Abstract
Objective
To explore the prognostic importance and preoperative predictors of lymph node metastasis in an effort to guide surgical decision making in patients with pancreatic neuroendocrine tumors (PNETs).
Background
PNETs are uncommon, and the natural history of the disease is not well described. As a result, there remains controversy regarding the optimal management of regional lymph nodes during resection of the primary tumor.
Methods
A retrospective review of a prospectively maintained database of patients who underwent surgery for locoregional PNET between 1994 and 2012 was performed. Logistic regression was used to identify predictors of nodal metastasis. Overall survival (OS) and disease-free survival (DFS) were calculated using Kaplan Meier method. Results were expressed as p- values and odds ratio estimates with 95% confidence intervals.
Results
One-hundred thirty six patients were identified, of whom 50 (38%) patients had nodal metastasis. The frequency of lymph node metastasis was higher for: larger tumors (> 1.5 cm (OR= 4.7), tumors of the head as compared to body-tail of the pancreas (OR= 2.8), tumors with Ki-67 > 20% (OR= 6.7), and tumors with lymph vascular invasion (OR= 3.6), (p value <0.05). Median DFS was lower for patients with nodal metastases (4.5 v 14.6 years, p < 0.0001).
Conclusions
Lymph node metastasis is predictive of poor outcomes in patients with PNETs. Preoperative variables are not able to reliably predict patients where the probability of lymph node involvement was less than 12%. These data support inclusion of regional lymphadenectmy in patients undergoing pancreatic resections for PNET.
Introduction
Pancreatic neuroendocrine tumors (PNET) are uncommon, accounting for only 1–2% of all pancreatic malignancies [1]. The incidence in the United States was 5.25 per 100,000 in 2004 compared to 1.09 in 1973 [2]. The rise in incidence is likely, in part, due to improvement of imaging techniques [3]. The biologic behavior and clinical outcomes of PNETs varies greatly, and while the majority are relatively benign, slow growing, and non-infiltrative, a subset is aggressive, rapidly metastasizing and locally invasive. PNETs can also be divided into functional and nonfunctional based on hypersecretion of biologically active hormones resulting in clinical syndromes [4]. The natural history of PNETs is consequently highly variable, with the majority having a more favorable outcome and longer OS after surgical resection compared to pancreatic adenocarcinoma [5]. Bilimoria, K.Y., et al reported 5 and 10 years OS after surgical resection of PNET as 59.3% and 37.7% respectively [6]. The mainstay of treatment for non-metastatic PNETs is surgery [7, 8].
The often indolent disease course and uncommon incidence of PNETs presents a considerable challenge to prospective study. Retrospective reviews with highly variable long term follow up have contributed to variable practice patterns. In addition many studies have inadequate or inconsistent lymph node sampling while others lack consistent pathological evaluation [9]. As a result the importance of lymph node metastasis on survival and recurrence remain uncertain. Some studies demonstrated the prognostic importance of nodal metastasis as a prognostic factor for PNET [10, 11], while others failed to find any such association [6, 12].
Pancreatic resections are associated with significant morbidity [13] and there is interest in minimizing the impact of surgery. Enucleation and central pancreatectomy in selected patients utilizing initially open and more recently with minimally invasive techniques has been reported [14–16]. These procedures have low morbidity and shorter hospital stay compared to standard pancreatectomy [14–16]. However, it remains difficult to predict which tumors are appropriate for enucleation because we don’t have a validated way to determine which tumors are unlikely to have metastasized. Prior studies reported controversial results regarding the predictors of nodal metastasis [17, 18]. As a result the surgical approach and indications for lymph nodes dissection in PNET remain unclear.
In this retrospective review of a prospectively maintained institutional database, we determined which pre-operative factors were associated with lymph nodes metastasis. We further determined associations between lymph node metastases and survival (DFS and OS). Our goal was to attempt to identify a low risk group where regional lymphadenectomy could be eliminated and limited resection (enucleation, central pancreatectomy, etc.) would be appropriate. Ninety-eight percent of the patients included in this study underwent regional lymph nodes sampling placing us in a unique position to look at this question.
Patients and methods
Patients who underwent surgery for primary PNETs between 1994 and 2012 were identified from a retrospective review of a prospectively maintained database at Barnes-Jewish Hospital, Washington University School of Medicine. Patients who were lost to follow up or had distant metastasis (M1 status) were excluded. Demographic and clinical data were collected, including age, gender, race, presence of multiple endocrine neoplasm and functional status. Tumor characteristics including tumor size, location, grade, Ki-67%, perineural, lymphovascular invasion, surgical margin and lymph node status were reviewed. In cases where Ki-67 % was not reported slides were cut, stained and reviewed by a dedicated pancreatic pathologist. This study was approved by the institutional review board at Washington University in St Louis and by the human studies committee of the Siteman Cancer Center.
Tumor size was determined by measuring the maximum tumor diameter in the pathological specimen. Tumor locations were coded as either proximal (head) or distal (body-tail). A tumor was considered functional if the patient was symptomatic with documented elevation of a hormone level and positive immunohistochemistry staining. Negative surgical margin (R0) was defined as macro- and microscopic absence of cancer cells at all resection margins on permanent section. Microscopic positive margin (R1) was defined as the microscopic presence of malignant cells <1mm from any margin. Macroscopic positive margin (R2) was defined as presence of gross residual cancer as defined by the American Joint Cancer Committee on Cancer [19]
DFS was defined as time from operation to the first disease recurrence. The date of recurrence was considered the first date of radiological evidence of disease recurrence. Patients who were alive and disease free were censored at the date of last contact. Overall survival was considered to be the time from surgery to time of death. Date of death was determined from the medical records and when not available from social security death index (SSDI). Patients who were alive at the date of last contact were censored at that date. Disease status was categorized as died of disease, died of other cause, alive with disease, and no evidence of disease.
Continuous variables were expressed as median and range or mean and standard deviation. Univariate logistic regression was used to model node involvement (N stage > 0) to identify factors predictive of lymph node metastasis. Multivariate logistic regression models were created to assess if any of the predictors of nodal metastasis were independent. To assess factors significantly affecting OS and DFS including lymph nodes metastasis, Kaplan-Meier models were created. Log-rank test was used to evaluate the significant difference. Multivariate Cox proportional hazard models were used to evaluate whether any of these factors were independent predictors of OS and DFS. The results were expressed as odds and hazard ratio estimates with 95% confidence intervals, and p-values were considered significant if ≤ 0.05. Statistical analyses were performed and figures were generated using SAS version 9.3 (SAS Institute Inc, Cary, NC) for Linux and GraphPad Prism version 5 (GraphPad Software, Inc, San Diego, CA).
Results
A total of 175 patients with primary PNET who underwent surgical resection between April 1, 1994 and October 1, 2012 were identified. Fourteen patients (8%) were excluded due to lack of data and insufficient follow up. Twenty-five patients (14%) with distant metastasis (stage IV) at presentation were also excluded from the study. Thus a total of 136 patients were included in the final analysis. Patient demographics and clinical characteristics can be found in table (1) and tumors characteristics in table (2). The median age of the patients was 57.2 years (range 24.3–83.9). The median follow up was 4.9 years (range 0.07–14.8). Lymph node resection was performed on 133 (98%) patients, 50 (37.6%) of whom were N1 while 83 (62.4%) were N0. The mean number ± SD of lymph node resected was 13.7 ± 11.5, and the number of positive lymph nodes in patients with N1 disease was 2.9 ± 2.5. There were no mortalities at 30 days and the overall complication rate was 39%. The most common complication was pancreatic leak at 7%. The majority of these were grade 1 leaks requiring prolonged drainage following left pancreatectomy. The most severe complications were myocardial infarction (2 patients), stroke (1 patient), and bleed (3 patients).
Table 1.
Demographic and clinical characteristics in 136 patients who underwent surgery for primary PNET between 1994 and 2012:
| Clinical Characteristic | Number (%) |
|---|---|
| Gender | |
| Male | 77 (56.6) |
| Female | 59 (43.4) |
| Race | |
| White | 117 (86) |
| Black | 14 (10.3) |
| Unknown | 5 (3.7) |
| Age at operation | |
| < 40 | 21(15.4) |
| 40–60 | 54(39.7) |
| > 60 | 61(44.9) |
| MEN- 1 | |
| Absent | 123 (90.4) |
| Present | 13 (9.6) |
| Functional status | |
| Nonfunctional | 127 (94.1) |
| Functional | 8 (5.9) |
Table 2.
Tumors characteristics in 136 patients who underwent surgery for primary PNET between 1994 and 2012:
| Tumor Characteristic | Number (%) |
|---|---|
| Size (cm) | |
| 0.02–1.0 | 17 (12.6) |
| 1.1–1.5 | 22 (16.3) |
| 1.6–2.0 | 21 (15.7) |
| > 2.0 | 75 (55.5) |
| Location | |
| Body-tail | 67 (50.4) |
| Head | 66 (49.6) |
| Grade | |
| Well differentiated | 70 (62.5) |
| Moderately differentiated | 32 (28.6) |
| Poorly differentiated | 10 (8.9) |
| PNI | |
| Absent | 42 (60.9) |
| Present | 27 (39.1) |
| LVI | |
| Absent | 64 (64) |
| Present | 36 (36) |
| Ki 67 index % | |
| 0.0–2.0 | 38 (31.4) |
| 2.1–20 | 68 (56.2) |
| > 20.0 | 15 (12.4) |
| Resection margin | |
| R0 | 120(88.9) |
| R1 | 15 (11.1) |
| Lymph node status | |
| N0 | 83 (62.4) |
| N1 | 50 (37.6) |
| Type of surgery | |
| Distal pancreatectomy | 74 (54.4) |
| Pancreatecodoudenectomy | 56 (41.2) |
| Total pancreatectomy | 3 (2.2) |
| Enucleation | 3 (2.2) |
PNI indicates perineural invasion; N0, no lymph node metastasis; N1, lymph node metastasis present; R0, negative surgical margin; R1, microscopic positive surgical margin.
Factors associated with lymph node metastasis
Tumor location, tumor size, LVI, and Ki-67 index were associated with lymph node metastasis. Two of these factors can be reliably identified preoperatively (tumor size and location). The others are measured from postoperative pathological evaluation (LVI and Ki-67) and cannot be reliably obtained from pre-operative biopsies. Ethnicity, gender, age at operation, functional status of the tumor, multiple endocrine neoplasia type 1 (MEN-1), margin status, tumor grade, and presence of perineural infiltration were not found to be associated with increased or decreased risk of nodal metastasis.
Tumor Size
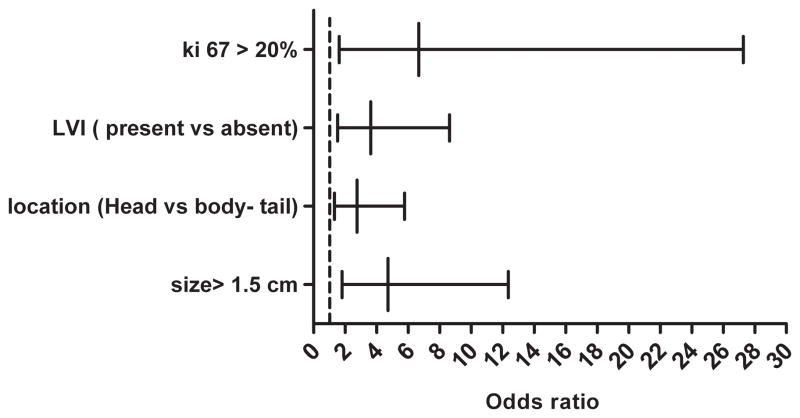
Tumor size was found to be associated with lymph node metastasis. Patients with tumor diameter >1.5 cm were 4.7 times likely to have lymph node metastasis as compared to those with smaller tumor diameters (95% CI, 1.81–12.35), (P value = 0.002) Fig (1). Tumor size was stratified into 4 different groups (≤ 1, 1.1–1.5, 1.6–2 and ≥2 cm; Fig 2A). The stratification showed that the proportion of patients with lymph node metastasis increased with larger tumor size. Interestingly 2 (12%) out of 17 patients with tumor size ≤1 cm and 5 (13%) out of 38 patients with tumor size ≤ 1.5 cm had lymph nodes metastases.
Figure 1.
Logistic regression, probability of lymph node metastasis Ki-67 > 20, LVI, tumor in the head vs. body-tail and size > 1.5 cm, were found to be associated with increase in probability of lymph node metastasis (P values= 0.008, 0.008, 0.004 and 0.002 respectively).
Figure 2.
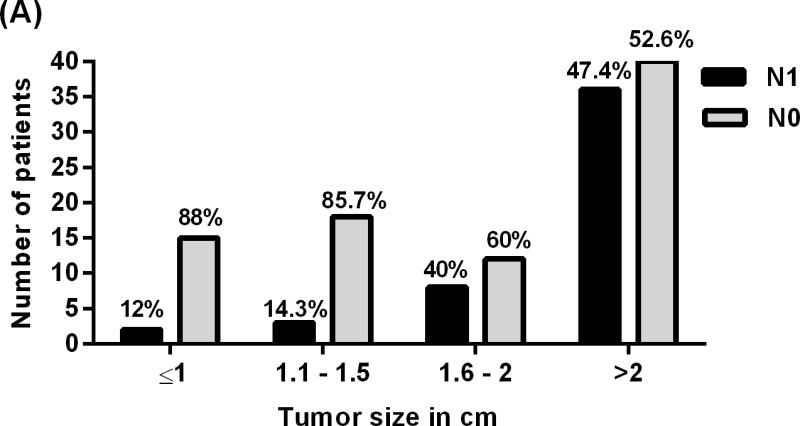
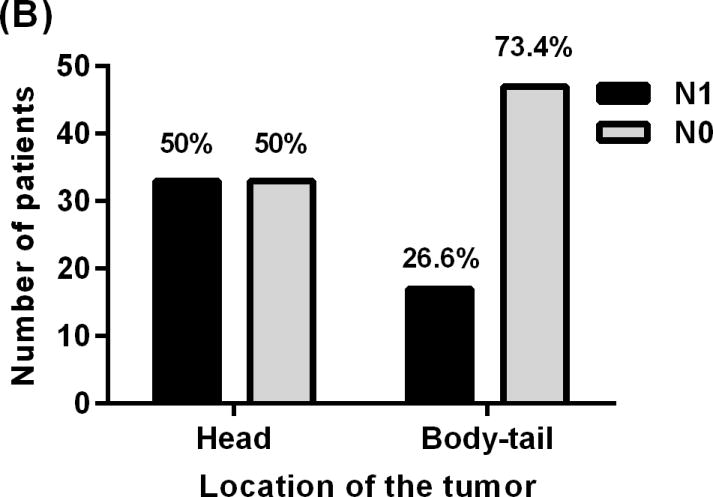
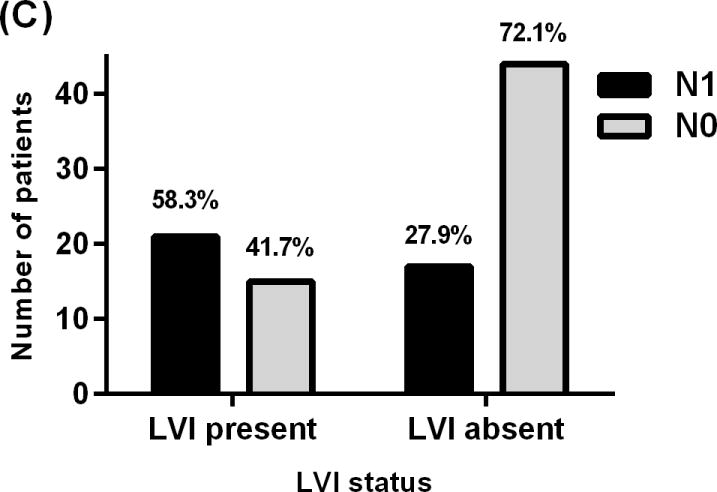
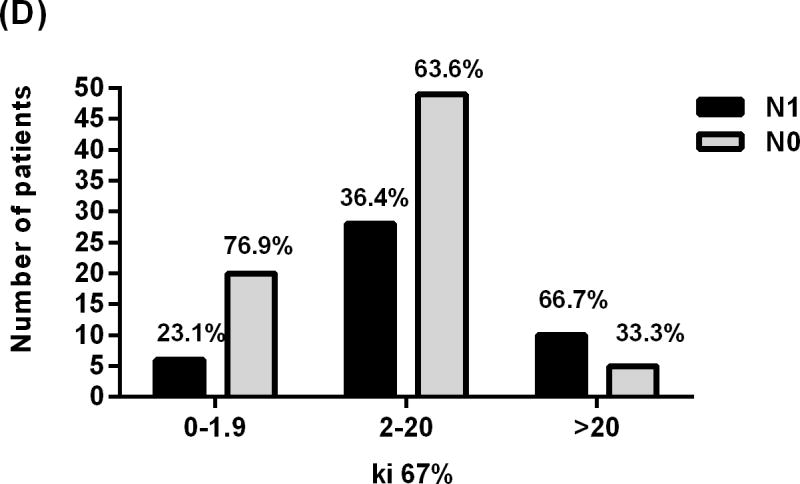
Showing relationship between lymph nodes status of patients with PNET and (A) tumor size, (B) Tumor location, (C) lymphovascular invasion, and (D) Ki-67 proliferative index.
Tumor location
PNETs in the head of the pancreas were 2.8 times more likely to metastasize to the regional lymph nodes when compared to tumors in the body and tail (95% CI, 1.3–5.8), (P value=0.007) Fig (1). Sixty-six patients had tumors in the head of the pancreas, 33 (50%) of whom were identified with nodal involvement (N1). Sixty-four patients had tumors in the body or tail of the pancreas 17 (26.6%) of whom were identified with nodal metastases (N1) status and 47 (73.4%) had no documented nodal metastases (N0) Fig (2B). The median number of lymph nodes examined in patients with tumors in the head and body-tail was 15 and 8 respectively, (p value = 0.0002). We evaluated if the increase in nodal metastasis of tumor in the head is due to the location or to the fact that more lymph nodes were examined. A model adjusted to the number of nodes examined was created. Results showed the odds ratio for N1 status of tumors in the head vs. body-tail after adjustment of number of nodes is about 2.5, (P value = 0.0006). Adding number of nodes examined did not significantly change the result.
Lymphvascular invasion
Patients with LVI were 3.6 times more likely to have nodal metastasis when compared to patients without (95% CI, 1.5–8.6), (P value= 0.004) Fig (1). LVI status was known for 97 patients, 36 of whom had LVI. Twenty one patients (58.3%) with LVI had N1 while 15 (41.7%) had N0 Fig (2C).
Tumor proliferation
Groups were stratified into three groups with respect to Ki-67 percent: < 2%, 2–20%, and > 20%. Patients with Ki-67 > 20% were found to have lymph node metastasis 6.7 times more frequently than those with Ki-67 < 2% (95% CI, 1.6–27.3), (p Value= 0.008) Fig (1). Fifteen patients with Ki-67 > 20% were identified, 10 (66.7%) of whom had N1 and 5 (33.3%) had N0 Fig (2D).
Preoperative prediction of nodal metastases
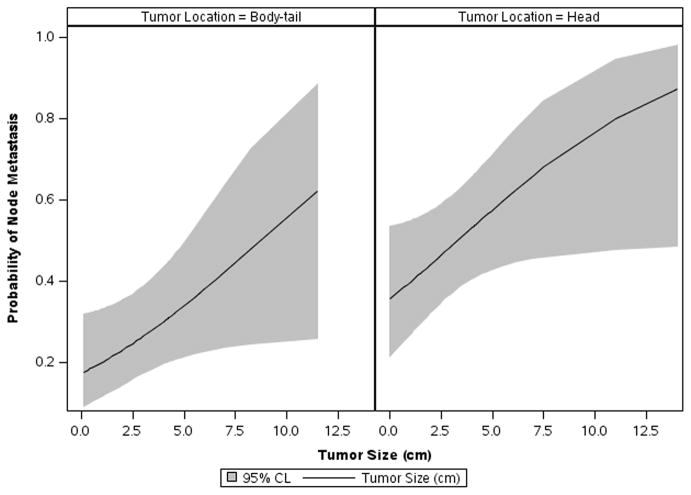
The two pre-operative variables associated with lymph node metastases (tumor location and tumor size) were analyzed for their ability to identify a group at low risk for nodal metastases. A probability plot for N stage > 0 versus tumor size for both tumors in the head and body tail of the pancreas was generated (Fig 3). In the pancreatic head even the smallest tumors had a probability that was unlikely to be less than 20% of having nodal metastasis (the lower bound of the CI is slightly below 20%). In the body-tail group, the smallest tumors had an estimated probability of nodal metastasis close to 20% (8.8% lower bound of 95% confidence interval). Multivariate analysis did not identify an independent predictor of lymph node metastasis.
Figure 3.
A probability plot for N stage > 0 versus tumor size for both tumors in the head and body-tail of the pancreas was generated. The smallest tumors in the pancreatic head have a probability that is very unlikely to be less 20% of having node metastasis; the lower bound of the CI is slightly below 20%. In tumors located at the body-tail the smallest tumors also have an estimated probability of node metastasis close to 20% with 8.8% lower bound of 95% CI.
Survival
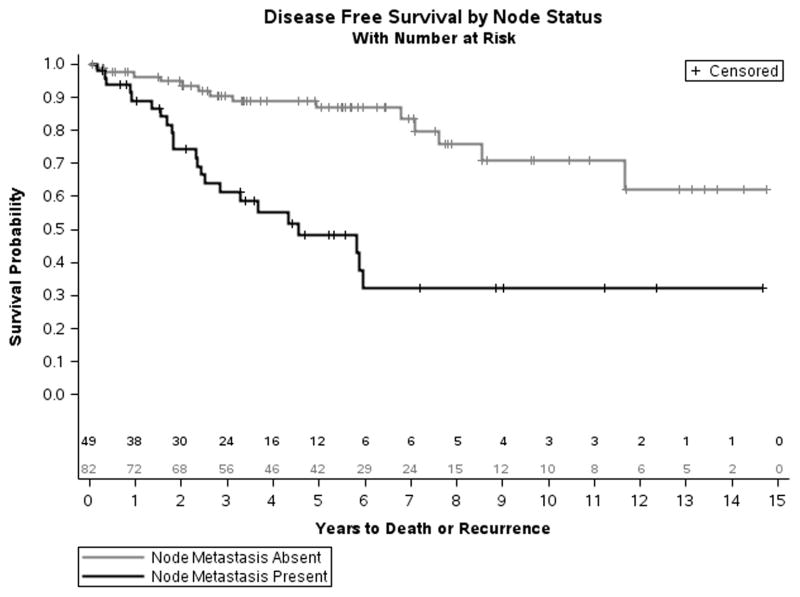
Disease free survival
The association between lymph node status and DFS was analyzed using a Kaplan-Meier model (Fig 4). Lymph node metastasis was found to be significantly associated with a decrease in DFS. The median of DFS was 4.5 years for patients with nodal metastasis compared to 14.6 years for patients without. The 5- and 10-year DFS was 86.8% and 70.8%, respectively, for N0 patients compared to 48.2% and 32.1%, respectively, for patients with N1 disease (Log-Rank < 0.0001). Median DFS in patients with Ki-67 < 2%, 2–20%, and > 20% was 11.7, > 5.9, and 2.6 years respectively. (Log-Rank =0.0004).
Figure 4.
Kaplan-Meier model of disease-free survival for patients with PNET, comparing patients with positive and negative lymph node metastasis (Log-Rank =0.0004).
Overall survival
Lymph node status
Shorter OS was observed in patients with nodal metastases (N1), but the difference was not statistically significant. Five- and 10 year-OS for patients with N1 disease were 77.1% and 55.4%, respectively, compared to 91.3% and 74.6% for patients with N0 disease. (Log-Rank= 0.1).
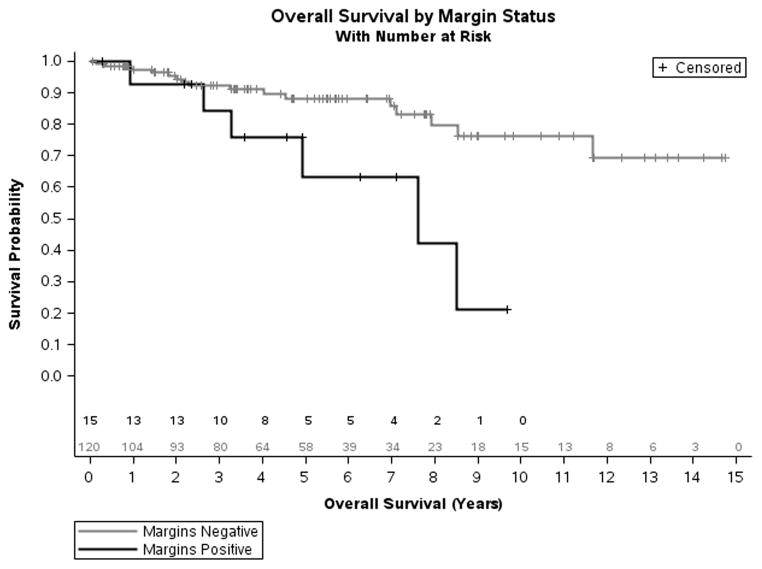
Surgical margin
Patients with positive surgical margins (R1 resections) were found to have shorter OS compared to patients with negative margins (Fig 5). The median OS for patients with positive margins was 7.6 years compared to > 11.7 years for patient with negative margins (Log-Rank=0.007). Five year actuarial survival rates were 63.3% (95% CI, 27.4–85.1) for patients with positive surgical margins and 88.2% (95% CI, 76.2–93.4) for those with negative margins.
Figure 5.
Kaplan-Meier model of overall survival for patients with PNET comparing patients with positive and negative surgical margins (Log-Rank= 0.007).
Tumor Grade
There was a statistically significant association between longer OS and improvement in tumor differentiation as demonstrated by the 5 and 10 year survival rates for poorly differentiated (64.3% and 21.4%), moderately differentiated (96.0% and 56.1%), and well differentiated tumors (88.6% and 81.8%, Log-Rank= 0.017).
Ki-67 index
Patients were stratified into three groups of Ki-67 percent < 2, 2–20, and > 20 as previously described [20, 21]. Median OS for patients with Ki-67 > 20% was 7.1 years compared to > 8.5 years for those with Ki-67 index < 2% (Log-Rank= 0.0004). Five year survival for patients with Ki-67 > 20% was 56.9%, for Ki-67 between 2–20% was 88.1%, and for Ki-67 < 2% was 90.5% (Log-Rank= 0.0004).
Multivariate analysis
Multivariate analysis showed N1 status and Ki-67 > 20% are independently associated with DFS, p value = 0.0002 and 0.003 respectively. It also showed positive surgical margin and Ki-67 > 20% are independently associated with OS, p value = 0.03 and 0.04 respectively.
Other factors
In some studies PNET associated with MEN-1 or functional tumors have been reported as having a better prognosis [6, 22]. Our data showed neither of these two factors significantly affected OS or DFS. The log-Ranks of OS and DFS for patients with MEN-1, compared to others were 0.3 and 0.1 respectively. Eight patients with functional tumors were identified, of these 5 were insulinomas, and 3 were gastrinomas. The log-Rank of OS and DFS for patients with functional, compared to non-functional tumors, were 0.2 and 0.1 respectively. MEN-1, functional tumors, ethnicity, gender, age at operation, and the presence of perineural infiltration, were not found to affect OS and DFS.
Discussion
Surgical resection is considered the mainstay of treatment with locoregional PNET and represents the only chance for cure [7, 8]. The optimal management for PNET is controversial as reported by National Comprehensive Cancer Network (NCCN) guidelines [23]. With increased utilization of cross-sectional imaging clinicians are identifying many more patients with incidental PNET. Treatment options span the spectrum from observation to oncologic resection. Observation seems quite reasonable to consider for small, well-differentiated PNET given the often indolent nature of the disease. This option is very sensible in the elderly patients and/or patients with significant comorbidities where the risks of surgery would outweigh the benefit of resection. At the other end of the spectrum is oncologic resection of the pancreas with regional lymphadenectomy. With increased technology the surgical options continue to expand and one can find several small series and case reports where local therapies including, enucleation, limited (central) pancreatectomy, and ablative procedures have been reported[14] [16] [24]. Historically all series of surgical innovations for PNET demonstrate favorable short term outcomes. However, given the indolent nature of many PNETs it will take several decades to determine if these local treatments have compromised long term outcomes for patients.
In some studies PNET associated with MEN-1 or functional tumors have been reported as having a better prognosis [6, 22]. In the current study MEN-1 and functional status did not affect OS and DFS. Thus they were included in the study. In our series, 98% of patients underwent resection inclusive of regional lymph nodes. Our institutional bias towards complete oncologic resection for PNET represents an advantage as the completeness of the data set exceeds that of previous reports.
We have separated the discussion to address two noteworthy questions. First, what is the clinical significance of positive lymph nodes? Second, which clinicopathologic factors might correlate with lymph node metastases? If we can better understand these two questions we might be able to better select patients for observation, local therapies, or oncologic resection with regional lymphadenectomy.
In the literature the importance of nodal metastasis in PNET remains controversial [6] [10–12]. For example, studies have concluded that nodal metastases are predictors of survival [10, 11], while others showed no association [6, 12]. Our data showed that lymph nodes metastasis was associated with decrease in DFS (Log- Rank < 0.0001). Shorter OS was observed in patients with nodal metastasis, but the data was not statistically significant (Log-Rank = 0.1). We interpret this data as supportive of regional lymphadenectomy because it yields important prognostic information about recurrence. It is also likely that with longer follow up many patients who recurred may yet proceed to die of their disease and that with more time OS survival will also become statistically significant.
This study found that positive surgical margin was associated with worse OS (Log-Rank = 0.007). Similar findings were reported by others [22, 25]. A positive margin can be left behind because one does a smaller operation for a similar tumor or because a tumor is somehow more aggressive and therefore a clear margin is more difficult to achieve. If we entertain that tumor left behind may have a negative impact, these data would also support an oncologic resection as it would be more likely to achieve a negative surgical margin. By extension it could be argued that if leaving tumor behind at a margin matters it should also matter if it is left behind in lymph nodes but this conclusion is more speculative.
Tumor size and location were identified as predictors of lymph nodes metastasis. We elected to jointly analyze these because they are reliably available to a surgeon pre-operatively. Tsutsumi, K., et al, reported increase prevalence of lymph node metastasis in gastrinoma patients and non gastrinoma patients with tumor size ≥ 1.5 cm [26]. They also found that 2 (8%) patients with gastrinoma out of 26 patients with tumor < 1.5 cm had nodal metastasis [26]. In contrast Parekh, J.R., et al reported that there was no difference in tumor size for patients with and without nodal metastasis, as 31% of patients with tumor < 3 cm had nodal metastasis [9]. Haynes, A.B., et al reported an association between tumor size > 2 cm with metastasis or progression of the disease; however 8% of the patients with smaller tumors developed metastasis [27]. The current study showed increased probability of nodal metastasis when the tumor size was >1.5 cm (p value= 0.002). However 2 patients out of 17 (12%) with a tumor size ≤ 1cm had nodal metastasis. We also found that tumors in the head of pancreas compared to the body-tail were more likely to be associated with nodal metastasis (p value= 0.007). However there were 17 patients out of 47 (26.6%) who had their tumors in the body-tail and had lymph node metastasis. The probability plot of nodal metastasis considering size and location revealed that even the smallest tumors in the body-tail have an estimated probability of node metastasis close to 20% with 8.8% lower bound of 95% confidence interval Fig (3). Tumor size and location together could not reliably predict a group of patients at low risk for nodal metastases.
Several authors have suggested surgical approaches based on tumor size [28–31]. Enucleation of PNET has becoming more common as minimally invasive techniques are more widely adapted. Yeo, C.J., et al considered enucleation as the procedure of choice for tumors < 2 cm that are not close to the main pancreatic duct [28]. Fernandez-Cruz, L. suggested enucleation for non functional tumor if the tumor size < 3cm, but he recommended lymph node sampling with frozen section and to proceed with enucleation and node dissection if malignancy is confirmed [29]. Sarmiento, J.M., et al suggested pancreaticoduodenectomy for tumors in the head in presence of malignancy or technical contraindication to enucleation [30]. Bettini, R., et al. reported strong association between tumor size and malignancy in nonfunctional PNET. He suggested nonoperative treatment for tumors ≤ 2cm [31]. Reviews from the literature demonstrated even small tumors < 1.5cm and < 3cm metastasize with a rate of 8% to 31% respectively [9, 26]. These results are consistent with our finding of 12% metastatic rate for tumors ≤ 1cm. Even if we accept that oncologic resection with regional lymphadenectomy is associated with an increased risk of death compared with enucleation it is unclear what the percent of acceptable missed metastases would be. If leaving metastases behind does not matter it may be correct that the primary tumor should be left alone. The authors interpret these data to suggest that if you are going to the operating room because the tumor has risk and your mortality rate for oncologic resection is acceptably low, then oncologic resection should be the standard operation for the majority of patients.
Additional predictors of nodal metastasis were LVI and Ki-67 index >20%. These predictors are less likely to help in planning the type of surgery because they are not reliably available pre-operatively. Ki-67 proliferative index can certainly be obtained on biopsy but it was reported that biopsy is not always reliable because tumors can be quite heterogeneous [32]. This additional procedure has risks and the 2 patients with tumor size ≤ 1 and nodal metastasis had Ki-67 < 20%. Further study is warranted as the combination of Ki-67 % and tumor size might ultimately help in combination with other factors to predict a low risk tumor that should be observed.
The retrospective nature of the data collection and the fact that this is a single institute experience are well appreciated limitations of this study. The patients for this study were selected from a surgical database and cannot include patients selected for observation of their tumors. In determining the optimal treatment for an indolent disease it is important to take into consideration the complication rates for the planned procedure as well as the potential longevity of the patient. The complication rates reported for these patients were lower than expected when compared with our own previously reported rates [33, 34] and the literature [35–38]. However these were obtained retrospectively and span 2 decades. Therefore it is highly likely that this represents an incomplete picture of the complications for oncologic resection for neuroendocrine tumor. The pancreas is often soft and complications are thought to be higher in this situation. We have maintained a prospective database of complications for patients undergoing a Whipple for the last two years. During this time span we performed 185 Whipple procedures of which 23 were performed for PNET. In this prospective cohort there was a 1.5% mortality rate with no deaths in the PNET group. There was an 17% pancreatic leak rate which was not statistically different from the PNET group at 26%. The great majority of leaks were grade 1 but oncologic resection clearly has associate risks. We are in support of further collaborative study to better understand the optimal management of PNET.
Conclusions
In summary, we believe that oncological resection with regional lymphadenectomy should be offered to the majority of patients undergoing surgery for PNETs. Patients with nodal metastasis demonstrated worse prognosis and this information adds prognostic value for both patients and treating physicians. Furthermore, lymph node status may be critically important to stratify patients for future trials investigating adjuvant treatment. Currently we lack accurate pre- operative methods to predict which tumors will be well behaved and which will progress to regional or distant metastasis. There was a decrease in OS with positive surgical margin and this is more likely to happen with enucleation compared with anatomic resection. The potential for surgical complications needs to be considered for each patient, however we interpret the continual improvements in mortality and morbidity for oncological resection in high volume centers [35–38] as supportive of the aggressive approach. We also recommend long periods of surveillance (10 years or more) and additional vigilance for patients with nodal involvement, lymphovascular invasion, Ki-67 >20%, poorly differentiated tumors, and positive surgical margins.
Acknowledgments
We acknowledge Nicholas Hamilton, MD and Cheryl A. Woolsey, PA-C, MMS for their contributions to our institutional PNET databases.
Footnotes
Disclosures:
There are no relevant conflicts of interest for this manuscript. Statistical support was provided by the Biostatistics Core, Siteman Comprehensive Cancer Center, and NCI Cancer Center Support Grant P30 CA091842. Salary support was provided for YH by the Washington University Surgical Oncology Training Grant (5T32CA00962124).
Contributor Information
Yassar M. Hashim, Department of Surgery and the Alvin J. Siteman Cancer Center, Barnes-Jewish Hospital and Washington University School of Medicine, St. Louis, MO.
Kathryn M. Trinkaus, Division of Biostatistics, Washington University School of Medicine, St. Louis, MO.
David C. Linehan, Department of Surgery and the Alvin J. Siteman Cancer Center, Barnes-Jewish Hospital and Washington University School of Medicine, St. Louis, MO.
Steven S. Strasberg, Department of Surgery and the Alvin J. Siteman Cancer Center, Barnes-Jewish Hospital and Washington University School of Medicine, St. Louis, MO.
Ryan C. Fields, Department of Surgery and the Alvin J. Siteman Cancer Center, Barnes-Jewish Hospital and Washington University School of Medicine, St. Louis, MO.
Dengfeng Cao, Department of Pathology and Immunology, Washington University School of Medicine, St Louis, MO, USA, and Department of Pathology, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital, Beijing, China.
William G. Hawkins, Email: hawkinsw@wudosis.wustl.edu, Department of Surgery and the Alvin J. Siteman Cancer Center, Barnes-Jewish Hospital and Washington University School of Medicine, St. Louis, MO”, 660 S. Euclid, Box 8109, Saint Louis, MO 63110, Office: (314) 362-7046, Fax: (314) 367-1943.
References
- 1.Milan SA, Yeo CJ. Neuroendocrine tumors of the pancreas. Curr Opin Oncol. 2012;24:46–55. doi: 10.1097/CCO.0b013e32834c554d. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”:epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Falconi M, Plockinger U, Kwekkeboom DJ, et al. Well-differentiated pancreatic nonfunctioning tumors/carcinoma. Neuroendocrinology. 2006;84:196–211. doi: 10.1159/000098012. [DOI] [PubMed] [Google Scholar]
- 4.Vagefi PA, Razo O, Deshpande V, et al. Evolving patterns in the detection and outcomes of pancreatic neuroendocrine neoplasms: the Massachusetts General Hospital experience from 1977 to 2005. Arch Surg. 2007;142:347–354. doi: 10.1001/archsurg.142.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fesinmeyer MD, Austin MA, Li CI, et al. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1766–1773. doi: 10.1158/1055-9965.EPI-05-0120. [DOI] [PubMed] [Google Scholar]
- 6.Bilimoria KY, Talamonti MS, Tomlinson JS, et al. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors:analysis of 3851 patients. Ann Surg. 2008;247:490–500. doi: 10.1097/SLA.0b013e31815b9cae. [DOI] [PubMed] [Google Scholar]
- 7.Burns WR, Edil BH. Neuroendocrine pancreatic tumors:guidelines for management and update. Curr Treat Options Oncol. 2012;13:24–34. doi: 10.1007/s11864-011-0172-2. [DOI] [PubMed] [Google Scholar]
- 8.Jarufe NP, Coldham C, Orug T, et al. Neuroendocrine tumours of the pancreas: predictors of survival after surgical treatment. Dig Surg. 2005;22:157–162. doi: 10.1159/000087148. [DOI] [PubMed] [Google Scholar]
- 9.Parekh JR, Wang SC, Bergsland EK, et al. Lymph node sampling rates and predictors of nodal metastasis in pancreatic neuroendocrine tumor resections: the UCSF experience with 149 patients. Pancreas. 2012;41:840–844. doi: 10.1097/MPA.0b013e31823cdaa0. [DOI] [PubMed] [Google Scholar]
- 10.Tomassetti P, Campana D, Piscitelli L, et al. Endocrine pancreatic tumors:factors correlated with survival. Ann Oncol. 2005;16:1806–1810. doi: 10.1093/annonc/mdi358. [DOI] [PubMed] [Google Scholar]
- 11.Bettini R, Boninsegna L, Mantovani W, et al. Prognostic factors at diagnosis and value of WHO classification in a mono-institutional series of 180 non-functioning pancreatic endocrine tumours. Ann Oncol. 2008;19:903–908. doi: 10.1093/annonc/mdm552. [DOI] [PubMed] [Google Scholar]
- 12.Kazanjian KK, Reber HA, Hines OJ. Resection of pancreatic neuroendocrine tumors:results of 70 cases. Arch Surg. 2006;141:765–769. doi: 10.1001/archsurg.141.8.765. discussion 769–770. [DOI] [PubMed] [Google Scholar]
- 13.Lermite E, Sommacale D, Piardi T, et al. Complications after pancreatic resection:Diagnosis, prevention and management. Clin Res Hepatol Gastroenterol. 2013;37:230–239. doi: 10.1016/j.clinre.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Berends FJ, Cuesta MA, Kazemier G, et al. Laparoscopic detection and resection of insulinomas. Surgery. 2000;128:386–391. doi: 10.1067/msy.2000.107413. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Cruz L, Herrera M, Saenz A, et al. Laparoscopic pancreatic surgery in patients with neuroendocrine tumours:indications and limits. Best Pract Res Clin Endocrinol Metab. 2001;15:161–175. doi: 10.1053/beem.2001.0133. [DOI] [PubMed] [Google Scholar]
- 16.DiNorcia J, Lee MK, Reavey PL, et al. One hundred thirty resections for pancreatic neuroendocrine tumor:evaluating the impact of minimally invasive and parenchyma-sparing techniques. J Gastrointest Surg. 2010;14:1536–1546. doi: 10.1007/s11605-010-1319-3. [DOI] [PubMed] [Google Scholar]
- 17.Ferrone CR, Tang LH, Tomlinson J, et al. Determining prognosis in patients with pancreatic endocrine neoplasms:can the WHO classification system be simplified? J Clin Oncol. 2007;25:5609–5615. doi: 10.1200/JCO.2007.12.9809. [DOI] [PubMed] [Google Scholar]
- 18.Franko J, Feng W, Yip L, et al. Non-functional neuroendocrine carcinoma of the pancreas:incidence, tumor biology, and outcomes in 2,158 patients. J Gastrointest Surg. 2010;14:541–548. doi: 10.1007/s11605-009-1115-0. [DOI] [PubMed] [Google Scholar]
- 19.Edge SBBD, Compton CC, et al., editors. AJCC Cancer Staging Manual. New York: New York, NY: Springer-Verlag; 2009. [Google Scholar]
- 20.Hamilton NA, Liu TC, Cavatiao A, et al. Ki-67 predicts disease recurrence and poor prognosis in pancreatic neuroendocrine neoplasms. Surgery. 2012;152:107–113. doi: 10.1016/j.surg.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kloppel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2011;18:S1–16. doi: 10.1530/ERC-11-0013. [DOI] [PubMed] [Google Scholar]
- 22.Phan GQ, Yeo CJ, Hruban RH, et al. Surgical experience with pancreatic and peripancreatic neuroendocrine tumors:Review of 125 patients. J Gastrointest Surg. 1998;2:473–482. doi: 10.1016/S1091-255X(98)80039-5. [DOI] [PubMed] [Google Scholar]
- 23.Kulke MH, Benson AB, 3rd, Bergsland E, et al. Neuroendocrine tumors. J Natl Compr Canc Netw. 2012;10:724–764. doi: 10.6004/jnccn.2012.0075. [DOI] [PubMed] [Google Scholar]
- 24.Hlavsa J, Prochazka V, Kala Z, et al. Radiofrequency ablation of pancreatic neuroendocrine tumor. Klin Onkol. 2011;24:209–215. [PubMed] [Google Scholar]
- 25.Madeira I, Terris B, Voss M, et al. Prognostic factors in patients with endocrine tumours of the duodenopancreatic area. Gut. 1998;43:422–427. doi: 10.1136/gut.43.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsutsumi K, Ohtsuka T, Mori Y, et al. Analysis of lymph node metastasis in pancreatic neuroendocrine tumors (PNETs) based on the tumor size and hormonal production. J Gastroenterol. 2012;47:678–685. doi: 10.1007/s00535-012-0540-0. [DOI] [PubMed] [Google Scholar]
- 27.Haynes AB, Deshpande V, Ingkakul T, et al. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: short-term and long-term patient outcomes. Arch Surg. 2011;146:534–538. doi: 10.1001/archsurg.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeo CJ, Wang BH, Anthone GJ, Cameron JL. Surgical experience with pancreatic islet-cell tumors. Arch Surg. 1993;128:1143–1148. doi: 10.1001/archsurg.1993.01420220063008. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Cruz L, Molina V, Vallejos R, et al. Outcome after laparoscopic enucleation for non-functional neuroendocrine pancreatic tumours. HPB (Oxford) 2012;14:171–176. doi: 10.1111/j.1477-2574.2011.00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarmiento JM, Farnell MB, Que FG, Nagorney DM. Pancreaticoduodenectomy for islet cell tumors of the head of the pancreas: long-term survival analysis. World J Surg. 2002;26:1267–1271. doi: 10.1007/s00268-002-6714-9. [DOI] [PubMed] [Google Scholar]
- 31.Bettini R, Partelli S, Boninsegna L, et al. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery. 2011;150:75–82. doi: 10.1016/j.surg.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 32.Tatsumoto S, Kodama Y, Sakurai Y, et al. Pancreatic neuroendocrine neoplasm: correlation between computed tomography enhancement patterns and prognostic factors of surgical and endoscopic ultrasound-guided fine-needle aspiration biopsy specimens. Abdom Imaging. 2013;38:358–366. doi: 10.1007/s00261-012-9953-8. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton NA, Porembka MR, Johnston FM, et al. Mesh reinforcement of pancreatic transection decreases incidence of pancreatic occlusion failure for left pancreatectomy:a single- blinded, randomized controlled trial. Ann Surg. 2012;255:1037–1042. doi: 10.1097/SLA.0b013e31825659ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strasberg SM, Drebin JA, Mokadam NA, et al. Prospective trial of a blood supply-based technique of pancreaticojejunostomy:effect on anastomotic failure in the Whipple procedure. J Am Coll Surg. 2002;194:746–758. doi: 10.1016/s1072-7515(02)01202-4. discussion 759–760. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-del Castillo C, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg. 1995;130:295–299. doi: 10.1001/archsurg.1995.01430030065013. discussion 299–300. [DOI] [PubMed] [Google Scholar]
- 36.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s:pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. doi: 10.1097/00000658-199709000-00004. discussion 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lillemoe KD, Kaushal S, Cameron JL, et al. Distal pancreatectomy:indications and outcomes in 235 patients. Ann Surg. 1999;229:693–698. doi: 10.1097/00000658-199905000-00012. discussion 698–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grobmyer SR, Pieracci FM, Allen PJ, et al. Defining morbidity after pancreaticoduodenectomy:use of a prospective complication grading system. J Am Coll Surg. 2007;204:356–364. doi: 10.1016/j.jamcollsurg.2006.11.017. [DOI] [PubMed] [Google Scholar]