Abstract
Background/Aims
We tried to investigate the expression characteristics of KAI1, a suppressor of wide-spectrum tumor metastasis, and vascular endothelial growth factor (VEGF), the most common angiogenesis factor, and then to analyze their diagnostic value for hepatocellular carcinoma (HCC).
Methods
The protein and mRNA expression levels of KAI1 or VEGF in HCC tissues and in self-controlled para-carcinoma tissues were analyzed by Western blot and real-time polymerase chain reaction, respectively. Serum levels of KAI1 and VEGF in the patients with HCC, benign liver disease or in healthy controls were quantitatively detected by enzyme-linked immunosorbent assay.
Results
The expression level of KAI1 was downregulated, while the expression level of VEGF was upregulated in the tissues or serum of the patients with HCC. The expression level of serum KAI1 in HCC patients was correlated with TNM staging, intrahepatic metastasis, lymph node or peritoneal metastasis, and portal vein thrombus. In addition to the factors that were correlated with KAI1 expression, VEGF expression was also closely related to the α-fetoprotein level of the patients. The area under the receiver operating characteristic curve for the diagnosis of HCC was 0.907 for KAI1 and 0.779 for VEGF. The sensitivity of serum KAI1 levels in the diagnosis of HCC was 86.96%; the accuracy was 83.06%, while the sensitivity, the accuracy and the negative predictive value were improved to 91.86%, 84.68%, and 78.79% according to the combined detection of KAI1 and VEGF, respectively.
Conclusions
A combined detection of KAI1 and VEGF may greatly improve the efficiency of diagnosis and form a reliable panel of diagnostic markers for HCC.
Keywords: KAI1, Vascular endothelial growth factor, Hepatocellular carcinoma, Clinicopathological characteristics, Diagnostic value
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most aggressive types of cancer worldwide and associated with high mortality and poor prognosis.1 Because of the obscure pathogenesis and rapid intrahepatic and extrahepatic metastasis, most HCC patients cannot be diagnosed until advanced stage, leading to a poor prognosis for this disorder. Metastasis and recurrence are two major determinants for the prognosis of HCC patients. Its early detection and treatment are an effective way to improve patients survival.2 Detection of circulating markers is the most effective method because of its easy to operate, accuracy and low price, but no ideal biomarker has been found so far.3
The KAI1 gene (Kangai1) was originally identified as a gene located on human chromosome 11p11.2. KAI1, also named as CD82, belongs to tetraspanin superfamily of transmembrane proteins, responsible for the specific inhibition of tumor metastasis.4 In malignant solid tumors, the presence of KAI1 predicts a better prognosis for cancer patients, and downregulation or loss of KAI1 expression is usually found in the clinically advanced stage.5 Decreased KAI1 expression is a useful marker for metastatic/invasive potential in a series of human tumor types,6 especially HCC.7
Tumor aggressiveness is mediated by angiogenic factors such as vascular endothelial growth factor (VEGF), which is a major factor involved in aggressive tumor behavior.8 Previous studies have shown that high serum VEGF levels predicted poor survival in HCC patients treated with hepatic resection, radiofrequency ablation, or transcatheter arterial chemoembolization.9,10
However, the clinicopathologic features of serum KAI1 and VEGF expression, and their diagnostic value for HCC have been unknown up to now. In this study, the expression levels of KAI1 and VEGF in serum and hepatic tissues of the patients with HCC were investigated and compared with those with benign liver diseases to evaluate diagnostic value for HCC.
MATERIALS AND METHODS
1. Collection of serum samples
We evaluated 86 HCC patients (47 men and 39 women) who were treated at The Third Affiliated Hospital of Wenzhou Medical College, Wenzhou, China. The patients’ ages ranged from 31 to 86 years (median, 46.7 years). All the HCC cases were confirmed by surgery and pathology.11 The other serum samples (38 cases of cirrhosis, 36 cases of hepatitis and 30 cases of healthy people) were collected at the same time in the hospital. All cases were diagnosed by biochemical tests, viral histology, and B-ultrasonic examination. About 3 mL of blood were collected with heparin in the morning and separated sera at once. α-Fetoprotein (AFP) level was detected by radiological method.12
2. Collection of liver specimens
The cancerous-, the self-matched adjacent cancerous (more than 3 cm to cancer focus), and the distant cancerous (more than 5 cm) specimens after surgical operation were respectively taken from 30 HCC patients who were treated at The Third Affiliated Hospital of Wenzhou Medical College, Wenzhou, China. The collected specimen was immediately frozen in liquid nitrogen for total RNA extraction and Western blotting. The diagnosis of HCC was based on the criteria proposed by the Ministry of Health of the People’s Republic of China.11 Prior written informed consent was obtained from all patients according to the World Medical Association Declaration of Helsinki, and the study received ethics board approval from The Third Affiliated Hospital of Wenzhou Medical College, Wenzhou, China.
3. Enzyme-linked immunosorbent assay
The level of serum KAI1 was detected by a human KAI1 enzyme-linked immunosorbent assay (ELISA) Kit (Cusabio Biotech Co., Ltd., Wuhan, China), and the level of serum VEGF was detected by a human VEGF ELISA kit (Cusabio Biotech Co., Ltd.) according to the manufacturer’s instructions. One hundred microliter of serum samples or standard were separately added into each well, and then 100 μL of Biotin-antibody was added and incubated for 1 hour at 37°C. Subsequently, 100 μL of HRP-avidin was added and incubated for 1 hour at 37°C. Then, 90 μL of TMB substrate was added and incubated for 25 minutes at 37°C. Finally, 50 μL of stop solution was added to each well, and absorbance was read at 450 nm. During the procedure, washing the plate was according to the ELISA routine method.
4. Total RNA isolation and synthesis of cDNA
Total RNA was isolated from 50 mg of liver tissue, using Trizol reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. The integrity of the total RNA was examined by 1% agarose gel electrophoresis, the quantity was determined based on absorbance at 260 nm (A260), and the purity was analyzed based on the absorbance ratio at 260 and 280 nm (A260/280) (Bio-Rad SmartspecTM plus; Bio-Rad Laboratories Inc., Hercules, CA, USA). cDNA was synthesized from 1 μg of total RNA using First Strand cDNA Synthesis Kit (Fermentas, Burlington, Canada) according to the manufacturer’s instructions.
5. Real-time quantitative polymerase chain reaction
The real-time quantitative polymerase chain reaction (qPCR) was run on an Applied Biosystems SteponeTM real-time PCR system (Life Technologies) according to the manufacturer’s recommendations. The reaction solution contained 25 μL 2×SYBR Premix Ex Taq (TaKaRa, Tokyo, Japan), 2 μL primer mix, 1 μL 50×ROX Reference Dye I, 4 μL cDNA, and 18 μL deionized water to make a total volume of 50 μL. Primers were as follows: KAI1 forward, 5′-CGGCACAAGCAGATGGACAGG-3′, and reverse, 5′-CGGCAACAGGACCCAGAGTG-3′;13 VEGF forward, 5′-CTCTACCTCCACCATGCCAAGT-3′, and reverse, 5′-TGATTCT-GCCCTCCTCCTTCT-3′;14 glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward, 5′-GGTGGTCTCCTCTGACTTCAAC-3′, and reverse, 5′-TCTCTCTTCCTCTTGTGTTCTTG-3′.13 GAPDH was used as an internal control, while no template control (H2O) was included in each reaction run. The optimized PCR conditions were as follows: 1 cycle at 95°C for 2 minutes; 40 cycles of 95°C for 10 seconds, 62°C for 1 minute and final extension at 60°C for 15 seconds. The relative quantitative analysis was performed by comparison of the 2−ΔΔCt values.
6. Western blotting
Liver tissues were homogenized in an ice-cold homogenization buffer containing many protease inhibitors. The homogenates were centrifuged at 800 g for 10 minutes at 4°C. The supernatants were collected, and total protein concentrations were determined by an enhanced bicinchoninic acid (BCA) protein assay kit (Beyotime Institute of Biotechnology, Haimen, China). Subsequently, western blotting was performed as described by Zhang et al.,12 with antibodies to 1) KAI1 (1:500, rabbit polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA, USA); 2) VEGF (1:500, rabbit polyclonal; Santa Cruz Biotechnology) and GAPDH (1:500, goat polyclonal; Santa Cruz Biotechnology). Immunoreactive bands were then subject to densitometry using ImageJ 2.1.4.7 software.
7. Statistical analysis
The data are expressed as mean±standard deviation. Differences between different groups were evaluated by using a Student t-test. Receiver operating characteristic (ROC) curves were constructed by calculating the sensitivities and specificities at several cutoff points. A p-value of less than 0.05 was considered to be statistically significant.
RESULTS
1. Expression level of KAI1 and VEGF in HCC tissues
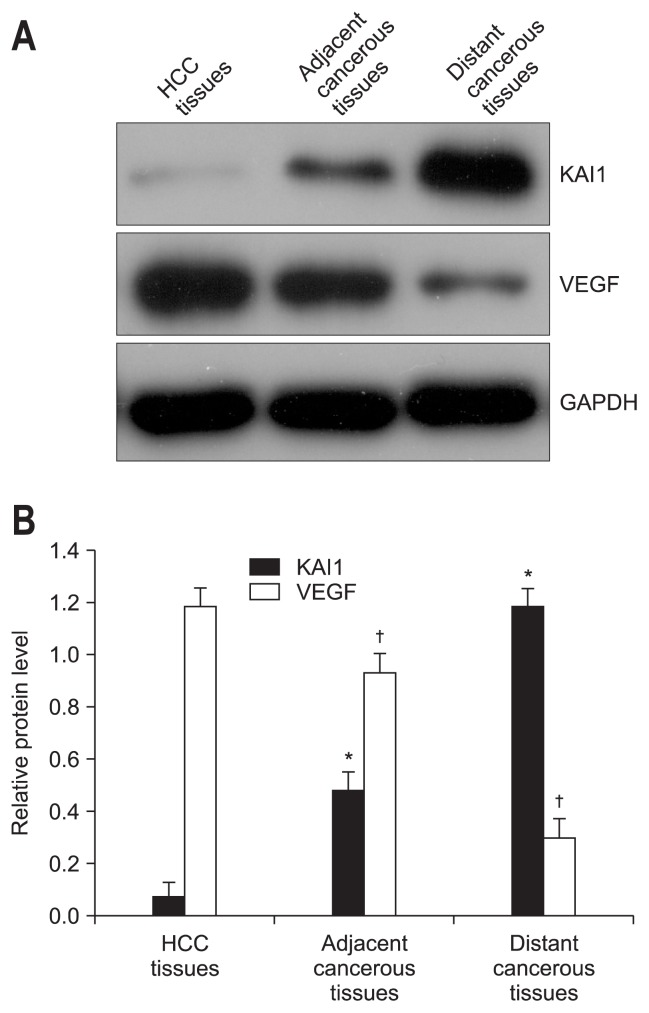
To analyse the expression levels of KAI1 and VEGF in 30 self-controlled HCC tissues, their matched adjacent- and distant-cancerous specimens, Western blotting and real-time PCR were carried out to detect their protein expression and mRNA levels, respectively. As shown in Fig. 1, the expression levels of KAI1 was obviously lower in HCC tissues than that in the self-controlled adjacent- and distant-cancerous specimens (p<0.001), while the expression levels of VEGF in HCC tissues, inversely, was distinctly higher than that in the self-controlled pericancerous tissues (p<0.001). The relative quantitative PCR analysis (Table 1) indicated that the level of KAI1 mRNA expression in the HCC tissues (2−ΔΔCt=0.078±0.037) was significantly lower (p<0.001) than that in the matched adjacent cancerous tissues (2−ΔΔCt=0.485±0.070) or the distant cancerous tissues (2−ΔΔCt=1.185±0.069), respectively. Similarly, the level of VEGF mRNA expression in the HCC tissues (2−ΔΔCt=1.192±0.067) was visibly higher (p<0.001) than that in the matched adjacent cancerous tissues (2−ΔΔCt=0.933±0.066) or the distant cancerous tissues (2−ΔΔCt=0.299±0.050). These results suggested that the expression of KAI1 was upregulated, while the level of VEGF was downregulated in HCC tissues, whether protein level or mRNA level.
Fig. 1.
The levels of KAI1 and vascular endothelial growth factor (VEGF) in liver tissues of individuals diagnosed with hepatocellular carcinoma (HCC). (A) Representative images of the Western blot. The levels of KAI1 and VEGF were detected, while β-actin served as the control. Three independent experiments were conducted, and the results are given as the mean±standard deviation. (B) The statistical results indicated that the expression level of KAI1 was significantly downregulated, while that of VEGF was strikingly upregulated in HCC tissues compared with the matched adjacent or distant cancerous tissues (p<0.05).
GAPDH, glyceraldehyde-3-phosphate dehydrogenase. *Versus the level of KAI1 in HCC tissues; †Versus the level of VEGF in HCC tissues.
Table 1.
Relative mRNA Levels of KAI1 and Vascular Endothelial Growth Factor in Hepatocellular Carcinoma, Adjacent-, and Distant-Cancerous Tissues
| Group | No. | KAI1 mRNA | VEGF mRNA | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 2−ΔΔCt | t | p-value | 2−ΔΔCt | t | p-value | ||
| HCC tissues | 30 | 0.078±0.037 | 1.192±0.067 | ||||
| Adjacent cancerous tissues | 30 | 0.485±0.070 | 28.155* | <0.001* | 0.933±0.066 | 15.084† | <0.001† |
| Distant cancerous tissues | 30 | 1.185±0.069 | 77.4423* | <0.001* | 0.299±0.050 | 58.507† | <0.001† |
VEGF, vascular endothelial growth factor; HCC, hepatocellular carcinoma.
Versus the level of KAI1 mRNA in HCC tissues;
Versus the level of VEGF mRNA in HCC tissues.
2. Serum KAI1 and VEGF level in patients with liver diseases
To investigate the expression levels of serum KAI1 and VEGF in 160 patients with HCC or benign liver disease, the levels of KAI1 and VEGF in peripheral blood were detected by ELISA analysis. As shown in Table 2, the expression level of serum KAI1 in HCC patients (45.391±6.376 pg/mL) was significantly lower (p<0.001) than that in the patients with liver cirrhosis (197.365±53.697 pg/mL), or hepatitis (221.597±49.768 pg/ mL), or control subjects (315.164±61.254 pg/mL), respectively. Inversely, the expression level of serum VEGF in HCC patients (276.894±73.547 pg/mL) was evidently higher (p<0.001) than that of liver cirrhosis (156.473±60.034 pg/mL), or hepatitis (97.394±21.765 pg/mL), or control subjects (54.368±9.847 pg/ mL), respectively.
Table 2.
Levels of Serum KAI1 and Vascular Endothelial Growth Factor in 160 Patients with Liver Diseases
| Group | No. | KAI1 | VEGF | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| χ̄ ±SD, pg/mL | t | p-value | χ̄ ±SD, pg/mL | t | p-value | ||
| HCC | 86 | 45.391±6.376 | 276.894±73.547 | ||||
| Cirrhosis | 38 | 197.365±53.697 | 25.966* | <0.001* | 156.473±60.034 | 8.866† | <0.001† |
| Hepatitis | 36 | 221.597±49.768 | 32.386* | <0.001* | 97.394±21.765 | 14.352† | <0.001† |
| Normal control | 30 | 315.164±61.254 | 40.542* | <0.001* | 54.368±9.847 | 16.475† | <0.001† |
VEGF, vascular endothelial growth factor; SD, standard deviation; HCC, hepatocellular carcinoma.
Versus the level of serum KAI1 in the HCC group;
Versus the level of serum VEGF in the HCC group.
3. Clinicopathologic features of serum KAI1 and VEGF expression in HCC patients
To study the clinicopathologic features of circulating KAI1 and VEGF expression in 86 HCC patients, the expression levels of serum KAI1 and VEGF were separately analyzed according to several clinical pathological parameters. As shown in Table 3, the expression level of serum KAI1 in HCC patients was correlated with TNM staging, intrahepatic metastasis, lymph node or peritoneal metastasis, portal vein thrombus, but not with patients’ sex, age, tumor size, HBsAg infection, differentiated grading, cirrhosis or serum AFP level. The expression level of serum VEGF in HCC patients was not only correlated with TNM staging, intrahepatic metastasis, lymph node or peritoneal metastasis, portal vein thrombus, but also with patients’ AFP level.
Table 3.
Pathologic Characteristics of KAI1 and Vascular Endothelial Growth Factor Expression in the Serum of 115 Patients with Hepatocellular Carcinoma
| Group | No. | KAI1 | VEGF | ||
|---|---|---|---|---|---|
|
|
|
||||
| χ̄ ±SD, pg/mL | p-value | χ̄ ±SD, pg/mL | p-value | ||
| Sex | |||||
| Male | 47 | 43.873±7.653 | 0.062 | 281.465±68.364 | 0.604 |
| Female | 39 | 47.197±8.638 | 273.597±71.595 | ||
| Age, yr | |||||
| ≥50 | 45 | 44.296±7.934 | 0.132 | 280.983±69.584 | 0.592 |
| <50 | 41 | 46.973±8.396 | 272.861±70.397 | ||
| Tumor size, cm | |||||
| ≥5 | 44 | 44.263±6.732 | 0.100 | 278.634±68.597 | 0.732 |
| <5 | 42 | 46.897±7.926 | 273.583±67.793 | ||
| α-Fetoprotein, ng/mL | |||||
| ≥400 | 46 | 43.469±7.286 | 0.100 | 296.794±69.537 | 0.023 |
| <400 | 40 | 46.197±7.938 | 261.867±70.184 | ||
| Cirrhosis | |||||
| With | 45 | 44.195±6.937 | 0.082 | 277.638±69.864 | 0.849 |
| Without | 41 | 46.934±7.496 | 274.737±70.613 | ||
| HBsAg | |||||
| Positive | 44 | 44.396±7.634 | 0.122 | 278.532±68.936 | 0.850 |
| Negative | 42 | 46.863±6.978 | 275.697±69.514 | ||
| Differentiated grading | |||||
| Well | 24 | 47.576±6.858 | 273.587±70.694 | ||
| Moderate | 32 | 45.793±7.315 | 0.358* | 276.932±68.453 | 0.859† |
| Poor | 30 | 43.984±6.598 | 0.056* | 278.634±69.529 | 0.794† |
| TNM stage | |||||
| I+II | 41 | 47.634±7.638 | 0.002 | 262.584±69.794 | 0.040 |
| III+IV | 45 | 42.587±6.853 | 293.653±68.537 | ||
| Intrahepatic metastasis | |||||
| With | 46 | 42.487±6.387 | <0.001 | 291.476±68.694 | 0.043 |
| Without | 40 | 48.386±7.536 | 260.763±69.836 | ||
| Lymph node or peritoneal metastasis | |||||
| With | 50 | 41.986±6.973 | <0.001 | 297.531±69.852 | 0.023 |
| Without | 36 | 47.834±6.534 | 262.476±68.597 | ||
| Portal vein thrombus | |||||
| With | 49 | 42.397±7.635 | <0.001 | 292.997±69.253 | 0.039 |
| Without | 37 | 47.932±6.866 | 261.534±68.597 | ||
VEGF, vascular endothelial growth factor; SD, standard deviation.
Versus the level of serum KAI1 in well-differentiated grading group;
Versus the level of serum VEGF in well-differentiated grading group.
4. Evaluation of serum KAI1 and VEGF levels for HCC diagnosis
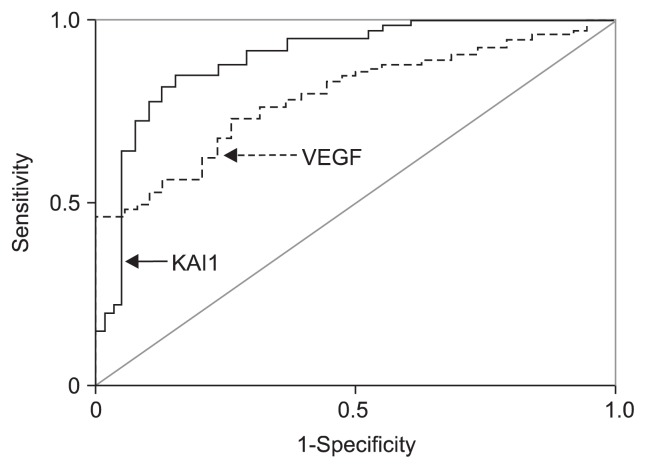
The evaluation of serum KAI1 and VEGF levels for HCC diagnosis is shown in Fig. 2. The comparative analysis of two markers for the whole range of sensitivities and specificities was 0.907 for KAI1 and 0.779 for VEGF according to the area under the ROC curve. The clinical evaluation of serum KAI1 or/and VEGF levels for HCC diagnosis is shown in Table 4. The sensitivity of serum KAI1 for HCC diagnosis was 86.96%, the accuracy was 83.06, while the sensitivity, accuracy, and negative predictive value were improved to 91.86%, 84.68%, and 78.79% according to the combination diagnosis of KAI1 and VEGF, respectively.
Fig. 2.
Analysis of the receiver operating characteristic curves of circulating KAI1 and vascular endothelial growth factor (VEGF) for the diagnosis of hepatocellular carcinoma. The area under the receiver operating characteristic was 0.907 for KAI1 and 0.779 for VEGF.
Table 4.
Evaluation of the Efficiency of Serum KAI1 or/and Vascular Endothelial Growth Factor Levels for the Diagnosis of Hepatocellular Carcinoma*
| Project of evaluation | KAI1, % | VEGF, % | Both, % |
|---|---|---|---|
| Sensitivity | 88.37 | 45.35 | 91.86 |
| Specificity | 71.05 | 97.37 | 68.42 |
| Accuracy | 83.06 | 61.29 | 84.68 |
| Positive predictive value | 87.36 | 97.50 | 86.81 |
| Negative predictive value | 72.97 | 44.05 | 78.79 |
VEGF, vascular endothelial growth factor.
The level of serum KAI1 <57.28 pg/mL or the level of serum VEGF >302.7 pg/mL was considered positive.
DISCUSSION
In this study, we found that the expression level of KAI1 was significantly downregulated, while VEGF expression was strikingly upregulated, whether in serum or in hepatic tissues of the patients with HCC. On the basis of this, we evaluated pathology characteristic of KAI1 and VEGF expression and their diagnostic value for HCC.
Previous researches showed that KAI1 acted as a suppressor of wide-spectrum tumor metastasis during tumor development.8 Compared to normal tissue, downregulation of KAI1 expression was found in the progression of different solid tumors including prostate,9 nonsmall cell lung cancer,10 endometriosis,15 and liver cancers.13 Serum level of VEGF in HCC patients was significantly higher than that in healthy control group; it was also significantly higher in recurrent group than that in nonrecurrent group. VEGF was expressed in cytoplasm of HCC specimens.16 Moreover, the serum VEGF level in patients with advanced HCC undergoing hepatic artery infusion chemotherapy was an important predictive factor for therapeutic effect and survival.17 On the basis of confirming the downregulation of KAI1 and upregulation of VEGF in HCC tissue at both protein (Fig. 1) and mRNA levels (Table 1), we further detected the expression levels of serum KAI1 and VEGF in the patients with HCC and benign liver diseases for the first time (Table 2). The expression level of KAI1 is gradually downregulated from normal control, hepatitis, cirrhosis to HCC patients, while VEGF expression is upregulated little by little, inversely, which is consistent with the detection results in HCC and its corresponding pericancerous tissues.
Recent studies showed that KAI1 affected cell cycle. Both KAI1 and CyclinD1 were involved in the occurrence and development of laryngeal squamous cell carcinoma (LSCC), and might provide clinical information for evaluation of invasiveness, metastasis and prognosis of LSCC.5 Loss of KAI1 expression was likely to predict metastasis and poor clinical outcome in gastric cancer patients. For the purpose of predicting the prognosis of gastric cancer patients, it was important to discriminate whether the carcinoma cells have loss of KAI1 expression.18 VEGF was the most well-characterized angiogenic factor closely associated with tumor progression and prognosis in several carcinogenesis.19 In HCC patients, VEGF was also associated with venous invasion and metastasis, serum of patients with remotely metastasized tumor showed much higher level of serum VEGF when compared with serum sample collected from HCC patients without metastasis.20 Herein, we found that the expression level of serum KAI1 in HCC patients was correlated with TNM staging, intrahepatic metastasis, lymph node or peritoneal metastasis, portal vein thrombus, while serum VEGF expression was not only correlated with TNM staging, intra-hepatic metastasis, lymph node or peritoneal metastasis, portal vein thrombus, but also with patients’ AFP level in HCC patients (Table 3), which was consistent with previous reports.
Metastasis was the final step in the tumor progression processes and involved increased invasiveness, extravasation into secondary organs, and angiogenesis.21 VEGF and hypoxia-inducible factor 1a (HIF-1a) expression were significantly inhibited by restoration of KAI1 in PC3 cells. In response to KAI1 expression, CUB-domain-containing protein 1 (CDCP1)-enhanced Src activation was downregulated and the level of von Hippel-Lindau (VHL) protein was significantly increased. In an in vivo xenograft model, KAI1 inhibited the expression of CDCP1 and HIF-1a. That is to say, KAI1 suppresses HIF-1a and VEGF expression by blocking CDCP1-enhanced Src activation in prostate cancer.22 Our results suggested that the expression of KAI1 was significantly negatively correlated with VEGF level in HCC patients (data not shown), which further supported the theory that KAI1 exerted profound metastasis-suppressor activity in the tumor malignancy process via inhibition of CDCP1-mediated Src activation, followed by VHL-induced HIF-1a degradation and, ultimately, decreased VEGF expression. KAI1 inhibited canonical Wnt signalling by controlling the cellular distribution of β-catenin in carcinoma cells.23
KAI1 was an excellent marker for distinguishing chromophobe renal cell carcinoma from other types of renal cell tumors, especially from renal oncocytomas with overlapping phenotype.24 The reduction of KAI1 expression was indicator for the metastatic potential of gastric carcinoma cells.25 Serum VEGF-C levels might provide additional information for distinguishing between the absence and presence of lymph node metastases in patients with lung carcinoma. The evaluation of serum VEGF-C was complementary to accurate lymph node staging in nonsmall cell lung cancer.26 High VEGF-expression in prostate cancer was a poor prognostic factor with statistical significance.27 Our results suggest that the sensitivity, accuracy and negative predictive value were improved according to the combination diagnosis of KAI1 and VEGF, although a good diagnostic value is presented by only KAI1 or VEGF for the first time (Table 4, Fig. 2).
In conclusion, the results presented herein suggest that the expression of KAI1 is downregulated, while VEGF expression is upregulated in the tissues or serum of the patients with HCC. The expression level of serum KAI1 in HCC patients is correlated with TNM staging, intrahepatic metastasis, lymph node or peritoneal metastasis, portal vein thrombus. Besides the correlation factors of KAI1, VEGF expression is also closely related to the patients’ AFP level. The area under the receiver operating characteristic is 0.907 for KAI1 and 0.779 for VEGF. The sensitivity, accuracy, and negative predictive value are improved to 91.86%, 84.68%, and 78.79% according to the combination diagnosis of KAI1 and VEGF, respectively. Only KAI1 or VEGF is insufficient to predict prognosis of liver cirrhosis to HCC, even though incidence of elevated serum VEGF level correlates with higher AFP and des-γ-carboxy prothrombin level in serum,13,16 combined diagnosis of KAI1 and VEGF maybe greatly improve the efficiency of diagnosis and form a reliable marker panel.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Program of Wenzhou Society Development (Y20110201).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Barraud H, Bronowicki JP. Curative treatment of hepatocellular carcinoma. Rev Prat. 2013;63:229–233. [PubMed] [Google Scholar]
- 2.Giannini EG, Cucchetti A, Erroi V, Garuti F, Odaldi F, Trevisani F. Surveillance for early diagnosis of hepatocellular carcinoma: how best to do it? World J Gastroenterol. 2013;19:8808–8821. doi: 10.3748/wjg.v19.i47.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez SA, Keeffe EB. Diagnosis of hepatocellular carcinoma: role of tumor markers and liver biopsy. Clin Liver Dis. 2011;15:297–306. vii–x. doi: 10.1016/j.cld.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Tsai YC, Weissman AM. Dissecting the diverse functions of the metastasis suppressor CD82/KAI1. FEBS Lett. 2011;585:3166–3173. doi: 10.1016/j.febslet.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik FA, Sanders AJ, Jiang WG. KAI-1/CD82, the molecule and clinical implication in cancer and cancer metastasis. Histol Histopathol. 2009;24:519–530. doi: 10.14670/HH-24.519. [DOI] [PubMed] [Google Scholar]
- 6.Zhang B, Liu W, Li L, et al. KAI1/CD82 and cyclinD1 as biomarkers of invasion, metastasis and prognosis of laryngeal squamous cell carcinoma. Int J Clin Exp Pathol. 2013;6:1060–1067. [PMC free article] [PubMed] [Google Scholar]
- 7.Yang JM, Peng ZH, Si SH, Liu WW, Luo YH, Ye ZY. KAI1 gene suppresses invasion and metastasis of hepatocellular carcinoma MHCC97-H cells in vitro and in animal models. Liver Int. 2008;28:132–139. doi: 10.1111/j.1478-3231.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 8.Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013;153:13–19. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanematsu M, Semelka RC, Osada S, Amaoka N. Magnetic resonance imaging and expression of vascular endothelial growth factor in hepatocellular nodules in cirrhosis and hepatocellular carcinomas. Top Magn Reson Imaging. 2005;16:67–75. doi: 10.1097/01.rmr.0000191133.91603.d2. [DOI] [PubMed] [Google Scholar]
- 10.Schoenleber SJ, Kurtz DM, Talwalkar JA, Roberts LR, Gores GJ. Prognostic role of vascular endothelial growth factor in hepatocellular carcinoma: systematic review and meta-analysis. Br J Cancer. 2009;100:1385–1392. doi: 10.1038/sj.bjc.6605017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ministry of Health of the People’s Republic of China. Updated standards for the diagnosis and treatment of primary liver cancer. Zhonghua Gan Zang Bing Za Zhi. 2012;20:419–426. [PubMed] [Google Scholar]
- 12.Zhang HJ, Yao DF, Yao M, et al. Expression characteristics and diagnostic value of annexin A2 in hepatocellular carcinoma. World J Gastroenterol. 2012;18:5897–5904. doi: 10.3748/wjg.v18.i41.5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo C, Liu QG, Zhang L, Song T, Yang X. Expression and clinical significance of p53, JunB and KAI1/CD82 in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2009;8:389–396. [PubMed] [Google Scholar]
- 14.Xue YJ, Xiao RH, Long DZ, et al. Overexpression of FoxM1 is associated with tumor progression in patients with clear cell renal cell carcinoma. J Transl Med. 2012;10:200. doi: 10.1186/1479-5876-10-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li MQ, Hou XF, Lv SJ, et al. CD82 gene suppression in endometrial stromal cells leads to increase of the cell invasiveness in the endometriotic milieu. J Mol Endocrinol. 2011;47:195–208. doi: 10.1530/JME-10-0165. [DOI] [PubMed] [Google Scholar]
- 16.Zhong C, Wei W, Su XK, Li HD, Xu FB, Guo RP. Serum and tissue vascular endothelial growth factor predicts prognosis in hepatocellular carcinoma patients after partial liver resection. Hepatogastroenterology. 2012;59:93–97. doi: 10.5754/hge10638. [DOI] [PubMed] [Google Scholar]
- 17.Niizeki T, Sumie S, Torimura T, et al. Serum vascular endothelial growth factor as a predictor of response and survival in patients with advanced hepatocellular carcinoma undergoing hepatic arterial infusion chemotherapy. J Gastroenterol. 2012;47:686–695. doi: 10.1007/s00535-012-0555-6. [DOI] [PubMed] [Google Scholar]
- 18.Knoener M, Krech T, Puls F, Lehmann U, Kreipe H, Christgen M. Limited value of KAI1/CD82 protein expression as a prognostic marker in human gastric cancer. Dis Markers. 2012;32:337–342. doi: 10.1155/2012/737132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sengupta B, Siddiqi SA. Hepatocellular carcinoma: important biomarkers and their significance in molecular diagnostics and therapy. Curr Med Chem. 2012;19:3722–3729. doi: 10.2174/092986712801661059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu L, Deng L, Li J, Zhang Y, Hu L. The prognostic value of vascular endothelial growth factor in ovarian cancer: a systematic review and meta-analysis. Gynecol Oncol. 2013;128:391–396. doi: 10.1016/j.ygyno.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Williams SC. Circulating tumor cells. Proc Natl Acad Sci U S A. 2013;110:4861. doi: 10.1073/pnas.1304186110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JJ, Jin YB, Lee YJ, et al. KAI1 suppresses HIF-1α and VEGF expression by blocking CDCP1-enhanced Src activation in prostate cancer. BMC Cancer. 2012;12:81. doi: 10.1186/1471-2407-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chigita S, Sugiura T, Abe M, et al. CD82 inhibits canonical Wnt signalling by controlling the cellular distribution of β-catenin in carcinoma cells. Int J Oncol. 2012;41:2021–2028. doi: 10.3892/ijo.2012.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yusenko MV, Kovacs G. Identifying CD82 (KAI1) as a marker for human chromophobe renal cell carcinoma. Histopathology. 2009;55:687–695. doi: 10.1111/j.1365-2559.2009.03449.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Gu S, Trojanowicz B, et al. Down-regulation of TM4SF is associated with the metastatic potential of gastric carcinoma TM4SF members in gastric carcinoma. World J Surg Oncol. 2011;9:43. doi: 10.1186/1477-7819-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Meng X, Zeng H, et al. Serum vascular endothelial growth factor-C levels: a possible diagnostic marker for lymph node metastasis in patients with primary non-small cell lung cancer. Oncol Lett. 2013;6:545–549. doi: 10.3892/ol.2013.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang K, Peng HL, Li LK. Prognostic value of vascular endothelial growth factor expression in patients with prostate cancer: a systematic review with meta-analysis. Asian Pac J Cancer Prev. 2012;13:5665–5669. doi: 10.7314/APJCP.2012.13.11.5665. [DOI] [PubMed] [Google Scholar]