Abstract
♦ Background: Encapsulating peritoneal sclerosis (EPS) is a severe complication of peritoneal dialysis (PD). Identification of patients at high risk for EPS (“EPS-prone”) and delivery of appropriate interventions might prevent its development. Our aim was to evaluate the clinical characteristics and outcomes of all EPS and EPS-prone patients diagnosed at our PD unit.
♦ Methods: For a 30-year period representing our entire PD experience, we retrospectively identified all patients with EPS (diagnosed according to International Society for Peritoneal Dialysis criteria) and all patients defined as EPS-prone because they met at least 2 established criteria (severe peritonitis, PD vintage greater than 3 years, severe hemoperitoneum, overexposure to glucose, and acquired ultrafiltration failure).
♦ Results: Of 679 PD patients, we identified 20 with EPS, for an overall prevalence of 2.9%. Mean age at diagnosis was 50.2 ± 16.4 years, with a median PD time of 77.96 months (range: 44.36 - 102.7 months) and a median follow-up of 30.91 months (range: 4.6 - 68.75 months). Of patients with EPS, 10 (50%) received tamoxifen, 10 (50%) received parenteral nutrition, and 2 (10%) underwent adhesiolysis, with 25% mortality related to EPS. Another 14 patients were identified as EPS-prone. Median follow-up was 54.05 months (range: 11.9 - 87.04 months). All received tamoxifen, and 5 (36%) received corticosteroids; none progressed to full EPS. We observed no differences in baseline data between the groups, but the group with EPS had been on PD longer (84 ± 53 months vs 39 ± 20 months, p = 0.002) and had a higher cumulative number of days of peritoneal inflammation from peritonitis (17.2 ± 11.1 days vs 9.8 ± 7.9 days, p = 0.015). Overall mortality was similar in the groups. The incidence of EPS declined during our three decades of experience (5.6%, 3.9%, and 0.3%).
♦ Conclusions: Being a serious, life-threatening complication of PD, EPS requires high suspicion to allow for prompt diagnosis and treatment. Early detection of EPS-prone states and delivery of appropriate intervention might prevent EPS development. Tamoxifen seems to be a key strategy in prevention, but caution should be used in interpreting our results. Additional randomized controlled studies are needed.
Keywords: Encapsulating peritoneal sclerosis, EPS, EPS-prone states, peritonitis, tamoxifen
Encapsulating peritoneal sclerosis (EPS) is a rare, but life-threatening complication of peritoneal dialysis (PD) that was originally described by Gandhi (1). Characterized by intraperitoneal inflammation, EPS leads to adhesive and inflammatory encapsulation over the small bowel, causing gastrointestinal symptoms and bowel obstruction leading to weight loss, malnutrition, and in some cases, death (2-5). Symptoms of EPS can develop during PD, but most patients become symptomatic after PD cessation and even after transplantation (2-4,6,7).
The prevalence of EPS is not well known, but it has been reported to range from 0.7% to 3.3%, increasing with duration of PD therapy (8-15). Very few data are available on the incidence of EPS in Spain. In 2007, Hospital Severo Ochoa in Madrid reported a 4.2% overall prevalence, with 51% 1-year survival in their population (15). The mortality reported for EPS is high (25% - 55%), especially during the first year after the diagnosis, and increases with longer duration on PD treatment (8,10,13,16).
Encapsulating peritoneal sclerosis is probably a multifactorial disease, with cumulative duration of exposure to bioincompatible PD fluids as the dominant risk factor, but younger age, frequent peritonitis, and kidney transplantation may also be risk factors (4,8,14,17,18). According to the two-hit theory, a second “hit” in concert with the peritoneal damage driven by PD treatment—for example infection (peritonitis), major surgery, discontinuation of PD, severe hemoperitoneum, or genetic predisposition—might trigger EPS development (3,18).
The natural history of EPS is not well understood, and unfortunately, there are no specific imaging or biochemical tests that allow for early detection, although some patients might show clinical, functional, and radiologic features that could be associated with an increased risk of EPS. Several markers in peritoneal effluent have been studied, but further investigations are needed (19).
The diagnosis of EPS is based on clinical, radiologic, and histologic findings (20). Once EPS has been diagnosed, transfer to HD is mandatory (4,20). No evidence-based treatment for EPS is available. Steroids have been shown to be beneficial, especially in the early “inflammatory” phase of the disease (8,21-23), and tamoxifen has also been reported to be effective (24-27), but prospective studies are lacking. When EPS reaches an advanced stage, total enterolysis is required (28-30).
Our PD program began in 1980, and 679 patients have received PD therapy in the program. We identified and treated several cases of EPS during those 30-plus years, and we believed that early detection of patients considered to be at increased risk for EPS (“EPS-prone”) and subsequent treatment of those patients could potentially lead to successful results. Based on that hypothesis, we identified several EPS-prone states, and for patients in those states, we applied interventions such as cessation of PD (when indicated), immediate pre-emptive treatment with tamoxifen, and close monitoring, to avoid progression to EPS.
The purpose of the present study was to compare the clinical characteristics of EPS and EPS-prone patients, so as to promote early detection and intervention for this malign disease.
Methods
Our study included all end-stage kidney disease patients who commenced PD in our unit between 1 January 1980 and 31 January 2012 (n = 679). Clinical records were reviewed in detail.
The data collected included demographics, cause of primary renal disease, prescribed dialysis solutions, time on PD, death, cause of death, complications, medications received, transfer to hemodialysis (HD), and kidney transplantation. Peritonitis was recorded both as episodes and as cumulative days of active peritoneal inflammation, defined as an effluent white blood cell count exceeding 100/μL, measured every other day until peritonitis resolution or catheter removal (31).
We regularly performed peritoneal equilibration tests (PETs), consisting of a 4-hour dwell with 3.86% or 4.25% glucose, taking 6 peritoneal effluent samples (at 0, 30, 60, 120, 180, and 240 minutes) and a blood sample to calculate the peritoneal mass transfer area coefficients (MTACs) for urea and creatinine in milliliters per minute, using a previously described mathematical model (31). The MTAC represents the isolated diffusive capacity of the membrane under a theoretic infinite dialysate flow (32). The patients fasted during each functional study. Data from the first to the last available PET were collected. Net ultrafiltration (UF) in milliliters was estimated based on the net negative balance after the 4-hour PET dwell (comparing the weight of the dialysate bag before and after drainage). The resulting value represents chiefly the convective transport capacity of the peritoneum. A negative balance lower than 400 mL was taken as an indicator of UF failure (UFF) (33). To quantify glucose exposure, we recorded the percentage of bags containing 2.27% and 3.86% glucose. More than 75% use of 2.27% bags or more than 25% use of 3.86% bags was considered high glucose exposure.
Diagnosis of EPS and EPS-prone States
Based on the 2009 guidelines from the International Society for Peritoneal Dialysis (20), a diagnosis of EPS was made when strong clinical suspicion was confirmed by radiologic, functional, or pathologic findings, alone or together. Diagnostic criteria included a combination of
clinical symptoms suggestive of obstruction;
changes in PD functional data (high or low transport associated with UFF diagnosed by PET);
radiologic findings (sclerosis, calcification, peritoneal thickening, or encapsulation of the intestine) on computed tomography (CT) or ultrasonography (US); and
anatomic criteria in cases of laparotomy or necropsy (adhesions, calcifications, or cocooning).
Treatment in these patients included adhesiolysis, PD cessation, and tamoxifen with or without other immunosuppressive drugs, as indicated by the physician.
Patients considered EPS-prone were those presenting with two or more of
time on PD longer than 3 years;
history of recurrent or severe peritonitis, including all episodes requiring catheter removal under active inflammatory conditions;
the presence of acquired UFF or high (“fast”) membrane transport;
high exposure to glucose PD fluids with high levels of glucose degradation products (indicated by poor volume control, with universal use of 2.27% or higher glucose); and
repeated and severe hemoperitoneum.
The foregoing criteria have been applied since the 1990s, when two new factors were proposed to influence outcomes in these patients: recognition of peritoneal toxicity for 3.86% and 4.25% glucose solutions, and the known usefulness of tamoxifen in other fibrotic processes. The therapeutic approach in EPS-prone patients was temporary or definitive PD cessation and oral tamoxifen 20 mg every 12 hours for a year, together with steroids for variable periods of time in cases of presumed inflammation.
This strategy was fully implemented in our unit after 1997, and we calculated the prevalence of EPS-prone patients by taking into account all patients who initiated PD after that year (n = 379). All patients at risk in the pre-tamoxifen era were omitted.
Statistical Analysis
All data were analyzed using the SPSS software program (version 15.0.1: SPSS, Chicago, IL, USA). The incidence of EPS was calculated as the number of EPS cases divided by the number of patients at risk. The level of significance was defined as p < 0.05, with the highest level of significance at p < 0.01. Group characteristics were compared using the Student t-test for independent samples, the Mann-Whitney test, and the Fisher exact probability test, as appropriate. Patient survival analyses were performed using the Kaplan-Meier approach.
Results
Our PD population during the study period was 679 patients, among whom 20 were diagnosed with EPS, and 14 were considered EPS-prone, for an overall EPS prevalence of 2.9% in our population. The prevalence declined over the three decades of the program from 5.6% in 1980-1990, to 3.9% in 1991-2000, and to 0.3% in subsequent years. The prevalence of EPS-prone patients from 1997 to 2012 was 3.69%.
EPS Group
Of the 20 identified patients with EPS (70% women; mean age: 50.2 years; Tables 1 and 2), 10 initiated PD in decade 1; 9, in decade 2; and 1, in decade 3 of the study. Peritonitis had occurred in 17 of the 20 patients (85%), and median time on PD in this group was 77.96 months (range: 44.36 - 102.7 months).
TABLE 1.
Patients with Encapsulating Peritoneal Sclerosis: Diagnostic Criteria, Treatment, and Outcomes
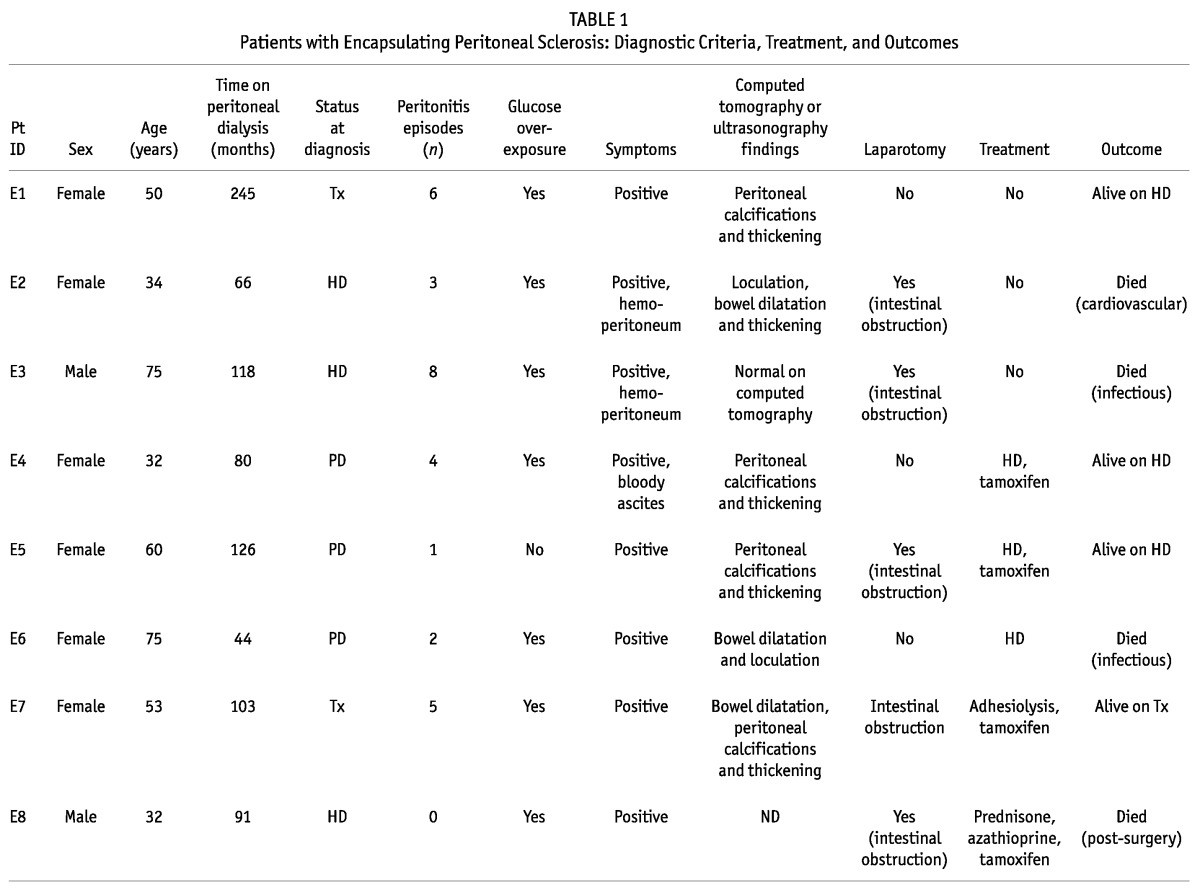
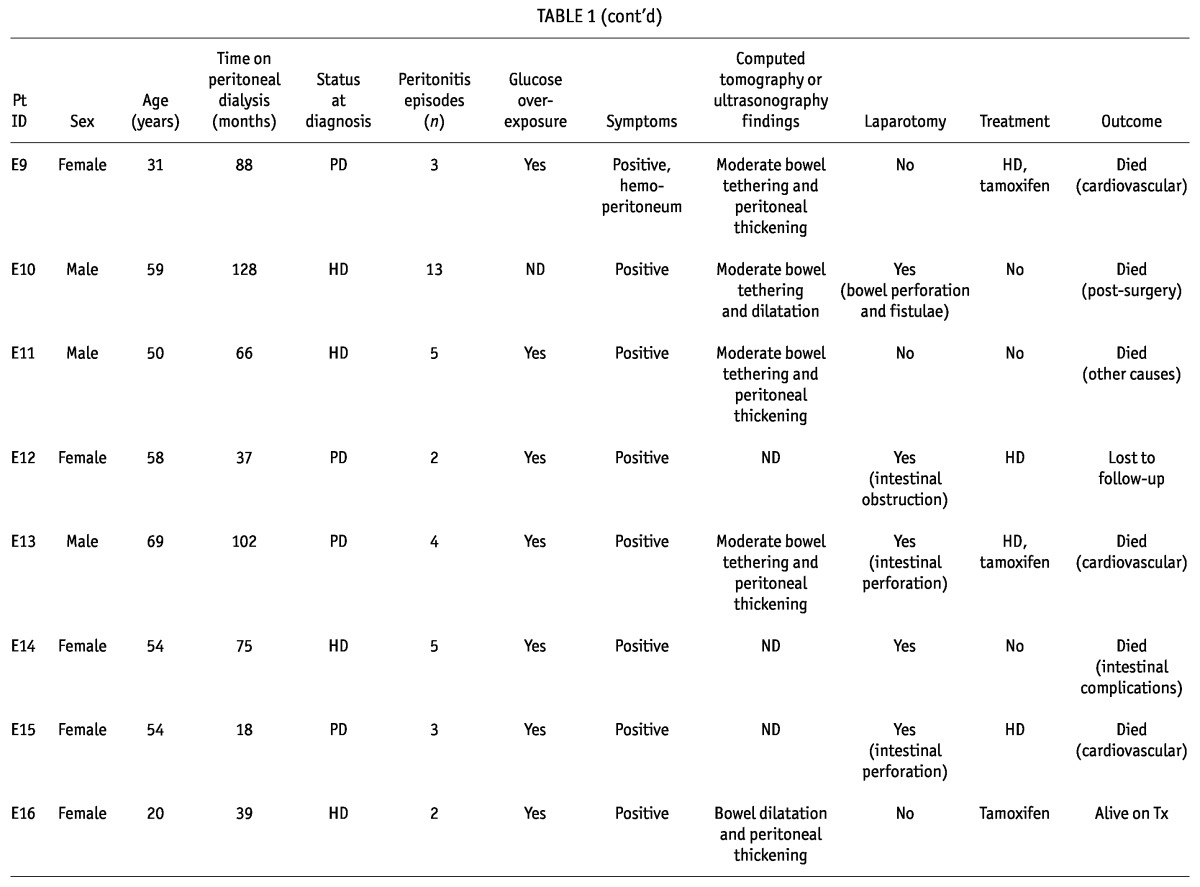
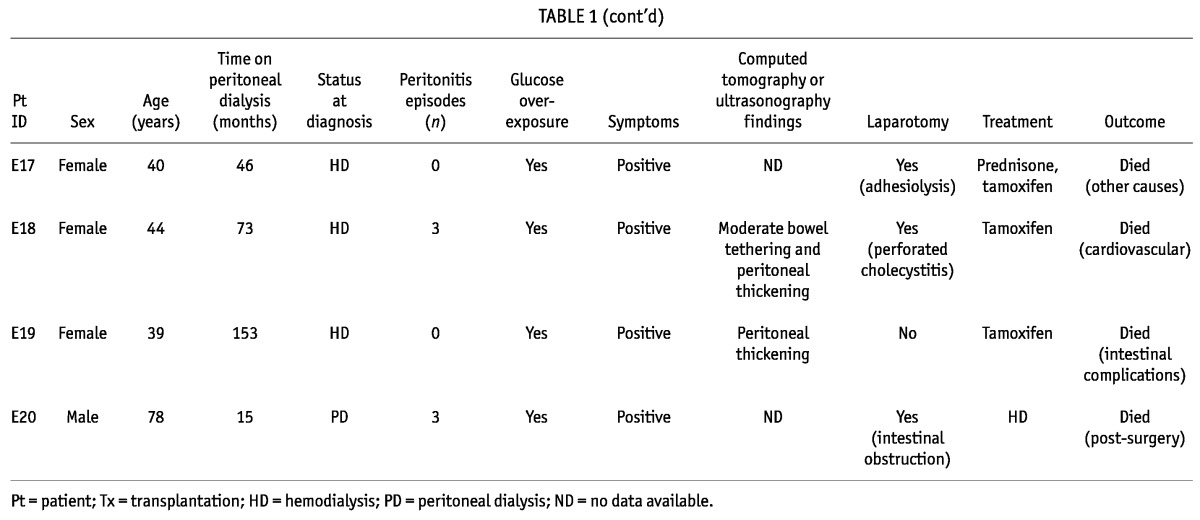
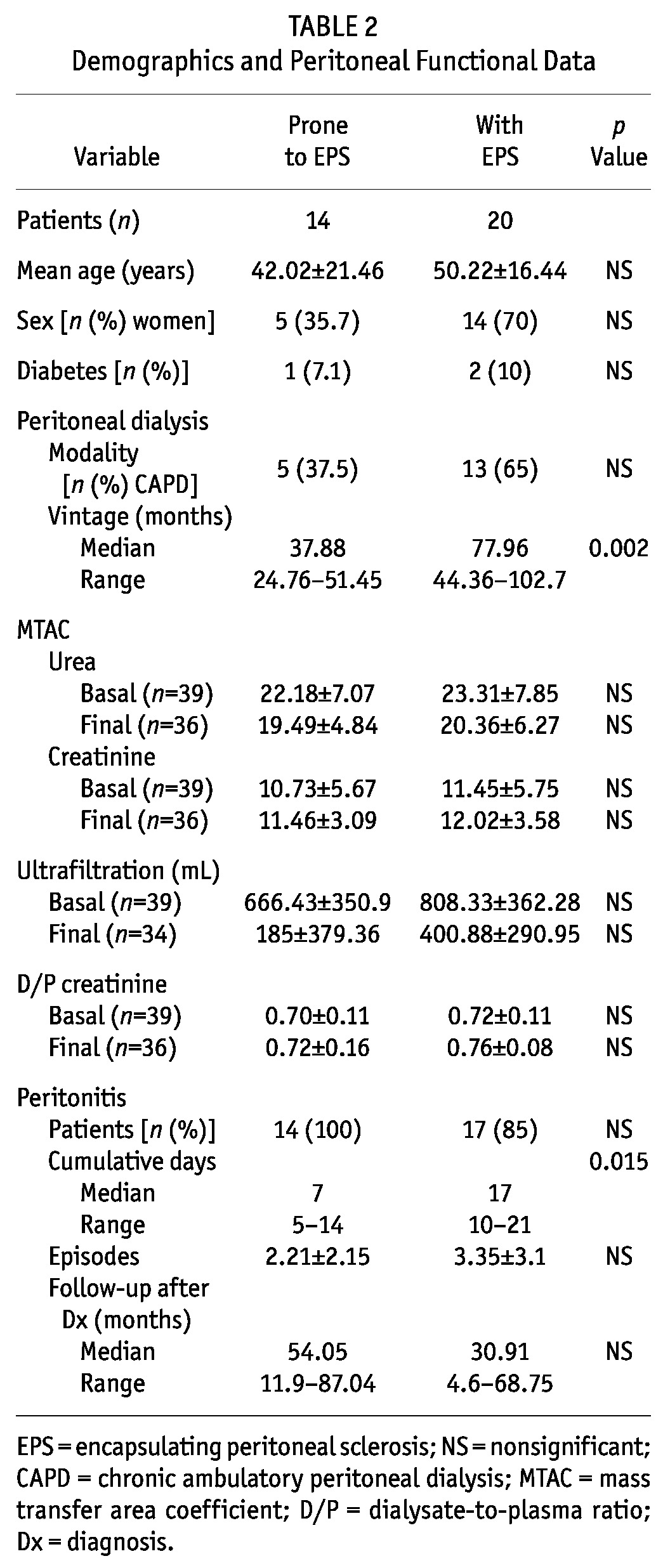
TABLE 2.
Demographics and Peritoneal Functional Data
All patients presented with clinical symptoms suggestive of EPS, and the diagnosis was confirmed in 12 patients (60%) by abnormalities on CT imaging; in 12 (60%), by laparotomy; in 1, by dynamic US; and in 2 (10%), by necropsy. At the time of diagnosis, 10 patients (50%) were already on HD, 8 (40%) remained on PD, and 2 (10%) had undergone transplantation.
In the peritoneal function studies, we observed that 9 patients (45%) had developed UFF, and 10 (50%), high transport status. Data from the baseline and the last available PET were compared, and UF was found to be significantly lower at the time of EPS diagnosis (808.3 ± 362.3 mL vs 400.9 ± 290.9 mL, p = 0.002). Immediately after the EPS diagnosis, 8 patients were switched to HD. Complications such as intestinal obstruction were also present in 7 patients (35%), and small-bowel perforation in 4 (20%), all of whom required surgery.
Tamoxifen (40 mg daily) was administered in 10 patients (50%); 10 (50%) received parenteral nutrition; 2 (10%) were administered prednisone (10 mg daily); and 1 also received azathioprine. Tamoxifen was given for a median of 12 months (range: 5.5 - 22 months). Adhesiolysis was performed in 2 patients (10%); only 1 remained alive during follow-up. After the diagnosis of EPS, patients were followed for a median of 30.91 months (range: 4.6 - 68.75 months), with 5 (25%) being alive at the time of writing, 14 (70%) having died, and 1 being lost to follow-up. Of the 14 deaths, 5 (25% of the EPS group) were related to EPS, 3 patients having died immediately after intra-abdominal surgery, and 2 because of intestinal complications.
EPS-prone Group
Of the 14 patients classified as EPS-prone [5 women (37.5%); mean age: 42.02 ± 21.46 years; Tables 2 and 3), 6 initiated PD in decade 2 of the study period, and 8, in decade 3.
TABLE 3.
Patients with Encapsulating Peritoneal Sclerosis: Peritoneal Dialysis Features, Radiologic Findings, Treatment, and Outcomes
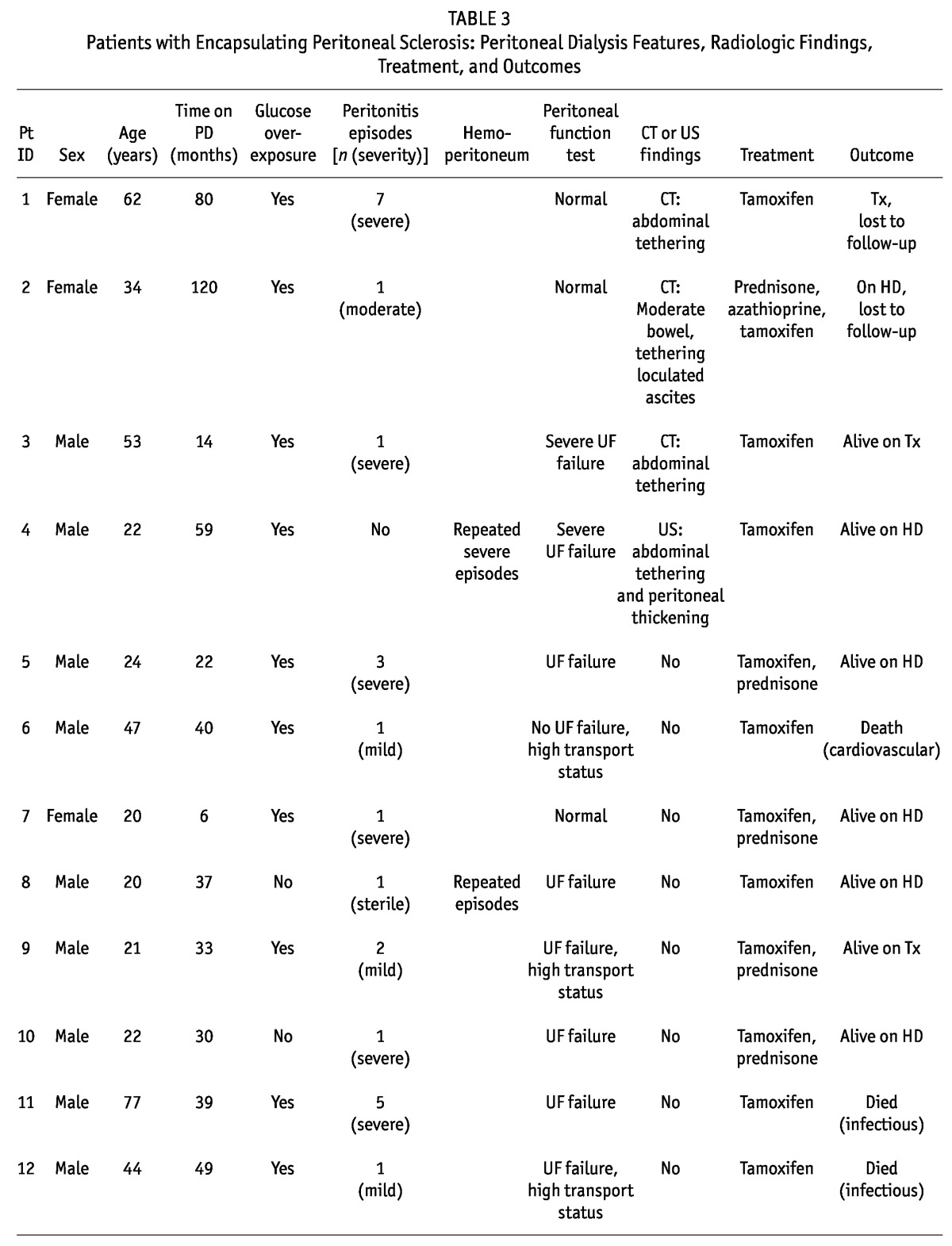
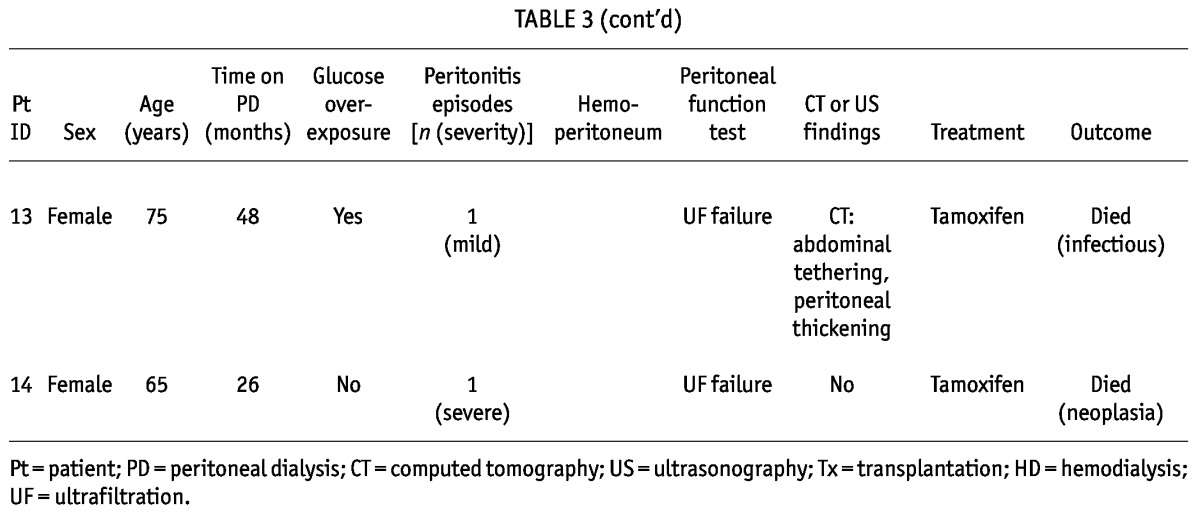
We identified at least 2 features of EPS in all these patients, with 71.4% having more than 2 features. Of the 14 patients, 11 (78.6%) presented with UFF, high transport status, or both; 4 (28.6%) showed abnormalities on CT or US; 11 (78.6%) had overexposure to glucose and glucose degradation products; 2 (14.3%) had experienced repeated and severe hemoperitoneum; 7 (50%) had experienced severe peritonitis episodes; and 8 (57.1%) had been on PD for more than 3 years. Median time on PD was 37.88 months (range: 24.76 - 51.4 months). All patients had experienced peritonitis (mean: 2.2 episodes; range: 1 - 13 episodes). Catheter removal was required in the 7 patients experiencing severe peritonitis, and only 2 patients returned to PD after the severe episode.
No patient presented symptoms suggestive of EPS, although 2 (14.3%) had an episode of small-bowel obstruction, and 1 (7.1%) had a small-bowel perforation. These patients also showed significantly lower UF from baseline and to final PET (p = 0.002), with no difference in other functional parameters.
After suspicions were raised that these patients were EPS-prone, 9 (64.3%) were switched to HD, but 5 (35.7%) remained on PD for a median of 11 months (range: 3 - 36 months). All received treatment with oral tamoxifen (20 mg every 12 hours) for a median of 12 months (range: 6.75 - 20.25 months), with 5 (35.7%) also receiving prednisone, and 1, azathioprine.
Patients were followed for 54.05 months (range: 11.9 - 87.04 months). During that time, 5 patients (35.7%) died, 7 (50%) remained alive, and 2 (14.3%) were lost to follow-up. No patient developed EPS during follow-up.
Comparison of EPS and EPS-prone Patients
The EPS and EPS-prone patients did not differ in baseline data (Table 2), although time on PD was significantly greater in patients with EPS (p = 0.002). The cumulative number of peritoneal inflammation days was also greater (p = 0.015) in the EPS group, but there were no differences in the number of peritonitis episodes. Survival was not different in the two groups.
Discussion
In the present study from a single center in Spain active since 1980, with 679 patients treated on PD, we have learned that the history of the most important PD complication, EPS, has developed partially depending on a clinical learning curve that may ultimately lead to successful management. Our initial EPS experiences subsequently led us to the identification of what we call an “EPS-prone” state. That identification started during our program’s second decade, and was fully implemented during the third decade, when we instituted pre-emptive or prophylactic treatment based on the use of tamoxifen. The lack of specific markers for this complex process requires the conjunction of a high index of suspicion based on the patient’s clinical context, together with proper interpretation of changes in peritoneal function at the appropriate time.
On the EPS Experience
The global incidence of EPS in our program is similar to that described in the literature (3.8% - 10.16%); 20 patients (2.9%) developed findings consistent with EPS. Our historical analysis led to the hypothesis that EPS is associated not only with long-term PD, but also with the decade in which PD took place (5.6% in the first decade, 3.9% in the second, and 0.3% in the third in our program)—those two factors being major determinants for the prevalence of this process. The effects of the new biocompatible solutions introduced during decade 3 are probably minimal. We believe it plausible that our intervention in the natural history of some EPS-prone patients might explain some of the difference in the EPS incidence in the second and third decades, although some of the differences might also be explained by shorter follow-up.
On the EPS-prone Experience
A novel aspect of the present study is our identification of EPS-prone patients. Although the natural history of EPS is not well known, and there are no clinical, radiologic, or biochemical tests for early diagnosis, the multifactorial nature of this disease—in which time on PD, exposure to glucose, young age, and cumulative or severe peritonitis episodes contribute to its development—offers an opportunity for intervention (4,8,14,17,18). A previously damaged peritoneal membrane experiencing minor injuries, peritonitis, hemoperitoneum, or surgery might also trigger EPS (34).
Patients defined as EPS-prone were found to be similar to EPS patients at PD commencement, but they were differentiated by a shorter time on PD and a lower cumulative number of peritoneal inflammation days even though they had no differences with respect to the number of peritonitis episodes. The EPS-prone group was followed for a median of 54.05 months (range 11.9 - 87.04 months) while remaining on PD or after transferring to HD or undergoing transplantation. During that period, no new EPS cases occurred in the group, although follow-up was shorter in some patients (including follow-up of less than 1 year in 2 patients because of death from non-EPS-related causes). We also found a significant decline in UF capacity relative to baseline data by the time of diagnosis (p = 0.002), with 9 patients (45%) reaching true UFF, and 10 (50%), fast small-solute transport. Although solute and fluid transport in PD have not been proposed in earlier studies to be useful for identifying patients at high EPS risk (9,35,36), and although neither UFF nor high transport status are diagnostic for EPS, there is some evidence that continuation of PD with UFF increases the risk of EPS development (35). The jump from UFF toward EPS might be triggered by continuation of PD therapy and exposure of the membrane to minor but important injuries. Even small amounts of high glucose exposure, required because of a lower UF capacity, might be determinant per se. Our group hypothesized that mesothelial-to-mesenchymal transition of mesothelial cells and sclerotic peritonitis syndromes, including EPS, might be part of the same process, with mesothelial-to-mesenchymal transition being one of the initial mechanisms of peritoneal fibrosis, whose perpetuation might lead to EPS (37).
On Interventions in EPS and EPS-prone States
No randomized controlled trials are available to guide EPS treatment, although case series have suggested that steroids and tamoxifen can help (24-26,38). Our 14 EPS-prone patients were closely followed and treated with tamoxifen. Some patients also initially received steroids, according to the clinician’s decision based on the situation (post-inflammatory). In our center, 50% of the patients diagnosed with EPS also received tamoxifen, and although that agent is associated with an increased risk of thromboembolic events and endometrial cancer, it was well-tolerated. Only 1 patient developed mild thrombocytopenia, which responded to dose adjustment. Tamoxifen is a synthetic estrogen that is effective in fibrotic diseases such as retroperitoneal fibrosis because of its effect on transforming growth factor β (39,40). Our experimental studies in a mouse model of PD recently demonstrated that tamoxifen blocks mesothelial-to-mesenchymal transition induced by transforming growth factor β, significantly reduces peritoneal thickness and angiogenesis, and improves peritoneal function (41).
The positive effect of tamoxifen in the treatment of EPS patients was previously confirmed by other small case series (24-26). A Dutch EPS study showed a decline in mortality among patients with EPS after treatment with tamoxifen (24). Our global experience in the empiric use of tamoxifen in 14 patients was reported in 2003. Comparing 14 EPS patients with a non-treated control group, significantly lower mortality (22% vs 71%, p = 0.03) was demonstrated with tamoxifen use (42). After that experience, we systematically identified patients at high risk of developing EPS, and we started pre-emptively administering tamoxifen for at least 1 year. It is plausible that, in all EPS-prone patients, the antifibrotic effects of tamoxifen, close monitoring, and early transfer to HD when indicated, might stop the pathophysiologic cascade of intraperitoneal events that ultimately lead to EPS.
Our findings in the present study should be considered in the context of certain limitations. Most important are the retrospective nature of the analysis and the lack of complete complementary—and particularly radiologic—imaging in all patients. A control group with whom to contrast EPS-prone treated patients is also lacking. Given that the observation period in our study was 31 years, the severity of EPS, clinical awareness, and medical and surgical management might all have changed over time. Although a decline in the EPS rate because of prophylactic tamoxifen use in high-risk EPS patients might represent a real phenomenon, the small number of patients included in our series and the short follow-up might also explain our results. Because symptomatic EPS can develop years or decades after PD cessation, it is difficult to know whether EPS-prone patients might still develop symptoms. Caution should be used when interpreting our results, and additional case-control studies are necessary to assess the role of tamoxifen in preventing EPS. In addition, markers have to be developed that will allow clinicians to detect EPS early. In this field, peritoneal effluent markers and genomic, proteomic, and metabolomic markers are currently being tested, with promising results. Meanwhile, only increased awareness will allow for prompt diagnosis (19,41-45). Use of the new biocompatible solutions might contribute to preservation of peritoneal membrane function and a lesser future incidence of EPS (34). Results from ongoing multicenter prospective observational studies—the NEXT-PD study is investigating this hypothesis—are still pending (45).
Conclusions
Being a serious, life-threatening complication of PD, EPS requires a high degree of suspicion to allow for prompt diagnosis and intervention. Treatment in specialized referral centers is recommended. The identification of patients who are EPS-prone could help in avoiding progression to EPS. Tamoxifen is potentially useful for that approach. Because EPS prevention in the PD population is clearly important, additional well-planned studies are needed.
Disclosures
The authors declare that no financial conflict of interest exists.
Acknowledgments
This study was supported by the Instituto Reina Sofia de Investigación Nefrológica (IRSIN), FIS PI 09/00641, and REDinREN (RETICS 12/0021).
References
- 1. Gandhi VC, Humayun HM, Ing TS, Daugirdas JT, Jablokow VR, Iwatsuki S, et al. Sclerotic thickening of the peritoneal membrane in maintenance peritoneal dialysis patients. Arch Intern Med 1980; 140:1201–3 [PubMed] [Google Scholar]
- 2. Nakamoto H. Encapsulating peritoneal sclerosis—a clinician’s approach to diagnosis and medical treatment. Perit Dial Int 2005; 25(Suppl 4):S30–8 [PubMed] [Google Scholar]
- 3. Korte M, Sampimon D, Betjes M, Krediet R. Encapsulating peritoneal sclerosis: the state of affairs. Nat Rev Nephrol 2011; 7:528–38 [DOI] [PubMed] [Google Scholar]
- 4. Habib SM, Betjes MG, Fieren MW, Boeschoten EW, Abrahams AC, Boer WH, et al. Management of encapsulating peritoneal sclerosis: a guideline on optimal and uniform treatment. Neth J Med 2011; 69:500–7 [PubMed] [Google Scholar]
- 5. Kawaguchi Y, Kawanishi H, Mujais S, Topley N, Oreopoulos DG. Encapsulating peritoneal sclerosis: definition, etiology, diagnosis, and treatment. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Perit Dial Int 2000; 20(Suppl 4):S43–55 [PubMed] [Google Scholar]
- 6. Korte MR, Habib SM, Lingsma H, Weimar W, Betjes MG. Posttransplantation encapsulating peritoneal sclerosis contributes significantly to mortality after kidney transplantation. Am J Transplant 2011; 11:599–605 [DOI] [PubMed] [Google Scholar]
- 7. Fieren MW, Betjes MG, Korte MR, Boer WH. Posttransplant encapsulating peritoneal sclerosis: a worrying new trend? Perit Dial Int 2007; 27:619–24 [PubMed] [Google Scholar]
- 8. Kawanishi H, Kawaguchi Y, Fukui H, Hara S, Imada A, Kubo H, et al. Encapsulating peritoneal sclerosis in Japan: a prospective, controlled, multicenter study. Am J Kidney Dis 2004; 44:729–37 [PubMed] [Google Scholar]
- 9. Balasubramaniam G, Brown EA, Davenport A, Cairns H, Cooper B, Fan SL, et al. The Pan-Thames EPS study: treatment and outcomes of encapsulating peritoneal sclerosis. Nephrol Dial Transplant 2009; 24:3209–15 [DOI] [PubMed] [Google Scholar]
- 10. Brown MC, Simpson K, Kerssens JJ, Mactier RA. on behalf of the Scottish Renal Registry. Encapsulating peritoneal sclerosis in the new millennium: a national cohort study. Clin J Am Soc Nephrol 2009; 4:1222–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee HY, Kim BS, Choi HY, Park HC, Kang SW, Choi KH, et al. Sclerosing encapsulating peritonitis as a complication of long-term continuous ambulatory peritoneal dialysis in Korea. Nephrology (Carlton) 2003; 8(Suppl):S33–9 [DOI] [PubMed] [Google Scholar]
- 12. Nomoto Y, Kawaguchi Y, Kubo H, Hirano H, Sakai S, Kurokawa K. Sclerosing encapsulating peritonitis in patients undergoing continuous ambulatory peritoneal dialysis: a report of the Japanese Sclerosing Encapsulating Peritonitis Study Group. Am J Kidney Dis 1996; 20:420–7 [DOI] [PubMed] [Google Scholar]
- 13. Summers AM, Clancy MJ, Syed F, Harwood N, Brenchley PE, Augustine T, et al. Single-center experience of encapsulating peritoneal sclerosis in patients on peritoneal dialysis for end-stage renal failure. Kidney Int 2005; 68:2381–8 [DOI] [PubMed] [Google Scholar]
- 14. Johnson DW, Cho Y, Livingston BE, Hawley CM, McDonald SP, Brown FG, et al. Encapsulating peritoneal sclerosis: incidence, predictors, and outcomes. Kidney Int 2010; 77:904–12 [DOI] [PubMed] [Google Scholar]
- 15. Herrero JC, Molina A, Lentisco C, García C, Ortiz M, Mon C, et al. Sclerosing encapsulating peritonitis: a latent threat. Changes of posture in surgical treatment [Spanish]. Nefrologia 2007; 27:729–36 [PubMed] [Google Scholar]
- 16. Nomoto Y, Kawaguchi Y, Kubo H, Hirano H, Sakai S, Kurokawa K. Sclerosing encapsulating peritonitis in patients undergoing continuous ambulatory peritoneal dialysis: a report of the Japanese Sclerosing Encapsulating Peritonitis Study Group. Am J Kidney Dis 1996; 28:420–7 [DOI] [PubMed] [Google Scholar]
- 17. Gayomali C, Hussein U, Cameron SF, Protopapas Z, Finkelstein FO. Incidence of encapsulating peritoneal sclerosis: a single-center experience with long-term peritoneal dialysis in the United States. Perit Dial Int 2011; 31:279–86 [DOI] [PubMed] [Google Scholar]
- 18. Korte MR, Sampimon DE, Lingsma HF, Fieren MW, Looman CW, Zietse R, et al. on behalf of the Dutch Multicenter EPS Study. Risk factors associated with encapsulating peritoneal sclerosis in Dutch EPS study. Perit Dial Int 2011; 31:269–78 [DOI] [PubMed] [Google Scholar]
- 19. Sampimon DE, Korte MR, Barreto DL, Vlijm A, de Waart R, Struijk DG, et al. Early diagnostic markers for encapsulating peritoneal sclerosis: a case-control study. Perit Dial Int 2010; 30:163–9 [DOI] [PubMed] [Google Scholar]
- 20. Brown EA, Van Biesen W, Finkelstein FO, Hurst H, Johnson DW, Kawanishi H, et al. Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis: position paper for ISPD. Perit Dial Int 2009; 29:595–600 [PubMed] [Google Scholar]
- 21. Imai H, Nakamoto H, Fukushima R, Yamanouchi Y, Ishida Y, Suzuki H. Glucocorticoid protects against the development of encapsulating peritoneal sclerosis on peritoneal dialysis. Adv Perit Dial 2002; 18:124–30 [PubMed] [Google Scholar]
- 22. Martins LS, Rodrigues AS, Cabrita AN, Guimaraes S. Sclerosing encapsulating peritonitis: a case successfully treated with immunosuppression. Perit Dial Int 1999; 19:478–81 [PubMed] [Google Scholar]
- 23. Mori Y, Matsuo S, Sutoh H, Toriyama T, Kawahara H, Hotta N. A case of a dialysis patient with sclerosing peritonitis successfully treated with corticosteroid therapy alone. Am J Kidney Dis 1997; 30:275–8 [DOI] [PubMed] [Google Scholar]
- 24. Korte MR, Fieren MW, Sampimon DE, Lingsma HF, Weimar W, Betjes MG. on behalf of the investigators of the Dutch Multicentre EPS Study. Tamoxifen is associated with lower mortality of encapsulating peritoneal sclerosis: results of the Dutch Multicentre EPS Study. Nephrol Dial Transplant 2011; 26:691–7 [DOI] [PubMed] [Google Scholar]
- 25. Evrenkaya TR, Atasoyu EM, Unver S, Basekim C, Baloglu H, Tulbek MY. Corticosteroid and tamoxifen therapy in sclerosing encapsulating peritonitis in a patient on continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant 2004; 19:2423–4 [DOI] [PubMed] [Google Scholar]
- 26. Thirunavukarasu T, Saxena R, Anijeet H, Pai P, Wong CF. Encapsulating peritoneal sclerosis presenting with recurrent ascites and tamoxifen: case reports and review of the literature. Ren Fail 2007; 29:775–6 [DOI] [PubMed] [Google Scholar]
- 27. Kawanishi H, Watanabe H, Moriishi M, Tsuchiya S. Successful surgical management of encapsulating peritoneal sclerosis. Perit Dial Int 2005; 25(Suppl 4):S39–47 [PubMed] [Google Scholar]
- 28. Kawanishi H, Moriishi M, Tsuchiya S. Experience of 100 surgical cases of encapsulating peritoneal sclerosis: investigation of recurrent cases after surgery. Adv Perit Dial 2006; 22:60–4 [PubMed] [Google Scholar]
- 29. Kawanishi H, Ide K, Yamashita M, Shimomura M, Moriishi M, Tsuchiya S, et al. Surgical techniques for prevention of recurrence after total enterolysis in encapsulating peritoneal sclerosis. Adv Perit Dial 2008; 24:51–5 [PubMed] [Google Scholar]
- 30. Kawanishi H, Shintaku S, Moriishi M, Dohi K, Tsuchiya S. Seventeen years’ experience of surgical options for encapsulating peritoneal sclerosis. Adv Perit Dial 2011; 27:53–8 [PubMed] [Google Scholar]
- 31. Selgas R, Fernandez-Reyes MJ, Bosque E, Bajo MA, Borrego F, Jimenez C, et al. Functional longevity of the human peritoneum: how long is continuous peritoneal dialysis possible? Results of a prospective medium long-term study. Am J Kidney Dis 1994; 23:64–73 [DOI] [PubMed] [Google Scholar]
- 32. Raderson DH, Farrel P. Mass transfer properties of human peritoneum. ASAIO J 1980; 3:140–6 [Google Scholar]
- 33. Krediet R. The peritoneal membrane in chronic peritoneal dialysis patients. Kidney Int 1999; 55:341–56 [DOI] [PubMed] [Google Scholar]
- 34. Siato A. Peritoneal dialysis in Japan: the issue of encapsulating peritoneal sclerosis and future challenges. Perit Dial Int 2005; 25(Suppl 4):S77–82 [PubMed] [Google Scholar]
- 35. Sampimon DE, Coester AM, Struijk DG, Krediet RT. The time course of peritoneal transport parameters in peritoneal dialysis patients who develop encapsulating peritoneal sclerosis. Nephrol Dial Transplant 2011; 26:291–8 [DOI] [PubMed] [Google Scholar]
- 36. Lambie ML, John B, Mushahar L, Huckvale C, Davies SJ. The peritoneal osmotic conductance is low well before the diagnosis of encapsulating peritoneal sclerosis is made. Kidney Int 2010; 78:611–18 [DOI] [PubMed] [Google Scholar]
- 37. Loureiro J, Gónzalez-Mateo G, Jimenez-Heffernan J, Selgas R, López-Cabrera M, Aguilera Peralta A. Are the mesothelial-to-mesenchymal transition, sclerotic peritonitis syndromes, and encapsulating peritoneal sclerosis part of the same process? Int J Nephrol 2013; 2013:263285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guest S. Tamoxifen therapy for encapsulating peritoneal sclerosis: mechanism of action and update on clinical experiences. Perit Dial Int 2009; 29:252–5 [PubMed] [Google Scholar]
- 39. Huang JW, Yen CJ, Wu HY, Chiang CK, Cheng HT, Lien YC, et al. Tamoxifen downregulates connective tissue growth factor to ameliorate peritoneal fibrosis. Blood Purif 2011; 31:252–8 [DOI] [PubMed] [Google Scholar]
- 40. Selgas R, Aguilera A, Bajo A, Loureiro J, Del Peso G, Aroeira L, et al. Effects of tamoxifen on epithelial-to-mesenchymal transition, fibrosis and angiogenesis of mesothelial cells [Abstract]. J Am Soc Nephrol 2006; (Suppl 3):S19 [Google Scholar]
- 41. Loureiro J, Sandoval P, Del Peso G, Gónzalez-Mateo G, Fernández-Millara V, Santamaria B, et al. Tamoxifen ameliorates peritoneal membrane damage by blocking mesothelial to mesenchymal transition in peritoneal dialysis. PLoS One 2013; 8:e1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. del Peso G, Bajo MA, Gil F, Aguilera A, Ros S, Costero O, et al. Clinical experience with tamoxifen in peritoneal fibrosing syndromes. Adv Perit Dial 2003; 19:32–5 [PubMed] [Google Scholar]
- 43. Summers AM, Abrahams AC, Alscher MD, Betjes M, Boeschoten EW, Braun N, et al. A collaborative approach to understanding EPS: the European perspective. Perit Dial Int 2011; 31:245–8 [DOI] [PubMed] [Google Scholar]
- 44. Dunn WB, Summers A, Brown M, Goodacre R, Lambie M, Johnson T, et al. Proof-of-principle study to detect metabolic changes in peritoneal dialysis effluent in patients who develop encapsulating peritoneal sclerosis. Nephrol Dial Transplant 2012; 27:2502–10 [DOI] [PubMed] [Google Scholar]
- 45. Kawanishi H, Nakayama M, Miyazaki M, Honda K, Tomo T, Kasai K, et al. Prospective multicenter observational study of encapsulating peritoneal sclerosis with neutral dialysis solution—the NEXT-PD study. Adv Perit Dial 2010; 26:71–4 [PubMed] [Google Scholar]


