Abstract
During wound healing, fibroblasts transition from quiescence to a migratory state, then to a contractile myofibroblast state associated with wound closure. We found that the myofibroblast phenotype, characterized by the expression of high levels of contractile proteins, suppresses the expression of the pro-migratory gene, MMP-2. Fibroblasts cultured in a 3-D collagen lattice and allowed to develop tension showed increased contractile protein expression and decreased MMP-2 levels in comparison to a stress-released lattice. In 2-D cultures, factors that promote fibroblast contractility, including serum or TGF-β, down-regulated MMP-2. Pharmacologically inducing F-actin disassembly or reduced contractility increased MMP-2 expression, while conditions that promote F-actin assembly suppressed MMP-2 expression. In all cases, changes in MMP-2 levels were inversely related to changes in the contractile marker, smooth muscle α-actin. To determine if the mechanisms involved in contractile protein gene expression play a direct role in MMP-2 regulation, we used RNAi-mediated knock-down of the myocardin-like factors, MRTF-A and MRTF-B, which induced the down-regulation of contractile protein genes by fibroblasts under both serum-containing and serum-free conditions. In the presence of serum or TGF-β, MRTF-A/B knock-down resulted in the up-regulation of MMP-2; serum-free conditions prevented this increased expression. Together, these results indicate that, while MMP-2 expression is suppressed by F-actin formation, its up-regulation is not simply a consequence of contractile protein down-regulation.
Keywords: Fibroblast, MMP-2, cell tension, contractility, myofibroblast
INTRODUCTION
Fibroblasts in uninjured tissue reside in a quiescent state, but in response to injury become activated to a migratory phenotype which enables these cells to migrate through tissue undergoing repair [1, 2]. These activated fibroblasts play an important role in extracellular matrix production and turnover during early wound healing. Over time, tension develops in the wound, and these cells undergo a phenotypic transition to become contractile myofibroblasts, which are responsible for wound closure and are characterized by large focal adhesions, prominent stress fibers, and high levels of contractile proteins including SM α-actin and SM-22α [1, 3]. During tissue repair, defects in myofibroblast differentiation delay healing. As wound healing progresses, the myofibroblast population disappears; failure to reduce myofibroblast populations can lead to pathological contractures and fibrosis [1, 4–6].
The myofibroblast both exerts tension on, and responds to tension in, its environment [7]. In early wounds, or in compliant 2-D or 3-D culture environments such as unattached collagen gels, fibroblasts are unable to exert significant force, inhibiting focal adhesion and stress fiber development [7]. As wounds progress, the matrix environment stiffens, facilitating the transition to the myofibroblast phenotype. Mechanistically, this involves a feed-forward mechanism that promotes an ever-increasing ability to exert tension; as tissue stiffens, the expression of contractile proteins is increased, thus enabling the development of more tension. Mechanical regulation of contractile gene expression is critical to phenotypic switching by fibroblasts. Recent studies have uncovered the links between changes in the actin cytoskeleton and subsequent changes in gene transcription relevant to the contractile phenotype [1]. Many of these contractile proteins, including smooth muscle (SM) α-actin, SM-22α, and calponin, are co-regulated at the gene level, along with the genes for other proteins involved in matrix attachment, cytoskeleton remodeling, and other processes, in a program of gene expression termed the “CArGome”, based on the presence of one or more functional CArG elements [CC(A/T)6GG] in their promoters [8]. CArG elements bind SRF, which itself associates with a number of coactivators and co-repressors [8–10]. A subset of CArG-containing genes is mechanically regulated by the actions of the SRF-binding, myocardin-related transcription factors, MRTF-A/MAL and MRTF-B [10–13]. MRTF-A and MRTF-B are sequestered through association with G-actin, and are liberated to facilitate the coordinated expression of contractile protein genes by F-actin formation [14, 15]. Thus, mechanical regulation of gene expression is of particular relevance to the phenotypic transition of fibroblasts to myofibroblasts. We have recently shown that myofibroblast differentiation is critically dependent on MRTF-A and MRTF-B [16].
Activated fibroblasts express a number of ECM proteins, as well as proteases that modify the extracellular matrix, including many of the MMP family of enzymes. MMPs have been generally associated with matrix turnover and cell invasion, but are now known to participate in a variety of other important functions that result from protein activation, inactivation, or cell-surface liberation [17, 18]. MMP-2, which is typically expressed by activated fibroblasts, was originally thought to be involved strictly in matrix turnover, but likely plays many other roles, given its wide range of biological substrates [18]. These roles include activation or inactivation of growth factors, release of cryptic factors from ECM, and shedding of adhesion proteins, all of which are important to cell migration. For example, MMP-2 dampens the inflammatory response during healing by cleaving a number of pro-inflammatory cytokines to inactivate them [17]. MMP-2 can also activate TGF-β [19, 20], which in turn can promote the fibroblast-to-myofibroblast transition. In VSMCs, increased MMP-2 expression is a hallmark of the transition to a migratory phenotype, particularly in response to PDGF-BB [21–24]. We have found that factors that oppose this phenotypic switch, including TGF-β, block MMP-2 up-regulation, and that the expression of MMP-2 is inversely related to that of contractile protein markers [23, 25]. Unlike VSMCs, fibroblasts do not maintain a contractile apparatus, but must be induced to express contractile proteins [1]. At present, it is not clear how MMP-2 expression by fibroblasts is controlled as they go through the phenotypic transitions associated with wound healing. We have previously shown that the activation of MMP-2 by fibroblasts can be accelerated by changes in the actin cytoskeleton [26], suggesting that MMP-2 may be mechanically regulated. Here, we investigated the hypothesis that, similar to CArG-containing genes, MMP-2 is also regulated by the actin cytoskeleton.
MATERIALS AND METHODS
Cell culture
Rat embryonic fibroblasts (REF-52), obtained from Dr. Boris Hinz (University of Toronto, Toronto, Canada), were cultured in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 1% sodium pyruvate (Sigma, St. Louis, MO), and antibiotic/antimycotic (Sigma). All experiments were performed using cells between 40 and 50 passages [27]. Myofibroblasts were generated by stimulating REF-52 cells in supplemented DMEM with 0.25 ng/mL TGF-β1 (Peprotech, Rocky Hill, NJ), as previously described [16]. Rat dermal fibroblasts (RDFs) were obtained from a 42-day old male Sprague-Dawley rat (Charles River Laboratories International, Inc., Wilmington, MA) using a primary explant technique [28] and stored in liquid nitrogen. Cell lines were initiated for culture by thawing an aliquot of cells and culturing them in DMEM supplemented with 10% fetal bovine serum supplemented with 1% sodium pyruvate and antibiotic/antimycotic. All experiments using RDFs were performed before passage 4.
For studies involving 3-D collagen lattices, RDFs were cultured in attached and released collagen lattices as previously described [26, 29, 30]. Briefly, a 1.0 mg/ml collagen solution (Millipore, Billerica, MA) containing 6.25 × 104 cells/ml was prepared, and 0.25 ml drops were deposited onto tissue culture plates (Techno Plastic Products AG, Switzerland). Lattices were cultured for 5 days in complete media with 1 ng/ml TGF-β1, with fresh TGF-β1 added at 2.5 days. After 5 days in culture, half of the lattices were left attached to the culture dish and half of the lattices were released to float in the medium. After 24 hours, total RNA was extracted from 4 – 5 lattices per treatment group using 1mL of TRIzol Reagent (Invitrogen).
For 2-D cultures, cells were cultured to confluence in serum-containing medium; cells grown on coverslips for immunocytochemistry were typically sub-confluent. In some cases, cells were serum-starved for 48 hours with DMEM containing 0.2% lactalbumin hydrolysate and antibiotic/antimycotic. This culture environment synchronizes the cell response to growth factor stimulation, which was performed using fresh serum-free medium supplemented with the growth factors or inhibitors of interest. Growth factors included recombinant human IGF-1 and CHO cell-derived TGF-β1 (both from PeproTech, Inc., Rocky Hill, NJ), α-thrombin and ET-1(EMD Chemicals, Inc., San Diego, CA), and S1P (Cayman Chemical, Ann Arbor, MI). Inhibitors included 0.5 µM Jasplakinolide and 10 µM Y-27632 (both from Calbiochem, San Diego, CA), 50 µM blebbistatin (Toronto Research Chemicals, North York, Ontario, Canada), and 1 µM latrunculin B (Lat B) (Biomol, Plymouth Meeting, PA).
Reverse transcription-PCR and real-time PCR
Total RNA was extracted from cultured cells using Trizol Reagent (Invitrogen) per the manufacturer’s protocol. RNA (5 µg) was reverse transcribed using the Superscript III cDNA Synthesis Kit (Invitrogen) primed with random hexamers plus oligo(dT). 4 µl of diluted cDNA was added per 10 µl of a mixture of PerfeCTa SYBR Green SuperMix Reaction Mix for iQ (Quanta Biosciences, Gaithersburg, MD) plus oligonucleotide primers for MMP-2, SM α-actin, and MRTF-A [16, 23, 25]. Amplification was performed in a Bio-Rad iCycler (Bio-Rad) and the comparative cycle at threshold (Ct) method was used to determine relative changes in mRNA levels.
siRNA transfections
REF-52 cells were pre-treated for 72 hours with TGF-β1 (0.25ng/mL; Peprotech, Inc., Rocky Hill, NJ) to promote myofibroblast differentiation [29]. Cells were then transiently transfected with 120 nM total On-Target Plus siRNA pools specific for MRTF-A and MRTF-B or a control Non-Targeting siRNA Pool (Dharmacon, Lafayette, CO), using Dharmafect 2 (Dharmacon) transfection reagent. The target sequences for the siRNA pools have been previously described [16].
Zymography and Western blots
Cells were lysed in SDS sample buffer, and lysates were evaluated for protein content by bicinchoninic acid assay (Thermo Scientific, Rockford, IL). Equal amounts of protein were assayed by Western blots to detect SM α-actin and β-tubulin, as described [16]. Zymography of conditioned medium was performed using gelatin SDS-PAGE gels, as previously described [23].
Immunolabeling and imaging
REF-52 cells grown on glass coverslips were fixed in 4% paraformaldehyde (Polysciences, Inc., Warrington, PA) for 60 minutes, rinsed, permeabilized with 0.5 % Triton-X 100 in PBS, then treated with 10% normal goat serum, 5% bovine serum albumin, 1% fish gelatin plus 0.5% Triton X-100 in Hank’s Buffered Saline Solution (pH 7.2) to block non-specific labeling. Cells were then incubated overnight at 4°C in rabbit anti-vinculin (diluted 1:400 in blocking solution; Sigma-Aldrich #V4139). Cells were rinsed and incubated in a combination of goat anti-rabbit conjugated to AlexaFluor488 (diluted 1:200 in blocking solution) and phalloidin conjugated to AlexaFluor568 (diluted 1:40; both from Invitrogen-Molecular Probes, Carlsbad, CA) for 75 min at room temperature. After rinsing, coverslips containing labeled cells were mounted onto glass slides using Prolong Gold + DAPI (Invitrogen-Molecular Probes). Labeled cells were imaged using an Olympus IX70 epifluorescence microscope (Olympus America, Center Valley, PA) and QICAM camera controlled by QCapture software (QImaging, Surrey, British Columbia, Canada). Figures were prepared by calibrating image scale, exporting to Photoshop (Adobe, Mountain View, CA), and adjusting brightness and contrast to highlight specific labeling.
RESULTS
Inducing actin cytoskeleton disassembly promotes MMP-2 expression
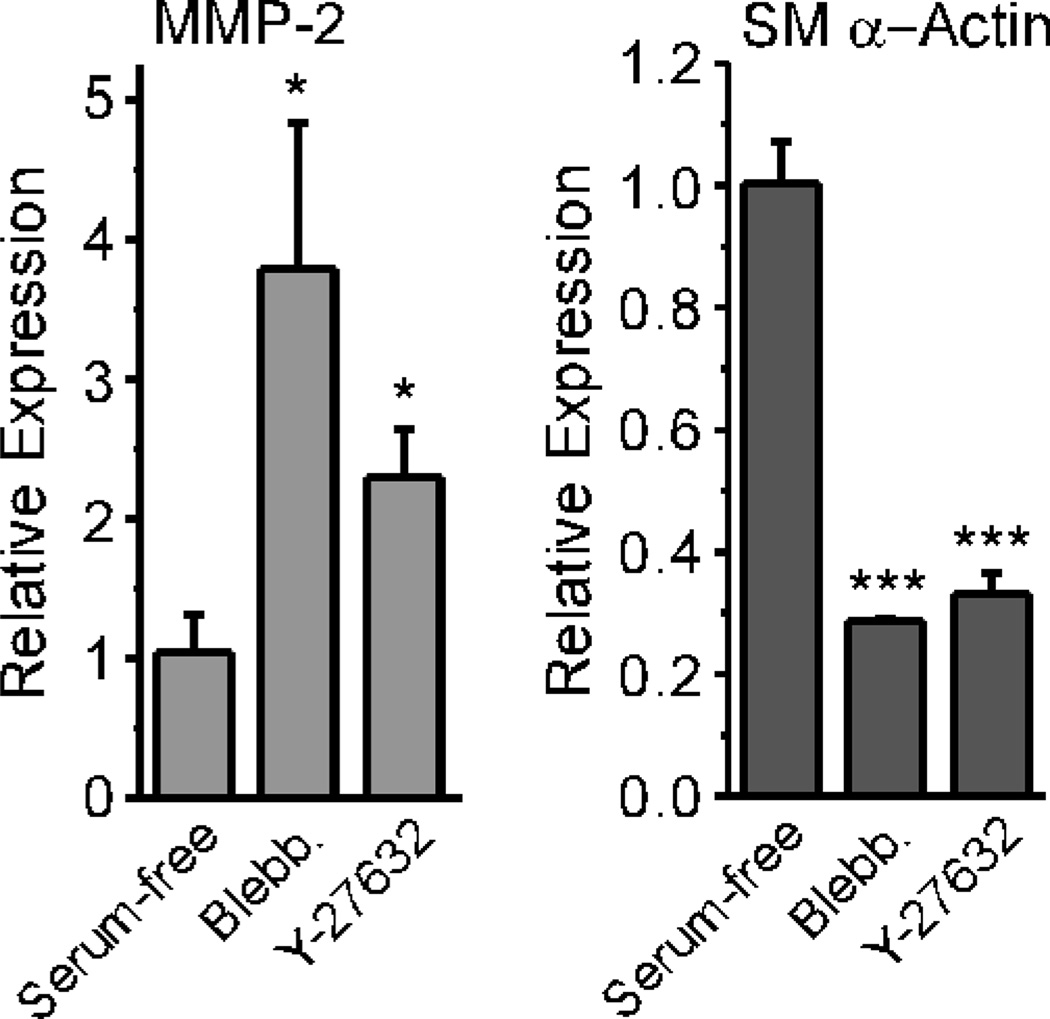
Cultured fibroblasts acquire the myofibroblast phenotype in the presence of serum and/or TGF-β [16, 29]. Myofibroblasts are characterized by large focal adhesions, prominent stress fibers, and enhanced expression of contractile marker proteins such as SM α-actin and SM-22α [1]. We have previously shown that MMP-2 expression is inversely related to contractile protein expression, at least in VSMCs [23]. To determine if a mechanistic link exists between MMP-2 and contractile protein regulation, we used cultured rat embryonic fibroblasts, REF-52, which we and others have used to study the mechanical regulation of gene expression in fibroblasts [16, 31]. We grew REF cultures to confluence in serum-containing medium, then stimulated them with Blebbistatin, an inhibitor of myosin light chain kinase, or Y-27632, a Rho kinase inhibitor, to determine how pharmacologically reducing force generation would influence MMP-2 message levels. We found that both inhibitors led to up-regulation of MMP-2 message and decreased SM α-actin message in serum-containing medium (Fig. 1), consistent with the changes in MMP-2 and SM α-actin message levels observed with RDFs cultured in collagen lattices and serum-free conditions.
Figure 1. Inhibiting Rho Kinase or Myosin Light Chain Kinase promotes MMP-2 expression in REFs cultured in serum-containing medium.
REFs were cultured to confluence in serum-containing medium, then challenged with Blebbistatin (50 µM) or Y-27632 (10 µM) for 24 hours in fresh serum-containing medium. Total RNA was analyzed by real-time PCR. Values represent the mean +/− average deviation from the mean; significance was determined by unpaired, two-tailed Student’s t-test (n=3).
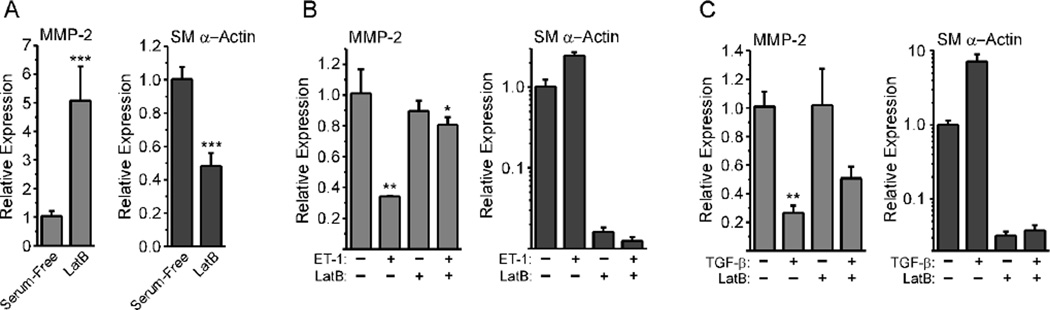
To determine if F-actin disassembly itself directly affected MMP-2 expression, we cultured REFs in serum-containing medium, then treated them with Lat B, which promotes actin fiber disassembly and G-actin formation [32]. Lat B increased MMP-2 mRNA expression 5-fold and decreased SM α-actin message levels by approximately 50% (Fig. 2A), consistent with the alterations in MMP-2 and SM α-actin mRNA levels observed in RDFs cultured in compliant collagen lattices. These findings confirmed that disassembly of F-actin increases MMP-2 mRNA levels. To determine if increasing cell contractility would suppress MMP-2 message expression as expected for a mechanically regulated gene, we cultured REFs under serum-free conditions, then challenged them with thrombin or ET-1, factors known to promote fibroblast contraction [33, 34].
Figure 2. Lat B reverses the down-regulation of MMP-2 induced by pro-contractile mediators.
A, REFs were grown to confluence in serum-containing medium, then challenged with Lat B for 24 hours in fresh serum-containing medium. Total RNA was analyzed by real-time PCR. Values represent the mean +/− average deviation from the mean; significance was analyzed by unpaired, two-tailed Student’s t-test (n=9). B–D, REFs were grown to confluence, serum-starved for 48 hours, then challenged with 3 ng/ml ET-1 (B; n = 3), thrombin (C; n = 6), or 10 ng/ml TGF-β (D; n = 6). Total RNA was collected after 24 hours for PCR analysis. Values are the mean +/− average deviation from the mean; significance was determined by one-way ANOVA with Tukey’s Multiple Comparison test.
ET-1 treatment down-regulated MMP-2 message by about 35% compared to serum-treated control (Fig. 2B). In contrast, treatment with Lat B did not alter MMP-2 message levels significantly. Treatment with ET-1 and Lat B together produced only a small, but statistically significant, reduction in MMP-2 message levels, indicating that preventing F-actin assembly also inhibited ET-1-driven reduction of MMP-2 message, consistent with actin-dependent regulation of MMP-2 mRNA levels. SM α-actin mRNA levels were not altered by ET-1; however, treatment with Lat B severely down-regulated SM α-actin mRNA levels. Stimulating cells with thrombin (Fig. 2C) or S1P (not shown), which induce Ca2+ flux and contraction similar to ET-1 [35], similarly down-regulated MMP-2 mRNA and up-regulated SM α-actin mRNA. TGF-β, which promotes the transition to the myofibroblast phenotype, strongly down-regulated MMP-2 message (Fig. 2D). Lat B only partially rescued MMP-2 message levels (Fig. 2D). As expected, TGF-β strongly up-regulated SM α-actin message, but was not able to up-regulate SM α-actin message in the presence of Lat B (Fig. 2D). This suggests that TGF-β acts partially through actin cytoskeleton-independent mechanisms to suppress MMP-2 mRNA levels. Together, these results suggest that promoting F-actin assembly and a more contractile, myofibroblast-like phenotype suppresses MMP-2 expression.
Rat dermal fibroblasts suppress MMP-2 expression when cultured in conditions that promote contractility
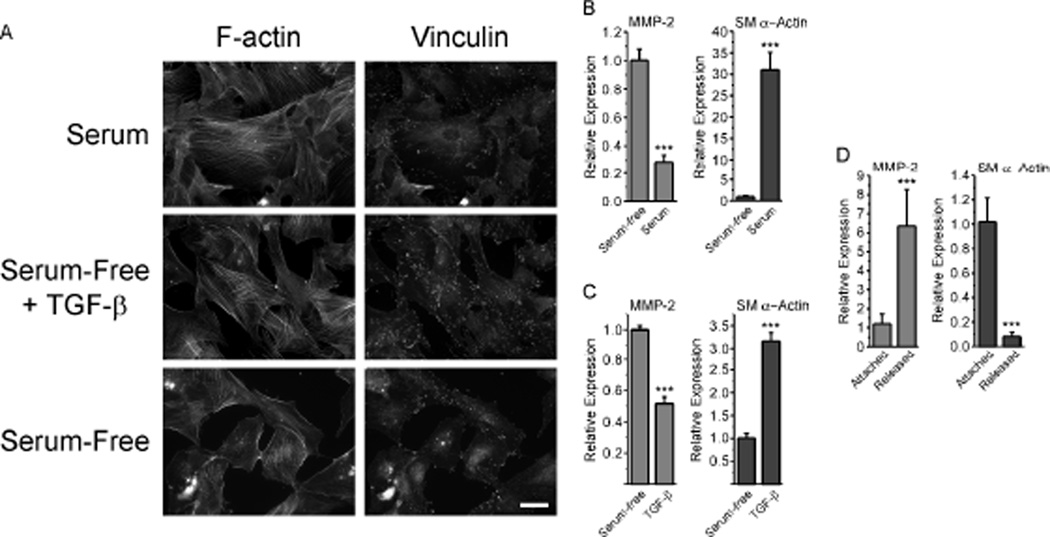
We next wished to determine if fibroblasts grown in a 3-D collagen lattice would alter MMP-2 expression in response to changes in lattice mechanical properties. To do this, we used rat dermal fibroblasts (RDFs), which we have found attach and spread well in lattices, something REFs do quite poorly. When cultured on plastic in serum-free conditions, RDFs had poorly organized stress fibers and few, small, vinculin-positive focal adhesions (Fig. 3A). In contrast, treatment of serum-starved cultures with serum-containing medium or serum-free medium plus 1 ng/ml TGF-β1 resulted in the formation of large, well-organized stress fibers and numerous, large, vinculin-positive focal adhesions (Fig. 3A). When we evaluated the expression of MMP-2 and SM α-actin under these conditions, we found that serum down-regulated MMP-2 message levels by over 70%, while SM α-actin message was up-regulated by over 30-fold (Fig. 3B). When cultured under serum-free conditions for 48 hours, then challenged with TGF-β, RDFs showed a 3-fold up-regulation of SM α-actin message within 24 hours, and a concomitant 50% decrease in MMP-2 message levels (Fig. 3C). When cultured in 3-D collagen lattices attached to the substratum, RDFs exert force that leads to increased tension on the collagen matrix [1, 29, 36]. When collagen lattices are released from the underlying substratum, this tension is dissipated after an initial contraction, and lattices then remain in a state of reduced stress. This change in the tension environment resulted in a 6-fold increase MMP-2 message levels and a concomitant 90% decrease in SM α-actin mRNA (Fig. 3D). Together, these results support the notion that environmental tension and the mechanical properties of fibroblasts have profound effects on MMP-2 expression, and that changes in MMP-2 mRNA expression are inversely related to changes in mRNA levels for SM α-actin, a key marker of the cell’s contractile state.
Figure 3. Pro-contractile conditions suppress MMP-2 expression by rat dermal fibroblasts.
A, RDFs were grown on glass coverslips, serum-starved for 24 hours, then treated with serum-free medium or serum-free medium supplemented with 10% serum or 1 ng/ml TGF-β1. RDFs were fixed 24 hours later, and stained with phalloidin and anti-vinculin antibody. For A–C, values represent the mean +/− average deviation from the mean; significance was determined by unpaired, two-tailed Student’s t-test. Scale bar = 50 µm. B, RDFs were grown to confluence, and cultures were replenished with fresh serum-containing medium or serum-free medium. After 24 hours total RNA was isolated for analysis by real-time PCR. (n = 4) C, RDFs were cultured to confluence, serum-starved for 48 hours, then switched to fresh serum-free medium plus 1 ng/ml TGF-β. Total RNA was isolated after 24 hours for PCR analysis (n = 3). D, RDFs were cultured in attached collagen lattices for 5 days and either allowed to remain attached for 24 hours or released and allowed to float in media for 24 hours. Total RNA was isolated from RDFs in collagen lattices for PCR analysis (n = 6).
F-actin assembly blocks IGF-1-induced MMP-2 up-regulation
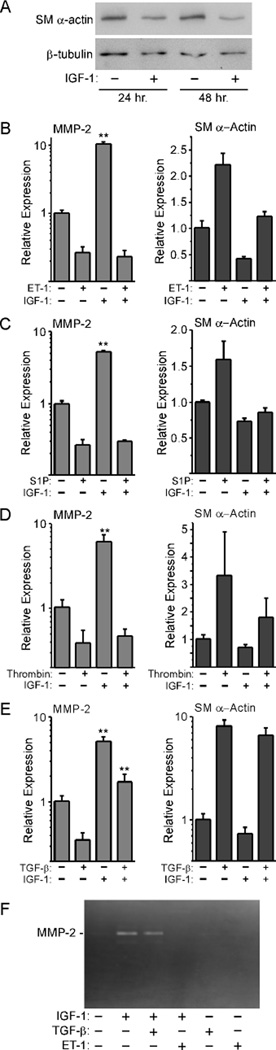
To determine if changes in the actin cytoskeleton could prevent growth factor-stimulated MMP-2 up-regulation, we stimulated serum-starved REFs with IGF-1, a known mediator of cell migration [37]. This resulted in a decrease in SM α-actin protein levels in lysates obtained from IGF-1-stimulated versus non-stimulated cells (Fig. 4A). This decrease was accompanied by a significant up-regulation of MMP-2 message levels, while SM α-actin message levels were somewhat reduced (Fig. 4B–E). This is in contrast to stimulation with ET-1, S1P, or thrombin, which suppressed MMP-2 and increased SM α-actin message levels. These G-protein-coupled receptor (GPCR) ligands promote fibroblast contraction through both Rho- and Ca2+-mediated actions [33, 38], which together should increase F-actin formation and contractile protein expression. Co-stimulation with IGF-1 plus any of these GPCR ligands blocked the IGF-1-stimulated up-regulation of MMP-2 message levels, and decreased MMP-2 message levels in comparison to treatment with IGF-1 alone. Co-treatment with TGF-β, which does not signal via a GPCR, showed little inhibition of the IGF-1 stimulated up-regulation of MMP-2 message. Zymography confirmed that release of MMP-2 into the culture medium paralleled the changes observed at the message level: ET-1 blocked the ability of IGF-1 to up-regulate MMP-2 expression, while TGF-β did not appreciably decrease MMP-2 expression (Fig. 4F).
Figure 4. Factors that promote contractile protein gene expression suppress IGF-1-mediated MMP-2 up-regulation.
A, REFs were cultured to confluence, serum-starved 48 hours, then co-stimulated in serum-free medium with 25 ng/ml IGF-1 for 24 and 48 hours. Lysates were evaluated by Western blots to detect SM α-actin and β-tubulin. REFs were cultured in the same way, then stimulated with IGF-1 plus 3 ng/ml ET-1 (B), 15 µM S1P (C), 3 nM thrombin (D), or 10 ng/ml TGF-β (E). Total RNA was isolated for real-time PCR analysis. In each case, MMP-2 up-regulation in response to IGF-1 was significant (P < 0.001) as assessed by one-way ANOVA with Tukey’s Multiple Comparison test; no significant differences were observed between IGF-1 + ET-1, thrombin, or S1P versus ET-1, thrombin, or S1P alone. Co-stimulation with IGF-1 + TGF-β resulted in a significant difference versus TGF-β alone or IGF-1 alone (P < 0.001 in both cases; n=6). F, MMP-2 in culture media was detected by gelatin zymography.
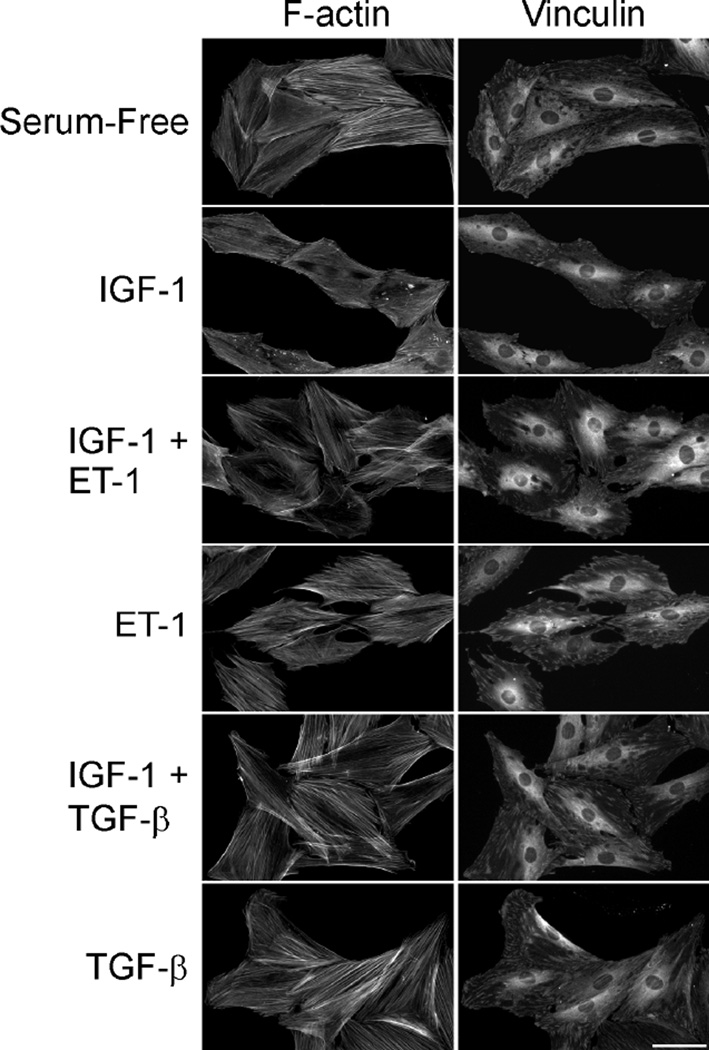
To confirm that the various growth factor treatments had the expected effects on the REF cytoskeleton, REFs were cultured under the various treatment conditions and then labeled using phalloidin and anti-vinculin to visualize F-actin stress fibers and focal adhesions, respectively (Fig. 5). IGF-1-stimulated cells showed a reduction in stress fibers and focal adhesions. Co-stimulation of cells with IGF-1 in combination with either ET-1 or TGF-β blocked these changes, with cells showing increased stress fibers and more prominent focal adhesions. Treatment of cells with either ET-1 or TGF-β alone promoted stress fiber formation and increased focal adhesion size, as expected. These findings are consistent with the known effects of these growth factors and the changes in MMP-2 message and protein levels described above. Taken together, these studies indicate that changes in the actin cytoskeleton regulate MMP-2 expression at the gene and protein levels, consistent with mechanical regulation of MMP-2. Furthermore, these studies also show that expression of MMP-2 is regulated inversely to SM α-actin, a well-known mechanically regulated gene. Finally, these studies indicate that growth factor signaling can further modulate MMP-2 expression.
Figure 5. IGF-1 stimulates a reduction in focal adhesion size and stress fibers in REFs.
Fibroblasts were grown on glass coverslips, serum-starved for 48 hours, then stimulated in fresh serum-free medium supplemented with 25 ng/ml IGF, 3 ng/ml ET-1, 10 ng/ml TGF-β, and combinations of factors. Cells were fixed 24 hours later, and stained with phalloidin and anti-vinculin antibody. Scale bar = 50 µm.
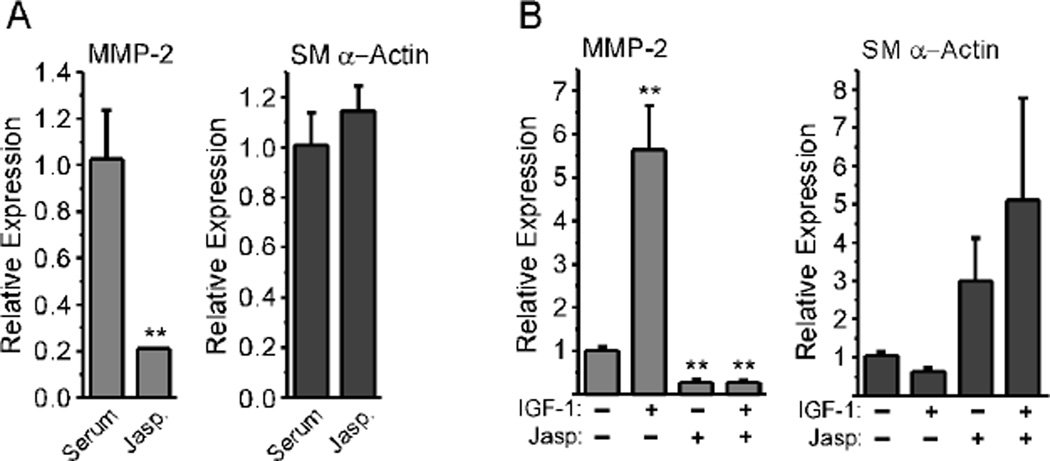
To more directly evaluate the role of F-actin in suppressing MMP-2 expression, we cultured REFs in serum-containing medium to promote the myofibroblast phenotype, then treated them with Jasplakinolide, a chemical enhancer of F-actin formation [39]. This resulted in an ~80% reduction in MMP-2 message levels (Fig. 6A). The already high SM α-actin levels did not increase significantly in response to Jasplakinolide. We next serum-starved REFs to promote a less contractile fibroblast phenotype, then stimulated cultures with IGF-1 in the presence of Jasplakinolide. We found that, with IGF-1 stimulation alone, MMP-2 message levels were strongly up-regulated, and SM α-actin message levels were down-regulated, as discussed above (Fig. 6B). Jasplakinolide suppressed MMP-2 message levels and elevated SM α-actin message levels, regardless of the presence of IGF-1. These results further support the idea that F-actin formation inhibits the up-regulation of MMP-2.
Figure 6. Jasplakinolide blocks IGF-1-mediated MMP-2 up-regulation.
A, REFs were grown to confluence in serum-containing medium, then incubated with Jasp (0.5 µM) for 24 hours in fresh, serum-containing medium. Total RNA was isolated for real-time PCR analysis. Values represent the mean +/− average deviation from the mean (n=3); significance was determined by unpaired, two-tailed Student’s t-test. B, REFs were grown to confluence, serum-starved for 48 hours, then pre-incubated with Jasp (0.5 µM) for 15 minutes in fresh serum-free medium. They were then stimulated for 24 hours with 25 ng/ml IGF-1. Total RNA was isolated for real-time PCR analysis. MMP-2 up-regulation was significantly different between IGF-1-stimulated and non-stimulated or Jasp-treated cultures (P < 0.001) as assessed by one-way ANOVA with Tukey’s Multiple Comparison test (n=9).
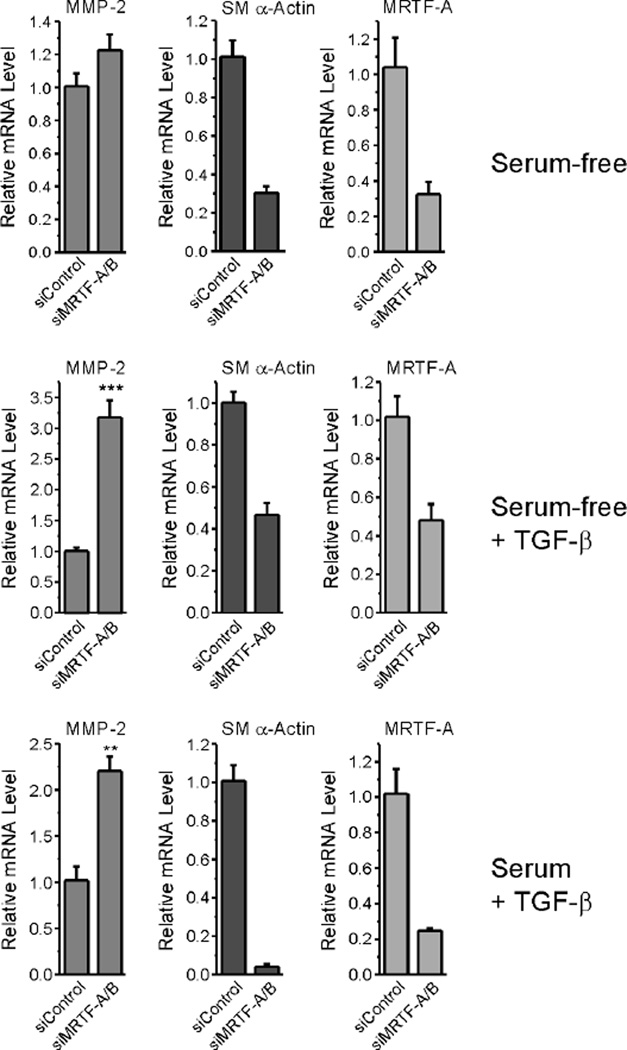
Increased contractility acts via a feed-forward mechanism to promote increased expression of contractile protein genes. This is directly mediated by the transcription factor, SRF, and its coactivators, MRTF-A and MRTF-B, which associate with G-actin, and are released upon the formation of F-actin [39]. Given that MMP-2 message was up-regulated under conditions that produced a concomitant decrease in SM α-actin expression and diminished F-actin stress fibers, we asked if there was a direct link between the mechanisms that regulate contractile proteins and those that suppress MMP-2 expression. We transfected REFs with an siRNA pool targeting both MRTF-A and B, then cultured cells in serum-free medium in the presence or absence of TGF-β. We have previously demonstrated that this strategy alters contractile protein expression in REFs [16]. For comparison, we cultured cells in parallel in serum-containing medium containing TGF-β to promote the transition to the myofibroblast phenotype. In all cases, MRTF-A message levels, which represent the majority of MRTFs expressed by REFs [16], were decreased by 70% or more, with a concomitant decrease in SM α-actin expression (Fig. 7). Cells treated with medium containing serum plus TGF-β, or treated with serum-free medium containing TGF-β, up-regulated MMP-2 message levels in response to MRTF-A/B knock-down. However, under serum-free conditions, MRTF-A/B knock-down resulted in no significant MMP-2 up-regulation. Taken together, these results indicate that, while actin dynamics regulate MMP-2 mRNA levels, this regulation is not directly mediated by the MRTFs alone.
Figure 7. Knockdown of MRTF-A/B increases expression of MMP-2 in fibroblasts stimulated with serum, but not in non-stimulated cultures.
REF-52 cell cultures were transfected with siRNA targeting MRTF-A and B or with control siRNA, then grown to confluence, serum-starved for 48 hours, and stimulated in fresh serum-free medium without (A) or with (B) TGF-β for 24 hours. P = 0.0002 by unpaired, two-tailed Student’s t-test. C, REF cultures were pre-treated with TGF-β1 (0.25 ng/mL) in the presence of 10% FBS for 72 hours to promote the myofibroblast phenotype, then transfected with MRTF-A/B or control siRNAs. In both experiments, total RNA was evaluated by real-time PCR to detect MMP-2, SM α-actin, and MRTF-A. P = 0.0052 by unpaired, two-tailed Student’s t-test. Error bars represent S.E.M.
DISCUSSION
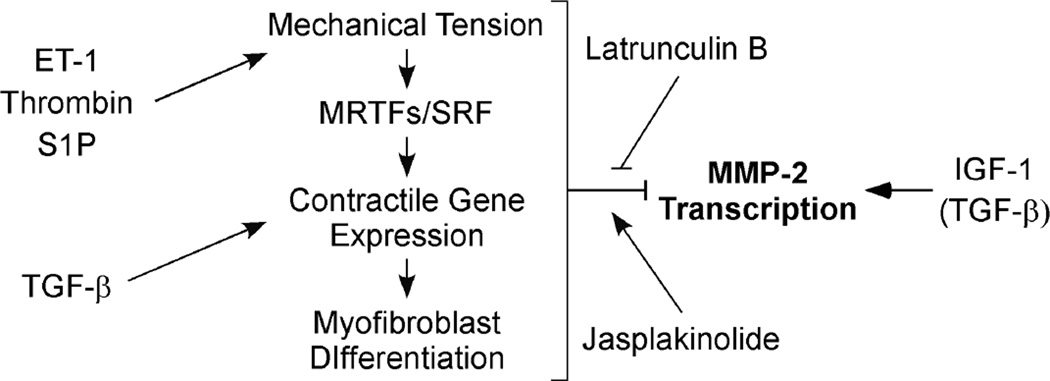
Mechanically regulated changes in contractile protein expression allow fibroblasts to fine-tune their responses to alterations in the mechanical properties of their extracellular environment during tissue repair. These same mechanical properties of the ECM may also influence the expression of genes associated with cell migration or matrix remodeling. Our studies examining the regulation of MMP-2, a factor important in modifying the extracellular environment during wound healing, suggest that this is, indeed, the case. Here, we demonstrate that conditions favoring contractile protein expression and the myofibroblast phenotype suppress MMP-2 expression, while conditions that reduce fibroblast contractility enhance MMP-2 expression. This suppression could be overcome by forcing the disassembly of F-actin, either through the addition of F-actin depolymerizing agents such as Lat B to 2-D cultures or by culturing cells in non-attached 3-D lattices that prevent the generation of force. In contrast, physiological factors that promote fibroblast contraction, like ET-1, thrombin, or S1P, all suppressed MMP-2 expression and blocked its up-regulation in response to IGF-1, a factor that promotes fibroblast migration. Jasplakinolide, which promotes F-actin assembly, also suppressed MMP-2 gene expression. However, while it appears that a highly contractile phenotype suppressed MMP-2 expression, the loss of contractility alone was not sufficient to promote MMP-2 up-regulation. Stimulating cells with the actin depolymerizing agent, Lat B, in the absence of other stimuli, caused contractile protein down-regulation, but did not up-regulate MMP-2 expression (see Fig. 2). In the presence of serum, which contains a variety of factors capable of up- or down-regulating MMP-2, Lat B increased MMP-2 expression, presumably by shifting the balance of signals away from contractility, leading to increased MMP-2 transcription. We propose the model depicted in Figure 8, in which mechanical regulation of actin dynamics modulates MMP-2 expression. Serum or pro-contractile GPCR ligands promote mechanical tension, which in turn promotes the increased expression MRTF-regulated contractile genes. This leads to conditions that favor F-actin formation and a myofibroblast phenotype. By a mechanism not yet clear, this phenotype suppresses MMP-2 expression. In contrast, conditions that favor F-actin depolymerization and thus increase G-actin levels, permit MMP-2 up-regulation, but only in the presence of appropriate stimulatory signals. IGF-1 can provide these signals. TGF-β acts as a mediator of contractile protein gene expression, but, as discussed below, may also stimulate MMP-2 expression under conditions that prevent myofibroblast formation.
Figure 8. Proposed model for mechanical regulation of MMP-2 by fibroblasts.
Select GPCR ligands promote cell contraction, which increases the mechanical tension exerted on the extracellular matrix. This increased tension is known to promote F-actin formation, which in turn liberates the SRF co-factors, MRTF-A and MRTF-B, from their association with G-actin. Free MRTF-A and MRTF-B up-regulate contractile protein gene expression, culminating in myofibroblast formation. By mechanisms not yet clear, these events suppress MMP-2 transcription. In contrast, IGF-1 and other pro-migration factors supply a critical signal that is required for MMP-2 up-regulation. However, this up-regulation is dependent on the reduction of the suppressive signals produced by the mechanical tension/SRF/myofibroblast axis. This can be modeled by the addition of Lat B, which promotes G-actin formation. Alternatively, promoting F-actin formation using Jasplakinolide restores MMP-2 suppression. TGF-β also promotes myofibroblast differentiation via pathways distinct from those activated by GPCR ligands. However, in the absence of MRTF-A and B, TGF-β promotes MMP-2 up-regulation, suggesting that the signaling pathways downstream of TGF-β are modulated by contractile protein gene expression.
Previous work by us and others strongly suggests that MMP-2 activation, and perhaps expression, is regulated by changes in the actin cytoskeleton. In our model, increased expression of MMP-2 required a decrease in cell tension; this decreased tension also promotes increased MMP-2 activation by fibroblasts, as we have reported earlier [26]. Others have shown, in endothelial cells, that increased Rho activation, which increases cell tension, leads to decreased MMP-2 expression and activation [40]. Co-culturing keratinocytes with fibroblasts leads to increased force generation and decreased MMP-2 expression by the fibroblasts [41]. In NIH3T3 cells, over-expression of the heat-shock protein, Hsp27, which promotes actin assembly and increased focal adhesion size, also decreases the amount of secreted MMP-2 [42]. In contrast, increased Rac activation, typically associated with reduced tension and increased migration, induces MMP-2 activation and cell invasion by HT1080 fibrosarcoma cells [43]. These studies support our model of F-actin-mediated suppression of MMP-2 up-regulation.
There are, however, a number of seemingly contradictory findings with regard to cell stress and MMP-2 expression. Other studies have shown, paradoxically, that Gα12/13 activation in NIH3T3 cells, which would be expected to promote Rho activation and stress fiber assembly, can increase MMP-2 expression [44]. Also in contrast to our study, ET-1 has been observed to increase MMP-2 expression and cell migration in colon cancer stromal fibroblasts [45]. Cyclic stretch in 2-D cultures induces MMP-2 expression by both fetal [46] and scleral fibroblasts [47]. Cyclic stress of cardiac fibroblast cultures increased cell proliferation, myofibroblast differentiation, and contractility, in parallel with increased MMP-2 expression [48]. These increases in MMP-2 expression appear to arise as the result of a mechanism(s) that is independent of changes in the actin cytoskeleton. For example, static stress up-regulates MMP-2 message levels in atrial myocytes, but this is dependent on the production of endogenous angiotensin, and can be inhibited by losartan, an inhibitor of Angiotensin-II type 1 receptors [49]. In 3-D collagen gels populated with lung airway fibroblasts, cyclic strain modestly increases both MMP-2 and SM α-actin expression [50]. While additional work will be needed to resolve the apparent differences seen in the various models, it should be appreciated that fibroblasts exposed to exogenous mechanical stresses respond very differently than do fibroblasts generating isometric contractile force on a non-deformable substratum [51]. Exogenous stretch activates mechanoreceptors and ion channels and promotes reorganization of the actin cytoskeleton, which does not occur with generation of isometric contractile force on a non-deformable substratum. In addition, fibroblasts in a 3-D tethered collagen lattice will, over time, reorganize the collagen matrix such that collagen fibrils align along lines of tension, resulting in a stabilized substratum and the generation of isometric contractile force. Again, this generates a very different response than exposure to exogenous stretch. As fibroblasts remodel a collagen matrix, they use tractional forces [36]. Dermal fibroblasts cultured in attached 3-D collagen lattices increase MMP-2 expression as matrix stiffness is increased during the early period of tractional remodeling [52]. Over time, they exert tension, and this results in the transition to a myofibroblast phenotype. In our studies, we used a 3-D lattice model to measure changes in MMP-2 levels in mature lattices five days after seeding. This results in the build-up of tension, which suppresses MMP-2 expression. Releasing such a lattice rapidly reduces tension, and promotes increased MMP-2 expression, as we demonstrate here.
Our findings are consistent with a mechanism by which the F-to-G-actin ratio governs MMP-2 message levels, much as it does for contractile protein markers such as SM α-actin. In fibroblasts, SM α-actin is co-regulated with a number of other contractile proteins in response to changes in MRTF-A and -B nuclear localization via a mechanism involving SRF [16, 39]. However, our data show that, while the suppression of MMP-2 levels hinges on the formation of F-actin, it does not appear to be directly linked to the mechanisms that regulate contractile protein expression. First, stimulating fibroblasts with Lat B resulted in the rapid loss of contractile protein expression, but caused only a modest increase in MMP-2 expression. Second, IGF-1 stimulated a rapid increase in MMP-2 expression, but elicited only a modest decrease in SM α-actin levels. Finally, knocking down the SRF co-factors, MRTF-A and B, consistently decreased expression of SM α-actin, but increased MMP-2 expression only in the presence of TGF-β. Furthermore, under serum-free conditions in the absence of TGF-β, MMP-2 levels were not increased in spite of the loss of SM α-actin. Together, these findings argue against a direct coupling of the SRF/MRTF axis and MMP-2 regulation, but rather suggest that MMP-2 expression is suppressed indirectly through the ability of the SRF-mediated signaling to influence the myofibroblast phenotype.
We have previously shown that MMP-2 up-regulation by VSMCs requires the activation of one or more positive signals and the loss of inhibitory signals [25]. In fibroblasts, one such inhibitory signal appears to depend on the actin cytoskeleton. However, our findings with TGF-β suggest that other, cytoskeleton-independent signals may also be involved. When we co-stimulated fibroblasts with IGF-1 plus TGF-β, we found that MMP-2 levels reflected a balance between positive and negative signals elicited by IGF-1 and TGF-β, respectively. In contrast, TGF-β-mediated increases in SM α-actin occurred regardless of the presence of IGF-1, indicating that TGF-β effects on SM α-actin expression are mediated by a mechanism distinct from TGF-β’s effects on MMP-2 expression. Previous work shows that contractile protein gene expression requires the presence of active TGF-β [7], and that TGF-β promotes the contractile phenotype in both myofibroblasts and VSMCs [1, 53]. In fibroblasts, we observe decreased MMP-2 expression in response to TGF-β. This is consistent with findings by others showing that endogenous TGF-β blocks TNF-α-stimulated up-regulation of MMP-2 expression in lung fibroblasts [54]. In VSMCs, decreased expression of TGF-β leads to increased MMP-2 expression [25]. Introducing a dominant negative TGFβRII construct into hepatic stellate cells decreases MMP-2 expression [55]. However, the effects of TGF-β on fibroblast phenotype can vary. Early studies linked TGF-β with increased MMP-2 expression by fibroblasts [56]. Fibroblasts stimulated with TGF-β in the presence of serum show increased SM α-actin and MMP-2 levels [57]. One possibility that could explain the diverse effects of TGF-β on MMP-2 production by fibroblasts is differential effects of Smad2 versus Smad3. Whereas Smad3 has been linked to fibrosis and matrix production, Smad2 is thought to oppose these actions and may itself promote MMP-2 expression in fibroblasts [58, 59].
Recently, it was suggested that TGF-β acts through a temporally delayed mechanism to stimulate myofibroblast formation [60]. This mechanism involves early Rho activation, which induces stress fiber formation, which in turn leads to increased MRTF-A-driven contractile protein expression. This same study found a more rapid induction of myofibroblast-like characteristics in response to GPCR ligands. This is similar to our findings that GPCR ligands such as ET-1, S1P, or thrombin consistently suppressed MMP-2 expression regardless of the presence of IGF-1. The ability of Lat B to completely reverse this suppression argues strongly for a mechanism linked to the actin cytoskeleton. TGF-β, on the other hand, only partially suppressed MMP-2 expression in the presence of IGF-1, and Lat B only partially reversed the down-regulation of MMP-2 expression induced by TGF-β alone. Thus, while both ET-1 and TGF-β reversed the loss of stress fibers and focal adhesions seen in IGF-1-stimulated fibroblasts (see Fig. 5), TGF-β appears to stimulate additional, cytoskeleton-independent signals that suppress MMP-2 expression. This is consistent with what is known about myofibroblast differentiation, which requires both force transduction plus TGF-β [51, 61]. Moreover, in the absence of MRTF-A/B, we found that TGF-β alone can promote MMP-2 expression (Fig. 7), suggesting that it can provide both positive and negative signals that influence MMP-2 expression. This may explain its pleiotropic effects, both in our hands and in previous studies by others. Clearly, additional studies into the mechanism of the mechanical regulation of MMP-2 are needed.
CONCLUSIONS
MMP-2 is up-regulated as fibroblasts transition away from a myofibroblast phenotype. The reduced expression of contractile proteins and the reduction of cell tension are prerequisites for MMP-2 up-regulation. Factors that promote cell tension, including serum factors, TGF-β, pro-contractile GPCR ligands, or culturing cells in collagen lattices under tension, down-regulate MMP-2. Forcing F-actin formation with Jasplakinolide results in down-regulation of MMP-2. In contrast, inducing actin depolymerization, either through the presence of the actin depolymerizing agent, Lat B, or by culturing fibroblasts in compliant collagen lattices, results in elevated MMP-2 expression. In most cases, the expression of the contractile protein marker, SM α-actin, is regulated inversely to MMP-2. Knocking down the expression of the SRF coactivators, MRTF-A and MRTF-B, results in the down-regulation of SM α-actin and the up-regulation of MMP-2, but this regulation is not seen in serum-free conditions. Therefore, the regulatory mechanisms that govern the expression of these two genes lead to reciprocal changes in expression, but do not appear to be directly linked. Rather, changes in the mechanical properties of the cell regulate MMP-2 expression through previously unidentified mechanisms. Why such a mechanism exists is not clear, but a mechanically mediated suppression of pro-migratory genes, particularly proteinases, might act as a fail-safe mechanism to prevent unwanted matrix turnover until cells are fully committed to a migratory phenotype. It would also make a migratory versus a contractile phenotype mutually exclusive.
HIGHLIGHTS.
Fibroblasts cultured in stiff versus compliant 3-D collagen lattices show reduced MMP-2 levels
Promoting contractile protein expression decreases MMP-2 expression
Factors that promote actin disassembly block the suppression of MMP-2 expression
TGF-β suppresses MMP-2 through multiple mechanisms
MRTF-A and MRTF-B suppress MMP-2 expression indirectly
Abbreviations
- DMEM
Dulbecco’s Modified Eagle’s Medium
- ECM
extracellular matrix
- ET-1
endothelin
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GPCR
G-protein-coupled receptor
- IGF-I
insulin-like growth factor-I
- Lat B
Latrunculin B
- MMP
matrix metalloproteinase
- MRTF
myocardin-like transcription factor
- PDGF
platelet-derived growth factor
- RDFs
rat dermal fibroblasts
- REFs
rat embryonic fibroblasts; SM α-actinsmooth muscle α-actin
- S1P
sphingosine-1-phosphate
- SRF
serum response factor
- TGF-β
transforming growth factor-β
- TIMP
tissue inhibitor of metalloproteinases
- VSMC
vascular smooth muscle cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 2.Schafer M, Werner S. Transcriptional control of wound repair. Annu Rev Cell Dev Biol. 2007;23:69–92. doi: 10.1146/annurev.cellbio.23.090506.123609. [DOI] [PubMed] [Google Scholar]
- 3.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 4.Hinz B. Masters and servants of the force: the role of matrix adhesions in myofibroblast force perception and transmission. Eur J Cell Biol. 2006;85:175–181. doi: 10.1016/j.ejcb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007;257:143–179. doi: 10.1016/S0074-7696(07)57004-X. [DOI] [PubMed] [Google Scholar]
- 6.Watsky MA, Weber KT, Sun Y, Postlethwaite A. New insights into the mechanism of fibroblast to myofibroblast transformation and associated pathologies. Int Rev Cell Mol Biol. 2010;282:165–192. doi: 10.1016/S1937-6448(10)82004-0. [DOI] [PubMed] [Google Scholar]
- 7.Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43:146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Sun Q, Chen G, Streb JW, Long X, Yang Y, Stoeckert CJ, Jr., Miano JM. Defining the mammalian CArGome. Genome Res. 2006;16:197–207. doi: 10.1101/gr.4108706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansen FE, Prywes R. Serum response factor: transcriptional regulation of genes induced by growth factors and differentiation. Biochim Biophys Acta. 1995;1242:1–10. doi: 10.1016/0304-419x(94)00014-s. [DOI] [PubMed] [Google Scholar]
- 10.Posern G, Treisman R. Actin' together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol. 2006;16:588–596. doi: 10.1016/j.tcb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Kumar MS, Owens GK. Combinatorial control of smooth muscle-specific gene expression. Arterioscler Thromb Vasc Biol. 2003;23:737–747. doi: 10.1161/01.ATV.0000065197.07635.BA. [DOI] [PubMed] [Google Scholar]
- 12.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 13.Pipes GC, Creemers EE, Olson EN. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev. 2006;20:1545–1556. doi: 10.1101/gad.1428006. [DOI] [PubMed] [Google Scholar]
- 14.Parmacek MS. Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ Res. 2007;100:633–644. doi: 10.1161/01.RES.0000259563.61091.e8. [DOI] [PubMed] [Google Scholar]
- 15.Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 2007;316:1749–1752. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]
- 16.Crider BJ, Risinger GM, Jr., Haaksma CJ, Howard EW, Tomasek JJ. Myocardin-Related Transcription Factors A and B Are Key Regulators of TGF-beta1-Induced Fibroblast to Myofibroblast Differentiation. J Invest Dermatol. 2011;131:2378–2385. doi: 10.1038/jid.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim Biophys Acta. 2010;1803:39–54. doi: 10.1016/j.bbamcr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Wu L, Derynck R. Essential role of TGF-beta signaling in glucose-induced cell hypertrophy. Dev Cell. 2009;17:35–48. doi: 10.1016/j.devcel.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 21.Hultgardh-Nilsson A, Lovdahl C, Blomgren K, Kallin B, Thyberg J. Expression of phenotype- and proliferation-related genes in rat aortic smooth muscle cells in primary culture. Cardiovasc Res. 1997;34:418–430. doi: 10.1016/s0008-6363(97)00030-8. [DOI] [PubMed] [Google Scholar]
- 22.Kenagy RD, Clowes AW. A possible role for MMP-2 and MMP-9 in the migration of primate arterial smooth muscle cells through native matrix. Ann N Y Acad Sci. 1994;732:462–465. doi: 10.1111/j.1749-6632.1994.tb24786.x. [DOI] [PubMed] [Google Scholar]
- 23.Risinger GM, Jr., Hunt TS, Updike DL, Bullen EC, Howard EW. Matrix metalloproteinase-2 expression by vascular smooth muscle cells is mediated by both stimulatory and inhibitory signals in response to growth factors. J Biol Chem. 2006;281:25915–25925. doi: 10.1074/jbc.M513513200. [DOI] [PubMed] [Google Scholar]
- 24.Uzui H, Lee JD, Shimizu H, Tsutani H, Ueda T. The role of protein-tyrosine phosphorylation and gelatinase production in the migration and proliferation of smooth muscle cells. Atherosclerosis. 2000;149:51–59. doi: 10.1016/s0021-9150(99)00295-6. [DOI] [PubMed] [Google Scholar]
- 25.Risinger GM, Jr., Updike DL, Bullen EC, Tomasek JJ, Howard EW. TGF-beta suppresses the upregulation of MMP-2 by vascular smooth muscle cells in response to PDGF-BB. Am J Physiol Cell Physiol. 2010;298:C191–C201. doi: 10.1152/ajpcell.00417.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomasek JJ, Halliday NL, Updike DL, Ahern-Moore JS, Vu TK, Liu RW, Howard EW. Gelatinase A activation is regulated by the organization of the polymerized actin cytoskeleton. J Biol Chem. 1997;272:7482–7487. doi: 10.1074/jbc.272.11.7482. [DOI] [PubMed] [Google Scholar]
- 27.Xie TX, Huang FJ, Aldape KD, Kang SH, Liu M, Gershenwald JE, Xie K, Sawaya R, Huang S. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66:3188–3196. doi: 10.1158/0008-5472.CAN-05-2674. [DOI] [PubMed] [Google Scholar]
- 28.Freshney IJ. Culture of Animal Cells: A Manual of Basic Technique. New York: Alan R. Liss, Inc; 1987. [Google Scholar]
- 29.Vaughan MB, Howard EW, Tomasek JJ. Transforming growth factor-beta1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res. 2000;257:180–189. doi: 10.1006/excr.2000.4869. [DOI] [PubMed] [Google Scholar]
- 30.Parizi M, Howard EW, Tomasek JJ. Regulation of LPA-promoted myofibroblast contraction: role of Rho, myosin light chain kinase, and myosin light chain phosphatase. Exp Cell Res. 2000;254:210–220. doi: 10.1006/excr.1999.4754. [DOI] [PubMed] [Google Scholar]
- 31.Guilluy C, Swaminathan V, Garcia-Mata R, O'Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011;13:722–727. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spector I, Shochet NR, Blasberger D, Kashman Y. Latrunculins--novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell Motil Cytoskeleton. 1989;13:127–144. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
- 33.Follonier Castella L, Gabbiani G, McCulloch CA, Hinz B. Regulation of myofibroblast activities: calcium pulls some strings behind the scene. Exp Cell Res. 2010;316:2390–2401. doi: 10.1016/j.yexcr.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 34.Bogatkevich GS, Tourkina E, Silver RM, Ludwicka-Bradley A. Thrombin differentiates normal lung fibroblasts to a myofibroblast phenotype via the proteolytically activated receptor-1 and a protein kinase C-dependent pathway. J Biol Chem. 2001;276:45184–45192. doi: 10.1074/jbc.M106441200. [DOI] [PubMed] [Google Scholar]
- 35.Jiang H, Rhee S, Ho CH, Grinnell F. Distinguishing fibroblast promigratory and procontractile growth factor environments in 3-D collagen matrices. Faseb J. 2008;22:2151–2160. doi: 10.1096/fj.07-097014. [DOI] [PubMed] [Google Scholar]
- 36.Grinnell F, Petroll WM. Cell Motility and Mechanics in Three-Dimensional Collagen Matrices. Annu Rev Cell Dev Biol. 2010 doi: 10.1146/annurev.cellbio.042308.113318. [DOI] [PubMed] [Google Scholar]
- 37.LeRoith D, Roberts CT., Jr. The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 38.Pyne S, Lee SC, Long J, Pyne NJ. Role of sphingosine kinases and lipid phosphate phosphatases in regulating spatial sphingosine 1-phosphate signalling in health and disease. Cell Signal. 2009;21:14–21. doi: 10.1016/j.cellsig.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 40.Ispanovic E, Serio D, Haas TL. Cdc42 and RhoA have opposing roles in regulating membrane type 1-matrix metalloproteinase localization and matrix metalloproteinase-2 activation. Am J Physiol Cell Physiol. 2008;295:C600–C610. doi: 10.1152/ajpcell.00460.2007. [DOI] [PubMed] [Google Scholar]
- 41.Wall IB, Bhadal N, Broad S, Whawell SA, Mudera V, Lewis MP. Force generation and protease gene expression in organotypic co-cultures of fibroblasts and keratinocytes. J Tissue Eng Regen Med. 2009;3:647–650. doi: 10.1002/term.206. [DOI] [PubMed] [Google Scholar]
- 42.Lee JW, Kwak HJ, Lee JJ, Kim YN, Park MJ, Jung SE, Hong SI, Lee JH, Lee JS. HSP27 regulates cell adhesion and invasion via modulation of focal adhesion kinase and MMP-2 expression. Eur J Cell Biol. 2008;87:377–387. doi: 10.1016/j.ejcb.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Zhuge Y, Xu J. Rac1 mediates type I collagen-dependent MMP-2 activation. role in cell invasion across collagen barrier. J Biol Chem. 2001;276:16248–16256. doi: 10.1074/jbc.m010190200. [DOI] [PubMed] [Google Scholar]
- 44.Kim ES, Lee KM, Noh DY, Moon A. Regulation of matrix metalloproteinases and invasion by G(alpha12/13) proteins in NIH3T3 mouse fibroblast cells. Oncol Res. 2011;19:297–301. doi: 10.3727/096504011x13021877989919. [DOI] [PubMed] [Google Scholar]
- 45.Knowles JP, Shi-Wen X, Haque SU, Bhalla A, Dashwood MR, Yang S, Taylor I, Winslet MC, Abraham DJ, Loizidou M. Endothelin-1 stimulates colon cancer adjacent fibroblasts. Int J Cancer. 2011 doi: 10.1002/ijc.26090. [DOI] [PubMed] [Google Scholar]
- 46.Hawwa RL, Hokenson MA, Wang Y, Huang Z, Sharma S, Sanchez-Esteban J. Differential Expression of MMP-2 and −9 and their Inhibitors in Fetal Lung Cells Exposed to Mechanical Stretch: Regulation by IL-10. Lung. 2011;189:341–349. doi: 10.1007/s00408-011-9310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shelton L, Rada JS. Effects of cyclic mechanical stretch on extracellular matrix synthesis by human scleral fibroblasts. Exp Eye Res. 2007;84:314–322. doi: 10.1016/j.exer.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dalla Costa AP, Clemente CF, Carvalho HF, Carvalheira JB, Nadruz W, Jr., Franchini KG. FAK mediates the activation of cardiac fibroblasts induced by mechanical stress through regulation of the mTOR complex. Cardiovasc Res. 2010;86:421–431. doi: 10.1093/cvr/cvp416. [DOI] [PubMed] [Google Scholar]
- 49.Saygili E, Rana OR, Meyer C, Gemein C, Andrzejewski MG, Ludwig A, Weber C, Schotten U, Kruttgen A, Weis J, Schwinger RH, Mischke K, Rassaf T, Kelm M, Schauerte P. The angiotensin-calcineurin-NFAT pathway mediates stretch-induced up-regulation of matrix metalloproteinases-2/−9 in atrial myocytes. Basic Res Cardiol. 2009;104:435–448. doi: 10.1007/s00395-008-0772-6. [DOI] [PubMed] [Google Scholar]
- 50.Choe MM, Sporn PH, Swartz MA. Extracellular matrix remodeling by dynamic strain in a three-dimensional tissue-engineered human airway wall model. Am J Respir Cell Mol Biol. 2006;35:306–313. doi: 10.1165/rcmb.2005-0443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiquet M, Gelman L, Lutz R, Maier S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim Biophys Acta. 2009;1793:911–920. doi: 10.1016/j.bbamcr.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 52.Karamichos D, Brown RA, Mudera V. Collagen stiffness regulates cellular contraction and matrix remodeling gene expression. J Biomed Mater Res A. 2007;83:887–894. doi: 10.1002/jbm.a.31423. [DOI] [PubMed] [Google Scholar]
- 53.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 54.Ye H, Cai PC, Zhou Q, Ma WL. Transforming growth factor-beta1 suppresses the up-regulation of matrix metalloproteinase-2 by lung fibroblasts in response to tumor necrosis factor-alpha. Wound Repair Regen. 2011;19:392–399. doi: 10.1111/j.1524-475X.2011.00680.x. [DOI] [PubMed] [Google Scholar]
- 55.Marquez-Aguirre A, Sandoval-Rodriguez A, Gonzalez-Cuevas J, Bueno-Topete M, Navarro-Partida J, Arellano-Olivera I, Lucano-Landeros S, Armendariz-Borunda J. Adenoviral delivery of dominant-negative transforming growth factor beta type II receptor up-regulates transcriptional repressor SKI-like oncogene, decreases matrix metalloproteinase 2 in hepatic stellate cell and prevents liver fibrosis in rats. J Gene Med. 2009;11:207–219. doi: 10.1002/jgm.1303. [DOI] [PubMed] [Google Scholar]
- 56.Overall CM, Wrana JL, Sodek J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-beta. J Biol Chem. 1989;264:1860–1869. [PubMed] [Google Scholar]
- 57.Simionescu A, Simionescu DT, Vyavahare NR. Osteogenic responses in fibroblasts activated by elastin degradation products and transforming growth factor-beta1: role of myofibroblasts in vascular calcification. Am J Pathol. 2007;171:116–123. doi: 10.2353/ajpath.2007.060930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meng XM, Huang XR, Chung AC, Qin W, Shao X, Igarashi P, Ju W, Bottinger EP, Lan HY. Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis. J Am Soc Nephrol. 2010;21:1477–1487. doi: 10.1681/ASN.2009121244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piek E, Ju WJ, Heyer J, Escalante-Alcalde D, Stewart CL, Weinstein M, Deng C, Kucherlapati R, Bottinger EP, Roberts AB. Functional characterization of transforming growth factor beta signaling in Smad2- and Smad3-deficient fibroblasts. J Biol Chem. 2001;276:19945–19953. doi: 10.1074/jbc.M102382200. [DOI] [PubMed] [Google Scholar]
- 60.Sandbo N, Lau A, Kach J, Ngam C, Yau D, Dulin NO. Delayed stress fiber formation mediates pulmonary myofibroblast differentiation in response to TGF-beta. Am J Physiol Lung Cell Mol Physiol. 2011;301:L656–L666. doi: 10.1152/ajplung.00166.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao XH, Laschinger C, Arora P, Szaszi K, Kapus A, McCulloch CA. Force activates smooth muscle alpha-actin promoter activity through the Rho signaling pathway. J Cell Sci. 2007;120:1801–1809. doi: 10.1242/jcs.001586. [DOI] [PubMed] [Google Scholar]