SUMMARY
Within neurons, Ca2+-dependent inactivation (CDI) of voltage-gated L-type Ca2+ channels shapes cytoplasmic Ca2+ signals. CDI is initiated by Ca2+ binding to channel-associated calmodulin and subsequent Ca2+/calmodulin activation of the Ca2+-dependent phosphatase, calcineurin (CaN), which is targeted to L channels by by the A-kinase anchoring protein, AKAP79/150. Here we report that CDI of neuronal L channels was abolished by inhibition of PKA activity or PKA anchoring to AKAP79/150, and that CDI was also suppressed by stimulation of PKA activity. Although CDI was reduced by positive or negative manipulation of PKA, interference with PKA anchoring or activity lowered Ca2+ current density whereas stimulation of PKA activity elevated it. In contrast, inhibition of CaN reduced CDI but had no effect on current density. These results suggest a model wherein PKA-dependent phosphorylation enhances neuronal L current, thereby priming channels to undergo CDI, and Ca2+/calmodulin-activated CaN actuates CDI by reversing PKA-mediated enhancement of channel activity.
INTRODUCTION
Voltage-gated Ca2+ channels convert patterns of electrical activity on the neuronal surface membrane into signals that can initiate intracellular signaling: rises in cytoplasmic Ca2+. Within neurons, Ca2+ can trigger release of neurotransmitter and changes in gene expression that contribute to modification of cell morphology and synaptic plasticity (Catterall, 2011). Residing at the interface between electrical and chemical signaling, Ca2+ channels represent natural points for regulation, with up-modulation and down-modulation of channel activity providing precise spatiotemporal control of cytoplasmic Ca2+ signals that specify which of various Ca2+-dependent processes are activated, and how strongly. Curbing Ca2+ channel activity is also critical in avoiding cytotoxicity arising from Ca2+ overload (Choi, 1994; Nägerl et al., 2000). One important mechanism that has evolved to limit Ca2+ entry via Ca2+ channels is Ca2+-dependent inactivation (CDI; Tillotson, 1979; Budde et al., 2002).
Calmodulin (CaM) has been identified as the Ca2+ sensor that initiates CDI (Zühlke et al., 1999; Peterson et al., 1999), and in the CaM-actuated model of CDI, Ca2+ ions entering the cytoplasm bind to calmodulin docked on the channel through which they have just passed; Ca2+/CaM undergoes a conformational change that is sensed by its associated channel; and the channel is nudged into an inactivated conformation incapable of conducting Ca2+ (Erickson et al., 2003). Despite the elegance of studies aimed at elucidating the mechanism of CaM-actuated CDI, they generally have had the major drawback of relying upon heterologous expression of voltage-gated Ca2+ channels in cells that naturally lack these channels and are also deficient in the scaffolding proteins that target enzymes like PKA and CaN to channels. Using a more intact and physiologically relevant system of cultured hippocampal neurons, we recently described experimental results strongly suggesting that Ca2+/CaM initiates CDI largely through activation of the natural Ca2+/CaM substrate, CaN (Oliveria et al., 2012). We found that CaN, anchored to CaV1.2 by the A-kinase anchoring protein AKAP79/150 (human/rodent), was essential for CDI of pharmacologically-isolated L-type Ca2+ current in hippocampal neurons.
Disruption of this anchoring protein prevented enhancement by PKA of L-current amplitude in cultured neurons, raising the possibility that PKA might enhance L-current by opposing CaM/CaN-mediated CDI. Modulation of CaV1.2 by PKA is one of the best-described forms of ion channel modulation, and has been identified in a variety of excitable cell types (Bean et al., 1984; Kalman et al., 1988; Hadley and Lederer, 1991; Rankovic et al., 2011).
Here, we report evidence from hippocampal neurons indicating that impairment of PKA anchoring or activity decreases L-type Ca2+ current density and abolishes CDI of these channels. Furthermore, neurons in which PKA activity was stimulated exhibited concomitant enhancement of current and diminution of CDI. These experimental results can be explained by a simple model of inverse control by PKA and CaN of L channel current and kinetics: PKA-dependent phosphorylation enhances L channel opening probability and primes channels for CDI, and Ca2+/CaM-activated CaN actuates CDI by reversing PKA-mediated enhancement. This mechanism readily accommodates the experimental observations that interference with the action of either PKA or CaN obstructs the normal process of CDI. More generally, these results expand the repertoire of L-channel-complexed proteins known to modulate Ca2+ signals in postsynaptic regions: channel-bound CaM and AKAP79/150-anchored CaN and PKA function coordinately to tune Ca2+ signals that regulate neuronal gene expression, as further explored in a companion paper (Murphy et al. submitted to Cell Reports).
RESULTS
Channel-localized PKA enhances current density and primes channels for CDI
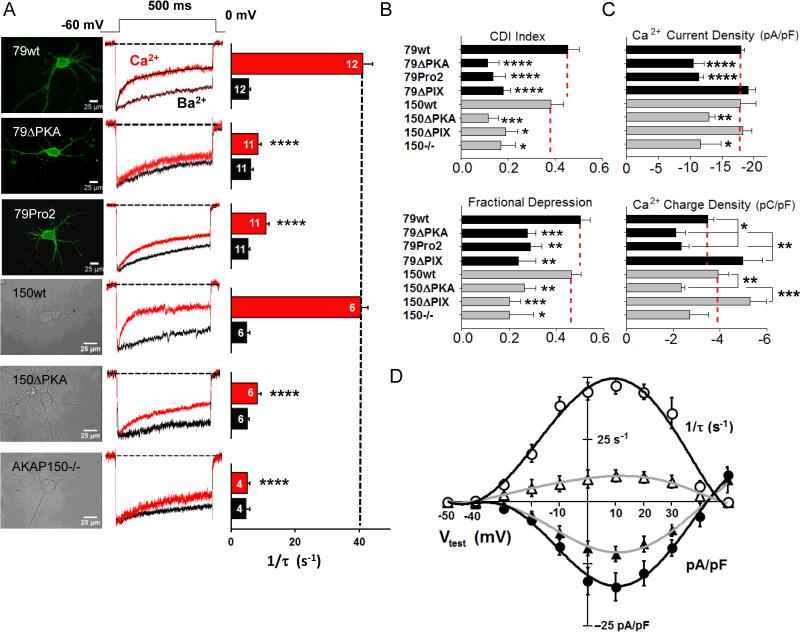
In rodent hippocampal pyramidal neurons grown in culture for up to 5 days, Ca2+ current carried by L-type channels exhibited two components of inactivation: fast, Ca2+-dependent inactivation (1/τ = 40.6 ± 2.1 s–1 in mice, Fig. 1A, red bars; 42.9 ± 2.0 s–1 in rats (Oliveria et al., 2012)) and slow, voltage-dependent inactivation that remains present when Ba2+ (black bars) is substituted for Ca2+ in the extracellular solution. The fast component—CDI—was virtually eliminated in AKAP150-knockout mice (AKAP150–/–; Fig. 1A), consistent with previously-reported results with RNAi-mediated knock-down of AKAP150 (150RNAi) in rat neurons (Oliveria et al., 2007; Oliveria et al., 2012). CDI was fully restored in 150RNAi knock-down neurons that were co-transfected with the 150RNAi-insensitive human ortholog of AKAP150, AKAP79 (79wt + 150RNAi), confirming the importance of AKAP79/150 in L-channel CDI.
Figure 1. CDI of neuronal L-type Ca2+ channels requires AKAP79/150-anchored PKA.
(A) (Left) Top three images, fluorescence images of cultured rat neurons transfected with 150RNAi and: wild-type AKAP79 (79wt), AKAP79ΔPKA (79ΔPKA), or AKAP79Pro2 (79Pro2). Bottom three images, differential interference contrast images of short-term cultured neurons from wild-type mice, AKAP150ΔPKA mice and AKAP150–/– mice. (Center) Superimposed, normalized records of pharmacologically-isolated L-channel Ca2+ (red) or Ba2+ (black) currents. (Right) Mean inactivation rates (1/τ). Number of cells recorded per condition (n) is noted on each bar.
(B) CDI index, calculated as the difference between r400 in Ba2+ and r400 in Ca2+. Black bars represent knockdown/replacement experiments in rat neurons and gray bars represent knockin mice, in all four panels. Fractional depression of divalent charge influx as a consequence of CDI, calculated as the difference between peak-normalized Ba2+ charge influx and peak-normalized Ca2+ charge influx during 500 ms depolarizations.
(C) Peak Ca2+ current density and Ca2+ charge density for each of the experimental manipulations in B. Dividing peak Ca2+ current (ICa, pA) by a measure of membrane surface area, cell capacitance (Cm, pF), yields the density of Ca2+ current (pA/pF). Ca2+ charge density (pC/pF) was calculated as the integrated Ca2+ current during 500-ms depolarization (pC) divided by cell capacitance (pF).
(D) For 150RNAi-transfected rat neurons, voltage-dependence of Ca2+ current density for 79wt (●) or 79ΔPKA (▲) co-transfected neurons, compared with the voltage-dependence of inactivation rate for Ca2+ current, for 79wt (○) or 79ΔPKA (△) co-transfected neurons. Error bars indicate standard error of the mean. Mean values were compared using ANOVA with a posthoc Bonferroni correction. Statistical significance marked as * p = 0.05, **p = 0.01, *** p = 0.005, and **** p = 0.001.
Knock-down or knockout of AKAP150 de-localizes both CaN and PKA from the L-channel nano-environment, which precludes identification of CaN- or PKA-specific effects on channel function. To probe more specifically whether AKAP-anchored PKA is involved in CDI, L currents were recorded from neurons that express an AKAP79/150 mutant incapable of anchoring PKA, owing to a selective deletion within the PKA-RII subunit binding domain (residues 391-400; designated 79ΔPKA) (Carr et al., 1992; Oliveria et al., 2003; Murphy et al., submitted to Cell Reports). Rat neurons co-transfected with AKAP79ΔPKA (79ΔPKA) and 150RNAi to knock down native AKAP150 exhibited greatly slowed CDI (Fig. 1A, row 2). Neurons cultured from AKAP150ΔPKA knockin mice (Murphy et al. submitted to Cell Reports) showed similarly-slowed CDI (Fig. 1A, row 5). CDI was also significantly slowed in neurons expressing 150RNAi and 79pro2 (Fig. 1A, row3)), an AKAP79 mutant bearing two proline substitutions (A396P and I404P) that disrupt the helical structure of the PKA binding site (Carr et al., 1992; Oliveria et al., 2007).
The fraction of peak current remaining 400 ms after the onset of step depolarization (r400) provides another view of inactivation, and the difference between Ba2+ and Ca2+ in fractional current remaining 400 ms after the onset of depolarization (r400,Ba – r400,Ca) provides a second useful index of CDI (Fig. 1B, top) (Kim et al., 2004; Oz et al., 2011). A third CDI metric estimates the fraction of Ca2+ prevented from entering the neuron as a consequence of CDI: peak Ba2+ and Ca2+ current amplitudes were normalized because Ba2+ permeates the channel more readily, then the peak-normalized Ba2+ and Ca2+ currents were integrated to obtain relative charge transfer, and the difference between these (ÂBa – ÂCa) approximates the fractional depression of Ca2+ entry by CDI (Fig. 1B, bottom). For the AKAP manipulations studied, the patterns for both CDI index and depression of Ca2+ entry (ÂBa – ÂCa) mirrored that for rate of CDI (1/τ) in Fig. 1A.
Deficits in AKAP79/150 anchoring of PKA (ΔPKA, pro2) that reduced CDI also reduced peak Ca2+ current density (Fig. 1C, top). In contrast, current density was not decreased in cells expressing mutant AKAP79/150 in which the CaN-binding PxIxIT-like motif (ΔPIX mutants) was deleted. The overall pattern for integrated Ca2+ current density, i.e. Ca2+ charge density (Fig. 1C, bottom), is similar to the peak current density pattern, but less pronounced: disruption of PKA anchoring moderately reduced charge density, whereas disruption of CaN anchoring increased L-channel charge density, albeit modestly. Peak current density and charge density provide different views of channel activity, but together, they suggest the idea that anchored PKA supports a basal degree of enhancement of L-channel activity that is limited by anchored CaN. Using Ca2+ imaging and phospho-immunoblotting, our companion paper in this issue (Murphy et al., submitted to Cell Reports) provides additional evidence that disruption of AKAP79/150-PKA anchoring leads to substantial decreases in basal L-channel phosphorylation and Ca2+ signaling in hippocampal neurons.
Inward Ca2+ current increases with increasing depolarization, and then declines to zero as membrane potential approaches the reversal potential for L channels; as a Ca2+-dependent process, the degree of CDI reflects the trajectory of this ICa-VM curve (Fig. 1D). L currents measured from 150RNAi/79ΔPKA neurons were smaller and inactivated more slowly than in 150RNAi/79wt control neurons, but the voltage-dependence of both current density and 1/τ for CDI showed the same form as for control, indicating that the decreased current density caused by disruption of PKA anchoring cannot be attributed to a shift in the channel's voltage-dependence of gating. Further, the large reduction in CDI caused by disruption of PKA anchoring cannot be explained by the more modest reduction in current density that was caused by this anchoring disruption.
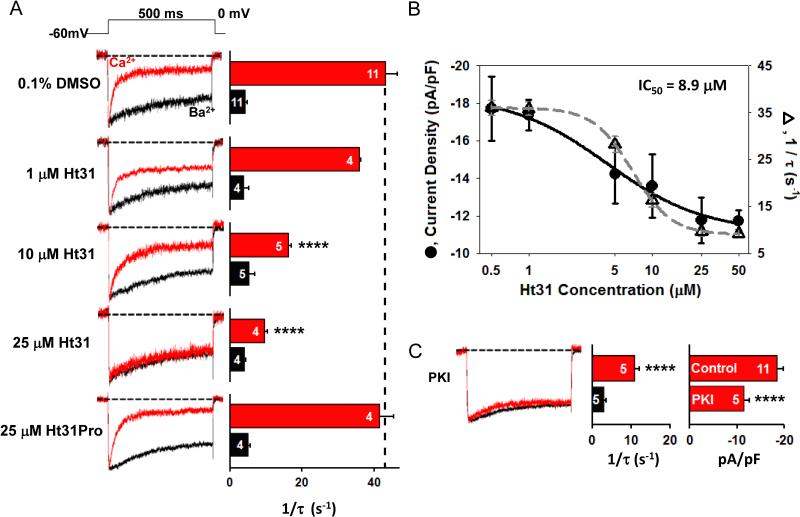
If our explanation of the effects of PKA on CDI was correct, we predicted that pharmacological antagonism of either PKA anchoring to the AKAP or of PKA enzymatic activity would diminish Ca2+ current denstity and CDI. To test this prediction, Ca2+ currents were measured when PKA anchoring was antagonized by Ht31, an AKAP-derived peptide that binds the RII regulatory subunit of PKA with nanomolar affinity and thereby competes with AKAPs for binding to RII (Carr et al., 1992). By incorporating Ht31 into the whole-cell pipet, we found that Ht31 dose-dependently reduced peak current density and CDI, with an IC50 of 5-10 μM (Fig. 2A, B). Current density and CDI were insensitive to a high concentration of a proline substitution mutant of Ht31 (Ht31-Pro) that cannot properly bind to PKA RII (Fig. 2A). In another set of experiments, PKA activity was inhibited by loading the PKA inhibitory peptide, PKI, into the whole-cell recording pipets. Inhibition of PKA activity also depressed Ca2+ current density and eliminated CDI (Fig. 2C). Thus competitive inhibitors of PKA anchoring and activity acted much like the genetic manipulations that impaired PKA anchoring, and as predicted, the competitive inhibitors reduced current density and attenuated CDI.
Figure 2. CDI of neuronal L-type Ca2+ channels is suppressed by acute inhibition of PKA anchoring or activity.
(A) Ht31 peptide, a competitive inhibitor of PKA anchoring to AKAP150, decreased CDI rate and current density of L-type Ca2+ channels in short-term cultured hippocampal neurons. Ht31 peptide was perfused into the cell through the patch pipet. (Left) Normalized Ca2+ (red) and Ba2+ currents (black), recorded from the same cell, were evoked by 500-ms step-depolarization from –60 to 0 mV. (Right) Inactivation rates for Ca2+ current (red bars) and Ba2+ current (black bars). Control rate measured with 0.1% DMSO in the patch Incorporation of Ht31-Pro in place of Ht31 in the patch pipet had no effect on current density or CDI. (B) Dose-response relationships for neurons treated with Ht31, (●) current density and (△) 1/τ. (C) Inhibition of PKA activity by internal perfusion with 5 μM PKI suppresses CDI and lowers current density. Error bars indicate standard error of the mean. Mean values were compared using ANOVA with a posthoc Bonferroni correction. Statistical significance marked as **** p = 0.001.
PKA versus CaN in CDI
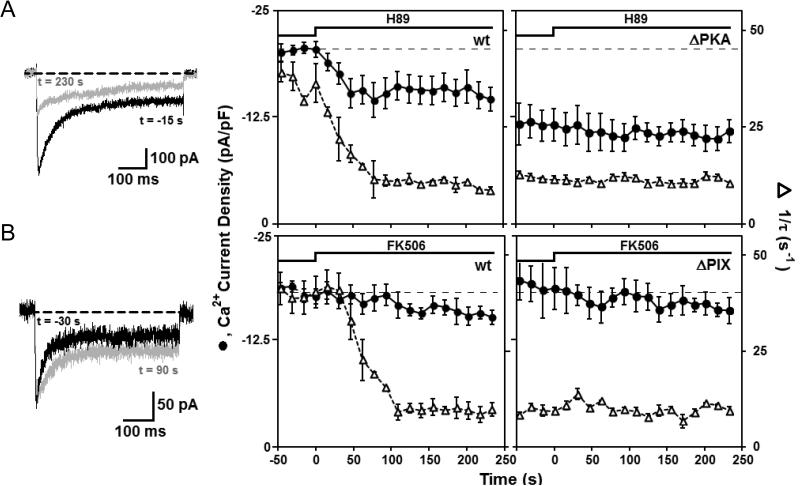
To examine the connection between PKA and CaN in CDI of neuronal L channels, we tested the effects on whole-cell Ca2+ currents of fast application of membrane-permeant inhibitors of PKA (H89) or CaN (FK506). After establishing a baseline for peak inward Ca2+ current density, the solution flowing over the neuron was quickly changed from control to 500 nM H89 (Ki = 135 nM; Davies et al., 2000). H89 application lowered both peak current density and CDI rate to new steady levels in control neurons (Fig. 3A, left), but not in 79ΔPKA neurons (Fig. 3A, right). The low density of current in 150RNAi/79ΔPKA neurons, near absence of CDI, and lack of effect of H89 are consistent with results in Figs. 1 and 2: in 79ΔPKA neurons, channels are already largely de-enhanced and inactivated. Antagonism of CaN via fast application of 5 μM FK506 to control neurons slowed CDI but had no significant effect on peak current density (Fig. 3B, left). In 150RNAi/79ΔPIX neurons, which have disrupted CaN anchoring, FK506 exerted no obvious effect on current density, or rate of CDI (Fig. 3B, right). But in this case, current density remained high, while CDI was low, as though channels were in an enhanced state but no longer subject to CDI. In both situations, the experimental results with drugs that altered PKA or CaN activity agreed with results obtained with AKAP mutants bearing bindng site deletions for PKA or CaN.
Figure 3. Time course of changes in current density and inactivation rate induced by inhibition of PKA or CaN.
(A) Response to fast perfusion of H89 (500 nM). (Left) Untransfected rat neurons. Inset, superimposed Ca2+ current records before (black) and during (gray) H89 application. (Right) Rat neurons expressing 79ΔPKA. (B) Response to fast application of FK506 (5 μM). (Left) Untransfected rat neurons. Inset, representative Ca2+ current records before (black) and during (gray) FK506 application. (Right) Rat neurons expressing 79ΔPIX. Error bars represent standard error of the mean.
Elevation of PKA activity suppresses CDI
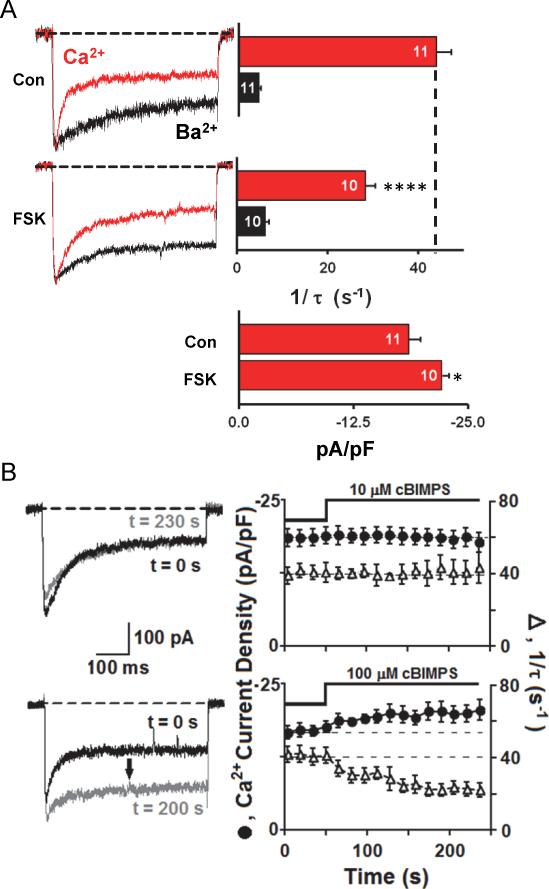
Compared to non-stimulated control neurons, forskolin-stimulated neurons with concomitant elevated PKA activity exhibited significantly less CDI and enhanced L current density (Fig. 4A). If attenuation of CDI reflected the ability of an elevated level of PKA activity to overcome CaN action, then other means of stimulating PKA were predicted to reduce CDI as well. We therefore tested the effects on L current of fast application of a potent and membrane-permeant cAMP analogue, Sp-5,6-dichloro-cBIMPS (Sandberg et al., 1991). At 100 μM, but not 10 μM, the cAMP analogue significantly increased peak L current density (~23%) and reduced CDI, as predicted (Fig. 4B).
Figure. 4. CDI of neuronal L-type Ca2+ channels is reduced by PKA pathway activation.
(A) Internal perfusion with forskolin slows Ca2+-dependent inactivation of L-type current and increases current density. Peak Ca2+ current amplitude (red) was normalized to peak Ba2+ current (black) amplitude. Bar graphs present inactivation rates for Ca2+ and Ba2+ currents; number of individual cells recorded (n) is marked on each bar.
(B) Bath application of 100 μM cAMP analog (Sp-5,6-dichloro-cBIMPS) (bottom), but not 10 μM Sp-5,6-dichloro-cBIMPS (top), enhances current density and slows Ca2+-dependent inactivation. Error bars indicate standard error of the mean. Mean values were compared using ANOVA with a Bonferroni posthoc correction. Statistical significance marked as * p = 0.05, **** p = 0.001.
DISCUSSION
For endogenous L-channel complexes in neurons, we have found that Ca2+/CaN-mediated CDI requires priming of L channels by PKA. A PKA-dependence for CDI was revealed by loss of CDI when either PKA activity was pharmacologically inhibited or PKA anchoring to AKAP79/150 was prevented. Three distinct experimental approaches to disrupt PKA anchoring—(i) 150ΔPKA knock-in, (ii) 150RNAi knockdown and replacement by PKA-binding-defective AKAP79ΔPKA or AKAP79pro2, or (iii) competitive inhibition of PKA anchoring by Ht31—abolished CDI, and thus we conclude that PKA anchoring by AKAP79/150 is a prerequisite for CDI (Fig. 1; Fig. 2A-B). Whether determined from rate of CDI, CDI index, or fractional depression of Ca2+ entry, all anchoring interventions strongly reduced CDI in cultured neurons.
Kinase activity of PKA is also a prerequisite for CDI of L channels in neurons: CDI was prevented either by a synthetic mimic of a highly-selective natural inhibitor of free PKA catalytic subunits, PKI-(6-22)-amide (Fig. 2C), or by a low concentration of a competitive inhibitor of ATP binding in the enzyme's catalytic site, 500 nM H89 (Fig. 3A) (Murray, 2008). H89 was without effect in knockdown/rescue experiments in which PKA anchoring was disrupted, indicating that H89 suppression of CDI is attributable specifically to antagonism of PKA that is anchored in the channel-AKAP complex.
CDI was efficaciously suppressed by the calcineurin inhibitor FK506 (Fig. 3B), directly in line with the idea that CDI of neuronal L channels is carried out by calcineurin (Chad and Eckert, 1986; Armstrong and Eckert, 1987; Oliveria et al., 2007; Oliveria et al., 2012). In calcineurin anchoring-defective 79ΔPIX neurons, the absence of calcineurin from L channel complexes resulted in large L channel Ca2+ currents with little CDI. FK506 had no effect on the (already-diminished) CDI of L channels in 79ΔPIX neurons, presumably because calcineurin, the target of FK506 action, was not present in the AKAP/L-channel complex.
Channel-localized PKA activity supports CaN-mediated CDI
Experimental manipulations that impaired either anchoring (ΔPKA or Ht31) or activity (PKI, H89) of PKA virtually abolished CDI, but they also decreased current density (from 18-20 pA/pF to 10-14 pA/pF). Was loss of CDI in these cases simply a consequence of smaller Ca2+ influx rather than decreased phosphorylation by PKA? Apparently not, because strong CDI was evident in WT currents recorded at –10 mV or +30 mV, and at these potentials WT currents were similar in amplitude to those of CDI-lacking 79ΔPKA currents recorded at the peak (+10 mV) of the current-voltage relationship (Fig. 1D). Therefore, loss of CDI upon disruption of PKA anchoring or block of PKA activity apparently arose as a direct consequence of the loss of channel-localized PKA activity.
PKI and disruption of PKA localization were very similar to one another in the size of their effects on current density (~30-40% depression) and CDI (abolishment). This similarity indicates that an intact pool of PKA anchored by AKAP79/150 to L channels has a basal level of activity in neurons, that this pool of PKA enhances L current, and that this degree of PKA-dependent enhancement is sufficient to support subsequent CaN-mediated CDI. For Ht31 treatment, de-enhancement of current and suppression of CDI displayed overlapping concentration-response relationships, suggesting that these two effects are directly related to one another (Fig. 2B). Regulation of L channels by basal activity of AKAP79/150-anchored PKA may be attributable to cAMP-independent kinase activity of catalytic subunits in type II PKA holoenzymes or to activity of catalytic subunits that may remain associated with RII subunits in cAMP-activated PKA holoenzymes (Yang et al., 1995; Smith et al., 2013).
Phosphorylation is a prerequisite for CaN-mediated, dephosphorylation-dependent CDI
We have found that stimulation of PKA activity (FSK, cBIMPS) in hippocampal neurons reduced CDI (Fig. 4). Similarly, other groups have found that the beta agonist isoproterenol, forskolin, mimics of cAMP or intracellular perfusion with PKA reduced CDI of voltage-gated Ca2+ channels in neurons (Kalman et al., 1988; You et al., 1995). Yet we have found that interference with PKA action (de-localization, block) also reduced CDI, much like stimulation of PKA did. Thus CDI was reduced by either positive or negative manipulation of PKA action. In contrast, the effects of PKA manipulation on current density were straightforward: interference with PKA localization or activity reduced current density whereas stimulation of PKA (by forskolin or cBIMPS) increased current density. How might the effects of PKA manipulation upon CDI and enhancement of L current be reconciled, and in particular, how might interference with localized PKA action reduce CDI?
Evidently, PKA-mediated enhancement and CaN-mediated CDI function as inverse operations at L channels: PKA enhances via phosphorylation, and CaN de-enhances – initiates CDI – via dephosphorylation. A natural conclusion is that PKA-dependent phosphorylation primes channels to undergo CDI, and Ca2+/CaM-activated CaN actuates CDI by reversing PKA-mediated enhancement. In this model, elevated PKA activity (FSK, cBIMPS) overcomes CaN phosphatase action and thereby reduces CDI and enhances current. Conversely, when channel-localized PKA action is impeded (ΔPKA, Pro2, Ht31, H89, PKI), the diminution of current density and of CDI reflect the facts that channel opening probability is reduced and fewer channels are phosphorylated and thus available to undergo CaN-dependent CDI. In essence, impediment of PKA action allows the CDI process to chronically dominate channel gating, an increased fraction of channels resides in a Ca2+-inactivated state, and fewer open channels remain available to undergo CDI. More complex alternative explanations are possible as well. The time course of the effects of H89 on L current particularly supports our simple interpretation (Fig. 3A). The time course of FK506 action can also be accommodated by this model: during FK506 inhibition of Ca2+/CaM-activated CaN, L-channel current density was maintained and presumably the number of phosphorylated, active L channels was preserved as well; at the same time, the Ca2+/CaN-mediated dephosphorylation of channels that subserves CDI was prevented, thus reducing rate and degree of CDI.
In summary, our work addresses the control of postsynaptic L-type Ca2+ signals, and particularly the mechanism of CDI in cultured hippocampal neurons. In these neurons, CDI requires prior priming of channels by PKA, with the actual process of CDI mediated by CaM and CaN (Chad and Eckert, 1986; Armstrong, 1989; Oliveria et al., 2007; Oliveria et al., 2012). In the PKA-CaN network that regulates L channel activity, PKA may be activated by an upstream input, for example by β2 adrenergic receptors (Gray and Johnston, 1987; Davare et al., 2001; Hoogland and Saggau, 2004), which provides a positive modulatory influence on L channels. PKA might also be activated by Ca2+/CaM-stimulated adenylyl cyclase activity, which would provide positive autoregulatory feedback onto L channels. Negative autoregulatory feedback is provided by Ca2+/CaM-activated CaN, in the form of CDI. Such integrated control by PKA and CaN of L-channel Ca2+ signals is also critical in regulation of gene expression in neurons. For example, a companion paper in this issue (Murphy et al., submitted to Cell Reports) demonstrates that anchoring of PKA within the AKAP/L channel complex is necessary to balance the inhibitory action on L channels of co-anchored-CaN, and thereby sustain proper neuronal excitation-transcription coupling.
EXPERIMENTAL PROCEDURES
Hippocampal pyramidal neurons obtained from neonatal rats and mice were cultured for up to 5 days, in order to reduce the more egregious loss of space-clamp that occurs in longer-term cultured neurons exhibiting extensive neuritic branching. Compared to older cultures, the short-term cultured neurons exhibited faster activation and deactivation of L-type currents, facilitating measurement of inactivation kinetics. Details regarding preparation of short-term cultured hippocampal pyramidal neurons, patch-clamp recording and data analysis are described in the Supplemental Experimental Procedures.
Supplementary Material
HIGHLIGHTS.
Ca2+/CaN-mediated CDI of neuronal L channels requires a basal level of PKA activity
CDI of neuronal L channels relies upon AKAP79/150-anchored PKA
CDI is attenuated by either elevation or obstruction of PKA action on L channels
L channels must be primed by PKA to undergo Ca2+/calcineurin-dependent inactivation
ACKNOWLEDGEMENTS
Support was provided by National Institutes of Health grants T32-AA007464, R01-MH080291 and R01-HL088548. AKAP150–/– mice were kindly provided by John D. Scott (Univ. of Washington). We thank John H. Caldwell for advice on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: PJD, MLD and WAS designed experiments; PJD carried out experiments; PJD, MLD and WAS wrote the manuscript.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, which can be found with this article online.
REFERENCES
- Armstrong D, Eckert R. Voltage-activated calcium channels that must be phosphorylated to respond to membrane depolarization. Proc. Natl. Acad. Sci. USA. 1987;84:2518–2522. doi: 10.1073/pnas.84.8.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DL. Calcium channel regulation by calcineurin, a Ca2+-activated phosphatase in mammalian brain. Trends Neurosci. 1989;12:117–122. doi: 10.1016/0166-2236(89)90168-9. [DOI] [PubMed] [Google Scholar]
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- Bauman AL, Goehring AS, Scott JD. Orchestration of synaptic plasticity through AKAP signaling complexes. Neuropharmacology. 2004;46:299–310. doi: 10.1016/j.neuropharm.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Bean BP, Nowycky MC, Tsien RW. β-Adrenergic modulation of calcium channels in frog ventricular heart cells. Nature. 1984;307:371–375. doi: 10.1038/307371a0. [DOI] [PubMed] [Google Scholar]
- Budde T, Meuth S, Pape HC. Calcium-dependent inactivation of neuronal calcium channels. Nat. Rev. Neurosci. 2002;3:873–883. doi: 10.1038/nrn959. [DOI] [PubMed] [Google Scholar]
- Carr DW, Hausken ZE, Fraser ID, Stofko-Hahn RE, Scott JD. Association of the type II cAMP-dependent protein kinase with a human thyroid RII-anchoring protein. Cloning and characterization of the RII-binding domain. J. Biol. Chem. 1992;267:13376–13382. [PubMed] [Google Scholar]
- Catterall WA. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad JE, Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J. Physiol. 1986;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Calcium and excitotoxic neuronal injury. Ann. N. Y. Acad. Sci. 1994;747:162–171. doi: 10.1111/j.1749-6632.1994.tb44407.x. [DOI] [PubMed] [Google Scholar]
- Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, Horne MC, Hoshi T, Hell JW. A β2 adrenergic receptor signaling complex assembled with the Ca2+ channel CaV1.2. Science. 2001;293:98–101. doi: 10.1126/science.293.5527.98. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MG, Liang H, Mori MX, Yue DT. FRET Two-hybrid mapping reveals function and location of L-Type Ca2+ channel CaM preassociation. Neuron. 2003;39:97–107. doi: 10.1016/s0896-6273(03)00395-7. [DOI] [PubMed] [Google Scholar]
- Gray R, Johnston D. Noradrenaline and β-adrenoceptor agonists increase activity of voltage-dependent calcium channels in hippocampal neurons. Nature. 1987;327:620–622. doi: 10.1038/327620a0. [DOI] [PubMed] [Google Scholar]
- Hadley RW, Lederer WJ. Ca2+ and voltage inactivate Ca2+ channels in guinea-pig ventricular myocytes through independent mechanisms. J. Physiol. 1991;444:257–268. doi: 10.1113/jphysiol.1991.sp018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogland TM, Saggau P. Facilitation of L-type Ca2+ channels in dendritic spines by activation of β2 adrenergic receptors. J. Neurosci. 2004;24:8416–8427. doi: 10.1523/JNEUROSCI.1677-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman D, O'Lague PH, Erxleben C, Armstrong DL. Calcium-dependent inactivation of the dihydropyridine-sensitive calcium channels in GH3 cells. J. Gen. Physiol. 1988;92:531–548. doi: 10.1085/jgp.92.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ghosh S, Nunziato DA, Pitt GS. Identification of the components controlling inactivation of voltage-gated Ca2+ channels. Neuron. 2004;41:745–754. doi: 10.1016/s0896-6273(04)00081-9. [DOI] [PubMed] [Google Scholar]
- Lee A, Scheuer T, Catterall WA. Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. J. Neurosci. 2000;20:6830–6838. doi: 10.1523/JNEUROSCI.20-18-06830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JG, Sanderson JL, Gorski JA, Scott JD, Catterall WA, Sather WA, Dell'Acqua ML. AKAP-anchored PKA maintains neuronal L-type calcium channel activity and NFAT transcriptional signaling. 2013. (submitted to Cell Reports) [DOI] [PMC free article] [PubMed]
- Murray AJ. Pharmacological PKA inhibition: all may not be what it seems. Sci. Signal. 2008;1:re4. doi: 10.1126/scisignal.122re4. [DOI] [PubMed] [Google Scholar]
- Nägerl UV, Mody I, Jeub M, Lie AA, Elger CE, Beck H. Surviving granule cells of the sclerotic human hippocampus have reduced Ca2+ influx because of a loss of calbindin-D(28k) in temporal lobe epilepsy. J. Neurosci. 2000;20:1831–1836. doi: 10.1523/JNEUROSCI.20-05-01831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria SF, Gomez LL, Dell'Acqua ML. Imaging kinase--AKAP79--phosphatase scaffold complexes at the plasma membrane in living cells using FRET microscopy. J. Cell. Biol. 2003;160:101–112. doi: 10.1083/jcb.200209127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria SF, Dell'Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria SF, Dittmer PJ, Youn DH, Dell'Acqua ML, Sather WA. Localized calcineurin confers Ca2+-dependent inactivation on neuronal L-type Ca2+ channels. J. Neurosci. 2012;32:15328–15337. doi: 10.1523/JNEUROSCI.2302-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz S, Tsemakhovich V, Christel CJ, Lee A, Dascal N. CaBP1 regulates voltage-dependent inactivation and activation of CaV1.2 (L-type) calcium channels. J. Biol. Chem. 2011;286:13945–13953. doi: 10.1074/jbc.M110.198424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- Pitt GS. Calmodulin and CaMKII as molecular switches for cardiac ion channels. Cardiovasc. Res. 2007;73:641–647. doi: 10.1016/j.cardiores.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Rankovic V, Landgraf P, Kanyshkova T, Ehling P, Meuth SG, Kreutz MR, Budde T, Munsch T. Modulation of calcium-dependent inactivation of L-type Ca2+ channels via beta-adrenergic signaling in thalamocortical relay neurons. PLoS One. 2011;6:e27474. doi: 10.1371/journal.pone.0027474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg M, Butt E, Nolte C, Fischer L, Halbrugge M, Beltman J, Jahnsen T, Genieser HG, Jastorff B, Walter U. Characterization of Sp-5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole- 3',5'-monophosphorothioate (Sp-5,6-DCl-cBiMPS) as a potent and specific activator of cyclic-AMP-dependent protein kinase in cell extracts and intact cells. Biochem. J. 1991;279:521–527. doi: 10.1042/bj2790521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FD, Reichow SL, Esseltine JL, Shi D, Langeberg LK, Scott JD, Gonen T. Intrinsic disorder within an AKAP-protein kinase A complex guides local substrate phosphorylation. eLife. 2013;2:e01319. doi: 10.7554/eLife.01319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc. Natl. Acad. Sci. USA. 1979;76:1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Fletcher WH, Johnson DA. Regulation of cAMP-dependent protein kinase: enzyme activation without dissociation. Biochemistry. 1995;34:6267–6271. doi: 10.1021/bi00019a002. [DOI] [PubMed] [Google Scholar]
- You Y, Pelzer DJ, Pelzer S. Trypsin and forskolin decrease the sensitivity of L-type calcium current to inhibition by cytoplasmic free calcium in guinea pig heart muscle cells. Biophys. J. 1995;69:1838–1846. doi: 10.1016/S0006-3495(95)80054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zühlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399:159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.