Abstract
Background
Pure autonomic failure (PAF) and Parkinson disease (PD) both are Lewy body diseases, and both entail substantia nigra dopaminergic, locus ceruleus noradrenergic, and cardiac sympathetic denervation. Multiple system atrophy (MSA) is a non-Lewy body disease in which alpha-synuclein accumulates in glial cells, with central catecholamine deficiency but preserved cardiac sympathetic innervation in most patients. PD is associated with more severe and consistent olfactory dysfunction than in MSA; whether PAF entails olfactory dysfunction has been unknown. In this study we assessed olfactory function in PAF in comparison with the two other synucleinopathies and whether olfactory dysfunction correlates with neuroimaging evidence of cardiac noradrenergic or nigrostriatal dopaminergic denervation.
Method
The University of Pennsylvania Smell Identification Test (UPSIT) was administered to 8 patients with PAF, 23 with PD, and 20 with MSA. 6-[18F]Fluorodopamine positron emission tomographic (PET) scanning was used to indicate cardiac noradrenergic innervation and the putamen:occipital cortex (PUT:OCC) and substantia nigra (SN):OCC ratios of 6-[18F]fluorodopa-derived radioactivity to indicate nigrostriatal dopaminergic innervation.
Results
The PAF group had a low mean UPSIT score (22 ± 3), similar to that in PD (20 ± 2) and lower than in MSA (31 ± 2, p = 0.004). Individual UPSIT scores correlated positively with cardiac 6-[18F]fluorodopamine-derived radioactivity (r = 0.63 in the septum, p < 0.0001; r = 0.64 in the free wall, p < 0.0001) but not with PUT:OCC or SN:OCC ratios of 6-[18F]fluorodopa-derived radioactivity.
Discussion
In synucleinopathies, olfactory dysfunction is related to Lewy body pathology and cardiac sympathetic denervation, independently of parkinsonism or striatal dopamine deficiency.
Keywords: Olfaction, Parkinson, Multiple system atrophy, Fluorodopa, Fluorodopamine, PET, Biomarker
1. Introduction
Pure autonomic failure (PAF) is a rare disease of the autonomic nervous system that is characterized by neurogenic orthostatic hypotension (NOH) without clinical evidence of central neurodegeneration, whereas Parkinson disease (PD) is a common neurodegenerative disease that is characterized by bradykinesia, cogwheel rigidity, and resting tremor. NOH in PAF is thought to result from loss of sympathetic nerves, and plasma levels of the sympathetic neurotransmitter, norepinephrine, are low, whereas the movement disorder in PD is thought to result from loss of dopamine (DA)-containing terminals in the corpus striatum, and never-treated PD patients have normal plasma norepinephrine levels [1]. PAF patients have no signs of parkinsonism and have neuroimaging evidence of intact striatal dopaminergic innervation [2], whereas PD patients have parkinsonism by definition and have loss of striatal dopaminergic innervation, as evidenced by decreased striatal uptake and by low putamen:occipital cortex (PUT:OCC) ratios of 6-[18F]fluorodopa-derived radioactivity [3].
Nevertheless, PAF and PD share several clinical and laboratory features. Both PAF and PD are Lewy body disorders. In PAF, Lewy bodies usually are present in the substantia nigra (SN)—a pathologic hallmark of PD [4]—and in PD, Lewy bodies usually are present in sympathetic ganglia [5]. About 40% of PD patients have orthostatic hypotension, and PD + NOH is associated with neuropharmacologic and neurochemical evidence of cardiac sympathetic and generalized noradrenergic denervation, as in PAF [6]. PAF patients can develop parkinsonism [7], and PAF and PD entail similarly reduced SN concentrations of 6-[18F]fluorodopa-derived radioactivity and similarly reduced cerebrospinal fluid concentrations of the dopamine metabolite, dihydroxyphenylacetic acid [8], neuroimaging and neurochemical indices of central dopamine deficiency.
Patients with PD often have olfactory dysfunction, which seems to occur independently of the severity or duration of the movement disorder and the cognitive deficits that can attend PD [9]. Deposition of alpha-synuclein in the olfactory bulb is one of the earliest pathologic findings in PD [10]. Recent reports have noted an association between loss of olfactory function and loss of cardiac noradrenergic innervation in PD [11]. Moreover, both loss of sense of smell and cardiac sympathetic denervation can precede the onset of motor symptoms, suggesting that the combination might provide a biomarker of risk of PD.
These findings led us to ask if PAF might entail olfactory dysfunction, as in PD. In this study we assessed whether University of Pennsylvania Smell Identification Test (UPSIT) scores were related to results of cardiac sympathetic neuroimaging, using 6-[18F]fluorodopamine positron emission tomographic (PET) scanning, or to PUT:OCC or SN:OCC ratios of 6-[18F]fluorodopa-derived radioactivity, in three forms of synucleinopathy—PAF, PD (with or without NOH), and multiple system atrophy (MSA). In MSA, a non-Lewy body disorder, alpha-synuclein is found in glial cytoplasmic inclusions. Thus, of the three synucleinopathies associated with NOH, PAF and PD + NOH are Lewy body disorders, and MSA is not. In MSA, sympathetic noradrenergic neurons are usually spared [12], and cardiac sympathetic neuroimaging results usually are normal [13]. MSA entails variable degrees of olfactory dysfunction, and at least some patients have a normal ability to distinguish among odors [14].
Whether olfactory dysfunction in the three synucleinopathies is related to cardiac noradrenergic or nigrostriatal dopaminergic denervation has not been assessed in the same study. In patients with early PD, olfactory function as gauged by the UPSIT has been reported to vary with concentrations of the dopamine transporter imaging agent, [99mTc]TRODAT-1 [15]. On the other hand, among PD or MSA patients there is no relationship between UPSIT scores and PUT:OCC ratios, although there is a relationship between UPSIT scores and cardiac 6-[18F]fluorodopamine-derived radioactivity [17].
If PAF patients were found to have olfactory dysfunction of similar severity to that in PD and more severe than in MSA, the findings would support the view that olfactory dysfunction in synucleinopathies is related to Lewy body pathology, even in patients without parkinsonism or striatal dopamine deficiency.
2. Methods
A total of 8 PAF, 23 PD, and 20 MSA patients were studied at the NIH Clinical Center. Each gave informed written consent before participating in protocols approved by the NINDS Institutional Review Board.
All patients were tested while on their usual medications, except that in some PD or MSA patients levodopa/carbidopa was withheld. The neurocirculatory abnormalities attending PD + NOH occur independently of levodopa treatment [16].
In our program, diagnosis of PAF or PD + OH requires neuroimaging and neurochemical or neuropharmacologic evidence of sympathetic noradrenergic denervation. Most patients with MSA have evidence of normal noradrenergic innervation [17,18].
Orthostatic hypotension was defined by a decrease in systolic blood pressure of at least 20 mmHg and in diastolic pressure at least 10 mmHg between supine rest for at least 15 min and upright posture for 5 min (unless symptomatic or rapid hypotension necessitated return to the supine position before 5 min upright). NOH was defined by orthostatic hypotension coupled with abnormal beat-to-beat blood pressure associated with performance of the Valsalva maneuver [19]. MSA was diagnosed based on previously published consensus statements [20].
Patients filled out the 4-booklet University of Pennsylvania Smell Identification Test (UPSIT). The results were scored using punched cards provided with the test (The Smell Identification Test Administration Manual: Third Edition, Sensonics, Inc., Haddon Heights, NJ).
For 6-[18F]fluorodopa brain PET scanning, the subject was placed supine head-first in a GE Advance scanner. Seven mCi of 6-[18F]fluorodopa was injected i.v. over 3 min using an automated syringe pump. Carbidopa pre-treatment was not used. A 15 min static scan was obtained in the GE Advance scanner, ending about 120 min from the time of injection of 6-[18F]fluorodopa.
For 6-[18F]fluorodopamine PET scanning, 1 mCi of the imaging agent was injected i.v. over 3 min. Dynamic data were obtained for 30 min.
6-[18F]Fluorodopa brain PET and MRI scans were fused using PMOD (PMOD Technologies Ltd., Zurich, Switzerland). Regions of interest were placed manually at the perimeters of the substantia nigra (SN), putamen (PUT), and occipital (OCC) cortex in the MRI scans of the same subjects. The sizes of the regions of interest therefore were not fixed. Tissue concentrations of 6-[18F]fluorodopa-derived radioactivity (in nCi/cc) were adjusted for the dose per unit body mass and expressed in units of nCi-kg/cc-mCi.
Data were analyzed by factorial analyses of variance, with Fisher’s PLSD post-hoc test, and linear regression, with calculation of Pearson correlation coefficients, using KaleidaGraph 4.01 (Synergy Software, Reading, PA).
3. Results
Most of the PAF, PD + NOH, and MSA patients were men (75, 63, and 70%). Mean subject ages were 67 ± 8, 70 ± 3, and 59 ± 2 years old in these patient groups.
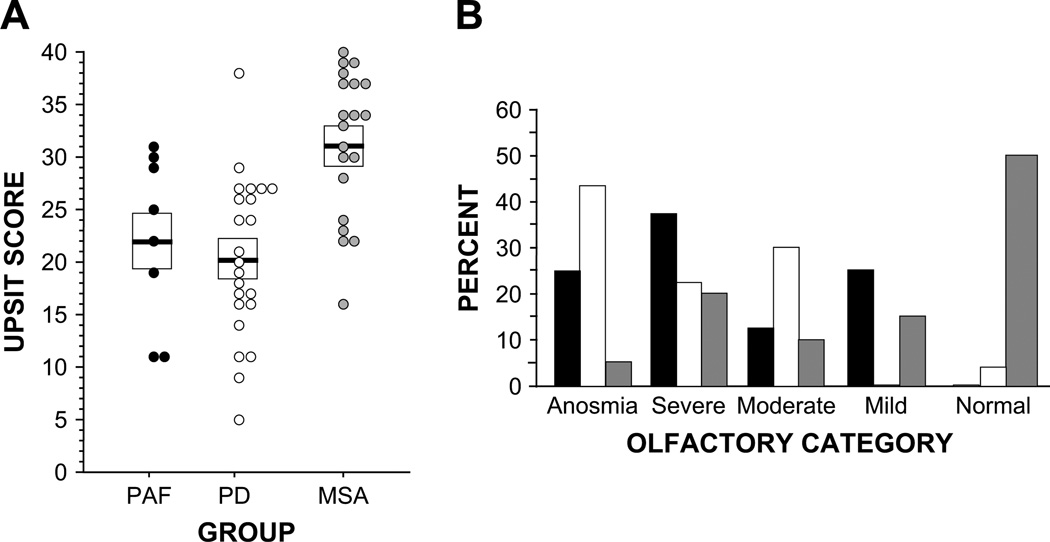
The PAF and PD groups had low mean UPSIT scores, compared to the MSA group (p = 0.005, p < 0.0001; Fig. 1A). The majority of PAF and PD patients were either anosmic or had severe microsmia, whereas the majority of MSA patients had either normal olfaction or mild microsmia (Fig. 1B). Among PD patients, those with NOH tended to have lower UPSIT scores (16 ± 2) than did those without NOH (23 ± 2, p = 0.07). Of the 8 PD + NOH patients, 6 (75%) were anosmic, whereas of the 15 PD patients without NOH, 4 (27%) were anosmic.
Fig. 1.
(A) University of Pennsylvania Smell Identification Test (UPSIT) scores in patients with pure autonomic failure (PAF, black), Parkinson disease (PD, white), or multiple system atrophy (MSA, gray). Horizontal bars show group mean values and boxes ± SEM. (B) Percents of patients in UPSIT olfactory categories. Note decreased UPSIT scores in PAF and PD compared to MSA, with the majority of PAF or PD patients having anosmia or severe microsmia and the majority of MSA patients having normal olfaction or mild microsmia.
There were no significant gender differences in UPSIT scores in the PAF, PD, or MSA groups. Across all patients, there was no relationship between UPSIT scores and the orthostatic change in systolic blood pressure (r = −0.02), and there were no significant relationships between UPSIT scores and the orthostatic change in systolic blood pressure in the PAF, PD, or MSA groups considered separately.
The PAF and PD groups had low mean 6-[18F]fluorodopamine-derived radioactivity in the interventricular septum (2564 ± 261 and 4881 ±621 nCi-kg/cc-mCi), compared to the MSA group (10,278 ± 410 and 9642 ± 329 nCi-kg/cc-mCi, p < 0.0001 each). In all 8 PAF patients, the left ventricular free wall was not distinguishable from the left ventricular chamber. Among PD patients, those with NOH had lower septal 6-[18F]fluorodopamine-derived radioactivity than did those without NOH (6262 ± 710 nCi-kg/cc-mCi, p < 0.0001).
Individual UPSIT scores were positively correlated with left ventricular myocardial concentrations of 6-[18F]fluorodopamine-derived radioactivity in the septum (r = 0.63, p < 0.0001) and free wall r = 0.64, p < 0.0001) but not with PUT:OCC ratios of 6-[18F]fluorodopa-derived radioactivity (Fig. 2) or with SN:OCC ratios (r = 0.16). Among patients with a Lewy body disorder (PD or PAF), UPSIT scores correlated positively with septal 6-[18F]fluorodopamine-derived radioactivity (r = 0.41, p = 0.02) and with the SN:OCC ratio (r = 0.54, p = 0.006) but not with the PUT:OCC ratio (r = 0.12). Among PD patients, UPSIT scores were positively correlated with septal 6-[18F]fluorodopamine-derived radioactivity (r = 0.54, p = 0.009) but not with the PUT:OCC ratio (r = 0.18) or SN:OCC ratio (r = 0.38, p = 0.11).
Fig. 2.
University of Pennsylvania Smell Identification Test (UPSIT) scores, expressed as a function of (A) cardiac septal 6-[18F]fluorodopamine-derived radioactivity and (B) the putamen:occipital cortex (PUT:OCC) ratio of 6-[18F]fluorodopa-derived radioactivity in patients with pure autonomic failure (PAF, black), Parkinson disease (PD, white), or multiple system atrophy (MSA, gray). Dashed line shows the line of best fit, with the linear regression equation displayed. Note positive correlation of UPSIT scores with cardiac septal 6-[18F]fluorodopamine-derived radioactivity but not with the PUT:OCC ratio.
Most PAF and PD patients had both an UPSIT score less than 30 and cardiac 6-[18F]fluorodopamine-derived radioactivity less than 7500 nCi-kg/cc-mCi, a combination not seen in any MSA patients, and most MSA patients had both an UPSIT score of at least 30 and cardiac 6-[18F]fluorodopamine-derived radioactivity more than 7500 nCi-kg/cc-mCi, a combination not seen in any PAF patients and in only 1 PD patient (Fig. 2).
We assessed whether olfactory function might have been related to drug treatment for orthostatic hypotension. The percentages of patients with PAF, PD + NOH, and MSA who were on midodrine were 57, 57, and 35%. In these three patient groups, mean UPSIT scores in patients on vs. not on midodrine were 15 vs. 18, 25 vs. 20, and 34 vs. 30. Analogously, mean UPSIT scores in patients on vs. not on fludrocortisone were 16 vs. 17, 24 vs. 20, and 32 vs. 30.
4. Discussion
In this study, patients with PAF had olfactory dysfunction that was about as severe as in PD and less severe than in MSA. These group differences suggest that in alpha-synucleinopathies, olfactory dysfunction is especially closely associated with Lewy body pathology. Consistent with this view, olfactory dysfunction is prevalent in dementia with Lewy bodies and in diffuse Lewy body disease, to a lesser extent than in Alzheimer’s disease [21,22].
The present results extend on our previous finding that in synucleinopathies, neuroimaging evidence for loss of striatal dopamine terminals occurs independently of evidence for loss of cardiac noradrenergic terminals, by noting olfactory deficiency in PAF, a Lewy body disorder that does not involve either parkinsonism or decreased striatal dopaminergic innervation [17]. Analogously, individuals with incidental Lewy body disease, established by post-mortem pathology, have been reported to have olfactory dysfunction in the absence of parkinsonism during life [23]. Olfactory function in the compared groups was not related to the magnitude or treatment of orthostatic hypotension.
Individual UPSIT scores correlated positively with the magnitude of cardiac 6-[18F]fluorodopamine-derived radioactivity, confirming a study by another group involving a different sympathoneural imaging agent, 123I-metaiodobenzylguanidine, and a different olfactory test, in patients with PD or MSA [11], as well as a previous report from our group that did not include PAF patients [17]. Thus, in alpha-synucleinopathies, the loss of ability to discriminate among odors seems to be related to the loss of cardiac sympathetic nerves. In contrast, individual UPSIT scores were unrelated to PUT:OCC ratios of 6-[18F]fluorodopa-derived radioactivity, either among all patients with alpha-synucleinopathies or specifically among PD patients. The latter finding seems inconsistent with the reported positive correlation between UPSIT scores and [99mTc]TRODAT-1-derived radioactivity in the PUT of patients with early PD [15]. All the PD patients in the present study had established disease, with a mean duration of 5 years from the time of onsent of the movement disorder until the time of study.
Pathologic accumulation of alpha-synuclein in the olfactory bulb and in autonomic and lower brainstem neurons occurs in the pre-motor phase of PD [24]. Although the olfactory bulb possesses relatively little dopaminergic innervation, it does possess extensive noradrenergic innervation, which is derived from the locus ceruleus. Olfactory stimulation releases norepinephrine in the olfactory bulb, and olfactory bulb ablation decreases norepinephrine contents in the piriform cortex. These findings suggest that a pathway from the locus ceruleus to the olfactory bulb and from there to olfactory cortical centers provides the circuitry for a modulatory role of norepinephrine in olfactory memory [25].
Although most research on catecholamine deficiency in PD has focused on the nigrostriatal dopamine system, the original description of brain tissue concentrations of catecholamines in PD noted decreased contents of both dopamine and norepinephrine [26], and PD involves loss of locus ceruleus neurons that can be as severe as the loss of dopaminergic nigral neurons [27], especially in PD patients with dementia. The present finding of an association between olfactory dysfunction and neuroimaging evidence of cardiac noradrenergic denervation in PAF and PD leads us to propose that the two disorders share a pathogenetic mechanism that produces loss of noradrenergic terminals in the olfactory bulb and heart relatively dissociated from loss of dopaminergic terminals in the striatum. To our knowledge, contents of catecholamines in the olfactory bulb have not yet been described in PAF, PD, or any other neurodegenerative disease.
What the etiologic basis might be of such a shared pathogenetic mechanism remains unknown. Herpes simplex type I virus inoculated into the nasal cavity leads to infection of locus ceruleus neurons [28] and loss of noradrenergic fibers in the olfactory bulb [29]. Retrograde transport of a neurotropic virus might lead concurrently to destruction of olfactory bulb noradrenergic terminals derived from the locus ceruleus and destruction of noradrenergic terminals derived from sympathetic ganglia. Japanese encephalitis virus can produce parkinsonism that is accompanied by SN lesions and decreased striatal dopaminergic neurons [30]; however, the status of the sympathetic nerves and olfactory bulb in this condition remains unknown, and there is no published evidence for sympathetic noradrenergic denervation produced by a virus.
In conclusion, PAF involves as severe olfactory deficiency as in PD. Since PAF patients do not have parkinsonism or decreased striatal dopaminergic innervation, and since cardiac noradrenergic denervation occurs in both diseases, we infer that pathogenetic mechanisms of olfactory dysfunction and cardiac noradrenergic denervation in Lewy body diseases differ from those producing parkinsonism and nigrostriatal dopaminergic denervation.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Institute of Neurological Disorders and Stroke.
Dr. Peter Herscovitch was the Authorized User for administration of 6-[18F]fluorodopa and 6-[18F]fluorodopamine to humans. Ms. Tereza Jenkins coordinated patient travel. Sandra Pechnik, RN, assisted with clinical procedures and scheduling. Drs. Basil Eldadah, Richard Imrich, and Yehonatan Sharabi served as post-doctoral Fellows when the work was done. Mr. Takuya Sato, Mr. Oladi Bentho, Dr. Jeffrey Moak, and Dr. Sheng-Ting Li carried out analyses of PET scans.
Footnotes
Financial support: Division of Intramural Research, NINDS, NIH.
References
- 1.Durrieu G, Senard JM, Rascol O, Tran MA, Lataste X, Rascol A, et al. Blood pressure and plasma catecholamines in never-treated Parkinsonian patients: effect of a selective D1 agonist (CY 208–243) Neurology. 1990;40:707–709. doi: 10.1212/wnl.40.4.707. [DOI] [PubMed] [Google Scholar]
- 2.Brooks DJ, Salmon EP, Mathias CJ, Quinn N, Leenders KL, Bannister R, et al. The relationship between locomotor disability, autonomic dysfunction, and the integrity of the striatal dopaminergic system in patients with multiple system atrophy, pure autonomic failure, and Parkinson’s disease, studied with PET. Brain. 1990;113:1539–1552. doi: 10.1093/brain/113.5.1539. [DOI] [PubMed] [Google Scholar]
- 3.Hoshi H, Kuwabara H, Leger G, Cumming P, Guttman M, Gjedde A. 6-[18F]fluoro-L-dopa metabolism in living human brain: a comparison of six analytical methods. J Cereb Blood Flow Metab. 1993;13:57–69. doi: 10.1038/jcbfm.1993.8. [DOI] [PubMed] [Google Scholar]
- 4.Hague K, Lento P, Morgello S, Caro S, Kaufmann H. The distribution of Lewy bodies in pure autonomic failure: autopsy findings and review of the literature. Acta Neuropathol (Berl) 1997;94:192–196. doi: 10.1007/s004010050693. [DOI] [PubMed] [Google Scholar]
- 5.Wakabayashi K, Takahashi H. Neuropathology of autonomic nervous system in Parkinson’s disease. Eur Neurol. 1997;38(Suppl 2):2–7. doi: 10.1159/000113469. [DOI] [PubMed] [Google Scholar]
- 6.Sharabi Y, Imrich R, Holmes C, Pechnik S, Goldstein DS. Generalized and neurotransmitter-selective noradrenergic denervation in Parkinson disease with orthostatic hypotension. Movement Dis. 2008;23:1725–1732. doi: 10.1002/mds.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufmann H, Nahm K, Purohit D, Wolfe D. Autonomic failure as the initial presentation of Parkinson disease and dementia with Lewy bodies. Neurology. 2004;63:1093–1095. doi: 10.1212/01.wnl.0000138500.73671.dc. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein DS, Holmes C, Sato T, Bernson M, Mizrahi N, Imrich R, et al. Central dopamine deficiency in pure autonomic failure. Clin Auton Res. 2008;18:166–171. doi: 10.1007/s10286-008-0457-0. [DOI] [PubMed] [Google Scholar]
- 9.Doty RL, Riklan M, Deems DA, Reynolds C, Stellar S. The olfactory and cognitive deficits of Parkinson’s disease: evidence for independence. Ann Neurol. 1989;25:166–171. doi: 10.1002/ana.410250210. [DOI] [PubMed] [Google Scholar]
- 10.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 11.Lee PH, Yeo SH, Kim HJ, Youm HY. Correlation between cardiac 123I-MIBG and odor identification in patients with Parkinson’s disease and multiple system atrophy. Mov Disord. 2006;21:1975–1977. doi: 10.1002/mds.21083. [DOI] [PubMed] [Google Scholar]
- 12.Orimo S, Oka T, Miura H, Tsuchiya K, Mori F, Wakabayashi K, et al. Sympathetic cardiac denervation in Parkinson’s disease and pure autonomic failure but not in multiple system atrophy. J Neurol Neurosurg Psychiatry. 2002;73:776–777. doi: 10.1136/jnnp.73.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein DS, Holmes C, Li ST, Bruce S, Metman LV, Cannon RO., 3rd Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med. 2000;133:338–347. doi: 10.7326/0003-4819-133-5-200009050-00009. [DOI] [PubMed] [Google Scholar]
- 14.Muller A, Mungersdorf M, Reichmann H, Strehle G, Hummel T. Olfactory function in Parkinsonian syndromes. J Clin Neurosci. 2002;9:521–524. doi: 10.1054/jocn.2001.1071. [DOI] [PubMed] [Google Scholar]
- 15.Siderowf A, Newberg A, Chou KL, Lloyd M, Colcher A, Hurtig HI, et al. [99mTc]TRODAT-1 SPECT imaging correlates with odor identification in early Parkinson disease. Neurology. 2005;64:1716–1720. doi: 10.1212/01.WNL.0000161874.52302.5D. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein DS, Eldadah BA, Holmes C, Pechnik S, Moak J, Saleem A, et al. Neurocirculatory abnormalities in Parkinson disease with orthostatic hypotension. Independence from levodopa treatment. Hypertension. 2005;46:1–7. doi: 10.1161/01.HYP.0000188052.69549.e4. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein DS, Holmes C, Bentho O, Sato T, Moak J, Sharabi Y, et al. Biomarkers to detect central dopamine deficiency and distinguish Parkinson disease from multiple system atrophy. Parkinsonism Relat Disord. 2008;14:600–607. doi: 10.1016/j.parkreldis.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein DS, Holmes C, Sharabi Y, Brentzel S, Eisenhofer G. Plasma levels of catechols and metanephrines in neurogenic orthostatic hypotension. Neurology. 2003;60:1327–1332. doi: 10.1212/01.wnl.0000058766.46428.f3. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein DS, Tack C. Non-invasive detection of sympathetic neurocirculatory failure. Clin Auton Res. 2000;10:285–291. doi: 10.1007/BF02281111. [DOI] [PubMed] [Google Scholar]
- 20.Gilman S, Low P, Quinn N, Albanese A, Ben-Shlomo Y, Fowler C, et al. Consensus statement on the diagnosis of multiple system atrophy. Clin Auton Res. 1998;8:359–362. doi: 10.1007/BF02309628. [DOI] [PubMed] [Google Scholar]
- 21.Olichney JM, Murphy C, Hofstetter CR, Foster K, Hansen LA, Thal LJ, et al. Anosmia is very common in the Lewy body variant of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2005;76:1342–1347. doi: 10.1136/jnnp.2003.032003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McShane RH, Nagy Z, Esiri MM, King E, Joachim C, Sullivan N, et al. Anosmia in dementia is associated with Lewy bodies rather than Alzheimer’s pathology. J Neurol Neurosurg Psychiatry. 2001;70:739–743. doi: 10.1136/jnnp.70.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross GW, Abbott RD, Petrovitch H, Tanner CM, Davis DG, Nelson J, et al. Association of olfactory dysfunction with incidental Lewy bodies. Mov Disord. 2006;21:2062–2067. doi: 10.1002/mds.21076. [DOI] [PubMed] [Google Scholar]
- 24.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Farooqui T. Octopamine-mediated neuronal plasticity in honeybees: implications for olfactory dysfunction in humans. Neuroscientist. 2007;13:304–322. doi: 10.1177/10738584070130040501. [DOI] [PubMed] [Google Scholar]
- 26.Ehringer H, Hornykiewicz O. Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Wien Klin Wochenschr. 1960;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- 27.Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol. 2003;60:337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- 28.Barnett EM, Cassell MD, Perlman S. Two neurotropic viruses, herpes simplex virus type 1 and mouse hepatitis virus, spread along different neural pathways from the main olfactory bulb. Neuroscience. 1993;57:1007–1025. doi: 10.1016/0306-4522(93)90045-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLean JH, Shipley MT, Bernstein DI, Corbett D. Selective lesions of neural pathways following viral inoculation of the olfactory bulb. Exp Neurol. 1993;122:209–222. doi: 10.1006/exnr.1993.1121. [DOI] [PubMed] [Google Scholar]
- 30.Hamaue N, Ogata A, Terado M, Ohno K, Kikuchi S, Sasaki H, et al. Brain catecholamine alterations and pathological features with aging in Parkinson disease model rat induced by Japanese encephalitis virus. Neurochem Res. 2006;31:1451–1455. doi: 10.1007/s11064-006-9197-5. [DOI] [PubMed] [Google Scholar]