Abstract
Objectives
Metastatic penile cancer typically comes to attention while the clinical extent of disease is limited to the inguinal or pelvic lymph nodes. Primary surgical management of lymph node metastases achieves tumor control and long-term survival for only a small percentage of these patients. To determine the optimal use of multimodality treatment in locally advanced penile cancer, we conducted a literature review.
Methods
Relevant English-language literature was identified with the use of Medline; additional cited works not detected on the initial search were also reviewed.
Results
There is an emerging strategy of preoperative (neoadjuvant) combination chemotherapy to improve the progression-free survival of penile cancer patients with bulky regional lymph node metastases. Radiotherapy for inguinal metastases and postoperative (adjuvant) radiation for selected patients has also been effective in this setting.
Conclusions
In patients with lymph node metastases, the benefit of ilioinguinal lymphadenectomy may be extended by the addition of neoadjuvant chemotherapy. Postoperative radiotherapy can be offered depending on the amount of residual disease after chemotherapy. Chemo-radiotherapy has been successful in squamous cell cancers from other sites (vulva and anal canal) and may be considered for unresectable penile cancer.
Keywords: Adjuvant radiotherapy, Cisplatin, Combined modality therapy, Penile neoplasms, Squamous cell carcinoma
Introduction
Squamous cell carcinoma of the penis is a rare but potentially fatal malignancy [1]. Its causes are not entirely understood, but the risk is almost completely eliminated by circumcision at birth. The incidence is higher in Africa, India, China, and parts of South America than it is in most Western countries, including the United States [2]. Other possible risk factors are smoking, sexually transmitted diseases, phimosis, and poor hygiene.
Tumors are often localized in the penis at the time of diagnosis, and they may be controlled by local excision or circumcision, laser ablation, brachytherapy, external radiation, or partial penectomy. Preservation of the penis is a concern for most patients, but more-proximal or advanced (stage T3) lesions require a total penectomy [3, 4]. With adequate treatment of the primary tumor, however, the rate of local recurrence is very low. For patients who have no palpable inguinal adenopathy when they are first seen, the risk of occult metastases is estimated as high as 40%, and is predicted by tumor grade [4] and the presence of lymphovascular invasion [5].
Of the patients who have palpable inguinal adenopathy when they are seen initially, approximately 50% have tumor metastases, and the other 50% have merely reactive lymph nodes. The prognosis for survival in patients with lymph node metastases is tightly correlated with the number and location of involved lymph nodes and the presence of extranodal extension [6].
The prognostic importance of lymph node metastases and the potential therapeutic effect of early resection led to the widely practiced procedure of staged ilioinguinal lymph node dissection [4]. The 5-year overall and disease-free survival rates are as high as 80% for unilateral, superficial inguinal lymph node involvement with no more than two nodes (stage N1 or limited N2), only 10–20% for bilateral or pelvic lymph nodes involved (stage N2/N3), and less than 10% in the presence of extranodal extension. Nearly all recurrences are detected within 2 years of surgery, and an aggressive, multimodality approach to the treatment of high-risk patients could result in better overall survival. In this review, we discuss the options for multimodality treatment of penile carcinoma in light of the published data.
Methods
Relevant English-language literature from 1966 through 2007 regarding chemotherapy, radiotherapy, and combined modality treatment of penile carcinoma was identified with the use of Medline; additional cited works not detected on the initial search were also reviewed. Moreover, the survival results from an ongoing clinical trial of neoadjuvant therapy [7] were updated (L.C·P.) and used in our analysis.
Results
In early chemotherapy studies, a number of candidate drugs for the treatment of penile carcinoma were identified on the basis of their antitumor activity in squamous cell carcinomas arising in other sites. These included cisplatin, vincristine, methotrexate, fluorouracil, mitomycin, and bleomycin. Responses to single-agent bleomycin were reported in 1969 [8] and to methotrexate, in 1972 [9].
Cisplatin and cisplatin-based combinations were studied in the 1980s [10]. In a multicenter study conducted by the Southwest Oncology Group (SWOG), 26 patients with metastatic penile cancer received single-agent cisplatin at a dosage of 50 mg/m2 on days 1 and 8 of each 28-day cycle [11]. Only four patients (15%) experienced responses that persisted for 1–3 months.
The combination of bleomycin, methotrexate, and cisplatin (BMP) was studied in phase II clinical trials. In a single-institution study conducted at The University of Texas M. D. Anderson Cancer Center [12], 30 patients with squamous cell carcinoma of the urinary tract, including 21 men with metastatic penile carcinoma, received bleomycin at a dosage of 50 mg/m2 on days 2–6, cisplatin at 20 mg/m2 on days 2–6, and methotrexate at 200 mg/m2 with leucovorin rescue on days 1, 15, and 22 of each 28-day cycle. There were 12 responses (55%) in the group with penile carcinoma, and the median duration of response was 4.7 months for the entire study. The frequency and duration of responses, although modest at best, appeared to be an improvement over those achieved with single-agent cisplatin.
A second phase II study of BMP, in which 40 patients received bleomycin at a dosage of 10 units/m2 on days 1 and 8, methotrexate at 25 mg/m2 on days 1 and 8, and cisplatin 75 mg/m2 on day 1 of each 21-day cycle, was performed by SWOG investigators [13]. To our knowledge, this is the largest prospective clinical trial in penile carcinoma whose results have been published. In that trial, there were five complete and eight partial responses, for an overall response rate of 32.5%, which exceeded the predetermined target rate of 30%. The median response duration was 16 weeks, and the estimated median survival time was 28 weeks. The toxicity of the regimen was considerable, however, with five treatment-related deaths. Nine additional patients withdrew from treatment because of toxicity. The most common toxic effects were gastrointestinal and hematologic, and the treatment-related deaths were due to bleomycin lung toxicity, other pulmonary causes, and infection.
A team of Italian investigators reported their experience with adjuvant or preoperative (neoadjuvant) bleomycin, vincristine, and methotrexate for patients with penile carcinoma and metastases confined to the inguinal lymph nodes [14]. Five patients with fixed inguinal nodes received weekly bleomycin (30 mg intramuscularly), vincristine (1 mg intravenously), and methotrexate (30 mg orally). Three of those five patients had enough of a partial response that they could undergo surgical consolidation, and they were reported to be alive and disease-free at 20, 27, and 72 months after surgery. The other two patients experienced less than partial responses, did not undergo surgery, and survived less than 12 months. The same investigators gave adjuvant bleomycin, vincristine, and methotrexate to 12 patients after inguinal lymphadenectomy, and 11 of them were free of relapse at their last follow-up. This study, although small, demonstrated that perioperative chemotherapy for locally advanced penile carcinoma was feasible.
More recently, a group from the Netherlands reported their retrospective analysis of 20 patients who had received preoperative chemotherapy to downstage unresectable disease [15]. Seventeen patients had had bulky lymph node metastases (Tx, N3), and the other three had had an advanced primary tumor (T3–4, N0–1). Twelve patients had experienced an objective tumor response; nine of the 12 had undergone surgery, and eight had achieved long-term disease-free survival. Two patients had had no residual tumor in the surgical specimen (pathologic complete response). The most commonly used regimens in the Dutch series, which spanned a 34-year period, had been BMP (10 patients); bleomycin, vincristine, and methotrexate (five patients); and single-agent bleomycin (three patients). Severe toxicity had occurred in four patients, including three treatment-related deaths.
Another retrospective study, this one from Germany and involving 13 patients who had received BMP for metastatic or locally advanced penile carcinoma, covered a 7-year period [16]. Eight patients had received adjuvant chemotherapy for radically resected local or nodal disease. Only three had survived; four had died of tumor progression, and one of treatment-related toxicity.
Bermejo and colleagues from M. D. Anderson Cancer Center also reported on a retrospective series of 10 patients who had received neoadjuvant chemotherapy. The reported experience spanned a 15-year period at one institution and was limited to patients who had actually undergone aggressive lymph node dissections after having experienced a response or stable disease after chemotherapy. The regimens given preoperatively had been BMP (three patients); paclitaxel and carboplatin (two patients); and paclitaxel, ifosfamide, and cisplatin (TIP) (five patients). Four patients had had complete responses and one had had a partial response to chemotherapy. Three patients had experienced a pathologic complete response in the lymph nodes, all of whom had received TIP and had had biopsy-confirmed metastases prior to chemotherapy. Four patients had experienced long-term disease-free survival, two of whom had received TIP and two, paclitaxel and carboplatin.
A phase II clinical trial of neoadjuvant TIP is in progress at M. D. Anderson. Eligible patients have penile carcinoma with stage Tx, N2–N3 lymph node disease, no evidence of distant metastases (M0), and no prior chemotherapy. Treatment consists of four courses of chemotherapy (Table 1) followed by bilateral inguinal lymph node dissections, unilateral or bilateral pelvic lymph node dissections, and surgical control of the primary tumor when appropriate. Preliminary analysis of data from the first 20 patients revealed an objective response rate of 55% and pathologic complete responses in 10% (two patients) [7]. Sixteen patients had residual disease at surgery, and two did not undergo surgery. Four patients (20%) were alive and disease-free at 23, 24, 31, and 32 months. Fifteen deaths occurred as a result of progressive disease and one from unknown causes. No deaths were treatment related.
Table 1.
A neoadjuvant chemotherapy regimen (TIP)
| Dose | Schedule | |
|---|---|---|
| Paclitaxela | 175 mg/m2 | Day 1 over 3 h |
| Ifosfamideb | 1200 mg/m2 | Days 1–3 |
| Cisplatinc | 25 mg/m2 | Days 1–3 |
The cycle is repeated every 21 days, usually with granulocyte colony-stimulating-factor prophylaxis beginning in the second cycle
Corticosteroid and antihistamines are given for hypersensitivity prophylaxis
Mesna is given for urothelial protection
Mannitol is given with hydration after cisplatin
Reports of radiotherapy used in the multimodality setting have included the primary treatment of lymph node metastases, postoperative radiotherapy, and chemo-radiotherapy [18–20]. In women with cancer of the vulva, a disease site that has natural history and nodal drainage similar to those of the penis, Hyde et al. [21] reported that node debulking was equally effective as full groin dissection when followed by adjuvant radiotherapy (50–54 Gy) to the groin. Moreover, Parthasarathy et al. [22] found that when a primary node dissection removed fewer than 12 nodes, even patients with a single positive node showed improved disease-free survival when they received adjuvant postoperative radiotherapy.
Discussion: when and which treatments?
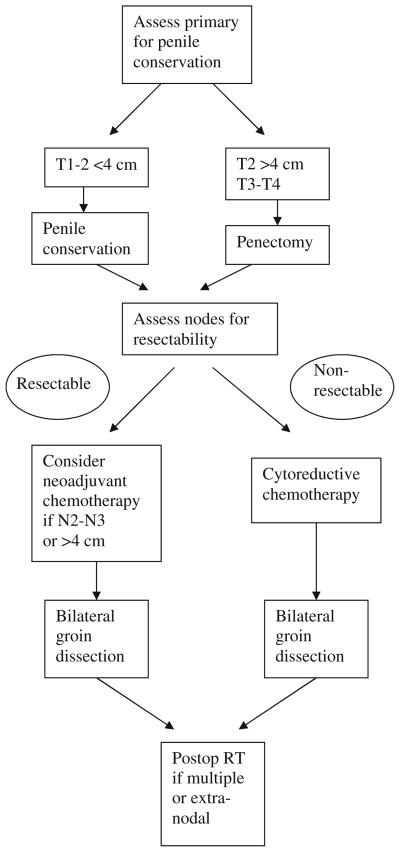
In patients who have concurrent adenopathy and a locally advanced primary tumor, penectomy with groin dissection is the treatment of choice because treatment with external radiotherapy is prolonged and the chances of local control with radiotherapy diminish markedly with bulky disease. In the case of unresectable nodes, although there is no definitive answer, a multimodality approach is desirable (Fig. 1). Possible strategies in this setting include neoadjuvant chemotherapy followed by surgery, or chemo-radiotherapy as the primary treatment.
Fig. 1.
Treatment algorithm for patients with regional lymph node metastases from penile cancer
The two prospective studies of BMP chemotherapy in metastatic penile cancer included some patients with regional lymph node disease who were potential candidates for aggressive consolidation. Four patients in the M. D. Anderson study had a clinical complete response to BMP, and one subsequently underwent post-chemotherapy lymph node dissection that revealed microscopic residual disease [12]. In the SWOG study, 8 of 13 responding patients had disease confined to regional lymph nodes, but whether they received consolidation surgery or radiotherapy was not reported [13]. Only three patients (7.5%) in the SWOG study survived 2 years or longer. The modest efficacy and prohibitive toxicity reported in these studies suggest that BMP is a poor choice for routine use in the adjuvant or neoadjuvant setting.
The regimen of bleomycin, vincristine, and methotrexate, given in low doses, was shown to be feasible for adjuvant or neoaduvant administration, but the only published prospective study [14] included just 17 patients and was not sufficient to establish efficacy. Toxicity was still a limiting factor, and the bleomycin dose was reduced 50% in the patients considered to be at risk for pulmonary toxicity. The results of one retrospective study [17] and preliminary results from an ongoing prospective study [7] suggested that four courses of neoadjuvant TIP chemotherapy was feasible, well tolerated, and yielded clinically meaningful responses in patients with bulky regional lymph node metastases. When the final results of this phase II trial are reported, they should be compared with the historical experience with surgery alone for clinical N2/N3 disease and with the published results on toxicity and response rates with bleomycin-containing regimens.
Radiotherapy may also have a role in the treatment of locally advanced penile cancer, particularly when inguinal adenopathy is initially unresectable. Although the evaluation of multimodality strategies is not readily accomplished with small numbers of patients, the experience in other uncommon squamous cell carcinomas of perineal origin such as cancer of the vulva and the anal canal is illuminating. Testing of hypotheses about efficacy in these diseases has depended on cooperative randomized trials with enough statistical power to detect the difference.
To establish the current standard of care for squamous carcinoma of the anal canal, there have been four key randomized trials. In 1996 the United Kingdom Coordinating Committee on Cancer Research reported the results of a Phase III trial comparing radiotherapy alone or combined with fluorouracil and mitomycin for 585 patients [23]. Surgery was reserved for those patients with a poor response at interim assessment. The combined modality arm achieved a 46% reduction in local failure as well as improved cancer specific survival (P = 0.02). The same year, the RTOG/ECOG published results of a randomized trial examining the contribution of mitomycin to the standard fluorouracil chemo-radiotherapy approach [24]. With 310 patients randomized, they demonstrated that despite higher toxicity with mitomycin, there was improved disease-free survival with the use of the combination of mitomycin and fluorouracil with radiotherapy (P = 0.0003). In parallel to these trials, the EORTC conducted a randomized trial for T3–4 or N1–3 anal cancer, looking at radiotherapy alone or combined with fluorouracil and mitomycin [25]. With 110 patients randomized, they reported a significant improvement (P = 0.03) in event-free survival (no loco-regional progression, colostomy, severe side effects or death) for the combined modality arm. The fourth and final landmark randomized trial defining optimal management in this disease site was again performed by the RTOG comparing the benefit of cisplatin-fluorouracil to mitomycin-fluorouracil when combined with radiotherapy [26]. Between 1998 and 2005, 682 patients were randomized of which 26% had clinically positive nodes. The results demonstrated 5-year disease-free survival rates of 54 and 60%, and 5-year overall survival of 70 and 75% (not statistically significant). These trials established chemoradiotherapy with mitomycin and fluorouracil as state-of-the-art management for anal cancer.
To define the optimal management of vulvar cancer, the Gynecologic Oncology Group (GOG) has conducted several multi-institutional trials. The first landmark study (GOG 37) was reported in 1986 [27]. Patients with positive inguinal nodes following radical vulvectomy and bilateral inguinal node dissection were randomized to subsequent pelvic node dissection versus postoperative radiotherapy. The trial was terminated early after 114 patients were accrued because interim analysis showed a significant survival advantage at 2 years for those in the radiotherapy arm (P = 0.03). A subsequent phase II trial for Stage III–IV squamous cell vulvar cancer not amenable to resection evaluated radiotherapy plus cisplatin and fluorouracil followed by surgical excision of the primary and bilateral inguinal femoral node dissection [28]. After completion of combined chemo-radiotherapy, only 2 of 71 women had residual unresectable disease. In a similar approach for N2/N3 disease, disease in the lymph nodes became resectable for 38/40 women [29].
The results from these cooperative trials set a challenging standard to meet in the study of penile carcinoma. Until such time as similar studies are performed for penile cancer, extrapolation from the experience with anal and vulvar cancer would suggest that in the treatment of advanced penile cancer, especially in the case of inoperable inguinal adenopathy, one could adopt an initial chemo-radiotherapy approach followed by node dissection.
In summary, chemo-radiotherapy or initial chemotherapy alone may render disease resectable in locally advanced penile cancer. Patients who present with bulky regional lymph node metastases are rarely cured by any one modality alone, but selected patients have achieved long-term survival after neoadjuvant chemotherapy and surgery. In the latter case, postoperative groin and/or pelvic radiotherapy can be offered, depending on the amount of residual disease after chemotherapy.
Footnotes
Conflict of interest statement There is no conflict of interest.
Contributor Information
Lance C. Pagliaro, Email: lpagliar@mdanderson.org, Department of Genitourinary Medical Oncology, Unit 1374, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA
Juanita Crook, The University of Toronto/Princess Margaret Hospital, 610 University Avenue, Toronto, ON M5G 2M9, Canada.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Misra S, Chaturvedi A, Misra NC. Penile carcinoma: a challenge for the developing world. Lancet Oncol. 2004;5:240. doi: 10.1016/S1470-2045(04)01427-5. [DOI] [PubMed] [Google Scholar]
- 3.Culkin DJ, Beer TM. Advanced penile carcinoma. J Urol. 2003;170:359. doi: 10.1097/01.ju.0000062829.43654.5e. [DOI] [PubMed] [Google Scholar]
- 4.Horenblas S, van Tinteren H, Delemarre JF, Moonen LM, Lustig V, van Waardenburg EW. Squamous cell carcinoma of the penis. III. Treatment of regional lymph nodes. J Urol. 1993;149:492. doi: 10.1016/s0022-5347(17)36126-8. [DOI] [PubMed] [Google Scholar]
- 5.Ficarra V, Zattoni F, Artibani W, Fandella A, Martignoni G, Novara G, Galetti TP, Zambolin T, Kattan MW. Nomogram predictive of pathological inguinal lymph node involvement in patients with squamous cell carcinoma of the penis. J Urol. 2006;175:1700. doi: 10.1016/S0022-5347(05)01003-7. [DOI] [PubMed] [Google Scholar]
- 6.de Kernion JB, Tynberg P, Persky L, Fegen JP. Proceedings: Carcinoma of the penis. Cancer. 1973;32:1256. doi: 10.1002/1097-0142(197311)32:5<1256::aid-cncr2820320534>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 7.Pagliaro L, Williams D, Daliani D, Tu S, Kincaid M, Heller L, Pettaway CA. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy prior to inguinal/pelvic lymphadenectomy for stage Tany, N2–3, M0 squamous carcinoma of the penis (abstract #602) J Urol. 2006;175:195. [Google Scholar]
- 8.Ichikawa T, Nakano I, Hirokawa I. Bleomycin treatment of the tumors of penis and scrotum. J Urol. 1969;102:699. doi: 10.1016/s0022-5347(17)62235-3. [DOI] [PubMed] [Google Scholar]
- 9.Mills EE. Intermittent intravenous methotrexate in the treatment of advanced epidermoid carcinoma. S Afr Med J. 1972;46:398. [PubMed] [Google Scholar]
- 10.Ahmed T, Sklaroff R, Yagoda A. Sequential trials of methotrexate, cisplatin and bleomycin for penile cancer. J Urol. 1984;132:465. doi: 10.1016/s0022-5347(17)49693-5. [DOI] [PubMed] [Google Scholar]
- 11.Gagliano RG, Blumenstein BA, Crawford ED, Stephens RL, Coltman CA, Jr, Costanzi JJ. cis-Diamminedichloroplatinum in the treatment of advanced epidermoid carcinoma of the penis: a Southwest Oncology Group Study. J Urol. 1989;141:66. doi: 10.1016/s0022-5347(17)40590-8. [DOI] [PubMed] [Google Scholar]
- 12.Corral DA, Sella A, Pettaway CA, Amato RJ, Jones DM, Ellerhorst J. Combination chemotherapy for metastatic or locally advanced genitourinary squamous cell carcinoma: a phase II study of methotrexate, cisplatin and bleomycin. J Urol. 1998;160:1770. [PubMed] [Google Scholar]
- 13.Haas GP, Blumenstein BA, Gagliano RG, Russell CA, Rivkin SE, Culkin DJ, Wolf M, Crawford ED. Cisplatin, methotrexate and bleomycin for the treatment of carcinoma of the penis: a Southwest Oncology Group study. J Urol. 1999;161:1823. [PubMed] [Google Scholar]
- 14.Pizzocaro G, Piva L. Adjuvant and neoadjuvant vincristine, bleomycin, and methotrexate for inguinal metastases from squamous cell carcinoma of the penis. Acta Oncol. 1988;27:823. doi: 10.3109/02841868809094366. [DOI] [PubMed] [Google Scholar]
- 15.Leijte JA, Kerst JM, Bais E, Antonini N, Horenblas S. Neo-adjuvant chemotherapy in advanced penile carcinoma. Eur Urol. 2007;52:488. doi: 10.1016/j.eururo.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Hakenberg OW, Nippgen JB, Froehner M, Zastrow S, Wirth MP. Cisplatin, methotrexate and bleomycin for treating advanced penile carcinoma. BJU Int. 2006;98:1225. doi: 10.1111/j.1464-410X.2006.06496.x. [DOI] [PubMed] [Google Scholar]
- 17.Bermejo C, Busby JE, Spiess PE, Heller L, Pagliaro LC, Pettaway CA. Neoadjuvant chemotherapy followed by aggressive surgical consolidation for metastatic penile squamous cell carcinoma. J Urol. 2007;177:1335. doi: 10.1016/j.juro.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 18.Modig H, Duchek M, Sjodin JG. Carcinoma of the penis. Treatment by surgery or combined bleomycin and radiation therapy. Acta Oncol. 1993;32:653. doi: 10.3109/02841869309092447. [DOI] [PubMed] [Google Scholar]
- 19.Ravi R. Correlation between the extent of nodal involvement and survival following groin dissection for carcinoma of the penis. Br J Urol. 1993;72:817. doi: 10.1111/j.1464-410x.1993.tb16273.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen MF, Chen WC, Wu CT, Chuang CK, Ng KF, Chang JT. Contemporary management of penile cancer including surgery and adjuvant radiotherapy: an experience in Taiwan. World J Urol. 2004;22:60. doi: 10.1007/s00345-003-0383-7. [DOI] [PubMed] [Google Scholar]
- 21.Hyde SE, Valmadre S, Hacker NF, Schilthuis MS, Grant PT, van der Velden J. Squamous cell carcinoma of the vulva with bulky positive groin nodes-nodal debulking versus full groin dissection prior to radiation therapy. Int J Gynecol Cancer. 2007;17:154. doi: 10.1111/j.1525-1438.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- 22.Parthasarathy A, Cheung MK, Osann K, Husain A, Teng NN, Berek JS, Kapp DS, Chan JK. The benefit of adjuvant radiation therapy in single-node-positive squamous cell vulvar carcinoma. Gynecol Oncol. 2006;103:1095. doi: 10.1016/j.ygyno.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 23.Anonymous. Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348:1049. [PubMed] [Google Scholar]
- 24.Flam M, John M, Pajak TF, Petrelli N, Myerson R, Doggett S, Quivey J, Rotman M, Kerman H, Coia L, Murray K. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 25.Bartelink H, Roelofsen F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, Peiffert D, van Glabbeke M, Pierart M. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 26.Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB, 3rd, Thomas CR, Jr, Mayer RJ, Haddock MG, Rich TA, Willett C. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299:1914. doi: 10.1001/jama.299.16.1914. [DOI] [PubMed] [Google Scholar]
- 27.Homesley HD, Bundy BN, Sedlis A, Adcock L. Radiation therapy versus pelvic node resection for carcinoma of the vulva with positive groin nodes. Obstet Gynecol. 1986;68:733. [PubMed] [Google Scholar]
- 28.Moore DH, Thomas GM, Montana GS, Saxer A, Gallup DG, Olt G. Preoperative chemoradiation for advanced vulvar cancer: a phase II study of the Gynecologic Oncology Group. Int J Radiat Oncol Biol Phys. 1998;42:79. doi: 10.1016/s0360-3016(98)00193-x. [DOI] [PubMed] [Google Scholar]
- 29.Montana GS, Thomas GM, Moore DH, Saxer A, Mangan CE, Lentz SS, Averette HE. Preoperative chemo-radiation for carcinoma of the vulva with N2/N3 nodes: a gynecologic oncology group study. Int J Radiat Oncol Biol Phys. 2000;48:1007. doi: 10.1016/s0360-3016(00)00762-8. [DOI] [PubMed] [Google Scholar]