Abstract
Background
Anti‐leukotrienes (5‐lipoxygenase inhibitors and leukotriene receptors antagonists) serve as alternative monotherapy to inhaled corticosteroids (ICS) in the management of recurrent and/or chronic asthma in adults and children.
Objectives
To determine the safety and efficacy of anti‐leukotrienes compared to inhaled corticosteroids as monotherapy in adults and children with asthma and to provide better insight into the influence of patient and treatment characteristics on the magnitude of effects.
Search methods
We searched MEDLINE (1966 to Dec 2010), EMBASE (1980 to Dec 2010), CINAHL (1982 to Dec 2010), the Cochrane Airways Group trials register, and the Cochrane Central Register of Controlled Trials (Dec 2010), abstract books, and reference lists of review articles and trials. We contacted colleagues and the international headquarters of anti‐leukotrienes producers.
Selection criteria
We included randomised trials that compared anti‐leukotrienes with inhaled corticosteroids as monotherapy for a minimum period of four weeks in patients with asthma aged two years and older.
Data collection and analysis
Two review authors independently assessed the methodological quality of trials and extracted data. The primary outcome was the number of patients with at least one exacerbation requiring systemic corticosteroids. Secondary outcomes included patients with at least one exacerbation requiring hospital admission, lung function tests, indices of chronic asthma control, adverse effects, withdrawal rates and biological inflammatory markers.
Main results
Sixty‐five trials met the inclusion criteria for this review. Fifty‐six trials (19 paediatric trials) contributed data (representing total of 10,005 adults and 3,333 children); 21 trials were of high methodological quality; 44 were published in full‐text. All trials pertained to patients with mild or moderate persistent asthma. Trial durations varied from four to 52 weeks. The median dose of inhaled corticosteroids was quite homogeneous at 200 µg/day of microfine hydrofluoroalkane‐propelled beclomethasone or equivalent (HFA‐BDP eq). Patients treated with anti‐leukotrienes were more likely to suffer an exacerbation requiring systemic corticosteroids (N = 6077 participants; risk ratio (RR) 1.51, 95% confidence interval (CI) 1.17, 1.96). For every 28 (95% CI 15 to 82) patients treated with anti‐leukotrienes instead of inhaled corticosteroids, there was one additional patient with an exacerbation requiring rescue systemic corticosteroids. The magnitude of effect was significantly greater in patients with moderate compared with those with mild airway obstruction (RR 2.03, 95% CI 1.41, 2.91 versus RR 1.25, 95% CI 0.97, 1.61), but was not significantly influenced by age group (children representing 23% of the weight versus adults), anti‐leukotriene used, duration of intervention, methodological quality, and funding source. Significant group differences favouring inhaled corticosteroids were noted in most secondary outcomes including patients with at least one exacerbation requiring hospital admission (N = 2715 participants; RR 3.33; 95% CI 1.02 to 10.94), the change from baseline FEV1 (N = 7128 participants; mean group difference (MD) 110 mL, 95% CI 140 to 80) as well as other lung function parameters, asthma symptoms, nocturnal awakenings, rescue medication use, symptom‐free days, the quality of life, parents' and physicians' satisfaction. Anti‐leukotriene therapy was associated with increased risk of withdrawals due to poor asthma control (N = 7669 participants; RR 2.56; 95% CI 2.01 to 3.27). For every thirty one (95% CI 22 to 47) patients treated with anti‐leukotrienes instead of inhaled corticosteroids, there was one additional withdrawal due to poor control. Risk of side effects was not significantly different between both groups.
Authors' conclusions
As monotherapy, inhaled corticosteroids display superior efficacy to anti‐leukotrienes in adults and children with persistent asthma; the superiority is particularly marked in patients with moderate airway obstruction. On the basis of efficacy, the results support the current guidelines' recommendation that inhaled corticosteroids remain the preferred monotherapy.
Plain language summary
Anti‐leukotriene agents compared to inhaled corticosteroids for people with asthma
In an asthma attack, the airways (passages to the lungs) narrow because of muscle spasms (bronchospasm), inflammation (swelling) and mucus secretion phlegm. The airway passage narrowing results in breathing problems, wheezing and coughing. Inhaled corticosteroids are considered the gold standard to reduce the airway inflammation in adults and children with asthma. Anti‐leukotrienes (5‐lipoxygenase inhibitors and leukotriene receptors antagonists) are anti‐inflammatory drugs that may have fewer adverse effects than inhaled corticosteroids. The review suggests that anti‐leukotrienes are safe, but less effective than a low dose of inhaled corticosteroids.
Background
Asthma is a condition that affects the airways, it is characterised by bronchoconstriction and underlying inflammation. Infiltration of bronchial airways with eosinophils and neutrophils with release of inflammatory mediators is characteristic of asthma (Murphy 1993). The cysteinyl leukotrienes are considered as the most potent inflammatory mediators in asthma. They are produced by the 5‐lipoxygenase pathway of the arachidonic acid metabolism. These mediators stimulate the production of airway secretions, cause micro vascular leakage and enhance eosinophilic migration in the airways; thus, leukotrienes are believed to play a pivotal role in mediating bronchoconstriction and inflammatory changes in the pathophysiology of asthma (Peters‐Golden 2007).
All recent consensus statements on asthma advocate aggressive treatment of airway inflammation (Australia 2006; NAEPP 2007; Lougheed 2010; GINA 2010; BTS 2011). Although several drugs such as ketotifen, sodium cromoglycate and sodium nedocromil have anti‐inflammatory properties, inhaled glucocorticoids remain the cornerstone of asthma management because of their efficacy, tolerability and rapid onset of action (Spahn 1996). Prolonged low dose administration of inhaled corticosteroids is generally considered safe, although there is considerable concern about the long‐term effects of steroids among some consumers (Elwyn 2010). However, when moderate or high doses are required to control symptoms, adverse effects such as growth stunting in children (Sharek 2000; Richard 2006), suppression of the adrenal axis (Bisgaard 1988; Phillip 1992; Padfield 1993; Zöllner 2007), and osteopenia (Todd 1996; Heuck 1997) may be observed.
Anti‐leukotrienes form a class of anti‐inflammatory drugs that interfere with leukotriene production (5‐lipoxygenase inhibitors) or with leukotriene receptors (leukotriene receptors antagonists, LTRAs). Anti‐leukotrienes have the advantage of being administered orally in a single or twice daily dose and importantly, seem to lack the adverse effects on growth, bone mineralization and adrenal axis, associated with long‐term or high‐dose systemic glucocorticoid therapy.
Why it is important to do this review
The previous version of this review (Ducharme 2004) summarised the accumulating evidence derived from 25 randomised controlled trials (three paediatric and 22 adult) and concluded that low doses of inhaled corticosteroids were superior in efficacy than anti‐leukotrienes. With the publications of several new randomised controlled trials especially in children, an update of the systematic review was deemed useful to review the safety and efficacy of anti‐leukotrienes as monotherapy as compared to inhaled corticosteroids and to provide better insight into the influence of patient and treatment characteristics on the magnitude of effects.
In addition, several national guidelines currently advocate their use as second choice monotherapy after inhaled corticosteroids in patients with mild asthma and as adjunct therapy with inhaled corticosteroids as an alternative of combination of long acting β2‐agonist and inhaled corticosteroids in patients with moderate asthma (Australia 2006; NAEPP 2007; Lougheed 2010; GINA 2010; BTS 2011).
Objectives
The aims of this systematic review were:
to compare the safety and efficacy of daily oral anti‐leukotrienes with that of inhaled corticosteroids;
to determine the dose of inhaled corticosteroids equivalent to the effect of anti‐leukotrienes in the management of asthma in adults and children; and
to explore different factors such as patients' age group, disease severity, anti‐leukotriene used, intervention duration, hydrofluoroalkane‐propelled beclomethasone or equivalent (HFA‐BDP eq) dose of inhaled corticosteroids, methodological quality, publication status and funding that could influence the magnitude of effect.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) conducted in adults and children, or both, in which anti‐leukotrienes were compared with inhaled corticosteroids.
Types of participants
We included children (aged two to 17 years) and adults (aged over 18 years) with chronic persistent asthma. We included participants who were either corticosteroid‐naive or had been taking maintenance inhaled corticosteroid therapy prior to randomisation.
Types of interventions
We included trials with interventions consisting of daily oral anti‐leukotrienes at usual licensed doses (see under 'Unit of analysis issues') compared to any type of daily inhaled corticosteroids. Interventions had to be administered for at least four weeks. We excluded trials that administered concomitant anti‐inflammatory or anti‐asthmatic drug such as sodium cromoglycate or theophylline. Only rescue medications, such as inhaled short‐acting β2‐agonists and short courses of oral corticosteroids were permitted and recorded.
Types of outcome measures
Primary outcomes
The primary outcome was the number of patients with at least one exacerbation requiring systemic corticosteroids.
Secondary outcomes
Other clinical outcomes reflecting the severity of asthma exacerbations (e.g. hospital admissions, acute care visits)
Clinical or physiologic outcomes reflecting chronic asthma control (e.g. pulmonary function tests, symptom score, β2‐agonist use, measures of functional status, quality of life, patient's and physician's satisfaction, etc.)
Biological markers of inflammation (e.g. eosinophil count in blood and sputum, leukotriene C4 in biological samples, expired nitric oxide, etc);
Clinical and biochemical adverse effects (e.g., elevation of liver enzymes, growth)
Withdrawal rates (overall withdrawals, withdrawals due to poor asthma control and withdrawals due to adverse effects)
In studies designed to identify the minimum effective dose of inhaled corticosteroids needed to achieve asthma control, and in which the control obtained with inhaled corticosteroids was similar to that obtained with anti‐leukotrienes, we aimed to report the median effective dose of inhaled corticosteroids; this dose may be taken to be equivalent in effect to that of the anti‐leukotrienes. We excluded trials that only documented compliance.
Search methods for identification of studies
Electronic searches
Trials were identified using the Cochrane Airways Group Specialised Register of trials (CAGR), which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED, and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see Appendix 1 for further details). All records in the CAGR coded as 'asthma' were searched using the following terms:
(leukotriene* OR anti‐leukotriene* OR leukotriene* antagonist* OR lukast*) AND [inhaled corticosteroids* OR steroid* OR corticosteroid* OR cortico‐steroid* OR beclomethasone* OR fluticasone* OR budesonide* OR triamcinolone* OR flunisolide* OR bronalide* OR becotide* OR azmacort* OR aerobid* OR flixotide* OR aerobec* OR flovent* OR becloforte* OR pulmicort* OR aerobid* OR beclovent* OR azmacort* OR vanceril* OR ciclesonide OR alvesco).
Searching other resources
Reference lists of all identified RCTs were checked to identify potentially relevant citations. In addition, between 1998 and 2003, we searched abstract books of the American Thoracic Society and the European Respiratory Society Meetings; we contacted the international headquarters of pharmaceutical companies producing anti‐leukotrienes; and enquiries regarding other published or unpublished studies known and/or supported by these companies or their subsidiaries were made so that these results could be included in our 2003 review. In 2010, we searched the websites of pharmaceutical companies that are producers or distributors of inhaled corticosteroids or anti‐leukotrienes for any posted report of potential relevant trials.
Data collection and analysis
Selection of studies
One review author (BFC) reviewed and annotated each title and abstract returned from the search as either 1) RCT; 2) clearly not an RCT; or 3) unclear. The full text publications of references annotated as clearly, or potentially, relevant RCTs were obtained and reviewed by BFC.
Data extraction and management
Both review authors extracted data independently (BFC and FMD) and we dealt with disagreement by consensus. We consulted authors and the funding pharmaceutical companies for confirmation of data extraction for all included trials where necessary.
Assessment of risk of bias in included studies
We assessed the methodological quality of the eligible studies using the Cochrane Collaboration's 'Risk of bias' tool (Higgins 2008). This instrument evaluates the reported quality of randomisation, allocation concealment, blinding, incomplete outcome data, selective reporting and other bias.
We previously used the five‐point scoring instrument proposed by with the Cochrane Classification 'Risk of bias' tool to assess study quality. Based on revised recommendations from the Cochrane Handbook for Systematic Reviews of Interventions, we assessed the risk of bias for each eligible study according to six pre‐specified domains (Higgins 2008). Both review authors performed this quality assessment independently. We resolved disagreement by consensus. We sought confirmation of methodology for included trials directly from the authors or the funding pharmaceutical companies. We assessed risk of bias against the following domains.
Allocation generation. The method used to allocate participants to treatment group (e.g. computer‐generated random number sequences).
Allocation concealment. The method used to conceal the sequence of treatment group assignment from study investigators and participants (e.g. assignment by date of birth, opaque sealed envelopes).
Blinding. The method by which knowledge of treatment group assignment was concealed from study investigators and participants after the study began (double‐blind, single‐blind).
Completeness of outcome data. The method for handling data from participants who withdrew from the study (e.g. intention‐to‐treat analysis, available case).
Selective reporting. Whether there was evidence that outcomes measured in the study were unreported (e.g. unreported harms, relevant efficacy data that were not available due for reporting and not statistical reasons).
Other sources of bias. Any other aspect of the study design which may be a source of bias.
We collected and reported information for each domain of bias and provided a judgment based on this information as high, low or unclear risk of bias. We considered reported data with low risk in bias for the three main parameters (randomisation, blinding and low withdrawal rate in both groups) as high quality data for sensitivity analysis (see below).
Measures of treatment effect
We summarised differences between groups in event rates, such as number of exacerbations in a specific period, using either a ratio of rates or relative risk when pertaining to "one or more" events per patient. In continuous outcomes, such as pulmonary function tests or symptom scores, we used the mean difference (MD) or standard mean difference (SMD) method as indicated to estimate the individual and pooled effect sizes. We reported all estimates with their 95% confidence interval and performed meta‐analysis using Reference Manager 5.1 (RevMan).
Studies designed to test equivalence in treatment efficacy require a different analytical approach to that used for trials in which the hypothesis under test is that one treatment has greater efficacy than its comparator. This is because small trials may favour the conclusion that there was no difference between treatments, since the confidence intervals for the two treatments will be wide and therefore more likely to include the line of no difference between the two treatments. We set limits of treatment efficacy at +/‐ 0.10 on either side of the no‐difference line for the number of patients with at least one exacerbation requiring systemic corticosteroids. The null hypothesis tested whether the confidence interval for the difference between the two treatments included one of these limits.
Unit of analysis issues
We referred to usual licensed doses of leukotriene receptor antagonists as: montelukast 4, 5, or 10 mg daily (patients aged two to five, six to 14 and > 15 years respectively); pranlukast 450 mg daily (children aged ≥ 12 years and adults), and zafirlukast 20 mg twice daily (children aged ≥ 12 years and adults). Doses of inhaled corticosteroids were converted to microfine HFA‐BDP eq based on 1 μg of fluticasone = 1 μg of microfine HFA‐propelled beclomethasone = 1 μg of ciclesonide = 1 μg of mometasone = 2 μg of budesonide = 2 μg of chlorofluorocarbon (CFC)‐propelled beclomethasone = 4 μg triamcinolone = 4 μg of flunisolide (NAEPP 2007). All doses of inhaled medications are reported based on ex‐valve, rather than ex‐inhaler, value.
Assessment of heterogeneity
We tested homogeneity of effect sizes between pooled studies using the DerSimonian and Laird method or the I2, which estimates the amount of statistical variation between the studies above what would be expected with the play of chance (Cochrane Handbook). We set values of P greater than 0.05 or I2 greater than 25% as providing an indication of significant heterogeneity. If heterogeneity was suggested by one or both statistical methods, we applied the DerSimonian and Laird random‐effects model to the summary estimates (available in RevMan). Unless specified otherwise we employed the fixed‐effect model.
Assessment of reporting biases
We used funnel plots to test for the presence of possible publication bias (Egger 1997).
Data synthesis
Meta‐analyses were performed using Reference Manager 5.1 (RevMan). We derived the number needed to treat to benefit (NNTB) or number needed to treat to harm (NNTH) from the pooled Odds Ratio using Visual Rx (www.nntonline.net). We used this method because the resulting NNTB or NNTH is independent of the way that the data are entered, which is not the case for relative risk (Cates 2002).
Subgroup analysis and investigation of heterogeneity
We performed subgroup analysis to explore possible reasons for heterogeneity of the primary outcome. All outcomes were stratified in children versus adults for the specific purpose of providing estimates particularly for these age groups and to explore a possible modifying effect of age. Additional a priori defined subgroups included:
anti‐leukotriene used (montelukast versus zafirlukast);
doses of inhaled corticosteroids in HFA‐BDP eq (100 to ‐150 µgversus 200 to ‐250 µg versus 400 to 500 µg HFA‐BDP eq);
intervention duration (four to eight weeks versus 12 to 16 weeks versus 24 to 26 weeks versus 36 to 52 weeks);
baseline severity of airway obstruction (moderate = FEV1 60 to 79% versus mild = FEV1 ≥80%);
publication status;
funding source.
We examined the difference in the magnitude of effect attributable to these subgroups with the residual Chi2 test from the Peto odds ratios (Deeks 2001).
Sensitivity analysis
For the primary outcome, we performed sensitivity analyses were performed to investigate the effect of methodological quality based on the reported quality of randomisation, concealment of allocation, blind assessment of outcomes, and description of withdrawals and dropouts. The fail‐safe N test will be used to assess the robustness of the results (Gleser 1996).
Results
Description of studies
Results of the search
The search strategy updated up to December 2010 yielded a total of 401 additional citations which combined to 658 previously identified citations lead to a total of 1053 citations. The data hereafter are presented as total exclusions (exclusions from latest search + exclusions from previous search). Of these 652 (319 + 333) citations were excluded for the following non‐mutually exclusive reasons: (1) duplicate references (31 + 162 = 193), (2) not a randomised controlled trial (31 + 253 = 284) or ongoing trials (23 + 1 = 24), (3) subjects were not asthmatics (3 + 17 = 20), (4) the tested intervention was not anti‐leukotrienes (82 + 17 = 99), (5) the control intervention was not inhaled corticosteroids (65 + 124 = 189), (6) use of higher than licensed doses of anti‐leukotrienes (from previous review = 1) (Korenblat 1998), (7) use of non permitted drugs (106 + 28 = 134), (8) the tested intervention was administered for less than 4 weeks (14 + 19 = 33), (9) acute care setting (3 + 1 = 4). Due to the large number of citations considered, the references and reasons for exclusion were provided only for full‐text randomised controlled trials in the last review up to October 2003, while reasons for exclusion for all references were included for 2003 to 2010. Some trials were excluded with more than one reason.
Included studies
Sixty‐five trials met the inclusion criteria for this review. There were nine (237 children and 241 adults) eligible clinical trials that did not contribute data to the review due to different format of presenting data than specified in the protocol or incomplete reports (Riccioni 2003; Basyigit 2004; Abadoglu 2005; Zeiger 2006; Lazarus 2007; Kanazawa 2007; Stelmach 2008; Khan 2008; Zedan 2009). The data presented hereafter pertains to only the 56 eligible trials representing total of 10,005 adults and 3,333 children with mild or moderate asthma that contributed data to meta‐analyses. Of these, most (N = 44) trials were published in full text; three were published as abstracts with additional unpublished report provided by the authors (Laitinen 1997; Hughes 1999 (FP); Hughes 1999 (BDP)) and the remaining nine citations were available only in abstract form (FLTA4031; FLTA4030; FMS40012; Dempsey 2002a; Sheth 2001; FPD40013; Jayaram 2002; NCT00442559; MK0479‐332). We described below the characteristics of the 56 trials that contributed to data analysis for this review.
Design: Sixteen paediatric and 33 adult trials had a parallel‐group design while three paediatric (Szefler 2005; Caffey 2005; Ng 2007) and four adult trials (Dempsey 2002a; Kanniess 2002; Jenkins 2005; Lu 2009) had a cross‐over design.
Participants: Thirteen trials involved children (Maspero 2001; FPD40013; Garcia Garcia 2005; Ostrom 2005; Peroni 2005; Caffey 2005; Ng 2007; Szefler 2007; Kumar 2007; Sorkness 2007; NCT00442559; Kooi 2008; Zielen 2010); five trials involved children and adolescents (Stelmach 2004; Stelmach 2005; Szefler 2005; Zeiger 2005; Stelmach 2007); one trial did not report the age of children (Peroni 2005); 19 trials involved adolescents and adults (FLTA4031; FLTA4030; Malmstrom 1999; Laviolette 1999; Bleecker 2000; Kim 2000; Nathan 2001; Busse 2001a; Busse 2001b; Meltzer 2002; Israel 2002; Brabson 2002; Baumgartner 2003; Jenkins 2005; Bousquet 2005; Koenig 2008; Lu 2009; Sheth 2001; Sheth 2001b); 10 trials involved adults (FMS40012; Riccioni 2001; Dempsey 2002a; Riccioni 2002b; Kanniess 2002; Yurdakul 2003; Overbeek 2004; Boushey 2005; Tamaoki 2008; MK0479‐332); and one trial involved adults and children (Peters 2007). Most trials described a gender ratio of 45% to 50% (range 18% to 81%) males. Almost half of the trials (N = 27) focused on asthmatics with mild airway obstruction, as defined as a baseline FEV1 ≥ 80% of predicted, 25 trials (Laitinen 1997; FLTA4031; FLTA4030; Malmstrom 1999; Laviolette 1999; Bleecker 2000; Nathan 2001; Busse 2001a; Busse 2001b; Meltzer 2002; FPD40013; Stelmach 2002a; Stelmach 2002b; Israel 2002; Kanniess 2002; Baumgartner 2003; Stelmach 2004; Stelmach 2005; Ostrom 2005; Jenkins 2005; Jayaram 2005; Kumar 2007; Koenig 2008; Lu 2009) focused on asthmatics with moderate airway obstruction, as defined as a baseline FEV1 60 to 79% of predicted; one trial reported mild to moderate airway obstruction (NCT00442559), while three trials (Jayaram 2002; Kooi 2008; MK0479‐332) did not reported the severity of airway obstruction at baseline. Asthma triggers were seldom reported, when atopy was reported. Intervention duration: Most paediatric and adult trials varied in the duration of intervention from four to eight weeks. Five paediatric trials (FPD40013; Ostrom 2005; Kumar 2007; NCT00442559; Kooi 2008) and 13 adult trials (FLTA4031; FLTA4030; Malmstrom 1999; Laviolette 1999; Bleecker 2000; Busse 2001a; Sheth 2001; Riccioni 2002a; Riccioni 2002b; Yurdakul 2003; Zeiger 2005; Peters 2007; Koenig 2008) were of 12 to 16 weeks; two paediatric trials (Maspero 2001; Stelmach 2005) and three adult trials (Busse 2001b; Meltzer 2002; MK0479‐332) were of 24 to 26 weeks; while three paediatric trials (Garcia Garcia 2005; Szefler 2007; Sorkness 2007) and two adult trials (Boushey 2005; Bousquet 2005) were of 36 to 52 weeks duration.
Intervention drugs were: montelukast 4, 5 or 10 mg per day, depending on age, for the 19 paediatric trials, montelukast 10 mg per day in 23 adult studies; pranlukast 450 mg per day in two trials (Yamauchi 2001; Tamaoki 2008), and zafirlukast 20 mg twice a day in 12 adult trials (Laitinen 1997; FLTA4031; FLTA4030; Bleecker 2000; Kim 2000; FMS40012; Nathan 2001; Riccioni 2001; Busse 2001b; Sheth 2001; Brabson 2002; Boushey 2005). One study tested two doses of anti‐leukotrienes, including a higher than licensed doses of zafirlukast (i.e. 80 mg per day) (Laitinen 1997).
The daily dose of inhaled corticosteroids (control intervention) in μg HFA‐BDP eq was relatively uniform across the 56 trial: eight trials tested a daily dose of 100 μg HFA‐BDP eq (FPD40013; Stelmach 2002a; Stelmach 2002b; Stelmach 2004; Ostrom 2005; Caffey 2005; Stelmach 2007; Tamaoki 2008); two trials tested a daily dose of 150 μg HFA‐BDP eq (Maspero 2001; Dempsey 2002a); 37 trials tested a daily dose of 200 μg HFA‐BDP eq.; two trials tested a daily dose of 250 μg HFA‐BDP eq (Jenkins 2005; Szefler 2007); three trials tested a daily dose of 400 μg HFA‐BDP eq (Riccioni 2001; Riccioni 2002a; Riccioni 2002b); two trials tested a daily dose of 500 μg HFA‐BDP eq (Jenkins 2005; MK0479‐332); and two trials did not mentioned the dose of inhaled corticosteroids (Jayaram 2002; NCT00442559). In all trials, the dose of inhaled corticosteroids was maintained throughout the intervention period; no trial tapered the dose of inhaled corticosteroids to the minimum effective dose.
Co‐intervention. No trials reported the use of additional anti‐asthmatic drugs other than rescue β2‐agonists and systemic corticosteroids.
Outcomes
Whenever possible, we considered outcomes measured at four to eight weeks, 12 to 16 weeks, 24 to 26 weeks and 36 to 52 weeks. The primary outcome, patients with at least one exacerbation requiring systemic corticosteroids, was documented in six (32%) paediatric and 15 (41%) adult trials; children contributed 23% of the weight of the main outcome. Other reported outcomes included patients with at least one exacerbation requiring admission, change from baseline FEV1 (forced expiratory volume in one second) (L), change from baseline FEV1 (%), change in FEV1 % of predicted, change in morning PEFR (peak expiratory flow rate) (L/min), change from baseline in daytime symptom scores, change from baseline in night‐time awakenings, change from baseline in mean daily use of β2‐agonists (puffs/day), change in proportion of symptom‐free days, change in rescue‐free days, change from baseline in quality of life, days with use of β2‐agonists, change from baseline in blood eosinophils, change in sputum eosinophils, leukotriene C4 concentration in nasal wash, % asthma control days during intervention period, change in PC20, % rescue‐free days, days off work or school, days with symptoms, days with β2‐agonist use (%), change in growth (cm), patient's satisfaction, physician's satisfaction, overall withdrawals, withdrawal due to poor asthma control/exacerbations, withdrawal due to adverse effects, overall adverse effects, elevated liver enzymes, upper respiratory tract infections, headache, nausea, oral candidiasis, hoarseness and death.
Risk of bias in included studies
Full details of the risk of bias for all 65 eligible trials can be found in the Characteristics of included studies tables with a graphical summary of in Figure 1.The following information pertains only to the 56 trials contributing data to the meta‐analysis.
1.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Although all trials were described as randomised, only 32 trials (10 paediatric and 22 adult trials) reported the method of randomisation. Therefore, we judged 32 trials to be at low risk of bias and 24 rest were unclear. Fifty‐two trials did not describe the method of concealment of treatment and were therefore judged at unclear risk of bias, while four trials reported the way the concealment was performed and were judged to be of low risk of bias.
Blinding
Forty‐one trials (13 paediatric and 28 adult trials) reported double‐blinding with convincing details, while nine trials used open label and six trials did not report sufficient information to ascertain blinding.
Incomplete outcome data
Forty‐two trials (14 paediatric and 28 adult trials) reported all data with balanced numbers in both groups, while seven trials failed to do so and seven trials were unclear.
Selective reporting
This bias refers to an outcome measured as part of the protocol but not reported in the publication. Judging from the reported methodology in the publication, most (N = 52) trials reported data without any apparent biasness, two trials were assessed as being at high risk of bias while the remaining two trials failed to report sufficient details for this assessment. With the similar proportion of paediatric (N = 6, 32%) and adult (N = 15, 41%) trials reporting the main outcome, there is no suspicion of a differential under reporting of this outcome in paediatric versus adult studies.
Other potential sources of bias
We did not encounter any other significant sources of bias in the included trials.
Effects of interventions
Primary outcome: people with at least one exacerbation requiring a course of oral corticosteroids
Twenty‐one (33%) trials on 6077 participants contributed to the primary end point; people with at least one exacerbation requiring systemic corticosteroids. Compared with inhaled corticosteroids, patients treated with anti‐leukotrienes had a 51% increased risk of experiencing one or more exacerbation requiring systemic corticosteroids (Risk ratio (RR) = 1.51, 95% confidence interval (CI) 1.17, 1.96; random‐effects model), that is, from 7% to 11% (Figure 2).
2.

Forest plot of comparison: 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), outcome: 1.1 Patients with at least one exacerbation requiring systemic steroids.
The number need to treat to prevent one more exacerbation requiring corticosteroid (NNT) is 28 (95% CI 15 to 82; Figure 3). There was no evidence of systematic bias identified by the test for funnel plot asymmetry (intercept 0.33, 95% CI ‐0.10 to 0.75; Figure 4). The fail‐safe N (the number of unpublished studies with null results needed to negate the current finding) was 180 trials. Selecting only one inhaled corticosteroid group (BDP or FP) as comparator in the three‐group Hughes trial fail to affect the overall estimate due to the absence of events in this study. There was heterogeneity between and within anti‐leukotrienes, the following subgroup and sensitivity analyses were performed on the main outcome to explore possible effect modifiers.
3.

In the control group (on ICS) 7 people out of 100 had at least one exacerbation requiring systemic steroids over 4 to 52 weeks, compared to 10 (95% CI 8 to 13) out of 100 for the active treatment group given LRTA.
4.

Funnel plot of comparison: 1 Anti‐leukotriene (AL) versus. Inhaled glucocorticoids (in HFC‐BDP equivalent), outcome: Patients with at least 1 exacerbation requiring systemic corticosteroids.
Subgroup analysis: Age group of subjects
Six (32%) paediatric trials on 1662 children and 15 (41%) adult trials on 4415 adults reported the primary outcome, number of patients with at least one exacerbation requiring systemic corticosteroids; children contributed 23% of the weight of the summary estimate. There was no significant group difference between paediatric (N = 6 trials, 1662 participants; RR 1.35; 95% CI 0.99 to 1.86) compared with adult, (N = 15 trials, 4415 participants; RR 1.61;95% CI 1.12 to 2.31) trials (Chi2 = 1.95 (1 df), P = 0.16; Analysis 1.1).
1.1. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 1 Patients with at least one exacerbation requiring systemic steroids.
Subgroup analysis: Anti‐leukotrienes
There was no significant difference in the risk of patients with at least one exacerbation requiring systemic corticosteroids according to the anti‐leukotriene used (montelukast versus zafirlukast) (Chi2 = 0.12 (1 df), P = 0.73); montelukast [N = 15 trials, 4352 participants: RR = 1.55 (95% CI: 1.14 to 2.12; Analysis 1.67)versus zafirlukast (N = 6 trials, 1725 participants; RR 1.92;95% CI 0.88 to 4.20).
1.67. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 67 Primary outcome ‐ stratified by anti‐leukotrienes.
Subgroup analysis: Duration of intervention
The duration of intervention was not a determinant of the magnitude of effect (Chi2 = 5.42 (3 df), P = 0.14): four to eight weeks (N = 9 trials, 2346 participants; RR 1.74; 95% CI 0.78 to 3.87; Analysis 1.68), 12 to 16 weeks (N = 7 trials, 1541 participants; RR 2.06; 95% CI 1.43 to 2.96), 24 to 26 weeks (N = 2 trials, 657 participants; RR 1.17; 95% CI 0.55 to 2.45), and 36 to 52 weeks (N = 3 trials, 1533 participants; RR = 1.29; 95% CI 0.87 to 1.91).
1.68. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 68 Primary outcome ‐ stratified by duration of intervention.
Subgroup analysis: Severity of airway obstruction
Baseline severity of airway obstruction played a significant role in the magnitude of the risk of exacerbation requiring systemic corticosteroid (Test for subgroup differences: Chi² = 4.59, (df = 1), P = 0.03, I² = 78.2%); baseline FEV1 between 60 to 79% of predicted (N = 11 trials, 3922 participants; RR = 2.03; 95% CI 1.41 to 2.91; Analysis 1.69) versus baseline FEV1 ≥ 80% of predicted (N = 10 trials, 2155; RR = 1.25; 95% CI 0.97 to 1.61).
1.69. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 69 Main outcome ‐stratified by severity of airway obstruction.
Subgroup analysis: Methodological quality
There was no significant group difference among the trials with high reported methodological quality (N = 11 trials, 4366 participants; RR = 1.62; 95% CI 1.29 to 2.03; Analysis 1.70) compared with hose with poor quality (N = 10 trials, 1695 participants; RR = 1.34; 95% CI 0.74 to 2.43) (Chi2 = 0.22 (1 df), P = 0.64).
1.70. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 70 Primary outcome ‐ stratified by methodological quality.
Subgroup analysis: Funding source
The source of funding did not significantly influence results (Chi2 = 3.61 (2 df), P = 0.16): funding from producers of inhaled corticosteroids (N = 9 trials, 2638 participants; RR = 1.71; 95% CI 1.05 to 2.80; Analysis 1.71), funding from producers of anti‐leukotrienes (N = 5 trials, 2797 participants; RR = 1.52; 95% CI 0.99 to 2.35) and no industry funding or not reported funding (N =7 trials, 642 participants; RR =1.22; 95% CI 0.90 to 1.66).
1.71. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 71 Primary outcome‐ stratified by funding source.
Subgroup analysis: HFC‐BDP equivalent
The comparative dose of inhaled corticosteroids did not significantly influence the magnitude of the risk of exacerbation requiring systemic corticosteroids (Chi2 = 1.97 (1 df), P = 0.16): 100 to 150 μg HFC‐BDP equivalent (N = 3 trials, 216 participants; RR = 0.74; 95% CI 0.26 to 2.08; Analysis 1.72), 200 to 250 μg HFC‐BDP equivalent (N = 15 trials, 5767 participants; RR = 1.75; 95% CI 1.29 to 2.38), and 400 to 500 μg HFC‐BDP equivalent (N = 3 trials, 94 participants; RR = 0.54; 95% CI 0.11 to 2.78).
1.72. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 72 Primary outcome ‐ stratified by HFC‐BDP equivalent.
Secondary outcomes reflecting the severity of asthma exacerbations
There was a three‐fold increase in the number of patients experiencing an exacerbation requiring hospital admission in the group treated with anti‐leukotrienes (N = 12 trials, 2715 participants; RR = 3.33; 95% CI: 1.02 to 10.94; Analysis 1.2) with no significant difference across the paediatric trials (N = 4 trials, 558 participants; RR = 3.04; 95% CI 0.12 to 73.93) and adult (N = 8 trials, 2157 participants; RR = 3.38; 95% CI 0.94 to 12.17) trials (Chi2 = 0.00 (1 df), P = 0.95).
1.2. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 2 Patients with at least one exacerbation requiring hospital admission.
Secondary outcomes reflecting asthma control
Lung function
Compared with inhaled corticosteroids, a significant group difference in the improvement from baseline in FEV1 (L) was observed at all points in time in disfavour of anti‐leukotrienes: at four to eight weeks (N = 12 trials, 3020 participants; mean difference (MD) ‐0.12 L; 95% CI ‐0.15 to ‐0.08, random‐effects model; Analysis 1.3), at 12 to 16 weeks (N = 9 trials, 1890 participants; MD ‐0.12 L; 95% CI ‐0.20 to ‐0.04, random‐effects model; Analysis 1.4), and at 24 to 26 weeks (N = 3 trials, 1178 participants; MD ‐0.13 L; 95% CI ‐0.22 to ‐0.04; random‐effects model; Analysis 1.5), at 36 to 52 weeks (N = 2 paediatric trials, 1040 participants; MD ‐0.03 L; 95% CI ‐0.07 to 0.00; model; Analysis 1.6). Similarly, a significant group difference in the per cent change from baseline FEV1 was observed in time in disfavour of anti‐leukotrienes: at 12 to 16 weeks (N = 2 adults trials, 603 participants; MD ‐5.70, 95% CI ‐9.81 to ‐1.59; Analysis 1.10) and at 24 to 26 weeks (N = 2 adult trials, 838 participants; MD ‐8.20%; 95% CI ‐10.85 to ‐5.55; Analysis 1.11), and only one trial reporting data at four to eight weeks and at 36 to 52 weeks each, precluding aggregation.
1.3. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 3 Change from baseline FEV1 (L) at 4 ‐ 8 weeks.
1.4. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 4 Change from baseline FEV1( L) 12 ‐ 16 weeks.
1.5. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 5 Change from baseline FEV1 (L) at 24 ‐ 26 weeks.
1.6. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 6 Change from baseline FEV1 (L) at 36 ‐ 52 weeks.
1.10. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 10 Change from baseline FEV1 (%) 12 ‐ 16 weeks.
1.11. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 11 Change from baseline FEV1 (%) at 24 ‐ 26 weeks.
No significant group difference was observed with regards to the change from baseline FEV1 % predicted at four to eight weeks (N = 2 trials, 219 participants; MD ‐2.58%; 95% CI ‐6.56 to 1.40; random‐effects model; Analysis 1.12), but a significant group difference was observed in disfavour of anti‐leukotrienes at 12 to 16 weeks (N = 3 trials, 948 participants; MD ‐3.76%; 95% CI ‐5.01 to ‐2.50; Analysis 1.13) and at 36 to 52 weeks (N = 3 paediatric trials, 1229 participants; MD ‐3.51%; 95% CI ‐7.14 to 0.12; random‐effects model; Analysis 1.14).
1.12. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 12 Change from baseline FEV1 % of predicted at 4 ‐ 8 weeks.
1.13. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 13 Change from baseline FEV1 % of predicated at 12 ‐ 16 weeks.
1.14. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 14 Change from baseline FEV1 % of predicated at 36 ‐ 52 weeks.
A significant group difference in the change from baseline morning PEFR in disfavour of anti‐leukotrienes was observed at four to eight weeks (N = 8 trials, 1926 participants; MD ‐15.12 L; 95% CI ‐20.80 to ‐9.44; random‐effects model; Analysis 1.15), at 12 to 16 weeks (N = 10 trials, 2713 participants; MD ‐19.07 L; 95% CI ‐25.86 to ‐12.27; random‐effects model; Analysis 1.16), 24 to 26 weeks (N = 3 trials, 1718 participants; MD ‐21.62 L; 95% CI ‐40.19 to ‐3.05; random‐effects model; Analysis 1.17) and at 36 to 52 weeks (N = 4 trials, 1652 participants; MD ‐5.34 L; 95% CI ‐9.35 to ‐1.34; random‐effects model; Analysis 1.18).
1.15. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 15 Change from baseline AM PEFR (L/min) at 4 ‐ 8 weeks.
1.16. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 16 Change from baseline AM PEFR (L/min) at 12 ‐ 16 weeks.
1.17. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 17 Change from baseline AM PEFR (L/min) at 24 ‐ 26 weeks.
1.18. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 18 Change from baseline AM PEFR (L/min) at 36 ‐ 52 weeks.
Symptom scores
A significant group difference in the improvement from baseline daytime symptom scores in favour of inhaled corticosteroids were observed at four to eight weeks (N = 6 trials, 1925 participants; standard mean difference (SMD) 0.20; 95% CI 0.08 to 0.32; random‐effects model; Analysis 1.19), at 24 to 26 weeks (N = 3 adult trials, 1719 participants; (SMD 0.22; 95% CI 0.02 to 0.42)SMD 0.25; 95% CI 0.18 to 0.33; random‐effects model; Analysis 1.21), but not at 12 to 16 weeks (N = 9 trials, 2650 participants; (SMD 0.25; 95% CI 0.18 to 0.33); random‐effects model; Analysis 1.20) or 36 to 52 weeks (N = 2 paediatric trials, 582 participants; SMD 0.16 95% CI ‐0.02 to 0.34; random‐effects model; Analysis 1.22).
1.19. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 19 Change from baseline daytime symptom scores at 4 ‐ 8 weeks.
1.21. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 21 Change from baseline daytime symptom scores at 24 ‐ 26 weeks.
1.20. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 20 Change from baseline daytime symptom scores at 12 ‐ 16 weeks.
1.22. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 22 Change from baseline daytime symptom scores at 36 ‐ 52 weeks.
Nightime awakening
A significant group difference in favour of inhaled corticosteroids was observed for change from baseline night‐time awakenings at 12 to 16 weeks (N = 9 adult trials, 2916 participants; SMD 0.18, 95% CI 0.11 to 0.26; Analysis 1.24), at 24 to 26 weeks (N = 2 trials, 1055 participants; SMD 0.23; 95% CI 0.11 to 0.35; Analysis 1.25), but not at four to eight weeks (N = 3 adult trials, 798 participants; SMD = 0.22; 95% CI ‐0.02 to 0.46; random‐effects model; Analysis 1.23); only one trial reported at 36 to 52 weeks, precluding aggregation.
1.24. Analysis.
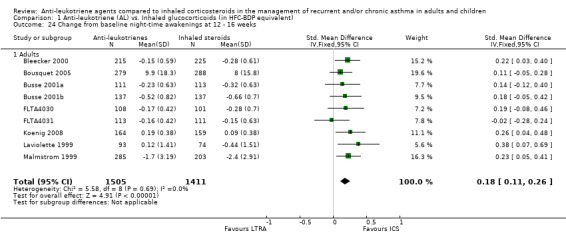
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 24 Change from baseline night‐time awakenings at 12 ‐ 16 weeks.
1.25. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 25 Change from baseline night‐time awakenings at 24 ‐ 26 weeks.
1.23. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 23 Change from baseline night‐time awakenings at 4 ‐ 8 week.
Rescue medication use
A significant group difference in the reduction from baseline mean daily use of β2‐agonists in favour of inhaled corticosteroids as observed at four to eight weeks (N = 10 trials, 3264 participants; SMD 0.20; 95% CI 0.07 to 0.34; random‐effects model; Analysis 1.26), at 12 to 16 weeks (N = 12 trials, 3479 participants; SMD 0.23; 95% CI 0.17 to 0.30; Analysis 1.27), at 24 to 26 weeks (N = 2 adult trials, 1055 participants; SMD 0.31; 95% CI 0.19 to 0.43; Analysis 1.28), only one paediatric trial reporting data at 36 to 52 weeks, precluding aggregation.
1.26. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 26 Change from baseline mean daily use of β2‐agonists at 4 ‐ 8 weeks.
1.27. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 27 Change from baseline mean daily use of β2‐agonists at 12 ‐ 16 weeks.
1.28. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 28 Change from baseline mean daily use of β2‐agonists at 24 ‐ 26 weeks.
No significant difference was observed in change from baseline rescue‐free days (%) at four to eight weeks (N = 5 trials, 1315 participants; MD ‐6.83 %; 95% CI ‐17.73 to 4.07; random‐effects model; Analysis 1.30), but a significant group difference was observed in disfavour of anti‐leukotrienes: at 12 to 16 weeks (N = 7 trials, 2304 participants; MD ‐9.64 %; 95% CI ‐13.71 to ‐5.56; random‐effects model; Analysis 1.31) and at 36 to 52 weeks (N = 3 trials, 1949 participants; MD ‐3.38 %; 95% CI ‐5.49 to ‐1.27; Analysis 1.33). Only one trial reported data at 24 to 26 weeks, precluding aggregation.
1.30. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 30 Change in rescue‐free days (%) at 4 ‐ 8 weeks.
1.31. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 31 Change in rescue‐free days (%) at 12 ‐ 16 weeks.
1.33. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 33 Change in rescue‐free days (%) at 36 ‐ 52 weeks.
Symptom‐free days
A significant group difference was observed in change in proportion of symptom‐free days (%) at all points in time in disfavour of anti‐leukotrienes at four to eight weeks (N = 3 adult trials, 1154 participants; MD ‐10.46%; 95% CI ‐14.56 to ‐6.36; Analysis 1.34), at 12 to 16 weeks (N = 9 trials, 2535 participants; MD ‐8.89%; 95% CI ‐11.92 to ‐5.87; Analysis 1.35), and at 36 to 52 weeks (N = 4 trials, 1805 participants; MD ‐5.71%; 95% CI ‐8.68 to ‐2.74; Analysis 1.36). Only one trial reported data at 24 to 26 weeks, precluding aggregation.
1.34. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 34 Change in proportion of symptom‐free days (%) at 4 ‐ 8 weeks.
1.35. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 35 Change in proportion of symptom‐free days (%) at 12 ‐ 16 weeks.
1.36. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 36 Change in proportion of symptom‐free days (%) at 24 ‐ 26 weeks.
Quality of life
A significant group difference was observed in the change in quality of life at all points in time in disfavour of anti‐leukotrienes: at 12 to 16 weeks (N = 2 adult trials, 1065 participants; MD ‐0.21; 95% CI ‐0.34 to ‐0.09; Analysis 1.39), at 24 to 26 weeks (N = 2 adult trials, 1028 participants; MD ‐0.38; 95% CI ‐0.54 to ‐0.21; Analysis 1.40), at 36 to 52 weeks (N = 2 trials, 1034; MD ‐0.19; 95% CI ‐0.31 to ‐0.07; Analysis 1.41). Only one trial reported data at four to eight weeks, precluding aggregation. Only one trial reported data on days with use of β2‐agonists at 36 to 52 weeks; Analysis 1.56, precluding aggregation.
1.39. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 39 Change from baseline quality of life (QOL) at 12 ‐ 16 weeks.
1.40. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 40 Change from baseline quality of life (QOL) at 24 ‐ 26 weeks.
1.41. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 41 Change from baseline quality of life (QOL) at 36 ‐ 52 weeks.
Airway inflammation
Although few trials examined indices of airway inflammation, there was a significant group difference in the reduction in blood eosinophils in favour of inhaled corticosteroids at four to eight weeks (N = 4 trials, 1294 participants; MD 0.06 x 109, 95% CI 0.02 to 0.10; random‐effects model; Analysis 1.43) but not at 12 to 16 weeks (N = 2 trials, 1013 participants; MD ‐0.0 x 109, 95% CI ‐0.03 to 0.02; Analysis 1.44) and only one trial reported at 36 to 52 weeks. However, no significant group difference was observed in sputum eosinophils at four to eight weeks (N = 2 trials, 117 participants; MD 0.71 x 109; 95% CI ‐2.06 to 3.47; random‐effects model; Analysis 1.45) and only one trial reported at 36 to 52 weeks. One paediatric trial examined the change in LTC4 concentration in nasal washes with no group difference observed at 12 to 24 weeks; Analysis 1.47.
1.43. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 43 Change from baseline blood eosinophils at 4 ‐ 8 weeks.
1.44. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 44 Change from baseline blood eosinophils at 12 ‐ 16 weeks.
1.45. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 45 % Change in sputum eosinophils at 4 ‐ 8 weeks.
1.47. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 47 LTC4 concentration (ng/mL) in nasal wash at 24 ‐ 26 weeks.
Asthma control days
Few trials evaluated the percentage of asthma control days during the intervention period; it was in disfavour of anti‐leukotrienes at four to eight weeks (N = 2 trials, 1293 participants; MD ‐5.72%; 95% CI ‐10.86 to ‐0.59; Analysis 1.48) and at 24 to 26 weeks (N = 2 trials, 1185 participants; MD ‐8.19%; 95% CI ‐19.46 to 3.07; random‐effects model; Analysis 1.49).
1.48. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 48 % Asthma control days during intervention period at 4 ‐ 8 weeks.
1.49. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 49 % Asthma control days during intervention period at 24 ‐ 26 weeks.
Bronchial challenge (PC20)
Only one trial reported PC20 at four to eight weeks, Analysis 1.50, precluding aggregation.
1.50. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 50 Change in PC20 at 4 ‐ 8 weeks.
There was no significant group difference in days off work or school at 24 to 26 weeks (N = 2 trials, 606 participants; MD 0.12; 95% CI ‐0.01 to 0.26; Analysis 1.52). Only one trial reported patient and physician's level of satisfaction, precluding aggregation.
1.52. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 52 Days off work or school at 24 ‐ 26 weeks.
Only one study (Sorkness 2007) reported change in height in paediatric patients at 48 weeks (Analysis 1.53).
1.53. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 53 Change in height (cm).
Withdrawals
Anti‐leukotriene therapy was associated with a 24% increased risk of overall withdrawals (N = 43 trials, 11,317 participants; RR 1.22; 95% CI 1.09 to 1.37; random‐effects model; Analysis 1.56) (Chi2 = 3.07 (1 df), P = 0.08).The withdrawals appeared to be attributable to an increased risk of withdrawals due to poor asthma control (N = 26 trials, 7669 participants; RR 2.56; 95% CI 2.01 to 3.27; P < 0.00001; Analysis 1.57) and with no statistically significant group difference due to adverse effects (N = 25 trials, 8518 participants, RR 1.24; 95% CI 0.95 to 1.63; P = 0.12; Analysis 1.58). Of note, there was no significant effect of age group on these withdrawal rates. The number needed to treat to harm (NNTH), that is, to observe one withdrawal due to poor asthma control is 31 (95% CI 22 to 47); in other words, 31 patients need to be treated with anti‐leukotrienes rather than inhaled corticosteroids to observe one more withdrawal due to poor asthma control (Figure 5).
1.56. Analysis.
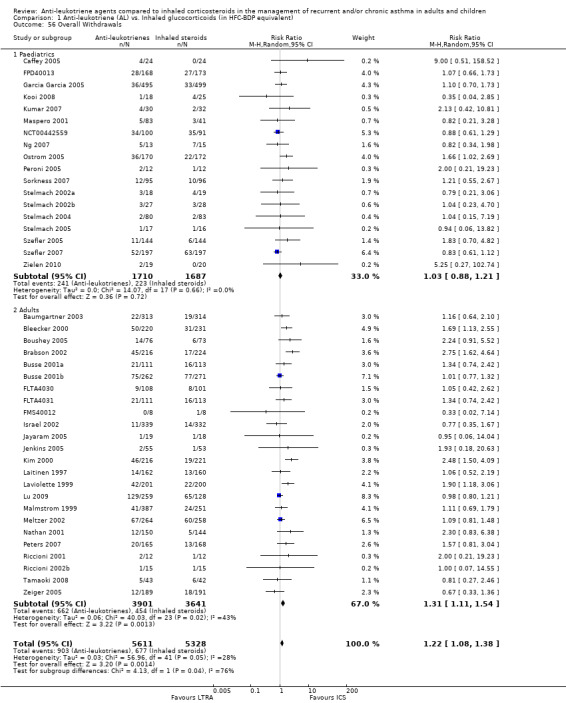
Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 56 Overall Withdrawals.
1.57. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 57 Withdrawal due to poor asthma control/exacerbations.
1.58. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 58 Withdrawals due to adverse effects.
5.

In the control group (on ICS) 2 people out of 100 had withdrawal due to poor control over 4 to 52 weeks, compared to 6 (95% CI 4 to 7) out of 100 for the active treatment group (given LRTA).
Adverse effects
There was no significant group difference in the number of patients who experienced "any adverse effects", (N = 22 trials, 7818 participants; RR 1.00; 95% CI 0.95 to 1.05; P = 0.90; Analysis 1.59), which met our definition of equivalence. There was also no significant group difference in elevation of liver enzymes (N = 7 trials, 1716 participants; RR 1.13; 95% CI 0.58 to 2.19; Analysis 1.60), upper respiratory infections (N = 8 trial, 2729 participants; RR 1.04; 95% CI 0.84 to 1.29; Analysis 1.61), headache (N = 24 trials, 8872 participants; RR 0.99; 95% CI 0.89 to 1.11; Analysis 1.62), nausea (N= 17 trials, 5563 participants; RR 0.83; 95% CI 0.64 to 1.08; Analysis 1.63), oral candidiasis (N = 3 trials, 865 participants; RR 0.25; 95% CI 0.05 to 1.19; Analysis 1.64), or death (N= 13 trials, 5489 participants; RR 3.05; 95% CI 0.32 to 29.26; Analysis 1.66) which was reported in only two trials both in anti‐leukotriene group. Only one trial reported growth in paediatric patients and the change in height between two groups could not achieve a statistical significant level Analysis 1.53; Sorkness 2007).
1.59. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 59 Overall Adverse effects.
1.60. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 60 Elevated liver enzymes.
1.61. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 61 Upper respiratory tract infections.
1.62. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 62 Headache.
1.63. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 63 Nausea.
1.64. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 64 Oral candidiasis.
1.66. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 66 Death.
Discussion
In adults and children with mild to moderate airway obstruction due to persistent asthma, four to 52 weeks of treatment with daily oral anti‐leukotrienes carries a 51% increased risk (from 7% to 11%) of an asthma exacerbation requiring systemic corticosteroids than treatment with inhaled corticosteroids at a median dose of 200 HFA‐BDP eq. Twenty‐eight people need to be treated with inhaled corticosteroids rather than anti‐leukotriene to prevent one person from experiencing an exacerbation requiring corticosteroid i.e. the NNT is 28. These data appear robust as 180 new trials with no group difference would be needed to reverse these findings.
The baseline severity of asthma obstruction significantly influenced the magnitude of response; indeed, the risk of exacerbation requiring rescue systemic steroids was increased by 'two‐fold' in patients with moderate airway obstruction treated with anti‐leukotrienes rather than inhaled corticosteroids, while the risk was increased by 25% in patients with mild asthma obstruction. The magnitude of the risk was not significantly influenced by age, choice of anti‐leukotrienes, duration of intervention, publication status, methodological quality, funding source and dose of inhaled corticosteroids in HFA‐BDP eq.
Secondary outcomes clearly favoured the use of inhaled corticosteroids over anti‐leukotriene agents in adults and children. There was a three‐fold increase in the risk of patients experiencing an exacerbation requiring hospital admission when treated with anti‐leukotriene agents compared with inhaled corticosteroids. With regards to other indicators of asthma control, inhaled corticosteroids were more effective than anti‐leukotrienes in improving lung function (FEV1 and PEFR), the percentage of symptom‐free and rescue‐free days, as well as in reducing symptoms, night‐time awakenings and rescue β2‐agonist use. These group differences were generally present at all points in time over four to 52 weeks of treatment.
The risk of overall adverse effects was similar in both treatment groups, meeting our a priori definition of equivalence. There was also no group difference in the following specific adverse effects, namely liver enzyme elevation, headaches, upper respiratory infections, oral candidiasis, nausea, and death. Anti‐leukotriene use was not associated with an increased risk of withdrawals due to adverse effects. However, adverse effects typically associated with inhaled corticosteroids such as growth suppression (in children), osteopenia and adrenal suppression were seldom measured except in one trial (Sorkness 2007, in which growth was measured), thus preventing a fair comparison of the safety of long‐term use of inhaled corticosteroids versus anti‐leukotrienes.
The increased risk of all‐cause withdrawals was significantly higher among patients treated with anti‐leukotriene agents as compared to inhaled corticosteroids. Most of the increased risk seems attributable to increased withdrawals due to poor asthma control with the use of anti‐leukotrienes which indirectly supports the superiority of inhaled corticosteroids over anti‐leukotrienes.
The results of this review pertain to asthmatic adults and children with a mild or moderate persistent asthma. The results can apply to school‐aged children; 19 trials with paediatric and adolescents contributed data to the review of which six trials (23% of the weight of the summary estimate) contributed to the primary outcome. The relatively modest number of trials could fall in the priori defined category of high methodological quality indicates either poor designing of trials or inadequate reporting of methodology. More long‐term trials are needed to compare the safety of anti‐leukotrienes versus inhaled corticosteroids as monotherapy in the treatment of paediatric asthma, as only one trial reported growth or effect of long‐term administration of inhaled corticosteroids in paediatrics.
This review summarises the best evidence available until December 2010. With a total of 56 trials (37 adult and 19 paediatric), it represents a significant update from the previous update in August 2003 which was based on 25 trials (22 adult and three paediatric). With 16 more paediatric trials than in the prior review, the current data more adequately represents children; paediatric trials represent now 23% of weight of the primary outcome compared with 6.7% in the prior review. While the results remain admittedly notably influenced by adults, the absence of a significant group difference between adults and children would support that the conclusion apply to both age groups. Twenty‐one (38%) of 56 trials contributing data to the meta‐analysis were of high reported methodological quality as per our predefined criteria using the Cochrane Classification 'Risk of bias' tool (29% of paediatric and 71% of adult trials); the impact of the large proportion of lower reported methodological quality on study results is unclear but could have led to an overestimation of the true effect. On the other hand, the robustness of the study results is supported by the fail‐safe N of 180 trials indicating the number of unpublished/future studies with no group difference in rescue oral corticosteroids needed to change the direction of the current findings. Consequently, in line with the previous version, the present review confirms the greater efficacy of inhaled corticosteroids administered at a median dose of 200 HFA‐BDP eq over anti‐leukotrienes in children and adult with mild and moderate persistent asthma.
Authors' conclusions
Implications for practice.
In symptomatic adults and children with mild or moderate asthma, anti‐leukotrienes are less effective than inhaled corticosteroids at a median dose of 200 HFA‐BDP eq for preventing exacerbations and achieving asthma control; the superiority of ICS is particularly marked in patients with moderateversus mild airway obstruction but does not appear influenced by age, duration of intervention, or anti‐leukotriene used. The use of anti‐leukotrienes is associated with a 51% increased risk of experiencing an exacerbation requiring systemic corticosteroids, a three‐fold increased hospital admission rate and more than a two‐fold increased risk of withdrawals due to poor asthma control compared to inhaled corticosteroids. The superiority of inhaled corticosteroids was also observed in lung function, symptoms, use of rescue β2‐agonists, quality of life, inflammatory markers and withdrawals including withdrawals due to poor asthma control. Although anti‐leukotrienes have a similar safety profile to that of inhaled corticosteroids, one must note that adverse effects typically associated with inhaled corticosteroids such as growth suppression (in children), osteopenia and adrenal suppression have not been measured in these trials. On the basis of efficacy, the results support the current guidelines' recommendation that inhaled corticosteroids remain the preferred monotherapy in adults and children with persistent asthma.
Implications for research.
There is little need for additional efficacy studies in this area, other perhaps than to determine the exact dose‐equivalence of anti‐leukotrienes, which is clearly less than 200 μg/day of HFA‐BDP eq or to compare the safety profile (on growth) in children. Acknowledging the low and potential differential adherence rate to daily controller therapies, one area of interest is certainly examining the real‐life effectiveness of low‐dose daily inhaled corticosteroids compared to anti‐leukotrienes,
Long‐term high methodological quality trials with adequate documentation of adverse effects associated with inhaled corticosteroids are needed to provide a fair comparison of the safety of both treatment options. Future trials should aim for the following design characteristics:
pragmatic effectiveness trials;
double blinding, adequate randomisation and complete reporting of withdrawals and drop outs with intention‐to‐treat analysis;
parallel‐group;
have a minimal intervention period of 24 to 52 weeks to assess the long‐term side effects of both interventions (anti‐leukotrienes and inhaled corticosteroids);
complete reporting of continuous (denominators, mean change and mean standard deviation of change) and dichotomous (denominators and rate) data;
specific reporting of exacerbations requiring systemic corticosteroids;
systematic documentation of reasons for withdrawals and adverse effects, including those associated with inhaled corticosteroids such as oral candidiasis, osteopenia, adrenal suppression, growth suppression, etc; and
compare different anti‐leukotriene agents (synthesis inhibitor and receptor antagonists).
Feedback
Data entry error
Summary
I believe there may be an error in Table 1.13. The numbers for Ostrom 2005 are exactly the same as in Table 1.10.
Reply
Response from Cochrane Airways editorial base: Thank you for bringing this error to our attention. The duplicate data has been removed from analysis 1.10.1 and the text updated in the results section to reflect this change. The impact on the results of this analysis is negligible.
Contributors
Stephanie Weinreich
Academic Medical Center, Amsterdam, The Netherlands
What's new
| Date | Event | Description |
|---|---|---|
| 8 December 2014 | Feedback has been incorporated | Feedback incorporated and typos corrected |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 3, 2000
| Date | Event | Description |
|---|---|---|
| 31 December 2010 | New search has been performed | New literature search run. |
| 31 December 2010 | New citation required but conclusions have not changed | 29 new studies included. Risk of bias has been updated across all studies. |
| 30 June 2008 | New search has been performed | Converted to new review format with added information. |
| 17 October 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We wish to thank Franco Di Salvio and Giselle Hicks for their participation in the assessment of methodology and data extraction, and diligent data entry in 2003 update. We are indebted to the following individuals who replied to our request for confirmation of methodology and data extraction, and graciously provided additional data whenever possible: Christopher Miller and Susan Shaffer from Astra‐Zeneca, USA in 2003; Ian Naya and Roger Metcalf for Astra‐Zeneca, Sweden; Theodore F Reiss and GP Noonan from Merck Frosst, USA; Frank Kanniess from the Pulmonary Research Institute, Germany; and Graziano Riccioni, Italy, Sept‐Oct 2003 . We are indebted to the Cochrane Airways Review Group, namely Toby Lasserson, Karen Blackhall, Dr Emma Welsh and Elizabeth Stovold for the literature search and ongoing support, and Paul Jones and Christopher Cates for their constructive comments. A special thanks to Mrs Anne James from the Consumer group for writing the original synopsis.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| CENTRAL (the Cochrane Library) | Quarterly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Hand‐searches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Data and analyses
Comparison 1. Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with at least one exacerbation requiring systemic steroids | 21 | 6077 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.17, 1.96] |
| 1.1 Paediatrics | 6 | 1662 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.99, 1.86] |
| 1.2 Adults | 15 | 4415 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [1.12, 2.31] |
| 2 Patients with at least one exacerbation requiring hospital admission | 12 | 2715 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [1.02, 10.94] |
| 2.1 Paediatrics | 4 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.12, 73.98] |
| 2.2 Adults | 8 | 2157 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.38 [0.94, 12.17] |
| 3 Change from baseline FEV1 (L) at 4 ‐ 8 weeks | 12 | 3020 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.15, ‐0.08] |
| 3.1 Paediatrics | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.69, 0.13] |
| 3.2 Adults | 11 | 2964 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.15, ‐0.08] |
| 4 Change from baseline FEV1( L) 12 ‐ 16 weeks | 8 | 1778 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.20, ‐0.04] |
| 4.1 Paediatrics | 2 | 179 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.13, 0.09] |
| 4.2 Adults | 6 | 1599 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.24, ‐0.05] |
| 5 Change from baseline FEV1 (L) at 24 ‐ 26 weeks | 3 | 1178 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.22, ‐0.04] |
| 5.1 Paediatrics | 1 | 123 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.14, 0.12] |
| 5.2 Adults | 2 | 1055 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.23, ‐0.11] |
| 6 Change from baseline FEV1 (L) at 36 ‐ 52 weeks | 2 | 1040 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.07, 0.00] |
| 6.1 Paediatrics | 2 | 1040 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.07, 0.00] |
| 7 FEV1 irrespective of time of treatment | 23 | 7016 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.14, ‐0.08] |
| 7.1 Paediatrics | 4 | 1398 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.07, 0.00] |
| 7.2 Adults | 19 | 5618 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.16, ‐0.09] |
| 8 Responders (defined as change from baseline in FEV1 >= 7.5% | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 9 Change from baseline FEV1 (%) at 4 ‐ 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Change from baseline FEV1 (%) 12 ‐ 16 weeks | 2 | 603 | Mean Difference (IV, Fixed, 95% CI) | ‐5.70 [‐9.81, ‐1.59] |
| 10.1 Adults | 2 | 603 | Mean Difference (IV, Fixed, 95% CI) | ‐5.70 [‐9.81, ‐1.59] |
| 11 Change from baseline FEV1 (%) at 24 ‐ 26 weeks | 2 | 838 | Mean Difference (IV, Fixed, 95% CI) | ‐8.20 [‐10.85, ‐5.55] |
| 11.1 Adults | 2 | 838 | Mean Difference (IV, Fixed, 95% CI) | ‐8.20 [‐10.85, ‐5.55] |
| 12 Change from baseline FEV1 % of predicted at 4 ‐ 8 weeks | 2 | 219 | Mean Difference (IV, Random, 95% CI) | ‐2.58 [‐6.56, 1.40] |
| 12.1 Paediatrics | 1 | 183 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐4.82, 3.74] |
| 12.2 Adults | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐4.6 [‐8.86, ‐0.34] |
| 13 Change from baseline FEV1 % of predicated at 12 ‐ 16 weeks | 3 | 948 | Mean Difference (IV, Fixed, 95% CI) | ‐3.76 [‐5.01, ‐2.50] |
| 13.1 Paediatrics | 1 | 335 | Mean Difference (IV, Fixed, 95% CI) | ‐6.02 [‐9.45, ‐2.59] |
| 13.2 Adults | 2 | 613 | Mean Difference (IV, Fixed, 95% CI) | ‐3.41 [‐4.76, ‐2.06] |
| 14 Change from baseline FEV1 % of predicated at 36 ‐ 52 weeks | 3 | 1229 | Mean Difference (IV, Random, 95% CI) | ‐3.51 [‐7.14, 0.12] |
| 14.1 Paediatrics | 3 | 1229 | Mean Difference (IV, Random, 95% CI) | ‐3.51 [‐7.14, 0.12] |
| 15 Change from baseline AM PEFR (L/min) at 4 ‐ 8 weeks | 8 | 1926 | Mean Difference (IV, Random, 95% CI) | ‐15.12 [‐20.80, ‐9.44] |
| 15.1 Paediatrics | 1 | 332 | Mean Difference (IV, Random, 95% CI) | ‐7.76 [‐13.43, ‐2.09] |
| 15.2 Adults | 7 | 1594 | Mean Difference (IV, Random, 95% CI) | ‐17.63 [‐22.56, ‐12.69] |
| 16 Change from baseline AM PEFR (L/min) at 12 ‐ 16 weeks | 9 | 2601 | Mean Difference (IV, Random, 95% CI) | ‐19.07 [‐25.86, ‐12.27] |
| 16.1 Paediatrics | 1 | 335 | Mean Difference (IV, Random, 95% CI) | ‐16.9 [‐28.54, ‐5.26] |
| 16.2 Adults | 8 | 2266 | Mean Difference (IV, Random, 95% CI) | ‐19.57 [‐27.27, ‐11.87] |
| 17 Change from baseline AM PEFR (L/min) at 24 ‐ 26 weeks | 3 | 1718 | Mean Difference (IV, Random, 95% CI) | ‐21.62 [‐40.19, ‐3.05] |
| 17.1 Adults | 3 | 1718 | Mean Difference (IV, Random, 95% CI) | ‐21.62 [‐40.19, ‐3.05] |
| 18 Change from baseline AM PEFR (L/min) at 36 ‐ 52 weeks | 3 | 1028 | Mean Difference (IV, Random, 95% CI) | ‐5.06 [‐10.58, 0.45] |
| 18.1 Adults | 3 | 1028 | Mean Difference (IV, Random, 95% CI) | ‐5.06 [‐10.58, 0.45] |
| 19 Change from baseline daytime symptom scores at 4 ‐ 8 weeks | 6 | 1925 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [0.08, 0.32] |
| 19.1 Paediatrics | 1 | 393 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.14, 0.26] |
| 19.2 Adults | 5 | 1532 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [0.11, 0.36] |
| 20 Change from baseline daytime symptom scores at 12 ‐ 16 weeks | 9 | 2650 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [0.18, 0.33] |
| 20.1 Paediatrics | 2 | 388 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.28 [0.08, 0.48] |
| 20.2 Adults | 7 | 2262 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [0.16, 0.33] |
| 21 Change from baseline daytime symptom scores at 24 ‐ 26 weeks | 3 | 1719 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.02, 0.42] |
| 21.1 Adults | 3 | 1719 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.02, 0.42] |
| 22 Change from baseline daytime symptom scores at 36 ‐ 52 weeks | 2 | 582 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.02, 0.34] |
| 22.1 Paediatrics | 2 | 582 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.02, 0.34] |
| 23 Change from baseline night‐time awakenings at 4 ‐ 8 week | 3 | 798 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.02, 0.46] |
| 23.1 Adults | 3 | 798 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.02, 0.46] |
| 24 Change from baseline night‐time awakenings at 12 ‐ 16 weeks | 9 | 2916 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [0.11, 0.26] |
| 24.1 Adults | 9 | 2916 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [0.11, 0.26] |
| 25 Change from baseline night‐time awakenings at 24 ‐ 26 weeks | 2 | 1055 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.23 [0.11, 0.35] |
| 25.1 Adults | 2 | 1055 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.23 [0.11, 0.35] |
| 26 Change from baseline mean daily use of β2‐agonists at 4 ‐ 8 weeks | 10 | 3264 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [0.07, 0.34] |
| 26.1 Paediatrics | 1 | 393 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.22, 0.17] |
| 26.2 Adults | 9 | 2871 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.10, 0.38] |
| 27 Change from baseline mean daily use of β2‐agonists at 12 ‐ 16 weeks | 11 | 3367 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.23 [0.17, 0.30] |
| 27.1 Paediatrics | 1 | 335 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.20, 0.23] |
| 27.2 Adults | 10 | 3032 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [0.19, 0.33] |
| 28 Change from baseline mean daily use of β2‐agonists at 24 ‐ 26 weeks | 2 | 1055 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [0.19, 0.43] |
| 28.1 Adults | 2 | 1055 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [0.19, 0.43] |
| 29 Change from baseline mean daily use of β2‐agonists at 36 ‐ 52 weeks | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 29.1 Paediatrics | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 30 Change in rescue‐free days (%) at 4 ‐ 8 weeks | 5 | 1315 | Mean Difference (IV, Random, 95% CI) | ‐6.83 [‐17.73, 4.07] |
| 30.1 Paediatrics | 1 | 393 | Mean Difference (IV, Random, 95% CI) | 2.72 [‐3.11, 8.55] |
| 30.2 Adults | 4 | 922 | Mean Difference (IV, Random, 95% CI) | ‐13.25 [‐18.11, ‐8.39] |
| 31 Change in rescue‐free days (%) at 12 ‐ 16 weeks | 7 | 2304 | Mean Difference (IV, Random, 95% CI) | ‐9.64 [‐13.71, ‐5.56] |
| 31.1 Paediatrics | 1 | 335 | Mean Difference (IV, Random, 95% CI) | ‐10.10 [‐18.97, ‐1.23] |
| 31.2 Adults | 6 | 1969 | Mean Difference (IV, Random, 95% CI) | ‐9.64 [‐14.39, ‐4.89] |
| 32 Change in rescue‐free days (%) at 24 ‐ 26 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 32.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 33 Change in rescue‐free days (%) at 36 ‐ 52 weeks | 2 | 1350 | Mean Difference (IV, Fixed, 95% CI) | ‐2.59 [‐4.97, ‐0.21] |
| 33.1 Paediatrics | 2 | 1350 | Mean Difference (IV, Fixed, 95% CI) | ‐2.59 [‐4.97, ‐0.21] |
| 33.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Change in proportion of symptom‐free days (%) at 4 ‐ 8 weeks | 3 | 1154 | Mean Difference (IV, Fixed, 95% CI) | ‐10.46 [‐14.56, ‐6.36] |
| 34.1 Adults | 3 | 1154 | Mean Difference (IV, Fixed, 95% CI) | ‐10.46 [‐14.56, ‐6.36] |
| 35 Change in proportion of symptom‐free days (%) at 12 ‐ 16 weeks | 8 | 2423 | Mean Difference (IV, Fixed, 95% CI) | ‐8.89 [‐11.92, ‐5.87] |
| 35.1 Paediatrics | 1 | 335 | Mean Difference (IV, Fixed, 95% CI) | ‐6.40 [‐15.82, 3.02] |
| 35.2 Adults | 7 | 2088 | Mean Difference (IV, Fixed, 95% CI) | ‐9.18 [‐12.38, ‐5.98] |
| 36 Change in proportion of symptom‐free days (%) at 24 ‐ 26 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 36.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 37 Change in proportion of symptom‐free days (%) at 36 ‐ 52 weeks | 3 | 1190 | Mean Difference (IV, Fixed, 95% CI) | ‐5.49 [‐9.06, ‐1.91] |
| 37.1 Paediatrics | 2 | 575 | Mean Difference (IV, Fixed, 95% CI) | ‐4.90 [‐9.73, ‐0.08] |
| 37.2 Adults | 1 | 615 | Mean Difference (IV, Fixed, 95% CI) | ‐6.20 [‐11.53, ‐0.87] |
| 38 Change from baseline quality of life (QOL) at 4 ‐ 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 38.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 39 Change from baseline quality of life (QOL) at 12 ‐ 16 weeks | 2 | 1065 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.34, ‐0.09] |
| 39.1 Adults | 2 | 1065 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.34, ‐0.09] |
| 40 Change from baseline quality of life (QOL) at 24 ‐ 26 weeks | 2 | 1028 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.54, ‐0.21] |
| 40.1 Adults | 2 | 1028 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.54, ‐0.21] |
| 41 Change from baseline quality of life (QOL) at 36 ‐ 52 weeks | 1 | 541 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.33, 0.07] |
| 41.1 Paediatrics | 1 | 541 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.33, 0.07] |
| 41.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 42 Days with use of β2‐agonists at 36 ‐ 52 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 42.1 Paediatrics | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 43 Change from baseline blood eosinophils at 4 ‐ 8 weeks | 4 | 1294 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.02, 0.10] |
| 43.1 Adults | 4 | 1294 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.02, 0.10] |
| 44 Change from baseline blood eosinophils at 12 ‐ 16 weeks | 2 | 1013 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.03, 0.02] |
| 44.1 Adults | 2 | 1013 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.03, 0.02] |
| 45 % Change in sputum eosinophils at 4 ‐ 8 weeks | 2 | 117 | Mean Difference (IV, Random, 95% CI) | 0.71 [‐2.06, 3.47] |
| 45.1 Adults | 2 | 117 | Mean Difference (IV, Random, 95% CI) | 0.71 [‐2.06, 3.47] |
| 46 % Change in sputum eosinophils at 36 ‐ 52 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 46.1 Paediatrics | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 47 LTC4 concentration (ng/mL) in nasal wash at 24 ‐ 26 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 47.1 Paediatrics | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 48 % Asthma control days during intervention period at 4 ‐ 8 weeks | 2 | 1293 | Mean Difference (IV, Fixed, 95% CI) | ‐5.72 [‐10.86, ‐0.59] |
| 48.1 Adults | 2 | 1293 | Mean Difference (IV, Fixed, 95% CI) | ‐5.72 [‐10.86, ‐0.59] |
| 49 % Asthma control days during intervention period at 24 ‐ 26 weeks | 2 | 1185 | Mean Difference (IV, Random, 95% CI) | ‐8.19 [‐19.46, 3.07] |
| 49.1 Adults | 2 | 1185 | Mean Difference (IV, Random, 95% CI) | ‐8.19 [‐19.46, 3.07] |
| 50 Change in PC20 at 4 ‐ 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 50.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 51 % rescue ‐ free days at 24 ‐ 26 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 51.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 52 Days off work or school at 24 ‐ 26 weeks | 2 | 606 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.01, 0.26] |
| 52.1 Paediatrics | 1 | 124 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐1.31, 0.83] |
| 52.2 Adults | 1 | 482 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.00, 0.26] |
| 53 Change in height (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 53.1 Paediatrics | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 54 Patient's satisfaction at 4 ‐ 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 54.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 55 Physician's satisfaction at 4 ‐ 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 55.1 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 56 Overall Withdrawals | 42 | 10939 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.08, 1.38] |
| 56.1 Paediatrics | 18 | 3397 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.88, 1.21] |
| 56.2 Adults | 24 | 7542 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.11, 1.54] |
| 57 Withdrawal due to poor asthma control/exacerbations | 26 | 7669 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [2.01, 3.27] |
| 57.1 Paediatrics | 7 | 1219 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.20, 3.94] |
| 57.2 Adults | 19 | 6450 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [2.02, 3.45] |
| 58 Withdrawals due to adverse effects | 25 | 8518 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.95, 1.63] |
| 58.1 Paediatrics | 8 | 2330 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.70, 2.33] |
| 58.2 Adults | 17 | 6188 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.91, 1.67] |
| 59 Overall Adverse effects | 22 | 7818 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.95, 1.05] |
| 59.1 Paediatrics | 3 | 1460 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.15] |
| 59.2 Adults | 19 | 6358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.94, 1.05] |
| 60 Elevated liver enzymes | 7 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.58, 2.19] |
| 60.1 Paediatrics | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.03, 7.68] |
| 60.2 Adults | 6 | 1643 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.60, 2.36] |
| 61 Upper respiratory tract infections | 8 | 2729 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.29] |
| 61.1 Paediatrics | 5 | 1514 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.81, 1.36] |
| 61.2 Adults | 3 | 1215 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.69, 1.50] |
| 62 Headache | 24 | 8872 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.89, 1.11] |
| 62.1 Paediatrics | 6 | 2589 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.81, 1.37] |
| 62.2 Adults | 18 | 6283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.10] |
| 63 Nausea | 17 | 5563 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.64, 1.08] |
| 63.1 Paediatrics | 2 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.28, 2.31] |
| 63.2 Adults | 15 | 5098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.64, 1.09] |
| 64 Oral candidiasis | 3 | 865 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.05, 1.19] |
| 64.1 Adults | 3 | 865 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.05, 1.19] |
| 65 Hoarseness | 2 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.24] |
| 65.1 Adults | 2 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.24] |
| 66 Death | 13 | 5489 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.32, 29.26] |
| 66.1 Paediatrics | 2 | 1114 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 73.46] |
| 66.2 Adults | 11 | 4375 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [0.13, 75.82] |
| 67 Primary outcome ‐ stratified by anti‐leukotrienes | 21 | 6077 | Odds Ratio (M‐H, Random, 95% CI) | 1.61 [1.20, 2.16] |
| 67.1 Monelukast | 15 | 4352 | Odds Ratio (M‐H, Random, 95% CI) | 1.55 [1.14, 2.12] |
| 67.2 Zafirlukast | 6 | 1725 | Odds Ratio (M‐H, Random, 95% CI) | 1.92 [0.88, 4.20] |
| 68 Primary outcome ‐ stratified by duration of intervention | 21 | 6077 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.17, 1.96] |
| 68.1 4‐8 weeks | 9 | 2346 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.78, 3.87] |
| 68.2 12‐16 weeks | 7 | 1541 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [1.43, 2.96] |
| 68.3 24‐26 weeks | 2 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.55, 2.45] |
| 68.4 36‐52 weeks | 3 | 1533 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.87, 1.91] |
| 69 Main outcome ‐stratified by severity of airway obstruction | 21 | 6077 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.17, 1.96] |
| 69.1 Mean FEV1 60‐80% of predicted | 11 | 3922 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.41, 2.91] |
| 69.2 Mean FEV1 ≥80% of predicted | 10 | 2155 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.97, 1.61] |
| 70 Primary outcome ‐ stratified by methodological quality | 21 | 6061 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.17, 1.96] |
| 70.1 High quality | 11 | 4366 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [1.29, 2.03] |
| 70.2 Poor quality | 10 | 1695 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.74, 2.43] |
| 71 Primary outcome‐ stratified by funding source | 21 | 6077 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.17, 1.96] |
| 71.1 Funded by producers of ICS | 9 | 2638 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [1.05, 2.80] |
| 71.2 Funded by producers of AL | 5 | 2797 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.99, 2.35] |
| 71.3 No industry funding or not reported | 7 | 642 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.90, 1.66] |
| 72 Primary outcome ‐ stratified by HFC‐BDP equivalent | 21 | 6077 | Odds Ratio (M‐H, Random, 95% CI) | 1.61 [1.20, 2.16] |
| 72.1 100‐150 μg HFA‐BDP equivalent | 3 | 216 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.26, 2.08] |
| 72.2 200‐250 μg HFA‐BDP equivalent | 15 | 5767 | Odds Ratio (M‐H, Random, 95% CI) | 1.75 [1.29, 2.38] |
| 72.3 400‐500 μg HFA‐BDP equivalent | 3 | 94 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.11, 2.78] |
1.7. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 7 FEV1 irrespective of time of treatment.
1.8. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 8 Responders (defined as change from baseline in FEV1 >= 7.5%.
1.9. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 9 Change from baseline FEV1 (%) at 4 ‐ 8 weeks.
1.29. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 29 Change from baseline mean daily use of β2‐agonists at 36 ‐ 52 weeks.
1.32. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 32 Change in rescue‐free days (%) at 24 ‐ 26 weeks.
1.37. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 37 Change in proportion of symptom‐free days (%) at 36 ‐ 52 weeks.
1.38. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 38 Change from baseline quality of life (QOL) at 4 ‐ 8 weeks.
1.42. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 42 Days with use of β2‐agonists at 36 ‐ 52 weeks.
1.46. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 46 % Change in sputum eosinophils at 36 ‐ 52 weeks.
1.51. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 51 % rescue ‐ free days at 24 ‐ 26 weeks.
1.54. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 54 Patient's satisfaction at 4 ‐ 8 weeks.
1.55. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 55 Physician's satisfaction at 4 ‐ 8 weeks.
1.65. Analysis.

Comparison 1 Anti‐leukotriene (AL) vs. Inhaled glucocorticoids (in HFC‐BDP equivalent), Outcome 65 Hoarseness.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abadoglu 2005.
| Methods | DESIGN: Randomised clinical study Confirmation of methodology: Not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 24 WITHDRAWALS: Not mentioned AGE in years ± SD: 35.65 ± 10.75 GENDER (male %): 20.83% ASTHMA SEVERITY: Mild persistent asthma ASTHMA DURATION:
MEAN ( ± SD) β2‐AGONIST USE (puffs/day): Not described DOSE OF inhaled corticosteroids AT STUDY ENTRY AND AT RUN‐IN: Not mentioned ATOPY (% of patients): 54.16% ELIGIBILITY CRITERIA: A history of recurrent symptoms of wheezing, shortness of breath, cough; Demonstration of objective signs of reversible airway obstruction by means of at least 12 % increase in FEV1 after 15 minutes with an inhalation of 200 μg salbutamol; A PC20 methacholine < 8 mg/mLas stated by the American Thoracic Society and International Asthma Guidelines. Asthma severity was determined by the frequency of asthma symptoms, pulmonary function tests. EXCLUSION CRITERIA: On inhaled corticosteroids, leukotriene receptor antagonists, theophylline and long acting β2‐agonists within the preceding 12 months of the study; airway infection for at least 6 weeks before investigation. |
|
| Interventions | PROTOCOL DURATION
TEST GROUP: Montelukast CONTROL GROUP: Fluticasone propionate DEVICE: Not mentioned CRITERIA FOR WITHDRAWAL FROM STUDY: Not mentioned |
|
| Outcomes | ANALYSIS: Not by intention‐to‐treat analysis OUTCOMES: Reported at 4 weeks; Report outcomes are reported as pre‐ and post‐values (not change from baseline) PULMONARY FUNCTION TESTS: Only pretreatment FEV1 was reported as not difference was observed after treatment FUNCTIONAL STATUS: Not reported INFLAMMATORY MEDIATORS: Eosinophils count; Apoptotic eosinophils; Apoptotic ratio ADVERSE EVENTS: Not mentioned WITHDRAWALS: Not mentioned |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 250 μg | |
| Notes | Full paper (2005) Funding not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There is no report as to whether some patients withdrew from the study after randomisation |
| Selective reporting (reporting bias) | Low risk | All primary and secondary data are presented |
| Other bias | Low risk | No apparent other bias |
Basyigit 2004.
| Methods | DESIGN: Randomised clinical study Confirmation of methodology: Not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 24 WITHDRAWALS: Not mentioned AGE in years (± SD): 35.65 ± 10.75 GENDER (male%): 20.83% ASTHMA SEVERITY: Mild persistent asthma ASTHMA DURATION:
MEAN ( ± SD) β2‐AGONIST USE (puffs per day): Not described DOSE OF inhaled corticosteroids AT STUDY ENTRY AND AT RUN‐IN: Not mentioned ATOPY (% of patients): 54.16% ELIGIBILITY CRITERIA:
EXCLUSION CRITERIA: Not mentioned |
|
| Interventions | PROTOCOL DURATION:
TEST GROUP: Zafirlukast TEST GROUP 2: Theophylline CONTROL GROUP: Budesonide DEVICE: Not mentioned CRITERIA FOR WITHDRAWAL FROM STUDY: Not mentioned |
|
| Outcomes | ANALYSIS (ITT) OUTCOMES: Reported at 8 weeks; report outcomes are reported as pre‐ and post‐values (not change from baseline) PULMONARY FUNCTION TESTS: Spirometry FUNCTIONAL STATUS: Not mentioned INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Not mentioned WITHDRAWALS: Not mentioned |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full paper (2004) Funding not available Confirmation of methodology and data extraction |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All data presented, withdrawal rate was not observed |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were not defined but no bias is observed |
| Other bias | Low risk | No apparent other bias |
Baumgartner 2003.
| Methods | DESIGN: Parallel‐group, multi‐centre, randomised, clinical trial. Confirmation of methodology obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 730 M: 313 BDP: 314 Placebo: 103 WITHDRAWALS: M: 22 (7%) BDP: 19 (6%) Placebo: 10 (10%) AGE in years ± SD: M: 35.9 ± 14.9 BDP:35.5 ± 15.0 Placebo:35.5 ± 14.6 GENDER (% male): M: 33% BDP: 38 % ASTHMA SEVERITY: Not described ASTHMA DURATION: Not reported % Pred. FEV1 % ± SD: M: 69 ± 12 BDP: 68 ± 11 Placebo: 68±12 MEAN ± SD β2‐AGONIST USE (puffs/day): M: 5.2 ± 3.7 BDP: 5.1 ± 3.3 Placebo: 5.5 ± 3.9 ATOPY: M: 69% BDP: 68% ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration
TEST GROUP: Montelukast 10 mg per day CONTROL GROUP 1: Beclomethasone 200 ug twice a day CONTROL GROUP 2: Placebo DEVICE: MDI (actuation inhaler) CO‐INTERVENTION: Not specified CRITERIA FOR WITHRAWAL FROM STUDY: Not described |
|
| Outcomes | ANALYSIS BY MODIFIED INTENTION‐TO‐TREAT OUTCOMES reported at 6 weeks PULMONARY FUNCTION TESTS: Change from baseline FEV1 SYMPTOM SCORES: Change in mean asthma score (6‐point) FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Change from baseline in serum eosinophils ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by Merck Research Labratories Confirmation of methodology and data extraction received from Dr Theodore Reiss |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated allocation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented with reasons for withdrawal by group and adverse effects by group, analysis wad done with intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary endpoint was described |
| Other bias | Low risk | No apparent other bias |
Bleecker 2000.
| Methods | DESIGN: Parallel‐group Confirmation of methodology obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 451 Z: 220 FP: 231 WITHDRAWALS: Z: 23 % FP: 13% AGE ( ± SD): Z: 31 (12‐66) FP: 31 (12‐68) GENDER (% male): Z: 49% FP: 52% ASTHMA SEVERITY: Not described % mean pred. FEV1: Z: 68 FP: 67 Mean ( ± SD) β2‐agonist use (puffs per day): Not reported ATOPY: Z: 43 % FP: 46% ASTHMA DURATION (years): 10 years ELIGIBILITY CRITERIA
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 8‐14 days Intervention Period: 12 weeks TEST GROUP: Zafirlukast 20 mg twice a day CONTROL GROUP: Inhaled Fluticasone 100 μg twice a day DEVICE: Not described CO‐INTERVENTION: None allowed |
|
| Outcomes | ANALYSIS ( ITT ) OUTCOMES reported at 12 weeks PULMONARY FUNCTION TESTS
SYMPTOM SCORES: Change in mean symptom score (average/week) FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by Glaxo Wellcome Confirmation of methodology and data extraction received from Gerry Hogan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation of block of 4 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported with intention‐to‐treat analysis, adverse events were reported |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
Boushey 2005.
| Methods | DESIGN: Parallel‐group, randomised, placebo controlled, clinical trial | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 149 Z: 76 BD: 73 WITHDRAWALS: Z: 18.42 % BD: 7.89 % AGE (years±SD): Z: 33.6 ± 11.1 BD: 33.2 ± 9.5 GENDER (% male): Z: 38 % BD: 34 % ASTHMA SEVERITY: Not described % Pred. FEV1 (mean ± SD) Z: 88.2 ± 14.4 BD: 90.5 ± 12.6 Mean ( ± SD) β2‐agonist use (puffs/day): Not reported ATOPY: Not reported ASTHMA DURATION (years): Z: 20.9 ± 13.1 BD: 17.1 ± 11.0 ELIGIBILITY CRITERIA
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 4 weeks Intervention Period: 52 weeks TEST GROUP: Zafirlukast 20 mg twice a day CONTROL GROUP: Inhaled Budesonide 200 μg twice a day DEVICE: Turbuhaler CO‐INTERVENTION: None allowed |
|
| Outcomes | ANALYSIS: Not reported OUTCOMES reported at 52 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Change in mean symptom score FUNCTIONAL STATUS
INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by National Institute of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: stratified adaptive randomisation to balance the effect of PC20, age, racial or ethnicity |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The withdrawal rate was unbalanced between groups: 8.2% and 18.4% in budesonide and zafirlukast group, respectively; the reasons for withdrawal are reported but not by group. There was no report of intention‐to‐treat analysis; although this was appropriate for the main outcome (time to event), it may have biased the interpretation of the some secondary outcomes |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were defined |
| Other bias | Low risk | No apparent other bias |
Bousquet 2005.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 645 M: 325 FP: 320 WITHDRAWALS: M: 24 (7.4%) FP: 17 (5.3)% AGE: years ± SD: M: 35.9 ± 14.3 FP: 36.6 ± 13.8 GENDER: n (% male): M: 126 (38.8%) FP: 113 (35.3%) ASTHMA SEVERITY: Mild persistent asthma as defined by Global Initiative for Asthma (GINA) guidelines ASTHMA DURATION: Not described % Pred. FEV1 ± SD M: 90.5 ± 12.2 FP: 88.2 ± 12.2 β2‐AGONIST USE (puffs per day): M: 1.28 ± 1.1 FP: 1.38 ± 1.3 ATOPY: n (%) M: 42 (12.9%) FP: 45 (14.1%) ELIGIBILITY CRITERIA
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 3 weeks Intervention Period: 12 weeks TEST GROUP: Montelukast 10 mg per day CONTROL GROUP: Fluticasone 100 μg twice a day DEVICE: Metered dose inhaler (MDI) |
|
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 12 weeks PULMONARY FUNCTION TESTS
SYMPTOM SCORES: Days with symptoms (%) FUNCTIONAL STATUS
INFLAMMATORY MEDIATORS: Eosinophil count (103/μL) ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by Merck & Co., Inc. Confirmation of methodology and data extraction: not obtained USER‐DEFINED ORDER: |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented; intention‐to‐treat analysis, reasons for withdrawal by group and adverse effects by group presented, |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were defined |
| Other bias | Low risk | No apparent other bias |
Brabson 2002.
| Methods | DESIGN: Parallel‐group, multi‐centre , randomised, double‐blind, double‐dummy, clinical trial Confirmation of methodology not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 440 Z: 216 FP: 224 WITHDRAWALS: Z: 45 (21%) FP: 17 (8)% AGE (± SD): Z: 35 (± 16) FP: 36 (± 14) GENDER (% male): Z: 75 (35%) FP: 90 (40%) ASTHMA SEVERITY: Not described ASTHMA DURATION: Not described % Pred. FEV1 (± SD): Z: 73 ± 7 FP: 73 ± 7 MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported DOSE OF inhaled corticosteroids AT STUDT ENTRY AND AT RUN‐IN: BDP 256 ± 80ug/d T 600 ± 213ug/d BDP271 ± 73 μg/d T 603 ± 169 μg/d ATOPY: Not described ELIGIBILITY CRITERIA
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 8 days Intervention Period: 6 weeks TEST GROUP: Zafirlukast 20 mg twice daily CONTROL GROUP: Fluticasone 100 μg twice a day DEVICE: Metered‐dose inhaler CRITERIA FOR WITHDRAWAL FROM STUDY
|
|
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 6 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES:
FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by Glaxo SmithKline Confirmation of methodology and data extraction: not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Details were not provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported including side effects; withdrawal rate with reasons by group |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
Busse 2001a.
| Methods | DESIGN: Parallel‐group, multi‐centre, randomised, clinical trial Confirmation of methodology obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 338 Z: 111 FP: 113 Placebo (not used):114 WITHDRAWALS: Z: 19% FP: 14% AGE (± SD): 12‐75 years Z: 33.8 ± 13.1 FP: 29.6 ± 11.4 GENDER (% male): 50% Z: 41 % FP: 58% ASTHMA SEVERITY: % moderate: Z: 82% FP: 77% % Pred. FEV1 (mean ± SD): 66‐67% Z: 69.1 ± 7.5 FP: 68.1 ± 8.3 Mean (± SD) β2‐agonist use (puffs/day) Z: 4.7 (SE 0.27) FP: 4.8 (SE:0.26) ATOPY: Z: 42% FP: 23% ASTHMA DURATION (years): at least 10 years Z:69% FP: 65% ELIGIBILITY CRITERIA
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 8‐14 days to establish baseline respiratory function Intervention Period: 12 weeks TEST GROUP: Zafirlukast 20 mg twice a day po CONTROL GROUP: Inhaled Fluticasone 100 μg twice a day (2 puffs of 50 μg twice a day) DEVICE: Metered dose inhaler (no spacer) CO‐INTERVENTION: None allowed other than rescue β2‐agonist and systemic corticosteroids |
|
| Outcomes | ANALYSIS ( ITT ) OUTCOMES reported at 6 and 12 weeks (also documented at 2, 4, 8 weeks) PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Weekly average symptom score (6‐point scale) FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by Glaxo Wellcome Confirmation of methodology and data extraction obtained from Shailesh Patel and Rob Pearson, Nov 2, 2001 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated in block of 6 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
Busse 2001b.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial Confirmation of methodology obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 533 M: 262 FP: 271 WITHDRAWALS: M: 29% FP: 28% AGE (± SD) years: M: 34.4 (15‐67) FP: 35.4 (15‐83) GENDER (% male): M: 42 % FP: 47% ASTHMA SEVERITY: not described % Pred. FEV1 (mean ± SD): M: 65.4 ± 8.2 FP: 65.6 ± 9.2 Mean (± SD) β2‐agonist use (puffs/day): Not reported ATOPY: Not reported ASTHMA DURATION (years): Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 8‐14 days Intervention Period: 24 weeks TEST GROUP: Montelukat 10 mg per day CONTROL GROUP: Inhaled Fluticasone 100 μg twice a day DEVICE: Metered dose inhaler CO‐INTERVENTION: None allowed |
|
| Outcomes | ANALYSIS ( ITT ) OUTCOMES reported at 4, 8, 12, 16, 24 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Change in mean symptom score/week (6‐point scale) FUNCTIONAL STATUS
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by Glaxo Wellcome Confirmation of methodology and data extraction and additional unpublished data obtained from and Shailesh Patel and Rob Pearson |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated in block of 4 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented including reasons for withdrawals and adverse effects, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were defined |
| Other bias | Low risk | No apparent other bias |
Caffey 2005.
| Methods | DESIGN: Three‐way cross‐over, placebo controlled, randomised, clinical trial | |
| Participants | MILD TO MODREATE PERSISTENT ASTHMA MEETING NAEPP CRITERIAS RANDOMISED: N = 24 WITHDRAWALS: 16.67% AGE: years(range): 8.92 (5‐12) GENDER (% male): Not reported ASTHMA SEVERITY: Mild to moderate as per NAEPP criteria % Pred. FEV1: mean(range)%: 88 (47‐111) % ATOPY: Not reported ASTHMA DURATION (years): Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 2 weeks Intervention Period: 4 weeks TEST GROUP: Montelukat 10 mg qd CONTROL GROUP: Inhaled Fluticasone 50 μg twice a day DEVICE: Aerochamber CO‐INTERVENTION: None allowed |
|
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 2, 4 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Daily PM asthma symptom score (6‐point scale) FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: *Fractional exhaled nitric oxide (FeNO) ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 100 μg | |
| Notes | Full‐text publication Funded by GlaxoSmithKline and by National Health Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, reasons for withdrawal by group and adverse effects by group presented, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were described |
| Other bias | Low risk | No apparent other bias |
Dempsey 2002a.
| Methods | DESIGN: Cross‐over, randomised, clinical trial Confirmation of methodology: Not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 21 WITHDRAWALS: Not mentioned AGE (years): N = 33.5 GENDER (% male): Not mentioned ASTHMA SEVERITY: Mild persistent atopic asthma ASTHMA DURATION: Not described % Pred. FEV1: N = 96.3% MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported DOSE OF inhaled corticosteroids AT STUDY ENTRY AND AT RUN‐IN: Not mentioned ATOPY: Not described ELIGIBILITY CRITERIA: Mild persistent asthma EXCLUSION: Not mentioned |
|
| Interventions | PROTOCOL Duration Run‐in Period: 1‐2 weeks Intervention Period: 4 weeks CONTROL GROUP: Hfa‐triamcinolone acetonide 450 μg od TEST GROUP: Montelukast 10 mg od DEVICE: Not mentioned Criteria for withdrawal from study: Not mentioned |
|
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 4 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Change in mean symptoms FUNCTIONAL STATUS: Change from baseline in night‐time use of rescue β2‐agonist use (puffs/day) INFLAMMATORY MEDIATORS
ADVERSE EVENTS: Not reported WITHDRAWALS: Not reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 112.5 μg | |
| Notes | Abs 2001 Funding not mentioned Confirmation of methodology and data extraction: not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
FLTA4030.
| Methods | DESIGN: Parallel‐group, multi‐centre, randomised, clinical trial. Confirmation of methodology: Not obtained |
|
| Participants | PARTICIPANTS WHO ARE RECEIVING β2‐AGONISTS ALONE RANDOMISED: N = 209 Z: 108 FP: 101 WITHDRAWALS: Z: 9 (8%) FP: 8 (8)% AGE (± SD): Z: 33.5 (± 12.8) FP: 32.9 (± 12.7) GENDER (% male): Z: 40.74% FP: 42.57% ASTHMA SEVERITY: Not described ASTHMA DURATION: Not described % Pred. FEV1 (±SD): Not described MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported DOSE OF INHALED CORTICOSTEROIDS AT STUDT ENTRY AND AT RUN‐IN: Not described ATOPY: Not described ELIGIBILITY CRITERIA
EXCLUSION: Not described |
|
| Interventions | PROTOCOL Duration Run‐in Period: 1‐2 weeks Intervention Period: 12 weeks CONTROL GROUP: Fluticasone propionate 88 μg twice a day TEST GROUP: Zafirlukast 20 mg twice a day DEVICE: Metered dose inhaler Criteria for withdrawal from study: Reported |
|
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 12 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES:
FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 | |
| Notes | Funding not reported but trials conducted by pharmaceutical company Confirmation of methodology and data extraction: not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
FLTA4031.
| Methods | DESIGN: Parallel‐group, multi‐centre, randomised, clinical trial. Confirmation of methodology: Not obtained |
|
| Participants | PARTICIPANTS WHO ARE RECEIVING β2‐AGONISTS ALONE RANDOMISED: N = 224 Z: 111 FP: 113 WITHDRAWALS: Z: 21 (19%) FP: 16 (14%) AGE (± SD): Z: 33.8 (± 13.1) FP: 29.6 (±11.4) GENDER (% male): Z: 40.54% FP: 58.41% ASTHMA SEVERITY: Not described ASTHMA DURATION: Not described FEV1 (± SD): Z: 2.41 (± 0.63) FP: 2.52 (± 0.53) MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported DOSE OF INHALED CORTICOSTEROIDS AT STUDT ENTRY AND AT RUN‐IN: Not described ATOPY: Not described ELIGIBILITY CRITERIA:
EXCLUSION: Not described |
|
| Interventions | PROTOCOL Duration Run‐in Period: 1‐2 weeks Intervention Period: 12 weeks CONTROL GROUP: Fluticasone propionate 88 μg twice a day TEST GROUP: Zafirlukast 20 mg twice a day DEVICE: Metered dose inhaler Criteria for withdrawal from study: Reported |
|
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 1, 2, 4, 6, 8, 12 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES:
FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 | |
| Notes | Funding not reported but trials conducted by pharmaceutical company Confirmation of methodology and data extraction: not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
FMS40012.
| Methods | DESIGN: Randomised clinical study | |
| Participants | PARTICIPANTS WITH MILD ASTHMA RANDOMISED: N = 16 Z: 8 FP: 8 WITHDRAWALS: Z: 1 (13%) FP: 0 (0%) AGE (± SD): Z: 26.3 (± 4.5) FP: 31.8 (± 12.2) GENDER (% male): Z: 87.5% FP: 62.5% ASTHMA SEVERITY: Not described ASTHMA DURATION: Not described FEV1 (± SD): Z: 4.06 (± 1.0) FP: 3.54 (± 0.94) MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported DOSE OF INHALED CORTICOSTEROIDS AT STUDT ENTRY AND AT RUN‐IN: Not described ATOPY: Described ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: not reported Intervention Period: 4 weeks CONTROL GROUP: Fluticasone propionate 50 μg twice a day TEST GROUP: Zafirlukast 20 mg twice a day DEVICE: Metered dose inhaler Criteria for withdrawal from study: Reported |
|
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 1, 4 weeks PULMONARY FUNCTION TESTS: FEV1 SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Not reported. INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 100 | |
| Notes | Funding not reported but trials conducted by pharmaceutical company Confirmation of methodology and data extraction: not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
FPD40013.
| Methods | DESIGN: Parallel‐group, multi centre, randomised, clinical trial. Confirmation of methodology: Not obtained |
|
| Participants | PARTICIPANTS WITH PERSISTENT ASTHMA RANDOMISED: N = 341 MON: 168 FP: 173 WITHDRAWALS: MON: 28 (17%) FP: 27 (16%) AGE (± SD): MON: 9.4 (± 1.9) FP: 9.5 (± 1.9) GENDER (% male): MON: 60.71% FP: 58.96% ASTHMA SEVERITY: Not described ASTHMA DURATION: Not described % Pred. FEV1 (± SD): MON: 75.7 (± 7.0) FP: 76.1 (± 6.6) MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported DOSE OF INHALED CORTICOSTEROIDS AT STUDT ENTRY AND AT RUN‐IN: Not described ATOPY: Not described ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: Not reported Intervention Period: 12 weeks CONTROL GROUP: Fluticasone propionate 50 μg twice a day TEST GROUP: Montelukast 5 mg QD DEVICE: Diskus Criteria for withdrawal from study: Reported |
|
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 12 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Change from baseline in percent of symptom‐free days FUNCTIONAL STATUS: Change from baseline in total albuterol use INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 100 | |
| Notes | Funding not reported but trials conducted by pharmaceutical company Confirmation of methodology and data extraction: not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
Garcia Garcia 2005.
| Methods | DESIGN: Parellel‐group, randomised, clinical trial. Confirmation of methodology: Not obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 994 M: 495 FP: 499 WITHDRAWALS: M:7.3% FP:6.6% AGE: years (range): M: 9 (6‐14) FP: 9 (5‐15) GENDER (% male): M: 64.8% FP: 58.5% ASTHMA SEVERITY: Mild persistent asthma at step 2 of GINA guidelines % Pred. FEV1 ± range: M: 86.8 (34.2‐129.0) FP: 87.7(51.8‐12.5‐0) β2‐agonist use (puffs per day): median (range) M: 5(0.0‐77.9) FP: 4.8 (0.0‐78.3) ATOPY: Not reported ASTHMA DURATION: (years): Not reported HISTORY OF ALLERGY:(%) M: 61.4 FP: 61.9 ELIGIBILITY CRITERIA:
(2) a positive methacholine or histamine provocative concentration causing a 20% decrease in FEV1 of 8 mg/mL; or (3) a decrease in FEV1 of 15% after an exercise challenge.
EXCLUSION: Not reported |
|
| Interventions | PROTOCOL Duration Run‐in Period: 4 weeks Intervention Period: 52 weeks CONTROL GROUP: Fluticasone 100 μg twice a day TEST GROUP: Montelukast 5‐mg oral tablet [10 mg if the patient turned 15 years of age during the study], once at bedtime DEVICE: Metered dose inhaler Criteria for withdrawal from study: Described |
|
| Outcomes | ANALYSIS ( ITT ) OUTCOMES reported at 52 weeks PULMONARY FUNCTION TESTS: Change from baseline FEV1 (L and %) SYMPTOM SCORES: Not reported FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Peripheral blood eosinophils level ADVERSE EVENTS: Described WITHDRAWALS: Described * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by Merck and Co. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: electronically generated randomisation using blocking factor of 4 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, reasons for withdrawal by group and adverse effects by group presented, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary, secondary and tertiary outcomes were described |
| Other bias | Low risk | No apparent other bias |
Hughes 1999 (BDP).
| Methods | DESIGN: Parallel‐group, clinical trial. Confirmation of methodology obtained (08/01) |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 71 M: 25 FP: 23 BDP: 23 WITHDRAWALS: M: 0 (0%) FP: 1 (4%) BDP:0 (0%) AGE (± SD): M: 29.0 ± 11.7 FP: 30.7 ± 10.7 BDP: 31.6 ± 9.6 GENDER (% male): M: 39% FP:60% BDP: 64% ASTHMA SEVERITY: Mild % Pred. FEV1 % ± SD: M: 83.0 ± 11.7 FP:85.3 ± 10.7 BDP:84.9 ± 11.0 Mean (± SD) β2‐agonist use (puffs/day): M: 1.2 ± 1.0 FP: 1.4 ± 1.5 BDP: 1.2 ± 1.5 ATOPY or ALLERGIC RHINITIS: M:84% FP:96% BUD:78% ELIGIBILITY CRITERIA:
EXCLUSION CRITERIA:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 2 weeks Intervention Period: 4 weeks TEST GROUP: Montelukast 10 mg CONTROL GROUP 1: Fluticasone 100 μg twice a day CONTROL GROUP 2: Budesonide 200 μg twice a day DEVICE: Aerosol inhaler for Fluticasone and Dry powder inhaler for Budesonide CO‐INTERVENTION: None allowed other than rescue B2‐agonist and systemic corticosteroids |
|
| Outcomes | ANALYSIS ( ITT)
(if received at least one study medication) OUTCOMES reported at 4 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Not reported FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Change from baseline in serum eosinophils ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Abstract & Poster (1999) Funded by Merck Letter, meth sent to Hughes December 1999 Confirmation of methodology, data extraction and additional data provided by Merck Laboratories. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation by opaque consecutive numbered envelopes containing assignment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, withdrawal and adverse effects, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary outcome was described |
| Other bias | Low risk | No apparent other bias |
Hughes 1999 (FP).
| Methods | as above | |
| Participants | as above | |
| Interventions | as above | |
| Outcomes | as above | |
| ICS dose in HFA beclomethasone ‐ equivalent | as above | |
| Notes | as above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation by opaque consecutive numbered envelopes containing assignment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, withdrawal and adverse effects, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary outcome was described |
| Other bias | Low risk | No apparent other bias |
Israel 2002.
| Methods | DESIGN: Parallel‐group, multi‐centre, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 782 M = 339 BDP = 332 Placebo = 111 WITHDRAWALS: n (%) M: 11 (3.2%) BDP: 14 (4.2%) Placebo: 5(4.5%) AGE: years (range): M = 33.5 (15‐71) BDP = 33.9 (15‐74) placebo = 33.3 (15‐64) GENDER: n (% male) M = 162 (47.8%) BDP = 156 (47%) Placebo = 54 (48.7%) ASTHMA SEVERITY: Not mentioned ASTHMA DURATION: Not mentioned % PRED. FEV1% ±SD: M = 67.4 ± 11.1 BDP = 65.9 ± 11.7 P = 66.8 ± 12.3 MEAN (± SD) β2‐AGONIST USE (puffs/day): M = 5.6 ± 2.9 BDP = 5.8 ± 2.9 Placebo=5.7 ± 2.6 DOSE OF inhaled corticosteroids AT STUDY ENTRY AND DURING RUN‐IN: Not reported ATOPY: Not described ELIGIBILITY CRITERIA:
EXCLUSION
|
|
| Interventions | PROTOCOL DURATION Run‐in: 2 weeks Intervention: 6 weeks TEST GROUP: Montelukast: 10 mg once daily CONTROL GROUP 1: BDP: 200 μg twice a day CONTROL GROUP 2: Placebo DEVICE: MDI + spacer CRITERIA FOR WITHDRAWAL FROM STUDY: Not described CO‐INTERVENTION: Short‐acting anti‐histamines |
|
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 6 weeks PULMINARY FUNCTION TESTS *Change from baseline FEV1 SYMPTOM SCORES: % of days with asthma control FUNCTIONAL STATUS
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported *primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by Merck Confirmation of methodology and data extraction not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: blinded allocation schedule produced by the study sponsor |
| Allocation concealment (selection bias) | Low risk | A block of allocation numbers that were assigned sequentially to consecutively randomised patients |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, withdrawal rate per group was mentioned |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were specified |
| Other bias | Low risk | No apparent other bias |
Jayaram 2002.
| Methods | DESIGN: Parallel‐group, multi‐centre, randomised, clinical trial. Confirmation of methodology: Not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 50 M = 19 FP = 18 WITHDRAWALS: Not mentioned AGE (years): Not mentioned GENDER (% female): Not mentioned ASTHMA SEVERITY: Not described ASTHMA DURATION: Not mentioned % PRED. FEV1% ± SD: Not mentioned MEAN ± SD β2‐AGONIST USE (puffs/day): Not mentioned DOSE OF inhaled corticosteroids AT STUDY ENTRY AND DURING RUN‐IN: Not reported ATOPY: Not described ELIGIBILITY CRITERIA:
EXCLUSION± Not mentioned |
|
| Interventions | PROTOCOL DURATION Run‐in: Not mentioned Intervention: 8 weeks TEST GROUP: Montelukast (dose un‐specified: probably 10 mg per day) CONTROL GROUP 1: Fluticasone (dose un‐specified) DEVICE: Not described CRITERIA FOR WITHDRAWAL FROM STUDY: Not described |
|
| Outcomes | ANALYSIS (ITT not specified) OUTCOMES ‐reported at 8 weeks Report outcomes are reported as pre‐ and post‐values (not change from baseline) PULMINARY FUNCTION TESTS
SYMPTOM SCORES: Change in symptoms FUNCTIONAL STATUS: Change from baseline in use of β2‐agonist INFLAMMATORY MEDULATOR: *Change in sputum Eo (%) ADVERSE EVENTS: Not reported WITHDRAWALS: Not reported *primary outcomes |
|
| ICS dose in HFA beclomethasone ‐ equivalent | Not reported | |
| Notes | Abstract 2002 Funding not mentioned Confirmation of methodology and data extraction: not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
Jayaram 2005.
| Methods | DESIGN: Parallel‐group, randomised, multicentric, placebo controlled, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED N = 37 M:19 FP:18 WITHDRAWALS: N (%) M: 1 (5.26%) FP: 1 (5.56%) AGE: years ± SD M: 31.4 ± 9.9 FP: 35.4 ± 13.9 GENDER: N (% male) M: 8 (42.11) FP:8 (44.44) ASTHMA SEVERITY: Persistent symptomatic asthma % Pred. FEV1 % (± SD) M: 76.7 (15.9) FP: 72.0 (16.0) β2‐agonist use (puffs per day) Mean (± SD): M: 3.3 (3.1) FP: 3.3 (2.6) ATOPY: M:84.21% FP:88.89% ELIGIBILITY CRITERIA:
|
|
| Interventions | PROTOCOL DURATION Run‐in: not mentioned Intervention: 8 weeks TEST GROUP: Montelukast (10 mg qd) CONTROL GROUP: Fluticasone (250 μg per day) DEVICE: Not described CRITERIA FOR WITHDRAWAL FROM STUDY: Not described |
|
| Outcomes | ANALYSIS: (ITT not specified) OUTCOMES Reported at 8 weeks Report outcomes are reported as pre‐ and post‐values and graph of change in values PULMINARY FUNCTION TESTS
SYMPTOM SCORES: Change in symptoms (5‐35 score) FUNCTIONAL STATUS: Pre and post use of β2‐agonist INFLAMMATORY MEDULATOR: *Change in sputum Eosinophils (%) ADVERSE EVENTS: Reported WITHDRAWALS: Reported *primary outcomes |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 250 μg | |
| Notes | Full‐text publication Funded by Glaxo Wellcome Inc; |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data on symptom severity, methacholine tests, PEF, skin prick test were mentioned in methodology but not described in results |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were defined |
| Other bias | Low risk | No apparent other bias |
Jenkins 2005.
| Methods | DESIGN: Cross‐over, active controlled, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 58 WITHDRAWALS: n(%): 4 (6.90) AGE: years (range): N = 38.5 (16–70) GENDER (% male): 60.35 ASTHMA SEVERITY: Mild to moderate asthma ASTHMA DURATION: Not described % Pred. FEV1: mean±SD: N = 76.1 ± 11.9 β2‐AGONIST USE: puffs per day (range): 3.0 (1.0–5.5) ATOPY: 93% ELIGIBILITY CRITERIA:
EXCLUSION CIRTERIA:
|
|
| Interventions | PROTOCOL DURATION Run‐in: 2 weeks Intervention: 6 weeks TEST GROUP: Montelukast (over‐encapsulated montelukast 10 mg tablet q.d.) CONTROL GROUP: Fluticasone propionate (Accuhaler/Diskus 250 mg twice a day) DEVICE: (Accuhaler/Diskus) CRITERIA FOR WITHDRAWAL FROM STUDY: Described |
|
| Outcomes | ANALYSIS: (ITT) OUTCOMES Reported at 6 weeks Report outcomes are reported as pre‐ and post‐values PULMINARY FUNCTION TESTS:
SYMPTOM SCORES: *Change in symptoms (1‐6 score) FUNCTIONAL STATUS:
INFLAMMATORY MEDULATOR: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported *primary outcomes |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 500 μg | |
| Notes | Full‐text publication Funding: Not reported; |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, intention‐to‐treat analysis,reasons for withdrawal by group and adverse effects by group described |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were defined |
| Other bias | Low risk | No apparent other bias |
Kanazawa 2007.
| Methods | DESIGN: Parallel‐group, active and placebo controlled, clinical trial. | |
| Participants | ASTHMA PATIENTS RANDOMISED: N = 30 M: 15 FP: 15 WITHDRAWALS: Not reported AGE: years ± SD M: 34.9 ± 5.5 FP: 36.1 ± 6.4 GENDER: % male M: 80% FP: 80% ASTHMA SEVERITY: Not mentioned % Pred. FEV1 % (± SD) M: 91.0 (± 5) FP: 90 (± 3) β2‐agonist use (puffs/day) Mean (± SD): Not reported ATOPY: Not reported Exhaled NO, parts per billion (± SD) M: 12.5 (4.8) FP: 13.4 (3.9) SPUTUM EOSINOPHILS, % (± SD) M: 11.0 (2.8) FP: 12.1 (3.9) SPUTUM ECP, ng/mL (± SD) M: 470 (170) FP: 500 (170) ELIGIBILITY CRITERIA:
|
|
| Interventions | PROTOCOL DURATION Run‐in: 2 weeks Intervention: 12 weeks TEST GROUP: Montelukast capsule (10 mg at night) CONTROL GROUP: Fluticasone propionate (200 μg twice a day) DEVICE: Not mentioned CRITERIA FOR WITHDRAWAL FROM STUDY: Not mentioned |
|
| Outcomes | ANALYSIS: (ITT not mentioned) OUTCOMES Reported at 12 weeks Outcomes are reported as pre‐ and post‐values PULMINARY FUNCTION TESTS:
SYMPTOM SCORES: Not reported FUNCTIONAL STATUS AND INFLAMMATORY MEDULATOR:
ADVERSE EVENTS: Not reported WITHDRAWALS: Not reported |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 400 μg | |
| Notes | Full‐text publication Funding: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were not defined but no bias is observed |
| Other bias | Low risk | No apparent other bias |
Kanniess 2002.
| Methods | DESIGN: Cross‐over, randomised, clinical trial. Confirmation of methodology: Not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 40 WITHDRAWALS: Not reported AGE mean years (range): 37 (18‐60) GENDER: 24 male (60%) ASTHMA SEVERITY: Moderate allergic bronchial asthma FEV1 % pred (Mean ±SD): 74.2 (10.6) MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported ATOPY: 100% of patients ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL DURATION Run‐in: 1‐2 weeks Treatment: 4 weeks Wash‐out: 3‐8 weeks TEST GROUP: Fluticasone 100 μg twice a day CONTROL GROUP: Montelukast 10 mg at night‐time DEVICE: Diskus CRITERIA FOR WITHDRAWAL FROM STUDY: Not described CO‐INTERVENTION: Not permitted |
|
| Outcomes | ANALYSIS (not by ITT) OUTCOMES Reported at 4 weeks PULMINARY FUNCTION TESTS:
SYMPTOM SCORES: Symptom score daytime and night‐time (range 0‐4) FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Reported WITHDRAWALS: Not reported PRIMARY OUTCOMES: Not reported |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by GlaxoSmithKline, d20354 Hamburg, Germany Confirmation of methodology and data extraction: not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | Not found |
| Other bias | Low risk | No apparent other bias |
Khan 2008.
| Methods | DESIGN: Parallel‐group, active controlled, randomised, clinical trial. | |
| Participants | ASTHMA PATIENTS RANDOMISED: N = 60 M: 30 BC: 30: WITHDRAWALS: Not reported AGE: years±SD: Not mentioned GENDER: % male M: 100% BC:100% ASTHMA SEVERITY: Not mentioned % Pred. FEV1 % (± SD): Not reported β2‐agonist use (puffs/day) Mean (± SD): Not reported ATOPY: Not reported ELIGIBILITY CRITERIA Not mentioned EXCLUSION CRITERIA:
|
|
| Interventions | PROTOCOL DURATION run‐in: Not mentioned treatment: 8 weeks TEST GROUP: Beclomethasone 250 μg daily CONTROL GROUP: Montelukast 10 mg at night‐time DEVICE: Not reported CRITERIA FOR WITHDRAWAL FROM STUDY: Not described CO‐INTERVENTION: Not permitted |
|
| Outcomes | ANALYSIS (ITT Not reported) OUTCOMES Reported at 8 weeks PULMINARY FUNCTION TESTS: Peak expiratory flow rate SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Not reported INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Not reported WITHDRAWALS: Not reported PRIMARY OUTCOMES: Not reported |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 125 μg | |
| Notes | Full‐text publication Funded : Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No proper statistical analysis was performed; severity of patient was not determined using any guideline, |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary outcomes were not defined but no bias is observed |
| Other bias | High risk | No details on ethical committee approval mentioned; no details on informed consent was mentioned |
Kim 2000.
| Methods | DESIGN: Parallel‐group, clinical trial. Confirmation of methodology: Obtained |
|
| Participants | ASYMPTOMATIC PARTICIPANTS RANDOMISED: N = 437 Z: 221 FP: 216 WITHDRAWALS: Z: 46 (21%) FP:19 (9%) AGE (years, range): Z: 32.9 (12‐71) FP: 35.5 (12‐81) GENDER (%male): Z: 19 % FP: 18 % ASTHMA SEVERITY: Mild stable FEV1 (Mean ± SD): Z: 2.53 ± 0.04 FP: 2.58 ± 0.04 Mean (± SD) β2‐agonist use (puffs/day): Not reported ATOPY: Z: 58% FP: 56% ASTHMA DURATION: 10 years ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 1 week Intervention Period: 6 weeks TEST GROUP: Zafirlukast 20 mg twice a day CONTROL GROUP: Fluticasone 100 μg twice a day DEVICE: MDI CO‐INTERVENTION: None |
|
| Outcomes | ANALYSIS BY INTENTION‐TO‐TREAT OUTCOMES reported at 6 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Change in mean symptom score (6‐point scale) FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by Glaxo Wellcome Confirmation of methodology and data extraction received from Gerry Hogan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated in block of 4 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented including withdrawal with reasons by group and adverse effects by group, analysis was done by intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary out come was presented |
| Other bias | Low risk | No apparent other bias |
Koenig 2008.
| Methods | DESIGN: Parellel‐group, randomised, clinical trial. | |
| Participants | ASTHMA PATIENTS RANDOMISED: N = 323 M: 164 FP: 159 WITHDRAWALS: Not reported AGE: years (SD): M: 40.1 (13.9) FP: 42.0 (14.5) GENDER (%male): M: 55 % FP: 57 % ASTHMA SEVERITY: Asthma patients with FEV1 between 40% and 85% of predicted normal FEV1 % of predicated: Mean: M: 72 FP: 74 ATOPY: Not reported ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 2 week Intervention Period: 16 weeks TEST GROUP: Montelukast 10 mg qd CONTROL GROUP: Fluticasone 100 μg twice a day DEVICE: Flovent Diskus CO‐INTERVENTION: None |
|
| Outcomes | ANALYSIS (ITT Not reported) OUTCOMES reported AM PEF: at every week up to 16 weeks FEV1 (L): at every 4 week up to 16 weeks Other parameters: baseline and at the end of 16 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Change in mean symptom score (0‐5 point scale) FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funding: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented; analysis by intention‐to‐treat; reasons for withdrawal and adverse effects described |
| Selective reporting (reporting bias) | Low risk | Primary and secondary end outcomes were described |
| Other bias | Low risk | No apparent other bias |
Kooi 2008.
| Methods | DESIGN: Parellel‐group, randomised, clinical trial. | |
| Participants | CHILDREN WITH ASTHMA LIKE SYMPTOMS RANDOMISED: N = 63 M: 18 FP: 25 WITHDRAWALS: Reported AGE: mean years ± SD: M: 3.8 ± 1.4 FP: 3.9 ± 1.1 GENDER (% male): M: 66.67 % FP: 52 % ASTHMA SEVERITY: Children with asthma‐like symptoms (wheeze, cough and/or shortness of breath) of sufficient severity to justify the use of prophylactic asthma treatment ATOPY: M: 89 % FP: 84 % ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 2 week Intervention Period: 12 weeks TEST GROUP: Montelukast 4 mg chewable tablet qd CONTROL GROUP: Fluticasone 100 μg twice a day DEVICE: Metered dose inhaler via a plastic spacer device (Aerochambers) CO‐INTERVENTION: None |
|
| Outcomes | ANALYSIS by intention‐to‐treat manner OUTCOMES reported at 12 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: *Symptom score (wheeze, cough, shortness of breath) as recorded by caregivers in a symptom diary card FUNCTIONAL STATUS: Rescue medication free days, INFLAMMATORY MEDIATORS: Blood eosinophils ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funding: from Merck Sharp and Dohme. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented; intention‐to‐treat analysis; reasons for withdrawal and adverse effects were described by group; |
| Selective reporting (reporting bias) | Low risk | Primary and secondary end outcomes were defined |
| Other bias | Low risk | No apparent other bias |
Kumar 2007.
| Methods | DESIGN: Parellel‐group, randomised, clinical trial. | |
| Participants | MILD PERSISTENT ASTHMA PATIENTS RANDOMISED: N = 62 M: 30 BD: 32 WITHDRAWALS: M: 4 BD: 2 AGE: years±SD M: 8.6 ± 2.14 BD: 9.87 ± 2.35 GENDER (%male): M: 83.33 % BD: 78.13 % ASTHMA SEVERITY: Mild persistent asthma was defined as asthma symptoms occurring more than two times in a week but < 1 episode per day and exacerbation may affect activity, night‐time symptoms more than two times a month and peak expiratory flow rate (PEFR) more than or equal to 80% of the predicted FEV1 % of predicted: Mean(range) M: 73.5 (66.55–81.44) BD: 76 (67.99–83.50) ATOPY: Not reported ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA
exacerbation may affect activity, night‐time symptoms more than two times a month
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: Not reported Intervention Period: 12 weeks TEST GROUP: Montelukast 10 mg qd CONTROL GROUP: Budesonide 400 μg per day DEVICE: Metered dose inhaler CO‐INTERVENTION: None |
|
| Outcomes | ANALYSIS (ITT Not reported) OUTCOMES reported: at every 4 weeks up to 12 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Symptom score FUNCTIONAL STATUS
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Not reported WITHDRAWALS: Reported |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funding: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Packets of drugs for each patient were labelled according to randomisation sequence by a person not involved in initial or subsequent assessment of the patients. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy, identical MDIs or tables of drugs were used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Total of 13.3% (4) and 6.25% (2) patients withdrew from montelukast and budesonide group, respectively and the reasons for withdrawals were reported by group. |
| Selective reporting (reporting bias) | Low risk | Analysis was not done with intention‐to‐treat analysis; the data on secondary outcomes was insufficiently reported to be aggregated; no data on visit to emergency or local practitioner. |
| Other bias | Low risk | No apparent other bias |
Laitinen 1997.
| Methods | DESIGN: Parallel‐group, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 481 Z80: 159 Z20: 162 BDP: 160 WITHDRAWALS: Z80:10 (6.3%) Z20:14 (8.6%) BDP:13 (8.1)% AGE: Z80:38.7 ± 12.6 Z20:37.8 ± 14.0 BDP:38.3 ± 13.6 GENDER (% male): Z80: 50.9% Z20: 53.1% BDP: 48.1% % Pred. FEV1 (mean ± SD): Z80: 79 ± 12.8 Z20:78.9 ±12.9 BDP:80.8 ± 12.7 Mean (± SD) β2‐agonist use (puffs/day): Not described ATOPY/ALLERGIC RHINITIS: Z80: 88 (55%) Z20: 86 (53%) BDP: 86 (54%) ASTHMA DURATION (Years, SD): Z80:13.4 ± 11 Z20:11.3 ± 10 BDP:13.3 ± 12 ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: not described Intervention Period: 6 weeks TEST GROUP 1: Zafirlukast (Accolate) 20 mg twice a day p.o. TEST GROUP 2: Zafirlukast (Accolate) 80 mg twice a day p.o. CONTROL GROUP: Inhaled beclomethasone dipropionate 200‐250 μg twice a day DEVICE: Not described CO‐INTERVENTION: Not specified |
|
| Outcomes | ANALYSIS (ITT not specified) OUTCOMES reported at 6 weeks PULMONARY FUNCTION TESTS
SYMPTOM SCORES: Change from baseline daytime symptom scores (max = 21) FUNCTIONAL STATUS
INFLAMMATORY MARKERS: Change in eosinophil counts ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 225 μg | |
| Notes | Abstract (1997) Funded by Zeneca Confirmation of methodology and data extraction |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation with block of 6 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, withdrawals and adverse effects were reported by groups, |
| Selective reporting (reporting bias) | Low risk | Not found |
| Other bias | Low risk | No apparent other bias |
Laviolette 1999.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 642 M: 201 BDP: 200 Placebo: 48 M+BDP: 193 WITHDRAWALS: M: 42 (21%) BDP: 22 (11%) AGE: M:38 (15‐75) BDP:39 (15‐78) GENDER (% male): M: 49% BDP: 52% % Pred. FEV1 (mean ±SD): M: 72 ± 12 BDP: 72 ± 12 Mean (± SD) β2‐agonist use (puffs/day): M: 3.5 ± 2.3 BDP: 3.5 ± 2.5 ATOPY/ALLERGIC RHINITIS: M: 74% BDP: 74% ELIGIBILITY CRITERIA:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 4 weeks Intervention Period: 16 weeks TEST GROUP 1: Montelukast 10 mg per day + Placebo (after blind beclomethasone removal) TEST GROUP 2: Montelukast 10 mg per day + inhaled beclomethasone 200 μg twice a day (not used in this review) CONTROL GROUP 1: Placebo + inhaled beclomethasone 200 μg twice a day CONTROL GROUP 2: Placebo + Placebo (not used in this review) DEVICE: Aero‐Chamber spacer device CO‐INTERVENTION: Not specified |
|
| Outcomes | ANALYSIS ( Intention‐to‐treat) OUTCOMES reported at 6 and 16 weeks PULMONARY FUNCTION TESTS
SYMPTOM SCORES: Change from baseline daytime asthma symptoms scores (6‐point scale) FUNCTIONAL STATUS:
INFLAMMATORY MARKERS: Change from baseline peripheral blood eosinophil counts ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full Text publication (1999) Funded by Merck Confirmation of methodology and data received from Reiss 01 Sept 1999 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation with block of 6 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All data presented, 20.9% and 11.0% withdrawal in montelukast and beclomethasone respectively, no intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Not found |
| Other bias | Low risk | No apparent other bias |
Lazarus 2007.
| Methods | DESIGN (ONLY DATA OF NONSMOKER PATIENTS ARE PRESENTED IN THIS REVIEW) Cross‐over, multicentric, randomised, clinical trial. | |
| Participants | CORTICOSTEROID‐NAIVE ASTHMATIC PATIENT RANDOMISED: N = 44 WITHDRAWALS: 1 (4.6%) AGE: mean years: 28.98 ± 5.92 GENDER (% male): Not reported % Pred. FEV1 (mean ± SD): 80.16 ± 9.16 Mean (±SD) β2‐agonist use (puffs/day): Not reported ATOPY/ALLERGIC RHINITIS: Not reported DURATION OF ASTHMA: More than 10 years ELIGIBILITY CRITERIA:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 2‐4 weeks Intervention Period: 16 weeks TEST GROUP 1: Montelukast 10 mg qd TEST GROUP 2: Inhaled beclomethasone 160 μg twice daily DEVICE: Inhaled (HFA)–beclomethasone dipropionate or QVAR CO‐INTERVENTION: Not specified |
|
| Outcomes | ANALYSIS (ITT‐ Not reported) OUTCOMES reported at 8 weeks PULMONARY FUNCTION TESTS
SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: AQOL average score INFLAMMATORY MARKERS:
ADVERSE EVENTS: Not reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 400 μg | |
| Notes | Full Text publication Funded: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: randomisation was performed online from the data coordinating centre |
| Allocation concealment (selection bias) | Low risk | Matching placebos were used; Treatment medication for each subject was packaged with unique number and distributed to the clinical centres |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Triple blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All data presented; analysis was not done using intention‐to‐treat analysis; data for drop out patients was not addressed |
| Selective reporting (reporting bias) | Low risk | Not found |
| Other bias | Low risk | No apparent other bias |
Lu 2009.
| Methods | DESIGN: Cross‐over, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 406 (including all other groups) M:46 BECLO: 63 WITHDRAWALS: Not reported group wise AGE: 34.11 ± 11.85 GENDER (% male): N: 48.0% % PRED FEV1 (mean, SD): Not reported Mean (± SD) β2‐agonist use (puffs/day): Not reported ATOPY: Not reported ASTHMA DURATION (years): Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 2 weeks Intervention Period: 6 weeks Wash‐out Period: 2 weeks TEST GROUP: Montelukast 10 mg per day at bedtime p.o. CONTROL GROUP 1: Beclomethasone 200 μg twice a day CONTROL GROUP 2: Placebo (not used in this review) DEVICE: Not reported |
|
| Outcomes | ANALYSIS BY INTENTION‐TO‐TREAT ANALYSIS OUTCOMES reported at 6 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Daytime asthma symptoms score FUNCTIONAL STATUS: Total daily β2‐agonist use INFLAMMATORY MARKERS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full Text publication Funded: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All data presented, intention‐to‐treat analysis, clinical and laboratory adverse experiences were reported however withdrawal were not reported by group |
| Selective reporting (reporting bias) | Low risk | Primary, secondary and tertiary outcomes were defined |
| Other bias | Low risk | No apparent other bias |
Malmstrom 1999.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. Confirmation of methodology obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 895 M:387 BDP:251 PLACEBO:257 WITHDRAWALS: M: 33 (8.5%) BDP:18 (7.2%) AGE: M: 35 (15‐78) BDP: 35 (15‐74) GENDER (% male): M: 40% BDP: 35% ‐%PRED FEV1 (mean, SD): M : 65 ± 10 BDP: 65 ± 10 Mean (± SD) β2‐agonist use (puffs/day): M: 5.4 ± 3.4 BDP: 5.5 ± 4.2 ATOPY: M: 62 % BDP:61 % ASTHMA DURATION years(range): M: 17 (1‐67) BDP: 18 (0.5‐65) ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 2 weeks Intervention Period: 12 weeks Wash‐out Period: 3 weeks TEST GROUP: Montelukast 10 mg per day at bedtime p.o. CONTROL GROUP 1: Beclomethasone 200 μg twice a day CONTROL GROUP 2: Placebo (not used in this review) DEVICE: Spacer for Beclomethasone CO‐INTERVENTION: Theophylline (10%) |
|
| Outcomes | ANALYSIS ( ITT not specified) OUTCOMES reported at 6 and 12 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Change from baseline daytime symptom scores FUNCTIONAL STATUS
INFLAMMATORY MARKERS: Change from baseline peripheral blood eosinophil counts ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full Text publication (1999) Funded by Merck Confirmation of methodology and data extraction obtained. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation with block of 7 |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, reasons for withdrawal and adverse effects were mentioned by group |
| Selective reporting (reporting bias) | Low risk | Not found |
| Other bias | Low risk | No apparent other bias |
Maspero 2001.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 124 M: 83 BDP: 41 WITHDRAWALS: M: 5 (6%) BDP: 3 (7%) AGE: 6‐11 years M: 9.5 ± 1.8 (6‐12) years BDP:9.7 ± 1.4 (7‐12) years GENDER (% male): M: 64 % BDP: 51% BASELINE SEVERITY: Moderate Mean % Predicted FEV1: M: 82 ± 13 BDP:82 ± 17 ATOPY/ALLERGEN TRIGGERS: M: 66% BDP:61% ASTHMA DURATION (years): Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: unspecified Primary RCT Period: 10 weeks Extension Period: 6 months TEST GROUP: Montelukast 5 mg per day at bedtime p.o. CONTROL GROUP: Inhaled BPD 100 μg tid DEVICE: MDI, spacer optional CO‐INTERVENTION: Not specified |
|
| Outcomes | ANALYSIS by Intention‐to‐treat OUTCOMES reported at 12, 24 weeks PULMONARY FUNCTION TESTS: Change from baseline FEV1 (L) SYMPTOM SCORES: Not reported FUNCTIONAL STATUS
INFLAMMATORY MEDIATORS: *LTC4 in nasal wash ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 150 μg | |
| Notes | Full‐text publication of primary and extension study (1999) Funded by Merck Frosst confirmation of methodology and data extraction obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label, the criteria for election of participants from last study not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Unclear risk | Peak expiratory flow rate was mentioned in method but data was not presented in results, primary and secondary outcomes not specified |
| Other bias | Low risk | No apparent other bias |
Meltzer 2002.
| Methods | DESIGN: Parallel‐group, multi‐centre, randomised, clinical trial. Confirmation of methodology: Not obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 522 M = 264 FP = 258 WITHDRAWALS; N (%) M: 67(25%) FP: 60 (23%) AGE: mean (range) M: 35.4 (15‐77) years FP: 36.2 (15‐73) years GENDER (% male): M: 41 % FP: 51 % BASELINE SEVERITY: Moderate % Predicted FEV1 (Mean ± SE): M: 65.9 ± 9.1 FP: 65.6 ± 8.9 ATOPY/ALLERGEN TRIGGERS: Not reported ASTHMA DURATION (years) M: 74% for >= 10 years FP: 78% for >= 10 years ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 8‐14 days Intervention: 24 weeks TEST GROUP: Montelukast 10 mg per day at bedtime p.o. CONTROL GROUP: Inhaled FP 100 μg twice a day DEVICE: MDI with spacer CO‐INTERVENTION: Rx permitted: antihistamines, nasal decongestants, intranasal medications (including corticosteroids) for the treatment of rhinitis |
|
| Outcomes | ANALYSIS by intention‐to‐treat OUTCOMES reported at 24 weeks or endpoint PULMONARY FUNCTION TESTS
SYMPTOM SCORES
FUNCTIONAL STATUS
INFLAMMATORY MARKERS: Not reported ADVERSE EVENTS: Not reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication (2002) Funded by GlaxoSmithKline Confirmation of methodology and data extraction: not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated in block of 4 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented including withdrawal with reasons by group and adverse effects by group, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were defined |
| Other bias | Low risk | No apparent other bias |
MK0479‐332.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. Confirmation of methodology: Not obtained |
|
| Participants | PARTICIPANTS WITH PERSISTENT ASTHMA RANDOMISED: N = 683 MON: 347 FP: 336 WITHDRAWALS: MON: 51 (14.70%) FP: 50 (14.88%) AGE (± SD): MON: 37.7 (± 10.4) FP: 38.4 (± 10.7) GENDER (% male): MON: 53.03% FP: 56.25% ASTHMA SEVERITY: Not described ASTHMA DURATION: Not described % Pred. FEV1 (±SD): MON: 75.7 (± 7.0) FP: 76.1 (± 6.6) MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported DOSE OF INHALED CORTICOSTEROIDS AT STUDT ENTRY AND AT RUN‐IN: Not described ATOPY: Not described ELIGIBILITY CRITERIA:
EXCLUSION:: Subjects with chronic obstructive pulmonary disease. |
|
| Interventions | PROTOCOL Duration Run‐in Period: not reported Intervention Period: 12 weeks CONTROL GROUP: Fluticasone propionate 250 twice a day TEST GROUP: Montelukast 10 mg QD DEVICE: Not reported Criteria for withdrawal from study: Reported |
|
| Outcomes | ANALYSIS: Not reported OUTCOMES reported at 24 weeks PULMONARY FUNCTION TESTS: Change from baseline in morning PEFR SYMPTOM SCORES: Change from baseline in mean day time symptom score FUNCTIONAL STATUS: *Percentage of asthma‐control days over the 6‐month treatment period, INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 500 | |
| Notes | Funding not reported but trials conducted by pharmaceutical company Confirmation of methodology and data extraction: not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation:not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
Nathan 2001.
| Methods | DESIGN: Parallel‐groups, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 294 Z = 150 FP = 144 WITHDRAWALS; N (%): Z: 12 (8%) FP: 5 (3%) AGE: Mean ( range) Z: 32 (12‐70) FP: 31 (12‐54) GENDER (% male): Z: 65 (43%) FP: 65 (45%) BASELINE SEVERITY: Not mentioned % Pred. FEV1 (Mean ± SD): Z: 68.9 ± 7.6 FP: 68 ± 8.5 MEAN (± SD) β2‐AGONIST USE (puffs/day): Z = 4.4 ± 2.7 FP = 4.4 ± 2.6 ATOPY: Not reported ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 7‐14 days Intervention: 4 weeks TEST GROUP: Zafirlukast 20 mg twice a day CONTROL GROUP: Fluticasone 100 μg twice a day DEVICE: Not reported CO‐INTERVENTION: None permitted |
|
| Outcomes | PER PROTOCOL ANALYSIS (not ITT) OUTCOMES reported at 4 and endpoint PULMONARY FUNCTION TESTS
SYMPTOM SCORES
FUNCTIONAL STATUS: % Change from baseline mean daily B2‐agonist use (puffs/day) INFLAMMATORY MARKERS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication (2001) Funded by: Not reported Confirmation of methodology and data extraction obtained/not requested/not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All data presented, adverse events by group were mentioned, 3% and 8% patients were withdrawn in fluticasone and zafirlukast, not a intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were not defined, but no bias was observed |
| Other bias | Low risk | No apparent other bias |
NCT00442559.
| Methods | DESIGN: Parallel‐groups, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 191 M = 100 ICS = 91 WITHDRAWALS; N (%): M: 34 (34%) ICS: 35 (38.5%) AGE: Mean ( years, SD): M: 5.4 (3.0) ICS: 6.1 (2.6) GENDER: n (% male): 39 (20.4%) BASELINE SEVERITY: Not mentioned % Pred. FEV1 (Mean ± SD): Not reported β2‐AGONIST USE (puffs per day): MEAN (± SD): Not reported ATOPY: Not reported ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Intervention: 12 weeks TEST GROUP: Montelukast 4 ‐ 5 mg per day CONTROL GROUP: ICS DEVICE: Not reported CO‐INTERVENTION: None permitted |
|
| Outcomes | PER PROTOCOL ANALYSIS (not ITT) OUTCOMES reported at 12 week endpoint PULMONARY FUNCTION TESTS: Not reported SYMPTOM SCORES:
FUNCTIONAL STATUS: Not reported INFLAMMATORY MARKERS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | Not reported | |
| Notes | Unpublised data (2008) Funded by: Merck |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High withdrawal rate but balanced in both groups |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported |
| Other bias | Low risk | No apparent other bias |
Ng 2007.
| Methods | DESIGN: Cross over, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N =19 M = 13 BD = 15 WITHDRAWALS; N (%) M: 5 (38%) BD: 7 (47%) AGE: Mean±SD M: 8.53 ± 2.17 BD: 8.39 ± 2.57 GENDER (% male): M: 8 (61.54%) BD: 9 (60%) BASELINE SEVERITY: Not mentioned % Pred. FEV1 (Mean ± SD): Not mentioned β2‐AGONIST USE (puffs per day): MEAN (± SD): Not mentioned ATOPY: Not mentioned ASTHMA DURATION: Not mentioned ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Intervention: 4 weeks TEST GROUP: Zafirlukast 20 mg twice a day CONTROL GROUP: Fluticasone 100 μg twice a day DEVICE: Not reported CO‐INTERVENTION: None permitted |
|
| Outcomes | ANALYSIS BY INTENTION‐TO‐TREAT ANALYSIS OUTCOMES reported at 8 week endpoint PULMONARY FUNCTION TESTS: *FEV‐1 % predicted SYMPTOM SCORES: Proportion of symptom‐free day FUNCTIONAL STATUS: % Change from baseline mean daily B2‐agonist use (puffs/day) INFLAMMATORY MARKERS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication (2007) Funded by: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: randomisation table was prepared by pharmacist |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Dobule blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented; intention‐to‐treat analysis was done; reason for drop outs by groups were mentioned |
| Selective reporting (reporting bias) | High risk | Primary outcome was presented; however the day time asthma symptoms and nocturnal awakenings (secondary outcomes) were not presented in details |
| Other bias | Low risk | No apparent other bias |
Ostrom 2005.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | ASTHMATIC PATIENTS WITH AT LEAST 6‐MONTH HISTORY OF CHRONIC ASTHMA AS PER ATS GUIDELINES RANDOMISED: N = 342 M = 170 FP = 172 WITHDRAWALS; N(%) M: 21% FP: 13% AGE: Mean years (range): M: 9.6 (6‐12) FP: 9.1 (5‐12) GENDER (% male): M: 68% FP: 63% BASELINE SEVERITY: ‐FEV1 of 60% to 85% of predicted % Pred. FEV1 (Mean ± SD) M: 76.4 ± 8.5 FP: 75.4 ± 9.4 MEAN ± SD) β2‐AGONIST USE (puffs/day) M=2.42 ± 0.12 FP=2.26 ± 0.12 ATOPY: Not reported ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 8‐14 days Intervention: 12 weeks TEST GROUP: Montelukast sodium chewable tablet 5 mg once daily CONTROL GROUP: Fluticasone propionate 50 μg twice daily DEVICE: DISKUS multi‐dose powder inhaler CO‐INTERVENTION: None permitted |
|
| Outcomes | ANALYSIS by intention‐to‐treat OUTCOMES reported at 12 weeks or endpoint PULMONARY FUNCTION TESTS
SYMPTOM SCORES
FUNCTIONAL STATUS
INFLAMMATORY MARKERS: Not reported ADVERSE EVENTS: Not reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 100 μg | |
| Notes | Full‐text publication Funded: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details were not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes are reported including withdrawal rate and reasons for withdrawals were well described by group, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary outcomes was defined |
| Other bias | Low risk | No apparent other bias |
Overbeek 2004.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | ATOPIC MILD ASTHMA PATIENTS RANDOMISED: N = 36 M = 19 BDP = 17 WITHDRAWALS; % Total: 0% AGE: mean (range) years M = 24.5 (19–49) BDP = 31.6 (19–57) GENDER (% male): 55.56% % Pred. FEV1 Mean ± SE M = 88.3 ± 13.6 BDP = 84.9 ± 9.7 ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 8 weeks Intervention: 8 weeks TEST GROUP: Montelukast sodium capsule (10 mg nocte) CONTROL GROUP: Fluticasone propionate (100 μg twice a day) by Diskus inhaler DEVICE: DISKUS multi‐dose inhaler CO‐INTERVENTION: None permitted |
|
| Outcomes | ANALYSIS by intention‐to‐treat. Not reported OUTCOMES reported at 8 weeks PULMONARY FUNCTION TESTS
SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Not reported INFLAMMATORY MARKERS:
ADVERSE EVENTS: Not reported WITHDRAWALS: Reported |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded by GlaxoSmithKline R&D, UK |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no withdrawal rate; all patients completed the study |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the manuscript are reported, including adverse events |
| Other bias | Low risk | No apparent other bias |
Peroni 2005.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | MILD ASTHMATIC CHILDREN ALLERGIC TO HOUSE DUST MITE RANDOMISED: N = 24 M = 12 BDP = 12 WITHDRAWALS; N(%): 3 (12.5%) AGE: range years: 6‐13 years GENDER (% male): 41.67% % Pred. FEV1 (Median%) M = 99% BDP = 108% ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Intervention: Not clear TEST GROUP: Montelukast (5 mg administered once a day in the evening), CONTROL GROUP: Budesonide (100 µg twice daily) DEVICE: Turbuhaler CO‐INTERVENTION: None permitted |
|
| Outcomes | ANALYSIS by intention‐to‐treat: Not reported PULMONARY FUNCTION TESTS
SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Not reported INFLAMMATORY MARKERS: Sputum eosinophil cell count (%) ADVERSE EVENTS: Not reported WITHDRAWALS: Reported |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funding source: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: used randomisation table prepared by a person not included in the study |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope were used to allocate drug however the concealment method is not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind; centralized randomisation table and placebo preparation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Analysis was not done by intention‐to‐treat; Data from 8.3% and 16.6% of patients were not evaluated due to lack of compliance to treatment. |
| Selective reporting (reporting bias) | Low risk | Primary outcome was not defined but not bias was observed |
| Other bias | Low risk | No apparent other bias |
Peters 2007.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | ASTHMATIC PATIENTS RANDOMISED: N = 500 M = 165 FP = 169 WITHDRAWALS; N (%) M = 20 FP = 13 AGE: Mean ± SD M = 32.4 ± 15.4 FP = 29.3 ± 14.6 GENDER (% male): M = 42.8 FP = 39.1 % of predicted value: mean ± SD At randomisation M = 91.9 ± 11.1 BDP = 92.8 ± 10.4 ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 4‐6 weeks Intervention: 16 weeks TEST GROUP: Montelukast (Singulair, GlaxoSmithKline), 5 mg once daily for children ages 6 to 14 years and 10 mg once daily for persons 15 years or older CONTROL GROUP: Inhaled fluticasone propionate (100 μg twice daily) DEVICE: Flovent Diskus CO‐INTERVENTION: None permitted |
|
| Outcomes | ANALYSIS by intention‐to‐treat OUTCOMES reported at 16 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES
FUNCTIONAL STATUS
INFLAMMATORY MARKERS: Not reported ADVERSE EVENTS: Reported WITHDRAWALS: Reported |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funding source: Unrestricted grant from GlaxoSmithKline, a grant from the American Lung Association. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: randomised using permuted‐block design stratified by clinic and age of patient. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo and matching montelukast tablets used for the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported in detail, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary and secondary data were well defined |
| Other bias | Low risk | No apparent other bias |
Riccioni 2001.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 48 Z = 12 BUD = 12 Z+BUD = 12 Control = 12 WITHDRAWALS; Z = 2 (16%) BUD = 1 (8%) Z+BUD = 2 (16%) AGE: mean (± SD) years Z: 33.75 (± 11.24) BUD: 32.15 (± 10.27) Z+BUD: 33.44 (± 11.12) Control: 29.15 (± 10.34) GENDER: N (% male): Z: 6 (50) BUD: 6 (50) Z+BUD: 6 (50) Control: 6 (50) BASELINE SEVERITY: Mild persistent % Predicted FEV1 (Mean ± SD) Z: 94.75 ± 7.68 BUD: 92.75 ± 9.87 Z+BUD: 92.16 ± 5.06 Control: 95.75 ± 5.84 ATOPY/ALLERGEN TRIGGERS: Not reported ASTHMA DURATION (years): 1 year ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 2 weeks Intervention: 8 weeks TEST GROUP: Zafirlukast 20 mg twice a day TEST GROUP 2: Zafirlukast 20 mg twice a day + Budesonide 400 μg twice a day CONTROL GROUP: Budesonide 400 μg twice a day CONTROL GROUP 2: Placebo DEVICE: Not reported CO‐INTERVENTION: Not reported |
|
| Outcomes | ANALYSIS PER PROTOCOL (not by ITT) OUTCOMES Reported at 8 weeks PULMONARY FUNCTION TEST
SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Not reported INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Not mentioned WITHDRAWALS: Not reported Primary outcome: Not specified. |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 400 μg | |
| Notes | Full‐text publication (2001) Funded by University Confirmation of methodology and data extraction: obtained from Graziano Riccioni, Oct 2003 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data on asthma diary, number of puffs used, compliance of treatment were mentioned in methodology but not addressed in the results |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were not defined, but no bias was observed |
| Other bias | Low risk | No apparent other bias |
Riccioni 2002a.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. Confirmation of methodology: Obtained (Oct 2003) |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 40 M = 20 BUD = 20 WITHDRAWALS: Reported AGE: years ± SD M = 25.16 ± 7.68 BUD = 26.18 ± 6.15 GENDER (% male): M = 11 (55) BUD = 10 (50) ASTHMA SEVERITY: Mild and persistent ASTHMA DURATION: Not mentioned % Pred. FEV1: M = 93.15 ± 12.17 BUD = 94.73 ± 10.18 Mean (± SD) β2‐agonist use (puffs per day): Not described ATOPY: M = 12 (60%) BUD = 10 (40%) PARTICIPANTS WITH ALLERGIC RHINITIS: M: 2 (10%) BUD: 2 (10%) ELIGIBILITY CRITERIA:
EXCLUSION CRITERIA:
|
|
| Interventions | PROTOCOL Duration Run‐in: 2 weeks Intervention: 16 weeks TEST GROUP: 10 mg Montelukast per day CONTROL GROUP: 400 μg Budesonide twice a day DEVICE: Turbuhaler CRITERIA FOR WITHDRAWAL: Mentioned CO‐INTERVENTION: Not permitted |
|
| Outcomes | ANALYSIS PER PROTOCOL
(no ITT analysis mentioned) OUTCOMES: Reported at 16 weeks PULMONARY FUNCTION TEST:
SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Not mentioned INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported (overall, not in details) WITHDRAWALS: Reported Primary outcome: not specified |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 400 μg | |
| Notes | Full‐text publication Funded by the University "G. D'Annunzio" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind (patient and assessor) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were not defined but no bias was observed |
| Other bias | Low risk | Not found |
Riccioni 2002b.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. CONFIRMATION OF METHODOLOGY: obtained (Oct 2003) |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 45 M = 15 BUD = 15 M+BUD = 15 WITHDRAWALS: M = 1 (7%) BUD = 1 (7%) M+BUD = 1 (7%) AGE: years ± SD M = 26.7 ± 8.6 BUD = 26.9 ± 12.3 M+BUD = 28.2 ± 10 GENDER: N (% male): M = 9 (60) BUD = 8 (53.3) M+BUD = 5(33.7) ASTHMA SEVERITY: Mild ASTHMA DURATION: 1 year % Pred. FEV1: mean (range): M = 97 (85‐123) BUD = 97 (76‐123) M+BUD = 99 (84‐131) Mean (± SD) β2‐agonist use (puffs/day): Not described ATOPY: 100% (Definition not mentioned) ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in: 4 weeks Intervention: 16 weeks TEST GROUP 1: 10 mg Montelukast once daily TEST GROUP 2: 10 mg Montelukast once daily + 400 μg Budesonide twice a day CONTROL GROUP: 400 μg Budesonide twice a day DEVICE: Turbo haler CRITERIA FOR WITHDRAWAL: Not described CO‐INTERVENTION: None permitted |
|
| Outcomes | ANALYSIS PER PROTOCOL: not by ITT OUTCOMES: Reported at 16 weeks PULMONARY FUNCTION TEST:
SYMPTOM SCORES: Not reported FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Not mentioned WITHDRAWALS: Reported Primary outcome: Not specified |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 400 μg | |
| Notes | Full‐text publication Funded by University Confirmation of methodology and data extraction: obtained by Graziono Riccioni, Oct 2003 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data on compliance of treatment and adverse effects were mentioned in methodology but not addressed in the results |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were not defined, but no bias was observed |
| Other bias | Low risk | No apparent other bias |
Riccioni 2003.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | ATOPIC NON‐SMOKING MILD‐PERSISTENT BRONCHIAL ASTHMATICS RANDOMISED: N = 45 N = 51 M = 12 BUD = 14 WITHDRAWALS: Not mentioned AGE: years ± SD: M = 26.16 ± 5.07 BUD = 25.85 ± 10.46 GENDER (% male): M = 58.33% BUD = 57.14% ASTHMA SEVERITY: Mild ASTHMA DURATION: 1 year % Pred. FEV1: mean ± SD: M=94.75 ± 7.68 BUD = 99.85 ± 12.92 ATOPY: 100% (Dermatophagoides Pteronyssinus) ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in: 2 weeks Intervention: 12 weeks TEST GROUP 1: 10 mg Montelukast once daily CONTROL GROUP: 400 μg Budesonide twice a day DEVICE: Not mentioned CRITERIA FOR WITHDRAWAL: Not described CO‐INTERVENTION: None permitted |
|
| Outcomes | ANALYSIS : Not mentioned OUTCOMES Reported at 12 weeks PULMONARY FUNCTION TEST:
SYMPTOM SCORES: Not reported INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Not mentioned WITHDRAWALS: Not reported Primary outcome: Not specified |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 400 μg | |
| Notes | Full‐text publication Funded: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There is no report as to whether some patients withdrew from the study after randomisation |
| Selective reporting (reporting bias) | High risk | The primary outcome was not specified. The data on several outcomes was insufficiently reported to be aggregated namely, number of puffs used each day; daily asthma diary; adverse events, FVC, compliance of treatment were not reported |
| Other bias | Low risk | No apparent other bias |
Sheth 2001.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. Confirmation of methodology: Not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: unclear if stratified randomisation on baseline FEV1 value FEV1 > 70% N = 152 Z = 55 FP = 51 Placebo = 46 FEV1 < 70% N = 177 Z = 52 FP = 60 Placebo = 65 WITHDRAWALS: Not reported AGE: mean (± SD) years: Not reported GENDER (% male): Not reported BASELINE SEVERITY: Not reported % Predicted FEV1: (FEV1 > 70%) Z: 75% FP:76% Placebo: 76% (FEV1 < 70%) Z: 63% FP: 62% Placebo: 63% ATOPY/ALLERGEN TRIGGERS: Not reported ASTHMA DURATION (years): Not reported ELIGIBILITY CRITERIA:
EXCLUSION: Not reported |
|
| Interventions | PROTOCOL Duration Intervention: 12 weeks TEST GROUP: Zafirlukast 20 mg twice a day CONTROL GROUP: Fluticasone 100 μg twice a day DEVICE: Not mentioned CRITERIA FOR WITHDRAWAL: Not mentioned CO‐INTERVENTION: Not mentioned |
|
| Outcomes | ANALYSIS (ITT) OUTCOMES reported at 12 weeks PULMONARY FUNCTION TESTS
SYMPTOM SCORES: Change in symptom‐free days (%) FUNCTIONAL STATUS: Change from baseline in rescue β2‐agonist use (puffs/day) INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Not reported WITHDRAWALS: Not reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Abstract Funding not mentioned, probably by GSK Confirmation of methodology and data extraction: Not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind ‐double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Primary outcome was defined |
| Other bias | Low risk | No apparent other bias |
Sorkness 2007.
| Methods | DESIGN: Parallel‐group, clinical trial. | |
| Participants | CHILDREN WITH MILD TO MODERATE PERSISTENT ASTHMA RANDOMISED: N = 285 M = 95 FP = 96 WITHDRAWALS: M: 12 (12.63%) FP: 10 (9.6%) AGE: mean ± SD years M: 9.6 ± 2.2 FP: 9.8 ± 2.2 GENDER: % male M: 60.0% FP: 59.4% ASTHMA SEVERITY: Mild to moderate % Pred. FEV1 (mean ± SD): M: 97.7 ± 13.6 FP:97.8 ± 12.2 ATOPY or ALLERGIC RHINITIS: M: 76.8% FP: 78.1% ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 2‐4 weeks Intervention Period: 486 weeks TEST GROUP: Montelukast 10 mg qd p.o. CONTROL GROUP 1: Fluticasone propionate 100 μcg morning and 100 μcg evening DEVICE: Flovent Diskus; GlaxoSmithKline CO‐INTERVENTION: None permitted |
|
| Outcomes | ANALYSIS by Intention‐to‐treat OUTCOMES reported at 48 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Asthma control questionnaire FUNCTIONAL STATUS:
INFLAMMATORY MEDIATORS: ENO, % ADVERSE EVENTS: Reported WITHDRAWALS: Reported *Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication Funded: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: randomisation was stratified by centre |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind; double‐dummy with identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Approximately 12% of withdrawal, balanced between groups but the reasons for withdrawal were not specified by group. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the manuscript are reported, including adverse events |
| Other bias | Low risk | No apparent other bias |
Stelmach 2002a.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. Confirmation of methodology: Obtained | |
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 91 M: 18 Triamcinolone: 19 Formoterol: 18 Placebo: 36 WITHDRAWALS: M: 3 (17%) Triamcinolone: 3 (17%) AGE: years ± SD: (without withdrawals): M: 11.1 ± 1.8 yr Triamcinolone:12.2 ± 2.1 yr GENDER (% male): M: 60% Triamcinolone:55% ASTHMA SEVERITY: Moderate % Pred. FEV1 % (± SD): M: 78.1 ± 3.1 Triamcinolone:73.5 ± 4.3 Mean (± SD) β2‐agonist use (puffs/day): Not described ALLERGIC RHINITIS: M: 15% Triamcinolone: 10% ATOPY (to dust mites): M: 100% Triamcinolone: 100% DURATION OF ASTHMA: M: 3.8 ± 0.6 Triamcinolone:3.7 ± 0.5 ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 4 weeks Intervention Period: 8 weeks TEST GROUP: Montelukast 5 mg per day if <=14 years (10 mg per day, otherwise) CONTROL GROUP 1: Inhaled Triamcinolone acetonide 100ug/day qid CONTROL GROUP 2: Placebo (not used in this review) CONTROL GROUP 3: Formoterol 12 μg twice a day (not used in this review) DEVICE: MDI (actuation inhaler) CO‐INTERVENTION: No other Rx allowed other than rescue b2‐agonists or rescue oral corticosteroids |
|
| Outcomes | ANALYSIS per protocol OUTCOMES reported at 4 weeks PULMONARY FUNCTION TESTS: Change from baseline FEV1 ‐Change from baseline in PC 20 SYMPTOM SCORES: Total score (3‐point for daytime symptoms + 3‐point for night‐time symptoms + 3 points for use of rescue β2‐agonists) FUNCTIONAL STATUS: Patients with exacerbations requiring systemic corticosteroids INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome not specified |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 100 μg | |
| Notes | Full Text publication (2002) Self‐funded by authors Confirmation of methodology and data obtained from I. Stelmach June 2003 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated allocation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, reasons for withdrawal were mentioned |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were not defined, but no bias was observed |
| Other bias | Low risk | No apparent other bias |
Stelmach 2002b.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. Confirmation of methodology obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N =154 M: 27 Triamcinolone: 28 Formoterol: 28 Nedocromil: 26 Placebo: 45 WITHDRAWALS (N = 14) M: 11% (3/27) Triamcinolone: 11 % (3/28) Formoterol: 11% (3/28) Nedocromil: 0% Placebo: 12% (40/45) AGE: years ± SD: M: 12.8 ± 1.7 yr Triamcinolone:13,1 ± 2.4 yr GENDER (% male): M: 48.1% Triamcinolone:53.6 % ASTHMA SEVERITY: Moderate asthma % Pred. FEV1 % ±SD: ‐ after withdrawals M: 73.7 ± 5.4 Triamcinolone:73.2 ± 3.8 Mean (± SD) β2‐agonist use (puffs/day): Not described ATOPY (described as chronic atopic): M: 100% Triamcinolone: 100% ALLERGIC RHINITIS: Montelukast: 7.1% Triamcinolone:10.7% DURATION OF ASTHMA (years): M: 3.8 ± 0.5 Triamcinolone: 3.7 ± 0.6 ASTHMA SYMPTOMS: M: 6.2 ± 1.0 Triamcinolone: 7.1 ± 0.9 (score of 0‐9) ELIGIBILITY CRITERIA:
EXCLUSION:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 4 weeks Intervention Period: 4 weeks TEST GROUP: Montelukast 5 mg per day if <= 14 years (10 mg per day, otherwise) CONTROL GROUP 1: Inhaled Triamcinolone acetonide 200ug twice a day CONTROL GROUP 2: Placebo (not used in this review) CONTROL GROUP 3: Formoterol 12 μg twice a day (not used in this review) CONTROL GROUP 4: Nedocromil 0.002 g/inhalation 2 inhalations qid (not used in this review) DEVICE: MDI (actuation inhaler) CO‐INTERVENTION: No other Rx allowed other than rescue β2‐agonist or rescue systemic corticosteroids. |
|
| Outcomes | ANALYSIS per protocol OUTCOMES reported at 4 weeks PULMONARY FUNCTION TESTS: Change from baseline FEV1 ‐Change from baseline in PC 20 SYMPTOM SCORES: Total score (3‐point for daytime symptoms + 3‐point for night‐time symptoms + 3 points for use of rescue β2‐agonists) FUNCTIONAL STATUS Patients with exacerbations requiring systemic corticosteroids INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Not reported WITHDRAWALS: Reported overall (not by group) Primary outcome: Not specified |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 100 μg | |
| Notes | Full Text publication (2002) Self‐Funded Confirmation of methodology and data obtained from I. Stelmach June 2003 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated allocation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, overall reasons for withdrawal were mentioned |
| Selective reporting (reporting bias) | Low risk | Primary outcome was not defined but no bias was observed |
| Other bias | Low risk | Not found |
Stelmach 2004.
| Methods | DESIGN: Three armed, parallel‐group, randomised, clinical trial. | |
| Participants | CHILDREN WITH HISTORY OF ASTHMA AND SENSITIVE TO HOUSE DUST MITES (DERMATOPHAGOIDES PTERONYSSINUS AND/OR DERMATOPHAGOIDES FARINE) RANDOMISED: N =250 M: 80 Triamcinolone: 83 WITHDRAWALS: N = 4 M: 2.5% Triamcinolone: 2.41% AGE: years ± SD: M: 12.6 ± 2.097 yr Triamcinolone:11.9 ± 1.51 yr GENDER (% male): M: 57.69 % Triamcinolone:54.32 % ASTHMA SEVERITY: Mild to moderate asthma % Pred. FEV1 % ±SD ‐ after withdrawals: M: 76.2 ± 5.16 Triamcinolone:74.3 ± 3.88 ATOPY (described as chronic atopic): M: 100% Triamcinolone: 100% DURATION OF ASTHMA (years): M: 3.9 ± 0.58 Triamcinolone: 3.7 ± 0.54 ASTHMA SYMPTOMS: M: 6.6 ± 1.05 Triamcinolone: 6.9 ± 0.99 ELIGIBILITY CRITERIA: Age= 6‐18 years
|
|
| Interventions | PROTOCOL Duration Run‐in Period: Not mentioned Intervention Period: 4 weeks TEST GROUP: Montelukast 5 mg tablets in children aged 6–14 yr or 10 mg tablets in children over 14 yr of age CONTROL GROUP 1: Inhaled Triamcinolone acetonide two puffs twice a day (400 μg/day), DEVICE: Azmacort, Aventis, USA CO‐INTERVENTION: No other Rx allowed other than rescue b2‐agonists or rescue oral corticosteroids |
|
| Outcomes | ANALYSIS BY INTENTION‐TO‐TREAT: Not reported OUTCOMES reported at 4 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Total asthma score INFLAMMATORY MEDIATORS: Eosinophil blood count ADVERSE EVENTS: Not reported WITHDRAWALS: Reported overall (not by group) Primary outcome not specified |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 100 μg | |
| Notes | Full Text publication Funded: Not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated allocation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open labelled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The withdrawal rate was low (2%) and balanced between groups and the reasons for withdrawal were specified by group. |
| Selective reporting (reporting bias) | High risk | The primary outcome was not specified and the results of the Paediatric asthma quality of life questionnaire and adverse effects were not presented. The data was not reported as analysed by intention‐to‐treat analysis. |
| Other bias | Low risk | No apparent other bias |
Stelmach 2005.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | CHILDREN WITH NEWLY DIAGNOSED ASTHMA AND SENSITIVE TO HOUSE‐DUST MITES (DERMATOPHAGOIDES PTERONYSSINUS AND/OR DERMATOPHAGOIDES FARINE) RANDOMISED: N = 51 M: 17 Medium dose BD (400 mg/day): 16 High dose BD (800 mg/day): 18 WITHDRAWALS: N = 2 M: 5.88% Medium dose BP (400 mg/day): 6.25% AGE: years ± SD M: 12.1 ± 1.6 Medium dose BD (400 mg/day): 11.6 ± 1.4 High dose BD (800 mg/day): 11.6 ± 1.8 GENDER: % male: M: 56.3 Medium dose BD (400 mg/day): 60 High dose BD (800 mg/day): 55.6 ASTHMA SEVERITY: Newly diagnosed asthma and sensitive to house‐dust mites (Dermatophagoides pteronyssinus or/and Dermatophagoides farinae) % Pred. FEV1 % ±SD ‐ after withdrawals: M: 83.4 ± 0.4 Medium dose BD (400 mg/day): 84.3 ± 0.5 High dose BD (800 mg/day): 83.4 ± 0.3 Mean (± SD) β2‐agonist use (puffs/day): Not described ATOPY (described as chronic atopic): Not described ALLERGIC RHINITIS: Not described DURATION OF ASTHMA (years): Not described ASTHMA SYMPTOMS: Not described ELIGIBILITY CRITERIA:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: Not mentioned Intervention Period: 6 months TEST GROUP: Montelukast sodium 5 mg tablets in children aged 6–14 yr or 10 mg tablets in children over 14 yr of age CONTROL GROUP 1 (Medium dose): Inhaled Budesonide 400 mg/day CONTROL GROUP 2 (High dose): Inhaled Budesonide 800 mg/day DEVICE: Dry powder capsule (Miflonide, Novartis, Switzerland) CO‐INTERVENTION:: No other Rx allowed other than rescue b2‐agonists |
|
| Outcomes | ANALYSIS BY INTENTION‐TO‐TREAT: Not reported OUTCOMES reported at 6 months PULMONARY FUNCTION TESTS: FEV1% of predicted SYMPTOM SCORES: Total asthma score INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Not reported WITHDRAWALS: Reported by group *Primary outcomes |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full Text publication Funded: Not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated allocation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Doubl blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The withdrawal rate was low, balanced between groups with reasons for withdrawal reported by group. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the manuscript are reported, including adverse events |
| Other bias | Low risk | No apparent other bias |
Stelmach 2007.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | CHILDREN WITH NEWLY DIAGNOSED ASTHMA AND SENSITIVE TO HOUSE‐DUST MITES (DERMATOPHAGOIDES PTERONYSSINUS AND/OR DERMATOPHAGOIDES FARINE) RANDOMISED: N = 51 M: 29 BD: 29 M + BD: 29 Formoterol + BD: 29 Placebo: 29 WITHDRAWALS: N = 2 M: 0 BD: 0 M + BD: 0 Formoterol + BD: 0 Placebo: 2 AGE: years ± SD M: 10.4 ± 2.9 BD: 12 ± 3.1 M + BD: 11 ± 3.2 Formoterol + BD: 8.79 ± 2.4 Placebo: 11.4 ± 3.3 GENDER: % male M: 62.1 BD: 69 M + BD: 69 Formoterol + BD: 58.6 Placeboe: 70.4 ASTHMA SEVERITY: Newly diagnosed asthma and sensitive to house‐dust mites (Dermatophagoides pteronyssinus or/and Dermatophagoides farinae) % Pred. FEV1 % ± SD: M: 95.5 ± 2.1 BD: 94.4 ± 1.8 M + BD: 94.8 ± 2.1 Formoterol + BD: 93.1 ± 2.3 Placebo: 94.8 ± 1.8 Mean (± SD) β2‐agonist use (puffs/day): Not described ATOPY (described as chronic atopic): Not described ALLERGIC RHINITIS: Not described DURATION OF ASTHMA (years ± SD): M: 3.85 ± 0.61 BD: 3.97 ± 0.52 M + BD: 3.79 ± 0.58 Formoterol + BD: 3.93 ± 0.63 Placebo: 3.88 ± 0.47 ASTHMA SYMPTOMS: Not described ELIGIBILITY CRITERIA: INCLUSION CRITERIAS:
EXCLUSION CRITERIAS:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: Not mentioned Intervention Period: 4 weeks TEST GROUP: Montelukast sodium 5 mg tablets in children aged 6–14 yr or 10 mg tablets in children over 14 yr of age CONTROL GROUP 1: Inhaled Budesonide 200 mg/day CONTROL GROUP 2: Montelukast 5 or 10 mg tablets and Inhaled Budesonide200 mg/day CONTROL GROUP 3: Formoterol mg/day and Inhaled Budesonide200 mg/day CONTROL GROUP 4: Placebo DEVICE: Turbuhaler |
|
| Outcomes | ANALYSIS BY INTENTION‐TO‐TREAT: Not reported OUTCOMES reported at 4 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Not described INFLAMMATORY MEDIATORS: Not described ADVERSE EVENTS: Not reported WITHDRAWALS: Reported by group |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 100 μg | |
| Notes | Full Text publication Funded: Grants from the Medical University of Lodz, Poland |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated allocation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind; double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, balanced numbers in all groups, reason for withdrawal by group reported, |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcome was not specified but no bias was observed |
| Other bias | Low risk | No apparent other bias |
Stelmach 2008.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | CHILDREN WITH SYMPTOMATIC ASTHMA AND SENSITIVE TO HOUSE‐DUST MITES (DERMATOPHAGOIDES PTERONYSSINUS AND/OR DERMATOPHAGOIDES FARINE) RANDOMISED: N =100 M: 20 BD: 20 M + BD: 20 Formoterol + BD: 20 Placebo: 20 WITHDRAWALS: N = 9 M: 3 BD: 0 M + BD: 3 Formoterol + BD: 2 Placebo: 1 AGE: years (range) M: 11.8 (7‐17) BD: 11.9 (6‐16) M + BD: 12.2 (7‐18) Formoterol + BD: 11.3 (6‐18) Placebo: 12.1 (7‐15) GENDER (% male): Not reported ASTHMA SEVERITY: Not reported % Pred. FEV1 % ±SD: M: 93.5 ± 11.4 BD:92.2 ± 13.5 M + BD: 90.2 ± 10.2 Formoterol + BD: 91.1 ± 11.2 Placebo: 92.4 12.7 Mean (± SD) β2‐agonist use (puffs/day): Not described ATOPY (described as chronic atopic): Not described ALLERGIC RHINITIS: Not described DURATION OF ASTHMA (years): Not reported ASTHMA SYMPTOMS: Not reported INCLUSION CRITERIAS:
EXCLUSION CRITERIAS:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: Not mentioned Intervention Period: 4 weeks TEST GROUP: Montelukast sodium 5 mg tablets in children aged 6–14 yr or 10 mg tablets in children over 14 yr of age CONTROL GROUP 1: Inhaled Budesonide 200 mg/day CONTROL GROUP 2: Montelukast 5 or 10 mg tablets and Inhaled Budesonide200 mg/day CONTROL GROUP 3: Formoterol mg/day and Inhaled Budesonide200 mg/day CONTROL GROUP 4: Placebo DEVICE: Turbuhaler |
|
| Outcomes | ANALYSIS BY INTENTION‐TO‐TREAT: Not reported OUTCOMES reported at 4 weeks PULMONARY FUNCTION TESTS: AUC0‐20min of FEV1 Maximum % fall in FEV1 SYMPTOM SCORES: Not described INFLAMMATORY MEDIATORS: Not described ADVERSE EVENTS: Not reported WITHDRAWALS: Reported |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 100 μg | |
| Notes | Full Text publication Funded: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated allocation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind; double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The overall withdrawal rate was 10% (N = 9) but it was not presented by group. This might be of importance as the only reason for withdrawal was poor efficacy. |
| Selective reporting (reporting bias) | Low risk | Primary outcome was not pre‐specified however no bias was observed |
| Other bias | Low risk | No apparent other bias |
Szefler 2005.
| Methods | DESIGN: Cross‐over, randomised, clinical trial. | |
| Participants | PATIENTS WITH MILD TO MODERATE PERSISTENT ASTHMA RANDOMISED: N = 144 WITHDRAWALS: no (%) N = 17, (11.81%) M: 11 (7.64%) FP: 6 (4.17%) AGE: years ± SD: Not reported GENDER (% male): Not reported ASTHMA SEVERITY: Not reported % Pred. FEV1 % ± SD: Not reported Mean (± SD) β2‐agonist use (puffs/day): Not reported ATOPY: Not reported ALLERGIC RHINITIS: Not reported DURATION OF ASTHMA (years): Not reported ASTHMA SYMPTOMS:Not reported INCLUSION CRITERIAS:
EXCLUSION CRITERIAS:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: not reported Intervention Period: 52 weeks TEST GROUP: Montelukast sodium 5‐mg chewable tablet for those 6 to 14 years of age and 10‐mg tablet for those 15 to 18 years of age CONTROL GROUP 1: Flovent Diskus, 100 μg per inhalation administered as one inhalation twice a day DEVICE: Diskus |
|
| Outcomes | ANALYSIS NOT BY INTENTION‐TO‐TREAT OUTCOMES reported at 8 weeks PULMONARY FUNCTION TESTS:
FUNCTIONAL TESTS:
SYMPTOM SCORES: Not reported INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Reported by groups WITHDRAWALS: Reported *Primary outcomes |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full Text publication Funded: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: by minimization method for several factors |
| Allocation concealment (selection bias) | Unclear risk | Nnot reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No intention‐to‐treat analysis, 8.4% and 15.1% of withdrawal in ICS and montelukast groups respectively |
| Selective reporting (reporting bias) | Low risk | Primary outcome was specified |
| Other bias | Low risk | No apparent other bias |
Szefler 2007.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | PATIENTS WITH MILD PERSISTENT ASTHMA RANDOMISED: N = 395 M: 198 BD: 197 WITHDRAWALS: no (%) N = 115, (29.1%) M: 52 (26.40%) BD: 63 (31.98%) AGE: years ± SD M: 4.7 ± 1.9 BD: 4.6 ± 2.0 GENDER (% male): M: 118 ± 59.9 BD: 122 ± 61.9 ASTHMA SEVERITY: Not reported % Pred. FEV1 % ± SD: M: 91.67 ± 18.07 BD:89.88 ± 17.75 Mean (± SD) β2‐agonist use (puffs/day): Not described ATOPY (described as chronic atopic): Not described ALLERGIC RHINITIS: Not described DURATION OF ASTHMA (years): Not reported ASTHMA SYMPTOMS: Not reported INCLUSION CRITERIAS:
EXCLUSION CRITERIAS:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 3‐21 days Intervention Period: 52 weeks TEST GROUP: Montelukast sodium 4 or 5 mg tablets CONTROL GROUP 1: Budesonide inhalation suspension 0.5 mg/day DEVICE: Not described |
|
| Outcomes | ANALYSIS BY INTENTION‐TO‐TREAT OUTCOMES reported at 52 weeks PULMONARY FUNCTION TESTS:
FUNCTIONAL TESTS:
SYMPTOM SCORES
INFLAMMATORY MEDIATORS: Not described ADVERSE EVENTS: Reported by groups WITHDRAWALS: Reported *Primary outcomes |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 250 μg | |
| Notes | Full Text publication Funded: AstraZeneca LP |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open labelled and study subjects could be discontinued at any time at the discretion of the investigators. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | the withdrawal data is presented by group, however the large withdrawal rate (29%), similar in both group, while acceptable for the main outcome (time to event) raise doubt about the validity of the secondary outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported |
| Other bias | Low risk | No apparent other bias |
Tamaoki 2008.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. | |
| Participants | NEWLY DIAGNOSED MILD INTERMITTENT ASTHMA PATIENTS RANDOMISED: N = 85 PRAN: 42 BD: 43 WITHDRAWALS: no (%) N = 11 (12.94%) PRAN: 6 (14.29%) BD: 5 (11.63%) AGE: years ± SD PRAN: 36 ± 3 BD: 38 ± 4 GENDER (% male) PRAN: 27.7 BD: 26.32 ASTHMA SEVERITY: Not reported % Pred. FEV1 % ± SD: PRAN: 84.6 ± 2.0 BD: 85.0 ± 2.1 Mean (±SD) β2‐agonist use (puffs/day): Not described ATOPY (described as chronic atopic): Not described ALLERGIC RHINITIS: Not described DURATION OF ASTHMA (years): PRAN: 1.6 ± 1.1 BD: 2.0 ± 1.2 ASTHMA SYMPTOMS SCORE: PRAN: 5.4 ± 0.4 BD: 5.7 ± 0.5 INCLUSION CRITERIAS:
EXCLUSION CRITERIAS:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 4 weeks Intervention Period: 8 weeks TEST GROUP: Pranlukast 225 mg twice a day CONTROL GROUP: Inhaled BDP 100 μg twice a day DEVICE: Metered‐dose inhaler using a spacing chamber |
|
| Outcomes | ANALYSIS BY INTENTION‐TO‐TREAT: Not reported OUTCOMES reported at 8 weeks PULMONARY FUNCTION TESTS:
FUNCTIONAL TESTS: Changes in the use of supplemental 2‐agonist SYMPTOM SCORES: Asthma symptom score INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Not reported WITHDRAWALS: Reported |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 100 μg | |
| Notes | Full Text publication Funded: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: randomised using balanced block of four |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, drop‐outs are reported by group and were balanced between the two groups |
| Selective reporting (reporting bias) | Low risk | No apparent other bias |
| Other bias | Low risk | No apparent other bias |
Yamauchi 2001.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. ‐Confirmation of methodology: Not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 30 P: 10 BDP: 10 Placebo: 10 (not used) WITHDRAWALS: Not reported AGE (± SD) years: P: 40.6 ± 2.02 BDP: 42.2 ± 3.32 GENDER (% male): M: 60% BDP: 70% ASTHMA SEVERITY: Mild persistent or mild intermittent % Pred. FEV1 (mean ± SD) P: 91.8 ± 3.21 BDP: 92.0 ±2.18 Mean (± SD) β2‐agonist use (puffs/day): Not described ATOPY: P: 60% BDP: 60% ASTHMA DURATION (years): Not reported ELIGIBILITY CRITERIA:
EXCLUSION CRITERIA: Not described. |
|
| Interventions | PROTOCOL Duration Run‐in Period: not described if any Intervention Period: 4 weeks TEST GROUP: Pranlukast 450 mg/day CONTROL GROUP: Inhaled Beclomethasone 400 μg/day DEVICE: Not reported CO‐INTERVENTION: Theophylline M: 11% BCP: 10% |
|
| Outcomes | ANALYSIS ( ITT not specified) OUTCOMES reported at 4 weeks PULMONARY FUNCTION TESTS: Change in morning and evening PEFR SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Not reported INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Not reported WITHDRAWALS: Not reported Primary outcome: Not specified |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication (2001) Funded by the Ministry of Education, Science, Sports and Culture in Japan Confirmation of data and methodology requested |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details on blinding provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All data presented, no intention‐to‐treat analysis and withdrawal rate is not reported |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were not defined, but no bias was observed |
| Other bias | Low risk | No apparent other bias |
Yurdakul 2003.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. Confirmation of methodology: Not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 74 M: 25 BDP: 25 THEO: 24 (not used) WITHDRAWALS: Reported AGE (years ± SD): M: 34.3 ± 5 BDP: 34.9 ± 5 THEO: 33.5 ± 5 GENDER (% male): M: 20% BDP: 20% THEO: 25% ASTHMA SEVERITY: Mild persistent % Pred. FEV1 (mean ± SD): M: 84.8 ± 5.3 BDP: 84.5 ± 4.1 THEO: 86.6 ± 5.5 Mean (± SD) β2‐agonist use (puffs/day): Not described ATOPY: Not reported ASTHMA DURATION (years): Not reported INCLUSION CRITERIAS:
EXCLUSION CRITERIAS:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: Not described if any Intervention Period: 4 weeks TEST GROUP: Pranlukast 450 mg/day CONTROL GROUP: Inhaled Beclomethasone 400 μg/day twice a day DEVICE: Not reported CO‐INTERVENTION: Theophylline M: 11% BCP: 10% |
|
| Outcomes | ANALYSIS ( ITT not specified) OUTCOMES reported at 12 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES:
FUNCTIONAL STATUS: Mean number of rescue inhalations, puffs/day INFLAMMATORY MEDIATORS: Not reported ADVERSE EVENTS: Reported exacerbation by groups WITHDRAWALS: Not reported *Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication (2003) Funded by: Not reported Confirmation of data and methodology: Not done |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: simple random sampling method according to random number table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No intention‐to‐treat analysis and no reported withdrawal rate |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the manuscript are reported, including adverse events by group |
| Other bias | Low risk | No apparent other bias |
Zedan 2009.
| Methods | DESIGN: Parallel‐group, randomised, clinical trial. Confirmation of methodology: Not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 56 M: 27 FP: 29 WITHDRAWALS: Reported AGE: years ± SD: N: 9.45 ± 2.42 GENDER (% male): 64.15% ASTHMA SEVERITY: Moderate persistent asthma % Pred. FEV1 (mean ± SD): Not reported Mean (±SD) β2‐agonist use (puffs/day): Not described ATOPY: Not reported ASTHMA DURATION (years): N: 5.36 ELIGIBILITY CRITERIAS:
|
|
| Interventions | PROTOCOL Duration Run‐in Period: 4 weeks Intervention Period: 4 weeks TEST GROUP: Montelukast 5 mg at bed time CONTROL GROUP: Fluticasone propionate (100 μg twice daily) DEVICE: Pressurized metered‐dose inhaler CO‐INTERVENTION: Short‐acting β2 agonists |
|
| Outcomes | ANALYSIS ( ITT not specified) OUTCOMES reported at 4 weeks PULMONARY FUNCTION TESTS: FEV1 SYMPTOM SCORES: Not reported FUNCTIONAL STATUS: Not reported INFLAMMATORY MEDIATORS:
ADVERSE EVENTS: Not reported WITHDRAWALS: Reported |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full‐text publication (2009) Funded by: Not reported Confirmation of data and methodology: Not done |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Non‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, withdrawal rate per group presented |
| Selective reporting (reporting bias) | Low risk | Not found |
| Other bias | Low risk | No apparent other bias |
Zeiger 2005.
| Methods | DESIGN: Parallel‐group, multi‐centre, randomised, clinical trial. Confirmation of methodology: Not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 380 M = 189 FP = 191 WITHDRAWALS; N (%): M: 25 (12.5%) FP: 28 (14%) AGE: Mean ( years ± SD): M: 33.9 ± 14.8 FP: 36.5 ± 13.8 GENDER (% male): M: 58 (30.2%) FP: 59 (30.9%) BASELINE SEVERITY: Mild and moderate % Pred. FEV1 ± SD: M: 93 ± 8.9 FP: 94.8 ± 10.8 MEAN (± SD) β2‐AGONIST USE (puffs/day): M = 3.4 ± 1.2 FP = 3.6 ± 1.4 ATOPY: Not reported ASTHMA DURATION: >=4 months ELIGIBILITY CRITERIA
EXCLUSION: Not reported |
|
| Interventions | PROTOCOL Duration Run‐in Period: 3 weeks Intervention: 12 weeks TEST GROUP: Montelukast 10 mg od CONTROL GROUP: Fluticasone 100 μg twice a day DEVICE: Not reported CO‐INTERVENTION: Not reported |
|
| Outcomes | ANALYSIS ( ITT not specified) OUTCOMES reported at 12 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Asthma symptoms FUNCTIONAL STATUS:
INFLAMMATORY MARKERS: Blood eosinophil count ADVERSE EVENTS: Reported WITHDRAWALS: Reported * Primary outcome |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full paper (2005) Funded by Merck & Co. Inc Confirmation of methodology and data extraction: Not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: computer‐generated allocation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind,double dummies: "appropriate matching placebo' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Modified intention‐to‐treat with low and similar withdrawal rate (6% LTRA versus 9% FP); |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome (rescue‐free days) reported; poorly reported clinical and laboratory adverse health events |
| Other bias | Low risk | No apparent other bias |
Zeiger 2006.
| Methods | DESIGN: Cross over, multi‐centre, randomised, clinical trial. Confirmation of methodology: Not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 144 WITHDRAWALS; N (%): M: 25 (12.5%) FP: 28 (14%) AGE: Mean years ± SD: N: 17 ± 11.80% GENDER (% male): Not reported BASELINE SEVERITY: Mild and moderate persistent asthma % Pred. FEV1 (Mean ± SD): Not reported MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported ATOPY: Not reported ASTHMA DURATION: Not reported ELIGIBILITY CRITERIA:
|
|
| Interventions | PROTOCOL Duration Intervention: 8 weeks TEST GROUP: Montelukast 5‐mg chewable tablet for those 6 to 14 years of age or as a 10‐mg for 15 to 18 years of age CONTROL GROUP: Fluticasone propionate 100 mg per inhalation administered as twice a day DEVICE: Flovent Diskus CO‐INTERVENTION: Not reported |
|
| Outcomes | ANALYSIS ( ITT not specified) OUTCOMES reported at 8 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES:
FUNCTIONAL STATUS
INFLAMMATORY MARKERS: eNO ADVERSE EVENTS: Not reported WITHDRAWALS: Reported |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full paper (2006) Funded by: Not reported Confirmation of methodology and data extraction: Not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Means of randomisation: minimization method |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind using double dummies: " placebo for the alternative drug" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No intention‐to‐treat analysis; withdrawal rate 12.5% and most withdrew because of treatment failure (i.e. requiring treatment with systemic corticosteroids with an imbalance between treatment period (2 FP and 10 in Montelukast) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No apparent other bias |
Zielen 2010.
| Methods | DESIGN: Parallel‐group, single centre, randomised, clinical trial. Confirmation of methodology: Not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 102 WITHDRAWALS; N M: 2 FP: 0 AGE: N: 4.94 GENDER (% male): N: 58.82% BASELINE SEVERITY: Episodic asthmatics % Pred. FEV1 (Mean ± SD): N: 98.8% MEAN (± SD) β2‐AGONIST USE (puffs/day): Not reported ATOPY: Not reported ASTHMA DURATION: Not reported INCLUSION CRITERIAS:
EXCLUSION CRITERIAS:
|
|
| Interventions | PROTOCOL Duration Intervention: 6 weeks TEST GROUP: Montelukast 4‐mg chewable tablet for those 4 to 6 years of age or as a 5‐mg for older than 6 years CONTROL GROUP: Inhaled fluticasone 100 mg twice daily DEVICE: By spacer CO‐INTERVENTION: Inhaled salbutamol |
|
| Outcomes | ANALYSIS ( ITT not specified) OUTCOMES reported at 6 weeks PULMONARY FUNCTION TESTS:
SYMPTOM SCORES: Asthma symptoms FUNCTIONAL STATUS: Number of exacerbations INFLAMMATORY MARKERS:
ADVERSE EVENTS: Reported WITHDRAWALS: Reported |
|
| ICS dose in HFA beclomethasone ‐ equivalent | 200 μg | |
| Notes | Full paper (2010) Funded by: Disclosed for all funds for authors Confirmation of methodology and data extraction: Not obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Means of randomisation: not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented, |
| Selective reporting (reporting bias) | Low risk | Primary outcome and secondary outcomes reported, withdrawal by group reported |
| Other bias | Low risk | No apparent other bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbott Pharma 1996 | Not a randomised controlled trial |
| Al Frayh 2008 | Intervention was not anti‐leukotriene |
| Allayee 2007 | Control intervention was not inhaled corticosteroid |
| Allen 1997 | Not a randomised controlled trial |
| Allen‐Ramey 2003 | Not a randomised controlled trial |
| Allen‐Ramey 2004 | Not a randomised controlled trial |
| Allen‐Ramey 2006 | Not a randomised controlled trial |
| Altman 1998k | Control intervention was not inhaled corticosteroid |
| Anonymous 1997 | Not a randomised controlled trial |
| Armour 2007 | Intervention was not anti‐leukotriene |
| Bacharier 2008 | Intervention was given < 4 weeks |
| Bai 2010 | Participants were not people with asthma |
| Balatsouras 2005 | Participants were not people with asthma Control intervention was not inhaled corticosteroid |
| Baren 2006 | Intervention was not anti‐leukotriene |
| Barnes 1996 | Control intervention was not inhaled corticosteroid |
| Barnes 1997 | Not a randomised controlled trial |
| Barnes 1997b | Control intervention was not inhaled corticosteroid |
| Barnes 2001 | Not a randomised controlled trial (meta‐analysis) |
| Barnes 2007 | Participants received additional non‐permitted co‐interventions (cetirizine with montelukast and intranasal beclomethasone with inhaled beclomethasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Barnes 2007a | Participants received additional non‐permitted co‐interventions (montelukast with inhaled budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Bartoli 2010 | Intervention was not anti‐leukotriene |
| Bateman 1995 | Control intervention was not inhaled corticosteroids |
| Bateman 2003 | Participants received additional non‐permitted co‐interventions (fluticasone with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Baumgartner 1999 | Duplicate of published paper in Eur Respir J 2003;21:123‐128. |
| Benitez 2005 | Participants received additional non‐permitted co‐interventions (budesonide with zafirlukast and oral loratadine/pseudoephedrine with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Berger 2006 | Intervention was not anti‐leukotriene |
| Bilancia 2000 | Participants received additional non‐permitted co‐interventions (combination of budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Bilderback 2004 | Duplicate of published paper in Journal of Allergy & Clinical Immunology 2007;119(4):916‐923. |
| Bilderback 2005 | Duplicate of published paper in Journal of Allergy & Clinical Immunology 2007;119(4):916‐923. |
| Bisgaard 1999 | Control intervention was not inhaled corticosteroids. Participants received additional non‐permitted co‐interventions (inhaled steroids) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid. Outcomes did not reflect control of asthma (airway inflammation). Intervention < 4 weeks |
| Bisgaard 2000 | Control intervention was not inhaled corticosteroid |
| Bisgaard 2005 | Control intervention was not inhaled corticosteroid |
| Bjermer 2002 | Control intervention was not placebo |
| Bjermer 2003 | Participants received additional non‐permitted co‐interventions (montelukast with fluticasone and salmeterole with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Bjermer 2004 | Participants received additional non‐permitted co‐interventions (montelukast with fluticasone and salmeterole with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Bleecker 2006 | Intervention was not anti‐leukotriene Control intervention was not inhaled corticosteroid |
| Borker 2005 | Participants received additional non‐permitted co‐interventions (montelukast with fluticasone and salmeterole with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Bousquet 2005a | Not a randomised controlled trial |
| Bousquet 2007 | Intervention was not anti‐leukotriene Control intervention was not inhaled corticosteroid |
| Brannan 2001 | Intervention administered < 4 weeks Control intervention was not inhaled corticosteroid |
| Brocks 1996 | Participants were not people with asthma |
| Bronsky 1997 | Participants were not people with asthma |
| Brown 2005 | Intervention was not anti‐leukotriene |
| Brown 2007 | Intervention was not anti‐leukotriene |
| Brown 2010 | Intervention was not anti‐leukotriene |
| Bruce 2002 | Outcome measures did not reflect asthma control |
| Buchvald 2002 | Duplicate of published paper in Annals of Allergy Asthma & Immunology 2003;91(3):309‐313. |
| Buchvald 2002a | Duplicate of published paper in Annals of Allergy Asthma & Immunology 2003;91(3):309‐313. |
| Buchvald 2003 | Control intervention was not inhaled corticosteroid |
| Buckstein 2003 | Not a randomised controlled trial |
| Burgess 2007 | Intervention was not anti‐leukotriene |
| Busse 1999 | Control intervention was not inhaled corticosteroid Participants received additional non‐permitted co‐interventions (inhaled steroids) other than short‐acting beta2‐agonists Outcomes did not reflect control of asthma (provocation challenge) |
| Buxton 2004 | Intervention was not anti‐leukotriene |
| Cakmak 2000 | Control intervention was not placebo |
| Cakmak 2004 | Participants received additional non‐permitted co‐interventions (zafirlukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Calhoun 1997 | Control intervention was not inhaled corticosteroid |
| Calhoun 2001 | Participants received additional non‐permitted co‐intervention other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Calhoun 2004 | Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Camargo 2002 | Acute asthma setting |
| Camargo 2003 | Control intervention was not inhaled corticosteroid Acute asthma setting |
| Canino 2008 | Not a randomised controlled trial Intervention was not anti‐leukotriene Control intervention was not inhaled corticosteroid |
| Capella 2001 | Participants were non‐asthmatics (atopic dermatitis) |
| Ceylan 2004 | Participants received additional non‐permitted co‐interventions (formoterol with budesonide and montelukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Chan 2003 | Not a randomised controlled trial |
| Chand 2005 | Participants received additional non‐permitted co‐interventions (montelukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Chanez 2010 | Intervention was not anti‐leukotriene Control intervention was not inhaled corticosteroids |
| Chen 2006 | Intervention was not anti‐leukotriene |
| Chiba 1997 | Not a randomised controlled trial |
| Choi 2003 | Intervention was not anti‐leukotriene |
| Chopra 2005 | Intervention was not anti‐leukotriene |
| Chuchalin 2002 | Intervention was not anti‐leukotriene |
| Chuchalin 2007 | Intervention was not anti‐leukotriene |
| Chung 2000 | Intervention was not anti‐leukotriene |
| Ciebiada 2009 | Participants received additional non‐permitted co‐interventions (fexofenadine with montelukast and fexofenadine with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Claesson 1998 | Not a randomised controlled trial |
| Cloud 1989 | Control intervention was not inhaled corticosteroid |
| Covar 2008 | Duplicate of published paper in Journal of Allergy & Clinical Immunology 2007;119(1):64‐72. |
| Cowan 2010 | Participants received additional non‐permitted co‐interventions (cromoglycate, formoterol with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid; Intervention was given < 4 weeks |
| Currie 2003 | Intervention was given < 4 weeks |
| Currie 2003a | Intervention was given < 4 weeks; Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Currie 2003b | Duplicate of published paper in Journal of Respiratory & Critical Care Medicine 2003;167(9):1232‐1238. |
| Cylly 2003 | Ongoing trial |
| Dahlén 2002 | Control intervention was not inhaled corticosteroid; Participants received additional non‐permitted co‐interventions (inhaled steroids) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Daikh 2003 | Participants were not people with asthma |
| Davies 2004 | Not a randomised controlled trial; All patients not received inhaled corticosteroid as controlled intervention |
| Daviskas 2007 | Control intervention was not inhaled corticosteroid |
| Dekhuijzen 2006 | Intervention was not anti‐leukotriene Control intervention was not inhaled corticosteroid |
| Delaronde 2005 | Intervention was not anti‐leukotriene Control intervention was not inhaled corticosteroid |
| Dempsey 1999 | Intervention administered for < 4 weeks |
| Dempsey 2000 | Intervention administered <4 weeks |
| Dempsey 2000a | Control intervention was not placebo |
| Dempsey 2002b | Control intervention was not placebo |
| Demuro‐Mercon 2001 | Control intervention was not inhaled corticosteroid |
| Dessanges 1999 | Participants received additional non‐permitted co‐interventions (inhaled steroids) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid Intervention lasted <4 weeks Outcomes did not reflect control of asthma (provocation challenge) |
| Deykin 2007 | Participants received additional non‐permitted co‐interventions (salmeterol with montelukast and salmeterol with beclomethasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Diamant 1997 | Control intervention was not inhaled corticosteroidervention administered for < 4 weeks Outcomes were solely the result of provocation |
| Diamant 2009 | Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Dicpinigaitis 2002 | Outcome measures did not reflect asthma control |
| Djukanovic 2010 | Participants received additional non‐permitted co‐interventions (fluticasone with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Dockhorn 2000 | Control intervention was not inhaled corticosteroid Intervention administered for < 4 weeks |
| Dorinsky 2001 | Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Dorinsky 2002 | Not a randomised controlled trial |
| Dorinsky 2004 | Intervention was not anti‐leukotriene |
| Edelman 2000 | Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| El Miedany 2006 | Intervention was not anti‐leukotriene Control intervention was not inhaled corticosteroid |
| Eliraz 2001 | Intervention was not anti‐leukotriene |
| Ensom 2003 | Not a randomised controlled trial Intervention was not anti‐leukotriene Control intervention was not inhaled corticosteroid |
| Fabbri 1996 | Not a randomised controlled trial |
| Fagerson 2003 | Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Failla 2006 | Intervention was not anti‐leukotriene |
| Fardon 2004 | Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Fardon 2007 | Not a randomised controlled trial. Intervention was not anti‐leukotriene. |
| Faul 2002 | Outcome measures did not reflect asthma control |
| Findlay 1992 | Control intervention was not inhaled corticosteroid. Intervention administered for < 4 weeks |
| Finkelstein 2005 | Not a randomised controlled trial. Intervention was not anti‐leukotriene |
| Finn 2000 | Control intervention was not inhaled corticosteroid (but placebo). A small number of placebo‐treated patients also received co‐intervention with inhaled steroids (N = 42), but co‐intervention with inhaled steroids was not randomly assigned. |
| Fischer 1995 | Control intervention was not inhaled corticosteroid. Intervention administered for < 4 weeks. Outcomes were solely the result of provocation. |
| Fischer 1997 | Control intervention was not inhaled corticosteroid |
| Fish 1997 | Control intervention was not inhaled corticosteroid |
| Fish 2000 | Participants received additional non‐permitted co‐interventions (salmeterol with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Fish 2001 | Co‐intervention with inhaled corticosteroid. Control intervention was not inhaled corticosteroid. |
| FitzGerald 2009 | Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Fogel 2010 | Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone and fluticasone with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Franzen 1994 | Not a randomised controlled trial |
| Fritsch 2006 | Intervention was not anti‐leukotriene |
| Fujimura 1993 | Control intervention was not inhaled corticosteroid |
| Gabrijelcic 2004 | Not a randomised controlled trial. Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. |
| Gaddy 1990 | Control intervention was not inhaled corticosteroid. Intervention administered for < 4 weeks, intravenously |
| Galbreath 2008 | Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. |
| Geha 2001 | Intervention was not anti‐leukotriene |
| Gelb 2008 | Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. |
| Georgiou 1997 | Control intervention was not inhaled corticosteroid. Participants were not asthmatics. Intervention administered for < 4 weeks. |
| Ghiro 2001 | Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Ghiro 2002 | Outcome measures did not reflect asthma control |
| Gold 2001 | Control intervention was not placebo |
| Gold 2001a | Control intervention was not placebo |
| Green 2002 | Control intervention was not placebo. Co‐intervention with inhaled steroids. |
| Green 2002a | Intervention was not anti‐leukotriene |
| Green 2006 | Participants received additional non‐permitted co‐interventions (montelukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Greenberger 2003 | Not a randomised controlled trial |
| Grosclaude 2003 | Participants received additional non‐permitted co‐interventions (montelukast with beclomethasone and salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Grossman 1995 | Control intervention was not inhaled corticosteroid |
| Grossman 1997 | Control intervention was not inhaled corticosteroid |
| Grzelewska‐Rzymowska 2003 | Intervention was not anti‐leukotriene |
| Grzelewski 2006 | Participants received additional non‐permitted co‐interventions (montelukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Guilbert 2004 | Intervention was not anti‐leukotriene |
| Gupta 1999 | Intervention was not anti‐leukotriene. Participants were not people with asthma. |
| Gupta 2007 | Participants received additional non‐permitted co‐interventions (fluticasone+salmeterol with montelukast and salmeterol+levocetirizine with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Gylfors 2005 | Duplicate of published paper in Journal of Allergy & Clinical Immunology 2006;118(1):78‐83. |
| Gyllfors 2003 | Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. |
| Gyllfors 2006 | Intervention was not anti‐leukotriene |
| Haahtela 1994 | Intervention was not anti‐leukotriene |
| Hakim 2007 | Control intervention was not inhaled corticosteroid |
| Hamilton 1998 | Control intervention was not inhaled corticosteroid. Intervention administered for < 4 weeks. Outcomes were solely the result of provocation. |
| Harmanci 2006 | Control intervention was not inhaled corticosteroid |
| Hartwig 2004 | Not a randomised controlled trial |
| Hassall 1998 | Control intervention was not inhaled corticosteroid |
| Havlucu 2005 | Participants received additional non‐permitted co‐interventions (formoterole with budesonide) other than short‐acting beta2‐agonists and/or short course of oral ccorticosteroid |
| Hay 1997 | Not a randomised controlled trial |
| Hendeles 2004 | Participants received additional non‐permitted co‐interventions (montelukast with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Henderson 1994 | Not a randomised controlled trial |
| Hernandez 2002 | Participants received additional non‐permitted co‐interventions (montelukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Hood 1999 | Participants were not people with asthma. Intervention was not anti‐leukotriene. Intervention administered for < 4 weeks. |
| Hothersall 2008 | Intervention was not anti‐leukotriene |
| Houghton 2004 | Intervention was not anti‐leukotriene |
| Howland 1994 | Control intervention was not inhaled corticosteroid |
| Hozawa 2009 | Intervention was not anti‐leukotriene |
| Hsieh 1996 | Intervention was not anti‐leukotriene |
| Huang 2003 | Participants received additional non‐permitted co‐interventions (zafirlukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Huang 2003a | Duplicate of published paper in Chang Gung Medical Journal. 2003;26(8):554‐560 |
| Hui K 1991 | Control intervention was not inhaled corticosteroid. Intervention administered for < 4 weeks. |
| Igde 2009 | Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Ikeda 1997 | Not a randomised controlled trial |
| Ilowite 2004 | Participants received additional non‐permitted co‐interventions (montelukast with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Inoue 2007 | Intervention was not anti‐leukotriene |
| Irvin 2003 | Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Irvin 2007 | Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Israel 1990 | Control intervention was not inhaled corticosteroid. Intervention administered for < 4 weeks. Outcomes were solely the result of provocation. |
| Israel 1992 | Control intervention was not inhaled corticosteroid |
| Israel 1993 | Control intervention was not inhaled corticosteroid |
| Israel 1996 | Control intervention was not inhaled corticosteroid |
| Jat 2006 | Participants received additional non‐permitted co‐interventions (montelukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Jayaram 2002a | Duplication |
| Jayaram 2005a | Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Jayaram 2006 | Intervention was not anti‐leukotriene |
| Johnson 1999 | Duplicate of published paper in J Fam Pract 2001;50:595‐602. |
| Johnston 2007 | Participants received additional non‐permitted co‐interventions (montelukast with other antihistaminc drugs) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Jones 2002 | Not a randomised controlled trial |
| Jonsson 2004 | Intervention was not anti‐leukotriene |
| Juniper 1995 | Control intervention was not inhaled corticosteroid |
| Kalberg 1999 | Control intervention was not inhaled corticosteroid |
| Kanazawa 2004 | Control intervention was not inhaled corticosteroid |
| Kane 1994 | Not a randomised controlled trial |
| Kanniess 2002a | Control intervention was not placebo |
| Karaman 2007 | Participants received additional non‐permitted co‐interventions (montelukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Karpel 2007 | Intervention was not anti‐leukotriene |
| Katial 2010 | Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Keith 2009 | Participants received additional non‐permitted co‐interventions (montelukast with inhaled coticosteroid and long acting beta2 agoinit with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Kemp 1995 | Control intervention was not inhaled corticosteroid |
| Kemp 1996 | Not a randomised controlled trial |
| Kemp 1998 | Control intervention was not inhaled corticosteroid. Intervention administered for < 4 weeks. |
| Kemp 1998a | Control intervention was not inhaled corticosteroid. Intervention administered for < 4 weeks. Outcomes were solely the result of provocation. |
| Kemp 1999 | Not a randomised controlled trial (meta‐analysis of RCTs) |
| Ketchell 2002 | Intervention was not anti‐leukotriene |
| Khayyal 2003 | Intervention was not anti‐leukotriene |
| Kippelen 2010 | Intervention was not anti‐leukotriene |
| Kips 1991 | Control intervention was not inhaled corticosteroid. Intervention administered for < 4 weeks, intravenously. |
| Knorr 1998 | Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Knorr 1999 | Control intervention were not placebo |
| Koenig 2004 | Duplication of paper in The Journal of Asthma 2008;45( 8):681‐687 |
| Kohrogi 1997 | Not a randomised controlled trial |
| Kondo 2006 | Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Kooi 2006 | Duplication of paper in Pulmonary Pharmacology and Therapeutics 2008;21(5):798‐804 |
| Korenblat 1998 | Use of higher than licensed dose of leukotriene receptor antagonists (Pranlukast 600 mcg/day vs. 450 mcg/day) |
| Kuna 1997 | Control intervention was not inhaled corticosteroid |
| Kylstra 1998 | Control intervention was not inhaled corticosteroid |
| Laitinen 1995 | Control intervention was not inhaled corticosteroid |
| Leaker 2010 | Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroids. |
| Lee 2004 | Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid Intervention administered for < 4 weeks |
| Lee 2004a | Intervention was not anti‐leukotriene |
| Lee 2004b | Control intervention was not inhaled corticosteroid |
| Lee 2004c | Duplication of paper in Chest 2004;125(4):1372‐1377 |
| Lee 2005 | Intervention was not anti‐leukotriene |
| Lee 2010 | Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroids. |
| Leff 1998 | Control intervention was not inhaled corticosteroid. Outcomes were solely the result of provocation. |
| Leibman 2002 | Duplication of paper in American Journal of Respiratory and Critical Care Medicine 2002. 165 (Suppl 8): B4 |
| Leibman 2002a | Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone and fluticasone with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Leigh 2002 | Intervention administered for < 4 weeks |
| Leigh 2002a | Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Lemanske 2010 | Participants received additional non‐permitted co‐interventions (montelukast with fluticasone) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Li 2001 | Control intervention was not placebo |
| Liebke 2001 | Control intervention was not placebo (it was sodium cromoglycate) |
| Lindemann 2009 | Intervention was not anti‐leukotriene |
| Lipworth 1999 | Ongoing trial |
| Lis 2001 | Control intervention was not inhaled corticosteroid. Intervention administered for < 4 weeks. |
| Liu 1996 | Control intervention was not inhaled corticosteroid |
| Lizaso 2003 | Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. |
| Lockey 1995 | Control intervention was not inhaled corticosteroid |
| Lofdahl 1999 | Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Luppo 2005 | Control intervention was not inhaled corticosteroid |
| Lyseng‐Williamson 2003 | Not a randomised controlled trial |
| Macfarlane 2000 | Not a randomised controlled trial |
| Magnussen 2008 | Intervention was not anti‐leukotriene |
| Majak 2010 | Participants received additional non‐permitted co‐interventions (ICS with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Malerba 2002 | Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Marchese 1998 | Not a randomised controlled trial |
| Margolskee 1991 | Control intervention was not inhaled corticosteroid |
| Marogna 2010 | Participants received additional non‐permitted co‐interventions (formoterol/fluticasone with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Maspero 2008 | Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Maspero 2008a | Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Maspero 2008b | Participants received additional non‐permitted co‐interventions (salmeterol with budesonide) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Mastruzzo 2010 | Intervention was given < 4 weeks |
| Matsunaga 2004 | Control intervention was not inhaled corticosteroid. Intervention administered for < 4 weeks. |
| McCarthy 2003 | Participants received additional non‐permitted co‐interventions (fluticasone with montelukast and salmeterol with fluticasone) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| McGill 1996 | Not a randomised controlled trial |
| Mclvor 2009 | Not a randomised controlled trial; Control intervention was not inhaled corticosteroid |
| Mehuys 2008 | Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. |
| Mendes 2004 | Intervention administered for < 4 weeks |
| Mendes 2004a | Intervention administered for < 4 weeks |
| Menendez 2001 | Duplicate of published paper in J Allergy Clin Immunol 2000;105:1123‐1129; Outcomes did not reflect control of chronic asthma |
| Meyer 2003 | Not a randomised controlled trial |
| Micheletto 1997 | Control intervention was not inhaled corticosteroid |
| Miraglia 2007 | Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Mitchell 2005 | Intervention was not anti‐leukotriene |
| Miyamoto 1999 | Control intervention was not placebo |
| Molitor 2005 | Intervention was not anti‐leukotriene. Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Montani 2007 | Duplicate of published paper in New Engl J of Med 2007;356(20):2027‐2039 |
| Moreira 2008 | Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. |
| Morris 2010 | Participants received additional non‐permitted co‐interventions (montelukast with other anti‐asthma therapies) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid. Acute asthma setting. |
| Mosnaim 2002 | Control intervention was not inhaled corticosteroid |
| Mosnaim 2008 | Intervention was not anti‐leukotriene |
| Murphy 2006 | Intervention was not anti‐leukotriene |
| Najberg 2008 | Intervention was not anti‐leukotriene |
| Nakagawa 1992 | Not a randomised controlled trial |
| Nakajima 2001 | Participants received additional non‐permitted co‐interventions (beclomethazone with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Nakazono 2004 | Control intervention was not inhaled corticosteroid |
| Nathan 1998 | Control intervention was not inhaled corticosteroid |
| Nathan 2000 | Participantsreceived additional non‐permitted co‐interventions (salmeterol with ICS and ICS with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Nathan 2004 | Duplication of paper in Chest 2005;128(4):1910‐1920 |
| Nathan 2005 | Participants received additional non‐permitted co‐interventions (fluticasone+salmeterol with montelukast) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Nayak 1998 | Control intervention was not inhaled corticosteroid |
| NCT00096954 | Test group is not anti‐leukotrienes. Control group is not ICS. |
| NCT00140881 | Ongoing and results are not available |
| NCT00196547 | Ongoing and results are not available |
| NCT00213252 | Ongoing and results are not available |
| NCT00299065 | Ongoing and results are not available |
| NCT00319488 | Ongoing and results are not available |
| NCT00395408 | Ongoing and results are not available |
| NCT00421018 | Ongoing and results are not available |
| NCT00462592 | Ongoing and results are not available |
| NCT00471809 | Terminated and results are not available |
| NCT00486343 | Terminated and results are not available |
| NCT00504946 | Ongoing and results are not available |
| NCT00545324 | Ongoing and results are not available |
| NCT00545844 | Control group received non‐permitted drugs (Montelukast and long‐acting beta 2 agonist) |
| NCT00575861 | Ongoing and results are not available |
| NCT00666679 | Test group received non‐permitted drug (Mometasone) |
| NCT00699062 | Ongoing and results are not available |
| NCT00755794 | Ongoing and results are not available |
| NCT00756418 | Ongoing and results are not available |
| NCT00913328 | Ongoing and results are not available |
| NCT00943397 | Control group is not ICS |
| NCT01055041 | Ongoing and results are not available |
| NCT01241084 | Ongoing and results are not available. Test group is not anti‐leukotrienes. |
| Negro 1997 | Not a randomised controlled trial |
| Neki 2006 | Not a randomised controlled trial |
| Nelson 2001 | Control intervention were not placebo |
| Nelson 2004 | Participants received additional non‐permitted co‐interventions (fluticasone+salmeterol with montelukast) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Nelson 2004a | Participants received additional non‐permitted co‐interventions (fluticasone+salmeterol with montelukast) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid. Participants were not people with asthma. |
| Nelson 2006 | Intervention was not anti‐leukotriene |
| Nishima 2005 | Control intervention was not inhaled corticosteroid |
| Nishimura 1999 | Control intervention were not placebo |
| Nishiyama 2006 | Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. |
| Nishizawa 2002 | Intervention was not anti‐leukotriene |
| Noonan 1998 | Control intervention was not inhaled corticosteroid |
| Noonan 1999 | Not a randomised controlled trial |
| Nsouli 2000 | Control intervention was not placebo |
| Nsouli 2001 | Control intervention was not placebo |
| O'Byrne 1997 | Not a randomised controlled trial |
| O'Byrne 1997a | Not a randomised controlled trial |
| O'Byrne 1997b | Not a randomised controlled trial |
| O'Connor 1994 | Not a randomised controlled trial |
| O'Connor 2004 | Participants received additional non‐permitted co‐interventions (montelukast with fluticasone) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| O'Connor 2006 | Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| O'Shaughnessy 1996 | Participants were not people with asthma Outcomes were solely the result of provocation |
| O'Sullivan 2002 | Duplicate of published abstract in European Respiratory Journal 2002;20(Suppl 38):388s |
| O'Sullivan 2002a | Participants received additional non‐permitted co‐interventions (montelukast with fluticasone) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| O'Sullivan 2003 | Control intervention was not placebo |
| Obase 2001 | Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Obata 1992 | Not a randomised controlled trial |
| Odjakova 2000 | Not a randomised controlled trial |
| Ohbayashi 2007 | Intervention was not anti‐leukotriene |
| Ohbayashi 2009 | Participants received additional non‐permitted co‐interventions (montelukast with fluticasone+salmeterold) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Ohbayashi 2009a | Participants received additional non‐permitted co‐interventions (pranlukast with fluticasone) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Ohkura 2009 | Participants received additional non‐permitted co‐interventions (pranlukast with inhaled corticosteroids+long acting beta2 agonists) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Ohta 2009 | Intervention was not anti‐leukotriene; Control intervention was not inhaled corticosteroid |
| Okudaira 1997 | Not a randomised controlled trial |
| Oosaki 1997 | Not a randomised controlled trial |
| Oosaki 1997a | Not a randomised controlled trial |
| Oppenheimer 2008 | Participants received additional non‐permitted co‐interventions (montelukast with fluticasone+salmeterold) other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Ostrom 2003 | Duplicate of published abstract in Journal of Pediatrics 2005;147(2):213‐220 |
| Overbeek 2002 | Outcomes measures did not reflect asthma control |
| Palmqvist 2003 | Intervention administered for < 4 weeks |
| Palmqvist 2005 | Intervention administered for < 4 weeks |
| Panettieri 1997 | Not a randomised controlled trial |
| Papadopoulos 2009 | Control intervention was not inhaled corticosteroid |
| Pasaoglu 2008 | Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Patel 2010 | Participants received additional non‐permitted co‐interventions (budesonide+formoterol with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Paterson 1999 | Intervention administered for < 4 weeks |
| Pavord 2007 | Participants received additional non‐permitted co‐interventions (montelukast with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Pearlman 1999 | Control intervention was not inhaled corticosteroid. Intervention administered for < 4 weeks. Outcomes were solely the result of provocation. |
| Pearlman 2002 | Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Pedersen 2007 | Intervention administered for < 4 weeks |
| Pereira 1989 | Participants were not people with asthma |
| Perng 2004 | Participants received additional non‐permitted co‐interventions (zafirlukast with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Peroni 2005a | Participants received additional non‐permitted co‐interventions (budesonide with montelukast and formoterol with budesonide) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Philip 2005 | Control intervention was not inhaled corticosteroid |
| Phipatanakul 2003 | Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroid) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Phipatanakul 2003a | Duplication of paper published in Annals of Allergy Asthma & Immunology 2003;91(1):49‐54 |
| Pieters 2005 | Participants received additional non‐permitted co‐interventions (montelukast with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Pizzichini 1999 | Control intervention was not inhaled corticosteroid |
| Plaza 2005 | Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. |
| Pogson 2008 | Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. |
| Pohl 2006 | Intervention was not anti‐leukotriene |
| Polos 2003 | Participants received additional non‐permitted co‐interventions (fluticasone with montelukast and salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Polos 2004 | Duplication of paper published in Pediatrics 2005;116(2):360‐369 |
| Ponce 2009 | Participants received additional non‐permitted co‐interventions (budesonide+salmeterol with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Price 1999 | Control intervention was not inhaled corticosteroid |
| Price 2002 | Control intervention were not placebo |
| Price 2003 | Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Price 2004 | Duplication; Participants received additional non‐permitted co‐interventions (eg. montelukast with budesonide) |
| Price 2006 | Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Pullerits 1999 | Participants were not people with asthma (allergic rhinitis) |
| Pullerits 2001 | Participants were not people with asthma |
| Pullerits 2002 | Participants were not people with asthma |
| Qaqundah 2006 | Intervention was not anti‐leukotriene; |
| Rachelefsky 1997 | Not a randomised controlled trial |
| Ragab 2001 | Not a randomised controlled trial |
| Ramsay 1997 | Control intervention was not inhaled corticosteroid |
| Ramsay 1998 | Control intervention was not inhaled corticosteroid |
| Rand 2004 | Duplication of full paper in Journal of Allergy & Clinical Immunology 2007;119(4):916‐923 |
| Ratner 2003 | Participants were not people with asthma |
| Reiss 1996 | Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid. Intervention administered for < 4 weeks. |
| Reiss 1997 | Control intervention was not inhaled corticosteroid. Duplication of full paper in Clin Exp Allergy 2001;31:1‐10. |
| Reiss 1997b | Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Reiss 1997c | Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid. Intervention administered for < 4 weeks. |
| Reiss 1998 | Control intervention was not inhaled corticosteroid |
| Reiss 2008 | Duplication of paper published in Journal of Asthma 2009;46(5):465‐469 |
| Riccioni 2001a | Duplication of International Journal of Immunopathology and Pharmacology 2001:14(2):87‐92 |
| Riccioni 2002 | Not a randomised controlled trial |
| Riccioni 2003a | Not a randomised controlled trial |
| Riccioni 2005 | Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Rickard 1999 | Control intervention was not placebo |
| Rickard 2001 | Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Ringdal 1997 | Outcomes did not reflect control of chronic or acute asthma |
| Ringdal 2003 | Participants received additional non‐permitted co‐interventions (fluticasone with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Robinson 2001 | Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid. Intervention administered for < 4 weeks. |
| Rosenhall 2003 | Intervention was not anti‐leukotriene |
| Rowe 2007 | Intervention was not anti‐leukotriene |
| Ruggins 2003 | Participants received additional non‐permitted co‐interventions (inhaled corticosteroids with montelukast and salmeterol with inhaled corticosteroids) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Sahn 1997 | Control intervention was not inhaled corticosteroid |
| Sano 2006 | Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. |
| Schneider 2008 | Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. |
| Schuh 2009 | Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. |
| Schwartz 1995 | Control intervention was not inhaled corticosteroid |
| SD‐004‐0216 | Participants received additional non‐permitted co‐interventions (budesonide with zafirlukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Shah 2003 | Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Shah 2004 | Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Shah 2006 | Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Sheth 2002 | Participants received additional non‐permitted co‐interventions other than short‐acting ß2‐agonists and/or short course of oral corticosteroid |
| Shimoda 2005 | Duplication |
| Shingo 2001 | Control intervention was not inhaled corticosteroid |
| Shoji 1999 | Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. |
| Simons 2001 | Control intervention was not inhaled corticosteroid |
| Simpson 2004 | Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. |
| Sims 2003 | Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroids) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid. Intervention administered for < 4 weeks. |
| Sims 2008 | Duplication |
| Smith 1993 | Control intervention was not inhaled corticosteroid. Intervention administered for < 4 weeks. |
| Smith 1997 | Treatments were administered for <4 weeks |
| Smith 1998 | Control intervention was not inhaled corticosteroid. Intervention administered for < 4 weeks. Outcomes were solely the result of provocation. |
| Smugar 2009 | Participants received additional non‐permitted co‐interventions (montelukast with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Smugar 2009a | Duplication of full paper in Annals of Allergy Asthma & Immunology 2010;104(6):511‐517 |
| Spahn 1996a | Not a randomised controlled trial |
| Spector 1992 | Control intervention was not inhaled corticosteroid |
| Spector 1994 | Control intervention was not inhaled corticosteroid |
| Spector 1995 | Control intervention was not inhaled corticosteroid |
| Spector 1996 | Not a randomised controlled trial |
| Stanford 2002 | Non‐permitted drugs |
| Stensrud 2006 | Not a randomised controlled trial |
| Stevenson 2005 | Intervention was not anti‐leukotriene |
| Sthoeger 2007 | Intervention was not anti‐leukotriene |
| Storms 2001 | Not a randomised controlled trial. Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. |
| Storms 2004 | Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroids) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Strauch 2003 | Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Strunk 2003 | Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. |
| Strunk 2008 | Participants received additional non‐permitted co‐interventions (budesonide+salmeterol with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Sugihara 2010 | Intervention was given < 4 weeks |
| Suguro 1997 | Not a randomised controlled trial |
| Suissa 1997 | Control intervention was not inhaled corticosteroid |
| Sutherland 2010 | Not a randomised controlled trial‐retrospective analysis |
| Suzuki 1997 | Not a randomised controlled trial |
| Svensson 1994 | Control intervention was not inhaled corticosteroid |
| Swern 2008 | Not a randomised controlled trial. Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. |
| Swernson 2003 | Participants received additional non‐permitted co‐interventions (ICS with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Tamaoki 1997 | Control intervention was not inhaled corticosteroid |
| Tan 2006 | Intervention was not anti‐leukotriene |
| Tashkin 1998 | Control intervention was not inhaled corticosteroid |
| Teper 2009 | Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Terzano 2001 | Intervention was not anti‐leukotriene |
| Thoma 2002 | Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Todi 2010 | Participants received additional non‐permitted co‐interventions (antiasthmatic drugs with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Tognella 2004 | Participants received additional non‐permitted co‐interventions (fluticasone with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Tohda 2002 | Control intervention was not placebo |
| Tomari 2001 | Control intervention was not placebo |
| Tomita 1999 | Participants received additional non‐permitted co‐interventions (inhaled and oral corticosteroids) other than short‐acting beta2‐agonists |
| Tonelli 2003 | Not a randomised controlled trial; Participants received additional non‐permitted co‐interventions (antihistaminic drugs) other than short‐acting beta2‐agonists |
| Townley 1995 | Control intervention was not inhaled corticosteroid |
| Trofor 2002 | Not a randomised controlled trial. Control intervention was not inhaled corticosteroid. |
| Tsai 2010 | Intervention was not anti‐leukotriene. Control intervention was not inhaled corticosteroid. |
| Tsuchida 2005 | Participants received additional non‐permitted co‐interventions (beclomethasone with pranlukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Tug 2007 | Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Tukiainen 2002 | Intervention was not anti‐leukotriene |
| Uh 2007 | Participants received additional non‐permitted co‐interventions (salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Ulrik 2009 | Duplication of work presented in Am J Respir Crit Care Med 2009;179:A2416 |
| Ulrik 2009a | Control intervention was not inhaled corticosteroid |
| Ulrik 2010 | Control intervention was not inhaled corticosteroid. Participants received additional non‐permitted co‐interventions (montelukast with inhaled corticosteroids) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid. |
| Van Adelsberg 2005 | Control intervention was not inhaled corticosteroid |
| Van Der Meer 2009 | Intervention was not anti‐leukotriene; Control intervention was not inhaled corticosteroid |
| Vaquerizo 2003 | Control intervention was not placebo |
| Vastagh 2003 | Intervention was not anti‐leukotriene |
| Verhoeven 2001 | Participants were not asthmatics (COPD) |
| Verini 2007 | Participants received additional non‐permitted co‐interventions (fluticasone with montelukast and salmeterol with fluticasone) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid. Intervention administered for < 4 weeks. |
| Vermeulen 2007 | Intervention was not anti‐leukotriene |
| Vethanayagam 2002 | Intervention administered for < 4 weeks |
| Vidal 2001 | Intervention administered for < 4 weeks |
| Vidal 2001a | Intervention administered for < 4 weeks |
| Virchow 1997 | Control intervention was not inhaled corticosteroid |
| Virchow 1997a | Duplication |
| Virchow 1997b | Control intervention was not inhaled corticosteroid |
| Virnig 2008 | Participants received additional non‐permitted co‐interventions (other antiasthmatic drugs with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Volovitz 1999 | Duplication of full paper in Curr Med Res Opin 2001;17(2):96‐104 |
| Von Berg 2002 | Intervention was not anti‐leukotriene |
| Wada 2000 | Participants received additional non‐permitted co‐interventions (inhaled steroids) other than short‐acting beta2‐agonists |
| Wada 2009 | Control intervention was not inhaled corticosteroid |
| Wahedna 1999 | Intervention administered for < 4 weeks. Outcomes were solely the result of provocation. |
| Warren 2003 | Ongoing clinical trial. |
| Wasserman 2006 | Intervention was not anti‐leukotriene |
| Wechsler 1998 | Not a randomised controlled trial |
| Weinberg 1998 | Outcomes did not reflect control of chronic or acute asthma |
| Weiss 2010 | Control intervention was not inhaled corticosteroids |
| Welch 1994 | Control intervention was not inhaled corticosteroid. Intervention administered for < 4 weeks. |
| Wen 2008 | Intervention was not anti‐leukotriene |
| Wenzel 1994 | Control intervention was not inhaled corticosteroid |
| Wenzel 1995 | Control participants were not people with asthma. Intervention administered for < 4 weeks. |
| Wenzel 1997 | Duplication of data published in American Journal of Respiratory & Critical Care Medicine 1998;157:A411 |
| Westbroek 1997 | Intervention was not anti‐leukotriene |
| Westbroek 1998 | Intervention administered for < 4 weeks |
| Westbroek 2000 | Intervention administered for < 4 weeks |
| Williams 2001 | Duplication of three studies |
| Wilson 1999 | Control intervention was not placebo |
| Wilson 2000 | Control intervention was not inhaled corticosteroid; Intervention administered for < 4 weeks |
| Wilson 2001 | Intervention administered for < 4 weeks |
| Wilson 2001a | Participants were not prople with asthma |
| Wilson 2001b | Intervention administered for < 4 weeks |
| Wilson 2001c | Outcome measures did not reflect asthma control |
| Wilson 2004 | Not a randomised controlled trial |
| Wilson 2010 | Not a randomised controlled trial |
| Wilson 2010a | Participants received additional non‐permitted co‐interventions (ICS with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Wise 2009 | Control intervention was not inhaled corticosteroid |
| Xiang 2001 | Control intervention was not placebo |
| Yaldiz 2000 | Participants received additional non‐permitted co‐intervention (ICS with montelukast) |
| Yamamoto 1994 | Control intervention was not inhaled corticosteroid. Intervention administered for < 4 weeks. Outcomes were solely the result of provocation. |
| Yildirim 2001 | Control intervention was not placebo |
| Yildirim 2004 | Participants received additional non‐permitted co‐interventions (budesonide with montelukast) other than short‐acting beta2‐agonists and/or short course of oral corticosteroid |
| Yoo 2001 | Participants received additional non‐permitted co‐interventions (inhaled corticosteroids) other than short‐acting beta2‐agonists |
| Yoshida 2000 | Intervention administered for < 4 weeks |
| Yoshida 2002 | Intervention administered for < 4 weeks |
| Zeiger 2004 | Not a randomised controlled trial |
| Zeiger 2005a | Duplication of full paper published in American Journal of Medicine 2005;118(6):649‐657 |
| Zeneca Accolate 1998 | Not a randomised controlled trial (product monograph) |
| Zhang 1999 | Control intervention was not inhaled corticosteroid |
| Zorc 2003 | Intervention was not anti‐leukotriene |
Differences between protocol and review
We revised the protocol to take account of the new methods in the Cochrane Handbook for Systematic Reviews of Interventions.
Updated the Cochrane Collaboration's 'Risk of bias' tool to include six domains
Contributions of authors
Dr Bhupendrasinh Chauhan reviewed the literature search from 2003 to December 2010, identified and reviewed all citations for relevance, reviewed all included trials for methodology and data extraction, verified all references, description of studies and data entry, analysed and interpreted results of the meta‐analysis, manuscript writing.
Prof Francine Ducharme conceived the protocol, requested the literature search, identified and contacted the corresponding authors and/or the pharmaceutical companies to solicit their collaboration in this review and in the identification of other possibly relevant trials, created the methodology and data extraction forms, reviewed all citations for relevance with research assistants, reviewed all included trials for methodology and data extraction, corresponded with authors or pharmaceutical companies to verify methodology and data extraction, verified all references, description of studies and data entry, analysed and interpreted results of the meta‐analysis.
Sources of support
Internal sources
No sources of support supplied
External sources
Francine Ducharme is supported by a National Researcher Award from the Fonds de la Santé du Québec, Canada.
-
Bhupendrasinh Chauhan, Canada.
received a postdoctoral scholarship from Canadian Institute of Health Research, Canada
Declarations of interest
Bhupendrasinh Chauhan ‐ none known.
Prof. Francine Ducharme has received travel support, research funds and fees for speaking from Glaxo SmithKline, Novartis, Nycomed, and Merck Frosst Inc.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Abadoglu 2005 {published data only}
- Abadoglu O, Mungan D, Aksu O, Erekul S, Misirligil Z. The effect of montelukast on eosinophil apoptosis: induced sputum findings of patients with mild persistent asthma. Allergol Immunopathol 2005;33(2):105‐11. [DOI] [PubMed] [Google Scholar]
Basyigit 2004 {published data only}
- Basyigit I, Yildiz F, Kacar Ozkara S, Boyaci H, Ilgazli A, Ozkarakas O. Effects of different anti‐asthmatic agents on induced sputum and eosinophil cationic protein in mild asthmatics. Respirology 2004;9(4):514‐20. [DOI] [PubMed] [Google Scholar]
Baumgartner 2003 {published data only}
Bleecker 2000 {unpublished data only}
- Bleecker ER, Welch MJ, Weinstein SF, Kalberg C, Johnson M, Edwards L, et al. Low‐dose inhaled fluticasone propionate versus oral zafirlukast in the treatment of persistent asthma. Journal of Allergy & Clinical Immunology 2000;105(6):1123‐9. [DOI] [PubMed] [Google Scholar]
Boushey 2005 {published data only}
- Boushey HA, Sorkness CA, King TS, Sullivan SD, Fahy JV, Lazarus SC, et al. Daily versus as‐needed corticosteroids for mild persistent asthma. New England Jounal of medicine 2005;352(15):1519‐1528. [DOI] [PubMed] [Google Scholar]
Bousquet 2005 {published data only}
- Bousquet J, Menten J, Tozzi CA, Polos PG. Oral montelukast sodium versus inhaled fluticasone propionate in adults with mild persistent asthma. Journal of Applied Research 2005;5(3):402‐14. [Google Scholar]
Brabson 2002 {published data only}
- Brabson JH, Clifford D, Kerwin E, Raphael G, Pepsin PJ, Edwards LD, et al. Efficacy and safety of low‐dose fluticasone propionate compared with zafirlukast in patients with persistent asthma. American Journal of Medicine 2002;113:15‐21. [DOI] [PubMed] [Google Scholar]
Busse 2001a {published data only}
- Busse W, Wolfe J, Storms W, Srebro S, Edwards L, Johnson M, et al. Fluticasone propionate compared to Zafirlukast in controlling persistent asthma: A randomised double‐blind, placebo‐controlled trial. Journal of Family Practice 2001;50:595‐602. [PubMed] [Google Scholar]
Busse 2001b {published data only}
- Busse W, Raphael GD, Galant S, Kalberg C, Goode‐Sellers S, Srebro S, et al. Low‐dose fluticasone propionate compared to montelukast for first‐line treatment of persistent asthma: a randomised controlled trial. Journal of Allergy & Clinical Immunology 2001;107(3):461‐8. [DOI] [PubMed] [Google Scholar]
Caffey 2005 {published data only}
- Caffey LF, Raissy HH, Marshik P, Kelly HW. A crossover comparison of fluticasone propionate and montelukast on inflammatory indices in children with asthma. Pediatric Asthma Allergy & Immunology 2005;18(3):123‐30. [Google Scholar]
Dempsey 2002a {published data only}
- Dempsey OJ, Wilson AM, Lipworth BJ. Comparative efficacy and anti‐inflammatory profile of once daily low dose hfa‐triamcinolone acetonide (taa) and montelukast (ml) in patients with mild persistent atopic asthma. Journal of Allergy and Clinical Immunology 2002;109(1):68‐74. [DOI] [PubMed] [Google Scholar]
FLTA4030 {unpublished data only}
- A randomized, double‐blind, double dummy, placebo‐controlled, parallel‐group, comparative study of inhaled fluticasone propionate (88 mcg bid) versus zafirlukast (20 mg bid) in subjects who are currently receiving beta agonists alone. www.gsk‐clinicalstudyregister.com.
FLTA4031 {unpublished data only}
- A randomized, double‐blind, double‐dummy, placebo‐controlled, parallel‐group, comparative study of inhaled fluticasone propionate (88 mcg bid) versus zafirlukast (20 mg bid) in subjects who are currently receiving beta agonists alone. www.gsk‐clinicalstudyregister.com.
FMS40012 {unpublished data only}
- Evaluation of the potential anti‐inflammatory action of leukotriene D4 receptor antagonists: comparison of zafirlukast, an LTD4 receptor antagonist with low‐dose fluticasone propionate, an inhaled steroid on sputum eosinophils in mild asthma. GlaxoSmithKline Clinical Trial Register 2005.
FPD40013 {unpublished data only}
- FPD40013. A randomized, double‐blind, double dummy, parallel group comparison of fluticasone propionate inhalation powder (50 mcg BID) via discus with oral montelukast (5 mg QD) chewable tablets in children 6‐12 years of age with persistent asthma. GlaxoSmithKline Clinical Trial Register 2005.
Garcia Garcia 2005 {published data only}
- Garcia Garcia ML, Wahn U, Gilles L, Swern A, Tozzi CA, Polos P. Montelukast, compared with fluticasone, for control of asthma among 6‐ to 14‐year‐old patients with mild asthma: the MOSAIC study. Pediatrics 2005;116(2):360‐9. [DOI] [PubMed] [Google Scholar]
Hughes 1999 (BDP) {unpublished data only}
- Hughes GL, Edelman JM, Turpin JA, Liss C, Weeks K. Randomized, open‐label pilot study comparing the effects of montelukast sodium tablets, fluticasone aerosol inhaler, and budesonide dry powder inhaler on asthma control in mild asthmatics. American Journal of Respiratory & Critical Care Medicine 1999;159:A641. [Google Scholar]
Hughes 1999 (FP) {unpublished data only}
- Hughes GL, Edelman JM, Turpin JA, Liss C, Weeks K. Randomized, open‐label pilot study comparing the effects of montelukast sodium tablets, fluticasone aerosol inhaler, and budesonide dry powder inhaler on asthma control in mild asthmatics. American Journal of Respiratory & Critical Care Medicine 1999;159:A641. [Google Scholar]
Israel 2002 {published data only}
- Israel E, Chervinsky PS, Friedman B, Bavel J, Skalky CS, Ghannam AF, et al for the Montelukast Beclomethasone Comparison Study Group. Effects of montelukast and beclomethasone on airway function and asthma control. Journal of Allergy and Clinical Immunology 2002;110(6):847‐54. [DOI] [PubMed] [Google Scholar]
Jayaram 2002 {published data only}
- Jayaram L, Pizzichini MMM, Hussack P, Efthimiadis A, Lemiere C, Cartier A, et al. First line anti‐inflammatory treatment for asthma; inhaled steroid or leukotriene antagonist?. Respirology 2002;7(A19):21. [Google Scholar]
Jayaram 2005 {published data only}
- Jayaram L, Pizzichini E, Lemiere C, Man SF, Cartier A, Hargreave FE, et al. Steroid naive eosinophilic asthma: anti‐inflammatory effects of fluticasone and montelukast. Thorax 2005;60(2):100‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jenkins 2005 {published data only}
- Jenkins CR, Thien FCK, Wheatley JR, Reddel HK. Traditional and patient‐centred outcomes with three classes of asthma medication. European Respiratory Journal 2005;26(1):36‐44. [DOI] [PubMed] [Google Scholar]
Kanazawa 2007 {published data only}
- Kanazawa H, Nomura S, Asai K. Roles of angiopoietin‐1 and angiopoietin‐2 on airway microvascular permeability in asthmatic patients. Chest 2007;131(4):1035‐41. [DOI] [PubMed] [Google Scholar]
Kanniess 2002 {published data only}
- Kanniess F, Richter K, Bohme S, Jorres RA, Magnussen H. Montelukast versus fluticasone: effects on lung function, airway responsiveness and inflammation in moderate asthma. European Respiratory Journal 2002;20:853‐8. [DOI] [PubMed] [Google Scholar]
Khan 2008 {published data only}
- Khan SA, Hashmi ZY. Comparison of therapeutic values between leukotriene receptor antagonist (Montelukast) and inhaled glucocorticoid (Beclomethasone propionate) in bronchial asthma of adults. Pakistan Journal of Medical Sciences 2008;24(3):399‐405. [Google Scholar]
Kim 2000 {published and unpublished data}
- Kim KT, Ginchansky EJ, Friedman BF, Srebro S, Pepsin PJ, Edwards L, et al. Fluticasone propionate versus zafirlukast: effect in patients previously receiving inhaled corticosteroid therapy. Annals of Allergy, Asthma, & Immunology 2000;85:398‐406. [DOI] [PubMed] [Google Scholar]
Koenig 2008 {published data only}
- Koenig SM, Ostrom N, Pearlman D, Waitkus‐Edwards K, Yancey S, Prillaman BA, et al. Deterioration in Asthma Control When Subjects Receiving Fluticasone Propionate/Salmeterol 100/50 mcg Diskus are "Stepped‐Down". Journal of Asthma 2008;45:681‐7. [DOI] [PubMed] [Google Scholar]
Kooi 2008 {published data only}
- Kooi EMW, Schokker S, Marike Boezen H, Vries TW, Vaessen‐Verberne AAPH, Molen T, et al. Fluticasone or montelukast for preschool children with asthma‐like symptoms: Randomized controlled trial. Pulmonary Pharmacology and Therapeutics 2008;21(5):798‐804. [DOI] [PubMed] [Google Scholar]
Kumar 2007 {published data only}
- Kumar V, Ramesh P, Lodha R, Pandey RM, Kabra SK. Montelukast vs. inhaled low‐dose budesonide as monotherapy in the treatment of mild persistent asthma: A randomized double blind controlled trial. Journal of Tropical Pediatrics 2007;53(5):325‐30. [DOI] [PubMed] [Google Scholar]
Laitinen 1997 {unpublished data only}
- Laitinen LA, Naya IP, Binks S, Harris A. Comparative efficacy of zafirlukast & low dose steroids in asthmatics on prn beta2‐agonists. European Respiratory Journal. 1997; Vol. 10, issue Suppl 25:419‐20, Abs. 2716.
Laviolette 1999 {published and unpublished data}
- Laviolette M, Malmstrom K, Lu S, Chervinsky P, Pujet J‐C, Peszek I, et al. Montelukast added to inhaled beclomethasone in treatment of asthma. American Journal of Respiratory & Critical Care Medicine 1999;160(6):1862‐8. [DOI] [PubMed] [Google Scholar]
Lazarus 2007 {published data only}
- Lazarus SC, Chinchilli VM, Rollings NJ, Boushey HA, Cherniack R, Craig TJ, et al. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. American Journal of Respiratory and Critical Care Medicine 2007;175(8):783‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2009 {published data only}
- Lu S, Liu N, Dass SB, Reiss TF. A randomized study comparing the effect of loratadine added to montelukast with montelukast, loratadine, and beclomethasone monotherapies in patients with chronic asthma. Journal of Asthma 2009;46(5):465‐9. [DOI] [PubMed] [Google Scholar]
Malmstrom 1999 {published and unpublished data}
- Malmstrom K, Rodriguez‐Gomez G, Guerra J, Villaran C, Pineiro A, Lynn X, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma: a randomized, controlled trial. Annals of Internal Medicine 1999;130(6):487‐95. [DOI] [PubMed] [Google Scholar]
Maspero 2001 {published data only}
- Maspero JF, Duenas‐Meza E, Volovitz B, Daza CP, Kosa L, Vrijens F, et al. Oral montelukast versus inhaled beclomethasone in 6 to 11‐year old children with asthma: results of an open‐label extension study evaluating long‐term safety, satisfaction, and adherence to therapy. Current Medical Research and Opinion 2001; Vol. 17, issue 2:96‐104. [PubMed]
Meltzer 2002 {published data only}
- Meltzer EO, Lockey RF, Friedman BF, Kalberg C, Goode‐Sellers S, Srebro S, et al for the Fluticasone Propionale Clinical Research Study Group. Efficacy and safety of low‐dose fluticasone propionate compared with montelukast for maintenance treatment of persistent asthma. Mayo Clinc Proceedings 2002;77:437‐5. [PubMed] [Google Scholar]
MK0479‐332 {unpublished data only}
- Merck. Montelukast asthmatic smoker study. Clinicaltrials.Gov 2006.
Nathan 2001 {published data only}
- Nathan RA, Bleecker ER, Kalberg C and the Fluticasone Propionate Study Group. A comparison of short‐term treatment with inhaled fluticasone propionate and zafirlukast for patients with persistent asthma. American Journal of Medicine 2001;111:195‐202. [DOI] [PubMed] [Google Scholar]
NCT00442559 {unpublished data only}
- Montelukast in mild asthmatic children with allergic rhinitis. Clinicaltrials.gov 2008.
Ng 2007 {published data only}
- Ng DKK, Chan CH, Wu S, Chow PY, Wong LSW, Fu YM, et al. Oral montelukast versus inhaled budesonide in children with mild persistent asthma: A pilot study. The Hong Kong Journal of Paediatrics 2007;12(1):3‐10. [Google Scholar]
Ostrom 2005 {published data only}
- Ostrom NK, Decotiis BA, Lincourt WR, Edwards LD, Hanson KM, Carranza Rosenzweig JR, et al. Comparative efficacy and safety of low‐dose fluticasone propionate and montelukast in children with persistent asthma. Journal of Pediatrics 2005;147((2):213‐20. [DOI] [PubMed] [Google Scholar]
Overbeek 2004 {published data only}
- Overbeek SE, O'Sullivan S, Leman K, Mulder PGH, Hoogsteden HC, Prins J‐B. Effect of montelukast compared with inhaled fluticasone on airway inflammation. Clinical & Experimental Allergy 2004;34(9):1388‐94. [DOI] [PubMed] [Google Scholar]
Peroni 2005 {published data only}
- Peroni D, Bodini A, Miraglia Del Giudice M, Loiacono A, Baraldi E, Boner AL, et al. Effect of budesonide and montelukast in asthmatic children exposed to relevant allergens. Allergy 2005;60(2):206‐10. [DOI] [PubMed] [Google Scholar]
Peters 2007 {published data only}
- Peters SP, Anthonisen N, Castro M, Holbrook JT, Irvin CG, Smith LJ, et al. Randomized comparison of strategies for reducing treatment in mild persistent asthma. New England Journal of Medicine 2007;356(20):2027‐39. [DOI] [PubMed] [Google Scholar]
Riccioni 2001 {published data only}
- Riccioni G, Castronuove M, Benedictis M, Pace‐Palitti V, Gioacchino M, Della Vecchia R, et al. Zafirlukast versus budesonide on bronchial reactivity in subjects with mild‐persistent asthma. International Journal of Immunopathology and Pharmacology 2001;14(2):87‐92. [PubMed] [Google Scholar]
Riccioni 2002a {published data only}
- Riccioni G, D'Orazio N, Ilio C, Della Vecchia R, Lorenzo A. Effectiveness and safety of montelukast versus budesonide at various doses on bronchial reactivity in subjects with mild persistent asthma [Efficacia e tollerabilita del montelukast verso budesonide a diverso dosaggio sulla reattivita bronchiale in soggetti con asma di grado lieve‐persistente]. La Clinica terapeutica 2002;153(5):317‐21. [PubMed] [Google Scholar]
Riccioni 2002b {published data only}
- Riccioni G, Ballone E, D'Orazio N, Sensi S, Nicola M, Mascio R, et al. Effectiveness of montelukast versus budesonide on quality of life and bronchial reactivity in subjects with mild‐persistent asthma. International Journal of Immunopathology & Pharmacology 2002;15(2):149‐55. [DOI] [PubMed] [Google Scholar]
Riccioni 2003 {published data only}
- Riccioni G, Vecchia RD, D'Orazio N, Sensi S, Guagnano MT. Comparison of montelukast and budesonide on bronchial reactivity in subjects with mild‐moderate persistent asthma. Pulmonary Pharmacology & Therapeutics 2003;16(2):111‐4. [DOI] [PubMed] [Google Scholar]
Sheth 2001 {published data only}
- Sheth K, Edwards L, Srebro S, Rickard K. Effects of baseline pulmonary function on treatment response: low‐dose fluticasone versus zafirlukast. Annals of Allergy, Asthma and IImmunology 2001;86:2001. [Google Scholar]
Sorkness 2007 {published data only}
- Sorkness CA, Lemanske Jr RF, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD. Long‐term comparison of 3 controller regimens for mild‐moderate persistent childhood asthma: The Pediatric Asthma Controller Trial. Journal of Allergy & Clinical Immunology 2007;119(1):64‐72. [DOI] [PubMed] [Google Scholar]
Stelmach 2002a {published data only}
- Stelmach I, Jerzynska J, Kuna P. A randomized, double‐blind trial of the effect of glucocorticoid, antileukotriene and beta‐agonist treatment on IL‐10 serum levels in children with asthma. Clinical & Experimental Allergy 2002;32(2):264‐9. [DOI] [PubMed] [Google Scholar]
Stelmach 2002b {published and unpublished data}
- Stelmach I, Grzelewski T, Stelmach W, Majak P, Jerzynska J, Gorski P, et al. Effect of triamcinolone acetonide, montelukast, nedocromil sodium and formoterol on eosinophil blood counts, ECP serum levels and clinical progression of asthma in children [Polish]. Polski Merkuriusz Lekarski 2002;12(69):208‐13. [PubMed] [Google Scholar]
Stelmach 2004 {published data only}
- Stelmach I, Majak P, Jerzynska J, Stelmach W, Kuna P. Comparative effect of triamcinolone, nedocromil and montelukast on asthma control in children: A randomized pragmatic study. Pediatric Allergy & Immunology 2004;15(4):359‐64. [DOI] [PubMed] [Google Scholar]
Stelmach 2005 {published data only}
- Stelmach I, Bobrowska‐Korzeniowska M, Majak P, Stelmach W, Kuna P. The effect of montelukast and different doses of budesonide on IgE serum levels and clinical parameters in children with newly diagnosed asthma. Pulmonary Pharmacology & Therapeutics 2005;18(5):374‐80. [DOI] [PubMed] [Google Scholar]
Stelmach 2007 {published data only}
- Stelmach I, Grzelewski T, Bobrowska‐Korzeniowska M, Stelmach P, Kuna P. A randomized, double‐blind trial of the effect of anti‐asthma treatment on lung function in children with asthma. Pulmonary Pharmacology and Therapeutics 2007;20(6):691‐700. [DOI] [PubMed] [Google Scholar]
Stelmach 2008 {published data only}
- Stelmach I, Grzelewski T, Majak P, Jerzynska J, Stelmach W, Kuna P. Effect of different antiasthmatic treatments on exercise‐induced bronchoconstriction in children with asthma. Journal of Allergy Clinical Immunology 2008;121(2):383‐9. [DOI] [PubMed] [Google Scholar]
Szefler 2005 {published data only}
- Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC. Characterization of within‐subject responses to fluticasone and montelukast in childhood asthma. Journal of Allergy and Clinical Immunology 2005;115(2):233‐42. [DOI] [PubMed] [Google Scholar]
Szefler 2007 {published data only}
- Szefler SJ, Baker JW, Uryniak T, Goldman M, Silkoff PE. Comparative study of budesonide inhalation suspension and montelukast in young children with mild persistent asthma. Journal of Allergy and Clinical Immunology 2007;120(5):1043‐50. [DOI] [PubMed] [Google Scholar]
Tamaoki 2008 {published data only}
- Tamaoki J, Isono K, Taira M, Tagaya E, Nakata J, Kawatani K, et al. Role of regular treatment with inhaled corticosteroid or leukotriene receptor antagonist in mild intermittent asthma. Allergy and Asthma Proceedings : the Official Journal of Regional and State Allergy Societies 2008;29(2):189‐96. [DOI] [PubMed] [Google Scholar]
Yamauchi 2001 {published data only}
- Yamauchi K, Tanifuji Y, Pan LH, Yoshida T, Sakurai S, Goto SI, et al. Effects of Pranlukast, a Leukotriene receptor antagonist, on airway inflammation in mild asthmatics. Journal of Asthma 2001;38(1):51‐7. [DOI] [PubMed] [Google Scholar]
Yurdakul 2003 {published data only}
- Yurdakul AS, Taci N, Eren A, Sipit T. Comparative efficacy of once‐daily therapy with inhaled corticosteroid, leukotriene antagonist or sustained‐release theophylline in patients with mild persistent asthma. Respiratory Medicine 2003;97(12):1313‐9. [DOI] [PubMed] [Google Scholar]
Zedan 2009 {published data only}
- Zedan M, Gamil N, El‐Chennawi F, Maysara N, Hafeez HA, Nasef N, et al. Evaluation of different asthma phenotype responses to montelukast versus fluticasone Treatment. Pediatric Asthma, Allergy and Immunology 2009;22(2):63‐9. [Google Scholar]
Zeiger 2005 {published data only}
- Zeiger RS, Bird SR, Kaplan MS, Schatz M, Pearlman DS, Orav EJ. Short‐term and long‐term asthma control in patients with mild persistent asthma receiving montelukast or fluticasone: a randomized controlled trial. American Journal of Medicine 2005;118(6):649‐57. [DOI] [PubMed] [Google Scholar]
Zeiger 2006 {published data only}
- Zeiger RS, Szefler SJ, Phillips BR, Schatz M, Martinez FD, Chinchilli VM. Response profiles to fluticasone and montelukast in mild‐to‐moderate persistent childhood asthma. Journal of Allergy & Clinical Immunology 2006;117(1):45‐52. [DOI] [PubMed] [Google Scholar]
Zielen 2010 {published data only}
- Zielen S, Christmann M, Kloska M, Dogan‐Yildiz G, Lieb A, Rosewich M. Predicting short term response to anti‐inflammatory therapy in young children with asthma. Current Medical Research and Opinion 2010;26(2):483‐92. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbott Pharma 1996 {published data only}
- Abbott Pharmaceuticals. Zyflo Filmtab (zileuton) product description. Abbott Laboratories 1996.
Al Frayh 2008 {published data only}
- Al Frayh A, Abba A, Iskandarani A, Shaker DS, Zaitoun F, Shahrabani L, et al. Establishing therapeutic bioequivalence of a generic salbutamol (Butalin) metered dose inhaler to Ventolin. Biomedical Research 2008;19(1):61‐8. [Google Scholar]
Allayee 2007 {published data only}
- Allayee H, Hartiala J, Lee W, Mehrabian M, Irvin CG, Conti DV, et al. The effect of montelukast and low‐dose theophylline on cardiovascular disease risk factors in asthmatics. Chest 2007;132(3):868‐74. [DOI] [PubMed] [Google Scholar]
Allen 1997 {published data only}
- Allen A, Georgiou P, Compton C, Walls C, Hibell M, Tomson DJ, et al. Lack of pharmacokinetic and pharmacodynamic interactions between pranlukast (Ultair) and terfenadine. American Thoracic Society. 1997:Abs C49.
Allen‐Ramey 2003 {published data only}
- Allen‐Ramey FC, Duong PT, Goodman DC, Sajjan SG, Nelsen LM, Santanello NC, et al. Treatment effectiveness of inhaled corticosteroids and leukotriene modifiers for patients with asthma: an analysis from managed care data. Allergy & Asthma Proceedings 2003;24(1):43‐51. [PubMed] [Google Scholar]
Allen‐Ramey 2004 {published data only}
- Allen‐Ramey FC, Anstatt DT, Riedel AA, Markson LE. Greater adherence in montelukast patients compared to those on fluticasone [Abstract]. Journal of Allergy and Clinical Immunology 2004;113(2 Suppl):S158. [Google Scholar]
Allen‐Ramey 2006 {published data only}
- Allen‐Ramey FC, Markson LE, Riedel AA, Sajjan S, Weiss KB. Patterns of asthma‐related health care resource use in children treated with montelukast or fluticasone. Current Medical Research & Opinion 2006;22(8):1453‐61. [DOI] [PubMed] [Google Scholar]
Altman 1998k {published data only}
- Altman LC, Munk Z, Seltzer J, Noonan N, Shingo S, Zhang J, et al. A placebo‐controlled, dose‐ranging study of montelukast, a cysteinyl leukotriene‐receptor antagonist. Journal of Allergy & Clinical Immunology 1998;102:50‐6. [DOI] [PubMed] [Google Scholar]
Anonymous 1997 {published data only}
- Anonymous. Zileuton for asthma. Medical Letter on Drugs & Therapeutics 1997;39(995):18‐9. [PubMed] [Google Scholar]
Armour 2007 {published data only}
- Armour C, Bosnic‐Anticevich S, Brillant M, Burton D, Emmerton L, Krass I, et al. Pharmacy Asthma Care Program (PACP) improves outcomes for patients in the community. Thorax 2007;62(6):496‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bacharier 2008 {published data only}
- Bacharier LB, Phillips BR, Zeiger RS, Szefler SJ, Martinez FD, Lemanske RF, et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate‐to‐severe intermittent wheezing. The Journal of Allergy and Clinical Immunology 2008;122(6):1127‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bai 2010 {published data only}
- Bai J, Xu PR. Montelukast in the treatment of bronchiolitis, a multi‐center, randomized, three‐blind, placebo‐controlled trial. Chinese Journal of Evidence‐Based Medicine 2010;10(9):1011‐5. [Google Scholar]
Balatsouras 2005 {published data only}
- Balatsouras DG, Eliopoulos P, Rallis E, Sterpi P, Korres S, Ferekidis E. Improvement of otitis media with effusion after treatment of asthma with leukotriene antagonists in children with co‐existing disease. Drugs Under Experimental & Clinical Research 2005;31(SUPPL):7‐10. [PubMed] [Google Scholar]
Baren 2006 {published data only}
- Baren JM, Boudreaux ED, Brenner BE, Cydulka RK, Rowe BH, Clark S, et al. Randomized controlled trial of emergency department interventions to improve primary care follow‐up for patients with acute asthma. Chest 2006;129(2):257‐65. [DOI] [PubMed] [Google Scholar]
Barnes 1996 {published data only}
- Barnes NC, Black B, Syrett N, Cohn J. Reduction of exacerbations of asthma in multi‐national clinical trials with zafirlukast (Accolate). Allergy 1996;51(Suppl 30):84. [Google Scholar]
Barnes 1997 {published data only}
- Barnes NC, Jong B, Miyamoto T. Worldwide clinical experience with the first marketed leukotriene receptor antagonist. Chest 1997;111 Suppl(2):52‐60. [DOI] [PubMed] [Google Scholar]
Barnes 1997b {published data only}
- Barnes NC, Pujet J‐C. Pranlukast, a novel leukotriene receptor antagonist: results of the first European, placebo controlled, multicentre clinical study in asthma. Thorax 1997;52:523‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnes 2001 {unpublished data only}
- Barnes N, Wei LX, Reiss TF, Leff JA, Shingo S, Yu C, et al. Analysis of montelukast in mild persistent asthmatic patients with near‐normal lung function. Respiratory Medicine 2001;95(5):379‐86. [DOI] [PubMed] [Google Scholar]
Barnes 2007 {published data only}
- Barnes ML, Menzies D, Fardon TC, Burns P, Wilson AM, Lipworth BJ. Combined mediator blockade or topical steroid for treating the unified allergic airway. Allergy 2007;62(1):73‐80. [DOI] [PubMed] [Google Scholar]
Barnes 2007a {published data only}
- Barnes N, Laviolette M, Allen D, Flood‐Page P, Hargreave F, Corris P, et al. Effects of montelukast compared to double dose budesonide on airway inflammation and asthma control. Respiratory Medicine 2007;101(8):1652‐8. [DOI] [PubMed] [Google Scholar]
Bartoli 2010 {published data only}
- Bartoli ML, Dente FL, Bancalari L, Bacci E, Cianchetti S, Franco A, et al. Beclomethasone dipropionate blunts allergen‐induced early increase in urinary LTE4. European Journal of Clinical Investigation 2010;40(6):566‐9. [DOI] [PubMed] [Google Scholar]
Bateman 1995 {published data only}
- Bateman ED, Holgate ST, Binks SM, Tarns IP. A multicentre study to assess the steroid‐sparing potential of accolate (zafirlukast; 20 mg bd). Allergy 1995;50 Suppl(26):320, Abs P‐0709. [Google Scholar]
Bateman 2003 {published data only}
- Bateman ED, Akveld M, Ho M. Greater responder rate to fluticasone propionate/salmeterol combination over montelukast plus fluticasone in asthma [abstract]. American Thoracic Society 99th International Conference 2003;B036:H90. [Google Scholar]
Baumgartner 1999 {published data only}
- Baumgartner RA, Polis A, Angner R, Bird S, Reiss TF. Comparison between montelukast and inhaled beclomethasone therapy in chronic asthma: a double blind, placebo controlled, parallel study in asthmatic patients. Merck Research Laboratories 1999.
Benitez 2005 {published data only}
- Benitez HH, Arvizu VM, Gutierrez DJ, Fogelbach GA, Castellanos Olivares A, Vazquez Nava F, et al. Nasal budesonide plus zafirlukast vs nasal budesonide plus loratadine‐pseudoephedrine for controlling the symptoms of rhinitis and asthma. Revista Alergia Mexico 2005;52(2):90‐5. [PubMed] [Google Scholar]
Berger 2006 {published data only}
- Berger WE, Milgrom H, Skoner DP, Tripp K, Parsey MV, Baumgartner RA. Evaluation of levalbuterol metered dose inhaler in pediatric patients with asthma: A double‐blind, randomized, placebo‐ and active‐controlled trial. Current Medical Research & Opinion 2006;22(6):1217‐26. [DOI] [PubMed] [Google Scholar]
Bilancia 2000 {published data only}
- Bilancia R, Margiotta D, Balacco D. Low doses of budesonide (B) plus montelukast (M) versus high doses of budesonide in asthmatic patients. American Journal of Respiratory and Critical Care Medicine 2000;161(supp 3):A197. [Google Scholar]
Bilderback 2004 {published data only}
- Bilderback A, Krishnan JA, Riekert KA, Schiller K, Rand CS. Patterns of use for oral montelukast and inhaled fluticasone in a clinical trial [Abstract]. American Thoracic Society 100th International Conference 2004;B39:C18. [Google Scholar]
Bilderback 2005 {published data only}
- Bilderback A, Rand C, Krishnan J, Riekert K, Schiller K, Zieger RS, et al. Adherence with montelukast or fluticasone and outcomes in a 1‐year clinical trial in patients with mild persistent asthma [Abstract]. American Thoracic Society International Conference 2005;D97:Poster 521. [Google Scholar]
Bisgaard 1999 {published data only}
- Bisgaard H, Loland L, Anhoj J. NO in exhaled air of asthmatic children is reduced by the leukotriene receptor antagonist montelukast. American Journal of Respiratory & Critical Care Medicine 1999;160:1227‐31. [DOI] [PubMed] [Google Scholar]
Bisgaard 2000 {published data only}
- Bisgaard H, Nielsen KG. Bronchoprotection with a leukotriene receptor antagonist in asthmatic preschool children. American Journal of Respiratory & Critical Care Medicine 2000;162(1):187‐90. [DOI] [PubMed] [Google Scholar]
Bisgaard 2005 {published data only}
- Bisgaard H, Zielen S, Garcia‐Garcia ML, Johnston SL, Gilles L, Menten J, et al. Montelukast reduces asthma exacerbations in 2‐ to 5‐year‐old children with intermittent asthma. American Journal of Respiratory & Critical Care Medicine 2005;171(1):315‐22. [DOI] [PubMed] [Google Scholar]
Bjermer 2002 {published data only}
- Bjermer L, Greening A, Haahtela T, Bousquet J, Holgate ST, Picado C, et al. IMPACT study group. Addition of montelukast or salmeterol to fluticasone in patients with uncontrolled asthma: results of the IMPACT trial.. Chest. 2002:434.
Bjermer 2003 {published data only}
- Bjermer L, Bisgaard H, Bousquet J, Fabbri LM, Greening AP, Haahtela T, et al. Montelukast and fluticasone compared with salmeterol and fluticasone in protecting against asthma exacerbation in adults: one year, double blind, randomised, comparative trial. BMJ 2003;327(7420):891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bjermer 2004 {published data only}
- Bjermer L, Kocevar VS, Zhang Q, Yin DD, Polos PG. Health care resource utilization following addition of montelukast or salmeterol in fluticasone in patients with inadequately controlled asthma (IMPACT trial) [Abstract]. European Respiratory Journal 2004;24(Suppl 48):127s. [Google Scholar]
Bleecker 2006 {published data only}
- Bleecker ER, Yancey SW, Baitinger LA, Edwards LD, Klotsman M, Anderson WH, et al. Salmeterol response is not affected by beta2‐adrenergic receptor genotype in subjects with persistent asthma. Journal of Allergy & Clinical Immunology 2006;118(4):809‐16. [DOI] [PubMed] [Google Scholar]
Borker 2005 {published data only}
- Borker R, Emmett A, Jhingran P, Rickard K, Dorinsky P. Determining economic feasibility of fluticasone propionate‐salmeterol vs montelukast in the treatment of persistent asthma using a net benefit approach and cost‐effectiveness acceptability curves. Annals of Allergy Asthma & Immunology 2005;95(2):181‐9. [DOI] [PubMed] [Google Scholar]
Bousquet 2005a {published data only}
- Bousquet J, Gaugris S, Kocevar VS, Zhang Q, Yin DD, Polos PG, et al. Increased risk of asthma attacks and emergency visits among asthma patients with allergic rhinitis: A subgroup analysis of the improving asthma control trial. Clinical & Experimental Allergy 2005;35(6):723‐7. [DOI] [PubMed] [Google Scholar]
Bousquet 2007 {published data only}
- Bousquet J, Rabe K, Humbert M, Chung KF, Berger W, Fox H, et al. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respiratory Medicine 2007;101(7):1483‐92. [DOI] [PubMed] [Google Scholar]
Brannan 2001 {published data only}
- Brannan JD, Anderson SD, Gomes K, King GG, Chan HK, Seale JP. Fenofenadine decreases sensitivity to and montelukast improves recovery from inhaled manitol. American Journal of Respiratory & Critical Care Medicine 2001;163(6):1420‐5. [DOI] [PubMed] [Google Scholar]
Brocks 1996 {published data only}
- Brocks DR, Upward JW, Georgiou P, Stelman G, Doyle E, Allen E, et al. The single and multiple dose pharmacokinetics of pranlukast in healthy volunteers. European Journal of Clinical Pharmacology 1996;51:303‐8. [DOI] [PubMed] [Google Scholar]
Bronsky 1997 {published data only}
- Bronsky E, Grossman J, Nathan RA, Jong B. Pranlukast (Ultair) reduces symptoms of seasonal allergic rhinitis: results of the first US double‐blind, placebo controlled trial in 484 patients. SmithKline Beecham Pharmaceuticals 1997.
Brown 2005 {published data only}
- Brown ES, Stuard G, Liggin JDM, Hukovic N, Frol A, Dhanani N, et al. Effect of phenytoin on mood and declarative memory during prescription corticosteroid therapy. Biological Psychiatry 2005;57(5):543‐8. [DOI] [PubMed] [Google Scholar]
Brown 2007 {published data only}
- Brown ES, Frol AB, Khan DA, Larkin GL, Bret ME. Impact of levetiracetam on mood and cognition during prednisone therapy. European Psychiatry 2007;22(7):448‐52. [DOI] [PubMed] [Google Scholar]
Brown 2010 {published data only}
- Brown ES, Denniston D, Gabrielson B, Khan DA, Khanani S, Desai S. Randomized, double‐blind, placebo‐controlled trial of acetaminophen for preventing mood and memory effects of prednisone bursts. Allergy and Asthma Proceedings 2010;31(4):331‐6. [DOI] [PubMed] [Google Scholar]
Bruce 2002 {published data only}
- Bruce C, Palmqvist M, Sjöstrand M, Aronsson B, Arvidsson P, Lotvall J. Greater attenuation of the late asthmatic reaction by fluticasone propionate compared to montelukast. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A215. [Google Scholar]
Buchvald 2002 {published data only}
- Buchvald FF, Bisgaard H. Comparison of add‐on of leukotriene receptor antagonist vs. long‐acting beta 2‐agonist of FeNO in asthmatic children on regular inhaled budesonide. European Respiratory Journal 2002;20(Suppl 38):431s. [Google Scholar]
Buchvald 2002a {published data only}
- Buchvald FF, Bisgaard H. Comparison of add‐on of leukotriene receptor antagonist vs. long‐acting beta2‐agonist on FeNO in asthmatic children on regular inhaled budesonide [abstract]. European Respiratory Society Annual Congress. 2002:P2736.
Buchvald 2003 {published data only}
- Buchvald F, Bisgaard H. Comparisons of the complementary effect on exhaled nitric oxide of salmeterol vs montelukast in asthmatic children taking regular inhaled budesonide. Annals of Allergy Asthma & Immunology 2003;91(3):309‐13. [DOI] [PubMed] [Google Scholar]
Buckstein 2003 {published data only}
- Bukstein DA, Luskin AT, Bernstein A. "Real‐world" effectiveness of daily controller medicine in children with mild persistent asthma. Annals of Allergy Asthma & Immunology 2003;90(5):543‐9. [DOI] [PubMed] [Google Scholar]
Burgess 2007 {published data only}
- Burgess SW, Sly PD, Cooper DM, Devadason SG. Novel spacer device does not improve adherence in childhood asthma. Pediatric Pulmonology 2007;42(8):736‐9. [DOI] [PubMed] [Google Scholar]
Busse 1999 {published data only}
- Busse W, Nelson H, Wolfe J, Kalberg C, Yancey SW, Rickard KA. Comparison of inhaled salmeterol and oral zafirlukast in patients with asthma. Journal of Allergy & Clinical Immunology 1999;103:1075‐80. [DOI] [PubMed] [Google Scholar]
Buxton 2004 {published data only}
- Buxton MJ, Sullivan SD, Andersson LF, Lamm CJ, Liljas B, Busse WW, et al. Country‐specific cost‐effectiveness of early intervention with budesonide in mild asthma. European Respiratory Journal 2004;24(4):568‐74. [DOI] [PubMed] [Google Scholar]
Cakmak 2000 {published data only}
- Cakmak G, Demir T, Aydemir A, Serdaroglu E, Erginoz E, Donma O, et al. The effect of adding zafirlukast to budesonide treatment on total antoxydant capacity. European Respiratory Journal 2000;16(Supplement 31):457s. [Google Scholar]
Cakmak 2004 {published data only}
- Cakmak G, Demir T, Gemicioglu B, Aydemir A, Serdaroglu E, Donma O. The effects of add‐on zafirlukast treatment to budesonide on bronchial hyperresponsiveness and serum levels of eosinophilic cationic protein and total antioxidant capacity in asthmatic patients. Tohoku Journal of Experimental Medicine 2004;204(4):249‐56. [DOI] [PubMed] [Google Scholar]
Calhoun 1997 {published data only}
- Calhoun WJ, Weisberg SC, Faiferman I, Stober PW. Pranlukast (Ultair) is effective in improving asthma: results of a 12‐week, multicenter, dose‐range study. Journal of Allergy & Clinical Immunology 1997;99:S318, Abs 1305. [Google Scholar]
Calhoun 2001 {published data only}
- Calhoun WJ, Nelson HS, Nathan RA, Pepsin PJ, Kalberg C, Emmett A, et al. Comparison of fluticasone propionate‐salmeterol combination therapy and montelukast in patients who are symptomatic on short‐acting beta(2)‐agonists alone. American Journal of Respiratory & Critical Care Medicine 2001;164(5):759‐63. [DOI] [PubMed] [Google Scholar]
Calhoun 2004 {published data only}
- Calhoun W, Sutton L, Emmett A, Dorinsky P. Asthma control with fluticasone propionate/salmeterol 100/50µg Diskus(r) versus montelukast in patients previously receiving short‐acting beta2‐agonists [Abstract]. Journal of Allergy and Clinical Immunology 2004;113(Suppl 2):S117. [Google Scholar]
Camargo 2002 {published data only}
Camargo 2003 {published data only}
- Camargo CA Jr, Smithline HA, Malice MP, Green SA, Reiss TF. A randomized controlled trial of intravenous montelukast in acute asthma. American Journal of Respiratory & Critical Care Medicine 2003;167(4):528‐33. [DOI] [PubMed] [Google Scholar]
Canino 2008 {published data only}
- Canino G, Vila D, Normand SLT, Acosta‐Perez E, Ramirez R, Garcia P, et al. Reducing asthma health disparities in poor Puerto Rican children: The effectiveness of a culturally tailored family intervention. Journal of Allergy and Clinical Immunology 2008;121(3):665‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Capella 2001 {published data only}
- Capella GL, Frigerio E, Altomare G. A randomized trial of leukotriene receptor antagonist montelukast in moderate‐to‐severe atopic dermatitis of adults. European Journal of Dermatology 2001;11(3):209‐13. [PubMed] [Google Scholar]
Ceylan 2004 {published data only}
- Ceylan E, Mehmet G, Sahin A. Addition of formoterol or montelukast to low‐dose budesonide: An efficacy comparison in short‐ and long‐term asthma control. Respiration 2004;71(6):594‐601. [DOI] [PubMed] [Google Scholar]
Chan 2003 {published data only}
- Chan T, Klassen TP. In children with asthma who are currently using inhaled corticosteroids, are anti‐leukotrienes more effective than placebo in improving clinical outcomes? Part A. Paediatrics & Child Health 2003;8(9):570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chand 2005 {published data only}
- Chand JG, Singh M, Mathew JL. Randomized controlled trial comparing montelukast plus low dose inhaled budesonide with conventional dose inhaled budesonide with conventional dose inhaled budesonide in childhood moderate persistent asthma [Abstract]. Indian Journal of Allergy Asthma and Immunology 2005;19(2):108. [Google Scholar]
Chanez 2010 {published data only}
- Chanez P, Contin‐Bordes C, Garcia G, Verkindre C, Didier A, Blay F, et al. Omalizumab‐induced decrease of FcRI expression in patients with severe allergic asthma. Respiratory Medicine 2010;104(11):1608‐17. [DOI] [PubMed] [Google Scholar]
Chen 2006 {published data only}
- Chen Y‐Z, Busse WW, Pedersen S, Tan W, Lamm C‐J, O'Byrne PM. Early intervention of recent onset mild persistent asthma in children aged under 11 yrs: The Steroid Treatment As Regular Therapy in early asthma (START) trial. Pediatric Allergy & Immunology 2006;17(Suppl 17):7‐13. [DOI] [PubMed] [Google Scholar]
Chiba 1997 {published data only}
- Chiba M, Xu X, Nishime JA, Balani SK, Lin JH. Hepatic microsomal metabolism of montelukast, a potent leukotriene D4 receptor antagonist, in humans. Drug Metabolism and Disposition 1997;25(9):1022‐31. [PubMed] [Google Scholar]
Choi 2003 {published data only}
- Choi IS, Koh YI. Effects of BCG revaccination on asthma. Allergy 2003;58(11):1114‐6. [DOI] [PubMed] [Google Scholar]
Chopra 2005 {published data only}
- Chopra N, Williams M, Rimmer M, Kahl L, Jenkins M. Salmeterol HFA is as effective as salmeterol CFC in children and adults with persistent asthma. Respiratory Medicine 2005;99(Suppl 1):S1‐S10. [DOI] [PubMed] [Google Scholar]
Chuchalin 2002 {published data only}
- Chuchalin AG, Ovcharenko SI, Goriachkina LA, Sidorenko IV, Tsoi AN, Alekseev VG, et al. The safety and efficacy of formoterol OxisRTurbuhalerR plus budesonide PulmicortRTurbuhaler in mild to moderate asthma: A comparison with budesonide Turbuhaler alone and current non‐corticosteroid therapy in Russia. International Journal of Clinical Practice 2002;56(1):15‐20. [PubMed] [Google Scholar]
Chuchalin 2007 {published data only}
- Chuchalin AG, Tsoi AN, Richter K, Krug N, Dahl R, Luursema PB, et al. Safety and tolerability of indacaterol in asthma: A randomized, placebo‐controlled 28‐day study. Respiratory Medicine 2007;101(10):2065‐75. [DOI] [PubMed] [Google Scholar]
Chung 2000 {published data only}
- Chung HL, Lee JJ, Kim SG. Effect of theophylline on urinary leukotriene B4 and C4 excretion in children with asthma. Korean Journal of Asthma, Allergy and Clinical Immunology 2000;20(5):710‐6. [Google Scholar]
Ciebiada 2009 {published data only}
- Ciebiada M, Gorska‐Ciebiada M, Gorski P. Fexofenadine with either montelukast or a low‐dose inhaled corticosteroid (fluticasone) in the treatment of patients with persistent allergic rhinitis and newly diagnosed asthma. Archives of Medical Science 2009;5(4):564‐9. [Google Scholar]
Claesson 1998 {published data only}
- Claesson HE, Dahlen SE. Asthma and leukotrienes: antileukotrienes as novel anti‐asthmatic drugs. Journal of Internal Medicine 1999;245(3):205‐27. [DOI] [PubMed] [Google Scholar]
Cloud 1989 {published data only}
- Cloud ML, Enas GC, Kemp J, Platts‐Mills T, Altman LC, Townley R, et al. A specific LTD4‐LTE4‐receptor antagonist improves pulmonary function in patients with mild, chronic asthma. American Review of Respiratory Disease 1989;140:1336‐9. [DOI] [PubMed] [Google Scholar]
Covar 2008 {published data only}
- Covar RA, Szefler SJ, Zeiger RS, Sorkness CA, Moss M, Mauger DT, et al. Factors associated with asthma exacerbations during a long‐term clinical trial of controller medications in children. Journal of Allergy and Clinical Immunology 2008;122(4):741‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cowan 2010 {published data only}
- Cowan DC, Hewitt RS, Cowan JO, Palmay R, Williamson A, Lucas SJE, et al. Exercise‐induced wheeze: Fraction of exhaled nitric oxide‐directed management. Respirology 2010;15(4):683‐90. [DOI] [PubMed] [Google Scholar]
Currie 2003 {published data only}
- Currie GP, Lee DK, Dempsey OJ, Fowler SJ, Cowan LM, Lipworth BJ. A proof of concept study to evaluate putative benefits of montelukast in moderate persistent asthmatics. British Journal of Clinical Pharmacology 2003;55(6):609‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Currie 2003a {published data only}
- Currie GP, Lee DK, Haggart K, Bates CE, Lipworth BJ. Effects of montelukast on surrogate inflammatory markers in corticosteroid‐treated patients with asthma. American Journal of Respiratory & Critical Care Medicine 2003;167(9):1232‐8. [DOI] [PubMed] [Google Scholar]
Currie 2003b {published data only}
- Currie GP, Lee DK, Haggart K, Bates CE, Lipworth BJ. Montelukast confers complimentary non‐steroid anti‐inflammatory activity in asthmatics receiving fluticasone alone and fluticasone/salmeterol combination [Abstract]. Journal of Allergy and Clinical Immunology 2003;111(Suppl 2):S146. [Google Scholar]
Cylly 2003 {published data only}
- Cylly A, Kara A, Ozdemir T, Ogus C, Gulkesen KH. Effects of oral montelukast on airway function in acute asthma. Respiratory Medicine 2003;97(5):533‐6. [DOI] [PubMed] [Google Scholar]
Dahlén 2002 {published data only}
- Dahlén SE, Malmstrom K, Nizankowska E, Dahlén B, Kuna P, Kowalski M, et al. Improvement of aspirin‐intolerant asthma by montelukast, a leukotriene antagonist. A randomised, double‐blind, placebo controlled trial. American Journal of Respiratory and Critical Care Medicine 2002;165(1):9‐14. [DOI] [PubMed] [Google Scholar]
Daikh 2003 {published data only}
- Daikh BE, Ryan CK, Schwartz RH. Montelukast reduces peripheral blood eosinophilia but not tissue eosinophilia or symptoms in a patient with eosinophilic gastroenteritis and esophageal stricture. Annals of Allergy, Asthma, & Immunology 2003;90(1):2327. [DOI] [PubMed] [Google Scholar]
Davies 2004 {published data only}
- Davies GM, Dasbach EJ, Santanello NC, Knorr BA, Bratton DL. The effect of montelukast versus usual care on health care resource utilization in children aged 2 to 5 years with asthma. Clinical Therapeutics 2004;26(11):1895‐1904. [DOI] [PubMed] [Google Scholar]
Daviskas 2007 {published data only}
- Daviskas E, Anderson SD, Young IH. Inhaled mannitol changes the sputum properties in asthmatics with mucus hypersecretion. Respirology 2007;12(5):683‐91. [DOI] [PubMed] [Google Scholar]
Dekhuijzen 2006 {published data only}
- Dekhuijzen PNR. Caution recommended in prescribing long‐acting beta2‐ adrenergic agonists to patients with asthma. Nederlands Tijdschrift voor Geneeskunde 2006;150(16):889‐91. [PubMed] [Google Scholar]
Delaronde 2005 {published data only}
- Delaronde S, Peruccio DL, Bauer BJ. Improving asthma treatment in a managed care population. American Journal of Managed Care 2005;11(6):361‐8. [PubMed] [Google Scholar]
Dempsey 1999 {published data only}
Dempsey 2000 {published data only}
- Dempsey OJ, Wilson AM, Sims EJ, Mistry C, Lipworth BJ. Additive bronchoprotective and bronchodilator effects with single doses of salmeterol and montelukast in asthmatic patients receiving inhaled corticosteroids. Chest 2000;117:950‐3. [DOI] [PubMed] [Google Scholar]
Dempsey 2000a {published data only}
- Dempsey OJ, Wilson AM, Sims EJ, Lipworth BJ. Additive anti‐inflammatory effects of montelukast but not salmeterol in asthmatics suboptimally controlled on inhaled steroids [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A198. [Google Scholar]
Dempsey 2002b {published data only}
- Dempsey OJ, Fowler SJ, Wilson A, Kennedy G, Lipworth BJ. Effects of adding either a leukotriene receptor antagonist or low‐dose theophylline to a low or medium dose of inhaled corticosteroid in patients with persistent asthma. Chest 2002;122(1):151‐9. [DOI] [PubMed] [Google Scholar]
Demuro‐Mercon 2001 {published data only}
- Demuro‐Mercon C, . Turpin J, Santanello N, Firriolo K, Edelman J. Montelukast improves asthma quality of life when added to fluticasone. Journal of Allergy and Clinical Immunology 2001;107(2):S248. [Google Scholar]
Dessanges 1999 {published data only}
- Dessanges JF, Prefaut C, Taytard A, Matran R, Naya I, Compagnon A, et al. The effect of zafirlukast on repetitive exercise‐induced bronchoconstriction: The possible role of leukotrienes in exercise‐induced refractoriness. Journal of Allergy & Clinical Immunology 1999;104(6):1155‐61. [DOI] [PubMed] [Google Scholar]
Deykin 2007 {published data only}
- Deykin A, Wechsler ME, Boushey HA, Chinchilli VM, Kunselman SJ, Craig TJ, et al. Combination therapy with a long‐acting beta‐agonist and a leukotriene antagonist in moderate asthma. American Journal of Respiratory & Critical Care Medicine 2007;175(3):228‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diamant 1997 {published data only}
- Diamant Z, et al. Effect of oral montelukast on allergen‐induced airway responses in asthmatic subjects. American Journal of Respiratory & Critical Care Medicine 1997.
Diamant 2009 {published data only}
- Diamant Z, Ulrik CS. Effect of add‐on montelukast to inhaled corticosteroids on excessive airway narrowing in adult asthmatics [Abstract]. American Thoracic Society International Conference. 2009:A2416.
Dicpinigaitis 2002 {published data only}
- Dicpinigaitis PV, Dobkin JB, Reichel J. Antitussive effect of the leukotriene receptor antagonist zafirlukast in subjects with cough‐variant asthma. Journal of Asthma 2002;39(4):291‐7. [DOI] [PubMed] [Google Scholar]
Djukanovic 2010 {published data only}
- Djukanovic R, Wilson SJ, Moore WC, Koenig SM, Laviolette M, Bleecker ER, et al. Montelukast added to fluticasone propionate does not alter inflammation or outcomes. Respiratory Medicine 2010;104(10):1425‐35. [DOI] [PubMed] [Google Scholar]
Dockhorn 2000 {published data only}
- Dockhorn RJ, Baumgartner RA, Leff JA, Noonan M, Vandormael K, Stricker W, et al. Comparison of the effects of intravenous and oral montelukast on airway function: A double blind, placebo controlled, three period, crossover study in asthmatic patients. Thorax 2000;55(4):260‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dorinsky 2001 {published data only}
- Dorinsky P, Kalberg C, Pepsin P, Emmett A, Bowers B, Rickard K. The fluticasone/salmeterol combination product is superior to montelukast as first‐line asthma control. European Respiratory Journal 2001;18(Suppl 33):263s. [Google Scholar]
Dorinsky 2002 {published data only}
- Dorinsky PM, Crim C, Yancey S, Edwards L, Rickard K. First line therapy with fluticasone propionate/salmeterol combination product provides superior asthma control versus montelukast or fluticasone propionate alone [Abstract]. Journal of Allergy Asthma and Immunolgy 2002;109(Suppl 1):Abstract No: 767. [Google Scholar]
Dorinsky 2004 {published data only}
- Dorinsky PM, Stauffer J, Waitkus‐Edwards K, Yancey S, Prillaman BA, Sutton L. "Stepping Down" from Fluticasone Propionate/Salmeerol 100/50mcg Diskus(R) Results in Loss of Asthma Control: Lack of Effect of Ethnic Origin [Abstract]. Chest 2004;126(Suppl 4):758S. [Google Scholar]
Edelman 2000 {published data only}
- Edelman JM, Ghannam A, Bird S, Dobbins T. Onset of action of montelukast and inhaled beclomethasone in achieving asthma control [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A202. [Google Scholar]
Eliraz 2001 {published data only}
- Eliraz A, Raminez‐Rivera A, Ferranti P, et al. Similar efficacy following four weeks treatment of asthmatics with formoterol 12 mcg bid delivered by two different dry powder inhalers: differences in inhaler handling. International Journal of Clinical Practice 2001;55(3):164‐70. [PubMed] [Google Scholar]
El Miedany 2006 {published data only}
- Miedany Y, Youssef S, Ahmed I, Gaafary M. Safety of etoricoxib, a specific cyclooxygenase‐2 inhibitor, in asthmatic patients with aspirin‐exacerbated respiratory disease. Annals of Allergy Asthma & Immunology 2006;97(1):105‐9. [DOI] [PubMed] [Google Scholar]
Ensom 2003 {published data only}
- Ensom MHH, Chong G, Beaudin B, Bai TR. Estradiol in severe asthma with premenstrual worsening. Annals of Pharmacotherapy 2003;37(11):1610‐3. [DOI] [PubMed] [Google Scholar]
Fabbri 1996 {published data only}
- Fabbri LM, Piattella M, Caramori G, Ciaccia A. Oral vs inhaled asthma therapy. Drugs 1996;52 Suppl(6):20‐8. [DOI] [PubMed] [Google Scholar]
Fagerson 2003 {published data only}
- Fagerspm GMH. Growth and adrenocortical function in children on high‐dose inhaled steroids versus low‐dose inhaled steroids plus montelukast. Tayside Research Consortium 2003.
Failla 2006 {published data only}
- Failla M, Biondi G, Provvidenza Pistorio M, Gili E, Mastruzzo C, Vancheri C, et al. Intranasal steroid reduces exhaled bronchial cysteinyl leukotrienes in allergic patients. Clinical & Experimental Allergy 2006;36(3):325‐30. [DOI] [PubMed] [Google Scholar]
Fardon 2004 {published data only}
- Fardon TC. Does Fluticasone/Salmeterol combination exhibit a putative in vivo anti‐inflammatory action during step‐down?. Tayside Research Consortium 2004.
Fardon 2007 {published data only}
- Fardon T, Haggart K, Lee DKC, Lipworth BJ. A proof of concept study to evaluate stepping down the dose of fluticasone in combination with salmeterol and tiotropium in severe persistent asthma. Respiratory Medicine 2007;101(6):1218‐28. [DOI] [PubMed] [Google Scholar]
Faul 2002 {published data only}
- Faul JL, Canfield JC, Gould MK, Wilson SR, Kischner WG. A randomized, double‐blind, placebo‐controlled, crossover study comparing the effects of inhaled fluticasone propionate (880 Micrograms per day) and montelukast (10 MG per day) on glucose control patients with diabetes and asthma. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A217. [Google Scholar]
Findlay 1992 {published data only}
- Findlay SR, Barden JM, Easley CB, Glass M. Effect of the oral leukotriene antagonist, ICI 204,219, on antigen‐induced bronchoconstriction in subjects with asthma. Journal of Allergy & Clinical Immunology 1992;89:1040‐5. [DOI] [PubMed] [Google Scholar]
Finkelstein 2005 {published data only}
- Finkelstein JA, Lozano P, Fuhlbrigge AL, Carey VJ, Inui TS, Soumerai SB, et al. Practice‐level effects of interventions to improve asthma care in primary care settings: The Pediatric Asthma Care Patient Outcomes Research Team. Health Services Research 2005;40(61):1737‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finn 2000 {published data only}
- Finn AF, Bonuccelli CM, Traxler BM, Beatty SE. Zafirlukast improves asthma control in children treated with and without inhaled corticosteroids. European Respiratory Journal 2000;16(Supp 31):307. [Google Scholar]
Fischer 1995 {published data only}
- Fischer AR, McFadden CA, Frantz R, Awni WM, Cohn J, Drazen JM, et al. Effect of chronic 5‐lipoxygenase inhibition on airway hyperresponsiveness in asthmatic subjects. American Journal of Respiratory & Critical Care Medicine 1995;152(4 part 1):1203‐07. [DOI] [PubMed] [Google Scholar]
Fischer 1997 {published data only}
- Fischer AR, Rosenberg MA, Roth M, Loper M, Jungerwirth S, Israel E. Effect of a novel 5‐lipoxygenase activating protein inhibitor, BAYx 1005, on asthma induced by cold air. Thorax 1997;52:1074‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fish 1997 {published data only}
- Fish JE, Kemp JP, Lockey RF, Glass M, Hanby L, Bonuccelli CM. Zafirlukast for symptomatic mild‐to‐moderate asthma: a 13‐week multicenter study. Clinical Therapeutics 1997;19(4):675‐90. [DOI] [PubMed] [Google Scholar]
Fish 2000 {published data only}
- Fish J, Boone R, Emmett A, Yancey S, Knobil K, Rickard K. Salmeterol added to inhaled corticosteroids (ICS) provides greater asthma control compared to montelukast [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 2):A203. [Google Scholar]
Fish 2001 {published data only}
- Fish JE, Israel E, Murray JJ, Emmett A, Boone R, Yancey SW, et al. Salmeterol powder provides significantly better benefit than montelukast in asthmatic patients receiving concomitant inhaled corticosteroid therapy. Chest 2001;120(2):423‐30. [DOI] [PubMed] [Google Scholar]
FitzGerald 2009 {published data only}
- FitzGerald JM, Foucart S, Coyle S, Sampalis J, Haine D, Psaradellis E, et al. Montelukast as add‐on therapy to inhaled corticosteroids in the management of asthma (the SAS trial). Canadian Respiratory Journal 2009;16(Suppl A):5A‐14A. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fogel 2010 {published data only}
- Fogel RB, Rosario N, Aristizabal G, Loeys T, Noonan G, Gaile S, et al. Effect of montelukast or salmeterol added to inhaled fluticasone on exercise‐induced bronchoconstriction in children. Annals of Allergy, Asthma and Immunology 2010;104(6):511‐7. [DOI] [PubMed] [Google Scholar]
Franzen 1994 {published data only}
- Franzen L, Kumlin M, Dahlen SE. Pharmacologic modulation of leukotriene and histamine release in the human lung. Abbott Laboratories 1994.
Fritsch 2006 {published data only}
- Fritsch M, Uxa S, Horak Jr F, Putschoegl B, Dehlink E, Szepfalusi Z, et al. Exhaled nitric oxide in the management of childhood asthma: A prospective 6‐months study. Pediatric Pulmonology 2006;41(9):855‐62. [DOI] [PubMed] [Google Scholar]
Fujimura 1993 {published data only}
- Fujimura M, Sakamoto S, Kamio Y, Matsuda T. Effect of a leukotriene antagonist, ONO‐1078, on bronchial hyperresponsiveness in patients with asthma. Respiratory Medicine 1993;87:133‐8. [DOI] [PubMed] [Google Scholar]
Gabrijelcic 2004 {published data only}
- Gabrijelcic J, Casas A, Rabinovich RA, Roca J, Barbera JA, Chung KF, et al. Formoterol protects against platelet‐activating factor‐induced effects in asthma. European Respiratory Journal 2004;23(1):71‐5. [DOI] [PubMed] [Google Scholar]
Gaddy 1990 {published data only}
- Gaddy J, Bush RK, Margolskee D, Williams VC, Busse W. The effects of a leukotriene D4 (LTD4) antagonist (MK‐571) in mild to moderate asthma. Journal of Allergy & Clinical Immunology 1990;85:197, Abs. 216. [Google Scholar]
Galbreath 2008 {published data only}
- Galbreath AD, Smith B, Wood PR, Inscore S, Forkner E, Vazquez M, et al. Assessing the value of disease management: Impact of 2 disease management strategies in an underserved asthma population. Annals of Allergy Asthma and Immunology 2008;101(6):599‐607. [DOI] [PubMed] [Google Scholar]
Geha 2001 {published data only}
- Geha RS. Desloratadine: a new, nonsedating, oral antihistamine. Journal of Allergy & Clinical Immunology 2001;107(4):751‐62. [DOI] [PubMed] [Google Scholar]
Gelb 2008 {published data only}
- Gelb AF, Karpel J, Wise RA, Cassino C, Johnson P, Conoscenti CS. Bronchodilator efficacy of the fixed combination of ipratropium and albuterol compared to albuterol alone in moderate‐to‐severe persistent asthma. Pulmonary Pharmacology and Therapeutics 2008;21(4):630‐6. [DOI] [PubMed] [Google Scholar]
Georgiou 1997 {published data only}
- Georgiou P, Compton C, Allen A, Hust R, Collie H. Pranlukast (Ultair) has no effect on cardiovascular parameters in healthy male subjects. American Thoracic Society. 1997:Abs C49.
Ghiro 2001 {published data only}
- Ghiro L, Zanconato S, Rampon O, Piovan V, Scollo M, Baraldi E. Effect of Montelukast added to inhaled steroids on exhaled NO in asthmatic children. European Respiratory Journal 2001;18(Suppl 33):40s. [DOI] [PubMed] [Google Scholar]
Ghiro 2002 {published data only}
- Ghiro L, Zanconato S, Rampon O, Piovan V, Pasquale MF, Baraldi E. Effect of montelukast added to inhaled corticosteroids on fractional exhaled nitric oxide in asthmatic children. European Respiratory Journal 2002;20(3):630‐4. [DOI] [PubMed] [Google Scholar]
Gold 2001 {published data only}
- Gold M, Jõgi R, Mulder PGH, Akveld MLM. Salmeterol/fluticasone propionate combination 50/100µg bid is more effective than fluticasone propionate 100µg bid plus montelukast 10 mg once daily in reducing exacerbations. European Respiratory Journal 2001;18(Supp 33):262s. [Google Scholar]
Gold 2001a {published data only}
- Gold M, Jõgi R, Mulder PGH, Akveld MLM. Salmeterol/fluticasone propionate combination 50/100µg bid is more effective than fluticasone propionate 100µg bid plus montelukast 10 mg once daily in reducing exacerbations. European Respiratory Journal 2001;18(Supp 33):262s. [Google Scholar]
Green 2002 {published data only}
- Green RH, Brightling S, Mckenna B, Hargadon B, Parker D, Wardlaw AJ, et al. A placebo controlled comparison of formoterol, montelukast or higher dose of inhaled corticosteroids in subjects with symptomatic asthma despite treatment with low dose inhaled corticosteroids. Thorax 2002;57(3):11. [Google Scholar]
Green 2002a {published data only}
- Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: A randomised controlled trial. Lancet 2002;360(9347):1715‐21. [DOI] [PubMed] [Google Scholar]
Green 2006 {published data only}
- Green RH, Brightling CE, McKenna S, Hargadon B, Neale N, Parker D, et al. Comparison of asthma treatment given in addition to inhaled corticosteroids on airway inflammation and responsiveness. European Respiratory Journal 2006;27(6):1144‐51. [DOI] [PubMed] [Google Scholar]
Greenberger 2003 {published data only}
- Greenberger PA. Therapy in the management of the rhinitis/asthma complex. Allergy & Asthma Proceedings 2003;24(6):403‐7. [PubMed] [Google Scholar]
Grosclaude 2003 {published data only}
- Grosclaude M, Cerruti JL, Delannay B, Hérent M, Spilthooren F, Desfougčres JL. A fixed combination of fluticasone and salmeterol permits better control of asthma than a beclomethasone dipropionate and montelukast combination. Allergie et immunologie 2003;35(9):356‐62. [PubMed] [Google Scholar]
Grossman 1995 {published data only}
- Grossman J, Bronsky E, Busse W, Montanaro A, Southern L, Tinkelman D, et al. A multicenter, double‐blind, placebo‐controlled study to evaluate the safety, tolerability and clinical activity of oral, twice‐daily LTA, Pranlukast (SB 205312) in patients with mild to moderate asthma. Journal of Allergy & Clinical Immunology 1995;95(1):352, Abs 846. [Google Scholar]
Grossman 1997 {published data only}
- Grossman J, Faiferman I, Dubb JW, Tompson DJ, Busse W, Bronsky E, et al. Results of the first U.S. double‐blind, placebo‐controlled, multicenter clinical study in asthma with pranlukast, a novel leukotriene receptor antagonist. Journal of Asthma 1997;34(4):321‐8. [DOI] [PubMed] [Google Scholar]
Grzelewska‐Rzymowska 2003 {published data only}
- Grzelewska‐Rzymowska I, Malolepszy J, Molina M, Sladek K, Zarkovic J, Siergiejko Z. Equivalent asthma control and systemic safety of inhaled budesonide delivered via HFA‐134a or CFC propellant in a broad range of doses. Respiratory Medicine 2003;97(Suppl D):S10‐S19. [DOI] [PubMed] [Google Scholar]
Grzelewski 2006 {published data only}
- Grzelewski T, Jerzynska J, Stelmach I. Budesonide and montelukast once daily inhibits exercise induced bronchoconstriction in 6 to 18 year old children with asthma. Journal of Allergy and Clinical Immunology 2006;117((2 Suppl 1)):S93. [DOI] [PubMed] [Google Scholar]
Guilbert 2004 {published data only}
- Guilbert TW, Morgan WJ, Krawiec M, Lemanske Jr RF, Sorkness C, Szefler SJ, et al. The Prevention of Early Asthma in Kids study: Design, rationale and methods for the Childhood Asthma Research and Education network. Controlled Clinical Trials 2004;25(3):286‐310. [DOI] [PubMed] [Google Scholar]
Gupta 1999 {published data only}
- Gupta RC, Dixit R, Khilnani G, Gupta N, Joshi N, Saluja M. Comparative efficacy of budesonide and azelastine nasal spray in nasobronchial allergy. Indian Journal of Allergy and Applied Immunology 1999;13(1):11‐6. [Google Scholar]
Gupta 2007 {published data only}
- Gupta S, Kansal AP, Kishan J. Comparative efficacy of combination of fluticasone and salmeterol; fluticasone, salmeterol and montelukast; fluticasone, salmeterol, and levocetirizine in moderate persistent asthma: a study of 120 patients. Chest 2007;132(4):512a. [Google Scholar]
Gylfors 2005 {published data only}
- Gylfors P, Dahlen SE, Larsson K, Kumlin M. Bronchial responsiveness to leukotriene D4 is resistant to inhaled fluticasone propionate [Abstract]. European Respiratory Journal 2005;26(Suppl 49):3687. [DOI] [PubMed] [Google Scholar]
Gyllfors 2003 {published data only}
- Gyllfors P, Bochenek G, Overholt J, Drupka D, Kumlin M, Sheller J, et al. Biochemical and clinical evidence that aspirin‐intolerant asthmatic subjects tolerate the cyclooxygenase 2‐selective analgetic drug celecoxib. Journal of Allergy & Clinical Immunology 2003;111(5):1116‐21. [DOI] [PubMed] [Google Scholar]
Gyllfors 2006 {published data only}
- Gyllfors P, Dahlen SE, Kumlin M, Larsson K, Dahlen B. Bronchial responsiveness to leukotriene D4 is resistant to inhaled fluticasone propionate. Journal of Allergy & Clinical Immunology 2006;118(1):78‐83. [DOI] [PubMed] [Google Scholar]
Haahtela 1994 {published data only}
- Haahtela T, Jarvinen M, Kava T, Kiviranta K, Koskinen S, Lehtonen K, et al. Effects of discontinuing inhaled budesonide in patients with mild asthma. New England Journal of Medicine 1994;331:700‐5. [DOI] [PubMed] [Google Scholar]
Hakim 2007 {published data only}
- Hakim F, Vilozni D, Adler A, Livnat G, Tal A, Bentur L. The effect of montelukast on bronchial hyperreactivity in preschool children. Chest 2007;131(1):180‐6. [DOI] [PubMed] [Google Scholar]
Hamilton 1998 {published data only}
Harmanci 2006 {published data only}
- Harmanci K, Bakirtas A, Turktas I, Degim T. Oral montelukast treatment of preschool‐aged children with acute asthma. Annals of Allergy Asthma & Immunology 2006;96(5):731‐5. [DOI] [PubMed] [Google Scholar]
Hartwig 2004 {published data only}
- Hartwig KC, DiNella JV, Chiappetta K, Clark MP, Ameredes BT, Calhoun WJ. Progressive improvement in airway hyperresponsiveness (AHR) over one year with fluticasone but not montelukast therapy in asthma: the OFTIRA trial [Abstract]. American Thoracic Society 100th International Conference 2004;A58:Poster K44. [Google Scholar]
Hassall 1998 {published data only}
- Hassell SM, Miller C, Harris A. Zafirlukast (Accolate) reduces the need for oral steroid bursts. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A411. [Google Scholar]
Havlucu 2005 {published data only}
- Havlucu Y, Yilmaz G, Goktan C, Yorgancioglu A. Usefulness of HRCT determining the distal airway inflammation in asthma [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 2072. [Google Scholar]
Hay 1997 {published data only}
- Hay DWP. Pharmacology of leukotriene receptor antagonists: more than inhibitors of bronchoconstriction. Chest 1997;111(2):35S‐45S. [DOI] [PubMed] [Google Scholar]
Hendeles 2004 {published data only}
- Hendeles L, Erb TA, Bird S, Hustard CM, Edelman JM. Post‐exercise response to albuterol after addition of montelukast or salmeterol to inhaled fluticasone [Abstract]. Journal of Allergy and Clinical Immunology 2004;113(Suppl 2):S34. [Google Scholar]
Henderson 1994 {published data only}
- Henderson WR. The role of leukotrienes in inflammation. Annals of Internal Medicine 1994;121(9):684‐97. [DOI] [PubMed] [Google Scholar]
Hernandez 2002 {published data only}
- Hernandez D, Namenyi M, Fiterman J, Price DB, Beeh KM, Fletcher CP, et al. Adding montelukast versus doubling the budesonide dose in persistent asthma: a subgroup analysis of the COMPACT study [abstract]. European Respiratory Society Annual Congress. 2002:P2406.
Hood 1999 {published data only}
- Hood PP, Cotter TP, Costello JF, Sampson AP. Effect of intravenous corticosteroid on ex vivo leukotriene generation by blood leucocytes of normal and asthmatic patients. Thorax 1999;54(12):1075‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hothersall 2008 {published data only}
- Hothersall EJ, Chaudhuri R, McSharry C, Donnelly I, Lafferty J, McMahon AD, et al. Effects of atorvastatin added to inhaled corticosteroids on lung function and sputum cell counts in atopic asthma. Thorax 2008;63(12):1070‐5. [DOI] [PubMed] [Google Scholar]
Houghton 2004 {published data only}
- Houghton CM, Langley SJ, Singh SD, Holden J, Monici Preti AP, Acerbi D, et al. Comparison of bronchoprotective and bronchodilator effects of a single dose of formoterol delivered by hydrofluoroalkane and chlorofluorocarbon aerosols and dry powder in a double blind, placebo‐controlled, crossover study. British Journal of Clinical Pharmacology 2004;58(4):359‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howland 1994 {published data only}
- Howland III W, Segal A, Glass M, Minkwitz MC. 6‐week therapy with the oral leukotriene‐receptor antagonist, ICI 204,219, in the treatment of asthma. Journal of Allergy & Clinical Immunology 1994;93(1 Part 2):259, Abs. 581. [Google Scholar]
Hozawa 2009 {published data only}
- Hozawa S, Haruta Y, Terada M, Yamakido M. Effects of the addition of beta2‐agonist tulobuterol patches to inhaled corticosteroid in patients with asthma. Allergology International 2009;58(4):509‐18. [DOI] [PubMed] [Google Scholar]
Hsieh 1996 {published data only}
- Hsieh, K‐H. Evaluation of efficacy of traditional chinese medicines in the treatment of childhood bronchial asthma: clinical trial, immunological tests and animal study. Pediatric Allergy & Immunology 1996;7:130‐40. [DOI] [PubMed] [Google Scholar]
Huang 2003 {published data only}
- Huang CJ, Wang CH, Liu WT, Yang MC, Lin HC, Yu CT, et al. Zafirlukast Improves Pulmonary Function in Patients with Moderate Persistent Asthma Receiving Regular Inhaled Steroids: A Prospective Randomized Control Study. Chang Gung Medical Journal 2003;26(8):554‐60. [PubMed] [Google Scholar]
Huang 2003a {published data only}
- Huang CJ, Wang CH, Lin HC, Yu CT, Shiao JR, Tan CG, et al. Zafirlukast improves pulmonary function in patients with moderate persistent asthma receiving regular inhaled steroids: a randomized control study [abstract]. American Thoracic Society 99th International Conference 2003;B036:Poster H80. [PubMed] [Google Scholar]
Hui K 1991 {published data only}
- Hui K, Barnes NC. Lung function improvement in asthma with a cysteinyl‐leukotriene receptor antagonist. Lancet 1991;337:1062‐3. [DOI] [PubMed] [Google Scholar]
Igde 2009 {published data only}
- Igde M, Anlar FY. The efficacy of montelukast monotherapy in moderate persistent asthmatic children. Iranian Journal of Allergy Asthma & Immunology 2009;8(3):169‐70. [PubMed] [Google Scholar]
Ikeda 1997 {published data only}
- Ikeda K, Hyashi M, Obata H, Fujita H, Nakanishi T, Izumi T. Two weeks' observation of pranlukast (ONO‐1078), leukotriene receptor antagonist) by peak expiratory flow rate (PEFR) was enough to evaluate clinical efficacies in severe chronic adult asthmatics. American Thoracic Society. 1997:Abs C49.
Ilowite 2004 {published data only}
- Ilowite J, Webb R, Friedman B, Kerwin E, Bird SR, Hustad CM, et al. Addition of montelukast or salmeterol to fluticasone for protection against asthma attacks: a randomized, double‐blind, multicenter study. Annals of Allergy Asthma & Immunology 2004;92(6):641‐8. [DOI] [PubMed] [Google Scholar]
Inoue 2007 {published data only}
- Inoue H, Komori M, Matsumoto T, Fukuyama S, Matsumura M, Nakano T, et al. Effects of salmeterol in patients with persistent asthma receiving inhaled corticosteroid plus theophylline. Respiration 2007;74(6):611‐6. [DOI] [PubMed] [Google Scholar]
Irvin 2003 {published data only}
- Irvin C, Anthonisen N, Castro M, Holbrook J, Kaminsky DA, Lima J, et al. Effectiveness of Low‐Dose Theophylline as Add‐on Therapy in the Treatment of Asthma: The LODO Trial [Abstract]. Chest 2003;124(4):3355. [Google Scholar]
Irvin 2007 {published data only}
- Irvin CG, Kaminsky DA, Anthonisen NR, Castro M, Hanania NA, Holbrook JT, et al. Clinical trial of low‐dose theophylline and montelukast in patients with poorly controlled asthma. American Journal of Respiratory & Critical Care Medicine 2007;175(3):235‐42. [DOI] [PubMed] [Google Scholar]
Israel 1990 {published data only}
- Israel E, Dermarkarkian R, Rosenberg M, Sperling R, Taylor G, Rubin P, et al. The effects of a 5‐lipoxygenase inhibitor on asthma induced by cold, dry air. New England Journal of Medicine 1990;323:1740‐4. [DOI] [PubMed] [Google Scholar]
Israel 1992 {published data only}
- Israel E, Drazen J, Pearlman H, Cohn J, Rubin P. A double‐blind multicenter study of zileuton, a potent 5‐lipoxygenase (5‐LO) inhibitor versus placebo in the treatment of spontaneous asthma in adults. Journal of Allergy & Clinical Immunology 1992;89:236, Abs. 368. [Google Scholar]
Israel 1993 {published data only}
- Israel E, Rubin P, Kemp JP, Grossman J, Pierson W, Siegel SC, et al. The effect of inhibition of 5‐lipoxygenase by zileuton in mild‐to‐moderate asthma. Annals of Internal Medicine 1993;119(11):1059‐66. [DOI] [PubMed] [Google Scholar]
Israel 1996 {published data only}
- Israel E, Cohn J, Dube L, Drazen JM. Effect of treatment with zileuton, a 5‐lipoxygenase inhibitor, in patients with asthma. JAMA 1996;275(12):931‐6. [PubMed] [Google Scholar]
Jat 2006 {published data only}
- Jat GC, Mathew JL, Singh M. Treatment with 400 mug of inhaled budesonide vs 200 mug of inhaled budesonide and oral montelukast in children with moderate persistent asthma: Randomized controlled trial. Annals of Allergy Asthma & Immunology 2006;97(3):397‐401. [DOI] [PubMed] [Google Scholar]
Jayaram 2002a {published data only}
- Jayaram L, Pizzichini M, Hussack P, Efthmiadis A, Goodwin S, Chaboillez S, et al. First line anti inflammatory treatment for asthma: inhaled steroid or leukotriene antagonist [Abstract]. Journal of Allergy Asthma and Immunolgy 2002;109(Suppl 1):Abstract No: 746. [Google Scholar]
Jayaram 2005a {published data only}
- Jayaram L, Duong M, Pizzichini MM, Pizzichini E, Kamada D, Efthimiadis A, et al. Failure of montelukast to reduce sputum eosinophilia in high‐dose corticosteroid‐dependent asthma. European Respiratory Journal 2005;25(1):41‐6. [DOI] [PubMed] [Google Scholar]
Jayaram 2006 {published data only}
- Jayaram L, Pizzichini MM, Cook RJ, Boulet LP, Lemiere C, Pizzichini E, et al. Determining asthma treatment by monitoring sputum cell counts: Effect on exacerbations. European Respiratory Journal 2006;27(3):483‐94. [DOI] [PubMed] [Google Scholar]
Johnson 1999 {unpublished data only}
- Johnson MC, Srebro S, Edwards L, Bowers B, Rickard K. Physician‐ and patient‐rated assessments correlate well with clinical efficacy measurements in a study comparing fluticasone and zafirlukast. Journal of Allergy & Clinical Immunology. 1999; Vol. 103, issue 1 Part 2:Abs. 882.
Johnston 2007 {published data only}
- Johnston NW, Mandhane PJ, Dai J, Duncan JM, Greene JM, Lambert K, et al. Attenuation of the September epidemic of asthma exacerbations in children: A randomized, controlled trial of montelukast added to usual therapy. Pediatrics 2007;120(3):e702‐e712. [DOI] [PubMed] [Google Scholar]
Jones 2002 {published data only}
- Jones C, Allen FC, Duong PT, Sajjan S, Nelsen L, Itzler R, et al. Similar health care outcomes for patients treated with fluticasone propionate (FP) and montelukast sodium (MON) monotherapy [Abstract]. Journal of Allergy Asthma and Immunolgy 2002;109(Suppl 1):Abstract No: 890. [Google Scholar]
Jonsson 2004 {published data only}
- Jonsson B, Berggren F, Svensson K, O'Byrne PM. An economic evaluation of combination treatment with budesonide and formoterol in patients with mild‐to‐moderate persistent asthma. Respiratory Medicine 2004;98(11):1146‐54. [DOI] [PubMed] [Google Scholar]
Juniper 1995 {published data only}
- Juniper EF, Dube L, Swanson LJ, Zileuton Study Group. The effect of zileuton, a 5‐lipoxygenase inhibitor, on asthma quality of life. Asthma. 1995.
Kalberg 1999 {published data only}
- Kalberg CJ, Yancey S, Emmett AH, Rickard K. A comparison of salmeterol versus zafirlukast in patients using inhaled corticosteroids. Journal of Allergy & Clinical Immunology 1999; Vol. 103, issue 1 part 2:abs 881.
Kanazawa 2004 {published data only}
- Kanazawa H, Yoshikawa T, Hirata K, Yoshikawa J. Effects of pranlukast administration on vascular endothelial growth factor levels in asthmatic patients. Chest 2004;125(5):1700‐05. [DOI] [PubMed] [Google Scholar]
Kane 1994 {published data only}
- Kane G, Pollice M, Tolino M, Cohn J, Dworski R, Murray J, et al. Effect of zileuton on eosinophil influx and leukotrienes in humans undergoing segmental antigen challenge. Abbott Laboratories 1994.
Kanniess 2002a {published and unpublished data}
- Kanniess F, Richter K, Janicki S, Schleiss MB, Jorres RA, Magnussen H. Dose reduction of inhaled corticosteroids under concomitant medication with montelukast in patients with asthma. European Respiratory Journal 2002;20:1080‐7. [DOI] [PubMed] [Google Scholar]
Karaman 2007 {published data only}
- Karaman O, Arli O, Uzuner N, Islekel H, Babayigit A, Olmez D, et al. The effectiveness of asthma therapy alternatives and evaluating the effectivity of asthma therapy by interleukin‐13 and interferon gamma levels in children. Allergy & Asthma Proceedings 2007;28(2):204‐9. [DOI] [PubMed] [Google Scholar]
Karpel 2007 {published data only}
- Karpel JP, Nayak A, Lumry W, Craig TJ, Kerwin E, Fish JE, et al. Inhaled mometasone furoate reduces oral prednisone usage and improves lung function in severe persistent asthma. Respiratory Medicine 2007;101(3):628‐37. [DOI] [PubMed] [Google Scholar]
Katial 2010 {published data only}
- Katial RK, Oppenheimer JJ, Ostrom NK, Mosnaim GS, Yancey SW, Waitkus‐Edwards KR, et al. Adding montelukast to fluticasone propionate/salmeterol for control of asthma and seasonal allergic rhinitis. Allergy and Asthma Proceedings 2010;31(1):68‐75. [DOI] [PubMed] [Google Scholar]
Keith 2009 {published data only}
- Keith PK, Koch C, Djandji M, Bouchard J, Psaradellis E, Sampalis JS, et al. Montelukast as add‐on therapy with inhaled corticosteroids alone or inhaled corticosteroids and long‐acting beta‐2‐agonists in the management of patients diagnosed with asthma and concurrent allergic rhinitis (the RADAR trial). Canadian Respiratory Journal 2009;16(Suppl A):17A‐31A. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kemp 1995 {published data only}
- Kemp JP, Glass M, Minkwitz MC. Onset of action of the leukotriene‐receptor antagonist, zafirlukast (Accolate), in patients with asthma. Journal of Allergy & Clinical Immunology 1995;95(1 Part 2):351, Abs. 844. [Google Scholar]
Kemp 1996 {published data only}
- Kemp JP. Zafirlukast, a selective leukotriene D4‐receptor antagonist: a new class of therapy for patients with asthma. Today's Therapeutic Trends 1996;14(2):89‐102. [Google Scholar]
Kemp 1998 {published data only}
- Kemp JP, Tinkelman D, Sublett J, Compton C, Georgiou P. Pranlukast (Ultair) pharmacokinetics in children consistent with that of adults. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A411. [Google Scholar]
Kemp 1998a {published data only}
- Kemp JP, Dockhorn RJ, Shapiro GG, Nguyen HH, Reiss TF, Seidenberg BC, et al. Montelukast once daily inhibits exercise induced bronchoconstriction in 6‐14 year old children with asthma. The Journal of Pediatrics 1998;133(3):424‐28. [DOI] [PubMed] [Google Scholar]
Kemp 1999 {published data only}
- Kemp JP, Minkwitz MC, Bonuccelli CM, Warren MS. Therapeutic effect of zafirlukast as monotherapy in steroid‐naive patients with severe persistent asthma. Chest 1999;115(2):336‐42. [DOI] [PubMed] [Google Scholar]
Ketchell 2002 {published data only}
- Ketchell RI, D'Amato M, Jensen MW, O'Connor BJ. Contrasting effects of allergen challenge on airway responsiveness to cysteinyl leukotriene D(4) and methocholine in mild asthma. Thorax 2002;57(7):575‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khayyal 2003 {published data only}
- Khayyal MT, el‐Ghazaly MA, el‐Khatib AS, Hatem AM, Vries PJ, el‐Shafei S, et al. A clinical pharmacological study of the potential beneficial effects of a propolis food product as an adjuvant in asthmatic patients. Fundamental & Clinical Pharmacology 2003;17(1):93‐102. [DOI] [PubMed] [Google Scholar]
Kippelen 2010 {published data only}
- Kippelen P, Larsson J, Anderson SD, Brannan JD, Delin I, Dahlen B, et al. Acute effects of beclomethasone on hyperpnea‐induced bronchoconstriction. Medicine & Science in Sports & Exercise 2010;42(2):273‐80. [DOI] [PubMed] [Google Scholar]
Kips 1991 {published data only}
- Kips JC, Joos GF, Lepeleire I, Margolskee DJ, Buntinx A, Pauwels RA, et al. MK‐571, a potent antagonist of leukotriene D4‐induced bronchoconstriction in the human. American Review of Respiratory Disease 1991;144:617‐21. [DOI] [PubMed] [Google Scholar]
Knorr 1998 {published data only}
- Knorr B, Matz J, Bernstein JA, Nguyen H, Seidenberg BC, Reiss TF, et al. Montelukast for chronic asthma in 6‐ to 14‐year‐old children. JAMA 1998;279(15):1181‐6. [DOI] [PubMed] [Google Scholar]
Knorr 1999 {published data only}
- Knorr B, Nguycn HH, Seidenberg BC, Reiss TF, Montelukast Pediatric Study Group. Montelukast, a leukotriene receptor antagonist, provides additional clinical benefit in asthmatic children aged 6 to 14 years using inhaled corticosteroids. European Respiratory Society. 1999:P363.
Koenig 2004 {published data only}
- Koenig S, Waitkus‐Edwards K, Yancey S, Prillman B, Dorinsky P. Loss of asthma control when patients receiving fluticasone propionate/salmeterol 100/50µg Diskus are "stepped‐down" to fluticasone propionate , salmeterol or montelukast alone [Abstract]. Journal of Allergy and Clinical Immunology 2004;113(Suppl 2):S94. [Google Scholar]
Kohrogi 1997 {published data only}
- Kohrogi H, Iwagoe H, Fujii K, Fukuda K, Kawano O, Hamamoto J, et al. The effect of leukotriene antagonist pranlukast on moderate and severe persistent asthma continues more than one year. American Journal of Respiratory & Critical Care Medicine 1997;155:A662. [Google Scholar]
Kondo 2006 {published data only}
- Kondo N, Katsunuma T, Odajima Y, Morikawa A. A randomized open‐label comparative study of montelukast versus theophylline added to inhaled corticosteroid in asthmatic children. Allergology International 2006;55(3):287‐93. [DOI] [PubMed] [Google Scholar]
Kooi 2006 {published data only}
- Kooi EMW, Schokker S, Boezen HM, Vries TW, Vanssen‐Verberne AAP, Molen T, et al. Effect of fluticasone and montelukast in preschool children with asthma like symptoms [Abstract]. European Respiratory Journal 2006;28(Suppl 50):709s [P4079]. [Google Scholar]
Korenblat 1998 {published data only}
- Korenblat P, Chervinsky P, Wenzel S, Faiferman I, Bakst A. Pranlukast (Ultair) reduces health care utilization and improves quality of life in adult patients with mild‐to‐moderate asthma. American Journal of Respiratory & Critical Care Medicine. 1998; Vol. 157, issue 3:A411.
Kuna 1997 {published data only}
- Kuna P, Malmstrom K, Dahlen SE, Nizankowska E, Kowalski M, Stevenson D, et al. Montelukast (MK‐0476), a cys‐LT1 receptor antagonist, improves asthma control in aspirin‐intolerant asthmatic patients. American Journal of Respiratory & Critical Care Medicine 1997;155(4):A975. [Google Scholar]
Kylstra 1998 {published data only}
- Kylstra JW, Sweitzer DE, Miller CJ, Bonuccelli CM. Zafirlukast (Accolate) in moderate asthma: patient‐reported outcomes and peripheral eosinophil data from a 13‐week trial. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A410. [Google Scholar]
Laitinen 1995 {published data only}
- Laitinen LA, Zetterstrom O, Holgate ST, Binks SM, Whitney JG. Effects of Accolate (Zafirlukast; 20 mg bd) in permitting reduced therapy with inhaled steroids: a multicenter trial in patients with doses of inhaled steroid optimised between 800 and 2000 mcg per day. Allergy 1995;50 Suppl(26):320, Abs. P‐0710. [Google Scholar]
Leaker 2010 {published data only}
- Leaker BR, Singh D, Barnes PJ, Imrie M, Hughes R, O'Connor B. The Effect Of The Novel Phosphodiesterase‐4 Inhibitor MEM 1414 On The Allergen‐induced Responses In mild Asthma [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181:A5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2004 {published data only}
- Lee DKC, Jackson CM, Haggart K, Lipworth BJ. Repeated dosing effects of mediator antagonists in inhaled corticosteroid‐treated atopic asthmatic patients. Chest 2004;125(4):1372‐7. [DOI] [PubMed] [Google Scholar]
Lee 2004a {published data only}
- Lee DK, Haggart K, Currie GP, Bates CE, Lipworth BJ. Effects of hydrofluoroalkane formulations of ciclesonide 400 microg once daily vs fluticasone 250 microg twice daily on methacholine hyper‐responsiveness in mild‐to‐moderate persistent asthma. British Journal of Clinical Pharmacology 2004;58(1):26‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2004b {published data only}
- Lee DKC, Haggart K, Robb FM, Lipworth BJ. Montelukast protects against nasal lysine‐aspirin challenge in patients with aspirin‐induced asthma. European Respiratory Journal 2004;24(2):226‐30. [DOI] [PubMed] [Google Scholar]
Lee 2004c {published data only}
- Lee DKC, Lipworth BJ. Anti‐inflammatory effects of histamine H1‐receptor antagonist and leukotriene CysLT1‐receptor antagonist in patients with atopic asthma maintained on inhaled corticosteroids [Abstract]. European Respiratory Journal 2004;24(Suppl 48):221s. [Google Scholar]
Lee 2005 {published data only}
- Lee DK, Fardon TC, Bates CE, Haggart K, McFarlane LC, Lipworth BJ. Airway and systemic effects of hydrofluoroalkane formulations of high‐dose ciclesonide and fluticasone in moderate persistent asthma. Chest 2005;127(3):851‐60. [DOI] [PubMed] [Google Scholar]
Lee 2010 {published data only}
- Lee RU, White AA, Ding D, Dursun AB, Woessner KM, Simon RA, et al. Use of intranasal ketorolac and modified oral aspirin challenge for desensitization of aspirin‐exacerbated respiratory disease. Annals of Allergy, Asthma and Immunology 2010;105(2):130‐5. [DOI] [PubMed] [Google Scholar]
Leff 1998 {unpublished data only}
- Leff JA, Busse WW, Pearlman D, Bronsky EA, Kemp J, Hendeles L, et al. Montelukast, a leukotriene‐receptor antagonist, for the treatment of mild asthma and exercise‐induced bronchoconstriction. New England Journal of Medicine 1998;339:147‐52. [DOI] [PubMed] [Google Scholar]
Leibman 2002 {published data only}
- Leibman C, Pathak D, Bowers B, Dorinsky PM, Pepsin P, Kalberg C, et al. Cost effectiveness analysis of fluticasone propionate/salmeterol combination versus montelukast in the treatment of adults with asthma [Abstract]. Journal of Allergy Asthma and Immunoly 2002;109(Suppl 1):Abstract No: 549. [Google Scholar]
Leibman 2002a {published data only}
- Leibman CW, Stanford R, Emmett A, Dorinsky PM, Rickard KA. Cost‐effectiveness of fluticasone propionate‐salmeterol combination versus fluticasone + montelukast in the treatment of persistent asthma. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):B4. [Google Scholar]
Leigh 2002 {published data only}
- Leigh R, Vethanayagam D, Yoshida M, Watson RM, Rerecich T, Inman MD, et al. Effects of montelukast and budesonide on airway responses and airway inflammation in asthma. American Journal of Respiratory and Critical Care Medicine 2002;166(9):1212‐7. [DOI] [PubMed] [Google Scholar]
Leigh 2002a {published data only}
- Leigh R, Vethanayagam D, Yoshida M, Watson RM, Rererich T, Killian K, et al. Effects of montelukast and budesonide alone or in combination on allergen induced early and late asthmatic responses, and post‐allergen airway hyperresponsiveness [abstract]. American Journal of Respiratory and Critical Care Medicine 2002;165(8 Suppl):A216. [Google Scholar]
Lemanske 2010 {published data only}
- Lemanske RF Jr, Mauger DT, Sorkness CA, Jackson DJ, Boehmer SJ, Martinez FD, et al. Step‐up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. New England Journal of Medicine 2010;362(11):975‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2001 {published data only}
- Li J, Zhang DH, Yang JY. Evaluation of the therapeutic effects of zafirlukast in asthma patients during reduction of high‐doses inhaled corticosteroid. Journal of Clinical Lung Section 2001;6(4):9‐11. [Google Scholar]
Liebke 2001 {published data only}
- Liebke C, Sommerfeld C, Wahn U, Niggemann B. [Preventive monotherapy with montelukast versus DNCG in children with mild asthma ‐ Results of an explorative pilot study]. Article in German. Pneumologie 2001;55(5):231‐7. [DOI] [PubMed] [Google Scholar]
Lindemann 2009 {published data only}
- Lindemann J, Pampe ED, Peterkin JJ, Orozco‐Cronin P, Belofsky G, Stull D. Clinical study of the effects on asthma‐related QOL and asthma management of a medical food in adult asthma patients. Current Medical Research and Opinion 2009;25(12):2865‐75. [DOI] [PubMed] [Google Scholar]
Lipworth 1999 {published data only}
- Lipworth BJ. Comparative potency and anti‐inflammatory profile of monotherapy with either montelukast or zafirlukast, patients with mild‐moderate asthma. Academic Publications (Ongoing trial) 1999.
Lis 2001 {published data only}
- Lis G, Cichocka Jarosz E, Glodzik I, Szczerbinski T, Bialoruska B. Montelukast in treatment mild chronic asthma. Pol Pneumonologia i Alergologia Polska 2001;69(5‐6):257‐64. [PubMed] [Google Scholar]
Liu 1996 {published data only}
- Liu MC, Dube LM, Lancaster J, Zileuton Study Group. Acute and chronic effects of a 5‐lipoxygenase inhibitor in asthma: a 6‐month randomized multicenter trial. Journal of Allergy & Clinical Immunology 1996;98:859‐71. [DOI] [PubMed] [Google Scholar]
Lizaso 2003 {published data only}
- Lizaso Bacaicoa M, Garcia B, Gomez B, Zabalegui A, Rodriguez M, Tabar A. Treatment of allergy to mushrooms. Anales Del Sistema Sanitario De Navarra 2003;26(Suppl 2):129‐37. [PubMed] [Google Scholar]
Lockey 1995 {published data only}
- Lockey RF, Lavins BJ, Snader L. Effects of 13 weeks of treatment with ICI 204,219 (Accolate) in patients with mild to moderate asthma. Journal of Allergy & Clinical Immunology 1995;95(Part 2):350, Abs. 839. [Google Scholar]
Lofdahl 1999 {published data only}
- Lofdahl CG, Reiss TF, Leff JA, Israel E, Noonan MJ, Finn AF, et al. Randomized, placebo controlled trial of a leukotriene receptor antagonist, montelukast, on tapering inhaled corticosteroids in asthmatic patients. BMJ 1999;318(7202):87‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luppo 2005 {published data only}
- Luppo A, Haag J, Maschio L, Alzua E, Nine LF, Lazaroni A. Evaluation of the variation of the PC 20 in patients treated with zafirlukast. Prensa Medica Argentina 2005;92(8):548‐9. [Google Scholar]
Lyseng‐Williamson 2003 {published data only}
- Lyseng‐Williamson KA. Plosker GL. Inhaled salmeterol/fluticasone propionate combination: A pharmacoeconomic review of its use in the management of asthma. Pharmacoeconomics 2003;21(13):951‐89. [DOI] [PubMed] [Google Scholar]
Macfarlane 2000 {published data only}
- Macfarlane AJ, Ying S, Smith SJ, Mannings P, Ryan M, Pavord I, et al. Inhibition of allergen‐induced late phase reactions by zafirlukast and beclomethasone may be mediated by attenuation of eotaxin‐ and rantes‐ mediated eosinophil recruitment. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A834. [Google Scholar]
Magnussen 2008 {published data only}
- Magnussen H, Bugnas B, Noord J, Schmidt P, Gerken F, Kesten S. Improvements with tiotropium in COPD patients with concomitant asthma. Respiratory Medicine 2008;102(1):50‐6. [DOI] [PubMed] [Google Scholar]
Majak 2010 {published data only}
- Majak P, Rychlik B, Pulaski L, Blauz A, Agnieszka B, Bobrowska‐Korzeniowska M, et al. Montelukast treatment may alter the early efficacy of immunotherapy in children with asthma. Journal of Allergy and Clinical Immunology 2010;125(6):1220‐7. [DOI] [PubMed] [Google Scholar]
Malerba 2002 {published data only}
- Malerba M, Radaeli A, Ceriani L, Amato M, Tomenzoli D, Nicolai P, et al. Comparison of oral montelukast and inhaled fluticasone in the treatment of asthma associated with chronic rhinopolyposis: A single‐blind, randomized, pilot study. Current Therapeutic Research, Clinical & Experimental 2002;63(6):355‐65. [Google Scholar]
Marchese 1998 {published data only}
Margolskee 1991 {published data only}
- Margolskee D, Bodman S, Dockhorn R, Irael E, Kemp J, Mansmann H, et al. The therapeutic effects of MK‐571, a potent and selective leukotriene (LT) D4 receptor antagonist, in patients with chronic asthma. Journal of Allergy & Clinical Immunology 1991;87(1 Part 2):309, Abs. 677. [Google Scholar]
Marogna 2010 {published data only}
- Marogna M, Colombo F, Spadolini I, Massolo A, Berra D, Zanon P, et al. Randomized open comparison of montelukast and sublingual immunotherapy as add‐on treatment in moderate persistent asthma due to birch pollen. Journal of Investigational Allergology & Clinical Immunology 2010;20(2):146‐52. [PubMed] [Google Scholar]
Maspero 2008 {published data only}
- Maspero J, Guerra F, Orozco S, Soto M, Gutierrez‐Schwanhauser J, Mechali DG, et al. The efficacy of inhaled salmeterol/fluticasone propionate diskus/accuhaler compared with oral montelukast in children with persistent asthma PEACE (PEdiatric Asthma Control Evaluation ) [Abstract]. American Thoracic Society International Conference. 2008:A709.
Maspero 2008a {published data only}
- Maspero J, Soto‐Ramos M, Guerra F, Chan R, Sharma R, Pedersen S. Improved asthma control and fewer exacerbations with inhaled salmeterol/fluticasone propionate compared with oral montelukast in children with persistent asthma: PEACE (Pediatric Asthma Control Evaluation) [Abstract]. Chest 2008;134(4):51001s. [Google Scholar]
Maspero 2008b {published data only}
- Maspero J, Guerra F, Cuevas F, Gutierrez JP, Soto‐Ramos M, Anderton S, et al. Efficacy and tolerability of salmeterol/fluticasone propionate versus montelukast in childhood asthma: A prospective, randomized, double‐blind, double‐dummy, parallel‐group study. Clinical Therapeutics 2008;30(8):1492‐504. [DOI] [PubMed] [Google Scholar]
Mastruzzo 2010 {published data only}
- Mastruzzo C, Contrafatto MR, Crimi C, Palermo F, Vancheri C, Crimi N. Acute additive effect of montelukast and beclomethasone on AMP induced bronchoconstriction. Respiratory Medicine 2010;104(10):1417‐24. [DOI] [PubMed] [Google Scholar]
Matsunaga 2004 {published data only}
- Matsunaga K, Nishimoto T, Hirano T, Nakanishi M, Yamagata T, Minakata Y, et al. Effect of a leukotriene receptor antagonist on the prevention of recurrent asthma attacks after an emergency room visit. Allergology International 2004;53(4):341‐7. [Google Scholar]
McCarthy 2003 {published data only}
- McCarthy TP, Woodcock AA, Pavord ID, Allen DJ, Parker D, Rice L. A comparison of the anti‐inflammatory and clinical effects of salmeterol 25mcg/fluticasone propionate 50mcg combination (SFC 50) with fluticasone propionate (FP) plus montelukast (M) in patients with mild to moderate asthma [abstract]. American Thoracic Society 99th International Conference 2003;B036:H89. [Google Scholar]
McGill 1996 {published data only}
- McGill K, Busse WW. Zileuton. Lancet 1996;348:519‐24. [DOI] [PubMed] [Google Scholar]
Mclvor 2009 {published data only}
- McIvor RA, Kaplan A, Koch C, Sampalis JS. Montelukast as an alternative to low‐dose inhaled corticosteroids in the management of mild asthma (the SIMPLE trial): an open‐label effectiveness trial. Canadian Respiratory Journal 2009;16(Suppl A):11A‐21A. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehuys 2008 {published data only}
- Mehuys E, Bortel L, Bolle L, Tongelen I, Remon JP, Annemans L, et al. Does pharmacist intervention lead to appropriate use of asthma medication and improved asthma control?. Farmaceutisch Tijdschrift Voor Belgie 2008;85(1):1‐9. [Google Scholar]
Mendes 2004 {published data only}
- Mendes ES, Campos MA, Hurtado A, Wanner A. Effect of montelukast and fluticasone propionate on airway mucosal blood flow in asthma. American Journal of Respiratory & Critical Care Medicine 2004;169(10):1131‐4. [DOI] [PubMed] [Google Scholar]
Mendes 2004a {published data only}
- Mendes ES, Campos MA, Hurtado A, Wanner A. Anti‐inflammatory actions of montelukast and fluticasone propionate as assessed by airway blood flow [Abstract]. American Thoracic Society 100th International Conference 2004;D31:Poster C6. [Google Scholar]
Menendez 2001 {published data only}
- Menendez R, Standford RH, Edwards L, Kalberg C, Rickard K. Cost‐efficacy analysis of fluticasone propionate versus zafirlukast in patients with persistent asthma. Pharmacoeconomics 2001;19(8):865‐74. [DOI] [PubMed] [Google Scholar]
Meyer 2003 {published data only}
- Meyer KA, Arduino JM, Santanello NC, Knorr BA, Bisgaard H. Response to montelukast among subgroups of children aged 2 to 14 years with asthma. Journal of Allergy & Clinical Immunology 2003;111(4):757‐62. [DOI] [PubMed] [Google Scholar]
Micheletto 1997 {published data only}
- Micheletto C, Turco P, Dal Negro R. Accolate 20 mg works as steroid sparing in moderate asthma. American Journal of Respiratory & Critical Care Medicine 1997;155(4):A664. [Google Scholar]
Miraglia 2007 {published data only}
- Miraglia del Giudice M, Piacentini GL, Capasso M, Capristo C, Maiello N, Boner AL, et al. Formoterol, montelukast, and budesonide in asthmatic children: Effect on lung function and exhaled nitric oxide. Respiratory Medicine 2007;101(8):1809‐13. [DOI] [PubMed] [Google Scholar]
Mitchell 2005 {published data only}
- Mitchell EA, Didsbury PB, Kruithof N, Robinson E, Milmine M, Barry M, et al. A randomized controlled trial of an asthma clinical pathway for children in general practice. Acta Paediatrica 2005;94(2):226‐33. [DOI] [PubMed] [Google Scholar]
Miyamoto 1999 {published data only}
- Miyamoto T, Accolate™ clinical trial committee. Effects of zafirlukast on symptoms and pulmonary function of asthmatic patients with and without corticosteroids. European Respiratory Society. 1999:P835.
Molitor 2005 {published data only}
- Molitor S, Liefring E, Traytmann M. Asthma control with the salmeterol‐fluticasone‐combination disc compared to standard treatment. Pneumologie 2005;59(3):167‐73. [DOI] [PubMed] [Google Scholar]
Montani 2007 {published data only}
- Montani D. Randomized comparison of strategies for reducing treatment in mild persistent asthma. Revue De Pneumologie Clinique 2007;63(6):390. [Google Scholar]
Moreira 2008 {published data only}
- Moreira A, Delgado L, Haahtela T, Fonseca J, Moreira P, Lopes C, et al. Physical training does not increase allergic inflammation in asthmatic children. European Respiratory Journal 2008;32(6):1570‐5. [DOI] [PubMed] [Google Scholar]
Morris 2010 {published data only}
- Morris CR, Becker AB, Pineiro A, Massaad R, Green SA, Smugar SS, et al. A randomized, placebo‐controlled study of intravenous montelukast in children with acute asthma. Annals of Allergy, Asthma and Immunology 2010;104(2):161‐71. [DOI] [PubMed] [Google Scholar]
Mosnaim 2002 {published data only}
- Mosnaim GS. The role of montelukast in the prevention of acute episodes of asthma in children ages 2 to 14 years [Dissertation]. Rush University 2002:75.
Mosnaim 2008 {published data only}
- Mosnaim GS, Cohen MS, Rhoads CH, Rittner SS, Powell LH. Use of MP3 players to increase asthma knowledge in inner‐city African‐American adolescents. International Journal of Behavioral Medicine 2008;15(4):341‐6. [DOI] [PubMed] [Google Scholar]
Murphy 2006 {published data only}
- Murphy K, Ververeli K, Harvey BM, Duke AL, Chapas‐Crilly J, Uryniak T, et al. Antibody response after varicella vaccination in children treated with budesonide inhalation suspension or non‐steroidal conventional asthma therapy. International Journal of Clinical Practice 2006;60(12):1548‐57. [DOI] [PubMed] [Google Scholar]
Najberg 2008 {published data only}
- Najberg E, Smorczewska‐Kiljan A, Ksiayk J, Kaczmarewicz E, Pludowski P, Jaworski M, et al. Effect of one year treatment with inhaled budesonide on bone density and mineralization in children with bronchial asthma. Alergia Astma Immunologia 2008;13(4):217‐26. [Google Scholar]
Nakagawa 1992 {published data only}
- Nakagawa N, Obata T, Kobayashi T, Okada Y, Nambu F, Terawaki T, et al. In vivo pharmacologic profile of ONO‐1078: a potent, selective and orally active peptide leukotriene (LT) antagonist. Japanese Journal of Pharmacology 1992;60(3):217‐25. [DOI] [PubMed] [Google Scholar]
Nakajima 2001 {published data only}
- Nakajima S, Tohda Y, Fujimura M, Taniguchi H, Takagi K, Igarashi T, et al. Effects of montelukast on tapering inhaled corticosteroids in patients with asthma. European Respiratory Journal 2001;18(Suppl 33):260s. [Google Scholar]
Nakazono 2004 {published data only}
- Nakazono H, Mukoyama T, Shinomiya N. The effects of the leukotriene receptor antagonist, pranlukast, on long‐term treatment in children with persistent asthma. Journal of the Medical Society of Toho University 2004;51(6):339‐46. [Google Scholar]
Nathan 1998 {published data only}
- Nathan RA, Bernstein JA, Bielory L, Bonuccelli CM, Calhoun WJ, Galant SP, et al. Zafirlukast improves asthma symptoms and quality of life in patients with moderate reversible airflow obstruction. Journal of Allergy & Clinical Immunology 1998;102:935‐42. [DOI] [PubMed] [Google Scholar]
Nathan 2000 {published data only}
- Nathan BA, Boone RI, Emmett AH, Knobil K, Yancey SW, Rickard KA. Salmeterol and inhaled corticosteroids provide greater asthma control than montelukast and inhaled corticosteroids [Abstract]. Chest 2000;118(Suppl 4):85S. [Google Scholar]
Nathan 2004 {published data only}
- Nathan RA, Philpot E, Faris M, Prillaman B, Yancey S, Dorinsky P. In patients taking fluticasone propionate/salmeterol 100/50µg Diskus(r) for asthma, the addition of fluticasone propionate nasal spray 200µg QD to treat concomitant allergic rhinitis has a safety profile comparable to the addition of montelukast 10mg QD or placebo [Abstract]. Journal of Allergy and Clinical Immunology 2004;113(Suppl 2):S202. [Google Scholar]
Nathan 2005 {published data only}
- Nathan RA, Yancey SW, Waitkus‐Edwards K, Prillaman BA, Stauffer JL, Philpot E, et al. Fluticasone propionate nasal spray is superior to montelukast for allergic rhinitis while neither affects overall asthma control. Chest 2005;128(4):1910‐20. [DOI] [PubMed] [Google Scholar]
Nayak 1998 {published data only}
- Nayak AS, Anderson P, Charous BL, Williams K, Simonson S. Equivalence of adding Zafirlukast versus double‐dose inhaled corticosteroids in asthmatic patients symptomatic on low‐dose inhaled corticosteroids. Journal of Allergy & Clinical Immunology 1998;101(1 pt 2):S233, Abs. 965. [Google Scholar]
NCT00096954 {unpublished data only}
- A Study to Evaluate the Efficacy of Xolair in Atopic Asthmatics (EXACT). ClinicalTrials.Gov 2004.
NCT00140881 {unpublished data only}
- A Study to Determine the Effect of Montelukast Sodium as an Episode Modifier in the Treatment of Infrequent Episodic Asthma in Children. ClinicalTrials.Gov.
NCT00196547 {unpublished data only}
- Montelukast in Modulating Exacerbations of Asthma in Children. ClinicalTrials.Gov 2005.
NCT00213252 {unpublished data only}
- Use of Montelukast to Treat Children With Mild to Moderate Acute Asthma. ClinicalTrials.Gov 2005.
NCT00299065 {unpublished data only}
- Safety Study of Zileuton Injection in Patients With Asthma. ClinicalTrials.Gov 2006.
NCT00319488 {unpublished data only}
- Childhood Asthma Research and Education (CARE) Network Trial ‐ Acute Intervention Management Strategies (AIMS). ClinicalTrial.Gov 2006.
NCT00395408 {unpublished data only}
- Linear Growth Study. ClinicalTrials.Gov 2006.
NCT00421018 {unpublished data only}
- Optimization of Asthma Treatment Through Exhaled NO for Increased Asthma‐Related Quality of Life (NOAK). ClinicalTrials.gov 2007.
NCT00462592 {unpublished data only}
- Comparison of Combination Therapy: Montelukast and Inhaled Steroid on Exercise Induced Bronchoconstriction. ClinicalTrials.Gov 2007.
NCT00471809 {unpublished data only}
- Childhood Asthma Research and Education (CARE) Network Trial ‐ Montelukast or Azithromycin for Reduction of Inhaled Corticosteroids in Childhood Asthma (MARS). ClinicalTrials.Gov 2007.
NCT00486343 {unpublished data only}
- Zileuton CR vs Placebo in Poorly Controlled Asthma Patients on Moderate Dose ICS. ClinicalTrials.Gov 2007.
NCT00504946 {unpublished data only}
- Pharmacological Modulations of Allergen‐Specific Immunotherapy. ClinicalTrials.Gov 2007.
NCT00545324 {unpublished data only}
- First Step With Singulair® Therapy (FIRST). ClinicalTrials.Gov 2007.
NCT00545844 {unpublished data only}
- Singulair(R) In Asthma And Allergic Rhinitis. ClinicalTrials.Gov 2007.
NCT00575861 {unpublished data only}
- Zileuton and Exhaled Nitric Oxide in Asthmatics. ClinicalTrials.Gov 2007.
NCT00666679 {unpublished data only}
- Study of Inhaled Corticosteroid Plus Montelukast Compared With Inhaled Corticosteroid Therapy Alone in Patients With Chronic Asthma. ClinicalTrials.Gov 2008.
NCT00699062 {unpublished data only}
- Effect of Montelukast on the Airway Remodeling. ClinicalTrials.Gov 2008.
NCT00755794 {unpublished data only}
- The Singulair® Add‐on Study Effectiveness of Adding Montelukast to Inhaled Corticosteroids in Adult Subjects With Uncontrolled Asthma (SAS). ClincalTrials.Gov 2008.
NCT00756418 {unpublished data only}
- Montelukast Post‐Marketing Comparative Study With Theophyline Added to Inhaled Corticosteroid. ClinicalTrials.Gov 2008.
NCT00913328 {unpublished data only}
- Effect of Add‐on Montelukast to Inhaled Corticosteroids on Airway Responsiveness (SINGDEN). ClinicalTrials.Gov 2009.
NCT00943397 {unpublished data only}
- Effect of Add‐on Montelukast to Inhaled Corticosteroids on Airway Responsiveness (SINGDEN). ClinicalTrials.Gov 2009.
NCT01055041 {unpublished data only}
- Controller Medications in the Management of Bronchial Asthma. ClinicalTrials.Gov 2010.
NCT01241084 {unpublished data only}
- Beneficial Effects of Lactobacillus Reuteri DSM 17938 Supplementation on Asthmatic Children. ClinicalTrials.Gov 2010.
Negro 1997 {published data only}
- Negro JM, Miralles JC, Ortiz JL, Funes E, Garcia A. Leukotrienes and its antagonists in allergic disorders. Allergologia et Immunopathologia 1997;25(2):104‐12. [PubMed] [Google Scholar]
Neki 2006 {published data only}
- Neki NS, Kazal HL. Comparative efficacy and safety profile of montelukast v/s beclomethasone in mild persistent asthma [Abstract]. Indian Journal of Allergy Asthma and Immunology 2006;20(2):124. [Google Scholar]
Nelson 2001 {published data only}
- Nelson HS, Nathan RA, Kalberg C, Yancey SW, Rickard KA. Comparison of inhaled salmeterol and oral zafirlukast in asthmatic patients using concomitant inhaled corticosteroids. Medgenmed Computer File: Medscape General Medicine 2001;3(4):3. [PubMed] [Google Scholar]
Nelson 2004 {published data only}
- Nelson H, Stauffer J, Yancey S, Prillaman B, Sutton L, Dorinsky. In patients with both uncontrolled asthma and allergic rhinitis, montelukast added to fluticasone propionate/salmeterol provides no additional clinical improvements in overall asthma control regardless of baseline asthma severity [Abstract]. American Thoracic Society 100th International Conference 2004;D36:A49. [Google Scholar]
Nelson 2004a {published data only}
- Nelson HS, Yancey S, Waitkus‐Edwards K, Prillaman B, Philpot E, Dorinsky P. In patients taking fluticasone propionate/salmeterol 100/50µg Diskus(r) for asthma, fluticasone propionate nasal spray 200µg QD is superior to montelukast 10mg QD in the treatment of allergic rhinitis in patients with coexistent allergic rhinitis: implication for the one airway hypothesis [Abstract]. Journal of Allergy and Clinical Immunology 2004;113(Suppl 2):S200. [Google Scholar]
Nelson 2006 {published data only}
- Nelson HS, Weiss ST, Bleecker EK, Yancey SW, Dorinsky PM. The salmeterol multicenter asthma research trial: A comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. chest 2006;129(1):15‐26. [DOI] [PubMed] [Google Scholar]
Nishima 2005 {published data only}
- Nishima S, Furusho K, Morikawa A, Mochizuki H, Akasaka T, Sugimoto H, et al. Pranlukast inhibits exercise‐induced bronchospasm in asthmatic children: A randomized, multicenter, double‐blind, placebo‐controlled two‐period crossover trial. Pediatric Asthma Allergy & Immunology 2005;18(1):5‐11. [Google Scholar]
Nishimura 1999 {published data only}
- Nishimura K, Hajiro T, Ishihara K, Hasegawa T, Taniguchi H, Katayama S, et al. Additive effect of pranlukast in combination with inhaled corticosteroid in the treatment of patients with chronic asthma. European Respiratory Journal. 1999:P837.
Nishiyama 2006 {published data only}
- Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Kato K, Kume H, et al. Comparison of the effects of tulobuterol patch and salmeterol in moderate to severe asthma. Clinical & Experimental Pharmacology & Physiology 2006;33(11):1016‐21. [DOI] [PubMed] [Google Scholar]
Nishizawa 2002 {published data only}
- Nishizawa Y, Greicy‐Goto H, Tanigaki Y, Fushiki S. Sparing effect of Saibokuto inhalation on inhaled beclomethasone dipropionate to halved of reduction of inhaled beclomethasone dipropionate‐dose: Well‐controlled comparative study of Saiboku‐to‐inhalation and sodium cromoglycate‐inhalation. Japanese. Oto‐Rhino‐Laryngology Tokyo 2002;45(Suppl 1):8‐15. [Google Scholar]
Noonan 1998 {published data only}
- Noonan MJ, Chervinsky P, Brandon M, Zhang J, Kundu S, McBurney J, et al. Montelukast, a potent leukotriene receptor antagonist, causes dose‐related improvements in chronic asthma. European Respiratory Journal 1998;11:1232‐9. [DOI] [PubMed] [Google Scholar]
Noonan 1999 {unpublished data only}
- Noonan G, Reiss TF, Shingo S, Nguyen HH, Knorr B. Montelukast (MK‐0476) maintains long‐term asthma control in adult and pediatric patients (aged >6 years). Merck Research Laboratories 1999.
Nsouli 2000 {published data only}
- Nsouli SM, McNutt WJ. The addition of montelukast to a low dose inhaled corticosteroid compared with a double‐dose of an inhaled corticosteroid in patients with persistent asthma. Annals of Allergy, Asthma & Immunology 2000;84:159. [Google Scholar]
Nsouli 2001 {published data only}
- Nsouli SM, McNutt WJ. The additive effects of montelukast and salmeterol in moderate asthmatics who are uncontrolled on a low dose on inhaled corticosteroids. Annals of Allergy, Asthma & Immunology 2001; 86:81.;86:81. [Google Scholar]
O'Byrne 1997 {published data only}
- O'Byrne PM. Leukotrienes in the pathogenesis of asthma. Chest 1997;111:27S‐34S. [DOI] [PubMed] [Google Scholar]
O'Byrne 1997a {published data only}
- O'Byrne PM, Barnes NC. Summary: the future promise of mediator inhibitors in 'New oral preventive therapy in asthma and oral leukotriene receptor antagonism'. European Respiratory Review 1997; Vol. 7, issue Review 46:274‐7.
O'Byrne 1997b {published data only}
- O'Byrne PM, Israel E, Drazen JM. Antileukotrienes in the treatment of asthma. Annals of Internal Medicine 1997;127:472‐80. [DOI] [PubMed] [Google Scholar]
O'Connor 1994 {published data only}
- O'Connor GT, Weiss ST. Clinical and symptom measures. American Journal of Respiratory & Critical Care Medicine 1994;149:S21‐8. [DOI] [PubMed] [Google Scholar]
O'Connor 2004 {published data only}
- O'Connor RD, Nelson H, Borker R, Emmett A, Jhingran P, Rickard K, et al. Cost effectiveness of fluticasone propionate plus salmeterol versus fluticasone propionate plus montelukast in the treatment of persistent asthma. Pharmacoeconomics 2004;22(12):815‐25. [DOI] [PubMed] [Google Scholar]
O'Connor 2006 {published data only}
- O'Connor RD, Gilmore AS, Manjunath R, Stanford RH, Legorreta AP, Jhingran PM. Comparing outcomes in patients with persistent asthma: a registry of two therapeutic alternatives. Current Medical Research & Opinion 2006;22(3):453‐61. [DOI] [PubMed] [Google Scholar]
O'Shaughnessy 1996 {published data only}
- O'Shaughnessy TC, Georgiou P, Howland K, Dennis M, Compton CH, Barnes NC. Effect of pranlukast, an oral leukotriene receptor antagonist, on leukotriene D4 (LTD4) challenge in normal volunteers. Thorax 1996;52:519‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Sullivan 2002 {published data only}
- O'Sullivan S, McWeeney M, Akveld M, Berelowitz KS, Burke CM, Poulter LW. Effect of the addition of montelukast to inhaled fluticasone propionate on immunopathology in bronchial biopsies from mild to moderate asthmatics [abstract]. European Respiratory Society Annual Congress. 2002:P2405.
O'Sullivan 2002a {published data only}
- O'Sullivan S, McWeeny M, Akveld M, Berelowitz KS, Burke CM, Poulter LW. Effect of the addition of montelukast to inhaled fluticasone propionate on immunopathology in bronchial biopsies from mild to moderate asthmatics. European Respiratory Journal 2002;20(Suppl 38):388s. [Google Scholar]
O'Sullivan 2003 {published data only}
- O'Sullivan S, Akveld M, Burke CM, Poulter LW. Effect of the addition of montelukast to inhaled fluticasone propionate on airway inflammation. American Journal of Respiratory and Critical Care Medicine 2003;167(5):745‐50. [DOI] [PubMed] [Google Scholar]
Obase 2001 {published data only}
- Obase Y, Shimoda T, Tomari S, Mitsuta K, Fukushima C, Kawano K, et al. Efficacy and safety of long‐term treatment of asthma in patients with pranlukast, a cysteinyl‐leukotriene‐receptor antagonist: a four‐year follow‐up study. Annals of Allergy, Asthma, & Immunology 2001;87(1):43‐7. [DOI] [PubMed] [Google Scholar]
Obata 1992 {published data only}
- Obata T, Okada Y, Motoishi M, Nakagawa N, Terawaki T, Aishita H. In vitro antagonism of ONO‐1078, a newly developed anti‐asthma agent, against peptide leukotrienes in isolated guinea pig tissues. Japanese Journal of Pharmacology 1992;60:227‐37. [DOI] [PubMed] [Google Scholar]
Odjakova 2000 {published data only}
- Odjakova TT, Peneva MA, Kissiova KP, Balev SS, Radkov JD. Changes in some lymphocyte markers after therapy with fluticasone propionate and montelukast in patients with bronchial asthma. European Respiratory Journal 2000;16(Suppl 31):163s. [Google Scholar]
Ohbayashi 2007 {published data only}
- Ohbayashi H, Adachi M, Ichinose M, Ohta K, Kokubu F, Sano Y, et al. A multicenter, open‐label, randomized comparison of suppressive effects on asthmatic inflammation of lower airways and improved effects on health‐related QOL between HFA‐BDP and fluticasone propionate. Japanese Journal of Allergology 2007;56(6):577‐86. [PubMed] [Google Scholar]
Ohbayashi 2009 {published data only}
- Ohbayashi H, Shibata N, Adachi M. The evaluation of the additional effect of pranlukast to salmeterol/fluticasone combination therapy using impulse oscillometry system in a randomized crossover study [Abstract]. American Thoracic Society International Conference 2009;A2764:Poster J32. [Google Scholar]
Ohbayashi 2009a {published data only}
- Ohbayashi H, Shibata N, Hirose T, Adachi M. Additional effects of pranlukast in salmeterol/fluticasone combination therapy for the asthmatic distal airway in a randomized crossover study. Pulmonary Pharmacology & Therapeutics 2009;22(6):574‐9. [DOI] [PubMed] [Google Scholar]
Ohkura 2009 {published data only}
- Ohkura N, Fujimura M, Tokuda A, Takato H, Tamori S, Waseda Y, et al. Additional effects of pranlukast on exhaled nitric oxide levels in patients with persistent asthma. Therapeutic Research 2009;30(8):1361‐6. [Google Scholar]
Ohta 2009 {published data only}
- Ohta K, Miyamoto T, Amagasaki T, Yamamoto M. Efficacy and safety of omalizumab in an Asian population with moderate‐to‐severe persistent asthma. Respirology 2009;14(8):1156‐65. [DOI] [PubMed] [Google Scholar]
Okudaira 1997 {published data only}
- Okudaira H. Challenge studies of a leukotriene receptor antagonist. Chest 1997;111:46S‐51S. [DOI] [PubMed] [Google Scholar]
Oosaki 1997 {published data only}
- Oosaki R, Mizushima Y, Kashii T, Kawasaki A, Kobayashi M. Therapeutic effect of pranlukast, a selective cysteinyl leukotriene receptor antagonist, on bronchial asthma. International Archives of Allergy & Immunology 1997;114(1):97‐100. [DOI] [PubMed] [Google Scholar]
Oosaki 1997a {published data only}
- Oosaki R, Mizushima Y, Kawasaki A, Kashii T, Mita H, Shida T, et al. Urinary excretion of leukotriene E4 and 11‐dehydrothromboxane B2 in patients with spontaneous asthma attacks. International Archives of Allergy & Immunology 1997;114:373‐8. [DOI] [PubMed] [Google Scholar]
Oppenheimer 2008 {published data only}
- Oppenheimer J, Mosnaim G, Waitkus‐Edwards K, Prillaman B, Ortega H. Fluticasone propionate/salmeterol via diskus is superior to montelukast in overall asthma control in subjects with both asthma and allergic rhinitis [Abstract]. Chest 2008;134(4):94002s. [Google Scholar]
Ostrom 2003 {published data only}
- Ostrom NK, DeCotiis BA, Lincourt WR, Edwards LD, Crim CC. A comparison of low does fluticasone propionate and montelukast in children 6‐12 years of age with persistent asthma [abstract]. American Thoracic Society 99th International Conference 2003;A117:D70. [Google Scholar]
Overbeek 2002 {published data only}
- Overbeek SE, O'Sullivan S, Leman K, Mulder PGM, Hoogsteden, Prins JB. Treatment with montelukast is less effective in reducing eosinophilic airway inflammation than fluticasone propionate in atopic asthmatics. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A215. [Google Scholar]
Palmqvist 2003 {published data only}
- Palmqvist M, Bruce C, Sjostrand M, Arvidsson P, Lotvall J. Inhibiton of allergen‐induced late asthmatics reaction by montelukast vs fluticasone propionate [Abstract]. European Respiratory Journal 2003;22(Suppl 45):2220. [Google Scholar]
Palmqvist 2005 {published data only}
- Palmqvist M, Bruce C, Sjostrand M, Arvidsson P, Lotvall J. Differential effects of fluticasone and montelukast on allergen‐induced asthma. Allergy 2005;60(1):65‐70. [DOI] [PubMed] [Google Scholar]
Panettieri 1997 {published data only}
- Panettieri RA, Tan EML, Ciocca V, Luttman MA, Leonard TB, Hay DWP. Effects of LTD4 on human airways smooth muscle cell proliferation, matrix expression and contraction in vitro: differential sensitivity to cysteinyl leukotriene receptor antagonists. SmithKline Beecham Pharmaceuticals 1997. [DOI] [PubMed]
Papadopoulos 2009 {published data only}
- Papadopoulos NG, Philip G, Giezek H, Watkins M, Smugar SS, Polos PG. The efficacy of montelukast during the allergy season in pediatric patients with persistent asthma and seasonal aeroallergen sensitivity. Journal of Asthma 2009;46(4):413‐420. [DOI] [PubMed] [Google Scholar]
Pasaoglu 2008 {published data only}
- Pasaoglu G, Mungan D, Abadoglu O, Misirligil Z. Leukotriene receptor antagonists: a good choice in the treatment of premenstrual asthma?. Journal of Asthma 2008;45(2):95‐99. [DOI] [PubMed] [Google Scholar]
Patel 2010 {published data only}
- Patel YA, Patel P, Bavadia H, Dave J, Tripathi CB. A randomized, open labelled, comparative study to assess the efficacy and safety of controller medications as add on to inhaled corticosteroid and long‐acting beta2 agonist in the treatment of moderate‐to‐severe persistent asthma. Journal of Postgraduate Medicine 2010;56(4):270‐274. [DOI] [PubMed] [Google Scholar]
Paterson 1999 {published data only}
- Paterson MC, Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. The effect of combination therapy with salmeterol and montelukast in asthmatic patients receiving inhaled corticosteroids. European Respiratory Society. 1999:P3490.
Pavord 2007 {published data only}
- Pavord I, Woodcock A, Parker D, Rice L, SOLTA study group. Salmeterol plus fluticasone propionate versus fluticasone propionate plus montelukast: a randomised controlled trial investigating the effects on airway inflammation in asthma. Respiratory Research 2007;8:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pearlman 1999 {published data only}
- Pearlman DS, Ostrom NK, Bronsky EA, Bonuccelli CM, Hanby LA. The leukotriene D4‐receptor antagonist zafirlukast attenuates exercise‐induced bronchoconstriction in children. Journal of Pediatrics 1999;134:273‐9. [DOI] [PubMed] [Google Scholar]
Pearlman 2002 {published data only}
- Pearlman DS, White MV, Lieberman AK, Pepsin PJ, Kalberg C, Emmett A, et al. Fluticasone propionate/salmeterol combination compared with montelukast for the treatment of persistent asthma. Annals of Allergy, Asthma & Immunology 2002;88(2):227‐35. [DOI] [PubMed] [Google Scholar]
Pedersen 2007 {published data only}
- Pedersen S, Agertoft L, Williams‐Herman D, Kuznetsova O, Reiss TF, Knorr B, et al. Placebo‐controlled study of montelukast and budesonide on short‐term growth in prepubertal asthmatic children. Pediatric Pulmonology 2007;42(9):838‐43. [DOI] [PubMed] [Google Scholar]
Pereira 1989 {published data only}
- Pereira CAdC, Brito VC, Olieveira VMC, Paitl CE, Nery LE, Romaldini H, et al. Effects of corticosteroids administered early by inhalation in stable chronic obstructive pulmonary diseases. American Review of Respiratory Disease 1989;15(52):1348‐53. [Google Scholar]
Perng 2004 {published data only}
- Perng DW, Huang HY, Lee YC, Perng RP. Leukotriene modifier vs inhaled corticosteroid in mild‐to‐moderate asthma: Clinical and anti‐inflammatory effects. Chest 2004;125(5):1693‐9. [DOI] [PubMed] [Google Scholar]
Peroni 2005a {published data only}
- Peroni DG, Piacentini GL, Bodini A, Ress M, Costella S, Boner AL. Montelukast versus formoterol as second‐line therapy in asthmatic children exposed to relevant allergens. Allergy & Asthma Proceedings 2005;26(4):283‐6. [PubMed] [Google Scholar]
Philip 2005 {published data only}
- Philip G, Nayak AS, Berger WE, Leynadier F, Vrijens F, Dass SB, et al. The effect of montelukast on rhinitis symptoms in patients with asthma and seasonal allergic rhinitis. Allergologie 2005;28(9):343‐54. [DOI] [PubMed] [Google Scholar]
Phipatanakul 2003 {published data only}
- Phipatanakul W, Greene C, Downes SJ, Cronin B, Eller TJ, Schneider LC, et al. Montelukast improves asthma control in asthmatic children maintained on inhaled corticosteroids. Annals of Allergy Asthma & Immunology 2003;91(1):49‐54. [DOI] [PubMed] [Google Scholar]
Phipatanakul 2003a {published data only}
- Phipatanakul W, Greene C, Downes S, Cronin B, Schneider L, Irani A. The efficacy of montelukast as an addictive and steroid sparing agent in children with asthma previously maintained on inhaled corticosteroid therapy [Abstract]. Journal of Allergy and Clinical Immunology 2003;111(Suppl 2):S146. [Google Scholar]
Pieters 2005 {published data only}
- Pieters WR, Wilson KK, Smith HC, Tamminga JJ, Sondhi S. Salmeterol/fluticasone propionate versus fluticasone propionate plus montelukast: a cost‐effective comparison for asthma. Treatments in Respiratory Medicine 2005;4(2):129‐38. [DOI] [PubMed] [Google Scholar]
Pizzichini 1999 {unpublished data only}
- Pizzichini E, Leff JA, Reiss TF, Hendeles L, Boulet L‐P, Wei LX, et al. Montelukast reduces airway eosinophilic inflammation in asthma: a randomized, controlled trial. European Respiratory Journal 1999;14(1):12‐8. [DOI] [PubMed] [Google Scholar]
Plaza 2005 {published data only}
- Plaza V, Cobos A, Ignacio‐Garcia JM, Molina J, Bergonon S, Garcia‐Alonso F, et al. Cost‐effectiveness of an intervention based on the Global INitiative for Asthma (GINA) recommendations using a computerized clinical decision support system: A physicians randomized trial. Spa Medicina Clinica 2005;124(6):201‐6. [DOI] [PubMed] [Google Scholar]
Pogson 2008 {published data only}
- Pogson ZEK, Antoniak MD, Pacey SJ, Lewis SA, Britton JR, Fogarty AW. Does a low sodium diet improve asthma control?: A randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2008;178(2):132‐8. [DOI] [PubMed] [Google Scholar]
Pohl 2006 {published data only}
- Pohl WR, Vetter N, Zwick H, Hrubos W. Adjustable maintenance dosing with budesonide/formoterol or budesonide: Double‐blind study. Respiratory Medicine 2006;100(3):551‐60. [DOI] [PubMed] [Google Scholar]
Polos 2003 {published data only}
- Polos PG. Montelukast or salmeterol added to fluticasone in uncontrolled asthma: a subgroup analysis of the IMPACT study [Abstract]. Journal of Allergy and Clinical Immunology 2003;111(Suppl 2):S126. [Google Scholar]
Polos 2004 {published data only}
- Polos P, Garcia Garcia ML, Gilles L, Tozzi CA, Knorr B, Reiss TF. Montelukast vs fluticasone in patients aged 6 to 14 with mild persistent asthma the MOSAIC study [Abstract]. European Respiratory Journal 2004;24(Suppl 48):377s. [Google Scholar]
Ponce 2009 {published data only}
- Ponce Castro H, Rodríguez Espino S, Rodríguez Orozco AR. Administration of budesonide (inhaled steroid) to children to control intermittent asthma. Revista Alergia Mexico 2009;26(1):9‐12. [PubMed] [Google Scholar]
Price 1999 {unpublished data only}
- Price DB, Wolfe S. Montelukast: real life effectiveness and cost‐effectiveness in primary care. Thorpe Medical Research 1999.
Price 2002 {published data only}
- Price DB, Hernandez D, Magyar P, Fiterman J, Beeh KM, James IG, et al. Adding montelukast is at least as efficacious as doubling the budesonide dose in persistent asthma: Results of the compact study. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl8):A216. [Google Scholar]
Price 2003 {published data only}
- Price DB, Hernandez D, Magyar P, Fiterman J, Beeh KM, James IG, et al. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax 2003;58(3):211‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Price 2004 {published data only}
- Price DB, Zhang Q, Kocevar VS, Polos PG, Yin DD. Healthcare resource use the following addition of montelukast to budesonide compared to doubling the dose of budesonide in patients with inadequately controlled asthma (COMPACT trial) [Abstract]. European Respiratory Journal 2004;24(Suppl 48):126s. [Google Scholar]
Price 2006 {published data only}
- Price DB, Swern A, Tozzi CA, Philip G, Polos P. Effect of montelukast on lung function in asthma patients with allergic rhinitis: analysis from the COMPACT trial. Allergy 2006;61(6):737‐42. [DOI] [PubMed] [Google Scholar]
Pullerits 1999 {published data only}
- Pullerits T, Praks L, Skoogh BE, Ani R, Lotvall J. Randomized placebo‐controlled study comparing a leukotriene receptor antagonist and a nasal glucocorticoid in seasonal allergic rhinitis. American Journal of Respiratory & Critical Care Medicine 1999;159(6):1814‐8. [DOI] [PubMed] [Google Scholar]
Pullerits 2001 {published data only}
- Pullerits T, Praks L, Baker R, Ani R, Lotvall J. Comparison of nasal fluticasone propionate, montelukast, and combined montelukast+loratadine in allergic rhinitis [abstract]. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A193. [Google Scholar]
Pullerits 2002 {published data only}
- Pullerits T, Praks L, Ristioja V, Lotvall J. Comparison of a nasal glucocorticoid, antileukotriene, and a combination of antileukotriene and antihistamine in the treatment of seasonal allergic rhinitis. Journal of Allergy & Clinical Immunology 2002;109(6):949‐55. [DOI] [PubMed] [Google Scholar]
Qaqundah 2006 {published data only}
- Qaqundah PY, Sugerman RW, Ceruti E, Maspero JF, Kleha JF, Scott CA, et al. Efficacy and safety of fluticasone propionate hydrofluoroalkane inhalation aerosol in pre‐school‐age children with asthma: A randomized, double‐blind, placebo‐controlled study. Journal of Pediatrics 2006;149(5):663‐70. [DOI] [PubMed] [Google Scholar]
Rachelefsky 1997 {published data only}
- Rachelefsky G. Childhood asthma and allergic rhinitis: the role of leukotrienes. Journal of Pediatrics 1997;131:348‐55. [DOI] [PubMed] [Google Scholar]
Ragab 2001 {published data only}
- Ragab S, Parikh A, Darby YC, Scadding GK. An open audit of montelukast, a leukotriene receptor antagonist, in nasal polyposis associated with asthma. Clinical & Experimental Allergy 2001;31(9):1385‐91. [DOI] [PubMed] [Google Scholar]
Ramsay 1997 {published data only}
- Ramsay CF, Kan CI, Nieman RB, Wang J, Krieken JHJM, Willems LNA, et al. The effects of oral pranlukast on airway immunopathology and clinical parameters in patients with asthma. American Thoracic Society. 1997:Abs C21.
Ramsay 1998 {published data only}
- Ramsay CF, Kan CI, Sterk PJ, Barnes NC. Pranlukast improves spirometry and bronchial hyperresponsiveness (BHR) in patients with mild asthma. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A411. [Google Scholar]
Rand 2004 {published data only}
- Rand CS, Bilderbank A, Krishnan J, Riekert K, Schiller K, MIAMI Study Research Group. Adherence with oral montelukast sodium or fluticasone propionate in a clinical trial [Abstract]. Journal of Allergy and Clinical Immunology 2004;113(Suppl 2):s157. [Google Scholar]
Ratner 2003 {published data only}
- Ratner PH, Howland WC, Arastu R, Philpot EE, Klein KC, Baidoo CA, et al. Fluticasone propionate aqueous nasal spray provided significantly greater improvement in daytime and nighttime nasal symptoms of seasonal allergic rhinitis compared with montelukast.[comment]. Annals of Allergy Asthma & Immunology 2003;90(5):536‐42. [DOI] [PubMed] [Google Scholar]
Reiss 1996 {published data only}
- Reiss TF, Altman LC, Chervinsky P, Bewtra A, Stricker WE, Noonan GP, et al. Effects of montelukast (MK‐0476), a new potent cysteinyl leukotriene (LTD4) receptor antagonist, in patients with chronic asthma. Journal of Allergy & Clinical Immunology 1996;98:528‐34. [DOI] [PubMed] [Google Scholar]
Reiss 1997 {unpublished data only}
- Reiss TF, White R, Noonan G, Korenblat P, Hess J, Shingo S. Montelukast (MK‐0476) a cys LT1 receptor antagonist improves the signs and symptoms of asthma over one year of treatment. European Respiratory Journal 1997;10 Suppl(25):437. [Google Scholar]
Reiss 1997b {published data only}
- Reiss TF, et al. Montelukast improves asthma outcomes over a 3‐month treatment period. American Thoracic Society. 1997.
Reiss 1997c {published data only}
- Reiss TF, Sorkness CA, Stricker W, Botto A, Busse WW, Kundu S, et al. Effects of montelukast (MK‐0476), a potent cysteinyl leukotriene receptor antagonist, on bronchodilation in asthmatic subjects treated with and without inhaled corticosteroids. Thorax 1997;52(1):45‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reiss 1998 {published data only}
- Reiss TF, Chervinsky P, Dockhorn RJ, Shingo S, Seidenberg B, Edwards TB. Montelukast, a once‐daily leukotriene receptor antagonist, in the treatment of chronic asthma. Archives of Internal Medicine 1998;158:1213‐20. [DOI] [PubMed] [Google Scholar]
Reiss 2008 {published data only}
- Reiss TF, Lu S, Liu N. A randomized study comparing concomitant montelukast and loratadine with montelukast, loratadine and beclomethasone in patients with chronic asthma [Abstract]. Chest 2008;134(4):93001s. [DOI] [PubMed] [Google Scholar]
Riccioni 2001a {published data only}
- Riccioni G, Guagnano MT, Castronuovo M, Benedicts M, Della R. Zafirlukast versus budesonide on bronchial reactivity in subjects with mild‐persistent asthma. European Respiratory Journal 2001;18(Suppl 33):263s. [PubMed] [Google Scholar]
Riccioni 2002 {published data only}
- Riccioni G, Santilli F, Guagnano MT, Vecchia RD. Montelukast versus budesonide on quality of life in asthmatic subjects [abstract]. European Respiratory Society Annual Congress. 2002:P757.
Riccioni 2003a {published data only}
- Riccioni G, D'Orazio N, Castronuovo M, Menna V, Lambo MS, Stefano F, et al. Dosage tapering of inhalatory budesonide in subjects with mild to moderate persistent asthma treated with montelukast [Abstract]. European Respiratory Journal 2003;22(Suppl 45):Abstract No: P713. [Google Scholar]
Riccioni 2005 {published data only}
- Riccioni G, Della Vecchia R, Castronuovo M, Ilio C, D'Orazio N. Tapering dose of inhaled budesonide in subjects with mild‐to‐moderate persistent asthma treated with montelukast: A 16‐week single‐blind randomized study. Annals of Clinical & Laboratory Science 2005;35(3):285‐9. [PubMed] [Google Scholar]
Rickard 1999 {published data only}
- Rickard KA, Yancey S, Emmet AH, Kalberg CJ. Salmeterol compared to zafirlukast when added to inhaled corticosteroid therapy in patients with persistent asthma. European Respiratory Society. 1999:P839.
Rickard 2001 {published data only}
- Rickard K, Dorinsky PM, Knobil K, Pepsin P, Akveld ML. The salmeterol/fluticasone propionate combination 50/100µg bid is more effective than oral montelukast 10mg od as a first line therapy in mild and moderate asthmatics. European Respiratory Journal 2001;18(Suppl 33):262s. [Google Scholar]
Ringdal 1997 {published data only}
- Ringdal N, Whitney JG, Summerton L. Problems with inhaler technique and patient preference for oral therapy tablet zafirlukast vs. inhaled beclomethasone. European Respiratory Journal 1997;10 Suppl(25):437, abs P2806. [Google Scholar]
Ringdal 2003 {published data only}
- Ringdal N, Eliraz A, Pruzinec R, Weber HH, Mulder PG, Akveld M, et al. The salmeterol/fluticasone combination is more effective than fluticasone plus oral montelukast in asthma. Respiratory Medicine 2003;97(3):234‐41. [DOI] [PubMed] [Google Scholar]
Robinson 2001 {published data only}
- Robinson DS, Campbell D, Barnes PJ. Addition of leukotriene antagonists to therapy in chronic persistent asthma: a randomised double‐blind placebo‐controlled trial. Lancet 2001;357(9273):2007‐11. [DOI] [PubMed] [Google Scholar]
Rosenhall 2003 {published data only}
- Rosenhall L, Elvstrand A, Tilling B, Vinge I, Jemsby P, Stahl E, et al. One‐year safety and efficacy of budesonide/formoterol in a single inhaler (Symbicort Turbuhaler ) for the treatment of asthma. Respiratory Medicine 2003;97(6):702‐8. [DOI] [PubMed] [Google Scholar]
Rowe 2007 {published data only}
- Rowe BH, Wong E, Blitz S, Diner B, Mackey D, Ross S, et al. Adding Long‐acting beta‐agonists to Inhaled Corticosteroids after Discharge from the Emergency Department for Acute Asthma: A Randomized Controlled Trial. Academic Emergency Medicine 2007;14(10):833‐40. [DOI] [PubMed] [Google Scholar]
Ruggins 2003 {published data only}
- Ruggins N. Pragmatic trial of add‐on therapy in Paediatric Asthma. National Research Register 2003.
Sahn 1997 {published data only}
- Sahn SA, Galant S, Murray J, Bronsky E, Spector S, Faiferman I, et al. Pranlukast (Ultair) improves FEV in patients with asthma: results of a 12‐week multicenter study vs. nedocromil. American Thoracic Society. 1997:Abs C49.
Sano 2006 {published data only}
- Sano Y, Adachi M, Kiuchi T, Miyamoto T. Effects of nebulized sodium cromoglycate on adult patients with severe refractory asthma. Respiratory Medicine 2006;100(3):420‐33. [DOI] [PubMed] [Google Scholar]
Schneider 2008 {published data only}
- Schneider A, Wensing M, Biessecker K, Quinzler R, Kaufmann‐Kolle P, Szecsenyi J. Impact of quality circles for improvement of asthma care: Results of a randomized controlled trial. Journal of Evaluation in Clinical Practice 2008;14(2):185‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schuh 2009 {published data only}
- Schuh S, Willan AR, Stephens D, Dick PT, Coates A. Can montelukast shorten prednisolone therapy in children with mild to moderate acute asthma? A randomized controlled trial. Journal of Pediatrics 2009;155(6):795‐800. [DOI] [PubMed] [Google Scholar]
Schwartz 1995 {published data only}
- Schwartz HJ, Petty T, Reed R, Dube LM, Swanson LJ, Zileuton Study Group. The comparative effects of zileuton, a 5‐lipoxygenase inhibitor, vs. theophylline in patients with moderate asthma: results from a 13‐week multicenter trial. ALA/ATS. 1995.
SD‐004‐0216 {published data only}
- Oxis Turbuhaler® (formoterol), Accolate® (zafirlukast) or placebo as add on treatment to Pulmicort Turbuhaler® (budesonide) in asthmatic patients on inhaled steroids. AstraZeneca Clinical Trials 2000.
Shah 2003 {published data only}
- Shah AR. Which Is More Steroid Sparing in Persistent Bronchial Asthma: Montelukast Or Theophylline? [Abstract]. Chest 2003;124(4):107. [Google Scholar]
Shah 2004 {published data only}
- Shah A, Solanki R, Shah K. Effect of add on therapy compared with doubling steroid inhalation in bronchial asthma [Abstract]. Thorax 2004;59(Suppl 2):ii70. [Google Scholar]
Shah 2006 {published data only}
- Shah AR, Sharples LD, Solanki RN, Shah KV. Double‐blind, randomised, controlled trial assessing controller medications in asthma. Respiration 2006;73(4):449‐56. [DOI] [PubMed] [Google Scholar]
Sheth 2002 {published data only}
- Sheth K, Borker R, Emmett A, Rickard K, Dorinsky P. Cost‐effectiveness comparison of salmeterol/fluticasone propionate versus montelukast in the treatment of adults with persistent asthma.. Pharmacoeconomics 2002;20(13):909‐18. [DOI] [PubMed] [Google Scholar]
Shimoda 2005 {published data only}
- Shimoda T, Kishikawa R, Shoji S, Nishima S. The efficacy of anti‐inflammatory treatment in mild intermittent bronchial asthma [Abstract]. Journal of Allergy & Clinical Immunology 2005;115(Suppl 2):S1. [Google Scholar]
Shingo 2001 {unpublished data only}
- Shingo S, Zhang J, Noonan N, Reiss TF, Leff JA. A standardized composite clinical score allows safe tapering of inhaled corticosteroids in an asthma clinical trial.. Unpublished data (Personal Communication:Theodore Reiss June 2001) 2001.
Shoji 1999 {published data only}
- Shoji T, Yoshida S, Sakamoto H, Hasegawa H, Nakagawa H, Amayasu H. Anti‐inflammatory effect of roxithromycin in patients with aspirin‐intolerant asthma. Clinical & Experimental Allergy 1999;9(7):950‐6. [DOI] [PubMed] [Google Scholar]
Simons 2001 {published data only}
- Simons FER, Villa JR, Lee BW, Teper AM, Lyttle B, Aristizabal G, et al. Montelukast added to budesonide in children with persistent asthma: a randomised, double‐blind, crossover study. Journal of Pediatrics 2001;138(5):694‐8. [DOI] [PubMed] [Google Scholar]
Simpson 2004 {published data only}
- Simpson MD, Burton DL, Burton MA, Gissing PM, Bowman SL. Pharmaceutical Care: Impact on Asthma Medication Use. Journal of Pharmacy Practice & Research 2004;34(1):26‐9. [Google Scholar]
Sims 2003 {published data only}
- Sims EJ, Jackson CM, Lipworth BJ. Add‐on therapy with montelukast or formoterol in patients with the glycine‐16 beta2‐receptor genotype. British Journal of Clinical Pharmacology 2003;56(1):104‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sims 2008 {published data only}
- Sims E, Freeman D, Kemp L, Musgrave S, Juniper L, Gilbert R, et al. Should guidelines be revised for add on therapy in asthma? A 2 year randomized pragmatic equivalence trail of leukotriene antagonist (LTRAs) and long acting beta agonists (LABAs) with inhaled corticosteroids (ICS) in primary care [Abstract]. European Respiratory Society Annual Congress. 2008:E233.
Smith 1993 {published data only}
- Smith LJ, Glass M, Minkwitz MC. Inhibition of leukotriene D4‐induced bronchoconstriction in subjects with asthma: a concentration‐effect study of ICI 204,219. Clinical Pharmacology & Therapeutics 1993;54(4):430‐6. [DOI] [PubMed] [Google Scholar]
Smith 1997 {published data only}
- Smith LJ, Hanby LA, Simonson MS, Simonson SG. Effect of zafirlukast on leukotriene D4 (LTD4) ‐ induced bronchoconstriction in asthmatic patients receiving inhaled corticosteroids [abstract]. European Respiratory Journal 1997;10(Suppl 25):437S. [Google Scholar]
Smith 1998 {published data only}
- Smith LJ, Hanby LA, Lavins BJ, Simonson SG. A single dose of zafirlukast reduces LTD4‐induced bronchoconstriction in patients on maintenance inhaled corticosteroid therapy. Annals of Allergy, Asthma, & Immunology 1998;81:43‐9. [DOI] [PubMed] [Google Scholar]
Smugar 2009 {published data only}
- Smugar SS, Fogel R, Aristizabal G, Rosario N, Loeys T, Gaile S, et al. Effect of montelukast or salmeterol added to inhaled fluticasone on response to albuterol in children with exercise‐induced bronchoconstriction [Abstract]. European Respiratory Society Annual Congress. 2009:P1221.
Smugar 2009a {published data only}
- Smugar SS, Fogel R, Rosario N, Aristizabal G, Loeys T, Gaile S, et al. Attenuation of exercise‐induced bronchoconstriction with montelukast or salmeterol added to inhaled fluticasone in children [Abstract]. European Respiratory Society Annual Congress. 2009:P1217.
Spahn 1996a {published data only}
- Spahn JD, Szefler SJ. The etiology and control of bronchial hyperresponsiveness in children. Current Opinion in Pediatrics 1996;8:591‐6. [DOI] [PubMed] [Google Scholar]
Spector 1992 {published data only}
- Spector SL, Glass M, Minkwitz MC, ICI Asthma Trial Group. The effect of six weeks of therapy with oral doses of ICI 204,219 in asthmatics. American Review of Respiratory Disease 1992;145(4):A16. [Google Scholar]
Spector 1994 {published data only}
- Spector SL, Smith LJ, Glass M. Effects of 6 weeks of therapy with oral doses of ICI 204,219, a leukotriene D4 antagonist, in subjects with bronchial asthma. American Journal of Respiratory & Critical Care Medicine 1994;150:618‐23. [DOI] [PubMed] [Google Scholar]
Spector 1995 {published data only}
- Spector S, Miller CJ, Glass M. 13‐week dose‐response study with Accolate (zafirlukast) in patients with mild to moderate asthma. American Journal of Respiratory & Critical Care Medicine 1995;151(4):A379. [Google Scholar]
Spector 1996 {published data only}
- Spector SL. Management of asthma with zafirlukast. Drugs 1996;52 Suppl(6):36‐46. [DOI] [PubMed] [Google Scholar]
Stanford 2002 {published data only}
- Stanford RH, Borker R, Dorinsky P, Pepsin P, Kalberg C, Emmett A, et al. The cost and efficacy of fluticasone propionate/salmeterol combination versus montelukast in the treatment of adults with persistent asthma. Chest. 2002:P422.
Stensrud 2006 {published data only}
- Stensrud T, Berntsen S, Carlsen KH. Humidity influences exercise capacity in subjects with exercise‐induced bronchoconstriction (EIB). Respiratory Medicine 2006;100(9):1633‐41. [DOI] [PubMed] [Google Scholar]
Stevenson 2005 {published data only}
- Stevenson DD, Mehra PK, White AA, Gupta S, Woessner KM, Simon RA. Failure of tacrolimus to prevent aspirin‐induced respiratory reactions in patients with aspirin‐exacerbated respiratory disease. Journal of Allergy & Clinical Immunology 2005;116(4):755‐60. [DOI] [PubMed] [Google Scholar]
Sthoeger 2007 {published data only}
- Sthoeger ZM, Eliraz A, Asher I, Berkman N, Elbirt D. The beneficial effects of Xolair (Omalizumab) as add‐on therapy in patients with severe persistent asthma who are inadequately controlled despite best available treatment (GINA 2002 step IV ‐ The Israeli arm of the INNOVATE study). Israel Medical Association Journal 2007;9(6):472‐5. [PubMed] [Google Scholar]
Storms 2001 {published data only}
- Storms W, Michele TM, Knorr B, Noonan G, Shapiro G, Zhang J, et al. Clinical safety and tolerability of montelukast, a leukotriene receptor antagonist, in controlled clinical trials in patients aged > or = 6 years. Clinical & Experimental Allergy 2001;31(1):77‐87. [DOI] [PubMed] [Google Scholar]
Storms 2004 {published data only}
- Storms W, Chervinsky P, Ghannam AF, Bird S, Hustad CM, Edelman JM, et al. A comparison of the effects of oral montelukast and inhaled salmeterol on response to rescue bronchodilation after challenge. Respiratory Medicine 2004;98(11):1051‐62. [DOI] [PubMed] [Google Scholar]
Strauch 2003 {published data only}
- Strauch E, Moske O, Thoma S, Van'S Gravesande KS, Ihorst G, Brandis M, et al. A randomized controlled trial on the effect of montelukast on sputum eosinophil cationic protein in children with corticosteroid‐dependent asthma. Pediatric Research 2003;54(2):198‐203. [DOI] [PubMed] [Google Scholar]
Strunk 2003 {published data only}
- Strunk RC, Szefler SJ, Phillips BR, Zeiger RS, Chinchilli VM, Larsen G, et al. Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. Journal of Allergy & Clinical Immunology 2003;112(5):883‐92. [DOI] [PubMed] [Google Scholar]
Strunk 2008 {published data only}
- Strunk RC, Bacharier LB, Phillips BR, Szefler SJ, Zeiger RS, Chinchilli VM, et al. Azithromycin or montelukast as inhaled corticosteroid‐sparing agents in moderate‐to‐severe childhood asthma study. The Journal of Allergy and Clinical Immunology 2008;122(6):1138‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sugihara 2010 {published data only}
- Sugihara N, Kanada S, Haida M, Ichinose M, Adachi M, Hosoe M, et al. 24‐h bronchodilator efficacy of single doses of indacaterol in Japanese patients with asthma: A comparison with placebo and salmeterol. Respiratory Medicine 2010;104(11):1629‐37. [DOI] [PubMed] [Google Scholar]
Suguro 1997 {published data only}
- Suguro H, Majima T, Ichimura K, Hashimoto N, Koyama S, Horie T. Effect of a leukotriene antagonist, pranlukast hydrate, on exercise‐induced bronchoconstriction. American Thoracic Society. 1997:Abs C49.
Suissa 1997 {published data only}
- Suissa S, Dennis R, Ernst P, Sheehy O, Wood‐Dauphinee S. Effectiveness of the leukotriene receptor antagonist zafirlukast for mild‐to‐moderate asthma: a randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 1997;126(3):177‐83. [DOI] [PubMed] [Google Scholar]
Sutherland 2010 {published data only}
- Sutherland ER, Camargo CA, Busse WW, Meltzer EO, Orgega HG, Yancey SW, et al. Comparative effect of body mass index on response to asthma controller therapy. Allergy and Asthma Proceedings 2010;31:20‐5. [DOI] [PubMed] [Google Scholar]
Suzuki 1997 {published data only}
- Suzuki N, Kudo K, Sano Y, Adachi M, Kanazawa M, Kudo S, et al. Efficacy of oral pranlukast, a leukotriene‐receptor antagonist, in the treatment of asthma: an open study in Tokyo. American Thoracic Society. 1997:Abs C49.
Svensson 1994 {published data only}
- Svensson C, Greiff L, Andersson M, Alkner U, Persson CGA. Bradykinin‐, leukotriene D4‐, and histamine‐induced mucosal exudation of plasma in human airways in vivo. Abbott Laboratories 1994.
Swern 2008 {published data only}
- Swern AS, Tozzi CA, Knorr B, Bisgaard H. Predicting an asthma exacerbation in children 2 to 5 years of age. Annals of Allergy Asthma and Immunology 2008;101(6):626‐30. [DOI] [PubMed] [Google Scholar]
Swernson 2003 {published data only}
- Swenson C, Fell S, Liu LY, Busse W. Effect of montelukast (mont) or placebo (PL) on asthma exacerbations and markers of airway inflammation with inhaled corticosteroid (ICS) reduction [abstract]. American Thoracic Society 99th International Conference 2003;B034:Poster H47. [Google Scholar]
Tamaoki 1997 {published data only}
- Tamaoki J, Kondo M, Sakai N, Nakata J, Takemura H, Nagai A, et al. Leukotriene antagonist prevents exacerbation of asthma during reduction of high‐dose inhaled corticosteroid. American Journal of Respiratory & Critical Care Medicine 1997;155:1235‐40. [DOI] [PubMed] [Google Scholar]
Tan 2006 {published data only}
- Tan WC, Lamm CJ, Chen YZ, O'Byrne PM, Pedersen S, Busse WW, et al. Effectiveness of early budesonide intervention in Caucasian versus Asian patients with asthma: 3‐Year results of the START study. Respirology 2006;11(6):767‐75. [DOI] [PubMed] [Google Scholar]
Tashkin 1998 {published data only}
- Tashkin DP, Minkwitz MC, Bonuccelli CM. Zafirlukast (Accolate) treatment results in better asthma control in patients with more moderate disease. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A410. [Google Scholar]
Teper 2009 {published data only}
- Teper A, Kofman C, Zaragoza S, Rodriguez V, Lubovich S, Eguiguren C, et al. Comparison of fluticasone propionate + salmeterol (FP+S) and montelukast (MK) on bronchial reactivity (BR), pulmonary function (PF) and clinical outcome in children with mild asthma [Abstract]. European Respiratory Society Annual Congress. 2009:[P1215].
Terzano 2001 {published data only}
- Terzano C, Allegra L, Barkai L, Cremonesi G. Beclomethasone dipropionate versus budesonide inhalation suspension in children with mild to moderate persistent asthma. European Review for Medical & Pharmacological Sciences 2001;5(1):17‐24. [PubMed] [Google Scholar]
Thoma 2002 {published data only}
- Thoma S. Anti‐inflammatory effect of montelukast on corticosteroid‐dependent children with asthma [Dissertation] [[Reduktion der bronchialen Inflammation bei mildem bis mittelschwerem Asthma bronchiale im Kindesalter unter Additivtherapie mit Montelukast und inhalativem Budesonid]]. Universitat Freiburg 2002.
Todi 2010 {published data only}
- Todi VK, Lodha R, Kabra SK. Effect of addition of single dose of oral montelukast to standard treatment in acute moderate to severe asthma in children between 5 and 15 years of age: A randomised, double‐blind, placebo controlled trial. Archives of Disease in Childhood 2010;95(7):540‐3. [DOI] [PubMed] [Google Scholar]
Tognella 2004 {published data only}
- Tognella S, Micheletto C, Visconti MP, Dal Negro RW. Additive Effects of Montelukast on Bronchial Hyperresponsiveness to MCh and LTE4 Urine Levels in Mild‐persistent Atopic Asthmatics Assuming ICS [Abstract]. Chest 2004;126(Suppl 4):814S. [Google Scholar]
Tohda 2002 {published and unpublished data}
- Tohda Y, Fujimura M, Taniguchi H, Takagi K, Igarashi T, Yasuhara H, et al. Leukotriene receptor antagonist, montelukast, can reduce the need for inhaled steroid while maintaining the clinical stability of asthmatic patients. Clinical & Experimental Allergy 2002;32:1180‐6. [DOI] [PubMed] [Google Scholar]
Tomari 2001 {published data only}
- Tomari S, Shimoda T, Kawano T, Mitsuta K, Obase Y, Fukushima C, et al. Effects of pranlukast, a cysteinyl leukotriene receptor 1 antagonist, combined with inhaled beclomethasone in patients with moderate or severe asthma. Annals of Allergy, Asthma & Immunology i 2001;87:156‐61. [DOI] [PubMed] [Google Scholar]
Tomita 1999 {published data only}
- Tomita K, Hashimoto K, Matsumoto S, Nakamoto N, Tokuyasu H, Yamasaki A, et al. [Pranlukast allows reduction of inhaled steroid dose without deterioration in lung function in adult asthmatics]. Article in Japanese. Arerugi ‐ Japanese Journal of Allergology 1999;48:459‐65. [PubMed] [Google Scholar]
Tonelli 2003 {published data only}
- Tonelli M, Zingoni M, Bacci E, Dente FL, Franco A, Giannini D, et al. Short‐term effect of the addition of leukotriene receptor antagonists to the current therapy in severe asthmatics. Pulmonary Pharmacology & Therapeutics 2003;16(4):237‐40. [DOI] [PubMed] [Google Scholar]
Townley 1995 {published data only}
- Townley R, Glass M, Minkwitz MC. 6‐week, dose‐escalation study with Accolate (zafirlukast) in patients with mild to moderate asthma. American Journal of Respiratory & Critical Care Medicine 1995;151(4):A379. [DOI] [PubMed] [Google Scholar]
Trofor 2002 {published data only}
- Trofor A, Rascarachi G, Popa E, Chiselita I. Clinical benefits of montelukast sodium treatment in chronic adult asthma. Revista Medico‐Chirurgicala a Societatii De Medici Si Naturalisti Din Iasi 2002;107(2):298‐302. [PubMed] [Google Scholar]
Tsai 2010 {published data only}
- Tsai TC, Lu JH, Chen SJ, Tang RB. Clinical efficacy of house dust mite‐specific immunotherapy in asthmatic children. Pediatrics and Neonatology 2010;51(1):14‐8. [DOI] [PubMed] [Google Scholar]
Tsuchida 2005 {published data only}
- Tsuchida T, Matsuse H, Machida I, Kondo Y, Saeki S, Tomari S, et al. Evaluation of theophylline or pranlukast, a cysteinyl leukotriene receptor 1 antagonist, as add‐on therapy in uncontrolled asthmatic patients with a medium dose of inhaled corticosteroids. Allergy & Asthma Proceedings 2005;26(4):287‐91. [PubMed] [Google Scholar]
Tug 2007 {published data only}
- Tug T, Godekmerdan A, Sari N, Karatas F, Erdem ES. Effects of supportive treatment such as antioxidant or leukotriene receptor antagonist drugs on inflammatory and respiratory parameters in asthma patients. Clinical Pharmacology & Therapeutics 2007;81(3):371‐6. [DOI] [PubMed] [Google Scholar]
Tukiainen 2002 {published data only}
- Tukiainen H, Rytila P, Hamalainen K M, Silvasti M S L, Keski‐Karhu J. Safety, tolerability and acceptability of two dry powder inhalers in the administration of budesonide in steroid‐treated asthmatic patients. Respiratory Medicine 2002;96(4):221‐9. [DOI] [PubMed] [Google Scholar]
Uh 2007 {published data only}
- Uh ST, Jung KS, Lee YC, Shim JJ, Park SK, Lee YS, et al. A comparison of the clinical efficacy and safety of salmeterol/fluticasone propionate versus leukotriene antagonist in moderate to severe asthmatics ‐ a sub‐analysis of the SUCCESS Study [Abstract]. Respirology 2007;12(Suppl 4):A148. [Google Scholar]
Ulrik 2009 {published data only}
- Ulrik CS, Diamant Z. Effect of add‐on montelukast to inhaled corticosteroids on excessive airway narrowing in adult asthmatics [Abstract]. European Respiratory Society Annual Congress. 2009:P1962.
Ulrik 2009a {published data only}
- Ulrik CS, Diamant Z. Effect of montelukast on excessive airway narrowing response to methacholine in adult asthmatic patients not on controller therapy. Allergy and Asthma Proceedings 2009;30(1):64‐8. [DOI] [PubMed] [Google Scholar]
Ulrik 2010 {published data only}
- Ulrik CS, Diamant Z. Add‐on montelukast to inhaled corticosteroids protects against excessive airway narrowing. Clinical and Experimental Allergy 2010;40(4):576‐81. [DOI] [PubMed] [Google Scholar]
Van Adelsberg 2005 {published data only}
- Adelsberg J, Moy J, Wei LX, Tozzi CA, Knorr B, Reiss TF. Safety, tolerability, and exploratory efficacy of montelukast in 6‐ To 24‐month‐old patients with asthma. Current Medical Research & Opinion 2005;21(6):971‐9. [DOI] [PubMed] [Google Scholar]
Van Der Meer 2009 {published data only}
- Meer V, Bakker MJ, Hout WB, Rabe KF, Sterk PJ, Kievit J, et al. Internet‐based self‐management plus education compared with usual care in asthma: A randomized trial. Annals of Internal Medicine 2009;151(2):110‐20. [DOI] [PubMed] [Google Scholar]
Vaquerizo 2003 {published data only}
- Vaquerizo MJ, Casan P, Castillo J, Perpina M, Sanchis J, Sobradillo V, et al. Effect of montelukast added to inhaled budesonide on control of mild to moderate asthma. Thorax 2003;58:204‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vastagh 2003 {published data only}
- Vastagh E, Kuna P, Calistru P, Bogdan MA. Efficacy and safety of inhaled budesonide delivered once or twice daily via HFA‐134a in mild to moderate persistent asthma in adult patients. Comparison with budesonide CFC. Respiratory Medicine 2003;97(Suppl D):S20‐S28. [DOI] [PubMed] [Google Scholar]
Verhoeven 2001 {published data only}
- Verhoeven GT, Garrelds IM, Hoogsteden HC, Zijlstra FJ. Effects of fluticasone propionate inhalation on levels of arachidonic acid metabolites in patients with chronic obstructive lung pulmonary disease. Mediators of Inflammation 2001;10(1):21‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verini 2007 {published data only}
- Verini M, Peroni DG, Piacentini GL, Nicodemo A, Rossi N, Bodini A, et al. Comparison of add‐on therapy to inhaled fluticasone propionate in children with asthma: residual volume and exhaled nitric oxide as outcome measures. Allergy & Asthma Proceedings 2007;28(6):691‐4. [DOI] [PubMed] [Google Scholar]
Vermeulen 2007 {published data only}
- Vermeulen JH, Gyurkovits K, Rauer H, Engelstatter R. Randomized comparison of the efficacy and safety of ciclesonide and budesonide in adolescents with severe asthma. Respiratory Medicine 2007;101(10):2182‐91. [DOI] [PubMed] [Google Scholar]
Vethanayagam 2002 {published data only}
- Vethanayagam D, Leigh R, Yoshida M, Watson RM, Rerecich T, Killian K, et al. Effects of montelukast and budesonide alone and together on allergen‐induced airway inflammation in asthma [abstract]. American Journal of Respiratory and Critical Care Medicine 2002;165(8 Suppl):A216. [DOI] [PubMed] [Google Scholar]
Vidal 2001 {published data only}
- Vidal C, Fernandez‐Ovide E, Pineiro J, Nuñez R, González‐Quintela A. Comparison of montelukast versus budesonide in the treatment of exercise‐induced bronchoconstriction. Annals of Allergy, Asthma, & Immunology 2001;86(6):655‐8. [DOI] [PubMed] [Google Scholar]
Vidal 2001a {published data only}
- Vidal C, Fernandez‐Ovide E, Pineiro J Nuñez R, González‐Quintela A. Comparison of montelukast versus budesonide in the treatment of exercise‐induced bronchoconstriction. Annals of Allergy, Asthma, & Immunology 2001;86(6):655‐8. [DOI] [PubMed] [Google Scholar]
Virchow 1997 {published data only}
- Virchow JC, Hassall SM, Summerton L, Harris A. Zafirlukast (Accolate) reduces the impact of asthma in patients taking inhaled corticosteroids. Journal of Allergy & Clinical Immunology 1997;9 Suppl(4):243, Abs. 888. [Google Scholar]
Virchow 1997a {published data only}
- Virchow CJ, Hassall SM, Summerton L, Harris A. Improved asthma control over 6 weeks with zafirlukast in patients on high dose inhaled corticosteroids [abstract]. European Respiratory Journal 1997;10(Suppl 25):437S. [Google Scholar]
Virchow 1997b {published data only}
- Virchow CJ, Hassall SM, Summerton L, Klim J, Harris A. Reduction of asthma exacerbations with zafirlukast in patients on inhaled corticosteroids [abstract]. European Respiratory Journal 1997;10(Suppl 25):420S. [Google Scholar]
Virnig 2008 {published data only}
- Virnig C, Gern JE. Attenuation of the september epidemic of asthma exacerbations in children: A randomized, controlled trial of montelukast added to usual therapy. Pediatrics 2008;122(Suppl 4):S218‐S219. [DOI] [PubMed] [Google Scholar]
Volovitz 1999 {published data only}
- Volovitz B, Tabachnik E, Nussinovitch M, Shtaif B, Blau H, Gil‐ad I, et al. Montelukast, a leukotriene receptor antagonist, reduces the concentration of leukotrienes in the respiratory tract of children with persistent asthma. Journal of Allergy & Clinical Immunology 1999;104:1162‐7. [DOI] [PubMed] [Google Scholar]
Von Berg 2002 {published data only}
- Berg A, Albrecht B, Darlath W, Vo H W, Berdel D. Intraindividual, randomised, double‐blind comparison between sodium cromoglycate and reproterol to assess the protective effect of the single drugs and their combination in children with exercise‐induced asthma. Allergologie 2002;25(11):557‐64. [Google Scholar]
Wada 2000 {published data only}
- Wada K, Minoguchi K, Kohno Y, Oda N, Matsuura T, Kawazu K, et al. Effect of a leukotriene receptor antagonist, pranlukast hydrate, on airway inflammation and airway hyperresponsiveness in patients with moderate to severe asthma. Allergology International 2000;49(1):63‐8. [Google Scholar]
Wada 2009 {published data only}
- Wada M, Nagata S, Kudo T, Shimizu T, Yamashiro Y. Effect of suplatast tosilate on antileukotriene non‐responders with mild‐to‐moderate persistent asthma. Allergology International 2009;58(3):389‐93. [DOI] [PubMed] [Google Scholar]
Wahedna 1999 {published data only}
- Wahedna I, Wisniewski AS, Tattersfield AE. Effect of RG 12525, an oral leukotriene D4 antagonist, on the airway response to inhaled leukotriene D4 in subjects with mild asthma. British Journal of Clinical Pharmacology 1991;32:512‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Warren 2003 {published data only}
- Warren L. Management of Asthma in School‐aged Children On Therapy ‐ MASCOT, Contract signed, not started. National Coordinating Centre for Health Technology Assessment (NCCHTA). http://www.hta.ac.uk/project/1599.asp,Ongoing.
Wasserman 2006 {published data only}
- Wasserman RL, Baker JW, Kim KT, Blake KV, Scott CA, Wu W, et al. Efficacy and safety of inhaled fluticasone propionate chlorofluorocarbon in 2‐ to 4‐year‐old patients with asthma: Results of a double‐blind, placebo‐controlled study. Annals of Allergy Asthma & Immunology 2006;96(6):808‐18. [DOI] [PubMed] [Google Scholar]
Wechsler 1998 {published data only}
- Wechsler ME, Garpestad E, Flier SR, Kocker O, Weiland DA, Polito AJ, et al. Pulmonary infiltrates, eosinophilia, and cardiomyopathy following corticosteroid withdrawal in patients with asthma receiving zafirlukast. JAMA 1998;279(6):455‐7. [DOI] [PubMed] [Google Scholar]
Weinberg 1998 {published data only}
- Weinberg EG, Summerton L, Harris A. Assessment of preference for oral zafirlukast vs inhaled beclomethasone in adolescent asthmatics. ERS Annual Congress. 1998; Vol. 12, issue 28:abs P0333.
Weiss 2010 {published data only}
- Weiss KB, Gern JE, Johnston NW, Sears MR, Jones CA, Jia G, et al. The back to school asthma study: The effect of montelukast on asthma burden when initiated prophylactically at the start of the school year [Abstract]. Annals of Allergy, Asthma and Immunology 2010;105(2):174‐81. [DOI] [PubMed] [Google Scholar]
Welch 1994 {published data only}
- Welch MJ, Nelson HS, Paull BR, Smith JA, Feiss G, Tobey RE. Effect of RG 12525, a new leukotriene antagonist, on pulmonary function of asthmatic adults. Annals of Allergy 1994;72:348‐52. [PubMed] [Google Scholar]
Wen 2008 {published data only}
- Wen CJ, Lai ST, Deng YC, Hung CH. Plasma levels of leukotriene E4 and 9alpha, 11 beta‐PGF2 in asthmatic young children during exacerbation and convalescence. Journal of Medical Sciences 2008;28(6):233‐7. [Google Scholar]
Wenzel 1994 {published data only}
- Wenzel S, Cohn J, Trudeau J, Wilson W, Martin R, Westcott J. Zileuton (Leutrol) decreases urine LTE4, BALF LTB4 and improves lung function in nocturnal asthmatics. Abbott Laboratories 1994.
Wenzel 1995 {published data only}
- Wenzel SE, Trudeau JB, Kaminsky DA, Cohn J, Martin RJ, Westcott JY. Effect of 5‐lipoxygenase inhibition on bronchoconstriction and airway inflammation in nocturnal asthma. American Journal of Respiratory & Critical Care Medicine 1995;152(3):897‐905. [DOI] [PubMed] [Google Scholar]
Wenzel 1997 {published data only}
- Wenzel S, Chervinsky P, Kerwin E, Silvers W, Faiferman I, Dubb J. Oral Pranlukast (Ultair) vs. inhaled beclomethasone: results of a 12‐week trial in patients with asthma. American Journal of Respiratory & Critical Care Medicine 1997;155:A203. [Google Scholar]
Westbroek 1997 {published data only}
- Westbroek J, Pasma HR. The effect of inhaled fluticasone propionate (FP) 100 µg bd compared with oral zafirlukast 20 mg bd on bronchial hyperresponsiveness in mild to moderate asthmatics [abstract]. European Respiratory Journal 1997;10(Suppl 25):243S. [Google Scholar]
Westbroek 1998 {published data only}
- Westbroek J, Pasma HR, James MH, Thwaites RMA. Inhaled fluticasone propionate and oral zafirlukast in moderate asthma: a clinical and cost comparison [abstract]. American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A416. [Google Scholar]
Westbroek 2000 {published data only}
- Westbroek J, Pasma HR. Effects of 2 weeks of treatment with fluticasone propionate 100 mcg b.d. by comparison with zafirlukast 20 mg b.d. on bronchial hyper‐responsiveness in patients with mild to moderate asthma. Respiratory Medicine 2000;94(2):112‐8. [DOI] [PubMed] [Google Scholar]
Williams 2001 {published and unpublished data}
- Williams B, Noonan G, Reiss TF, Knorr B, Guerra J, White R, et al. Long‐term asthma control with oral montelukast and inhaled beclomethasone. Clinical & Experimental Allergy 2001;31:1‐10. [DOI] [PubMed] [Google Scholar]
Wilson 1999 {published data only}
- Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. A comparison of salmeterol and montelukast as second‐line therapy in asthmatic patients receiving inhaled corticosteroids. European Respiratory Society. 1999:P3486.
Wilson 2000 {published data only}
- Wilson AM, Orr LC, Sims EJ, Dempsey OJ. Antiasthmatic effects of mediator block versus topical corticosteroids in allergic rhinitis and asthma. American Journal of Respiratory & Critical Care Medicine 2000;162(4 part 1):1297‐301. [DOI] [PubMed] [Google Scholar]
Wilson 2001 {published data only}
- Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. Evaluation of salmeterol or montelukast as second‐line therapy for asthma not controlled with inhaled corticosteroids. Chest 2001;119(4):1021‐6. [DOI] [PubMed] [Google Scholar]
Wilson 2001a {published data only}
- Wilson AM, Orr LC, Sims EJ, Lipworth BJ. Effects of monotherapy with intra‐nasal corticosteroid or combined oral histamine and leukotriene receptor antagonists in seasonal allergic rhinitis. Clinical & Experimental Allergy 2001;31(1):61‐8. [PubMed] [Google Scholar]
Wilson 2001b {published data only}
- Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. A comparison of topical budesonide and oral montelukast in seasonal allergic rhinitis and asthma. Clinical & Experimental Allergy 2001;31(4):616‐24. [DOI] [PubMed] [Google Scholar]
Wilson 2001c {published data only}
- Wilson AM, Sims EJ, Orr LC, Coutie WJ, White PS, Gardiner Q, et al. Effects of topical corticosteroid and combined mediator blockade on domiciliary and laboratory measurements of nasal function in seasonal allergic rhinitis. Annals of Allergy, Asthma, & Immunology 2001;87(4):344‐9. [DOI] [PubMed] [Google Scholar]
Wilson 2004 {published data only}
- Wilson A. Are antihistamines useful in managing asthma?. Advanced Studies in Medicine 2004;4(7A):S514‐S519. [Google Scholar]
Wilson 2010 {published data only}
- Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. American Journal of Respiratory and Critical Care Medicine 2010;181(6):566‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2010a {published data only}
- Wilson ECF, Price D, Musgrave SD, Sims EJ, Shepstone L, Murdoch J, et al. Cost effectiveness of leukotriene receptor antagonists versus long‐acting beta‐2 agonists as add‐on therapy to inhaled corticosteroids for asthma: A pragmatic trial. Pharmacoeconomics 2010;28(7):597‐608. [DOI] [PubMed] [Google Scholar]
Wise 2009 {published data only}
- Wise RA, Bartlett SJ, Brown ED, Castro M, Cohen R, Holbrook JT, et al. Randomized trial of the effect of drug presentation on asthma outcomes: The American Lung Association Asthma Clinical Research Centers. Journal of Allergy and Clinical Immunology 2010;124(3):436‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xiang 2001 {published data only}
- Xiang X D, Zhou R, Chen Y. Influence of zafirlukast on pulmonary functions and quality of life in patients with asthma. Bulletin of Hunan Medical University 2001;26(3):251‐3. [PubMed] [Google Scholar]
Yaldiz 2000 {published data only}
- Yaldiz E, Özkan G, Özdemir A, Gür A, Çamsari G. The role of montelukast in the reduction of high dose inhaled steroid in asthmatic patients. European Respiratory Journal 2000;16(Supp 31):457s. [Google Scholar]
Yamamoto 1994 {published data only}
- Yamamoto H, Nagata M, Kuramitsu K, Tabe K, Kiuchi H, Sakamoto Y, et al. Inhibition of analgesic‐induced asthma by leukotriene receptor antagonist ONO‐1078. American Journal of Respiratory & Critical Care Medicine 1994;150:254‐7. [DOI] [PubMed] [Google Scholar]
Yildirim 2001 {published data only}
- Yildirim Z, Ozlu T, Bulbul Y. Montelukast and budesonide vs double dose budesonide in moderate asthma. European Respiratory Journal 2001;18(Supp 33):261s. [Google Scholar]
Yildirim 2004 {published data only}
- Yildirim Z, Ozlu T, Bulbul Y, Bayram H. Addition of montelukast versus double dose of inhaled budesonide in moderate persistent asthma. Respirology 2004;9(2):243‐8. [DOI] [PubMed] [Google Scholar]
Yoo 2001 {published data only}
- Yoo SH, Park SH, Song JS, Kang KH, Park CS, Yoo JH, et al. Representing Korea Pranlukast Study Group. Clinical effects of pranlukast, an oral leukotriene receptor antagonist, in mild‐to‐moderate asthma: a 4‐week randomized multicentre controlled trial. Respirology 2001;6(1):15‐21. [DOI] [PubMed] [Google Scholar]
Yoshida 2000 {published data only}
- Yoshida S, Sakamoto H, Ishizaki Y, Onuma K, Shoji T, Nakagawa H, et al. Efficacy of leukotriene receptor antagonist in bronchial hyperresponsiveness and hypersensitivity to analgesic in aspirin‐intolerant asthma. Clinical & Experimental Allergy 2000;30(1):64‐70. [DOI] [PubMed] [Google Scholar]
Yoshida 2002 {published data only}
- Yoshida M, Vethanayagam D, Leigh R, Matsumoto K, Watson RM, Reerich T, et al. A comparison montelukast and budesonide effects on interleukin‐10 profiles in peripheral blood lymphocytes. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A217. [Google Scholar]
Zeiger 2004 {published data only}
- Zeiger RS, Baker JW, Kaplan MS, Pearlman DS, Schatz M, Bird S, et al. Variability of symptoms in mild persistent asthma: baseline data from the MIAMI study. Respiratory Medicine 2004;98(9):898‐905. [DOI] [PubMed] [Google Scholar]
Zeiger 2005a {published data only}
- Zeiger RS, Edelman JM, Bird SR, Kaplan MS, Schatz M, Pearlman DS, et al. Short‐term and long‐term asthma control in patients with mild persistent asthma receiving montelukast or fluticasone: a randomised controlled trial [Abstract]. Journal of Allergy & Clinical Immunology 2005;115(Suppl 2):S151. [DOI] [PubMed] [Google Scholar]
Zeneca Accolate 1998 {published data only}
- Zeneca Pharmaceuticals. Zafirlukast (Accolate) product monograph. Zeneca Pharmaceuticals 1998.
Zhang 1999 {unpublished data only}
- Zhang J, Chang Y, Reiss TF. Predicting future response using patient baseline variables and early responses to montelukast, a potent cysLT1 antagonist. Merck Research Laboratories 1999.
Zorc 2003 {published data only}
- Zorc JJ, Scarfone RJ, Li Y, Hong T, Harmelin M, Grunstein L, et al. Scheduled follow‐up after a pediatric emergency department visit for asthma: A randomized trial. Pediatrics 2003;111(3):495‐502. [DOI] [PubMed] [Google Scholar]
Additional references
Australia 2006
- National Asthma Council. Asthma Management Handbook 2006. Asthma Management Handbook 2006. Vol. 6th edition, Melbourne: National Asthma Campaign, 2006 (http://www.nationalasthma.org.au/cms/images/stories/amh2006_web_5.pdf). [Google Scholar]
Bisgaard 1988
- Bisgaard H, Nielsen MD, Andersen B, Andersen P, Foged N, Fuglsang G, et al. Adrenal function in children with bronchial asthma treated with beclomethasone dipropionate or budesonide. Journal of Allergy & Clinical Immunology 1988;81(6):1088‐95. [DOI] [PubMed] [Google Scholar]
BTS 2011
Cates 2002
- Cates CJ. Simpson's paradox and calculation of number needed to treat from meta‐analysis. Bio Med Central 2002;BMC Medical Research Methodology 2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Smith GD, Altman DG editor(s). Systematic reviews in health care: meta‐analysis in context. London: BMJ Publishing, 2001:285‐312. [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elwyn 2010
- Elwyn G, Crowe S, Fenton M, Firkins L, Versnel J, Walker S, et al. Identifying and prioritizing uncertainties: patient and clinician engagement in the identification of research questions. Journal of Evaluation in Clinical Practice 2010;16(3):627‐31. [DOI] [PubMed] [Google Scholar]
GINA 2010
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. NIH Publication. Bethesda, MD: National Heart, Lung and Blood Institute, 2002 (http://www.ginasthma.org/pdf/GINA_Report_2010.pdf).
Heuck 1997
- Heuck C, Wolthers OD, Hansen M, Kollerup G. Short‐term growth and collagen turnover in asthmatic adolescents treated with the inhaled glucocorticoid budesonide. Steroids 1997;62(10):659‐64. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, Available from www.cochrane‐handbook.org, 2008. [Google Scholar]
Lougheed 2010
- Lougheed MD, Lemiere C, Ducharme FM, FitzGerald M, Leigh R, Licskai C, et al. Canadian Thoracic Society Asthma Management Continuum – 2010 Consensus Summary for children six years of age and over and adults. Canadian Respiratory Journal 2010;17:15‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 1993
- Murphy S, Kelly HW. Asthma, inflammation, and airway hyper responsiveness in children.Asthma, inflammation, and airway hyper responsiveness in children. Current Opinion in Pediatrics 1993;5(3):255‐65. [DOI] [PubMed] [Google Scholar]
NAEPP 2007
- National Heart, Lung and Blood Institute. National Asthma Education and Prevention Program. NAEPP Expert Panel Report Guidelines for the Diagnosis and Management of Asthma. NIH Publication. National Heart, Lung and Blood Institute. Bethesda, MD: National Institute of Health, 2007 (http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf).
Padfield 1993
- Padfield PL, Teelucksingh S. Inhaled corticosteroids: the endocrinologist's view. European Respiratory Review 1993;3(15):494‐500. [Google Scholar]
Peters‐Golden 2007
- Peters‐Golden M, Henderson W. Leukotrienes. New England Journal of Medicine 2007;357:1841‐54. [DOI] [PubMed] [Google Scholar]
Phillip 1992
- Phillip M, Aviram M, Leiberman E, Zadik Z, Giat Y, Levy J, et al. Integrating plasma cortisol concentration in children with asthma receiving long‐term inhaled corticosteroids. Pediatric Pulmonology 1992;12:84‐9. [DOI] [PubMed] [Google Scholar]
RevMan [Computer program]
- Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan) Version 5.1. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2008.
Richard 2006
- Irwin RS, Richardson ND. Side effects with inhaled corticosteroids: The physician's perception. Chest 2006;130:41S‐53S. [DOI] [PubMed] [Google Scholar]
Sharek 2000
- Sharek PJ, Bergman D, Ducharme FM. Beclomethasone for asthma in children: effects on linear growth. Cochrane Database of Systematic Reviews 2000;Issue 1:Art. No.: CD001282. DOI: 10.1002/14651858.CD001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spahn 1996
- Spahn JD, Leung DYM. The role of glucocorticoids in the management of asthma. Allergy & Asthma Proceedings 1996;17(6):341‐50. [DOI] [PubMed] [Google Scholar]
Todd 1996
- Todd G, Dunlop K, McNaboe J, Ryan MF, Carson D, Shields MD. Growth and adrenal suppression in asthmatic children treated with high‐dose fluticasone propionate. Lancet 1996;348:27‐9. [DOI] [PubMed] [Google Scholar]
Zöllner 2007
- Zöllner E. Hypothalamic‐pituitary‐adrenal axis suppression in asthmatic children on inhaled corticosteroids (Part 2)‐‐the risk as determined by gold standard adrenal function tests: a systematic review. Pediatric Allergy and Immunology 2007;18:469‐74. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ducharme 2004
- Ducharme F, Salvio F. Anti‐leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002314.pub2] [DOI] [PubMed] [Google Scholar]


