Abstract
♦ Background: Peritonitis rate has been reported to be associated with technique failure and overall mortality in previous literatures. However, information on the impact of the timing of the first peritonitis episode on continuous ambulatory peritoneal dialysis (CAPD) patients is sparse. The aim of this research is to study the influence of time to first peritonitis on clinical outcomes, including technique failure, patient mortality and dropout from peritoneal dialysis (PD).
♦ Methods: A retrospective observational cohort study was conducted over 10 years at a single PD unit in Taiwan. A total of 124 patients on CAPD with at least one peritonitis episode comprised the study subjects, which were dichotomized by the median of time to first peritonitis into either early peritonitis patients or late peritonitis patients. Cox proportional hazard model was used to analyze the correlation of the timing of first peritonitis with clinical outcomes.
♦ Results: Early peritonitis patients were older, more diabetic and had lower serum levels of creatinine than the late peritonitis patients. Early peritonitis patients were associated with worse technique survival, patient survival and stay on PD than late peritonitis patients, as indicated by Kaplan-Meier analysis (log-rank test, p = 0.04, p < 0.001, p < 0.001, respectively). In the multivariate Cox regression model, early peritonitis was still a significant predictor for technique failure (hazard ratio (HR), 0.54; 95% confidence interval (CI), 0.30 - 0.98), patient mortality (HR, 0.34; 95% CI, 0.13 - 0.92) and dropout from PD (HR, 0.50; 95% CI, 0.30 - 0.82). In continuous analyses, a 1-month increase in the time to the first peritonitis episode was associated with a 2% decreased risk of technique failure (HR, 0.98; 95% CI, 0.97 - 0.99), a 3% decreased risk of patient mortality (HR, 0.97; 95% CI, 0.95 - 0.99), and a 2% decreased risk of dropout from PD (HR, 98%; 95% CI, 0.97 - 0.99). Peritonitis rate was inversely correlated with time to first peritonitis according to the Spearman analysis (r = -0.64, p < 0.001).
♦ Conclusions: Time to first peritonitis is significantly correlated with clinical outcomes of peritonitis patients with early peritonitis patients having poor prognosis. Patients with shorter time to first peritonitis were prone to having a higher peritonitis rate.
Keywords: Continuous ambulatory peritoneal dialysis (CAPD), early peritonitis, patient survival, peritonitis rate, technique failure, time to first peritonitis
Continuous ambulatory peritoneal dialysis (CAPD) was first described as a renal replacement therapy in the late 1970s (1). Peritonitis is the most common serious complication of peritoneal dialysis (PD) and the leading cause of technique failure necessitating a switch to hemodialysis (HD), accounting for 42.6% of cases in Scotland, and 41.7% in London (2,3). Fortunately, the mortality rate directly associated with peritonitis is below 4 - 6 % (4,5) and greatly depends on the causative organisms, with the highest mortality rate for fungal peritonitis, followed by those due to gram-negative organisms in general (4-7). Technical improvements in PD systems, including double-bag system and Y-set, and better understanding of risk factors predisposing to peritonitis episodes have led to a significant decrease in peritonitis rates (8).
Peritonitis rates vary from center to center and are largely influenced by factors such as patient selection, quality of PD training, nursing experience and the use of nasal mupirocin (9-11). Many other factors, on the other hand, have been inconsistently linked with PD peritonitis, such as older age, female sex, race, nasal carriage of Staphylococcus aureus, diabetes mellitus, use of standard lactate dialysate compared with novel biocompatible solution, hypoalbuminemia, low residual renal function and transfer from HD (2,12-18). Nonetheless, knowledge of risk factors for peritonitis with the subsequent implementation of preventive strategies may be helpful in reducing peritonitis occurrence. Guidelines from the International Society for Peritoneal Dialysis (ISPD) suggest that the peritonitis rate should not exceed 1 episode in 18 patient-months (19).
A high peritonitis rate is associated with inferior technique and patient survival. However, few studies have extensively examined whether the timing of the first peritonitis episode has an impact on technique survival or patient survival of PD patients. We thus conducted a retrospective study of incident CAPD patients over 10 years at a single medical center in Taiwan, with the aim of determining the influence of the timing of the first peritonitis episode on clinical outcomes among peritonitis patients.
Patients and Methods
This was a retrospective observational cohort study. Incident CAPD patients aged 18 years or older at Changhua Christian Hospital in Taiwan between January 1, 2001, and December 31, 2010, were screened for study eligibility. A total of 391 incident CAPD patients with a mean age of 55.8 ± 16 years and 44.2% being male commenced dialysis therapy during the period. The overall peritonitis rate was 0.196 episodes per patient-year over a total observation period of 969.5 patient-years. Of the 391 CAPD patients, 124 patients developing at least one episode of peritonitis were enrolled in our study. The median time from CAPD commencement to the first peritonitis onset was 20.28 months for those patients. Based on the median time to first peritonitis, 124 patients were dichotomized into an early peritonitis group (EP) (< 20.28 months, n = 62) and a late peritonitis group (LP) (> 20.28 months, n = 62). All patients were followed till death, renal transplant and switch to HD or the end of the study on December 31, 2010. The data collected included demographic data, body mass index, educational status, the cause of end-stage renal disease (ESRD), relevant biochemical data, comorbid conditions at the start of dialysis therapy (coronary artery disease, cerebrovascular disease, peripheral vascular disease, diabetes mellitus, and hypertension), relevant PD related parameters, such as membrane transport status, the clearance of the peritoneum and kidneys, as well as microbiologic characteristics of peritonitis episodes. Cardiovascular comorbidity was defined as a history of coronary artery disease, cerebrovascular or peripheral vascular disease.
The outcomes measured in the study were all-cause mortality, technique failure, and dropout from CAPD. Patient survival was the probability of patients surviving on PD, while only death was considered an end-point event; other reasons for dropouts, such as transfer to HD, transplantation, or renal function recovery, were the censored observations. Technique failure was defined as permanent transfer to HD due to inadequate dialysis, peritonitis, ultrafiltration failure, exit-site infection, and tunnel infection, mechanical or operational problems; transplantation, death and recovery of renal function were the censored observations. In the “stay on PD” (the combined endpoint of patient survival and technique survival) analysis, death on PD or technique failure was the event of interest; transplantation was a censored observation. The causes of death were categorized as cardiovascular, malignancy, neurological, infection/sepsis, gastrointestinal bleeding, intracranial hemorrhage, and others. Patients were classified as peritonitis-related deaths if they met the following criteria: (1) death from sepsis secondary to peritonitis, (2) death within 14 days of peritonitis onset, or (3) death during a hospitalization due to peritonitis.
Statistical Analysis
Results were expressed as means and standard deviation (SD) for continuous variables. Categorical variables were expressed as frequency (n) and percentage (%). Gender, age group, icodextrin prescription, comorbidity diseases, creatinine D/P 4-hour, follow-up outcomes and microbiologic characteristics between the EP and LP groups were analyzed by chi-square test (or Fisher’s exact test). Student t-test or the Wilcoxon rank sum test was used to evaluate age, body mass index (BMI), biochemical measurements and peritoneal equilibration test (PET) between the two groups. The Kaplan-Meier survival curve and log-rank statistic were conducted for the analysis of “stay on PD”, patient survival, and technique survival. The analyzed variables included time to first peritonitis, gender, age, BMI, icodextrin use, diabetes mellitus, hypertension, cardiovascular disease, serum albumin, Ca × P, total Kt/V urea, residual glomerular filtration rate (GFR) and dialysate to plasma ratio of creatinine (D/P creatinine). Significant variables from a univariate analysis were incorporated into a multivariate Cox regression model to determine the most significant predictors of clinical outcomes. To maximize statistical power to examine the relationship between time to first peritonitis and outcomes of interest, continuous variable analyses were conducted with hazard ratios presented per 1-month increase in the time to first peritonitis. A two-tailed p value less than 0.05 was considered statistically significant. All statistical analyses were conducted using the statistical package for Windows, SAS 9.2 (SAS Institute Inc, Cary, NC, USA) and SPSS 16.0 (SPSS, Chicago, IL, USA).
Results
Population and Microbiologic Characteristics
Table 1 shows the baseline characteristics at the commencement of CAPD. A total of 124 patients (EP, n = 62; LP, n = 62) were analyzed in our study with an average age of 56.9 years (male, 41.3%). No patient in the EP group had the first peritonitis episode within the first 3 months after commencing CAPD. There were 34 patients (54.8%) who had the first peritonitis episode within the first 12 months. Most of them had low educational level (illiteracy or junior high school, 53.2%), 33.1% had icodextrin prescription and 86.3% had hypertension. Chronic glomerulonephritis, diabetes mellitus and hypertension accounted for the three most common causes of ESRD either in EP or in LP patients.
TABLE 1.
Demographic, Clinical and Biochemical Data at Baseline
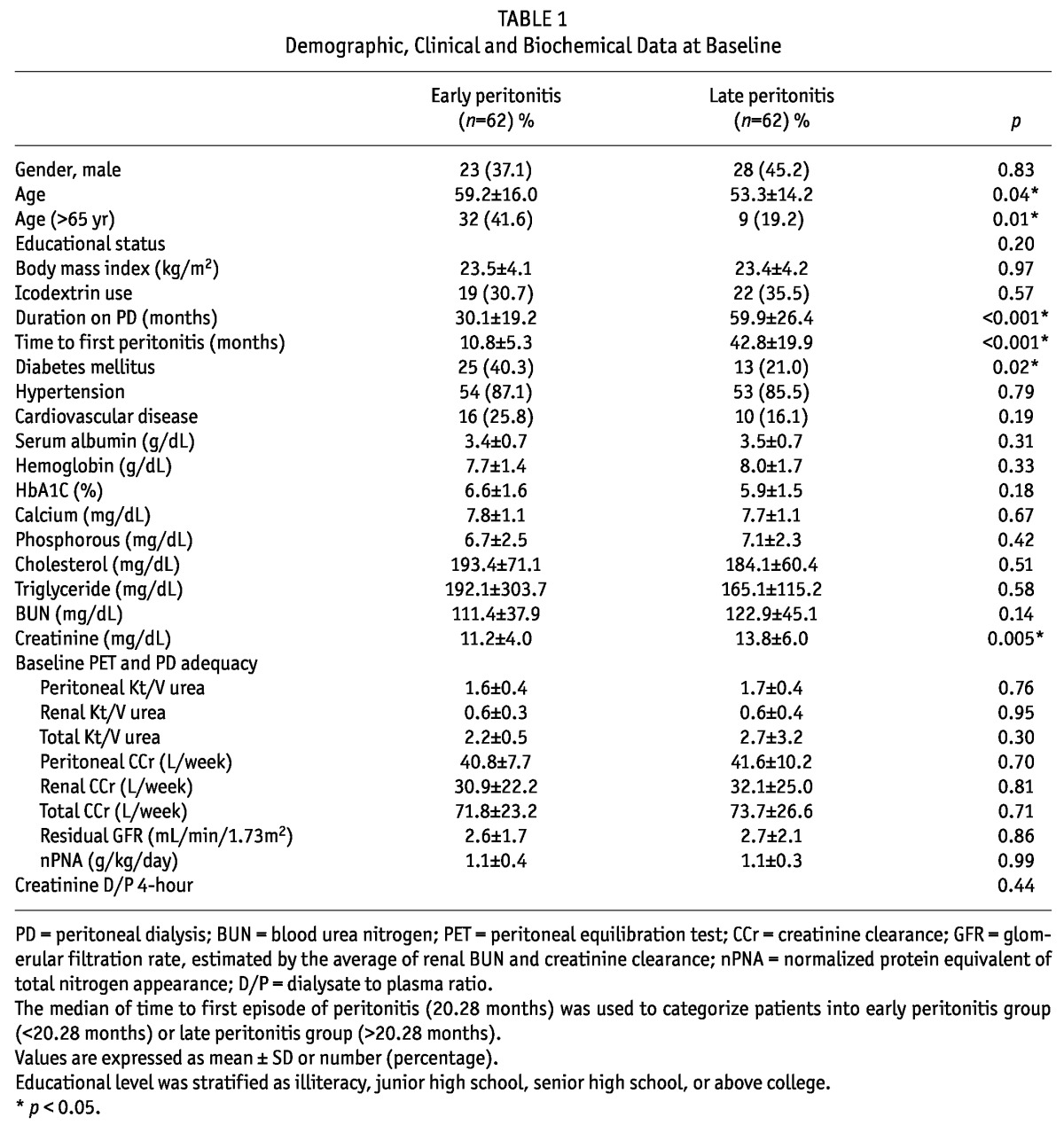
The EP patients were more likely to be older in age and have diabetes, as well as lower serum levels of creatinine. There was no significant difference in gender, educational level, BMI, icodextrin prescription, hypertension, cardiovascular disease, serum level of albumin, hemoglobin, HbA1c, calcium, phosphorus, cholesterol, triglyceride, blood urea nitrogen (BUN) and PD-related parameters between the EP and LP groups. At the end of the observation period, 39 patients (31.5%) were still on CAPD, 27 (21.8%) died, 53 (42.7%) switched to HD, and 5 (4%) received renal transplantation. Furthermore, the EP group had more death events and fewer switches to HD than the LP group (29% vs 14.5%, p = 0.05; 32.3% vs 53.2%, p = 0.02, respectively). No significant differences were noted between the EP group and LP group in terms of continuing PD and undergoing renal transplantation (32.3% vs 30.7%, p = 0.85; 6.5% vs 1.6%, p = 0.36, respectively).
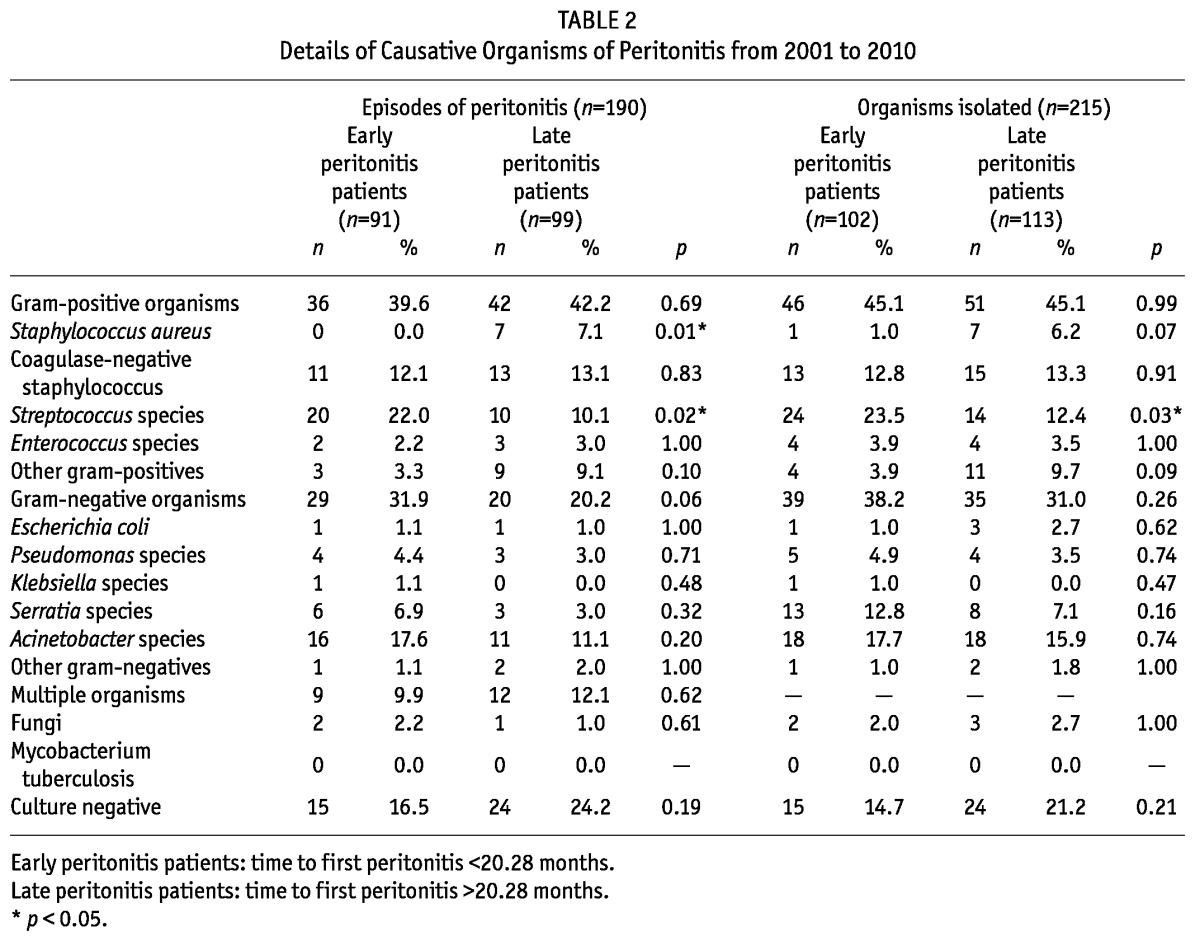
There were 190 episodes of peritonitis with 215 organisms isolated during the observational period. Table 2 shows the microbiologic spectrum for early peritonitis and late peritonitis patients. Streptococcus species were the more common organisms isolated in the EP group compared to those in the LP group (p = 0.03). Moreover, 49 episodes (25.8%) were due to gram-negative organisms, 78 episodes (41.1%) due to gram-positive organisms and 39 episodes (20.4%) culture-negative. Gram-positive organisms were the most common isolates, comprising 45.1%, whereas gram-negative organisms comprised 34.4% of the organisms with Escherichia coli being the most common pathogen (16.7%). Fungal organisms comprised 2.3% and no mycobacterium species were isolated. Finally, 39 episodes of peritonitis remained culture-negative.
TABLE 2.
Details of Causative Organisms of Peritonitis from 2001 to 2010
The Influence of Time to First Peritonitis on Technique Survival, Patient Survival and “Stay on PD”
Technique survival: Figure 1A describes technique survival using the Kaplan-Meier method. LP patients had longer technique survival than EP patients (log-rank test, p = 0.04). In a univariate Cox regression analysis, the LP group had a 45% reduction in technique failure compared with the EP group (HR 0.55; 95% CI 0.31 - 0.96, p = 0.04; Table 3). After controlling for icodextrin prescription, there was a 46% reduction in technique failure in the LP group as compared with the EP group (p = 0.04; Table 3). Of the 53 patients switching to HD (20 in the EP group, 33 in the LP group), peritonitis (13 in the EP group, 27 in the LP group) and ultrafiltration failure (3 in the EP group, 2 in the LP group) were the two most common causes (65% vs 81.8%, p = 0.2; 15% vs 6.1%, p = 0.35, respectively). The other causes of technique failure included dialysis inadequacy (2 in the EP group), operational or mechanical problems (2 in the EP group, 4 in the LP group).
Figure 1 —
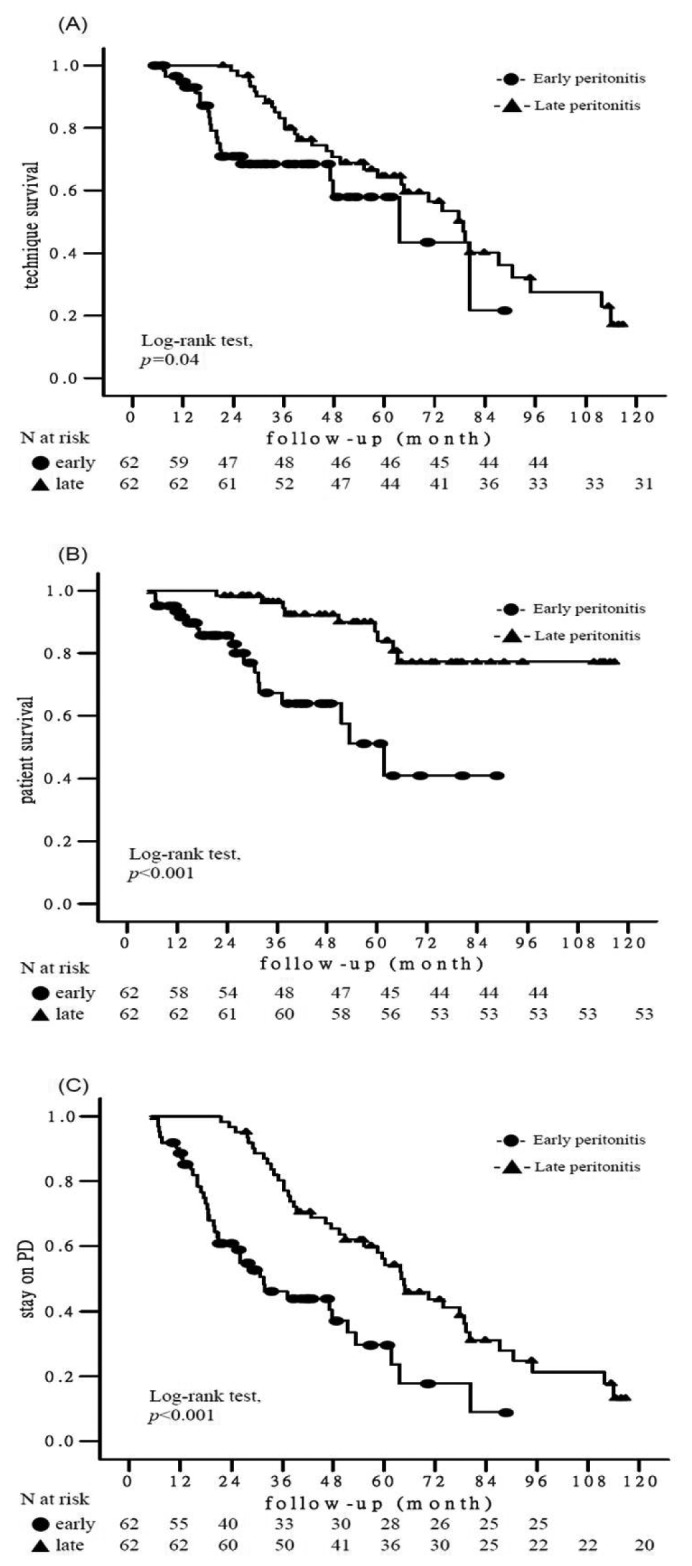
Kaplan-Meier survival analysis of clinical outcomes according to the timing of the first peritonitis episode. (A) technique survival (p = 0.04); (B) patient survival (p < 0.001); (C) stay on PD (p < 0.001). Stay on PD is the combined end point of technique survival and patient survival. PD = peritoneal dialysis.
TABLE 3.
Cox Proportional Hazard Model for Clinical Outcomes
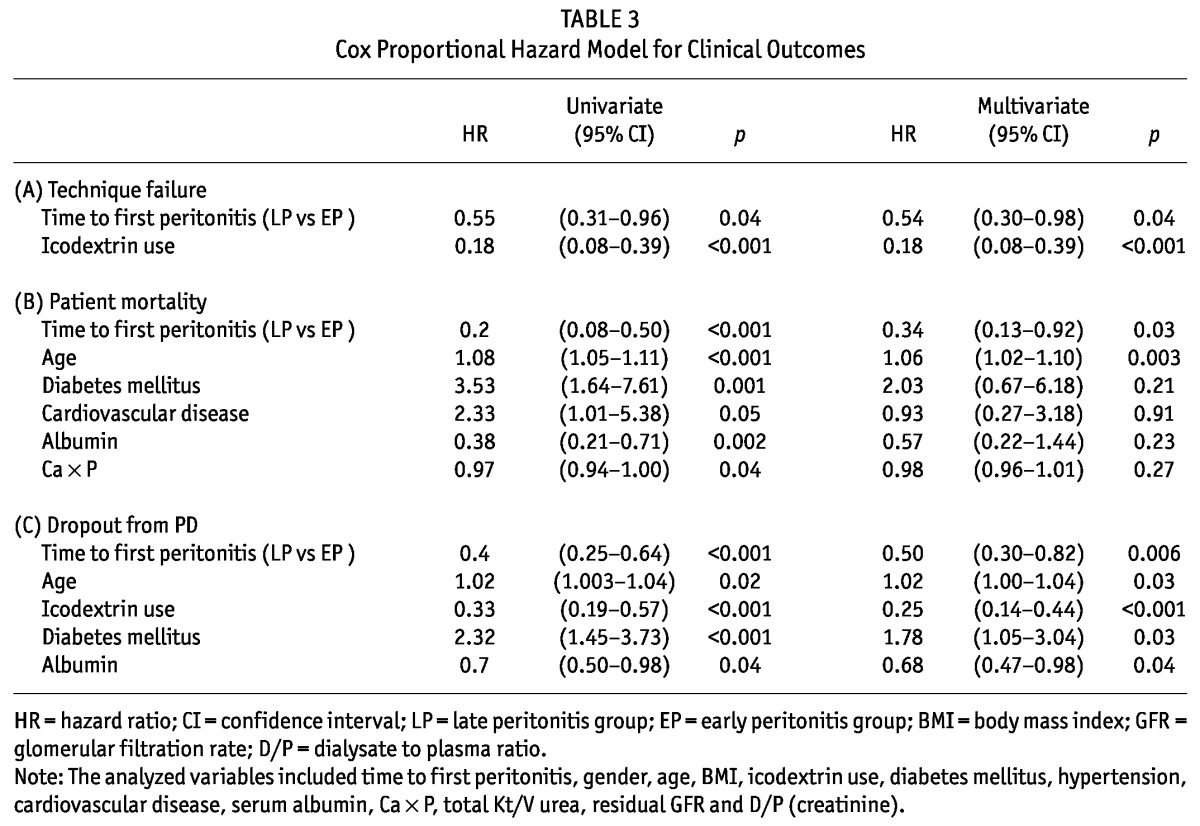
Analyses were repeated with the time to first peritonitis as a continuous variable. The longer time to first peritonitis was associated with decreased risk of technique failure in the unadjusted model (HR per 1-month increase in the time to first peritonitis, 0.98; 95% CI, 0.97 - 0.99, p = 0.02) or fully adjusted model (HR 0.98; 95% CI, 0.97 0.99, p = 0.002).
Patient survival: The patient survival was 46.51±3.01 months for the EP patients and 61.78±1.29 months for the LP patients. The Kaplan-Meier analysis revealed that LP patients were significantly associated with better patient survival than EP patients (log-rank test, p < 0.001; Figure 1B). Infection was the leading cause of patient mortality (9 in the EP group, 7 in the LP group; 50% vs 77.8%, p = 0.23) in the whole cohort (n = 124). The other causes of patient mortality included cardiovascular disease (8 in the EP group), neurological events (1 in the EP group), malignancy (1 in the LP group) and cachexia (1 in the LP group). In the multivariate Cox regression model, the LP group was associated with a 66% reduction in patient mortality compared with the EP group (HR 0.34; 95% CI 0.13 - 0.92, p = 0.03; Table 3).
Analyses were repeated with the time to first peritonitis as a continuous variable. The longer time to first peritonitis was associated with decreased risk of patient mortality in the unadjusted model (HR per 1-month increase in the time to first peritonitis, 0.96; 95% CI, 0.93 - 0.98, p < 0.001) or fully adjusted model (HR 0.97; 95% CI, 0.95 - 0.99, p = 0.02).
“Stay on PD” survival: The “stay on PD” survival (the combined endpoint of patient survival and technique survival) was 39.64±3.73 months for EP patients and 67.8±4.22 months for LP patients. The Kaplan-Meier analysis for “stay on PD” survival for the EP and LP patients is depicted in Figure 1C. The LP patients were significantly associated with higher probability of “stay on PD” than the EP patients (log-rank test, p < 0.001). Using a univariate Cox regression analysis, the LP group was associated with a 60% reduction in “dropout from PD” compared with the EP group (HR 0.40; 95% CI 0.25 - 0.64, p < 0.001; Table 3). In the multivariate Cox regression analysis adjusted for age, icodextrin prescription, diabetes mellitus and serum albumin, the LP group was still associated with a 50% reduction in “dropout from PD” compared with the EP group (HR 0.50; 95% CI 0.30 - 0.82, p = 0.006; Table 3).
Analyses were repeated with the time to first peritonitis as a continuous variable. The longer time to first peritonitis was associated with decreased risk of dropout from PD in the unadjusted model (HR per 1-month increase in the time to first peritonitis, 0.98; 95% CI, 0.97 - 0.99, p = 0.02) or fully adjusted model (HR 0.98; 95% CI, 0.97 - 0.99, p = 0.001).
The Correlation of Peritonitis Rate with Time to First Peritonitis by Spearman’s Correlation Test
Peritonitis rate was inversely correlated with the time to first peritonitis for the whole cohort, for both EP patients and LP patients (r = -0.64, r = -0.31, r = -0.60, p < 0.001, respectively).
Discussion
Peritonitis is one of the major complications of PD and, despite reductions in overall peritonitis rates, remains the primary reason patients switch from PD to HD. In the present study, we analyzed the data of CAPD patients complicated by peritonitis and found that the timing of the first peritonitis episode was an independent predictor of technique failure, all-cause mortality, and dropout from PD.
Nowadays, the focus has been on strategies to improve peritonitis outcomes in addition to lowering the peritonitis incidence. Prognostic factors for peritonitis outcome have been found to include age, gender, race, diabetes, residual renal function, time on PD, concurrent exit-site infection, cytokines, inflammatory markers, albumin, causative organisms, recent antibiotic therapy and recurrent peritonitis, some of which have been contradictorily reported in the literature (20-26). Awareness of these risk factors can influence decision-making during the treatment of peritonitis, and, on the basis of this motivation, we are able to identify early peritonitis occurrence as a risk factor for long-term outcomes in this study.
Peritonitis is associated with significant morbidity, PD catheter loss, and switch to HD, transient or permanent ultrafiltration failure, possible permanent membrane damage, and even death (27-29). Reports from the United States have also shown that the mortality rate directly from peritonitis is less than 5% and related to specific pathogens (4,5). In one retrospective Spanish study of 565 patients, 41 of the observed 693 episodes of peritonitis resulted in death (5.9%) (28). Similarly for our cohort, peritonitis resulted in death in 4.2% of the episodes (8 died out of 190 episodes), which is also comparable to that reported in other studies (1 - 6%) (30,31).
Peritonitis was attributed as the cause of technique failure in 42.6% of adult PD patients in a nationwide audit in Scotland, matching the 41.7% figure for a London-based study (2,3). In a further study conducted in Japan, the major reason for the transfer to HD from 2005 to 2007 in the Tokai area was peritonitis (27%), followed by dialysis failure (21.3%) (32). Of the 391 incident CAPD patients during the 10-year observation period in our unit, among the 92 patients switching to HD, 42 of the cases (45.7%) were due to peritonitis.
Peritonitis rate is always chosen as an independent factor for the study of the impact of peritonitis on PD outcomes. However, to date there have been few studies conducted investigating the role of the timing of the first peritonitis espisode in CAPD. A Greek study of 47 CAPD or automated peritoneal dialysis (APD) patients by Fourtounas et al. reported that patients who developed peritonitis during the first year after starting PD were more prone to not only repeated episodes of peritonitis but also decreased technique survival compared with those presenting with the first peritonitis episode after at least 24 months on PD (33). The mean overall time to the first episode of peritonitis after commencement of PD was 26.4 ± 22 months in the entire cohort, similar to that in our study (26.8 ± 21.6 months). Fourtounas et al. also found that the early occurrence of peritonitis in PD patients was usually caused by gram-positive organisms, presumably due to mistakes during connection. Another retrospective Canadian study compared technique and patient outcomes between early-onset peritonitis (defined as peritonitis occurring within 3 months of PD catheter implantation) and a matched control group of PD patients (34). They observed that the early-onset cohort was younger and had a higher peritonitis rate than the control group, with gram-positive organisms, particularly coagulase-negative staphylococci, being the most common causative agents. The dropout rate of PD in the early-onset group was almost twice that of the control group. In our study, Streptococcus species were the more common organisms in the EP patients compared with the LP patients (p = 0.03). EP patients were significantly older, had a higher rate of peritonitis incidence, technique failure, patient mortality, and dropout than the LP patients.
On the other hand, the direct correlation between time to first peritonitis and PD-related clinical outcomes, including technique survival, dropout from PD, and patient survival, has never been reported. The strong correlation between peritonitis rate and time to first peritonitis by Spearman analysis showed that late peritonitis onset is associated with a low peritonitis rate. Indeed, a high peritonitis rate is conducive to adverse outcomes, as is attested in the literature. For example, peritonitis rate was a predictor for mortality in whites (p = 0.005), non-diabetes (p = 0.006), and those over the age of 60 (p = 0.009) (30) and for every 0.5 episodes per year increase in the peritonitis rate, the risk of mortality increased 10% in whites, 4% in non-diabetes and 11% in those over the age of 60. In a Spanish study, the incidence of peritonitis was a strong predictor of peritonitis-related death and showed a clear trend to predict overall mortality (28). Peritonitis rate also predicted technique failure and mortality in a Turkish report (RR = 3.22, p < 0.001; RR=1.87, p < 0.001, respectively) (29). This phenomenon between the timing of the first peritonitis episode and peritonitis rate could partly explain why patients with late peritonitis are linked with better patient survival, technique survival, and stay on PD compared with those with early onset.
Elderly patients with ESRD have a much greater disease burden than younger patients. The age-related comorbidities include impaired vision, deafness, immobility, cognitive problems and depression due to loss of independence or bereavement (35). They are often socially isolated, live in poor accommodations, and have financial problems. The finding of early peritonitis occurrence in elderly patients may be related to an increased risk of inadvertent microbial contamination during CAPD exchanges due to poor visual acuity or impaired dexterity in the absence of assistance. Diabetes mellitus is associated with faster decline of residual renal function in PD patients (36-38). When residual renal function declines, the dialysis adequacy may be maintained only by more exchanges (enhancing the risk of peritonitis) or increased fill volumes. In addition, altered immunity of peritoneal defenses in diabetes is evidenced by impaired migration of phagocytes into the peritoneum and suppression of phagocytosis by advanced glycation end products (39). Furthermore, intestinal bacterial overgrowth due to low intestinal motility or slower colonic transit in diabetes may also increase the risk of peritonitis. The above reasons or mechanisms could partly explain why the early peritonitis group tended to be elderly and more diabetic in our study.
We also recognize the limitations of our study. First, although the analytic model incorporated several potentially important factors implicated with clinical outcomes, we can not exclude the possibility of residual confounding from variables not included in the present study, such as socioeconomic condition, the rate of decline of residual renal function, and the distance from the center. Second, our single-center study results cannot be extrapolated to other centers until confirmed by a study of a sufficiently large sample size. These findings may not be applied to other patient populations.
In conclusion, to the best of our knowledge, this study is the largest to explore the relationship between the timing of the first peritonitis episode and outcomes in CAPD patients with peritonitis. We demonstrate that the time to first peritonitis is an important predictor for technique survival, patient survival and stay on PD for incident CAPD patients. The EP patients tended to be elderly, have diabetes mellitus and lower serum creatinine levels at enrollment compared to the LP patients. Furthermore, patients with early peritonitis onset had a high peritonitis rate. Training by nurses with advanced experience predicted the longest period free of first-episode gram-positive peritonitis (10). Early identification of patients at risk for early peritonitis, such as the diabetic or elderly, coupled with intensive and detailed training and additional support, may be the solution for preventing PD withdrawal and improving patient survival. Whether switching early peritonitis patients to HD leads to better outcomes, as compared with those remaining on CAPD, still needs to be investigated in the future.
Disclosures
The authors have no financial or other conflicts of interest to declare.
Acknowledgments
We thank all the PD nursing teams for their help in collecting data.
References
- 1. Oreopoulos DG, Robson M, Faller B, Ogilvie R, Rapoport A, deVeber GA. Continuous ambulatory peritoneal dialysis: a new era in the treatment of chronic renal failure. Clin Nephrol 1979; 11:125–8 [PubMed] [Google Scholar]
- 2. Kavanagh D, Prescott GJ, Mactier R. Peritoneal dialysis associated peritonitis in Scotland 1999-2002. Nephrol Dial Transplant 2004; 19:2584–91 [DOI] [PubMed] [Google Scholar]
- 3. Davenport A. Peritonitis remains the major clinical complication of peritoneal dialysis: the London (UK) peritonitis audit 2002-2003. Perit Dial Int 2009; 29:297–302 [PubMed] [Google Scholar]
- 4. Mujais S. Microbiology and outcomes of peritonitis in North America. Kidney Int 2006; 70(Suppl 103):S55–62 [DOI] [PubMed] [Google Scholar]
- 5. Bunke CM, Brier ME, Golper TA. Outcomes of single organism peritonitis in peritoneal dialysis: gram negatives versus gram positives in the Network 9 Peritonitis Study. Kidney Int 1997; 52(2):524–9 [DOI] [PubMed] [Google Scholar]
- 6. Valdés F. Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int 2005; 25(3):274–84 [PubMed] [Google Scholar]
- 7. Kim DK, Yoo TH, Ryu DR. Changes in causative organisms and their antimicrobial susceptibilities in CAPD peritonitis: a single center’s experience over one decade. Perit Dial Int 2004; 24(5):424–32 [PubMed] [Google Scholar]
- 8. Daly CD, Campbell MK, MacLeod AM, Cody DJ, Vale LD, Grant AM, et al. Do the Y-set and double bag systems reduce the incidence of CAPD peritonitis? Nephrol Dial Transplant 2001; 16:341–7 [DOI] [PubMed] [Google Scholar]
- 9. Wong SS, Chu KH, Cheuk A, Tsang WK, Fung SK, Chan HW, et al. Prophylaxis against gram-positive organisms causing exit-site infection and peritonitis in continuous ambulatory peritoneal dialysis patients by applying mupirocin ointment at the catheter exit site. Perit Dial Int 2003; 23(Suppl 2):S153–8 [PubMed] [Google Scholar]
- 10. Yang Z, Xu R, Zhuo M, Dong J. Advanced nursing experience is beneficial for lowering the peritonitis rate in patients on peritoneal dialysis. Perit Dial Int 2012; 32(1):60–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chow KM, Szeto CC, Law MC, Fun Fung JS, Kam-Tao Li P. Influence of peritoneal dialysis training nurses’ experience on peritonitis rates. Clin J Am Soc Nephrol 2007; 2(4):647–52 [DOI] [PubMed] [Google Scholar]
- 12. Han SH, Lee SC, Ahn SV, Lee JE, Kim DK, Lee TH, et al. Reduced residual renal function is a risk of peritonitis in continuous ambulatory peritoneal dialysis patients. Nephrol Dial Transplant 2007; 22:2653–8 [DOI] [PubMed] [Google Scholar]
- 13. Ahmad S, Sehmi JS, Ahmad-Zakhi KH, Clemenger M, Levy JB, Brown EA. Impact of new dialysis solutions on peritonitis rates. Kidney Int 2006; (Suppl 103):S63–6 [DOI] [PubMed] [Google Scholar]
- 14. Furkert J, Zeier M, Schwenger V. Effects of peritoneal dialysis solutions low in GDPs on peritonitis and exit-site infection rates. Perit Dial Int 2008; 28:637–40 [PubMed] [Google Scholar]
- 15. Montenegro J, Saracho R, Gallardo I, Martínez I, Muñoz R, Quintanilla N. Use of pure bicarbonate-buffered peritoneal dialysis fluid reduces the incidence of CAPD peritonitis. Nephrol Dial Transplant 2007; 22:1703–8 [DOI] [PubMed] [Google Scholar]
- 16. Lim CT, Wong KS, Foo MW. The impact of topical mupirocin on peritoneal dialysis infection rates in Singapore General Hospital. Nephrol Dial Transplant 2005; 20:1702–6 [DOI] [PubMed] [Google Scholar]
- 17. Nessim SJ, Bargman JM, Austin PC, Nisenbaum R, Jassal SV. Predictors of peritonitis in patients on peritoneal dialysis: results of a large, prospective Canadian database. Clin J Am Soc Nephrol 2009; 4:1195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rabindranath KS, Adams J, Ali TZ, Daly C, Vale L, Macleod AM. Automated vs continuous ambulatory peritoneal dialysis: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2007; 22:2991–8 [DOI] [PubMed] [Google Scholar]
- 19. Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, et al. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int 2005; 25:107–31 [PubMed] [Google Scholar]
- 20. Krishnan M, Thodis E, Ikonomopoulos D, Vidgen E, Chu M, Bargman JM, et al. Predictors of outcome following bacterial peritonitis in peritoneal dialysis. Perit Dial Int 2002; 22(5):573–81 [PubMed] [Google Scholar]
- 21. Yang CY, Chen TW, Lin YP, Lin CC, Ng YY, Yang WC, et al. Determinants of catheter loss following continuous ambulatory peritoneal dialysis peritonitis. Perit Dial Int 2008; 28(4):361–70 [PubMed] [Google Scholar]
- 22. Golper TA, Brier ME, Bunke M, Schreiber MJ, Bartlett DK, Hamilton RW, et al. Risk factors for peritonitis in long-term peritoneal dialysis: the Network 9 peritonitis and catheter survival studies. Am J Kid Dis 1996; 28:428–36 [DOI] [PubMed] [Google Scholar]
- 23. Troidle L, Gorban-Brennan N, Kliger AS, Finkelstein FO. Effect of duration of chronic peritoneal dialysis therapy on the development of peritonitis. Perit Dial Int 1999; 19:376–9 [PubMed] [Google Scholar]
- 24. Elsurer R, Afsar B, Sezer S, Ozdemir FN: Peritoneal cells at admission: do they have prognostic significance in peritonitis? Ren Fail 2010; 32:335–42 [DOI] [PubMed] [Google Scholar]
- 25. Chow KM, Szeto CC, Cheung KK, Leung CB, Wong SS, Law MC, et al. Predictive value of dialysate cell counts in peritonitis complicating peritoneal dialysis. Clin J Am Soc Nephrol 2006; 1:768–73 [DOI] [PubMed] [Google Scholar]
- 26. Szeto CC, Wong TY, Chow KM, Leung CB, Li PK. The clinical course of culture-negative peritonitis complicating peritoneal dialysis. Am J Kidney Dis 2003; 42(3):567–74 [DOI] [PubMed] [Google Scholar]
- 27. Woodrow G, Turney JH, Brownjohn AM. Technique failure in peritoneal dialysis and its impact on patient survival. Perit Dial Int 1997; 17(4):360–4 [PubMed] [Google Scholar]
- 28. Pérez Fontan M, Rodríguez-Carmona A, García-Naveiro R, Rosales M, Villaverde P, Valdés F. Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int 2005; 25:274–84 [PubMed] [Google Scholar]
- 29. Sipahioglu MH, Aybal A, Unal A, Tokgoz B, Oymak O, Utas C. Patient and technique survival and factors affecting mortality on peritoneal dialysis in Turkey: 12 years’ experience in a single center. Perit Dial Int 2008; 28(3):238–45 [PubMed] [Google Scholar]
- 30. Fried LF, Bernardini J, Johnston JR, Piraino B. Peritonitis influences mortality in peritoneal dialysis patients. J Am Soc Nephrol 1996; 7:2176–82 [DOI] [PubMed] [Google Scholar]
- 31. Tzamaloukas AH. Decreasing morbidity and mortality in long term peritoneal dialysis patients. Semin Dial 1995; 8:397–400 [Google Scholar]
- 32. Mizuno M, Ito Y, Tanaka A, Suzuki Y, Hiramatsu H, Watanabe M, et al. Peritonitis is still an important factor for withdrawal from peritoneal dialysis therapy in the Tokai area of Japan. Clin Exp Nephrol 2011; 15(5):727–37 [DOI] [PubMed] [Google Scholar]
- 33. Fourtounas C, Savidaki E, Dousdabanis P, Hardalias A, Kalliakmani P, Papachristou E, et al. Peritonitis during the first year after commencement of peritoneal dialysis has an impact on technique survival and patient morbidity. Adv Perit Dial 2006;22:50–4 [PubMed] [Google Scholar]
- 34. Harel Z, Wald R, Bell C, Bargman JM. Outcome of patients who develop early-onset peritonitis. Adv Perit Dial 2006; 22:46–9 [PubMed] [Google Scholar]
- 35. Brown EA. Should older patients be offered peritoneal dialysis? Perit Dial Int 2008; 28:444–8 [PubMed] [Google Scholar]
- 36. Liao CT, Shiao CC, Huang JW, Hung KY, Chuang HF, Chen YM, et al. Predictors of faster decline of residual renal function in Taiwanese peritoneal dialysis patients. Perit Dial Int 2008; 28(Suppl 3):S191–5 [PubMed] [Google Scholar]
- 37. Johnson DW, Mudge DW, Sturtevant JM, Hawley CM, Campbell SB, Isbel NM, et al. Predictors of decline of residual renal function in new peritoneal dialysis patients. Perit Dial Int 2003; 23:276–83 [PubMed] [Google Scholar]
- 38. Singhal MK, Bhaskaran S, Vidgen E, Bargman JM, Vas SI, Oreopoulos DG. Rate of decline of residual renal function in patients on continuous peritoneal dialysis and factors affecting it. Perit Dial Int 2000; 20:429–38 [PubMed] [Google Scholar]
- 39. Liu BF, Miyata S, Kojima H, Uriuhara A, Kusunoki H, Suzuki K, et al. Low phagocytic activity of resident peritoneal macrophages in diabetic mice: relevance to the formation of advanced glycation end products. Diabetes 1999; 48:2074–82 [DOI] [PubMed] [Google Scholar]


