Abstract
Purpose of review
A strong male bias in autism spectrum disorder (ASD) prevalence has been observed with striking consistency, but no mechanism has yet to definitively account for this sex difference. Toward the pursuit of a more complete understanding of the biological basis for sex-differential risk, this review explores the current status of epidemiological, genetic, and neuroendocrinological work addressing ASD prevalence and liability in males and females.
Recent findings
Recent studies continue to report a male bias in ASD prevalence, but also suggest that sex differences in phenotypic presentation, including fewer restricted and repetitive behaviors and externalizing behavioral problems in females, may contribute to this bias. Genetic studies demonstrate that females are protected from the effects of heritable and de novo ASD risk variants, and compelling work suggests that sex chromosomal genes and/or sex hormones, especially testosterone, may modulate the effects of genetic variation on the presentation of an autistic phenotype.
Summary
ASDs affect females less frequently than males, and several sex-differential genetic and hormonal factors may contribute. Future work to determine the mechanisms by which these factors confer risk and protection to males and females is essential.
Keywords: autism spectrum disorders, sex differences, hormones, sex chromosomes, genetic liability
Introduction
Sexually dimorphic disease prevalence is well recognized, but poorly understood. For example, many disorders with autoimmune etiologies, such as multiple sclerosis and systemic lupus erythematosis, are female predominant [1], whereas some neurodevelopmental disorders, such as attention deficit hyperactivity disorder and language impairment show a male bias [2,3,4]. Autism spectrum disorders (ASDs) are prototypical in this regard, as they show a striking male bias in prevalence, with approximately 4 affected males for every 1 affected female. The consistency of this observation across time and populations strongly implicates the involvement of sex-specific biological factors in ASD etiology. However, we have yet to definitively identify the underlying mechanism through which these pathways interact to give rise to the male preponderance among individuals with ASDs. In recent years, increased priority has been placed on the inclusion and study of autistic females, while geneticists have made considerable headway in identifying novel genetic risk variants for ASD, putting us now in a position to assess relationships between genetic risk factors, hormones, and observed patterns of sex-differential vulnerability to ASDs. Toward this goal, here we review patterns of sex bias in ASD prevalence and phenotypic presentation, and evaluate the evidence for several hypotheses that could explain the biological basis of the male bias in ASD. We also identify areas of research where additional work is needed to advance our understanding of the interactions between sex-differential biology and risk factors for ASD.
ASD prevalence in males and females
From the first published descriptions of autism, it has been a male-typical disorder: 8 of the 11 cases described by Kanner [5], and all 4 cases described by Asperger [6], were male. Prevalence surveys conducted since have reported a range of male biases from 1.33:1 male:female (M:F) to 15.7:1 [7], and a commonly referenced consensus ratio of ~4:1. Intelligence level affects this sex ratio: males are substantially over-represented among high-functioning cases, and males and females are more equally represented among cases with severe intellectual disability (ID) [8,9,10,11]; a 1999 review reported median sex ratios of 6:1 among normal-functioning subjects and 1.7:1 among cases with moderate to severe ID [11].
Several biological factors could explain this relationship between IQ and the sex ratio, but it should also be recognized that this could also, at least partially, reflect ascertainment bias. Co-morbid ID increases females’ likelihood of acquiring an ASD diagnosis, and conversely high-functioning females may go undiagnosed. The wide variation in the sex ratio reported by different sites in the Autism and Developmental Disabilities Monitoring (ADDM) Network and findings from large-scale population screening for ASD in a South Korean community where clinically ascertained samples show higher M:F ratios than less biased population screening are consistent with this hypothesis [12**,13**]. Generally, high sex ratios have been found by studies that predominantly identified subjects via treatment facilities or disability registries [8,9,14], including more recent studies of records from Boston area hospital records [15*] and Taiwanese disability registries [16*]. In contrast, low sex ratios between 1.7:1 in high-functioning ASD cases, and 2.3:1 in cases with ASD and co-morbid ID were found in an epidemiological, population-screening study for ASDs in Finland [17*], although the same trend was not found in England, where a 9 to 1 M:F ratio was observed in high functioning individuals with an ASD [18*]. Overall, prevalence studies demonstrate that ASD is consistently over-represented in males as compared to females. But, we currently do not understand the extent of this over-representation, or the degree to which this male bias in prevalence is related to intellectual functioning or ascertainment methods in addition to the influence of sex-differential genetic or hormonal factors.
Presentation of ASD symptoms and related phenotypes in males and females
In contrast with the higher proportion of diagnosed autistic females than males with ID, many studies find no sex differences in overall composite ASD severity as measured on several standard assessment tools [16,19,20,21*,22*,23**]. This suggests that among those who meet diagnostic criteria, females are not more severely affected. However, differences emerge when each core symptom domain of ASD is considered separately, and sex differences observed in cases tend to reflect sex differences observed in the typical population. Males with ASD are found to show more externalizing behavior problems than females, such as aggressive behavior, hyperactivity, reduced prosocial behavior, and increased repetitive/restricted behaviors and interests [24*,25*,26**,27*,28]. Females with ASD show greater internalizing symptoms than boys, including anxiety, depression, and other emotional symptoms as reported by parents [25*,29*]; parents also more frequently endorse the item “avoids demands” for female cases on the Autism Spectrum Screening Questionnaire (ASSQ) [19], perhaps reflecting girls’ tendency to misbehave passively, as opposed to acting out. The observed sex differences raise the possibility that male-typical externalizing behaviors are more disruptive in the home or school setting than female-typical internalizing behaviors, preferentially prompting evaluation and diagnosis for boys, especially as compared to high-functioning girls. For girls then, ID may be more likely the secondary issue prompting evaluation and diagnosis. This scenario further implies that some proportion of the sex difference in ASD prevalence is attributable to biases inherent in the diagnostic process.
A recent study from the UK addressed this potential diagnosis gap by characterizing children with high autistic traits who met or fell short of the threshold for ASD diagnosis [30**]. A significantly smaller proportion of high-scoring girls met full ASD diagnostic criteria than males (38% versus 56%) whereas ASD-diagnosed girls had a higher mean total problem score (hyperactivity, anxiety, and conduct, peer, and prosocial problems) and a higher frequency of low IQ than ASD-diagnosed boys. Girls without diagnoses showed increased communication difficulties, but reduced social impairments as compared to non-diagnosed boys. Thus, it may be that relatively higher levels of social ability in females preclude full diagnosis of ASD, particularly for those who are high-functioning. Nevertheless, whether the male-skewed prevalence of ASD is due to biased diagnosis of sex-differential presentations of the disease or to true sex differences in prevalence (or both), sex-specific biology is likely to play a role. For the remainder of this review, we discuss the relationships between ASD and the two major drivers of sex-specific biology: genetics and hormones.
Sex differences in genetic contributions to ASD risk
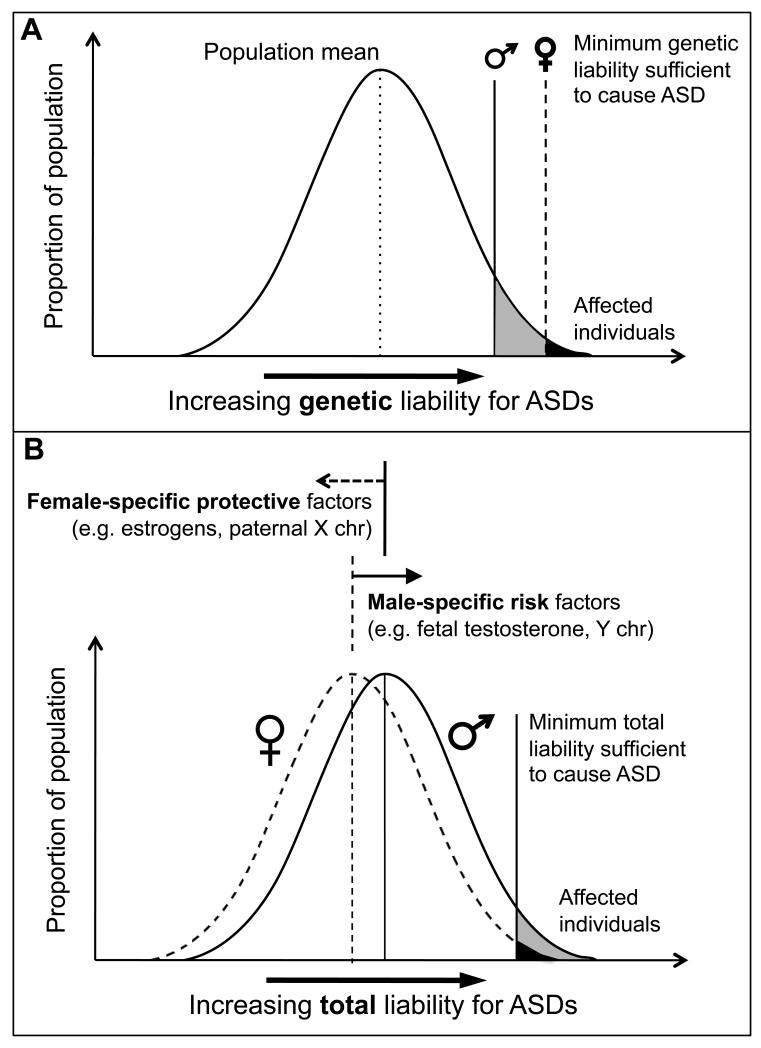
Biological theories for the sex difference in ASD prevalence most frequently take the form of a multiple-threshold multifactorial liability model [31], in which females have a higher threshold for reaching affection status than males (Figure 1A). Thus, genetic studies operating under this model hypothesize that females with ASD are likely to be carrying a higher heritable mutational “load” than affected males. This model predicts that relatives of female probands should be at increased risk for ASD as compared with relatives of male probands, which is supported by a recent twin study [32]. In contrast, other studies have failed to support the genetic loading hypothesis, including a study of 882 families [33] and another recent study of high risk siblings of autistic probands that found that only the sex of the sibling was a significant predictor of their future ASD status [34**]. However, a new study of more than 9000 dizygotic twin pairs from population-based cohorts provides the most conclusive demonstration of female-protective factors to date, showing that siblings of autistic females exhibit significantly greater autistic impairments than siblings of autistic males (Angelica Ronald, December 13, 2012). This finding also supports a role for heritable variation in ASD liability under the threshold model.
Figure 1.
Multifactorial liability models for ASDs. A) Multiple-threshold model in which genetic liability for ASD is normally distributed in the population and the minimum genetic liability sufficient to cause ASD (liability threshold) in females is greater than in males. B) Multifactorial liability model in which total liability for ASD, including contributions from genetic variation, environment, and other biological factors, is distributed in the population; female-specific factors shift females’ total liability distribution away from, and male-specific factors shift males’ distribution toward, a single threshold. Figure adapted from Reich et al. [31]
There is additional experimental evidence for heritable loci with sex-differential penetrance. In one approach, multiplex family samples are divided into two groups for analysis: those with only affected male children (“male-only”), and those with at least one affected female child (“female-containing”) to identify sex-differential genetic variation at several loci in male-only and female-containing families [35,36,37,38,39,40]. However, only the male-only linkage signal at 17q21 has been successfully replicated, and the exact risk genes or variants responsible for these linkage peaks remain unknown. Other approaches have identified more defined sex related risk loci. For example, Lu and Cantor used a case-pseudocontrol genome-wide association test with sex as a factor to find two genome-wide significant single nucleotide polymorphisms (SNPs) within genes RYR2 (Ryanodine receptor 2) and UPP2 (uridine phosphorylase 2) [41*]. Also, a study of rare copy number variants (CNVs) in ASD identified the first inherited autosomal variant with clear male-biased penetrance: males carrying a microdeletion in SHANK1 had high-functioning autism, while female relatives carrying the same microdeletion showed anxiety but did not meet diagnostic criteria for ASD [42**]. While they cannot fully explain the male bias in ASD, these sex-differential linkage peaks, SNPs, and the SHANK1 microdeletion represent promising starting points for further work to elucidate the mechanism by which these inherited variants confer sex-differential ASD risk.
Aside from heritable variation, it is also plausible that some of females’ hypothesized higher genetic load is caused by de novo variation [43*] and is therefore not shared with relatives. In fact, close to 90% of ASD families have only one affected member [44], suggesting that de novo variants of large effect may contribute significantly to ASD liability. This is supported by studies of chromosomal structural variation that indicate that a higher proportion of female cases carry a de novo CNV than male cases and that these CNVs disrupt a greater number of genes than those from males [45,46*,47*]. An elevated rate of de novo single nucleotide variants (SNVs) is also observed in exome sequences from autistic females [48**,49**], especially the most deleterious SNVs (nonsense, splice site, some missense) [50**,51**]. One study also observed a trend towards increased de novo CNV rate in unaffected female siblings [47*], consistent with the hypothesis that females can withstand more significant mutations than males before being affected with ASD. Taken together with studies of familial recurrence rate, it appears that regardless of whether risk variants are inherited or de novo, in the face of a comparable degree of genetic liability, males are at increased risk for and females are protected from manifesting ASD symptoms that meet diagnostic criteria.
Sex chromosomal genes have been proposed to be key players in molecular mechanisms driving females’ protection from ASD liability conferred by specific risk loci and/or by genome-wide mutational load (Figure 1B). An early theory proposed that ASD might be an X-linked disorder, in which females are protected from deleterious effects of X chromosomal mutations by compensatory transcription from their intact, second X chromosome. However, ASD transmission in most families does not follow an X-linked pattern, and while several ASD risk genes have been identified on the X chromosome (e.g. FMRP, MECP2, NLGN3, NLGN4X), all cause significant ID, indicating a more general role for X chromosome gene dosage in neural development.
Although ASD may not be X-linked in the Mendelian sense, sex chromosome complement may still modulate ASD risk. Sex chromosome aneuploidies provide test cases for this hypothesis, with an increased rate of ASD diagnosis in Turner syndrome (TS, XO, ~3% ASD) [52,53,54], Klinefelter syndrome (KS, XXY, ~10% ASD) [55,56*], and 47,XYY syndrome (~20% ASD), but no increased rate in X chromosome trisomy [57,58**]. In addition to the general association of aneuploidy with ID, these observations suggest several intriguing possibilities: 1) the Y chromosome is a risk factor for ASD, and 2) a second X chromosome is protective, possibly via genes that escape X-inactivation. Interestingly, the reported TS cases with co-morbid ASD predominantly carry an intact maternal X chromosome, which led Skuse and colleagues to propose the theory that imprinted genes expressed only from the paternal X protect against ASD [59]. Since 40-50% of KS cases arise from maternal nondisjunction of the X, this subset of cases also lacks putative protective paternally expressed X genes, as do all 47,XYY cases. In combination with the presence of a Y chromosome, this lack of paternal X expression could then raise ASD risk for both syndromes, and for a higher proportion of XYY cases as is observed. However, larger epidemiological studies are needed to more accurately establish ASD prevalence in aneuploid individuals, and the parental origin of all sex chromosomes and patterns of escape from X-inactivation must be determined to better assess the validity of this model.
Sex hormonal contributors to ASD risk
One major theory that invokes a broad role for testosterone in ASD etiology is the Extreme Male Brain theory, which proposes that ASD arises from hypermasculinization of the brain [60]. A theory born from cognitive-behavioral observations, this masculinization is conceptualized along two cognitive dimensions: 1) empathizing, the drive to perceive others’ feelings and thoughts and respond appropriately, and 2) systemizing, the drive to interact with and understand rule-based systems. Early work convincingly demonstrated that typical females score significantly higher on measures of empathizing and value placed on meaningful relationships with others [61,62], whereas typical males score significantly higher on measures of systemizing [63]. In these studies, high-functioning ASD cases scored lower than typical males on measures of empathy and friendship, and higher than typical males on measures of systemizing.
Given that testosterone secreted by fetal testes during gestation drives human sexual differentiation to the male phenotype, Baron-Cohen and colleagues have proposed that fetal testosterone (FT) levels may also drive cognitive hypermasculinization in ASD. Findings of significant positive correlations between FT levels and measures of systemizing [64] and autistic traits [65], and negative correlations with measures of empathizing [66] and the quality of social relationships [67] are consistent with this hypothesis. Recent work has even found a correlation between increasing FT and volume of sexually dimorphic brain regions, specifically increased volume of the right temporoparietal junction/posterior superior temporal sulcus and decreased volume of the planum temporale/parietal operculum and posterior lateral orbitofrontal cortex volume [68*]. These results suggest that increased FT levels predispose the differentiating brain to a hypermasculine cognitive and neuroanatomical phenotype.
Interestingly, work from other investigators have suggested that testosterone beyond fetal development may also play a role in ASD pathophysiology. For example, levels of testosterone and its precursors were found to be significantly elevated in a sample of ASD cases, with 57 of the 70 subjects having at least one androgen metabolite measuring above the upper limit of sex- and age-matched reference ranges [69]. Subsequent studies have found increased androstenedione in serum from adults with ASD compared to controls irrespective of sex [70*], and a higher free androgen index in females with Asperger’s syndrome versus controls [71*], although a study of unaffected Japanese adults found no correlation between salivary testosterone levels and autistic-like traits [72]. These findings were recently reviewed in detail by Geier et al. [73*], collectively suggesting that hyperandrogenism may be a significant risk factor for ASD, and that more frequent assessment of testosterone levels in ASD cases is warranted to determine how prevalent this risk factor may be.
One potential pathway by which testosterone influences ASD risk may involve RORA (retinoic acid-related orphan receptor-alpha), a gene down-regulated in ASD lymphoblastoid cell lines [74]. RORA regulates expression of aromatase, the enzyme that converts androgens to estrogens, and is reciprocally activated by estradiol and inhibited by testosterone [75*]. These regulatory relationships may create a feedback loop that further elevates testosterone levels, but it may have more specific effects on brain as well, since RORA has a role in cerebellar and Purkinje cell development, and neuroprotection from oxidative stress. Another potential mechanism may involve immune system functioning in the brain, as a co-expression module of genes involved in immune system and glial function was observed to be up-regulated in adult autistic cortex [76**], and sex hormones, particularly estradiol, have been shown to affect glial-neuronal interactions [77*,78]. Thus, it may not be the absolute levels of androgens or estrogens, but the balance between them that influences ASD risk.
Sex hormones are attractive candidates for sex-biased ASD risk and protective factors in that they raise the possibility for the development of treatments that cut across individuals’ specific genetic liability. However, much work remains to determine the precise cellular and molecular mechanisms by which testosterone interacts with neurodevelopmental pathways and genetic risk loci to increase liability for autistic behavior, so that future treatments may specifically target these interactions. For example, in addition to the liability conferred directly to neural development by sex chromosomal genes, it also should be noted that sex chromosomal abnormalities frequently affect gonadal function. In fact, gonadal dysgenesis is common in TS and KS, causing abnormally low postnatal estrogen and testosterone levels, respectively, whereas testosterone levels are normal prenatally in KS and throughout life in 47,XYY cases [79,80]. While this more male-like hormonal environment may contribute to increased ASD risk in TS, hypogonadism in XXY males in the face of a nearly 10-fold increase in ASD prevalence suggests that the role of the hormonal milieu in ASD liability is likely complex and may be mediated by other risk factors.
Conclusion
ASD prevalence remains highly biased toward males, although more recent population screens have identified a higher proportion of autistic females relative to males than past work on clinically ascertained samples. Discussion continues as to whether females present the autistic phenotype differently than males, and further work is needed to determine if currently undiagnosed females would benefit from standard ASD services, and if diagnostic criteria need to be adjusted to effectively identify these girls. ASD risk is likely to be multifactorial, with many different genetic variants and environmental factors contributing to liability, and still other sex-differential genetic and hormonal factors acting to potentiate risk to males and/or attenuate risk to females (Figure 1B). Evidence suggests that sex chromosomal gene dosage and sex hormone levels may be involved in setting sex-specific liability thresholds, but much future work is needed to definitively identify the most critical players at hand and to elucidate the precise mechanisms by which these sex-specific factors modulate presentation of the ASD phenotype.
Key points.
The prevalence of ASDs is strongly male-biased, affecting 4 times as many males as females, on average.
Little attention has been paid to understanding if ASD in females differs from that in males.
Sex differences in behavior, or the presentation of autistic symptoms and co-morbid intellectual disability may contribute to the male bias in diagnoses.
There is evidence for a greater “genetic load” in females with ASD, consistent with the idea of female protective factors.
The potential role of sex hormones in modulating ASD risk warrants further study.
References
- 1.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2(9):777–80. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 2.Szatmari P, Offord DR, Boyle MH. Ontario Child Health Study: prevalence of attention deficit disorder with hyperactivity. J Child Psychol Psychiatry. 1989;30(2):219–30. doi: 10.1111/j.1469-7610.1989.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 3.Barbaresi WJ, Katusic SK, Colligan RC, et al. How common is attention-deficit/hyperactivity disorder? Incidence in a population-based birth cohort in Rochester, Minn. Arch Pediatr Adolesc Med. 2002;156(3):217–24. doi: 10.1001/archpedi.156.3.217. [DOI] [PubMed] [Google Scholar]
- 4.Viding E, Spinath FM, Price TS, et al. Genetic and environmental influence on language impairment in 4-year-old same-sex and opposite-sex twins. J Child Psychol Psychiatry. 2004;45(2):315–25. doi: 10.1111/j.1469-7610.2004.00223.x. [DOI] [PubMed] [Google Scholar]
- 5.Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–50. [PubMed] [Google Scholar]
- 6.Asperger H. The “autistic psychopathy” in childhood. Archiv Fur Psychiatrie Und Nervenkrankheiten. 1944;117(1):76–136. [Google Scholar]
- 7.Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res. 2009;65(6):591–8. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- 8.Volkmar FR, Szatmari P, Sparrow SS. Sex differences in pervasive developmental disorders. J Autism Dev Disord. 1993;23(4):579–91. doi: 10.1007/BF01046103. [DOI] [PubMed] [Google Scholar]
- 9.Yeargin-Allsopp M, Rice C, Karapurkar T, et al. Prevalence of autism in a US metropolitan area. JAMA. 2003;289(1):49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]
- 10.Banach R, Thompson A, Szatmari P, et al. Brief Report: Relationship between non-verbal IQ and gender in autism. J Autism Dev Disord. 2009;39(1):188–93. doi: 10.1007/s10803-008-0612-4. [DOI] [PubMed] [Google Scholar]
- 11.Fombonne E. The epidemiology of autism: a review. Psychol Med. 1999;29(4):769–86. doi: 10.1017/s0033291799008508. [DOI] [PubMed] [Google Scholar]
- **12.Kim YS, Leventhal BL, Koh YJ, et al. Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry. 2011;168(9):904–12. doi: 10.1176/appi.ajp.2011.10101532. The first population screening for ASD prevalence in children in South Korea. In addition to finding a higher-than-expected rate of ASDs overall (2.64%), this screening also identified a high proportion of autistic females in a general population (non-clinical) sample (M:F 2.5:1).
- **13.Prevalence of autism spectrum disorders--Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61(3):1–19. The most recent report from the US CDC on ASD prevalence, reporting an overall prevalence of 1 in 88 (1.1%), up from 1 in 110 in 2009. High variability in the prevalence and sex ratio across 14 surveillance sites using different record sources to identify cases (health and/or educational) suggest that ascertainment methods may influence ASD prevalence estimates by sex.
- 14.Lord C, Schopler E. Differences in sex ratios in autism as a function of measured intelligence. J Autism Dev Disord. 1985;15(2):185–93. doi: 10.1007/BF01531604. [DOI] [PubMed] [Google Scholar]
- *15.Kohane IS, McMurry A, Weber G, et al. The co-morbidity burden of children and young adults with autism spectrum disorders. PLoS One. 2012;7(4):e33224. doi: 10.1371/journal.pone.0033224. Large-scale prevalence estimates for co-morbidities in 14,000 ASD cases from retrospective examination of hospital records; 3.84 M:F also reported for this clinical sample.
- *16.Lai DC, Tseng YC, Hou YM, Guo HR. Gender and geographic differences in the prevalence of autism spectrum disorders in children: analysis of data from the national disability registry of Taiwan. Res Dev Disabil. 2012;33(3):909–15. doi: 10.1016/j.ridd.2011.12.015. ASD cases documented in Taiwanese government registry found to be more prevalent in males (M:F near 6:1) and in urban areas (urban:rural about 2.5:1).
- *17.Mattila ML, et al. Autism spectrum disorders according to DSM-IV-TR and comparison with DSM-5 draft criteria: an epidemiological study. J Am Acad Child Adolesc Psychiatry. 2011;50(6):583–592. e11. doi: 10.1016/j.jaac.2011.04.001. Population screening study in Finland finds DSM-5 draft criteria to be less sensitive for diagnosing cases with high-functioning ASDs and Asperger’s syndrome, and a low M:F of 2:1.
- *18.Brugha TS, McManus S, Bankart J, et al. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry. 2011;68(5):459–65. doi: 10.1001/archgenpsychiatry.2011.38. Population-based survey for ASDs among adults living in households (non-institutionalized) in England finds prevalence of 0.98% and M:F of 9:1.
- 19.Kopp S, Gillberg C. The Autism Spectrum Screening Questionnaire (ASSQ)-Revised Extended Version (ASSQ-REV): an instrument for better capturing the autism phenotype in girls? A preliminary study involving 191 clinical cases and community controls. Res Dev Disabil. 2011;32(6):2875–88. doi: 10.1016/j.ridd.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Carter AS, Black DO, Tewani S, et al. Sex differences in toddlers with autism spectrum disorders. J Autism Dev Disord. 2007;37(1):86–97. doi: 10.1007/s10803-006-0331-7. [DOI] [PubMed] [Google Scholar]
- *21.Lai MC, Lombardo MV, Pasco G, et al. A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PLoS One. 2011;6(6):e20835. doi: 10.1371/journal.pone.0020835. A comparison of males and females with ASDs finds some differences in symptom subdomains and related behaviors but no difference in overall childhood symptom severity as measured by the ADI-R.
- *22.Mayes SD, Calhoun SL. Impact of IQ, age, SES, gender, and race on autistic symptoms. Res Autism Spectr Disord. 2011;5(2):749–757. Examination of the relationship between IQ, age, SES, sex, and race and autistic symptoms in 777 children, only IQ and age are found to have significant effects on ASD severity.
- **23.Zwaigenbaum L, Bryson SE, Szatmari P, et al. Sex differences in children with autism spectrum disorder identified within a high-risk infant cohort. J Autism Dev Disord. 2012;42(12):2585–96. doi: 10.1007/s10803-012-1515-y. Prospective study of infant siblings of autistic probands which identifies a higher than expected proportion of high functioning female ASD cases among probands’ siblings, possibly due to the rigorous surveillance of ASD symptoms in this sample, again suggesting ascertainment contributes to the identification of autistic females.
- *24.Hattier MA, Matson JL, Tureck K, Horovitz M. The effects of gender and age on repetitive and/or restricted behaviors and interests in adults with autism spectrum disorders and intellectual disability. Res Dev Disabil. 2011;32(6):2346–51. doi: 10.1016/j.ridd.2011.07.028. Evaluation of restricted interests and repetitive behaviors in 140 adults with ASD finds males exhibit more repetitive and restricted behaviors and interests than females regardless of age.
- *25.Mandy W, Chilvers R, Chowdhury U, et al. Sex differences in autism spectrum disorder: evidence from a large sample of children and adolescents. J Autism Dev Disord. 2012;42(7):1304–13. doi: 10.1007/s10803-011-1356-0. Assessment of ASD symptom severity, IQ, and behavioral problems finds sex differential expression of these phenotypes, including increased repetitive stereotyped behaviors in males.
- **26.Szatmari P, Liu XQ, Goldberg J, et al. Sex differences in repetitive stereotyped behaviors in autism: implications for genetic liability. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(1):5–12. doi: 10.1002/ajmg.b.31238. Characterization of 970 Caucasian multiplex families finds significantly higher repetitive and restricted behaviors and interests scores in males than females with ASD, and also in brothers of female probands, supporting the multiple threshold model for ASD liability in which affected females have a higher genetic liability.
- *27.Bolte S, Duketis E, Poustka F, Holtmann M. Sex differences in cognitive domains and their clinical correlates in higher-functioning autism spectrum disorders. Autism. 2011;15(4):497–511. doi: 10.1177/1362361310391116. Comparison of cognitive performance in ASD by sex finds higher scores for stereotypic behavior and interests in males, and a potential association between these behaviors and performance on executive function tasks.
- 28.Giarelli E, Wiggins LD, Rice CE, et al. Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disabil Health J. 2010;3(2):107–16. doi: 10.1016/j.dhjo.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *29.Solomon M, Miller M, Taylor SL, et al. Autism symptoms and internalizing psychopathology in girls and boys with autism spectrum disorders. J Autism Dev Disord. 2012;42(1):48–59. doi: 10.1007/s10803-011-1215-z. Parental accounts report a greater degree of internalizing behaviors and depressive symptoms in females than males with ASDs.
- **30.Dworzynski K, Ronald A, Bolton P, Happe F. How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J Am Acad Child Adolesc Psychiatry. 2012;51(8):788–97. doi: 10.1016/j.jaac.2012.05.018. The first study to explicitly evaluate sex differences in the presentation of ASD symptoms and related traits between cases exhibiting autistic traits but who either meet or do not meet standing criteria for ASD diagnosis. Results show that among individuals without co-morbid intellectual or behavioral problems, females are less likely to be diagnosed than males.
- 31.Reich R, Cloninger CR, Guze SB. The multifactorial model of disease transmission: I. Description of the model and its use in psychiatry. Br J Psychiatry. 1975;127:1–10. doi: 10.1192/bjp.127.1.1. [DOI] [PubMed] [Google Scholar]
- **32.Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68(11):1095–102. doi: 10.1001/archgenpsychiatry.2011.76. A recent, large, population-based twin study of ASD concordance. Findings of ~60% concordance rate in monozygotic and ~25% concordance in dizygotic twin pairs have recalibrated heritability estimates for ASD, and suggest that the gestational environment play a significant role in ASD susceptibility.
- 33.Goin-Kochel RP, Abbacchi A, Constantino JN. Lack of evidence for increased genetic loading for autism among families of affected females: a replication from family history data in two large samples. Autism. 2007;11(3):279–86. doi: 10.1177/1362361307076857. [DOI] [PubMed] [Google Scholar]
- **34.Ozonoff S, Young GS, Carter A, et al. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics. 2011;128(3):e488–95. doi: 10.1542/peds.2010-2825. Prospective study finds a recurrence risk of 18.7% for siblings of autistic probands, but no effect of proband sex on this recurrence risk; recurrence rate in siblings is 26.2% for males and 9.1% for females, regardless of the sex of their older autistic sibling.
- 35.Stone JL, Merriman B, Cantor RM, et al. Evidence for sex-specific risk alleles in autism spectrum disorder. Am J Hum Genet. 2004;75(6):1117–23. doi: 10.1086/426034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cantor RM, Kono N, Duvall JA, et al. Replication of autism linkage: fine-mapping peak at 17q21. Am J Hum Genet. 2005;76(6):1050–6. doi: 10.1086/430278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szatmari P, Paterson AD, Zwaigenbaum L, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39(3):319–28. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamb JA, Barnby G, Bonora E, et al. Analysis of IMGSAC autism susceptibility loci: evidence for sex limited and parent of origin specific effects. J Med Genet. 2005;42(2):132–7. doi: 10.1136/jmg.2004.025668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schellenberg GD, Dawson G, Sung YJ, et al. Evidence for multiple loci from a genome scan of autism kindreds. Mol Psychiatry. 2006;11(11):1049–60. doi: 10.1038/sj.mp.4001874. [DOI] [PubMed] [Google Scholar]
- 40.Weiss LA, Arking DE, Daly MJ, Chakravarti A. A genome-wide linkage and association scan reveals novel loci for autism. Nature. 2009;461(7265):802–8. doi: 10.1038/nature08490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.Lu AT, Cantor RM. Allowing for sex differences increases power in a GWAS of multiplex Autism families. Mol Psychiatry. 2012;17(2):215–22. doi: 10.1038/mp.2010.127. The inclusion of sex as a factor in a genome-wide association test for ASD in families facilitates the identification of two risk loci.
- **42.Sato D, Lionel AC, Leblond CS, et al. SHANK1 Deletions in Males with Autism Spectrum Disorder. Am J Hum Genet. 2012;90(5):879–87. doi: 10.1016/j.ajhg.2012.03.017. The first identified autosomal risk variant for ASD with clear male-specific penetrance; males with a microdeletion in SHANK1 are autistic, while related female carriers are not affected with ASD.
- *43.Berg JM, Geschwind DH. Autism genetics: searching for specificity and convergence. Genome Biol. 2012;13(7):247. doi: 10.1186/gb-2012-13-7-247. Comprehensive review of the current state of genetics as relates to ASD.
- 44.Constantino JN, Zhang Y, Frazier T, et al. Sibling recurrence and the genetic epidemiology of autism. Am J Psychiatry. 2010;167(11):1349–56. doi: 10.1176/appi.ajp.2010.09101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sebat J, Lakshmi B, Malhotra D, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316(5823):445–9. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *46.Levy D, Ronemus M, Yamrom B, et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70(5):886–97. doi: 10.1016/j.neuron.2011.05.015. Identification of rare, de novo CNVs in sporadic autistic cases from nearly 1000 simplex families supports a role for these rare variants in ASD risk, and also finds an increased CNV rate in females versus males.
- *47.Sanders SJ, Ercan-Sencicek AG, Hurs V, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70(5):863–85. doi: 10.1016/j.neuron.2011.05.002. This study identifies rare, de novo CNVs in sporadic ASD cases, finding several CNV events that recur independently in unrelated cases, thereby implicating specific CNV loci in ASD liability.
- **48.Neale BM, Kou Y, Liu L, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485(7397):242–5. doi: 10.1038/nature11011. This study and references 49-51 present the first identifications of rare SNVs in exome sequences from autistic cases, finding a handful of genes that are recurrently hit by these rare variants. Together, results from these papers demonstrate a significant role for rare SNVs in genetic liability for ASD, and also that the loci hit by these SNVs are highly heterogeneous.
- **49.O’Roak BJ, Vives L, Girirajan S, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485(7397):246–50. doi: 10.1038/nature10989. See 48.
- **50.Sanders SJ, Murtha MT, Gupta AR, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485(7397):237–41. doi: 10.1038/nature10945. See 48.
- **51.Iossifov I, Ronemus M, Levy D, et al. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012;74(2):285–99. doi: 10.1016/j.neuron.2012.04.009. See 48.
- 52.Donnelly SL, Wolport CM, Menold MM, et al. Female with autistic disorder and monosomy X (Turner syndrome): parent-of-origin effect of the X chromosome. Am J Med Genet. 2000;96(3):312–6. doi: 10.1002/1096-8628(20000612)96:3<312::aid-ajmg16>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 53.Skuse DH, James RS, Bishop DV, et al. Evidence from Turner’s syndrome of an imprinted X-linked locus affecting cognitive function. Nature. 1997;387(6634):705–8. doi: 10.1038/42706. [DOI] [PubMed] [Google Scholar]
- 54.Creswell C, Skuse D. Autism in association with Turner syndrome: Genetic implications for male vulnerability to pervasive developmental disorders. Neurocase. 1999;5:511–518. [Google Scholar]
- 55.Jha P, Sheth D, Ghaziuddin M. Autism spectrum disorder and Klinefelter syndrome. Eur Child Adolesc Psychiatry. 2007;16(5):305–8. doi: 10.1007/s00787-007-0601-8. [DOI] [PubMed] [Google Scholar]
- *56.van Rijn S, Bierman M, Bruining H, Swaab H. Vulnerability for autism traits in boys and men with an extra X chromosome (47,XXY): the mediating role of cognitive flexibility. J Psychiatr Res. 2012;46(10):1300–6. doi: 10.1016/j.jpsychires.2012.06.004. Finds a negative correlation between presentation of autistic traits and a measurement of cognitive flexibility in cases with Klinefelter syndrome.
- 57.Bishop DV, Jacobs PA, Lachlan K, et al. Autism, language and communication in children with sex chromosome trisomies. Arch Dis Child. 2011;96(10):954–9. doi: 10.1136/adc.2009.179747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **58.Ross JL, Roeltgen DP, Kushner H, et al. Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Pediatrics. 2012;129(4):769–78. doi: 10.1542/peds.2011-0719. Characterization of behaviors and autistic traits in males with sex chromosome aneuploidies finds a high rate of autistic traits in both KS and 47,XYY, with the highest rate (50% of 26 subjects) in 47,XYY cases. Behavioral problems were also found to be more severe in the 47,XYY group.
- 59.Skuse DH. Imprinting, the X-chromosome, and the male brain: explaining sex differences in the liability to autism. Pediatr Res. 2000;47(1):9–16. doi: 10.1203/00006450-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn Sci. 2002;6(6):248–254. doi: 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- 61.Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord. 2004;34(2):163–75. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- 62.Baron-Cohen S, Wheelwright S. The Friendship Questionnaire: an investigation of adults with Asperger syndrome or high-functioning autism, and normal sex differences. J Autism Dev Disord. 2003;33(5):509–17. doi: 10.1023/a:1025879411971. [DOI] [PubMed] [Google Scholar]
- 63.Baron-Cohen S, Richler J, Bisarya D, et al. The systemizing quotient: an investigation of adults with Asperger syndrome or high-functioning autism, and normal sex differences. Philos Trans R Soc Lond B Biol Sci. 2003;358(1430):361–74. doi: 10.1098/rstb.2002.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Auyeung B, Baron-Cohen S, Chapman E, et al. Foetal testosterone and the child systemizing quotient. European Journal of Endocrinology. 2006;155:S123–S130. [Google Scholar]
- 65.Auyeung B, Baron-Cohen S, Ashwin E, et al. Fetal testosterone and autistic traits. Br J Psychol. 2009;100(Pt 1):1–22. doi: 10.1348/000712608X311731. [DOI] [PubMed] [Google Scholar]
- 66.Chapman E, Baron-Cohen S, Auyeung B, et al. Fetal testosterone and empathy: evidence from the empathy quotient (EQ) and the “reading the mind in the eyes” test. Soc Neurosci. 2006;1(2):135–48. doi: 10.1080/17470910600992239. [DOI] [PubMed] [Google Scholar]
- 67.Knickmeyer R, Baron-Cohen S, Raggatt P, Taylor K. Foetal testosterone, social relationships, and restricted interests in children. J Child Psychol Psychiatry. 2005;46(2):198–210. doi: 10.1111/j.1469-7610.2004.00349.x. [DOI] [PubMed] [Google Scholar]
- *68.Lombardo MV, Ashwin E, Auyeung B, et al. Fetal testosterone influences sexually dimorphic gray matter in the human brain. J Neurosci. 2012;32(2):674–80. doi: 10.1523/JNEUROSCI.4389-11.2012. Correlation of males’ fetal testosterone level with volume of brain regions found to be sexually dimorphic in size from a large data set of structural MRI images of brains from male and female children.
- 69.Geier DA, Geier MR. A prospective assessment of androgen levels in patients with autistic spectrum disorders: biochemical underpinnings and suggested therapies. Neuro Endocrinol Lett. 2007;28(5):565–73. [PubMed] [Google Scholar]
- *70.Ruta L, Ingudomnukul E, Taylor K, et al. Increased serum androstenedione in adults with autism spectrum conditions. Psychoneuroendocrinology. 2011;36(8):1154–63. doi: 10.1016/j.psyneuen.2011.02.007. Assessment of circulating androgens in high-functioning autistic adults finds higher levels of androstenedione in males and females with ASD than in sex-matched controls.
- *71.Schwarz E, Guest PC, Rahmoune H, et al. Sex-specific serum biomarker patterns in adults with Asperger’s syndrome. Mol Psychiatry. 2011;16(12):1213–20. doi: 10.1038/mp.2010.102. Measurement of molecules in blood from individuals with Asperger’s syndrome identifies different sets of significant biomarkers in males and females.
- 72.Takagishi H, Takahashi T, Yamagishi T, et al. Salivary testosterone levels and autism-spectrum quotient in adults. Neuro Endocrinol Lett. 2010;31(6):837–41. [PubMed] [Google Scholar]
- *73.Geier DA, Kern JK, King PG, et al. An evaluation of the role and treatment of elevated male hormones in autism spectrum disorders. Acta Neurobiol Exp (Wars) 2012;72(1):1–17. doi: 10.55782/ane-2012-1876. Comprehensive review of the evidence linking testosterone and ASDs.
- 74.Nguyen A, Rauch TA, Pfeifer GP, Hu VW. Global methylation profiling of lymphoblastoid cell lines reveals epigenetic contributions to autism spectrum disorders and a novel autism candidate gene, RORA, whose protein product is reduced in autistic brain. FASEB J. 2010;24(8):3036–51. doi: 10.1096/fj.10-154484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *75.Sarachana T, Xu M, Wu RC, Hu VW. Sex hormones in autism: androgens and estrogens differentially and reciprocally regulate RORA, a novel candidate gene for autism. PLoS One. 2011;6(2):e17116. doi: 10.1371/journal.pone.0017116. Finding of integral role for RORA, a gene downregulated in ASD, in sex hormone metabolism; transcription of RORA is activated by testosterone and inhibited by estrogen, and RORA stimulates expression of aromatase.
- **76.Voineagu I, Wang X, Johnston P, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474(7351):380–4. doi: 10.1038/nature10110. Characterization of cortical gene expression patterns in adults with ASD finds diminished regional specificity of gene expression patterns in ASD, and two ASD-associated co-expression modules that may represent expression changes caused by primary effects of genetic risk, and secondary effects of downstream pathophysiology.
- *77.Schwarz JM, Bilbo SD. Sex, glia, and development: interactions in health and disease. Horm Behav. 2012;62(3):243–53. doi: 10.1016/j.yhbeh.2012.02.018. Review of the interactions between sex-specific biological factors and glial function in neural development.
- 78.McCarthy MM, Todd BJ, Amateau SK. Estradiol modulation of astrocytes and the establishment of sex differences in the brain. Ann N Y Acad Sci. 2003;1007:283–97. doi: 10.1196/annals.1286.027. [DOI] [PubMed] [Google Scholar]
- 79.Price WH, van der Molen HJ. Plasma testosterone levels in males with the 47,XYY karyotype. J Endocrinol. 1970;47(1):117–22. doi: 10.1677/joe.0.0470117. [DOI] [PubMed] [Google Scholar]
- 80.Ratcliffe SG, Read G, Pan H, et al. Prenatal testosterone levels in XXY and XYY males. Horm Res. 1994;42(3):106–9. doi: 10.1159/000184157. [DOI] [PubMed] [Google Scholar]