Summary
Allergies are widely considered to be misdirected type 2 immune responses, in which IgE antibodies are produced against any of a broad range of seemingly harmless antigens. However, components of insect venoms also can sensitize individuals to develop severe IgE-associated allergic reactions, including fatal anaphylaxis, upon subsequent venom exposure. We found that mice injected with amounts of honeybee venom similar to that which could be delivered in one or two stings developed a specific type 2 immune response which increased their resistance to subsequent challenge with potentially lethal amounts of the venom. Our data indicate that IgE antibodies and the high affinity IgE receptor, FcεRI, were essential for such acquired resistance to honeybee venom. The evidence that IgE-dependent immune responses against venom can enhance survival in mice supports the hypothesis that IgE, which also contributes to allergic disorders, has an important function in protection of the host against noxious substances.
Keywords: allergy, Th2 cell immunity, type 2 immunity, toxin hypothesis, honeybee, Apis mellifera, phospholipase A2, Russell's viper, Daboia russelli, venom, noxious substance, IgE, FcεRIα, C57BL/6, BALB/c, mouse
Introduction
Allergies, which afflict 20–30% of people worldwide, are detrimental immune responses against any of a large variety of environmental antigens (Pawankar et al., 2012). Such antigens (called allergens) share the ability to elicit acquired type 2 immune responses which are orchestrated by CD4+ T helper type (Th)2 cells and include the production of allergen-specific IgE antibodies (Galli and Tsai, 2012; Paul and Zhu, 2010; Pulendran and Artis, 2012). During infections with helminthes and certain other parasites, IgE-associated Th2 cell-mediated responses are thought to contribute to host defense (Finkelman et al., 2004; Fitzsimmons and Dunne, 2009; Pulendran and Artis, 2012; Stetson et al., 2004). However, when IgE-associated immune responses are directed against other types of allergens, such as those in foods or medicines, allergen exposure of certain sensitized individuals can induce anaphylaxis, an acute, catastrophic, and potentially fatal systemic reaction (Finkelman, 2007; Portier and Richet, 1902). Because most allergens do not represent a threat to the non-sensitized host, Th2 cell responses resulting in antigen-specific IgE antibodies to such allergens are widely considered to be misdirected and maladaptive immune responses (Artis et al., 2012; Holgate and Polosa, 2008).
In 1991, Margie Profet suggested that the common feature of most allergens is their origin from sources (such as nuts, seafood, or venoms) which either might (e.g., foods) or always (e.g., venoms) contain toxins (Profet, 1991). Profet also proposed that allergic reactions (manifested as immediately occurring symptoms, such as coughing or diarrhea) evolved as a defense mechanism which allows the sensitized host to respond immediately to, and to neutralize and/or avoid, noxious substances which might be indicative of potentially life-threatening situations (Profet, 1991). However, until recently (Palm et al., 2012), Profet’s “toxin hypothesis” was largely ignored by the scientific community and, to date, supporting experimental evidence has been missing.
In mammals, venoms provoke an innate inflammatory response and pathology related to the biological activities of the toxic components (Habermann, 1972; Mukherje et al., 2000; Risch et al., 2009). Venoms also can induce allergic sensitization and development of specific IgE antibodies (Annila, 2000; Charavejasarn et al., 1975; Jarisch et al., 1979; Saelinger and Higginbotham, 1974; Wadee and Rabson, 1987), which bind to the high affinity IgE receptor, FcεRI, on tissue mast cells (MCs) and blood basophils, priming them to release mediators of allergy and anaphylaxis upon subsequent venom exposure (Charavejasarn et al., 1975; Finkelman, 2007; Galli and Tsai, 2012; Oettgen and Geha, 1999; Saelinger and Higginbotham, 1974). MCs also can be activated directly by certain venoms, in the absence of specific IgE, and work in mice indicates that innate functions of MCs, including degradation of venom toxins by MC-derived proteases, can enhance host resistance to the venoms of certain arthropods (including the honeybee) and reptiles (Akahoshi et al., 2011; Metz et al., 2006; Schneider et al., 2007). However, it is not known whether acquired type 2 immunity against venoms also can enhance host defense.
We found that a type 2 immune response and associated IgE antibodies against honeybee venom were able to increase host resistance to challenge with a potentially lethal dose of venom, an effect that was mediated by FcεRI. Our data indicate that one function of IgE, which is best known for its role in allergic reactions, is to protect the host against noxious substances.
Results
Mice Develop an Antigen-Specific Th2 Cell Response After Immunization with Honeybee Venom
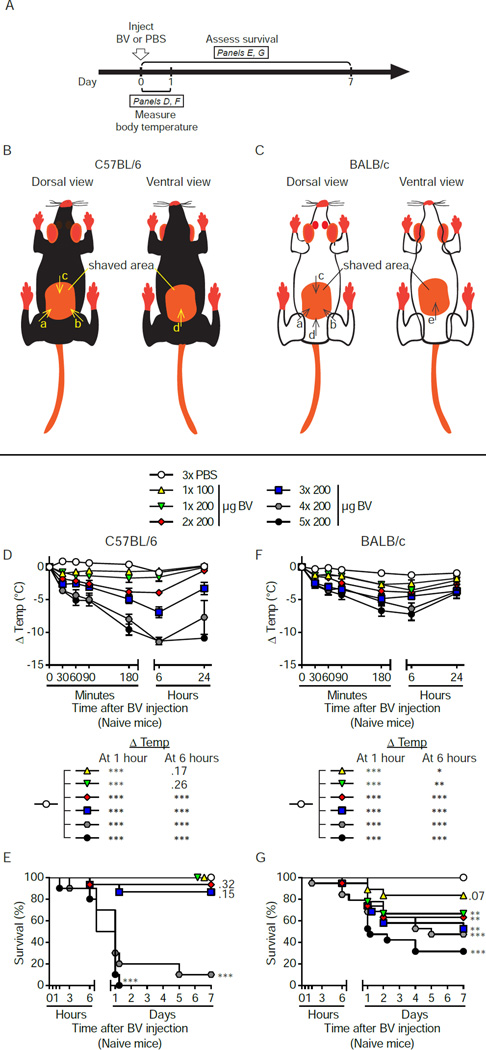
Honeybee (Apis mellifera) venom (BV) consists of a mixture of cytolytic peptides (e.g., melittin), enzymes (e.g., phospholipase A2 [PLA2; considered the main allergen in BV]), hyaluronidase, neurotoxins and bioactive amines (Habermann, 1972), and accounts for the majority of venom allergies in humans (Bilo et al., 2005). To assess their susceptibility to BV, we injected naive wild-type (WT) Th1 cell response-prone C57BL/6 mice and Th2 cell response-prone BALB/c mice subcutaneously (s.c.) with increasing total amounts of complete, unprocessed BV at 1 to 5 different sites. Each injection contained 100 to 200 µg of BV, mimicking the amount of venom typically found in individual honeybees (our immunization dosages therefore correspond to ~ 1 to 5–10 honeybee stings (Metz et al., 2006; Schmidt, 1995)) (Figure 1A to 1C). Acute reactions to BV were characterized by dose-dependent reductions in both body temperature (Figure 1D and 1F) and survival (Figure 1E and 1G). Similar results were obtained with Russell‧s viper venom, which also induced dose-dependent toxicity (Figure S1A to S1G).
Figure 1. Acute systemic responses of naive C57BL/6 and BALB/c mice to increasing doses of BV.
(A) Experimental outline. (B and C) Schematic showing sites of s.c. injection of up to 4 or 5 doses of BV (or PBS) in (B) C57BL/6 and (C) BALB/c mice. Arrowheads indicate the injection sites in the shaved area(s). Injections were carried out in alphabetical order, depending on the number of injections. In studies of innate responses to BV, C57BL/6 and BALB/c mice received 1–5 doses of BV or PBS (x3) (see D–G below). In experiments in which mice were challenged with high dose BV, C57BL/6 mice always got 4 injections of BV (200 µg each, as shown in B) and BALB/c mice always got 5 injections of BV (200 µg each, as shown in C). Female (D and E) C57BL/6 and (F and G) BALB/c WT mice were treated s.c. with the indicated doses of BV. Mock-treated control mice received 3 injections of PBS. Mice receiving 1–3 injections were treated in the back only (see B and C, arrows a–c); mice injected 4 times received 3 injections in the back and 1 in the belly (see B, arrows a-d); mice injected 5 times received 4 injections in the back and 1 in the belly (see C, arrows a–e). (D and F) Changes in body temperature (Δ Temp [mean ± SEM]) and (E and G) survival (% of live animals) were monitored at the indicated times. P values are versus PBS-treated mice and were calculated by (D and F) Student’s t test or (E and G) Mantel-Cox test. (D – G) Data are pooled from 2 (for groups receiving 4× or 5× 200 µg BV) or 3 (all other groups) independent experiments (n=10–19/group). *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus PBS; numbers in D, E and G are the P values for comparisons to PBS that were not significant (P > 0.05). See also Figures S1 for a similar set of experiments with Russell’s viper venom.
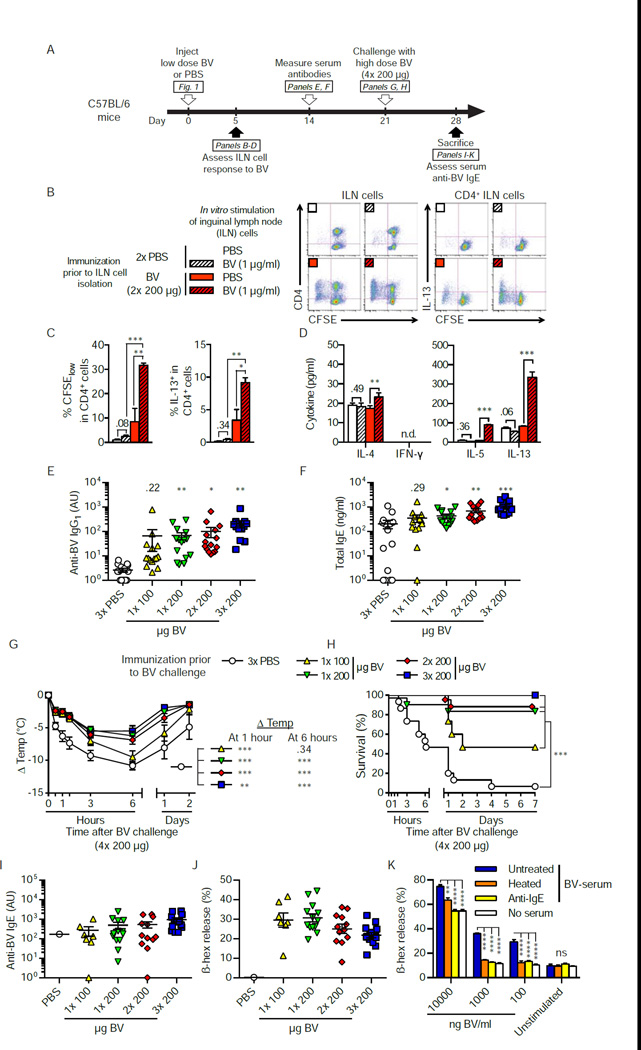
Honeybee stings can induce a Th2 cell-mediated immune response associated with BV-specific IgE antibodies, which can prime some individuals to exhibit anaphylaxis in response to a subsequent sting (Annila, 2000). Mice can develop Th2 cell-mediated responses to BV when they are immunized with BV admixed with adjuvants such as Freund’s complete adjuvant (Saelinger and Higginbotham, 1974) or aluminum hydroxide (Charavejasarn et al., 1975). We tested whether injections of whole BV (without added adjuvants) also could induce type 2 immunity in mice (Figure 2A; Figure S2A).
Figure 2. Injection of a sub-lethal dose of BV induces a Th2 cell immune response that can increase the resistance of C57BL/6 mice to the hypothermia and mortality caused by subsequent challenge with a potentially lethal dose of BV.
(A) Experimental outline For assessment of the ILN cell response in B-D, mice were injected s.c. with 2× 200 µg BV or PBS. In panels E-J, mice were injected with PBS, 1× 100, 1× 200, 2× 200 or 3× 200 µg BV and challenged 3 weeks later with 4× 200 µg BV. (B and C) Flow cytometry analysis of CFSE-labeled ILN cells stimulated for 4 days with 1 µg/ml BV or PBS. (B) Representative dot plots and (C) quantification (pooled from 3 independent experiments) of proliferation (% CFSElow) and intracellular IL-13 (% IL-13+) of CD4+ ILN cells. (D) IL-4, −5, −13, and IFN-y in supernatants of CFSE-labeled ILN cells after 4 days of BV or PBS stimulation in vitro. (E) BV-specific IgG1 and (F) total IgE antibody levels in serum obtained two weeks after BV immunization or mock immunization with PBS. (G) Changes in body temperature (A Temp) and (H) survival (% of live animals) of mice challenged with 4× 200 µg BV three weeks after BV immunization. (I) Titers of BV-specific IgE in sera collected 7 days after venom challenge from all surviving mice shown in H. (J) C57BL/6 BMCMCs were sensitized with sera of individual mice, stimulated with 1 µg/ml BV, and the amount of β-hexosaminidase (β-hex) in the cell supernatant was measured to assess BMCMC degranulation. (K) Pooled serum of BV-immunized and challenged C57BL/6 mice (BV-serum, which was either heated to eliminate the ability of IgE to bind to FcεRI [heated], treated with rat anti-mouse IgE [anti-IgE], or mock-treated with PBS [untreated]) was used to sensitize C57BL/6 BMCMCs, which were then stimulated with different concentrations of BV (100 – 10,000 ng/ml) or an equivalent volume of PBS (unstimulated). (C–F) Data are pooled from 3 independent experiments (n=13–15/group). (C to G, I and J): Values are mean ± or + SEM (E, F, I and J also show values for individual mice). (K) Values shown are mean + SD from one representative of 3 independent experiments, P values are versus (C and D) in vitro PBS-treated cells or (E–H) PBS-injected mice or (K) cells sensitized with untreated BV-serum, by (C-G, K) Student’s t test or (H) Mantel-Cox test. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (C, D and K) for the indicated comparisons or (E–H) versus PBS; the number in C-G are the P values for the comparisons that were not significant (P > 0.05). n.d., not detectable; ns, not significant. See also Figure S2 for data from a similar set of experiments performed in BALB/c mice.
We assessed responses to BV of primary T cells from draining inguinal lymph nodes (ILNs) from C57BL/6 or BALB/c mice 5 days after injection of either PBS or a sub-lethal dose of BV (i.e., one not resulting in the death of significant numbers of animals [P=0.32 and P=0.07 in Figure 1E and 1G, respectively]). CD4+ ILN cells from PBS-injected mice did not proliferate upon BV stimulation, whereas stimulation with whole BV (Figure 2B and 2C; Figures S2B and S2C) or with purified phospholipase A2 (PLA2 [data not shown]) induced substantial proliferation of CD4+ ILN cells from BV-injected mice, as reflected by an increased percentage of CFSElow cells. Moreover, upon BV stimulation, ILN cells from BV-, versus PBS-, injected mice produced increased amounts of the Th2 cell-associated cytokines interleukin (IL)-13 (Figures 2B and 2C; Figure S2B and S2C), IL-4 and IL-5, but no, or only low amounts of, the Th1 cell-associated cytokine interferon (IFN)-y (Figure 2D; Figure S2D).
Two weeks after injection of sub-lethal amounts of BV, we measured serum titers of BV-specific IgG1 and total IgE, the two antibody isotypes associated with Th2 cell immune responses in mice. One, 2 or 3 s.c. injections of 200 µg BV induced significant elevation of BV-specific IgG1 (P<0.05, Figure 2E) and total IgE (P<0.05, Figure 2F) antibodies in C57BL/6 mice. Mice on the Th2 cell-prone BALB/c background also developed marked increases in BV-specific IgG1 (but without significant increases in total IgE) when immunized with BV (Figure S2E and S2F). Together, our results show that mice can develop type 2 immune responses after exposure to amounts of BV that mimic actual bee stings.
Type 2 Immunity to Honeybee Venom Can Confer Increased Resistance to a High Dose of Venom
Because both IgE and IgG1 antibodies can orchestrate anaphylaxis and other allergic reactions in mice (Finkelman, 2007; Miyajima et al., 1997; Oettgen and Geha, 1999; Oettgen et al., 1994), we speculated that a BV-induced Th2 cell response might prime mice to exhibit exacerbated reactions to subsequent BV challenge. In contrast, we found that 3 weeks after they had received a sub-lethal dose of BV, such mice exhibited markedly increased resistance to challenge with a potentially lethal dose of BV, i.e., 4× 200 µg and 5× 200 µg BV for C57BL/6 and BALB/c mice, respectively (which caused death in 90% or 70%, respectively, of 6–8 week old naive WT animals [Figure 1E and 1G]) (Figure 2G and 2H; Figures S2G and S2H). For example, C57BL/6 mice which had been immunized with as little as 1× 200 µg BV exhibited significantly less hypothermia (P<0.001 at 1 and 6 hours, Figure 2G) and markedly enhanced survival after potentially lethal BV challenge (Figure 2H). In BALB/c mice, even immunization with 1× 100 µg BV induced enhanced protection (Figure S2G and S2H).
Individual sera of protected mice obtained a week after BV challenge (Figure 2A; Figure S2A) contained BV-specific IgE antibodies specific for BV (Figure 2I; Figure S2I) and for the major BV allergen PLA2 (data not shown), and such sera also markedly increased the ability of bone marrow-derived cultured mast cells (BMCMCs) to degranulate in response to BV stimulation (Figure 2J; Figure S2J). Pre-treatment of such pooled immune serum (collected from immunized and challenged mice) by heating (to ablate IgE function (Ishizaka et al., 1986; Prouvost-Danon et al., 1977; Strait et al., 2006)) or by using an anti-mouse IgE antibody decreased its ability to sensitize BMCMCs to degranulate in response to BV (i.e., 100 ng/ml and 1,000 ng/ml; Figure 2K; Figure S2K), confirming that BV immunization induced production of functionally active BV-specific IgE antibodies.
Both C57BL/6 and BALB/c mice injected with sub-lethal amounts of BV developed a Th2 cell immune response and exhibited increased resistance to challenge with a potentially lethal dose of BV. This finding is especially remarkable given that: 1) BV consists of a variety of active toxins with different mechanisms of action (Habermann, 1972; Schmidt, 1995) and 2) type 2 immune responses against venoms (i.e., development of anti-venom IgG1 and IgE antibodies) are classically thought to exacerbate the outcome of subsequent venom exposure (Annila, 2000; Bilo et al., 2005; Charavejasarn et al., 1975; Jarisch et al., 1979; Reimers et al., 2000; Saelinger and Higginbotham, 1974; Wadee and Rabson, 1987).
Acquired Resistance to High Dose Honeybee Venom Challenge Is Dependent on Functional IgE Antibodies
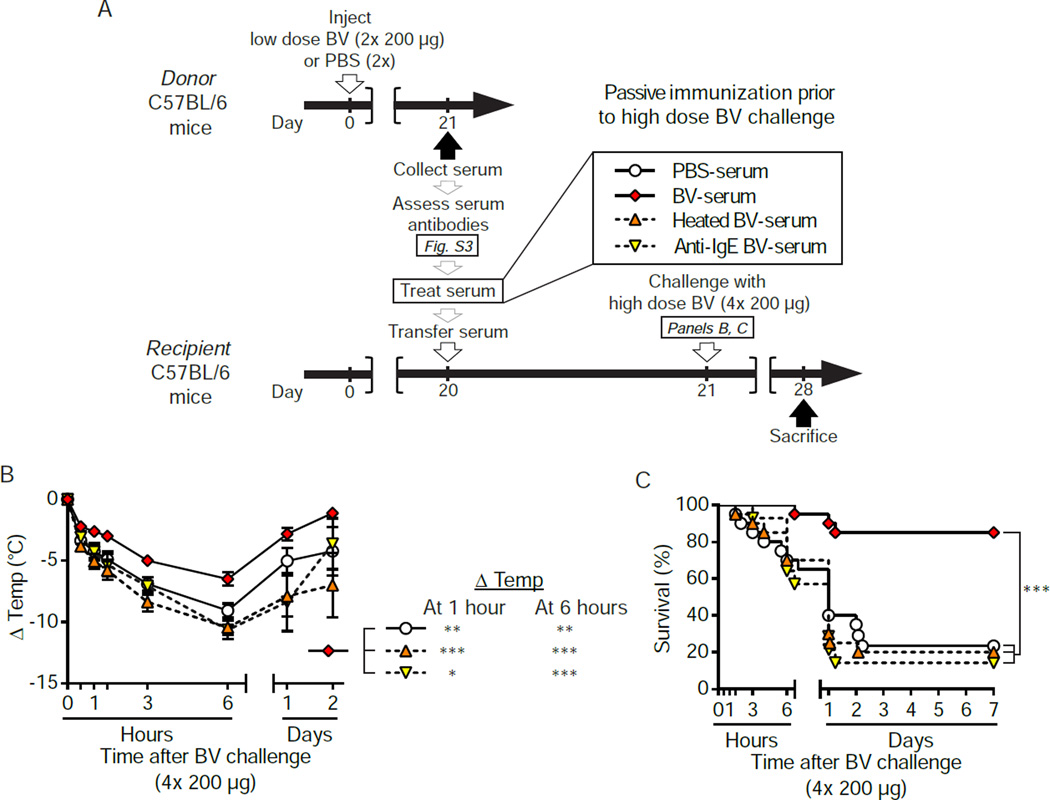
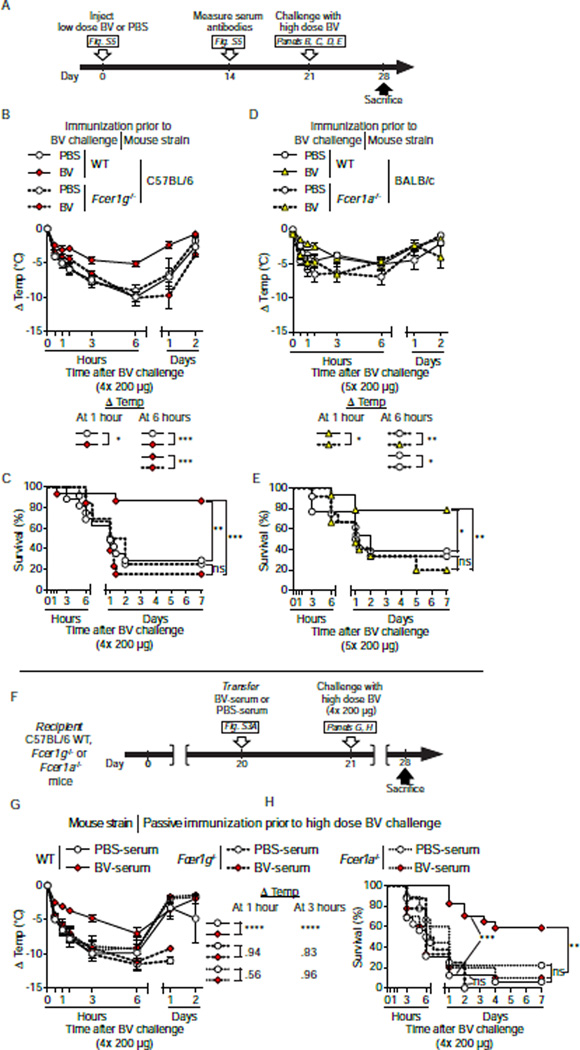
In mice, passive transfer of either antigen-specific IgE or IgG1 antibodies can confer anaphylactic reactivity to naive animals (Ando et al., 1993; Finkelman, 2007; Miyajima et al., 1997). We used passive immunization to evaluate whether the protective effect of BV immunization might be antibody-mediated (Figure S3A). Briefly, we immunized C57BL/6 mice with 2× 200 µg BV (a sub-lethal dose [Figure 1E] which enhanced resistance of C57BL/6 mice to challenge with a high dose of BV [Figure 2G and 2H]) or injected them with PBS. Three weeks later, we confirmed the presence of increased serum titers of BV-specific IgG1 and total IgE antibodies in BV-immunized mice (Figure S3B and S3C). We also confirmed that serum of BV-immunized mice contained IgE antibodies that promoted BV binding to BMCMCs via FcεRIα, the IgE-binding component of the receptor (Figure S3D to S3F). In parallel, some actively immunized mice were challenged with a high dose of BV (4× 200 µg) to confirm successful immunization (Figure S3G and S3H, dashed lines). We then transferred pooled sera (derived from mice mock-immunized with PBS [“PBS-serum”] or immunized with BV [“BV-serum”]) i.v. into age-matched naive WT mice and challenged them 22 h later with 4× 200 µg B V.
Transfer of BV-serum (compared to transfer of PBS-serum) conferred significantly increased resistance to high dose BV challenge, as assessed by body temperature (P<0.01, Figure S3G) or survival (P<0.001, Figure S3H). Remarkably, passive immunization was as effective as active immunization (Figure S3G and S3H). We concluded that soluble factors in the serum of BV-immunized mice are sufficient to increase host resistance to a potentially lethal dose of BV.
We next used two approaches in WT mice to assess the contribution of IgE antibodies to the protection conferred by immune serum (Figure 3A). First, we treated the BV-serum with an anti-IgE antibody (i.e., “anti-IgE BV-serum”) before passive immunization. This approach is straightforward, but risks inducing anaphylaxis due to formation of antibody immune-complexes which in turn activate effector cells. Indeed, 5 of the 20 mice which received anti-IgE BV-serum developed severe hypothermia and died soon after the transfusion; the other 15 mice appeared normal and were used for the experiments shown in Figure 3. We also passively immunized mice with heated BV-serum (Figure 3A). Heating results in a loss of the ability of IgE to induce passive cutaneous anaphylaxis (Prouvost-Danon et al., 1977), while the function of other antibody classes, including IgG1, is not affected (Strait et al., 2006).
Figure 3. The increased resistance of passively immunized C57BL/6 WT mice to challenge with a potentially lethal dose of BV is dependent on functional serum IgE.
(A) Experimental outline. Donor mice were injected with 2× 200 µg BV or PBS. Three weeks later, sera from PBS-or BV-injected mice ([PBS-serum] or [BV-serum], respectively) were collected (see also Figure S2), and pooled BV-serum was processed in vitro to neutralize IgE function, either by heating or anti-mouse IgE treatment ([heated BV-serum] or [anti-IgE BV-serum], respectively). Age-matched recipient mice were anesthetized and transfused i.v. with serum 22 h before challenge with 4× 200 µg BV. (B) Changes in body temperature after challenge (Δ Temp [mean ± SEM]) and (C) survival (% of live animals) were monitored at indicated time points. Data are pooled from experiments obtained with 2 different pools of serum and a total of 4 independent experiments (n=14–20/group). P values are calculated by (B) Student’s t test and (C) Mantel-Cox test. *, P<0.05; **, P < 0.01; ***, P < 0.001 for the indicated comparisons. See also Figure S3.
Notably, either heating (Figure 3B and 3C; orange triangles) or anti-IgE antibody treatment (Figures 3B and 3C; inverted yellow triangles) essentially abolished the protective potential of BV-serum (Figures 3B and 3C; red diamonds) in mice challenged with 4× 200 µg BV. These results support the conclusion that IgE antibodies substantially contribute to, or may fully account for, the immune serum’s protective effect.
Honeybee Venom Immunized IgE-deficient Mice Do Not Develop Enhanced Resistance to the Venom
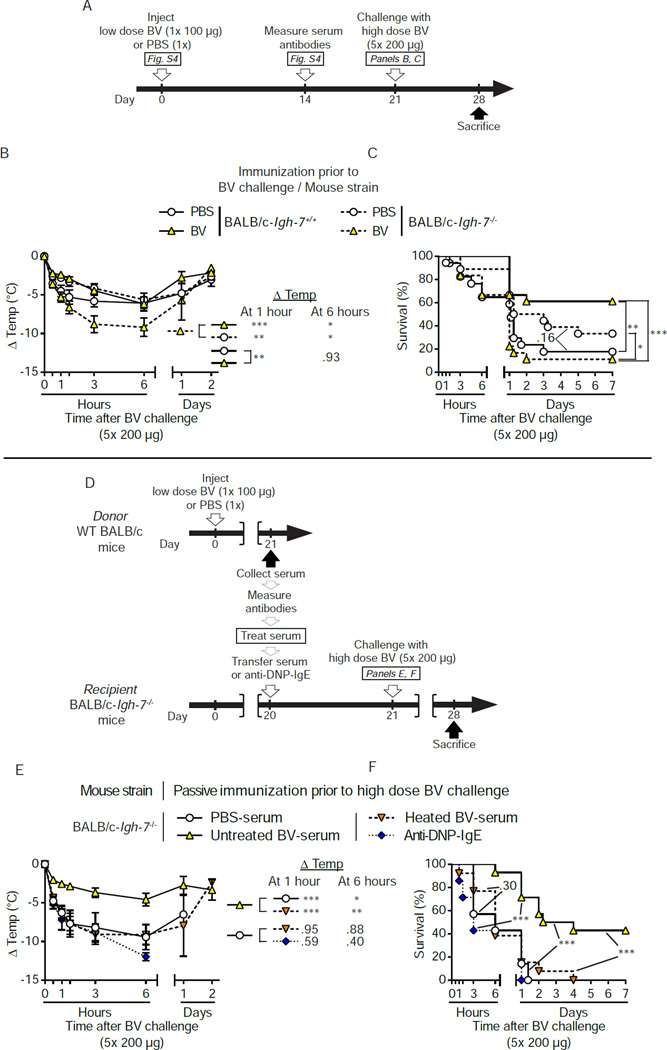
In order to confirm the role of IgE antibodies in acquired host resistance against BV using a genetic approach, we attempted active immunization of mice genetically lacking IgE (BALB/c-Igh-7−/−) (Figure S4A to S4C; Figure 4A). Fourteen days after immunization with 1× 100 µg BV, the serum of IgE-deficient animals contained specific IgG1 antibodies at titers comparable to WT controls and, as expected, no detectable IgE (Figure S4D and S4E). As observed in prior experiments (Figure S2G and S2H), BV-immunized WT mice were significantly more resistant to challenge with 5× 200 µg BV than were PBS mock-immunized mice (P<0.01, Figures 4B and 4C). In contrast, BV-immunized IgE-deficient mice were less resistant to high dose BV challenge than were PBS mock-immunized IgE-deficient mice (Figure 4B and 4C). Notably, IgE-deficient mice that were passively immunized with untreated BALB/c BV-serum (that had been confirmed to confer protection in BALB/c WT mice challenged with 5× 200 µg BV [data not shown]) developed less hypothermia and exhibited lower mortality than mice that had received heated BV-serum or PBS-serum (Figure 4D to 4F). By contrast, transfer of dinitrophenyl (DNP)-specific monoclonal IgE antibody (that was functionally active in both IgE-deficient and WT mice [Figure S4F to S4H]), in amounts equivalent to the amounts of IgE transferred in BV-serum, did not influence the resistance of recipient IgE-deficient mice to BV challenge (Figure 4D to 4F).
Figure 4. IgE, but not IgG antibodies, are necessary for the protective effect of the adaptive response to BV.
(A) Experimental outline for panels B and C. (B and C) IgE-deficient (Igh-7−/−) or IgE-sufficient (Igh-7+/+) BALB/c mice mice were injected with 1× 100 µg BV or PBS. Three weeks later, mice were challenged with 5× 200 µg BV. (B) Changes in body temperature (Δ Temp) and (C) survival (% of live animals) were monitored at the indicated times. (D) Experimental outline for panels E and F. WT BALB/c donor mice were injected with 1× 100 µg BV or PBS. Three weeks later, sera from PBS- or BV-injected mice ([PBS-serum] or [BV-serum], respectively) were collected, and pooled BV-serum was processed in vitro to neutralize IgE function by heating (heated BV serum). Age-matched IgE-deficient recipient mice were anesthetized and transfused i.v. with serum or PBS containing monoclonal anti-DNP-IgE (an amount equivalent to the total IgE content of transferred BV-serum) 22 h before challenge with 5× 200 µg BV. (E) Changes in body temperature after challenge (Δ Temp) and (F) survival (% of live animals) were monitored at the indicated times. Data are pooled from (B and C) 4 independent experiments (n=17–19/group) or (E and F) from experiments obtained with 2 different pools of serum and a total of 3 independent experiments (n=8–14/group). (B and E) Values are mean ± SEM. P values are calculated by (B and E) Student’s t test or (C and F) Mantel-Cox test. *, P < 0.05; **, P < 0.01; ***, P < 0.001 for the indicated comparisons; the numbers in B, C, E and F are the P values for indicated comparisons that were not significant (P > 0.05). See also Figure S4.
Together with the results of our serum transfer experiments in WT C57BL/6 mice (Figure 3) these findings support the conclusion that type 2 immune responses can enhance host resistance to honeybee venom by IgE-dependent mechanisms.
FcεRIγ and FcεRIα Are Required for IgE-Mediated Resistance to High Dose Honeybee Venom Challenge
Antigen- and IgE-dependent activation of MCs and basophils by FcεRI is considered the key mechanism underlying acute allergic reactions (Kawakami and Kitaura, 2005; Kinet, 1999; Rivera et al., 2008). FcεRI contains one IgE-binding α-chain, one β-chain (that can function as a signal amplifier), and two signal-transducing γ-chains (referred to as FcεRIα, -β, and -γ, respectively) (Kinet, 1999; Rivera et al., 2008); FcεRIγ also participates in the signaling of the IgG receptors FcγRI, FcγRIII and FcγRIV (Bruhns, 2012; Kinet, 1999). To elucidate the function of antibodies in host defense against BV, we used a genetic approach targeting cell-mediated effects of IgG and IgE.
First, we actively immunized mice lacking FcεRIγ (C57BL/6-Fcer1g−/−) and C57BL/6 WT controls with 2× 200 µg BV (a sub-lethal dose [Figure 1E] which enhanced resistance of C57BL/6 mice to challenge with a high dose of BV [Figure 2G and 2H]) or injected them with PBS (Figure S5A; Figure 5A). Naive Fcer1g−/− and WT animals exhibited similar acute systemic innate responses to initial BV injection (Figure S5B and S5C), and elevated serum levels of BV-specific IgG1 and total IgE 2 weeks later (Figure S5D and S5E). However, in contrast to WT mice, BV immunization did not protect Fcer1g−/− mice against challenge with a potentially lethal dose of BV (Figures 5B and 5C). These results demonstrate the importance of IgE and/or IgG-Fc-receptor signaling via the FcεRIγ-chain for the protective effect of this venom-specific type 2 immune response.
Figure 5. FcεRIoα IgE binding and FcεRIγ signaling are required for BV-immunized mice to exhibit increased resistance against a potentially lethal dose of BV.
(A) Experimental outline for panels B to E. (B and C) C57BL/6 Fcer1g−/− and WT mice and (D and E) BALB/c Fcer1a−/− and WT mice were injected with 2× 200 µg BV or PBS (2x), or 1× 100 µg BV or PBS (1x), respectively. Three weeks later, (B and C) mice of C57BL/6 background were challenged with 4× 200 µg BV and (D and E) mice of BALB/c background were challenged with 5× 200 µg BV. (B and D) Changes in body temperature (Δ Temp) and (C and E) survival (% of live animals) were monitored at indicated time points. (F) Experimental outline for panels G and H. PBS-serum or BV-serum from WT C57BL/6 “donor” mice (see Figure S2A) were transferred i.v. into recipient C57BL/6 WT, Fcer1g−/− or Fcer1a−/− mice 22 h before challenge with 4× 200 µg BV. (G) Changes in body temperature (Δ Temp) and (H) survival (% of live animals) were monitored at the indicated times. Data are pooled (B–E) from 3 independent experiments (n=11–15/group) or (G and H) from experiments obtained with 2 different pools of serum and a total of 3–4 independent experiments (n=8–17/group). (B–D, G) Values are mean ± SEM. P values are calculated by (B, D and G) Student’s t test, and (C, E and H) Mantel-Cox test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P< 0.0001 for the indicated comparisons; the numbers in B, D and G are the P values for the indicated comparisons that were not significant (P > 0.05); ns, not significant. See also Figure S5.
We then assessed whether BV immunization could enhance resistance to high dose BV challenge in BALB/c mice lacking only the IgE-binding FcεRIα chain (BALB/c-Fcer1a−/−; Figure S5A; Figure 5A). Fcer1a−/− and WT mice responded similarly to 1× 100 µg BV immunization (a low dose which conferred enhanced protection to BALB/c mice upon challenge with a high dose of BV [Figure S2G and S2H]) (Figure S5F and S5G) and developed a BV-specific type 2 antibody response (Figure S5H and S5I). However, in comparison to WT counterparts (Figures 5D and 5E), BV-immunized Fcer1a−/− mice developed increased hypothermia and much higher mortality upon challenge with a potentially lethal dose (5 × 200 µg) of BV 3 weeks after immunization (Figure 5D and 5E). Notably, 2 of the 3 BV-immunized WT mice which did not have detectable serum IgE (but did develop increased BV-specific IgG1, Figure S5I) were not protected and died within 24 h. The importance of FcεRIα- and FcεRIγ-related signaling in the acquired resistance to BV was supported further by passive immunization experiments in which BV-serum derived from C57BL/6 WT mice, as compared to PBS-serum, improved neither hypothermia nor survival when transferred into C57BL/6-Fcer1g−/− or C57BL/6-Fcer1a−/− mice (Figures 5F to 5H).
Together, our results highlight the importance of IgE binding to FcεRIα and signaling via FcεRIy in the type 2 response-mediated host resistance against BV.
Evidence for a Role for Mast Cells in IgE-Mediated Resistance to High Dose Honeybee Venom Challenge
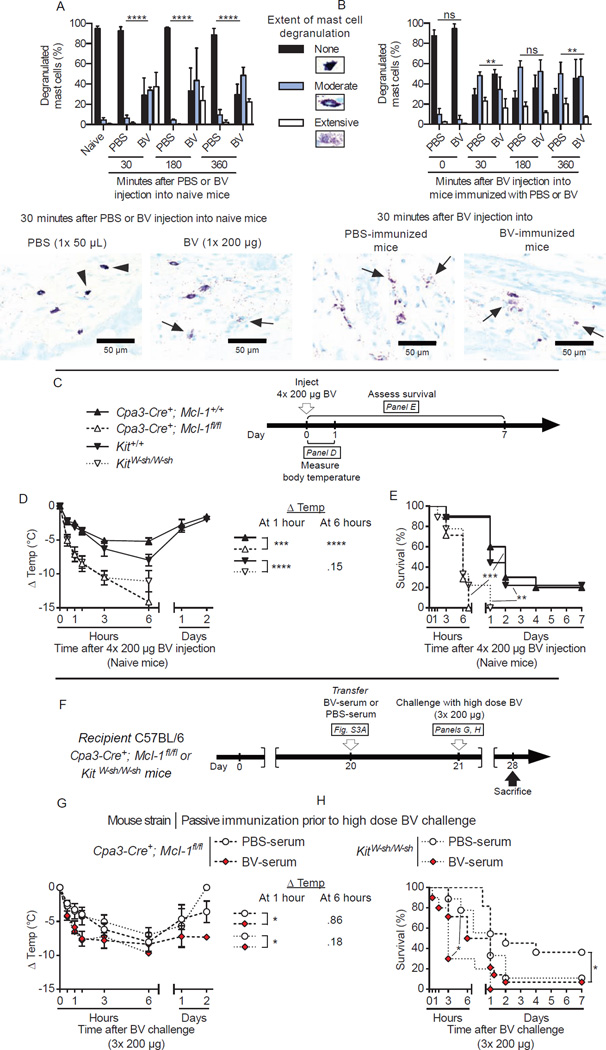
Our experiments indicate that IgE and signaling via FcεRI are critical components of type 2 immune responses that increase resistance to potentially lethal BV doses. MCs are major FcεRI-expressing IgE effector cells (Galli and Tsai, 2012; Kinet, 1999; Oettgen and Geha, 1999; Rivera et al., 2008), and previous reports by our lab (Akahoshi et al., 2011; Metz et al., 2006) and others (Schneider et al., 2007) indicate that MCs importantly contribute to the innate resistance to certain venoms (including BV [(Metz et al., 2006)]) and venom components (Akahoshi et al., 2011; Metz et al., 2006; Schneider et al., 2007). We therefore speculated that MCs may also play an important role in acquired resistance to BV. Consistent with a previous report (Higginbotham and Karnella, 1971) and our in vitro experiments (Figure 2K and S2K), a single BV injection (1× 200 µg) induced extensive MC degranulation at injection sites in both naive mice (Figure 6A) and mice injected 3 weeks earlier either with PBS or BV (Figure 6B). Consistent with our previous findings (Metz et al., 2006), MC-deficient C57BL/6-KitW-sh/W-sh mice (whose MC deficiency is caused by a mutation affecting expression of Kit, the receptor for the MC survival and maturation factor stem cell factor) and C57BL/6-Cpa3-Cre+-Mcl-1fl/fl mice (whose marked MC deficiency and decreased numbers of basophils are not due to c-kit mutations (Lilla et al., 2011)) were more susceptible to challenge with 4× 200 µg BV than their respective controls (i.e., Kit+/+ and Cpa3-Cre+-Mcl-1+/+ mice) (Figures 6C to 6E).
Figure 6. Passive immunization with serum of BV-immunized WT mice fails to increase the resistance against challenge with a potentially lethal dose of BV in MC-deficient mice.
(A–B) Extent of MC degranulation (quantified as % of none, moderate and extensive degranulated MCs) at the site of injection (back skin) following (A) injection of PBS (1x) or BV (1× 200 µg) in naive mice or (B) challenge of BV (4× 200 µg) in mice previously immunized with PBS or BV, and representative Toluidine Blue-stained back skin sections from the 30 minutes time point; arrowheads indicate some non-degranulated MCs and arrows indicate some of the extensively degranulated MCs. In (A), PBS injection did not cause significant MC degranulation compared to naive animals. In (B), “0 minutes” refers to baseline values in back skin of mice in the two groups obtained prior to injections of BV; BV injection caused significant MC degranulation after 30, 180 or 360 minutes in PBS or BV groups compared to the corresponding “0” values. (C) Experimental outline for panels D and E. KitWsh/Wsh and Kit+/+controls, Cpa3-Cre+-Mcl-1fl/fl and Cpa3-Cre+-Mcl-1+/+ controls were injected with 4× 200 µg BV. (D) Changes in body temperature (Δ Temp) and (E) survival (% of live animals) were monitored at the indicated times. (F) Experimental outline for panels G and H. PBS-serum or BV-serum from WT C57BL/6 “donor” mice (see Figure S2A) were transferred i.v. into MC-deficient recipient KitWsh/Wsh and Cpa3-Cre+-Mcl-1fl/fl mice 22 h before challenge with 3× 200 µg BV. (G) Changes in body temperature after challenge (Δ Temp) and (H) survival (% of live animals) were monitored at the indicated times. Data are pooled from (C–E) 2 independent experiments (n=7–10/group) and (F–H) 2–3 independent experiments obtained with 3 different pools of serum (n=9–13/group). (A,B) Values are mean ± SD. (D, G) Values are mean ± SEM. P values are calculated by (A, B) Chi-square, (D, G) Student’s t or (E and H) Mantel-Cox tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001 for the indicated comparisons; ns, not significant (P > 0.05).
The important role of MCs in innate resistance to BV complicates assessment of the contribution of MCs as potential IgE effector cells in acquired increased resistance to BV. To deal with this issue, we decreased the challenge dose for MC-deficient mice to 3× 200 µg BV (this dose caused death in 60–90% of naive MC-deficient mice [data not shown]) and performed serum transfer experiments. We found that naive MC-deficient mice that received C57BL/6 BV-serum (from the same pools of BV-serum that conferred protection in C57BL/6 WT mice challenged with 4× 200 µg BV [Figure S3G and S3H, 5G and 5H]) exhibited worse survival upon BV challenge than those passively immunized with PBS-serum (Figures 6F to 6H), suggesting that MCs can contribute to IgE-mediated resistance against BV.
Russell’s Viper Venom Can Induce a Protective Type 2 Immune Response Against a High Venom Dose
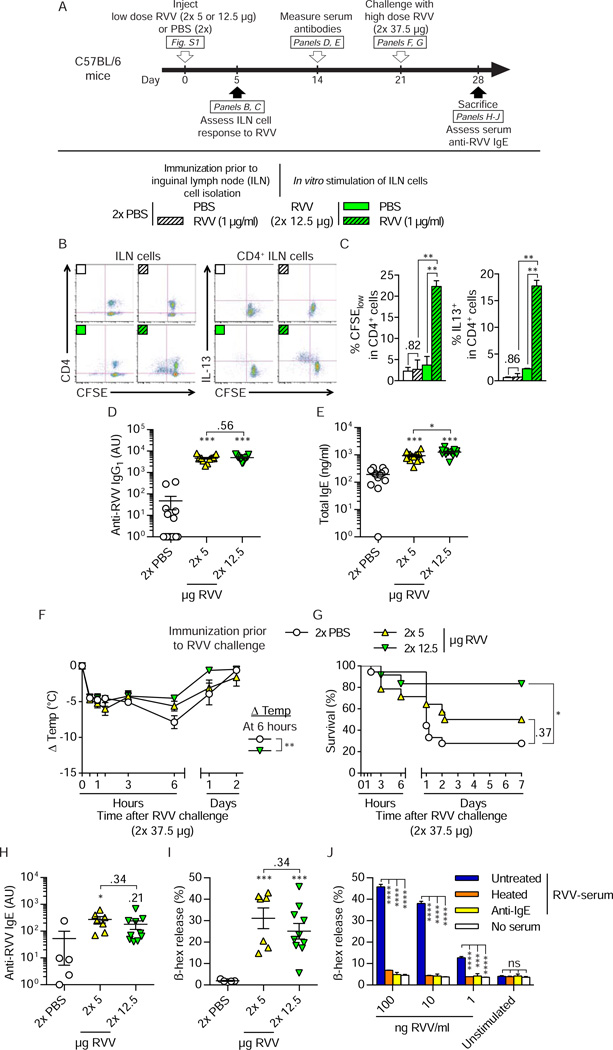
Finally, we were curious whether protective type 2 immunity could be induced by another type of venom. Venomous reptiles account for considerable morbidity and mortality in people (Theakston et al., 2003). Together with the Indian cobra (Naja naja), the krait (Bungarus caerulaeus), and the saw scaled viper (Echis carinatus), the Russell’s viper (Daboia russelli) composes the group of snakes referred to as the “Big Four”, which accounts for the majority of snake bite-related deaths in India (Simpson and Norris, 2007). Russell’s viper venom (RVV) is a complex mixture of growth factors and enzymes with pro-coagulant and neurotoxic activities (Risch et al., 2009).
We defined sub-lethal and potentially lethal doses of RVV in C57BL/6 and BALB/c mice, using 2 s.c. injections in the back (mimicking a snake bite) containing 5 – 50 µg of RVV per each 50 µl injection (Figure S1A to S1C). Five and 12.5 µg of RVV per injection (i.e., 10 µg and 25 µg of total RVV, respectively), which did not cause death in a substantial number of C57BL/6 animals, were chosen as the immunizing doses (Figure S1D to S1G). Like with BV, immunization with RVV induced a type 2 immune response characterized by the development of RVV-specific T cells with a Th2 cell profile (Figures 7A to 7C; Figures S6A to S6C), as well as increased serum levels of RVV-specific IgG1 (Figure 7D; Figure S6D) and total IgE (Figure 7E; Figure S6E). Three weeks after immunization, the mice were challenged with a potentially lethal dose of RVV (i.e., 2× 37.5 µg RVV, which caused death in 100% of 6–8 week old naive WT animals [Figure S1E and S1G]). Compared to PBS-injected mice, C57BL/6 and BALB/c mice that were immunized with 2× 12.5 µg RVV exhibited less hypothermia (Figure 7F; Figure S6F) and markedly improved survival (Figure 7G; Figure S6G). When we tested mice a week after RVV challenge, we detected functionally active RVV-specific IgE antibodies in the serum of the protected mice (Figure 7H to 7J; Figure S6H to S6J). These results show that IgE-associated type 2 immune responses induced by a reptile venom, i.e. Russell’s viper venom, also can enhance resistance of the host to challenge with a high dose of venom.
Figure 7. Injection of a sub-lethal dose of RVV induces a type 2 immune response that can increase the resistance of C57BL/6 mice to the hypothermia and mortality caused by subsequent challenge with a potentially lethal dose of RVV.
(A) Experimental outline For assessment of the ILN cell response in B and C, mice were injected s.c. with 2× 12.5 µg RVV or PBS. In panels D–I, mice were injected with PBS, 2× 5 µg RVV or 2× 12.5 µg RVV and challenged 3 weeks later with 2× 37.5 µg RVV. (B and C) Flow cytometry analysis of CFSE-labeled ILN cells stimulated for 4 days with 1 µg/ml RVV or PBS. (B) Representative dot plots and (C) quantification (pooled from 2 independent experiments) of proliferation (% CFSElow) and intracellular IL-13 (% IL-13+) of CD4+ ILN cells. (D) RVV-specific IgG1 and (E) total IgE antibody levels in serum obtained two weeks after RVV immunization or mock immunization with PBS. (F) Changes in body temperature (Δ Temp) and (G) survival (% of live animals) of mice challenged with 2× 37.5 µg RVV three weeks after immunization with RVV or mock immunization with PBS. (H) Titers of RVV-specific IgE in sera collected 7 days after the challenge from all surviving mice whose data are reported in G. (I) C57BL/6 BMCMCs were sensitized with sera of individual mice, stimulated with 1 µg/ml RVV, and the amount of β-hexosaminidase (β-hex) in the cell supernatant was measured to assess BMCMC degranulation. (J) Pooled serum of RVV-immunized and challenged C57BL/6 mice (RVV-serum, which was either heated to eliminate the ability of IgE to bind to FcεRI [heated], treated with rat anti-mouse IgE [anti-IgE], or mock-treated with PBS [untreated]) was used to sensitize C57BL/6 BMCMCs, which were then stimulated with different concentrations of RVV (1 – 100 ng/ml) or an equivalent volume of PBS (unstimulated). (C–I) Data are pooled from 3 independent experiments ([C–G], n=12–15/group; [H and I], n=5–10/group). (C–F, H and I) Values are mean ± or + SEM (D, E, H and I also show values for individual mice). (J) Values shown are mean + SD of triplicate values from one representative of 3 independent experiments. P values are versus (C) in vitro PBS-treated cells and (D–F, H, I) PBS-injected mice or (J) cells sensitized with untreated RVV-serum. (C–F and H–J) Student’s t or (G) Mantel-Cox test. * P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 for the indicated comparisons (C–E and J) or versus PBS (E–J); the numbers in C–E, H and I and J are the P values for comparisons that were not significant (P > 0.05). ns, not significant. See also Figure S6 for data from a similar set of experiments performed in BALB/c mice.
Discussion
We found that injecting mice with whole honeybee venom or Russell’s viper venom can induce the differentiation of BV- or RVV-specific Th2 cells and the production of venom-specific IgG1 and IgE antibodies. This was true in mice with genetic biases to develop either type 1 (C57BL/6) or type 2 (BALB/c) immune responses, suggesting that BV and RVV have strong intrinsic abilities to induce a Th2 cell-mediated immune response in mice. Notably, these BV- or RVV-induced IgE-associated type 2 immune responses enhanced host resistance to the toxicity of that venom. To our knowledge, our findings represent the first experimental evidence in support of Profet’s “toxin hypothesis”, which proposes that one key function of what are now known as Th2 cell-type immune responses, that in some clinical settings contribute to disease, is to protect the host against venoms and other environmental toxins (Palm et al., 2012; Profet, 1991).
We previously reported that MCs can reduce the toxicity of certain arthropod (including BV) and reptile venoms in mice (Akahoshi et al., 2011; Metz et al., 2006). This MC-related enhanced innate resistance to venom toxicity depends at least in part on compounds released by activated MCs, such as proteases (including carboxypeptidase A3 and the chymase mMCP-4) which can degrade and neutralize certain venom components (Akahoshi et al., 2011; Metz et al., 2006; Schneider et al., 2007). It is tempting to speculate that the occurrence of large numbers of MCs in the skin (a frequent site of envenomation) and around blood vessels (representing key “access points” that can facilitate the systemic distribution of venom components initially deposited locally at the site of stings or bites, thereby increasing their systemic toxicity) in part reflects evolutionary pressure to position MCs at sites where they can rapidly respond to venoms and thereby help to limit venom-induced pathology.
Our data, which need extension by future studies, suggest that MCs can contribute not only to innate resistance but also to IgE-related acquired resistance to BV. Moreover, we showed that venom-specific IgE antibodies permitted MCs to be activated by lower amounts of venom than are required to activate MCs via antibody-independent innate mechanisms. It therefore seems reasonable to propose that the release of proteases that can degrade venom components (Akahoshi et al., 2011; Schneider et al., 2007), and perhaps the secretion of other MC mediators such as heparin (Higginbotham and Karnella, 1971), by MCs activated via venom-specific IgE can contribute to the acquired, IgE-associated enhanced resistance to bee venom.
It will be interesting to see how many additional examples of “protective Th2 cell responses” to toxins or other noxious substances will be identified. It also will be important to try to understand why some conditions of venom immunization and challenge result in venom-directed, IgE-associated Th2 cell responses that enhance host resistance to that venom whereas others prime certain unfortunate individuals to exhibit serious and potentially fatal allergic responses, i.e., anaphylaxis, upon subsequent venom exposure (Annila, 2000; Bilo et al., 2005; Reimers et al., 2000; Wadee and Rabson, 1987). In considering this question, it should be noted that some people who develop Th2 cell responses to BV do not exhibit anaphylactic reactivity despite having venom-specific IgE antibodies (Antonicelli et al., 2002). Also, there is abundant evidence that Th2 cell-mediated responses are subject to immune regulation which can diminish pathology related to IgE-dependent reactivity to the inducing antigen, including BV (Meiler et al., 2008; Muller, 2005; Ozdemir et al., 2011). These findings, taken together with those reported herein, raise the possibility that anaphylaxis represents only the most extreme and maladaptive end of a spectrum of acquired Th2 cell-mediated immunity to venom and that includes, at the other end of the spectrum, appropriately regulated Th2 cell immune responses which can enhance resistance to such venoms. It therefore will be of interest to assess the extent that genetic and environmental factors, as well as differences in the conditions, timing, and type of exposure to venoms or other potentially toxic antigens, can determine whether the Th2 cell-mediated responses induced by such antigens confer net benefit or harm.
Although these and other questions raised by our findings remain to be answered, our experiments demonstrate that Th2 cell-mediated immune responses and the production of IgE antibodies, in addition to contributing to host defense against certain parasites, can confer a survival advantage in animals exposed to venoms.
Experimental Procedures
Mice
All animal care and experiments were carried out in accord with current National Institutes of Health guidelines and the approval of the Stanford University Institutional Animal Care and Use Committee. Six to 8 week-old female C57BL/6J or BALB/cJ mice were used in experiments involving solely WT mice. Transgenic mouse strains were bred and housed with the respective control mice in the local animal facilities. Transgenic C57BL/6-Fcer1g−/− mice were purchased from Taconic. BALB/c-Fcer1a−/− mice were originally obtained from Jean-Pierre Kinet (Harvard Medical School, MA, USA). C57BL/6-Fcer1a−/− mice were purchased from Jackson Laboratories. IgE-deficient BALB/c (Igh-7−/−) mice were generated as described (Oettgen et al., 1994). Mast-cell deficient C57BL/6-KitW-sh/W-sh mice extensively backcrossed onto the C57BL/6J background (Piliponsky et al., 2010) and MC- and basophil-deficient C57BL/6-Cpa3-Cre; Mcl-1fl/fl mice (Lilla et al., 2011) were as described. Age-matched male and female transgenic mice were used for experiments (with comparable gender distribution within the groups). See Supplemental Experimental Procedures for additional information.
Reagents
Honeybee venom (BV) was from ALK Abello Source Materials, Inc.; Russell’s viper venom (RVV) was from Sigma. Additional information on venoms and other reagents are in the Supplemental Experimental Procedures.
Venom injections and active immunization
Fig. 2A outlines the active immunization protocol (see Supplemental Experimental Procedures for details). Briefly, 6 to 8 week old WT or transgenic mice were shaved at injection sites 24 h before injections and always received, in the morning, one or several s.c. injections of PBS alone or containing 100 µg or 200 µg BV. For experiments using RVV, mice were immunized by two s.c. injections of PBS alone or PBS containing 5 – 50 µg of RVV per injection. On day 21, mice on the C57BL/6 or BALB/c background were challenged s.c. (without anesthesia) by 4 or 5 injections of 200 µg BV or with 2 s.c. injections of 37.5 µg RVV (in back skin). Body temperature was measured with a rectal thermometer immediately before and at various times after challenge.
Measurement of serum antibodies
Serum antibody levels were measured using ELISA (see Supplemental Experimental Procedures for details).
BMCMC generation and analysis of serum for functional IgE
Passive immunization – serum preparation and transfer
Fig. S2A outlines the passive immunization protocol (see Supplemental Experimental Procedures for details). Briefly, for experiments in Figure 3, Figure 6 to 8 week-old female C57BL/6J “donor” mice were immunized by 2 s.c. injections of 200 µg BV or with PBS alone; 3 weeks later, serum antibody levels were measured. Aliquots of pooled serum derived from “donor” mice which had been injected with either PBS or BV are referred to as PBS-serum or BV-serum, respectively. BV-serum was either supplemented with a rat anti-mouse IgE antibody (Amiri et al., 1994; Haak-Frendscho et al., 1998) for at least 30 min at room temperature (anti-IgE BV-serum) or was supplemented with a rat isotype control antibody and left untreated (untreated BV-serum) or was heated 60 min at 56°C (heated BV-serum). PBS-serum also was supplemented with the rat isotype control antibody at the time of the transfer. Aliquots of these modified sera were transfused i.v. into 10 to 11 week-old female C57BL/6 “recipient” mice. The next day, “recipient” mice were challenged with 4 s.c. injections (3× back, 1× belly) of 200 µg BV. We measured body temperature at indicated times and assessed survival over one week.
Flow cytometry
Statistical analysis
Statistical tests were performed using the software GraphPad PRISM 6. Two-tailed Student’s t-test (unpaired), 2-way ANOVA, Chi-square or Mantel-Cox tests were performed as noted in the respective figure legends. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; n.s. = not significant (P > 0.05).
Supplementary Material
Highlights.
Th2 cell immunity can enhance mouse resistance to honeybee or Russell’s viper venoms.
IgE and FcεRI contribute to such acquired increased resistance to honeybee venom.
IgE-associated immune responses can protect the host against noxious substances.
Acknowledgements
We thank Brit B. Turnbull for statistical advice, all members of the Galli lab for discussions, and Chen Liu and Mariola Liebersbach for technical assistance. T.M. is supported by a Belgium American Educational Foundation fellowship and a Marie Curie International Outgoing Fellowship for Career Development: European Union’s Seventh Framework Programme (FP7-PEOPLE-2011-IOF), 299954; P.S. is supported by a Max Kade Fellowship of the Max Kade Foundation and the Austrian Academy of Sciences and a Schroedinger Fellowship of the Austrian Science Fund (FWF): J3399-B21; L.L.R. is supported by fellowships from the French “Fondation pour la Recherche Médicale FRM” and the Stanford Pediatric Research Fund of the Lucile Packard Foundation for Children’s Health and the Stanford CTSA (National Institutes of Health grant UL1 RR025744) and by grant #SPO106496 from the Arthritis National Research Foundation. This work was supported by the German Research Foundation (grant DFG Me 2668/2-1, to M.M.) and by National Institutes of Health grants AI023990, CA072074 and AI070813 (to S.J.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
S.J.G. and M.M. conceived the project. T.M., P.S., M.M., J.K., M.T., and S.J.G. were involved in experimental design. T.M. and P.S. performed most experiments, compiled the data and contributed equally to this work. L.L.R., J.K., and M.M. helped with experiments. All authors participated in analyzing the data and writing or editing the paper.
References
- Akahoshi M, Song CH, Piliponsky AM, Metz M, Guzzetta A, Abrink M, Schlenner SM, Feyerabend TB, Rodewald HR, Pejler G, et al. Mast cell chymase reduces the toxicity of Gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice. J. Clin. Invest. 2011;121:4180–4191. doi: 10.1172/JCI46139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri P, Haak-Frendscho M, Robbins K, McKerrow JH, Stewart T, Jardieu P. Anti-immunoglobulin E treatment decreases worm burden and egg production in Schistosoma mansoni-infected normal and interferon gamma knockout mice. J. Exp. Med. 1994;180:43–51. doi: 10.1084/jem.180.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando A, Martin TR, Galli SJ. Effects of chronic treatment with the c-kit ligand, stem cell factor, on immunoglobulin E-dependent anaphylaxis in mice. Genetically mast cell-deficient Sl/Sld mice acquire anaphylactic responsiveness, but the congenic normal mice do not exhibit augmented responses. J. Clin. Invest. 1993;92:1639–1649. doi: 10.1172/JCI116749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annila I. Bee venom allergy. Clin. Exp. Allergy. 2000;30:1682–1687. doi: 10.1046/j.1365-2222.2000.00885.x. [DOI] [PubMed] [Google Scholar]
- Antonicelli L, Bilo MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr. Opin. Allergy Clin. Immunol. 2002;2:341–346. doi: 10.1097/00130832-200208000-00008. [DOI] [PubMed] [Google Scholar]
- Artis D, Maizels RM, Finkelman FD. Forum: Immunology: Allergy challenged. Nature. 2012;484:458–459. doi: 10.1038/484458a. [DOI] [PubMed] [Google Scholar]
- Bilo BM, Rueff F, Mosbech H, Bonifazi F, Oude-Elberink JN. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339–1349. doi: 10.1111/j.1398-9995.2005.00963.x. [DOI] [PubMed] [Google Scholar]
- Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119:5640–5649. doi: 10.1182/blood-2012-01-380121. [DOI] [PubMed] [Google Scholar]
- Charavejasarn CC, Reisman RE, Arbesman CE. Reactions of anti-bee venom mouse reagins and other antibodies with related antigens. Int. Arch. Allergy Appl. Immunol. 1975;48:691–697. doi: 10.1159/000231356. [DOI] [PubMed] [Google Scholar]
- Finkelman FD. Anaphylaxis: lessons from mouse models. J. Allergy Clin. Immunol. 2007;120:506–515. doi: 10.1016/j.jaci.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF., Jr Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol. Rev. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons CM, Dunne DW. Survival of the fittest: allergology or parasitology? Trends. Parasitol. 2009;25:447–451. doi: 10.1016/j.pt.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat. Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haak-Frendscho M, Saban R, Shields RL, Jardieu PM. Anti-immunoglobulin E antibody treatment blocks histamine release and tissue contraction in sensitized mice. Immunology. 1998;94:115–121. doi: 10.1046/j.1365-2567.1998.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann E. Bee and wasp venoms. Science. 1972;177:314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- Higginbotham RD, Karnella S. The significance of the mast cell response to bee venom. J. Immunol. 1971;106:233–240. [PubMed] [Google Scholar]
- Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat. Rev. Immunol. 2008;8:218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- Ishizaka T, Helm B, Hakimi J, Niebyl J, Ishizaka K, Gould H. Biological properties of a recombinant human immunoglobulin µ-chain fragment. Proc. Natl. Acad. Sci. U S A. 1986;83:8323–8327. doi: 10.1073/pnas.83.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarisch R, Yman L, Boltz A, Sandor I, Janitsch A. IgE antibodies to bee venom, phospholipase A, melittin and wasp venom. Clin. Allergy. 1979;9:535–541. doi: 10.1111/j.1365-2222.1979.tb02518.x. [DOI] [PubMed] [Google Scholar]
- Kawakami T, Kitaura J. Mast cell survival and activation by IgE in the absence of antigen: a consideration of the biologic mechanisms and relevance. J. Immunol. 2005;175:4167–4173. doi: 10.4049/jimmunol.175.7.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinet JP. The high-affinity IgE receptor (FcεRI): from physiology to pathology. Annu. Rev. Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- Lilla JN, Chen CC, Mukai K, BenBarak MJ, Franco CB, Kalesnikoff J, Yu M, Tsai M, Piliponsky AM, Galli SJ. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood. 2011;118:6930–6938. doi: 10.1182/blood-2011-03-343962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiler F, Zumkehr J, Klunker S, Ruckert B, Akdis CA, Akdis M. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J. Exp. Med. 2008;205:2887–2898. doi: 10.1084/jem.20080193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, Tsai M, Galli SJ. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–530. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and FcyRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J. Clin. Invest. 1997;99:901–914. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherje AK, Ghosal SK, Maity CR. Some biochemical properties of Russell’s viper (Daboia russelli) venom from Eastern India: correlation with clinico-pathological manifestation in Russell’s viper bite. Toxicon. 2000;38:163–175. doi: 10.1016/s0041-0101(99)00125-7. [DOI] [PubMed] [Google Scholar]
- Muller UR. Bee venom allergy in beekeepers and their family members. Curr. Opin. Allergy Clin. Immunol. 2005;5:343–347. doi: 10.1097/01.all.0000173783.42906.95. [DOI] [PubMed] [Google Scholar]
- Oettgen HC, Geha RS. IgE in asthma and atopy: cellular and molecular connections. J. Clin. Invest. 1999;104:829–835. doi: 10.1172/JCI8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- Ozdemir C, Kucuksezer UC, Akdis M, Akdis CA. Mechanisms of immunotherapy to wasp and bee venom. Clin. Exp. Allergy. 2011;41:1226–1234. doi: 10.1111/j.1365-2222.2011.03812.x. [DOI] [PubMed] [Google Scholar]
- Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–472. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul WE, Zhu J. How are TH2-type immune responses initiated and amplified? Nat. Rev. Immunol. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawankar R, Canonica GW, Holgate ST, Lockey RF. Allergic diseases and asthma: a major global health concern. Curr. Opin. Allergy Clin. Immunol. 2012;12:39–41. doi: 10.1097/ACI.0b013e32834ec13b. [DOI] [PubMed] [Google Scholar]
- Piliponsky AM, Chen CC, Grimbaldeston MA, Burns-Guydish SM, Hardy J, Kalesnikoff J, Contag CH, Tsai M, Galli SJ. Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW-sh/W-sh mice. Am. J. Pathol. 2010;176:926–938. doi: 10.2353/ajpath.2010.090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portier MM, Richet C. De l’action anaphylactique de certains venims. C. R. Soc. Biol. 1902;54:170–172. [Google Scholar]
- Profet M. The function of allergy: immunological defense against toxins. Quart. Rev. Biol. 1991;66:23–62. doi: 10.1086/417049. [DOI] [PubMed] [Google Scholar]
- Prouvost-Danon A, Binaghi RA, Abadie A. Effect of heating at 56 degrees C on mouse IgE antibodies. Immunochemistry. 1977;14:81–84. doi: 10.1016/0019-2791(77)90284-1. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers AR, Weber M, Muller UR. Are anaphylactic reactions to snake bites immunoglobulin E-mediated? Clin. Exp. Allergy. 2000;30:276–282. doi: 10.1046/j.1365-2222.2000.00697.x. [DOI] [PubMed] [Google Scholar]
- Risch M, Georgieva D, von Bergen M, Jehmlich N, Genov N, Arni RK, Betzel C. Snake venomics of the Siamese Russell’s viper (Daboia russelli siamensis) - relation to pharmacological activities. J. Proteomics. 2009;72:256–269. doi: 10.1016/j.jprot.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Rivera J, Fierro NA, Olivera A, Suzuki R. New insights on mast cell activation via the high affinity receptor for IgE. Adv. Immunol. 2008;98:85–120. doi: 10.1016/S0065-2776(08)00403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saelinger CB, Higginbotham RD. Hypersensitivity responses to bee venom and the mellitin. Int. Arch. Allergy Appl. Immunol. 1974;46:28–37. doi: 10.1159/000231110. [DOI] [PubMed] [Google Scholar]
- Schmidt JO. Toxinology of venoms from the honeybee genus Apis . Toxicon. 1995;33:917–927. doi: 10.1016/0041-0101(95)00011-a. [DOI] [PubMed] [Google Scholar]
- Schneider LA, Schlenner SM, Feyerabend TB, Wunderlin M, Rodewald HR. Molecular mechanism of mast cell mediated innate defense against endothelin and snake venom sarafotoxin. J. Exp. Med. 2007;204:2629–2639. doi: 10.1084/jem.20071262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson ID, Norris RL. Snakes of Medical Importance in India: Is the Concept of the “Big 4” Still Relevant and Useful? Wild. Env. Med. 2007;18:2–9. doi: 10.1580/06-weme-co-023r1.1. [DOI] [PubMed] [Google Scholar]
- Stetson DB, Voehringer D, Grogan JL, Xu M, Reinhardt RL, Scheu S, Kelly BL, Locksley RM. Th2 cells: orchestrating barrier immunity. Adv. Immunol. 2004;83:163–189. doi: 10.1016/S0065-2776(04)83005-0. [DOI] [PubMed] [Google Scholar]
- Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and FcyRIIb cross-linking. J. Clin. Invest. 2006;116:833–841. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theakston RD, Warrell DA, Griffiths E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon. 2003;41:541–557. doi: 10.1016/s0041-0101(02)00393-8. [DOI] [PubMed] [Google Scholar]
- Wadee AA, Rabson AR. Development of specific IgE antibodies after repeated exposure to snake venom. J. Allergy Clin. Immunol. 1987;80:695–698. doi: 10.1016/0091-6749(87)90289-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.