Abstract
Background
Although pharmacological and psychological interventions are both effective for major depression, antidepressant drugs remain the mainstay of treatment in primary and secondary care settings. During the last 20 years, antidepressant prescribing has risen dramatically in western countries, mainly because of the increasing consumption of selective serotonin reuptake inhibitors (SSRIs) and newer antidepressants, which have progressively become the most commonly prescribed antidepressants. Escitalopram is the pure S-enantiomer of the racemic citalopram.
Objectives
To assess the evidence for the efficacy, acceptability and tolerability of escitalopram in comparison with tricyclics, other SSRIs, heterocyclics and newer agents in the acute-phase treatment of major depression.
Search methods
Electronic databases were searched up to July 2008. Trial databases of drug-approving agencies were hand-searched for published, unpublished and ongoing controlled trials.
Selection criteria
All randomised controlled trials comparing escitalopram against any other antidepressant (including non-conventional agents such as hypericum) for patients with major depressive disorder (regardless of the diagnostic criteria used).
Data collection and analysis
Data were entered by two review authors (double data entry). Responders and remitters to treatment were calculated on an intention-to-treat basis. For dichotomous data, odds ratios (ORs) were calculated with 95% confidence intervals (CI). Continuous data were analysed using standardised mean differences (with 95% CI) using the random effects model.
Main results
Fourteen trials compared escitalopram with another SSRI and eight compared escitalopram with a newer antidepressive agent (venlafaxine, bupropion and duloxetine). Escitalopram was shown to be significantly more effective than citalopram in achieving acute response (OR 0.67, 95% CI 0.50 to 0.87). Escitalopram was also more effective than citalopram in terms of remission (OR 0.53, 95% CI 0.30 to 0.93). Significantly fewer patients allocated to escitalopram withdrew from trials compared with patients allocated to duloxetine, for discontinuation due to any cause (OR 0.62, 95% CI 0.38 to 0.99).
Authors’ conclusions
Some statistically significant differences favouring escitalopram over other antidepressive agents for the acute phase treatment of major depression were found, in terms of efficacy (citalopram and fluoxetine) and acceptability (duloxetine). There is insufficient evidence to detect a difference between escitalopram and other antidepressants in early response to treatment (after two weeks of treatment). Cost-effectiveness information is also needed in the field of antidepressant trials. Furthermore, as with most standard systematic reviews, the findings rely on evidence from direct comparisons. The potential for overestimation of treatment effect due to sponsorship bias should also be borne in mind.
Medical Subject Headings (MeSH): Antidepressive Agents [*therapeutic use], Citalopram [*therapeutic use], Depression [*drug therapy], Randomized Controlled Trials as Topic, Serotonin Uptake Inhibitors [*therapeutic use]
MeSH check words: Humans
BACKGROUND
Description of the condition
Major depression is generally diagnosed when a persistent and unreactive low mood and loss of all interest and pleasure are accompanied by a range of symptoms including appetite loss, insomnia, fatigue, loss of energy, poor concentration, psychomotor symptoms, inappropriate guilt and morbid thoughts of death (APA 1994). It was the third leading cause of burden among all diseases in the year 2002 and it is expected to show a rising trend during the coming 20 years (Murray 1997). This condition is associated with marked personal, social and economic morbidity, loss of functioning and productivity, and creates significant demands on service providers in terms of workload (NICE 2007).
Description of the intervention
Although pharmacological and psychological interventions are both effective for major depression, in primary and secondary care settings antidepressant drugs remain the mainstay of treatment (APA 2000; Ellis 2004; NICE 2007) (see below for other references to the relevant evidence). Amongst antidepressants many different agents are available, including tricyclics (TCAs), monoamine oxidase inhibitors (MAOIs), selective serotonin re-uptake inhibitors (SSRIs), serotonin-noradrenaline reuptake inhibitors (SNRIs, such as venlafaxine, duloxetine and milnacipran), and other newer agents (mirtazapine, reboxetine, bupropion). During the last 20 years, consumption of antidepressant has risen dramatically in western countries, mainly because of the increasing consumption of SSRIs and newer antidepressants, which have progressively become the most commonly prescribed antidepressants (Ciuna 2004; Guaiana 2005). SSRIs are generally better tolerated than TCAs (Barbui 2000), and there is evidence of similar efficacy (Anderson 2000; Geddes 2000; Williams 2000). However, head-to-head comparison have provided contrasting findings. Amitriptyline, for example, may have the edge over SSRIs in terms of efficacy (Guaiana 2003), and individual SSRIs and SNRIs may differ in terms of efficacy and tolerability (Puech 1997; Smith 2002; Hansen 2005; Cipriani 2006).
Escitalopram is the pure S-enantiomer of the racemic citalopram. As for all other antidepressants belonging to the SSRIs class, the mechanism of antidepressant action of escitalopram is presumed to be linked to potentiation of serotonergic activity in the central nervous system resulting from its inhibition of neuronal re-uptake of serotonin. Escitalopram is at least 100 fold more potent than the R-enantiomer with respect to inhibition of 5-HT reuptake and inhibition of 5-HT neuronal firing rate. Escitalopram has no or very low affinity for other receptors (alpha- and beta-adrenergic, dopamine (D1-5), histamine (H1-3), muscarinic (M1-5), and benzodiazepine receptors). The single- and multiple-dose pharmacokinetics of escitalopram are linear and dose-proportional in a dose range of 10 to 30 mg/day. In vitro studies indicated that CYP3A4 and −2C19 are the primary enzymes involved in the metabolism of escitalopram, however these studies did not reveal an inhibitory effect of escitalopram on CYP2D6.
How the intervention might work
The efficacy of escitalopram as a treatment for major depressive disorder was established in three, 8-week, placebo-controlled studies conducted in outpatients between 18 and 65 years of age who met DSM-IV criteria for major depressive disorder (www.fda.gov). The primary outcome in all three studies was change from baseline to endpoint in the Montgomery Asberg Depression Rating Scale (MADRS) (Montgomery 1979). The 10 mg/day and 20 mg/day escitalopram treatment groups showed significantly greater mean improvement compared to placebo on the MADRS. Analyses of the relationship between treatment outcome and age, gender, and race did not suggest any differential responsiveness on the basis of these patient characteristics. Among the 715 depressed patients who received escitalopram in placebo-controlled trials, 6% discontinued treatment due to an adverse event, compared to 2% of 592 patients receiving placebo. The rate of discontinuation for adverse events in patients assigned to a fixed dose of 20 mg/day escitalopram was 10%, which was significantly different from the rate of discontinuation for adverse events in patients receiving 10 mg/day escitalopram (4%) and placebo (3%). The most commonly observed adverse events in escitalopram patients (incidence of approximately 5% or greater and approximately twice the incidence as in placebo patients) were insomnia, ejaculation disorder (primarily ejaculatory delay), nausea, increased sweating, fatigue, and somnolence.
Why it is important to do this review
To shed light on the field of antidepressant trials and treatment of major depressive disorder, a group of researchers agreed to join forces under the rubric of the Meta-Analyses of New Generation Antidepressants Study Group (MANGA Study Group) to systematically review all available evidence for each specific newer antidepressant. As of October 2008, we have completed an individual review for fluoxetine (Cipriani 2005), and published the protocols for venlafaxine (Cipriani 2007a), sertraline (Malvini 2006), fluvoxamine (Omori 2006), citalopram (Imperadore 2007), duloxetine (Nose 2007), milnacipran (Nakagawa 2007), paroxetine (Cipriani 2007b) and mirtazapine (Watanabe 2006). Thus, the aim of the present review is to assess the evidence for the efficacy and tolerability of escitalopram in comparison with TCAs, heterocyclics, other SSRIs and newer antidepressants, including non-conventional agents such as herbal products like hypericum (Linde 2008), in the acute-phase treatment of major depression.
OBJECTIVES
To determine the efficacy of escitalopram in comparison with other antidepressive agents for depression in alleviating the acute symptoms of major depressive disorder.
To investigate the acceptability of escitalopram in comparison with other antidepressive agents for depression.
To investigate the adverse effects of escitalopram in comparison with other antidepressive agents for depression.
METHODS
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials were included. Quasi-randomised trials, such as those allocating by using alternate days of the week, were excluded.
Types of participants
Patients aged 18 or older, of both sexes, with a primary diagnosis of major depression. Studies adopting any standardised criteria to define patients suffering from unipolar major depression were included. Studies from the 1990s onwards were likely to have used DSM-IV (APA 1994) or ICD-10 (WHO 1992) criteria. Earlier studies may had used ICD-9 (WHO 1978), DSM-III (APA 1980) / DSM-III-R (APA 1987) or other diagnostic systems. ICD-9 is not operationalised criteria, because it has only disease names and no diagnostic criteria, so studies using ICD-9 were excluded. However, studies using Feighner criteria or Research Diagnostic Criteria were included. Studies in which less than 20% of the participants might be suffering from bipolar depression were included, but the validity of this decision was examined in a sensitivity analysis. A concurrent secondary diagnosis of another psychiatric disorder was not considered as an exclusion criterion. A concurrent primary diagnosis of Axis I or II disorders was an exclusion criterion. Antidepressant trials in depressive patients with a serious concomitant medical illness were also excluded.
Types of interventions
Experimental intervention
Escitalopram (as monotherapy). No restrictions on dose, frequency, intensity and duration were applied.
Comparator interventions
All other antidepressive agents in the treatment of acute depression, including:
conventional tricyclic ADs (TCAs)
heterocyclic antidepressants (e.g. maprotiline)
SSRIs (fluoxetine, fluvoxamine, citalopram, paroxetine, sertraline)
newer antidepressants (SNRIs such as venlafaxine, duloxetine, milnacipran; MAOIs or newer agents such as mirtazapine, bupropion, reboxetine; and non-conventional ADs, such as herbal products - e.g. hypericum).
No restrictions on dose, frequency, intensity and duration were applied.
Other types of psychopharmacological agent such as anxiolytics, anticonvulsants, antipsychotics or mood-stabilizers were excluded. Trials in which escitalopram was used as an augmentation strategy were also excluded
Types of outcome measures
Primary outcomes
Number of patients who responded to treatment, showing a reduction of at least 50% on the Hamilton Depression Scale (HAM-D) (Hamilton 1960) or MADRS (Montgomery 1979), or any other depression scale, or “much or very much improved” (score 1 or 2) on CGI-Improvement (Guy 1970). All response rates were calculated out of the total number of randomised patients. Where more than one criterion was provided, we preferred the HAM-D for judging response. We used the HAM-D whenever possible, even when we needed to impute SDs or response rates according to the procedures described in the Methods section below.
When studies reported response rates at various time points of the trial, we decided a priori to subdivide the treatment indices as follows:
Early response: between 1 and 4 weeks, the time point closest to 2 weeks was given preference.
Acute phase treatment response: between 6 and 12 weeks, the time point given in the original study as the study endpoint was given preference.
Follow-up response: between 4 and 6 months, the time point closest to 24 weeks was given preference.
The acute phase treatment response, i.e. between 6 and 12 weeks, was our primary outcome of interest.
Secondary outcomes
Number of patients who achieved remission. The cut-off point for remission was set a priori (i) at 7 or less on the 17-item HAM-D and at 8 or less for all the other longer versions of HAM-D, or (ii) at 12 or less on the MADRS (Zimmerman 2004), or (iii) “not ill or borderline mentally ill” (score 1 or 2) on CGI-Severity (Guy 1970). All remission rates was calculated out of the total number of randomised patients. Where two or more were provided, we preferred the HAM-D for judging remission.
Change scores from baseline to the time point in question (early response, acute phase response, or follow-up response as defined above) on HAM-D, or MADRS, or any other depression scale. We applied a looser form of ITT analysis, whereby all the patients with at least one post-baseline measurement were represented by their last observations carried forward.
Social adjustment, social functioning, including the Global Assessment of Function (Luborsky 1962) scores
Health-related quality of life: we limited ourselves to SF-12/SF-36 (Ware 1993), HoNOS (Wing 1994) and WHOQOL (WHOQOL Group 1998).
Costs to health care services
-
Acceptability
Acceptability was evaluated using the following outcome measures:- Number of patients who dropped out during the trial as a proportion of the total number of randomised patients - Total drop out rate
- Number of patients who dropped out due to inefficacy during the trial as a proportion of the total number of randomised patients
-
-Drop out rates due to inefficacy
-
-
- Number of patients who dropped out due to side effects during the trial as a proportion of the total number of randomised patients
-
-Drop out rates due to side effects.
-
-
Tolerability
Tolerability was evaluated using the following outcome measures:
Total number of patients experiencing at least some side effects.
- Total number of patients experiencing the following specific side effects were sought for:
- Agitation/anxiety
- Constipation
- Diarrhoea
- Dry mouth
- Hypotension
- Insomnia
- Nausea
- Sleepiness/drowsiness
- Urination problems
- Vomiting
- Deaths, suicide and suicidality
In order not to miss any relatively rare or unexpected yet important side effects, in the data extraction phase, we collected all side effects data reported in the literature and discussed ways to summarise them post hoc.
Search methods for identification of studies
Electronic searches
See: Cochrane Collaboration Depression, Anxiety and Neurosis Group (CCDAN) methods used in reviews.
Electronic Databases
CCDANCTR-Studies were searched using the following search strategy:
Diagnosis = Depress* or Dysthymi* or “Adjustment Disorder*” or “Mood Disorder*” or “Affective Disorder” or “Affective Symptoms” and Intervention = Escitalopram
CCDANCTR-References were searched using the following search strategy:
Keyword = Depress* or Dysthymi* or “Adjustment Disorder*” or “Mood Disorder*” or “Affective Disorder” or “Affective Symptoms” and Free-Text = Escitalopram
An additional Medline search was carried out (update: July 2008). Trial databases of the following drug-approving agencies - the Food and Drug Administration (FDA) in the USA, the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK, the European Medicines Agency (EMEA) in the EU, the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan, the Therapeutic Goods Administration (TGA) in Australia) and ongoing trial registers (clinicaltrials.gov in the USA, ISRCTN and National Research Register in the UK, Nederlands Trial Register in the Netherlands, EUDRACT in the EU, UMIN-CTR in Japan and the Australian Clinical Trials Registry in Australia) were searched for published, unpublished and ongoing controlled trials (update: July 2008).
Searching other resources
1) Handsearches
Appropriate journals and conference proceedings relating to escitalopram treatment for depression were hand-searched and incorporated into the CCDANCTR databases.
2) Personal communication
Pharmaceutical companies and experts in this field were asked if they knew of any study which met the inclusion criteria of this review.
3) Reference checking
Reference lists of the included studies, previous systematic reviews and major textbooks of affective disorder written in English were checked for published reports and citations of unpublished research. The references of all included studies were checked via Science Citation Index for articles which had cited the included study.
Data collection and analysis
Selection of studies
Studies relating to escitalopram generated by the electronic search of CCDANCTR-Studies were scanned by one review author (HMG). Those studies that met the following criteria constituted the preliminary list and their full texts were retrieved:.
The rough inclusion criteria were:
Randomised trial
Comparing escitalopram against any other antidepressant
Patients with major depression, regardless of the diagnostic criteria used.
Studies relating to escitalopram generated by the search strategies of CCDANCTR-References and the other complementary searches were checked independently by the CCDAN Trials Search Coordinator (HMG), who is an author of this review, and another review author (AC, AS or CS) to see if they met the rough inclusion criteria, firstly based on the title and abstracts. All the studies rated as possible candidates by either of the two reviewers were added to the preliminary list and the full texts were retrieved. All the full text articles in this preliminary list was then assessed by two review authors (AC, AS or CS) independently to see if they met the strict inclusion criteria. If the raters disagreed, the final rating were made by consensus with the involvement (if necessary) of another member of the review group. Non-congruence in selection of trials was reported as percentage disagreement. Considerable care was taken to exclude duplicate publications.
Data extraction and management
One review author (CS) first extracted data concerning participant characteristics (age, sex, depression diagnosis, comorbidity, depression severity, antidepressant treatment history for the index episode, study setting), intervention details (intended dosage range, mean daily dosage actually prescribed, co-intervention if any, escitalopram as investigational drug or as comparator drug, sponsorship) and outcome measures of interest from the included studies. The results were compared with those in the completed reviews of individual antidepressants in the Cochrane Library. If there were any discrepancies, a second review author (AC) intervened and the agreed-upon results were used in the review as well as fed back to the authors of the completed reviews.
Assessment of risk of bias in included studies
We used the Cochrane risk-of-bias tool as recommended in RevMan 5.0.0. This instrument consists of six items. Two of the items assess the strength of the randomisation process in preventing selection bias in the assignment of participants to interventions: adequacy of sequence generation and allocation concealment. The third item (blinding) assesses the influence of performance bias on the study results. The fourth item assesses the likelihood of incomplete outcome data, which raise the possibility of bias in effect estimates. The fifth item assesses selective reporting, the tendency to preferentially report statistically significant outcomes. It requires a comparison of published data with trial protocols, when such are available. The final item refers to other sources of bias that are relevant in certain circumstances, for example, in relation to trial design (methodologic issues such as those related to crossover designs and early trial termination) or setting.
Two review authors (AC, CB) assessed risk of bias in each trial independently, in accordance with the Cochrane Handbook (Higgins 2008). Where inadequate details of allocation concealment and other characteristics of trials were provided, the trial authors were contacted in order to obtain further information. If the raters disagreed the final rating was made by consensus with the involvement (if necessary) of another member of the review group. The ratings were also compared with those in the completed reviews of individual antidepressants in the Cochrane Library. If here were any discrepancies, they were fed back to the authors of the completed reviews.
Measures of treatment effect
Data were checked and entered into Review Manager 5 software by two review authors (AC, CS) (double data entry). For dichotomous, or event-like data, odds ratios (OR) were calculated with 95% confidence intervals. Continuous data were analysed using weighted mean differences (with 95% confidence intervals) or standardised mean differences (where different measurement scales are used) using the random effects model.
Unit of analysis issues
For trials which had a crossover design only results from the first randomisation period were considered. If the trial was a three (or more)-armed trial involving a placebo arm, the data were extracted from the placebo arm as well.
Dealing with missing data
Responders and remitters to treatment were calculated on the intention-to-treat (ITT) basis: drop-outs were always included in this analysis. Where participants had withdraw from the trial before the endpoint, it was assumed they would have experienced the negative outcome by the end of the trial (e.g. failure to respond to treatment). When there were missing data and the method of “last observation carried forward” (LOCF) had been used to do an ITT analysis, then the LOCF data were used, with due consideration of the potential bias and uncertainty introduced. When dichotomous or continuous outcomes were not reported, trial authors were asked to supply the data.
When only the SE or t-statistics or p values were reported, SDs were calculated according to Altman (Altman 1996). In the absence of supplemental data from the authors, the SDs of the HAM-D (or any other depression scale) and response/remission rates were calculated according to validated imputation methods (Furukawa 2005; Furukawa 2006). We examined the validity of these imputations in sensitivity analyses.
Assessment of heterogeneity
Skewed data and non-quantitative data were presented descriptively. An outcome whose minimum score is zero could be considered skewed when the mean was smaller than twice the SD. Heterogeneity between studies was investigated by the I-squared statistic (Higgins 2003) (I-squared equal to or more than 50% was considered indicative of heterogeneity) and by visual inspection of forest plots.
Assessment of reporting biases
Funnel plot analysis was performed to check for existence of small study effects, including publication bias.
Data synthesis
The primary analysis used a random effects model OR, which had the highest generalisability in our empirical examination of summary effect measures for meta-analyses (Furukawa 2002a). The robustness of this summary measure was routinely examined by checking the fixed effect model OR and the random effects model risk ratio (RR). Material differences between the models were reported. Fixed effect analyses were done routinely for the continuous outcomes as well, to investigate the effect of the choice of method on the estimates. Material differences between the models were reported.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were performed and interpreted with caution because multiple analyses can lead to false positive conclusions (Oxman 1992). However, we performed the following subgroup analyses, where possible, for the following a priori reasons:
Escitalopram dosing (fixed low dosage, fixed standard dosage, fixed high dosage; flexible low dosage, flexible standard dosage, flexible high dosage), because there was evidence to suspect that low dosage antidepressant might be associated with better outcomes both in terms of effectiveness and side effects than standard or high dosage antidepressants (Bollini 1999; Furukawa 2002b) and also because fixed versus flexible dosing schedule might affect estimates of treatment effectiveness (Khan 2003). In the case of escitalopram, based on the Defined Daily Dosage by World Health Organisation (WHO), low dosage referred to <10, standard dosage to >10 but <20, and high dosage to >20 mg/day.
Comparator dosing (low effective range, medium to high effective range), as it was easy to imagine that there were greater chances of completing the study on the experimental drug than on the comparator drug that was increased to the maximum dosage.
Depression severity (severe major depression, moderate/mild major depression).
Treatment settings (psychiatric inpatients, psychiatric outpatients, primary care).
Elderly patients (>65 years of age), separately from other adult patients.
Sensitivity analysis
The following sensitivity analyses were planned a priori. By limiting included studies to those with higher quality, we examined if the results changed, and checked for the robustness of the observed findings.
Excluding trials with unclear concealment of random allocation and/or unclear double blinding.
Excluding trials whose drop out rate was greater than 20%.
Performing the worst case scenario ITT (all the patients in the experimental group experience the negative outcome and all those allocated to the comparison group experience the positive outcome) and the best case scenario ITT (all the patients in the experimental group experience the positive outcome and all those allocated to the comparison group experience the negative outcome).
Excluding trials for which the response rates had to be calculated based on the imputation method (Furukawa 2005) and those for which the SD had to be borrowed from other trials (Furukawa 2006).
Examination of “wish bias” (also called “optimism bias”) by comparing escitalopram as investigational drug vs escitalopram as comparator, as there was evidence to suspect that a new antidepressant might perform worse when used as a comparator than when used as an experimental agent (Barbui 2004).
Excluding studies funded by the pharmaceutical company marketing escitalopram. This sensitivity analysis was particularly important in view of the recent repeated findings that funding strongly affects outcomes of research studies (Als-Nielsen 2003; Bhandari 2004; Lexchin 2003; Montgomery 2004; Perlis 2005; Procyshyn 2004) and because industry sponsorship and authorship of clinical trials were increasing over 20 years (Buchkowsky 2004).
If subgroups within any of the subgroup or sensitivity analyses turned out to be significantly different from one another, we ran meta-regression for exploratory analyses of additive or multiplicative influences of the variables in question. Our routine application of random effects and fixed effect models as well as our secondary outcomes of remission rates and continuous severity measures may be considered additional forms of sensitivity analyses.
RESULTS
Description of studies
See: Characteristics of included studies.
Results of the search
The search yielded 52 references of potentially eligible studies. After exclusion of papers that were not relevant (because they mainly were non-randomised studies or reviews), 19 randomised controlled trials were included in the present review. Three further randomised controlled trials were found in the web-based clinical trial register of the pharmaceutical industry manufacturing escitalopram and were included in the pool of included studies. Therefore, a total of 22 trials were included in the review.
In the presentation of the following analyses, a post-hoc decision was made to present all SSRIs (with sub-totals) together in one group, and SNRIs and newer antidepressant agents (without subtotals) together in a second group (see graphs). Fourteen trials (64%) compared escitalopram with another SSRI and eight (36%) compared escitalopram with a newer antidepressant (venlafaxine, bupropion and duloxetine). Neither trials comparing escitalopram with TCAs or MAOIs nor cross-over design studies were retrieved by the comprehensive search. In this review all studies were multicentre, randomised, double-blind trials (nine were three-arm, placebo-controlled trials).
Included studies
Design
Length of the studies
Escitalopram versus other SSRIs
In 11 studies the follow-up was 8 weeks (Alexopoulos 2004; Baldwin 2006; Burke 2002; Kasper 2005; Kennedy 2005; Lepola 2003; Mao 2008; Moore 2005; SCT-MD-02; SCT-MD-09; Ventura 2007). One study was a 6-week trial (Yevtushenko 2007) and in two studies the follow-up lasted up to 24 weeks (Boulenger 2006; Colonna 2005).
Escitalopram versus newer antidepressants
Seven studies were 8-week trials (Bielski 2004; Clayton (AK130926); Clayton (AK130927); Khan 2007; Montgomery 2004; Nierenberg 2007; SCT-MD-35) and one study was a 24-week trial (Wade 2007).
Sample size
Escitalopram versus other SSRIs
The mean of participants per study was 280.8 (SD 103.9), with a minimum sample size of 30 (SCT-MD-09) and a maximum of 459 (Boulenger 2006).
Escitalopram versus newer antidepressants
The mean of participants was 307.1 (SD 101.3), ranging between 202 (Bielski 2004) and 547 (Nierenberg 2007).
Setting
Escitalopram versus other SSRIs
In 11 studies the participants were outpatients (Alexopoulos 2004; Baldwin 2006; Boulenger 2006; Burke 2002; Colonna 2005; Kennedy 2005; Moore 2005; SCT-MD-02; SCT-MD-09; Ventura 2007; Yevtushenko 2007). In one study (Lepola 2003) participants were recruited in primary care. In two studies (Kasper 2005; Mao 2008) both outpatients and inpatients were eligible. In Kasper 2005, patients were recruited both in general practice and specialist settings.
Escitalopram versus other antidepressants
In 7 studies the participants were outpatients (Bielski 2004; Clayton (AK130926); Clayton (AK130927); Khan 2007; Nierenberg 2007; SCT-MD-35; Wade 2007). In Montgomery 2004 patients were recruited in primary care.
Participants
Age
Escitalopram versus other SSRIs
In eight studies patients over 65 years were excluded (Alexopoulos 2004; Baldwin 2006; Burke 2002; Colonna 2005; Lepola 2003; Mao 2008; SCT-MD-09; Yevtushenko 2007). Five studies included patients over 65 years (Boulenger 2006; Kennedy 2005; Moore 2005; SCT-MD-02; Ventura 2007). One study (Kasper 2005) included only elderly patients (over 65 years).
Escitalopram versus newer antidepressants
In two studies patients over 65 years were excluded (Bielski 2004; Wade 2007). Four studies included patients over 65 years (Khan 2007; Montgomery 2004; Nierenberg 2007; SCT-MD-35). In two studies age range was not specified (Clayton (AK130926); Clayton (AK130927)).
Diagnosis
All the studies enrolled patients suffering from DSM-IV criteria for major depressive disorder.
Interventions
Escitalopram versus other SSRIs
In six studies escitalopram was compared with citalopram (Burke 2002; Colonna 2005; Lepola 2003; Moore 2005; SCT-MD-02; Yevtushenko 2007), in four with fluoxetine (Kasper 2005; Kennedy 2005; Mao 2008; SCT-MD-09), in two with paroxetine (Baldwin 2006; Boulenger 2006) and in the remaining two with sertraline (Alexopoulos 2004; Ventura 2007). Five studies included a placebo arm (Alexopoulos 2004; Burke 2002; Kasper 2005; Lepola 2003; SCT-MD-02). One study (Burke 2002) presented a comparison between four arms: escitalopram 10mg/day, escitalopram 20mg/day, citalopram and placebo.
Escitalopram versus newer antidepressants
Three studies compared escitalopram with bupropion XR (Clayton (AK130926); Clayton (AK130927); SCT-MD-35), three with duloxetine (Khan 2007; Nierenberg 2007; Wade 2007) and two with venlafaxine XR (Bielski 2004; Montgomery 2004). Four studies included a placebo arm (Clayton (AK130926); Clayton (AK130927); Nierenberg 2007; SCT-MD-35). One study (SCT-MD-35) presented a comparison between four arms: escitalopram 4mg/day, bupropion XR 150mg/day, placebo and a combination of escitalopram 4mg/day and bupropion XR 150mg/day.
Dosage of study drugs
In 21 out of 22 studies the dosage of escitalopram was within the therapeutic dosage (10 to 20 mg/day). In one study (SCT-MD-35) the escitalopram dosage was set at 4 mg/day (fixed dose). Eleven trials used a fixed- and the remaining eleven a flexible-dosage regimen. The use of a fixed- or a flexible-dose regimen was consistent among comparisons within the same study in the great majority of included trials. However, in three out of 22 studies one of the two compounds used a fixed-dose while the other used a flexible-dose design (Burke 2002; Khan 2007; Ventura 2007).
Primary Outcomes
Escitalopram versus other SSRIs
The primary outcome used in the great majority of studies was change from baseline on MADRS. One study (Mao 2008) used the change on the HAM-D-17 total score and another trial (SCT-MD-09) evaluated the effects of escitalopram and fluoxetine on sleep in depressed patients using the number of awakenings (polysomnogram) as the primary outcome. The latter study lasted only five weeks, did not evaluate efficacy and has been included in the present review only for early discontinuation and tolerability outcomes (side-effect profile).
Escitalopram versus other antidepressants
Five studies used the change from baseline on MADRS (Bielski 2004; Khan 2007; Montgomery 2004; SCT-MD-35; Wade 2007) as the primary outcome and one used change on HAMD-17 (Nierenberg 2007). In studies by Clayton (Clayton (AK130926); Clayton (AK130927)) sexual functioning was considered the primary outcome and depression was rated as mean change on HAMD-17.
Response Rate
Escitalopram versus other SSRIs
In nine studies a decrease from baseline to endpoint of at least 50% in rating scale total score (either on MADRS or on HAM-D) was used to define “response” (Baldwin 2006; Boulenger 2006; Burke 2002; Colonna 2005; Kasper 2005; Lepola 2003; Mao 2008; Moore 2005; Ventura 2007). The four remaining studies (Alexopoulos 2004; Kennedy 2005; SCT-MD-02; SCT-MD-09) provided only continuous data and therefore response rates were imputed (see Methods).
Escitalopram versus newer antidepressants
In four studies, a decrease from baseline to endpoint of at least 50% in MADRS total score was used to define “response” (Bielski 2004; Khan 2007; Montgomery 2004; Wade 2007). In three studies a decrease from baseline to endpoint of at least 50% in HAMD-17 total score was used for defining “response” (Clayton (AK130926); Clayton (AK130927); Nierenberg 2007). In one study (SCT-MD-35) only continuous data were available and therefore response rate was imputed (see Methods).
Remission Rate
Escitalopram versus other SSRIs
Six studies used MADRS to assess remission rate (Baldwin 2006; Boulenger 2006; Colonna 2005; Kasper 2005; Lepola 2003; Moore 2005). One study used HAMD-17 (Ventura 2007). Five studies did not report remission rate (Alexopoulos 2004; Burke 2002; Kennedy 2005; SCT-MD-02; SCT-MD-09). However, considering that continuous outcomes were available, remission rates were imputed (see Methods)
Escitalopram versus newer antidepressants
Six studies used HAMD-17 (Bielski 2004; Clayton (AK130926); Clayton (AK130927); Khan 2007; Nierenberg 2007; Wade 2007) and one used MADRS (Montgomery 2004) to assess remission rate. One study (SCT-MD-35) did not report dichotomous data remission rate, so remission rate was imputed using continuous outcomes (see Methods)
Sponsorship
Escitalopram versus other SSRIs
All the studies were sponsored by the drug company marketing escitalopram.
Escitalopram versus newer antidepressants
Five studies were sponsored by the drug company marketing escitalopram (Bielski 2004; Khan 2007; Montgomery 2004; SCT-MD-35; Wade 2007). Two studies were sponsored by the drug company marketing bupropion XR (Clayton (AK130926); Clayton (AK130927)). One study was sponsored by the drug company marketing duloxetine (Nierenberg 2007).
Excluded studies
Thirty-three references of potentially eligible studies were excluded after checking titles and abstracts. Of those, 21 were reviews, nine were non-randomised studies and three were duplicates.
Risk of bias in included studies
See Figure 1 and Figure 2 for a graphical summary of methodological quality for the 22 included studies, based on the six risk of bias domains.
Figure 1. Methodological quality graph: review authors’ judgements about each methodological quality item presented as percentages across all included studies.
Figure 2. Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Allocation
Only four studies reported sufficient details on allocation concealment (Baldwin 2006; Boulenger 2006; Colonna 2005; Wade 2007).
Blinding
All studies were reported to be double-blind trials, however only five studies reported sufficient details on blinding (Baldwin 2006; Boulenger 2006; Colonna 2005; Wade 2007; Yevtushenko 2007)
Incomplete outcome data
Only three studies reported incomplete outcome data (Clayton (AK130926); Clayton (AK130927); SCT-MD-09).
Selective reporting
Only eight studies were indicated to be free from selective reporting (Alexopoulos 2004; Bielski 2004; Boulenger 2006; Clayton (AK130926); Clayton (AK130927); Khan 2007; Ventura 2007; Yevtushenko 2007).
Other potential sources of bias
The large majority of included studies were sponsored by the manufacturer of escitalopram.
Effects of interventions
Some statistically significant differences in efficacy, acceptability and tolerability were found and details are listed below. The results are reported comparison by comparison (dividing SSRIs from newer antidepressants) and the forest plots are organised according to the relevance of outcomes, as reported in the review protocol. For adverse events, all the information about adverse events specified in the review protocol are reported (either statistically or non-statistically significant). However, remaining adverse events are only reported when statistically significant (non-statistically significant results about adverse events are presented in Table 1).
Table 1. Adverse events.
| Adverse event | Study | Escitalopram | Comparator | Odds Ratio, Random [95% CI] | ||
|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||
| Escitalopram versus citalopram | ||||||
| Accidental overdose | Burke 2002 | 0 | 244 | 1 | 125 | 0.17 [0.01, 4.20] |
| Aggressive behaviour | Moore 2005 | 1 | 142 | 0 | 152 | 3.23 [0.13, 80.02] |
| Anaphylaxis | Burke 2002 | 1 | 244 | 0 | 125 | 1.55 [0.06, 38.23] |
| Anorexia | Yevtushenko 2007 | 0 | 108 | 1 | 108 | 0.33 [0.01, 8.20] |
| Asthenia | Moore 2005 | 2 | 142 | 2 | 152 | 1.07 [0.15, 7.71] |
| Bronchitis | Colonna 2005, Lepola 2003 | 13 | 330 | 11 | 342 | 1.24 [0.54, 2.83] |
| Coma | Burke 2002 | 0 | 244 | 1 | 125 | 0.17 [0.01, 4.20] |
| Concentration decrease | Moore 2005 | 0 | 142 | 1 | 152 | 0.35 [0.01, 8.77] |
| Death fetal | Lepola 2003 | 0 | 116 | 1 | 111 | 0.32 [0.01, 7.84] |
| Dehydration | SCT-MD-02 | 0 | 125 | 1 | 123 | 0.33 [0.01, 8.06] |
| Depression | Moore 2005 | 0 | 142 | 1 | 152 | 0.35 [0.01, 8.77] |
| Dermatological problems | Moore 2005, Yevtushenko 2007 | 1 | 250 | 3 | 260 | 0.44 [0.06, 3.02] |
| Disease Of Liver And Hepatic Duct | SCT-MD-02 | 0 | 125 | 1 | 123 | 0.33 [0.01, 8.06] |
| Dizziness | Burke 2002, Moore 2005, SCT-MD-02 | 31 | 511 | 15 | 400 | 1.43 [0.58, 3.49] |
| Dyspepsia | Yevtushenko 2007 | 0 | 108 | 1 | 108 | 0.33 [0.01, 8.20] |
| Enuresis | Moore 2005 | 1 | 142 | 0 | 152 | 3.23 [0.13, 80.02] |
| Forgetfulness | Moore 2005 | 0 | 142 | 2 | 152 | 0.21 [0.01, 4.44] |
| Hot flashes | Moore 2005 | 1 | 142 | 0 | 152 | 3.23 [0.13, 80.02] |
| Inflicted injury | Burke 2002 | 8 | 244 | 3 | 125 | 1.38 [0.36, 5.29] |
| Non-accidental overdose | SCT-MD-02 | 1 | 125 | 0 | 123 | 2.98 [0.12, 73.76] |
| Ophtalmological problems | Moore 2005 | 0 | 142 | 2 | 152 | 0.21 [0.01, 4.44] |
| Oppression | Moore 2005 | 0 | 142 | 1 | 152 | 0.35 [0.01, 8.77] |
| Palpitation | Moore 2005 | 1 | 142 | 0 | 152 | 3.23 [0.13, 80.02] |
| Panic attack | Moore 2005 | 0 | 142 | 1 | 152 | 0.35 [0.01, 8.77] |
| Pelvic inflammation | Lepola 2003 | 1 | 116 | 0 | 111 | 2.90 [0.12, 71.85] |
| Pharyngitis | Burke 2002, Lepola 2003, Moore 2005 | 18 | 541 | 12 | 437 | 0.92 [0.41, 2.06] |
| Pregnancy unintended | Lepola 2003 | 1 | 116 | 0 | 111 | 2.90 [0.12, 71.85] |
| Rhinitis | Burke 2002, Colonna 2005, Lepola 2003, SCT-MD-02 | 46 | 699 | 33 | 590 | 1.19 [0.73, 1.91] |
| Sexual problems | Burke 2002, Lepola 2003, SCT-MD-02, Yevtushenko 2007 | 19 | 283 | 15 | 267 | 1.14 [0.53, 2.44] |
| Sinusitis | Lepola 2003 | 6 | 155 | 4 | 160 | 1.57 [0.43, 5.68] |
| Subjects with non-fatal severe adverse events | Burke 2002, Lepola 2003, SCT-MD-02 | 6 | 524 | 4 | 408 | 1.12 [0.31, 4.08] |
| Tachycardia | SCT-MD-02 | 1 | 125 | 0 | 123 | 2.98 [0.12, 73.76] |
| Tinnitus | Moore 2005 | 0 | 142 | 1 | 152 | 0.35 [0.01, 8.77] |
| Tremor | Moore 2005 | 3 | 142 | 0 | 152 | 7.65 [0.39, 149.47] |
| Trismus | Moore 2005 | 0 | 142 | 1 | 152 | 0.35 [0.01, 8.77] |
| Upper respiratory tract infection | Burke 2002, Lepola 2003, SCT-MD-02 | 31 | 524 | 16 | 408 | 1.59 [0.85, 2.98] |
| Weight gain | Colonna 2005, Moore 2005 | 4 | 317 | 14 | 334 | 0.38 [0.06, 2.37] |
| Escitalopram versus bupropion XR | ||||||
| Accidental overdose | Clayton (AK130926) | 0 | 143 | 1 | 135 | 0.31 [0.01, 7.74] |
| Decreased appetite | Clayton (AK130927) | 13 | 138 | 9 | 141 | 1.53 [0.63, 3.69] |
| Disease Of Liver And Hepatic Duct | Clayton (AK130927) , SCT-MD-35 | 2 | 269 | 0 | 275 | 3.09 [0.32, 29.89] |
| DIzziness | Clayton (AK130926) , Clayton (AK130927) | 15 | 281 | 18 | 276 | 0.84 [0.33, 2.12] |
| Dyspepsia | Clayton (AK130926) | 5 | 143 | 5 | 135 | 0.94 [0.27, 3.33] |
| Flatulence | Clayton (AK130926) | 8 | 143 | 4 | 135 | 1.94 [0.57, 6.60] |
| Irritability | Clayton (AK130926) , Clayton (AK130927) | 3 | 281 | 14 | 276 | 0.26 [0.06, 1.04] |
| Musculoskeletal disorder | SCT-MD-35 | 10 | 131 | 9 | 134 | 1.15 [0.45, 2.92] |
| Nasal congestion | Clayton (AK130926), Clayton (AK130927) | 11 | 281 | 6 | 276 | 1.86 [0.68, 5.11] |
| Non-accidental overdose | Clayton (AK130927) | 1 | 138 | 0 | 141 | 3.09 [0.12, 76.44] |
| Palpitation | Clayton (AK130926) | 3 | 143 | 4 | 135 | 0.70 [0.15, 3.20] |
| Pharyngitis | Clayton (AK130927) | 7 | 138 | 9 | 141 | 0.78 [0.28, 2.17] |
| Subjects with non-fatal severe adverse events | Clayton (AK130926) , Clayton (AK130927) , SCT-MD-35 | 5 | 410 | 5 | 412 | 0.95 [0.24, 3.72] |
| Tooth ache | Clayton (AK130926) | 3 | 143 | 2 | 135 | 1.43 [0.23, 8.66] |
| Tremor | Clayton (AK130926) | 3 | 143 | 6 | 135 | 0.46 [0.11, 1.88] |
| Upper respiratory tract infection | Clayton (AK130926) , SCT-MD-35 | 20 | 274 | 14 | 269 | 1.45 [0.68, 3.08] |
| Escitalopram versus duloxetine | ||||||
| Accidental overdose | Khan 2007 | 0 | 137 | 1 | 133 | 0.32 [0.01, 7.96] |
| Chest pressure | Khan 2007 | 0 | 137 | 1 | 133 | 0.32 [0.01, 7.96] |
| Decreased appetite | Nierenberg 2007 | 13 | 274 | 22 | 273 | 0.57 [0.28, 1.15] |
| Depression | Khan 2007 | 0 | 137 | 2 | 133 | 0.19 [0.01, 4.02] |
| Dizziness | Khan 2007, Nierenberg 2007, Wade 2007 | 39 | 554 | 64 | 557 | 0.59 [0.39, 0.90] |
| Dyspepsia | Wade 2007 | 9 | 143 | 4 | 151 | 2.47 [0.74, 8.20] |
| Hypertension | Khan 2007 | 0 | 137 | 1 | 133 | 0.32 [0.01, 7.96] |
| Musculoskeletal disorder | Khan 2007 | 18 | 137 | 12 | 133 | 1.53 [0.70, 3.30] |
| Pharyngitis | Wade 2007 | 15 | 143 | 11 | 151 | 1.49 [0.66, 3.37] |
| Sexual problems | Khan 2007 | 5 | 56 | 7 | 48 | 0.57 [0.17, 1.94] |
| Subjects with non-fatal severe adverse events | Khan 2007, Nierenberg 2007 | 4 | 411 | 9 | 406 | 0.45 [0.07, 2.80] |
| Upper respiratory tract infection | Khan 2007, Nierenberg 2007 | 57 | 411 | 48 | 406 | 1.21 [0.79, 1.85] |
| Escitalopram versus fluoxetine | ||||||
| Anorexia | Kasper 2005 | 2 | 173 | 4 | 164 | 0.47 [0.08, 2.59] |
| Depression | Kasper 2005 | 2 | 173 | 4 | 164 | 0.47 [0.08, 2.59] |
| Dermatological problems | SCT-MD-09 | 3 | 16 | 2 | 14 | 1.38 [0.20, 9.77] |
| Disease Of Liver And Hepatic Duct | Mao 2008 | 2 | 123 | 6 | 117 | 0.31 [0.06, 1.55] |
| DIzziness | Kasper 2005, Kennedy 2005, Mao 2008 | 20 | 394 | 22 | 380 | 0.88 [0.46, 1.67] |
| Dyspepsia | Kasper 2005 | 4 | 173 | 7 | 164 | 0.53 [0.15, 1.85] |
| Hypertension | Kasper 2005 | 4 | 173 | 4 | 164 | 0.95 [0.23, 3.85] |
| Indigestion | Kennedy 2005 | 2 | 98 | 6 | 99 | 0.32 [0.06, 1.64] |
| Orthostatic symptoms | Kasper 2005 | 2 | 173 | 1 | 164 | 1.91 [0.17, 21.23] |
| Rhinitis | SCT-MD-09 | 1 | 16 | 3 | 14 | 0.24 [0.02, 2.68] |
| Sexual problems | Kennedy 2005 | 4 | 37 | 1 | 32 | 3.76 [0.40, 35.49] |
| Subjects with non-fatal severe ad-verse events | Kasper 2005, Kennedy 2005, Mao 2008 | 8 | 394 | 16 | 380 | 0.48 [0.20, 1.14] |
| Tooth ache | SCT-MD-09 | 1 | 16 | 2 | 14 | 0.40 [0.03, 4.96] |
| Vertigo | Kasper 2005 | 3 | 173 | 7 | 164 | 0.40 [0.10, 1.56] |
| Escitalopram versus paroxetine | ||||||
| DIzziness | Boulenger 2006 | 21 | 229 | 20 | 225 | 1.03 [0.54, 1.97] |
| Erectile dysfunction | Boulenger 2006 | 4 | 75 | 4 | 68 | 0.90 [0.22, 3.75] |
| Sexual problems | Boulenger 2006 | 2 | 75 | 6 | 68 | 0.28 [0.06, 1.45] |
| Subjects with non-fatal severe adverse events | Boulenger 2006 | 7 | 229 | 3 | 225 | 2.33 [0.60, 9.14] |
| Escitalopram versus sertraline | ||||||
| Decreased appetite | Ventura 2007 | 8 | 104 | 6 | 108 | 1.42 [0.47, 4.23] |
| Depression | Alexopoulos 2004 | 1 | 134 | 0 | 137 | 3.09 [0.12, 76.52] |
| Flatulence | Alexopoulos 2004 | 6 | 134 | 6 | 137 | 1.02 [0.32, 3.26] |
| Indigestion | Alexopoulos 2004 | 3 | 134 | 7 | 137 | 0.43 [0.11, 1.68] |
| Rhinitis | Alexopoulos 2004 | 6 | 134 | 7 | 137 | 0.87 [0.28, 2.66] |
| Sexual problems | Alexopoulos 2004, Ventura 2007 | 23 | 162 | 23 | 172 | 0.91 [0.48, 1.72] |
| Subjects with non-fatal severe adverse events | Alexopoulos 2004 | 3 | 134 | 1 | 137 | 3.11 [0.32, 30.32] |
| Upper respiratory tract infection | Alexopoulos 2004, Ventura 2007 | 21 | 238 | 26 | 245 | 0.82 [0.44, 1.50] |
| Escitalopram versus venlafaxine | ||||||
| Cardiac failure | Montgomery 2004 | 0 | 146 | 1 | 143 | 0.32 [0.01, 8.03] |
| Colon Obstruction | Bielski 2004 | 1 | 98 | 0 | 100 | 3.09 [0.12, 76.83] |
| Decreased weight | Montgomery 2004 | 5 | 146 | 10 | 143 | 0.47 [0.16, 1.42] |
| Dermatological problems | Montgomery 2004 | 0 | 146 | 1 | 143 | 0.32 [0.01, 8.03] |
| Dizziness | Montgomery 2004 | 7 | 146 | 8 | 143 | 0.85 [0.30, 2.41] |
| Gastritis | Montgomery 2004 | 0 | 146 | 3 | 143 | 0.14 [0.01, 2.68] |
| Inflicted injury | Bielski 2004 | 0 | 98 | 1 | 100 | 0.34 [0.01, 8.37] |
| Non-accidental overdose | Bielski 2004 | 0 | 98 | 1 | 100 | 0.34 [0.01, 8.37] |
| Sexual problems | Bielski 2004 | 2 | 30 | 12 | 53 | 0.24 [0.05, 1.18] |
| Subjects with non-fatal severe adverse events | Bielski 2004, Montgomery 2004 | 4 | 244 | 8 | 243 | 0.51 [0.14, 1.77] |
| Transitory Ischaemic Attack | Montgomery 2004 | 0 | 146 | 1 | 143 | 0.32 [0.01, 8.03] |
| Vertigo | Montgomery 2004 | 7 | 146 | 6 | 143 | 1.15 [0.38, 3.51] |
1. ESCITALOPRAM versus OTHER SSRIs
A. ESCITALOPRAM versus CITALOPRAM
PRIMARY OUTCOME
EFFICACY - Number of patients who responded to treatment
a) Acute phase treatment (6 to 12 weeks)
There was a statistically significant difference with escitalopram being more effective than citalopram (OR 0.67, 95% CI 0.50 to 0.89, p = 0.006; 6 studies, 1823 participants) (see Figure 3).
Figure 3. Forest plot of comparison: 1 Failure to respond at endpoint (6-12 weeks), outcome: 1.1 Escitalopram versus other SSRIs.
b) Early response (1 to 4 weeks)
No data available.
c) Follow-up response (16 to 24 weeks)
There was no statistically significant difference between escitalopram and citalopram (OR 0.96, 95% CI 0.60 to 1.56, p = 0.88; 1 study, 357 participants) (Analysis 3.1).
SECONDARY OUTCOMES
1) EFFICACY - Number of patients who achieved remission
a) Acute phase treatment (6 to 12 weeks)
There was a statistically significant difference with escitalopram being more effective than citalopram (OR 0.57, 95% CI 0.36 to 0.90, p = 0.02; 6 studies, 1823 participants) (see Figure 4). Test for heterogeneity was statistically significant: Tau2 = 0.25; Chi2 = 25.12, df = 5 (P<0.00001); I2=80%. The heterogeneity arose because of the extreme result in the Yevtushenko 2007 trial.
Figure 4. Forest plot of comparison: 4 Failure to remission at endpoint (6-12 weeks), outcome: 4.1 Escitalopram versus other SSRIs.
b) Early response (1 to 4 weeks)
No data available.
c) Follow-up response (16 to 24 weeks)
No data available.
2) EFFICACY - Mean change from baseline
a) Acute phase treatment: between 6 and 12 weeks
Escitalopram was found to be more efficacious than citalopram in reduction of depressive symptoms (SMD −0.17, 95% CI −0.30 to −0.04, p = 0.009; 5 studies, 1392 participants) (see Figure 5).
Figure 5. Forest plot of comparison: 6 Standardised mean difference at endpoint (6-12 weeks), outcome: 6.1 Escitalopram versus other SSRIs.
3) to 5) EFFICACY- Social adjustment, social functioning, health-related quality of life, costs to health care services
No data available.
6) ACCEPTABILITY - Drop out rate
No statistically significant difference was found in terms of discontinuation due to any cause (OR 0.78, 95% CI 0.56 to 1.10, p = 0.16; 6 studies, 1823 participants) (see Figure 6).
No statistically significant difference was found in terms of discontinuation due to inefficacy (OR 0.74, 95% CI 0.27 to 2.03, p = 0.56; 5 studies, 1604 participants) (see Figure 7).
No statistically significant difference was found in terms of discontinuation due to side effects (OR 0.79, 95% CI 0.47 to 1.31, p = 0.36; 5 studies, 1604 participants) (see Figure 8).
Figure 6. Forest plot of comparison: 7 Failure to complete (any cause), outcome: 7.1 Escitalopram vs. other SSRIs.
Figure 7. Forest plot of comparison: 8 Failure to complete (due to inefficacy), outcome: 8.1 Escitalopram versus other SSRIs.
Figure 8. Forest plot of comparison: 9 Failure to complete (due to side effects), outcome: 9.1 Escitalopram vs. other SSRIs.
7) TOLERABILITY
Total number of patients experiencing at least one side effect
There was no evidence that escitalopram was associated with a smaller or higher rate of adverse events than citalopram (OR 0.79, 95% CI 0.58 to 1.07, p = 0.12; 6 studies, 1802 participants) (Analysis 10.1).
Total number of patients experiencing a specific side effect (only figures for statistically significant differences were reported in the text)
a) Agitation/Anxiety
There was no evidence that escitalopram was associated with a higher or less rate of participants experiencing agitation/anxiety than citalopram (Analysis 11.1).
b) Constipation
No difference was found between escitalopram and citalopram in terms of number of participants experiencing constipation (Analysis 12.1)
c) Diarrhoea
No difference was found between escitalopram and citalopram in terms of number of participants experiencing diarrhoea (Analysis 13.1).
d) Dry mouth
No difference was found between escitalopram and citalopram in terms of number of participants experiencing dry mouth (Analysis 14.1).
e) Hypotension
A single case of hypotension was reported in one study (Moore 2005) and so there was no statistically significant difference between escitalopram and citalopram (Analysis 15.1).
f) Insomnia
There was no evidence that escitalopram was associated with a higher or less rate of participants experiencing insomnia than citalopram (Analysis 16.1).
g) Nausea
There was no evidence that escitalopram was associated with a higher or less rate of participants experiencing nausea than citalopram (Analysis 17.1).
h) Urination problems
No data reported.
i) Sleepiness/drowsiness
There was no evidence that escitalopram was associated with a higher or less rate of participants experiencing sleepiness than citalopram (Analysis 18.1).
j) Vomiting
There was no evidence that escitalopram was associated with a higher or lower rate of participants experiencing vomiting than citalopram (Analysis 19.1).
k) Deaths, suicide and suicidality
One patient developed suicidal ideation/tendency (in the escitalopram group) (Analysis 31.1), a total of nine patients attempted suicide (six with escitalopram and three with citalopram) (Analysis 31.2) and one patient died (in the citalopram group) (Analysis 31.4), which was by suicide (Analysis 31.3). However, none of these differences were statistically significant.
l) Other adverse events
Escitalopram was associated with a lower rate of participants experiencing jitteriness than citalopram (OR 0.16, 95% CI 0.03 to 0.82, p = 0.03; 1 trial, 369 participants (Analysis 25.1). No statistically significant differences were found for dizziness (Analysis 20.1), fatigue (Analysis 21.1), flu syndrome (Analysis 22.1), headache (Analysis 23.1), impotence (Analysis 24.1), lethargy/sedation (Analysis 26.1), decreased libido (Analysis 27.1), pain (Analysis 28.1, Analysis 28.2, Analysis 28.3), increased sweating (Analysis 29.1) or yawning (Analysis 30.1).
B. ESCITALOPRAM versus FLUOXETINE
PRIMARY OUTCOME
EFFICACY - Number of patients who responded to treatment
a) Acute phase treatment (6 to 12 weeks)
There was no evidence that escitalopram was more or less efficacious than fluoxetine in the acute phase of treatment (OR 0.81, 95% CI 0.60 to 1.10, p = 0.17; 3 studies, 783 participants) (see Figure 3).
b) Early response (1 to 4 weeks)
Only one trial reported data on the early phase of treatment and the difference was not statistically significant (OR 1.15, 95% CI 0.52 to 2.56, p = 0.73; 1 study, 240 participants) (Analysis 2.1).
c) Follow-up response (16 to 24 weeks)
No data available
SECONDARY OUTCOMES
1) EFFICACY - Number of patients who achieved remission
a) Acute phase treatment (6 to 12 weeks)
There was no evidence that escitalopram was more or less efficacious than fluoxetine in terms of remission of symptoms (OR 0.86, 95% CI 0.65 to 1.15, p = 0.32; 3 studies, 783 participants) (see Figure 4).
b) Early response (1 to 4 weeks)
No data available.
c) Follow-up response (16 to 24 weeks)
No data available.
2) EFFICACY - Mean change from baseline
a) Acute phase treatment: between 6 and 12 weeks
Escitalopram was found to be more efficacious than fluoxetine in reduction of depressive symptoms (SMD −0.17, 95% CI −0.32 to −0.03, p = 0.02; 3 studies, 759 participants) (see Figure 5).
3) to 5) EFFICACY- Social adjustment, social functioning, health-related quality of life, costs to health care services
No data available.
6) ACCEPTABILITY - Drop out rate
No statistically significant difference was found in terms of discontinuation due to any cause (OR 0.89, 95% CI 0.51 to 1.55, p = 0.68; 4 studies, 813 participants) (see Figure 6).
No statistically significant difference was found in terms of discontinuation due to inefficacy (OR 0.57, 95% CI 0.15 to 2.15, p = 0.41; 4 studies, 813 participants) (see Figure 7).
No statistically significant difference was found in terms of discontinuation due to side effects (OR 0.75, 95% CI 0.44 to 1.28, p = 0.29; 4 studies, 813 participants) (see Figure 8).
7) TOLERABILITY
Total number of patients experiencing at least one side effect
There was no evidence that escitalopram was associated with a less or higher rate of adverse events than fluoxetine (OR 0.80, 95% CI 0.59 to 1.07, p = 0.13; 4 studies, 804 participants) (Analysis 10.1).
Total number of patients experiencing specific side effects (only figures for statistically significant differences are reported in the text - for full details see graphs)
a) Agitation/Anxiety
There was no evidence that escitalopram was associated with a higher or lower rate of participants experiencing agitation/anxiety than fluoxetine (Analysis 11.1).
b) Constipation
No difference was found between escitalopram and fluoxetine in terms of number of participants experiencing constipation (Analysis 12.1).
c) Diarrhoea
No difference was found between escitalopram and fluoxetine in terms of number of participants experiencing diarrhoea (Analysis 13.1).
d) Dry mouth
No difference was found between escitalopram and fluoxetine in terms of number of participants experiencing dry mouth (Analysis 14.1).
e) Hypotension
No data available
f) Insomnia
There was no evidence that escitalopram was associated with a higher or lower rate of participants experiencing insomnia than fluoxetine (Analysis 16.1).
g) Nausea
There was no evidence that escitalopram was associated with a higher or lower rate of participants experiencing nausea than fluoxetine (Analysis 17.1).
h) Urination problems
No data reported.
i) Sleepiness/drowsiness
There was no evidence that escitalopram was associated with a higher or lower rate of participants experiencing sleepiness than fluoxetine (Analysis 18.1).
j) Vomiting
No data reported.
k) Deaths, suicide and suicidality
Two patients attempted suicide (one with escitalopram and one with fluoxetine) (Analysis 31.2). Neither of these differences were statistically significant. Overall three patients died, two in the fluoxetine group and one in the escitalopram group (Analysis 31.4) (this patient committed suicide) (Analysis 31.3). No data about suicidal tendency/ideation were reported.
l) Other adverse events
No statistically significant differences were found for dizziness (Analysis 20.1), fatigue (Analysis 21.1), headache (Analysis 23.1), impotence (Analysis 24.1), libido decreased (Analysis 27.1) or pain (Analysis 28.1, Analysis 28.2).
C. ESCITALOPRAM versus PAROXETINE
PRIMARY OUTCOME
EFFICACY - Number of patients who responded to treatment
a) Acute phase treatment (6 to 12 weeks)
There was no evidence that escitalopram was more or less efficacious than paroxetine in terms of response to treatment (OR 0.89, 95% CI 0.61 to 1.32, p = 0.57; 2 studies, 784 participants) (see Figure 3).
b) Early response (1 to 4 weeks)
No data available.
c) Follow-up response (16 to 24 weeks)
There was no statistically significant difference between escitalopram and paroxetine (OR 0.73, 95% CI 0.47 to 1.15, p = 0.17; 1 study, 459 participants) (Analysis 3.1).
SECONDARY OUTCOMES
1) EFFICACY - Number of patients who achieved remission
a) Acute phase treatment (6 to 12 weeks)
There was no evidence that escitalopram was more or less efficacious than paroxetine in terms of remission of symptoms (OR 0.87, 95% CI 0.45 to 1.68, p = 0.67; 2 studies, 784 participants) (see Figure 4).
b) Early response (1 to 4 weeks)
No data available.
c) Follow-up response (16 to 24 weeks)
No data available.
2) EFFICACY - Mean change from baseline
(a) Acute phase treatment: between 6 and 12 weeks
There was no evidence that escitalopram was more or less efficacious than paroxetine in reduction of depressive symptoms (SMD −0.05, 95% CI −0.36 to 0.26, p = 0.76; 2 studies, 776 participants) (see Figure 5).
3) to 5) EFFICACY- Social adjustment, social functioning, health-related quality of life, costs to health care services
No data available.
6) ACCEPTABILITY - Drop out rate
No statistically significant difference was found in terms of discontinuation due to any cause (OR 0.68, 95% CI 0.36 to 1.29, p = 0.24; 2 studies, 784 participants) (see Figure 6).
No statistically significant difference was found in terms of discontinuation due to inefficacy (OR 1.39, 95% CI 0.17 to 11.44, p = 0.76; 2 studies, 784 participants) (see Figure 7).
No statistically significant difference was found in terms of discontinuation due to side effects (OR 0.70, 95% CI 0.25 to 1.96, p = 0.50; 2 studies, 784 participants) (see Figure 8).
7) TOLERABILITY
Total number of patients experiencing at least one side effect
There was no evidence that escitalopram was associated with a smaller or larger rate of adverse events than paroxetine (OR 0.78, 95% CI 0.52 to 1.17, p = 0.23; 1 study, 454 participants) (Analysis 10.1).
Total number of patients experiencing a specific side effect (only figures for statistically significant differences were reported in the text)
a) Agitation/Anxiety
No data reported.
b) Constipation
No difference was found between escitalopram and paroxetine in terms of number of participants experiencing constipation (Analysis 12.1).
c) Diarrhoea
No difference was found between escitalopram and paroxetine in terms of number of participants experiencing diarrhoea (Analysis 13.1).
d) Dry mouth
No difference was found between escitalopram and paroxetine in terms of number of participants experiencing dry mouth (Analysis 14.1).
e) Hypotension
No data reported.
f) Insomnia
No difference was found between escitalopram and paroxetine in terms of number of participants experiencing insomnia (Analysis 16.1).
g) Nausea
There was no evidence that paroxetine was associated with a higher or lower rate of participants experiencing nausea than escitalopram (Analysis 17.1).
h) Urination problems
No data reported.
i) Sleepiness/drowsiness
No data reported.
j) Vomiting
No data reported.
k) Deaths, suicide and suicidality
Neither deaths, nor completed or attempted suicides were reported.
l) Other adverse events
No statistically significant differences were found for dizziness (Analysis 20.1), headache (Analysis 23.1) or increased sweating (Analysis 24.1).
D. ESCITALOPRAM versus SERTRALINE
PRIMARY OUTCOME
EFFICACY - Number of patients who responded to treatment
a) Acute phase treatment (6 to 12 weeks)
There was no evidence that escitalopram was less or more efficacious than sertraline in terms of response to treatment in the acute phase (OR 1.06, 95% CI 0.73 to 1.53, p = 0.76; 2 studies, 489 participants) (see Figure 3).
b) Early response (1 to 4 weeks)
No data available.
c) Follow-up response (16 to 24 weeks)
No data available.
SECONDARY OUTCOMES
1) EFFICACY - Number of patients who achieved remission
a) Acute phase treatment (6 to 12 weeks)
There was no evidence that escitalopram was less or more efficacious than sertraline in terms of remission of symptoms (OR 1.16, 95% CI 0.81 to 1.67, p = 0.42; 2 studies, 489 participants (see Figure 4).
b) Early response (1 to 4 weeks)
No data available.
c) Follow-up response (16 to 24 weeks)
No data available.
2) EFFICACY - Mean change from baseline
a) Acute phase treatment: between 6 and 12 weeks
There was no evidence that escitalopram was less or more efficacious than sertraline in reduction of depressive symptoms (SMD 0.02, 95% CI −0.16 to 0.20, p = 0.85; 2 studies, 477 participants) (see Figure 5).
3) to 5) EFFICACY- Social adjustment, social functioning, health-related quality of life, costs to health care services
No data available.
6) ACCEPTABILITY - Drop out rate
No statistically significant difference was found in terms of discontinuation due to any cause (OR 1.24, 95% CI 0.78 to 1.97, p = 0.37; 2 studies, 489 participants) (see Figure 6).
No statistically significant difference was found in terms of discontinuation due to inefficacy (OR 3.09, 95% CI 0.32 to 30.08, p = 0.33; 1 study, 274 participants) (see Figure 7).
No statistically significant difference was found in terms of discontinuation due to side effects (OR 1.08, 95% CI 0.35 to 3.37, p = 0.89; 2 studies, 489 participants) (see Figure 8).
6) TOLERABILITY
Total number of patients experiencing at least one side effects
There was no evidence that escitalopram was associated with a smaller or larger rate of adverse events than sertraline (OR 0.62, 95% CI 0.33 to 1.19, p = 0.15; 2 studies, 483 participants) (Analysis 10.1).
Total number of patients experiencing a specific side effect (only figures for statistically significant differences were reported in the text)
a) Agitation/Anxiety
No data reported.
b) Constipation
No data reported.
c) Diarrhoea
There was evidence that escitalopram was associated with a lower rate of participants experiencing diarrhoea than sertraline (OR 0.49, 95% CI 0.28 to 0.84, p = 0.009; 2 trials, 483 participants) (Analysis 13.1).
d) Dry mouth
No difference was found between escitalopram and sertraline in terms of number of participants experiencing dry mouth (Analysis 14.1).
e) Hypotension
No data reported.
f) Insomnia
No difference was found between escitalopram and sertraline in terms of number of participants experiencing insomnia (Analysis 16.1).
g) Nausea
There was no evidence that escitalopram was associated with a higher or lower rate of participants experiencing nausea than sertraline (Analysis 17.1).
h) Urination problem
No data reported.
i) Sleepiness/drowsiness
There was no evidence that escitalopram was associated with a higher or lower rate of participants experiencing sleepiness/drowsiness than sertraline (Analysis 18.1).
j) Vomiting
No data reported.
k) Deaths, suicide and suicidality
Neither deaths, nor completed or attempted suicides were reported.
l) Other adverse events
Although not statistically significant, there was some evidence in one study (Ventura 2007) that escitalopram was associated with a higher rate of participants experiencing lethargy/sedation than sertraline (OR 3,72, 95% CI 0.99 to 13.94, p = 0.05; 1 trial, 212 participants). No statistically significant differences were found for fatigue (Analysis 21.1), headache (Analysis 22.1), impotence (Analysis 23.1), lethargy/sedation (Analysis 26.1), decreased libido (Analysis 27.1) or increased sweating (Analysis 29.1).
2) ESCITALOPRAM versus NEWER ANTIDEPRESSANTS
A. ESCITALOPRAM versus BUPROPION
PRIMARY OUTCOME
EFFICACY - Number of patients who responded to treatment
a) Acute phase treatment (6 to 12 weeks)
There was no evidence that escitalopram was more efficacious than bupropion in terms of response to treatment in the acute phase (OR 0.91, 95% CI 0.69 to 1.20, p = 0.50; 3 studies, 842 participants) (see Figure 9).
Figure 9. Forest plot of comparison: 1 Failure to respond at endpoint (6-12 weeks), outcome: 1.2 Escitalopram versus newer ADs.
b) Early response (1 to 4 weeks)
No data available.
c) Follow-up response (16 to 24 weeks)
No data available.
SECONDARY OUTCOMES
1) EFFICACY - Number of patients who achieved remission
a) Acute phase treatment (6 to 12 weeks)
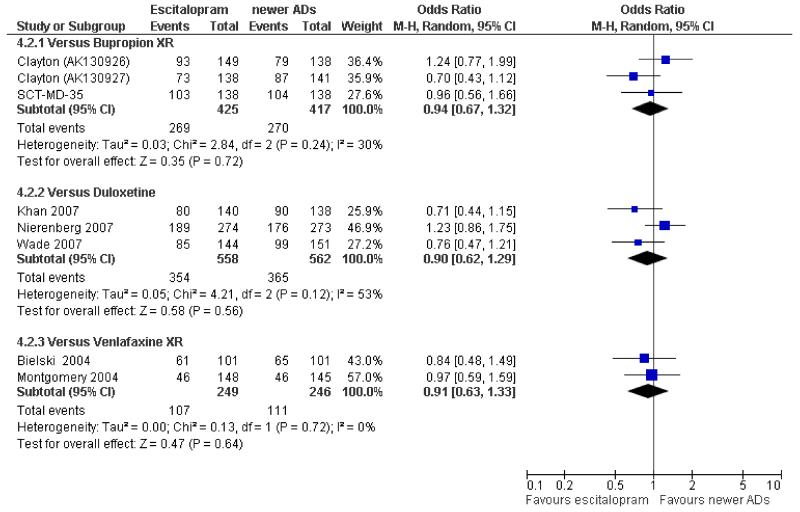
There was no evidence that escitalopram was more efficacious than bupropion in terms of remission of depressive symptoms (OR 0.94, 95% CI 0.67 to 1.32, p = 0.72; 3 studies, 842 participants) (see Figure 10).
Figure 10. Forest plot of comparison: 4 Failure to remission at endpoint (6-12 weeks), outcome: 4.2 Escitalopram versus newer ADs.
b) Early response (1 to 4 weeks)
No data available.
c) Follow-up response (16 to 24 weeks)
No data available.
2) EFFICACY - Mean change from baseline
a) Acute phase treatment: between 6 and 12 weeks
There was no evidence that escitalopram was more efficacious than bupropion in reduction of depressive symptoms (SMD −0.08, 95% CI −0.22 to 0.05, p = 0.23; 3 studies, 793 participants) (see Figure 11).
Figure 11. Forest plot of comparison: 6 Standardised mean difference at endpoint (6-12 weeks), outcome: 6.2 Escitalopram versus newer ADs.
3) to 5) EFFICACY- Social adjustment, social functioning, health-related quality of life, costs to health care services
No data available.
6) ACCEPTABILITY - Drop out rate
No statistically significant difference was found in terms of discontinuation due to any cause (OR 1.02, 95% CI 0.75 to 1.39, p = 0.90; 3 studies, 842 participants) (see Figure 12)
No statistically significant difference was found in terms of discontinuation due to inefficacy (OR 0.11, 95% CI 0.01 to 2.02, p = 0.14; 1 study, 276 participants) (see Figure 13)
No statistically significant difference was found in terms of discontinuation due to side effects (OR 0.65, 95% CI 0.25 to 1.65, p = 0.36; 3 studies, 842 participants) (see Figure 14)
Figure 12. Forest plot of comparison: 7 Failure to complete (any cause), outcome: 7.2 Escitalopram versus newer ADs.
Figure 13. Forest plot of comparison: 8 Failure to complete (due to inefficacy), outcome: 8.2 Escitalopram versus newer ADs.
Figure 14. Forest plot of comparison: 9 Failure to complete (due to side effects), outcome: 9.2 Escitalopram versus newer ADs.
7) TOLERABILITY
Total number of patients experiencing at least one side effect
There was no evidence that escitalopram was associated with a smaller rate of adverse events than bupropion (OR 0.77, 95% CI 0.55 to 1.07, p = 0.12; 3 studies, 822 participants) (Analysis 10.2).
Total number of patients experiencing a specific side effect (only figures for statistically significant differences were reported in the text)
a) Agitation/Anxiety
No difference was found between escitalopram and bupropion in terms of number of participants experiencing agitation/anxiety (Analysis 11.2).
b) Constipation
There was evidence that escitalopram was associated with a lower rate of participants experiencing constipation than bupropion (OR 0.32, 95% CI 0.15 to 0.69, p = 0.004; 2 studies, 557 participants (Analysis 12.2).
c) Diarrhoea
No difference was found between escitalopram and bupropion in terms of number of participants experiencing diarrhoea (Analysis 13.2).
d) Dry mouth
There was evidence that escitalopram was associated with a lower rate of participants experiencing dry mouth than bupropion (OR 0.58, 95% CI 0.39 to 0.87, p = 0.007; 3 studies, 822 participants) (Analysis 14.2).
e) Hypotension
No data reported.
f) Insomnia
There was evidence that escitalopram was associated with a lower rate of participants experiencing insomnia than bupropion (OR 0.55, 95% CI 0.33 to 0.92, p = 0.02; 3 studies, 822 participants) (Analysis 16.2).
g) Nausea
There was no evidence that escitalopram was associated with a higher or lower rate of participants experiencing nausea than bupropion (Analysis 17.2).
h) Urination problem
No data reported.
i) Sleepiness/drowsiness
There was no evidence that escitalopram was associated with a higher or lower rate of participants experiencing sleepiness than bupropion (Analysis 18.2).
j) Vomiting
No data reported.
k) Deaths, suicide and suicidality
Two patients developed suicidal ideation/tendency (both in the escitalopram group), however this difference was not statistically significant (Analysis 31.1). Neither deaths, nor completed or attempted suicides were reported.
l) Other adverse events
There was evidence that escitalopram was associated with a higher rate of participants experiencing fatigue (OR 3.48, 95%CI 1.77 to 6.84, p = 0.0003; 2 studies, 557 participants) (Analysis 21.2) and yawning (OR 7.71, 95%CI 1.75 to 34.05, p = 0.007; 2 studies, 557 participants) (Analysis 30.2) than bupropion. Although not statistically significant, there was some evidence that irritability was less frequent in patients randomised to escitalopram than in patients allocated to bupropion (OR 0.26, 95% CI 0.06 to 1.04, p = 0.06; 2 trials, 557 participants). No statistically significant differences were found for dizziness (Analysis 20.2), headache (Analysis 23.2), or pain (Analysis 28.1, Analysis 28.2, Analysis 28.3, Analysis 28.4).
B. ESCITALOPRAM versus DULOXETINE
PRIMARY OUTCOME
EFFICACY - Number of patients who responded to treatment
a) Acute phase treatment (6 to 12 weeks)
There was no evidence that escitalopram was more or less efficacious than duloxetine in terms of response to treatment in the acute phase (OR 0.72, 95% CI 0.43 to 1.20, p = 0.21; 3 studies, 1120 participants) (see Figure 9).
b) Early response (1 to 4 weeks)
No data available.
c) Follow-up response (16 to 24 weeks)
There was no evidence that escitalopram was more or less efficacious than duloxetine in terms of response to treatment at 24 weeks (OR 0.72, 95% CI 0.42 to 1.25, p = 0.25; 1 study, 295 participants) (Analysis 3.2).
SECONDARY OUTCOMES
1) EFFICACY - Number of patients who achieved remission
a) Acute phase treatment (6 to 12 weeks)
There was no evidence that escitalopram was more or less efficacious than duloxetine in terms of remission of depressive symptoms during the acute phase treatment (OR 0.90, 95% CI 0.62 to 1.29, p = 0.56; 3 studies, 1120 participants) (see Figure 10).
b) Early response (1 to 4 weeks)
No data available.
c) Follow-up response (16 to 24 weeks)
There was no evidence that escitalopram was more or less efficacious than duloxetine in terms of remission of depressive symptoms at 24 weeks (OR 0.72, 95% CI 0.45 to 1.16, p = 0.18; 1 study, 295 participants) (Analysis 5.1).
2) EFFICACY - Mean change from baseline
a) Acute phase treatment: between 6 and 12 weeks
There was no evidence that escitalopram was more or less efficacious than duloxetine in reduction of depressive symptoms (SMD −0.10, 95% CI −0.30 to 0.09, p = 0.28; 3 studies, 1096 participants) (see Figure 11).
3) to 5) EFFICACY- Social adjustment, social functioning, health-related quality of life, costs to health care services
One study (Wade 2007) used the SF-36 as a measure of general health status. Ratings from eight subscales were reported and no statistically significant differences between escitalopram and duloxetine were found (data not shown here but available from authors).
6) ACCEPTABILITY - Drop out rate
There was a statistically significant difference with fewer patients allocated to escitalopram withdrawing from study than duloxetine for discontinuation due to any cause (OR 0.62, 95% CI 0.38 to 0.99, p = 0.05; 3 studies, 1120 participants) (see Figure 12).
No statistically significant difference was found in terms of discontinuation due to inefficacy (OR 0.95, 95% CI 0.21 to 4.25, p = 0.95; 3 studies, 1120 participants) (see Figure 13)
No statistically significant difference was found in terms of discontinuation due to side effects (OR 0.49, 95% CI 0.18 to 1.29, p = 0.15; 3 studies, 1120 participants) (see Figure 14)
7) TOLERABILITY
Total number of patients experiencing at least one side effect.
No statistically significant difference was found in terms of rate of adverse events (OR 0.96, 95% CI 0.67 to 1.38, p = 0.82; 3 studies, 1111 participants) (Analysis 10.2).
Total number of patients experiencing a specific side effect (only figures for statistically significant differences were reported in the text)
a) Agitation/Anxiety
There was no evidence that escitalopram was associated with a higher or lower rate of participants experiencing agitation/anxiety than duloxetine (Analysis 11.2).
b) Constipation
There was no evidence that escitalopram was associated with a higher or lower rate of participants experiencing constipation than duloxetine (Analysis 12.2).
c) Diarrhoea
No difference was found between escitalopram and duloxetine in terms of number of participants experiencing diarrhoea (Analysis 13.2).
d) Dry mouth
There was evidence that escitalopram was associated with a lower rate of participants experiencing dry mouth than duloxetine (OR 0.55, 95% CI 0.39 to 0.79, p = 0.001; 3 trials, 1111 participants) (Analysis 14.2).
e) Hypotension
No data reported.
f) Insomnia
Even though not statistically significant, there was some evidence that insomnia was less frequent in patients treated with escitalopram than in patients randomised to duloxetine (OR 0.58, 95% CI 0.33 to 1.02, p = 0.06; 3 trials, 1111 participants) (Analysis 16.2).
g) Nausea
There was evidence that escitalopram was associated with a lower rate of participants experiencing nausea than duloxetine (OR 0.56, 95% CI 0.42 to 0.75, p = 0.0001; 3 trials, 1111 participants) (Analysis 17.2).
h) Urination problem
No data reported.
i) Sleepiness/drowsiness
There was no evidence that escitalopram was associated with a higher or lower rate of participants experiencing sleepiness than duloxetine (Analysis 18.2).
j) Vomiting
There was no evidence that escitalopram was associated with a higher or lower rate of participants experiencing vomiting than duloxetine (Analysis 19.2).
k) Deaths, suicide and suicidality
Overall two patients died, one in the escitalopram group and one in the duloxetine group (this patient committed suicide), and these differences were not statistically significant (Analysis 31.3, Analysis 31.4). No data about attempted suicide or suicidal tendency/ideation were reported.
l) Other adverse events
Escitalopram was associated with a lower rate of participants experiencing dizziness than duloxetine (OR 0.59, 95%CI 0.39 to 0.90, p = 0.01; 3 trials, 1111 participants). (Analysis 20.2). Though not statistically significant, there was some evidence that irritability was less frequent in patients randomised to escitalopram than in patients treated with duloxetine (OR 0.39, 95% CI 0.15 to 1.01, p = 0.05; 1 trials, 547 participants. No statistically significant differences were found for fatigue (Analysis 21.2), flu syndrome (Analysis 22.2), headache (Analysis 23.2), decreased libido (Analysis 27.2), pain (Analysis 28.2), increased sweating (Analysis 29.2) and yawning (Analysis 30.2).
C. ESCITALOPRAM versus VENLAFAXINE
PRIMARY OUTCOME
EFFICACY - Number of patients who responded to treatment
a) Acute phase treatment (6 to 12 weeks)
There was no evidence that escitalopram was more or less efficacious than venlafaxine in terms of response to treatment in the acute phase (OR 0.86, 95% CI 0.53 to 1.39, p = 0.53; 2 studies, 495 participants) (see Figure 9).
b) Early response (1 to 4 weeks)
Only one trial reported data on the early phase of treatment and the difference was not statistically significant (OR 0.85, 95% CI 0.47 to 1.55, p = 0.60; 1 study, 293 participants) (Analysis 2.2).
c) Follow-up response (16 to 24 weeks)
No data available.
SECONDARY OUTCOMES
1) EFFICACY - Number of patients who achieved remission
a) Acute phase treatment (6 to 12 weeks)
There was no evidence that escitalopram was more or less efficacious than venlafaxine in terms of remission of depressive symptoms during the acute phase treatment (OR 0.91, 95% CI 0.63 to 1.33, p = 0.64; 2 studies, 495 participants) (see Figure 10).
b) Early response (1 to 4 weeks)
No data available.
c) Follow-up response (16 to 24 weeks)
No data available.
2) EFFICACY - Mean change from baseline
a) Acute phase treatment: between 6 and 12 weeks
There was no evidence that escitalopram was more or less efficacious than venlafaxine in reduction of depressive symptoms (SMD −0.07, 95% CI −0.38 to 0.25, p = 0.68; 5 studies, 283 participants) (see Figure 11).
3) to 5) EFFICACY- Social adjustment, social functioning, health-related quality of life, costs to health care services
No data available.
6) ACCEPTABILITY - Drop out rate
No statistically significant difference was found in terms of discontinuation due to any cause (OR 0.90, 95% CI 0.58 to 1.39, p = 0.62; 2 studies, 495 participants) (see Figure 12).
No statistically significant difference was found in terms of discontinuation due to inefficacy (OR 9.06, 95% CI 0.48 to 169.85, p = 0.14; 1 study, 293 participants) (see Figure 13).
There was no evidence that escitalopram was statistically significantly better than venlafaxine in terms of discontinuation due to side effects (OR 0.41, 95% CI 0.14 to 1.17, p = 0.09; 2 studies, 495 participants) (see Figure 14).
7) TOLERABILITY
Total number of patients experiencing at least one side effect
There was no evidence that escitalopram was associated with a smaller rate of adverse events than venlafaxine (OR 0.58, 95% CI 0.28 to 1.23, p = 0.16; 2 studies, 487 participants) (Analysis 10.2).
Total number of patients experiencing a specific side effect (only figures for statistically significant differences were reported in the text)
a) Agitation/Anxiety
There was no evidence that escitalopram was associated with a higher or lower rate of participants experiencing agitation/anxiety than venlafaxine (Analysis 11.2).
b) Constipation
There was no evidence that escitalopram was associated with a higher or lower rate of participants experiencing constipation than venlafaxine (Analysis 12.2).
c) Diarrhoea
No difference was found between escitalopram and venlafaxine in terms of number of participants experiencing diarrhoea (Analysis 13.2).
d) Dry mouth
There was no evidence that escitalopram was associated with a higher or lower rate of participants experiencing dry mouth than venlafaxine (Analysis 14.2).
e) Hypotension
No data reported.
f) Insomnia
No difference was found between escitalopram and venlafaxine in terms of number of participants experiencing insomnia (Analysis 16.2).
g) Nausea
There was evidence that escitalopram was associated with a lower rate of participants experiencing nausea than venlafaxine (OR 0.37, 95% CI 0.14 to 0.99, p = 0.05; 2 trials, 487 participants) (Analysis 17.2).
h) Urination problem
No data reported.
i) Sleepiness/drowsiness
No difference was found between escitalopram and venlafaxine in terms of number of participants experiencing sleepiness (Analysis 18.2).
j) Vomiting
No data reported.
k) Deaths, suicide and suicidality
One patient developed suicidal ideation/tendency (in the escitalopram group) (Analysis 31.1) and two patients attempted suicide (both randomised to escitalopram) (Analysis 31.2). One patient died in the venlafaxine group (Analysis 31.4) but no completed suicide was reported in both the comparisons. None of these differences were statistically significant.
l) Other adverse events
Escitalopram was associated with a lower rate of participants experiencing increased sweating than venlafaxine (OR 0.45, 95%CI 0.23 to 0.87, p = 0.02; 2 trials, 487 participants (Analysis 29.2). No statistically significant differences were found for dizziness (Analysis 20.2), fatigue (Analysis 21.2), headache (Analysis 23.2), impotence (Analysis 24.2), lethargy/sedation (Analysis 26.2), decreased libido (Analysis 27.2) and pain (Analysis 28.1, Analysis 28.2).
SUBGROUP ANALYSES
1) Escitalopram dosing
All studies used escitalopram within the standard therapeutic range (10 to 20 mg/day), with the exception of only one unpublished study (SCT-MD-35) where escitalopram dose was set at 4 mg/day. Therefore, it was not meaningful to carry out this pre-planned sub-group analysis.
2) Comparator dosing
All comparator doses were within the therapeutic range, with the exception of two studies (SCT-MD-35; Yevtushenko 2007). Due to the small number of trials without the therapeutic range, it was not considered meaningful to carry out this pre-planned subgroup analysis.
3) Depression severity
All studies reported a mean baseline score corresponding to moderate to severe major depression, with the exception of three studies where the mean baseline score corresponded to a mild major depression (Montgomery 2004; Nierenberg 2007; SCT-MD-09). Therefore, it was not meaningful to carry out this pre-planned sub-group analysis.
4) Treatment settings
Only one study selectively recruited patients in general practice (Lepola 2003) and no studies enrolled only inpatients, therefore it was not considered meaningful to carry out this pre-planned subgroup analysis.
5) Elderly patients
As only one study specifically recruited elderly patients (Kasper 2005), it was not meaningful to carry out this pre-planned subgroup analysis.
FUNNEL PLOT ANALYSIS
As stated in the protocol, analyses were carried out as head-to head comparisons. The presence of publication bias was not examined because there were insufficient trials to allow meaningful formal assessment using funnel plots.
SENSITIVITY ANALYSES
1) Excluding trials with unclear concealment of random allocation and/or unclear double blinding
Although technically possible to carry out these sensitivity analyses, they were not performed, because they would not have contributed useful information due to the small number of studies (only four trials) reporting clear details on concealment of random allocation (Baldwin 2006; Boulenger 2006; Wade 2007; Colonna 2005).
2) Excluding trials whose dropout rate was greater than 20%
Referring to other SSRIs, a dropout rate greater than 20% was found for two studies comparing escitalopram with citalopram (Burke 2002; SCT-MD-02), one with fluoxetine (Kennedy 2005) and one with paroxetine (Boulenger 2006). Among newer antidepressants, a dropout rate greater than 20% was found for all the three studies comparing escitalopram with bupropion (Clayton (AK130926); Clayton (AK130927); SCT-MD-35), two with duloxetine (Nierenberg 2007; Wade 2007) and one with venlafaxine (Bielski 2004). Three studies had only one arm reporting a dropout rate greater than 20% (Colonna 2005; Kasper 2005; Khan 2007). Therefore, sensitivity analyses were carried out only for the comparisons between (i) escitalopram and citalopram, and (ii) escitalopram and fluoxetine.
a) escitalopram vs citalopram
Results from the sensitivity analyses were still statistically significant in favour of escitalopram, not only when trials whose dropout rate was greater than 20% in both arms were excluded (OR 0.56, 95% CI 0.40 to 0.79, p = 0.0009; 4 studies, 1187 participants) but also when trials whose dropout rate was greater than 20% in only one arm were additionally excluded (OR 0.49, 95% CI 0.34 to 0.72, p = 0.0002; 3 studies, 830 participants).
b) escitalopram vs fluoxetine
Results from the sensitivity analyses were still not statistically significant when trials whose dropout rate was greater than 20% in both arms were excluded (OR 0.80, 95% CI 0.56 to 1.13, p = 0.20; 2 studies, 578 participants).
3) Performing the worst and best-case scenario analysis
Results from these sensitivity analyses did not materially change the main findings (full details available on request from authors)
4) Excluding trials for which the imputation methods were used
a) Imputed response rate
Excluding trials for which the response rate had to be calculated based on the imputation method, results for all comparisons did not materially change.
b) Imputed remission rate
Excluding trials for which the remission rate had to be calculated based on the imputation method, results for all comparisons did not materially change.
c) Borrowed SDs
Excluding trials for which the SD had to be borrowed from other trials, the two previously statistically significant results (namely, escitalopram versus citalopram and escitalopram versus fluoxetine) became no more statistically significant. The remaining outcomes did not materially change.
5) Examination of “wish bias” and exclusion of studies funded by the pharmaceutical company marketing escitalopram
These pre-planned sensitivity analyses were not carried out because there were insufficient trials run by manufacturers other than the one marketing escitalopram to allow meaningful formal assessment. In almost all the included studies (19 out of 22) escitalopram was the investigational drug, with the exception of two studies carried out by the bupropion manufacturer (Clayton (AK130926); Clayton (AK130927)), one by the duloxetine manufacturer (Nierenberg 2007) where escitalopram was used as reference compound.
DISCUSSION
Summary of main results
Twenty two trials were included in this review. Some statistically significant differences favouring escitalopram over other antidepressants for the acute phase treatment of major depression were found in terms of efficacy (citalopram and fluoxetine) and acceptability (duloxetine). The included studies did not report on all the outcomes that were pre-specified in the protocol of this review. Outcomes of clear relevance to patients and clinicians, in particular, patients’ and their carers’ attitudes to treatment, their ability to return to work and resume normal social functioning, were not reported in the included studies. Almost one third of trials used citalopram as the comparator and only a small number of trials per comparison were found for most of the remaining antidepressants (with the exception of fluoxetine). This limits the power of the review to detect moderate, but clinically meaningful differences between the drugs. The dataset of the present review collected insufficient randomised evidence to detect a difference in early response to treatment (after two weeks of treatment). Looking at the data reported in the trials included in this systematic review, the question on comparative efficacy of early onset response has yet to be proven and remains a matter of ongoing debate (Gourion 2008).
Overall completeness and applicability of evidence
It has long been argued that placebo controlled trials are required to adequately demonstrate the efficacy of novel antidepressant drugs (Kupfer 2002), however, in the present review we focused only on the comparison between escitalopram and other active treatments. Retrieved randomised evidence compared escitalopram with a small selection of possible comparator antidepressants and no randomised trials comparing escitalopram with fluvoxamine (amongst SSRIs), or with mirtazapine, reboxetine, milnacipran or hypericum (amongst the newest antidepressants), or with any of the first generation antidepressants (such as TCAs or MAOIs) were found. Although the search was thorough, it is still possible that there are unpublished studies that have not been identified but the small number of trials identified per comparison hinders the detection of any publication bias. As in all systematic reviews and meta-analyses, in the present study the main concern is about the amount of the included information. The more information that is pooled together, the more precise and accurate is the estimate (Higgins 2005). We are realistically aware that a possibly significant piece of information has not been published and thus is not contributing to the true treatment estimate we were seeking. Although we did our very best to retrieve as many data as possible, through asking pharmaceutical companies and study authors to supply all available information, we can assume that data from some trials are still lacking, most of which are likely to be studies with negative findings. We are also aware of the possibility that a number of further randomised controlled trials comparing escitalopram with other antidepressant drugs are currently being conducted and will be included in future updates of the review.
Quality of the evidence
In this review the mean sample size was bigger than mean sample size of antidepressant studies included in a similar systematic review comparing fluoxetine versus other antidepressants (Cipriani 2005). Escitalopram is a relatively new compound and the quality of trials in psychiatry may have improved in the last years. Notwithstanding the increased sample size, the great majority of trials still do not report adequate information about randomisation and allocation concealment. The reporting of the outcomes in the included studies was often unclear or incomplete and the figures used for the analyses not immediately understandable. All antidepressant studies included in the review were very similar in design and conduct, and the scant information about randomisation and allocation concealment may be matter of reporting in the text than real defects in study design. However, sometimes there were some discrepancies between published reports and unpublished data available on the websites of the pharmaceutical industries. Dealing with summary statistics, the quality of information is important. Meta-analyses of poor quality studies may be seriously misleading, because the bias associated with defects in the conduct of primary studies (randomised trials) can seriously affect overall estimates of intervention. Systematic reviewers (not only within the Cochrane Collaboration) should routinely assess the risk of bias in the results of trials, and should report meta-analyses restricted to trials at low risk of bias (Wood 2008).
Potential biases in the review process
There is unanimous evidence of presence of sponsorship bias in medicine and long-standing concern exists about the potential influence of financial interests on medical literature (Djulbegovic 2000). In the field of antidepressant trials sponsorship can play an important role (Bekelman 2003). In this review the great majority of the trials were sponsored by the escitalopram manufacturer. Because nearly all the trials were sponsored by the drug company marketing escitalopram, it was not possible to formally examine the potential influence of sponsorship bias in sensitivity analyses. For this reason, the potential for an overestimation of the effect size favouring escitalopram need be considered. Limitations of the primary trials and potential confounders may affect the validity of the findings. Readers cannot fully appreciate a study’s meaning without acknowledging the subtle biases in design and interpretation that may arise when a sponsor stands to gain from the report (Schwartz 2008). Such associations should be made clear to let anyone judge the relevance of findings.
To clinically assess comparative treatment effectiveness, in this study we used both dichotomous and continuous outcomes (the number of patients who responded and the mean change between baseline and endpoint on standardised rating scales for depressive symptoms, respectively). It has been claimed that a very small difference in symptom score can translate into a very large difference in the proportion of patients who responded (Moncrieff 2005), however in this review we found that a statistically significant difference in symptom score did not translate into a significant difference in terms of dichotomous outcome. We used an imputation method to impute missing SDs. As reported in the result section, a sensitivity analysis excluding trials for which the SD had to be borrowed was pre-planned and carried out, and the two previously statistically significant results became no more statistically significant. For the fluoxetine comparison, this might be due to the imputation method which could have allowed us to use possibly narrower SDs; by contrast, for the citalopram versus escitalopram studies the difference might be due to the reduction of the overall sample size and, therefore, to the consequent loss of statistical power. However, even though no more statistically significant, the ORs in the sensitivity analysis were consistent with the primary overall analysis. A substantial limitation of some trials was the high rate of patients lost on follow-up (more than 30%). High withdrawal rates reduce the reliability of the assessment of other outcomes. A further limitation of this analysis is that by making multiple comparisons we might have committed a type 1 error, that is, reporting a spurious association. Therefore, results should be interpreted with caution.
Agreements and disagreements with other studies or reviews
In terms of side effect profile, different tolerability profiles for different antidepressants were found. This is an important issue from a clinical point of view and results from this review are consistent with previous findings (Cipriani 2005; Hansen 2005). However, a full description of tolerability profile of drugs cannot rely solely on randomised evidence (Etminan 2005). Furthermore, adverse effects were inconsistently reported in the studies in the present review, thus hampering cross-study comparisons. Standardisation in the reporting of adverse effects is needed, and patients’ subjective experience of medication should be given more consideration. The issue about tolerability is clinically important because during the evidence-based decision-making process, clinicians should take into consideration and inform patients of different side effect profiles among antidepressants. However, it has been shown that randomised controlled trials might not be the most effective tool to identify possible causal relationship between antidepressants and even severe adverse events (De Abajo 2008). This is true for class-related adverse event, but might also be true for each specific compound. As it happens for the second generation antipsychotics (Kumra 2008), newer antidepressants need more reliable and longer-term studies before knowing the real impact and burden on treated patients in terms of tolerability.
Dosage is another compelling issue when dealing with pharmacological treatments (Barbui 2004). In this review almost all studies used treatments within the therapeutic range. Half the studies used a fixed-dose regimen design and the remaining eleven a flexibledose one. Among the included studies there was no evidence of imbalance in terms of dose design or disease severity favouring the investigational drugs.
AUTHORS’ CONCLUSIONS
Implications for practice
Escitalopram appears to be suitable as first-line antidepressant treatment for moderate to severe major depression. It has been compared with only a few other antidepressants and so we are unable to say whether it is better, worse or the same as many of the other drugs used in practice. As for all other new investigational compounds, the potential for overestimation of treatment effect due to sponsorship bias should be borne in mind. Results reported for comparative efficacy have therefore to be viewed with caution.
Cost effectiveness evaluation of pharmacological treatments is extremely important for health policy purposes. Health economic models have been constructed to assess the cost-utility of antidepressant treatments (Sobocki 2008) and new modelling has been developed to provide methodological benefits in depicting evolution of major depressive disorder, as well (Le Lay 2006). By contrast, even though NICE guidelines suggested to prescribe antidepressant with generic formulation (NICE 2007), physicians infrequently discuss medication cost and acquisition issues when prescribing new medications (Tarn 2006). Some antidepressants have a lower acquisition cost and these are the non-patented drugs. Nowadays only two antidepressants, escitalopram and duloxetine, are still on patent in the US and in Europe. In this analysis we did not perform a full economic analysis. To simply rely on lower cost is not justified by the literature, because the acquisition cost may differ country by country and because several other costs associated with the use of antidepressants should be considered (Le Lay 2006). However, acquisition cost of antidepressants may play a important role in impacting on the economic burden of drug prescription for such a common mental health disorder as depression, most of all in developing countries (Patel 2007).
Implications for research
Future studies should focus to a greater extent on outcomes of clear relevance to patients and clinicians, in particular, patients’ and carers’ attitudes to treatment, their ability to return to work and resume normal social functioning. Cost-effectiveness information is also needed in the field of antidepressant trials. Recognising the importance of addressing cost and acquisition issues with patients, appropriate economic analysis independent from pharmaceutical industry considering both costs and clinical outcomes should be carried out in the field of antidepressant trials, to improve physician knowledge about helping patients achieve affordable medication regimens.
The main methodological limitation of standard systematic reviews is that they can rely only on evidence from direct comparisons. However, given the wide spectrum of available comparisons for the treatment of major depression, the use of the methodology of multiple treatments meta-analysis (MTM) may help overcome this limitation (Lumley 2002; Lu 2006; Salanti 2008). MTM (also known as network meta-analysis) is a statistical method that enables to integrate data from direct comparisons (when treatments are compared within a randomised trial) and indirect comparisons (when treatments are compared between trials by combining results on how effective they are against a common comparator treatment) involving diverse regimens, and to assess the strength and consistency of the evidence. Many new drugs have been introduced for the treatment of depressive disorder over the last twenty years, many of which are structurally related and share similar putative mechanisms of action. It is unclear to what extent these agents vary in terms of efficacy and tolerability and some of the more recently introduced drugs (e.g. escitalopram) appear to be chemically similar to existing drugs with expiring patents rather than genuine advances in treatment. Some systematic reviews have found inconsistent differences in efficacy between second-generation antidepressants both within and between classes. MTM has already been used in other fields of medicine (Psaty 2003; Elliott 2007) and the review of a MTM comparing a group of antidepressants has been recently published (Cipriani 2009). MTM may provide a clinically useful summary of the results that can be used to guide treatment decisions.
PLAIN LANGUAGE SUMMARY.
Escitalopram versus other antidepressive agents for depression
Although pharmacological and psychological interventions are both effective for major depression, antidepressant drugs remain the mainstay of treatment. During the last 20 years, selective serotonin reuptake inhibitors (SSRIs) have progressively become the most commonly prescribed antidepressants. Escitalopram, the last SSRI introduced in the market, is the pure S-enantiomer of the racemic citalopram. In the present review we assessed the evidence for the efficacy, acceptability and tolerability of escitalopram in comparison with all other antidepressants in the acute-phase treatment of major depression. Twenty-two randomised controlled trials (about 4000 participants) were included in the present review. Escitalopram appears to be suitable as first-line antidepressant treatment for people with moderate to severe major depression. It has been compared with only a few other antidepressants and so we are unable to say whether it is better, worse or the same as many of the other drugs used in practice. However, it did perform better than citalopram when we brought together the results of six studies in nearly two thousand patients
ACKNOWLEDGEMENTS
This review is one publication of the Meta-Analyses of New Generation Antidepressants (MANGA) project in which a group of researchers within the Cochrane Collaboration Depression, Anxiety and Neurosis Group agreed to conduct a systematic review of all available evidence for 12 new generation antidepressants to inform clinical practice and mental health policies. We are grateful to the Fondazione Cariverona, who provided a three-year Grant to the WHO Collaborating Centre for Research and Training in Mental Health and Service Organisation at the University of Verona, directed by Professor Michele Tansella. The authors would also like to acknowledge and thank Dr Vivien Hunot for her excellent editorial input on this and other MANGA reviews.
SOURCES OF SUPPORT
Internal sources
Department of Medicine and Public Health, Section of Psychiatry and Clinical Psychology, University of Verona, Italy.
External sources
No sources of support supplied
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Eight-week, double-blind, randomised, multicentre study. | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder and having a minimum score of 22 on Montgomery-Asberg Depression Ration Scale. Age range: 18-65 years. |
|
| Interventions | Escitalopram: 136 participants. Sertraline: 138 participants. Escitalopram dose range: 10-20 mg/day. Sertraline dose range: 50-200 mg/day. |
|
| Outcomes | Primary Outcome: Change from baseline to week 8 in Montgomery-Asberg Depression Ration Scale. Secondary Outcomes: Hamilton Depression Rating Scale - 24 Item, Clinical Global Impression - Improvement, Clinical Global Impression - Severity |
|
| Notes | Only unpublished data. This study was funded by escitalopram manufacturer |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “randomized”. Probably done. |
| Allocation concealment? | Unclear | No information provided. |
| Blinding? All outcomes |
Unclear | No information provided. |
| Incomplete outcome data addressed? All outcomes |
Yes | Quote: “ITT population, which included patients who had at least one post-baseline assessment of MADRS” |
| Free of selective reporting? | Yes | |
| Methods | Eight-week, double-blind, randomised, multicentre study . Patients demonstrating evidence of clinical improvement (CGI-I <= 2) at week 8 entered a 19-week, double-blind continuation period. At the end of the continuation period, patients entered a 1- or 2-week down-tapering period randomly scheduled to start between weeks 28 and 31. All patients received placebo during week 32 and no study product thereafter | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder and having a total score on Montgomery-Asberg Depression Rating Scale (MADRS) between 22 and 40. Age range: 18 years or older. Exclusion criteria: another Axis I disorder within the previous 6 months, learning disability or other cognitive disorder, serious risk of suicide (such as when the patient was rated >= 5 on MADRS item 10), previous failure to respond or hypersensitivity to citalopram and/or paroxetine, a history of severe drug allergy or hypersensitivity, a history of lactose intolerance, use of a psychoactive drug within the past 2 weeks (5 weeks for fluoxetine) , use of triptans, oral anticoagulants, sildenafil citrate, cimetidine, type 1 c anti-arrhythmics, cardiac glycosides, narcotic analgesic or an investigational drug within the past 3 months, formal psychotherapy |
|
| Interventions | Escitalopram: 166 participants. Paroxetine: 159 participants. Escitalopram dose range: 10-20 mg/day. Paroxetine dose range: 20-40 mg/day. Benzodiazepines were allowed if the dose had been stable for the previous 6 months and remained fixed during the study |
|
| Outcomes | Primary Outcome: Change from baseline to week 8 in Montgomery-Asberg Depression Rating Scale. Secondary Outcomes: Hamilton Rating Scale for Depression - 24 Item, Clinical Global Impression - Improvement, Clinical Global Impression - Severity |
|
| Notes | Remission: a score equal or less than 12 on MADRS. This study was funded by escitalopram manufacturer |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “computer-generated randomization list” |
| Allocation concealment? | Yes | Quote: “sealed opaque envelopes” |
| Blinding? All outcomes |
Yes | Quote: “all study personnel and participants were blinded to treatment assignment for the duration of the study” |
| Incomplete outcome data addressed? All outcomes |
Yes | |
| Free of selective reporting? | Unclear | Missing information on standard deviation |
| Free of other bias? | Yes | |
| Methods | Eight-week, double-blind, randomised, multicentre study, followed by a two week, double-blind, down-titration period | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder and having a minimum score of 20 on Hamilton Depression Rating Scale. Age range: 18-65 years. Exclusion criteria: abnormal results of physical examinations, laboratory tests and electrocardiograms (ECG), pregnancy, lactation, any Axis I disorder other than major depressive disorder, substance abuse or dependence within the past 6 months, risk of suicide, any clinically significant medical illness that had not been stable for at least 1 year, use of a depot neuroleptic within the past 6 months, use of any neuroleptic, antidepressant or anxiolytic medication within the past 2 weeks (5 weeks for fluoxetine), previous treatment with either escitalopram or venlafaxine, previous failure to respond to adequate trials of 2 or more antidepressants, concomitant use of any psychoactive drug (or any drug with a psychotropic component) |
|
| Interventions | Escitalopram: 101 participants. Venlafaxine XR: 101 participants. Escitalopram dose: 20 mg/day. Venlafaxine XR dose: 225 mg/day. Zolpidem or Zaleplon were allowed for sleep. |
|
| Outcomes | Primary Outcome: Change from baseline to week 8 in Montgomery-Asberg Depression Rating Scale Secondary Outcomes: Hamilton Depression Rating Scale - 24 Item, Clinical Global Impression - Improvement, Clinical Global Impression - Severity |
|
| Notes | A greater proportion of patients randomly assigned to receive escitalopram were female (69,4%) compared with the venlafaxine XR group (47,0%; p<,01). This study was funded by escitalopram manufacturer. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote: “randomly assigned”. Probably done. |
| Allocation concealment? | Unclear | No information provided |
| Blinding? All outcomes |
Unclear | No information provided |
| Incomplete outcome data addressed? All outcomes |
Yes | Quote: “ITT population, that is those who had received at least 1 dose of double-blind study medication and had at least one post-baseline MADRS assessment” |
| Free of selective reporting? | Yes | |
| Methods | 24-week, double-blind, randomised, multicentre study. | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder and having a minimum score of 30 on Montgomery-Asberg Depression Rating Scale (MADRS) . Age range: 18-75 years. Exclusion criteria: bipolar disorder, psychotic disorder or features, obsessive-compulsive disorder, current eating disorders, mental retardation, any pervasive developmental disorder or cognitive disorder, or alcohol or drug abuse-related disorders within the past 12 months, a serious risk of suicide (a minimum score of 5 on item 10 of the MADRS) , concomitant behaviour therapy or systematic psychotherapy, pregnancy or breastfeeding, a history of lactose intolerance, a history of hypersensitivity or non-response to citalopram or escitalopram or duloxetine or with increased intra-ocular pressure, risk of acute narrow-angle glaucoma, use within the past 2 weeks of MAOI or RIMA, SSRIs (fluoxetine within 5 weeks), SNRIs, tricyclic antidepressants, tryptophan, psychoactive herbal remedies, any drug used for augmentation of antidepressant action or any other antidepressant drugs, oral antipsychotics and anti-manic drugs, dopamine antagonists, anxiolytics, any anticonvulsant drug, serotoninergic agonists, narcotic analgesics, cardiac glycosides, type 1c anti-arrythmics, oral anticoagulants, cimetidine, potent inhibitors of CYP2C19, CYP1A2, or medicinal products with a narrow therapeutic index predominantly metabolised by CYP2D6, use of ECT within the past 6 months |
|
| Interventions | Escitalopram: 232 participants. Paroxetine: 227 participants. Escitalopram dose: 20 mg/day. Paroxetine dose: 40 mg/day. Zolpidem, zolpiclone or zaleplon used episodically for insomnia were allowed |
|
| Outcomes | Primary Outcome: Change from baseline to week 24 in Montgomery-Asberg Depression Rating Scale Secondary Outcomes: Hamilton Depression Scale - 24 and 17 item, Hamilton Anxiety Scale, Clinical Global Impression - Improvement, Clinical Global Impression - Severity |
|
| Notes | Remission: a score equal or less than 12 on MADRS. This study was funded by escitalopram manufacturer |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “Patients …. were assigned to 24 weeks of double-blind treatment in a 1:1 ratio … according to a computer-generated randomisation list.” |
| Allocation concealment? | Yes | Quote: “The details of the randomisation series were unknown to any of the investigators and were contained in a set of sealed opaque envelopes. At each study centre, sequentially enrolled patients were assigned the lowest randomisation number available in blocks of 4.” |
| Blinding? All outcomes |
Yes | Quote: “The medication was given as capsules of identical appearance, taste and smell. All study personnel and participants were blinded to treatment assignment for the duration of the entire study.” |
| Incomplete outcome data addressed? All outcomes |
Yes | The prospectively defined primary end-point was the adjusted mean change in MADRS total score from baseline to Week 24, based on the intent-to-treat set (ITT) and using last-observation-carried-forward (LOCF) analysis |
| Free of selective reporting? | Yes | |
| Methods | Eight-week, double-blind, randomised, multicentre study. | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder, having a minimum score of 22 on Montgomery-Asberg Depression Rating Scale (MADRS) and a minimum score of 2 on Item 1 of Hamilton Depression Rating Scale. Age range: 18-65 years. Exclusion criteria: any DSM-IV Axis I disorder other than major depression, any personality disorder, a history of substance abuse, a suicide attempt within the past year or evidence of active suicidal ideation (as indicated by a score of at least 5 on item 10 of the MADRS), pregnancy, lactation, women of childbearing potential if they didn’t agree to use a medically acceptable method of contraception, concomitant psychotropic medication |
|
| Interventions | Escitalopram: 252. Citalopram: 127. Escitalopram dose range: 10-20 mg/day. Citalopram dose: 40 mg/day. Zolpidem for insomnia was allowed (no more than 3 times per week) |
|
| Outcomes | Primary Outcome: Change from baseline to week 8 in Montgomery-Asberg Depression Rating Scale. Secondary Outcomes: Hamilton Depression Rating Scale, HAMD Depressed Mood Item, Clinical Global Impression - Improvement, Clinical Global Impression - Severity |
|
| Notes | This study was funded by escitalopram manufacturer | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “randomly assigned”. Probably done |
| Allocation concealment? | Unclear | No information provided |
| Blinding? All outcomes |
Unclear | No clear information provided |
| Incomplete outcome data addressed? All outcomes |
Yes | Quote: “Last-observation-carried-forward approach that included patients who had received at least 1 dose of double-blind study medication and had at least one post-baseline MADRS assessment” |
| Free of selective reporting? | No | Only graphs and a few side effects reported |
| Methods | Eight-week, double-blind, double-dummy, randomised, multicentre study | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder, having a minimum score of 19 on a 17-item Hamilton Rating Scale for Depression, with normal orgasm function, engaging in sexual activity leading to orgasm at least once every 2 weeks. Age range: 18 years or older. Exclusion criteria: any sexual dysfunction except sexual desire disorder related to depression, history of anorexia nervosa, bulimia, seizure disorder, brain injury, diagnosis of panic disorder, obsessive-compulsive disorder, posttraumatic stress disorder or acute stress disorder within the past 12 months, diagnosis of bipolar I or II disorder, schizophrenia or other psychotic disorders, history of attempted suicide within the past 6 months, use of medications that might affect sexual functioning |
|
| Interventions | Escitalopram: 138. Bupropion XR: 141. Escitalopram dose range: 10-20 mg/day. Bupropion XR dose range: 300-450 mg/day. |
|
| Outcomes | Primary Outcome: Percentage of subjects with orgasm dysfunction and change from baseline to week 8 in 17-item Hamilton Depression Rating Scale. Secondary Outcomes: Percentage of subjects with worsened sexual function, percentage of subjects satisfied with their sexual functioning, 17-item Hamilton Depression Scale |
|
| Notes | This study was funded by burpopion manufacturer. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “randomised”. Probably done |
| Allocation concealment? | Unclear | No information reported |
| Blinding? All outcomes |
Unclear | No information reported |
| Incomplete outcome data addressed? All outcomes |
No | ITT population defined as all randomised patients who took at least 1 dose of study medication, had no orgasm dysfunction reported from the sexual functioning assessment at rendomization, had a HAM-D assessment completed at randomization and provided at least 1 post-randomization HAMD-17 and orgasm function assessment |
| Free of selective reporting? | Yes | |
| Methods | Eight-week, double-blind, double-dummy, randomised, multicentre study | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder, having a minimum score of 19 on a 17-item Hamilton Rating Scale for Depression, with normal orgasm function, engaging in sexual activity leading to orgasm at least once every 2 weeks. Age range: 18 years or older. Exclusion criteria: any sexual dysfunction except sexual desire disorder related to depression, history of anorexia nervosa, bulimia, seizure disorder, brain injury, diagnosis of panic disorder, obsessive-compulsive disorder, posttraumatic stress disorder or acute stress disorder within the past 12 months, diagnosis of bipolar I or II disorder, schizophrenia or other psychotic disorders, history of attempted suicide within the past 6 months, use of medications that might affect sexual functioning |
|
| Interventions | Escitalopram: 149. Bupropion XR: 138. Escitalopram dose range: 10-20 mg/day. Bupropion XR dose range: 300-450 mg/day. |
|
| Outcomes | Primary Outcome: Percentage of subjects with orgasm dysfunction and change from base’line to week 8 in 17-item Hamilton Depression Rating Scale. Secondary Outcomes: Percentage of subjects with worsened sexual function, percentage of subjects satisfied with their sexual functioning, 17-item Hamilton Depression Scale |
|
| Notes | This study was funded by burpopion manufacturer. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “randomised”. Probably done |
| Allocation concealment? | Unclear | No information reported |
| Blinding? All outcomes |
Unclear | No information reported |
| Incomplete outcome data addressed? All outcomes |
No | ITT population defined as all randomised patients who took at least 1 dose of study medication, had no orgasm dysfunction reported from the sexual functioning assessment at rendomization, had a HAM-D assessment completed at randomization and provided at least 1 post-randomization HAMD-17 and orgasm function assessment |
| Free of selective reporting? | Yes | |
| Methods | 24-week, double-blind, randomised, multicentre study. | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder, having a total score between 22 and 40 on Montgomery-Asberg Depression Rating Scale (MADRS). Age range: 18-65 years. Exclusion criteria: other serious illnesses on the basis of medical history and the screening results of a physical examination, electrocardiogram (ECG) and clinical laboratory tests, pregnancy, breast-feeding, non adequate contraception at time of screening, mania or any bipolar disorder, schizophrenia or any psychotic disorder, obsessive-compulsive disorder, eating disorders, mental retardation or any pervasive developmental or cognitive disorder, MADRS score >= 5 on item 10, concomitant treatment with antipsychotics, antidepressants, hypnotics, anxiolytics, antiepileptics, barbiturates, chloral hydrate, 5-HT receptor agonists, electroconvulsive treatment, behaviour therapy or psychotherapy, use of any investigational drug within the past 30 days, history of schizophrenia, psychotic disorder or drug abuse, history of severe drug allergy or hypersensitivity (including to citalopram), a lack of response to more than one antidepressant treatment (including citalopram) during the present depressive episode |
|
| Interventions | Escitalopram: 175 participants. Citalopram: 182 participants. Escitalopram dose: 10 mg/day. Citalopram dose: 20 mg/day. Benzodiazepines in low doses for insomnia were allowed. |
|
| Outcomes | Primary Outcome: Change from baseline in the mean of the Montgomery-Asberg Depression Rating Scale during the 24 weeks. Secondary Outcomes: MADRS single items, Clinical Global Impression - Improvement, Clinical Global Impression - Severity |
|
| Notes | Remission: a score equal or less than 12 on MADRS. This study was funded by escitalopram manufacturer |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “computer-generated randomization list” |
| Allocation concealment? | Yes | Quote: “sealed opaque envelopes” |
| Blinding? All outcomes |
Yes | Quote: “all study personnel and participants were blinded” |
| Incomplete outcome data addressed? All outcomes |
Yes | Quote: “ITT population included all randomised patients who took at least one dose of double-blind study product and who had at least one valid post-baseline MADRS assessment.” |
| Free of selective reporting? | No | Missing standard deviations. |
| Free of other bias? | Yes | |
| Methods | Eight-week, double-blind, randomised, multicentre study. | |
| Participants | In- and out-patients meeting DSM-IV criteria for Major Depression Disorder, having a total score between 22 and 40 on Montogmery-Asberg Depression Rating Scale (MADRS) and a minimum score of 22 on Mini Mental State Examination (MMSE). Age range: 65 years or older. Criteria exclusion: DSM-IV criteria for mania or any bipolar disorder, schizophrenia or any psychotic disorder, obsessive-compulsive disorder, eating disorders, mental retardation or pervasive developmental or cognitive disorder, a MADRS score >= 5 on Item 10, concomitant treatment with antipsychotics, antidepressants, hypnotics, anxiolytics, antiepileptics, barbiturates, chloral hydrate, antiparkinsonian drugs, diuretics, 5-HT receptor agonists, lithium, sodium valproate, carbamazepine, electroconvulsive treatment, behaviour therapy or psychotherapy, use of an investigational drug within the past 30 days, a history of schizophrenia or psychotic disorder or drug abuse, a history of severe allergy or hypersensitivity (including to citalopram), a lack of response to more than one antidepressant treatment (including citalopram) during the present depressive episode |
|
| Interventions | Escitalopram: 174 participants. Fluoxetine: 164 participants. Escitalopram dose: 10 mg/day. Fluoxetine dose: 20 mg/day. Oxazepam (maximum 30 mg/day), temazepam (maximum 10 mg/day), zopiclone (maximum 3,75 mg/day), zolpidem (maximum 5 mg/day) were allowed |
|
| Outcomes | Change from baseline to final assessment in Montogmery-Asberg Depression Rating Scale | |
| Notes | Settings: both general practice and specialist. Remission: a score equal or less than 12 on MADRS. This study was funded by escitalopram manufacturer |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “randomized”. Probably done |
| Allocation concealment? | Unclear | No information provided |
| Incomplete outcome data addressed? All outcomes |
Yes | Quote: “ITT including all randomized patients who took at least one dose of double-blind study medication and who had at least one post-baseline assessment of the MADRS total score” |
| Methods | Eight-week, double-blind, randomised, multicentre study. | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder, having a minimum score of 22 on Montgomery-Asberg Depression Rating Scale. Age range: 18-80 years. |
|
| Interventions | Escitalopram: 102 participants. Fluoxetine: 103 participants. Escitalopram dose range: 10-20 mg/day. Fluoxetine: 20-40 mg/day. |
|
| Outcomes | Primary Outcome: Change from baseline to week 8 in Montgomery-Asberg Depression Rating Scale. Secondary Outcomes: Hamilton Depression Rating Scale, Hamilton Anxiety Scale, Clinical Global Impression - Improvement, Clinical Global Impression - Severity, Center of Epidemiologic Studies - Depression Scale, Quality of Life Scale |
|
| Notes | Only unpuplished data. This study was funded by escitalopram manufacturer. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “randomized”. Probably done |
| Blinding? All outcomes |
Unclear | Quote: “double-blind”. |
| Incomplete outcome data addressed? All outcomes |
Yes | Quote: ITT analysis (“all patients with at least one post-baseline assessment of MADRS”) |
| Methods | Eight-week, double-blind, randomised, multicentre study followed by a 1 week, double-blind, down-titration period | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder, having a minimum score of 26 on Montgomery-Asberg Depression Rating Scale (MADRS) and a minimum score of 4 on the Clinical Global Impression-Severity. Age range: 18-80 years. Exclusion criteria: any Axis I disorder than MDD, a recent history or current diagnosis of drug or alcohol dependence, current suicidal ideation (a score of 5 or 6 on item 10 of the MADRS) or suicide attempt within the past year, history of any psychotic disorder or psychotic features, any personality disorder of sufficient severity to interfere with the participation in the study, a history of seizure disorder or any condition that predisposes to the risk of seizure, any history of narrow-angle glaucoma, a history of inappropriate antidiuretic hormone secretion syndrome, a current diagnosis or history of any clinically significant medical illness that had not been stable for at least the past year, pregnancy or breast feeding, women of childbearing potential that were not using a medically accepted form of contraception, use of a depot antipsychotic within the past 6 months, use of Benzodiazepines within 4 weeks, any antipsychotic, antidepressant or anxiolytic medication within the past 2 weeks (5 weeks for fluoxetine), participation in a previous clinical study or failure to respond to treatment with either escitalopram or duloxetine, failure to respond to adequate trials of two or more antidepressants, treatment with an investigational drug within the past month, electroconvulsive therapy within the past 3 months, initiation or termination of any type of psychotherapy within the past 3 months, concomitant use of any psychoactive drug (or any drug with a psychotropic component) |
|
| Interventions | Escitalopram: 140 participants. Duloxetine: 138 participants. Escitalopram dose range: 10-20 mg/day. Duloxetine dose: 60 mg/day. Zolpidem or zaleplon for sleep were allowed. |
|
| Outcomes | Primary Outcome: Change from baseline to week 8 in Montgomery-Asberg Depression Rating Scale. Secondary Outcome: Hamilton Depression Rating Scale (24 item) |
|
| Notes | This study was funded by escitalopram manufacturer. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “randomized”. Probably done |
| Blinding? All outcomes |
Unclear | No information provided |
| Incomplete outcome data addressed? All outcomes |
Yes | Quote: “ITT population, which included patients who had at least one post-baseline assessment on the MADRS total score” |
| Free of selective reporting? | Yes | |
| Methods | Eight-week, double-blind, randomised, multicentre study. | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder and having a total score on Montgomery-Asberg Depression Rating Scale (MADRS) between 22 and 40. Age range: 18-65 years. Exclusion criteria: mania or any bipolar disorder, schizophrenia or any psychotic disorder, obsessive-compulsive disorder, eating disorder, mental retardation, any pervasive developmental disorder or cognitive disorder (according to DSM-IV criteria), MADRS score >= 5 on item 10, treatment with antipsychotics, antidepressants, hypnotics, anxiolytics, barbiturates, chloral hydrate or other 5-hydroxytryptamine receptor agonists, electroconvulsive treatment, treatment with behaviour therapy or psychotherapy |
|
| Interventions | Escitalopram: 156 participants. Citalopram: 161 participants. Escitalopram dose range: 10-20 mg/day. Citalopram dose range: 20-40 mg/day. Benzodiazepines for insomnia were allowed. |
|
| Outcomes | Primary Outcome: Change from baseline to week 8 in Montgomery-Asberg Depression Rating Scale. Secondary Outcomes: Clinical Global Impression - Improvement, Clinical Global Impression - Severity, MADRS Individuals Items (apparent sadness, reported sadness, inner tension, reduced sleep, reduced appetite, concentration difficulties, lassitude, inability to feel, pessimistic thoughts, suicidal thoughts) |
|
| Notes | Remission: a score equal or less than 12 on MADRS. This study was funded by escitalopram manufacturer. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “randomized”. Probably done |
| Allocation concealment? | Unclear | No information provided |
| Blinding? All outcomes |
Unclear | No information provided |
| Incomplete outcome data addressed? All outcomes |
Yes | Quote: “ITT population included all randomized patients who took at least one dose of double-blind study product and who had at least one valid post-baseline MADRS assessment.” |
| Free of selective reporting? | No | |
| Methods | Eight-week, double-blind, randomised, multicentre study | |
| Participants | Outpatients and inpatients of six psychiatric hospitals in China, enrolled from November 2003 to July 2004. Patients were between 18 and 65 years of age, meeting criteria for major depressive disorder as defined by DSM-IV. In addition, patients were required to have both a Clinical Global Impression of Severity (CGI-S) rating more than 4 and a HAM-D-17 total score more than 18 at both the screening and baseline study visits for inclusion. Patients were excluded if they had any current primary DSM-IV Axis I diagnosis other than MDDmajor depression or any anxiety disorder as a primary diagnosis within the year preceding enrolment; any previous diagnosis of bipolar disorder, psychosis, or schizoaffective disorder; a history of substance abuse or dependence (not including nicotine dependence) within the past year; serious suicidal risk; or a serious medical illness (cardiovascular, hepatic, renal, respiratory, hematological, endocrinological, or neurological disease, or clinically significant laboratory abnormality), or if they were currently taking St. John’s wort (Hypericum perforatum) or other Chinese herbal medicine for depression | |
| Interventions | All patients had been drug-free for at least 14 days before starting treatment with escitalopram or fluoxetine. After the washout period, patients were randomly assigned in a 1:1 ratio to escitalopram (10 mg/day) plus placebo fluoxetine or fluoxetine (20 mg/day) plus placebo escitalopram (both administered once daily between 9:00 and 10:00 A.M.) | |
| Outcomes | The primary outcome measure was change in the HAM-D-17 total score. The secondary outcome measure was change in the MADRS total score. Remission was defined as a HAM-D-17 total score less than 7, and response was defined as at least a 50% decrease from baseline. For the MADRS, remission was defined as a MADRS score equal to or less than 12, and response was defined by at least a 50% decrease from baseline | |
| Notes | The hospitals and locations for the trials were Beijing Anding Hospital (Beijing), the Mental Health Institute of Peking University (Beijing), Beijing Chaoyang Hospital (Beijing), Guangzhou Brain Hospital (Guangzhou, Guandong Province), Huzhou Mental Health Center (Huzhou, Zhejiang Province), and Suzhou Guangji Hospital (Suzhou, Jiangsu Province). Escitalopram tablets were produced by H. Lundbeck A/S (Denmark). Fluoxetine tablets were produced by the Fourth Pharmaceutical Company (Changzhou) . Baseline symptom severity scores indicated a moderately to severely depressed patient population, with mean baseline HAM-D-17 scores of 24.7 in the escitalopram group and 24.1 in the fluoxetine group of patients (by contrast, MADRS mean baseline scores were 30.1 and 31.2,respectively) | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote: “…patients were randomly assigned in a 1:1 ratio to…”. Comment: probably not done |
| Incomplete outcome data addressed? All outcomes |
Yes | |
| Free of selective reporting? | No | |
| Free of other bias? | Unclear | Quote: “…all statistical analyses were performed by Shanghai Apex medical research company” |
| Methods | Eight-week, double-blind, randomised, multicentre study followed by a 1 week, single-blind, down-titration period | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder, having a minimum score of 18 on Montgomery-Asberg Depression Rating Scale (MADRS). Age range: 18-85 years. Exclusion criteria: history of mania or any bipolar disorder, schizophrenia or any psychotic disorder, currently obsessive-compulsive disorder, eating disorders, mental retardation, any pervasive development disorder or cognitive disorder, MADRS score >= 5 on item 10, alcohol or drug abuse problems within the past 12 months, concomitant treatment with antipsychotics, antidepressants, psychotropics, serotonin receptor agonists, lithium, carbamazepine, valproate, valpromide, electroconvulsive treatment, behaviour treatment or psychotherapy, pregnancy, breast feeding, use of medications thought likely to interfere with the study |
|
| Interventions | Escitalopram: 148 participants. Venlafaxine XR: 145 participants. Escitalopram dose range: 10-20 mg/day. Venlafaxine XR dose range: 75-150 mg/day. Zolpidem or stable low doses of Benzodiazepines for insomnia were allowed |
|
| Outcomes | Primary Outcome: Change from baseline to week 8 in Montgomery-Asberg Depression Rating Scale. Secondary Outcomes: Hamilton Depression Rating Scale (24 item and 17 item), HAMD Subscale Scores (anxiety/somatization, psychomotor retardation, sleep disturbance, cognitive disturbance and melancholia), Clinical Global Impression - Improvement, Clinical Global Impression - Severity |
|
| Notes | Remission: a score equal or less than 12 on MADRS. This study was funded by escitalopram manufacturer. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “randomized”. Probably done |
| Allocation concealment? | Unclear | No information provided |
| Blinding? All outcomes |
Unclear | No information provided |
| Incomplete outcome data addressed? All outcomes |
Yes | Quote: “ITT population included all randomized patients who took at least one dose of double-blind study product and who had at least one valid post-baseline MADRS assessment.” |
| Free of selective reporting? | No | |
| Methods | Eight-week, double-blind, randomised, multicentre study. | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder, having a minimum score of 30 on Montgomery-Asberg Depression Rating Scale (MADRS). Age range: 18-65 years. Exclusion criteria: primary diagnoses for any axis I disorder other than MDD, history of mania, bipolar disorder, schizophrenia or other psychotic disorder, substance abuse or dependence within the past 12 months, use of a depot antipsychotic within the past 6 months, any antipsychotic, anxiolytic or anticonvulsant medications within the past 2 weeks |
|
| Interventions | Escitalopram: 142 participants. Citalopram: 152 participants. Escitalopram dose: 20 mg/day. Citalopram dose: 40 mg/day. |
|
| Outcomes | Primary Outcome: Change from baseline to end-of-study in Montgomery-Asberg Depression Rating Scale. Secondary Outcome: Clinical Global Impression - Severity. |
|
| Notes | Remission: a score equal or less than 12 on MADRS. This study was funded by escitalopram manufacturer. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “patients were randomly assigned”. Probably done |
| Allocation concealment? | Unclear | No information provided. |
| Incomplete outcome data addressed? All outcomes |
Yes | Efficacy analysis on ITT population (all patients who took at least one dose of study medication and who had at least one valid post-baseline MADRS assessment |
| Free of selective reporting? | No | |
| Methods | Eight-week, double-blind, randomisation, multicentre study. | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder, having a minimum score of 22 on Montgomery-Asberg Depression Rating Scale (MADRS) and a minimum score of 4 on Clinical Global Impression - Severity. Age range: 18 years and over. Exclusion criteria: pregnancy, lactation, primary Axis I disorder other than MDD, an Axis II disorder considered likely to interfere with compliance to the study protocol, substance dependence (excluding nicotine and caffeine) within the past 6 months, a significant risk of suicide, clinically significant laboratory abnormalities or co-morbid medical conditions if would likely require intervention, hospitalisation or use of an excluded medication during the course of the study, a lack of response to two or more courses of antidepressant medication in the current episode with each trial at least 4 weeks in duration and at a clinically appropriate dose, a history of non-response in association with a previous adequate trial of duloxetine, escitalopram or citalopram therapy, use of monoamine oxidase inhibitor within 14 days or of fluoxetine within the past 30 days, electroconvulsive treatment (ECT) or transcranial magnetic stimulation within the past year |
|
| Interventions | Escitalopram: 274 participants. Duloxetine: 273 participants. Escitalopram dose: 10 mg/day. Duloxetine dose : 60 mg/day. Episodic use of benzodiazepines and certain hypnotics or sedatives (e.g., chloral hydrate or zolpidem) was allowed, provided that use occurred on no more than 50% of the total days between visits. Psychotherapy was permitted but initiating, stopping or changing frequency or modality after study entry was prohibited |
|
| Outcomes | Primary Outcome: Decrease from baseline in the Hamilton Rating Scale for Depression (17-item) Maier subscale (includes HAMD17 Items 1, 2, 7, 8, 9 and 10). Secondary Outcomes: Hamilton Rating Scale for Depression (17-item) total scores, HAMD17 subscales (core, anxiety/somatization, retardation/somatization, sleep), Hamilton Rating Scale for Anxiety total score, Clinical Global Impression - Severity, Patient Global Impression of Improvement |
|
| Notes | This study was funded by duloxetine manufacturer | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “randomized”. Probably done |
| Allocation concealment? | Yes | Quote: “..via the Interactive Voice Response System”. Probably done |
| Incomplete outcome data addressed? All outcomes |
Yes | |
| Free of selective reporting? | No | Many graphs and some unclear reporting (i.e. re. sexual functioning) |
| Methods | Eight-week, double-blind, randomised, multicentre study. | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder and having a minimum score of 22 on Montgomery-Asberg Depression Rating Scale and a minimum score of 2 on Item 1 of Hamilton Depression Rating Scale. Age range: 18-80 years. |
|
| Interventions | Escitalopram: 129 participants. Citalopram: 128 participants. Escitalopram dose range: 10-20 mg/day. Citalopram dose range: 20-40 mg/day. |
|
| Outcomes | Primary Outcome: Change from baseline to week 8 in Montgomery-Asberg Depression Rating Scale Secondary Outcomes: Hamilton Depression Rating Scale, HAMD Depressed Mood Item, Clinical Global Impression-Improvement, Clinical Global Impression-Severity |
|
| Notes | Only unpublished data. This study was funded by escitalopram manufacturer. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “randomized”. Probably done |
| Blinding? All outcomes |
Unclear | Quote: “double-blind”. |
| Incomplete outcome data addressed? All outcomes |
Yes | Quote: ITT analysis (“all patients with at least one post-baseline assessment of MADRS”) |
| Methods | Five-week, double-blind, randomised, forced-titration, single-centre study | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder and having a minimum score of 18 on the Hamilton Depression Rating Scale (HAMD) with a score of at least 1 on the HAMD sleep disturbance subscale. Age range: 18-55 years. |
|
| Interventions | Escitalopram: 16 participants. Fluoxetine: 14 participants. Escitalopram dose range: 10-20 mg/day. Fluoxetine dose range: 20-40 mg/day. |
|
| Outcomes | Primary Outcome: Change from baseline to day 1 and to endpoint (mean of days 33 and 34) in the number of awakenings (Polysomnogram) Secondary Outcomes: Polysomnogram (Percent Time Awake, Sleep Efficiency, Percent REM Sleep, REM Latency, REM Epochs with Eye Movements, non-REM Epochs with Eye Movements), Sleep Diary, Pittsburgh Sleep Quality Index |
|
| Notes | Only unpublished data. This study was funded by escitalopram manufacturer. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote: “randomized”. Probably done |
| Blinding? All outcomes |
Unclear | Quote:“double-blind” |
| Incomplete outcome data addressed? All outcomes |
No | |
| Free of other bias? | No | |
| Methods | Eight-week, double-blind, randomised, multicentre study. | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder and having a minimum score of 22 on Montgomery-Asberg Depression Rating Scale. Age range: 18-80 years. |
|
| Interventions | Escitalopram: 138 participants. Bupropion XR: 147 participants. Escitalopram dose: 4 mg/day. Bupropion XR dose: 150 mg. |
|
| Outcomes | Primary Outcome: Change from baseline to week 8 in Montgomery-Asberg Depression Rating Scale. Secondary Outcome: Hamilton Depression Rating Scale - 24 item |
|
| Notes | Only unpublished data. This study was funded by escitalopram manufacturer |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “randomized”. Probably done |
| Allocation concealment? | Unclear | No information provided. |
| Blinding? All outcomes |
Unclear | Quote: “double-blind”. |
| Incomplete outcome data addressed? All outcomes |
Yes | Quote: ITT analysis (“all patients with at least one post-baseline assessment of MADRS”) |
| Free of selective reporting? | Unclear | No information provided. |
| Free of other bias? | Unclear | No information provided. |
| Methods | Eight-week, double-blind, randomised, multicentre study. | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder with an ongoing episode and having a minimum score of 22 on Montgomery-Asberg Depression Rating Scale (MADRS). Age range: 18-80 years. Exclusion criteria: significant abnormalities from physical examination, laboratory tests and electrocardiogram (ECG), pregnancy, female patients of childbearing potential that were not using a medically accepted form of contraception, lactation, a primary Axis I disorder other than MDD, a history of any DSM-IV-definied psychotic disorder, substance abuse or dependency, risk of suicide, any personality disorder considered to be of sufficient severity to interfere with participation in the study, use of a depot neuroleptic within the past 6 months, use of any neuroleptic, antidepressant or anxiolytic medication within the past 2 weeks (5 weeks for fluoxetine), previous treatment with either escitalopram or sertraline, previous failure to respond to adequate trials of any two SSRIs, previous participation in an investigational study within the past month or previous treatment with an investigational drug within the past month (or five half-lives of the drug, whichever was longer), concomitant use of any psychotropic drug (or any drug with a psychotropic component) |
|
| Interventions | Escitalopram: 107 participants. Sertraline: 108 participants. Escitalopram dose: 10 mg/day. Sertraline dose range: 50-200 mg/day. Zolpidem or zaleplon for sleep were allowed. |
|
| Outcomes | Primary Outcome: Change from baseline to week 8 in Montgomery-Asberg Depression Rating Scale. Secondary Outcomes: Hamilton Depression Scale- 24 item, Clinical Global Impression - Improvement, Clinical Global Impression - Severity |
|
| Notes | This study was funded by escitalopram manufacturer. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “randomized”. Probably done |
| Incomplete outcome data addressed? All outcomes |
Yes | ITT population (at least one dose of study medication and at least one post-baseline MADRS assessment) using the LOCF approach |
| Free of selective reporting? | Yes | |
| Methods | 24-week, double-blind, randomised, multicentre study. | |
| Participants | Outpatients meeting DSM-IV criteria for Major Depressive Disorder, having a minimum score of 26 on Montgomery-Asberg Depression Rating Scale (MADRS) and a minimum score of 4 on Clinical Global Impression-Scale. Age range: 18-65 years. Exclusion criteria: bipolar disorder, psychotic disorder or features, current eating disorders, mental retardation, any pervasive developmental disorder or cognitive disorder, or alcohol or drug abuse-related disorders within the past 12 months, obsessive-compulsive disorder, post-traumatic stress disorder, panic disorder, serious suicide risk (a minimum score of 5 on item 10 of the MADRS) concomitant behaviour therapy or systematic psychotherapy, pregnancy or breastfeeding, a history of lactose intolerance, a history of hypersensitivity or non-response to citalopram or escitalopram or duloxetine or with increased intra-ocular pressure, risk of acute narrow-angle glaucoma, use within the past 2 weeks of MAOI or RIMA, SSRIs (fluoxetine within 5 weeks), SNRIs, tricyclic antidepressants, tryptophan, psychoactive herbal remedies, any drug used for augmentation of antidepressant action or any other antidepressant drugs, oral antipsychotics and anti-manic drugs, dopamine antagonists, anxiolytics, any anticonvulsant drug, serotoninergic agonists, narcotic analgesics, cardiac glycosides, type 1c anti-arrhythmics, oral anticoagulants, cimetidine, potent inhibitors of CYP2C19, CYP1A2, or medicinal products with a narrow therapeutic index predominantly metabolised by CYP2D6, use of ECT within the past 6 months |
|
| Interventions | Escitalopram: 144 participants. Duloxetine: 151 participants. Escitalopram dose: 10 mg/day. Duloxetine dose: 60 mg/day. Zolpidem, zolpiclone or zaleplon used episodically for insomnia were allowed |
|
| Outcomes | Primary Outcome: Change from baseline to week 24 in Montgomery-Asberg Depression Rating Scale (MADRS). Secondary Outcomes: Hamilton Depression Scale - 17 item, Hamilton Anxiety Scale, Clinical Global Impression - Improvement, Clinical Global Impression - Severity |
|
| Notes | This study was funded by escitalopram manufacturer. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “..computer-generated randomization list” |
| Allocation concealment? | Yes | Quote: “..sealed opaque envelopes …” |
| Blinding? All outcomes |
Yes | Quote:, “all personnel and participants were blinded to treatment assignment..” |
| Incomplete outcome data addressed? All outcomes |
Yes | ITT analysis comprising all patients who took at least one dose of study medication and had at least one valid post-baseline MADRS assessment |
| Free of selective reporting? | No | Some missing standard deviations |
| Methods | 6-week, prospective, randomised, double-blind, active-controlled trial was conducted at 8 psychiatric outpatient clinics across the Federation of Russia | |
| Participants | Outpatients, aged 25 (this minimum age limit was a requirement of one of the ethics committees) to 45 years, with a diagnosis of major depressive disorder, as defined in the DSM-IV and a total score more than or equal to 25 on MADRS. Patients were not eligible if they met DSM-IV criteria for mania or any bipolar disorder, schizophrenia, or any psychotic disorder, or displayed any psychotic features, obsessive-compulsive disorder, mental retardation or any pervasive developmental disorder, eating disorder (anorexia nervosa or bulimia nervosa), dementia, or alcohol or drug abuse within the previous12 months, a history of severe drug allergy or hypersensitivity, other serious illness or sequela of serious illness, citalopram or escitalopram treatment within 60 days prior to inclusion. Patients were also excluded if they had received an oral antipsychotic drug or monoamine oxidase inhibitor within 2 weeks prior to inclusion; a depot antipsychotic preparation within 6 months prior to inclusion; an SSRI (except fluoxetine), a serotonin-noradrenaline reuptake inhibitor, or a TCA within 1 week prior to inclusion; or fluoxetine within 5 weeks before inclusion; an antiparkinsonian compound, barbiturate, chloral hydrate, lithium, anticonvulsant, or hypnotic and anxiolytic (except for benzodiazepines used for insomnia at a stable dose for the previous 6 months or used episodically at a lower recommended dose). Women who were pregnant or breast feeding were also excluded from the study | |
| Interventions | Using equal (~110 patients per group) block randomization, patients were assigned to receive a once-daily fixed dose of escitalopram 10 mg (109 participants), citalopram10 mg (111 participants), or citalopram 20 mg (110 participants) for 6 weeks | |
| Outcomes | The primary efficacy measure was the change in the MADRS total score from baseline to end of study. Secondary efficacy measures were changes from baseline in MADRS total score in a subgroup of severely depressed patients (MADRS total score more than or equal to 35), MADRS core depression subscale score (in the overall population and severely depressed subgroup), CGI-S, andCGI-I. In addition, the proportions of patients classified a priori as responders (decrease in MADRS total score by at least 50% of the baseline value) or remitters (primary definition, MADRS total score less than or equal to 12; secondary definition, less than or equal to 10) were analysed | |
| Notes | The present study was part of the S-citalopram development program for approval in some European countries through a bridging procedure using results from studies of the racemate, citalopram. Care and medication were free of charge for the patients enrolled in the trial. This study was specifically designed a priori as a superiority study. The sample size was calculated using Singer’s method. The largest between-group difference was estimated at 5 points, with an SD of 12. Given this assumption, and with an a level of 5% (2-tailed) and a b level of 20%, it was calculated that 100 patients per arm would be needed to achieve sufficient power. Assuming a 10% withdrawal rate, 10 additional patients per arm were included in the design to ensure sufficient power, giving 110 patients per arm (330 patients in total). This research was sponsored by OOO ARBACOM (Moscow, Federation of Russia) (it’s unclear the relationship with the escitalopram manufacturer) |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “…Eight block randomizations were generated, 1 per center.” Probably done |
| Blinding? All outcomes |
Yes | Quote: “To maintain blinding, all study medication was provided in capsules (tablets were encapsulated in a lactose powder) that were identical in appearance, taste, and odor.Investigators and patients were blinded to treatment.” |
| Incomplete outcome data addressed? All outcomes |
Yes | LOCF method of replacing missing values |
| Free of selective reporting? | Yes | |
DATA AND ANALYSES
Comparison 1. Failure to respond at endpoint (6-12 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 13 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 6 | 1823 | Odds Ratio (M-H, Random, 95% CI) | 0.67 [0.50, 0.89] |
| 1.2 Versus Fluoxetine | 3 | 783 | Odds Ratio (M-H, Random, 95% CI) | 0.81 [0.60, 1.10] |
| 1.3 Versus Paroxetine | 2 | 784 | Odds Ratio (M-H, Random, 95% CI) | 0.89 [0.61, 1.32] |
| 1.4 Versus Sertraline | 2 | 489 | Odds Ratio (M-H, Random, 95% CI) | 1.06 [0.73, 1.53] |
| 2 Escitalopram versus newer ADs | 8 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 3 | 842 | Odds Ratio (M-H, Random, 95% CI) | 0.91 [0.69, 1.20] |
| 2.2 Versus Duloxetine | 3 | 1120 | Odds Ratio (M-H, Random, 95% CI) | 0.72 [0.43, 1.20] |
| 2.3 Versus Venlafaxine XR | 2 | 495 | Odds Ratio (M-H, Random, 95% CI) | 0.86 [0.53, 1.39] |
Comparison 2. Failure to respond (at 1-4 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 1 | 240 | Odds Ratio (M-H, Random, 95% CI) | 1.15 [0.52, 2.56] |
| 1.1 Versus Fluoxetine | 1 | 240 | Odds Ratio (M-H, Random, 95% CI) | 1.15 [0.52, 2.56] |
| 2 Escitalopram versus newer ADs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Venlafaxine XR | 1 | 293 | Odds Ratio (M-H, Random, 95% CI) | 0.85 [0.47, 1.55] |
Comparison 3. Failure to respond (at 16-24 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 1 | 357 | Odds Ratio (M-H, Random, 95% CI) | 0.96 [0.60, 1.56] |
| 1.2 Versus Paroxetine | 1 | 459 | Odds Ratio (M-H, Random, 95% CI) | 0.73 [0.47, 1.15] |
| 2 Escitalopram versus newer ADs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Duloxetine | 1 | 295 | Odds Ratio (M-H, Random, 95% CI) | 0.72 [0.42, 1.25] |
Comparison 4. Failure to remission at endpoint (6-12 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 13 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 6 | 1823 | Odds Ratio (M-H, Random, 95% CI) | 0.57 [0.36, 0.90] |
| 1.2 Versus Fluoxetine | 3 | 783 | Odds Ratio (M-H, Random, 95% CI) | 0.86 [0.65, 1.15] |
| 1.3 Versus Paroxetine | 2 | 784 | Odds Ratio (M-H, Random, 95% CI) | 0.87 [0.45, 1.68] |
| 1.4 Versus Sertraline | 2 | 489 | Odds Ratio (M-H, Random, 95% CI) | 1.16 [0.81, 1.67] |
| 2 Escitalopram versus newer ADs | 8 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 3 | 842 | Odds Ratio (M-H, Random, 95% CI) | 0.94 [0.67, 1.32] |
| 2.2 Versus Duloxetine | 3 | 1120 | Odds Ratio (M-H, Random, 95% CI) | 0.90 [0.62, 1.29] |
| 2.3 Versus Venlafaxine XR | 2 | 495 | Odds Ratio (M-H, Random, 95% CI) | 0.91 [0.63, 1.33] |
Comparison 5. Failure to remission (at 16-24 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus newer ADs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Duloxetine | 1 | 295 | Odds Ratio (M-H, Random, 95% CI) | 0.72 [0.45, 1.16] |
Comparison 6. Standardised mean difference at endpoint (6-12 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 12 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only’ | |
| 1.1 Versus Citalopram | 5 | 1392 | Std. Mean Difference (IV, Random, 95% CI) | −0.17 [−0.30, −0.04] |
| 1.2 Versus Fluoxetine | 3 | 759 | Std. Mean Difference (IV, Random, 95% CI) | −0.17 [−0.32, −0.03] |
| 1.3 Versus Paroxetine | 2 | 772 | Std. Mean Difference (IV, Random, 95% CI) | −0.05 [−0.36, 0.26] |
| 1.4 Versus Setraline | 2 | 477 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [−0.16, 0.20] |
| 2 Escitalopram versus newer ADs | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 3 | 793 | Std. Mean Difference (IV, Random, 95% CI) | −0.08 [−0.22, 0.05] |
| 2.2 Versus Duloxetine | 3 | 1096 | Std. Mean Difference (IV, Random, 95% CI) | −0.10 [−0.30, 0.09] |
| 2.3 Versus Venlafaxine XR | 2 | 483 | Std. Mean Difference (IV, Random, 95% CI) | −0.07 [−0.38, 0.25] |
Comparison 7. Failure to complete (any cause).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram vs. other SSRIs | 14 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 6 | 1823 | Odds Ratio (M-H, Random, 95% CI) | 0.78 [0.56, 1.10] |
| 1.2 Versus Fluoxetine | 4 | 813 | Odds Ratio (M-H, Random, 95% CI) | 0.89 [0.51, 1.55] |
| 1.3 Versus Paroxetine | 2 | 784 | Odds Ratio (M-H, Random, 95% CI) | 0.68 [0.36, 1.29] |
| 1.4 Versus Sertraline | 2 | 489 | Odds Ratio (M-H, Random, 95% CI) | 1.24 [0.78, 1.97] |
| 2 Escitalopram versus newer ADs | 8 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 3 | 842 | Odds Ratio (M-H, Random, 95% CI) | 1.02 [0.75, 1.39] |
| 2.2 Versus Duloxetine | 3 | 1120 | Odds Ratio (M-H, Random, 95% CI) | 0.62 [0.38, 0.99] |
| 2.3 Versus Venlafaxine XR | 2 | 495 | Odds Ratio (M-H, Random, 95% CI) | 0.90 [0.58, 1.39] |
Comparison 8. Failure to complete (due to inefficacy).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 12 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 5 | 1604 | Odds Ratio (M-H, Random, 95% CI) | 0.74 [0.27, 2.03] |
| 1.2 Versus Fluoxetine | 4 | 812 | Odds Ratio (M-H, Random, 95% CI) | 0.57 [0.15, 2.15] |
| 1.3 Versus Paroxetine | 2 | 784 | Odds Ratio (M-H, Random, 95% CI) | 1.39 [0.17, 11.44] |
| 1.4 Versus Sertraline | 1 | 274 | Odds Ratio (M-H, Random, 95% CI) | 3.09 [0.32, 30.08] |
| 2 Escitalopram versus newer ADs | 5 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 1 | 276 | Odds Ratio (M-H, Random, 95% CI) | 0.11 [0.01, 2.02] |
| 2.2 Versus Duloxetine | 3 | 1120 | Odds Ratio (M-H, Random, 95% CI) | 0.95 [0.21, 4.25] |
| 2.3 Versus Venlafaxine XR | 1 | 293 | Odds Ratio (M-H, Random, 95% CI) | 9.06 [0.48, 169.85] |
Comparison 9. Failure to complete (due to side effects).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram vs. other SSRIs | 13 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 5 | 1604 | Odds Ratio (M-H, Random, 95% CI) | 0.79 [0.47, 1.31] |
| 1.2 Versus Fluoxetine | 4 | 813 | Odds Ratio (M-H, Random, 95% CI) | 0.75 [0.44, 1.28] |
| 1.3 Versus Paroxetine | 2 | 784 | Odds Ratio (M-H, Random, 95% CI) | 0.70 [0.25, 1.96] |
| 1.4 Versus Sertraline | 2 | 489 | Odds Ratio (M-H, Random, 95% CI) | 1.08 [0.35, 3.37] |
| 2 Escitalopram versus newer ADs | 8 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 3 | 842 | Odds Ratio (M-H, Random, 95% CI) | 0.65 [0.25, 1.65] |
| 2.2 Versus Duloxetine | 3 | 1020 | Odds Ratio (M-H, Random, 95% CI) | 0.49 [0.18, 1.29] |
| 2.3 Versus Venlafaxine XR | 2 | 495 | Odds Ratio (M-H, Random, 95% CI) | 0.41 [0.14, 1.17] |
Comparison 10. SE - Subjects with at least one TEAE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 13 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 6 | 1802 | Odds Ratio (M-H, Random, 95% CI) | 0.79 [0.58, 1.07] |
| 1.2 Versus Fluoxetine | 4 | 804 | Odds Ratio (M-H, Random, 95% CI) | 0.80 [0.59, 1.07] |
| 1.3 Versus Paroxetine | 1 | 454 | Odds Ratio (M-H, Random, 95% CI) | 0.78 [0.52, 1.17] |
| 1.4 Versus Sertraline | 2 | 483 | Odds Ratio (M-H, Random, 95% CI) | 0.62 [0.33, 1.19] |
| 2 Escitalopram versus newer ADs | 8 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 3 | 822 | Odds Ratio (M-H, Random, 95% CI) | 0.77 [0.55, 1.07] |
| 2.2 Versus Duloxetine | 3 | 1111 | Odds Ratio (M-H, Random, 95% CI) | 0.96 [0.67, 1.38] |
| 2.3 Versus Venlafaxine XR | 2 | 487 | Odds Ratio (M-H, Random, 95% CI) | 0.58 [0.28, 1.23] |
Comparison 11. SE - Agitation / anxiety.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 3 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 1 | 294 | Odds Ratio (M-H, Random, 95% CI) | 4.38 [0.48, 39.64] |
| 1.2 Versus Fluoxetine | 2 | 534 | Odds Ratio (M-H, Random, 95% CI) | 0.62 [0.26, 1.47] |
| 2 Escitalopram versus newer ADs | 5 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 2 | 557 | Odds Ratio (M-H, Random, 95% CI) | 0.55 [0.06, 5.32] |
| 2.2 Versus Duloxetine | 2 | 817 | Odds Ratio (M-H, Random, 95% CI) | 0.43 [0.15, 1.19] |
| 2.3 Versus Venlafaxine XR | 1 | 289 | Odds Ratio (M-H, Random, 95% CI) | 0.72 [0.24, 2.14] |
Comparison 12. SE - Constipation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 5 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 2 | 663 | Odds Ratio (M-H, Random, 95% CI) | 0.78 [0.29, 2.09] |
| 1.2 Versus Fluoxetine | 2 | 534 | Odds Ratio (M-H, Random, 95% CI) | 0.50 [0.19, 1.28] |
| 1.3 Versus Paroxetine | 1 | 454 | Odds Ratio (M-H, Random, 95% CI) | 0.40 [0.14, 1.14] |
| 2 Escitalopram versus newer ADs | 6 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 2 | 557 | Odds Ratio (M-H, Random, 95% CI) | 0.32 [0.15, 0.69] |
| 2.2 Versus Duloxetine | 3 | 1111 | Odds Ratio (M-H, Random, 95% CI) | 0.69 [0.34, 1.40] |
| 2.3 Versus Venlafaxine XR | 1 | 289 | Odds Ratio (M-H, Random, 95% CI) | 0.23 [0.05, 1.12] |
Comparison 13. SE - ire.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 10 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 4 | 1226 | Odds Ratio (M-H, Random, 95% CI) | 0.85 [0.55, 1.30] |
| 1.2 Versus Fluoxetine | 3 | 564 | Odds Ratio (M-H, Random, 95% CI) | 0.76 [0.30, 1.93] |
| 1.3 Versus Paroxetine | 1 | 454 | Odds Ratio (M-H, Random, 95% CI) | 0.62 [0.31, 1.21] |
| 1.4 Versus Setraline | 2 | 483 | Odds Ratio (M-H, Random, 95% CI) | 0.49 [0.28, 0.84] |
| 2 Escitalopram versus newer ADs | 8 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 3 | 822 | Odds Ratio (M-H, Random, 95% CI) | 1.45 [0.86, 2.45] |
| 2.2 Versus Duloxetine | 3 | 1111 | Odds Ratio (M-H, Random, 95% CI) | 1.10 [0.75, 1.63] |
| 2.3 Versus Venlafaxine XR | 2 | 487 | Odds Ratio (M-H, Random, 95% CI) | 0.96 [0.35, 2.59] |
Comparison 14. SE - Dry mouth.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 12 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 5 | 1442 | Odds Ratio (M-H, Random, 95% CI) | 0.98 [0.61, 1.58] |
| 1.2 Versus Fluoxetine | 4 | 804 | Odds Ratio (M-H, Random, 95% CI) | 0.92 [0.47, 1.83] |
| 1.3 Versus Paroxetine | 1 | 454 | Odds Ratio (M-H, Random, 95% CI) | 0.69 [0.35, 1.36] |
| 1.4 Versus Sertraline | 2 | 483 | Odds Ratio (M-H, Random, 95% CI) | 0.52 [0.15, 1.84] |
| 2 Escitalopram versus newer ADs | 8 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 3 | 822 | Odds Ratio (M-H, Random, 95% CI) | 0.58 [0.39, 0.87] |
| 2.2 Versus Duloxetine | 3 | 1111 | Odds Ratio (M-H, Random, 95% CI) | 0.55 [0.39, 0.79] |
| 2.3 Versus Venlafaxine XR | 2 | 487 | Odds Ratio (M-H, Random, 95% CI) | 0.65 [0.34, 1.26] |
Comparison 15. SE - Hypotension.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 1 | 294 | Odds Ratio (M-H, Random, 95% CI) | 3.23 [0.13, 80.02] |
Comparison 16. SE - ns.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 9 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 4 | 1226 | Odds Ratio (M-H, Random, 95% CI) | 1.20 [0.77, 1.86] |
| 1.2 Versus Fluoxetine | 2 | 534 | Odds Ratio (M-H, Random, 95% CI) | 0.79 [0.43, 1.45] |
| 1.3 Versus Paroxetine | 1 | 454 | Odds Ratio (M-H, Random, 95% CI) | 0.92 [0.46, 1.84] |
| 1.4 Versus Sertraline | 2 | 483 | Odds Ratio (M-H, Random, 95% CI) | 1.07 [0.59, 1.93] |
| 2 Escitalopram versus newer ADs | 8 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 3 | 822 | Odds Ratio (M-H, Random, 95% CI) | 0.55 [0.33, 0.92] |
| 2.2 Versus Duloxetine | 3 | 1111 | Odds Ratio (M-H, Random, 95% CI) | 0.58 [0.33, 1.02] |
| 2.3 Versus Venlafaxine XR | 2 | 487 | Odds Ratio (M-H, Random, 95% CI) | 0.85 [0.46, 1.60] |
Comparison 17. SE - Nausea.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 13 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 6 | 1799 | Odds Ratio (M-H, Random, 95% CI) | 1.04 [0.72, 1.48] |
| 1.2 Versus Fluoxetine | 4 | 804 | Odds Ratio (M-H, Random, 95% CI) | 1.20 [0.70, 2.03] |
| 1.3 Versus Paroxetine | 1 | 454 | Odds Ratio (M-H, Random, 95% CI) | 0.95 [0.63, 1.46] |
| 1.4 Versus Sertraline | 2 | 483 | Odds Ratio (M-H, Random, 95% CI) | 1.07 [0.67, 1.70] |
| 2 Escitalopram versus newer ADs | 8 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 3 | 822 | Odds Ratio (M-H, Random, 95% CI) | 1.17 [0.78, 1.75] |
| 2.2 Versus Duloxetine | 3 | 1111 | Odds Ratio (M-H, Random, 95% CI) | 0.56 [0.42, 0.75] |
| 2.3 Versus Venlafaxine XR | 2 | 487 | Odds Ratio (M-H, Random, 95% CI) | 0.37 [0.14, 0.99] |
Comparison 18. SE - Sleepiness/drowsiness.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 10 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 5 | 1442 | Odds Ratio (M-H, Random, 95% CI) | 1.55 [0.88, 2.72] |
| 1.2 Versus Fluoxetine | 3 | 774 | Odds Ratio (M-H, Random, 95% CI) | 1.28 [0.62, 2.63] |
| 1.3 Versus Sertraline | 2 | 483 | Odds Ratio (M-H, Random, 95% CI) | 1.31 [0.54, 3.21] |
| 2 Escitalopram versus newer ADs | 7 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 2 | 557 | Odds Ratio (M-H, Random, 95% CI) | 1.57 [0.39, 6.39] |
| 2.2 Versus Duloxetine | 3 | 1111 | Odds Ratio (M-H, Random, 95% CI) | 1.62 [0.89, 2.94] |
| 2.3 Versus Venlafaxine XR | 2 | 487 | Odds Ratio (M-H, Random, 95% CI) | 1.00 [0.22, 4.47] |
Comparison 19. SE - itin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 2 | 609 | Odds Ratio (M-H, Random, 95% CI) | 1.74 [0.41, 7.48] |
| 2 Escitalopram versus newer ADs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Duloxetine | 2 | 841 | Odds Ratio (M-H, Random, 95% CI) | 0.46 [0.18, 1.21] |
Comparison 20. SE - Dizziness.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 7 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 3 | 911 | Odds Ratio (M-H, Random, 95% CI) | 1.43 [0.58, 3.49] |
| 1.2 Versus Fluoxetine | 3 | 774 | Odds Ratio (M-H, Random, 95% CI) | 0.88 [0.46, 1.67] |
| 1.3 Versus Paroxetine | 1 | 454 | Odds Ratio (M-H, Random, 95% CI) | 1.03 [0.54, 1.97] |
| 2 Escitalopram versus newer ADs | 6 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 2 | 557 | Odds Ratio (M-H, Random, 95% CI) | 0.84 [0.33, 2.12] |
| 2.2 Versus Duloxetine | 3 | 1111 | Odds Ratio (M-H, Random, 95% CI) | 0.59 [0.39, 0.90] |
| 2.3 Versus Venlafaxine XR | 1 | 289 | Odds Ratio (M-H, Random, 95% CI) | 0.85 [0.30, 2.41] |
Comparison 21. SE - Fatigue.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 8 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 4 | 1148 | Odds Ratio (M-H, Random, 95% CI) | 1.44 [0.62, 3.33] |
| 1.2 Versus Fluoxetine | 2 | 227 | Odds Ratio (M-H, Random, 95% CI) | 0.71 [0.23, 2.14] |
| 1.3 Versus Sertraline | 2 | 483 | Odds Ratio (M-H, Random, 95% CI) | 1.10 [0.54, 2.25] |
| 2 Escitalopram versus newer ADs | 7 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 2 | 557 | Odds Ratio (M-H, Random, 95% CI) | 3.48 [1.77, 6.84] |
| 2.2 Versus Duloxetine | 3 | 1111 | Odds Ratio (M-H, Random, 95% CI) | 1.04 [0.66, 1.62] |
| 2.3 Versus Venlafaxine XR | 2 | 487 | Odds Ratio (M-H, Random, 95% CI) | 0.51 [0.11, 2.29] |
Comparison 22. SE - Flu Syndrome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 3 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 3 | 932 | Odds Ratio (M-H, Random, 95% CI) | 0.71 [0.40, 1.27] |
| 2 Escitalopram versus newer ADs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Duloxetine | 1 | 294 | Odds Ratio (M-H, Random, 95% CI) | 1.96 [0.64, 6.00] |
Comparison 23. SE - Headache.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 13 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 6 | 1799 | Odds Ratio (M-H, Random, 95% CI) | 0.78 [0.58, 1.04] |
| 1.2 Versus Fluoxetine | 4 | 804 | Odds Ratio (M-H, Random, 95% CI) | 1.05 [0.66, 1.68] |
| 1.3 Versus Paroxetine | 1 | 454 | Odds Ratio (M-H, Random, 95% CI) | 1.26 [0.81, 1.96] |
| 1.4 Versus Sertraline | 2 | 483 | Odds Ratio (M-H, Random, 95% CI) | 0.92 [0.57, 1.48] |
| 2 Escitalopram versus newer ADs | 8 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 3 | 822 | Odds Ratio (M-H, Random, 95% CI) | 0.87 [0.53, 1.41] |
| 2.2 Versus Duloxetine | 3 | 1111 | Odds Ratio (M-H, Random, 95% CI) | 1.13 [0.84, 1.52] |
| 2.3 Versus Venlafaxine XR | 2 | 487 | Odds Ratio (M-H, Random, 95% CI) | 1.26 [0.73, 2.17] |
Comparison 24. SE - Impotence.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 4 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 2 | 382 | Odds Ratio (M-H, Random, 95% CI) | 1.59 [0.09, 28.02] |
| 1.2 Versus Fluoxetine | 1 | 69 | Odds Ratio (M-H, Random, 95% CI) | 4.58 [0.21, 98.95] |
| 1.3 Versus Sertraline | 1 | 122 | Odds Ratio (M-H, Random, 95% CI) | 0.30 [0.06, 1.59] |
| 2 Escitalopram versus newer ADs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Venlafaxine XR | 2 | 164 | Odds Ratio (M-H, Random, 95% CI) | 3.67 [0.69, 19.67] |
Comparison 25. SE - Jitteriness.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 1 | 369 | Odds Ratio (M-H, Random, 95% CI) | 0.16 [0.03, 0.82] |
Comparison 26. SE - Lethargy/Sedation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 1 | 294 | Odds Ratio (M-H, Random, 95% CI) | 3.23 [0.13, 80.02] |
| 1.2 Versus Sertraline | 1 | 212 | Odds Ratio (M-H, Random, 95% CI) | 3.72 [0.99, 13.94] |
| 2 Escitalopram versus newer ADs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Venlafaxine XR | 1 | 198 | Odds Ratio (M-H, Random, 95% CI) | 6.46 [0.76, 54.65] |
Comparison 27. SE - Decreased libido.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 4 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 1 | 248 | Odds Ratio (M-H, Random, 95% CI) | 0.87 [0.32, 2.32] |
| 1.2 Versus Fluoxetine | 1 | 197 | Odds Ratio (M-H, Random, 95% CI) | 1.83 [0.52, 6.45] |
| 1.3 Versus Setraline | 2 | 483 | Odds Ratio (M-H, Random, 95% CI) | 1.19 [0.34, 4.15] |
| 2 Escitalopram versus newer ADs | 2 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Duloxetine | 1 | 547 | Odds Ratio (M-H, Random, 95% CI) | 0.77 [0.34, 1.74] |
| 2.2 Versus Venlafaxine XR | 1 | 198 | Odds Ratio (M-H, Random, 95% CI) | 0.66 [0.23, 1.93] |
Comparison 28. SE - Pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abdominal Pain | 4 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Bupropion XR | 1 | 278 | Odds Ratio (M-H, Random, 95% CI) | 0.78 [0.23, 2.61] |
| 1.2 Versus Citalopram | 1 | 294 | Odds Ratio (M-H, Random, 95% CI) | 0.35 [0.01, 8.77] |
| 1.3 Versus Fluoxetine | 1 | 337 | Odds Ratio (M-H, Random, 95% CI) | 1.05 [0.43, 2.53] |
| 1.4 Versus Venlafaxine XR | 1 | 289 | Odds Ratio (M-H, Random, 95% CI) | 1.39 [0.43, 4.49] |
| 2 Back Pain | 8 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 1 | 278 | Odds Ratio (M-H, Random, 95% CI) | 2.91 [0.58, 14.69] |
| 2.2 Versus Citalopram | 4 | 1289 | Odds Ratio (M-H, Random, 95% CI) | 0.84 [0.38, 1.83] |
| 2.3 Versus Duloxetine | 1 | 270 | Odds Ratio (M-H, Random, 95% CI) | 2.93 [0.12, 72.67] |
| 2.4 Versus Fluoxetine | 1 | 337 | Odds Ratio (M-H, Random, 95% CI) | 1.94 [0.57, 6.57] |
| 2.5 Versus Venlafaxine XR | 1 | 289 | Odds Ratio (M-H, Random, 95% CI) | 0.98 [0.38, 2.54] |
| 3 Chest Pain | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | ||
| 3.1 Versus Bupropion XR | 1 | 265 | Odds Ratio (M-H, Random, 95% CI) | 0.34 [0.01, 8.38] |
| 3.2 Versus Citalopram | 1 | 294 | Odds Ratio (M-H, Random, 95% CI) | 0.35 [0.01, 8.77] |
| 4 Pain In Extremity | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Versus Bupropion XR | 1 | 278 | Odds Ratio (M-H, Random, 95% CI) | 4.79 [0.23, 100.64] |
| 5 Pharyngolaryngeal Pain | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only |
Comparison 29. SE - Increased sweating.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 6 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 4 | 1226 | Odds Ratio (M-H, Random, 95% CI) | 1.39 [0.72, 2.68] |
| 1.2 Versus Paroxetine | 1 | 454 | Odds Ratio (M-H, Random, 95% CI) | 0.67 [0.37, 1.23] |
| 1.3 Versus Setraline | 1 | 271 | Odds Ratio (M-H, Random, 95% CI) | 0.66 [0.26, 1.67] |
| 2 Escitalopram versus newer ADs | 4 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Duloxetine | 2 | 841 | Odds Ratio (M-H, Random, 95% CI) | 0.59 [0.33, 1.06] |
| 2.2 Versus Venlafaxine XR | 2 | 487 | Odds Ratio (M-H, Random, 95% CI) | 0.45 [0.23, 0.87] |
Comparison 30. SE - Yawning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 1 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 1 | 294 | Odds Ratio (M-H, Random, 95% CI) | 3.23 [0.13, 80.02] |
| 2 Escitalopram versus newer ADs | 3 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 2 | 557 | Odds Ratio (M-H, Random, 95% CI) | 7.71 [1.75, 34.05] |
| 2.2 Versus Duloxetine | 1 | 547 | Odds Ratio (M-H, Random, 95% CI) | 0.39 [0.15, 1.01] |
Comparison 31. Deaths, suicide and suicidality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Suicide - Tendency/Ideation | 3 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 1 | 248 | Odds Ratio (M-H, Random, 95% CI) | 2.98 [0.12, 73.76] |
| 1.2 Versus Bupropion XR | 1 | 279 | Odds Ratio (M-H, Random, 95% CI) | 5.18 [0.25, 108.95] |
| 1.3 Versus Venlafaxine XR | 1 | 198 | Odds Ratio (M-H, Random, 95% CI) | 3.09 [0.12, 76.83] |
| 2 Suicide - Attempted | 6 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Citalopram | 3 | 974 | Odds Ratio (M-H, Random, 95% CI) | 1.48 [0.40, 5.45] |
| 2.2 Versus Fluoxetine | 2 | 437 | Odds Ratio (M-H, Random, 95% CI) | 0.98 [0.10, 9.50] |
| 2.3 Versus Venlafaxine XR | 1 | 289 | Odds Ratio (M-H, Random, 95% CI) | 4.97 [0.24, 104.34] |
| 3 Suicide - Completed | 3 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 Versus Citalopram | 1 | 294 | Odds Ratio (M-H, Random, 95% CI) | 0.35 [0.01, 8.77] |
| 3.2 Versus Fluoxetine | 1 | 337 | Odds Ratio (M-H, Random, 95% CI) | 2.86 [0.12, 70.73] |
| 3.3 Versus Duloxetine | 1 | 294 | Odds Ratio (M-H, Random, 95% CI) | 0.35 [0.01, 8.65] |
| 4 Deaths | 5 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 Versus Citalopram | 1 | 294 | Odds Ratio (M-H, Random, 95% CI) | 0.35 [0.01, 8.77] |
| 4.2 Versus Fluoxetine | 1 | 337 | Odds Ratio (M-H, Random, 95% CI) | 1.91 [0.17, 21.23] |
| 4.3 Versus Duloxetine | 2 | 841 | Odds Ratio (M-H, Random, 95% CI) | 1.03 [0.11, 9.90] |
| 4.4 Versus Venlafaxine XR | 1 | 293 | Odds Ratio (M-H, Random, 95% CI) | 0.32 [0.01, 8.03] |
Comparison 32. Sensitivity analyses - Excluding trials whose dropout rate was greater than 20%.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs (dropout rate greater than 20% in both arms) | 6 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 4 | 1187 | Odds Ratio (M-H, Random, 95% CI) | 0.56 [0.40, 0.79] |
| 1.2 Versus Fluoxetine | 2 | 578 | Odds Ratio (M-H, Random, 95% CI) | 0.80 [0.56, 1.13] |
| 2 Escitalopram versus other SSRIs (dropout rate greater than 20% in only one arm) | 3 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Citalopram | 3 | 830 | Odds Ratio (M-H, Random, 95% CI) | 0.49 [0.34, 0.72] |
Comparison 33. Sensitivity analyses - Excluding trials for which the imputation methods were used - RESPONSE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 9 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 5 | 1566 | Odds Ratio (M-H, Random, 95% CI) | 0.62 [0.46, 0.84] |
| 1.2 Versus Fluoxetine | 2 | 578 | Odds Ratio (M-H, Random, 95% CI) | 0.80 [0.56, 1.13] |
| 1.3 Versus Paroxetine | 1 | 325 | Odds Ratio (M-H, Random, 95% CI) | 1.11 [0.70, 1.78] |
| 1.4 Versus Sertraline | 1 | 215 | Odds Ratio (M-H, Random, 95% CI) | 0.84 [0.47, 1.53] |
| 2 Escitalopram versus newer ADs | 7 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 2 | 566 | Odds Ratio (M-H, Random, 95% CI) | 0.93 [0.60, 1.45] |
| 2.2 Versus Duloxetine | 3 | 1120 | Odds Ratio (M-H, Random, 95% CI) | 0.72 [0.43, 1.20] |
| 2.3 Versus Venlafaxine XR | 2 | 495 | Odds Ratio (M-H, Random, 95% CI) | 0.86 [0.53, 1.39] |
Comparison 34. Sensitivity analyses - Excluding trials for which the imputation methods were used - REMISSION.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 9 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 4 | 1187 | Odds Ratio (M-H, Random, 95% CI) | 0.40 [0.18, 0.88] |
| 1.2 Versus Fluoxetine | 2 | 578 | Odds Ratio (M-H, Random, 95% CI) | 0.83 [0.55, 1.25] |
| 1.3 Versus Paroxetine | 2 | 784 | Odds Ratio (M-H, Random, 95% CI) | 0.87 [0.45, 1.68] |
| 1.4 Versus Sertraline | 1 | 215 | Odds Ratio (M-H, Random, 95% CI) | 1.06 [0.62, 1.81] |
| 2 Escitalopram versus newer ADs | 6 | Odds Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 2 | 566 | Odds Ratio (M-H, Random, 95% CI) | 0.93 [0.53, 1.64] |
| 2.2 Versus Duloxetine | 2 | 825 | Odds Ratio (M-H, Random, 95% CI) | 0.96 [0.56, 1.63] |
| 2.3 Versus Venlafaxine XR | 2 | 495 | Odds Ratio (M-H, Random, 95% CI) | 0.91 [0.63, 1.33] |
Comparison 35. Sensitivity analyses - Excluding trials for which the imputation methods were used - STANDARD DEVIATION.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Escitalopram versus other SSRIs | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Versus Citalopram | 3 | 773 | Std. Mean Difference (IV, Random, 95% CI) | −0.19 [−0.43, 0.05] |
| 1.2 Versus Fluoxetine | 2 | 425 | Std. Mean Difference (IV, Random, 95% CI) | −0.10 [−0.29, 0.09] |
| 1.3 Versus Paroxetine | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
| 1.4 Versus Setraline | 2 | 477 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [−0.16, 0.20] |
| 2 Escitalopram versus newer ADs | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Versus Bupropion XR | 3 | 793 | Std. Mean Difference (IV, Random, 95% CI) | −0.08 [−0.22, 0.05] |
| 2.2 Versus Duloxetine | 2 | 809 | Std. Mean Difference (IV, Random, 95% CI) | −0.06 [−0.32, 0.20] |
| 2.3 Versus Venlafaxine XR | 2 | 483 | Std. Mean Difference (IV, Random, 95% CI) | −0.07 [−0.38, 0.25] |
Analysis 1.1. Comparison 1 Failure to respond at endpoint (6-12 weeks), Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 1 Failure to respond at endpoint (6-12 weeks)
Outcome: 1 Escitalopram versus other SSRIs
 |
 |
Analysis 1.2. Comparison 1 Failure to respond at endpoint (6-12 weeks), Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 1 Failure to respond at endpoint (6-12 weeks)
Outcome: 2 Escitalopram versus newer ADs
 |
 |
Analysis 2.1. Comparison 2 Failure to respond (at 1-4 weeks), Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 2 Failure to respond (at 1-4 weeks)
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 2.2. Comparison 2 Failure to respond (at 1-4 weeks), Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 2 Failure to respond (at 1-4 weeks)
Outcome: 2 Escitalopram versus newer ADs
 |
Analysis 3.1. Comparison 3 Failure to respond (at 16-24 weeks), Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 3 Failure to respond (at 16-24 weeks)
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 3.2. Comparison 3 Failure to respond (at 16-24 weeks), Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 3 Failure to respond (at 16-24 weeks)
Outcome: 2 Escitalopram versus newer ADs
 |
Analysis 4.1. Comparison 4 Failure to remission at endpoint (6-12 weeks), Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 4 Failure to remission at endpoint (6-12 weeks)
Outcome: 1 Escitalopram versus other SSRIs
 |
 |
Analysis 4.2. Comparison 4 Failure to remission at endpoint (6-12 weeks), Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 4 Failure to remission at endpoint (6-12 weeks)
Outcome: 2 Escitalopram versus newer ADs
 |
 |
Analysis 5.1. Comparison 5 Failure to remission (at 16-24 weeks), Outcome 1 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 5 Failure to remission (at 16-24 weeks)
Outcome: 1 Escitalopram versus newer ADs
 |
Analysis 6.1. Comparison 6 Standardised mean difference at endpoint (6-12 weeks), Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 6 Standardised mean difference at endpoint (6-12 weeks)
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 6.2. Comparison 6 Standardised mean difference at endpoint (6-12 weeks), Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 6 Standardised mean difference at endpoint (6-12 weeks)
Outcome: 2 Escitalopram versus newer ADs
 |
Analysis 7.1. Comparison 7 Failure to complete (any cause), Outcome 1 Escitalopram vs. other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 7 Failure to complete (any cause)
Outcome: 1 Escitalopram vs. other SSRIs
 |
 |
Analysis 7.2. Comparison 7 Failure to complete (any cause), Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 7 Failure to complete (any cause)
Outcome: 2 Escitalopram versus newer ADs
 |
 |
Analysis 8.1. Comparison 8 Failure to complete (due to inefficacy), Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 8 Failure to complete (due to inefficacy)
Outcome: 1 Escitalopram versus other SSRIs
 |
 |
Analysis 8.2. Comparison 8 Failure to complete (due to inefficacy), Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 8 Failure to complete (due to inefficacy)
Outcome: 2 Escitalopram versus newer ADs
 |
Analysis 9.1. Comparison 9 Failure to complete (due to side effects), Outcome 1 Escitalopram vs. other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 9 Failure to complete (due to side effects)
Outcome: 1 Escitalopram vs. other SSRIs
 |
 |
Analysis 9.2. Comparison 9 Failure to complete (due to side effects), Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 9 Failure to complete (due to side effects)
Outcome: 2 Escitalopram versus newer ADs
 |
 |
Analysis 10.1. Comparison 10 SE - Subjects with at least one TEAE, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 10 SE - Subjects with at least one TEAE
Outcome: 1 Escitalopram versus other SSRIs
 |
 |
Analysis 10.2. Comparison 10 SE - Subjects with at least one TEAE, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 10 SE - Subjects with at least one TEAE
Outcome: 2 Escitalopram versus newer AD
 |
Analysis 11.1. Comparison 11 SE - Agitation / anxiety, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 11 SE - Agitation / anxiety
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 11.2. Comparison 11 SE - Agitation / anxiety, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 11 SE - Agitation / anxiety
Outcome: 2 Escitalopram versus newer ADs
 |
Analysis 12.1. Comparison 12 SE - Constipation, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 12 SE - Constipation
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 12.2. Comparison 12 SE - Constipation, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 12 SE - Constipation
Outcome: 2 Escitalopram versus newer AD
 |
Analysis 13.1. Comparison 13 SE - Diarrhoea, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 13 SE - Diarrhoea
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 13.2. Comparison 13 SE - Diarrhoea, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 13 SE - Diarrhoea
Outcome: 2 Escitalopram versus newer ADs
 |
Analysis 14.1. Comparison 14 SE - Dry mouth, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 14 SE - Dry mouth
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 14.2. Comparison 14 SE - Dry mouth, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 14 SE - Dry mouth
Outcome: 2 Escitalopram versus newer AD
 |
Analysis 15.1. Comparison 15 SE - Hypotension, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 15 SE - Hypotension
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 16.1. Comparison 16 SE - Insomnia, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 16 SE - Insomnia
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 16.2. Comparison 16 SE - Insomnia, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 16 SE - Insomnia
Outcome: 2 Escitalopram versus newer ADs
 |
Analysis 17.1. Comparison 17 SE - Nausea, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 17 SE - Nausea
Outcome: 1 Escitalopram versus other SSRIs
 |
 |
Analysis 17.2. Comparison 17 SE - Nausea, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 17 SE - Nausea
Outcome: 2 Escitalopram versus newer ADs
 |
 |
Analysis 18.1. Comparison 18 SE - Sleepiness/drowsiness, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 18 SE - Sleepiness/drowsiness
Outcome: 1 Escitalopram versus other SSRIs
 |
 |
Analysis 18.2. Comparison 18 SE - Sleepiness/drowsiness, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 18 SE - Sleepiness/drowsiness
Outcome: 2 Escitalopram versus newer ADs
 |
Analysis 19.1. Comparison 19 SE - Vomiting, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 19 SE - Vomiting
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 19.2. Comparison 19 SE - Vomiting, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 19 SE - Vomiting
Outcome: 2 Escitalopram versus newer ADs
 |
Analysis 20.1. Comparison 20 SE - Dizziness, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 20 SE - Dizziness
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 20.2. Comparison 20 SE - Dizziness, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 20 SE - Dizziness
Outcome: 2 Escitalopram versus newer ADs
 |
Analysis 21.1. Comparison 21 SE - Fatigue, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 21 SE - Fatigue
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 21.2. Comparison 21 SE - Fatigue, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 21 SE - Fatigue
Outcome: 2 Escitalopram versus newer ADs
 |
Analysis 22.1. Comparison 22 SE - Flu Syndrome, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 22 SE - Flu Syndrome
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 22.2. Comparison 22 SE - Flu Syndrome, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 22 SE - Flu Syndrome
Outcome: 2 Escitalopram versus newer AD
 |
Analysis 23.1. Comparison 23 SE - Headache, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 23 SE - Headache
Outcome: 1 Escitalopram versus other SSRIs
 |
 |
Analysis 23.2. Comparison 23 SE - Headache, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 23 SE - Headache
Outcome: 2 Escitalopram versus newer ADs
 |
 |
Analysis 24.1. Comparison 24 SE - Impotence, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 24 SE - Impotence
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 24.2. Comparison 24 SE - Impotence, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 24 SE - Impotence
Outcome: 2 Escitalopram versus newer ADs
 |
Analysis 25.1. Comparison 25 SE - Jitteriness, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 25 SE - Jitteriness
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 26.1. Comparison 26 SE - Lethargy/Sedation, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 26 SE - Lethargy/Sedation
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 26.2. Comparison 26 SE - Lethargy/Sedation, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 26 SE - Lethargy/Sedation
Outcome: 2 Escitalopram versus newer ADs
 |
Analysis 27.1. Comparison 27 SE - Decreased libido, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 27 SE - Decreased libido
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 27.2. Comparison 27 SE - Decreased libido, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 27 SE - Decreased libido
Outcome: 2 Escitalopram versus newer ADs
 |
Analysis 28.1. Comparison 28 SE - Pain, Outcome 1 Abdominal Pain.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 28 SE - Pain
Outcome: 1 Abdominal Pain
 |
Analysis 28.2. Comparison 28 SE - Pain, Outcome 2 Back Pain.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 28 SE - Pain
Outcome: 2 Back Pain
 |
Analysis 28.3. Comparison 28 SE - Pain, Outcome 3 Chest Pain.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 28 SE - Pain
Outcome: 3 Chest Pain
 |
Analysis 28.4. Comparison 28 SE - Pain, Outcome 4 Pain In Extremity.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 28 SE - Pain
Outcome: 4 Pain In Extremity
 |
Analysis 28.5. Comparison 28 SE - Pain, Outcome 5 Pharyngolaryngeal Pain.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 28 SE - Pain
Outcome: 5 Pharyngolaryngeal Pain
 |
Analysis 29.1. Comparison 29 SE - Increased sweating, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 29 SE - Increased sweating
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 29.2. Comparison 29 SE - Increased sweating, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 29 SE - Increased sweating
Outcome: 2 Escitalopram versus newer ADs
 |
Analysis 30.1. Comparison 30 SE - Yawning, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 30 SE - Yawning
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 30.2. Comparison 30 SE - Yawning, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 30 SE - Yawning
Outcome: 2 Escitalopram versus newer ADs
 |
Analysis 31.1. Comparison 31 Deaths, suicide and suicidality, Outcome 1 Suicide - Tendency/Ideation.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 31 Deaths, suicide and suicidality
Outcome: 1 Suicide - Tendency/Ideation
 |
Analysis 31.2. Comparison 31 Deaths, suicide and suicidality, Outcome 2 Suicide - Attempted.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 31 Deaths, suicide and suicidality
Outcome: 2 Suicide - Attempted
 |
Analysis 31.3. Comparison 31 Deaths, suicide and suicidality, Outcome 3 Suicide - Completed.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 31 Deaths, suicide and suicidality
Outcome: 3 Suicide - Completed
 |
Analysis 31.4. Comparison 31 Deaths, suicide and suicidality, Outcome 4 Deaths.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 31 Deaths, suicide and suicidality
Outcome: 4 Deaths
 |
Analysis 32.1. Comparison 32 Sensitivity analyses - Excluding trials whose dropout rate was greater than 20%, Outcome 1 Escitalopram versus other SSRIs (dropout rate greater than 20% in both arms).
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 32 Sensitivity analyses - Excluding trials whose dropout rate was greater than 20%
Outcome: 1 Escitalopram versus other SSRIs (dropout rate greater than 20% in both arms)
 |
Analysis 32.2. Comparison 32 Sensitivity analyses - Excluding trials whose dropout rate was greater than 20%, Outcome 2 Escitalopram versus other SSRIs (dropout rate greater than 20% in only one arm).
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 32 Sensitivity analyses - Excluding trials whose dropout rate was greater than 20%
Outcome: 2 Escitalopram versus other SSRIs (dropout rate greater than 20% in only one arm)
 |
Analysis 33.1. Comparison 33 Sensitivity analyses - Excluding trials for which the imputation methods were used - RESPONSE, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 33 Sensitivity analyses - Excluding trials for which the imputation methods were used - RESPONSE
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 33.2. Comparison 33 Sensitivity analyses - Excluding trials for which the imputation methods were used - RESPONSE, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 33 Sensitivity analyses - Excluding trials for which the imputation methods were used - RESPONSE
Outcome: 2 Escitalopram versus newer ADs
 |
Analysis 34.1. Comparison 34 Sensitivity analyses - Excluding trials for which the imputation methods were used - REMISSION, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 34 Sensitivity analyses - Excluding trials for which the imputation methods were used - REMISSION
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 34.2. Comparison 34 Sensitivity analyses - Excluding trials for which the imputation methods were used - REMISSION, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 34 Sensitivity analyses - Excluding trials for which the imputation methods were used - REMISSION
Outcome: 2 Escitalopram versus newer ADs
 |
Analysis 35.1. Comparison 35 Sensitivity analyses - Excluding trials for which the imputation methods were used - STANDARD DEVIATION, Outcome 1 Escitalopram versus other SSRIs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 35 Sensitivity analyses - Excluding trials for which the imputation methods were used - STANDARD DEVIATION
Outcome: 1 Escitalopram versus other SSRIs
 |
Analysis 35.2. Comparison 35 Sensitivity analyses - Excluding trials for which the imputation methods were used - STANDARD DEVIATION, Outcome 2 Escitalopram versus newer ADs.
Review: Escitalopram versus other antidepressive agents for depression
Comparison: 35 Sensitivity analyses - Excluding trials for which the imputation methods were used - STANDARD DEVIATION
Outcome: 2 Escitalopram versus newer ADs
 |
WHAT’S NEW
Last assessed as up-to-date: 31 May 2008.
| Date | Event | Description |
|---|---|---|
| 13 May 2009 | Amended | Contact details changed |
HISTORY
Protocol first published: Issue 2, 2007
Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 10 July 2008 | Amended | Converted to new review format and last update of the search |
| 13 March 2007 | New citation required and conclusions have changed | Substantive amendment |
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
In the analyses, the cut-off point for remission was set at 12 or less on the MADRS (instead of 10), because all studies included in the present review used this cut-off point for defining remission.
Footnotes
DECLARATIONS OF INTEREST
AC, CS, AS, CB, AN, CRC, HMG: none
TAF has received research funds and speaking fees from Asahi Kasei, Astellas, Dai-Nippon Sumitomo, Eisai, Eli Lilly, GlaxoSmithKline, Janssen, Kyowa Hakko, Meiji, Nikken Kagaku, Organon, Otsuka, Pfizer and Yoshitomi. The Japanese Ministry of Education, Science and Technology, and the Japanese Ministry of Health, Labour and Welfare have also funded TAFs research.
References to studies included in this review
- Alexopoulos 2004 {unpublished data only} .Alexopoulos G, Gordon J, Zhang D. A placebo-controlled trial of escitalopram and sertraline in the treatment of major depressive disorder; Poster presented at the Annual Meeting of the American College of Neuropsychopharmacology; 2004.Dec, [Google Scholar]
- Baldwin 2006 {published and unpublished data} .Baldwin S, Cooper JA, Huusom AKT, Hindmarch I. A double-blind, randomized, parallel-group, flexible-dose study to evaluate the tolerability, efficacy and effects of treatment discontinuation with escitalopram and paroxetine in patients with major depressive disorder. International Clinical Psychopharmacology. 2006;21(3):159–169. doi: 10.1097/01.yic.0000194377.88330.1d. [DOI] [PubMed] [Google Scholar]
- Bielski 2004 {published and unpublished data} .Bielski RJ, Ventura D, Chung-Chi Chang. A double-blind comparison of escitalopram and venlafaxine extended release in the treatment of major depressive disorder. Journal of Clinical Psychiatry. 2004;65(9):1190–1196. doi: 10.4088/jcp.v65n0906. [DOI] [PubMed] [Google Scholar]
- Boulenger 2006 {published and unpublished data} .Boulenger J-P, Huusom AKT, Florea I, Baekdal T, Sarchiapone M. A comparative study of the efficacy of long-term treatment with escitalopram and paroxetine in severly depressed patients. Current Medical Research and Opinion. 2006;22(7):1331–1341. doi: 10.1185/030079906X115513. [DOI] [PubMed] [Google Scholar]
- Burke 2002 {published and unpublished data} .Burke WJ, Gergel I, Bose A. Fixed-dose trial of the single isomer SSRI escitalopram in depressed outpatients. Journal of Clinical Psychiatry. 2002;63(4):331–336. doi: 10.4088/jcp.v63n0410. [DOI] [PubMed] [Google Scholar]
- Clayton (AK130926) {published and unpublished data} . www.gsk.com/research/clinical/clinicalreg.html.
- Clayton (AK130927) {published and unpublished data} . www.gsk.com/research/clinical/clinicalreg.html.
- Colonna 2005 {published and unpublished data} .Colonna L, Andersen HF, Reines EH. A randomized, double-blind, 24-week study of escitalopram (10 mg/day) versus citalopram (20 mg/day) in primary care patients with major depressive disorder. Current Medical Research and Opinion. 2005;21(10):1659–1668. doi: 10.1185/030079905X65484. [DOI] [PubMed] [Google Scholar]
- Kasper 2005 {published and unpublished data} .Kasper S, de Swart H, Andersen HF. Escitalopram in the treatment of depressed elderly patients. American Journal of Geriatric Psychiatry. 2005;13(10):884–891. doi: 10.1176/appi.ajgp.13.10.884. [DOI] [PubMed] [Google Scholar]
- Kennedy 2005 {unpublished data only} .Kennedy SH, Anderson HF, Lam RW. A pooled analysis of selective serotonin reuptake inhibitors and venlafaxine; Poster presented at the American Psychiatric Association; 2005.May, [Google Scholar]
- Khan 2007 {published and unpublished data} .Khan A, Bose A, Alexopoulos GS, Gommoll C, Li D, Gandhi C. Double-blind comparison of escitalopram and duloxetine in the acute treatment of major depressive disorder. Clinical Drug Investigation. 2007;27(7):481–492. doi: 10.2165/00044011-200727070-00005. [DOI] [PubMed] [Google Scholar]
- Lepola 2003 {published and unpublished data} .Lepola UM, Loft H, Reines EH. Escitalopram (10-20 mg/day) is effective and well tollerated in a placebo-controlled study in depression in primary care. International Clinical Psychopharmacology. 2003;18(4):211–217. doi: 10.1097/00004850-200307000-00003. [DOI] [PubMed] [Google Scholar]
- Mao 2008 {published data only} .Mao PX, Tang YL, Jiang F, Shu L, Gu X, Li M, Qian M, Ma C, Mitchell PB, Cai ZJ. Escitalopram in major depressive disorder: a multicenter, randomized, double-blind, fixed-dose, parallel trial in a Chinese population. Depress Anxiety. 2008;25(1):46–54. doi: 10.1002/da.20222. [DOI] [PubMed] [Google Scholar]
- Montgomery 2004 {published and unpublished data} .Montgomery SA, Huusom AKT, Bothmer J. A randomised study comparing escitalopram with venlafaxine XR in primary care patients with major depressive disorder. Neuropsychobiology. 2004;50:57–64. doi: 10.1159/000078225. [DOI] [PubMed] [Google Scholar]
- Moore 2005 {published data only} .Moore N, Verdoux H, Fantino B. Prospective, multicentre, randomized, double-blind study of the efficacy of escitalopram versus citalopram in outpatient treatment of major depressive disorder. International Clinical Psychopharmacology. 2005;20(3):131–137. doi: 10.1097/00004850-200505000-00002. [DOI] [PubMed] [Google Scholar]
- Nierenberg 2007 {published and unpublished data} .Nierenberg AA, Greist JH, Mallinckrodt CH, Prakash A, Sambunaris A, Tollefson G, Wohlreich MM. Duloxetine versus escitalopram and placebo in the treatment of patients with major depressive disorder: onset of antidepressant action, a non-inferiority study. Current Medical Research and Opinion. 2007;23(2):401–416. doi: 10.1185/030079906X167453. [DOI] [PubMed] [Google Scholar]
- SCT-MD-02 {unpublished data only} .Flexible-dose comparison of the safety and efficacy of Lu 26-054 (escitalopram), citalopram, and placebo in the treatment of major depressive disorder. www.forestclinicaltrials.com.
- SCT-MD-09 {unpublished data only} .Double-blind comparison of the effects of Lu 26-054 (escitalopram) and fluoxetine on sleep in depressed patients. www.forestclinicaltrials.com.
- SCT-MD-35 {unpublished data only} .Fixed-dose comparison of escitalopram combination in adult patients with major depressive disorder. www.forestclinicaltrials.com.
- Ventura 2007 {published and unpublished data} .Ventura D, Armstrong EP, Skrepnek GH, Erder MH. Escitalopram versus sertraline in the treatment of major depressive disorder: a randomized clinical trial. Current Medical Research and Opinion. 2007;23(2):245–250. doi: 10.1185/030079906X167273. [DOI] [PubMed] [Google Scholar]
- Wade 2007 {published and unpublished data} .Wade A, Gembert K, Florea I. A comparative study of the efficacy of acute and continuation treatment with escitalopram versus duloxetine in patients with major depressive disorder. Current Medical Research and Opinion. 2007;23(7):1605–1614. doi: 10.1185/030079907x210732. [DOI] [PubMed] [Google Scholar]
- Yevtushenko 2007 {published data only} .Yevtushenko VY, Belous AI, Yevtushenko YG, Gusinin SE, Buzik OJ, Agibalova TV. Efficacy and tolerability of escitalopram versus citalopram in major depressive disorder: a 6-week, multicenter, prospective, randomized, doubleblind, active-controlled study in adult outpatients. Clinical Therapeutics. 2007;29(11):2319–32. doi: 10.1016/j.clinthera.2007.11.014. [DOI] [PubMed] [Google Scholar]
Additional references
- Als-Nielsen 2003 .Als-Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events? JAMA. 2003;290:921–8. doi: 10.1001/jama.290.7.921. [DOI] [PubMed] [Google Scholar]
- Altman 1996 .Altman DG, Bland JM. Detecting skewness from summary information. BMJ. 1996;313:1200. doi: 10.1136/bmj.313.7066.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson 2000 .Anderson IM, Nutt DJ, Deakin JF. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 1993 British Association for Psychopharmacology guidelines. Journal of Psychopharmacology. 2000;14:3–20. doi: 10.1177/026988110001400101. [DOI] [PubMed] [Google Scholar]
- APA 1980 .American Psychiatric Association . Diagnostical and Statistical Manual of Mental Disorders (DSM-III) 3rd Edition APA; Washington, DC: 1980. [Google Scholar]
- APA 1987 .American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R) 3rd Edition American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- APA 1994 .American Psychiatric Association . Diagnostical and Statistical Manual of Mental Disorders (DSM-IV) 4rd Edition American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- APA 2000 .American Psychiatric Association Practice guideline for the treatment of patients with major depressive disorder (revision) American Journal of Psychiatry. 2000;147:1–45. [PubMed] [Google Scholar]
- Barbui 2000 .Barbui C, Hotopf M, Freemantle M, Boynton J, Churchill R, Eccles MP. Selective serotonin reuptake inhibitors versus tricyclic and heterocyclic antidepressants: comparison of drug adherence. Cochrane Database of Systematic Reviews. 2000;(1) doi: 10.1002/14651858.CD002791. DOI: 10.1002/14651858.CD002791.pub2. [DOI] [PubMed] [Google Scholar]
- Barbui 2004 .Barbui C, Cipriani A, Brambilla P, Hotopf M. “Wish bias” in antidepressants drug trials? Journal of Clinical Pharmacology. 2004;24:126–130. doi: 10.1097/01.jcp.0000115665.45074.0d. [DOI] [PubMed] [Google Scholar]
- Bekelman 2003 .Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA. 2003;289:454–65. doi: 10.1001/jama.289.4.454. [DOI] [PubMed] [Google Scholar]
- Bhandari 2004 .Bhandari M, Busse JW, Jackowski D, Montori VM, Schunemann H, Sprague S. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trial. CMAJ. 2004;170:477–480. [PMC free article] [PubMed] [Google Scholar]
- Bollini 1999 .Bollini P, Pampallona S, Tibaldi G, Kupelnick B, Munizza C. Effectiveness od antidepressants. Meta-analysis of dose-effect relationships in randomised clinical trials. British Journal of Psychiatry. 1999;174:297–303. doi: 10.1192/bjp.174.4.297. [DOI] [PubMed] [Google Scholar]
- Buchkowsky 2004 .Buchkowsky SS, Jewesson PJ. Industry sponsorship and authorship of clinical trials over 20 years. Annals of Pharmacotherapy. 2004;38:579–85. doi: 10.1345/aph.1D267. [DOI] [PubMed] [Google Scholar]
- Cipriani 2005 .Cipriani A, Brambilla P, Furukawa T, Geddes J, Gregis M, Hotopf M, Malvini L, Barbui C. Fluoxetine versus other types of pharmacotherapy for depression. Cochrane Database of Systematic Reviews. 2005;(4) doi: 10.1002/14651858.CD004185.pub2. DOI: 10.1002/14651858.CD004185.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani 2006 .Cipriani A, Barbui C, Brambilla P, Furukawa TA, Hotopf M, Geddes JR. Are all antidepressants really the same? The case of fluoxetine: A systematic review. Journal of Clinical Psychiatry. 2006;67:850–64. doi: 10.4088/jcp.v67n0601. [DOI] [PubMed] [Google Scholar]
- Cipriani 2007a .Cipriani A, Signoretti A, Furukawa TA, Churchill R, Tomelleri S, Omori IM, McGuire HF, Barbui C, for the Meta-Analysis of New Generation Antidepressants (MANGA) Study Group Venlafaxine versus other antidepressive agents for depression. Cochrane Database of Systematic Reviews. 2007;(2) doi: 10.1002/14651858.CD006530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani 2007b .Cipriani A, Furukawa TA, Veronese A, Watanabe N, Churchill R, McGuire HF, Barbui C, for the Meta-Analysis of New Generation Antidepressants (MANGA) Study Group Paroxetine versus other anti-depressive agents for depression. Cochrane Database of Systematic Reviews. 2007;(2) doi: 10.1002/14651858.CD006531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani 2009 .Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JPT, Churchill R, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. The Lancet. 2009;373(9665):746–58. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- Ciuna 2004 .Ciuna A, Andretta M, Corbari L, Levi D, Mirandola M, Sorio A, Barbui C. Are we going to increase the use of antidepressants up to that of benzodiazepines? European Journal of Clinical Pharmacology. 2004;60:629–34. doi: 10.1007/s00228-004-0810-8. [DOI] [PubMed] [Google Scholar]
- De Abajo 2008 .de Abajo FJ, García-Rodríguez LA. Risk of upper gastrointestinal tract bleeding associated with selective serotonin reuptake inhibitors and venlafaxine therapy: interaction with nonsteroidal anti-inflammatory drugs and effect of acid-suppressing agents. Arch Gen Psychiatry. 2008;65(7):795–803. doi: 10.1001/archpsyc.65.7.795. [DOI] [PubMed] [Google Scholar]
- Djulbegovic 2000 .Djulbegovic B, Lacevic M, Cantor A, Fields KK, Bennett CL, Adams JR, Kuderer NM, Lyman GH. The uncertainty principle and industry-sponsored research. Lancet. 2000;356:635–38. doi: 10.1016/S0140-6736(00)02605-2. [DOI] [PubMed] [Google Scholar]
- Elliott 2007 .Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007;369(9557):201–7. doi: 10.1016/S0140-6736(07)60108-1. [DOI] [PubMed] [Google Scholar]
- Ellis 2004 .Ellis P. Australian and New Zealand practice guidelines for the treatment of depression. Australian and New Zealand Journal of Psychiatry. 2004;38:389–407. doi: 10.1080/j.1440-1614.2004.01377.x. [DOI] [PubMed] [Google Scholar]
- Etminan 2005 .Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63. doi: 10.1136/bmj.38302.504063.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa 2002a .Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the “number needed to treat”? An empirical study of summary effect measures in meta-analyses. International Journal of Epidemiology. 2002;31:72–6. doi: 10.1093/ije/31.1.72. [DOI] [PubMed] [Google Scholar]
- Furukawa 2002b .Furukawa TA, McGuire H, Barbui C. Meta-analysis of effects and side-effects of low dosage tricyclic antidepression in depression: systematic review. BMJ. 2002;325:991–5. doi: 10.1136/bmj.325.7371.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa 2005 .Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N. Imputing response rate from means and standard deviations in meta-analysis. International Clinical Psychopharmacology. 2005;20:49–52. doi: 10.1097/00004850-200501000-00010. [DOI] [PubMed] [Google Scholar]
- Furukawa 2006 .Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology. 2006;59:7–10. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Geddes 2000 .Geddes JR, Freemantle N, Mason J, Eccles MP, Boynton J. SSRIs versus other antidepressants for depressive disorder. Cochrane Database of Systematic Reviews. 2000;(2) doi: 10.1002/14651858.CD001851. DOI: 10.1002/14651858.CD001851.pub2. [DOI] [PubMed] [Google Scholar]
- Gourion 2008 .Gourion D. Antidepressants and their onset of action: a major clinical, methodological and pronostical issue. Encephale. 2008;34(1):73–81. doi: 10.1016/j.encep.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Guaiana 2003 .Guaiana G, Barbui C, Hotopf M. Amitriptyline versus other types of pharmacotherapy for depression. Cochrane Database of Systematic Reviews. 2003;(2) doi: 10.1002/14651858.CD004186. DOI: 10.1002/14651858.CD004186.pub2. [DOI] [PubMed] [Google Scholar]
- Guaiana 2005 .Guaiana G, Andretta M, Corbari L, Mirandola M, Sorio A, D’Avanzo B, Barbui C. Antidepressant drug consumption and public health indicators in Italy. Journal of Clinical Psychiatry. 2005;66:750–5. doi: 10.4088/jcp.v66n0612. [DOI] [PubMed] [Google Scholar]
- Guy 1970 .Guy W, Bonato RR. Manual for the ECDEU Assessment Battery 2. National Institute of Mental Health; Chevy Chase, MD: 1970. [Google Scholar]
- Hamilton 1960 .Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen 2005 .Hansen RA, Gartlehner G, Lohr KN, Gaynes BN, Carey TS. Efficacy and safety of second-generation antidepressants in the treatment of major depressive disorder. Ann Intern Med. 2005;143(6):415–26. doi: 10.7326/0003-4819-143-6-200509200-00006. [DOI] [PubMed] [Google Scholar]
- Hansen 2005a .Hansen RA, Gartlehner G, Lohr KN, Gaynes BN, Carey TS. Efficacy and safety of second-generation of antidepressants in the treatment of major depressive disorder. Annals of Internal Medicine. 2005;143:415–26. doi: 10.7326/0003-4819-143-6-200509200-00006. [DOI] [PubMed] [Google Scholar]
- Higgins 2003 .Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2005 .Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, Ltd; Chichester: 2005. [Google Scholar]
- Higgins 2008 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008] The Cochrane Collaboration; 2008. Available from www.cochrane-handbook.org. [Google Scholar]
- Imperadore 2007 .Imperadore G, Cipriani A, Signoretti A, Furukawa TA, Watanabe N, Churchill R, McGuire HF, Barbui C, for the Meta-Analysis of New Generation Antidepressants (MANGA) Study Group Citalopram versus other antidepressive agents for depression. Cochrane Database of Systematic Reviews. 2007;(2) [Google Scholar]
- Khan 2003 .Khan A, Khan SR, Walens G, Kolts R, Giller EL. Frequency of positive studies among fixed and flexible dose antidepressant clinical trials: an analysis of the food and drug administration summary basis of approval reports. Neuropsychopharmacologiy. 2003;28:552–7. doi: 10.1038/sj.npp.1300059. [DOI] [PubMed] [Google Scholar]
- Kumra 2008 .Kumra S, Oberstar JV, Sikich L, Findling RL, McClellan JM, Vinogradov S, Charles Schulz S. Efficacy andtolerability of second-generation antipsychotics in children and adolescents with schizophrenia. Schizophr Bull. 2008;34(1):60–71. doi: 10.1093/schbul/sbm109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer 2002 .Kupfer DJ, Frank E. Placebo in clinical trials for depression: complexity and necessity. JAMA. 2002;287:1853–54. doi: 10.1001/jama.287.14.1853. [DOI] [PubMed] [Google Scholar]
- Le Lay 2006 .Le Lay A, Despiegel N, François C, Duru G. Can discrete event simulation be of use in modelling major depression? Cost Eff Resour Alloc. 2006;4:19. doi: 10.1186/1478-7547-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexchin 2003 .Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ. 2003;326:1167–70. doi: 10.1136/bmj.326.7400.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde 2008 .Linde K, Berner MM, Kriston L. St John’s wort for major depression. Cochrane Database of Systematic Reviews. 2008;(4) doi: 10.1002/14651858.CD000448.pub3. Art. No.: CD000448. DOI: 10.1002/14651858.CD000448.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu 2006 .Lu M, Ades T. Assessing evidence consistency in mixed treatment comparisons. Journal of The American Statistical Association. 2006;101:447–59. [Google Scholar]
- Luborsky 1962 .Luborsky L. Clinician’s judgements of mental health. Archives of General Psychiatry. 1962;7:407–17. doi: 10.1001/archpsyc.1962.01720060019002. [DOI] [PubMed] [Google Scholar]
- Lumley 2002 .Lumley S. Network meta-analysis for indirect treatment comparisons. Statistics in Medicine. 2002;21:2313–24. doi: 10.1002/sim.1201. [DOI] [PubMed] [Google Scholar]
- Malvini 2006 .Malvini L, Cipriani A, Furukawa TA, McGuire H, Churchill R, Barbui C, for the MANGA Study Sertraline versus other antidepressive agents for depression. (Protocol) Cochrane Database of Systematic Reviews. 2006;(3) DOI: 10.1002/14651858.CD006117. [Google Scholar]
- Moncrieff 2005 .Moncrieff J, Kirsch I. Efficacy of antidepressants in adults. BMJ. 2005;331:155–57. doi: 10.1136/bmj.331.7509.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery 1979 .Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Montgomery JH 2004 .Montgomery JH, Byerly M, Carmody T, Li B, Miller DR, Varghese F. An analysis of the effect of funding source in randomized clinical trials of second generation antipsychotics for the treatment of schizophrenia. Controlled Clinical Trials. 2004;25:598–612. doi: 10.1016/j.cct.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Murray 1997 .Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- Nakagawa 2007 .Nakagawa A, Watanabe N, Omori IM, Cipriani A, Barbui C, McGuire H, Churchill R, Furukawa TA, for the MANGA study group Milnacipran versus other antidepressive agents for depression. Cochrane Database of Systematic Reviews. 2007;(2) doi: 10.1002/14651858.CD006529.pub2. DOI: 10.1002/14651858.CD006529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE 2007 .National Institute for Health and Clinical Excellence . Depression: management of depression in primary and secondary care (amended) National Institute for Health and Clinical Excellence; London: 2007. [Google Scholar]
- Nose 2007 .Nose M, Cipriani A, Furukawa TA, Omori IM, Churchill R, McGuire HF, Barbui C, for the Meta-Analysis of New Generation Antidepressants (MANGA) Study Group Duloxetine versus other anti-depressive agents for depression. Cochrane Database of Systematic Reviews. 2007;(2) [Google Scholar]
- Omori 2006 .Omori I, Watanabe N, Cipriani A, Barbui C, McGuire H, Churchill R, Furukawa TA, for the MANGA Study Fluvoxamine versus other anti-depressive agents for depression. (Protocol) Cochrane Database of Systematic Reviews. 2006;(3) doi: 10.1002/14651858.CD006114.pub2. DOI: 10.1002/14651858.CD006114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxman 1992 .Oxman AD, Guyatt GH. A consumer’s guide to subgroup analyses. Annals of Internal Medicine. 1992;116:78–84. doi: 10.7326/0003-4819-116-1-78. [DOI] [PubMed] [Google Scholar]
- Patel 2007 .Patel V, Araya R, Chatterjee S, Chisholm D, Cohen A, De Silva M, Hosman C, McGuire H, Rojas G, van Ommeren M. Treatment and prevention of mental disorders in low-income and middle-income countries. Lancet. 2007;370(9591):991–1005. doi: 10.1016/S0140-6736(07)61240-9. [DOI] [PubMed] [Google Scholar]
- Perlis 2005 .Perlis RH, Perlis CS, Wu Y, Hwang C, Joseph M, Nierenberg AA. Industry sponsorship and financial conflict interest the reporting of clinical trials in psychiatry. American Journal of Psychiatry. 2005;162:1957–60. doi: 10.1176/appi.ajp.162.10.1957. [DOI] [PubMed] [Google Scholar]
- Procyshyn 2004 .Procyshyn RM, Chau A, Fortin P, Jenkins W. Prevalence and outcomes of pharmaceutical industry-sponsored clinical trials involving clozapine, risperidone, or olanzapine. Canadian Journal of Psychiatry - Revue Canadienne De Psychiatrie. 2004;49:601–6. doi: 10.1177/070674370404900905. [DOI] [PubMed] [Google Scholar]
- Psaty 2003 .Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, Weiss NS. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA. 2003;289(19):2534–44. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- Puech 1997 .Puech A, Montgomery SA, Prost JF, Solles A, Briley M. Milnacipran, a new serotonin and noradrenaline reuptake inhibitor: an overview of its antidepressant activity and clinical tolerability. International Clinical Psychoharmacology. 1997;12:99–108. doi: 10.1097/00004850-199703000-00005. [DOI] [PubMed] [Google Scholar]
- Salanti 2008 .Salanti G, Higgins JP, Ades A, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res. 2008;17:279–301. doi: 10.1177/0962280207080643. [DOI] [PubMed] [Google Scholar]
- Schwartz 2008 .Schwartz RS, Curfman GD, Morrissey S, Drazen JM. Full Disclosure and the Funding of Biomedical Research. N Engl J Med. 2008;358:1850–51. doi: 10.1056/NEJMe0802618. [DOI] [PubMed] [Google Scholar]
- Smith 2002 .Smith D, Dempster C, Glanville J, Freemantle N, Anderson I. Efficacy and tolerability of venlafaxine compared with selective serotonin reuptake inhibitors and other antidepressants: a meta-analysis. British Journal of Psychiatry. 2002;180:396–404. doi: 10.1192/bjp.180.5.396. [DOI] [PubMed] [Google Scholar]
- Sobocki 2008 .Sobocki P, Ekman M, Ovanfors A, Khandker R, Jönsson B. The cost-utility of maintenance treatment with venlafaxine in patients with recurrent major depressive disorder. Int J Clin Pract. 2008;62(4):623–32. doi: 10.1111/j.1742-1241.2008.01711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarn 2006 .Tarn DM, Paterniti DA, Heritage J, Hays RD, Kravitz RL, Wenger NS. Physician communication about the cost and acquisition of newly prescribed medications. Am J Manag Care. 2006;12(11):657–64. [PubMed] [Google Scholar]
- Ware 1993 .Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpetation Guide. New England Medical Centre; Boston, MA: 1993. [Google Scholar]
- Watanabe 2006 .Watanabe N, Barbui C, Churchill R, Cipriani A, Furukawa TA, McGuire HF, Omori I, for the MANGA Study Mirtazapine versus other anti-depressive agents for depression (Protocol) Cochrane Database of Systematic Reviews. 2006;(3) doi: 10.1002/14651858.CD006114.pub2. DOI: 10.1002/14651858.CD006528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO 1978 .World Health Organization . The Ninth Revision of the International Classification of Diseases and Related Health Problems (ICD-9) World Health Organisation; Geneva: 1978. [Google Scholar]
- WHO 1992 .World Health Organization . The Tenth Revision of the International Classification of Diseases and Related Health Problems (ICD-10) World Health Organisation; Geneva: 1992. [Google Scholar]
- WHOQOL Group 1998 .WHOQOL Group The World Health Organization quality of life assessment (WHOQOL): Development and general psychometric properties. Social Science and Medicine. 1998;46:1569–85. doi: 10.1016/s0277-9536(98)00009-4. [DOI] [PubMed] [Google Scholar]
- Williams 2000 .Williams JW, Jr, Mulrow CD, Chiquette E, Noel PH, Aguilar C, Cornell J. A systematic review of newer pharmacoterapies for depression in adults: evidence report summary. Annals of Internal Medicine. 2000;132:743–56. doi: 10.7326/0003-4819-132-9-200005020-00011. [DOI] [PubMed] [Google Scholar]
- Wing 1994 .Wing J. Outcomes into Clinical Practice. BMJ publishing; London: 1994. Measuring mental health outcomes: a perspective from the Royal College of Psychiatrists. [Google Scholar]
- Wood 2008 .Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, Gluud C, Martin RM, Wood AJ, Sterne JA. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336(7644):601–5. doi: 10.1136/bmj.39465.451748.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman 2004 .Zimmerman M, Posternak MA, Chelminski I. Derivation of a definition of remission on Montgomery-Asberg depression rating scale corresponding to the definition of remission on the Hamilton rating scale for depression. Journal of Psychiatric Research. 2004;38:577–82. doi: 10.1016/j.jpsychires.2004.03.007. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study