Abstract
Purpose
To review the patient and clinical characteristics of patients with Fuchs endothelial corneal dystrophy (FECD).
Methods
Review of records for every patient who presented to the Bascom Palmer Eye Institute between 2003 and 2009 whose visit was coded for endothelial corneal dystrophy (International Classification of Diseases, Ninth Revision [ICD9] 371.57), bullous keratopathy (ICD9 371.23), or who underwent a corneal surgery with or without cataract extraction. Demographic, clinical, and ancillary testing data were collected from the time of presentation, diagnosis, and follow-up, and the use, timing, and type of surgical interventions was documented, with 6-month and final visual acuities recorded.
Results
A total of 2,370 charts were included in this study, of which 966 patients had a diagnosis of FECD. Of these, 197 patients (21%) received a corneal transplantation procedure. The surgery most often performed was penetrating keratoplasty with or without cataract extraction (66%), followed by endothelial keratoplasty with or without cataract extraction (34%). The risk factors for surgery include worse visual acuity at presentation (20/60 Snellen visual acuity in surgical patients versus 20/40 Snellen visual acuity in nonsurgical patients, P<0.001), greater average central corneal thickness (635 μm versus 592 μm, P<0.001), loss of visual acuity over time (two lines lost versus zero lines lost, P<0.001), increasing age (P<0.001), and male sex (P=0.008). Over half of patients (52%) did not receive surgery despite poor vision.
Conclusion
During this time period, FECD did not have a consistent pattern for management or treatment, and despite advances in surgical techniques, most patients were still managed without surgery.
Keywords: Fuchs corneal dystrophy, bullous keratopathy, penetrating keratoplasty, endothelial keratoplasty
Introduction
Fuchs endothelial corneal dystrophy (FECD) is a degenerative disorder of the cornea characterized by the accumulation of guttae on the inner layer of the cornea, thickening of Descemet membrane, and loss of endothelial cells.1–3 Over time, as the cell loss increases, the remaining endothelium is unable to sufficiently deturgesce the corneal stroma, leading to fluid accumulation, clouding of the central cornea, decreased visual acuity, and bullae, which can be painful.4
Less-severe disease often can be managed conservatively, either with observation or with topical hypertonic saline to dehydrate microcystic epithelial edema externally. For more advanced disease, surgeons treat FECD with corneal transplantation, either full-thickness penetrating keratoplasty (PK), or, more recently, Descemet-stripping automated endothelial keratoplasty (DSAEK) and Descemet membrane endothelial keratoplasty.5–7
This study reviews the patient and clinical characteristics of patients with FECD at the Bascom Palmer Eye Institute (BPEI) between 2003 and 2009. This manuscript represents a large-scale review of patients with FECD at an academic referral center, and offers an opportunity to update the patient features and clinical management patterns.
Methods
After approval for this study was given by the Institutional Review Board of the University of Miami Miller School of Medicine, the charts of every patient who presented to the BPEI between 2003 and 2009 with a diagnosis of endothelial corneal dystrophy (International Classification of Diseases, Ninth Revision [ICD9] code 371.57) or bullous keratopathy (ICD9 code 371.23), or who underwent a PK, DSAEK, PK combined with cataract extraction and lens implantation, or DSAEK combined with cataract extraction and lens implantation, were reviewed retrospectively.
After individually reviewing each chart, only patients with a diagnosis of FECD were included in this analysis. Patients who had FECD diagnosed in a pseudophakic eye simultaneously with a phakic eye were classified as FECD patients for the purposes of analysis, though the underlying mechanism of corneal edema in the pseudophakic eye could not be determined with certainty.
The data collected included: the age and sex of the patient; the date of first presentation to BPEI with the visual acuity and best-corrected visual acuity when available; the diagnosis; the presence and grading of endothelial guttae as documented in the chart and not based on any standardized grading system; the presence of an intraocular lens; and the pachymetry if performed. Specular microscopy was performed on a small subset of patients and was not analyzed in this review. Subsequent visits were recorded prior to any intraocular surgery, with the above clinical features recorded if performed. The type and date of surgery performed were recorded, as well as the postoperative visual acuity at 6 months. The final visual acuity was also recorded for the last visit at BPEI during the study period.
Statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Proportions were compared with the Fisher’s exact test, means were compared with the two-sample two-tailed t-test, and visual acuities were compared with the Mann–Whitney test. Time to surgery analyses included Kaplan–Meier and Cox proportional hazards regression.
Results
Of the 2,370 reviewed charts, 966 patients had a diagnosis of FECD, including 21 patients who had a corneal transplant prior to presentation at BPEI. See Table 1 for a summary of the baseline characteristics of patients with FECD. Nearly all patients (957; 99%) had bilateral disease, of which 953 (99%) were diagnosed bilaterally on the same visit. The patients in this review first presented to BPEI between May 1981 and February 2009 (though all were seen at some point between 2003 and 2009, as stated above), and had a median follow-up of 2.1 years (range: 0–25 years).
Table 1.
Characteristics of patients with Fuchs endothelial corneal dystrophy at BPEI, 2003–2009
| Number of patients | 966 |
| Age at diagnosis, average (SD) | 66.6 years (12) |
| Median follow-up, years | 2.1 |
| Number (percent) | |
| Women | 692 (74%) |
| Men | 274 (26%) |
| Diabetes | 109 (12%) |
| Hypertension | 435 (45%) |
| Pseudophakic in one eye | 139 (15%) |
Abbreviations: BPEI, Bascom Palmer Eye Institute; SD, standard deviation.
Clinical characteristics of FECD patients
The median visual acuity at the time of diagnosis was 20/30 with a range of 20/15 to hand motion (HM) in both right and left eyes.
Corneal thickness was measured in one or both eyes at the time of diagnosis in 222 (23%) patients. The right eye pachymetry mean (standard deviation [SD] [range]) was 603.2 (59.7 [388–860]) μm, and the left eye mean was 596.5 (57.9 [471–883]) μm. Postdiagnosis measurements were made in one or both eyes in 209 (21.6%) patients, and showed a mean central corneal thicknesses in the right and left eye of 603.2 (75.6 [191–843]) and 602.5 (67.0 [217–797]) μm, respectively. Only 46 (5%) patients had pachymetry measured both at and after diagnosis. The average grading of guttae is presented in Table 2. Specular microscopy was performed on 137 patients (14%) at some point during their management at BPEI.
Table 2.
Guttae grading at the time of presentation
| Number (percent) | |
|---|---|
| “0” or “1+” | 72 (7.5%) |
| “2+” or “3+” | 545 (56.4%) |
| “4+” | 320 (33.1%) |
| Not recorded | 29 (3.0%) |
Of the 958 patients with bilateral FECD, 139 patients (15%) had FECD diagnosed in a pseudophakic eye simultaneously with a phakic eye. Among 1,780 phakic eyes diagnosed with FECD, 640 (36%) underwent a subsequent cataract extraction. The median preoperative Snellen visual acuity among these eyes was 20/60, which improved to 20/30 during the 1st year after cataract surgery (median [range] follow-up =7 months [3–12 months]). Of the 214 first eyes that progressed to corneal surgery, 192 also had a cataract extraction: 70 eyes (40%) prior to PK or DSAEK, 92 (52%) in conjunction with a PK or DSAEK (a “triple” procedure), and 14 (8%) after a PK or DSAEK.
Corneal surgery: risk factors and results
Among the 945 patients who had no history of PK or DSAEK prior to their initial presentation, 748 (79%) did not undergo corneal surgery while under management at BPEI. The remaining 197 patients did receive a corneal transplantation procedure: 96 (49%) underwent a PK, 35 (18%) underwent a PK/triple procedure, 35 (18%) underwent a DSAEK, and 31 (16%) underwent a DSAEK/triple procedure. The average time from presentation to surgery was 31 months (range: <1 month to 14.5 years, standard deviation: 41 months), though 74 patients (38%) had only the presenting visit prior to surgery. See Figure 1 for the cumulative risk for corneal surgery over time.
Figure 1.
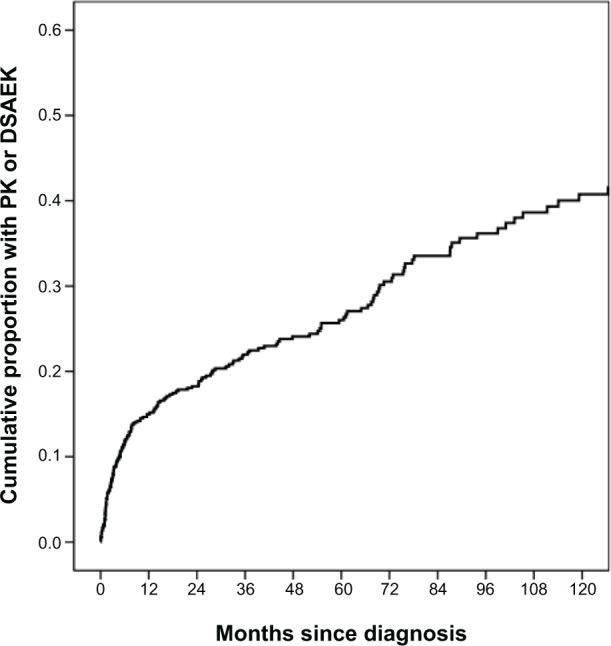
Kaplan–Meier cumulative risk of corneal surgery over time among patients diagnosed with Fuchs endothelial corneal dystrophy.
Notes: The Kaplan–Meier cumulative risk for corneal transplant surgery was 15% at year 1 from diagnosis (SE: 1%), 26% by year 5 (SE: 2%), and 40% by year 10 (SE: 3%).
Abbreviations: DSAEK, Descemet-stripping automated endothelial keratoplasty; PK, penetrating keratoplasty; SE, standard error.
The median visual acuity at presentation for the patients who ultimately underwent surgery was 20/60, compared to a median visual acuity of 20/40 at presentation in the worse eye of patients who did not receive surgery (P-value <0.001, Mann–Whitney test). The 74 patients who had only a single presurgery visit had median acuity in the operated eye of 20/90 compared to a median presenting visual acuity of 20/50 in the operated eyes of those who underwent surgery later.
The median visual acuity at the time of surgery was 20/80 (range 20/20 to HM), while the median worst visual acuity throughout the follow-up period for the nonsurgical patients was 20/50 (range 20/15 to HM, P<0.001 by Mann–Whitney test). The median number of lines of Snellen visual acuity lost for patients between presentation and immediately prior to corneal transplantation was one, compared to zero lines lost among nonsurgical patients (P<0.001, Mann–Whitney test). Figure 2 shows the distribution of preoperative visual acuity in surgical patients and the worst-recorded visual acuity at any time point in nonsurgical patients.
Figure 2.
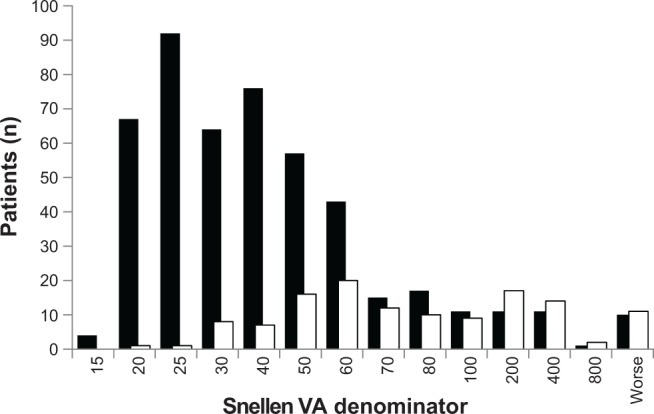
Preoperative visual acuity among surgical and nonsurgical patients with Fuchs endothelial corneal dystrophy.
Notes: The distribution of the nonsurgical (worst recorded vision over study period) and surgical (last recorded vision prior to surgery) cohort Snellen visual acuities. Patients with worse visual acuity were more likely to undergo corneal surgery (P<0.001). Nonsurgical patients are represented by the solid black bars, surgical patients by the white bars.
Abbreviation: VA, Snellen visual acuity.
The average (SD) initial corneal thickness was 592 (48.0) μm for the nonsurgical patients versus 634.5 (68.3) μm for the patients who ultimately underwent corneal transplantation surgery (P<0.001, two-sample t-test). Of patients who ultimately underwent surgery, 85% (n=185) had guttae graded 3+ or 4+ at diagnosis in at least one eye compared to 63% (n=454) of patients who did not proceed to corneal transplantation surgery during the study period (P<0.001, chi-squared test). See Table 3 for a comparison of baseline characteristics between surgical and nonsurgical patients with FECD.
Table 3.
Comparison of surgical (those undergoing PK and DSAEK, with or without cataract surgery) and nonsurgical patients with Fuchs endothelial corneal dystrophy
| Surgical | Nonsurgical | P-value | |
|---|---|---|---|
| Number | 197 (21%) | 748 (79%) | |
| PK | 96 (48.7%) | ||
| PK/CE/IOL | 35 (17.8%) | ||
| DSAEK | 35 (17.8%) | ||
| DSAEK/CE/IOL | 31 (15.7%) | ||
| Median VA, presentation | 20/60 | 20/40 | <0.001* |
| Median VA, worst‡ | 20/80 | 20/50 | <0.001* |
| Median lines of VA lost | 1 | 0 | <0.001* |
| Average (SD) corneal thickness | 634.5 (68.3) μm | 592 (48.0) μm | <0.001** |
| “3+” or “4+” corneal guttae | 185 (85%) | 454 (63%) | <0.001*** |
Notes:
Recorded VA for surgical patients at the last visit prior to surgery, compared with the worst-recorded visual acuity throughout the follow-up period for the nonsurgical patients.
Mann–Whitney test.
Two-sample t-test.
Chi-squared test.
Abbreviations: CE, cataract extraction; DSAEK, descemet-stripping automated endothelial keratoplasty; IOL, intraocular lens implantation; PK, penetrating keratoplasty; SD, standard deviation; VA, Snellen visual acuity.
Among surgical patients, 45 (23%) of the 197 surgical cases had equal vision in both eyes at the time of diagnosis. Of the 152 patients with unequal visual acuity at diagnosis, the worse-seeing eye was operated on first in 87% of cases. Of the 26 patients who had surgery first in the better-seeing eye, 16 (62%) had only one line of Snellen visual acuity difference between the two eyes. The risk of corneal surgery increased with worse visual acuity at diagnosis (P<0.001). The relative risk (RR) of surgery for three lines worse acuity increased by 1.7 (95% confidence interval [CI]: 1.5–2.0) in the best eye and 1.3 (95% CI: 1.26–1.43) in the worst eye.
By Cox proportional hazards survival regression, other risk factors for corneal surgery among FECD patients include visual acuity worse than 20/40 (RR: 3.6, 95% CI: 2.5–5.2, P<0.001), guttae graded 3+ or 4+ at diagnosis (RR: 3.7, 95% CI: 2.5–5.5, P<0.001), increasing age (RR: 1.31 for one decade of age, 95% CI: 1.1–1.5, P<0.001), and male sex (RR: 1.5, 95% CI: 1.1–2.0, P=0.008). Diabetes (RR: 1.2, 95% CI: 0.8–1.7, P=0.44), hypertension (RR: 1.2, 95% CI: 0.9–1.6, P=0.16), and a reported family history (RR: 0.9, 95% CI: 0.4–2.0, P=0.87) were not found to be significant risk factors for corneal surgery.
In a multiple variable Cox regression model, the risk of corneal surgery increased with worse acuity in the better-seeing eye at diagnosis (RR: 2.2, 95% CI: 1.2–4.1 per LogMAR unit), worse acuity in the worse-seeing eye at diagnosis (RR: 1.8, 95% CI: 1.3–2.5), acuity of 20/40 or worse at diagnosis (RR: 2.4, 95% CI: 1.6–3.7), guttae graded 3+ or 4+ at diagnosis (RR: 3.4, 95% CI: 2.3–5.1), male sex (RR: 1.9, 95% CI: 1.4–2.6), and number of Snellen lines lost during follow-up or until time of surgery (RR: 1.1, 95% CI: 1.04–1.16 per line lost). See Table 4 for a summary of the risk factors and RR for corneal surgery in FECD.
Table 4.
Risk factors for undergoing corneal surgery among patients with Fuchs endothelial corneal dystrophy
| RR for surgery | 95% CI | P-value† | |
|---|---|---|---|
| Univariable regression | |||
| VA <20/40 | 3.6 | 2.5–5.2 | <0.001 |
| Guttae “3+” or “4+” | 3.7 | 2.5–5.5 | <0.001 |
| Increasing age, per decade | 1.31 | 1.1–1.5 | <0.001 |
| Male sex | 1.5 | 1.1–2.0 | 0.008 |
| Diabetes | 1.2 | 0.8–1.7 | 0.44 |
| Hypertension | 1.2 | 0.9–1.6 | 0.16 |
| Reported family history | 0.9 | 0.4–2.0 | 0.87 |
| Multivariable regression | |||
| VA by LogMAR unit, better eye* | 2.2 | 1.2–4.1 | |
| VA by LogMAR unit, worse eye** | 1.8 | 1.3–2.5 | |
| VA 20/40 or worse | 2.4 | 1.6–3.7 | |
| Guttae “3+” or “4+” | 3.4 | 2.3–5.1 | |
| VA lines lost | 1.1 | 1.04–1.16 | |
| Male sex | 1.9 | 1.4–2.6 | |
Notes:
Cox proportion regression.
The relative risk for surgery for each LogMAR unit decrease in Snellen visual acuity of the better-seeing eye at the time of diagnosis.
The relative risk for surgery for each LogMAR unit decrease in Snellen visual acuity of the worse-seeing eye at the time of diagnosis.
Abbreviations: CI, confidence interval; RR, relative risk; VA, Snellen visual acuity.
Excluding patients who required cataract surgery, the visual acuity among corneal surgery patients improved by an average of three lines (SD = three) at 6 months after surgery. However, 23 (28%) patients were within one line of their preoperative visual acuity, and five (6%) had visual acuity more than one-line worse at postoperative month 6.
Discussion
FECD is a bilateral corneal endothelial dystrophy whose pathogenesis is poorly understood, despite new genetic components of the disease that have recently been elucidated.8 This review corroborates previously published estimates demonstrating that FECD is more common in women, though why this sex preference exists is not known.4,9 Its appearance and clinical characteristics can vary broadly, with a range in presenting visual acuities, degree of guttae formation, and central corneal thickness.10
During this time period, most patients with FECD, even at a tertiary referral center, were not managed with corneal surgery. Only 21% of patients received a PK, DSAEK, or combined procedure, and the median time from presentation to surgery among these patients was over 2.5 years. During this time, the visual acuity had deteriorated on average by one line on a Snellen chart, compared to a median zero lines lost among nonsurgical patients. This demonstrates that FECD is a slow-progressing disease, and perhaps a longer follow-up period would show an increased rate of corneal surgery.
Not surprisingly, worse visual acuity, a thicker central cornea, and increasing age were all risk factors for corneal surgery. Despite the importance of corneal thickness as a risk factor for surgery and as an objective measure of disease progression, in this review only 40% of patients received even one pachymetry measurement, and only 5% received more than one documented pachymetry measurement. This rate may be artificially low as some patients were certainly referred specifically for corneal surgery, though with a median time to surgery of 2.5 years, the documented rate of pachymetry was strikingly low. Specular microscopy was used even less frequently: only 14% of patients received a specular microscopy evaluation, despite the fact that this may be the gold-standard for diagnosis without histopathological evaluation.11 The use of these tools may be even lower in community settings that lack readily available instrumentation found at BPEI.
Advancing age and time elapsed from diagnosis were both also significant risk factors for corneal surgery in FECD. Though the disease is more common in women, male sex was a risk factor for corneal surgery (P=0.008), which may represent the possibility that men were more likely to present or be diagnosed later in the course of their disease.
It is striking how many patients (79%) did not undergo surgical treatment despite significant deterioration in visual acuity. As Table 5 illustrates, a large number of patients had poor vision, and several-fold more have some deterioration in visual acuity, yet did not undergo surgical treatment. Several factors may explain this observation. First, surgery, whether PK or DSAEK, is still considered a major intervention with significant risks, long visual rehabilitations, and variable outcomes.12–17 With the advent of DSAEK, and perhaps now Descemet membrane-only keratoplasties, surgery is being considered earlier in the therapeutic algorithm,18 though still a large number of patients remain untreated. At this institution, the first DSAEK was performed in 2005, and many surgeons were still learning this new technique during the study period, which may delayed the decision to operate on nonsurgical patients.19 The average gain of visual acuity among surgical patients (three lines in this review) is consistent with prior reports.7,12,17
Table 5.
Visual characteristics and change in vision among nonsurgical patients
| Worst eye acuity range | N (%) patients |
|---|---|
| 20/15 to 20/20 | 171 (23%) |
| 20/30 to 20/40 | 185 (25%) |
| 20/50 to 20/100 | 288 (39%) |
| <20/100 to 20/200 | 43 (6%) |
| <20/200 to > CF | 49 (7%) |
| CF or worse | 12 (2%) |
| ≥2 Snellen lines lost since diagnosis | 275 (37%) |
Notes: The majority of patients who did not undergo corneal surgery had a worse-eye Snellen visual acuity worse than 20/40. Over one-third of nonsurgical patients lost two or more lines of vision during the study period.
Abbreviation: CF, counting fingers.
Other reasons may delay surgical intervention which may not be captured in this review, including other vision-limiting disease pathology and availability of suitable tissue (though this has generally not been a problem in this region or at BPEI). Some patients may have received treatment after 2009 or at another location, and this information was not captured in our review.
Other limitations with this study include its retrospective nature. The data and analyses were extracted from what was documented in the clinical chart, which was not uniform for each patient. For example, clinicians may have scored corneal guttae differently in their clinical exam, or not documented it at all. Additionally, corneal guttae exist in non-Fuchs patients, and while ancillary testing can be helpful, the diagnosis of FECD is largely a clinical one;20–22 this review relied on the documentation and judgment of individual clinicians.
In 15% of cases, the diagnosis of FECD was made in a patient who was already pseudophakic in one eye. This “retrospective” diagnosis suggests that many patients with FECD may go undiagnosed until more symptomatic corneal edema and clinically-obvious guttae are present. Alternatively, cataract surgery may exacerbate FECD and make the corneal problems more obvious. Not only is this a limitation of our review, but it is a limitation with the current clinical diagnostic methods used to diagnosis FECD. The lack of simple, objective testing may lead to an underestimation of the disease prevalence.23,24 and may impact recommendations for the timing of cataract surgery in patients with FECD.25–27
The risk factors for surgical intervention in FECD in this review included decreased vision, increased age, and increased corneal thickness. While in general clinicians proceed from observation to the use of hypertonic saline followed by surgical intervention, no standardized approach to disease monitoring or when to proceed down the therapeutic algorithm exists. New interventions such as DSAEK, and now Descemet membrane endothelial keratoplasty, have expanded the surgical options for the treatment of FECD, though large numbers of patients do not progress to the point of requiring surgical intervention.
Footnotes
Disclosure
RAG has stock in Emmetrope Ophthalmics. JLG is a consultant for Alcon and for Quark, and has stock in Emmetrope Ophthalmics. The other authors report no conflicts of interest in this work.
References
- 1.Elhalis H, Azizi B, Jurkunas UV. Fuchs endothelial corneal dystrophy. Ocul Surf. 2010;8(4):173–184. doi: 10.1016/s1542-0124(12)70232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamis AP, Filatov V, Tripathi BJ, Tripathi RC. Fuchs’ endothelial dystrophy of the cornea. Surv Ophthalmol. 1993;38(2):149–168. doi: 10.1016/0039-6257(93)90099-s. [DOI] [PubMed] [Google Scholar]
- 3.Wilson SE, Bourne WM. Fuchs’ dystrophy. Cornea. 1988;7(1):2–18. [PubMed] [Google Scholar]
- 4.Borboli S, Colby K. Mechanisms of disease: Fuchs’ endothelial dystrophy. Ophthalmol Clin North Am. 2002;15(1):17–25. doi: 10.1016/s0896-1549(01)00016-5. [DOI] [PubMed] [Google Scholar]
- 5.Nanavaty MA, Shortt AJ. Endothelial keratoplasty versus penetrating keratoplasty for Fuchs endothelial dystrophy. Cochrane Database Syst Rev. 2011;(7):CD008420. doi: 10.1002/14651858.CD008420.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Price MO, Price FW., Jr Endothelial keratoplasty – a review. Clin Experiment Ophthalmol. 2010;38(2):128–140. doi: 10.1111/j.1442-9071.2010.02213.x. [DOI] [PubMed] [Google Scholar]
- 7.Price MO, Price FW. Descemet’s stripping endothelial keratoplasty. Curr Opin Ophthalmol. 2007;18(4):290–294. doi: 10.1097/ICU.0b013e3281a4775b. [DOI] [PubMed] [Google Scholar]
- 8.Baratz KH, Tosakulwong N, Ryu E, et al. E2-2 protein and Fuchs’s corneal dystrophy. N Engl J Med. 2010;363(11):1016–1024. doi: 10.1056/NEJMoa1007064. [DOI] [PubMed] [Google Scholar]
- 9.Kenyon KR, Hersh PS, Starck T, Fogle JA. Corneal Dysgeneses, Dystrophies, and Degenerations. In: Tasman W, Jaeger EA, editors. Duane’s Clinical Ophthalmology. 15th ed. Philadelphia: Lippincott Williams & Wilkins; 2009. [Accessed August 20, 2014]. chap 16. Available at: http://80.36.73.149/almacen/medicina/oftalmologia/enciclopedias/duane/pages/v4/v4c016.html. [Google Scholar]
- 10.Bergmanson JP, Sheldon TM, Goosey JD. Fuchs’ endothelial dystrophy: a fresh look at an aging disease. Ophthalmic Physiol Opt. 1999;19(3):210–222. doi: 10.1046/j.1475-1313.1999.00408.x. [DOI] [PubMed] [Google Scholar]
- 11.Chiou AG, Kaufman SC, Beuerman RW, Ohta T, Soliman H, Kaufman HE. Confocal microscopy in cornea guttata and Fuchs’ endothelial dystrophy. Br J Ophthalmol. 1999;83(2):185–189. doi: 10.1136/bjo.83.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee WB, Jacobs DS, Musch DC, Kaufman SC, Reinhart WJ, Shtein RM. Descemet’s stripping endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116(9):1818–1830. doi: 10.1016/j.ophtha.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Price FW, Jr, Price MO. Descemet’s stripping with endothelial keratoplasty in 200 eyes: early challenges and techniques to enhance donor adherence. J Cataract Refract Surg. 2006;32(3):411–418. doi: 10.1016/j.jcrs.2005.12.078. [DOI] [PubMed] [Google Scholar]
- 14.Price MO, Fairchild KM, Price DA, Price FW., Jr Descemet’s stripping endothelial keratoplasty five-year graft survival and endothelial cell loss. Ophthalmology. 2011;118(4):725–729. doi: 10.1016/j.ophtha.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Price MO, Gorovoy M, Benetz BA, et al. Descemet’s stripping automated endothelial keratoplasty outcomes compared with penetrating keratoplasty from the Cornea Donor Study. Ophthalmology. 2010;117(3):438–444. doi: 10.1016/j.ophtha.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hjortdal J, Ehlers N. Descemet’s stripping automated endothelial keratoplasty and penetrating keratoplasty for Fuchs’ endothelial dystrophy. Acta Ophthalmol. 2009;87(3):310–314. doi: 10.1111/j.1755-3768.2008.01492.x. [DOI] [PubMed] [Google Scholar]
- 17.Beckingsale P, Mavrikakis I, Al-Yousuf N, Mavrikakis E, Daya SM. Penetrating keratoplasty: outcomes from a corneal unit compared with national data. Br J Ophthalmol. 2006;90(6):728–731. doi: 10.1136/bjo.2005.086272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krader CG. Endothelial keratoplasty outcomes continue to improve: climbing transplant rates fueled by evolving endothelial keratoplasty techniques. Ophthalmology Times. 2010;35(9):26–28. [Google Scholar]
- 19.Suh LH, Yoo SH, Deobhakta A, et al. Complications of Descemet’s stripping with automated endothelial keratoplasty: survey of 118 eyes at one institute. Ophthalmology. 2008;115(9):1517–1524. doi: 10.1016/j.ophtha.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues MM, Krachmer JH, Hackett J, Gaskins R, Halkias A. Fuchs’ corneal dystrophy. A clinicopathologic study of the variation in corneal edema. Ophthalmology. 1986;93(6):789–796. [PubMed] [Google Scholar]
- 21.Rosenblum P, Stark WJ, Maumenee IH, Hirst LW, Maumenee AE. Hereditary Fuchs’ Dystrophy. Am J Ophthalmol. 1980;90(4):455–462. doi: 10.1016/s0002-9394(14)75011-1. [DOI] [PubMed] [Google Scholar]
- 22.Zoega GM, Fujisawa A, Sasaki H, et al. Prevalence and risk factors for cornea guttata in the Reykjavik Eye Study. Ophthalmology. 2006;113(4):565–569. doi: 10.1016/j.ophtha.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Lorenzetti DW, Uotila MH, Parikh N, Kaufman HE. Central cornea guttata. Incidence in the general population. Am J Ophthalmol. 1967;64(6):1155–1158. [PubMed] [Google Scholar]
- 24.Cross HE, Maumenee AE, Cantolino SJ. Inheritance of Fuchs’ endothelial dystrophy. Arch Ophthalmol. 1971;85(3):268–272. doi: 10.1001/archopht.1971.00990050270002. [DOI] [PubMed] [Google Scholar]
- 25.Seitzman GD, Gottsch JD, Stark WJ. Cataract surgery in patients with Fuchs’ corneal dystrophy: expanding recommendations for cataract surgery without simultaneous keratoplasty. Ophthalmology. 2005;112(3):441–446. doi: 10.1016/j.ophtha.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 26.Eghrari AO, Daoud YJ, Gottsch JD. Cataract surgery in Fuchs corneal dystrophy. Curr Opin Ophthalmol. 2010;21(1):15–19. doi: 10.1097/ICU.0b013e328333e9d6. [DOI] [PubMed] [Google Scholar]
- 27.Yamazoe K, Yamaguchi T, Hotta K, et al. Outcomes of cataract surgery in eyes with a low corneal endothelial cell density. J Cataract Refract Surg. 2011;37(12):2130–2136. doi: 10.1016/j.jcrs.2011.05.039. [DOI] [PubMed] [Google Scholar]


