pathway requires activation of the mitochondrial pore-forming proteins BAK or BAX. Activation can be effected by the activator BH3-only proteins BID or BIM, which have been considered to be functionally redundant in this role. Herein, we show that significant activation preferences exist between these proteins: BID preferentially activates BAK while BIM preferentially activates BAX. Furthermore, we find that cells lacking BAK are relatively resistant to agents that require BID activation for maximal induction of apoptosis including topoisomerase inhibitors and TRAIL. Consequently, patients with tumors that harbor a loss of BAK1 exhibit an inferior response to topoisomerase inhibitor treatment in the clinic. Therefore, BID and BIM have non-overlapping roles in the induction of apoptosis via BAK and BAX, affecting chemotherapy response.
INTRODUCTION
Apoptosis is a highly regulated form of cell death that is essential for normal growth and development of an organism and for culling damaged, dysfunctional, or superfluous cells(Jacobson, 1997). Apoptosis can be triggered via the extrinsic pathway which involves activation of cell surface death receptors or the intrinsic pathway which requires mitochondrial outer membrane permeabilization (MOMP)(Tait and Green, 2010). Activation of either pathway culminates in the activation of the executioner caspases, caspase 3 and caspase 7, for dismantling of the cell(Tait and Green, 2010). Notably, receptor-mediated cell death requires MOMP in most cell types(Tait and Green, 2010). The mitochondrial apoptotic pathway, which contributes to the anti-tumor effects of many chemotherapies(Johnstone et al., 2002), is controlled by the pro- and anti-apoptotic proteins of the BCL-2 family. Members of this family can be divided into three classes based on their sequence homology and function. One class includes the anti-apoptotic proteins BCL-2, BCL-w, MCL-1, BFL-1, and BCL-XL which contain all four BCL-2 homology domains (BH1–4). The proteins in this class prevent apoptosis by binding and sequestering their pro-apoptotic counterparts. The second class includes the pro-apoptotic proteins PUMA, BIM, BID, BAD, BIK, NOXA, and BMF, which contain only the BH3 domain (BH3-only). The final class contains BCL-2-associated X protein (BAX) and BCL-2 antagonist or killer (BAK) which contain domains BH1–3. BAX and BAK, when activated, oligomerize and directly cause mitochondrial outer membrane permeabilization (MOMP), a critical event during apoptosis. Cytochrome c and other factors are released during MOMP and associate with several cytosolic proteins to activate caspases for cell death.
Of the pro-apoptotic BH3-only proteins, BIM and BID have been shown to directly activate BAX and BAK with a particularly high potency and are consequently termed “activator” BH3-only proteins(Czabotar et al., 2013; Kim et al., 2006; Leshchiner et al., 2013; Letai et al., 2002; Wei et al., 2000). BIM and BID also share similar binding profiles for the anti-apoptotic members of the BCL-2 family since they are both able to bind and inactivate all five of the major anti-apoptotic proteins listed above(Certo et al., 2006).
Although BIM and BID have been considered to be functionally redundant with regard to BAX and BAK activation, several differences in function and physiological roles have emerged for these two proteins. BIM and BID have non-overlapping physiological roles in maintenance of homeostasis and distinct expression patterns(Farrow et al., 1995; Kiefer et al., 1995; Krajewski et al., 1996; O'Connor et al., 1998). BIM, but not BID, has been linked to cell death following growth factor withdrawal(Biswas and Greene, 2002) and to the apoptosis that is required for proper regulation of the immune system(Bouillet et al., 1999). In contrast, BID, but not BIM, is cleaved and activated by caspases 8 and 10 to trigger cell death in response to activation of cell surface death receptors by Fas, TNF and TRAIL (Li et al., 1998; Luo et al., 1998). BID has also been implicated in apoptosis following treatment with DNA damaging agents, including topoisomerase inhibitors(Kamer et al., 2005; Slee et al., 2000; Zinkel et al., 2005). Specifically, topoisomerase I and/or II inhibitors require BID cleavage to induce maximal apoptosis(Maas et al., 2011; Slee et al., 2000; Werner et al., 2004). These functional differences are generally attributed to differences in stress-induced regulation of BID and BIM rather than to any difference in activation of BAK or BAX.
BH3 profiling is an assay that allows detailed study of interactions between BCL-2 family members. In this assay, mitochondria in permeabilized cells are stained with a potential-sensitive fluorescent dye, JC1, to monitor mitochondrial membrane integrity. Mitochondrial transmembrane potential (ΔΨm), measured by JC1 fluorescence, is lost following mitochondrial outer membrane permeabilization (MOMP) as a secondary effect following BAX and BAK activation and oligomerization(Bossy-Wetzel et al., 1998). BH3 profiling enables monitoring of mitochondrial integrity following perturbation with different pro-death stimuli including BH3 peptides or recombinant proteins. We have previously utilized BH3 profiling to identify cellular dependence on specific anti-apoptotic proteins and to measure overall mitochondrial apoptotic priming which is predictive of responses to chemotherapy in vitro and in patients(Davids et al., 2012; Deng et al., 2007; Ni Chonghaile et al., 2011; Vo et al., 2012).
In this study, we show that although both BID and BIM can activate both BAK and BAX, they exhibit differential activation preferences: BID preferentially activates BAK and BIM preferentially activates BAX. The activation preference of BID for BAK is generally stronger than that of BIM for BAX but both preferences were observed consistently across mouse and human cells in assays using either recombinant proteins or BH3 domain peptides. These preferences are responsible for relative protection of BAK-deficient cells from apoptosis induced by TRAIL or by topoisomerase inhibitors, both of which require BID activation for maximal apoptosis. Consequently, BAK deficiency leads to inferior clinical responses in patients being treated with topoisomerase inhibitors.
RESULTS
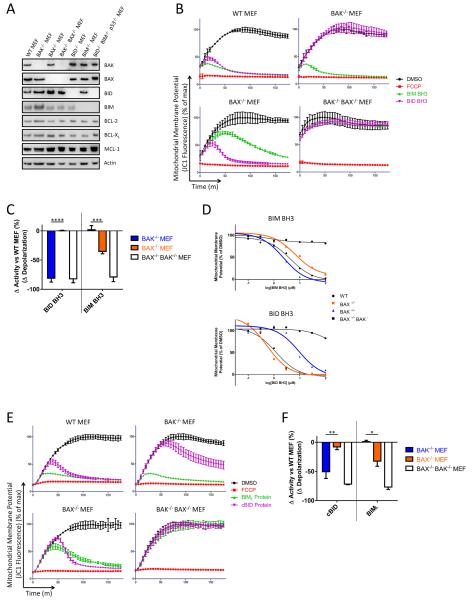
We had previously observed that the BID BH3 peptide was more effective than the BIM BH3 peptide at inducing cytochrome release in mouse liver mitochondria, which contain detectable levels of BAK but not BAX(Letai et al., 2002). We therefore hypothesized that BIM and BID may not be fully redundant in their ability to activate BAX and BAK. To systematically assess the abilities of BIM and BID to activate BAX and BAK, we utilized mouse embryonic fibroblasts (MEFs) from mice that were either wild type (WT), or lacking either BAX, or BAK, or both (Figure 1A). The levels of expression of other major BCL-2 family members in these MEFs were similar (less than 50% difference) (Figure 1A and S1A). To accurately identify any differences in the abilities of BIM and BID peptides to activate BAX and BAK, we used the BH3 profiling assay which allowed us to monitor mitochondrial potential after peptide treatment. DMSO was used as vehicle control and FCCP, a proton ionophore, was used as a positive control to measure the background fluorescence of mitochondria that unable to maintain a potential gradient. Peptide titration experiments showed that both BIM and BID BH3 peptides could induce mitochondrial depolarization (indicative of MOMP) in WT MEFs (Figures 1B and S2) with slightly higher activity of the BID versus the BIM peptide. Similar and near-complete mitochondrial depolarization was observed with 3μM BID peptide and 10μM BIM peptide (Figure 1B, upper left panel) which prompted us to use these doses to assess the effect of loss of BAK or BAX. Strikingly, in MEFs lacking BAK and expressing only BAX, response to BID peptide was reduced as compared to WT MEFs while the response to BIM peptide was unaffected (Figures 1B and 1C). In contrast, the response of MEFs lacking BAX and expressing only BAK to BID peptide was comparable to that of WT MEFs but response to BIM peptide was significantly attenuated. MEFs lacking both BAX and BAK exhibited no mitochondrial depolarization in response to either the BIM or BID peptides. To exclude the possibility that the activation preferences we observed were due to differences in the expression of anti-apoptotic proteins among the cell lines instead of direct activation of BAK and BAX, we inhibited the major anti-apoptotic proteins prior to peptide treatment and continued to observe the same activation preferences (Figure S3). These data suggest that BID more efficiently activates BAK while BIM more efficiently activates BAX.
Figure 1.
BID and BIM preferentially activate BAK and BAX, respectively, in mouse embryonic fibroblasts (MEFs). (A) Western blotting was performed for the indicted proteins. Representative, 3 IEs (independent experiments). (B) Digitonin-permeabilized MEFs were treated with the mitochondrial potential-sensitive dye JC1 and mitochondrial potential was monitored over 180 minutes in the presence of BIM (10μM) or BID (3μM) BH3 peptides. DMSO served as a negative control (fully polarized mitochondria) while FCCP served as positive control (fully depolarized mitochondria). Representative, 3 IEs. (C) The difference in mitochondrial depolarization by peptides was calculated from (B) and compared. Mean ± SE, 3 IEs. (D) Dose-response curves for BIM and BID BH3 peptide treatment of MEFs for calculation of EC50. Representative, 3 IEs. (E) Mitochondrial polarization was monitored in MEFs in the presence of BIML (30nM) or cBID (30nM) proteins. Representative, 3 IEs. (F) The difference in mitochondrial depolarization by proteins was calculated from (E) and compared. Mean ± SE, 3 IEs. See also Figures S1, S2, and S3 and Table S1.
In MEFs, differences in responses to BIM and BID peptides were evident across a range of peptide concentrations (Figure 1D) yielding EC50 values that differed almost three-fold for the BIM peptide (2.3μM in BAK−/− and 6.7μM in BAX−/−) and more than ten-fold for BID peptide (9.3μM in BAK−/− and 0.54μM in BAX−/−) (Table S1). To obtain a more quantitative understanding of the relative differences among interactions between BIM, BID, BAX and BAK, we performed mathematical modeling of the mitochondrial depolarization observed (see Methods for details). From this modeling, we obtained comparisons of rate (k) and maximal effect (Fmax) (Table S1). The parameters indicate that BID BH3 can activate MOMP in BAX−/− mitochondria significantly faster than BIM BH3, (k = 0.115 vs 0.036% depolarized μM−1 min−1). For BAK−/− mitochondria, BID BH3 is only slightly slower than BIM BH3 (k = 0.021 vs. 0.017% depolarized μM−1 min−1) but significantly less efficient, with an estimated EC50 for the Fmax of 4.5μM for BID BH3 and 0.7μM for BIM BH3 These data confirm a substantial difference in BIM- and BID-mediated activation of BAX and BAK.
We next tested if preferential activation would be also observed for full-length, recombinant BIML and activated, cleaved BID (cBID) proteins. We observed a close correlation in the data for peptides and whole proteins (Figures 1E and 1F): BAK loss inhibited cBID-induced MOMP while having no effect on BIML-induced MOMP. Conversely, loss of BAX had more of an effect on BIML-induced MOMP than cBID-induced MOMP, though the distinction was less absolute than for BAK loss (Figure 1E and 1F). MEFs lacking both BAX and BAK were again unresponsive to both recombinant BH3-only proteins.
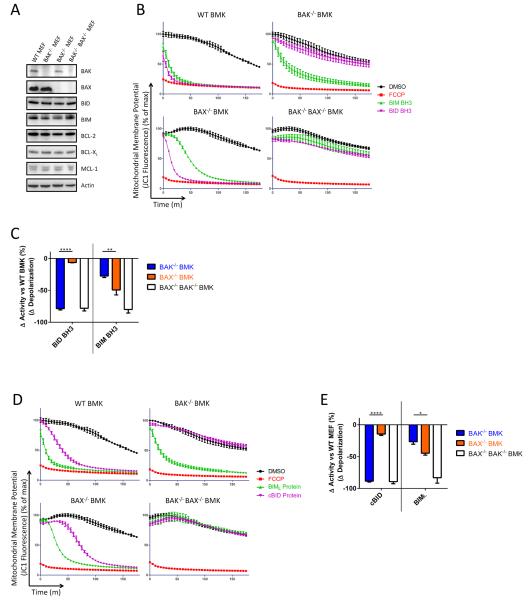
To determine whether preferential activation of BAK by BID and BAX by BIM were evident in an alternate cell line of epithelial origin, we repeated the MEF experiments with baby mouse kidney (BMK) cells having the relevant genotypes. These cells were immortalized in parallel using E1A and dominant-negative p53(Degenhardt et al., 2002) and ostensibly differ only in their expression of BAK and BAX (Figure 2A and S1). The activation preferences evident in the MEFs were observed in the BMKs using peptides (Figure 2A – 2C) or recombinant cBID and BIML and BIMS proteins (Figures 2D and 2E, BIMS data not shown).
Figure 2.
BID and BIM preferentially activate BAK and BAX, respectively, in baby mouse kidney (BMK) epithelial cells. (A) Western blotting was performed for the indicted proteins. Representative, 2 IEs. (B) Mitochondrial polarization was monitored in digitonin-permeabilized BMKs in the presence of BIM (100μM) or BID (100μM) BH3 peptides. Representative, 3 IEs. (C) The difference in mitochondrial depolarization by peptides was calculated from (B) and compared. Mean ± SE, 3 IEs (D) Mitochondrial polarization was monitored in BMKs of indicated genotype in the presence of BIML (100nM) or cBID (30nM) proteins. Representative, 3 IEs. (E) The difference in mitochondrial depolarization by proteins was calculated from (D) and compared. Mean ± SE, 3 IEs. See also Figure S1.
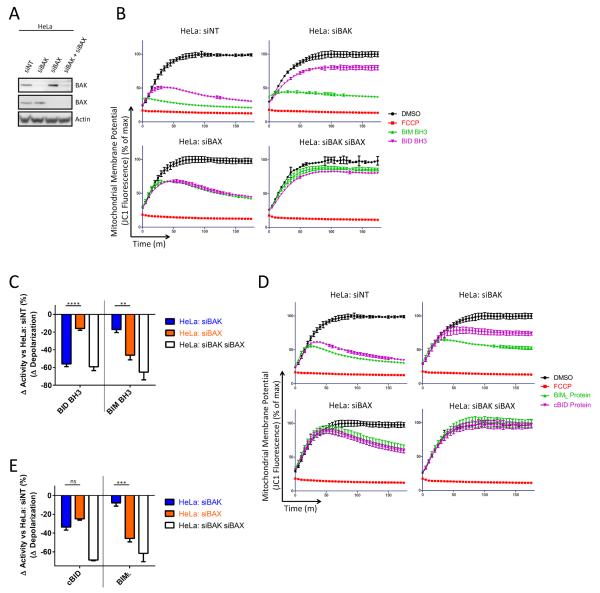
To confirm differential activation of BAX and BAK by BIM and BID in human cells we utilized siRNA to knock down expression of BAX and BAK (Figure 3A). As in MEFs, the ability of BIM BH3 peptide to induce mitochondrial depolarization was most attenuated by loss of BAX while the ability of BID BH3 to induce MOMP was most attenuated by loss of BAK (Figure 3B–C). HeLa cells treated with recombinant BIML and cBID gave similar results as the BH3 peptides, though in this case BAX and BAK loss roughly equally affected cBID-induced MOMP (Figure 3D–E). Overall, the differences in sensitivities to BIM and BID in the HeLa cells with knockdowns of BAX and BAK were less categorical than those observed in MEFs and BMKs, possibly due to far lower concentrations of BAX and BAK in HeLa cells (Figure S4) or to less than complete knockdown of BAX and BAK by RNAi. However, we observe the same trend in all cell types with loss of BAX preferentially reducing BIM response, and loss of BAK preferentially reducing BID response.
Figure 3.
BID and BIM induce preferential activation of BAK and BAX, respectively, in human cancer cells. (A) HeLa cells were transfected with siRNA that was non-targeting (siNT) or specific for BAX or BAK. Western blotting was used to confirm knockdown of target proteins. Representative, 3 IEs. (B) Mitochondrial polarization was monitored in digitonin-permeabilized HeLa cells in the presence of BIM (100μM)or BID (100μM) BH3 peptides. Representative, 3 IEs. (C) The difference in mitochondrial depolarization by peptides was calculated from (B) and compared. Mean ± SE, 3 IEs (D) Mitochondrial polarization was monitored in HeLa cells in the presence of BIML (100nM) or cBID (30nM) proteins. Representative, 3 IEs. (E) The difference in mitochondrial depolarization by proteins was calculated from (D) and compared. Mean ± SE, 3 IEs. See also Figure S4.
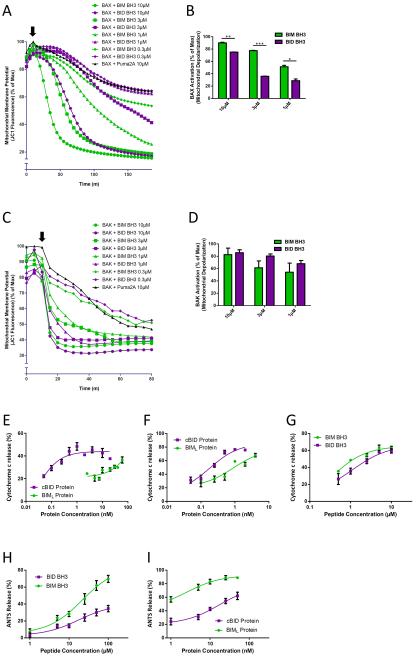
The experiments described above show that BIM and BID possess distinct activation preferences for endogenously expressed BAX and BAK. Next we sought to determine whether such preferences would also be evident with exogenous BAX and BAK. We therefore incubated digitonin-permeabilized BAX−/− BAK−/− MEFs with recombinant, full-length BAX protein for 10 minutes, added BIM or BID BH3 peptides at a range of concentrations and monitored mitochondrial potential (Figures 4A and 4B). BAX, upon activation by BID or BIM, quickly and efficiently depolarized mitochondria lacking endogenous BAX and BAK. In agreement with our previous findings, we observed that the BIM peptide was more efficient at activating BAX protein than the BID peptide as evidenced by the faster and more complete depolarization of mitochondria at several concentrations. Although the ideal complementary experiment would utilize bacterially-produced and purified BAK, no laboratory to our knowledge has successfully produced full-length, wild-type BAK in bacteria. As an alternative, we used in vitro transcription and translation (IVTT) to produce BAK and then examined its activation by BID and BIM. Although the results did not reach statistical significance, BID peptide was more efficient at activating BAK than BIM peptide (Figures 4C and 4D). To rule out potential effects of the IVTT buffer on the activity of the peptides, we tested the ability of BIM and BID peptides to activate recombinant BAX in IVTT buffer; the results were consistent with those shown in Figures 4A and 4B (Figure S5). Thus BID more efficiently activated BAK while BIM more efficiently activated BAX when all components were recombinant.
Figure 4.
BID preferentially activates BAK while BIM preferentially activates BAX. (A) Mitochondrial polarization was monitored in digitonin-permeabilized BAX−/− BAK−/− MEFs while initially treated over 10 minutes in the presence of recombinant BAX. BIM or BID BH3 peptides were then spiked in (arrow) and mitochondrial polarization continued to be monitored. Addition of PUMA2A peptide served as negative control for background depolarization caused by BAX. Representative, 3 IEs. (B) Percent depolarization was calculated from (A) for comparison of BAX activation induced by BIM and BID BH3 peptides. Mean ± SE, 3 IEs. (C) Mitochondrial polarization was monitored in digitonin-permeabilized BAX−/− BAK−/− MEFs while initially treated for 10 minutes in the presence of recombinant BAK. BIM or BID BH3 peptides were then spiked in (arrow) and mitochondrial polarization continued to be monitored. Representative, 2 IEs. (D) Percent depolarization was calculated from (C) for comparison of BAK activation induced by BIM and BID BH3 peptides. Mean ± SE, 2 IEs. (E) Heavy membranes, including mitochondria, were isolated from WT mouse livers which express BAK, but not BAX. Mitochondria were treated with the indicated concentrations of cBID and BIML protein and cytochrome c release was measured. Mean ± SE, 3 IEs. (F–G) Heavy membranes, including mitochondria, were isolated from BAK−/− mouse livers which do not contain BAX or BAK. Mitochondria were then incubated in the presence of recombinant BAX and indicated concentrations of cBID and BIML proteins (F) or BID and BIM BH3 peptides (G) and cytochrome c release was measured. Mean ± SE, 3 IEs. (H–I) ANTS release was monitored in liposomes in the presence of recombinant BAX and indicated concentrations of BID and BIM BH3 peptides (H) or cBID and BIML proteins (I). Addition of only BAX or BIM/BID alone at highest doses shown yielded a background ANTS release of less than 5%. Mean ± SE, 3 IEs. See also Figure S5.
Our experiments thus far showed that when treating cells that were lacking either BAX or BAK with BIM or BID, differences in preference for activation were evident using an indirect readout for MOMP (loss of mitochondrial membrane potential). During apoptosis, release of cytochrome c from mitochondria is a direct consequence of MOMP and we therefore confirmed the activation preferences described above using cytochrome c translocation as an assay endpoint. Isolation of heavy membranes from mouse livers yields mitochondria that contain BAK but not BAX(Letai et al., 2002). It has previously been shown that these BAK-containing mitochondria release cytochrome c more readily in response to BID peptide than BIM(Letai et al., 2002) but the activity of full-length pro-apoptotic proteins has not been explored in this setting. We found that cBID was more efficient at inducing cytochrome c release than BIML protein across a range of concentrations (Figure 4E), consistent with our previous data. Next we isolated mitochondria that lack detectable BAX or BAK from BAK−/− mice(Hsu, 1997) and added exogenous, recombinant BAX followed by cBID or BIML. Mitochondria in this assay released cytochrome c at much lower concentrations of BIML than mitochondria from WT mice, but cBID protein was still more efficient than BIML (Figure 4F). MTCH2 has recently been identified as a powerful facilitator of tBID recruitment to the mitochondrial membrane of liver cells where it activates BAX or BAK(Zaltsman et al., 2010), which may explain why cBID was a more efficient activator of BAX in this model. To test this, we treated these BAX-containing mitochondria with BH3 peptides instead of full-length proteins since BID BH3 lacks the MTCH2 binding domain(Katz et al., 2012). Consistent with our previous data, we observed that BIM BH3 was more efficient as an activator of BAX than BID BH3 (Figure 4G). Finally, we used a cell-free, liposome-based system to assay BAX activation, once again observing that BIM BH3 and BIML are more efficient activators of recombinant BAX than the BID peptide or protein (Figures 4H and 4I).
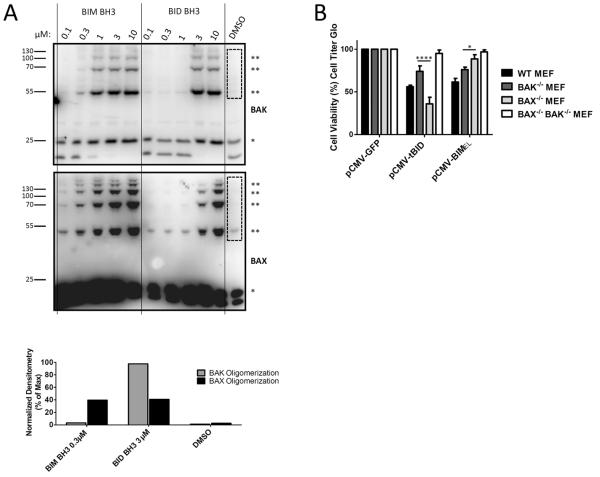
In apoptosis, activation and homo-oligomerization of BAX and BAK by BIM or BID precedes MOMP and can be assayed directly. By comparing BAX and BAK oligomerization in the same experimental sample we were also able to directly compare the preference of BID and BIM for activation of BAK and BAX. We exposed mitochondria to BIM and BID peptides and monitored BAX and BAK oligomerization by crosslinking the activated, oligomerized BAX and BAK proteins. We utilized BIM−/−, BID−/−, p53−/− MEFs for these experiments to reduce any interference from the endogenously expressed BIM, BID or p53 (a regulator of PUMA protein which potentially activates BAX and/or BAK(Kim et al., 2009)) proteins present in WT MEFs. As can be seen in Figure 5A, BIM BH3 induces BAX oligomerization at a lower concentration (0.3μM) than it does BAK oligomerization (1.0μM). Conversely, BID BH3 induced maximal BAK oligomerization at a lower concentration (3μM) than it did BAX oligomerization (10μM). While the selectivity of the interactions is clearly not absolute, the lesson holds that BIM preferentially induces BAX oligomerization, while BID preferentially induces BAK oligomerization.
Figure 5.
BID preferentially crosslinks and activates BAK while BIM preferentially crosslinks and activates BAX. (A) Heavy membranes including mitochondria were isolated from MEFs lacking BIM, BID and p53 and were treated with the indicated concentrations of BH3 peptides. Samples were then treated with the crosslinking agent BMH and analyzed by western blotting for crosslinking of BAX and BAK. * = monomeric BAK and BAX; ** = oligomerized BAK and BAX. Densitometry was performed in area indicated by dashed rectangle for comparison of oligomerization efficiency. Representative, 2 IEs. (B) MEFs of the indicated genotype were plated in a 96-well plate and transfected with plasmids (0.1μg/well) encoding either GFP, or untagged tBID or BIMEL in the pCMV vector. After 24 hours, cell viability was assessed using CellTiterGlo. Mean ± SE, 4 IEs. See also Figure S6.
We next tested whether the same preferences would be observed with cells ectopically expressing BIM and BID from plasmid vectors. MEFs were transfected with either GFP as a control, untagged tBID, or untagged BIMEL; cell viability was measured 24 hr later. We observed similar levels of transfection efficiency and protein expression in the BAK−/− and BAX−/− MEFs with the GFP plasmid (Figure S6).. As expected, MEFs lacking BAX had significantly reduced sensitivity to BIMEL overexpression as compared to MEFs lacking BAK (Figure 5B). Conversely, loss of BAK reduced sensitivity to tBID overexpression, while loss of BAX afforded no protection. These results were consistent with our earlier findings.
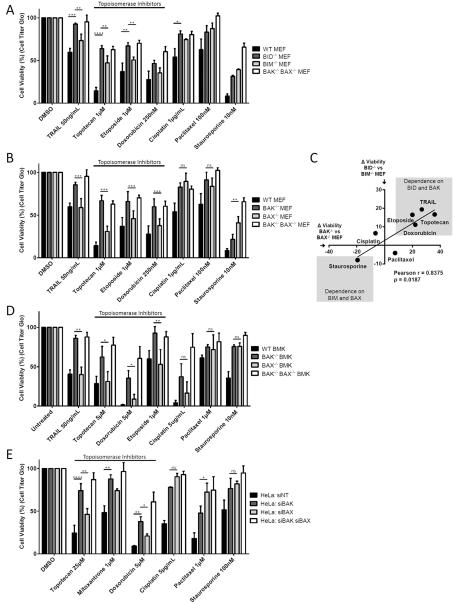
The preferences we observed for activation of BAK by BID and activation of BAX by BIM are more quantitative than absolute, particularly in the latter case. We asked whether these preferences had biological consequences nonetheless. We therefore tested whether differences in BAX and BAK expression might have a predictable impact on sensitivity to certain classes of apoptosis-inducing agents. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis requires BID cleavage to induce MOMP(Yamada et al., 1999) and we therefore tested the sensitivity of WT, BID−/− and BIM−/− MEFs to TRAIL. As expected, loss of BID protected MEFs from TRAIL-induced apoptosis to a greater extent than loss of BIM (Figure 6A). Topoisomerase inhibitors have been used in the clinic for over 20 years to treat a wide range of malignancies and are among the most highly prescribed anti-cancer drugs(Baldwin and Osheroff, 2005). These inhibitors trigger apoptosis by inducing DNA double-strand breaks regardless of whether they target topoisomerase I (topotecan and irinotecan) or topoisomerase II (etoposide, mitoxantrone and doxorubicin)(Baldwin and Osheroff, 2005; Kaina, 2003; Strumberg et al., 2000). Efficient induction of apoptosis by topoisomerase II inhibitors requires BID cleavage(Friesen et al., 1996; Fulda and Strauss, 2000; Slee et al., 2000). It has also been shown that topotecan-induced apoptosis can be blocked by caspase 8 inhibition (the enzyme that cleaves Bid to tBid) or by upregulation of BCL-2 and BCL-XL indicating that cleavage of BID may be a necessary step in induction of apoptosis by topoisomerase I inhibitors as well(Ferreira et al., 2000). We confirmed that BID is cleaved and activated in MEFs treated with topoisomerase inhibitors but not cisplatin (Figure S7A). When we compared the sensitivity of WT, BID−/− and BIM−/− MEFs to topoisomerase inhibitors we observed that BID−/− MEFs were resistant to etoposide, topotecan, and doxorubicin, in agreement with the previous reports (Figure 5A). BIM−/− MEFs also exhibited some resistance to this class of chemotherapies although loss of BIM provided significantly less resistance than loss of BID. Notably, BID−/− MEFs did not exhibit any significant differences in sensitivity to other chemotherapies as compared to BIM−/− MEFs, including the DNA-crosslinking agent cisplatin, the microtubule inhibitor paclitaxel, or the kinase inhibitor staurosporine. These data confirm the selective importance of BID in topoisomerase inhibitor-induced apoptosis.
Figure 6.
BID and BAK are necessary for maximal effectiveness of topoisomerase inhibitors. (A–B) MEFs were treated with the indicated agents and after 24 hours (TRAIL) or 48 hours (all other agents), viability was assessed by measuring total ATP levels with CellTiterGlo reagent. Mean ± SE, 4 IEs. (C) The dependence of chemotherapeutic agents on BIM, BID, BAX and BAK (difference in viability observed in MEFs from [A] and [B]) was compared. (D) BMK cells were treated with the indicated agents and after 24 hours (TRAIL, doxorubicin and cisplatin) or 48 hours (all other agents), viability was assessed using CellTiterGlo. Mean ± SE, 3 IEs. (E) HeLa cells were transfected with the indicated siRNAs and, 48 hours later, treated with the indicated agents. Viability was assessed at 24 hours for all agents except topotecan and paclitaxel (48 hours) using CellTiterGlo. Mean ± SE 3 IEs. See also Figure S7, S8 and S9.
Since we observed a preference for BID to activate BAK over BAX, we hypothesized that cells lacking BAK would be less sensitive to TRAIL- and topoisomerase-induced apoptosis. As expected, BAK−/− MEFs were less sensitive to these agents than BAX−/− MEFs while exhibiting similar sensitivity to cisplatin and paclitaxel (Figure 6B). BAX−/− MEFs exhibited significantly more resistance to the kinase inhibitor staurosporine than BAK−/− MEFs which is interesting considering the higher, but not significant, resistance of BIM−/− MEFs as compared to BID−/− MEFs to this agent (Figure 6A). By utilizing Annexin V and propidium iodide staining and flow cytometric analysis we observed that differences observed in the viability assay were due to the induction of classical apoptosis by chemotherapeutic agents and not a unrelated effect on cell growth (Figure S8). Overall, we concluded that there was a correlation between an agent's killing being more dependent on BAK and its killing being more dependent on BID (Figure 6C).
We performed a similar analysis of chemosensitivity in BMK and HeLa cells to determine if the differences in chemosensitivity would also be present in epithelial and human cancer cells, respectively. The same agents were utilized with one exception: HeLa cells are extremely resistant to treatment with the topoisomerase II inhibitor etoposide (data not shown) and the topoisomerase II inhibitor mitoxantrone was therefore used instead. As with the MEFs, we observed protection from TRAIL and topoisomerase inhibitors in BMK cells lacking BAK as compared to those lacking BAX (Figure 6D). The protection was not evident with agents that induced apoptosis via alternate mechanisms that are less distinctly BID-dependent. Finally, we found that even transient, siRNA-based knock down of BAK in HeLa cells prevented much of the apoptosis induced by topoisomerase inhibitors and slowed TRAIL-induced caspase activation (Figure 6E and S9) while knock down of BAX had a significantly smaller effect. These data are consistent with the fact that BID cleavage and activation was observed in HeLa cells upon treatment with topoisomerase inhibitors but not other chemotherapeutic agents (Figure S7B and S7C). Conversely, BAX knockdown was either equivalent or superior to BAK knockdown in affording protection from the other chemotherapies tested (Figure 6E). The chemosensitivity assays performed on MEFs, BMKs and HeLa cells showed that topoisomerase inhibitors require BID and BAK for maximal induction of apoptosis.
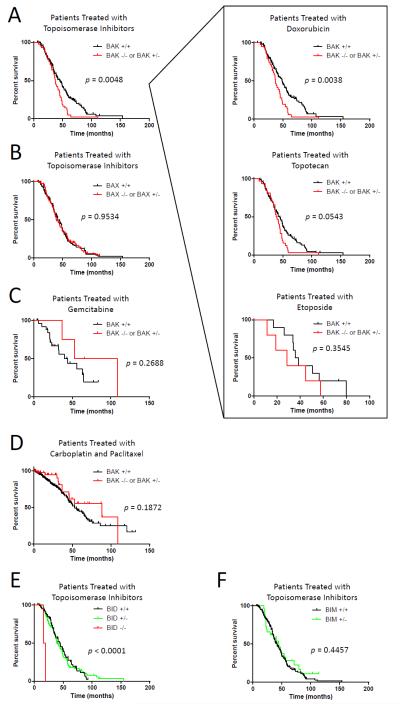
The strength of the protection seen in BAK−/− and BID−/− cells from apoptosis induced by topoisomerase inhibitors suggested that BAK deficiency in tumors may be particularly detrimental to patients receiving one of these agents in the clinic. Inhibitors of topoisomerases I or II are used frequently to treat both hematopoietic and solid tumors including lung, ovarian and cervical cancers as well as lymphomas, leukemias and glioblastomas. Recently, The Cancer Genome Atlas (TCGA) project examined DNA copy number alterations and clinical outcomes in a series of 489 high-grade serous ovarian adenocarcinomas(Cancer Genome Atlas Research Network, 2011). Topoisomerase inhibitors are frequently used as second-line drugs for this disease if a patient has relapsed following initial treatment with carboplatin and paclitaxel. We therefore examined whether loss of BAK expression in these patients would affect responses to topoisomerase inhibitors. A subset of 185 patients in the study were treated with topoisomerase inhibitors (topotecan, etoposide, doxorubicin and mitoxantrone), and a large percentage of those patients exhibited a homozygous or heterozygous loss of BAK1 (23.2%). Patients lacking one or both alleles of BAK1 exhibited an inferior overall survival as compared to patients whose tumors maintained both alleles of BAK1 (Figure 7A) (p=0.0048). Even when analyzing each topoisomerase inhibitor separately, tumors with BAK1 loss either showed a significantly inferior overall survival (doxorubicin) or were trending in that manner (topotecan, etoposide) (Figure 7A inset).
Figure 7.
Patients treated with topoisomerase inhibitors respond poorly when exhibiting a loss of BAK. (A–D) DNA copy number analysis was performed on patients with a confirmed diagnosis of high-grade serous ovarian adenocarcinoma as part of the TCGA study. (A) Overall survival (OS) was compared in patients treated with topoisomerase inhibitors (topotecan, etoposide, mitoxantrone and doxorubicin) that exhibited a loss of one or both alleles of BAK (n=43 patients) and those that had not (n=142). OS was also compared in patients being treated with each topoisomerase inhibitor separately (inset): doxorubicin (BAK+/+ n=113; BAK+/− or BAK−/− n=38), topotecan (BAK+/+ n=88; BAK+/− or BAK−/− n=32), or etoposide (BAK+/+ n=15; BAK+/− or BAK−/− n=5). OS was not compared in patients treated with mitoxantrone due to low number of patients receiving this therapy (n=1). (B) OS was compared in patients treated with topoisomerase inhibitors that exhibited a loss of one or both alleles of BAX (n=85) and those that had not (n=100). (C) OS was compared in patients treated with gemcitabine that exhibited a loss of one or both alleles of BAK (n=4) and those that had not (n=30). (D) OS was compared in patients treated with carboplatin and paclitaxel but not topoisomerase inhibitors that exhibited a loss of one or both alleles of BAK (n=47) and those that had not (n=281). (E) OS was compared in patients treated with topoisomerase inhibitors that exhibited a loss of one (n=102) or both (n=2) alleles of BID and those that had not (n=81). (F) OS was compared in patients treated with topoisomerase inhibitors that exhibited a loss of one allele of BIM (n=29) and those that had not (n=156). No patients exhibited a loss of both alleles of BIM. See also Table S2.
Since topoisomerase inhibitors are given as therapy only after the tumor becomes resistant to carboplatin and paclitaxel, the topoisomerase inhibitors were administered at a median of 25.6 months after initial surgical resection (Day 0 on Kaplan-Meier survival curves). This timepoint coincides with the separation of the survival curves for the two groups of patients. In contrast to loss of BAK1, losing one or both alleles of BAX had no effect on survival in patients treated with topoisomerase inhibitors as would be expected based on our results (Figure 7B). Furthermore, patients treated with a different class of second-line chemotherapy for recurrent disease, gemcitabine (a nucleoside analog), did not show any reduction in overall survival due to heterozygous or homozygous loss of BAK1 (Figure 7C). The same held true for patients treated solely with carboplatin and paclitaxel and not receiving topoisomerase inhibitors (Figure 7D). We observed no major differences in the tumor staging or minimum residual disease post tumor resection when comparing the patients in these two groups implying that the patients had similarly aggressive disease and that divergent survival was primarily a consequence of differences in drug response (Table S2).
Finally, we examined the effect of BID and BIM loss on responses to topoisomerase inhibitors. Although the number of patients exhibiting a homozygous loss of BID was limited, complete loss of BID resulted in extremely poor overall survival in patients treated with topoisomerase inhibitors (p < 0.0001) (Figure 7E). We did not observe any adverse effects of heterozygous loss of BID (Figure 7E). This is perhaps not surprising since prevailing models of BAK and BAX activation propose that once BIM and BID activate BAX and BAK, the oligomerization and mitochondrial permeabilization processes can continue independently of the activators, thus freeing BIM and BID to continue activating further BAX and BAK proteins(Wei et al., 2000). Additionally, it was recently shown that full activation of BAK can be achieved with ratios of tBID:BAK as low as 1:25(Leshchiner et al., 2013). Heterozygous loss of BIM had no effect on responses to these agents and no patients were identified with a loss of both BIM alleles (Figure 7F). Overall, these data highlight the clinical importance of BID and BAK expression specifically in topoisomerase inhibitor-induced apoptosis.
DISCUSSION
Apoptosis is an evolutionarily conserved form of cell death that, when deregulated, can lead to life-threatening diseases such as cancer, neurodegeneration and immune disorders (reviewed in (Fuchs and Steller, 2011)). At its most basic level, apoptosis involves the activation of the mitochondrial pore-forming proteins BAX and BAK by activator proteins, especially BIM and BID. For the most part, BIM and BID have been considered to be functionally redundant with respect to their ability to activate BAX and BAK. Here we showed that BID preferentially activates BAK while BIM preferentially activates BAX in both mouse and human cells. Although both of these preferences were consistently observed, their magnitude varied, as might be expected, across different model systems, and the preference of BID for BAK was generally stronger than BIM for BAX. Our findings are in agreement with the observations in mouse liver mitochondria that prompted this study(Letai et al., 2002). Furthermore, we have demonstrated that cancer cells and patients with tumors lacking BAK respond poorly to topoisomerase inhibitors due to the importance of BID in the apoptotic signaling induced by this class of chemotherapeutic agents.
The preferential activation of BAX and BAK by BIM and BID, respectively, may shed light on some observations previously reported in the literature. For example, several independent groups have shown that the apoptosis induced by c-Myc activation is entirely dependent on BAX but not BAK(Dansen et al., 2006; Eischen and Roussel, 2001; Sarosiek et al., 2010). It has been also been reported that c-Myc-induced apoptosis is triggered via upregulation of BIM(Egle et al., 2004). Our data showing that BIM can more readily activate BAX would thus provide an explanation for the BAX-dependence of c-Myc-induced cell death. Similarly, Cartron et al. has reported that BAK deficient glioblastoma cells are completely resistant to Fas-mediated cell death (Cartron et al., 2003). Because Fas ligand elicits death receptor signaling and leads to caspase 8 activation and subsequent BID cleavage and activation (Li et al., 1998), the dependency of this apoptotic pathway on BAK is potentially explained by our findings. Additional reports of either BAX or BAK dependence for apoptosis induced by various insults abound and may potentially be explained by our study(Von Haefen et al., 2004; Handrick et al., 2010; Letai et al., 2002; Zhang et al., 2000).
Previous studies may provide some basis for the evolution of the differential affinities we observed among BCL-2 family proteins BIM has been shown to be more important than BID as a regulator of life and death decisions in the hematopoietic system since its loss leads to an accumulation of lymphoid and myeloid cells(Bouillet et al., 1999), a finding that does not hold true for BID loss(Yin et al., 1999). Interestingly, BAX knockout mice exhibit a similar hyperplasia of B cells(Knudson et al., 1995) while BAK knockout mice do not(Lindsten et al., 2000). It is possible that the increased affinity of BIM for BAX is the product of co-evolution due to both proteins' vital and delicate roles in tightly regulating the hematopoietic system.
The preferential partnership between BID and BAK has an equally plausible mode of co-evolution. Specifically, it has been shown that death induced in mice by in vivo administration of Fas ligand (FasL) is due to severe liver damage induced by activation of Fas receptor which is expressed at high levels on hepatocytes (Hao et al., 2004; Ogasawara and Watanabe-Fukunaga, 1993). Furthermore, pro-death FasL signaling is integral in maintaining liver homeostasis as evidenced by the development of hepatomegaly in Fas knockout mice(Adachi et al., 1995). FasL, as mentioned previously, induces apoptosis via activation of caspase 8 and subsequent cleavage and activation of BID(Luo et al., 1998) which explains how BID−/− mice are protected from the liver damage induced by in vivo administration of FasL(Yin et al., 1999). Based on our study, hepatocytes are particularly well suited to respond to FasL and BID signaling since their mitochondria contain BAK but not BAX protein(Korsmeyer et al., 2000; Letai et al., 2002). In fact, we would expect that BAK−/−, but not BAX−/− mice would be protected from BID-mediated, FasL-induced hepatocyte apoptosis, as recently confirmed experimentally(Hikita et al., 2011). The preferential activation of BAK by BID may therefore be born of the necessity to regulate liver homeostasis via the Fas/FasL system with a high degree of specificity and customization.
Our report focused on the dynamics of BAK and BAX activation by BID and BIM and its potential functional and clinical relevance yet the mechanisms responsible for these preferences remain unknown. Notably, these preferences were observed with unstructured BH3 domain peptides as well as full-length proteins which suggests that the preferences are predominantly based on the amino acid sequences of the two proteins. There are many differences between BID and BIM in amino acid sequence across their roughly 20-mer BH3 domains. Elegant mutational studies have elucidated key residues within the BH3 domains of both BID and BIM that are vital for binding BAK and BAX(Gavathiotis et al., 2008; Leshchiner et al., 2013); those studies may be extended to identify the components of the BH3 domains that are responsible for their activation preferences.
Other factors may also influence the selectivity we observed. For instance, recent studies have shown that proteins such as MTCH2 may modulate activation of BAX and BAK by BID and BIM and thus provide another means by which to control these effectors in certain cell types(Katz et al., 2012). Future studies may identify additional modes of regulation and provide a more complex and nuanced picture of how cell fate is determined by these critical BCL-2 family proteins.
The importance of BID for topoisomerase-induced cell death has been extensively reported(Kamer et al., 2005; Maas et al., 2011; Slee et al., 2000; Werner et al., 2004; Zinkel et al., 2005). Our observation that BID preferentially activates BAK and that, consequently, BAK deficiency protects cells from apoptosis induced by these agents sheds additional light on mechanisms of resistance to these often-used chemotherapies. Our results suggest that guiding chemotherapy decisions based on alterations in BAK and BID may be beneficial clinically.
EXPERIMENTAL PROCEDURES
BH3 profiling using whole cells
Proliferating cells that were 30–50% confluent were harvested and counted for BH3 profiling. 15 μL of BH3 peptides or recombinant proteins (see below for peptide sequences) in T-EB (300 mM Trehalose [Sigma-Aldrich, St. Louis MO], 10 mM Hepes-KOH pH 7.7 [Sigma-Aldrich], 80 mM KCl [Sigma-Aldrich], 1 mM EGTA [Sigma-Aldrich], 1 mM EDTA [Sigma-Aldrich], 0.1% BSA [Sigma-Aldrich], and 5 mMμ succinate[Sigma-Aldrich]) was deposited into each wellμ in a nontreated black 384-well plate, 1 treatment per well, in triplicate for each independent experiment. Single cell suspensions were washed once with T-EB before beingμ resuspended at 4X their final density of 6.75 × 105 cells/mL. One volume of the 4Xμ cell suspension was added to one volume of a 4X dye solutionμ containing 4 μM JC-1 (Enzo Life Sciences, Farmingdale NY), 40 μg/ml oligomycin (Sigma-Aldrich), 0.02%μ digitonin (Sigma-Aldrich), and 20 mM 2-mercaptoethanol (Life Technologies) in T-EB. The resultingμ 2X cell/dye solution was kept at room temperatureμ for 5 min to allow cell permeabilization and dye equilibration. 15 μL of the 2X cell/dye mix was then added to each treatmentμ well of the 384-well plate, shaken for 15 seconds inside the plateμ reader, and the fluorescence at 590 nM was measured every 5 minμ at room temperature. Peptide treatments that were used corresponded to the BH3 domains of the BCL-2 family proteins, and their respective sequences are as follows: BIM: MRPEIWIAQELRRIGDEFNA; BID: EDIIRNIARHLAQVGDSMDR (New England Peptide, Gardner MA). Relative mitochondrial depolarization was defined as the magnitude of mitochondrial depolarization resulting from BH3 peptide treatment as compared to vehicle DMSO (Sigma-Aldrich) and positive control FCCP (p-trifluoromethoxy carbonyl cyanide phenyl hydrazone) (Sigma-Aldrich). The percentage of mitochondrial depolarization was calculated by comparing the JC1 signal (mitochondrial polarization) in cell lines treated with each peptide or protein concentration in the following manner:
where R(t) is the fluorescence value in the reference sample (DMSO), F(t) is the fluorescence value in the test sample (peptide or protein) and FCCP(t) is the fluorescence value in the positive control sample (FCCP) at a time (t) and averaged over the dynamic portion of depolarization curves.
Western blotting
Western blotting was performed as previously described(Sarosiek et al., 2009). Antibodies used are listed in Supplement.
Ovarian cancer copy number alterations, treatment history and survival
Treatment history and survival data (clinical follow-up) for all TCGA high-grade serous ovarian adenocarcinoma cases was obtained from the TCGA data portal on August 1, 2012. Full procedures are included in supplement.
Supplementary Material
HIGHLIGHTS.
-
-
BID and BIM preferentially activate BAK and BAX, respectively.
-
-
BID-dependent cell death correlates with BAK-dependent cell death.
-
-
Patients with tumors lacking BAK respond poorly to topoisomerase inhibitors.
ACKNOWLEDGEMENTS
We kindly thank Dr. John C. Reed for providing us with the pcDNA3-myc-BAK plasmid. We gratefully acknowledge funding from the American Cancer Society Postdoctoral Fellowship 121360-PF-11-256-01-TBG (K.A.S.), Women's Cancers Program at the Dana-Farber Cancer Institute (K.A.S.), and NIH grants RO1CA129974, and P01CA139980. A.L. is a Leukemia and Lymphoma Society Scholar.
Footnotes
The authors have no conflict of interest to declare.
REFERENCES
- Adachi M, Suematsu S, Kondo T. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nature Genetics. 1995 doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]
- Baldwin EL, Osheroff N. Etoposide, Topoisomerase II and Cancer. Current Medicinal Chemistry - Anti-Cancer Agents. 2005;5:10. doi: 10.2174/1568011054222364. [DOI] [PubMed] [Google Scholar]
- Biswas SC, Greene LA. Nerve growth factor (NGF) down-regulates the Bcl-2 homology 3 (BH3) domain-only protein Bim and suppresses its proapoptotic activity by phosphorylation. The Journal of Biological Chemistry. 2002;277:49511–49516. doi: 10.1074/jbc.M208086200. [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Newmeyer DD, Green DR. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. The EMBO Journal. 1998;17:37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Köntgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science (New York, N.Y.) 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network, T. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartron P, Juin P, Oliver L, Martin S. Nonredundant role of Bax and Bak in Bid-mediated apoptosis. Molecular and Cellular Biology. 2003;23 doi: 10.1128/MCB.23.13.4701-4712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong S. a, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Czabotar PE, Westphal D, Dewson G, Ma S, Hockings C, Fairlie WD, Lee EF, Yao S, Robin AY, Smith BJ, et al. Bax Crystal Structures Reveal How BH3 Domains Activate Bax and Nucleate Its Oligomerization to Induce Apoptosis. Cell. 2013;152:519–531. doi: 10.1016/j.cell.2012.12.031. [DOI] [PubMed] [Google Scholar]
- Dansen TB, Whitfield J, Rostker F, Brown-Swigart L, Evan GI. Specific requirement for Bax, not Bak, in Myc-induced apoptosis and tumor suppression in vivo. The Journal of Biological Chemistry. 2006;281:10890–10895. doi: 10.1074/jbc.M513655200. [DOI] [PubMed] [Google Scholar]
- Davids MS, Deng J, Wiestner A, Lannutti BJ, Wang L, Wu CJ, Wilson WH, Brown JR, Letai A. Decreased mitochondrial apoptotic priming underlies stroma-mediated treatment resistance in chronic lymphocytic leukemia. Blood. 2012 doi: 10.1182/blood-2012-02-414060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt K, Sundararajan R, Lindsten T, Thompson C, White E. Bax and Bak independently promote cytochrome C release from mitochondria. The Journal of Biological Chemistry. 2002;277:14127–14134. doi: 10.1074/jbc.M109939200. [DOI] [PubMed] [Google Scholar]
- Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6164–6169. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eischen C, Roussel M. Bax loss impairs Myc-induced apoptosis and circumvents the selection of p53 mutations during Myc-mediated lymphomagenesis. And Cellular Biology. 2001 doi: 10.1128/MCB.21.22.7653-7662.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow S, White J, Martinou I, Raven T. Cloning of a bcl-2 homologue by interaction with adenovirus E1B 19K. Nature. 1995 doi: 10.1038/374731a0. [DOI] [PubMed] [Google Scholar]
- Ferreira CG, Span SW, Peters GJ, Kruyt F. a, Giaccone G. Chemotherapy triggers apoptosis in a caspase-8-dependent and mitochondria-controlled manner in the non-small cell lung cancer cell line NCI-H460. Cancer Research. 2000;60:7133–7141. [PubMed] [Google Scholar]
- Friesen C, Herr I, Krammer PH, Debatin KM. Involvement of the CD95 (APO-1/FAS) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nature Medicine. 1996;2:574–577. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Strauss G. Functional CD95 ligand and CD95 death-inducing signaling complex in activation-induced cell death and doxorubicin-induced apoptosis in leukemic T cells. Blood. 2000:301–308. [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu H-C, Kim H, Cheng EH-Y, Tjandra N, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Haefen C, Gillissen B, Hemmati PG, Wendt J, Güner D, Mrozek A, Belka C, Dörken B, Daniel PT. Multidomain Bcl-2 homolog Bax but not Bak mediates synergistic induction of apoptosis by TRAIL and 5-FU through the mitochondrial apoptosis pathway. Oncogene. 2004;23:8320–8332. doi: 10.1038/sj.onc.1207971. [DOI] [PubMed] [Google Scholar]
- Handrick R, Ontikatze T, Bauer K-D, Freier F, Rübel A, Dürig J, Belka C, Jendrossek V. Dihydroartemisinin induces apoptosis by a Bak-dependent intrinsic pathway. Molecular Cancer Therapeutics. 2010;9:2497–2510. doi: 10.1158/1535-7163.MCT-10-0051. [DOI] [PubMed] [Google Scholar]
- Hao C, Song JH, Hsi B, Lewis J, Song DK, Petruk KC, Tyrrell DLJ, Kneteman NM. TRAIL inhibits tumor growth but is nontoxic to human hepatocytes in chimeric mice. Cancer Research. 2004;64:8502–8506. doi: 10.1158/0008-5472.CAN-04-2599. [DOI] [PubMed] [Google Scholar]
- Hikita H, Takehara T, Kodama T, Shimizu S, Shigekawa M, Hosui A, Miyagi T, Tatsumi T, Ishida H, Li W, et al. Delayed-onset caspase-dependent massive hepatocyte apoptosis upon Fas activation in Bak/Bax-deficient mice. Hepatology (Baltimore, Md.) 2011;54:240–251. doi: 10.1002/hep.24305. [DOI] [PubMed] [Google Scholar]
- Hsu Y-T. Nonionic Detergents Induce Dimerization among Members of the Bcl-2 Family. Journal of Biological Chemistry. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- Jacobson M. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- Johnstone RW, Ruefli AA, Lowe SW, Victoria EM. Apoptosis : A Link between Cancer Genetics and Chemotherapy Defects in apoptosis underpin both tumorigenesis and. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Kaina B. DNA damage-triggered apoptosis: critical role of DNA repair, double-strand breaks, cell proliferation and signaling. Biochemical Pharmacology. 2003;66:1547–1554. doi: 10.1016/s0006-2952(03)00510-0. [DOI] [PubMed] [Google Scholar]
- Kamer I, Sarig R, Zaltsman Y, Niv H, Oberkovitz G, Regev L, Haimovich G, Lerenthal Y, Marcellus RC, Gross A. Proapoptotic BID is an ATM effector in the DNA-damage response. Cell. 2005;122:593–603. doi: 10.1016/j.cell.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Katz C, Zaltsman-Amir Y, Mostizky Y, Kollet N, Gross A, Friedler A. Molecular basis of the interaction between proapoptotic truncated BID (tBID) protein and mitochondrial carrier homologue 2 (MTCH2) protein: key players in mitochondrial death pathway. The Journal of Biological Chemistry. 2012;287:15016–15023. doi: 10.1074/jbc.M111.328377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M, Brauer M, Powers V, Wu J. Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature. 1995 doi: 10.1038/374736a0. [DOI] [PubMed] [Google Scholar]
- Kim H, Rafiuddin-Shah M, Tu H-C, Jeffers JR, Zambetti GP, Hsieh JJ-D, Cheng EH-Y. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nature Cell Biology. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- Kim H, Tu H-C, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ-D, Cheng EH-Y. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Molecular Cell. 2009;36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science (New York, N.Y.) 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Proapoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death and Differentiation. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- Krajewski S, Krajewska M, Reed J. Immunohistochemical analysis of in vivo patterns of Bak expression, a proapoptotic member of the Bcl-2 protein family. Cancer Research. 1996:2849–2855. [PubMed] [Google Scholar]
- Leshchiner ES, Braun CR, Bird GH, Walensky LD. Direct activation of full-length proapoptotic BAK. Proceedings of the National Academy of Sciences of the United States of America. 2013:1–10. doi: 10.1073/pnas.1214313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by Caspase 8 Mediates the Mitochondrial Damage in the Fas Pathway of Apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Lindsten T, Ross a J., King a, Zong WX, Rathmell JC, Shiels H. a, Ulrich E, Waymire KG, Mahar P, Frauwirth K, et al. The combined functions of pro-apoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Molecular Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Maas C, De Vries E, Tait SWG, Borst J. Bid can mediate a pro-apoptotic response to etoposide and ionizing radiation without cleavage in its unstructured loop and in the absence of p53. Oncogene. 2011;30:3636–3647. doi: 10.1038/onc.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Chonghaile T, Sarosiek K. a, Vo T-T, Ryan J. a, Tammareddi A, Moore VDG, Deng J, Anderson KC, Richardson P, Tai Y-T, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science (New York, N.Y.) 2011;334:1129–1133. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara J, Watanabe-Fukunaga R. Lethal effect of the anti-Fas antibody in mice. Nature. 1993 doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- O'Connor L, Strasser a, O'Reilly L. a, Hausmann G, Adams JM, Cory S, Huang DC. Bim: a novel member of the Bcl-2 family that promotes apoptosis. The EMBO Journal. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarosiek K. a, Nechushtan H, Lu X, Rosenblatt JD, Lossos IS. Interleukin-4 distinctively modifies responses of germinal centre-like and activated B-cell-like diffuse large B-cell lymphomas to immuno-chemotherapy. British Journal of Haematology. 2009;147:308–318. doi: 10.1111/j.1365-2141.2009.07851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarosiek KAK, Malumbres R, Nechushtan H, Gentles a. J., Avisar E, Lossos IS. Novel IL-21 signaling pathway up-regulates c-Myc and induces apoptosis of diffuse large B-cell lymphomas. Blood. 2010;115:570–580. doi: 10.1182/blood-2009-08-239996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee E. a, Keogh S. a, Martin SJ. Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release. Cell Death and Differentiation. 2000;7:556–565. doi: 10.1038/sj.cdd.4400689. [DOI] [PubMed] [Google Scholar]
- Strumberg D, Pilon AA, Smith M, Hickey R, Malkas L, Pommier Y. Conversion of Topoisomerase I Cleavage Complexes on the Leading Strand of Ribosomal DNA into 5'-Phosphorylated DNA Double-Strand Breaks by Replication Runoff. Molecular and Cellular Biology. 2000;20:3977–3987. doi: 10.1128/mcb.20.11.3977-3987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait SWG, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nature Reviews. Molecular Cell Biology. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- Vo T-T, Ryan J, Carrasco R, Neuberg D, Ross DJ, Stone R, DeAngelo DJ, Frattini MG, Letai A. Relative Mitochondrial Priming of Malignant Myeloblasts and Normal HSCs Determines Chemotherapeutic Success in AML. Cell. 2012 doi: 10.1016/j.cell.2012.08.038. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Lindsten T, Mootha V, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes & …. 2000:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- Werner AB, Tait SWG, De Vries E, Eldering E, Borst J. Requirement for aspartate-cleaved bid in apoptosis signaling by DNA-damaging anti-cancer regimens. The Journal of Biological Chemistry. 2004;279:28771–28780. doi: 10.1074/jbc.M400268200. [DOI] [PubMed] [Google Scholar]
- Yamada H, Tada-Oikawa S, Uchida a, Kawanishi S. TRAIL causes cleavage of bid by caspase-8 and loss of mitochondrial membrane potential resulting in apoptosis in BJAB cells. Biochemical and Biophysical Research Communications. 1999;265:130–133. doi: 10.1006/bbrc.1999.1641. [DOI] [PubMed] [Google Scholar]
- Yin X, Wang K, Gross A, Zhao Y, Zinkel S. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:2–7. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- Zaltsman Y, Shachnai L, Yivgi-Ohana N, Schwarz M, Maryanovich M, Houtkooper RH, Vaz FM, De Leonardis F, Fiermonte G, Palmieri F, et al. MTCH2/MIMP is a major facilitator of tBID recruitment to mitochondria. Nature Cell Biology. 2010;12:553–562. doi: 10.1038/ncb2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science (New York, N.Y.) 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- Zinkel SS, Hurov KE, Ong C, Abtahi FM, Gross A, Korsmeyer SJ. A role for proapoptotic BID in the DNA-damage response. Cell. 2005;122:579–591. doi: 10.1016/j.cell.2005.06.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.