Abstract
Receptor tyrosine kinase regulation of phospholipase C-ɛ (PLC-ɛ), which is under the control of Ras-like and Rho GTPases, was studied with HEK-293 cells endogenously expressing PLC-coupled epidermal growth factor (EGF) receptors. PLC and Ca2+ signaling by the EGF receptor, which activated both PLC-γ1 and PLC-ɛ, was specifically suppressed by inactivation of Ras-related GTPases with clostridial toxins and expression of dominant-negative Rap2B. EGF induced rapid and sustained GTP loading of Rap2B, binding of Rap2B to PLC-ɛ, and Rap2B-dependent translocation of PLC-ɛ to the plasma membrane. GTP loading of Rap2B by EGF was inhibited by chelation of intracellular Ca2+ and expression of lipase-inactive PLC-γ1 but not of PLC-ɛ. Expression of RasGRP3, a Ca2+/diacylglycerol-regulated guanine nucleotide exchange factor for Ras-like GTPases, but not expression of various other exchange factors enhanced GTP loading of Rap2B and PLC/Ca2+ signaling by the EGF receptor. EGF induced tyrosine phosphorylation of RasGRP3, but not RasGRP1, apparently caused by c-Src; inhibition of c-Src interfered with EGF-induced Rap2B activation and PLC stimulation. Collectively, these data suggest that the EGF receptor triggers activation of Rap2B via PLC-γ1 activation and tyrosine phosphorylation of RasGRP3 by c-Src, finally resulting in stimulation of PLC-ɛ.
Stimulation of phospholipase C (PLC) enzymes, resulting in the production of the second messengers (diacylglycerol [DAG] and inositol 1,4,5-trisphosphate [IP3]) and subsequent activation of protein kinase C isoforms and Ca2+ release from intracellular stores, plays a major role in diverse early and late cellular responses to activation of various membrane receptors, including many G protein-coupled receptors (GPCRs) and receptor tyrosine kinases (2, 19). The 12 mammalian PLC isoforms identified so far (PLC-β1-4, PLC-γ1-2, PLC-δ1-4, PLC-ɛ, and PLC-ζ) contain family-specific regulatory domains and take differential positions in membrane receptor signaling (8, 24, 26). PLC isoforms share the structure of the archetypal PLC-δ enzymes, including an X and Y domain to form the catalytic core, an N-terminal pleckstrin homology domain, two EF hands, and a C2 domain. PLC-δ enzymes are tightly regulated by capacitative Ca2+ entry, but their activation by membrane receptors is only poorly understood (14, 18).
PLC-ζ (the PLC most recently identified), which was identified in sperm and which lacks the pleckstrin homology domain, induces typical Ca2+ oscillations in eggs and may represent the molecular trigger for embryo development (28). PLC-β enzymes are activated by GPCRs (either via GTP-loaded α subunits of the Gq class of G proteins or by Gβγ dimers liberated from Gi-type G proteins). On the other hand, PLC-γ enzymes, which contain Src homology 2 domains, are activated by receptor tyrosine kinases (such as those for the epidermal growth factor [EGF] and platelet-derived growth factor [PDGF]) by recruitment to the autophosphorylated receptor and subsequent tyrosine phosphorylation (8, 24, 26). PLC-ɛ bears an N-terminal CDC25 guanine nucleotide exchange factor (GEF) domain for Ras GTPases and two C-terminal Ras-binding (RA) domains, of which the RA2 domain interacts with GTP-bound Ras-like GTPases (10, 12, 15, 33). Coexpression studies revealed that PLC-ɛ can be activated by Gα12-type G proteins and Gβγ dimers (15, 37), Ras-like GTPases (such as H-Ras, Rap1A, and Rap2B) (12, 33, 34), and Rho GTPases (specifically, RhoA-C) (38). Some of these signaling molecules may even act upstream and downstream of PLC-ɛ; however, none of these molecules is apparently sufficient to directly stimulate PLC-ɛ activity in vitro, suggesting that a highly organized signaling complex is required to achieve PLC-ɛ activation.
Kataoka and colleagues reported that the EGF receptor induces subcellular translocation of PLC-ɛ in a H-Ras- and Rap1A-dependent manner (10, 33). Furthermore, this group demonstrated that ectopically expressed PLC-ɛ is activated by a PDGF receptor mutant deficient with respect to activation of PLC-γ1 and that this activation is mediated by H-Ras and Rap1A (34). Similarly, stimulation of ectopically expressed PLC-ɛ by the EGF receptor was reported to be mediated by Ras and Rap GTPases (13). On the other hand, Schmidt et al. have found that two typical adenylyl cyclase-coupled GPCRs, the β2-adrenergic receptor and a receptor for prostaglandin E1, can induce PLC-ɛ stimulation and that this PLC and Ca2+ signaling pathway is dependent on cyclic AMP (cAMP) formation and subsequent activation of Rap2B by Epac1, a cAMP-regulated Rap-specific GEF (32). Evellin et al. have furthermore reported that the M3 muscarinic acetylcholine receptor, which stimulates PLC-β1 via Gαq-type G proteins (27), can additionally stimulate PLC-ɛ by Gs-dependent cAMP formation and activation of Rap2B (7). The aim of the present study was to analyze whether the EGF receptor stimulates two distinct PLC isoforms, i.e., PLC-γ1 and PLC-ɛ, as well as to identify the mechanisms of PLC-ɛ activation, particularly whether and how PLC-γ1, which is directly activated by the EGF receptor (8, 24, 26, 29), contributes to activation of PLC-ɛ. Using HEK-293 cells endogenously expressing PLC-coupled EGF receptors and PLC-ɛ (7, 17, 31) as a cellular model, we report here that the EGF receptor stimulates both PLC-γ1 and PLC-ɛ and that stimulation of PLC-ɛ is mediated by Rap2B activated by the Ca2+/DAG-regulated GEF RasGRP3 in response to PLC-γ1 activation and tyrosine phosphorylation of RasGRP3 by c-Src.
MATERIALS AND METHODS
Materials, expression plasmids, and transfection.
AG1478, BAPTA/AM, Gö 6976, PP2, and recombinant c-Src were from Calbiochem-Merck Biosciences. Fura-2/AM and Alexa-488 goat anti-mouse immunoglobulin G (heavy plus light chains) conjugate were from Molecular Probes, MoBiTec. A23187, EGF, and ionomycin were from Biomol, and 8-(4-chlorophenylthio)-2′-O-methyl cAMP (8-pCPT-2Me-cAMP) was from Biolog Life Science Institute. The antibodies against C3G, Rap1, Rap2, H-Ras, PLC-γ1, and phosphotyrosine (PY20) were from Santa Cruz, and the antihemagglutinin (anti-HA) (12CA5) and anti-c-myc (9E10) antibodies were from Roche Molecular Biochemicals. The clostridial toxins used were kind gifts of C. von Eichel-Streiber. cDNAs encoding PLC-β1 and PLC-γ1 (each subcloned into pRK5), H335Q PLC-γ1 (subcloned into pCMV2), and PLC-δ1, PLC-ɛ, and H1144L PLC-ɛ (each subcloned into pcDNA3) were kindly provided by D. Illenberger, A. Ullrich, P.-G. Suh, and J. W. Lomasney. cDNAs encoding the mutants of the Ras-like GTPases were kindly provided by J. L. Bos, J. de Gunzburg, and H. Rehmann. cDNAs encoding C3G (121 kDa; subcloned into pcDNA3) and PDZ (200 kDa), Repac (64 kDa), Epac1 (99 kDa), RasGRP1 (90 kDa), GRP2 (69 kDa), and RasGRP3 (78 kDa; each subcloned into pMT2-HA) were kindly provided by J. L. Bos and J. de Rooij. cDNAs encoding K298M c-Src and K457A Pyk2 (each subcloned into pRK5) were kindly provided by J. T. Parsons and A. Blaukat. Transfection of HEK-293 cells grown to near confluence on 145-mm-diameter culture dishes with the indicated amounts of plasmid DNA or the corresponding empty vectors was performed with the calcium phosphate method, reaching a transfection efficiency of at least 50% (32). Expression of the encoded proteins was verified by the immunoblotting of cell lysates with specific antibodies. Assays were performed 48 h after transfection.
Detection of RasGRPs by reverse transcriptase PCR.
Genomic DNA and total cellular RNA were prepared from HEK-293 cells with a QIAamp DNA Mini kit and an RNeasy Mini kit (Qiagen), respectively. Reverse transcription was performed with an oligo(dT15) primer and SuperScript II reverse transcriptase (Invitrogen). Subsequently, PCR was carried out with a Taq PCR Master Mix kit (Qiagen) with 2 μl of cDNA and primer pairs encompassing one single intron each of RasGRP1 (forward primer, 5′-ACTAACACCTTCAAAGCCACCAGTAG-3′; reverse, 5′-TCCTGAGAAATGTATCCATCCTGGTC-3′), GRP2 (forward, 5′-GAAGCGGCAGGTGACTCAGCGGAAC-3′; reverse, 5′-AGAGGGAGATGAACCGCTCCAGGAC-3′), and RasGRP3 (forward, 5′-GTGGAGCCAATTGTCACAAACAGTG-3′; reverse, 5′-GCCTGTAACCAGTGTGATGGCTCTG-3′), respectively. PCR without template DNA served as a negative control, and PCR with 2 μl of genomic DNA served as a positive control. The sizes for the corresponding genomic amplicons amounted to 1,004, 416, and 515 bp and to 153, 212, and 206 bp for the amplified transcripts of RasGRP1, GRP2, and RasGRP3, respectively. Sequence information was derived from the GenBank database (accession numbers NT_010194, 033903, and 022184).
Measurement of PLC activity and [Ca2+]i.
For measurement of PLC activity, HEK-293 cells were serum starved for 36 h and incubated for the indicated periods of time at 37°C with or without 100 ng of EGF/ml followed by IP3 mass determination (32). Intracellular free Ca2+ concentrations ([Ca2+]i) were determined in cell suspensions with the fluorescent Ca2+ indicator dye Fura-2 in a Hitachi spectrofluorometer (7). To study the effects of clostridial toxins, the cells were treated for 24 h without or with the toxins at the indicated concentrations followed by IP3 and [Ca2+]i measurements.
Activation of Rap2B.
Activation of Rap2B was determined in serum-starved HEK-293 cells transfected with wild-type Rap2B and stimulated for the indicated periods of time at 37°C without or with EGF followed by extraction of the activated Rap2B from the cell lysates with glutathione S-transferase (GST)-tagged RalGDS-RBD (Rap-binding domain of the Ral guanine nucleotide dissociation stimulator) bound to glutathione Sepharose beads and immunoblotting with an anti-Rap2 antibody as described before (32). Densitometric analysis of the bands was performed with ImageQuant software (Molecular Dynamics).
Confocal laser scanning microscopy.
Cells were transfected with a combination of expression plasmids as indicated in the corresponding figure legend and grown on coverslips pretreated with 0.1 mg of poly-d-lysine/ml (9). Serum-starved cells were rinsed with Moscona containing 13.6 mM NaCl, 4 mM KCl, 12 mM NaHCO3, 10 mM d-glucose, 0.36 mM NaH2PO4, and 0.18 mM KH2PO4 (pH 7.4) and incubated without or with EGF for 5 min at 37°C. Thereafter, the cells were fixed and permeabilized with ethanol-acetone (1/1) for 10 min at room temperature followed by two washes in Moscona. Unspecific binding was blocked by incubation of the cells for 15 min with Moscona-bovine serum albumin (BSA) (Moscona supplemented with 0.5% BSA). The cells were rinsed and incubated for 1 h at room temperature with the anti-c-myc antibody. Afterwards, the cells were rinsed three times with Moscona-BSA and incubated for 1 h in darkness with a fluorescence-conjugated secondary antibody (Alexa-488; dilution, 1:200). The secondary antibody was washed out in darkness with Moscona-BSA, and the cell specimens were mounted in Moscona supplemented with 90% glycerol and 1.0% p-phenylenediamine. Confocal immunofluorescence imaging was performed with a Zeiss LSM 510 Axiovert 100 M confocal laser scanning microscopy system (Plan-neofluor 40×/1.3 oil objective, 488- to 633-nm excitation). Confocal laser microscopy images were processed by ImageProPlus software (version 4.5; Media Cybernatics) with a Gaussian filter module (1).
Immunoprecipitation.
Cells were transfected with the expression plasmids indicated in the corresponding figure legends and grown on 60-mm-diameter culture dishes in serum-free medium. Cells were rinsed in Hank's balanced salt solution and incubated without or with EGF for 5 min at 37°C. Then, the cells were washed twice with ice-cold phosphate-buffered saline containing 137 mM NaCl, 2.7 mM KCl, 6.5 mM NaH2PO4, 1.5 mM KH2PO4, 0.9 mM CaCl2, 0.5 mM MgCl2, and 100 μM orthovanadate (pH 7.2). Meanwhile, the anti-HA antibody (2 μg) or the anti-c-myc antibody (2 μg) was gently mixed with protein A-Sepharose (20 μl/reaction tube) in immunoprecipitation buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 10 mM sodium pyrophosphate, 50 mM NaF, 1 mM EGTA, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS) for 1 h at 4°C. Cells were scraped in 500 μl of ice-cold immunoprecipitation buffer supplemented with 1 mM orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin/ml, and 25 μg of aprotinin/ml, transferred to precooled reaction tubes, vortexed for 10 s, and incubated on ice for at least 10 min. Lysates were clarified by centrifugation, the supernatants (1 mg of protein/reaction tube) were gently mixed for 2 h at 4°C, and precipitates were washed four times with immunoprecipitation buffer. Precipitated proteins were incubated in Laemmli buffer for 10 min at 95°C and separated by SDS-polyacrylamide gel electrophoresis (PAGE). Tyrosine phosphorylation and bound Rap2B were detected by immunoblotting with specific antibodies.
Purification of GST-tagged RasGRP3 from Sf9 cells.
For generation of a RasGRP3 baculovirus construct, pMT2-HA-RasGRP3 (KIAA0846; GenBank accession number AB020653) was digested by SalI/NotI and subcloned into the restriction sites XhoI and NotI of the transfer vector pAcGHLT-C (Pharmingen). Purified transfer vector construct (1.0 μg) was cotransfected with 0.25 μg of linearized BaculoGold DNA (Pharmingen) into Sf9 cells with Lipofectamine (Invitrogen). Recombinant RasGRP3-encoding baculovirus was amplified by infection of Sf9 cell monolayers followed by plaque purification and identification of the heterologous RasGRP3 insert within the genome of the plaque-purified recombinant virus. RasGRP3 baculovirus-infected Sf9 cells were harvested 1 day postinfection by centrifugation, resuspended in preparation buffer containing 50 mM Tris-HCl (pH 7.5), 2 mM EDTA, 1 mM dithiothreitol, 250 mM sucrose, 10 μM phenylmethylsulfonyl fluoride, and 0.5 μg of leupeptin/ml, and lysed by sonification. The cell lysate was incubated for 30 min at 4°C with Triton X-100 (1% final concentration). After centrifugation, the supernatant (containing GST-tagged RasGRP3) was gently mixed with glutathione Sepharose beads, washed three times with the preparation buffer, and stored at 4°C. The immobilized GST-tagged RasGRP3 was analyzed by SDS-PAGE and immunoblotting with an anti-GST antibody.
In vitro c-Src assay.
GST or GST-tagged RasGRP3 (2 to 3 μg) was incubated for 10 min at 30°C with or without 2 U of recombinant c-Src. Assays were carried out in kinase reaction buffer containing 50 mM MgCl2, 15 mM MnCl2, 5 mM sodium orthovanadate (buffered at pH 7.5 with 100 mM HEPES), and [γ-32P]ATP (0.75 MBq/reaction tube) or 1 mM unlabeled ATP. The reaction was stopped by addition of Laemmli buffer and heating for 10 min at 95°C. Phosphorylated proteins were separated by SDS-PAGE and visualized by Coomassie staining and autoradiography or by immunoblotting with an antiphosphotyrosine antibody.
Immunoblot analysis.
For detection of c-myc, PLC-γ1, Rap1A, Rap2A, Rap2B, HA, phosphotyrosine, and PLC-ɛ, equal amounts of protein from cell lysates were separated by SDS-PAGE on 5 or 15% acrylamide gels. After a transfer to nitrocellulose membranes and a 10-h incubation with the appropriate antibodies, the proteins were visualized by enhanced chemiluminescence.
Data presentation.
Data shown in figures or given in the text are means ± standard errors of the means (SEM) of the results of n independent experiments (each performed in triplicate). Comparisons between means were performed with Student's paired t test or by a one-way analysis of variance test; a difference at P < 0.05 was regarded as significant.
RESULTS
Activation of PLC-γ1 and PLC-ɛ by the EGF receptor.
We have recently reported that stimulation of cellular PLC activity by a GPCR, the M3 muscarinic acetylcholine receptor, is a composite action of Gαq-mediated PLC-β1 and Rap2B-mediated PLC-ɛ stimulation (7). Thus, we wondered whether the EGF receptor (known to activate PLC-γ1) (8, 24, 26) might stimulate PLC-ɛ in addition as well. For this, we first studied the effects of wild-type PLC-γ1 and PLC-ɛ and their lipase-inactive mutants, H335Q PLC-γ1 and H1144L PLC-ɛ (15), respectively, in HEK-293 cells endogenously expressing PLC-coupled EGF receptors and PLC-ɛ (7, 17, 31). As expected, overexpression of wild-type PLC-γ1 largely enhanced (by about twofold) IP3 formation induced by EGF (100 ng/ml), leaving basal IP3 levels unaffected whereas expression of the lipase-inactive H335Q PLC-γ1 reduced EGF-stimulated IP3 formation by about 50% (Fig. 1A, left panel). Corresponding changes were observed when measuring EGF-induced [Ca2+]i levels, which were increased from 127 ± 15 nM to 186 ± 23 nM (n = 10 to 12; P < 0.0001) by overexpression of wild-type PLC-γ1 and decreased from 117 ± 16 nM to 65 ± 15 nM (n = 6 to 8; P < 0.001) by expression of H335Q PLC-γ1 (Fig. 1B, left panel).
FIG. 1.
Effects of PLC-γ1 and PLC-ɛ on EGF-induced PLC and Ca2+ signaling in HEK-293 cells. HEK-293 cells on 145-mm-diameter culture dishes were transfected with empty vector (Vector, V), PLC-γ1, PLC-ɛ, PLC-β1, PLC-δ1 (wild type each), lipase-inactive H335Q PLC-γ1, and H1144L PLC-ɛ or the combination of lipase-inactive H335Q PLC-γ1 and H1144L PLC-ɛ. Transfection was with 100 μg of DNAs encoding lipase-inactive PLCs and with 25 μg of DNAs encoding wild-type PLCs. At 48 h after transfection, IP3 formation measured for 1 min in the absence (Basal) or presence of 100 ng of EGF/ml (A) and EGF-induced [Ca2+]i increases (B) were determined. Data in panel A represent means ± SEM (n = 4 to 5); in panel B, superimposed tracings of [Ca2+]i are shown. The insets show immunoblot detection of the PLCs.
Very similar changes in these EGF responses were observed with the PLC-ɛ enzymes. Overexpression of wild-type PLC-ɛ increased IP3 formation induced by EGF by about twofold, while expression of lipase-inactive H1144L PLC-ɛ reduced EGF-induced IP3 formation by about 50% (Fig. 1A, left panel). Furthermore, the EGF-induced [Ca2+]i rise was increased from 156 ± 26 nM to 285 ± 21 nM (n = 8 to 10; P < 0.0001) by overexpression of wild-type PLC-ɛ and reduced to 113 ± 15 nM (n = 6 to 8; P < 0.001) by expression of lipase-inactive PLC-ɛ (Fig. 1B, middle panel). Coexpression of lipase-inactive PLC-γ1 and PLC-ɛ nearly fully abolished EGF-induced IP3 formation and [Ca2+]i increases (Fig. 1). In contrast to the results seen with PLC-γ1 and PLC-ɛ, overexpression of PLC-β1 (which increased PLC stimulation by the M3 muscarinic acetylcholine receptor) (7) did not change basal levels of IP3 or its stimulation by EGF. Overexpression of PLC-δ1 increased basal IP3 levels by about twofold, whereas EGF-stimulated IP3 formation was not changed (Fig. 1A, right panel). Together, these data suggested that stimulation of PLC activity by the EGF receptor in HEK-293 cells is a composite action of PLC-γ1 and PLC-ɛ.
Involvement of Rap2B in EGF receptor-mediated PLC stimulation.
PLC-ɛ activity is controlled by small GTPases of the Ras and Rho families (10, 12, 13, 15, 33, 34, 38). To study whether and which types of GTPases are involved in PLC stimulation by the EGF receptor, we first examined the effects of several clostridial toxins inactivating these small GTPases. Treatment of HEK-293 cells with Clostridium difficile toxin B, which inactivates RhoA, Rac1, and Cdc42 (11), did not alter EGF-induced IP3 formation and [Ca2+]i increases (Fig. 2A). In contrast, treatment of the cells with C. difficile toxin B-1470 (known to inactivate Rac, Rap, and Ral GTPases) or C. sordellii lethal toxin (which inactivates Ras in addition) (4, 30) strongly reduced levels of IP3 formation induced by EGF (Fig. 2A, left panel). This inhibition was paralleled by an attenuation of EGF-induced [Ca2+]i rises, which were significantly (P < 0.001) decreased from 148 ± 25 nM (n = 7) to 102 ± 17 nM (n = 5) and 114 ± 15 nM (n = 4) by the presence of toxin B-1470 and lethal toxin, respectively (Fig. 2A, right panel). Inhibition of PLC and Ca2+ signaling by the toxins was not due to a fall in PLC substrate levels (7). Furthermore, the toxins did not alter the expression of EGF receptors (as determined by immunoblotting with an EGF receptor antibody) or EGF-induced phosphorylation of the mitogen-activated protein kinases (ERK1 and ERK2) (36) (data not shown).
FIG.2.
Effects of clostridial toxins and Ras-like GTPase mutants on EGF-induced PLC and Ca2+ signaling. (A) HEK-293 cells were treated for 24 h without (Control) or with 100 pg of toxin B/ml, 300 pg of toxin B-1470/ml, or 100 ng of lethal toxin/ml followed by determination of IP3 formation for 1 min in the absence (Basal) or presence of 100 ng of EGF/ml (left panel) and of EGF-induced [Ca2+]i increases (right panel). (B) HEK-293 cells were transfected with empty vector (Control), S17N Ras, S17N Rap1A, or S17N Rap2B (100 μg of DNA each). At 48 h after transfection, levels of IP3 formation measured for the indicated periods of time without (Basal) or with 100 ng of EGF/ml (left panel) and EGF-induced [Ca2+]i increases (right panel) were determined. (C) HEK-293 cells were transfected with empty vector (Control), G12V Rap1A, G12V Rap2A, or G12V Rap2B (25 μg of DNA each). At 48 h after transfection, IP3 formation was measured for 1 min without (Basal) or with 100 ng of EGF/ml. Inset: immunoblot detection of the GTPases in lysates of cells transfected with empty vectors (V) or the indicated GTPases. Data in the left panels represent means ± SEM (n = 3 to 6); in the right panels, superimposed tracings of [Ca2+]i are shown. n.s., not significant.
We then examined which specific Ras-related GTPase (serving as a toxin substrate) is involved in EGF receptor signaling. For this, we studied the effects of various GTPase mutants on IP3 formation and [Ca2+]i increase by the EGF receptor. In control cells EGF caused a rapid and transient increase in IP3, with a peak level at 1 to 2 min (Fig. 2B, left panel). Expression of dominant-negative S17N H-Ras, which interfered with EGF signaling to phospholipase D in these cells (36), or S17N Rap1A did not alter the extent or time course of IP3 formation. In contrast, expression of S17N Rap2B strongly reduced the EGF-induced IP3 formation. In line with their distinct effects on IP3 formation, expression of S17N Rap2B, but not of S17N H-Ras or S17N Rap1A, reduced the EGF-induced [Ca2+]i increase from 162 ± 25 nM to 116 ± 14 nM (n = 6 to 8; P < 0.0001) (Fig. 2B, right panel). Furthermore, expression of constitutively active G12V Rap2B, but not of G12V Rap1A or G12V Rap2A, significantly enhanced basal IP3 accumulation in unstimulated cells (Fig. 2C). EGF-induced IP3 formation was not further increased by expression of G12V Rap2B.
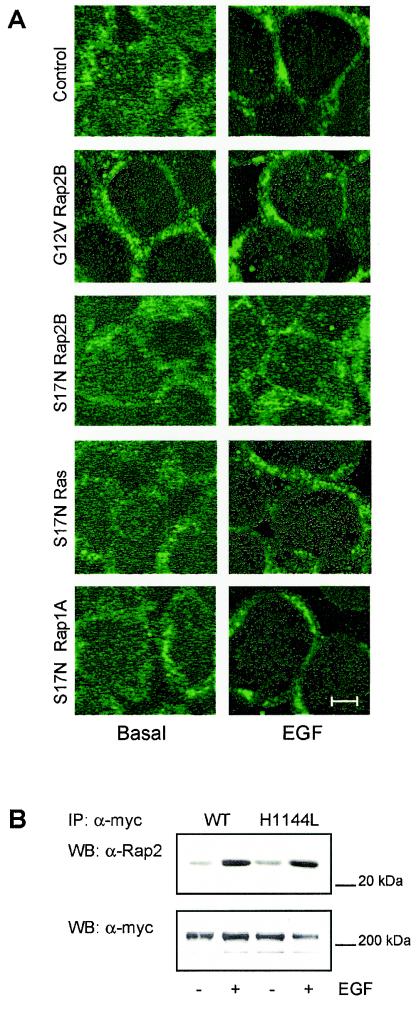
Recently, Kataoka and colleagues reported that EGF induced translocation of PLC-ɛ to the plasma membrane in COS-7 cells in a Ras-dependent manner and that Rap1A induced translocation of PLC-ɛ to perinuclear regions (10, 33). Therefore, we studied whether EGF alters the subcellular distribution of PLC-ɛ in HEK-293 cells as well as whether such a process is under the control of Rap2B (apparently involved in PLC stimulation by the EGF receptor). As illustrated in Fig. 3A, EGF induced translocation of PLC-ɛ to the plasma membrane (as analyzed by immunofluorescence laser confocal microscopy of HEK-293 cells expressing c-myc-tagged PLC-ɛ). This receptor effect was mimicked by expression of constitutively active G12V Rap2B. The subcellular redistribution of PLC-ɛ induced by EGF was fully eliminated upon coexpression of dominant-negative Rap2B but not of dominant-negative H-Ras or Rap1A (Fig. 3A). These data suggested that Rap2B interacts with PLC-ɛ. Indeed, Rap2B was coimmunoprecipitated with PLC-ɛ from lysates of HEK-293 cells coexpressing c-myc-tagged PLC-ɛ and Rap2B, demonstrating their in vivo interaction (Fig. 3B). Most important, stimulation of the EGF receptor strongly enhanced the interaction of PLC-ɛ and Rap2B. Similar data were obtained with the lipase-inactive H1144L PLC-ɛ, implying that the lipase activity is not required for the interaction of PLC-ɛ and Rap2B. In line with the missing effect of dominant-negative Rap1A on EGF-induced subcellular translocation of PLC-ɛ (Fig. 3A), EGF did not lead to coimmunoprecipitation of Rap1A with PLC-ɛ (data not shown). Collectively, these data suggested that the EGF receptor induces stimulation of PLC-ɛ by specific binding of Rap2B and translocation of the lipase to the plasma membrane.
FIG. 3.
Interaction of Rap2B with PLC-ɛ. (A) HEK-293 cells were transfected with c-myc-tagged PLC-ɛ alone (Control) or together with G12V Rap2B (25 μg of DNA) or with S17N Rap2B, S17N Ras, or S17N Rap1A (100 μg of DNA each). After 48 h, the cells were treated for 5 min without (Basal) or with 100 ng of EGF/ml, stained with anti-c-myc antibody, and analyzed by immunofluorescence laser confocal microscopy. Bar, 5 μm. (B) HEK-293 cells were transfected with wild-type Rap2B (50 μg of DNA) and either c-myc-tagged wild-typePLC-ɛ (WT) or H1144L PLC-ɛ (H1144L) (25 μg of DNA each). After 48 h, the cells were treated for 5 min without (−) or with (+) 100 ng of EGF/ml followed by cell lysis and immunoprecipitation with an anti-c-myc-antibody. The immunoprecipitates (IP) of c-myc-tagged PLC-ɛ were resolved by SDS-PAGE and probed with anti-Rap2 (α-Rap2) or anti-c-myc antibodies (α-myc) as indicated. The results shown are representative of three to four experiments. WB, Western blot.
Involvement of RasGRP3 in EGF receptor-mediated PLC stimulation and Rap2B activation.
GTPases of the Rap family are activated by various GEFs, such as C3G, Ca2+/DAG-regulated GEFs, PDZ-GEFs, Repac-GEFs, and Epac-GEFs (5, 23). To identify the specific GEF involved in Rap2B-mediated signaling of the EGF receptor to the PLC-ɛ, we expressed various of these GEFs to comparable levels (Fig. 4A) and analyzed their effects on EGF-induced PLC stimulation, [Ca2+]i increase, and Rap2B activation. The GEFs differed strikingly in their effects on PLC and Ca2+ signaling. Expression of PDZ, C3G, Epac1, Repac, GRP2, and RasGRP1 did not change basal or EGF-induced IP3 formation results (Fig. 4B). In contrast, expression of RasGRP3 strongly enhanced (by about twofold) the EGF-induced IP3 formation (Fig. 4B). Coexpression of dominant-negative Rap2B fully suppressed the potentiation of EGF-induced IP3 formation caused by RasGRP3 (from 185 ± 15 pmol mg−1 to 56 ± 10 pmol mg−1 [n = 6]; P < 0.0001). In line with their distinct effects on PLC stimulation, only expression of RasGRP3, but not of C3G, PDZ, Epac1, or GRP2, greatly enhanced the EGF-induced [Ca2+]i level from 155 ± 25 nM to 295 ± 29 nM (n = 8 to 10; P < 0.0001) (Fig. 4C). As RasGRP3 seemed to specifically mediate PLC signaling, we investigated whether HEK-293 cells endogenously express this GEF. Reverse transcriptase PCR with RasGRP-specific oligonucleotide primer pairs demonstrated that HEK-293 cells express (in addition to specific mRNA transcripts encoding RasGRP1 and GRP2) a specific mRNA transcript for RasGRP3 (Fig. 4D).
FIG. 4.
Effects of various GEFs for Ras-like GTPases on EGF-induced PLC and Ca2+ signaling. (A to C) HEK-293 cells were transfected with empty vector (Vector, V), PDZ, C3G, Epac1, Repac, GRP2, RasGRP1, or RasGRP3 (25 μg of DNA each). At 48 h after transfection, levels of IP3 formation measured for 1 min in the absence (Basal) or presence of 100 ng of EGF/ml (B) and EGF-induced [Ca2+]i increases (C) were determined. (A) Immunoblot detection of the HA-tagged GEFs in lysates of transfected cells. Inset in panel B: immunoblot detection of C3G. (D) Detection of RasGRPs in HEK-293 cells by reverse transcriptase PCR (RT-PCR). Data in panel B represent means ± SEM (n = 3 to 5); in panel C, superimposed tracings of [Ca2+]i are shown.
EGF treatment of HEK-293 cells caused a rapid, rather long-lasting activation of Rap2B, with a maximum level at 5 min (as determined by extraction of GTP-loaded Rap2B from cell lysates with immobilized RalGDS-RBD) (Fig. 5A). Expression of PDZ, C3G, Epac1, Repac (data not shown), GRP2, or RasGRP1 had no effect on activation of Rap2B by EGF. In contrast, expression of RasGRP3 (which by itself had no effect) strongly enhanced (by about twofold) the stimulatory effect of EGF on Rap2B activation (Fig. 5B). PDZ, C3G, Repac, GRP2, and RasGRP1, however, were not inactive; these GEFs enhanced EGF-induced GTP loading of Rap1A and H-Ras, respectively (data not shown). Thus, RasGRP3, a member of the Ca2+/DAG-regulated GEF family known to induce GTP loading of members of the Ras and Rap families both in vitro and in vivo (20, 25, 39), is apparently specifically involved in EGF receptor-mediated Rap2B activation and subsequent PLC-ɛ stimulation.
FIG. 5.
Rap2B activation by the EGF receptor and its regulation by RasGRP3, intracellular Ca2+, and PLC-γ1. (A) HEK-293 cells were transfected with wild-type Rap2B (50 μg of DNA). At 48 h after transfection, the cells were stimulated for the indicated periods of time without (−) or with (+) 100 ng of EGF/ml. (B) HEK-293 cells were transfected with Rap2B alone (Control) and with the indicated GEFs (25 μg of DNA each). At 48 h after transfection, the cells were stimulated for 5 min without (−) or with (+) 100 ng of EGF/ml. (C) HEK-293 cells were transfected with Rap2B. At 48 h after transfection, the cells were first treated for 30 min without (Control) or with 100 nM Gö 6976 or 20 μM BAPTA/AM and then stimulated for 5 min without (Basal) or with 100 ng of EGF/ml (left panel) or with 1 μM A23187 or 1 μM ionomycin (right panel). (D) HEK-293 cells were transfected with Rap2B alone (Control) or with H1144L PLC-ɛ or H335Q PLC-γ1 (100 μg of DNA each). At 48 h after transfection, the cells were stimulated for 5 min without (Basal) or with 100 ng of EGF/ml. GTP-loaded Rap2B was extracted with RalGDS-RBD, separated by SDS-PAGE, and immunoblotted with an anti-Rap2 antibody. The results shown are representative of three to six experiments.
Mechanisms of RasGRP3-mediated Rap2B activation and PLC-ɛ stimulation by the EGF receptor.
The GEF activity of RasGRP3 is dependent on the presence of PLC-derived second messengers Ca2+ and DAG, the latter acting independently (at least in part) of protein kinase C (3, 5, 16, 23). In line with this notion, treatment of HEK-293 cells with Gö 6976 (100 nM), an inhibitor of conventional protein kinase C isoforms, did not alter EGF-induced Rap2B activation (Fig. 5C, left panel). In contrast, chelation of intracellular Ca2+ by treatment of the cells with BAPTA/AM (20 μM) strongly inhibited (by about 80%) activation of Rap2B by EGF (Fig. 5C, left panel) whereas the Ca2+ ionophores (A23187 and ionomycin) (1 μM each) mimicked the activation of Rap2B by EGF (Fig. 5C, right panel). As PLC stimulation (and thus [Ca2+]i increase and DAG formation induced by the EGF receptor) is apparently a composite action of PLC-γ1 and PLC-ɛ (Fig. 1), it was of major interest to know which of the two PLC isoforms is involved in EGF-induced Rap2B activation. Expression of lipase-inactive H1144L PLC-ɛ did not alter the EGF-induced Rap2B activation. In contrast, expression of lipase-inactive H335Q PLC-γ1 strongly reduced (by 40 to 50%) activation of Rap2B by the EGF receptor (Fig. 5D), suggesting that Rap2B activation by the EGF receptor is a consequence of PLC-γ1 stimulation.
The EGF receptor activates PLC-γ1 by recruitment of the lipase to autophosphorylated tyrosine residues of the receptor and subsequent tyrosine phosphorylation (8, 24, 26, 29). As stimulation of PLC-ɛ by the EGF receptor was apparently mediated by RasGRP3, we examined whether EGF might induce tyrosine phosphorylation of this GEF. Figure 6A illustrates that the activated EGF receptor in fact causes tyrosine phosphorylation of RasGRP3, but not of RasGRP1, detected in immunoprecipitates from cells expressing HA-tagged RasGRP3 with an antiphosphotyrosine antibody. The EGF-induced tyrosine phosphorylation of RasGRP3 was fully eliminated by the EGF receptor kinase inhibitor AG1478 (10 μM). As we failed to coimmunoprecipitate RasGRP3 with the EGF receptor, we then studied whether c-Src, a cytosolic tyrosine kinase activated by the EGF receptor (26), triggers phosphorylation of RasGRP3. Treatment of HEK-293 cells with PP2 (10 μM), an inhibitor of c-Src, completely suppressed tyrosine phosphorylation of RasGRP3 (Fig. 6B). Furthermore, EGF-induced tyrosine phosphorylation of RasGRP3 was inhibited by expression of kinase-deficient K298M c-Src but not of kinase-deficient K457A Pyk2. Finally, we purified recombinant GST-tagged RasGRP3 from Sf9 cells and subjected the protein to in vitro phosphorylation by recombinant c-Src. c-Src caused incorporation of 32P into the GST-RasGRP3 fusion protein (Fig. 6C) but not into the GST protein (data not shown). In vitro tyrosine phosphorylation of GST-tagged RasGRP3 by c-Src, which was suppressed by PP2 (data not shown), was confirmed by immunoblotting with an antiphosphotyrosine antibody (Fig. 6C).
FIG. 6.
EGF receptor-mediated tyrosine phosphorylation of RasGRP3 by c-Src. (A) HEK-293 cells were transfected with HA-tagged RasGRP1 or RasGRP3 (25 μg of DNA each). After 48 h, the cells were incubated for 30 min without (−) or with (+) 10 μM AG1478 and then stimulated for 5 min without (−) or with (+) 100 ng of EGF/ml as indicated. WB, Western blot; α-HA, anti-HA. (B) HEK-293 cells were transfected with RasGRP3 (25 μg of DNA) alone or with kinase-deficient K298M c-Src or kinase-deficient K457A Pyk2 (75 μg of DNA each). After 48 h, the cells were incubated for 30 min without (−) or with (+) 10 μM PP2 and then stimulated for 5 min without (−) or with (+) 100 ng of EGF/ml as indicated. After cell lysis, the HA-tagged RasGRPs were immunoprecipitated, separated by SDS-PAGE, and probed with antiphosphotyrosine (α-PY) antibodies. Expression of K298M c-Src and K457A Pyk2 was confirmed by immunoblot experiments with specific antibodies (data not shown). (C) Purified GST-tagged RasGRP3 was subjected to in vitro kinase assays without (−) or with (+) recombinant c-Src in the presence of [γ-32P]ATP or unlabeled ATP. Proteins were separated by SDS-PAGE, and RasGRP3 was detected by Coomassie staining, by autoradiography (32P), or by immunoblotting with an antiphosphotyrosine antibody (α-PY). The results are representative of three to five experiments.
We next sought to determine whether the EGF receptor-mediated tyrosine phosphorylation of RasGRP3 by c-Src triggers activation of Rap2B. As illustrated in Fig. 7A (left panel), treatment of the cells with PP2 (10 μM) strongly reduced (by about 80%) activation of Rap2B by EGF and did so almost as effectively as treatment with the EGF receptor inhibitor, AG1478 (10 μM). The c-Src inhibitor PP2 (10 μM) also strongly reduced (by about 60%) activation of Rap2B by A23187 (Fig. 7A, middle panel). The inhibitory effect of PP2 on Rap2B activation by the EGF receptor was mimicked by expression of kinase-deficient c-Src but not of kinase-deficient Pyk2 (Fig. 7A, right panel). Finally, we studied the effects of inhibition of Ca2+ and c-Src on the EGF receptor-mediated PLC stimulation. Treatment of the cells with BAPTA/AM (20 μM) or PP2 (10 μM) had no major effect on the initial rapid IP3 rise but strongly reduced the prolonged increase in IP3 formation in response to stimulation by EGF, and the combination of BAPTA/AM plus PP2 further reduced IP3 formation (Fig. 7B, left panel). In contrast, PP2 did not reduce PLC stimulation induced by the Epac-specific cAMP analog, 8-pCPT-2Me-cAMP (10 μM) (6), which increased IP3 formation in HEK-293 cells by 75 ± 10 pmol mg−1 and 68 ± 8 pmol mg−1 (n = 4; P > 0.05) in the absence and presence of 10 μM PP2, respectively, indicating that c-Src is specifically involved in the EGF receptor responses. Finally, expression of kinase-deficient K298M c-Src, but not of kinase-deficient K457A Pyk2, mimicked the inhibitory effect of PP2 on PLC stimulation by EGF (Fig. 7B, right panel).
FIG. 7.
Effects of Ca2+ chelation and c-Src inhibition on Rap2B activation and PLC stimulation by the EGF receptor. (A) HEK-293 cells were transfected with wild-type Rap2B (50 μg of DNA) alone (Control) or with K457A Pyk2 or K298M c-Src (75 μg of DNA each). At 48 h after transfection, the cells were treated for 30 min without (Control) or with 10 μM AG1478 or 10 μM PP2 and then stimulated for 5 min without (Basal) or with 100 ng of EGF/ml or 1 μM A23187. GTP-loaded Rap2B was extracted with RalGDS-RBD, separated by SDS-PAGE, and immunoblotted with an anti-Rap2 antibody. Immunoblots shown are representative of four to six experiments. (B) HEK-293 cells were transfected with empty vector (Control), K457A Pyk2, or K298M c-Src (75 μg of DNA each). After 48 h, the cells were first treated for 30 min without (Control) or with 10 μM PP2, 20 μM BAPTA/AM, or PP2 plus BAPTA/AM (Both). IP3 formation was measured for the indicated periods of time without (Basal) or with 100 ng of EGF/ml. Data in panel B represent means ± SEM (n = three to six).
DISCUSSION
Receptor tyrosine kinases (such as the EGF and PDGF receptors) are known to stimulate PLC-γ1 by recruitment of the lipase to the activated receptor and subsequent tyrosine phosphorylation (8, 24, 26, 29). Recent data demonstrated that PLC-ɛ, which is under the control of small GTPases (10, 12, 13, 15, 33, 34, 38), can be regulated by these receptors as well. Specifically, it was shown that the EGF receptor and a PDGF receptor mutant deficient in its ability to activate PLC-γ1 can induce stimulation of ectopically expressed PLC-ɛ. These EGF and PDGF receptor actions were apparently mediated by H-Ras and Rap1A GTPases (13, 34). An aim of the present study was to analyze the mechanisms of PLC-ɛ stimulation by the EGF receptor in a cellular setting (in which a possible interaction of PLC-γ1 and PLC-ɛ can be assessed). For this, we used HEK-293 cells endogenously expressing PLC-coupled EGF receptors as well as PLC-γ1 and PLC-ɛ (7, 17, 31). We report here that the EGF receptor induces stimulation of PLC-ɛ in these cells in a Rap2B-dependent manner and that this process apparently involves PLC-γ1, c-Src, and the Ras/Rap-GEF RasGRP3.
Expression of lipase-inactive mutants of PLC-γ1 and PLC-ɛ reduced PLC and Ca2+ signaling by the EGF receptor to comparable extents, and coexpression of both lipase-inactive PLC mutants nearly fully suppressed these receptor responses, suggesting that both PLC isoforms contribute to EGF receptor signaling. That the EGF receptor in fact stimulates PLC-γ1 and PLC-ɛ, but not PLC-β1 or PLC-δ1, was confirmed by overexpression of the lipases. As PLC-ɛ, but not PLC-γ1, is under the control of small GTPases of the Ras and Rho families, different approaches were used to identify the specific GTPase involved in the EGF receptor action. When clostridial toxins with distinct sensitivities to these GTPases were used, (Ras and Rap but not Rho) GTPases appeared to be the most likely candidates. Of these GTPases, H-Ras, Rap1A, and Rap2B were previously shown to mediate PLC-ɛ stimulation by receptors (7, 13, 32, 34). IP3 formation and [Ca2+]i increase induced by the EGF receptor in HEK-293 cells were specifically suppressed by expression of dominant-negative Rap2B but not by dominant-negative mutants of H-Ras and Rap1A. Furthermore, only expression of constitutively active Rap2B (but not of the close relatives, Rap1A and Rap2A) strongly enhanced IP3 formation in unstimulated cells, suggesting that PLC-ɛ stimulation by the EGF receptor in HEK-293 cells is mediated by Rap2B. The use of ectopically expressed PLC-ɛ corroborated the specific role of Rap2B. EGF receptor activation induced translocation of PLC-ɛ to the plasma membrane. This receptor action was mimicked by expression of constitutively active Rap2B and prevented by expression of dominant-negative Rap2B but not of dominant-negative mutants of H-Ras and Rap1A. Furthermore, EGF strongly enhanced association of Rap2B, but not Rap1A, to PLC-ɛ, as revealed by coimmunoprecipitation of PLC-ɛ and Rap2B. Overall, these data strongly suggest that the EGF receptor induces PLC-ɛ stimulation in HEK-293 cells by activation of Rap2B and binding of the activated GTPase to PLC-ɛ, probably to its RA2 domain (13, 34), which then leads to translocation of the lipase to the plasma membrane.
This conclusion differs from the reported involvement of GTPases in regulation of ectopically expressed PLC-ɛ by the EGF and PDGF receptors in other cell types. Whereas expression of dominant-negative S17N H-Ras suppressed EGF-induced translocation of PLC-ɛ to the plasma membrane in COS-7 cells (33) as well as PDGF- and EGF-induced PLC-ɛ stimulation in BaF3 and COS-7 cells, respectively (13, 34), expression of S17N H-Ras in HEK-293 cells did not affect PLC and Ca2+ signaling by the EGF receptor or EGF-induced translocation of PLC-ɛ to the plasma membrane (effects strongly reduced by expression of S17N Rap2B). Also, Rap1A has been suggested to affect PLC-ɛ signaling in COS-7 cells (10, 33, 34); using dominant-negative and constitutively active mutants of Rap1A and analyzing a possible association of PLC-ɛ with Rap1A in response to EGF, however, we found no evidence for an involvement of Rap1A in EGF-induced PLC-ɛ activation. Thus, although EGF activated not only Rap2B but also H-Ras and Rap1A in HEK-293 cells (data not shown) the latter two GTPases apparently do not play a role in PLC-ɛ stimulation by the EGF receptor.
Evellin et al. and Schmidt et al. previously reported that Rap2B activation and subsequent PLC-ɛ stimulation by GPCRs in HEK-293 cells is mediated by the cAMP-activated Rap-GEF Epac1 (7, 32). However, overexpression of Epac1 had no effect on EGF-induced Rap2B and PLC activation. In line with this, EGF did not increase cAMP levels and inhibition of adenylyl cyclase by 2′,5′-dideoxyadenosine did not alter PLC stimulation by EGF (data not shown). Other GEFs (PDZ, C3G, Repac1, GRP2, and RasGRP1) for Rap GTPases (5, 23) were similarly ineffective. In contrast, expression of RasGRP3 strongly increased Rap2B activation as well as IP3 formation and [Ca2+]i rise induced by the EGF receptor (an effect fully blocked by coexpression of dominant-negative Rap2B). RasGRP3, which is most abundant in kidney, brain, lung, and heart (5, 20, 23, 25, 39), is expressed in HEK-293 cells, as revealed by reverse transcriptase PCR. As RasGRP3 is a Ca2+/DAG-regulated GEF for Ras and Rap GTPases, we assumed that these second messengers and the enzyme responsible for their accumulation, i.e., a PLC, are involved in Rap2B activation by the EGF receptor. Indeed, expression of lipase-inactive PLC-γ1 and chelation of intracellular Ca2+ with BAPTA/AM largely reduced Rap2B activation by the EGF receptor whereas Ca2+ ionophores mimicked the receptor response. As lipase-inactive PLC-ɛ reduced EGF-induced PLC stimulation as well as lipase-inactive PLC-γ1 but did not interfere with Rap2B activation, we conclude that PLC-γ1 stimulation by the EGF receptor is upstream of RasGRP3-mediated Rap2B activation and PLC-ɛ stimulation. Although a role for PLC-ɛ for sustained Rap2B activation cannot be excluded, it was recently reported that once activated, Rap2 (in contrast to Rap1) remains active for a rather long period of time, most likely due to the low level of sensitivity of Rap2 proteins towards Rap-specific GTPase-activating proteins (21). In line with our findings, it was recently shown that PLC-γ2 acts upstream of RasGRP3 in B cell receptor-induced Ras activation in DT40 cells (most likely due to the formation of DAG) (22). We found that Ca2+ plays an essential role in Rap2B activation by the EGF receptor in HEK-293 cells; this finding, however, does not exclude an additional contribution of DAG to this apparently RasGRP3-mediated receptor action.
PLC-γ2-derived DAG is apparently necessary, but may not be sufficient, for RasGRP3 activation by the B-cell receptor (22). Indeed, RasGRP3 has recently been reported to be phosphorylated by protein kinase C isoforms in Ramos B cells (35). We report here that in addition to PLC-γ1-derived signals, activation of Rap2B by the EGF receptor in HEK-293 cells apparently requires tyrosine phosphorylation of RasGRP3. RasGRP3 was not phosphorylated by the EGF receptor tyrosine kinase itself but by the nonreceptor tyrosine kinase c-Src, as shown in intact cells and in vitro. Importantly, pharmacological inhibition of c-Src and expression of kinase-deficient c-Src, but not of the Ca2+-activated tyrosine kinase Pyk2, inhibited not only phosphorylation of RasGRP3 but also EGF-induced Rap2B activation and PLC stimulation whereas PLC stimulation induced by cAMP-activated Epac proteins was not affected by inhibition of c-Src. Thus, Rap2B activation by RasGRP3 is apparently under the dual control of two direct EGF receptor effectors, PLC-γ1 and c-Src (Fig. 8), suggesting that the activated EGF receptor functions as a platform, first assembling and activating PLC-γ1 and c-Src, which then recruit and activate RasGRP3, Rap2B, and PLC-ɛ to a signaling complex at the plasma membrane for rapid and efficient signal transduction.
FIG. 8.
Model of EGF receptor-mediated stimulation of PLC-ɛ. For further details, see text.
In conclusion, we report here that PLC and Ca2+ signaling by the EGF receptor is a composite action of PLC-γ1 and PLC-ɛ. Furthermore, evidence is provided that stimulation of PLC-ɛ is apparently initiated by two direct EGF receptor effectors, PLC-γ1 and c-Src, which then activate (via second messenger formation and tyrosine phosphorylation, respectively) the exchange factor RasGRP3, catalyzing GTP/GDP exchange and hence activation of Rap2B. Activated Rap2B finally binds to PLC-ɛ and translocates the lipase to its substrate at the plasma membrane.
Acknowledgments
We thank K. Baden, M. Hagedorn, H. Geldermann, D. Petermeyer, and A. Kötting-Dorsch for expert technical assistance, C. Rimmbach for assistance in the PCR experiments, C. Heneweer for advice on the microscopic analysis, and A. Blaukat, J. L. Bos, C. von Eichel-Streiber, J. de Gunzburg, D. Illenberger, J. W. Lomasney, J. T. Parsons, H. Rehmann, J. de Rooij, P.-G. Suh, and A. Ullrich for providing toxins and cDNA constructs.
This work was supported by the Deutsche Forschungsgemeinschaft, the Interne Forschungsförderung Essen, and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Adelmann, H. G. 1997. A frequency-domain Gaussian filter module for quantitative and reproducible high-pass, low-pass and band-pass filtering of images. Am. Lab. 29:27-33. [Google Scholar]
- 2.Berridge, M. J., P. Lipp, and M. D. Bootman. 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1:11-21. [DOI] [PubMed] [Google Scholar]
- 3.Brose, N., and C. Rosenmund. 2002. Move over protein kinase C, you've got company: alternative cellular effectors of diacylglycerol and phorbol esters. J. Cell Sci. 115:4399-4411. [DOI] [PubMed] [Google Scholar]
- 4.Chaves-Olarte, E., P. Löw, E. Freer, T. Norlin, M. Weidmann, C. von Eichel-Streiber, and M. Thelestam. 1999. A novel cytotoxin from Clostridium difficile serogroup F is a functional hybrid between two other large cytotoxins. J. Biol. Chem. 274:11046-11052. [DOI] [PubMed] [Google Scholar]
- 5.Cullen, P. J., and P. J. Lockyer. 2002. Integration of calcium and Ras signalling. Nat. Rev. Mol. Cell Biol. 3:339-348. [DOI] [PubMed] [Google Scholar]
- 6.Enserink, J. M., A. E. Christensen, J. de Rooij, M. van Triest, F. Schwede, H. G. Genieser, S. O. Døskeland, J. L. Blank, and J. L. Bos. 2002. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat. Cell Biol. 4:901-906. [DOI] [PubMed] [Google Scholar]
- 7.Evellin, S., J. Nolte, K. Tysack, F. vom Dorp, M. Thiel, P. A. Oude Weernink, K. H. Jakobs, E. J. Webb, J. W. Lomasney, and M. Schmidt. 2002. Stimulation of phospholipase C-ɛ by the M3 muscarinic acetylcholine receptor mediated by cyclic AMP and the GTPase Rap2B. J. Biol. Chem. 277:16805-16813. [DOI] [PubMed] [Google Scholar]
- 8.Fukami, K. 2002. Structure, regulation, and function of phospholipase C isozymes. J. Biochem. 131:293-299. [DOI] [PubMed] [Google Scholar]
- 9.Heneweer, C., L. H. Kruse, F. Kindhäuser, M. Schmidt, K. H. Jakobs, H.-W. Denker, and M. Thie. 2002. Adhesiveness of human uterine epithelial RL95-2 cells to trophoblast: Rho protein regulation. Mol. Hum. Reprod. 8:1014-1022. [DOI] [PubMed] [Google Scholar]
- 10.Jin, T.-G., T. Satoh, Y. Liao, C. Song, X. Gao, K. Kariya, C.-D. Hu, and T. Kataoka. 2001. Role of the CDC25 homology domain of phospholipase Cɛ in amplification of Rap1-dependent signaling. J. Biol. Chem. 276:30301-30307. [DOI] [PubMed] [Google Scholar]
- 11.Just, I., J. Selzer, M. Wilm, C. von Eichel-Streiber, M. Mann, and K. Aktories. 1995. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 375:500-503. [DOI] [PubMed] [Google Scholar]
- 12.Kelley, G. G., S. E. Reks, J. M. Ondrako, and A. V. Smrcka. 2001. Phospholipase Cɛ: a novel Ras effector. EMBO J. 20:743-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelley, G. G., S. E. Reks, and A. V. Smrcka. 2003. Hormonal regulation of phospholipase Cɛ through distinct and overlapping pathways involving G12 and Ras family G proteins. Biochem. J. 378:129-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, Y.-H., T.-J. Park, Y. H. Lee, K. J. Baek, P.-G. Suh, S. H. Ryu, and K.-T. Kim. 1999. Phospholipase C-δ1 is activated by capacitative calcium entry that follows phospholipase C-β activation upon bradykinin stimulation. J. Biol. Chem. 274:26127-26134. [DOI] [PubMed] [Google Scholar]
- 15.Lopez, I., E. C. Mak, J. Ding, H. E. Hamm, and J. W. Lomasney. 2001. A novel bifunctional phospholipase C that is regulated by Gα12 and stimulates the Ras/mitogen-activated protein kinase pathway. J. Biol. Chem. 276:2758-2765. [DOI] [PubMed] [Google Scholar]
- 16.Lorenzo, P. S., J. W. Kung, D. A. Bottorff, S. H. Garfield, J. C. Stone, and P. M. Blumberg. 2001. Phorbol ester modulates the Ras exchange factor RasGRP3. Cancer Res. 61:943-949. [PubMed] [Google Scholar]
- 17.Meyer zu Heringdorf, D., H. Lass, I. Kuchar, R. Alemany, Y. Guo, M. Schmidt, and K. H. Jakobs. 1999. Role of sphingosine kinase in Ca2+ signalling by the epidermal growth factor receptor. FEBS Lett. 461:217-222. [DOI] [PubMed] [Google Scholar]
- 18.Murthy, S. N. P., J. W. Lomasney, E. C. Mak, and L. Lorand. 1999. Interactions of Gh/transglutaminase with phospholipase Cδ1 and with GTP. Proc. Natl. Acad. Sci. USA 96:11815-11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishizuka, Y. 1995. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 9:484-496. [PubMed] [Google Scholar]
- 20.Ohba, Y., N. Mochizuki, S. Yamashita, A. M. Chan, J. W. Schrader, S. Hattori, K. Nagashima, and M. Matsuda. 2000. Regulatory proteins of R-Ras, TC21/R-Ras2, and M-Ras/R-Ras3. J. Biol. Chem. 275:20020-20026. [DOI] [PubMed] [Google Scholar]
- 21.Ohba, Y., N. Mochizuki, K. Matsuo, S. Yamashita, M. Nakaya, Y. Hashimoto, M. Hamaguchi, T. Kurata, K. Nagashima, and M. Matsuda. 2000. Rap2 as a slowly responding molecular switch in the Rap1 signaling cascade. Mol. Cell. Biol. 20:6074-6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh-hora, M., S. Johmura, A. Hashimoto, M. Hikida, and T. Kurosaki. 2003. Requirement for Ras guanine nucleotide releasing protein 3 in coupling phospholipase C-γ2 to Ras in B cell receptor signaling. J. Exp. Med. 198:1841-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quilliam, L. A., J. F. Rebhun, and A. F. Castro. 2002. A growing family of guanine nucleotide factors is responsible for activation of Ras-family GTPases. Prog. Nucleic Acid Res. 71:391-444. [DOI] [PubMed] [Google Scholar]
- 24.Rebecchi, M. J., and S. N. Pentyala. 2000. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol. Rev. 80:1291-1335. [DOI] [PubMed] [Google Scholar]
- 25.Rebhun, J. F., A. F. Castro, and L. A. Quilliam. 2000. Identification of guanine nucleotide exchange factors (GEFs) for the Rap1 GTPase. Regulation of MR-GEF by M-Ras-GTP interaction. J. Biol. Chem. 275:34901-34908. [DOI] [PubMed] [Google Scholar]
- 26.Rhee, S. G. 2001. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 70:281-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rümenapp, U., M. Asmus, H. Schablowski, M. Woznicki, L. Han, K. H. Jakobs, M. Fahami-Vahid, C. Michalek, T. Wieland, and M. Schmidt. 2001. The M3 muscarinic acetylcholine receptor expressed in HEK-293 cells signals to phospholipase D via G12 but not Gq-type G proteins. Regulators of G proteins as tools to dissect pertussis toxin-resistant G proteins in receptor-effector coupling. J. Biol. Chem. 276:10168-10174. [DOI] [PubMed] [Google Scholar]
- 28.Saunders, C. M., M. G. Larman, J. Parrington, L. J. Cox, J. Royse, L. M. Blayney, K. Swann, and F. A. Lai. 2002. PLCζ: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development 129:3533-3544. [DOI] [PubMed] [Google Scholar]
- 29.Schlessinger, J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103:211-225. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt, M., M. Voβ, M. Thiel, B. Bauer, A. Grannaβ, E. Tapp, R. H. Cool, J. de Gunzburg, C. von Eichel-Streiber, and K. H. Jakobs. 1998. Specific inhibition of phorbol ester-stimulated phospholipase D by Clostridium sordellii lethal toxin and Clostridium difficile toxin B-1470 in HEK-293 cells. J. Biol. Chem. 273:7413-7422. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt, M., M. Frings, M.-L. Mono, Y. Guo, P. A. Oude Weernink, S. Evellin, L. Han, and K. H. Jakobs. 2000. G protein-coupled receptor-induced sensitization of phospholipase C stimulation by receptor tyrosine kinases. J. Biol. Chem. 275:32603-32610. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt, M., S. Evellin, P. A. Oude Weernink, F. vom Dorp, H. Rehmann, J. W. Lomasney, and K. H. Jakobs. 2001. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat. Cell Biol. 3:1020-1024. [DOI] [PubMed] [Google Scholar]
- 33.Song, C., C.-D. Hu, M. Masago, K. Kariya, Y. Yamawaki-Kataoka, M. Shibatohge, D. Wu, T. Satoh, and T. Kataoka. 2001. Regulation of a novel human phospholipase C, PLCɛ, through membrane targeting by Ras. J. Biol. Chem. 276:2752-2757. [DOI] [PubMed] [Google Scholar]
- 34.Song, C., T. Satoh, H. Edamatsu, D. Wu, M. Tadano, X. Gao, and T. Kataoka. 2002. Differential roles of Ras and Rap1 in growth factor-dependent activation of phospholipase Cɛ. Oncogene 21:8105-8113. [DOI] [PubMed] [Google Scholar]
- 35.Teixeira, C., S. L. Stang, Y. Zheng, N. S. Beswick, and J. C. Stone. 2003. Integration of DAG signaling systems mediated by PKC-dependent phosphorylation of RasGRP3. Blood 102:1414-1420. [DOI] [PubMed] [Google Scholar]
- 36.Voβ, M., P. A. Oude Weernink, S. Haupenthal, U. Möller, R. H. Cool, B. Bauer, J. H. Camonis, K. H. Jakobs, and M. Schmidt. 1999. Phospholipase D stimulation by receptor tyrosine kinases mediated by protein kinase C and a Ras/Ral signaling cascade. J. Biol. Chem. 274:34691-34698. [DOI] [PubMed] [Google Scholar]
- 37.Wing, M. R., D. Houston, G. G. Kelley, J. D. Channing, D. P. Siderovski, and T. K. Harden. 2001. Activation of phospholipase C-ɛ by heterotrimeric G protein βγ-subunits. J. Biol. Chem. 276:48257-48261. [DOI] [PubMed] [Google Scholar]
- 38.Wing, M. R., J. T. Snyder, J. Sondek, and T. K. Harden. 2003. Direct activation of phospholipase C-ɛ by Rho. J. Biol. Chem. 278:41253-41258. [DOI] [PubMed] [Google Scholar]
- 39.Yamashita, S., N. Mochizuki, Y. Ohba, M. Tobiume, Y. Okada, H. Sawa, K. Nagashima, and M. Matsuda. 2000. CalDAG-GEFIII activation of Ras, R-Ras, and Rap1. J. Biol. Chem. 275:25488-25493. [DOI] [PubMed] [Google Scholar]