Abstract
Several forms of chronic autonomic failure manifest as neurogenic orthostatic hypotension, including autoimmune autonomic ganglionopathy (AAG) and pure autonomic failure (PAF). AAG and PAF are thought to differ in pathogenesis, AAG reflecting decreased ganglionic neurotransmission due to circulating antibodies to the neuronal nicotinic receptor and PAF being a Lewy body disease with prominent loss of sympathetic noradrenergic nerves. AAG therefore would be expected to differ from PAF in terms of clinical laboratory findings indicating postganglionic noradrenergic denervation. Both diseases are rare. Here we report preliminary observations about clinical physiologic, neuropharmacologic, neurochemical, and neuroimaging data that seem to fit with the hypothesized pathogenetic difference between AAG and PAF. Patients with either condition have evidence of baroreflex–sympathoneural and baroreflex–cardiovagal failure. Both disorders feature low plasma levels of catecholamines during supine rest, but plasma levels of the other endogenous catechols, dihydroxyphenylalanine (DOPA), dihydroxyphenylacetic acid (DOPAC), and dihydroxyphenylglycol (DHPG), seem to be lower in PAF than in AAG, probably reflecting decreased norepinephrine synthesis and turnover in PAF, due to diffuse sympathetic noradrenergic denervation. PAF entails cardiac sympathetic denervation, whereas cardiac sympathetic neuroimaging by thoracic 6-[18F]fluorodopamine scanning indicates intact myocardial sympathetic innervation in AAG.
Keywords: Autonomic failure, Dysautonomia, Sympathetic nervous system, Norepinephrine, Fluorodopamine
Orthostatic hypotension (OH), defined as a persistent, consistent orthostatic fall in systolic blood pressure of at least 20 mm Hg or diastolic pressure of at least 10 mm Hg by 3 min of standing up (Kaufmann, 1996), occurs especially commonly in the elderly, with a prevalence in the US of about 12% (Applegate et al., 1991). As the US population ages, the prevalence of OH and the incidence of OH-related morbidity are likely to increase, because the rate of hospitalization for non-acute OH increases exponentially with age (Shibao et al., 2007). Better understanding of mechanisms and pathophysiology-based treatments should decrease the public health burden of OH.
There are many causes of OH. Probably the most common are medications, hypovolemia, dehydration, cardiac pump failure, and diseases that are associated with autonomic neuropathies (e.g., diabetes mellitus, chronic renal failure, and amyloidosis).
Even after extensive evaluation, however, about one-third of patients with persistent, consistent OH have no identified cause (Robertson and Robertson, 1994). For these the term, idiopathic OH, has been used. In virtually all such cases, OH is associated with an abnormality of reflexive regulation of the circulation by the sympathetic noradrenergic system— that is, the OH is neurogenic.
Neurogenic OH (NOH) is a major manifestation of several chronic autonomic failure syndromes. About 40% of patients with Parkinson disease (PD) have NOH (Goldstein et al., 2005). Multiple system atrophy (MSA), pure autonomic failure (PAF), familial dysautonomia, and dopamine-beta-hydroxylase deficiency also cause NOH.
In 2000, investigators at the Mayo Clinic reported conditions called “autoimmune autonomic neuropathies,” (Vernino et al., 2000) in which the serum of the affected patients was found to contain an antibody to the neuronal nicotinic acetylcholine receptor (nAChR), which mediates ganglionic neurotransmission. That study did not report such an antibody in patients with PAF. Subsequently, a case report by our group described a patient, thought initially to have PAF, who had evidence of decreased neurotransmission to intact sympathetic post-ganglionic nerves and whose plasma contained a high titer of nAChR antibody (Goldstein et al., 2002). This condition has come to be called “autoimmune autonomic ganglionopathy,” or AAG.
AAG and PAF both are rare disorders, and clinical and laboratory tests to differentiate AAG from PAF have not yet been assessed comprehensively. Here we report results of physiologic, neuropharmacologic, neurochemical, and neuroimaging testing in patients with AAG, PAF, and two other forms of chronic autonomic failure manifesting with NOH—multiple system atrophy (MSA) and Parkinson disease with neurogenic orthostatic hypotension (PD+NOH).
1. Methods
Three patients with AAG and high titers of nAChR antibodies underwent autonomic function testing at the NIH Clinical Center, after giving written informed consent to participate in protocols approved by the Intramural Research Board of the National Institute of Neurological Disorders and Stroke.
The first AAG patient was a 78 year old black woman, the second a 65 year old white man, and the third a 74 year old white woman. All three patients reported severe orthostatic intolerance, dry mouth, and absent sweating. The first patient's chief symptoms were dry mouth, constipation, and orthostatic intolerance. The male patient carried a diagnosis of Sjogren's syndrome and had significant urinary retention that required frequent self-catheterization to void. The third patient had drymouth treated with pilocarpine, dry eyes treated with artificial tears, and neck ache treated with prednisone. A lip biopsy had revealed mild Sjogren's syndrome. She had urinary urgency, frequency, and nocturia but not urinary retention.
Results in the AAG patients were compared to those in a total of 76 other patients with NOH (17 PAF, 43 MSA, 16 PD+NOH) who were studied approximately concurrently.
The diagnostic sequence employed for PAF was as follows. First, a history of chronic, persistent, consistent orthostatic hypotension, without evidence of central neurodegeneration, was obtained and confirmed with the patients as inpatients at the NIH Clinical Center. Second, orthostatic hypotension was determined to be neurogenic, by a progressive fall in beat-to-beat systolic blood pressure during Phase II of the Valsalva maneuver, delayed recovery of blood pressure in Phases III–IV, and absence of an overshoot of blood pressure in Phase IV. All PAF patients also had a small (less than 30 pg/mL) increment in the plasma norepinephrine concentration between supine rest and upright posture. Third, neurogenic orthostatic hypotension was shown to be associated with sympathetic post-ganglionic noradrenergic denervation, as indicated by a low concentration of 6-[18F]fluorodopamine-derived radioactivity in the interventricular septal myocardium and by a low plasma concentration of dihydroxyphenylglycol (DHPG), the main neuronal metabolite of norepinephrine.
The diagnostic sequence employed for AAG was as follows. First, a history of chronic, persistent, consistent orthostatic hypotension, without evidence of central neurodegeneration, and with evidence of cholinergic failure (dry mouth, dry eyes, constipation, decreased sweating) was obtained and confirmed with the patients as inpatients at the NIH Clinical Center. Second, orthostatic hypotension was determined to be neurogenic, based on abnormal beat-to-beat blood pressure responses to the Valsalva maneuver. Third, no convincing neuroimaging or neurochemical evidence of post-ganglionic denervation was obtained. Fourth, high titers of nAChR antibodies were found.
In contrast with patients who had AAG or PAF, patients with PD+NOH or MSA had unequivocal symptoms and signs of chronic, progressive central neurodegeneration, such as parkinsonism or cerebellar ataxia.
1.1. Quantitative sudomotor axon reflex test
The quantitative sudomotor axon reflex test was done on the right forearm. Briefly, acetylcholine (100 mg/mL)was delivered by iontophoresis at 2.0 mA for 5 min, and the total volume of sweat production in 10 min was measured using an Atlas device and Q-Sweat software (WR Medical Electronics, Stillwater, MN).
1.2. Valsalva maneuver
Beat-to-beat blood pressure was measured non-invasively using a Finometer device (Finapres Medical Systems, Amsterdam, The Netherlands). The patient blew into tubing connected to a sphygmomanometer at a pressure of 30 mm Hg for 12 s (Goldstein and Tack, 2000). The maneuver was repeated as necessary until a satisfactory recording was obtained. Rarely, in the event of inability to perform a technically inadequate maneuver by this means, the subject performed the Valsalva maneuver against a closed glottis.
1.3. Orthostasis
The patient was tilted passively upright using a motorized tilt table. Hemodynamic measures and arm venous blood for neurochemical assays were obtained during supine rest and at 5 min of orthostasis.
1.4. 6-[18F]fluorodopamine PET scanning
For 6-[18F]fluorodopamine positron emission tomographic (PET scanning), 1 mCi of the imaging agent was injected i.v. over 3 min (Goldstein et al., 2000). Data are reported for the interventricular septum for the 5-minute interval between 5 and 10 min after initiation of scanning (midpoint about 8 min after initiation of injection).
1.5. Plasma catechols
Plasma catechols, including the catecholamines norepinephrine (NE), epinephrine (EPI), and dopamine (DA) and the non-catecholamines dihydroxyphenylalanine (DOPA), dihydroxyphenylacetic acid (DOPAC), and dihydroxyphenylglycol (DHPG), were measured in arm venous blood obtained through an indwelling i.v. catheter or needle after at least 15 min of supine rest. Catechols were assayed in the Clinical Neurochemistry Laboratory of the Clinical Neurocardiology Section, by liquid chromatography with electrochemical detection after batch alumina extraction, as described previously (Holmes et al., 1994).
1.6. Data analysis and statistics
Data were analyzed by factorial analyses of variance, with Fisher's PLSD post-hoc test, using KaleidaGraph 4.0.1 (Synergy Software, Reading, PA). A p value less than 0.05 defined statistical significance.
2. Results
2.1. Valsalva maneuver
Subjects in the four patient groups (AAG, PAF, MSA, PD+NOH) had the same abnormal pattern of beat-to-beat blood pressure during and after performance of the Valsalva maneuver, with a progressive fall in systolic pressure in Phase II, delayed return of blood pressure in Phases III–IV (Vogel et al., 2005), and absence of pressure overshoot over the baseline pressure in Phase IV. Baroreflex–cardiovagal gain, calculated from the slope of the line of best fit for the relationship between interbeat interval and systolic blood pressure during Phase II of the Valsalva maneuver, was also low in all four groups (−0.28±0.33, 1.30±0.31, 1.51±0.45, and 0.89±0.13 ms/mm Hg).
2.2. Orthostatic vital signs
By definition, all four patient groups had orthostatic hypotension, with similar mean falls in systolic blood pressure during orthostasis (77±28, 68±7, 59±7, and 55±4 mm Hg).
2.3. QSART
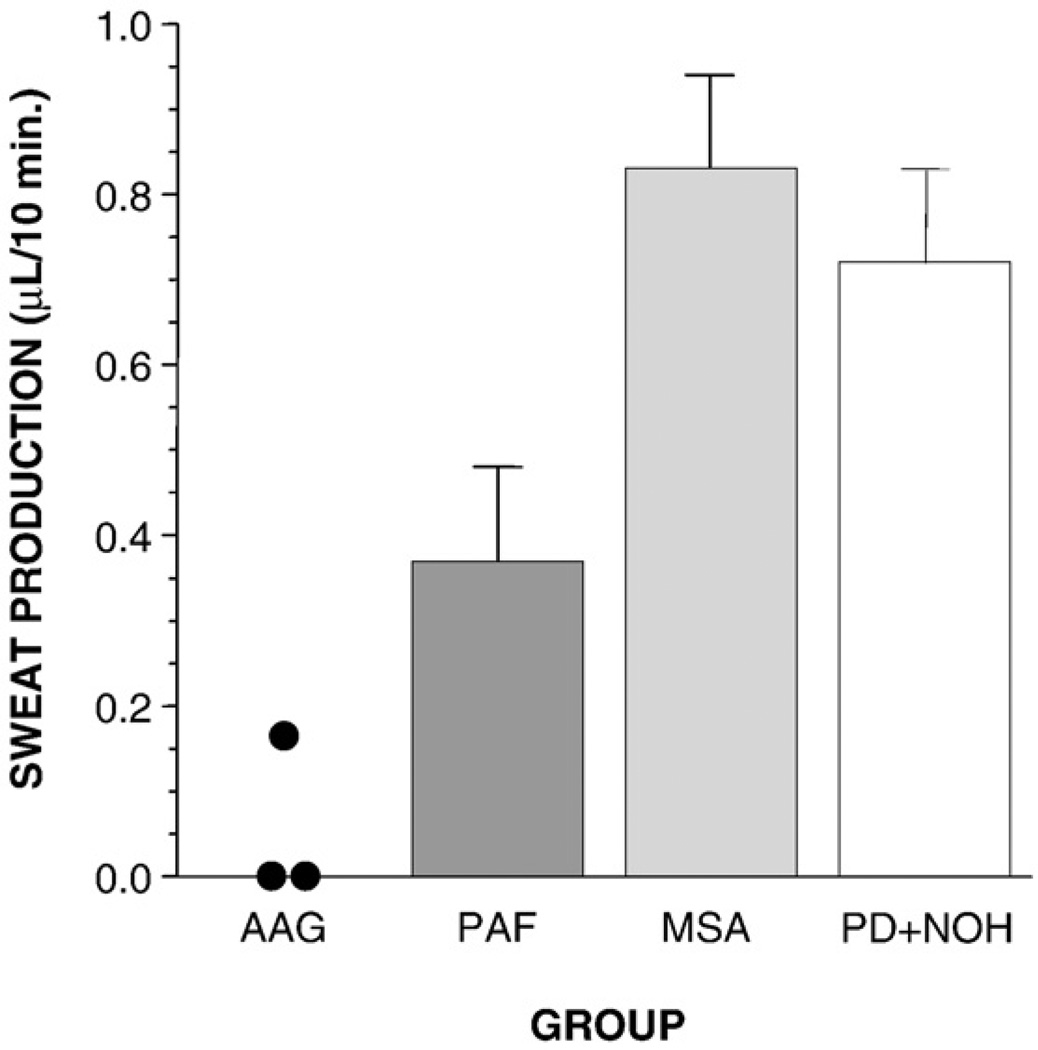
Sweat production in the QSART differentiated the four patient groups with chronic autonomic failure (Fig. 1). Of the 8 PAF patients with QSART data, 4 were men and 4 women. Of the 4 men, 2 had subnormal QSART responses, and 2 had responses that were within the published normal range for men. Of the 4 women, all 4 had QSART responses that were within the published normal range for women. Thus, among PAF patients, QSART responses were often within the normal range, after taking gender into account. In contrast, in all 3 AAG patients, QSART responses were remarkably subnormal. Indeed, in the 2 female AAG patients, there was no detectable QSART response at all.
Fig. 1.
Mean (±SEM) values for sweat production in the quantitative sudomotor axon reflex test in patients with autoimmune autonomic ganglionopathy (AAG), pure autonomic failure (PAF), multiple system atrophy (MSA), or Parkinson disease with neurogenic orthostatic hypotension (PD+NOH).
2.4. Cardiac 6-[18F]fluorodopamine-derived radioactivity
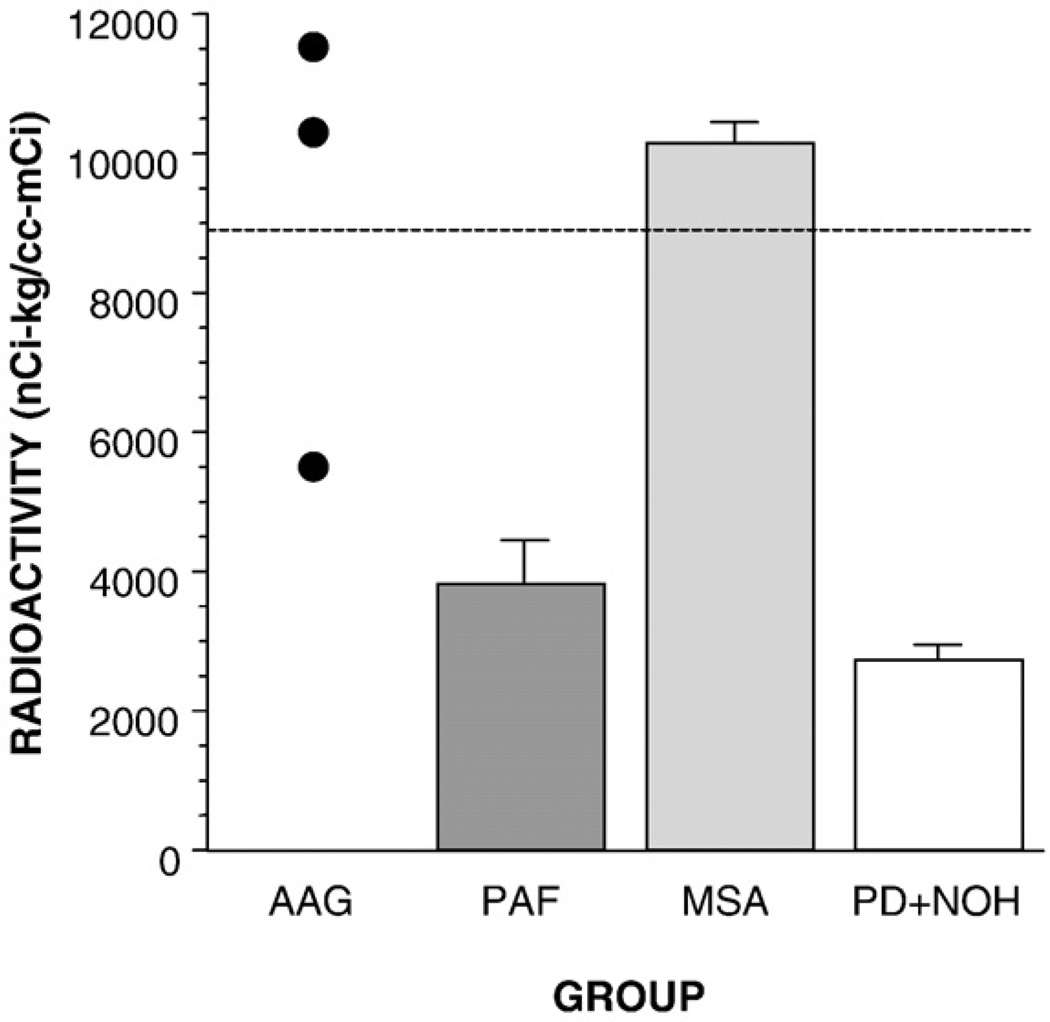
6-[18F]fluorodopamine-derived radioactivity in the interventricular septum was normal in the AAG and MSA groups and low in the PAF and PD+NOH groups (Fig. 2). The mean value in the AAG group was significantly higher than that in the PAF group.
Fig. 2.
Mean (±SEM) values for 6-[18F]flurodopamine-derived radioactivity in the interventricular septum in patients with autoimmune autonomic ganglionopathy (AAG), pure autonomic failure (PAF), multiple system atrophy (MSA), or Parkinson disease with neurogenic orthostatic hypotension (PD+NOH). Horizontal dashed line shows normal mean value.
2.5. Plasma catechols
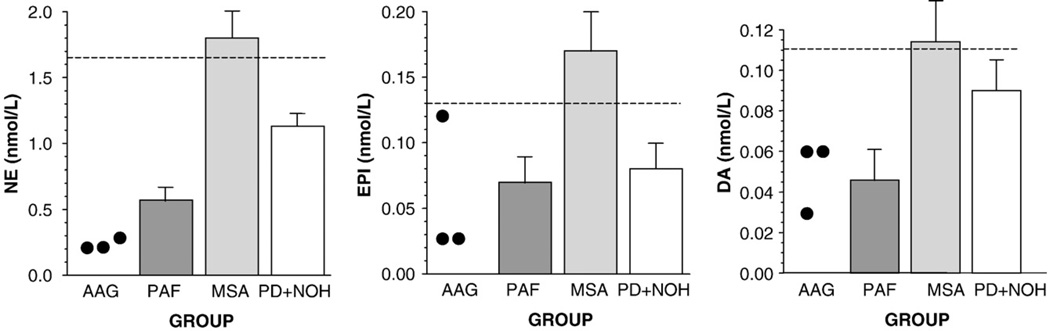
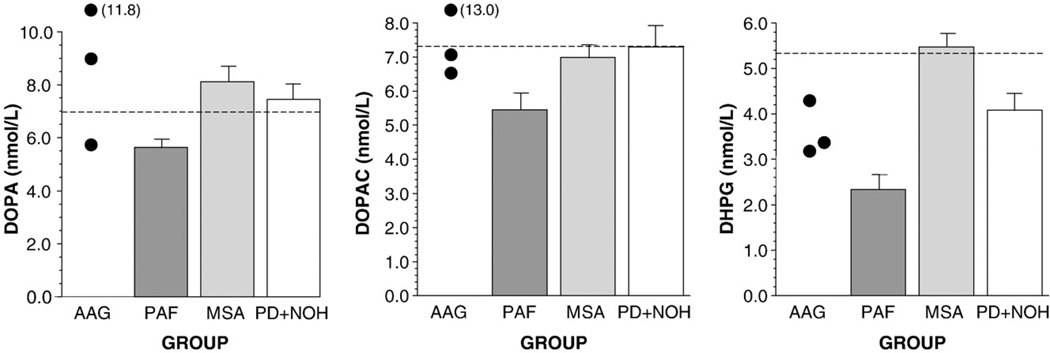
Plasma levels of catecholamines (NE, EPI, and DA) seemed low in the AAG and PAF groups and approximately normal in the MSA and PD+NOH groups (Fig. 3). Plasma levels of non-catecholamine catechols (DOPA, DOPAC, and DHPG) seemed normal in the AAG, MSA, and PD+NOH groups, in contrast with low levels in the PAF group (Fig. 4).
Fig. 3.
Mean (±SEM) values for plasma levels of catecholamines (norepinephrine, NE; epinephrine, EPI; dopamine, DA) in patients with autoimmune autonomic ganglionopathy (AAG), pure autonomic failure (PAF), multiple system atrophy (MSA), or Parkinson disease with neurogenic orthostatic hypotension (PD+NOH).
Fig. 4.
Mean (±SEM) values for plasma levels of non-catecholamine catechols (L-3,4-dihydroxyphenylalanine, DOPA; dihydroxyphenylacetic acid, DOPAC; dihydroxyphenylglycol, DHPG) in patients with autoimmune autonomic ganglionopathy (AAG), pure autonomic failure (PAF), multiple system atrophy (MSA), or Parkinson disease with neurogenic orthostatic hypotension (PD+NOH).
The PAF, MSA, and PD+NOH groups had attenuated orthostatic increments in plasma NE levels (71±15, 128±50, and 28±12 pg/mL; normal 286±34 pg/mL). Of the 3 AAG patients, 2 had clear attenuation of the orthostatic increment in plasma NE levels (−6 and 20 pg/mL), and 1 had a normal increment.
3. Discussion
The clinical laboratory findings in this study provide preliminary evidence that AAG and PAF can be distinguished by indices of postganglionic sympathetic, noradrenergic innervation.
In AAG the lesion is at the level of nicotinic receptors on postganglionic neurons and adrenomedullary chromaffin cells. Therefore, plasma levels of catecholamines are low. This finding does not distinguish AAG from PAF, because PAF features loss of sympathetic noradrenergic nerves and adrenomedullary chromaffin cells and therefore also low plasma catecholamine levels.
PAF patients, but not AAG patients, had low plasma levels of the noncatecholamine catechols, DOPA, DOPAC, and DHPG (Goldstein et al., 2003a). Plasma DOPA and DOPAC levels are related to catecholamine biosynthesis, and plasma DHPG is related to the turnover of NE stores in sympathetic nerves (Goldstein et al., 2003b). Normal plasma DOPA and DOPAC levels in AAG suggest intact catecholamine biosynthesis. In PAF, low levels of DOPA, DOPAC, and DHPG are consistent with decreased catecholaminergic cell populations.
Plasma DHPG mainly reflects NE turnover due to net leakage from storage vesicles into the cytoplasm and metabolic conversion of NE to dihydroxyphenylglycolaldehyde catalyzed by monoamine oxidase in the outer mitochondrial membrane and then conversion of dihydroxyphenylglycolaldehyde to DHPG catalyzed by aldose/aldehyde reductase (Kawamura et al., 1997). Plasma DHPG is also influenced by turnover due to reuptake and intraneuronal metabolism of NE that is released in response to sympathetic nerve traffic (Goldstein et al., 1988). In AAG, interference with ganglionic neurotransmission may decrease NE release and consequently NE reuptake, resulting in decreased plasma DHPG levels. The DHPG levels in AAG, however, seem higher than in PAF, because the latter involves both decreased vesicular stores and decreased neuronal reuptake of NE.
Consistent with post-ganglionic sympathetic denervation in PAF but not in AAG, patients with PAF had low myocardial concentrations of 6-[18F]fluorodopamine-derived radioactivity, whereas patients with AAG had normal radioactivity. These differences seem analogous to those between PD+NOH and MSA, because essentially all patients with PD+NOH have remarkably low myocardial concentrations of 6-[18F]fluorodopamine-derived radioactivity, whereas most MSA patients have normal radioactivity (Goldstein et al., 2000), in line with the findings of profoundly decreased tyrosine hydroxylase immunoreactivity in epicardial nerves of patients with PAF or PD (Orimo et al., 2002; Amino et al., 2005).
The cholinergic receptors on sweat glands are muscarinic, not nicotinic. Virtually absent QSART results in AAG patients therefore suggest either a trophic decrease in responsiveness of sweat glands or tonically decreased acetylcholine release from sympathetic cholinergic postganglionic neurons, as a result of impaired ganglionic neurotransmission.
Since the diagnosis of AAG required a high anti-AChR antibody titer, we did not include patients who might have AAG and negative anti-AChR titers. Such patients were probably not mistakenly included in the PAF group, however, because of the requirement of evidence for post-ganglionic sympathetic denervation in order to diagnose PAF.
Taken together, these preliminary results hold promise for development of clinical neurochemical and neuroimaging methods to distinguish AAG from PAF.
References
- Amino T, Orimo S, Takahashi A, Uchihara T, Mizusawa H. Profound cardiac sympathetic denervation occurs in Parkinson disease. Brain Pathol. 2005;15:29–34. doi: 10.1111/j.1750-3639.2005.tb00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate WB, Davis BR, Black HR, Smith WM, Miller ST, Burlando AJ. Prevalence of postural hypotension at baseline in the Systolic Hypertension in the Elderly Program (SHEP) cohort. J. Am. Geriatr. Soc. 1991;39(11):1057–1064. doi: 10.1111/j.1532-5415.1991.tb02869.x. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Tack C. Non-invasive detection of sympathetic neurocirculatory failure. Clin. Auton. Res. 2000;10:285–291. doi: 10.1007/BF02281111. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Eisenhofer G, Stull R, Folio CJ, Keiser HR, Kopin IJ. Plasma dihydroxyphenylglycol and the intraneuronal disposition of norepinephrine in humans. J. Clin. Invest. 1988;81:213–220. doi: 10.1172/JCI113298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Holmes C, Li ST, Bruce S, Metman LV, Cannon RO., 3rd Cardiac sympathetic denervation in Parkinson disease. Ann. Intern. Med. 2000;133(5):338–347. doi: 10.7326/0003-4819-133-5-200009050-00009. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Holmes C, Dendi R, Li ST, Brentzel S, Vernino S. Pandysautonomia associated with impaired ganglionic neurotransmission and circulating antibody to the neuronal nicotinic receptor. Clin. Auton. Res. 2002;12:281–285. doi: 10.1007/s10286-002-0020-3. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Holmes C, Sharabi Y, Brentzel S, Eisenhofer G. Plasma levels of catechols and metanephrines in neurogenic orthostatic hypotension. Neurology. 2003a;60(8):1327–1332. doi: 10.1212/01.wnl.0000058766.46428.f3. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Eisenhofer G, Kopin IJ, et al. Sources and significance of plasma levels of catechols and their metabolites in humans. J. Pharmacol. Exp. Ther. 2003b;305(3):800–811. doi: 10.1124/jpet.103.049270. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Eldadah BA, Holmes C, et al. Neurocirculatory abnormalities in Parkinson disease with orthostatic hypotension. Independence from levodopa treatment. Hypertension. 2005;46:1–7. doi: 10.1161/01.HYP.0000188052.69549.e4. [DOI] [PubMed] [Google Scholar]
- Holmes C, Eisenhofer G, Goldstein DS. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J. Chromatogr., B, Biomed. Appl. 1994;653:131–138. doi: 10.1016/0378-4347(93)e0430-x. [DOI] [PubMed] [Google Scholar]
- Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin. Auton. Res. 1996;6(2):125–126. doi: 10.1007/BF02291236. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Kopin IJ, Kador PF, Sato S, Tjurmina O, Eisenhofer G. Effects of aldehyde/aldose reductase inhibition on neuronal metabolism of norepinephrine. J. Auton. Nerv. Syst. 1997;66:145–148. doi: 10.1016/s0165-1838(97)00086-6. [DOI] [PubMed] [Google Scholar]
- Orimo S, Oka T, Miura H, et al. Sympathetic cardiac denervation in Parkinson's disease and pure autonomic failure but not in multiple system atrophy. J. Neurol. Neurosurg. Psychiatry. 2002;73:776–777. doi: 10.1136/jnnp.73.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Robertson RM. Causes of chronic orthostatic hypotension. Arch. Intern. Med. 1994;154(14):1620–1624. [PubMed] [Google Scholar]
- Shibao C, Grijalva CG, Raj SR, Biaggioni I, Griffin MR. Orthostatic hypotensionrelated hospitalizations in the United States. Am. J. Med. 2007;120(11):975–980. doi: 10.1016/j.amjmed.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N. Engl. J. Med. 2000;343(12):847–855. doi: 10.1056/NEJM200009213431204. [DOI] [PubMed] [Google Scholar]
- Vogel ER, Sandroni P, Low PA. Blood pressure recovery from Valsalva maneuver in patients with autonomic failure. Neurology. 2005;65(10):1533–1537. doi: 10.1212/01.wnl.0000184504.13173.ef. [DOI] [PubMed] [Google Scholar]