Abstract
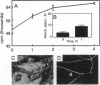
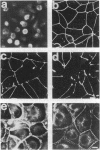
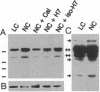
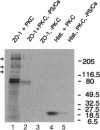
We have previously shown that protein phosphorylation plays an important role in the sorting and assembly of tight junctions. We have now examined in detail the role of protein kinases in intercellular junction biogenesis by using a combination of highly specific and broad-spectrum inhibitors that act by independent mechanisms. Our data indicate that protein kinase C (PKC) is required for the proper assembly of tight junctions. Low concentrations of the specific inhibitor of PKC, calphostin C, markedly inhibited development of transepithelial electrical resistance, a functional measure of tight-junction biogenesis. The effect of PKC inhibitors on the development of tight junctions, as measured by resistance, was paralleled by a delay in the sorting of the tight-junction protein, zona occludens 1 (ZO-1), to the tight junction. The assembly of desmosomes and the adherens junction were not detectably affected, as determined by immunocytochemical analysis. In addition, ZO-1 was phosphorylated subsequent to the initiation of cell-cell contact, and treatment with calphostin C prevented approximately 85% of the phosphorylation increase. Furthermore, in vitro measurements indicate that ZO-1 may be a direct target of PKC. Moreover, membrane-associated PKC activity more than doubled during junction assembly, and immunocytochemical analysis revealed a pool of PKC zeta that appeared to colocalize with ZO-1 at the tight junction. A preformed complex containing ZO-1, ZO-2, p130, as well as 330- and 65-kDa phosphoproteins was detected by coimmunoprecipitation in both the presence and absence of cell-cell contact. Identity of the 330- and 65-kDa phosphoproteins remains to be determined, but the 65-kDa protein may be occludin. The mass of this complex and the incorporation of ZO-1 into the Triton X-100-insoluble cytoskeleton were not PKC dependent.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1992 May 11;20 (Suppl):2013–2018. doi: 10.1093/nar/20.suppl.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda M. S., Gonzalez-Mariscal L., Matter K., Cereijido M., Anderson J. M. Assembly of the tight junction: the role of diacylglycerol. J Cell Biol. 1993 Oct;123(2):293–302. doi: 10.1083/jcb.123.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987 Feb 15;47(4):936–942. [PubMed] [Google Scholar]
- Flint A. J., Paladini R. D., Koshland D. E., Jr Autophosphorylation of protein kinase C at three separated regions of its primary sequence. Science. 1990 Jul 27;249(4967):408–411. doi: 10.1126/science.2377895. [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Lowenkopf T., Apatira D. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3460–3464. doi: 10.1073/pnas.88.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Watanabe M., Kobayashi R. Properties and use of H-series compounds as protein kinase inhibitors. Methods Enzymol. 1991;201:328–339. doi: 10.1016/0076-6879(91)01029-2. [DOI] [PubMed] [Google Scholar]
- Howarth A. G., Singer K. L., Stevenson B. R. Analysis of the distribution and phosphorylation state of ZO-1 in MDCK and nonepithelial cells. J Membr Biol. 1994 Feb;137(3):261–270. doi: 10.1007/BF00232594. [DOI] [PubMed] [Google Scholar]
- Itoh M., Nagafuchi A., Yonemura S., Kitani-Yasuda T., Tsukita S., Tsukita S. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol. 1993 May;121(3):491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M., Yonemura S., Nagafuchi A., Tsukita S., Tsukita S. A 220-kD undercoat-constitutive protein: its specific localization at cadherin-based cell-cell adhesion sites. J Cell Biol. 1991 Dec;115(5):1449–1462. doi: 10.1083/jcb.115.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesaitis L. A., Goodenough D. A. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J Cell Biol. 1994 Mar;124(6):949–961. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi E., Nakano H., Morimoto M., Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989 Mar 15;159(2):548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Meza I., Sabanero M., Stefani E., Cereijido M. Occluding junctions in MDCK cells: modulation of transepithelial permeability by the cytoskeleton. J Cell Biochem. 1982;18(4):407–421. doi: 10.1002/jcb.1982.240180403. [DOI] [PubMed] [Google Scholar]
- Nigam S. K., Brenner B. M. Toward an understanding of epithelial morphogenesis in health and disease. Curr Opin Nephrol Hypertens. 1992 Dec;1(2):187–191. doi: 10.1097/00041552-199212000-00001. [DOI] [PubMed] [Google Scholar]
- Nigam S. K., Denisenko N., Rodriguez-Boulan E., Citi S. The role of phosphorylation in development of tight junctions in cultured renal epithelial (MDCK) cells. Biochem Biophys Res Commun. 1991 Dec 16;181(2):548–553. doi: 10.1016/0006-291x(91)91224-z. [DOI] [PubMed] [Google Scholar]
- Nigam S. K., Rodriguez-Boulan E., Silver R. B. Changes in intracellular calcium during the development of epithelial polarity and junctions. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6162–6166. doi: 10.1073/pnas.89.13.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosin J. M., Keramidas M., Souvignet C., Chambaz E. M. Differential inhibition of protein kinase C subtypes. Biochem Biophys Res Commun. 1990 Jun 29;169(3):1040–1048. doi: 10.1016/0006-291x(90)91999-9. [DOI] [PubMed] [Google Scholar]
- Quick J., Ware J. A., Driedger P. E. The structure and biological activities of the widely used protein kinase inhibitor, H7, differ depending on the commercial source. Biochem Biophys Res Commun. 1992 Sep 16;187(2):657–663. doi: 10.1016/0006-291x(92)91245-l. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Nelson W. J. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989 Aug 18;245(4919):718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Stappenbeck T. S., Green K. J. The desmoplakin carboxyl terminus coaligns with and specifically disrupts intermediate filament networks when expressed in cultured cells. J Cell Biol. 1992 Mar;116(5):1197–1209. doi: 10.1083/jcb.116.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart R. O., Sun A., Panichas M., Hebert S. C., Brenner B. M., Nigam S. K. Critical role for intracellular calcium in tight junction biogenesis. J Cell Physiol. 1994 Jun;159(3):423–433. doi: 10.1002/jcp.1041590306. [DOI] [PubMed] [Google Scholar]
- Willott E., Balda M. S., Fanning A. S., Jameson B., Van Itallie C., Anderson J. M. The tight junction protein ZO-1 is homologous to the Drosophila discs-large tumor suppressor protein of septate junctions. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7834–7838. doi: 10.1073/pnas.90.16.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]