Abstract
Background/Aims
Doublecortin and CaM kinase-like-1 (DCAMKL1) is a marker of stem cells expressed predominantly in the crypt base in the intestine. However, DCAMKL1-positive cells have been shown to be differentiated tuft cells rather than quiescent progenitors. Tuft cells are the only epithelial cells that express cyclooxygenase 2 (COX-2) in the normal intestinal epithelium. We previously generated Cdx2-transgenic mice as model mice for intestinal metaplasia and gastric carcinoma. In the current study, we investigated the association between COX-2 and DCAMKL1 in gastric carcinoma.
Methods
We examined the association between COX-2 and DCAMKL1 expression in gastric carcinomas in clinical samples (early gastric well-differentiated adenocarcinoma) and Cdx2-transgenic mice; and the DCAMKL1-transgenic mouse stomach using immunohistochemistry and quantitative real-time polymerase chain reaction.
Results
The COX-2-expressing cells were scattered, not diffusely expressed, in gastric carcinomas from humans and Cdx2-transgenic mice. DCAMKL1-positive cells were also scattered in the gastric carcinomas, indicating that tuft cells could still be present in gastric carcinoma. COX-2 was expressed in DCAMKL1-positive tuft cells in Cdx2- and DCAMKL1-transgenic mouse stomachs, whereas the Sox9 transcription factor was ubiquitously expressed in gastric carcinomas, including COX-2-positive cells.
Conclusions
COX-2 is expressed in DCAMKL1-expressing quiescent tuft cells in gastric carcinoma.
Keywords: Sox9, Cdx2-transgenic mice, Stem cell marker
INTRODUCTION
Doublecortin and CaM kinase-like-1 (DCAMKL1) expression has been demonstrated in nondividing cells in the lower crypt of the small intestine, indicating that DCAMKL1 may represent a putative intestinal stem cell marker. The absence of cell division, however, does not provide definitive proof of its role as an intestinal stem cell marker. Furthermore, DCAMKL1 has also been detected within epithelial cells of intestinal villi, raising the question of intestinal stem cell specificity.1 It is controversial whether DCAMKL1 is a marker of progenitor cells. In fact, it has recently been suggested that these cells are differentiated tuft cells rather than quiescent progenitors.1,2 We have recently shown that Sox9 is diffusely expressed in human intestinal metaplasia and gastric carcinoma.3 Sox9 is expressed in proliferating stem/progenitor cells found at the bottom third of Lieberkühn crypts throughout the length of the intestine.4,5
Tuft cells are found in the gastrointestinal tract and respiratory organs.6 They are reliably distinguished from other epithelial cells by their apical tuft of stiff microvilli that protrudes in the gut lumen. They also express molecular markers, such as DCAMKL1 and cyclooxygenase 2 (COX-2) enzyme.7
COX-2, an inducible cyclooxygenase, catalyzes the rate-limiting reaction of production of prostaglandin E2 from arachidonate. Accumulated evidence indicates that COX-2 expression is correlated with gastric cancer progression. It is upregulated in gastric cancer8–11 and associated with the depth of invasion, lymphatic vessel invasion, lymph node metastasis, and poor prognosis of human gastric carcinoma.12–15 Therefore, it has been suggested that COX-2 is a potential therapeutic target for prevention and treatment of gastric carcinoma.15–22
Helicobacter pylori induces chronic, persistent inflammatory responses that accelerate remodeling of the gastric epithelium and glandular loss (gastric atrophy) followed by intestinal metaplasia, dysplasia, and progression to gastric carcinoma.23,24 We have previously generated Cdx2-transgenic mice as model mice for intestinal metaplasia. The Cdx2-transgenic mice specifically express Cdx2 in the gastric mucosa by the rat H+/K+-ATPase β-subunit (Atp4b) gene promoter.25 The gastric mucosa of these mice is completely changed to intestinal metaplastic mucosa.25,26 Gastric carcinoma spontaneously develops from the intestinal metaplasia in all stomachs of Cdx2-transgenic mice examined.27
COX-2 is expressed in tumor epithelial cells and their surrounding stromal cells, while DCAMKL1 is expressed in epithelial cells, but not in their surrounding stromal cells. DCAMKL1 is a cell surface expression protein, and a specific antibody against the extracellular domain of DCAMKL1 has been used for immunohistochemistry in mice.2 However, an adequate antibody for DCAMKL1 for immunohistochemistry for human tissue is currently unavailable.
The purpose of this study is to evaluate whether DCAMKL1-positive epithelial cells express COX-2 because COX-2 plays an important role in mediating inflammation and carcinogenesis in the gastric epithelium. Therefore, we first examined whether the expression pattern of COX-2 in human gastric carcinoma is the same as that of the gastric carcinoma of Cdx2-transgenic mice. We then examined the association between COX-2 and DCAMKL1 expression in the gastric carcinoma of Cdx2-transgenic mice using DCAMKL1 antibody, which is available in mouse tissue. Since both chronic H. pylori-associated gastritis and Cdx2-transgenic mouse stomach develop intestinal metaplasia and finally gastric carcinoma, we used Cdx2-transgenic mice as the model for gastric carcinoma.
MATERIALS AND METHODS
1. Clinical samples
Formalin-fixed, paraffin-embedded samples for immunohistochemistry were obtained from gastric tumors and adjacent tissues of 46 patients with primary early gastric well-differentiated carcinoma who had undergone endoscopic submucosal dissection between January 2002 and December 2003, at Jichi Medical University Hospital, Japan. The diagnoses were confirmed by at least two pathologists. The degree of differentiation was evaluated by a pathologist and well-differentiated adenocarcinoma samples with mucosal invasion were used for the current study. All of the samples including gastric cancer samples were fixed in paraffin. Approval from the Institute Research Ethics Committee was obtained for the use of clinical materials described in the current study.
2. Cdx2-transgenic mice and DCAMKL1-transgenic mice
Cdx2-transgenic mice, with stomach-specific expression of Cdx2 under the control of the rat H+/K+-ATPase β-subunit (Atp4b) gene promoter, were used.25 The gastric mucosa of these mice is completely changed to intestinal metaplastic mucosa.25,26 We generated DCAMKL1-transgenic mice with stomach-specific expression of DCAMKL1 under the control of the rat H+/K+-ATPase β-subunit (Atp4b) gene promoter. Mice had free access to standard food and drinking water and were maintained on a 12-hour light/12-hour dark cycle. All experiments in this study were performed in accordance with the Jichi Medical University Guide for Laboratory Animals.
3. Immunohistochemistry
Gastric specimens were sectioned at a thickness of 3 μm and used for immunohistochemistry as described previously.25,26 Primary antisera were diluted in phosphate-buffered saline (PBS) and incubated overnight at 4°C. The next day, slides were washed in PBS and incubated with secondary antibody at 37°C for 30 minutes. Cy3 Donkey Anti-Goat IgG, Cy3 Donkey Anti-Rabbit IgG, Alexa Fluor 488 Anti-Rabbit IgG, and Alexa Fluor 488 Anti-Mouse IgG (Molecular Probes Inc., Eugene, OR, USA) were used as the secondary antibodies.
The panel of primary antisera included anti-COX-2 antibody (sc-1747: dilution of 1:200, goat polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-DCAMKL1 antibody (dilution of 1:30, rabbit polyclonal; Abgent, San Diego, CA, USA), anti-proliferating cell nuclear antigen (PCNA) antibody (dilution of 1:2,000, mouse monoclonal; Sigma-Aldrich, St. Louis, MO, USA), and anti-Sox9 antibody (dilution of 1:1,000, rabbit polyclonal; Millipore, Temecula, CA, USA).
The number of positive stained cells for COX-2 or DCAMKL1 was counted in five microscopic fields and then the mean value was calculated.
4. RNA isolation and quantitative real-time polymerase chain reaction
Total RNA was extracted from gastric mucosa (normal mice) and gastric carcinoma (Cdx2-transgenic mice) using the guanidinium isothiocyanate/phenol method (Isogen; Nippon Gene, Tokyo, Japan) according to the manufacturer’s instructions. Total RNA (1 μg) was reverse transcribed at 37°C for 1 hour in a final volume of 20 μL of reverse transcription buffer (50 mM Tris-HCl, pH 8.3, 50 mM KCl, 10 mM MgCl2, 0.5 mM spermidine, and 10 mM dithiothreitol) containing reverse transcriptase (ReverTra Ace; Toyobo, Osaka, Japan), 200 pmol random primers, and 1 mM dNTPs (Sigma-Aldrich). The cDNA (100 ng) was then used in each real-time polymerase chain reaction (PCR) to determine the expression levels for each specific gene. PCR was performed by ready-to-use Assay-on-Demand gene expression products (Applied Biosystems, Foster City, CA, USA): DCAMKL1, Mm00444950_m1; Cox2, Mm03294838_g1. Each Assay-on-Demand gene expression product contains target-specific primers and probes and a TaqMan Gene Expression Master Mix containing AmpErase uracil-N-glycosylase (Applied Biosystems) to prevent reamplification of carryover PCR products. PCR amplification and fluorescence data collection were performed with an ABI PRISM 7900HT sequence detection system (Applied Biosystems), using the following conditions: 50°C for 2 minutes, 95°C for 10 minutes, and then 40 cycles of amplification (95°C for 15 seconds, 60°C for 1 minute). All PCRs were performed in 96-well plates using a final volume of 20 μL, and each gene was studied in triplicate. To normalize RNA transcript abundance for each gene, the housekeeping gene β-actin (Pre-Developed TaqMan Assay Reagents; Applied Biosystems) was used to calculate the ΔCT (CT [target]/CT [actin]). The CT values for β-actin for normal mouse gastric tissues and Cdx2-transgenic mouse gastric carcinoma tissues were similar with no specific pattern of spatial or temporal variation detected (data not shown). A relative quantification approach was used in the current study to describe the change in expression of the target gene in a test sample relative to a calibrator sample (reference) as described previously.28 The normal mouse stomach was used as the reference for DCAMKL1 and Cox2 expression. Finally, the fold difference (relative abundance) was calculated using the formula 2−ΔΔCT and was plotted as the mean (n=6).29
5. Statistical analysis
Statistical analysis was performed by the Student t-test. A p<0.05 was considered to be statistically significant.
RESULTS
1. COX-2-expressing cells are scattered in human gastric carcinoma
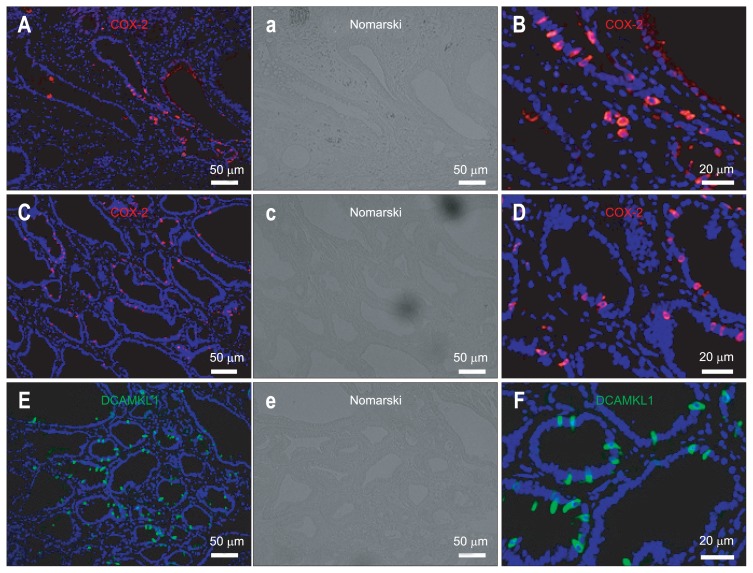
COX-2 expression in human gastric carcinomas was oberved using clinical specimens (gastric tumors) resected under esophagogastroduodenoscopy (Fig. 1A and B). COX-2-expressing cells were scattered and not diffusely expressed in human gastric carcinoma (Fig. 1A and B).
Fig. 1.
Cyclooxygenase 2 (COX-2)-positive cells are scattered in gastric carcinomas from human (A, B) and Cdx2-transgenic mouse stomachs (C, D). Doublecortin and CaM kinase-like-1 (DCAMKL1)-positive cells are scattered in the gastric carcinomas of Cdx2-transgenic mouse stomachs (E, F). (A) and (B) show immunofluorescence staining for COX-2 (Cy3) in human gastric carcinoma. (C) and (D) show immunofluorescence staining for COX-2 (Cy3) in the gastric carcinoma of Cdx2-transgenic mouse stomachs. (E) and (F) show immunofluorescence staining for DCAMKL1 (Alexa 488) in gastric carcinomas from Cdx2-transgenic mouse stomachs. COX-2-positive epithelial cells are scattered, not diffuse. DCAMKL1-positive epithelial cells are scattered, not diffuse. A portion of (A), (C), and (E) is magnified in panels (B), (D), and (F), respectively. The nuclei are stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Panels (a), (c), and (e) are Nomarski of panels (A), (C), and (E), respectively.
2. COX-2-expressing cells are scattered in gastric carcinoma of Cdx2-transgenic mouse stomach
COX-2 expression in the gastric carcinoma of Cdx2-transgenic mouse stomach was investigated (Fig. 1C and D). COX-2-expressing cells were also scattered, and not diffusely expressed in gastric carcinoma of Cdx2-transgenic mouse stomach (Fig. 1C and D). The expression pattern of COX-2 in the gastric carcinoma of Cdx2-transgenic mice was similar to that of humans. Because coexpression of COX-2 and DCAMKL1 has recently been shown in the normal intestinal epithelium,2 the association between COX-2 and DCAMKL1 in gastric carcinoma was analyzed. The following experiments for clarifying the association between COX-2 and DCAMKL1 were performed using Cdx2-transgenic mouse stomach because an adequate antibody for DCAMKL1 for immunohistochemistry for human tissues is currently unavailable.
3. DCAMKL1-expressing cells are scattered in gastric carcinoma of Cdx2-transgenic mouse stomach
To clarify the association between COX-2- and DCAMKL1-expressing cells, the expression of DCAMKL1 in gastric carcinoma of Cdx2-transgenic mouse stomach was evaluated first (Fig. 1E and F). DCAMKL1-expressing cells were scattered in gastric carcinoma of Cdx2-transgenic mouse stomach. The expression pattern of DCAMKL1 was similar to that of COX-2 in the gastric carcinoma of the human and Cdx2-transgenic mouse stomach.
4. COX-2 colocalizes with DCAMKL1 in the gastric carcinoma
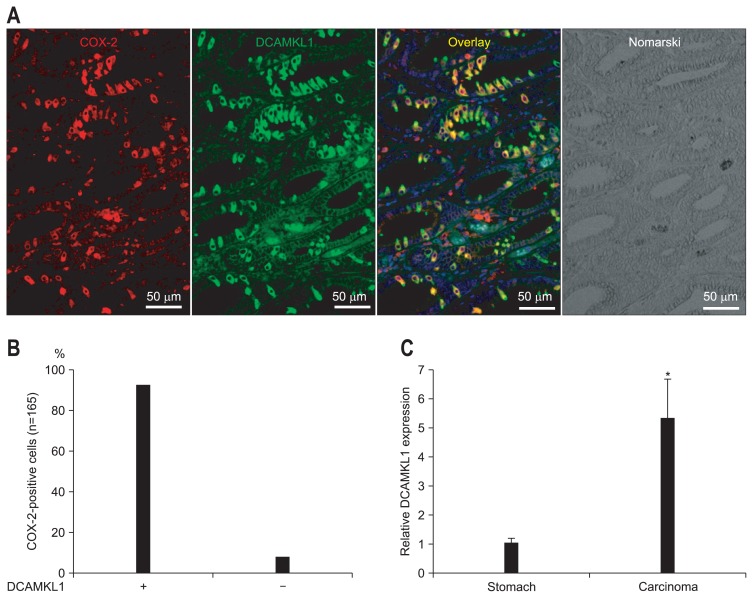
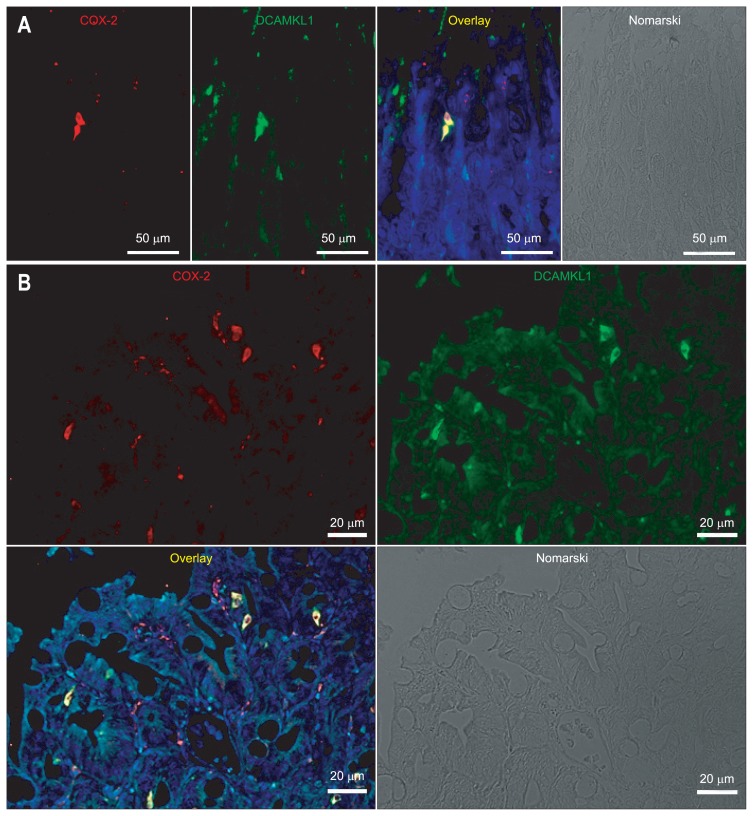
To investigate an association between COX-2- and DCAMKL1-expressing cells, costaining experiments in the gastric carcinoma were performed. COX-2-expressing cells were positive for DCAMKL1 in the gastric carcinoma of the Cdx2-transgenic mouse stomach (Fig. 2A).
Fig. 2.
Coexpression of cyclooxygenase 2 (COX-2) and doublecortin and CaM kinase-like-1 (DCAMKL1) in gastric carcinomas from Cdx2-transgenic mouse stomachs. (A) Immunofluorescence staining for COX-2 (Cy3; red) and DCAMKL1 (Alexa 488; green) in gastric carcinoma is shown. COX-2 and DCAMKL1 are colocalized (merged image). The nuclei are stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). (B) The number of DCAMKL1-positive cells was 152 among the COX-2-positive cells (n=165), indicating that 92% of the DCAMKL1-positive cells were positive for COX-2. (C) Quantitative real-time polymerase chain reaction analysis of DCAMKL1 expression. Messenger RNA levels of DCAMKL1 were compared between normal mouse stomachs and gastric carcinomas (Cdx2-transgenic mouse stomach). Each column indicates the means±SD of 6 tissue samples. *p<0.01 versus normal stomach values.
The number of COX-2- and DCAMKL1-positive cells was counted in the gastric carcinoma of Cdx2-transgenic mice. Ninety-two percent of COX-2-positive cells were DCAMKL1-positive (n=165) (Fig. 2B) and 89% of DCAMKL1-positive cells were COX-2-positive (n=170).
5. COX-2 and DCAMKL1 mRNA expression in gastric carcinoma of Cdx2-transgenic mouse stomach
For further investigation, COX-2 and DCAMKL1 expression in the gastric carcinoma of Cdx2-transgenic mouse stomach using quantitative real-time PCR was evaluated (Fig. 2C). Gastric carcinoma of Cdx2-transgenic mouse stomach exhibited a 25-fold increase in COX-2 mRNA expression compared with that in normal gastric mucosa (p<0.01). Gastric carcinoma of Cdx2-transgenic mouse stomach exhibited a 5-fold increase in DCAMKL1 mRNA expression compared with that in normal gastric mucosa (p<0.01) (Fig. 2C).
6. Proliferation status of DCAMKL1- and COX-2-expressing cells
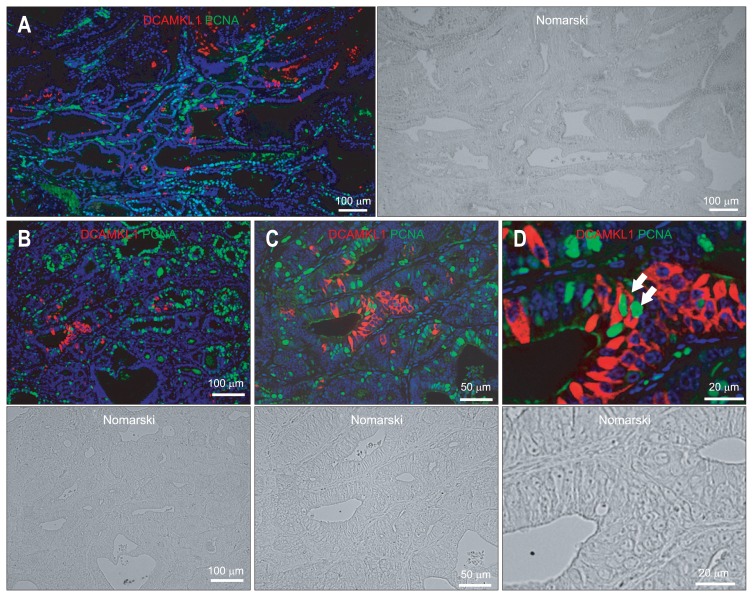
It has been shown that DCAMLK1-positive cells are never observed in a proliferative state.2,30,31 Cancer stem cells display several key characteristics of normal tissue stem cells, such as self-renewal and unlimited proliferative and differentiation capacity. Therefore, we confirmed whether DCAMKL1-positive cells in the gastric carcinoma are in a proliferative state using PCNA (Fig. 3). Almost all of the DCAMKL1-positive cells in the gastric carcinoma were not in an active cycle because no DCAMKL1 (Cy3; red) colocalization with PCNA (Alexa 488; green) was observed (Fig. 3). However, 0.5% of DCAMKL1-expressing cells were PCNA-positive (n=362) (Fig. 3D).
Fig. 3.
(A–D) Immunofluorescence staining for doublecortin and CaM kinase-like-1 (DCAMKL1) (Cy3; red) and PCNA (Alexa 488; green) in gastric carcinoma. The arrows in (D) indicate cells positive for both DCAMKL1 and proliferating cell nuclear antigen (PCNA). (C) A magnified image of (B). (D) A magnified image of (C). The nuclei are stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue).
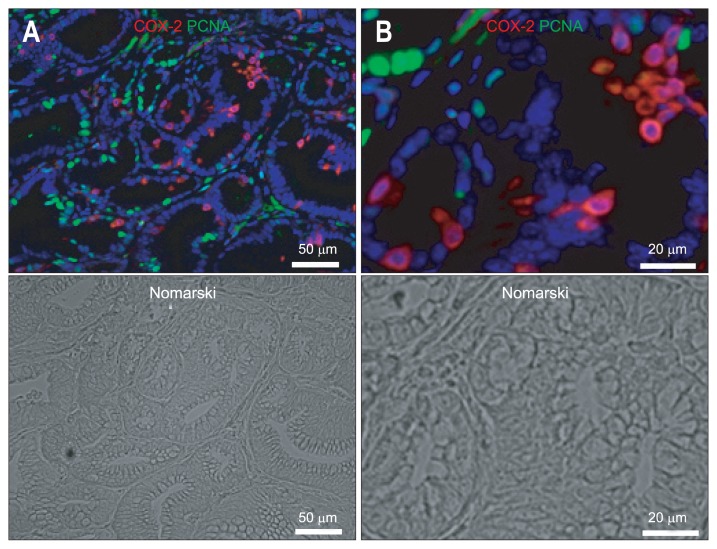
On the other hand, none of the COX-2-expressing cells in the gastric carcinoma were actively cycling because no COX-2 (Cy3; red) colocalization with PCNA (Alexa 488; green) was observed (Fig. 4).
Fig. 4.
(A, B) Immunofluorescence staining for cyclooxygenase 2 (COX-2) (Cy3; red) and proliferating cell nuclear antigen (PCNA) (Alexa 488; green) in gastric carcinoma. (B) A magnified image of (A). COX-2 (Cy3; red) does not colocalize with PCNA (Alexa 488; green). The nuclei are stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue).
7. COX-2-expressing cells are positive for DCAMKL1 in normal gastric mucosa
The expression of COX-2 and DCAMKL1 in normal mouse gastric mucosa was examined (Fig. 5A). COX-2 and DCAMKL1 were coexpressed in the isthmus/neck region of normal mouse gastric mucosa (Fig. 5A). COX-2-positive cells expressed DCAMKL1 (Fig. 5A).
Fig. 5.
Immunofluorescence staining for cyclooxygenase 2 (COX-2) (Cy3; red) and doublecortin and CaM kinase-like-1 (DCAMKL1) (Alexa 488; green) in normal gastric mucosa (A) and intestinal metaplasia (B). COX-2 and DCAMKL1 colocalize (merged image). The nuclei are stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue).
8. COX-2-expressing cells are positive for DCAMKL1 in intestinal metaplasia
Next, the expression of COX-2 and DCAMKL1 in the pre-malignant intestinal metaplastic mucosa was examined using Cdx2-transgenic mouse stomach (Fig. 5B). COX-2 and DCAMKL1 were coexpressed in intestinal metaplastic mucosa of Cdx2-transgenic mouse stomach (Fig. 5B). The cells positive for COX-2 and DCAMKL1 were scattered in the intestinal metaplastic mucosa, similar to the scattered but not diffuse expression, in the gastric carcinoma. COX-2-expressing cells were positive for DCAMKL1 in the intestinal metaplasia of the Cdx2-transgenic mouse stomach (Fig. 5B).
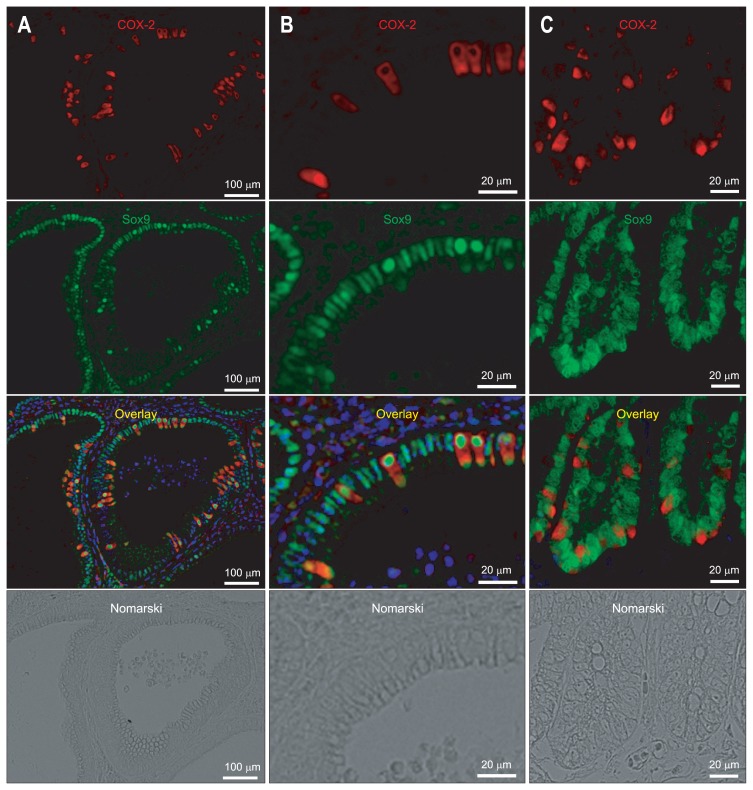
9. Sox9 expression in the gastric carcinoma
The association between Sox9 and COX-2 in gastric carcinoma of Cdx2-transgenic mice was examined (Fig. 6A and B). In contrast to DCAMKL1, Sox9 was diffusely expressed in epithelial cells of the gastric carcinoma. All of the gastric epithelial cells including COX-2-positive cells expressed Sox9 (Fig. 6A and B). Also, the association between Sox9 and COX-2 in human gastric carcinoma was examined. All of the gastric epithelial cells including COX-2-positive cells also expressed Sox9 in human gastric carcinoma (Fig. 6C).
Fig. 6.
Immunofluorescence staining for cyclooxygenase 2 (COX-2) (Cy3; red) and Sox9 (Alexa 488; green) in gastric carcinomas in Cdx2-transgenic mice (A, B) and humans (C). All of the gastric epithelial cells, including COX-2-positive cells, express Sox9. The nuclei are stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue).
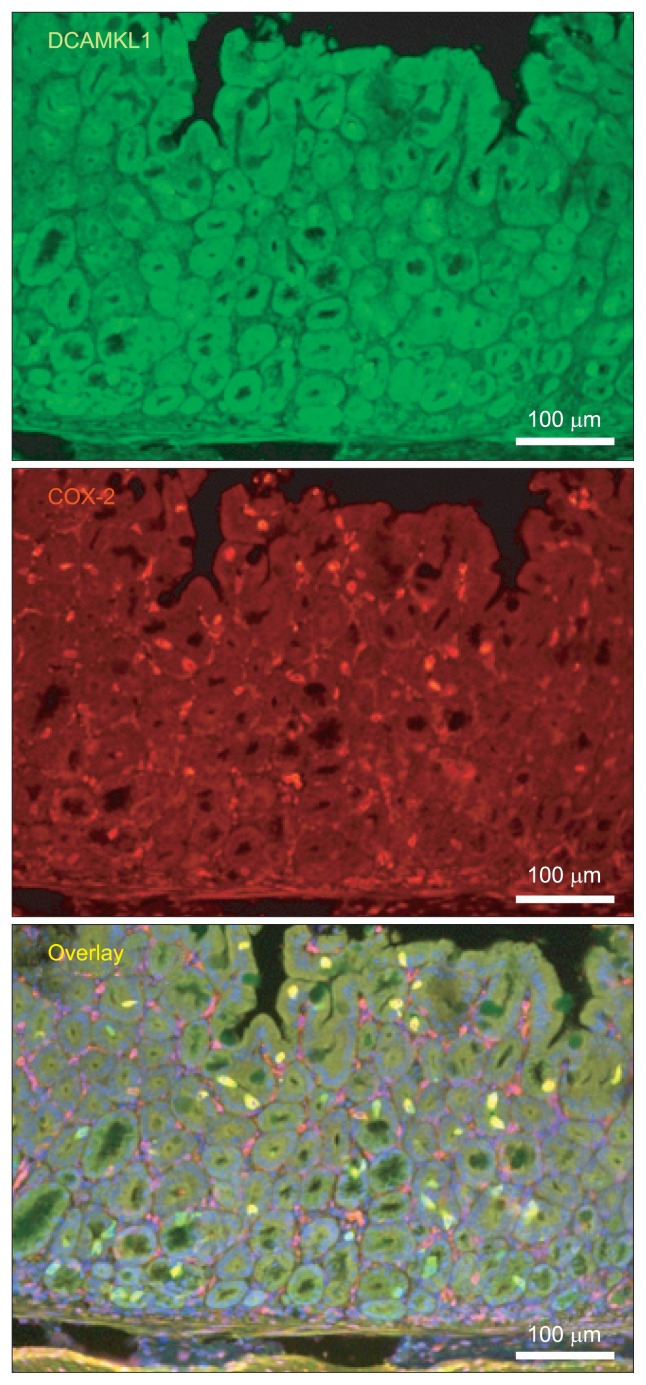
10. DCAMKL1 and COX-2 expression in gastric mucosa of DCAMKL1-transgenic mouse stomach
To clarify the association between DCAMKL1 and COX-2, DCAMKL1-transgenic mice with stomach specific expression of DACMKL1 under the control of the rat H+/K+-ATPase β-subunit (Atp4b) gene promoter was generated. DCAMKL1-expressing cells were scattered in gastric mucosa of DCAMKL1-transgenic mouse stomach (Fig. 7). DCAMKL1-expressing cells were positive for COX-2 in the gastric mucosa of the DCAMKL1-transgenic mouse stomach (Fig. 7).
Fig. 7.
Immunofluorescence staining for doublecortin and CaM kinase-like-1 (DCAMKL1) (Alexa 488; green) and cyclooxygenase 2 (COX-2) (Cy3; red) in DCAMKL1-transgenic mouse stomachs. DCAMKL1 and COX-2 colocalize (merged image). The nuclei are stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue).
DISCUSSION
We investigated gastric carcinoma of humans and Cdx2-transgenic mice to determine the association of COX-2-expressing cells with DCAMKL1-expressing cells. The current findings indicate that COX-2, which has been shown to be correlated with gastric carcinogenesis, is expressed in DCAMKL1-expressing specific quiescent tuft cells in gastric carcinoma.
DCAMKL1 has been reported to be a putative intestinal and gastric stem cell marker.30,32 In the current study, very few DCAMKL1-expressing cells were actively proliferating in the gastric carcinoma, similar to the finding that DCAMKL1 is expressed in quiescent cells located at the stem cell niche of mouse small intestine.30 DCAMKL1-expressing cells, isolated from the adult mouse small intestine by fluorescence-activated cell sorting (FACS), self-renew and ultimately form spheroids that form glandular epithelial structures, which express multiple markers of gut epithelial lineage.31 A previous study also reported DCAMKL1 expression in the isthmus/neck region of the corpus where putative stem cells reside in the normal gastric mucosa.30 Furthermore, these solitary DCAMKL1-positive cells do not express biomarkers associated with differentiating members of enteroendocrine, parietal, or pit cell lineages.30 However, in the current study, DCAMKL1-positive cells in the gastric carcinoma expressed COX-2. DCAMKL1-positive cells in normal gastric mucosa also expressed COX-2, indicating that DCAMKL1-positive cells express biomarkers associated with differentiation. These results indicate that DCAMKL1 may not be a stem cell marker in normal gastric mucosa.
Quantitative real-time PCR showed that COX-2 was increased in gastric carcinoma compared with that in the normal stomach. These results are compatible with previous reports that COX-2 is related to carcinogenesis.8–11 COX-2-positive cells, which express DCAMKL1, were scattered, not diffusely expressed, indicating that gastric carcinoma cells are not homogeneous. Many solid tumors contain cancer stem cells which are a small subpopulation of cells that can give rise to tumor mass.33,34 Also, DCAMKL1-positive cells were scattered, which indicates that a subpopulation among gastric carcinoma cells expresses DCAMKL1, suggesting that DCAMKL1-positive cells may be cancer stem cells. However, DCAMKL1-positive cells express the differentiation marker COX-2, which does not support the notion that DCAMKL1 is a cancer stem cell marker. COX-2 expressed in scattered DCAMKL1-positive cells in gastric carcinoma may be related to gastric carcinogenesis.
The finding that COX-2-expressing gastric carcinoma cells of humans and mice were scattered, not diffusely expressed, coincides with that of human colon adenoma and adenocarcinoma.2 COX-2-positive cells are also scattered in colon tumors from Apc- or K-Ras-mutated mice.2,35,36 In both cases, COX-2-positive cells invariably stained negative for expression of the PCNA proliferation marker. DCAMKL1 has been shown to be overexpressed in various human cancers including stomach, colon, pancreas, prostate, and breast cancers.37 According to the current results, the observed increase in DCAMKL1 indicates an increase of COX-2-expressing cells. Increased DCAMKL1 expression may be related to carcinogenesis through upregulation of COX-2 expression in tuft cells. Further clarification is needed to determine whether DCAMKL1 participates or not in the regulation of COX-2 expression.
DCAMKL-1 was identified by Giannakis et al.’s group30,38 as a marker of adult gut stem cells by the comparison of gut epithelial progenitors with their descendant cells. In the current study, when counting COX-2-expressing cells among DCAMKL1-positive cells in the gastric carcinoma, we found that approximately 10% of DCAMKL1-positive cells were DCAMKL1-positive and COX-2-negative cells (Fig. 2B). Gerbe et al.2 also showed that 98.1% of DCAMKL1-positive cells in the normal intestine were Cox1-positive, and that the rare DCAMKL1-positive and Cox1-negative cells were mainly found in the lower half of the crypts. Therefore, it cannot be ruled out that a small amount of DCAMKL1-positive and COX-2-negative cells, isolated from the adult mouse small intestine by FACS, may self-renew and ultimately form spheroids that form glandular epithelial structures.
In conclusion, the presence of tuft cells in gastric carcinoma as well as coexpression of COX-2 and DCAMKL1 in gastric carcinoma was demonstrated in this study. Overexpression of DCAMKL1 in gastric carcinoma may be associated with overexpression of COX-2, which is associated with gastric carcinogenesis. In order to determine whether DCAMKL1 participates or not in the regulation of COX-2 expression, we have generated DCAMKL1-transgenic mice that express DCAMKL1 specifically in the stomach. In the DCAMKL1-transgenic mouse, DCAMKL1 induced COX-2 in vivo. Currently, the DCAMKL1-transgenic mice is being analyzed in detail. The data obtained from DCAMKL1-transgenic mice may lead to the clarification of the role of DCAMKL1 in the gastric carcinogenesis from the viewpoint of the association between DCAMKL1 and COX-2.
ACKNOWLEDGEMENTS
This work was supported in part by a Grant-in-Aid for Scientific Research (C) (21590793 and 21590794 to H.M.) and a Grant-in-Aid for Scientific Research on Innovative Areas (22114002 to H.M.) from the Japan Society for the Promotion of Science. This publication was subsidized by JKA through its promotion funds from KEIRIN RACE.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Gerbe F, Brulin B, Makrini L, Legraverend C, Jay P. DCAMKL-1 expression identifies Tuft cells rather than stem cells in the adult mouse intestinal epithelium. Gastroenterology. 2009;137:2179–2180. doi: 10.1053/j.gastro.2009.06.072. [DOI] [PubMed] [Google Scholar]
- 2.Gerbe F, van Es JH, Makrini L, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011;192:767–780. doi: 10.1083/jcb.201010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sashikawa Kimura M, Mutoh H, Sugano K. SOX9 is expressed in normal stomach, intestinal metaplasia, and gastric carcinoma in humans. J Gastroenterol. 2011;46:1292–1299. doi: 10.1007/s00535-011-0443-5. [DOI] [PubMed] [Google Scholar]
- 4.Blache P, van de Wetering M, Duluc I, et al. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol. 2004;166:37–47. doi: 10.1083/jcb.200311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jay P, Berta P, Blache P. Expression of the carcinoembryonic antigen gene is inhibited by SOX9 in human colon carcinoma cells. Cancer Res. 2005;65:2193–2198. doi: 10.1158/0008-5472.CAN-04-1484. [DOI] [PubMed] [Google Scholar]
- 6.Sato A. Tuft cells. Anat Sci Int. 2007;82:187–199. doi: 10.1111/j.1447-073X.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- 7.Bezençon C, Fürholz A, Raymond F, et al. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J Comp Neurol. 2008;509:514–525. doi: 10.1002/cne.21768. [DOI] [PubMed] [Google Scholar]
- 8.Ristimäki A, Honkanen N, Jänkälä H, Sipponen P, Härkönen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997;57:1276–1280. [PubMed] [Google Scholar]
- 9.Uefuji K, Ichikura T, Mochizuki H, Shinomiya N. Expression of cyclooxygenase-2 protein in gastric adenocarcinoma. J Surg Oncol. 1998;69:168–172. doi: 10.1002/(SICI)1096-9098(199811)69:3<168::AID-JSO9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto H, Itoh F, Fukushima H, Hinoda Y, Imai K. Overexpression of cyclooxygenase-2 protein is less frequent in gastric cancers with microsatellite instability. Int J Cancer. 1999;84:400–403. doi: 10.1002/(SICI)1097-0215(19990820)84:4<400::AID-IJC12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 11.Lin PT, Gleeson JG, Corbo JC, Flanagan L, Walsh CA. DCAMKL1 encodes a protein kinase with homology to doublecortin that regulates microtubule polymerization. J Neurosci. 2000;20:9152–9161. doi: 10.1523/JNEUROSCI.20-24-09152.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohno R, Yoshinaga K, Fujita T, et al. Depth of invasion parallels increased cyclooxygenase-2 levels in patients with gastric carcinoma. Cancer. 2001;91:1876–1881. doi: 10.1002/1097-0142(20010515)91:10<1876::AID-CNCR1209>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 13.Shi H, Xu JM, Hu NZ, Xie HJ. Prognostic significance of expression of cyclooxygenase-2 and vascular endothelial growth factor in human gastric carcinoma. World J Gastroenterol. 2003;9:1421–1426. doi: 10.3748/wjg.v9.i7.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murata H, Kawano S, Tsuji S, et al. Cyclooxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol. 1999;94:451–455. doi: 10.1111/j.1572-0241.1999.876_e.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen JH, Wu CW, Kao HL, et al. Effects of COX-2 inhibitor on growth of human gastric cancer cells and its relation to hepatocyte growth factor. Cancer Lett. 2006;239:263–270. doi: 10.1016/j.canlet.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Yeh TS, Wu CW, Hsu KW, et al. The activated Notch1 signal pathway is associated with gastric cancer progression through cyclooxygenase-2. Cancer Res. 2009;69:5039–5048. doi: 10.1158/0008-5472.CAN-08-4021. [DOI] [PubMed] [Google Scholar]
- 17.Hsu KW, Hsieh RH, Wu CW, et al. MBP-1 suppresses growth and metastasis of gastric cancer cells through COX-2. Mol Biol Cell. 2009;20:5127–5137. doi: 10.1091/mbc.E09-05-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu PJ, Yu J, Zeng ZR, et al. Chemoprevention of gastric cancer by celecoxib in rats. Gut. 2004;53:195–200. doi: 10.1136/gut.2003.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee DS, Moss SF. COX-2 inhibition and the prevention of gastric cancer. Digestion. 2006;74:184–186. doi: 10.1159/000100502. [DOI] [PubMed] [Google Scholar]
- 20.Jiang XH, Wong BC. Cyclooxygenase-2 inhibition and gastric cancer. Curr Pharm Des. 2003;9:2281–2288. doi: 10.2174/1381612033453983. [DOI] [PubMed] [Google Scholar]
- 21.Fujimura T, Ohta T, Oyama K, Miyashita T, Miwa K. Role of cyclooxygenase-2 in the carcinogenesis of gastrointestinal tract cancers: a review and report of personal experience. World J Gastroenterol. 2006;12:1336–1345. doi: 10.3748/wjg.v12.i9.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saukkonen K, Tomasetto C, Narko K, Rio MC, Ristimäki A. Cyclooxygenase-2 expression and effect of celecoxib in gastric adenomas of trefoil factor 1-deficient mice. Cancer Res. 2003;63:3032–3036. [PubMed] [Google Scholar]
- 23.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process: first American Cancer Society Award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 24.Correa P, Fox J, Fontham E, et al. Helicobacter pylori and gastric carcinoma: serum antibody prevalence in populations with contrasting cancer risks. Cancer. 1990;66:2569–2574. doi: 10.1002/1097-0142(19901215)66:12<2569::AID-CNCR2820661220>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 25.Mutoh H, Hakamata Y, Sato K, et al. Conversion of gastric mucosa to intestinal metaplasia in Cdx2-expressing transgenic mice. Biochem Biophys Res Commun. 2002;294:470–479. doi: 10.1016/S0006-291X(02)00480-1. [DOI] [PubMed] [Google Scholar]
- 26.Mutoh H, Satoh K, Kita H, et al. Cdx2 specifies the differentiation of morphological as well as functional absorptive enterocytes of the small intestine. Int J Dev Biol. 2005;49:867–871. doi: 10.1387/ijdb.052016hm. [DOI] [PubMed] [Google Scholar]
- 27.Mutoh H, Sakurai S, Satoh K, et al. Development of gastric carcinoma from intestinal metaplasia in Cdx2-transgenic mice. Cancer Res. 2004;64:7740–7747. doi: 10.1158/0008-5472.CAN-04-1617. [DOI] [PubMed] [Google Scholar]
- 28.Mutoh H, Hayakawa H, Sashikawa M, Sakamoto H, Sugano K. Direct repression of Sonic hedgehog expression in the stomach by Cdx2 leads to intestinal transformation. Biochem J. 2010;427:423–434. doi: 10.1042/BJ20091177. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Giannakis M, Stappenbeck TS, Mills JC, et al. Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J Biol Chem. 2006;281:11292–11300. doi: 10.1074/jbc.M512118200. [DOI] [PubMed] [Google Scholar]
- 31.May R, Sureban SM, Hoang N, et al. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells. 2009;27:2571–2579. doi: 10.1002/stem.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montgomery RK, Breault DT. Small intestinal stem cell markers. J Anat. 2008;213:52–58. doi: 10.1111/j.1469-7580.2008.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 34.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 35.Janssen KP, el-Marjou F, Pinto D, et al. Targeted expression of oncogenic K-ras in intestinal epithelium causes spontaneous tumorigenesis in mice. Gastroenterology. 2002;123:492–504. doi: 10.1053/gast.2002.34786. [DOI] [PubMed] [Google Scholar]
- 36.Colnot S, Niwa-Kawakita M, Hamard G, et al. Colorectal cancers in a new mouse model of familial adenomatous polyposis: influence of genetic and environmental modifiers. Lab Invest. 2004;84:1619–1630. doi: 10.1038/labinvest.3700180. [DOI] [PubMed] [Google Scholar]
- 37.Sureban SM, May R, Mondalek FG, et al. Nanoparticle-based delivery of siDCAMKL-1 increases MicroRNA-144 and inhibits colorectal cancer tumor growth via a Notch-1 dependent mechanism. J Nanobiotechnol. 2011;9:40. doi: 10.1186/1477-3155-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giannakis M, Chen SL, Karam SM, Engstrand L, Gordon JI. Helicobacter pylori evolution during progression from chronic atrophic gastritis to gastric cancer and its impact on gastric stem cells. Proc Natl Acad Sci U S A. 2008;105:4358–4363. doi: 10.1073/pnas.0800668105. [DOI] [PMC free article] [PubMed] [Google Scholar]