Abstract
Background
This is an update of a Cochrane review that was first published in Issue 1, 2009. Gestational trophoblastic neoplasia (GTN) is a rare but curable disease arising in the fetal chorion during pregnancy. Most women with low-risk GTN will be cured by evacuation of the uterus with or without single-agent chemotherapy. However, chemotherapy regimens vary between treatment centres worldwide and the comparable benefits and risks of these different regimens are unclear.
Objectives
To determine the efficacy and safety of first-line chemotherapy in the treatment of low-risk GTN.
Search methods
In September 2008, we electronically searched the Cochrane Gynaecological Cancer Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL Issue 3, 2008), MEDLINE and EMBASE. In addition, we searched online trial registers, conference proceedings and reference lists of identified studies. We re-ran these searches in February 2012 for this updated review.
Selection criteria
For the original review, we included randomised controlled trials (RCTs), quasi-RCTs and non-RCTs that compared first-line chemotherapy for the treatment of low-risk GTN. For this updated version of the review, we included only RCTs.
Data collection and analysis
Two review authors independently assessed studies for inclusion and extracted data to a pre-designed data extraction form. Meta-analysis was performed by pooling the risk ratio (RR) of individual trials.
Main results
We included five moderate to high quality RCTs (517 women) in the updated review. These studies all compared methotrexate with dactinomycin. Three studies compared weekly intramuscular (IM) methotrexate with bi-weekly pulsed intravenous (IV) dactinomycin (393 women), one study compared five-day IM methotrexate with bi-weekly pulsed IV dactinomycin (75 women) and one study compared eight-day IM methotrexate-folinic acid (MTX-FA) with five-day IV dactinomycin (49 women).
Overall, dactinomycin was associated with significantly higher rates of primary cure than methotrexate (five studies, 513 women; RR 0.64, 95% Confidence Interval (CI) 0.54 to 0.76). Methotrexate was associated with significantly more treatment failure than dactinomycin (five studies, 513 women; RR 3.81, 95% CI 1.64 to 8.86). We consider this evidence to be of a moderate quality.
There was no significant difference between the two groups with respect to nausea (four studies, 466 women; RR 0.61, 95% CI 0.29 to 1.26) or any of the other individual side-effects reported, although data for all of these outcomes were insufficient and too heterogeneous to be conclusive. No severe adverse effects (SAEs) occurred in either group in three out of the five included studies and there was no significant difference in SAEs between the groups overall (five studies, 515 women; RR 0.35, 95% CI 0.08 to 1.66; I2 = 60%), however, there was a trend towards fewer SAEs in the methotrexate group. We considered this evidence to be of a low quality due to substantial heterogeneity and low consistency in the occurrence/reporting of SAEs between trials.
Authors’ conclusions
Dactinomycin is more likely to achieve a primary cure in women with low-risk GTN, and less likely to result in treatment failure, compared with methotrexate. There is limited evidence relating to side-effects, however, the pulsed dactinomycin regimen does not appear to be associated with significantly more side-effects than the low-dose methotrexate regimen and therefore should compare favourably to the five- and eight-day methotrexate regimens in this regard.
We consider pulsed dactinomycin to have a better cure rate than, and a side-effect profile at least equivalent to, methotrexate when used for first-line treatment of low-risk GTN. Data from a large ongoing trial of pulsed dactinomycin compared with five- and eight-day methotrexate regimens is likely to have an important impact on our confidence in these findings.
Medical Subject Headings (MeSH): Antineoplastic Agents [*administration & dosage; adverse effects], Case-Control Studies, Cohort Studies, Dactinomycin [*administration & dosage; adverse effects], Drug Administration Schedule, Gestational Trophoblastic Disease [*drug therapy], Leucovorin [administration & dosage], Methotrexate [*administration & dosage; adverse effects], Randomized Controlled Trials as Topic, Risk, Vitamin B Complex [administration & dosage]
MeSH check words: Female, Humans, Pregnancy
BACKGROUND
This is an updated version of this original review first published in the Cochrane Database of Systematic Reviews, 2009, Issue 1.
Description of the condition
Gestational trophoblastic disease (GTD) is a rare disease of pregnancy arising in the fetal chorion. It encompasses five main clinicopathologic forms: hydatidiform mole (complete and partial), invasive mole, choriocarcinoma, placental site trophoblastic tumour (PSTT) and epithelioid trophoblastic tumour (ETT). The term gestational trophoblastic neoplasia (GTN) refers only to the invasive and malignant forms of GTD i.e. invasive mole, choriocarcinoma, PSTT and ETT. GTN may develop after a molar or non-molar pregnancy, irrespective of the site and gestational age, as a consequence of autonomous overgrowth of one of the three cell layers of the trophoblast.
The incidence of GTD varies between different regions of the world, with higher rates reported in Indonesia (around 10 per 1000 pregnancies), Mexico (4.6 per 1000) and Japan (two per 1000) and lower rates reported in North America and Europe (less than one per 1000); however, rates differ according to whether studies are population-based or hospital-based and may vary between areas within the same country (Lee 2009). Newer data from North America and Asia suggest that rates of GTD are declining (Lee 2009). The aetiology of GTD is poorly understood; a previous molar pregnancy and advanced or very young maternal age are associated with an increased risk of GTD; however, other factors including ethnicity, poor nutrition, viral infections and environmental factors may play a role (Lee 2009).
Complete moles (CMs) usually arise as a consequence of duplication of the haploid sperm following fertilisation of an ‘empty’ ovum, and are therefore diploid and androgenic in origin, with no evidence of fetal tissue. Partial moles (PMs) are typically triploid in origin with two sets of paternal haploid and one set of maternal haploid genes (Fisher 2009). In most cases, moles resolve spontaneously following one or more uterine evacuations without a need for chemotherapy, however, in approximately 16% of CMs and 0.5% of PMs the disease persists and chemotherapy is required (Seckl 2009). Molar transformation to GTN results in an enlarging uterine mass that may invade locally, metastasize to other sites (most ominously, the liver or brain), and lead to death if left untreated. The most common clinical manifestations of post-molar GTN are vaginal bleeding, uterine and ovarian enlargement, and raised human chorionic gonadotrophin (hCG) levels (Lurain 2010).
Moles are considered to have undergone transformation to GTN if four or more hCG values indicate a plateau over a period of at least three weeks, if there is a rise in at least three consecutive hCG values by at least 10% over a two-week period, if hCG values are raised six months after evacuation, or if there is a histological diagnosis of choriocarcinoma (Kohorn 2009). Urine hCG levels may be helpful in predicting malignant transformation (Alazzam 2011). Furthermore, a recent randomised controlled trial (RCT) has shown that Vitamin A prophylaxis may reduce the risk of malignant transformation (Andrijono 2010).
Various staging and scoring systems have been developed over the years (Hammond 1973; Bagshawe 1976; WHO 1983; Nagan 2002). The system described by Bagshawe 1976 formed the basis of the WHO Prognostic scoring System (WHO 1983) that included age, antecedent pregnancy, interval since antecedent pregnancy, hCG level, ABO blood group, largest tumour site(s) of metastases, site of metastases and previous chemotherapy. This system was subsequently modified and adapted by FIGO (see Table 1; Table 2) (Nagan 2002; FIGO 2009). The modified WHO-FIGO system differs from the WHO system in that the ABO blood group risk factor has been eliminated and the risk factor for liver metastases has been upgraded from two to four. A score of six or less defines ‘low-risk’ due to the merging of the old intermediate risk group (previously described by scores of five and six) with the existing low risk category (score zero to four). A score of seven or more defines ‘high-risk’.
Table 1.
FIGO anatomical staging *
| Stage I | Disease confined to the uterus |
| Stage II | GTN extends outside of the uterus, but is limited to the genital structures (adnexae, vagina, broad ligament) |
| Stage III | GTN extends to the lungs with or without known genital tract involvement |
| Stage IV | All other metastatic sites |
Table 2.
Modified WHO Prognostic Scoring System as adapted by FIGO for GTN
| Scores | 0 | 1 | 2 | 4 |
| Age (years) | < 40 | ≥ 40 | - | - |
| Antecedent pregnancy | mole | abortion | term | - |
| Interval months from index pregnancy | < 4 | 4-6 | 7-12 | > 12 |
| Pretreatment serum hCG (iu/1) | < 103 | 103-104 | 104-105 | > 105 |
| Largest tumour size (including uterus) | < 3 | 3-4 cm | ≥ 5 cm | - |
| Site of metastases | lung | spleen, kidney | gastrointestinal | liver, brain |
| Number of metastases | - | 1-4 | 5-8 | > 8 |
| Previous failed chemotherapy | - | - | single drug | ≥ 2 drugs |
| To stage and allot a risk factor score, a patient’s diagnosis is allocated to a stage as represented by a Roman numeral I, II, III, and IV. This is then separated by a colon from the sum of all the actual risk factor scores expressed in Arabic numerals, i.e., stage II:4, stage IV:9. This stage and score will be allotted for each patient. (FIGO 2009). A score ≤ 6 indicates low-risk; > 6 indicates high-risk. | ||||
hCG = human chorionic gonadotrophin
Low-risk GTN includes invasive moles and choriocarcinomas that receive a low-risk score. Once the uterus has been evacuated (preferably by suction curettage to minimise the chance of uterine perforation) and a diagnosis of low-risk GTN has been made, either histologically or following serial hCG measurements, treatment with single agent chemotherapy is usually commenced. Indications for commencing chemotherapy vary, with some clinicians preferring a conservative approach. In the UK, the indications for commencing chemotherapy include (Seckl 2010):
a plateaued or rising hCG concentration after evacuation;
heavy vaginal bleeding or evidence of gastrointestinal or intraperitoneal haemorrhage;
evidence of metastatic disease;
serum hCG equal to or greater than 20 000 IU/L four weeks or more after evacuation; and
a raised hCG six months after evacuation.
Description of the intervention
There are many effective chemotherapeutic regimens used worldwide for the treatment of low-risk GTN, mostly involving methotrexate and dactinomycin. The first report of methotrexate therapy for GTN was in 1956 (Hertz 1956). By 1971, methotrexate had been reported in conjunction with folinic acid as “rescue” from the severe marrow and gestational toxicities seen with high-dose methotrexate given alone (Bagshawe 1976) and dactinomycin had been reported as a drug of choice for initial therapy (Goldstein 1972).
The most commonly used first-line regimens for treating low-risk GTN are as follows:
Methotrexate eight-day regimen (1 mg/kg intramuscular (IM), days one, three, five and seven) with folinic acid rescue (days two, four six and eight), repeated every 14 to 16 days (Bagshawe 1989; McNeish 2002); also know as the Charing Cross or Modified Bagshawe regimen;
Low-dose (30 to 50 mg/m2) IM methotrexate, repeated weekly (Homesley 1988; Homesley 1990);
Five-day low-dose methotrexate (intravenous (IV) or IM); maximum of 25mg/m2 daily for five days, repeated every 14 days (Soper 1994; Roberts 1996);
Pulsed IV dactinomycin (1.25 mg/m2 to a maximum 2 mg single dose), repeated every 14 days (Twiggs 1983; Schlaerth 1984; Petrilli 1987; Osborne 2011); and
Five-day dactinomycin (0.5 mg IV), repeated every 14 days (Osathanondh 1975; Kohorn 1996).
Other regimens that have been described are included in Table 3. Most women with low-risk GTN will be cured by chemotherapy regardless of the regimen used; however, reported primary remission rates vary and up to 40% per cent of patients may require additional drug therapy to effect a cure (Homesley 1988; Soper 1994; Lurain 1995; McNeish 2002; Khan 2003). In a recent analysis of 359 patients with low-risk GTN treated between 1979 and 2006 at the Brewer Trophoblastic Center (Chicago), approximately 80% of women were cured with first-line single agent therapy (mainly methotrexate), an additional 10% responded to sequential single-agent therapy and approximately 10% needed multi-agent therapy (Lurain 2011).
Table 3.
Other first line chemotherapy regimens described
| Drug | Study | Comment |
|---|---|---|
| Intravenous (IV) methotrexate (100, 150, or 300 mg/m2) with folinic acid rescue 24 hours later, repeated weekly | Bagshawe 1976 | The original Baghawe regimen. |
| Bolus (100mg/m2 IV or IM) and 12-hour continuous methotrexate infusion (200mg/m2) with folinic acid rescue 24 hours later, repeated fortnightly | Garrett 2002 | |
| Combined 5-day methotrexate (day 1 to 5) and 5-day dactinomycin (day 15 to 19) , repeated every 28 days | Abrao 2008; Smith 1975; Rose 1989 | Associated with a high incidence of toxicity. |
| High dose methotrexate (600mg/m2) | Elit 1994 | Did not affect a higher cure than other methotrexate regimens |
| Etoposide (oral and parenteral) | Hitchins 1988; Wong 1984; Wong 1986 | Reported to be highly effective but has not gained acceptance for low-risk GTN due to the high incidence of side-effects |
| Fluorouracil | Sung 1984; Song 1998 | Used in China for several decades, mainly because of its low cost, but is not favoured elsewhere |
| Intra-lesional methotrexate infusion | Su 2001 | Not favoured in Europe or North America. |
| Chinese preparations | Wang 1998 | Not favoured in Europe or North America. |
Due to the chemosensitive nature of this disease and its’ low prevalence, the choice of treatment regimen depends more on geographic location and clinician’s experience/preference than high quality evidence relating to the relative efficacy and side-effects of the various regimens. In Europe and North America, there is a preference for the five-day or eight-day methotrexate regimens. On these regimens, women are usually hospitalised for the first cycle due to concerns regarding potential haemorrhage from arteriovenous malformations, and cycles are usually continued for at least three weeks once hCG is normal.
Historically, five-day dactinomycin has been associated with severe alopecia and nausea; therefore, in many centres, it is reserved as salvage therapy in cases of methotrexate resistance or toxicity. However, pulsed dactinomycin every 14 days is reported to be effective, with minimal side-effects, when used as salvage therapy (Covens 2006), and as effective as weekly methotrexate when used as first-line therapy (Twiggs 1983; Schlaerth 1984; Petrilli 1987). In addition, this pulsed dactinomycin regimen has potential advantages over the other regimens in terms of convenience and cost. Drug resistance and toxic side-effects leading to discontinuation and switching to an alternative regimen may occur with either drug. Predictors of resistance to single-agent treatment in low-risk GTN include non-molar antecedent pregnancy, a histological diagnosis of choriocarcinoma (Hammond 1973; Lurain 1995), higher pre-treatment hCG levels (Yarandi 2008; McGrath 2010) and higher risk scores (Osborne 2011).
Why it is important to do this review
We embarked on this systematic review and meta-analysis because various treatment regimens are used for the first-line treatment of low-risk GTN, yet the comparative benefits and risks of these regimens were unclear.
OBJECTIVES
To assess the efficacy and safety of the various first-line chemotherapy regimens in the treatment of women with low-risk GTN.
METHODS
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing first-line chemotherapy regimens for the treatment of low-risk GTN.
Types of participants
Inclusions
All women with low-risk GTN (as defined by any of the known risk scoring systems), who received primary chemotherapy. Studies that did not provide complete information about the risk scoring system, or that did not distinguish a low-risk group were excluded from the review.
Exclusions
Women with high-risk GTN, placental site trophoblastic tumour (PSTT) or epithelial trophoblastic tumour (ETT).
Types of interventions
Any chemotherapeutic agent used in the first-line treatment of GTN (e.g. methotrexate, dactinomycin, fluorouracil, etoposide) in any dose, duration, frequency and setting.
Types of outcome measures
Primary outcomes
Primary cure (remission)
Failure of first-line therapy
Overall survival (OS)
Death due to toxicity
Death due to disease
Secondary outcomes
Mean number of courses or time to first-line cure
Mean number of courses or time to first-line failure (failure was defined as change in regimen due to drug resistance or toxicity, or surgery for drug resistance)
Quality of life (QoL), measured by a validated scale
Secondary tumours due to chemotherapy
Toxicity due to chemotherapy
Grades of toxicity were extracted according to CTCAE 2010:
(a) haematological (anaemia, neutropenia, abnormal liver function);
(b) gastrointestinal (pain, nausea, vomiting);
(c) genitourinary (vaginal bleeding);
(d) skin (stomatitis, mucositis, alopecia, allergy);
(e) neurological (peripheral and central);
(f) respiratory (pain, shortness of breath, pleural effusion).
Search methods for identification of studies
Electronic searches
The original search included Cochrane Gynaecological Cancer Group Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL, Issue 3 2008), Ovid MEDLINE (1950 to 2008) and EMBASE (1980 to 2008) in September 2008. For this revised review, these database searches were extended to February 2012.
The search strategy was broad and we adapted the key words in the strategies in the databases listed in Searching other resources, as appropriate. We considered papers in all languages. For the original search strategies see Appendix 1 and for the 2012 update see Appendix 2 .
Searching other resources
In addition to electronic searches, we searched the following for ongoing trials: National Research Register, National Cancer Institute, Metaregister of Controlled Trials and the Medical Research Council Clinical Trial Directory. We searched reference lists of identified studies for additional articles.
Data collection and analysis
Selection of studies
Two review authors independently selected articles on the basis of title and/or abstract for full text scrutiny (Mo’iad Alazzam [MA] and John Tidy [JT] for the original review; MA and Tess Lawrie [TL] for the update). We excluded those studies that clearly did not meet the inclusion criteria and obtained the full text of the others. MA and JT independently assessed each article to determine whether it met the review eligibility criteria. This was done by MA and TL for the update. Differences were resolved by discussion between the two review authors or by involving a third review author.
Data extraction and management
For the original review, MA and JT independently extracted data using a pre-designed data extraction form; for the update, this was performed by MA and TL. We included the following information from each study.
Design: description of randomisation method, blinding, number of study centres, study duration and number of study withdrawals.
Participants: number, mean age, mean risk score.
Intervention; name of chemotherapy agents used, dose, route of administration and schedule.
Outcomes: Where possible data was extracted to allow intention-to-treat (ITT) analysis. For dichotomous outcomes (e.g. primary cure, adverse events, and number of patients who relapsed or died), we abstracted the number of patients in each treatment arm who experienced the outcome of interest, in order to estimate a risk ratio (RR). For continuous outcomes (e.g. QoL measures and duration of treatment) we extracted the arithmetic mean and standard deviation (SD) of the outcome of interest in each treatment arm. For dichotomous and continuous data, we abstracted the number of patients assessed at the endpoint. If reported, we extracted median and range data too. We noted any scoring systems (e.g. FIGO, WHO, NCI) used.
Where the data were insufficient or missing from a trial, we contacted authors for more information. Differences between the review authors were resolved by discussion or by referral to a third review author.
Assessment of risk of bias in included studies
We assessed the risk of bias of included studies according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) including the following.
Selection bias (random sequence generation and allocation concealment).
Detection bias (blinding of outcome assessment).
Attrition bias (incomplete outcome data, loss to follow-up).
Reporting bias (selective reporting of outcomes).
Other possible sources of bias.
For more details of risk of bias assessment, see Appendix 3.
Measures of treatment effect
We used the following measures of the effect of treatment.
For dichotomous outcomes, we present results as summary RR with 95% confidence intervals (CI).
For continuous outcomes, we present results as the mean difference (MD) between treatment arms with the associated SD.
Dealing with missing data
We attempted to extract data on the outcomes only among participants who were assessed at endpoint. We did not impute missing outcome data for the primary outcome. If data were missing or only imputed data were reported, we contacted trial authors to request data on the outcomes only among the participants who were assessed.
Assessment of heterogeneity
We assessed heterogeneity between studies by visual inspection of forest plots and by measuring the statistical variation between combined studies using the I2 statistic (Deeks 2001; Higgins 2003). In addition, we applied random-effects modelling (REM) to all pooled effect estimates. When heterogeneity was found, we tried to determine the potential reasons for it by examining individual study characteristics.
Assessment of reporting biases
As the largest meta-analysis included only five studies we did not assess funnel plots.
Data synthesis
Studies were grouped into those comparing similar chemotherapy regimens. Within these groups, we performed meta-analyses if there were sufficient trials using the RevMan 2011 software.
For dichotomous outcomes, the RR was calculated for each study. Statistics from all studies were pooled.
For continuous outcomes, the MD between the treatment arms at the end of follow-up were pooled using the MD method.
Random-effects method was used for all meta-analyses (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
In the protocol and for the original review, we did not perform subgroup analyses but grouped studies into individual comparisons based on the comparative interventions and regimens tested. For the revised review, we compared interventions, subgrouping trials by regimen. Therefore, for trials comparing methotrexate with dactinomycin, we considered the following subgroups where possible.
Weekly IM methotrexate versus bi-weekly pulsed IV dactinomycin.
Five-day IM methotrexate versus bi-weekly pulsed IV dactinomycin.
Eight-day IM methotrexate-folinic acid (MTX-FA) versus five-day IV dactinomycin.
Five-day IM methotrexate versus five-day IV dactinomycin.
Eight-day IM MTX-FA versus bi-weekly pulsed IV dactinomycin.
Weekly IM methotrexate versus five-day pulsed dactinomycin.
Although subgroup analyses were not pre-specified, these ‘subgroups’ were analysed in the original review, where possible, as individual comparisons.
Sensitivity analysis
We performed sensitivity analysis to assess the robustness of the meta-analyses by comparing the results using all trials and then excluding trials of lower methodological quality or those considered to be at a higher risk of bias.
RESULTS
Description of studies
See Characteristics of included studies; Characteristics of excluded studies
Results of the search
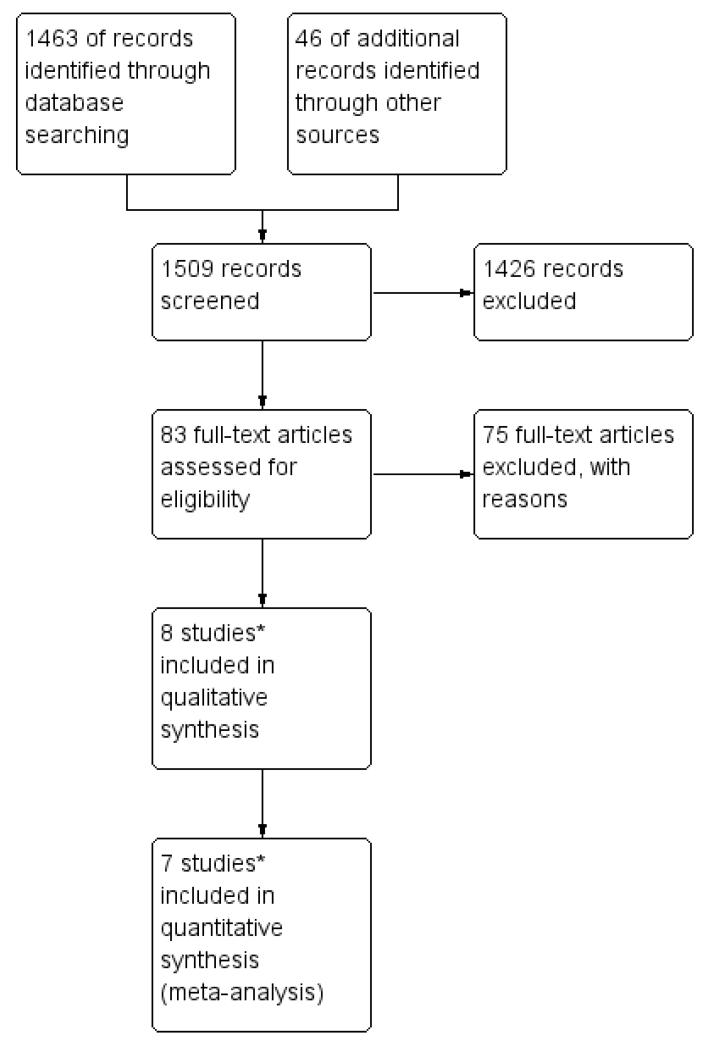
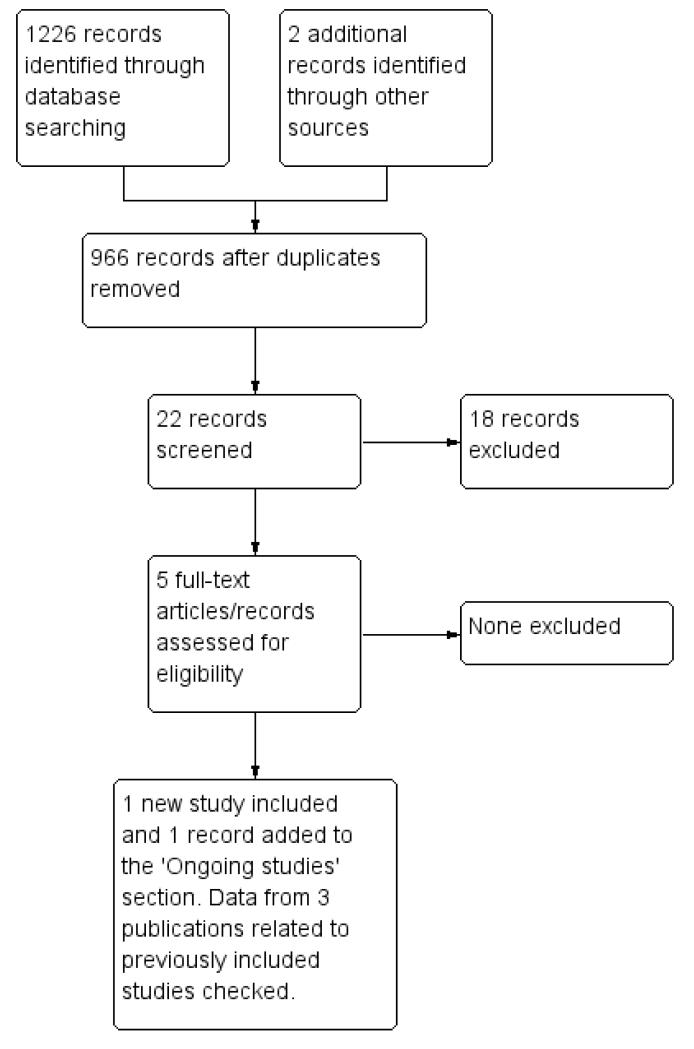
For the original review we identified 14 potentially eligible studies and, of these, we included eight studies and excluded six (see Figure 1). For the revised review, only four of these originally included studies met our inclusion criteria (Gilani 2005; Yarandi 2008; Lertkhachonsuk 2009; Osborne 2011). From the updated search (see Figure 2), we identified one new study for inclusion (Mousavi 2012), three additional records relating to the previously included studies (Rahimi-Moghaddam 2004; Lertkhachonsuk 2009a; Osborne 2011a) and one ongoing study (GOG 0275). Thus, altogether, we included data from five RCTs (with eight references) in this revised review.
Figure 1.
Study flow diagram of the original 2009 review*The original 2009 review Included four non-RCTs (Abrao 2008; Kohorn 1996; Smith 1982; Wong 1985) in the qualitative and three (Abrao 2008 not included) in the quantitative meta-analysis). These non-RCTs were excluded in the updated review.
Figure 2.
Study flow diagram of the updated search conducted from January 2010 to February 2012.
Included studies
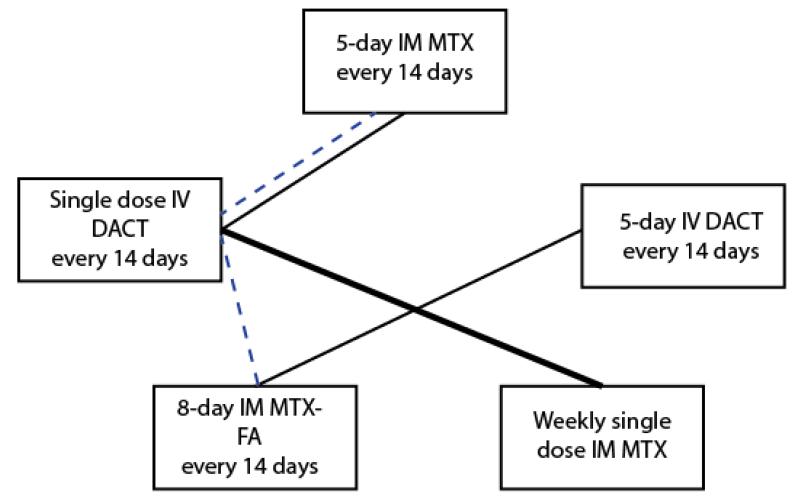
Investigators recruited a total of 541 women with low-risk GTN to five RCTs. Low-risk GTN was defined by either the earlier WHO/FIGO 2000 scoring system (Gilani 2005; Lertkhachonsuk 2009) or the modified WHO scoring system (Table 2; Yarandi 2008; Mousavi 2012). One study (Osborne 2011) defined low-risk as a score of less than or equal to four for women recruited before June 2002, and as less than or equal to six for women recruited from July 2002 and February 2007, following modification of the WHO scoring system. The included RCTs evaluated the following comparisons (see Figure 3):
Figure 3.
Chemotherapy regimens tested in RCTs for low-risk GTN (Dotted line = ongoing trial)
1. Weekly IM methotrexate versus bi-weekly pulsed IV dactinomycin
Three trials (Gilani 2005; Yarandi 2008; Osborne 2011) compared a weekly IM methotrexate regimen with fortnightly pulsed IV dactinomycin. All three trials used the same protocol of treatment (weekly IM methotrexate at 30 mg/m2 versus bi-weekly pulsed IV dactinomycin at 1.25 mg/m2). Gilani 2005 (46 women) and Yarandi 2008 (131 women) randomised participants in a methotrexate:dactinomycin ratio of 1.5:1 for ‘economic reasons’; all participants were evaluated. Osborne 2011 randomised 240 participants in a 1:1 ratio, of whom 214 were evaluable.
2. Five-day IM methotrexate versus bi-weekly pulsed IV dactinomycin
Mousavi 2012 randomised 75 participants in a ratio of 1:2 to receive five-day methotrexate (0.4 mg/kg daily IM) or dactinomycin (1.25 mg/m2 IV bolus) respectively, repeated every 14 days until normal hCG levels were obtained. All participants were evaluated.
3. Eight-day IM methotrexate-folinic acid versus five-day IV dactinomycin
Lertkhachonsuk 2009 randomised 49 participants to receive either five-day dactinomycin (10 mcg/kg; N = 22) or eight-day methotrexate-folinic acid (MTX-FA) (methotrexate 1mg/kg, alternate days and folinic acid 0.1 mg/kg alternate days; N = 27). Two participants in each group were not evaluable for the primary outcome.
Women were followed up in all trials for one year after last treatment. See Characteristics of included studies for further details. For details of the ongoing trial, GOG 0275, see Characteristics of ongoing studies.
Excluded studies
For the original review, we excluded six studies (Berkowitz 1979; Petrilli 1980; Gleeson 1993; Roberts 1996; Matsui 1998; Matsui 2005). All these studies were non-RCTs, excluded mainly due to a high-risk of bias. (Characteristics of excluded studies).
For the updated review, we excluded a further four non-RCTs, that had been classified as ‘included’ in the original review (see Differences between protocol and review), on the basis that they were not RCTs. These case-control studies evaluated the following comparisons:
1. Eight-day methotrexate-folinic acid versus five-day methotrexate
Smith 1982 and Wong 1985 compared eight-day MTX-FA with the five-day methotrexate regimen. Both studies used the same treatment protocol (methotrexate at 1 mg/kg days one, three, five and seven and folinic acid at 0.1 mg/kg days two, four, six and eight; OR methotrexate 0.4 mg/kg on days one to five). Ninety-seven participants received MTX-FA and 72 participants received five-day methotrexate. Remission rates in Wong 1985 were 82% versus 79% respectively; and 82% versus 72% in Smith 1982.
2. ‘Pulsed’ dactinomycin versus five-day dactinomycin
Kohorn 1996 compared pulsed dactinomycin (1.25 mg/m2; N = 18) with the five-day dactinomycin (12 mcg/kg; N = 43). Complete response was achieved in 14 out of 18 (77%) and 38/43 (88%) respectively, with a mean number of 4.6 (SD 5.4) versus 2.7 (SD 1.3) courses. Toxicity was reported as ‘minimal’ in both groups with no further details.
3. Five-day methotrexate versus five-day dactinomycin versus combination of both
Abrao 2008 compared three different regimens; five-day methotrexate (20 mg/m2, N = 42), five-day dactinomycin (12 mcg/kg, N = 42) and the combination of five-day methotrexate and dactinomycin (methotrexate 20 mg/day & dactinomycin 500 mcg, N = 24). Remission rates were 69%, 61% and 79% respectively. Adverse effects occurred most frequently in the combined treatment group (62.5%) and least frequently in the dactinomycin group.
Risk of bias in included studies
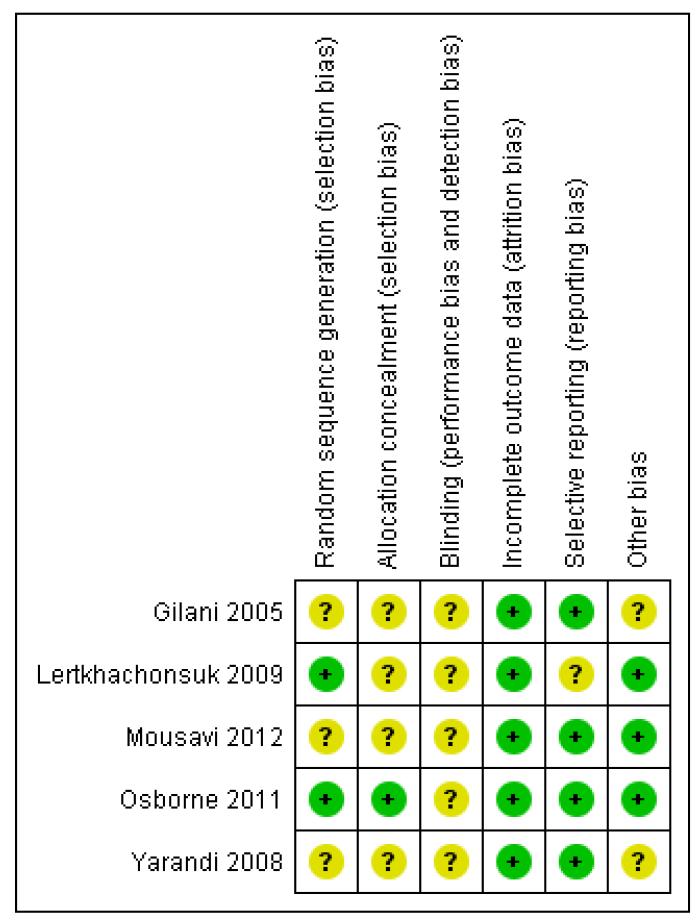
The ‘Risk of bias’ assessment of included studies is graphically represented in Figure 4.
Figure 4.
‘Risk of bias’ summary: review authors’ judgements about each risk of bias item for each included study.
Allocation
The method of randomisation was described in only two trials: Lertkhachonsuk 2009 (random number tables) and Osborne 2011 (central randomisation). Only one trial described allocation concealment (Osborne 2011).
Blinding
Neither patients nor physicians were blind to the allocated treatment in Osborne 2011. Blinding was not described in any of the other studies.
Incomplete outcome data
Loss to follow-up was low (less than 20% for all assessable outcomes) in one trial (Lertkhachonsuk 2009) and balanced between treatment arms. The other four trials reported complete follow-up (Gilani 2005; Yarandi 2008; Osborne 2011; Mousavi 2012).
Selective reporting
The five included studies reported all pre-specified and most expected outcomes. Toxicity and adverse effects were insufficiently reported in Gilani 2005 but were described as ‘minimal’.
Other potential sources of bias
It is not clear why the data from Gilani 2005 and Yarandi 2008 were not combined by the investigators, since these trials were conducted in consecutive years by the same investigators, compared the same interventions and applied the same methodology. For this reason, we performed sensitivity analyses, by excluding the Gilani 2005 data, where applicable.
Effects of interventions
See: Summary of findings for the main comparison
Five RCTs evaluated 517 women who were randomly allocated to receive methotrexate or dactinomycin for low-risk GTN.
1. Methotrexate versus Dactinomycin
1.1 Primary cure (remission)
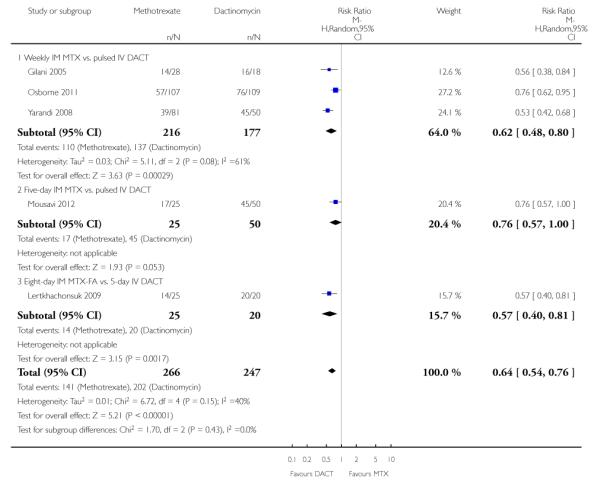
Irrespective of the type of regimen used, dactinomycin was significantly more likely to effect a primary cure than methotrexate in women with low-risk GTN (five trials, 513 participants; risk ratio (RR) 0.64, 95% confidence interval (CI) 0.54 to 0.76, P < 0.00001; I2 = 40%; Analysis 1.1).
Due to concerns about potential bias (see Other potential sources of bias), we performed sensitivity analysis, excluding Gilani 2005, and obtained similar results (RR 0.65, 95% CI 0.54 to 0.79). Tests for subgroup differences indicated no heterogeneity between subgroups for this outcome (I2 = 0%).
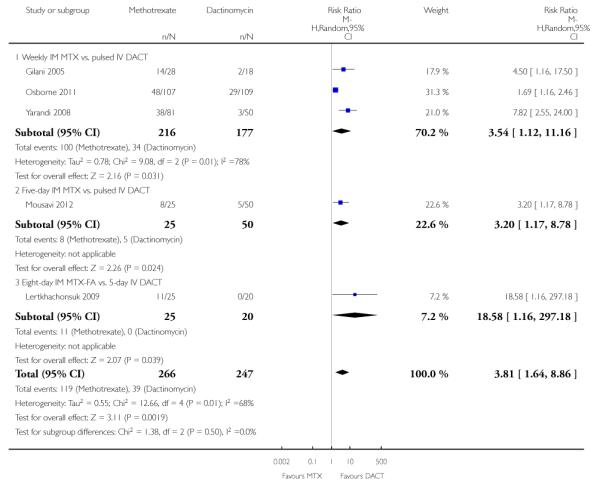
1.2 Failure of first-line therapy
First-line therapy was significantly more likely to fail in the methotrexate group than the dactinomycin group (five trials, 513 participants; RR 3.81, 95% CI 1.64 to 8.86, P = 0.002; I2 = 68%; Analysis 1.2). As in Analysis 1.1, a sensitivity analysis, excluding Gilani 2005, produced similar results.
Tests for subgroup differences indicated no heterogeneity between subgroups for this outcome (I2 = 0%). The Lertkhachonsuk 2009 data included six women in the MTX-FA group who were changed to second-line therapy due to chemotoxicity. When we excluded these women, the results were similar.
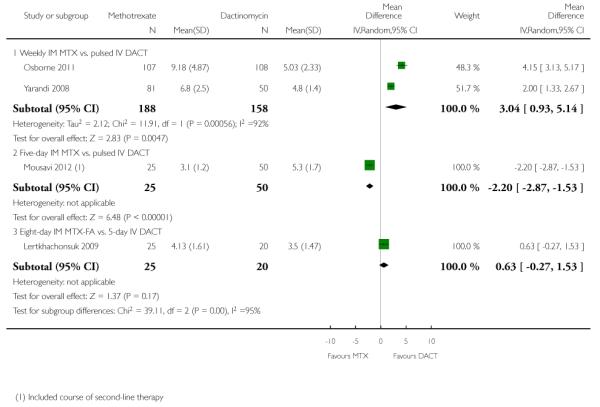
1.3 Chemotherapy cycles to primary cure
The combined data for this outcome was substantially heterogenous and subgroup differences were significant (I2 = 75.6%, P = 0.04), therefore, we present these results as subtotals only.
For the subgroup of trials comparing weekly IM methotrexate versus bi-weekly pulsed IV dactinomycin, fewer cycles of dactinomycin were needed to effect a primary cure (two trials, 346 participants; mean difference (MD) 3.04, 95% CI 0.93 to 5.14, P = 0.005; Analysis 1.3). There was substantial heterogeneity between the two trials included in this subgroup (I2 = 92%).
The other subgroups included only one trial for this outcome (Analysis 1.3):
Mousavi 2012 reported that significantly fewer cycles were necessary in the five-day IM methotrexate group than in the pulsed IV DACT group, however, this trial included secondary treatment cycles in these data.
In Lertkhachonsuk 2009, there was no significant difference between the eight-day MTX-FA group and the five-day IV dactinomycin group with regard to the number of cycles.
1.4 to 1.14 Adverse effects
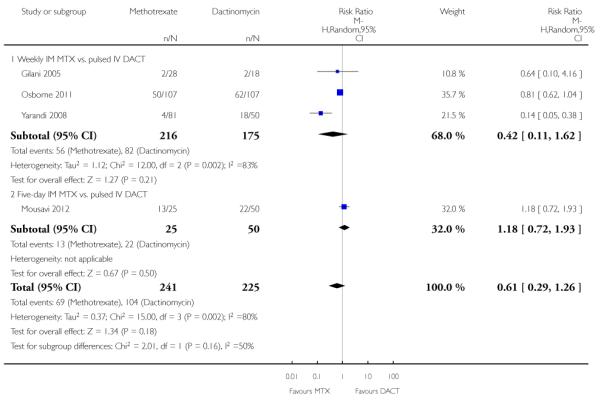
The most commonly occurring side-effects in both groups were nausea, fatigue (constitutional) and anaemia. There were no significant differences, overall or for subgroup analyses, in any of the following,
Nausea (four trials, 466 participants; RR 0.61, 95% CI 0.29 to 1.26; Analysis 1.4; I2 = 80%).
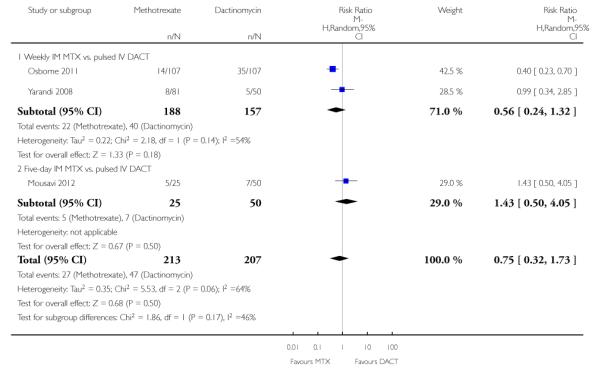
Vomiting (three trials, 420 participants; RR 0.75, 95% CI 0.32 to 1.73; Analysis 1.5; I2 = 64%).
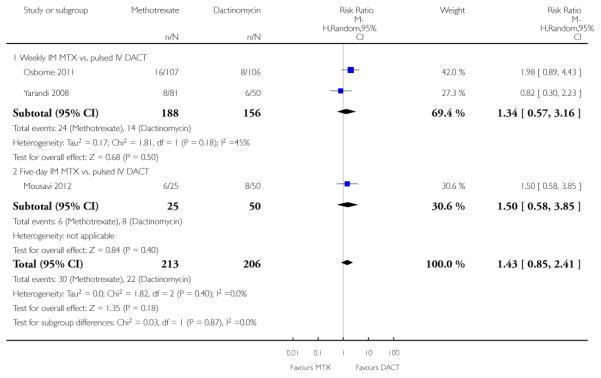
Diarrhoea (three trials, 419 participants; RR 1.43, 95% CI 0.85 to 2.41; Analysis 1.6; I2 = 0%).
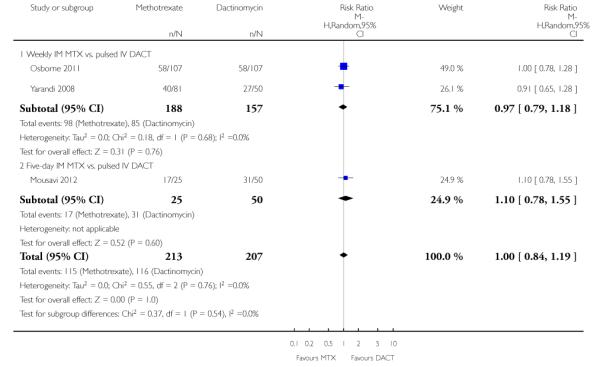
Constitutional (three trials, 420 participants; RR 1.00, 95% CI 0.84 to 1.19; Analysis 1.7; I2 = 0%).
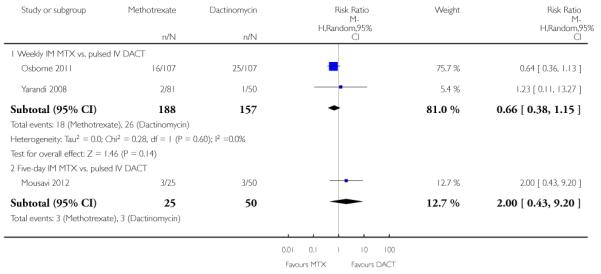
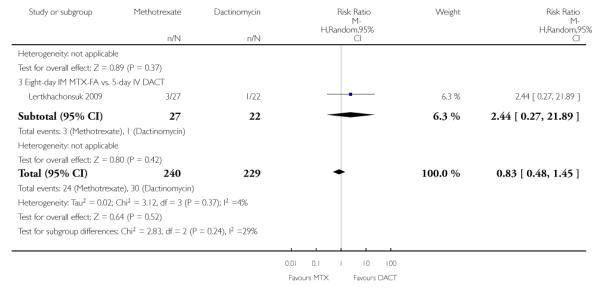
Neutropenia (four trials, 469 participants; RR 0.83, 95% CI 0.48 to 1.45; Analysis 1.11 I2 = 4%).
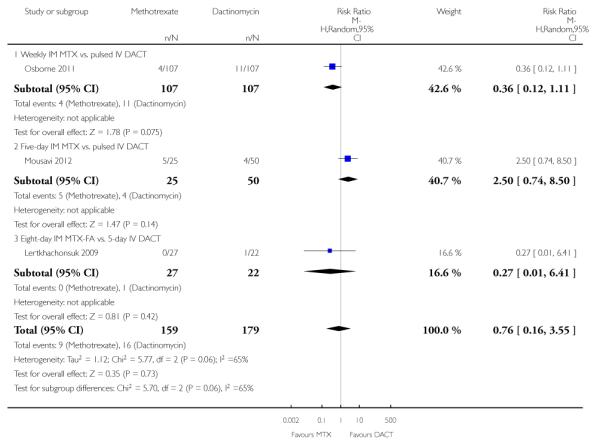
Thrombocytopenia (three trials, 338 participants; RR 0.76, 95% CI 0.16 to 3.55; Analysis 1.12; I2 = 65%).
Anaemia (one trial, 214 participants; Analysis 1.13).
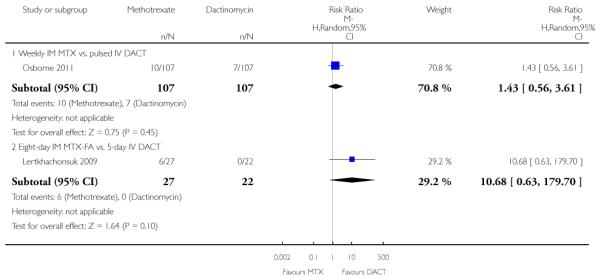
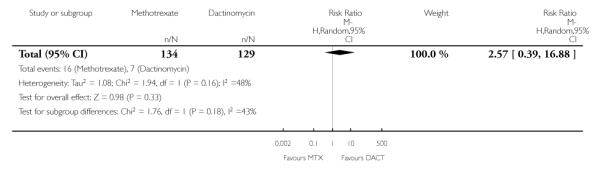
Hepatotoxicity (two trials, 263 participants; RR 2.57, 95% CI 0.39 to 16.88; Analysis 1.14).
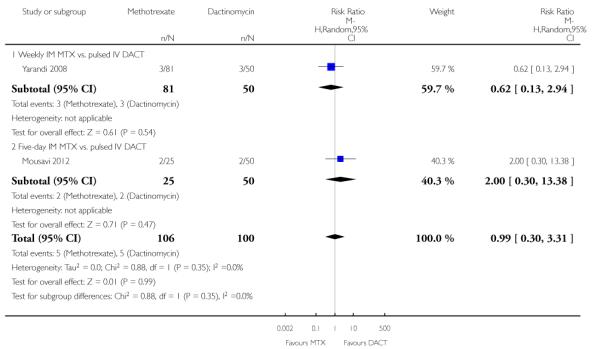
Haemoptysis (two trials, 206 participants; RR 0.99, 95% CI 0.30 to 3.31; Analysis 1.15).
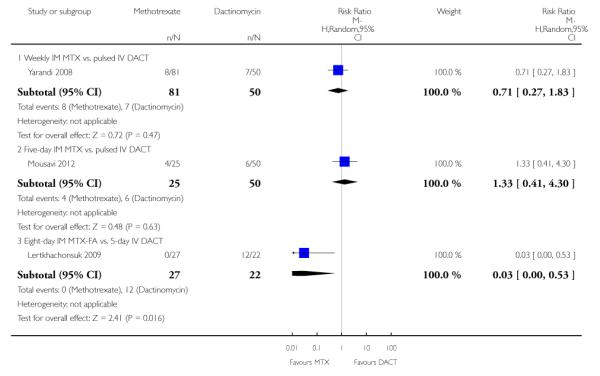
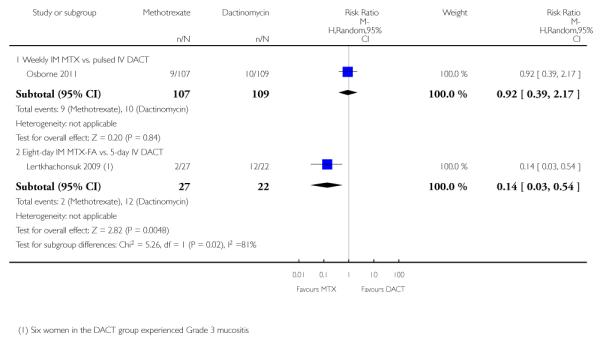
Three studies reported alopecia. There was no significant difference in the rate of women experiencing alopecia in the two studies that compared methotrexate (weekly or five-day IM) with pulsed IV dactinomycin (206 participants; RR 0.91, 95% CI 0.43 to 1.90; I2 = 0%; Analysis 1.8). However, in the one study that compared eight-day IM MTX-FAmethotrexate-folinic acid with five-day IV dactinomycin (Lertkhachonsuk 2009), significantly more women in the dactinomycin group experienced alopecia (49 participants; RR 0.03, 95% CI 0.00 to 0.53; Analysis 1.8). Subgroup differences for this outcome were significant when the Lertkhachonsuk 2009 data were included (P = 0.05; I2 = 65.7%) therefore, we did not pool these data.
Similarly, mucositis occurred significantly more frequently in the five-day IV dactinomycin group than the eight-day MTX-FA treatment group of Lertkhachonsuk 2009. These data were significantly different from the only other study reporting this outcome and in which intervention groups were not significantly different (Osborne 2011). Tests for subgroup differences were significant (P = 0.02; I2 = 81%), therefore, we did not pool these data (Analysis 1.9).
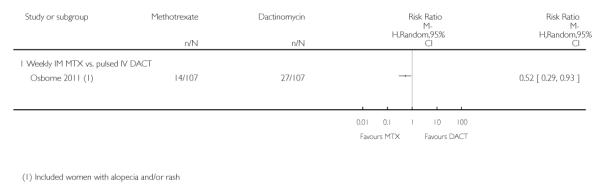
Dermatological adverse effects (rash or alopecia) occurred significantly more frequently in the dactinomycin group, in the one study reporting this outcome (Osborne 2011; RR 0.52, 95% CI 0.29 to 0.93; Analysis 1.10), However, these adverse effects were all CTCAE 2010 grade one, except for one grade two effect in the methotrexate arm.
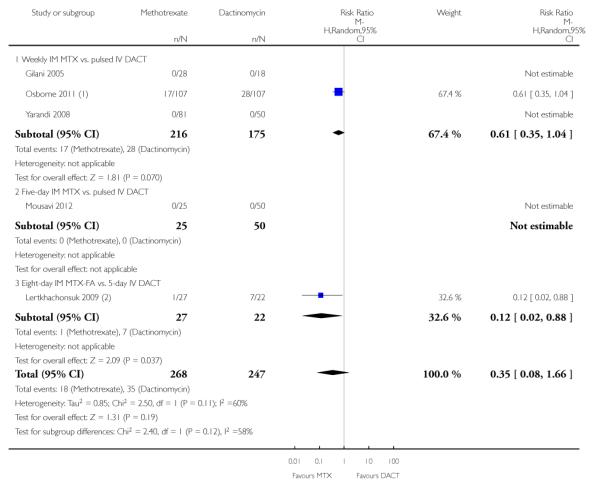
Severe adverse events (SAEs; CTCAE 2010 or Gynecologic Oncology Group (GOG) ≥ grade three) were experienced by participants of two out of the five studies (Lertkhachonsuk 2009; Osborne 2011). All reported SAEs were grade three except for two women in the DACT group of the Osborne 2011 study who experienced grade four haematological SAEs. There was no significant difference between the methotrexate and dactinomycin groups overall, although the point estimate favoured the methotrexate group (five trials, 515 participants; RR 0.35, 95% 0.08 to 1.66; Analysis 1.16; I2 = 60%). With regard to subgroup analyses, participants in the five-day dactinomycin group experienced significantly more SAEs than the eight-day MTX-FA group in the one study that made this comparison (Lertkhachonsuk 2009).
No women in any of the trials comparing methotrexate with pulsed dactinomycin had to have the allocated treatment discontinued due to drug-related toxicity, however, six women in the Lertkhachonsuk 2009 had the allocated treatment (MTX-FA) discontinued due to hepatotoxicity. No deaths occurred in any of the trials.
Reproductive data were scarce: only Yarandi 2008 reports that “no ovarian failure” occurred.
DISCUSSION
Summary of main results
See Summary of findings for the main comparison.
Five RCTs evaluating 517 women were included. All five trials compared methotrexate with dactinomycin. Dactinomycin was associated with a significantly higher primary cure rate than methotrexate, irrespective of the treatment regimens compared, and significantly less first-line treatment failure.
Side-effects were reported to be mild (CTCAE 2010 or GOG grade one to two) or minimal in three of the five included trials. Overall, there were no significant differences in side-effects, however in the subgroup comparing the five-day DACT regimen with the eight-day methotrexate-folinic acid regimen (MTX-FA) (one study; Lertkhachonsuk 2009), dactinomycin was associated with significantly more alopecia and mucositis than methotrexate.
Participants of two trials experienced SAEs. Overall, there was no significant difference in SAEs between dactinomycin and methotrexate. Data from these two studies was substantially heterogeneous, however, the point estimate favoured methotrexate. Furthermore, the five-day DACT regimen was significantly more likely to result in SAEs than the eight-day MTX-FA regimen (one study; Lertkhachonsuk 2009).
Overall completeness and applicability of evidence
From the evidence, it appears that dactinomycin is superior to methotrexate in achieving a primary cure in women with low-risk GTN, and that primary treatment with dactinomycin is less likely to fail. However, three out of five included trials used a weekly IM dose of methotrexate and it has been argued that this regimen is not as effective as the five- or eight-day regimens (Aghajanian 2011). Hence, further research is needed comparing pulsed dactinomycin with these more commonly used methotrexate regimens.
Although most included studies reported minimal side-effects and there were no significant differences, the relative side-effect profiles of the two drugs is still not clear. This is largely because data were frequently heterogeneous. Furthermore, in the two studies that reported the occurrence of severe adverse events (SAEs) (mainly grade three) (Lertkhachonsuk 2009; Osborne 2011), more SAEs occurred in the dactinomycin arms. As side-effects and SAEs play an important role in treatment choice, more evidence on the relative side-effects is necessary. However, since the efficacy of dactinomycin does not appear to be adversely affected by the lower, pulsed bi-weekly dosage, which is associated with fewer side-effects than the five-day regimen, pulsed dactinomycin may compare favourably in terms of relative side-effects to the five- and eight-day methotrexate regimens. This was shown in the one small included study (Mousavi 2012) in which no significant differences were found between these two groups in terms of side-effects, and no SAEs occurred. We await the completion of a large, ongoing Gynecologic Oncology Group (GOG) study of five-day or eight-day methotrexate compared with pulsed dactinomycin to corroborate or refute these findings (GOG 0275).
These results may not be applicable to women with WHO risk scores of five to six and/or those with histologically confirmed choriocarcinoma. Yarandi 2008 excluded women with histologically confirmed choriocarcinoma from their trial, and Osborne 2011 found that these higher scoring, low-risk lesions (previously classified as intermediate risk) were significantly less likely to respond to either drug as single-agent therapy. In the latter study, the primary response rate for these higher scoring lesions was 9% and 42% for methotrexate and dactinomycin respectively. This needs further investigation.
Women undergoing treatment for low-risk GTN may wish to bear children in the future. Follow-up in the included studies was continued for one year after the last treatment cycle and only one mentioned ovarian failure as an potential adverse outcome (Yarandi 2008). Reproductive data should be included in future studies of treatment for low-risk GTN.
As health economics play an increasingly important role in determining treatment guidelines, particularly where several different treatment regimens result in similar cure rates and similar/low rate of side-effects, the relative cost of treatment and treatment failure needs to be examined. Unfortunately, this was beyond the scope of this review.
Quality of the evidence
We downgraded the evidence for primary outcomes from high quality to moderate quality because more than 64% of the data came from three trials using a low-dose methotrexate regimen that may be less effective than the five- or eight-day regimens (see Summary of findings for the main comparison). We assessed the quality of evidence for side-effects and adverse events to be moderate to low, mainly due to the heterogeneity of the data.
Potential biases in the review process
To our knowledge there were no potential biases in the review process. We included all the relevant RCTs identified by the search. Where there were concerns regarding the quality of included trials, we contacted the investigators and performed sensitivity analyses.
Agreements and disagreements with other studies or reviews
The largest contributing subgroup of trials used the weekly methotrexate regimen. This regimen has been criticised as being less effective than the more commonly used methotrexate treatment regimens, namely the five- or eight-day regimens (Aghajanian 2011). However, efficacy data on the various methotrexate regimens has come mainly from retrospective (Bagshawe 1989; Soper 1994; McNeish 2002) and case-control studies (Smith 1982; Wong 1985) which may be subject to high levels of bias; furthermore, there have been no RCTs comparing the weekly methotrexate regimen with the five- and eight-day regimens (Figure 3).
To our knowledge, this is the first attempt to pool data from randomised trials of treatment for low-risk GTN. From the evidence, dactinomycin appears to be highly effective in achieving a primary cure in women with low-risk GTN (82% cured in the DACT group compared with 53% with MTX overall). with similar efficacy achievable with the less intensive, and more convenient, pulsed regimen (77% cured with DACT versus 50% with MTX). More data, including long-term reproductive data, are needed with respect to relative side-effects. We anticipate that completion of the GOG 0275 trial comparing pulsed dactinomycin with the five- and eight-day methotrexate regimens will determine whether pulsed dactinomycin becomes widely accepted as the treatment of choice.
AUTHORS’ CONCLUSIONS
Implications for practice
Dactinomycin treatment is more likely to achieve a primary cure in women with GTN and is less likely to result in treatment failure compared with methotrexate. There is insufficient evidence relating to side-effects, however, the pulsed dactinomycin regimen does not appear to be associated with significantly more side-effects than the low-dose methotrexate regimen. Therefore, we consider bi-weekly pulsed dactinomycin at least equivalent in efficacy and safety to methotrexate for first-line treatment of low-risk GTN. Further research will establish whether it becomes the treatment of choice.
Implications for research
At the time of writing, recruitment of 384 women with low-risk GTN to GOG 0275 had just begun, with results expected in 2016. This trial randomises participants to methotrexate (eight-day MTX-FA or five-day MTX) or pulsed IV dactinomycin. The primary outcome is ‘complete response’ with secondary outcomes of post protocol surgery, post-protocol multi-agent treatment, severe adverse events and quality of life. This trial should provide the important (missing) information on the comparable effects of these more commonly used methotrexate regimens with dactinomycin. For further information, see Characteristics of ongoing studies.
More research is needed to determine whether higher scoring, low-risk lesions (scores of five/six) are best treated with single-agent therapy, and whether future reproduction is affected in the long-term by the allocated intervention.
PLAIN LANGUAGE SUMMARY.
First-line treatment with anti-cancer drugs for low risk gestational trophoblastic neoplasia
Gestational trophoblastic neoplasia (GTN) is a rare but curable disease whereby a malignant tumour develops in the womb after a normal or molar pregnancy (where tissue develops in the womb instead of a baby). Women with GTN are classified as having lowor high-risk GTN using a specific scoring system. Virtually all women with low-risk GTN are cured by treatment with chemotherapy (anti-cancer drugs) after undergoing dilatation and curettage (D&C) of the womb. Methotrexate and dactinomycin are the two most commonly used drugs for first-line treatment of low-risk GTN, although methotrexate is favoured in Europe and North America. Sometimes the first-line treatment fails to cure the disease or has side-effects that require it be discontinued, and a secondary treatment has to be used. If methotrexate is the first drug used, dactinomycin is usually the secondary treatment, and vice versa. We undertook this review as it was not clear which drug, if any, was more likely to cure low-risk disease in the first instance. Furthermore, it was not clear which, if any, caused more side-effects.
This review included five studies of moderate to high quality comparing three different treatment regimens of dactinomycin and methotrexate that differed by drug dose and dosing frequency. Overall, and for each treatment regimen compared, dactinomycin was much more likely to achieve a cure in the first instance than methotrexate, and much less likely to fail.
More evidence is needed on the relative side-effects of these drugs for low-risk GTN. The most commonly experienced side-effects in both groups were nausea, fatigue and anaemia. Overall, side-effects were relatively mild in both groups but there was a trend to more severe side-effects in women treated with dactinomycin, especially with the five-day treatment. Since pulsed dactinomycin achieved similar rates of cure to higher doses of dactinomycin but with milder side-effects, pulsed dactinomycin is preferable to the five-day dactinomycin regimen for first-line treatment of GTN. In addition, since the side-effects are modest and comparable to ‘low-dose’ methotrexate, we consider pulsed dactinomycin to be at least as good as methotrexate (low-and higher dose regimens), the more commonly used drug, for first-line treatment of low-risk GTN.
A large trial is underway, comparing the more conventional five- and eight-day methotrexate treatment schedules with pulsed dactinomycin, that will add to this body of evidence and may change our conclusions.
ACKNOWLEDGEMENTS
We thank the following people:
Clare Jess, Gail Quinn, Jane Hayes, Andy Bryant and the team at the Cochrane Gynaecological Cancer Review Group in Bath for their advice and support throughout the review process;
Alison Little librarian (Sheffield University) and Anne Oestmann (Cochrane Gynaecological Cancer Collaborative Review Group) for their assistance in developing the search strategy process and literature search for the original review; and
The authors of Lertkhachonsuk 2009 and Yarandi 2008 for providing additional data.
This review update received methodological and statistical support as part of the 10/4001/12 NIHR Cochrane Programme Grant Scheme - Optimising care, diagnosis and treatment pathways to ensure cost effectiveness and best practice in gynaecological cancer: improving evidence for the NHS
SOURCES OF SUPPORT
Internal sources
Sheffield Teaching Hospitals NHS Trust, UK.
External sources
10/4001/12 National Institute for Health Research (NIHR) Cochrane Programme Grant Scheme, UK., UK.
This review received methodological and statistical support as part of the 10/4001/12 NIHR Cochrane Programme Grant Scheme - Optimising care, diagnosis and treatment pathways to ensure cost effectiveness and best practice in gynaecological cancer: improving evidence for the NHS.
SUMMARY OF FINDINGS FOR THE MAIN COMPARISON
Dactinomycin compared with methotrexate for low-risk gestational trophoblastic neoplasia
Patient or population: women withe low-risk GTN
Settings: outpatient or hospital
Intervention: dactinomycin (DACT)
Comparison: methotrexate (MTX)
| Outcomes | Illustrative Assumed risk* (MTX) | Illustrative Corresponding risk* (DACT) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
|---|---|---|---|---|---|---|
| Primary cure (remission) | 530 per 1000 | 828 per 1000 (697 to 981) |
RR 0.64 (0.54 to 0.76) | 513 women (5 studies) | ⊕⊕⊕○ moderate |
DACT is significantly more likely to achieve a primary cure than MTX We downgraded this evidence because 64% of the data came from comparisons of weekly IM MTX which may be less effective than the 5- or 8- day regimens |
| Failure of first-linetherapy | 447 per 1000 | 115 per 1000 (50 to 268) |
RR 3.81 (1.64 to 8.86) | 513 women (5 studies) | ⊕⊕⊕○ moderate |
DACT as a first-line treatment is significantly less likely to fail than MTX We downgraded this evidence because 70% of the data came from comparisons of weekly IM MTX which may be less effective than the 5- or 8-day regimens |
| Severe adverse events (≥ grade 3) | 102 per 1000 (23 to 481) |
290 per 10001 | RR 0.35 (0.08 to 1.66) | 515 women (5 studies) | ⊕⊕○○ low |
There was no significant difference between interventions overall, however, the point estimate and subgroup analyses favoured MTX. We downgraded this evidence due to the low consistency and heterogeneity (I2 = 60%). SAEs occurred in only 2 out of 5 studies |
| Nausea | 280 per 1000 | 171 per 1000 81 to 353) |
RR 0.61 (0.29 to 1.26) | 466 women (4 studies) | ⊕⊕⊕○ moderate |
There is no significant difference between the interventions in the rate of nausea. We downgraded this evidence due to the heterogeneity (I2 = 80%) |
| Alopecia | Subtotals only | ⊕⊕○○ low |
Data not pooled due to substantial subgroup differences. Five-day DACT was associated with significantly more alopecia than MTX, but there was no significant difference in alopecia between groups in the studies that used pulsed DACT |
The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk Ratio
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Assumed risk calculated for DACT (not MTX) using the median control group risk across included studies.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Single centre RCT. Study duration: 2001 to 2003. |
|
| Participants | Low-risk GTN. Number randomised: 46. Number evaluable:46. Randomisation ratio of 1.5 MTX:1 DACT applied due to economic limitations |
|
| Interventions | Group1: MTX, IM, 30mg/m2 repeated every week. Group2: ACT, IV, 1.25mg/m2 repeated every 2 weeks. |
|
| Outcomes | Efficacy: remission rate, number of cycles to remission, duration of treatment, need for second-line chemotherapy Adverse effects: nausea. |
|
| Notes | Risk scoring: WHO/FIGO 2000. Non-response defined as < 10% decrease in hCG over 3 weeks, more than 20% rise in hCG over 2 consecutive weeks or the appearance of new metastatic disease Remission defined as hCG < 5 IU/L. One additional cycle was given after remission |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Blinding not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 100% of participants analysed. |
| Selective reporting (reporting bias) | Low risk | All pre-specified outcomes reported. Toxicity not reported in detail but said to be ‘minimal’ in both groups |
| Other bias | Unclear risk | This trial has the same authors, location and protocol as Yarandi 2008, with consecutive enrolment dates, yet the later Yarandi 2008 study does not refer to Gilani 2005. It is not clear why. Attempts to clarify this with Dr Yarandi have been unsuccessful |
| Methods | Single centre RCT. Study duration: 1994 to 2005. Follow-up: 1 year. |
|
| Participants | Low-risk GTN (FIGO stage 1). Number randomised: 49. Number evaluable: 45. |
|
| Interventions | Group 1: DACT IV 10 μg/kg/day (D1 to D5) repeated every two weeks Group 2: methotrexate-folinic acid (MTX-FA): MTX IM 1mg/kg/day (days 1, 3, 5, 7) and FA IM 0.1mg/kg/day (days 2, 4, 6, 8), repeated every two weeks |
|
| Outcomes | Efficacy: remission rate, number of cycles to remission, need for second-line chemotherapy Adverse effects: liver toxicity, neutropenia, skin pigmentation, alopecia and mucositis |
|
| Notes | Risk scoring: FIGO. Two participants in each arm of treatment were lost to follow-up and were excluded from the analysis in the reporting article Six participants in the MTX-FA group were switched to DACT due to rising levels of liver enzymes. The investigators excluded these participants from analyses of remission rates (i.e. not ITT analyses), however we have added these data back. ITT analysis gives a remission rate of 14/25 in the MTX group, not 14/19 as reported |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Blinding not reported. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Four lost to follow-up, two in each group. Therefore 92% analysed : 20/22 (91%) of 5-day DACT arm and 25/27 (92.6%) of the MTX-FA arm |
| Selective reporting (reporting bias) | Unclear risk | Not ITT analysis. Six women in MTX-FA who were switched to DACT due to hepatotoxicity were excluded from final analysis; therefore remission rate was reported as 14/19 instead of 14/25 |
| Other bias | Low risk | No evidence of other bias. |
| Methods | RCT conducted in Iran; randomisation ratio not stated but appears to be 1 MTX:2 DACT Accrual dates: Jan 2008 to Dec 2010 Follow-up: 1 year |
|
| Participants | 75 women with FIGO stage I-III; modified WHO risk scores ≤ 6; ; a rise in hCG of >10% in the 3 weeks post-termination of pregnancy or a hCG plateau of 4 weeks Exclusion criteria were prior CT or hysterectomy. |
|
| Interventions | Group 1: 5-day IM MTX (0.4mg/kg) repeated every two weeks vs Group 2: bi-weekly IV DACT (1.25mg/m2 bolus) |
|
| Outcomes | Primary remission; need for second-line CT; duration of treatment; toxicity | |
| Notes | Non-response defined as an hCG plateau or rising hCG titres for 2 consecutive weeks No major adverse events were reported in either group (classified according to the GOG grading system). Baseline characteristic similar. Mucositis not reported | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Randomised’; no other details provided. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No loss to follow-up. |
| Selective reporting (reporting bias) | Low risk | All pre-specified and expected outcomes were reported. |
| Other bias | Low risk | None evident. Baseline characteristic similar. |
| Methods | Multicentre RCT (US, Japan, Canada and South Africa) with central randomisation through the GOG Centre Study duration: 1999 to 2007. Follow-up: 1 year. |
|
| Participants | Low-risk GTN (see notes). Number randomised: 240. Number ineligible:24. Number evaluable: 216. |
|
| Interventions | Group 1 = MTX, IM, 30mg/m2, weekly. Group 2= ACT, IV, 1.25mg/m2, every two weeks. |
|
| Outcomes | Primary: Complete response (CR) defined as a normal hCG sustained over four weekly measurements Secondary: number of CT cycles to remission, treatment failure/need for second-line CT Adverse effects. |
|
| Notes | Baseline characteristics were similar between the two groups. Risk scoring: WHO/FIGO 2000 WHO risk score 0 to 4 was used between June 1999 to June 2002, then modified score 0 to 6 was used from July 2002 to February 2007. Twenty-seven women had WHO scores of 0 (balanced between groups) which may have inflated the CR rate Two women did not receive their allocated treatment, therefore, they were included in ITT analysis but not in analysis of toxicity. Twenty-four women deemed ineligible: 13 did not meet the entry criteria for persistent disease, 4 did not have GTN, 4 had inadequate documentation of the disease and 3 had a centrally re-calculated risk WHO risk score > 6 Non-response (NR) defined as any set of three consecutive assay results that declined by < 10%. Eleven NR women (5 MTX and 6 DACT) continued to receive their allocated treatment and went on to achieve CR No women had to have allocated treatment terminated because of toxicity Alopecia coded as dermatological toxicity. 11 women continued on the allocated regimen after being assessed as non-responders and attained CR. If these women had been included in the analyses of CR the percentage of responders would have been 63% for MTX and 79% for DACT (compared with 53% and 70% respectively) |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Central randomisation and allocation of treatment. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Neither participants nor treatment providers were blinded. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 90% of participants were analysed : 107/120 (89%) of weekly methotrexate arm and 108/120 (90%) of “pulsed” dactinomycin arm |
| Selective reporting (reporting bias) | Low risk | All pre-specified and expected outcomes reported. Analysis by ITT |
| Other bias | Low risk | No evidence of other bias. |
| Methods | Single centre RCT. Study duration: 09/2003 to 09/2006. Follow-up: 1 year. |
|
| Participants | Low-risk GTN. Number eligible:131. Number evaluable:131. Participants randomised into two groups: group 1 = 81 and group 2 = 50 (randomisation ratio of 1.5 MTX:1 DACT applied). Reasons given for this were economic limitations Excluded patients with choriocarcinoma. |
|
| Interventions | ||
| Outcomes | ||
| Notes | Risk scoring: FIGO 2000. Six women ( 4 in group 1 and 2 in group 2) did not complete their first-line chemotherapy, but were considered in the ITT analysis |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding (performance bias and detection bias) All outcomes |
Unclear risk | Blinding not described. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 100% of randomised participants were analysed. |
| Selective reporting (reporting bias) | Low risk | All pre-specified and expected outcomes were reported. Analysis was by ITT |
| Other bias | Unclear risk | See ‘Risk of bias’ comment for Gilani 2005. |
Abbreviations: CR = Complete response; CT = chemotherapy; DACT = Dactinomycin or Actinomycin D; FIGO = International Federation of Gynecology and Obstetrics; GOG = Gynecologic Oncology Group; GTN = Gestational trophoblastic neoplasia; hCG = human chorionic gonadotrophin; IM = intramuscular; ITT = Intention-to-treat; IV = intravenous; MTX = Methotrexate; MTX-FA = methotrexate-folinic acid; RCT = randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abrao 2008 | Not an RCT. In this case-control study, 108 women were treated with 5-day MTX (42 women) or 5-day DACT (42 women) or MTX/DACT combined (24 women). The combined intervention was stopped due to high rates of toxicity |
| Berkowitz 1979 | Not an RCT. The study may have high-risk patients. It was not clear if patients were balanced for demographic variables It was not clear if all patients between 1976 to 1978 were treated with MTX @ 6mg/kg or if the decision to use this dose was left to attending physician |
| Gleeson 1993 | Not an RCT. High risk of selection bias “ the choice of treatment was at the discretion of the attending oncologist” Follow-up period not clear. |
| Kohorn 1996 | Not an RCT. In this case-control study, women were treated with a 5-day DACT regimen (43 women) or a pulsed DACT regimen (18 women) |
| Matsui 1998 | High risk of selection bias; patients were not matched for the potential confounding variables in the different treatment groups Included in subsequent publication. |
| Matsui 2005 | High risk of selection bias; study did not provide information about patient characteristics and if they were matched for the potential confounding variables in the different treatment groups |
| Petrilli 1980 | High risk of selection bias: study did not provide information about the characteristics of patients and if they were matched for potential confounding variables in the treatment groups |
| Roberts 1996 | Case series rather than case-control study; 61 patients received MTX, 4 ACT and 5 MACT Risk of selection bias; patients were not matched for the potential confounding variables |
| Smith 1982 | Not an RCT. In this case-control study, 39 women received MTX and 29 women received MTX-FA |
| Wong 1985 | Not an RCT. In this case-control study, 33 women received MTX and 68 women received MTX-FA |
DACT = Dactinomycin or Actinomycin D; MTX = Methotrexate; MTX-FA = methotrexate-folinic acid; RCT = randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Methotrexate or dactinomycin in treating patients with low-risk gestational trophoblastic neoplasia (NCT01535053) |
| Methods | Multicentre phase III RCT; open label |
| Participants | 384 |
| Interventions | Arm I: methotrexate IM on days 1, 3, 5, and 7 and leucovorin calcium PO on days 2, 4, 6, and 8 OR single to agent methotrexate IV on days 1 to 5 Arm II: dactinomycin IV over 15 minutes on day 1. Cycles repeated every 14 days for up to 13 courses in the absence of disease progression or unacceptable toxicity. Women receive 3 courses after hCG < 5 mIU/Ml |
| Outcomes | Primary: Complete response rate Secondary: post protocol surgery; post-protocol multi-agent treatment; severe adverse events; QoL |
| Starting date | January 2012. Estimated completion August 2016 |
| Contact information | Dr Julian Schink Robert H. Lurie Cancer Center, Northwestern University, Chicago, Illinois, United States, 60611 Ph: 312-472-4684 |
| Notes |
IM = intramuscular; IV = intravenous; PO = by mouth; QoL = quality of life; RCT = randomised controlled trial
DATA AND ANALYSES
Comparison 1.
Methotrexate vs. Dactinomycin
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary cure (remission) | 5 | 513 | Risk Ratio (M-H, Random, 95% CI) | 0.64 [0.54, 0.76] |
| 1.1 Weekly IM MTX vs. pulsed IV DACT | 3 | 393 | Risk Ratio (M-H, Random, 95% CI) | 0.62 [0.48, 0.80] |
| 1.2 Five-day IM MTX vs. pulsed IV DACT | 1 | 75 | Risk Ratio (M-H, Random, 95% CI) | 0.76 [0.57, 1.00] |
| 1.3 Eight-day IM MTX-FA vs. 5-day IV DACT | 1 | 45 | Risk Ratio (M-H, Random, 95% CI) | 0.57 [0.40, 0.81] |
| 2 Failure of first line therapy | 5 | 513 | Risk Ratio (M-H, Random, 95% CI) | 3.81 [1.64, 8.86] |
| 2.1 Weekly IM MTX vs. pulsed IV DACT | 3 | 393 | Risk Ratio (M-H, Random, 95% CI) | 3.54 [1.12, 11.16] |
| 2.2 Five-day IM MTX vs. pulsed IV DACT | 1 | 75 | Risk Ratio (M-H, Random, 95% CI) | 3.2 [1.17, 8.78] |
| 2.3 Eight-day IM MTX-FA vs. 5-day IV DACT | 1 | 45 | Risk Ratio (M-H, Random, 95% CI) | 18.58 [1.16, 297.18] |
| 3 Chemotherapy cycles to primary cure | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Weekly IM MTX vs. pulsed IV DACT | 2 | 346 | Mean Difference (IV, Random, 95% CI) | 3.04 [0.93, 5.14] |
| 3.2 Five-day IM MTX vs. pulsed IV DACT | 1 | 75 | Mean Difference (IV, Random, 95% CI) | −2.20 [−2.87, −1.53] |
| 3.3 Eight-day IM MTX-FA vs. 5-day IV DACT | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.63 [−0.27, 1.53] |
| 4 Adverse effects: Nausea | 4 | 466 | Risk Ratio (M-H, Random, 95% CI) | 0.61 [0.29, 1.26] |
| 4.1 Weekly IM MTX vs. pulsed IV DACT | 3 | 391 | Risk Ratio (M-H, Random, 95% CI) | 0.42 [0.11, 1.62] |
| 4.2 Five-day IM MTX vs. pulsed IV DACT | 1 | 75 | Risk Ratio (M-H, Random, 95% CI) | 1.18 [0.72, 1.93] |
| 5 Adverse effects: Vomiting | 3 | 420 | Risk Ratio (M-H, Random, 95% CI) | 0.75 [0.32, 1.73] |
| 5.1 Weekly IM MTX vs. pulsed IV DACT | 2 | 345 | Risk Ratio (M-H, Random, 95% CI) | 0.56 [0.24, 1.32] |
| 5.2 Five-day IM MTX vs. pulsed IV DACT | 1 | 75 | Risk Ratio (M-H, Random, 95% CI) | 1.43 [0.50, 4.05] |
| 6 Adverse effects: Diarrhoea | 3 | 419 | Risk Ratio (M-H, Random, 95% CI) | 1.43 [0.85, 2.41] |
| 6.1 Weekly IM MTX vs. pulsed IV DACT | 2 | 344 | Risk Ratio (M-H, Random, 95% CI) | 1.34 [0.57, 3.16] |
| 6.2 Five-day IM MTX vs. pulsed IV DACT | 1 | 75 | Risk Ratio (M-H, Random, 95% CI) | 1.5 [0.58, 3.85] |
| 7 Adverse effects: Constitutional | 3 | 420 | Risk Ratio (M-H, Random, 95% CI) | 1.00 [0.84, 1.19] |
| 7.1 Weekly IM MTX vs. pulsed IV DACT | 2 | 345 | Risk Ratio (M-H, Random, 95% CI) | 0.97 [0.79, 1.18] |
| 7.2 Five-day IM MTX vs. pulsed IV DACT | 1 | 75 | Risk Ratio (M-H, Random, 95% CI) | 1.10 [0.78, 1.55] |
| 8 Adverse effects: Alopecia | 3 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 8.1 Weekly IM MTX vs. pulsed IV DACT | 1 | 131 | Risk Ratio (M-H, Random, 95% CI) | 0.71 [0.27, 1.83] |
| 8.2 Five-day IM MTX vs. pulsed IV DACT | 1 | 75 | Risk Ratio (M-H, Random, 95% CI) | 1.33 [0.41, 4.30] |
| 8.3 Eight-day IM MTX-FA vs. 5-day IV DACT | 1 | 49 | Risk Ratio (M-H, Random, 95% CI) | 0.03 [0.00, 0.53] |
| 9 Adverse effects: Mucositis/stomatitis | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 9.1 Weekly IM MTX vs. pulsed IV DACT | 1 | 216 | Risk Ratio (M-H, Random, 95% CI) | 0.92 [0.39, 2.17] |
| 9.2 Eight-day IM MTX-FA vs. 5-day IV DACT | 1 | 49 | Risk Ratio (M-H, Random, 95% CI) | 0.14 [0.03, 0.54] |
| 10 Adverse effects: Dermatological | 1 | Risk Ratio (M-H, Random, 95% CI) | Totals not selected | |
| 10.1 Weekly IM MTX vs. pulsed IV DACT | 1 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Adverse effects: Neutropenia | 4 | 469 | Risk Ratio (M-H, Random, 95% CI) | 0.83 [0.48, 1.45] |
| 11.1 Weekly IM MTX vs. pulsed IV DACT | 2 | 345 | Risk Ratio (M-H, Random, 95% CI) | 0.66 [0.38, 1.15] |
| 11.2 Five-day IM MTX vs. pulsed IV DACT | 1 | 75 | Risk Ratio (M-H, Random, 95% CI) | 2.0 [0.43, 9.20] |
| 11.3 Eight-day IM MTX-FA vs. 5-day IV DACT | 1 | 49 | Risk Ratio (M-H, Random, 95% CI) | 2.44 [0.27, 21.89] |
| 12 Adverse effects: Thrombocytopenia | 3 | 338 | Risk Ratio (M-H, Random, 95% CI) | 0.76 [0.16, 3.55] |
| 12.1 Weekly IM MTX vs. pulsed IV DACT | 1 | 214 | Risk Ratio (M-H, Random, 95% CI) | 0.36 [0.12, 1.11] |
| 12.2 Five-day IM MTX vs. pulsed IV DACT | 1 | 75 | Risk Ratio (M-H, Random, 95% CI) | 2.5 [0.74, 8.50] |
| 12.3 Eight-day IM MTX-FA vs. 5-day IV DACT | 1 | 49 | Risk Ratio (M-H, Random, 95% CI) | 0.27 [0.01, 6.41] |
| 13 Adverse effects: Anaemia | 1 | Risk Ratio (M-H, Random, 95% CI) | Totals not selected | |
| 13.1 Weekly IM MTX vs. pulsed IV DACT | 1 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Adverse effects: Hepatotoxicity | 2 | 263 | Risk Ratio (M-H, Random, 95% CI) | 2.57 [0.39, 16.88] |
| 14.1 Weekly IM MTX vs. pulsed IV DACT | 1 | 214 | Risk Ratio (M-H, Random, 95% CI) | 1.43 [0.56, 3.61] |
| 14.2 Eight-day IM MTX-FA vs. 5-day IV DACT | 1 | 49 | Risk Ratio (M-H, Random, 95% CI) | 10.68 [0.63, 179.70] |
| 15 Adverse effects: Haemoptysis | 2 | 206 | Risk Ratio (M-H, Random, 95% CI) | 0.99 [0.30, 3.31] |
| 15.1 Weekly IM MTX vs. pulsed IV DACT | 1 | 131 | Risk Ratio (M-H, Random, 95% CI) | 0.62 [0.13, 2.94] |
| 15.2 Five-day IM MTX vs. pulsed IV DACT | 1 | 75 | Risk Ratio (M-H, Random, 95% CI) | 2.0 [0.30, 13.38] |
| 16 Severe adverse events (≥G3) | 5 | 515 | Risk Ratio (M-H, Random, 95% CI) | 0.35 [0.08, 1.66] |
| 16.1 Weekly IM MTX vs. pulsed IV DACT | 3 | 391 | Risk Ratio (M-H, Random, 95% CI) | 0.61 [0.35, 1.04] |
| 16.2 Five-day IM MTX vs. pulsed IV DACT | 1 | 75 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Eight-day IM MTX-FA vs. 5-day IV DACT | 1 | 49 | Risk Ratio (M-H, Random, 95% CI) | 0.12 [0.02, 0.88] |
Analysis 1.1. Comparison 1 Methotrexate vs. Dactinomycin, Outcome 1 Primary cure (remission)
Review: First-line chemotherapy in low-risk gestational trophoblastic neoplasia
Comparison: 1 Methotrexate vs. Dactinomycin
Outcome: 1 Primary cure (remission)
 |
Analysis 1.2. Comparison 1 Methotrexate vs. Dactinomycin, Outcome 2 Failure of first line therapy
Review: First-line chemotherapy in low-risk gestational trophoblastic neoplasia
Comparison: 1 Methotrexate vs. Dactinomycin
Outcome: 2 Failure of first line therapy
 |
Analysis 1.3. Comparison 1 Methotrexate vs. Dactinomycin, Outcome 3 Chemotherapy cycles to primary cure
Review: First-line chemotherapy in low-risk gestational trophoblastic neoplasia
Comparison: 1 Methotrexate vs. Dactinomycin
Outcome: 3 Chemotherapy cycles to primary cure
 |
Analysis 1.4. Comparison 1 Methotrexate vs. Dactinomycin, Outcome 4 Adverse effects: Nausea
Review: First-line chemotherapy in low-risk gestational trophoblastic neoplasia
Comparison: 1 Methotrexate vs. Dactinomycin
Outcome: 4 Adverse effects: Nausea
 |
Analysis 1.5. Comparison 1 Methotrexate vs. Dactinomycin, Outcome 5 Adverse effects: Vomiting
Review: First-line chemotherapy in low-risk gestational trophoblastic neoplasia
Comparison: 1 Methotrexate vs. Dactinomycin
Outcome: 5 Adverse effects: Vomiting
 |
Analysis 1.6. Comparison 1 Methotrexate vs. Dactinomycin, Outcome 6 Adverse effects: Diarrhoea
Review: First-line chemotherapy in low-risk gestational trophoblastic neoplasia
Comparison: 1 Methotrexate vs. Dactinomycin
Outcome: 6 Adverse effects: Diarrhoea
 |
Analysis 1.7. Comparison 1 Methotrexate vs. Dactinomycin, Outcome 7 Adverse effects: Constitutional
Review: First-line chemotherapy in low-risk gestational trophoblastic neoplasia
Comparison: 1 Methotrexate vs. Dactinomycin
Outcome: 7 Adverse effects: Constitutional
 |
Analysis 1.8. Comparison 1 Methotrexate vs. Dactinomycin, Outcome 8 Adverse effects: Alopecia
Review: First-line chemotherapy in low-risk gestational trophoblastic neoplasia
Comparison: 1 Methotrexate vs. Dactinomycin
Outcome: 8 Adverse effects: Alopecia
 |
Analysis 1.9. Comparison 1 Methotrexate vs. Dactinomycin, Outcome 9 Adverse effects: Mucositis/stomatitis
Review: First-line chemotherapy in low-risk gestational trophoblastic neoplasia
Comparison: 1 Methotrexate vs. Dactinomycin
Outcome: 9 Adverse effects: Mucositis/stomatitis
 |
Analysis 1.10. Comparison 1 Methotrexate vs. Dactinomycin, Outcome 10 Adverse effects: Dermatological
Review: First-line chemotherapy in low-risk gestational trophoblastic neoplasia
Comparison: 1 Methotrexate vs. Dactinomycin
Outcome: 10 Adverse effects: Dermatological
 |
Analysis 1.11. Comparison 1 Methotrexate vs. Dactinomycin, Outcome 11 Adverse effects: Neutropenia
Review: First-line chemotherapy in low-risk gestational trophoblastic neoplasia
Comparison: 1 Methotrexate vs. Dactinomycin
Outcome: 11 Adverse effects: Neutropenia
 |
 |
Analysis 1.12. Comparison 1 Methotrexate vs. Dactinomycin, Outcome 12 Adverse effects: Thrombocytopenia
Review: First-line chemotherapy in low-risk gestational trophoblastic neoplasia
Comparison: 1 Methotrexate vs. Dactinomycin
Outcome: 12 Adverse effects: Thrombocytopenia
 |
Analysis 1.13. Comparison 1 Methotrexate vs. Dactinomycin, Outcome 13 Adverse effects: Anaemia
Review: First-line chemotherapy in low-risk gestational trophoblastic neoplasia
Comparison: 1 Methotrexate vs. Dactinomycin
Outcome: 13 Adverse effects: Anaemia
 |
Analysis 1.14. Comparison 1 Methotrexate vs. Dactinomycin, Outcome 14 Adverse effects: Hepatotoxicity
Review: First-line chemotherapy in low-risk gestational trophoblastic neoplasia
Comparison: 1 Methotrexate vs. Dactinomycin
Outcome: 14 Adverse effects: Hepatotoxicity
 |
 |
Analysis 1.15. Comparison 1 Methotrexate vs. Dactinomycin, Outcome 15 Adverse effects: Haemoptysis
Review: First-line chemotherapy in low-risk gestational trophoblastic neoplasia
Comparison: 1 Methotrexate vs. Dactinomycin
Outcome: 15 Adverse effects: Haemoptysis
 |
Analysis 1.16. Comparison 1 Methotrexate vs. Dactinomycin, Outcome 16 Severe adverse events (≥G3)
Review: First-line chemotherapy in low-risk gestational trophoblastic neoplasia
Comparison: 1 Methotrexate vs. Dactinomycin
Outcome: 16 Severe adverse events (≥G3)
 |
Appendix 1. Search strategies 2008
MEDLINE search strategies
Phase I
RANDOMIZED CONTROLLED TRIAL.pt.
CONTROLLED CLINICAL TRIAL.pt.
RANDOMIZED CONTROLLED TRIALS.sh.
RANDOM ALLOCATION.sh.
DOUBLE BLIND METHOD.sh.
SINGLE BLIND METHOD.sh.
1 OR 2 OR 3 OR 4 OR 5 OR 6
ANIMALS.sh. not HUMANS.sh.
7 not 8
Phase II
10 CLINICAL TRIAL.pt.
11 exp CLINICAL TRIALS/
12 (clin$ adj25 trial$).ti,ab.
13 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab.
14 PLACEBOS.sh.
15 placebo$.ti,ab.
16 random$.ti,ab.
17 RESEARCH DESIGN.sh.
18 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17
19 18 not 8
20 19 not 9
Phase III
21 COMPARATIVE STUDY.pt
22 exp EVALUATION STUDIES/
23 FOLLOW UP STUDIES.sh
24 PROSPECTIVE STUDIES.sh.
25 (control$ or prospectiv$ or volunteer$).ti,ab.
26 21 OR 22 OR 23 OR 24 OR 25
27 26 not 8
28 27 not (9 or 20)
29 9 or 20 or 28
Phase IV (gestational trophoblastic tumours)
30 exp gestational trophoblastic neoplasm
31 exp gestational trophoblastic disease
32 invasive mole
33 choriocarcinoma
34 gestational trophoblastic tumo$
35 gestational trophoblastic disease
36 gestational trophoblastic neoplasm$
37 hydatidiform mole
38 persistent trophoblastic disease
39 GTT
40 GTD
41 GTN
42 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41
Phase V (chemotherapy)
43 dt.fs
44 tu.fs
45 exp drug therapy
46 exp antineoplastic agents
47 chemo$
48 methotrexate
49 dactinomycin
50 etoposide
51 cyclophosphamide
52 cisplatin
53 vincristine
54 chlorambucil
55 doxorubicin
56 melphalan
57 hydroxyurea
58 CHAMOCA
59 EMA-CO
60 MAC
61 EMA
62 VPB
63 EMACE
64 5-FU-Adria
65 43 OR 44 OR 45 OR 46 OR 47 OR 48 OR 49 OR 50 OR 51 OR 52 OR 53 OR 54 OR 55 OR 56 OR 57 OR 58 OR 59 OR 60 OR 61 OR 62 OR 63 OR 64
Phase VI ( combining all previous phases)
66 29 AND 42 AND 65
CENTRAL search strategy
#1 GTT
#2 GTD
#3 GTN
#4 (GESTATIONAL AND TROPHOBLASTIC)AND TUMO*
#5 (GESTATIONAL AND TROPHOBLASTIC) AND DISEASE
#6 (GESTATIONAL AND TROPHOBLASTIC) AND NEOPLAS*
#7INVASIVE MOLE
#8 CHORIOCRACINOMA
#9 HYDATIDIFORM
#10 PERSISTENT TROPHOBLASTIC DISEASE
#11 (OR/ #1-#10)
#12 METHOTREXATE
#13 DACTINOMYCIN
#14 ETOPSIDE
#15 CYCLOPHOSPHAMIDE
#16 CISPLATIN
#17 VINCRISTINE
#18 CHLORAMBUCIL
#19 DOXORUBICIN
#20 MELPHALAN
#21 HYDROXYUREA
#22 CHAMOCA
#23 EMA-CO
#24 MAC
#25 EMA
#26 VPB
#27 EMACE
#28 5-FU-ADRIA
#29 CHEMO*
#30 THERAPY
#31 TREATMENT
#32 (OR/ #12-#31)
#33 (#11 AND #32)
EMBASE search strategy
Study identification
#1 Clinical trial/
#2 Randomized controlled trials/
#3 Random Allocation/
#4 Single-Blind Method/
#5 Double-Blind Method/
#6 Cross-Over Studies/
#7 Placebos/
#8 Randomi?ed controlled trial$.tw.
#9 RCT.tw.
#10 Random allocation.tw.
#11 Randomly allocated.tw.
#12 Allocated randomly.tw.
#13 (allocated adj2 random).tw.
#14 Single blind$.tw.
#15 Double blind$.tw.
#16 ((treble or triple) adj blind$).tw.
#17 Placebo$.tw.
#18 Prospective Studies/
#19 or/1-18
#20 Case study/
#21 Case report.tw.
#22 Abstract report/ or letter/
#23 or/20-22
#24 19 not 23
#25 animal/
#26 human/
#27 25 not 26
#28 24 not 27
Location of gestational trophoblastic tumours
#29 exp trophoblastic tumours
#30 exp trophoblastic disease
#31 invasive mole
#32 choriocarcinoma
#33 gestational trophoblastic tumo$
#34 gestational trophoblastic disease
#35 gestational trophoblastic neoplasm$
#36 hydatidiform mole
#37 persistent trophoblastic disease
#38 GTT
#39 GTD
#40 GTN
#41 or/ 29-40
Location of chemotherapy
#42 exp cancer chemotherapy
#43 exp antineoplastic agents
#44 DT.FS
#45 TU.FS
#46 chemo*
#47 methotrexate
#48 dactinomycin
#49 etopside
#50 cyclophosphamide
#51 cisplatin
#52 vincristine
#53 chlorambucil
#54 doxorubicin
#55 melphalan
#56 hydroxyurea
#57 CHAMOCA
#58 EMA-CO
#59 MAC
#60 EMA
#61 VPB
#62 EMACE
#63 5-FU-Adria
#64 or/42-63
Combining phases
#65 #28 and #41and #64
Appendix 2. Search strategies 2012 update
CENTRAL Issue 1 2012
#1 MeSH descriptor Trophoblastic Neoplasms explode all trees
#2 (trophoblastic near/5 (cancer* or neoplas* or tumor* or tumour* or disease*))
#3 choriocarcinoma*
#4 ((hydatid* or invasive) near/5 mole*)
#5 molar near/5 pregnanc*
#6 (#1 OR #2 OR #3 OR #4 OR #5)
#7 Any MeSH descriptor with qualifier: DT
#8 MeSH descriptor Antineoplastic Agents explode all trees
#9 MeSH descriptor Antineoplastic Combined Chemotherapy Protocols explode all trees
#10 chemotherap*
#11 (methotrexate or dactinomycin or etoposide or cyclophosphamide or cisplatin or vincristine or chlorambucil or doxorubicin or melphalan or hydroxyurea or CHAMOCA or EMA or EMA-CO or MAC or VPB or EMACE or 5-FU* or 5-fluorouracil)
#12 (#7 OR #8 OR #9 OR #10 OR #11
#13 (#6 AND #12)
MEDLINE 2008 to Feb week 1 2012
exp Trophoblastic Neoplasms/
(trophoblastic adj5 (cancer* or neoplas* or tumor* or tumour* or disease*)).mp.
choriocarcinoma*.mp.
((hydatid* or invasive) adj5 mole*).mp.
(molar adj5 pregnanc*).mp.
1 or 2 or 3 or 4 or 5
drug therapy.fs.
exp Antineoplastic Agents/
Antineoplastic Combined Chemotherapy Protocols/
chemotherap*.mp.
(methotrexate or dactinomycin or etoposide or cyclophosphamide or cisplatin or vincristine or chlorambucil or doxorubicin or melphalan or hydroxyurea or CHAMOCA or EMA or EMA-CO or MAC or VPB or EMACE or 5-FU* or 5-fluorouracil).mp.
7 or 8 or 9 or 10 or 11
6 and 12
exp animals/ not humans.sh.
13 not 14
key:
mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier
EMBASE Ovid 2008 to 2012 week 06
exp trophoblastic tumor/
(trophoblastic adj5 (cancer* or neoplas* or tumor* or tumour* or disease*)).mp.
choriocarcinoma*.mp.
((hydatid* or invasive) adj5 mole*).mp.
(molar adj5 pregnanc*).mp.
1 or 2 or 3 or 4 or 5
exp chemotherapy/
exp antineoplastic agent/
chemotherap*.mp.
(methotrexate or dactinomycin or etoposide or cyclophosphamide or cisplatin or vincristine or chlorambucil or doxorubicin or melphalan or hydroxyurea or CHAMOCA or EMA or EMA-CO or MAC or VPB or EMACE or 5-FU* or 5-fluorouracil).mp.
7 or 8 or 9 or 10
6 and 11
(exp Animal/ or Nonhuman/ or exp Animal Experiment/) not Human/
12 not 13
key:
mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword
Appendix 3. Risk of bias assessment for included studies
We assessed the risk of bias of included RCTs in accordance with guidelines in the Cochrane Handbook (Higgins 2011) as follows:.
Randomisation
The method of randomisation was noted on the data extraction form. We assessed the randomisation as:
Low risk of bias: e.g. a computer-generated random sequence or a table of random numbers.
High risk of bias: e.g. date of birth, clinic id-number or surname.
Unclear risk of bias: e.g. details not reported.
Allocation concealment
We assessed the concealment of allocation sequence from treatment providers and participants as:
Low risk of bias: e.g. where the allocation sequence could not be foretold.
High risk of bias: e.g. the computer-generated random sequence was displayed so treatment providers could see which arm of the trial the next participant was assigned to, or kept in a sealed opaque envelope.
Unclear risk of bias: allocation concealment not reported.
Blinding
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes and assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
Incomplete outcome data
We recorded the proportion of participants whose outcomes were not reported at the end of the study and we noted if loss to follow-up was not reported.
We assessed methods as:
Low risk of bias, if fewer than 20% of patients were lost to follow-up and reasons for loss to follow-up were similar in both treatment arms.
High risk of bias, if more than 20% of patients were lost to follow-up or reasons for loss to follow-up differed between the treatment arms.
Unclear risk of bias if loss to follow-up was not reported.
Selective reporting
We assessed the methods of outcome reporting as:
low risk of bias (where it is clear that all of the study’s pre-specified outcomes and all expected outcomes of interest have been reported);
high risk of bias (where not all the study’s pre-specified outcomes were reported; one or more reported primary outcomes were not pre-specified; outcomes of interest were reported incompletely and so could not be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
Other bias
We described for each included study any important concerns we had about other possible sources of bias. We assessed whether each study was free of other problems that could put it at risk of bias and assessed the risk as follows:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
HISTORY
Protocol first published: Issue 2, 2008
Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 28 March 2012 | New citation required and conclusions have changed | Five records were identified from the updated search: one new study was included (Mousavi 2012); three records were added to previously included studies (Lertkhachonsuk 2009a; Rahimi-Moghaddam 2004; Osborne 2011a); one record was added to ‘Ongoing studies’ section (GOG 0275). Previously included non-RCTs (Abrao 2008; Kohorn 1996; Smith 1982; Wong 1985) were excluded. Conclusions updated. |
| 14 February 2012 | New search has been performed | Electronic database search updated rendering 964 records after de-duplication |
| 11 February 2009 | Amended | Acknowledgements amended. |
| 14 April 2008 | Amended | Converted to new review format. |
| 27 January 2008 | New citation required and major changes | Substantive amendment |
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
In the protocol and the original 2009 review, we included non-RCTs as well as RCTs. For the updated and revised review, we included only RCTs.
WHAT’S NEW
Last assessed as up-to-date: 30 May 2012.
| Date | Event | Description |
|---|---|---|
| 27 March 2014 | Amended | Contact details updated. |
Footnotes
DECLARATIONS OF INTEREST Ray Osborne was an primary investigator of a study that has been included in this review (Osborne 2011). He was not involved in the consideration or assessment of this study for inclusion.
References to studies included in this review
- Gilani 2005 {published data only} .Gilani MM, Yarandi F, Eftekhar Z, Hanjani P. Comparison of pulse methotrexate and pulse dactinomycin in the treatment of low-risk gestational trophoblastic neoplasia. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2005;Vol. 45(issue 2):161–4. doi: 10.1111/j.1479-828X.2005.00366.x. [DOI] [PubMed] [Google Scholar]
- Lertkhachonsuk 2009 {published and unpublished data} .Lertkhachonsuk A, Tangtrakul S, Israngura N, Wilailak S. Actinomycin D versus methotrexate-folinic acid as the treatment of stage 1, low-risk gestational trophoblastic neoplasia. Conference abstract from the International Society for the Study of Trophoblastic Disease (ISSTD) conference; 2007; [DOI] [PubMed] [Google Scholar]; *; Lertkhachonsuk AA, Israngura N, Wilailak S, Tangtrakul S. Actinomycin d versus methotrexate-folinic acid as the treatment of stage I, low-risk gestational trophoblastic neoplasia: a randomized controlled trial. International Journal of Gynecological Cancer. 2009;19(5):985–8. doi: 10.1111/IGC.0b013e3181a8333d. [DOI] [PubMed] [Google Scholar]
- Mousavi 2012 {published data only} .Mousavi A, Cheraghi F, Yarandi F, Gilani MM, Shojaei H. Comparison of pulsed actinomycin D versus 5-day methotrexate for the treatment of low-risk gestational trophoblastic disease. International Journal of Gynecology and Obstetrics. 2012;116(1):39–42. doi: 10.1016/j.ijgo.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Osborne 2011 {published and unpublished data} .Osborne R, Filiaci V, Schink J, Mannel R, Provencher D, Alvarez-Secord A, et al. A randomized phase III trial comparing weekly parenteral methotrexate and “pulsed” dactinomycin as primary management for low-risk gestational trophoblastic neoplasia: A Gynecologic Oncology Group study. Gynecologic Oncology. 2008;Vol. 108:S2–S31. doi: 10.1200/JCO.2010.30.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]; *; Osborne RJ, Filiaci V, Schink JC, Mannel RS, Alvarez Secord A, Kelley JL, et al. Phase III trial of weekly methotrexate or pulsed dactinomycin for low-risk gestational trophoblastic neoplasia: a Gynecologic Oncology Group study. Journal of Clinical Oncology. 2011;29(7):825–31. doi: 10.1200/JCO.2010.30.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarandi 2008 {published and unpublished data} .Rahimi-Moghaddam P, Eftekhar Z, Yarandi F. Single-agent therapy for low risk gestational trophoblastic tumor: a comparison between pulse-methotrexate versus pulse-actinomycin [abstract] International Journal of Gynecological Cancer. 2004;14(Suppl 1):96. [Google Scholar]; *; Yarandi F, Eftekhar Z, Shojaei H, Kanani S, Sharifi A, Hanjani P. Pulse methotrexate versus pulse actinomycin D in the treatment of low-risk gestational trophoblastic neoplasia. International Journal of Gynecology and Obstetrics. 2008;Vol. 103(issue 1):33–7. doi: 10.1016/j.ijgo.2008.05.013. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Abrao 2008 {published data only} .Abrao RA, de Andrade JM, Tiezzi DG, Marana HR, Candido dos Reis FJ, Clagnan WS. Treatment for low-risk gestational trophoblastic disease: comparison of single-agent methotrexate, dactinomycin and combination regimens. Gynecologic Oncology. 2008;Vol. 108(issue 1):149–53. doi: 10.1016/j.ygyno.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Berkowitz 1979 {published data only} .Berkowitz RS, Goldstein DP. Methotrexate with citrovorum factor rescue for nonmetastatic gestational trophoblastic neoplasms. Obstetrics and Gynecology. 1979;Vol. 54(issue 6):725–8. [PubMed] [Google Scholar]
- Gleeson 1993 {published data only} .Gleeson NC, Finan MA, Fiorica JV, Robert WS, Hoffman MS, Wilson J. Nonmetastatic gestational trophoblastic disease. Weekly methotrexate compared with 8-day methotrexate-folinic acid. European Journal of Gynaecological Oncology. 1993;Vol. 14(issue 6):461–5. [PubMed] [Google Scholar]
- Kohorn 1996 {published data only} .Kohorn EI. Decision making for chemotherapy administration in patients with low risk gestational trophoblastic neoplasia. International Journal of Gynecological Cancer. 1996;6:279–85. [Google Scholar]
- Matsui 1998 {published data only} .Matsui H, Iitsuka Y, Seki K, Sekiya S. Comparison of chemotherapies with methotrexate, VP-16 and actinomycin-D in low-risk gestational trophoblastic disease. Remission rates and drug toxicities. Gynecologic and Obstetric Investigation. 1998;Vol. 46(issue 1):5–8. doi: 10.1159/000009987. [DOI] [PubMed] [Google Scholar]
- Matsui 2005 {published data only} .Matsui H, Suzuka K, Yamazawa K, Tanaka N, Mitsuhashi A, Seki K, et al. Relapse rate of patients with low-risk gestational trophoblastic tumour initially treated with single-agent chemotherapy. Gynecologic Oncology. 2005;Vol. 96(issue 3):616–20. doi: 10.1016/j.ygyno.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Petrilli 1980 {published data only} .Petrilli ES, Morrow CP. Actinomycin D toxicity in the treatment of trophoblastic disease. Gynecologic Oncology. 1980;9:18–22. doi: 10.1016/0090-8258(80)90004-9. [DOI] [PubMed] [Google Scholar]
- Roberts 1996 {published data only} .Roberts JP, Lurain JR. Treatment of low-risk metastatic gestational trophoblastic tumors with single-agent chemotherapy. American Journal of Obstetrics and Gynecology. 1996;Vol. 174(issue 6):1917–23. doi: 10.1016/s0002-9378(96)70229-6. [DOI] [PubMed] [Google Scholar]
- Smith 1982 {published data only} .Smith EB, Weed JC, Jr, Tyrey L, Hammond CB. Treatment of nonmetastatic gestational trophoblastic disease: results of methotrexate alone versus methotrexate--folinic acid. American Journal of Obstetrics and Gynecology. 1982;144(1):88–92. doi: 10.1016/0002-9378(82)90400-8. [DOI] [PubMed] [Google Scholar]
- Wong 1985 {published data only} .Wong LC, Choo YC, Ma HK. Methotrexate with citrovorum factor rescue in gestational trophoblastic disease. American Journal of Obstetrics and Gynecology. 1985;Vol. 152(issue 1):59–62. doi: 10.1016/s0002-9378(85)80178-2. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- GOG 0275 {published data only} .Schink JC, DiSaia PJ, the Gynecologic Oncology Group [14 Feb, 2012];Methotrexate or dactinomycin in treating patients with low-risk gestational trophoblastic neoplasia. www.clinicaltrials.gov/ct2/show/NCT01535053.
Additional references
- Aghajanian 2011 .Aghajanian C. Treatment of low-risk gestational trophoblastic neoplasia. Journal of Clinical Oncology. 2011;29(7):786–8. doi: 10.1200/JCO.2010.31.0151. [DOI] [PubMed] [Google Scholar]
- Alazzam 2011 .Alazzam M, Young T, Coleman R, Hancock B, Drew D, Wilson P, et al. Predicting gestational trophoblastic neoplasia (GTN): is urine hCG the answer? Gynecologic Oncology. 2011;122(3):595–9. doi: 10.1016/j.ygyno.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Andrijono 2010 .Andrijono A, Muhilal M. Prevention of post-mole malignant trophoblastic disease with vitamin A. Asian Pacific Journal of Cancer Prevention. 2010;11(2):567–70. [PubMed] [Google Scholar]
- Bagshawe 1976 .Bagshawe KD. Risk and prognostic factors in trophoblastic neoplasia. Cancer. 1976;38(3):1373–85. doi: 10.1002/1097-0142(197609)38:3<1373::aid-cncr2820380342>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Bagshawe 1989 .Bagshawe KD, Dent J, Newlands ES, Begent RH, Rustin GJ. The role of low-dose methotrexate and folinic acid in gestational trophoblastic tumours (GTT) British Journal of Obstetrics and Gynaecology. 1989;96(7):795–802. doi: 10.1111/j.1471-0528.1989.tb03318.x. [DOI] [PubMed] [Google Scholar]
- Covens 2006 .Covens A, Fialici VL, Burger RA, Osborne R, Chen MD. Phase II trial of pulse dactinomycin as salvage therapy for failed low-risk gestational trophoblastic neoplasia. Cancer. 2006;107(6):1280–6. doi: 10.1002/cncr.22118. [DOI] [PubMed] [Google Scholar]
- CTCAE 2010 .Common Terminology Criteria for Adverse Events (CTCAE) Vol. V4.0. National Cancer Institute; [June 14, 2010]. available at: http://evs.nci.nih.gov/ftp1/CTCAE/About.html. [Google Scholar]
- Deeks 2001 .Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors. Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd edition BMJ Publication Group; London: 2001. [Google Scholar]
- DerSimonian 1986 .DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Elit 1994 .Elit L, Covens A, Osborne R, Gerulath A, Murphy J, Rosen B, et al. High-dose methotrexate for gestational trophoblastic disease. Gyncologic Oncology. 1994;54(3):282–7. doi: 10.1006/gyno.1994.1211. [DOI] [PubMed] [Google Scholar]
- FIGO 2009 .FIGO Committee on Gynecologic Oncology Current FIGO staging for cancer of the vagina, fallopian tube, ovary and gestational trophoblastic neoplasia. International Journal of Gynaecology and Obstetrics. 2009;105:3–4. doi: 10.1016/j.ijgo.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Fisher 2009 .Fisher R. Genetics. In: Hancock BW, Seckl M, Berkowitz RS, Cole LA, editors. Gestational trophoblastic disease. International Society for the Study of Trophoblastic Diseases. 3rd Edition ISSTD; 2009. [Google Scholar]
- Garrett 2002 .Garrett AP, Garner EO, Goldstein DP, Berkowitz RS. Methotrexate infusion and folinic acid as primary therapy for nonmetastatic and low-risk metastatic gestational trophoblastic tumors. 15 years of experience. Journal of Reproductive Medicine. 2002;47(5):355–62. [PubMed] [Google Scholar]
- Goldstein 1972 .Goldstein DP, Winig P, Shirley RL. Actinomycin D as initial therapy of gestational trophoblastic disease. A reevaluation. Obstetrics & Gynecology. 1972;39(3):341–5. [PubMed] [Google Scholar]
- Hammond 1973 .Hammond CB, Borchert LG, Tyrey L, Creaman WT, Parker RT. Treatment of metastatic trophoblastic disease: good and poor prognosis. American Journal of Obstetrics & Gynecology. 1973;115(4):451–7. doi: 10.1016/0002-9378(73)90389-x. [DOI] [PubMed] [Google Scholar]
- Hertz 1956 .Hertz R, Li MC, Spencer DB. Effect of methotrexate therapy upon choriocarcinoma and chorioadenoma. Proceedings of the Society of Experimental Biology and Medicine. 1956;93(2):361–6. doi: 10.3181/00379727-93-22757. [DOI] [PubMed] [Google Scholar]
- Higgins 2003 .Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2011 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. 5.1. The Cochrane Collaboration; [updated March 2011]. 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- Hitchins 1988 .Hitchins RN, Holden L, Newlands ES, Begent RH, Rustin GJ, Bagshawe KD. Single agent etoposide in gestational trophoblastic tumours. Experience at Charing Cross Hospital 1978-1987. European Journal of Cancer and Clinical Oncology. 1988;24(6):1041–6. doi: 10.1016/0277-5379(88)90156-3. [DOI] [PubMed] [Google Scholar]
- Homesley 1988 .Homeseley HD, Blessing JA, Rettenmaier M, Capizzi RL, Major FJ, Twiggs LB. Weekly intramuscular methotrexate for nonmetastatic gestational trophoblastic disease. Obstetrics and Gynecology. 1988;72(3):413–8. [PubMed] [Google Scholar]
- Homesley 1990 .Homesley HD, Blessing JA, Schlaerth J, Rettenmaier M, Major FJ. Rapid escalation of weekly intramuscular methotrexate for nonmetastatic gestational trophoblastic disease: a Gynecologic Oncology Group study. Gynecologic Oncology. 1990;39(3):305–8. doi: 10.1016/0090-8258(90)90257-l. [DOI] [PubMed] [Google Scholar]
- Khan 2003 .Khan F, Everard J, Ahmed S, Coleman RE, Aitkin M, Hancock BW. Low-risk persistent trophoblastic disease treated with low-dose methotrexate: efficacy, acute and long term effects. British Journal of Cancer. 2003;89(12):2197–201. doi: 10.1038/sj.bjc.6601422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn 2009 .Kohorn EI. Hancock BW, Seckl MJ, Berkowitz RS, Cole LA, editors. Chapter 7: Gestational Trophoblastic Neoplasia. The FIGO 2000 staging and risk factor scoring system for gestational trophoblastic neoplasia: a critical analysis. (3rd Edition) 2009 www.isstd.org.
- Lee 2009 .Lee C, Smith HO, Kim SJ. Chapter 3: Gestational Trophoblastic Diseases. In: Hancock BW, Newlands ES, Berkowitz RS, Cole LA, editors. Epidemiology. 3rd Edition International Society for the Study of Trophoblastic Diseases; 2009. pp. 49–96. [Google Scholar]
- Lertkhachonsuk 2009a .Lertkhachonsuk AA, Israngura N, Wilailak S, Tangtrakul S. Actinomycin d versus methotrexate-folinic acid as the treatment of stage I, low-risk gestational trophoblastic neoplasia: a randomized controlled trial. International Journal of Gynecological Cancer. 2009;19(5):985–8. doi: 10.1111/IGC.0b013e3181a8333d. [DOI] [PubMed] [Google Scholar]
- Lurain 1995 .Lurain JR, Elfstrand EP. Single-agent methotrexate chemotherapy for the treatment of nonmetastatic gestational trophoblastic tumors. American Journal of Obstetrics and Gynaecology. 1995;172:574–9. doi: 10.1016/0002-9378(95)90575-8. [DOI] [PubMed] [Google Scholar]
- Lurain 2010 .Lurain JR. Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. American Journal of Obstetrics & Gynecology. 2010 Dec;:531–9. doi: 10.1016/j.ajog.2010.06.073. [DOI] [PubMed] [Google Scholar]
- Lurain 2011 .Lurain JR. Gestational trophoblastic disease II: classification and management of gestational trophoblastic neoplasia. American Journal of Obstetrics & Gynecology. 2011 Jan;:11–18. doi: 10.1016/j.ajog.2010.06.072. [DOI] [PubMed] [Google Scholar]
- McGrath 2010 .Mcgrath S, Short D, Harvey R, Schmid P, Savage PM, Seckl MJ. The management and outcome of women with post-hydatidiform mole ‘low-risk’ gestational trophoblastic neoplasia, but hCG levels in excess of 100 000 IUI−1. British Journal of Cancer. 2010;102:810–4. doi: 10.1038/sj.bjc.6605529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeish 2002 .McNeish IA, Strickland S, Holden L, Rustin GJ, Foskett M, Seckl MJ, et al. Low-risk persistent gestational trophoblastic disease: outcome after initial treatment with low-dose methotrexate and folinic acid from 1992 to 2000. Journal of Clinical Oncology. 2002;20(7):1838–44. doi: 10.1200/JCO.2002.07.166. [DOI] [PubMed] [Google Scholar]
- Nagan 2002 .Nagan HY. The FIGO staging for gestational trophoblastic neoplasia 2000, FIGO Committee Report. International Journal of Gynecology and Obstetrics. 2002;77:285–7. doi: 10.1016/s0020-7292(02)00063-2. [DOI] [PubMed] [Google Scholar]
- Osathanondh 1975 .Osathanondh R, Goldstein DP, Pastorfide GB. Actinomycin D as the primary agent for gestational trophoblastic disease. Cancer. 1975;36(3):863–6. doi: 10.1002/1097-0142(197509)36:3<863::aid-cncr2820360306>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Osborne 2011a .Osborne RJ, Filiaci V, Schink JC, Mannel RS, Alvarez Secord A, Kelley JL, et al. Phase III trial of weekly methotrexate or pulsed dactinomycin for low-risk gestational trophoblastic neoplasia: a Gynecologic Oncology Group study. Journal of Clinical Oncology. 2011;29(7):825–31. doi: 10.1200/JCO.2010.30.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli 1987 .Petrilli ES, Twiggs LB, Blessing JA, Teng NH, Curry S. Single-dose actinomycin-D treatment for nonmetastatic gestational trophoblastic disease. A prospective phase II trial of the Gynecologic Oncology Group. Cancer. 1987;60(9):2173–6. doi: 10.1002/1097-0142(19871101)60:9<2173::aid-cncr2820600910>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Rahimi-Moghaddam 2004 .Rahimi-Moghaddam P, Eftekhar Z, Yarandi F. Single-agent therapy for low risk gestational trophoblastic tumor: a comparison between pulse-methotrexate versus pulse-actinomycin [abstract] International Journal of Gynecological Cancer. 2004;14(Suppl 1):96. [Google Scholar]
- RevMan 2011 .The Nordic Cochrane Centre. The Cochrane Collaboration . Review Manager (RevMan) [Computer program] Version 5.1 The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2011. [Google Scholar]
- Rose 1989 .Rose PG, Piver MS. Alternating methotrexate and dactinomycin in nonmetastatic gestational trophoblastic disease. Journal of Surgical Oncology. 1989;41(3):148–52. doi: 10.1002/jso.2930410304. [DOI] [PubMed] [Google Scholar]
- Schlaerth 1984 .Schlaerth JB, Morrow CP, Nalick RH, Gaddis O., Jr Single-dose actinomycin D in the treatment of postmolar trophoblastic disease. Gyncologic Oncology. 1984;19(1):53–6. doi: 10.1016/0090-8258(84)90157-4. [DOI] [PubMed] [Google Scholar]
- Seckl 2009 .Seckl MJ. Presentation and management of persistent gestational trophoblastic disease (GTD) and gestational trophoblastic tumours (GTT) in the United Kingdom. In: Hancock BW, Seckl M, Berkowitz RS, Cole LA, editors. Gestational trophoblastic disease, International Society for the Study of Trophoblastic Diseases. 3rd Edition ISSTD; 2009. [Google Scholar]
- Seckl 2010 .Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet. 2010;376:717–29. doi: 10.1016/S0140-6736(10)60280-2. [DOI] [PubMed] [Google Scholar]
- Smith 1975 .Smith JP. Chemotherapy in gynecologic cancer. Clinical Obstetrics and Gynecology. 1975;65:113–6. doi: 10.1097/00003081-197512000-00009. [DOI] [PubMed] [Google Scholar]
- Song 1998 .Song HZ, Yang XY, Xiang Y. Forty-five year’s experience of the treatment of choriocarcinoma and invasive mole. International Journal of Gynaecology and Obstetrics. 1998;60(Suppl 1):S77–83. [PubMed] [Google Scholar]
- Soper 1994 .Soper JT, Clarke-Pearson DL, Berchuck A, Rodriguez G, Hammond CB. 5-day methotrexate for women with metastatic gestational trophoblastic disease. Gynecological Oncology. 1994;54(1):76–9. doi: 10.1006/gyno.1994.1169. [DOI] [PubMed] [Google Scholar]
- Su 2001 .Su WH, Wang PH, Chang SP. Successful treatment of a persistent mole with myometrial invasion by direct injection of methotrexate. European Journal of Gynaecological Oncology. 2001;22(4):283–6. [PubMed] [Google Scholar]
- Sung 1984 .Sung HC, Wu PC, Wang YB. Re-evaluation of 5-fluorouracil as a single agent for gestational malignant trophoblastic neoplasms. Advances in Experimental Medicine and Biology. 1984;176:355–67. doi: 10.1007/978-1-4684-4811-5_21. [DOI] [PubMed] [Google Scholar]
- Twiggs 1983 .Twiggs LB. Pulse actinomycin D scheduling in nonmetastatic gestational trophoblastic neoplasia: cost-effective chemotherapy. Gynecologic Oncology. 1983;16(2):190–5. doi: 10.1016/0090-8258(83)90093-8. [DOI] [PubMed] [Google Scholar]
- Wang 1998 .Wang Y, Jiu L, Guan Y, Qiu D. Chemotherapy using 5-fluorouracil and nitrocaphanum in malignant trophoblastic tumor. Gynecologic Oncology. 1998;71(3):416–9. doi: 10.1006/gyno.1998.5196. [DOI] [PubMed] [Google Scholar]
- WHO 1983 .WHO Gestatational trophoblastic diseases. WHO Technical report Sens. 1983;692 [Google Scholar]
- Wong 1984 .Wong LC, Choo YC, Ma HK. Oral etoposide in gestational trophoblastic disease. Cancer Treatment Reports. 1984;68(5):775–7. [PubMed] [Google Scholar]
- Wong 1986 .Wong LC, Choo YC, Ma HK. Primary oral etoposide therapy in gestational trophoblastic disease. An update. Cancer. 1986;58(1):14–7. doi: 10.1002/1097-0142(19860701)58:1<14::aid-cncr2820580104>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Alazzam 2009 .Alazzam M, Tidy J, Hancock B, Osborne R. First-line chemotherapy in low risk gestational trophoblastic neoplasia. Cochrane Database of Systematic Reviews. 2009;(Issue 1) doi: 10.1002/14651858.CD007102.pub2. [DOI: 10.1002/14651858.CD007102.pub2] [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study