Abstract
The genetic background of rheumatoid arthritis (RA) is only partly understood, and several genes seem to be involved. The matrix metalloproteinases MMP1 (interstitial collagenase) and MMP3 (stromelysin 1) are thought to be important in destructive joint changes seen in RA. In the present study, functional relevant promoter polymorphisms of MMP1 and MMP3 were genotyped in 308 patients and in 110 controls, to test whether the polymorphisms contribute to the severity of the disease measured by radiographic progression of joint destruction. For comparison, the shared epitope of HLA DR4 and DR1 (SE) was determined by polymerase chain reaction. There was no association of MMP polymorphisms with susceptibility to RA. However, a strong linkage disequilibrium was observed between the 1G/2G (MMP1) and the 5A/6A (MMP3) polymorphisms (P << 10-6; linkage disequilibrium index D' = 0.46). In factorial regression, the degree of radiographic joint destruction correlated significantly with the 1G-5A haplotype (P = 0.0001) and the interaction term 'estimated number of 1G-5A haplotypes × duration of disease' (P = 0.0007). This association was phasic, indicating that possession of the 1G-5A haplotype has a protective effect over a period of about 15 years of RA, but might be associated with a more pronounced radiographic progression later on. Similar results were also found with the 1G allele of MMP1 alone (P = 0.015) and with the interaction term 'estimated number of 1G alleles × duration of disease' (P = 0.014). The correlation of SE with the Ratingen score was comparable (0.044). The regression model of MMP haplotypes explained 35% of the variance of the radiographic score, whereas the SE explained 29%. The 1G-5A haplotype across the closely linked MMP1 and MMP3 gene loci is a newly described genetic factor strongly associated with the progression of joint damage in RA. Our findings suggest that there are haplotypes in a MMP cluster region that modify the joint destruction in RA in a phasic manner.
Keywords: allelic polymorphism, matrix metalloproteinase, radiographic progression, rheumatoid arthritis
Introduction
Rheumatoid arthritis is an inflammatory joint disease with considerable variability. The clinical course ranges from mild joint swelling to severe polyarthritis with progressive destruction of cartilage and bone. In recent years, research has focused on the identification of genes that influence the susceptibility as well as the severity of this disorder. The shared epitope (SE), a common peptide sequence on the antigen-binding regions of some HLA DR4 subtypes and on HLA DR1, is associated with an increased prevalence and severity of rheumatoid arthritis (RA) [1,2]. In addition, promoter polymorphisms of tumor necrosis factor α (TNF-α) are associated with a more aggressive disease [3]. However, allelic polymorphisms of these genes can only partly explain the variance of the clinical course, because the genetic background of RA involves multiple genes [4].
The destruction of cartilage and bone in RA is mediated by proteolytic enzymes secreted by an inflammatory synovial tissue [5]. Because destructive enzymes of the matrix metalloproteinase (MMP) family are involved in this process, allelic polymorphisms of MMP genes could possibly influence the course of RA.
MMP1 (collagenase) and MMP3 (stromelysin) belong to the most intensely studied proteases in RA: MMP1 and MMP3 are secreted on stimulation by inflammatory cytokines; they degrade key components of cartilage and bone matrix [6]. MMP1 expression occurs in cells of the invading front of the activated RA synovial tissue [7] and is correlated with erosive arthritis [8,9]. Integrated MMP1 levels are correlated with the number of new joint erosions [10]. Levels of both MMPs are elevated in the serum and synovial fluid of patients with RA and other inflammatory joint diseases [10-13]. Serum MMP3 levels are correlated with markers of inflammation [10] and with the radiographic damage in early RA [9,14,15].
The genes MMP1 and MMP3 are both located at the long arm of chromosome 11 [16], in a cluster together with five other MMP genes (MMP7 and MMP10-13). In recent years, functional relevant polymorphisms of both enzymes have been detected. The promoter region of MMP1 contains a guanine insertion/deletion polymorphism (1G/2G polymorphism) at position -1607 [17]. The 2G allele results in increased transcriptional activity [17] because the guanine insertion creates a binding site for a member of the ETS transcription factor family [18]. The 2G allele may contribute to increased invasiveness of colorectal tumors [19] and to the development of ovarian cancer [20] and also of lung cancer [21].
The promoter region of MMP3 is characterized by a 5A/6A promoter polymorphism at position -1171 in which one allele has six adenosines (6A) and the second has five adenosines (5A) [22]. The 6A allele has a lower promoter activity than the 5A allele in vitro [23]. This polymorphism is of influence in conditions involving the deposition of extracellular matrix such as primary sclerosing cholangitis [24] and coronary arteriosclerosis [23,25].
In the study presented here, the association of promoter polymorphisms of both MMP1 and MMP3 on the radiographic progression was investigated in a cohort of 308 patients with RA, considering also the association of the shared epitope. Because of the location of both MMP genes on the same chromosome, linkage disequilibrium was also investigated.
Materials and methods
Patients and controls
The study was approved by the Ethics Committee of the Medical Faculty of the MLU Halle-Wittenberg. All patients attended the study after giving written informed consent. Inclusion criteria were the presence of definite RA as defined in the American College of Rheumatology criteria [26], current treatment with disease-modifying antirheumatic drugs (DMARDs) (see Table 1), continuous treatment by a single rheumatologist (GH) for at least 4 years and the presence of at least two sequential radiographs of hands and feet, for the assessment of the radiographic progression of joint destruction. A group of 110 unrelated healthy Caucasian volunteers matched for age and for sex ratio served as the control group (mean age 50 years, with 79.3% females).
Table 1.
Characteristics of 308 patients with mild and severe rheumatoid arthritis (RA)
| Parameter | Mild RA (n = 170) | Severe RA (n = 138) | All patients |
| Age (years) | 64 (31–88) | 65.5 (34–82) | 65 (31–88) |
| Sex (% female) | 88.8 | 79.7 | 82 |
| Disease duration (years) | 12 (4–37) | 18.5 (7–44) | 14 (4–44) |
| Ratingen score | 9 (0–24) | 52 (25–179) | 20 (0–179) |
| Swollen joint count | 1 (0–27) | 4 (0–28) | 2 (0–28) |
| IgM RF-positive patients | 86 (50.5%) | 110 (79.7%) | 196 (63.6%) |
| SE-positive patients (%) | 55.6 | 72.4 | 64.3 |
| CRP (mg/dl) | 2 (0.1–94) | 3 (0.2–135) | 2.0 (0.1–135) |
| Number of failed DMARDs | 1 (0–3) | 2 (0–9) | 1 (0–9) |
Values are given as percentages or as medians (range), with the exception of 'IgM RF-positive patients'. CRP, C-reactive protein; DMARDs, disease-modifying antirheumatic drugs; RF, rheumatoid factor; SE, shared epitope of HLA DR4 and DR1.
Clinical markers of disease activity
In each patient, 28 peripheral joints were examined for soft tissue swelling. Erythrocyte sedimentation rate and C-reactive protein were determined. The modified disease activity score was calculated as described [27].
Radiographic analysis
Radiographic damage of hands and feet was assessed by the Ratingen score [28], a modification of the Larsen score. It evaluates 38 joints separately (all proximal interphalangeal and metacarpophalangeal joints, four sites in the wrists, interphalangeal joints of the great toes, and metatarsophalangeals 2 to 5). The amount of joint surface destruction is graded on a 0 to 5 scale for each joint, providing a maximum score of 190. Each grade represents 20% of joint surface destruction. All radiographs were scored by one investigator (RR) who was unaware of the results of the genetic analyses.
At the Rheumaklinik Ratingen, radiographs of both hands and feet are routinely obtained for all RA patients at disease onset and every 2 years thereafter, to evaluate radiographic progression. For our study we chose sequential radiographs that were at least 4 years apart, to detect more marked differences in the radiographic course. Two sequential radiographs were available for evaluation in all except five patients. In 228 patients, three sequential radiographs were scored. The first radiograph was obtained after a median of 1 year, the second after a median of 6 years and the third after a median of 14 years after disease onset. The total Ratingen score refers to the last radiograph obtained in each patient. In addition, the radiographic progression per year was determined for each patient and for each radiograph by division of the Ratingen score by the disease duration at the time that the radiograph was taken.
DNA isolation
Blood samples containing EDTA as anti-coagulant were obtained from patients and controls. Genomic DNA was extracted from peripheral blood leukocytes by using the QIAamp® DNA Blood Mini-Kit (Qiagen, Hilden, Germany). The plasma was collected for determination of the concentration of MMP1 and MMP3 and stored at -80°C (see below).
Genotyping
MMP1 and MMP3 promoter polymorphisms were determined by single-strand conformation polymorphism analysis. Polymerase chain reaction (PCR) was performed with forward and reverse oligonucleotide primers that were labelled with Cy5 fluorescent dyes. The 1G/2G polymorphism of MMP1 was identified by using the primers 5'-GTT ATG CCA CTT AGA TGA GG-3' and 5'-TTC CTC CCC TTA TGG ATT CC-3'. To screen for the 5A/6A polymorphism of MMP3, the primers 5'-GGT TCT CCA TTC CTT TGA TG-3' and 5'-TCC TGG AAT TCA CAT CAC TG-3' were used.
The reaction was performed in a total volume of 20 μl containing 100 ng of genomic DNA, 15 pmol of each primer, 200 μM dNTPs, 1 × PCR buffer and 0.75 U of Taq polymerase (Roche Molecular Biochemicals, Mannheim, Germany). The solution was incubated for 2.5 min at 94°C, followed by 30 PCR cycles, each for 1 min at 94°C, 45 s at 60°C and 90 s at 72°C, with a final extension for 7 min at 72°C.
The PCR products (1 μl) were mixed with 19 μl of formamide loading dye, denatured for 4 min at 85°C, then cooled directly on ice; 2 μl of this mixture was subjected to electrophoresis on a non-denaturing polyacrylamide gel. Differences in the electrophoretic mobility, based on specific folding effects induced by the sequence variability, were detected with an automated sequencer (Alf-express; Pharmacia, Peapack, NJ, USA).
The validity of the method was checked by sequence analysis. For all possible allelic forms of MMP1 and MMP3, PCR products were purified from agarose gels and sequenced in both directions, using the Thermo Sequenase Dye Terminator Cycle Sequencing Kit (Amersham Pharmacia Biotech, Freiburg, Germany) and an ABI Prism 377 DNA Sequencer (Perkin-Elmer).
Determination of shared epitope
The DR4 subtyping was performed in a sample of 104 randomly chosen patients as described previously [29]. Genomic DNA was amplified with primers DR86AMP-GR (5'-CTGCACTGTGAAGCTCTCAC-3'; codons 86–92) and DR86AMP-VR (5'-CTGCACTGTGAAGCTCTCCA-3'; codons 86–92) as 3' primers and DRB AMP-4 (5'-GTTTCTTGGAGCAGGTTAAAC-3'; codons 6–13) at the 5' end. DR4-specific amplification was achieved by 10 cycles of denaturation at 94°C for 60 s and annealing and extension at 55°C for 60 s, followed by 30 three-temperature cycles (20 s at 94°C, 20 s at 55°C and 30 s at 72°C). Differentiation of DR4 alleles was performed in accordance with the XI.IHWC protocol by hybridisation with sequence-specific oligonucleotide, using non-radioactive labelling and detection as described [29]. With this method the DRB1 alleles from *0401 to *0419, except 0415, could be identified. In all cases of DR4 homozygosity, direct sequencing of PCR products was performed for confirmation.
Measurement of MMP1 and MMP3 concentrations in patient plasma
The protein concentration was determined in a subgroup of 120 patients with defined alleles of MMP1 and MMP3. Patients were selected to obtain equal proportions of homozygous and heterozygous individuals in each group. MMP concentrations were measured by enzyme-linked immunosorbent assay (sandwich ELISA) (BIOTRAK-ELISA-System; Amersham International, Little Chalfont, UK) as described previously [13]. The ELISA for MMP3 is specific for free MMP3, pro-MMP3 and MMP3 bound to tissue inhibitor of metalloproteinases-1 (TIMP-1). The ELISA for MMP1 recognizes human MMP1 and MMP1 bound to TIMP-1, but not MMP1 bound to the non-specific proteinase inhibitor α2-macroglobulin. It cross-reacts with pro-MMP1 but not with TIMP-1.
Statistical analysis
For statistical analysis, two groups of patients were formed: one group with mild disease (n = 170) and a second group with severe disease (n = 138). The distinction between the two groups was made by the Ratingen score, which had to exceed 24 after 4 years in the group with severe disease. The hypothesis that a joint haplotype across the genes MMP3 and MMP1 shows an association with severe RA was tested with a likelihood ratio test, which tested the frequencies of allele differences and of a given haplotype in severe and mild cases. Estimation of haplotype frequencies and allele frequencies was performed with the program ASSOCIAT.EXE (J Ott, http://linkage.rockefeller.edu).
Regression analyses were performed with STATISTICA v 6. The target phenotype (severity of disease as measured by the Ratingen score) was used as a quantitative measure (Ratingen score) and analysed by factorial regression. All P values from regression analyses were also checked empirically by means of a bootstrap as well as a permutation procedure.
Calculation of odds ratios (ORs)
The regression analysis suggested an early increase in the Ratingen score in patients with no 1G-5A haplotype, with a shoulder at 15 years of RA (see Fig. 1). For further analysis, radiographs were selected to form two different samples. One sample contained the radiographs obtained less than 15 years after disease onset (n = 282; median 9 years; interquartile range [IQR] 4). The latest radiograph was chosen if more than one radiograph was available per patient. The other sample contained one radiograph per patient after 15 or more years after disease onset (n = 123, median 18 years, IQR 4). In each sample, ORs were calculated for the MMP polymorphisms and for the SE to be associated with a Ratingen score above the median.
Figure 1.
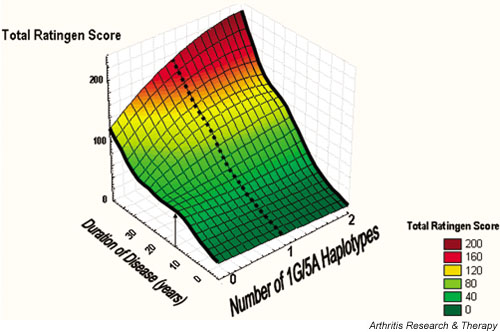
Three-dimensional surface plot showing the main effects for a multiple regression of total Ratingen score versus duration of disease and number of 1G(MMP1)-5A(MMP3) haplotypes. The plot was created by distance-weighted least-squares interpolation. Black lines represent patients with either none or two 1G-5A haplotypes. The broken line, representing patients with one haplotype, is an approximation because it was not possible to determine exactly the number of patients with one 1G-5A haplotype. The arrow indicates the maximum increase in the Ratingen score after 15 years of rheumatoid arthritis in patients with no 1G-5A haplotype (see the text for further explanations). MMP, matrix metalloproteinase.
For comparison between groups, the Mann–Whitney U-test and the Kruskal–Wallis test were applied. Comparisons within groups were performed with the Wilcoxon test. The correlation of plasma concentrations of MMP1 and MMP3 with the Ratingen score was tested by Spearman rank correlation.
Results
Radiographic progression
In the patient group as a whole, the median of the total Ratingen score was 25 (IQR 41), with a median of radiographic progression per year of 1.73 (IQR 2.39). The radiographic progression decreased significantly over time. The yearly progression between disease onset and the first radiograph (median 3.0) was significantly higher than the progression between onset of disease and the second (median 1.8) and third radiograph (median 1.7), respectively (for all comparisons, P < 0.001).
Allelic distribution and deviation of MMP1 and MMP3 alleles from Hardy–Weinberg equilibrium
No deviations from Hardy–Weinberg equilibrium were found for the MMP1 or MMP3 alleles, respectively. The allele frequencies of MMP1 and MMP3 were not different between RA patients and controls (see Table 2).
Table 2.
Allele frequencies (percentages) of the 1G/2G (MMP1) polymorphism and the 5A/6A (MMP3) polymorphism
| Locus | Allele | Controls | RA total | Severe RA | Mild RA |
| MMP1 | 1G | 53.4 | 50.8 | 47.8 | 53.7 |
| 2G | 46.6 | 49.2 | 52.2 | 46.3 | |
| MMP3 | 5A | 46.8 | 49.6 | 47.1 | 51.2 |
| 6A | 53.2 | 50.4 | 52.9 | 48.8 |
MMP, matrix metalloproteinase; RA, rheumatoid arthritis.
Allelic association of MMP1 and MMP3
A statistically significant association between alleles at the MMP1 and MMP3 loci was observed in the group of all patients. Specifically, a combination of 1G (MMP1) and 5A (MMP3) as well as 2G (MMP1) and 6A (MMP3) was significantly over-represented in comparison with random expectations (P << 10-6). Actually, most haplotypes were found to be either 1G-5A (36.4%, expected frequency 25.2%) or 2G-6A (35.0%, expected 24.8%), with only few 1G-6A (12.8%, expected 25.6%) or 2G-5A (15.8%, expected 24.4%) allelic combinations (see Table 3). The linkage disequilibrium index D' was 0.45 in patients and in controls. As a consequence we also defined a quantitative variable, 'estimated number of 1G-5A haplotypes', to capture the haplotypic information. This was in consideration of the fact that it was not possible to determine exactly how many patients had one 1G-5A haplotype: in only 84% of all patients who had one 1G (MMP1) and one 5A (MMP3) allele were both alleles located on the same chromosome.
Table 3.
Allelic combinations of the 1G/2G (MMP1) polymorphism and the 5A/6A (MMP3) polymorphism
| MMP3 | ||||||
| Rheumatoid arthritis patients | Controls | |||||
| MMP1 | 5A/5A | 5A/6A | 6A/6A | 5A/5A | 5A/6A | 6A/6A |
| 1G/1G | 13.2 | 11.1 | 2.1 | 13.4 | 16.4 | 0.9 |
| 1G/2G | 10.0 | 28.2 | 10.0 | 7.3 | 26.4 | 15.5 |
| 2G/2G | 2.1 | 8.6 | 14.6 | 2.7 | 6.4 | 10.9 |
Results are observed frequencies of all rheumatoid arthritis patients and controls (percentages). MMP, matrix metalloproteinase.
Regression analysis of MMP polymorphisms
In univariate analyses, the factor that was most strongly correlated with the Ratingen score was the duration of disease (P < 10-6). We therefore included this variable in the analyses. In factorial regression, a highly significant positive correlation was detected between the Ratingen score and 'estimated number of 1G-5A haplotypes' per se (P = 0.0001). In addition, significant correlation was detected between the Ratingen score and the interaction term 'estimated number of 1G-5A haplotypes × duration of disease' (P = 0.0007) (Fig. 1).
In total, the regression model was found to explain 35% of the variance of the Ratingen score.
Similar, but less significant, results were found with the MMP1 system alone (number of 1G alleles, P = 0.015) and with the interaction 'number of 1G alleles × duration of disease' (P = 0.014). P values for the MMP3 system alone were not significant at the 5% level.
Regression analysis of the SE
In factorial regression, there also was a correlation of the SE with the Ratingen score (P = 0.044). The presence or absence of the SE explained 29% of the variance of the Ratingen score, independently of the location at DR4, indicating an association of the SE with radiological progression that was comparable to the effect of the 1G-5A haplotype. The direct comparison between SE-negative and SE-positive patients by non-parametric testing indicated a higher Ratingen score in the SE-positive group (P = 0.026). The presence of the SE on HLA DR4 was not associated with a more pronounced radiographic progression, compared with the whole group of SE-positive individuals.
Time-dependent effect of different genotypes on the Ratingen score
The data in Fig. 1 suggest that the association of the 1G-5A haplotype with the Ratingen score is phasic. Patients without this haplotype show the most rapid increase in the Ratingen score within the first years of disease, reaching a peak at about 15 years. In contrast, patients with two 1G-5A haplotypes seem to develop more pronounced radiographic damage after more than 15 years of RA.
To analyse this phenomenon further, two samples of radiographs were examined separately. Radiographs obtained after less than 15 years of RA revealed a median Ratingen score of 18. The ORs to achieve a Ratingen score above this median are given in Table 4, showing significant associations of a higher Ratingen score with the SE. Patients homozygous for the MMP1 2G allele had a significantly increased risk in comparison with patients with the 1G-1G genotype. The absence of the 1G-5A haplotype was also associated with a higher Ratingen score than in patients with two haplotypes. No increased risk for higher radiographic damage was seen with respect to the MMP3 alleles.
Table 4.
Odds ratio (OR) and 95% confidence interval (CI) for the achievement of a Ratingen score above the median
| Sample 1 | Sample 2 | |||||||
| Parameter | OR | 95% CI | P | n | OR | 95% CI | P | n |
| No 1G/5A haplotypes vs two haplotypes | 2.85 | 1.08–7.52 | 0.032 | 74 | 0.75 | 0.23–2.7 | n.s. | 37 |
| MMP1 2G/2G vs MMP1 1G/1G | 2.41 | 1.20–4.84 | 0.012 | 138 | 0.31 | 0.11–0.86 | 0.023 | 64 |
| MMP3 5A/5A vs MMP3 6A/6A | 1.69 | 0.86–3.32 | n.s. | 144 | 1.76 | 0.66–4.68 | n.s. | 69 |
| SE-positive vs SE-negative | 3.03 | 1.24–7.39 | 0.013 | 104 | 4.6 | 1.34–15.8 | 0.012 | 54 |
P values given are asymptotic P values. Sample 1: radiographs taken earlier than 15 years after disease onset. Sample 2: radiographs taken after more than 15 years of rheumatoid arthritis. MMP, matrix metalloproteinase; SE, shared epitope of HLA DR 4 and DR1.
Radiographs taken after more than 15 years of RA had a median Ratingen score of 38. Calculation of ORs confirmed the association of the SE with a Ratingen score above this median. Of interest, the presence of the homozygous MMP1 2G genotype was now associated with a significantly lower risk for radiographic damage than in the homozygotes for the 1G allele. In this sample, no significant ORs were found in association with the 1G-5A haplotypes.
In both samples, ORs of homozygous patients in comparison with heterozygotes did not reach statistical significance, with respect to either the MMP alleles or the 1G-5A haplotype.
Regression analysis of clinical and laboratory data
In factorial regression there was a significant correlation between the Ratingen score and the disease activity score (P = 0.0001), the C-reactive protein level (P = 0.0053) and the presence of rheumatoid factor (P < 0.0001). Neither the MMP haplotype nor the SE was correlated significantly with the disease activity score or the laboratory parameters.
Plasma concentrations of MMP1 and MMP3 and MMP polymorphisms
The plasma concentrations of MMP1 and MMP3 were not significantly different between patient groups with defined MMP alleles and did not correlate with the Ratingen score (data not shown).
Discussion
In recent years, analysis of the inheritable factors of RA has largely focused on components of the immune system, such as the SE and the cytokine network [1,2,30]. In the cohort investigated here, the association of the SE with radiographic progression was seen, as it has in previous investigations [1,2]. A more prominent radiographic progression in patients who carried the SE on HLA DR4 could not be detected, in contrast with other studies [29].
Our data stress the significance of inheritable factors that affect joint destruction downstream of the inflammatory cascade. MMP1 and MMP3 are involved in processes of tissue remodelling, including wound healing and angiogenesis, but also in cancer invasion and inflammatory joint destruction [31,32].
Similarly to other recent investigations [33,34], our study failed to detect any connection of the MMP1 or MMP3 polymorphism with the susceptibility to RA. This is not surprising, given the widely accepted perception of RA as a disease that is dependent on, if not initiated by, T cell-driven antigen-dependent mechanisms, labelling tissue-destructive processes as a secondary phenomenon. However, functional relevant allelic polymorphisms of MMP genes, specifically the MMP1 polymorphism, could influence the severity of the disease. The 2G allele of MMP1 is associated with a higher promoter activity in vitro [17,18], which leads to the production of increased amounts of MMP1 protein [35].
Our study shows for the first time a significant linkage disequilibrium between the 1G/2G MMP1 and the 5A/6A MMP3 polymorphism. This phenomenon can be attributed to the proximity of the MMP1 and MMP3 genes. Both genes have been mapped to the long arm of chromosome 11 in the region 11q22.3 [16], with a distance between them of 37.64 kilobases. The biological function of this phenomenon is still unknown. However, our observation illustrates the tight interrelationship of both enzymes. MMP1 and MMP3 are often coordinately expressed, and their promoters contain similar regulatory elements, for example activator protein-1 (AP-1) and ETS [36]. On the transcriptional level, they are activated by similar factors such as interleukin-1 [37], whereas plasmin and trypsin activate the precursors of both proteins [38]. In addition, MMP1 and MMP3 interact at the protein level. MMP3 activates latent MMP1, enhancing MMP1 activity in vitro up to 12-fold [39].
Another novel finding of this study is a significant association of the 1G-5A haplotype with radiographic damage. The 1G-5A haplotype explained the variability of the Ratingen score in the same order of magnitude as the SE. Our data suggest that this association is phasic. The possession of the 1G-5A haplotype had a protective effect over a period of about 15 years of RA that faded in later stages. In fact, the biphasic association of the homozygous MMP1 1G genotype with radiographic progression in late RA suggests that the 1G-5A haplotype will even promote radiographic destruction after more than 15 years of disease. However, this assumption could not be proved with clarity, owing to the small number of late RA cases with two 1G-5A haplotypes.
The data presented in Table 4 indicate that this time-dependent association is due mainly to the contribution of the MMP1 polymorphism. Patients homozygous for the MMP1 2G allele had an OR of 3.41 for more pronounced radiographic damage in the first period of RA. In later years, the OR of 0.31 points to inversion into a significant protective effect.
Our data contrast with those of other investigations reporting the lack of association between this polymorphism of MMP1 and radiographic progression in a sample of 103 patients with early RA [33]. However, the data provided show a tendency towards higher radiographic scores in the groups with either one or two 2G alleles, in a comparable manner to our data. However, this was not significant, perhaps because of the smaller number of patients. In addition, the previous studies of MMP1 and MMP3 polymorphisms [33,34] had a shorter observation period.
A link between the 6A promoter polymorphism of MMP3 and radiographic progression has recently been published [34]. That study included patients with early RA and observed the radiographic progression over 4 years. Interestingly, our data do not confirm this strong association between the 6A polymorphism of MMP3 and radiographic damage as shown in [34]. Our findings suggest that this association might be an indirect one, caused by the linkage disequilibrium between MMP1 and MMP3 polymorphisms.
There is currently no proven explanation for the phasic nature of the association between the haplotype and joint destruction. Radiographic damage is modulated by genetic factors and by the response to DMARD therapy alike, but genes can also influence the long-term response to therapy. In addition, it can be speculated that the processes of destruction in earlier arthritis are distinct from those in late RA [40].
Our data stress the relative importances of MMP1 and MMP3 with respect to joint destruction. This agrees with another publication that describes the correlation of integrated MMP1, but not MMP3, levels with the number of new joint erosions [10]. In addition, the radiological arrest of patients with successful DMARD treatment is accompanied by a reduction of MMP1 expression but not that of MMP3 [41]. In contrast, others have described baseline MMP3 levels as a predictor for the development of joint erosions in a longitudinal study [9].
Conclusions
Taken together, our findings suggest that there are haplotypes in a MMP cluster region that modify the joint destruction in RA in a phasic manner. In our study, the association of the 1G-5A haplotype with radiographic damage was comparable with that of the SE. In addition, our data indicate that this association is due mainly to the contribution of MMP1. Interestingly, this association was biphasic, indicating that the 1G/2G (MMP1) polymorphism that is correlated with more marked joint destruction in the first 15 years might be associated with less damage later on.
For further investigation of the variability of the genetic background with respect to disease outcome, prospective cohorts are required that have been observed in the long term and that are large enough to mirror the complex interrelation between genetic and environmental factors.
Competing interests
None declared.
Author contributions
SD and NL established all experimental methods and performed the experiments. Both wrote the Materials and methods section.
RR and GH took care of all patients. They collected clinical data and all blood samples and radiographs and scored them by means of the Ratingen score.
UW carried out the HLA typing.
BM-M performed the statistical analysis.
IH supervised all experimental work and validated the methods.
GK conceived the study, wrote the grant application, organized the cooperation and wrote the paper except the Materials and methods section.
Abbreviations
DMARD = disease-modifying antirheumatic drug; ELISA = enzyme-linked immunosorbent assay; IQR = interquartile range; MMP = matrix metallo-proteinase; OR = odds ratio; PCR = polymerase chain reaction; RA = rheumatoid arthritis; SE = shared epitope of HLA DR4 and DR1; TIMP = tissue inhibitor of metalloproteinases.
Acknowledgments
Acknowledgements
This study was supported by a grant of the Department of Culture of Saxony-Anhalt, Germany (3224A/0020M). SD was supported by a scholarship of the German Kompetenznetzwerk Rheumatologie. We thank Professor Regine Wittkowski, Charité Berlin, Germany, for helpful discussions, and Professor Steffen Gay, University of Zürich, Switzerland, for carefully reviewing the manuscript.
References
- Mattey DL, Hassell AB, Dawes PT, Cheung NT, Poulton KV, Thomson W, Hajeer AH, Ollier WE. Independent association of rheumatoid factor and the HLA-DRB1 shared epitope with radiographic outcome in rheumatoid arthritis. Arthritis Rheum. 2001;44:1529–1533. doi: 10.1002/1529-0131(200107)44:7<1529::AID-ART275>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- Kaijzel EL, van Krugten MV, Brinkman BM, Huizinga TW, van der Straaten T, Hazes JM, Ziegler-Heitbrock HW, Nedospasov SA, Breedveld FC, Verweij CL. Functional analysis of a human tumor necrosis factor alpha (TNF-alpha) promoter polymorphism related to joint damage in rheumatoid arthritis. Mol Med. 1998;4:724–733. [PMC free article] [PubMed] [Google Scholar]
- Jawaheer D, Gregersen PK. Rheumatoid arthritis. The genetic components. Rheum Dis Clin North Am. 2002;28:1–15. doi: 10.1016/s0889-857x(03)00066-8. [DOI] [PubMed] [Google Scholar]
- Gay S, Gay RE, Koopman WJ. Molecular and cellular mechanisms of joint destruction in rheumatoid arthritis: two cellular mechanisms explain joint destruction? Ann Rheum Dis. 1993;52 Suppl 1:39–47. doi: 10.1136/ard.52.suppl_1.s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNaul KL, Chartrain N, Lark M, Tocci MJ, Hutchinson NI. Discoordinate expression of stromelysin, collagenase, and tissue inhibitor of metalloproteinases-1 in rheumatoid human synovial fibroblasts. Synergistic effects of interleukin-1 and tumor necrosis factor-alpha on stromelysin expression. J Biol Chem. 1990;265:17238–17245. [PubMed] [Google Scholar]
- Xue C, Takahashi M, Hasunuma T, Aono H, Yamamoto K, Yoshino S, Sumida T, Nishioka K. Characterisation of fibroblast-like cells in pannus lesions of patients with rheumatoid arthritis sharing properties of fibroblasts and chondrocytes. Ann Rheum Dis. 1997;56:262–267. doi: 10.1136/ard.56.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnane G, Fitzgerald O, Hummel KM, Youssef PP, Gay RE, Gay S, Bresnihan B. Synovial tissue protease gene expression and joint erosions in early rheumatoid arthritis. Arthritis Rheum. 2001;44:1744–1753. doi: 10.1002/1529-0131(200108)44:8<1744::AID-ART309>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Green MJ, Gough AK, Devlin J, Smith J, Astin P, Taylor D, Emery P. Serum MMP-3 and MMP-1 and progression of joint damage in early rheumatoid arthritis. Rheumatology (Oxford) 2003;42:83–88. doi: 10.1093/rheumatology/keg037. [DOI] [PubMed] [Google Scholar]
- Cunnane G, Fitzgerald O, Beeton C, Cawston TE, Bresnihan B. Early joint erosions and serum levels of matrix metalloproteinase 1, matrix metalloproteinase 3, and tissue inhibitor of metalloproteinases 1 in rheumatoid arthritis. Arthritis Rheum. 2001;44:2263–2274. doi: 10.1002/1529-0131(200110)44:10<2263::AID-ART389>3.3.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Case JP, Lafyatis R, Remmers EF, Kumkumian GK, Wilder RL. Transin/stromelysin expression in rheumatoid synovium. A transformation-associated metalloproteinase secreted by phenotypically invasive synoviocytes. Am J Pathol. 1989;135:1055–1064. [PMC free article] [PubMed] [Google Scholar]
- Ishiguro N, Ito T, Obata K, Fujimoto N, Iwata H. Determination of stromelysin-1, 72 and 92 kDa type IV collagenase, tissue inhibitor of metalloproteinase-1 (TIMP-1), and TIMP-2 in synovial fluid and serum from patients with rheumatoid arthritis. J Rheumatol. 1996;23:1599–1604. [PubMed] [Google Scholar]
- Keyszer G, Lambiri I, Nagel R, Keysser C, Keysser M, Gromnica-Ihle E, Franz J, Burmester GR, Jung K. Circulating levels of matrix-metalloproteinases MMP-3 and MMP-1, tissue inhibitor of metalloproteinases-1 (TIMP-1) and MMP-1/TIMP-1 complex in rheumatic disease. Correlation with clinical activity of rheumatoid arthritis in comparison to other surrogate markers. J Rheumatol. 1999;26:251–258. [PubMed] [Google Scholar]
- Posthumus MD, Limburg PC, Westra J, van Leeuwen MA, van Rijswijk MH. Serum matrix metalloproteinase 3 in early rheumatoid arthritis is correlated with disease activity and radiological progression. J Rheumatol. 2000;27:2761–2768. [PubMed] [Google Scholar]
- Posthumus MD, Limburg PC, Westra J, van Leeuwen MA, van Rijswijk MH. Serum matrix metalloproteinase 3 levels in comparison to C-reactive protein in periods with and without progression of radiological damage in patients with early rheumatoid arthritis. Clin Exp Rheumatol. 2003;21:465–472. [PubMed] [Google Scholar]
- Spurr NK, Gough AC, Gosden J, Rout D, Porteous DJ, van Heyningen V, Docherty AJ. Restriction fragment length polymorphism analysis and assignment of the metalloproteinases stromelysin and collagenase to the long arm of chromosome 11. Genomics. 1988;2:119–127. doi: 10.1016/0888-7543(88)90093-6. [DOI] [PubMed] [Google Scholar]
- Rutter JL, Mitchell TI, Buttice G, Meyers J, Gusella JF, Ozelius LJ, Brinckerhoff CE. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter creates an Ets binding site and augments transcription. Cancer Res. 1998;58:5321–5325. [PubMed] [Google Scholar]
- Tower GB, Coon CC, Benbow U, Vincenti MP, Brinckerhoff CE. Erk 1/2 differentially regulates the expression from the 1G/2G single nucleotide polymorphism in the MMP-1 promoter in melanoma cells. Biochim Biophys Acta. 2002;1586:265–274. doi: 10.1016/S0925-4439(01)00105-3. [DOI] [PubMed] [Google Scholar]
- Ghilardi G, Biondi ML, Mangoni J, Leviti S, DeMonti M, Guagnellini E, Scorza R. Matrix metalloproteinase-1 promoter polymorphism 1G/2G is correlated with colorectal cancer invasiveness. Clin Cancer Res. 2001;7:2344–2346. [PubMed] [Google Scholar]
- Kanamori Y, Matsushima M, Minaguchi T, Kobayashi K, Sagae S, Kudo R, Terakawa N, Nakamura Y. Correlation between expression of the matrix metalloproteinase-1 gene in ovarian cancers and an insertion/deletion polymorphism in its promoter region. Cancer Res. 1999;59:4225–4227. [PubMed] [Google Scholar]
- Zhu Y, Spitz MR, Lei L, Mills GB, Wu X. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter enhances lung cancer susceptibility. Cancer Res. 2001;61:7825–7829. [PubMed] [Google Scholar]
- Ye S, Watts GF, Mandalia S, Humphries SE, Henney AM. Preliminary report: genetic variation in the human stromelysin promoter is associated with progression of coronary atherosclerosis. Br Heart J. 1995;73:209–215. doi: 10.1136/hrt.73.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Eriksson P, Hamsten A, Kurkinen M, Humphries SE, Henney AM. Progression of coronary atherosclerosis is associated with a common genetic variant of the human stromelysin-1 promoter which results in reduced gene expression. J Biol Chem. 1996;271:13055–13060. doi: 10.1074/jbc.271.22.13055. [DOI] [PubMed] [Google Scholar]
- Satsangi J, Chapman RW, Haldar N, Donaldson P, Mitchell S, Simmons J, Norris S, Marshall SE, Bell JI, Jewell DP, Welsh KI. A functional polymorphism of the stromelysin gene (MMP-3) influences susceptibility to primary sclerosing cholangitis. Gastroenterology. 2001;121:124–130. doi: 10.1053/gast.2001.25527. [DOI] [PubMed] [Google Scholar]
- Terashima M, Akita H, Kanazawa K, Inoue N, Yamada S, Ito K, Matsuda Y, Takai E, Iwai C, Kurogane H, Yoshida Y, Yokoyama M. Stromelysin promoter 5A/6A polymorphism is associated with acute myocardial infarction. Circulation. 1999;99:2717–2719. doi: 10.1161/01.cir.99.21.2717. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Prevoo ML, 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- Rau R, Wassenberg S, Herborn G, Stucki G, Gebler A. A new method of scoring radiographic change in rheumatoid arthritis. J Rheumatol. 1998;25:2094–2107. [PubMed] [Google Scholar]
- Wagner U, Kaltenhauser S, Sauer H, Arnold S, Seidel W, Hantzschel H, Kalden JR, Wassmuth R. HLA markers and prediction of clinical course and outcome in rheumatoid arthritis. Arthritis Rheum. 1997;40:341–351. doi: 10.1002/art.1780400219. [DOI] [PubMed] [Google Scholar]
- Cantagrel A, Navaux F, Loubet-Lescoulie P, Nourhashemi F, Enault G, Abbal M, Constantin A, Laroche M, Mazieres B. Interleukin-1beta, interleukin-1 receptor antagonist, interleukin-4, and interleukin-10 gene polymorphisms: relationship to occurrence and severity of rheumatoid arthritis. Arthritis Rheum. 1999;42:1093–1100. doi: 10.1002/1529-0131(199906)42:6<1093::AID-ANR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res. 2001;7:14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- Ye S. Polymorphism in matrix metalloproteinase gene promoters: implication in regulation of gene expression and susceptibility of various diseases. Matrix Biol. 2000;19:623–629. doi: 10.1016/S0945-053X(00)00102-5. [DOI] [PubMed] [Google Scholar]
- Constantin A, Lauwers-Cances V, Navaux F, Abbal M, van Meerwijk J, Mazieres B, Cambon-Thomsen A, Cantagrel A. Collagenase-1 (MMP-1) and HLA-DRB1 gene polymorphisms in rheumatoid arthritis: a prospective longitudinal study. J Rheumatol. 2002;29:15–20. [PubMed] [Google Scholar]
- Constantin A, Lauwers-Cances V, Navaux F, Abbal M, van Meerwijk J, Mazieres B, Cambon-Thomsen A, Cantagrel A. Stromelysin 1 (matrix metalloproteinase 3) and HLA-DRB1 gene polymorphisms: Association with severity and progression of rheumatoid arthritis in a prospective study. Arthritis Rheum. 2002;46:1754–1762. doi: 10.1002/art.10336. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Parry S, Urbanek M, Sammel M, Macones G, Kuivaniemi H, Romero R, Strauss JF., III A single nucleotide polymorphism in the matrix metalloproteinase-1 (MMP-1) promoter influences amnion cell MMP-1 expression and risk for preterm premature rupture of the fetal membranes. J Biol Chem. 2002;277:6296–6302. doi: 10.1074/jbc.M107865200. [DOI] [PubMed] [Google Scholar]
- White LA, Maute C, Brinckerhoff CE. ETS sites in the promoters of the matrix metalloproteinases collagenase (MMP-1) and stromelysin (MMP-3) are auxiliary elements that regulate basal and phorbol-induced transcription. Connect Tissue Res. 1997;36:321–335. doi: 10.3109/03008209709160231. [DOI] [PubMed] [Google Scholar]
- Hui A, Min WX, Tang J, Cruz TF. Inhibition of activator protein 1 activity by paclitaxel suppresses interleukin-1-induced collagenase and stromelysin expression by bovine chondrocytes. Arthritis Rheum. 1998;41:869–876. doi: 10.1002/1529-0131(199805)41:5<869::AID-ART15>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rao CN, Mohanam S, Puppala A, Rao JS. Regulation of ProMMP-1 and ProMMP-3 activation by tissue factor pathway inhibitor-2/matrix-associated serine protease inhibitor. Biochem Biophys Res Commun. 1999;255:94–98. doi: 10.1006/bbrc.1999.0153. [DOI] [PubMed] [Google Scholar]
- Murphy G, Cockett MI, Stephens PE, Smith BJ, Docherty AJ. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987;248:265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein GS, Zvaifler NJ. How important are T cells in chronic rheumatoid synovitis? II. T cell-independent mechanisms from beginning to end. Arthritis Rheum. 2002;46:298–308. doi: 10.1002/art.502. [DOI] [PubMed] [Google Scholar]
- Katrib A, Smith MD, Ahern MJ, Slavotinek J, Stafford L, Cuello C, Bertouch JV, McNeil HP, Youssef PP. Reduced chemokine and matrix metalloproteinase expression in patients with rheumatoid arthritis achieving remission. J Rheumatol. 2003;30:10–21. [PubMed] [Google Scholar]


