Abstract
Recent studies have proposed nomenclatures of type 1 autoimmune pancreatitis (AIP) (IgG4-related pancreatitis), IgG4-related sclerosing cholangitis (IgG4-SC), IgG4-related cholecystitis, and IgG4-related hepatopathy as IgG4-related disease (IgG4-RD) in the hepato-bilio-pancreatic system. In IgG4-related hepatopathy, a novel concept of IgG4-related autoimmune hepatitis (AIH) with the same histopathological features as AIH has been proposed. Among organs involved in IgG4-RD, associations with pancreatic and biliary lesions are most frequently observed, supporting the novel concept of “biliary diseases with pancreatic counterparts.” Targets of type 1 AIP and IgG4-SC may be periductal glands around the bile and pancreatic ducts. Based on genetic backgrounds, innate and acquired immunity, Th2-dominant immune status, regulatory T (Treg) or B cells, and complement activation via a classical pathway may be involved in the development of IgG4-RD. Although the role of IgG4 remains unclear in IgG4-RD, IgG4-production is upregulated by interleukin 10 from Treg cells and by B cell activating factor from monocytes/basophils with stimulation of toll-like receptors/nucleotide-binding oligomerization domain-like receptors. Based on these findings, we have proposed a hypothesis for the development of IgG4-RD in the hepato-bilio-pancreatic system. Further studies are necessary to clarify the pathogenic mechanism of IgG4-RD.
Keywords: IgG4-related disease, Autoimmune pancreatitis, IgG4-related sclerosing cholangitis, IgG4-related hepatopathy
INTRODUCTION
In 1961, Sarles et al.1 observed a case of particular pancreatitis with hypergammaglobulinaemia, which is supposed to be a prototype of autoimmune pancreatitis (AIP) (Table 1). In 1995, Yoshida et al.2 proposed a novel concept of AIP, which has been accepted as type 1 AIP (IgG4-related pancreatitis), the pancreatic manifestation of IgG4-related disease (IgG4-RD).3 IgG4-RD is recognized worldwide as a novel clinical entity following the epoch-making evidence of increased serum levels of IgG4 in the history of AIP.4 The histopathological findings are characterized by the periductal localization of predominantly CD4 positive T cells, IgG4-positive plasma cells, storiform fibrosis with acinar cell atrophy, and obliterative fibrosis,5,6 which is also called lymphoplasmacytic sclerosing pancreatitis (LPSP).7 On the other hand, mainly in the Western countries, histological analyses using resected pancreatic samples in patients with chronic non-alcoholic pancreatitis demonstrated a different histological pattern of pancreatitis from LPSP, so called idiopathic duct-centric pancreatitis (IDCP) or AIP with granulocytic epithelial lesion. In 2003, Kamisawa et al.8 first suggested that AIP showing LPSP is a systemic sclerosing disease based on the concept of multifocal fibrosclerosis proposed by Comings et al.,9 because the pancreas and other involved organs have fibrosis with abundant infiltration of IgG4-positive plasma cells. On the other hand, patients with IDCP, rarely observed in Japan, are not associated with either serum IgG4 elevation or with other organ involvement typically seen in LPSP. AIP is subclassified according to the International Consensus of Diagnostic Criteria for AIP as either type 1 (LPSP) or type 2 (IDCP).10 Type 2 AIP, unlike type 1 AIP, is thought to be a specific pancreatic disease with occasional coexistence with ulcerative colitis.10,11
Table 1.
Transition of the Concept of IgG4-Related Disease
| Author (Year) | Evidences/Contents |
|---|---|
| Mikulicz (1892)12 | Mikulicz’s disease (Z Chir Fesrschr) |
| Sarles et al. (1961)1 | Hypergammaglobulinemia in CP (Am J Dig Dis) |
| Comings et al. (1967)9 | Familial multifocal fibrosclerosis (Ann Intern Med) |
| Küttner (1972)13 | Küttner tumor (Beitr Klin Chir) |
| Kawaguchi et al. (1991)7 | Lymphoplasmacytic sclerosing pancreatitis (Hum Pathol) |
| Yoshida et al. (1995)2 | Autoimmune pancreatitis (Dig Dis Sci) |
| Hamano et al. (2001)4 | High IgG4 levels in sclerosing pancreatitis (N Eng J Med) |
| Kamisawa et al. (2003)8 | IgG4-related sclerosing disease (J Gastroenterol) |
| Kamisawa et al. (2006)14 | IgG4-related sclerosing disease (J Gastroenterol) |
| Yamamoto et al. (2006)15 | IgG4-related plasmacytic disease (Mod Rheumatol) |
| Masaki et al. (2009)16 | IgG4-multiorgan lymphoproliferative syndrome (MOLPS) (Ann Rheum Dis) |
| Shimosegawa et al. (2011)11 | International Consensus Diagnostic Criteria for AIP (Pancreas) |
| Umehara et al.3,17 | Concept and comprehensive diagnostic criteria for IgG4-related disease (Mod Rheumatol) |
| Deshpande et al. (2012)18 | International Pathological Consensus for IgG4-RD (Mod Pathol) |
| Stone et al. (2012)19 | Nomenclatures of individual organ manifestation of IgG4-RD (Arthritis Rheum) |
CP, chronic pancreatitis; AIP, autoimmune pancreatitis.
On the other hand, in 1892, Mikulicz12 first observed a patient with symmetrical swelling of the lachrymal, parotid and submandibular glands, with massive infiltration of mononuclear cells. The condition was called Mikulicz’s disease; however, it has since been classified as an atypical type of Sjögren’s syndrome, which also presents with bilateral, painless, and symmetrical swelling of the lachrymal, parotid, and submandibular glands. Küttner13 reported a tumor-like enlargement of the submandibular gland that was sometimes a result of stones in the Wharton duct. These patients, lacking anti-SS-A/Ro or anti-SS-B/La antibodies, often show other systemic organ involvement with elevated serum levels of IgG4, infiltration of IgG4-positive plasma cells into the glands, and recovery of secretion with steroid treatment similar to AIP.4–6 Referring to the original concept of multifocal fibrosclerosis, recent studies led us to develop a novel concept of a systemic disease such as IgG4-related systemic sclerosing disease,14 systemic IgG4-related plasmacytic syndrome,15 or IgG4-positive multiorgan lymphoproliferative syndrome,16 all of which may refer to the same conditions. Based on these findings, although it is unclear whether the pathogenetic mechanisms in individual organs are same or not,3,17 the comprehensive term “IgG4-related disease IgG4-RD,” which was internationally endorsed with the proposal of nomenclatures for individual organ lesions as well as pathological consensus, and diagnostic criteria have been proposed from the Japanese investigators.17 In this review, we discussed the current concepts of hepato-bilio-pancreatic lesions and recent advances in our understanding of the pathogenesis of IgG4-RD.
CURRENT CONCEPTS OF IgG4-RD IN THE HEPATO-BILIO-PANCREATIC SYSTEM
The patients with IgG4-RD show diffuse or focal organ enlargement and mass-forming or nodular/thickened lesions in various organs, either synchronously or metachronously. This is due to the prominent infiltration of lymphocytes and plasmacytes with fibrosis.3,5,14 The causes are still unclear; however, some abnormal immunological mechanisms are involved. The organs known to be affected include the pancreas, biliary duct, lacrimal/salivary glands, retroperitoneum, central nervous system, thyroid gland, lungs, liver, gastrointestinal tracts, kidneys, prostate gland, and lymph nodes.5,14–19 Clinical symptoms vary depending on the organ in which the lesions are located, but many cases are treated effectively by steroid therapy. All of them show similar pathological findings with abundant infiltration of IgG4-positive cells and fibrosis, and international minimum histological consensus was proposed (Table 2). Although the infiltration of IgG4-positive cells and increased serum levels of IgG4 are characteristic in IgG4-RD, the severity of fibrosis seems to be different among the individual involved organs.18 Storiform fibrosis and obliterative phlebitis are characteristic in pancreatic and biliary tract lesions, but rarely observed in the salivary or lymphnodes.18 Although most patients have multi-organ lesions synchronously or metachronously, about 10% to 20% of the patients have solitary organ involvement.20 Therefore, it is unclear whether the pathogenetic mechanism is same among individual organs or not. Type 1 AIP (IgG4-related pancreatitis), IgG4-related sclerosing cholangitis (IgG4-SC), IgG4-related cholecystitis, and IgG4-related hepatopathy are recommended as the nomenclatures of IgG4-RD in the hepato-bilio-pancreatic system.19
Table 2.
The Three Major Histopathological Features Associated with IgG4-Related Disease and the Minimal Criteria in a New Organ/Site in the International Pathological Consensus18
The three major histopathological features associated with IgG4-RD
|
IgG4-RD, IgG4-related disease.
1. Type 1 AIP (IgG4-related pancreatitis)
AIP is a distinct form of pancreatitis clinically characterized by frequent presentation with obstructive jaundice with or without a pancreatic mass, histologically by a lymphoplasmacytic infiltrate and fibrosis and therapeutically by a dramatic response to steroids.5,21 Recent studies have suggested that “AIP” manifests two distinct subtypes, type 1 and type 2 AIP (Table 3).10,11 Type 1 AIP (IgG4-related pancreatitis) is more prevalent in Japan and Korea, whereas type 2 AIP, with granulocytic epithelial lesion, is more commonly observed in Europe and the United States.
Table 3.
Subtypes of Autoimmune Pancreatitis
| Subtype of AIP | Type 1 | Type 2 |
|---|---|---|
| Other nomenclatures | AIP without GEL | AIP with GEL |
| IgG4-related | IgG4-unrelated | |
| LPSP | IDCP | |
| Prevalence | Asia>USA, EU | EU>USA>Asia |
| Age | High aged | Younger |
| Gender | Male>>Female | Male=Female (NS) |
| Symptoms | ||
| Obstructive jaundice | Often | Often |
| Abdominal pain | Rare | Common |
| Pancreas swelling | Common | Common |
| Serology | High serum IgG, IgG4, autoAbs (+) | Normal IgG, normal IgG4, autoAbs (−) |
| OOI | Sclerosing cholangitis | Unrelated with OOI |
| Sclerosing sialadenitis | ||
| Reteroperitoneal fibrosis | ||
| Others | ||
| Ulcerative colitis | Rare | Often |
| Steroid | Responsive | Responsive |
| Relapse | High rate | Rare |
AIP, autoimmune pancreatitis; GEL, granulocytic epithelial lesion; LPSP, lymphoplasmacytic sclerosing pancreatitis; IDCP, idiopathic duct-centric chronic pancreatitis; NS, not significant; OOI, other organ involvement.
In type 1 AIP, the pancreatic histopathology shows the following characteristic features of LPSP: 1) abundant infiltration of plasma cells (IgG4+ cells; >10/hpf, 40%>IgG4/IgG cells) and lymphocytes, 2) peculiar storiform or swirling fibrosis, and 3) perivenular infiltration with lymphocytes and plasma cells often leading to obliterative phlebitis. Clinically, it is characterized by swelling of the pancreas, elevated serum IgG4 levels and extra-pancreatic lesions (e.g., sclerosing cholangitis, sclerosing sialadenitis, and retroperitoneal fibrosis) associated with infiltration of abundant IgG4+ plasma cells. Patients with type 1 AIP often have obstructive jaundice in elderly males, and the pancreatic and extrapancreatic manifestations respond to steroid therapy.21 Therefore, it is a pancreatic manifestation of a systemic disorder, IgG4-RD.19,21
2. IgG4-SC
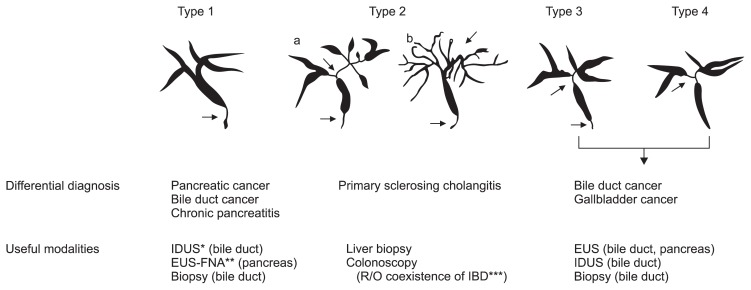
About 60% to 80% of patients with type 1 AIP are associated with IgG4-SC,5,20–22 in which cholangiographic features are similar to those of primary sclerosing cholangitis (PSC), pancreatic cancer, and cholangiocarcinoma. The steroid responses and the prognoses of IgG4-SC differ from patients with PSC, which suggests different pathological conditions.5,20–22 Four types of the characteristic cholangiographic features of IgG4-SC have been proposed based on the regions of stricture (Fig. 1).22 IgG4-SC with only stenosis of the distal common bile duct (type 1) is difficult to differentiate from pancreatic cancer. This stricture might be due to both the thickening of bile duct and the effect of inflammation and/or edema of pancreas without wall thickness. IgG4-SC with diffuse stenosis throughout the intrahepatic/proximal bile ducts (type 2) is similar to PSC. IgG4-SC with stenosis in the hilar hepatic bile duct (type 3 and 4) is difficult to differentiate from hepatic hilar colangiocarcinoma.22 In addition to stenosis of bile ducts, circular and symmetric thickening of the bile duct wall, smooth outer and inner margin, and homogenous internal echo demonstrated by abdominal ultrasonography, abdominal computed tomography, abdominal magnetic resonance imaging, endoscopic ultrasonography, and intraductal ultrasonography are most characteristic images.22 These characteristic features are recognized not only in the stenotic areas or occasionally in the gallbladder but also in areas without stenosis that appear normal in cholangiogram. Most cases of IgG4-SC (80% to 90%) are associated with AIP.20–22 It is particularly difficult to accurately diagnose IgG4-SC without AIP. In contrast to PSC, inflammatory bowel disease is rarely observed in the patients with IgG4-SC.20–22
Fig. 1.
Classification of cholangiography in IgG4-related sclerosing cholangitis (IgG4-SC). The characteristic features of IgG4-SC can be classified into four types, based on the regions of stricture as revealed by cholangiography and differential diagnosis. Type 1 IgG4-SC shows stenosis only in the lower part of the common bile duct, which should be differentiated from chronic pancreatitis, pancreatic cancer, or cholangiocarcinoma. Type 2 IgG4-SC, in which stenosis is diffusely distributed throughout the intrahepatic and extrahepatic bile ducts, should be differentiated from primary sclerosing cholangitis. Type 2 is further subdivided into two types. Type 2a has a narrowing of the intrahepatic bile ducts with prestenotic dilation, and Type 2b has a narrowing of the intrahepatic bile ducts without prestenotic dilation and reduced bile duct branches, caused by marked lymphocytic and plasmacyte infiltration into the peripheral bile ducts. Type 3 IgG4-SC is characterized by stenosis in both the hilar hepatic lesions and the lower portion of the common bile duct. Type 4 IgG4-SC shows strictures of the bile duct only in the hilar hepatic lesions. Cholangiographic findings of types 3 and 4 need to be discriminated from those of cholangiocarcinoma. From Ohara H, et al. J Hepatobiliary Pancreat Sci 2012;19:536–542, with permission from Springer.22
IDUS, intraductal ultrasonography; EUS, endoscopic ultrasonography; EUS-FNA, EUS-guided fine-needle aspiration; IBD, inflammatory bowel disease.
Histopathologically, similar to LPSP in type 1 AIP, massive infiltration of IgG4-positive plasma cells, storiform fibrosis and/or obliterative phlebitis in the bile duct wall are characteristic and called as lymphoplasmacytic sclerosing cholangitis.19,22 Such fibroinflammatory involvement is mainly observed in the submucosa of the bile duct wall, whereas the epithelium of the bile duct is intact.23 Endoscopic transpapillary bile duct biopsy or cytological examinations are useful for differential diagnosis of cholangiocarcinoma, although it is difficult to take enough biopsy samples for characteristic histopathological findings of IgG4-SC.22 Liver biopsy is sometimes useful in the diagnosis of IgG4-SC in cases of intrahepatic bile duct involvement.22
3. IgG4-related hepatopathy
Liver dysfunction is frequently observed in AIP patients and most of them show various pathological changes with infiltration of IgG4-bearing plasma cells in the liver; portal inflammation with or without interface hepatitis, large bile duct obstructive features, portal sclerosis, lobular hepatitis, and canalicular cholestasis.24 As a very few of IgG4-RD patients without AIP or IgG4-SC show the same histological features as autoimmune hepatitis (AIH), a novel concept of IgG4-related AIH has been proposed.25,26 To establish the concept of IgG4-related AIH, further studies are required.
RECENT ADVANCES IN THE PATHOGENIC MECHANISMS OF IgG4-RD IN THE HEPATO-BILIO-PANCREATIC SYSTEM
1. Immunogenic backgrounds
Although immunogenic backgrounds of IgG4-RD are not well understood, Japanese patients with AIP, most of whom are IgG4-related, may be associated with class II antigen haplotype of the major histocompatibility complex (HLA-DRB1*0405-DQB1*0401),27 polymorphism of nuclear factor-κB and Fc-receptor-like 3 genes expressed on B cells.28 An inhibitory molecule, cytotoxic T lymphocyte antigen-4 (CTLA-4; CD152) expressed on the activated memory T cells or CD4+CD25+ regulatory T cells (Tregs), was independently reported as a susceptibility factor.29,30 Based on immunogenic backgrounds, abnormal conditions of immune responses may be involved in the development of type 1 AIP, although the precise pathogenic mechanisms remain unclear.5
2. Innate immunity
Recently, abnormal innate immunity has been demonstrated in some patients with IgG4-RD.5,21 Activation of NOD-2 and TLR ligands on monocytes or basophils from patients with IgG4-related AIP enhances IgG4 responses via B cell activating factor (BAFF) and interleukin (IL)-13, although specific pathogens still remain unclear.31,32 In animal models, activation of TLR3 by polyinosinic:polycytidylic acid or TLR4 by lipopolysaccharide can induce immune-mediated cholangitis, pancreatitis and sialadenitis similar to human IgG4-RD.33
3. Possible roles of IgG4 in IgG4-RD
Although the association of IgE-mediated allergy and IgG4 antibodies is well known, IgG4 characteristics are still poorly understood. IgG4 is involved in an immune process referring to as ‘Fab-arm exchange,’ which is a swapping of a heavy chain and attached light chain (half-molecule) with a heavy-light chain pair from another molecule; this usually results in asymmetric antibodies with two different antigen-combining sites.34 While these modified antibodies are hetero-bivalent, they behave as monovalent antibodies. Another aspect of IgG4 is that it mimics IgG rheumatoid factor activity by interacting with IgG, namely Fc-mediated aggregation.35 IgG4 seems to be associated with a pathogenic effect in a few situations. In pemphigus, recognition of skin autoantigens (desmogleins) by IgG4 is at the origin of the disease process.36 A most recent study of structural determinants of human IgG4-Fc by crystallography suggested that Fc-Fc interactions are compatible with intact IgG4 molecules and may provide a model for the formation of aggregates of IgG4 that can cause disease pathology in the absence of antigen.37
Another recent data on regulation of IgG4 showed that IgG4-RD may reflect an excessive production of anti-inflammatory cytokines such as IL-10 that triggers an overwhelming expansion of IgG4-producing plasma cells.38–42 Increased peripheral inducible-memory Tregs are positively correlated with serum levels of IgG4.39 In addition, prominent infiltration of Tregs up-regulated IL-10 in livers of the patients with IgG4-SC.40 These findings suggest that IgG4 do not act as a pathogenic factor, but as an anti-inflammartory factor in IgG4-RD. Further studies are necessary to clarify the precise role of IgG4 in IgG4-RD.
4. The complement system
Patients in active stages of AIP occasionally show decreased complement (C3, C4) with elevated circulating immune complex as well as serum levels of IgG4 and the IgG4 subclass of immune complexes.43 However, a previous study showed that the classical pathway of complement activation through IgG1 may be involved in the development of AIP rather than mannose-binding lectin or alternative pathways through IgG4.43
5. Autoantibodies and candidate of target antigens
Although some patients with IgG4-RD have nonspecific antibodies such as an antinuclear antibody, there is scarce association of IgG4-RD. From the view of IgG4 function, the big mystery is whether IgG4-RD is an autoimmune or an allergic disease. Although disease specific targets are unknown, the occasional coexistence of multiorgan involvements leads us to consider that there may be common target antigens. Among candidate antigens previously reported, lactoferrin (LF),44,45 carbonic anhydrase (CA)-II,44–47 CA-IV,48 and pancreatic secretory trypsin inhibitor (PSTI)49 are expressed in the pancreas, salivary glands, biliary duct, lungs, renal tubules, and so forth. Immunization with CA-II or LF induced systemic lesions such as pancreatitis, sialadenitis, cholangitis, interstitial nephritis in the mice models similar to human IgG4-RD.50 Amylase α-2A,51 HSP-10,52 and Helicobacter pylori53–56 are also candidates of disease-associated antigens. Among the involved organs in IgG4-RD, recent studies suggest an extremely high association of pancreatic and biliary lesions.5,20,21 As both peribiliary glands in the biliary tract and pancreatic duct glands associated with pancreatic ducts in human are intermingled with small amounts of pancreatic exocrine acini,57 and the biliary tree-derived stem cells may be involved in a pancreatic organogenesis in mice.58 Nakanuma et al.59 have proposed a new concept of the “biliary diseases with pancreatic counterparts,” in which targets of type 1 AIP and IgG4-SC may be periductal glands around the bile and pancreatic ducts. Further studies of the biliary tract’s patho-physiology based on its similarity to pancreatic counterparts are warranted.
6. Role of B cells
In addition to steroid and immune-modulators, the B cell depletion by rituximab, which reduces only IgG4, but not IgG1, IgG2, or IgG3, is useful in the therapeutic strategy in IgG4-RD.60,61 A recent study showed expansion of IgG4+ B cell receptor clones in blood and tissue of patients with active IgG4-chol-angiopathy, and disappearance by corticosteroid treatment.62 A recent study showed that the increased CD19+CD24highCD38high Bregs may suppress the disease activity of type 1 AIP, whereas the decreased CD19+CD24highCD27+ Bregs might be involved in the development of type 1 AIP.63 These findings suggest that specific B cell responses may have a pivotal role in the pathogenesis of IgG4-RD such as type 1 AIP and IgG4-SC.
7. Th1 and Th2 immune balance
The effector cells in IgG4-RD have been poorly understood. The CD4+ T cells differentiate from naive T cells (Th0) to Th1, Th2, Th17, and Treg cells. In the livers of IgG4-SC patients, a Th2 type immune reaction38,42 is induced in addition to the Th1 responses.45,50 Th2 cytokines may be involved in the progression of the disease process, especially the maturation and proliferation of local B cells and plasmacytes.
8. Tregs
Foxp3 is a member of the forkhead/winged-helix family of transcriptional regulators, and functions as the master regulator in the development and function of CD4+CD25+ Tregs classified as naturally occurring naive-Tregs originating in the thymus and adaptively induced memory-Tregs in the periphery by different antigens.64 In type 1 AIP, circulatory naive (CD45RA+) Tregs are significantly decreased in the peripheral blood, whereas memory (CD45RA−)-Tregs are significantly increased.39 In addition, prominent infiltration of Tregs with upregulation of IL-10 is observed in the liver of type 1 AIP and IgG4-SC patients.40,41 These findings suggest that increased memory-Tregs in the periphery and local tissues may be an inhibitory immune response against inflammation, although decreased naive Tregs may be pathogenic.
9. Our hypothesis for the pathogenesis of IgG4-SC
The neonatally thymectomized (nTx)-BALB/c mice models showed that immunization with CA-II or LF induced pancreatitis, cholangitis, and sialadenitis similar to human IgG4-RD.50 These findings suggest that depletion of naive Tregs may induce macrophage/T cell activation and further proinflammatory reactions during the early stage of the disease as direct cytotoxicity effects through Fas ligand expression. WBN/Kob rat models with congenital decreased peripheral Tregs spontaneously develop sclerotic cholangitis, sialadenitis, thyroiditis, and tubulointerstitial nephritis.65 These animal models suggest that CD4+/CD8+ T cells play major roles in the development of primary lesions similarly to human IgG4-RD; however, the counterpart of IgG4 in mice IgG subclasses has not been identified.
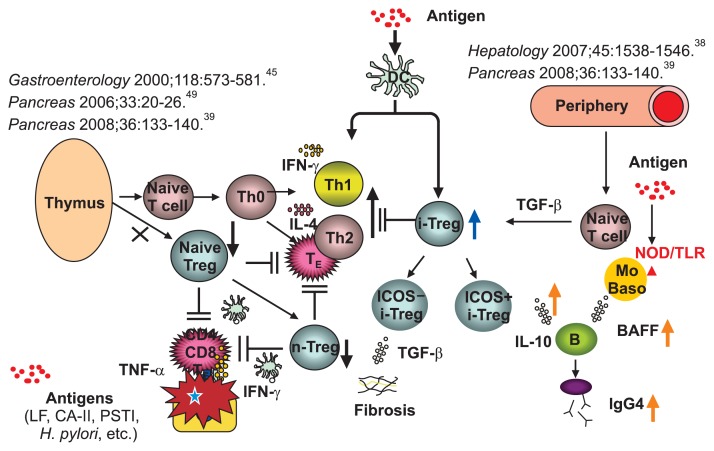
Based on these findings, we proposed the pathogenesis of type 1 AIP (Fig. 2).5 The basic concept is the biphasic mechanism of “induction” and “progression.” An initial response to unknown disease specific antigens including self-antigens (LF, CA-II, CA-IV, and PSTI) or microorganisms (bacteria or virus) might be induced by decreased naive-Tregs followed by a Th1 type immune response with the release of proinflammatory cytokines (interferon γ, IL-1β, IL-2, tumor necrosis factor α). In progression, Th2 type immune responses producing IgG, IgG4, and autoantibodies may be involved in pathophysiology. IgG4 and fibrosis may be regulated by increased IL-10 and transforming growth factor β secreted from inducible T cell co-stimulator (ICOS)-positive and ICOS-negative inducible adaptive Tregs, respectively. Production of IgG4 may be also upregulated by BAFF from monocytes and basophils.
Fig. 2.
Hypothesis for the pathogenesis of autoimmune pancreatitis (AIP) and IgG4-related disease. In central tolerance, naturally occurring naive regulatory T cells (n-Tregs) derived from the thymus suppress autoreactive CD4 or CD8 cells in the normal state. In the IgG4-related disease, the basic concept is a biphasic mechanism of “induction” and “progression.” Initial response to antigens (lactoferrin [LF], carbonic anhydrase II [CA-II], CA-IV, pancreatic secretory trypsin inhibitor [PSTI], α-amylase, plasminogen binding protein peptide of Helicobacter pylori, etc.) might be induced by decreased n-Tregs. Th2 immune responses were followed by Th1-type immune responses, with releases of proinflammatory cytokines (interferon γ [IFN-γ], interleukin [IL]-1b, IL-2, tumor necrosis factor α [TNF-α]). In progression, Th2-type immune responses producing IgG, IgG4 and autoantibodies may be involved in pathophysiology. IgG4 and fibrosis may be regulated by increased IL-10 and transforming growth factor β (TGF-β) secreted from inducible memory-Tregs (i-Tregs), respectively. However, activation of nucleotide-binding oligomerization domain (NOD) receptor or TLRs on monocytes or basophils increases IgG4 via the upregulation of B cell activating factor belonging to the tumor necrosis factor family (BAFF) and IL-13. From Okazaki K, et al. J Gastroenterol 2011;46:277–288, with permission from Springer.5
DC, ductal cell; TE, effector T cell.
CONCLUSIONS
Recent advances support the concept of IgG4-RD, a unique clinical entity, in the hepato-bilio-pancreas system. Although the pathogenic mechanism remains unclear, innate and acquired immunity, Tregs, and B cells may be involved in the development of these lesions. Further studies are necessary to clarify the pathogenesis including genetic backgrounds, disease-specific antigens, and the role of IgG4.
ACKNOWLEDGEMENTS
This study was partially supported by 1) Grant-in-Aid for Scientific Research (C) of the Ministry of Culture and Science of Japan (20590810, 24591020, 12008507); 2) the Research Program on Intractable Diseases, from the Ministry of Labor and Welfare of Japan; and 3) grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan, from CREST Japan Science, and Technology Agency.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Sarles H, Sarles JC, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreas: an autonomous pancreatic disease? Am J Dig Dis. 1961;6:688–698. doi: 10.1007/BF02232341. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality: proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561–1568. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]
- 3.Umehara H, Okazaki K, Masaki Y, et al. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol. 2012;22:1–14. doi: 10.3109/s10165-011-0508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 5.Okazaki K, Uchida K, Koyabu M, Miyoshi H, Takaoka M. Recent advances in the concept and diagnosis of autoimmune pancreatitis and IgG4-related disease. J Gastroenterol. 2011;46:277–288. doi: 10.1007/s00535-011-0386-x. [DOI] [PubMed] [Google Scholar]
- 6.Pickartz T, Mayerle J, Lerch MM. Autoimmune pancreatitis. Nat Clin Pract Gastroenterol Hepatol. 2007;4:314–323. doi: 10.1038/ncpgasthep0837. [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi K, Koike M, Tsuruta K, Okamoto A, Tabata I, Fujita N. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: a variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol. 1991;22:387–395. doi: 10.1016/0046-8177(91)90087-6. [DOI] [PubMed] [Google Scholar]
- 8.Kamisawa T, Funata N, Hayashi Y, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982–984. doi: 10.1007/s00535-003-1175-y. [DOI] [PubMed] [Google Scholar]
- 9.Comings DE, Skubi KB, Van Eyes J, Motulsky AG. Familial multi-focal fibrosclerosis: findings suggesting that retroperitoneal fibrosis, mediastinal fibrosis, sclerosing cholangitis, Riedel’s thyroiditis, and pseudotumor of the orbit may be different manifestations of a single disease. Ann Intern Med. 1967;66:884–892. doi: 10.7326/0003-4819-66-5-884. [DOI] [PubMed] [Google Scholar]
- 10.Chari ST, Kloeppel G, Zhang L, et al. Histopathologic and clinical subtypes of autoimmune pancreatitis: the Honolulu consensus document. Pancreas. 2010;39:549–554. doi: 10.1097/MPA.0b013e3181e4d9e5. [DOI] [PubMed] [Google Scholar]
- 11.Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis. Guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352–358. doi: 10.1097/MPA.0b013e3182142fd2. [DOI] [PubMed] [Google Scholar]
- 12.Mikulicz J. Über eine eigenartige symmetrishe Erkrankung der Tränen und Mundspeicheldrüsen. Stuttgart: Beitr z Chir Fesrschr f Theodor Billroth; 1892. pp. 610–630. [Google Scholar]
- 13.Küttner H. Über entzündiche Tumoren der submaaxillären Speicheldrüse. Beitr Klin Chir. 1896;15:815–834. [Google Scholar]
- 14.Kamisawa T, Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. J Gastroenterol. 2006;41:613–625. doi: 10.1007/s00535-006-1862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto M, Takahashi H, Ohara M, et al. A new conceptualization for Mikulicz’s disease as an IgG4-related plasmacytic disease. Mod Rheumatol. 2006;16:335–340. doi: 10.3109/s10165-006-0518-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masaki Y, Dong L, Kurose N, et al. Proposal for a new clinical entity, IgG4-positive multiorgan lymphoproliferative syndrome: analysis of 64 cases of IgG4-related disorders. Ann Rheum Dis. 2009;68:1310–1315. doi: 10.1136/ard.2008.089169. [DOI] [PubMed] [Google Scholar]
- 17.Umehara H, Okazaki K, Masaki Y, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22:21–30. doi: 10.3109/s10165-011-0571-z. [DOI] [PubMed] [Google Scholar]
- 18.Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181–1192. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 19.Stone JH, Khosroshahi A, Deshpande V, et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012;64:3061–3067. doi: 10.1002/art.34593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okazaki K, Uchida K, Matsushita M, Takaoka M. How to diagnose autoimmune pancreatitis by the revised Japanese clinical criteria. J Gastroenterol. 2007;42( Suppl 18):32–38. doi: 10.1007/s00535-007-2049-5. [DOI] [PubMed] [Google Scholar]
- 21.Okazaki K, Kawa S, Kamisawa T, et al. Amendment of the Japanese consensus guidelines for autoimmune pancreatitis, 2013 I. Concept and diagnosis of autoimmune pancreatitis. J Gastroenterol. 2014;49:567–588. doi: 10.1007/s00535-014-0942-2. [DOI] [PubMed] [Google Scholar]
- 22.Ohara H, Okazaki K, Tsubouchi H, et al. Clinical diagnostic criteria of IgG4-related sclerosing cholangitis 2012. J Hepatobiliary Pancreat Sci. 2012;19:536–542. doi: 10.1007/s00534-012-0521-y. [DOI] [PubMed] [Google Scholar]
- 23.Nakanuma Y, Zen Y. Pathology and immunopathology of immunoglobulin G4-related sclerosing cholangitis: the latest addition to the sclerosing cholangitis family. Hepatol Res. 2007;37( Suppl 3):S478–S486. doi: 10.1111/j.1872-034X.2007.00243.x. [DOI] [PubMed] [Google Scholar]
- 24.Umemura T, Zen Y, Hamano H, Kawa S, Nakanuma Y, Kiyosawa K. Immunoglobin G4-hepatopathy: association of immunoglobin G4-bearing plasma cells in liver with autoimmune pancreatitis. Hepatology. 2007;46:463–471. doi: 10.1002/hep.21700. [DOI] [PubMed] [Google Scholar]
- 25.Umemura T, Zen Y, Hamano H, et al. IgG4 associated autoimmune hepatitis: a differential diagnosis for classical autoimmune hepatitis. Gut. 2007;56:1471–1472. doi: 10.1136/gut.2007.122283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Umemura T, Zen Y, Hamano H, et al. Clinical significance of immunoglobulin G4-associated autoimmune hepatitis. J Gastroenterol. 2011;46( Suppl 1):48–55. doi: 10.1007/s00535-010-0323-4. [DOI] [PubMed] [Google Scholar]
- 27.Kawa S, Ota M, Yoshizawa K, et al. HLA DRB10405-DQB10401 haplotype is associated with autoimmune pancreatitis in the Japanese population. Gastroenterology. 2002;122:1264–1269. doi: 10.1053/gast.2002.33022. [DOI] [PubMed] [Google Scholar]
- 28.Umemura T, Ota M, Hamano H, Katsuyama Y, Kiyosawa K, Kawa S. Genetic association of Fc receptor-like 3 polymorphisms with autoimmune pancreatitis in Japanese patients. Gut. 2006;55:1367–1368. doi: 10.1136/gut.2006.095059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umemura T, Katsuyama Y, Hamano H, et al. Association analysis of Toll-like receptor 4 polymorphisms with autoimmune pancreatitis. Hum Immunol. 2009;70:742–746. doi: 10.1016/j.humimm.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Chang MC, Chang YT, Tien YW, et al. T-cell regulatory gene CTLA-4 polymorphism/haplotype association with autoimmune pancreatitis. Clin Chem. 2007;53:1700–1705. doi: 10.1373/clinchem.2007.085951. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe T, Yamashita K, Fujikawa S, et al. Involvement of activation of toll-like receptors and nucleotide-binding oligomerization domain-like receptors in enhanced IgG4 responses in autoimmune pancreatitis. Arthritis Rheum. 2012;64:914–924. doi: 10.1002/art.33386. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe T, Yamashita K, Sakurai T, et al. Toll-like receptor activation in basophils contributes to the development of IgG4-related disease. J Gastroenterol. 2013;48:247–253. doi: 10.1007/s00535-012-0626-8. [DOI] [PubMed] [Google Scholar]
- 33.Yamashina M, Nishio A, Nakayama S, et al. Comparative study on experimental autoimmune pancreatitis and its extrapancreatic involvement in mice. Pancreas. 2012;41:1255–1262. doi: 10.1097/MPA.0b013e31824a0e58. [DOI] [PubMed] [Google Scholar]
- 34.van der Neut Kolfschoten M, Schuurman J, Losen M, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317:1554–1557. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- 35.Kawa S, Kitahara K, Hamano H, et al. A novel immunoglobulin-immunoglobulin interaction in autoimmunity. PLoS One. 2008;3:e1637. doi: 10.1371/journal.pone.0001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishii K, Amagai M, Hall RP, et al. Characterization of autoantibodies in pemphigus using antigen-specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J Immunol. 1997;159:2010–2017. [PubMed] [Google Scholar]
- 37.Davies AM, Rispens T, Ooijevaar-de Heer P, et al. Structural determinants of unique properties of human IgG4-Fc. J Mol Biol. 2014;426:630–644. doi: 10.1016/j.jmb.2013.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zen Y, Fujii T, Harada K, et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology. 2007;45:1538–1546. doi: 10.1002/hep.21697. [DOI] [PubMed] [Google Scholar]
- 39.Miyoshi H, Uchida K, Taniguchi T, et al. Circulating naive and CD4+CD25high regulatory T cells in patients with autoimmune pancreatitis. Pancreas. 2008;36:133–140. doi: 10.1097/MPA.0b013e3181577553. [DOI] [PubMed] [Google Scholar]
- 40.Koyabu M, Uchida K, Miyoshi H, et al. Analysis of regulatory T cells and IgG4-positive plasma cells among patients of IgG4-related sclerosing cholangitis and autoimmune liver diseases. J Gastroenterol. 2010;45:732–741. doi: 10.1007/s00535-010-0199-3. [DOI] [PubMed] [Google Scholar]
- 41.Kusuda T, Uchida K, Miyoshi H, et al. Involvement of inducible costimulator- and interleukin 10-positive regulatory T cells in the development of IgG4-related autoimmune pancreatitis. Pancreas. 2011;40:1120–1130. doi: 10.1097/MPA.0b013e31821fc796. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka A, Moriyama M, Nakashima H, et al. Th2 and regulatory immune reactions contribute to IgG4 production and the initiation of Mikulicz disease. Arthritis Rheum. 2012;64:254–263. doi: 10.1002/art.33320. [DOI] [PubMed] [Google Scholar]
- 43.Muraki T, Hamano H, Ochi Y, et al. Autoimmune pancreatitis and complement activation system. Pancreas. 2006;32:16–21. doi: 10.1097/01.mpa.0000188308.75043.e4. [DOI] [PubMed] [Google Scholar]
- 44.Uchida K, Okazaki K, Konishi Y, et al. Clinical analysis of auto-immune-related pancreatitis. Am J Gastroenterol. 2000;95:2788–2794. doi: 10.1111/j.1572-0241.2000.03187.x. [DOI] [PubMed] [Google Scholar]
- 45.Okazaki K, Uchida K, Ohana M, et al. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology. 2000;118:573–581. doi: 10.1016/S0016-5085(00)70264-2. [DOI] [PubMed] [Google Scholar]
- 46.Nishi H, Tojo A, Onozato ML, et al. Anti-carbonic anhydrase II antibody in autoimmune pancreatitis and tubulointerstitial nephritis. Nephrol Dial Transplant. 2007;22:1273–1275. doi: 10.1093/ndt/gfl672. [DOI] [PubMed] [Google Scholar]
- 47.Aparisi L, Farre A, Gomez-Cambronero L, et al. Antibodies to carbonic anhydrase and IgG4 levels in idiopathic chronic pancreatitis: relevance for diagnosis of autoimmune pancreatitis. Gut. 2005;54:703–709. doi: 10.1136/gut.2004.047142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishimori I, Miyaji E, Morimoto K, Nagao K, Kamada M, Onishi S. Serum antibodies to carbonic anhydrase IV in patients with auto-immune pancreatitis. Gut. 2005;54:274–281. doi: 10.1136/gut.2004.049064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asada M, Nishio A, Uchida K, et al. Identification of a novel auto-antibody against pancreatic secretory trypsin inhibitor in patients with autoimmune pancreatitis. Pancreas. 2006;33:20–26. doi: 10.1097/01.mpa.0000226881.48204.fd. [DOI] [PubMed] [Google Scholar]
- 50.Uchida K, Okazaki K, Nishi T, et al. Experimental immune-mediated pancreatitis in neonatally thymectomized mice immunized with carbonic anhydrase II and lactoferrin. Lab Invest. 2002;82:411–424. doi: 10.1038/labinvest.3780435. [DOI] [PubMed] [Google Scholar]
- 51.Endo T, Takizawa S, Tanaka S, et al. Amylase alpha-2A autoanti-bodies: novel marker of autoimmune pancreatitis and fulminant type 1 diabetes. Diabetes. 2009;58:732–737. doi: 10.2337/db08-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takizawa S, Endo T, Wanjia X, Tanaka S, Takahashi M, Kobayashi T. HSP 10 is a new autoantigen in both autoimmune pancreatitis and fulminant type 1 diabetes. Biochem Biophys Res Commun. 2009;386:192–196. doi: 10.1016/j.bbrc.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 53.Kountouras J, Zavos C, Gavalas E, Tzilves D. Challenge in the pathogenesis of autoimmune pancreatitis: potential role of helicobacter pylori infection via molecular mimicry. Gastroenterology. 2007;133:368–369. doi: 10.1053/j.gastro.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 54.Kountouras J, Zavos C, Chatzopoulos D. A concept on the role of Helicobacter pylori infection in autoimmune pancreatitis. J Cell Mol Med. 2005;9:196–207. doi: 10.1111/j.1582-4934.2005.tb00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guarneri F, Guarneri C, Benvenga S. Helicobacter pylori and autoimmune pancreatitis: role of carbonic anhydrase via molecular mimicry? J Cell Mol Med. 2005;9:741–744. doi: 10.1111/j.1582-4934.2005.tb00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frulloni L, Lunardi C, Simone R, et al. Identification of a novel antibody associated with autoimmune pancreatitis. N Engl J Med. 2009;361:2135–2142. doi: 10.1056/NEJMoa0903068. [DOI] [PubMed] [Google Scholar]
- 57.Nakanuma Y. A novel approach to biliary tract pathology based on similarities to pancreatic counterparts: is the biliary tract an incomplete pancreas? Pathol Int. 2010;60:419–429. doi: 10.1111/j.1440-1827.2010.02543.x. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Lanzoni G, Carpino G, et al. Biliary tree stem cells, precursors to pancreatic committed progenitors: evidence for possible life-long pancreatic organogenesis. Stem Cells. 2013;31:1966–1979. doi: 10.1002/stem.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakanuma Y, Harada K, Sasaki M, Sato Y. Proposal of a new disease concept “biliary diseases with pancreatic counterparts”: anatomical and pathological bases. Histol Histopathol. 2014;29:1–10. doi: 10.14670/HH-29.1. [DOI] [PubMed] [Google Scholar]
- 60.Topazian M, Witzig TE, Smyrk TC, et al. Rituximab therapy for refractory biliary strictures in immunoglobulin G4-associated cholangitis. Clin Gastroenterol Hepatol. 2008;6:364–366. doi: 10.1016/j.cgh.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 61.Khosroshahi A, Carruthers MN, Deshpande V, Unizony S, Bloch DB, Stone JH. Rituximab for the treatment of IgG4-related disease: lessons from 10 consecutive patients. Medicine (Baltimore) 2012;91:57–66. doi: 10.1097/MD.0b013e3182431ef6. [DOI] [PubMed] [Google Scholar]
- 62.Maillette de Buy Wenniger LJ, Doorenspleet ME, Klarenbeek PL, et al. Immunoglobulin G4+ clones identified by next-generation sequencing dominate the B cell receptor repertoire in immunoglobulin G4 associated cholangitis. Hepatology. 2013;57:2390–2398. doi: 10.1002/hep.26232. [DOI] [PubMed] [Google Scholar]
- 63.Sumimoto K, Uchida K, Kusuda T, et al. The role of CD19+ CD-24high CD38high and CD19+ CD24high CD27+ regulatory B cells in patients with type 1 autoimmune pancreatitis. Pancreatology. 2014;14:193–200. doi: 10.1016/j.pan.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 64.Valencia X, Lipsky PE. CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nat Clin Pract Rheumatol. 2007;3:619–626. doi: 10.1038/ncprheum0624. [DOI] [PubMed] [Google Scholar]
- 65.Sakaguchi Y, Inaba M, Tsuda M, et al. The Wistar Bonn Kobori rat, a unique animal model for autoimmune pancreatitis with extrapancreatic exocrinopathy. Clin Exp Immunol. 2008;152:1–12. doi: 10.1111/j.1365-2249.2008.03588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]