Abstract
Background/Aims
This study aimed to compare the outcomes of endoscopic submucosal dissection (ESD) and gastrectomy based on the two sets of indications for ESD, namely guideline criteria (GC) and expanded criteria (EC).
Methods
Between January 2004 and July 2007, 213 early gastric cancer (EGC) patients were enrolled in this study. Of these patients, 142 underwent ESD, and 71 underwent gastrectomy. We evaluated the clinical outcomes of these patients according to the criteria.
Results
The complication rates in the ESD and gastrectomy groups were 8.5% and 28.2%, respectively. The duration of hospital stay was significantly shorter in the ESD group than the gastrectomy group according to the GC and EC (p<0.001 and p<0.001, respectively). There was no recurrence in the ESD and gastrectomy groups according to the GC, and the recurrence rates in the ESD and gastrectomy groups were 4.7% and 0.0% according to the EC, respectively (p=0.279). The occurrence rates of metachronous cancer in the ESD and gastrectomy groups were 5.7% and 5.0% according to the GC (p=1.000) and 7.5% and 0.0% according to the EC (p=0.055), respectively.
Conclusions
Based on safety, duration of hospital stay, and long-term outcomes, ESD may be an effective and safe first-line treatment for EGC according to the EC and GC.
Keywords: Endoscopic submucosal dissection, Gastrectomy, Long-term outcome, Indication
INTRODUCTION
Early gastric cancer (EGC) is defined as gastric cancer that is confined to the mucosa or submucosa, irrespective of the presence of regional lymph node metastases.1 EGC is associated with an excellent prognosis, with reports indicating a 5-year survival rate above 90% worldwide.2 Surgical resection has been performed as the conventional treatment for EGC. However, in selected cases, endoscopic resection is widely accepted due to its minimal invasiveness, low cost, patient tolerance, and better quality of life after the procedure.3 The criteria for endoscopic submucosal dissection (ESD) according to the Japanese Gastric Cancer Association are strictly limited to the absolute indication.1,4 Endoscopic resection has been limited to EGC with differentiated histopathologic features, without submucosal extension, that is smaller than 2 cm and not associated with ulceration.5,6 However, owing to the development of endoscopic techniques and devices, the rate of en bloc resection and complete resection (CR) has increased. ESD has been performed as the primary treatment for selected EGCs. Gotoda et al.5 reported the expanded criteria for ESD. In this report, cases in accordance with the expanded criteria displayed a low probability of lymph node metastasis and a high probability of complete recovery. Therefore, ESD represents a main treatment option for selected EGC cases.
The aim of this study was to compare the long-term outcomes of ESD and gastrectomy according to the guidelines and expanded criteria for ESD.
MATERIALS AND METHODS
1. Patients
Between January 2004 and July 2007, 413 EGCs of 379 patients were treated using ESD or gastrectomy at Soonchunhyang University Bucheon Hospital. Exclusion criteria were patients who did not meet the guideline criteria (GC) and expanded criteria (EC) based on histology after ESD or gastrectomy and were not followed for less than 60 months.
The EGC patients treated with ESD or gastrectomy were classified into the GC and EC groups. Characteristics of GC included: 1) differentiated adenocarcinoma confined to the mucosa; and 2) elevated lesions ≤2 cm and depressed lesions ≤1 cm without ulceration.7 Characteristics of EC included: 1) nonulcerated differentiated intramucosal cancers without limitation of tumor size; 2) ulcerated differentiated intramucosal cancers measuring ≤ 3 cm; 3) differentiated minute submucosal cancer ≤3 cm (SM1, ≤500 μm); and 4) nonulcerated undifferentiated intramucosal cancers ≤2 cm.5
The records of all patients with EGC treated by ESD or gastrectomy were analyzed retrospectively. All EGCs were classified according to histopathological findings after the procedure. This study was approved by the Ethics Committee and Institutional Review Board of our institution.
2. ESD
Patients were sedated with midazolam (2.0 to 6.0 mg) and propofol (20 to 200 mg) administered intravenously. After observation of the lesion, circumferential markings were made 5mm from the lateral side of the tumor margin with argon plasma coagulation. Normal saline mixed with a small volume of indigo carmine and diluted epinephrine (1:100,000) was injected into the lesion. Cutting was done along the outside of the marked area with a knife (Hook Knife or IT Knife; Olympus, Tokyo, Japan). Submucosal dissection of the lesion was performed after incision for the mucosa. Following resection of the lesion, all visible vessels on the ulcer floor were coagulated with a Coagrasper (FD-410LR; Olympus) and VIO 300D or ICC 200 (ERBE, Tübingen, Germany). If bleeding occurred during the procedure, hemostatic clips (HX-600-090L; Olympus) or a Coagrasper was applied to control the bleeding.
3. Gastrectomy
In the gastrectomy group, EGC was treated by open or laparoscopic gastrectomy according to surgeon or patient preference. The extent of gastrectomy (total/distal/proximal) was determined based on the location and size of the tumor. After total gastrectomy (seven patients), the Roux-en-Y esophagojejunostomy and jejuno-jejunostomy were performed.
4. Assessment of therapeutic efficacy
The location and shape of the lesions were reviewed using endoscopic images and reports. Other characteristics, such as the exact carcinoma size, degree of differentiation, depth of tumor and presence of lymphovascular invasion, were analyzed using histopathological examination. Differentiated adenocarcinoma included well and moderately differentiated tubular adenocarcinoma and papillary adenocarcinoma. Undifferentiated adenocarcinoma comprised the following: poorly differentiated tubular adenocarcinoma, signet ring cell carcinoma, or other histological types.8 The short-term outcomes were evaluated on the basis of the rate of en bloc resection, histological CR, the complication rate, and hospital days after procedure. The definition of CR in this study was the cancer-free lateral margin of at least 2 mm and the cancer free vertical margin of at least 0.1 mm, regardless of whether en bloc resection or multi-fragment resection had been performed.9 Incomplete resection was defined as when the cancer-free lateral margin was less than 2 mm, the cancer-free vertical margin less than 0.1 mm regardless of submucosal invasion, or the presence of lymphovascular invasion. Significant bleeding was defined as a hemorrhage resulting in hematemesis, melena, or hemoglobin drop >2 g/dL that required endoscopic treatment or transfusion. Perforation was defined as a gross defect or the presence of free air on radiography following the procedure.
5. Endoscopic and radiologic surveillance for determining the long-term outcome
Scheduled surveillance endoscopy was performed every 6 months for the first 2 years and annually thereafter. Radiologic surveillance using computerized tomography and chest X-ray were performed on an annual basis. Frequent short-term endoscopic follow-up was applied for patients with incomplete resection in the ESD group. Local recurrence was defined as a cancer detected in the follow-up forceps biopsy of previous ESD scar or anastomosis sites, regardless of the period from ESD or gastrectomy. Tumor recurrence was defined as local recurrence, lymph node metastasis, and distant metastasis. Metachronous cancer was defined as a newly developed cancer after 1 year of ESD. Procedure-related mortality was defined as any death within 30 days after ESD.10 The overall survival rate was defined as the proportion of patients who had survived from causes of gastric-cancer-related and unrelated death after ESD or gastrectomy. The disease-free survival rate was defined as the proportion of patients who had survived without any sign or symptoms of gastric cancer since ESD or gastrectomy had been performed.
6. Statistical analyses
Continuous variables were assessed by Student t-test and are presented as means±SD. The chi-square test was used for categorical variables. The overall survival rate and the disease-free survival rate were determined by the Kaplan-Meier plot and log-rank test. A p<0.05 was considered to indicate statistical significance. All analyses were performed using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Clinical and endoscopic characteristics
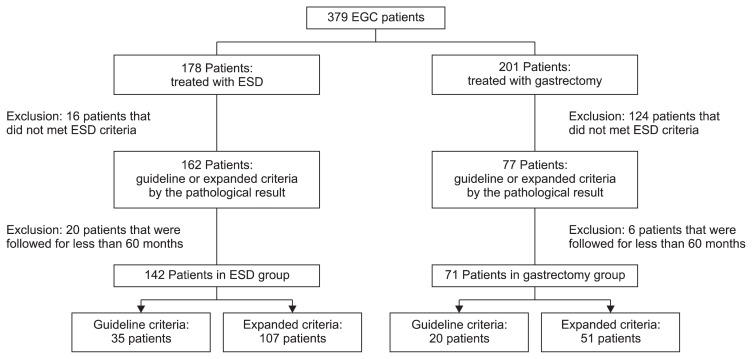
Based on the histology after ESD or gastrectomy, 213 EGC patients were enrolled in this study. Of these patients, 142 underwent ESD and 71 underwent gastrectomy during the study period. In the ESD group, 15 patients had two lesions and one patient had three lesions. All lesions were synchronous. Among the patients who underwent ESD, 35 were in the GC group and 107 were in the EC group. Among those patients who underwent gastrectomy, 20 were in the GC group and 51 in the EC group (Fig. 1).
Fig. 1.
Flowchart of enrolled patients.
EGC, early gastric cancer; ESD, endoscopic submucosal dissection.
The follow-up periods (mean±SD) of the ESD and gastrectomy groups were 76.7±16.5 and 65.5±16.5 months, respectively. The ages (mean±SD) of the patients treated by ESD and gastrectomy were 62.0±10.3 and 56.7±12.0 years, respectively (p=0.001). The male to female ratios of those who underwent ESD and gastrectomy were 66.2% (94/48) and 81.7% (58/13), respectively (p=0.018). The most common location of the lesions was the lower third (47.9% in the ESD group and 87.3% in the gastrectomy group). The clinical and endoscopic characteristics of the enrolled patients are shown in Table 1.
Table 1.
Clinical and Endoscopic Characteristics
| Characteristic | ESD group (n=142) | Gastrectomy group (n=71) | p-value |
|---|---|---|---|
| Age, yr | 62.0±10.3 | 56.7±12.0 | 0.001 |
| Sex, male/female | 94/48 | 58/13 | 0.018 |
| Concomitant ulceration | 47 (33.1) | 14 (19.7) | 0.042 |
| Gross type | |||
| Depressed | 33 (23.2) | 26 (36.6) | 0.075 |
| Flat | 46 (32.4) | 23 (32.4) | |
| Elevated | 63 (44.4) | 22 (31.0) | |
| Size, cm | |||
| ≤1 | 24 (16.9) | 11 (15.5) | 0.589 |
| ≤2 | 70 (72.7) | 39 (54.9) | |
| ≤3 | 30 (21.1) | 10 (14.1) | |
| >3 | 18 (12.7) | 11 (15.5) | |
| Location | |||
| Upper 1/3 | 9 (6.3) | 8 (11.3) | |
| Mid 1/3 | 65 (45.8) | 1 (1.4) | |
| Lower 1/3 | 68 (47.9) | 62 (87.3) | <0.001 |
| Follow-up, mo | 76.7±16.5 | 65.5±16.5 | <0.001 |
Data are presented as mean±SD or number (%).
ESD, endoscopic submucosal dissection.
2. Histopathological characteristics
Histology revealed that 89.4% of the ESD group and 76.1% of the gastrectomy group had a differentiated-type tumor (p=0.01). SM invasion of the tumor was more frequent in the gastrectomy group than the ESD group (4.9% vs 12.7%, p=0.04). Based on the histology from ESD or surgical specimens, each group was further divided into GC and EC subgroups. Histopathological assessment is shown in Table 2.
Table 2.
Histopathological Characteristics
| Characteristic | ESD group (n=142) | Gastrectomy group (n=71) | p-value |
|---|---|---|---|
| Histology | 0.01 | ||
| Differentiated | 127 (89.4) | 54 (76.1) | |
| Undifferentiated | 15 (10.6) | 17 (23.9) | |
| SM1 invasion | 7 (4.9) | 9 (12.7) | 0.04 |
| Criteria | |||
| Guideline | 35 (24.6) | 20 (28.2) | 0.58 |
| Expanded | 107 (75.4) | 51 (71.8) |
Data are presented as number (%).
ESD, endoscopic submucosal dissection; SM, submucosa.
3. Short-term outcomes
In the ESD group, the rates of en bloc resection and CR were 97.1% and 97.1% for GC, 86.4% and 81.8% for EC, respectively. No differences between the selection of operative methods between the open and laparoscopic gastrectomy groups were observed. Proximal gastrectomy was performed in two patients in the laparoscopic gastrectomy group, due to the patient’s preference. D2 lymph node dissection was performed in 65 patients (91.5%), and six patients underwent D1+β lymph node dissection. After distal gastrectomy (62 patients), gastroduodenostomy was performed in 20 patients and gastrojejunostomy was performed in 42 patients. In two cases of proximal gastrectomy, esophagogastrostomy using circular staplers was performed after proximal gastrectomy.
The rates of significant bleeding were 3.5% in the ESD group and 15.5% in the gastrectomy group (p=0.004). One patient underwent gastrectomy due to post-ESD bleeding, and other bleeding events were successfully managed using endoscopic clipping or coagulation therapy with a Coagrasper. The rate of perforation after ESD was 4.9% (7/142). All perforations were managed by endoscopic closure with clipping and conservative treatment, including intravenous antibiotics. The rate of wound complication after gastrectomy was 5.6% (4/71). Other complications that occurred after gastrectomy included duodenal leakage, ileus and hepatic dysfunction in GC, and pancreatic leakage and duodenal stump leakage in EC. The bleeding and pancreatic leakage patients underwent reoperations. In addition, the duodenal stump leakage patient underwent pigtail catheter insertion. All other complications were controlled by conservative treatment. The complication rates of ESD and gastrectomy were 8.5% (12/145) and 28.2% (20/71), respectively. The hospital stay durations (mean±SD) of the ESD and gastrectomy groups were 6.1±2.4 and 13.0±7.3 days, respectively, for GC (p<0.001), 6.6±3.0 and 13.5±17.5 days, respectively, for EC (p<0.001). Procedure-related complications and clinical outcomes are shown in Tables 3 and 4.
Table 3.
Complications of Endoscopic Submucosal Dissection and Gastrectomy
| Total (n=213) | ESD group (n=142) | Gastrectomy group (n=71) | p-value | |
|---|---|---|---|---|
| Significant bleeding | 16 (7.5) | 5 (3.5) | 11 (15.5) | 0.004 |
| Perforation | 7 (3.3) | 7 (4.9) | 0 | 0.135 |
| Wound complication | 4 (1.9) | 0 | 4 (5.6) | 0.020 |
| Others* | 5 (2.3) | 0 | 5 (7.0)* | 0.006 |
| Total | 32 (15.0) | 12 (8.5) | 20 (28.2) | <0.01 |
Data are presented as number (%).
ESD, endoscopic submucosal dissection.
Others: duodenal leakage, ileus, hepatic dysfunction, pancreatic leakage, duodenal stump leakage.
Table 4.
Clinical Outcomes Following Endoscopic Submucosal Dissection and Gastrectomy according to the Criteria
| Guideline criteria | Expanded criteria | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| ESD group (n=35) | Gastrectomy group (n=20) | p-value | ESD group (n=107) | Gastrectomy group (n=51) | p-value | |
| Hospital stay after procedure, day | 6.1±2.4 | 13.0±7.3 | <0.001 | 6.6±3.0 | 13.5±17.5 | <0.001 |
| Recurrence | 0 | 0 | - | 5 (4.7) | 0 | 0.279 |
| Metachronous cancer | 2 (5.7) | 1 (5.0) | 1.000 | 8 (7.5) | 0 | 0.055 |
Data are presented as mean±SD or number (%).
ESD, endoscopic submucosal dissection.
4. Long-term outcomes
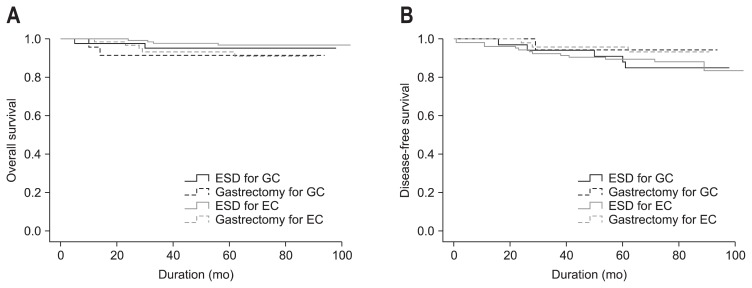
In the GC group treated with ESD or gastrectomy, there was no recurrence. In contrast, the recurrence rates of the EC groups treated with ESD and gastrectomy were 4.7% and 0.0%, respectively (p=0.279). The occurrence rates of metachronous cancer after ESD and gastrectomy were 5.7% and 5.0% for GC (p=1.000), and 7.5% and 0.0% in EC (p=0.055), respectively (Table 4). The overall survival of the ESD and gastrectomy groups were 93.4±3.2 and 85.8±5.5 months, respectively, for GC and 100.5±1.3 and 84.9±2.6 months, respectively, for EC (p=0.397) (Fig. 2A). The disease-free survival of the ESD and gastrectomy groups were 89.7±3.6 and 90.4±3.5 months, respectively, for GC and 93.6±2.5 and 87.6±2.0 months, respectively, for EC (p=0.597) (Fig. 2B).
Fig. 2.
Long-term outcomes. (A) The overall survival following endoscopic submucosal dissection (ESD) and gastrectomy according to the criteria (p=0.397). (B) The disease-free survival following ESD and gastrectomy according to the criteria (p=0.597).
EC, expanded criteria; GC, guideline criteria.
DISCUSSION
EGC is defined as when tumor invasion is confined to the mucosa or submucosa (T1 cancer), irrespective of the presence of lymph node metastasis.1 Radical gastrectomy can achieve adequate oncological clearance with wide resection margins and nodal dissection, but can result in significant perioperative morbidity and compromise long-term gastrointestinal function as well as quality of life.10 With the low risk of lymph node metastasis, methods of endoscopic resection were pioneered for the treatment of EGC.11 En bloc resection facilitates accurate histological assessment and confirms the need for CR. To improve the en bloc resection rate, ESD methods were introduced and developed.12 Several studies showed ESD enables a high en bloc resection rate (above 90%) in the treatment of EGC.13–17 ESD also allows the resection of large lesions. Further, endoscopic treatment enables gastric function to be preserved and allows the patient to maintain a better quality of life. In comparison with endoscopic treatment and surgery in the treatment of EGC, ESD displays lower mortality and complication rates than surgery.18,19 Based on the advantages of ESD, it has emerged as the main treatment of EGC without lymph node metastasis in the place of surgery. As reported recently, ESD showed acceptable results in the treatment of EGC. Isomoto et al.20 reported that the 5-year overall and disease-specific survival rates were 97.1% and 100%, respectively. Nakamoto et al.21 also reported an overall 5-year recurrence-free rate of 100%. As mentioned, indications for ESD are strictly limited to the GC. According to the National Cancer Center of Japan, the 5-year survival rate is 99% in patients with EGCs confined to the mucosa and 96% in those with EGCs confined to the submucosa.22 Thus, it has been proposed that the indication for ESD in the treatment of EGC should be expanded to cancer patients with a minimal risk of lymph node metastasis.5
Choi et al.23 reported that the complication rates of EGC treated by endoscopic mucosal resection and gastrectomy were similar, despite those treated by endoscopic mucosal resection being older and displaying more frequent comorbidities. Chiu et al.24 reported that the overall complication rate of gastrectomy was significantly higher than that of ESD (32.5% and 5.4%). The overall complication rates of ESD and gastrectomy in our hospital were 8.5% (12/142) and 28.2% (20/71). The significant bleeding rates of gastrectomy were higher than those of ESD, but ESD had the risk of perforation during the procedure. Several other complications of gastrectomy also occurred, but the total complication rates could not be directly compared due to procedure differences and a variety of complications of gastrectomy. Duodenal leakage, ileus and hepatic dysfunction occurred in GC, while pancreatic leakage and duodenal stump leakage occurred after gastrectomy. Several reports regarding the safety of ESD, in terms of bleeding and perforation, have been published; the rate of bleeding related to ESD was approximately 7% and the rate of perforation was 4%.12,13,25,26 The significant bleeding rates after ESD in this study were 3.5%. In the gastrectomy group, the significant bleeding rates in this study were 15.5%, which are higher than ESD patients. The overall rate of perforation after ESD was 4.9%, which is similar to previous reports.
The CR rates ranged from 81.8% to 95.9% of ESD for the GC group,21,26–28 88.4% to 89.9% for the EC group.27,28 In this study, CR rates of ESD for GC were 97.1%, which was above average. CR rates of ESD for EC were 81.8% representing an acceptable result. High en bloc resection rates (97.1% in GC and 86.4% in EC) allowed increased CR rates. These high CR rates in turn resulted in lower recurrence rates. The recurrence rates of ESD for GC and EC were 0% and 4.7%, respectively.
The results of this study demonstrated that there were no significant differences in recurrence rates, occurrence rates of metachronous cancer, and 5-year survival rates between EC and GC groups that underwent ESD or gastrectomy when assessing long-term outcomes. However, complication rates were less frequent and the hospital stay durations were shorter in patients treated with ESD than with gastrectomy.
This study has several limitations. First, this was a single-center study and the number of EGC patients was relatively small, particularly in the surgery group. Thus, we were unable to determine the factors that affected the prognosis of this group. Second, the results of this study were evaluated by retrospective methods and some selection bias occurred. Patients in the surgery group may have had more severe cancer than those in the ESD group. From the histopathological characteristics of the EGCs for EC, the surgery group possessed a more undifferentiated histology and submucosal invasion than the ESD group. In spite of these limitations, this study is meaningful as it included long-term follow-up. In addition, treatment outcomes of this study did not differ from those of former EGC reports. We therefore suggest that the long-term outcome of patients that undergo ESD due to GC and EC is not inferior to that of those who undergo gastrectomy. Considering its safety, duration of hospital stay and long-term outcomes, ESD may be an effective and safe first-line treatment for EGC in EC as well as GC.
ACKNOWLEDGEMENTS
This work was supported in part by the Soonchunhyang University Research Fund (2013-0002).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma: 2nd English edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/PL00011681. [DOI] [PubMed] [Google Scholar]
- 2.Everett SM, Axon AT. Early gastric cancer in Europe. Gut. 1997;41:142–150. doi: 10.1136/gut.41.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc. 2012;76:763–770. doi: 10.1016/j.gie.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi N, Isomoto H, Fukuda E, et al. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer by indication criteria. Digestion. 2009;80:173–181. doi: 10.1159/000215388. [DOI] [PubMed] [Google Scholar]
- 5.Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/PL00011720. [DOI] [PubMed] [Google Scholar]
- 6.Yamao T, Shirao K, Ono H, et al. Risk factors for lymph node metastasis from intramucosal gastric carcinoma. Cancer. 1996;77:602–606. doi: 10.1002/(SICI)1097-0142(19960215)77:4<602::AID-CNCR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 7.Shimada Y JGCA (The Japan Gastric Cancer Association) Gastric cancer treatment guidelines. Jpn J Clin Oncol. 2004;34:58. [PubMed] [Google Scholar]
- 8.International Agency for Research on Cancer. Pathology and genetics of tumours of the digestive system. Lyon: IARC Press; 2000. [Google Scholar]
- 9.Nagano H, Ohyama S, Fukunaga T, et al. Indications for gastrectomy after incomplete EMR for early gastric cancer. Gastric Cancer. 2005;8:149–154. doi: 10.1007/s10120-005-0328-5. [DOI] [PubMed] [Google Scholar]
- 10.Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from large consecutive series. Dig Endosc. 2005;17:54–58. doi: 10.1111/j.1443-1661.2005.00459.x. [DOI] [Google Scholar]
- 11.Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc. 2003;57:567–579. doi: 10.1067/mge.2003.130. [DOI] [PubMed] [Google Scholar]
- 12.Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–942. doi: 10.1007/s00535-006-1954-3. [DOI] [PubMed] [Google Scholar]
- 13.Onozato Y, Ishihara H, Iizuka H, et al. Endoscopic submucosal dissection for early gastric cancers and large flat adenomas. Endoscopy. 2006;38:980–986. doi: 10.1055/s-2006-944809. [DOI] [PubMed] [Google Scholar]
- 14.Gotoda T. A large endoscopic resection by endoscopic submucosal dissection procedure for early gastric cancer. Clin Gastroenterol Hepatol. 2005;3(7 Suppl 1):S71–S73. doi: 10.1016/S1542-3565(05)00251-X. [DOI] [PubMed] [Google Scholar]
- 15.Imagawa A, Okada H, Kawahara Y, et al. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy. 2006;38:987–990. doi: 10.1055/s-2006-944716. [DOI] [PubMed] [Google Scholar]
- 16.Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228–1235. doi: 10.1016/j.gie.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 17.Lee H, Yun WK, Min BH, et al. A feasibility study on the expanded indication for endoscopic submucosal dissection of early gastric cancer. Surg Endosc. 2011;25:1985–1993. doi: 10.1007/s00464-010-1499-7. [DOI] [PubMed] [Google Scholar]
- 18.Bonenkamp JJ, Songun I, Hermans J, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345:745–748. doi: 10.1016/S0140-6736(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 19.Sasako M. Risk factors for surgical treatment in the Dutch Gastric Cancer Trial. Br J Surg. 1997;84:1567–1571. doi: 10.1111/j.1365-2168.1997.02842.x. [DOI] [PubMed] [Google Scholar]
- 20.Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331–336. doi: 10.1136/gut.2008.165381. [DOI] [PubMed] [Google Scholar]
- 21.Nakamoto S, Sakai Y, Kasanuki J, et al. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy. 2009;41:746–750. doi: 10.1055/s-0029-1215010. [DOI] [PubMed] [Google Scholar]
- 22.Sasako S, Kinoshita T, Maruyama K. Prognosis of early gastric cancer. Stomach Intest. 1993;28:139–146. [Google Scholar]
- 23.Choi KS, Jung HY, Choi KD, et al. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc. 2011;73:942–948. doi: 10.1016/j.gie.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 24.Chiu PW, Teoh AY, To KF, et al. Endoscopic submucosal dissection (ESD) compared with gastrectomy for treatment of early gastric neoplasia: a retrospective cohort study. Surg Endosc. 2012;26:3584–3591. doi: 10.1007/s00464-012-2371-8. [DOI] [PubMed] [Google Scholar]
- 25.Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225–229. doi: 10.1136/gut.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oda I, Saito D, Tada M, et al. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262–270. doi: 10.1007/s10120-006-0389-0. [DOI] [PubMed] [Google Scholar]
- 27.Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc. 2011;74:485–493. doi: 10.1016/j.gie.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 28.Park CH, Shin S, Park JC, et al. Long-term outcome of early gastric cancer after endoscopic submucosal dissection: expanded indication is comparable to absolute indication. Dig Liver Dis. 2013;45:651–656. doi: 10.1016/j.dld.2013.01.014. [DOI] [PubMed] [Google Scholar]