Abstract
Background/Aims
We investigated the treatment outcomes and prognostic factors of hepatocellular carcinoma (HCC) with obstructive jaundice.
Methods
Among 2,861 patients newly diagnosed with HCC between 2002 and 2011, a total of 63 patients who initially presented with obstructive jaundice were analyzed. Only four patients presented with resectable tumors and underwent curative resection. In the other patients who presented with unresectable tumors, 5, 8, 9, and 18 patients received transarterial chemoembolization (TACE), chemotherapy, radiotherapy, and combined treatment, respectively. Both the clinical and the treatment factors that affect overall survival (OS) were analyzed.
Results
The median OS was 4 months, and the 1-year OS rate was 23%. Patients who received treatment for HCC had a significantly improved OS rate compared with the patients who received supportive care only (1-year OS, 32% vs 0%; p<0.01). Responders to treatment showed a better OS than nonresponders (1-year OS, 52% vs 0%; p<0.01). TACE and radiotherapy resulted in relatively good treatment responses of 64% and 67%, respectively. In multivariate analyses, treatment of HCC (p=0.02) and the normalization of serum bilirubin by biliary drainage (p=0.02) were significantly favorable prognostic factors that affected the OS.
Conclusions
Unresectable HCC with obstructive jaundice has a poor prognosis. However, effective biliary drainage and treatment of HCC such as with TACE or radiotherapy improves survival.
Keywords: Transarterial chemoembolization, Drug therapy, Radiotherapy
INTRODUCTION
Jaundice is a common complication in patients with hepatocellular carcinoma (HCC), and is observed in 19% to 40% of patients at the time of diagnosis.1–3 The most frequent cause of jaundice associated with HCC is the hepatic insufficiency due to underlying liver cirrhosis or extensive hepatic parenchymal destruction due to a massive liver tumor.4 Obstructive jaundice caused by HCC is rare and associated with a poor prognosis. Obstructive jaundice, presented in 1% to 12% of patients with HCC,5 is caused by tumor invasion or blood clots in the biliary tract.6 Previous studies have shown that curative resection can improve survival.7–10 However, the prognosis for patients, who cannot undergo surgery, is dismal with the median survival of 2 to 4 months.11,12 Thus, proper management for HCC with obstructive jaundice remains unclear, especially in patients with unresectable HCC. In this study, we analyzed the treatment outcomes in patients with HCC accompanying obstructive jaundice due to bile duct tumor invasion treated by surgery, transarterial chemoembolization (TACE), radiotherapy, chemotherapy, or a combination of these treatments. In addition, we investigated predictive factors of survival in patients with HCC accompanying obstructive jaundice for survival to identify the patients who require intensive treatments.
MATERIALS AND METHODS
1. Study population
Between 2002 and 2011, 5,086 consecutive patients with HCC were treated at Severance Hospital, Yonsei University College of Medicine. Among these patients, 2,861 patients were newly diagnosed with HCC. Of these, 307 patients presented with jaundice at the time of diagnosis. Of these 307 patients, 63 (2.2%), who presented with obstructive jaundice due to bile duct tumor invasion at the time of diagnosis, were analyzed in this study. The diagnosis of HCC was made based on the guidelines proposed by the Korea Liver Cancer Study Group.13 The clinical characteristics and biochemical values of patients at the time of diagnosis were analyzed. These included the etiology of HCC, serum chemistry, the presence of ascites and hepatic encephalopathy, Child-Pugh’s classification, and Barcelona Clinic Liver Cancer (BCLC) Stage.14 To distinguish obstructive jaundice from jaundice caused by hepatic insufficiency, serum γ-glutamyl transpeptidase (γ-GT) and the finding of bile duct dilation in imaging studies (dynamic computed tomography [CT], magnetic resonance imaging, or endoscopic retrograde cholangiopancreatography [ERCP]) were evaluated. HCC was classified as nodular or diffuse-infiltrative HCC by following the classification of Yuki with some modifications.15 Tumor size was measured on CT images based on the longest diameter of the tumor. The Institutional Review Board of Yonsei University Health System approved this study.
2. Treatment
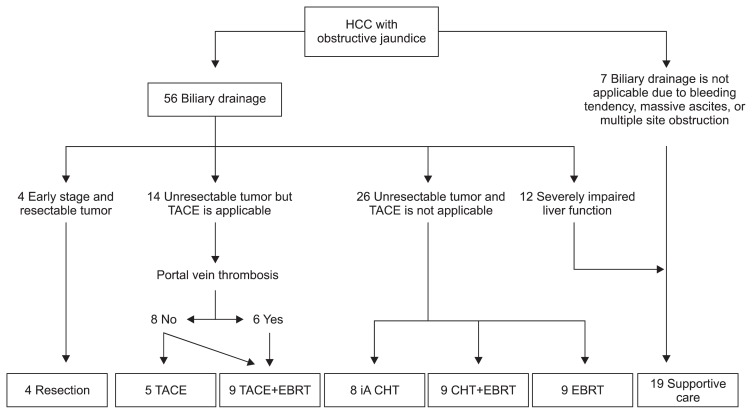
All patients were evaluated by a multidisciplinary team including hepatobiliary surgeons, radiation oncologists, hepatologists, oncologists, and interventional radiologists at Yonsei Liver Cancer Special Clinic, and treatment was determined based on both patient and tumor characteristics (Fig. 1). After the diagnosis of HCC with obstructive jaundice, biliary drainage, either by percutaneous transhepatic biliary drainage (PTBD) or ERCP with biliary stenting, was performed in 56 patients. The other seven patients could not undergo biliary drainage due to ascites, a bleeding tendency, or multiple sites of obstruction in the biliary tract. Median time interval between biliary drainage and initiation of treatment for HCC was 14 days (range, 1 to 43 days). Four patients who had surgically resectable tumors, Child-Pugh class A status, and the retention rate of indocyanine green at 15 minutes after injection (ICG-R15) less than 25% underwent curative resection; segmental resection of the liver with choledochoduodenostomy for one patient, and hemihepatectomy for three patients. TACE was performed in 14 patients by infusion of a mixture of 5 mL lipiodol (Guerbet, Aunay-sous-Bois, France) and 50 mg adriamycin and followed by subsequent embolization of feeding arteries by gelatin sponge particles. Among these 14 patients, six patients who presented portal vein tumor thrombosis (PVTT), received radiotherapy followed by TACE. Seventeen patients received chemotherapy, nine, six, and two patients received intra-arterial cisplantin, intra-arterial 5-fluorouracil, and systemic chemotherapy, respectively. Intra-arterial chemotherapy was performed using an implantable port system. Radiotherapy was conducted in 27 patients. All patients underwent CT simulation and were treated with 3-dimensional-conformal radiotherapy (3D-CRT) planned by the AcQsim® or the Pinnacle3 system (Philips Healthcare, Andover, MA, USA). The median radiation dose was 45 Gy (range, 8.4 to 62 Gy), and the median dose per fraction was 1.8 Gy (range, 1.2 to 3.1 Gy). Radiotherapy was delivered with megavoltage beams (6 or 10 MV X-ray), and the planning target volumes included radiographically visible gross tumor with 2 to 3 cm margins to compensate for setup error and internal organ motion. Median time of treatment sessions was 2, ranged from 1 to 9, and 1 treatment session means 1 time of intra-arterial chemotherapy, 1 time of TACE, 1 cycle of systemic chemotherapy, or overall planned course of radiotherapy.
Fig. 1.
Treatment scheme according to the clinical characteristics of the patients.
HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; EBRT, external beam radiotherapy; iA, intra-arterial; CHT, chemotherapy.
3. Statistical analysis
Treatment response was evaluated 1 to 3 months after the completion of planned treatment, according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST).16 On the basis of mRECIST criteria, patients who showed complete and partial responses were classified as responders, and patients who showed stable or progressive disease were classified as nonresponders. The primary endpoint was overall survival (OS), which was defined as the time from the date of diagnosis to date of death resulting from any cause, or it was measured at the date of the last follow-up visit for survivors. Differences in biochemical values before and after treatment were analyzed using paired sample t-tests. Survival was evaluated using Kaplan-Meier estimates, and the log-rank test was used to assess the equality of the survival function across the group. Significant variables on univariate analyses (log-rank test) were input into the multivariate analyses using Cox regression analysis. Statistical analyses were conducted using SPSS version 20 (SPSS Inc., Chicago, IL, USA). A p-value of less than 0.05 was considered statistically significant.
RESULTS
1. Patients’ characteristics
Patients’ clinical characteristics according to the effectiveness of biliary drainage are summarized in Table 1, and the details of patients’ characteristics are follows. The median patient age was 54 years (range, 34 to 79 years), and 81% of patients were male. Chronic hepatitis B virus infection was the most common etiology (76%), followed by chronic hepatitis C virus infection (6%). Twenty-seven patients (43%) presented with nodular HCC, while the other 36 patients (57%) presented with diffuse-infiltrative HCC. Fifty-one patients (81%) had tumors larger than 5 cm, and 33 patients (52%) had biliary tract obstruction at common hepatic duct. Obstruction at single site of the biliary tract was observed in 38 patients (60%), and obstruction at multiple sites was observed in remaining 25 patients. PVTT was observed in 37 patients (59%), and most PVTT was located in the main or first branch of the portal vein. Forty patients (64%) had multiple tumors, and 27 patients (43%) were categorized as Child-Pugh class B. Five (8%), 21 (33%), and 37 (59%) patients were BCLC stage A, B, and C, respectively. Patients who did not undergo successful biliary drainage showed higher frequencies of common hepatic duct obstruction and multiple tumors than patients who underwent successful biliary drainage.
Table 1.
Clinical and Laboratory Characteristics of the Patients
| Characteristic | Unsuccessful BD* (n=48) | Sucessful BD (n=15) | p-value |
|---|---|---|---|
| Age, yr | 57 (39–79) | 52 (34–73) | 0.10 |
| Male gender | 41 (85) | 10 (67) | 0.14 |
| Etiology | |||
| HBV | 36 (75) | 12 (80) | 1.00 |
| HCV | 4 (8) | 0 | 0.56 |
| Others | 8 (17) | 3 (20) | 0.71 |
| Tumor type | |||
| Nodular | 19 (40) | 8 (53) | 0.38 |
| Diffuse-infiltrative | 29 (60) | 7 (27) | |
| Tumor size, cm | |||
| ≤5 | 8 (17) | 4 (27) | 0.46 |
| >5 | 40 (83) | 11 (73) | |
| Site of biliary obstruction | |||
| CHD | 29 (60) | 4 (27) | 0.04 |
| 1st branch of HD | 8 (17) | 8 (53) | 0.01 |
| 2nd branch of HD | 11 (23) | 3 (20) | 1.00 |
| Biliary obstruction | |||
| Single site | 38 (79) | 10 (67) | 0.76 |
| Multiple sites | 20 (21) | 5 (33) | |
| PVTT | |||
| None | 20 (42) | 6 (40) | 1.00 |
| Main or first branch | 26 (54) | 8 (53) | 1.00 |
| Second or third branch | 2 (4) | 1 (7) | 0.56 |
| Multiplicity of tumor | |||
| Solitary | 16 (33) | 10 (67) | 0.04 |
| Multiple | 32 (67) | 5 (33) | |
| Child-Pugh Class at diagnosis | |||
| A | 23 (48) | 13 (87) | 0.02 |
| B | 25 (52) | 2 (13) | |
| BCLC stage | |||
| A | 2 (4) | 3 (20) | 0.08 |
| B | 18 (38) | 3 (20) | 0.35 |
| C | 28 (58) | 9 (60) | 1.00 |
| Albumin, g/dL | 3.2 (2.2–5.5) | 3.3 (2.7–4.7) | 0.26 |
| Prothrombin time, INR | 1.13 (0.8–2.48) | 1.14 (0.95–2.13) | 0.51 |
| γ-GT, IU/L | 203 (25–787) | 242 (42–929) | 0.88 |
| Alkaline phosphatase, IU/L | 192.5 (80–970) | 236 (65–566) | 0.76 |
| Total bilirubin, mg/dL | |||
| Initial | 10.2 (2.2–28.5) | 3.9 (2.1–15.5) | <0.01 |
| Pretreatment | 8.1 (2.6–27) | 1.2 (0.2–1.9) | <0.01 |
| Posttreatment | 5.6 (1.2–47.6) | 1.0 (0.3–6.7) | <0.01 |
| AFP, ng/mL | |||
| Pretreatment | 701 (1.4–83,000) | 48.1 (1.4–2,736) | <0.01 |
| Posttreatment | 351 (3.4–83,000) | 10.8 (1.5–1,488) | <0.04 |
| PIVKA-II, mAU/mL† | |||
| Pretreatment | 2,000 (22–2,000) | 1,849 (15–2,000) | 0.57 |
| Posttreatment | 272 (10–2,000) | 197 (10–2,000) | 0.56 |
Data are presented as median (range) or number (%).
BD, biliary drainage; HBV, hepatitis B virus; HCV, hepatitis C virus; CHD, common hepatic duct; PVTT, portal vein tumor thrombosis; BCLC, Barcelona Clinic Liver Cancer; GT, glutamyl transpeptidase; AFP, α-fetoprotein; PIVKA, protein induced by vitamin K antagonist or absence.
Patients who did not undergo biliary drainage were included in this group;
p<0.01 in paired sample t-test between pretreatment values and post-treatment values.
Laboratory values of the patients are also shown in Table 1. The median serum albumin level was 3.3 g/dL, and 39 patients (62%) had a serum albumin level lower than 3.5 g/dL. Prothrombin time was elevated by more than 1.2 INR in 25 patients (40%). The median initial total bilirubin level was 7.9 mg/dL (range, 2.1 to 28.5 mg/dL), and it decreased to 2.9 mg/dL (range, 0.3 to 47.6 mg/dL) after treatments. Serum total bilirubin level decreased in 29 patients (46%) after biliary drainage or treatment of HCC, while it was elevated in 15 patients (24%). Median AFP level was 602 ng/mL (range, 1.4 to 83,000 ng/mL), and was higher than 200 ng/mL in 36 patients (57%). Median protein induced by vitamin K absence or antagonist-II (PIVKA-II) level was 1,849 mAU/mL (range, 15 to 2,000 mAU/mL) and serum PIVKA-II levels were elevated (>40 mAU/mL) in 49 patients (78%). PIVKA-II values were significantly decreased after treatment, and a treatment-induced decrease in AFP or PIVKA-II level was noted in 29 patients (46%). In addition, AFP or PIVKA-II level decreased in 21 patients (21/27, 78%) who underwent radiotherapy, and in eight patients (8/17, 47%) who were treated using other treatment modalities. Total bilirubin and AFP levels were significantly lower in patients who underwent successful biliary drainage compared with the patients who did not.
2. Survival
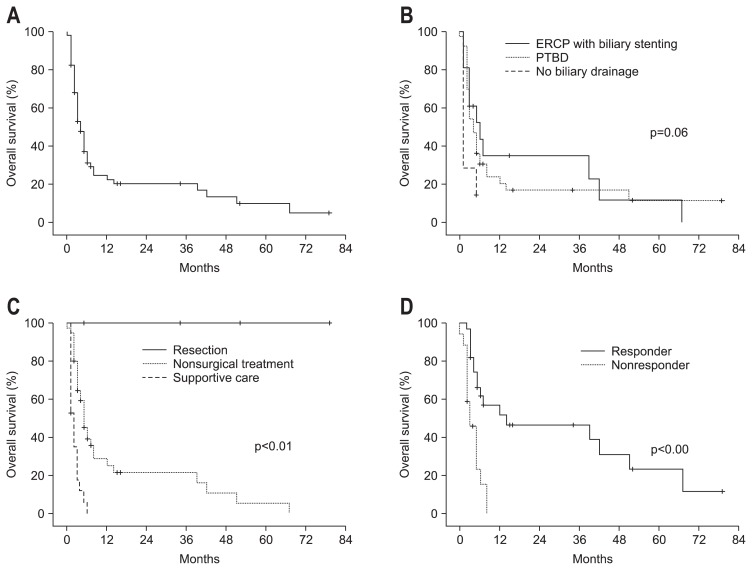
During the follow-up period, a total of 50 deaths (79%) occurred; most patients died of jaundice-related complications such as hepatic failure (20 patients) or biliary sepsis (nine patients). For all patients, the median survival was 4 months, and the 1-year survival rate was 23% (Fig. 2A). Biliary drainages, including PTBD and ERCP with stenting, were performed in 40 and 16 patients, respectively. Among these patients, nine and six patients who underwent PTBD and ERCP with stenting, respectively, had normalized total bilirubin levels after biliary drainage; those cases were defined as having effective biliary drainage. Biliary drainage significantly improved the 1-year survival rate (24% vs 0%, p=0.02) (Fig. 2B). In addition, the 1-year survival rate was not statistically different between patients who underwent PTBD and ERCP with stenting (21% vs 35%, p=0.66). Cumulative OS according to treatment modality is shown in Fig. 2C. Treatment of HCC significantly improved the 1-year survival rates compared with supportive care only (22% vs 0%, p<0.01). None of the four patients who underwent curative resection died, resulting in a 1-year survival rate of 100%. In the patients treated with nonsurgical treatment options including TACE, chemotherapy, radiotherapy, or combined treatment, the 1-year survival rates were not significantly different among treatment modalities. However, those patients who received nonsurgical treatment had an improved 1-year OS rate than patients who were treated by supportive care only (25% vs 0%, p<0.01).
Fig. 2.
Kaplan-Meier plot of (A) overall survival for all patients, (B) overall survival depending on the method of biliary drainage, (C) overall survival according to the treatment for hepatocellular carcinoma (HCC), (D) survival of the 44 patients who received treatment for HCC according to the modified Response Evaluation Criteria in Solid Tumors.
ERCP, endoscopic retrograde cholangiopancreatography; PTBD, percutaneous transhepatic biliary drainage.
3. Treatment responses
Forty-four patients who received treatment of HCC were evaluated according to mRECIST criteria. Twenty-seven patients (61%) were classified as responders, and the other 17 patients were classified as nonresponders (Table 2). All patients who underwent surgery were classified as responders. Treatment response rates for the patients who received TACE, TACE combined with radiotherapy, intra-arterial chemotherapy, combined chemoradiotherapy, and radiotherapy alone were 60%, 67%, 25%, 67%, and 67%, respectively. Responders had an improved 1-year OS compared with nonresponders (52% vs 0%, p<0.01) (Table 2, Fig. 2D). In addition, Child-Pugh class A patients who received radiotherapy showed an excellent response rate of 92% (data not shown).
Table 2.
Overall Survival for the 44 Patients Who Received Treatment for Hepatocellular Carcinoma according to the mRECIST Response
| Treatment | No. of patient (%) | 1-Year survival, % | p-value* |
|---|---|---|---|
| All patients | |||
| Responder | 27 (61) | 51.7 | <0.01 |
| Nonresponder | 17 (39) | 0 | |
| Curative resection | |||
| Responder | 4 (100) | 100 | NA |
| Nonresponder | 0 | 0 | |
| TACE | |||
| Responder | 3 (60) | 33 | 0.04 |
| Nonresponder | 2 (40) | 0 | |
| TACE+Radiotherapy | |||
| Responder | 6 (67) | 50 | 0.07 |
| Nonresponder | 3 (33) | 0 | |
| Intra-arterial chemotherapy | |||
| Responder | 2 (25) | 100 | 0.10 |
| Nonresponder | 6 (75) | 0 | |
| Chemotherapy+Radiotherapy | |||
| Responder | 6 (67) | 33 | 0.30 |
| Nonresponder | 3 (33) | 0 | |
| Radiotherapy alone | |||
| Responder | 6 (67) | 33 | 0.41 |
| Nonresponder | 3 (33) | 0 | |
mRECIST, modified Response Evaluation Criteria in Solid Tumors; NA, not applicable; TACE, transarterial chemoembolization.
Log-rank test.
4. Prognostic factors
To determine prognostic factors affecting OS, several variables related to clinical features and treatment modalities were evaluated by univariate and multivariate analyses. On univariate analysis, diffuse-infiltrative tumor (p=0.01), the presence of PVTT on main or first branch of portal vein (p<0.01), multiple tumors (p<0.01), Child-Pugh class B (p=0.01), and prolonged prothrombin time (p<0.01) were significantly unfavorable prognostic factors affecting OS. Treatment of HCC (p<0.01), curative resection (p<0.01), and effective biliary drainage (p<0.01) were significant prognostic factors improving OS, respectively. Multivariate analysis found treatment of HCC (p=0.02) and effective biliary drainage (p=0.02) were significantly favorable prognostic factors, respectively. These data are shown in Table 3.
Table 3.
Prognostic Factors for Overall Survival
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
|
|
|
||||
| 1-Year OS, % | p-value* | HR | 95% CI | p-value† | |
| Age, yr | 0.84 | ||||
| ≤50 | 25.2 | ||||
| >50 | 20.4 | ||||
| Gender | 0.42 | ||||
| Male | 23.8 | ||||
| Female | 16.7 | ||||
| Active HBV infection | 0.91 | ||||
| Yes | 37.0 | ||||
| No | 19.6 | ||||
| Tumor type | 0.01 | 1.13 | 0.56–2.26 | 0.74 | |
| Diffuse infiltrative tumor | 10.3 | ||||
| Nodular tumor | 39.3 | ||||
| Tumor size, cm | 0.16 | ||||
| ≤5 | 36.5 | ||||
| >5 | 19.1 | ||||
| PVTT on main or 1st branch of PV | <0.01 | 1.72 | 0.79–3.73 | 0.17 | |
| Yes | 11.3 | ||||
| No | 39.3 | ||||
| Site of biliary obstruction | 0.84 | ||||
| CHD | 17.2 | ||||
| Below CHD | 28.5 | ||||
| Multiplicity of tumor | <0.01 | 1.18 | 0.53–2.59 | 0.69 | |
| Solitary | 46.4 | ||||
| Multiple | 6.4 | ||||
| Child-Pugh class | 0.01 | 0.99 | 0.48–2.04 | 0.98 | |
| A | 39.2 | ||||
| B | 0 | ||||
| PT prolongation | <0.01 | 1.63 | 0.78–3.42 | 0.20 | |
| Yes | 0 | ||||
| No | 38.7 | ||||
| Treatment for HCC | <0.01 | 0.42 | 0.21–0.85 | 0.02 | |
| Yes | 32.1 | ||||
| No | 0 | ||||
| Curative resection of tumor | <0.01 | <0.01 | NA | 0.97 | |
| Yes | 100 | ||||
| No | 17.1 | ||||
| TACE | 0.99 | ||||
| Yes | 26.8 | ||||
| No | 21.0 | ||||
| Radiotherapy | 0.30 | ||||
| Yes | 26.9 | ||||
| No | 18.4 | ||||
| Chemotherapy | 0.20 | ||||
| Yes | 22.2 | ||||
| No | 21.7 | ||||
| Effective BD | <0.01 | 0.26 | 0.09–0.78 | 0.02 | |
| Yes | 58.2 | ||||
| No | 8.8 | ||||
OS, overall survival; HR, hazard ratio; CI, confidence interval; HBV, hepatitis B virus; PVTT, portal vein tumor thrombosis; CHD, common hepatic duct; PT, prothrombin time; HCC, hepatocellular carcinoma; NA, not applicable; TACE, transarterial chemoembolization; BD, biliary drainage.
Log-rank test;
Cox regression analysis.
DISCUSSION
We investigated the treatment outcomes and prognostic factors for overall survival in HCC patients with obstructive jaundice. The prognosis of patients with HCC accompanying obstructive jaundice was very poor with a median OS of 4 months. Nevertheless, treatment of HCC and biliary drainage significantly increased survivals in multivariate analysis (Table 3). As a result, 11 patients survived for longer than 1 year. Among these patients, the lives of eight patients were prolonged by radiotherapy, TACE, chemotherapy, or combined treatment, despite the fact that none of these patients were eligible for curative resection (Table 4).
Table 4.
Clinical and Tumor Characteristics of Patients Who Survived Longer than 1 Year
| Patient, age/sex | Tumor | Biochemical values | Treatment | Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Type | Size, cm | PVT | Initial T. bil | Post-Tx T. bil | γ-GT | PT, INR | |||
| 47/M | DI | 10 | PV1 | 7.5 | 2.9 | 137 | 1.33 | PTBD, 45 Gy EBRT with systemic CHT | DOD at 12 mo |
| 38/M | DI | 10 | PV1 | 2.8 | 1.5 | 311 | 1.19 | PTBD, 45 Gy EBRT | DOD at 14 mo |
| 76/F | DI | 5 | No | 9.7 | 5.6 | 660 | 0.96 | ERCP with stenting, iA CHT | Alive at 15 mo |
| 52/M | SN | 5 | PV1 | 15.5 | 0.6 | 261 | 1.04 | PTBD, 54 Gy EBRT | Alive at 16 mo |
| 52/M | SN | 3 | No | 5.6 | 0.8 | 99 | 1.19 | PTBD, curative resection | Alive at 34 mo |
| 34/M | SN | 4 | PV2 | 2.5 | 0.3 | 184 | 1.00 | ERCP with stenting, 45 Gy EBRT with TACE | Alive at 39 mo |
| 57/M | DI | 8 | No | 4.7 | 0.6 | 503 | 1.02 | ERCP with stenting, 50 Gy EBRT with TACE | DOD at 42 mo |
| 45/M | SN | 9 | No | 9.9 | 1.0 | 929 | 1.05 | PTBD, 42.4 Gy EBRT with concurrent iA CHT | DOD at 51 mo |
| 54/M | SN | 2.8 | No | 8.4 | 0.5 | 418 | 0.98 | PTBD, curative resection | Alive at 52 mo |
| 47/F | SN | 5 | No | 2.6 | 0.5 | 42 | 0.95 | ERCP with stenting, TACE | Alive at 67 mo |
| 62/M | SN | 6 | No | 26.2 | 3.8 | 755 | 0.81 | PTBD, curative resection | Alive at 79 mo |
PVT, portal vein thrombosis; T. bil, total bilirubin; Post-Tx, posttreatment; γ-GT, γ-glutamyl transpeptidase; PT, prothrombin time; M, male; DI, diffuse infiltrative; PV1, first or main branch of portal vein; PTBD, percutaneous transhepatic biliary drainage; EBRT, external beam radiotherapy; CHT, chemotherapy; DOD, died of disease; F, female; ERCP, endoscopic retrograde cholangiopancreatography; iA, intra-arterial; PV2, second or third branch of portal vein; SN, single nodular; TACE, transarterial chemoembolization.
HCC with tumor invasion to the bile duct causing obstructive jaundice was classified as “icteric type hepatoma” by Lin et al.17 or “cholestatic type of HCC” by Kunio.18 In the present study, among a total of 2,861 patients in this study cohort, the frequency of patients with icteric type HCC was 2.2%. A previous study in Taiwan reported that the frequency of icteric HCC was 0.53%,19 while a Japanese study reported a frequency of 9.3%.6 Another Japanese study reported that nine (1.66%) out of 542 cases of HCC treated surgically had macroscopic bile duct thrombi.20 The differences in frequency of icteric HCC according to country or institution could result from differences in environmental and genetic factors, the etiology of HCC, or study design. In the present study, we included the patients only who presented symptomatic obstructive jaundice, while the patients who had incidentally discovered bile duct invasion in surgical specimens were excluded.
Previous studies have shown that biliary drainage procedures, such as PTBD and ERCP with biliary stenting have a beneficial effect on survival in patients with HCC accompanying obstructive jaundice.21,22 Consistent with these previous studies, we showed that effective biliary drainage is an important factor for OS in multivariate analysis. In addition, prolonged prothrombin time, which indicates impaired liver function, exacerbated OS in univariate analysis (Table 3). Moreover, many patients with HCC accompanying obstructive jaundice at the time of diagnosis have preserved liver function, while jaundice due to disease progression or hepatic insufficiency is associated with impaired liver function.23,24 In this study, 36 patients (57%) presented the Child-Pugh class A at the time of diagnosis. As a result, early and effective biliary drainage might be essential to preserve patients’ liver function and improve survival. However, effective biliary drainage for patients with HCC accompanying obstructive jaundice is a challenging problem as many patients present with multiple sites of biliary obstruction and diffuse-infiltrative tumors. Hence, investigation and improvement of intervention techniques such as PTBD and ERCP with biliary stenting are required to enhance patients’ survival.
In the present study, those patients who underwent curative resection showed better survival than patients who could not undergo surgery (1-year OS, 100% vs. 17%, respectively, p<0.01) as reported in previous studies.7–10 However, most HCC patients with obstructive jaundice present with surgically unresectable tumors due to advanced stage or are medically inoperable due to hepatic insufficiency caused by accompanying cholangitis.20,25 In the present study, 57% and 64% of patients presented diffuse-infiltrative and multiple tumors, respectively. In a previous autopsy-based study that investigated pathologic features of HCC with bile duct tumor invasion, 58% of cases had infiltrative tumors.6 Thus, curative resection is not applicable in most patients with this type of HCC; therefore, nonsurgical treatments such as TACE, chemotherapy, and radiotherapy are also important to improve patients’ survival. Although the survival rate of patients who received nonsurgical treatment was poor with 1-year survival rate of 25%, those patients who received nonsurgical treatment including TACE, chemotherapy, radiotherapy, or combined treatment had a better 1-year OS than patients treated by supportive care only (25% vs 0%, p<0.01). Although nonsurgical treatment did not improve outcomes in patients who could not receive effective biliary drainage, the 1-year survival rate of patients who received both effective biliary drainage and nonsurgical treatments improved to 56%.
PVTT is one of the reliable indicators of a poor prognosis for patients with HCC and also an important parameter in the BCLC staging system.14,26 A previous study reported that PVTT in 88% of patients with icteric HCC;6 in the present study, 61% of patients presented with PVTT. In addition, the presence of PVTT is generally considered to be a relative contraindication for TACE due to the potential risk of ischemic liver injury.27 Moreover, one study reported that PVTT in the first branches of the portal vein or portal trunk decreased quality of life and reduced the success rate of endoscopic biliary drainage in the patients with HCC accompanying obstructive jaundice.22 Therefore, PVTT could exacerbate the prognosis by preventing effective biliary drainage as well as further TACE treatment.
Previous studies reported that TACE could prolong the survivals of patients with unresectable HCC accompanying obstructive jaundice.19,28 However, many patients with HCC accompanying obstructive jaundice present with PVTT as described above, thus, the application of TACE could be limited in these patients. Recent studies showed that radiotherapy combined with TACE is an effective and safe treatment option for HCC with PVTT.29,30 In the present study, 14 patients underwent TACE. Among these patients, three patients without PVTT and six patients with PVTT received radiotherapy followed by TACE. Both TACE alone and radiotherapy followed by TACE resulted in relatively good treatment responses of 60% and 67%, respectively. As a result, two patients who received TACE alone and TACE combined with radiotherapy, respectively, survived for more than 1 year (Table 4). Consequently, TACE or radiotherapy followed by TACE could be effective treatment options for patients with HCC accompanying obstructive jaundice. Especially, radiotherapy followed by TACE may be a good treatment option when PVTT is present.
In the present study, a total of 27 patients received radiotherapy at a median radiation dose of 45 Gy, and 21 patients completed radiotherapy as initially planned without significant radiation-associated toxicities. In addition, 17 patients who received radiotherapy were categorized as BCLC stage 3. However, radiotherapy resulted in a relatively respectable response rate of 67% and 1-year survival rate of 27% despite the advance stages of HCC in these patients. Moreover, nine patients who received effective biliary drainage and radiotherapy had relatively good survivals with a median survival of 39 months and a 1-year survival rate of 65%. Six patients who received radiotherapy alone and radiotherapy combined with TACE or chemotherapy, survived for longer than a year (Table 4). Thus, radiotherapy combined with TACE is a potential treatment option in patients with PVTT, and radiotherapy alone is a potential treatment option in patients who are not able to undergo surgery or TACE due to advanced stage HCC.
Hepatic toxicities related to treatments such as TACE, chemotherapy, or radiotherapy can worsen patients’ cholangitis or hepatitis associated with obstructive jaundice. Therefore, the use of modulators of hepatic and biliary toxicity, such as amifostine31 could help to improve patients’ survival; further studies of new therapeutic agents to reduce treatment-related toxicities and preserve patients’ liver function are therefore critical.
In conclusion, we evaluated treatment outcomes in patients with HCC accompanying obstructive jaundice due to bile duct tumor invasion, and investigated prognostic factors for overall survival. Although, the prognosis of HCC patient with obstructive jaundice is dismal, our data suggest that effective biliary drainage and treatment of HCC including surgery, TACE, and radiotherapy can improve survival. Techniques to improve biliary drainage and preserve liver function from jaundice-associated toxicities are required to improve the prognosis of HCC patients with obstructive jaundice due to bile duct tumor invasion.
ACKNOWLEDGEMENTS
This study was supported by a grant (0620390) from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Ihde DC, Sherlock P, Winawer SJ, Fortner JG. Clinical manifestations of hepatoma: a review of 6 years’ experience at a cancer hospital. Am J Med. 1974;56:83–91. doi: 10.1016/0002-9343(74)90753-0. [DOI] [PubMed] [Google Scholar]
- 2.Shiu W, Dewar G, Leung N, et al. Hepatocellular carcinoma in Hong Kong: clinical study on 340 cases. Oncology. 1990;47:241–245. doi: 10.1159/000226823. [DOI] [PubMed] [Google Scholar]
- 3.Lau W, Leung K, Leung TW, et al. A logical approach to hepatocellular carcinoma presenting with jaundice. Ann Surg. 1997;225:281–285. doi: 10.1097/00000658-199703000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lau WY, Leung JW, Li AK. Management of hepatocellular carcinoma presenting as obstructive jaundice. Am J Surg. 1990;160:280–282. doi: 10.1016/S0002-9610(06)80023-1. [DOI] [PubMed] [Google Scholar]
- 5.Kew MC, Paterson AC. Unusual clinical presentations of hepatocellular carcinoma. Trop Gastroenterol. 1985;6:10–22. [PubMed] [Google Scholar]
- 6.Kojiro M, Kawabata K, Kawano Y, Shirai F, Takemoto N, Nakashima T. Hepatocellular carcinoma presenting as intrabile duct tumor growth: a clinicopathologic study of 24 cases. Cancer. 1982;49:2144–2147. doi: 10.1002/1097-0142(19820515)49:10<2144::AID-CNCR2820491026>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 7.Yeh CN, Jan YY, Lee WC, Chen MF. Hepatic resection for hepatocellular carcinoma with obstructive jaundice due to biliary tumor thrombi. World J Surg. 2004;28:471–475. doi: 10.1007/s00268-004-7185-y. [DOI] [PubMed] [Google Scholar]
- 8.Hu J, Pi Z, Yu MY, Li Y, Xiong S. Obstructive jaundice caused by tumor emboli from hepatocellular carcinoma. Am Surg. 1999;65:406–410. [PubMed] [Google Scholar]
- 9.Wang HJ, Kim JH, Kim JH, Kim WH, Kim MW. Hepatocellular carcinoma with tumor thrombi in the bile duct. Hepatogastroenterology. 1999;46:2495–2499. [PubMed] [Google Scholar]
- 10.Peng SY, Wang JW, Liu YB, et al. Surgical intervention for obstructive jaundice due to biliary tumor thrombus in hepatocellular carcinoma. World J Surg. 2004;28:43–46. doi: 10.1007/s00268-003-7079-4. [DOI] [PubMed] [Google Scholar]
- 11.Lau WY, Leung KL, Leung TW, et al. Obstructive jaundice secondary to hepatocellular carcinoma. Surg Oncol. 1995;4:303–308. doi: 10.1016/S0960-7404(10)80042-8. [DOI] [PubMed] [Google Scholar]
- 12.Huang GT, Sheu JC, Lee HS, Lai MY, Wang TH, Chen DS. Icteric type hepatocellular carcinoma: revisited 20 years later. J Gastroenterol. 1998;33:53–56. doi: 10.1007/PL00009966. [DOI] [PubMed] [Google Scholar]
- 13.Korean Liver Cancer Study Group; National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 14.Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 15.Yuki K, Hirohashi S, Sakamoto M, Kanai T, Shimosato Y. Growth and spread of hepatocellular carcinoma: a review of 240 consecutive autopsy cases. Cancer. 1990;66:2174–2179. doi: 10.1002/1097-0142(19901115)66:10<2174::AID-CNCR2820661022>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 16.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 17.Lin TY, Chen KM, Chen YR, Lin WS, Wang TH, Sung JL. Icteric type hepatoma. Med Chir Dig. 1975;4:267–270. [PubMed] [Google Scholar]
- 18.Kunio O. Clinical aspects of hepatocellular carcinoma: analysis of 134 cases. In: Kunio O, Peters RL, editors. Hepatocellular carcinoma. New York: John Wiley; 1976. pp. 387–436. [Google Scholar]
- 19.Huang JF, Wang LY, Lin ZY, et al. Incidence and clinical outcome of icteric type hepatocellular carcinoma. J Gastroenterol Hepatol. 2002;17:190–195. doi: 10.1046/j.1440-1746.2002.02677.x. [DOI] [PubMed] [Google Scholar]
- 20.Ueda M, Takeuchi T, Takayasu T, et al. Classification and surgical treatment of hepatocellular carcinoma (HCC) with bile duct thrombi. Hepatogastroenterology. 1994;41:349–354. [PubMed] [Google Scholar]
- 21.Lee JW, Han JK, Kim TK, et al. Obstructive jaundice in hepatocellular carcinoma: response after percutaneous transhepatic biliary drainage and prognostic factors. Cardiovasc Intervent Radiol. 2002;25:176–179. doi: 10.1007/s00270-001-0100-0. [DOI] [PubMed] [Google Scholar]
- 22.Matsueda K, Yamamoto H, Umeoka F, et al. Effectiveness of endoscopic biliary drainage for unresectable hepatocellular carcinoma associated with obstructive jaundice. J Gastroenterol. 2001;36:173–180. doi: 10.1007/s005350170125. [DOI] [PubMed] [Google Scholar]
- 23.Ikenaga N, Chijiiwa K, Otani K, Ohuchida J, Uchiyama S, Kondo K. Clinicopathologic characteristics of hepatocellular carcinoma with bile duct invasion. J Gastrointest Surg. 2009;13:492–497. doi: 10.1007/s11605-008-0751-0. [DOI] [PubMed] [Google Scholar]
- 24.Wang YD, Xue HZ, Jiang QF, et al. Surgical operation and re-operation for hepatocellular carcinoma with bile duct thrombosis. Chin Med J (Engl) 2010;123:2163–2170. [PubMed] [Google Scholar]
- 25.Chen MF, Jan YY, Jeng LB, Hwang TL, Wang CS, Chen SC. Obstructive jaundice secondary to ruptured hepatocellular carcinoma into the common bile duct: surgical experiences of 20 cases. Cancer. 1994;73:1335–1340. doi: 10.1002/1097-0142(19940301)73:5<1335::AID-CNCR2820730505>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 26.Liver Cancer Study Group of Japan. Primary liver cancer in Japan: clinicopathologic features and results of surgical treatment. Ann Surg. 1990;211:277–287. [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148:397–401. doi: 10.1148/radiology.148.2.6306721. [DOI] [PubMed] [Google Scholar]
- 28.Takagi H, Yamada S, Abe T, et al. A case report of transcatheter arterial embolization of cholestatic type of hepatoma. Gastroenterol Jpn. 1989;24:315–319. doi: 10.1007/BF02774330. [DOI] [PubMed] [Google Scholar]
- 29.Yoon SM, Lim YS, Won HJ, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82:2004–2011. doi: 10.1016/j.ijrobp.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Yamada K, Izaki K, Sugimoto K, et al. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:113–119. doi: 10.1016/S0360-3016(03)00434-6. [DOI] [PubMed] [Google Scholar]
- 31.Fiorentini G, Giovanis P, Leoni M, et al. Amifostine (Ethyol) as modulator of hepatic and biliary toxicity from intraarterial hepatic chemoembolization: results of a phase I study. Hepatogastroenterology. 2001;48:313–316. [PubMed] [Google Scholar]