Abstract
Background/Aims
Malnutrition and growth retardation are important issues in treating pediatric Crohn’s disease (CD). Thus, we aimed to investigate the prevalence of various nutritional and growth parameters at the time of diagnosis in Korean children with CD.
Methods
Seventy-one children (<18 years) were enrolled. We analyzed the Z-scores of height-for-age (HAZ), weight-for-height (WHZ), body mass index for age (BMIZ), bone mineral density for age (BMDZ), and the biochemical markers measured at the time of diagnosis.
Results
At diagnosis, HAZ <−2 was observed in three patients (4%), WHZ <−2 in 20 patients (28%), BMIZ <−2 in 19 patients (27%), and BMDZ <−2 in 11 patients (18%). The HAZ was significantly lower in females and patients with extraintestinal manifestations, and the WHZ and BMIZ were significantly lower in patients with stricturing and penetrating disease. Subnormal serum levels were highly prevalent for hemoglobin, albumin, iron, ferritin, calcium, magnesium, folate, vitamin B12, and zinc. There was a significant correlation between nutritional status, growth retardation, and disease activity.
Conclusions
Abnormal nutritional status was highly prevalent in Korean children with CD at the time of diagnosis and was associated with the extent, behavior, and activity of the disease.
Keywords: Crohn’s disease, Pediatrics, Malnutrition, Growth disorder, Bone density
INTRODUCTION
Crohn’s disease (CD) is a chronic inflammatory bowel disease (IBD) that can affect any portion of the gastrointestinal tract from the mouth to the perianal area. The incidence and prevalence of CD in Western countries are higher than in Asian countries. However, the latest studies have reported a gradually increasing incidence of CD in Asian populations.1,2 The incidence of CD in Korean adults has been increasing,3 and it has been suggested that the incidence in children is also increasing.4
Malnutrition and impaired growth are the major complications of pediatric CD, with growth retardation occurring in 15% to 40% of patients.5,6 The etiology of growth retardation in children with CD is multifactorial and poorly understood. Decreased oral intake, malabsorption, increased enteral loss and energy requirements, and increased production of inflammatory cytokines are believed to be the major determinants.7 In pediatrics, growth is integral to quality of life because growth retardation and developmental delays can have devastating psychological consequences in children. Thus, growth and nutrition are key priorities in the management of pediatric CD, and effective disease control can optimize growth potential and pubertal development.8 An accurate evaluation of each patient’s nutritional status is essential for planning the appropriate treatments that can reverse growth retardation. However, a comprehensive picture of the nutritional status of children with CD has not been properly investigated in Korea, which is known as a low-incidence area for CD.
We could not perform a population-based study in Korea, instead we attempted to identify the prevalence of growth retardation and malnutrition in children with CD at a single tertiary center in Korea.
MATERIALS AND METHODS
1. Patients
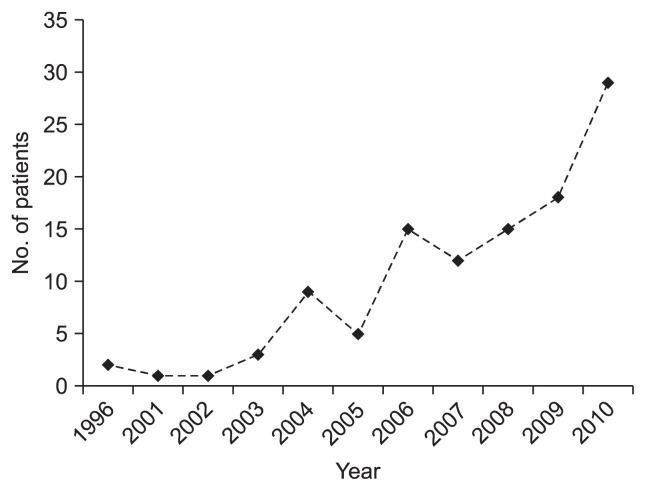
Between January 1996 and March 2011, a total of 123 patients with CD that manifested at <18 years of age were newly registered at the Department of Pediatrics, Asan Medical Center Children’s Hospital, a tertiary medical center in Seoul, Korea (Fig. 1). Fifty-two patients who were referred from primary clinics or other hospitals during the management were excluded. Therefore, 71 patients (51 males and 20 females) who were newly diagnosed and underwent management of definite CD at Asan Medical Center Children’s Hospital were included in this study.
Fig. 1.
Annual number of newly diagnosed pediatric Crohn’s disease (CD) patients at Asan Medical Center between 1996 and 2010. Starting in 2000, we noted that the number of children with CD rapidly increased.
2. CD characteristics and definitions
Data gathered at the time of diagnosis and during follow-up were retrospectively obtained from the medical records of consecutive CD cases. We evaluated baseline demographic and clinical characteristics, including sex, age, symptoms, extraintestinal manifestations, medical history, status of perianal lesions, medications, and disease location, behavior, and activity.
Diagnosis of CD was based on the conventional clinical, radiologic, endoscopic, and histopathologic criteria.9,10 Patients with indeterminate colitis were excluded from this study. Infectious enteritis and colitis were excluded by stool cultures for Salmonella, Shigella, Yersinia, Campylobacter, and Clostridium difficile, stool tests for parasites, and tuberculosis was excluded by the Mantoux skin test, interferon-γ release assays (QuantiFERON®-TB Gold In-Tube; Cellestis Ltd., Carnegie, Australia), acid-fast bacilli staining, culturing, and polymerase chain reaction of tissue samples, and chest X-ray. All the patients underwent colonoscopy, and all the patients with suspected CD underwent esophagogastroduodenoscopy at diagnosis and were studied using more than one radiographic methods (e.g., small bowel series, barium enema, abdominal computed tomography, abdominal ultrasonography, magnetic resonance imaging) and transperineal ultrasonography11 to determine lesion location and behavior according to the Porto criteria.9
After confirming the diagnosis of CD, we classified the disease status according to the Paris Classification.12 CD location and behavior were determined at diagnosis and during follow-up. The presence of any macroscopic abnormality, such as mucosal ulceration, fistula, stricture, or abscess, was considered to indicate regional involvement. The perianal lesions of the CD patients included skin tags, fissures, fistulas, and abscesses. CD activity was assessed at the time of diagnosis according to the Pediatric Crohn’s Disease Activity Index (PCDAI), which is based on symptoms (30%), physical examination (30%), laboratory parameters (20%), and growth data (20%).13 PCDAI scores range from 0 to 100, and were categorized as follows: no disease activity (<10), mild disease activity (11–30), and moderate to severe disease activity (≥30).14
3. Anthropometry and laboratory studies
Growth is the best indicator of nutritional status, and the use of growth curves remains the simplest way to assess nutritional status in children.15 The assessment of growth involves the accurate measurement of height and weight, and these were retrospectively obtained from the medical records. Both height (cm) and weight (kg) were measured to 1 decimal place, and body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. The standard deviation scores (SDS; Z-score) were used to evaluate and compare the anthropometric measurements of CD children of various ages and both sexes. SDS is a standardized value based on data from a normal pediatric population, and it is calculated using values appropriate for the child’s age and sex. Z-score for height-for-age (HAZ), weight-for-height (WHZ), and BMI (BMIZ) after taking into account age and sex were calculated to 2 decimal places for every patient. These deviations were used for between-group comparisons of the patients and the normal pediatric population and references to growth standards for Korean children and adolescents.16 Growth retardation was defined as a Z-score <−2 SD, and severe malnutrition was defined as BMI <−2 SD.15
Bone mineral density (BMD) was measured at the anterior-posterior lumbar spine (L1–4) and at the nondominant femoral neck by dual energy X-ray absorptiometry using Lunar equipment (Lunar Prodigy; GE Healthcare, Madison, WI, USA). BMD results are expressed in g/cm2. Each value was converted to a Z-score (BMDZ), which were determined by subtracting the mean height-, age- and sex-specific BMD values from the measured BMD, and the result was divided by the normative standard deviation.
Laboratory studies included the determination of serum hemoglobin (g/dL), erythrocyte sedimentation rate (mm/hr), albumin (g/dL), iron (μg/dL), calcium (mg/dL), magnesium (mg/dL), folate (ng/mL), and zinc (μg/dL). These biochemical markers were measured at the clinical laboratory of Asan Medical Center Children’s Hospital using standard techniques.
4. Statistics
The continuous variables, such as age and follow-up duration, are expressed as the median values and ranges. Continuous variables, including HAZ, WHZ, BMIZ, and BMDZ, are expressed as the mean±SD. Categorical variables, including sex, disease characteristics, growth retardation rates, and prevalence of subnormal serum levels of the various biochemical markers, are reported as numbers and percentages. Differences between groups of measurements were assessed using the Student t-test or one way ANOVA for normally distributed variables and using the Mann-Whitney U test or the Kruskal-Wallis test for non-normally distributed variables. In addition, subgroup analyses of PCDAI at baseline (moderate to severe ≥30, mild to quiescent <30) and disease activity of CD were performed.
Statistical analyses were performed using SPSS version 14.0 for Windows (SPSS Inc., Chicago, IL, USA), and a p-value <0.05 was considered statistically significant.
RESULTS
1. Demographic characteristics
Seventy-one children <18 years of age with newly diagnosed CD were identified at Asan Medical Center Children’s Hospital. The male to female sex ratio was 2.5:1 (51 boys and 20 girls). The median age at the time of diagnosis was 13 years (range, 0 to 17 years). The median period from symptom onset to diagnosis was 5.5 months (range, 0.7 to 73.1 months). The demographic characteristics of the patients at diagnosis are summarized in Table 1.
Table 1.
Patient Characteristics at the Time of Diagnosis (n=71)
| Characteristic | No. (%) |
|---|---|
| Median age (range), yr | 13 (12–15) |
| Sex | |
| Male | 51 (72) |
| Female | 20 (28) |
| Symptoms | |
| Abdominal pain | 47 (66) |
| Diarrhea | 46 (65) |
| Hematochezia | 22 (31) |
| Weight loss | 40 (56) |
| Anorexia | 40 (56) |
| Extraintestinal manifestations | 14 (20) |
| Oral ulcers | 10 (14) |
| Joint symptoms | 3 (4) |
| Erythema nodosum | 1 (1) |
| Disease behavior | |
| Inflammatory (B1) | 64 (90) |
| Stricturing (B2) | 3 (4) |
| Penetrating (B3) | 3 (4) |
| Both penetrating and stricturing (B2B3) | 1 (1) |
| Disease location | |
| L1 | 12 (17) |
| L2 | 7 (10) |
| L3 | 52 (73) |
| Upper GI (L4) | 38 (51) |
| L4a: upper disease proximal to ligament of Treitz | 24 (34) |
| L4b: upper disease distal to ligament of Treitz and proximal to the distal third of the ileum | 7 (10) |
| L4aL4b | 7 (10) |
| PCDAI | |
| No active disease (<10) | 4 (6) |
| Mild (11–30) | 22 (31) |
| Moderate to severe (≥30) | 45 (63) |
| Perianal lesions | 55 (77) |
| Perianal fistula | 42 (59) |
| Perianal abscess | 10 (14) |
| Perianal fissures | 19 (27) |
| Anal skin tag | 27 (38) |
GI, gastrointestinal; PCDAI, Pediatric Crohn’s Disease Activity Index.
2. Disease characteristics at diagnosis
The common presenting symptoms were abdominal pain (66%), diarrhea (65%), weight loss (56%), anorexia (56%), hematochezia (31%), and extraintestinal manifestations (14%). Disease behavior at diagnosis was inflammatory (B1) in 64 patients (90%), stricturing (B2) in three patients (4%), penetrating (B3) in three patients (4%), and both penetrating and stricturing (B2B3) in one patient. Perianal disease was present in 55 patients (77%), including perianal fistulas in 42 patients (59%), perianal abscesses in 10 patients (14%), perianal fissures in 19 patients (27%), and skin tags in 27 patients (38%).
At the time of diagnosis, 12 patients (17%) had disease located in distal third of the ileum and/or cecum alone (L1), seven patients (10%) had disease in the colon alone (L2), and 52 patients (73%) had disease in both the small bowel and colon (L3). Upper gastrointestinal tract involvement above the distal ileum was observed in 38 patients (54%) and classified as L4a (upper disease proximal to the ligament of Treitz) in 24 patients (34%), L4b (disease distal to ligament of Treitz a proximal to the distal third of the ileum) in seven patients (10%), and both L4a and L4b in seven patients (10%). The PCDAI score at diagnosis was <10 (no activity) in four patients (6%), 10 to 30 (mild activity) in 22 patients (31%), and ≥30 (moderate to severe activity) in 45 patients (63%). The disease characteristics at diagnosis are summarized in Table 1.
3. Assessment of nutritional parameters and growth at the diagnosis of CD
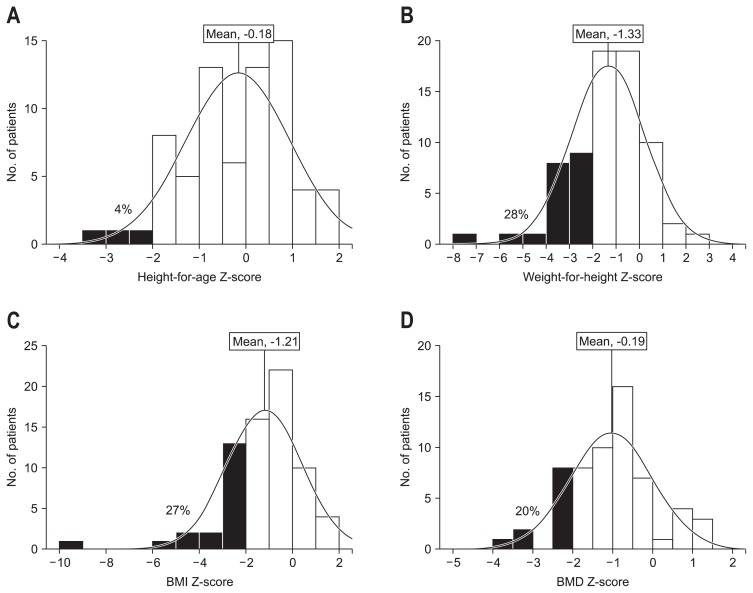
At diagnosis, HAZ (mean, −0.18), WHZ (mean, −1.33), and BMIZ (mean, −1.21) were significantly lower than the referenced general population (p<0.001) (Fig. 2). Three patients (4%) demonstrated growth retardation as defined by HAZ <−2 SD, 20 patients (28%) had WHZ <−2 SD, and 19 patients (27%) had BMIZ <−2 SD (Table 2, Fig. 2). Sixteen patients (23%) were short, as defined by a HAZ <−1 SD, 39 patients (55%) had WHZ <−1 SD, and 35 patients (49%) had BMIZ <−1 SD (Table 2, Fig. 2).
Fig. 2.
Distribution and mean of Z-scores for height-for-age (A), weight-for-height (B), body mass index (BMI) (C), and bone mineral density (BMD) (D) in patients with Crohn’s disease at the time of diagnosis and percentages (in black) of children with values less than −2 standard deviation.
Table 2.
Distribution of the Z-Scores of Various Growth Parameters in Patients with Crohn’s Disease at the Time of Diagnosis
| Anthropometrical data | Z-score <−1 SD | Z-score <−2 SD |
|---|---|---|
| Height-for-age (Z-score) | 16 (23) | 3 (4) |
| Weight-for-height (Z-score) | 39 (55) | 20 (28) |
| Body mass index (Z-score) | 35 (49) | 19 (27) |
Data are presented as number (%).
SD, standard deviation.
Among 71 patients with CD, BMD was measured in 60 patients (47 males and 13 females), and the BMDZ values are shown in Table 3. Females with CD demonstrated significantly lower BMDZ than males with CD (p=0.02). Twenty percent of patients (15% of males and 39% of females) had moderate to severe BMD as defined by a BMDZ ≤−2 SD, and 35% of patients (34% of males and 39% of females) demonstrated mildly decreased BMD (−1.99≤Z-score≤−1 SD). Forty-five percent of patients had BMDZ values within the normal range (>−1 SD).
Table 3.
Z-Scores for the Bone Mineral Density Values Determined Using Dual Energy X-Ray Absorptiometry in Patients with Crohn’s Disease
| All (n=60) | Male (n=47) | Female (n=13) | |
|---|---|---|---|
| Z-score | −1.09±1.04 | −0.92±1.00* | −1.68±1.03* |
| Z-score ≤−2 SD | 12 (20) | 7 (15) | 5 (39) |
| −2< Z-score ≤−1 SD | 21 (35) | 16 (34) | 5 (39) |
| Z-score >−1.0 SD | 27 (45) | 24 (51) | 3 (23) |
Data are presented as mean±SD or number (%).
SD, standard deviation.
p=0.02, significant for the bone mineral density Z-scores grouped by sex.
Table 4 shows the prevalence of subnormal serum levels of the examined biochemical markers. Anemia was observed in 62% and hypoalbuminemia was observed in 61% of patients. Iron deficiency, as determined by the serum iron or transferrin saturation, was observed in 77% and 72% of patients, respectively. In addition, there was a high prevalence of micronutrient deficiencies. Serum calcium, magnesium, folate, and zinc levels of <8.8 mg/dL, <1.8 mg/dL, <5 ng/mL, and <70 μg/dL, respectively, were considered indicative of a deficiency in each the respective micronutrients. About 35%, 10%, 40%, and 51% of CD patients were deficient in calcium, magnesium, folate, and zinc, respectively.
Table 4.
Prevalence of Subnormal Serum Levels of Various Biochemical Markers (n=71)
| Biochemical marker | No. (%) |
|---|---|
| Hemoglobin* | 44/71 (62) |
| Albumin (<3.5 g/dL) | 43/70 (61) |
| Iron (<50 μg/dL) | 49/64 (77) |
| Transferrin saturation (<16%) | 46/64 (72) |
| Ferritin (<100 μg/L) | 45/65 (69) |
| Calcium (<8.8 mg/dL) | 24/68 (35) |
| Magnesium (<1.8 mg/dL) | 6/61 (10) |
| Folate (<5 ng/mL) | 25/63 (40) |
| Vitamin B12 (<211 pg/mL) | 1/62 (2) |
| Zinc (<70 μg/dL) | 26/51 (51) |
Hemoglobin levels used to define anemia: children 6 months to 5 years, 11.0 g/dL; children 5 to 11 years, 11.5 g/dL; children 12 to 13 years, 12.0 g/dL; men, 13.0 g/dL; nonpregnant women, 12.0 g/dL.
4. Predictive factors of nutritional parameters and growth
The influences of the various demographic and clinical parameters on height-for-age, weight-for-height, and BMI on the diagnosis of CD in 71 children are shown in Table 5. HAZ was significantly lower in females (p=0.002), whereas WHZ and BMIZ were not affected by sex. There were no significant differences in HAZ, WHZ, and BMIZ between the two age groups according to the Paris classification at diagnosis (age, <10 years [A1a] vs ≥10 years [A1b and A2]). WHZ and BMIZ were significantly lower in patients with stricturing and penetrating diseases (p=0.015 and p=0.016, respectively). There were no significant differences in HAZ, WHZ, and BMIZ in terms of disease location or upper gastrointestinal location. HAZ was significantly lower in the patients with extraintestinal manifestations (p=0.039).
Table 5.
Influence of Demographic and Clinical Parameters on Height, Weight, and Body Mass Index at the Time of Diagnosis (Expressed as the Mean Z-scores) in 71 Children Diagnosed with Crohn’s Disease
| HAZ | p-value | WHZ | p-value | BMIZ | p-value | |
|---|---|---|---|---|---|---|
| Sex | 0.002 | 0.136 | 0.085 | |||
| Male (n=51) | 0.09 | −1.15 | −1.00 | |||
| Female (n=20) | −0.88 | −1.79 | −1.75 | |||
| Age, yr | 0.274 | 0.772 | 0.291 | |||
| A1a, ≤10 (n=6) | −0.67 | −1.52 | −0.52 | |||
| A1bA2, 10–17 (n=65) | −1.39 | −1.31 | −1.27 | |||
| Disease behavior at diagnosis | 0.327 | 0.015 | 0.016 | |||
| B1 inflammatory (n=64) | −0.20 | −1.11 | −1.02 | |||
| B2 stricturing (n=3) | 0.46 | −4.63 | −3.78 | |||
| B3 penetrating (n=3) | 0.12 | −1.95 | −1.70 | |||
| B2B3 both penetrating and stricturing (n=1) | −1.84 | −3.62 | −4.22 | |||
| Disease location at diagnosis | 0.359 | 0.867 | 0.808 | |||
| L1 (n=12) | 0.09 | −1.53 | −1.27 | |||
| L2 (n=7) | −0.68 | −1.13 | −0.82 | |||
| L3 (n=52) | −0.18 | −1.31 | −1.25 | |||
| Upper GI location at diagnosis | 0.730 | 0.970 | 0.868 | |||
| Presence (n=38) | −0.14 | −1.32 | −1.24 | |||
| Absence (n=33) | −0.23 | −1.34 | −1.17 | |||
| Extraintestinal manifestations at diagnosis | 0.039 | 0.619 | 0.817 | |||
| Presence (n=14) | −0.91 | −1.14 | −1.04 | |||
| Absence (n=57) | −0.01 | −1.38 | −1.25 |
HAZ, height-for-age Z-score; WHZ, weight-for-height Z-score; BMIZ, body mass index Z-score; GI, gastrointestinal.
Nutritional parameters were more severely impaired in patients with moderate to severe disease. Moderate to severe CD demonstrated lower anthropometric and laboratory data compared with mild to quiescent CD (Table 6). Children with severe to moderate CD demonstrated significantly lower HAZ, WHZ, and BMIZ values than patients with mild CD.
Table 6.
Correlation between Various Parameters and Activity of Crohn’s Disease
| PCDAI ≤ 30 | PCDAI >30 | p-value | |
|---|---|---|---|
| Anthropometrical data | |||
| Height-for-age (Z-score) | 0.21 | −0.40 | 0.029 |
| Weight-for-height (Z-score) | −0.69 | −1.68 | 0.001 |
| BMI (Z-score) | −0.56 | −1.56 | 0.002 |
| Laboratory data | |||
| Hemoglobin, g/dL | 12.80 | 11.10 | <0.001 |
| Albumin, g/dL | 3.90 | 3.00 | <0.001 |
| Iron, μg/dL | 55.20 | 24.60 | <0.001 |
| Calcium, mg/dL | 9.10 | 8.70 | 0.001 |
| Magnesium, mg/dL | 2.30 | 2.10 | 0.005 |
| Folate, ng/mL | 8.70 | 5.90 | 0.018 |
| Zinc, μg/dL | 80.20 | 67.90 | 0.030 |
PCDAI, Pediatric Crohn’s Disease Activity Index; BMI, body mass index.
According to the analysis of the laboratory markers and disease location, serum iron and albumin are significantly lower patients with disease in L3 versus L1 or L2 (p=0.004 and p=0.007, respectively).
DISCUSSION
This study showed that the growth retardation and malnutrition are highly present at time of diagnosis in Korea pediatric CD patients. Twenty percent of children presented with severely low BMD, and about 35%, 10%, 40%, and 51% of children were deficient in calcium, magnesium, folate, and zinc, respectively. The Korean National Growth Charts (2007) were used as a reference for height-for-age, weight-for-height, and BMI. The Korean National Growth Charts are especially relevant when assessing Korean ethnic groups.17
The proportions of children with severe malnutrition and growth retardation at diagnosis were lower than the rates of 19% to 23% that had been previously reported,5,18 possibly due to the shorter interval to diagnosis from the onset of symptom that resulted from more effective diagnoses. The previous reports are originated from foreign country, therefore, there would be the limitations on direct comparison actually.5,18 In this study, the median interval from symptom onset to diagnosis was 5.5 months (range, 0.7 to 73.1 months), whereas the median diagnostic delay was 11.7 months in the previous study.18 Likewise, an earlier study in Korean children reported a high incidence of growth retardation (58% in CD patients), which was defined as being at <3 SD from the mean growth percentile when measured at 18-month intervals.19
Sawczenko and Sandhu20 reported that there is a significant negative correlation between the length of any developmental delays and height in CD patients, suggesting that earlier diagnosis might minimize growth retardation by rectifying the growth-inhibiting effects of malnutrition and inflammatory cytokines. Therefore, early diagnosis prevents abnormal growth, thus reducing the duration of symptoms before treatment, which is an important goal for improving treatment.
There was a high prevalence of anemia and malnutrition, including micronutrient deficiencies. Anemia in IBD patients can be the result of multiple causes, with iron deficiency being the most prevalent due to dietary restrictions, malabsorption, or intestinal bleeding.21 Approximately 30% to 80% of patients with CD develop anemia.22 In this study, 62% of the patients were diagnosed with anemia, similar to previous studies.23,24 The diagnostic criteria for iron deficiency depend on the level of inflammation, but appropriate criteria are typically serum ferritin <100 μg/L or transferrin saturation <16% with the presence of inflammation.21 Using this definition, which was agreed upon by the international IBD Working Group, about 70% of our patients presented with iron deficiency at diagnosis. However, the results of this study may overestimate or underestimate the true prevalence of iron deficiency. More extensive evaluation of anemia, such as erthrocyte zinc protoporphyrin or tranferrin receptor testing, may be beneficial. There are several mechanisms that may precipitate malnutrition in patients with CD.25 Decreased nutrient intake, malabsorption, increased nutrient loss, and increased energy requirements are several causes of malnutrition. In addition, diarrhea causes the loss of electrolytes, including calcium, magnesium, and zinc. Magnesium deficiency is common and related to gastrointestinal loss and inadequate intake.26 Zinc loss is correlated with the volume of diarrhea, and the loss of zinc increases with steatorrhea, as are the losses of other divalent cations (e.g., calcium, magnesium, copper) and fat soluble vitamins.25 Serum vitamin D and vitamin B6 were not measured in this study, but these may be valuable parameters to measure when assessing CD patients in future research.
It is likely that the extent of disease and intensity of inflammation have deleterious influences on growth and nutritional status.27 One important observation of this study was the association between stricturing and penetrating disease and reduced weight-for-height and BMI at diagnosis. In addition, most of the nutritional and growth parameters were more severely impaired in patients with moderate to severe activity compared with patients with mild activity. The presence of extraintestinal manifestations at diagnosis was associated with low height. The inflammatory process presumably plays important deleterious roles against growth.28 Complex interactions exist between nutritional status, growth retardation, and inflammation. The growth-inhibiting effects of the proinflammatory cytokines released from the inflamed intestines and chronically inadequate nutritional intake may cause a variety of responses that affect growth. Decreased circulating insulin-like growth factor 1 is found in active CD patients.29 Tumor necrosis factor-α appears to play a pivotal role in cytokine-mediated growth retardation and can affect growth through multiple pathways, including anorexia, loss of skeletal muscle, and cachexia.30 Because inflammation is closely associated with growth failure, tight control of the inflammatory process appears to be of paramount importance when treating pediatric CD. In this study, the disease location did not seem to influence growth or nutritional status, although a recent study by Sawczenko et al.31 reported that linear growth is associated with the site of disease. In their study, they noticed that involvement of the ileum and jejunum is associated with being at high risk for growth retardation.
The growth patterns observed during childhood and adolescence are different between males and females.32 In our study, females demonstrated impaired linear growth (height) and lower BMD at diagnosis compared with males. In healthy children, the average age at peak height velocity is 11.5 years in females, whereas in males the average age is 13.5 years.32 The mean age at diagnosis for females in our study was 11.4 years; 13.2 years was the mean age for males. Although the Tanner stage at diagnosis was not determined, both sexes tended to develop CD just before the average age at peak height velocity. Children who acquire CD just before the reaching peak height velocity may be at greater risk for impaired linear growth and warrant special attention to growth issues during the treatment of CD. Therefore, the time of onset of CD in relation to age at peak height velocity may, in part, explain the sex differences regarding the disease’s impact on growth. However, in the present study, the assessment of puberty at diagnosis was only available for a minority of patients. Because pubertal status was not taken into account in this study, the differences between “prepubertal” and “pubertal” effects on growth cannot be stated with accuracy.
To the best of our knowledge, this study is the first to examine the growth and nutritional status of children with CD in Korea by documenting the effects of this disease on growth at diagnosis and prior to the initiation of corticosteroid therapy and nutritional supplementation. Therefore, the growth retardation and nutritional deficits seen in the CD patients in this study reflect the cumulative effects of inflammation, malnutrition/malabsorption, and pubertal delays.
This study has some limitations, including the lack of Tanner stage evaluations and dietary and physical data. In addition, the levels of various inflammatory cytokines, such as tumor necrosis factor-α, are not available even though they may correlate with both growth and nutritional status. Future studies are needed that measure resting energy expenditure, physical activity, dietary intake, and growth hormones in order to identify potential etiologies. Although this study was based on the experiences of a single hospital, differences from hospital-based studies in Western countries are probably due to variations in the health delivery systems. In Korea, every hospital has its own outpatient clinic, and patients can visit the clinics of tertiary referral centers without being referred by their primary physicians. Furthermore, we analyzed data only from patients who were first diagnosed at our hospital, excluding those referred by other physicians.
In conclusion, malnutrition and growth retardation are generally present at the time of diagnosis in Korean children with CD. It is likely that extent of disease, behavior, and activity have deleterious influences on growth and nutritional status. One cannot address growth failure if it is not recognized, and early detection is key. Therefore, in clinical practice, growth should be taken into account as an important criterion for the management of CD in children and should be considered as an important marker of therapeutic efficiency. Pediatric patients with CD should have height, weight, and BMI measured and plotted on a growth chart at each visit. The physical exam should include above growth parameters as well as Tanner stage, and physical signs of malnutrition.
Future treatment strategies must be developed to control the inflammatory process during childhood in order to minimize its long-term deleterious consequences on growth and nutritional status. Additionally, future prospective population-based studies are necessary and must be performed at the national level.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Leong RW, Lau JY, Sung JJ. The epidemiology and phenotype of Crohn’s disease in the Chinese population. Inflamm Bowel Dis. 2004;10:646–651. doi: 10.1097/00054725-200409000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Thia KT, Loftus EV, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008;103:3167–3182. doi: 10.1111/j.1572-0241.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- 3.Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986–2005: a KASID study. Inflamm Bowel Dis. 2008;14:542–549. doi: 10.1002/ibd.20310. [DOI] [PubMed] [Google Scholar]
- 4.Kim BJ, Song SM, Kim KM, et al. Characteristics and trends in the incidence of inflammatory bowel disease in Korean children: a single-center experience. Dig Dis Sci. 2010;55:1989–1995. doi: 10.1007/s10620-009-0963-5. [DOI] [PubMed] [Google Scholar]
- 5.Motil KJ, Grand RJ, Davis-Kraft L, Ferlic LL, Smith EO. Growth failure in children with inflammatory bowel disease: a prospective study. Gastroenterology. 1993;105:681–691. doi: 10.1016/0016-5085(93)90883-e. [DOI] [PubMed] [Google Scholar]
- 6.Markowitz J, Grancher K, Rosa J, Aiges H, Daum F. Growth failure in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1993;16:373–380. doi: 10.1097/00005176-199305000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Dieleman LA, Heizer WD. Nutritional issues in inflammatory bowel disease. Gastroenterol Clin North Am. 1998;27:435–451. doi: 10.1016/S0889-8553(05)70012-1. [DOI] [PubMed] [Google Scholar]
- 8.Heuschkel R, Salvestrini C, Beattie RM, Hildebrand H, Walters T, Griffiths A. Guidelines for the management of growth failure in childhood inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:839–849. doi: 10.1002/ibd.20378. [DOI] [PubMed] [Google Scholar]
- 9.Escher JC, Dias JA, Bochenek K, et al. Inflammatory bowel disease in children and adolescents: recommendations for diagnosis: the Porto criteria. J Pediatr Gastroenterol Nutr. 2005;41:1–7. doi: 10.1097/01.MPG.0000163736.30261.82. [DOI] [PubMed] [Google Scholar]
- 10.Bousvaros A, Antonioli DA, Colletti RB, et al. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J Pediatr Gastroenterol Nutr. 2007;44:653–674. doi: 10.1097/MPG.0b013e31805563f3. [DOI] [PubMed] [Google Scholar]
- 11.Maconi G, Ardizzone S, Greco S, Radice E, Bezzio C, Bianchi Porro G. Transperineal ultrasound in the detection of perianal and rectovaginal fistulae in Crohn’s disease. Am J Gastroenterol. 2007;102:2214–2219. doi: 10.1111/j.1572-0241.2007.01441.x. [DOI] [PubMed] [Google Scholar]
- 12.Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314–1321. doi: 10.1002/ibd.21493. [DOI] [PubMed] [Google Scholar]
- 13.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439–447. doi: 10.1097/00005176-199105000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Hyams J, Markowitz J, Otley A, et al. Evaluation of the pediatric Crohn disease activity index: a prospective multicenter experience. J Pediatr Gastroenterol Nutr. 2005;41:416–421. doi: 10.1097/01.mpg.0000183350.46795.42. [DOI] [PubMed] [Google Scholar]
- 15.Joosten KF, Hulst JM. Malnutrition in pediatric hospital patients: current issues. Nutrition. 2011;27:133–137. doi: 10.1016/j.nut.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Aiges H, Markowitz J, Rosa J, Daum F. Home nocturnal supplemental nasogastric feedings in growth-retarded adolescents with Crohn’s disease. Gastroenterology. 1989;97:905–910. doi: 10.1016/0016-5085(89)91496-0. [DOI] [PubMed] [Google Scholar]
- 17.Moon JS, Lee SY, Nam CM, et al. 2007 Korean National Growth Charts: review of developmental process and an outlook. Korean J Pediatr. 2008;51:1–25. doi: 10.3345/kjp.2008.51.1.1. [DOI] [Google Scholar]
- 18.Spray C, Debelle GD, Murphy MS. Current diagnosis, management and morbidity in paediatric inflammatory bowel disease. Acta Paediatr. 2001;90:400–405. doi: 10.1111/j.1651-2227.2001.tb00439.x. [DOI] [PubMed] [Google Scholar]
- 19.Seo JK, Yeon KM, Chi JG. Inflammatory bowel disease in children: clinical, endoscopic, radiologic and histopathologic investigation. J Korean Med Sci. 1992;7:221–235. doi: 10.3346/jkms.1992.7.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawczenko A, Sandhu BK. Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch Dis Child. 2003;88:995–1000. doi: 10.1136/adc.88.11.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gasche C, Berstad A, Befrits R, et al. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1545–1553. doi: 10.1002/ibd.20285. [DOI] [PubMed] [Google Scholar]
- 22.Semrin G, Fishman DS, Bousvaros A, et al. Impaired intestinal iron absorption in Crohn’s disease correlates with disease activity and markers of inflammation. Inflamm Bowel Dis. 2006;12:1101–1106. doi: 10.1097/01.mib.0000235097.86360.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson A, Reyes E, Ofman J. Prevalence and outcomes of anemia in inflammatory bowel disease: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):44S–49S. doi: 10.1016/j.amjmed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Burbige EJ, Huang SH, Bayless TM. Clinical manifestations of Crohn’s disease in children and adolescents. Pediatrics. 1975;55:866–871. [PubMed] [Google Scholar]
- 25.Shamir R, Phillip M, Levine A. Growth retardation in pediatric Crohn’s disease: pathogenesis and interventions. Inflamm Bowel Dis. 2007;13:620–628. doi: 10.1002/ibd.20115. [DOI] [PubMed] [Google Scholar]
- 26.Galland L. Magnesium and inflammatory bowel disease. Magnesium. 1988;7:78–83. [PubMed] [Google Scholar]
- 27.Sawczenko A, Azooz O, Paraszczuk J, et al. Intestinal inflammation-induced growth retardation acts through IL-6 in rats and depends on the −174 IL-6 G/C polymorphism in children. Proc Natl Acad Sci U S A. 2005;102:13260–13265. doi: 10.1073/pnas.0503589102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanof ME, Lake AM, Bayless TM. Decreased height velocity in children and adolescents before the diagnosis of Crohn’s disease. Gastroenterology. 1988;95:1523–1527. doi: 10.1016/s0016-5085(88)80072-6. [DOI] [PubMed] [Google Scholar]
- 29.Corkins MR, Gohil AD, Fitzgerald JF. The insulin-like growth factor axis in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2003;36:228–234. doi: 10.1097/00005176-200302000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Ladner KJ, Caligiuri MA, Guttridge DC. Tumor necrosis factor-regulated biphasic activation of NF-kappa B is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem. 2003;278:2294–2303. doi: 10.1074/jbc.M207129200. [DOI] [PubMed] [Google Scholar]
- 31.Sawczenko A, Ballinger AB, Savage MO, Sanderson IR. Clinical features affecting final adult height in patients with pediatric-onset Crohn’s disease. Pediatrics. 2006;118:124–129. doi: 10.1542/peds.2005-2931. [DOI] [PubMed] [Google Scholar]
- 32.Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107:317–329. doi: 10.1016/S0022-3476(85)80501-1. [DOI] [PubMed] [Google Scholar]